Introduction
Among the top leading causes of death in the world,
cancer represents a major public health concern accounting for more
than 19 million new cases and nearly 10 million death cases
worldwide in 2020, according to the International Agency for
Research on Cancer (https://gco.iarc.fr/today/home accessed on Dec 25th
2021). The severity of cancer arises from the capacity of cancerous
cells, harboring oncogenic mutations, to divide chaotically, clump
to form tumors, destroy surrounding tissues and metastatically
invade distant organs. The burden of cancer is thus a major problem
in developing countries and aging populations. Fighting cancer thus
necessitates i) a thorough understanding of cancer biology and
driving intrinsic and extrinsic stimulators such as lifestyle and
environmental factors, ii) alleviated awareness of preventive
measures, iii) early detection and iv) efficient and targeted
treatments.
Glioblastoma multiforme (GBM) is a grade IV
malignant glioma and is the most frequent and aggressive brain
tumor associated with a very poor patient prognosis (1). According to the Central Brain Tumor
Registry of the United States, GBM was reported as the most
commonly occurring malignant brain tumor from 2014-2018. Among
brain and other central nervous system (CNS) tumors, GBM accounts
for 14.3% of all tumors and 49.1% of malignant tumors (2). Due to its high tumor cell
proliferative nature and neovascularization, GBM can infiltrate
crucial structures in the brain, thus increasing the possibility of
tumor recurrence even after conventional treatment (3). In 2007, the World Health Organization
(WHO) reported that the median survival rate of patients diagnosed
with GBM is significantly low, ranging between 1 to 2 years
(4). This low survival rate
suggests that more efficient treatment methods are needed to
increase the lifespan of the patients.
Massive research has been conducted in the field of
cancer therapies since the discovery of X-rays in 1896 and the
emergence of radiotherapy as a main source for cancer treatment
(5). Chemotherapy, which involves
the use of cytotoxic drugs that target proliferating cells, is the
second conventional modality widely used for cancer treatment. Both
radio- and chemotherapy are considered as a ‘two-edged sword’. This
is because these treatments are often associated with damage or
genetic modifications to normal tissues, nonspecific drug
distribution, and multidrug resistance (MDR). Progress in these
standard treatments is built on evidence-based medicine where
clinical achievements are evaluated from results of randomized
trials.
The drawbacks of conventional therapies necessitate
the identification of specific genetic modifications and molecular
signaling events responsible for tumor cell formation. This paved
the way for new therapeutic interventions that specifically target
tumors, providing an advantage over toxicity and
lack-of-specificity associated with conventional therapies.
Immunotherapy and targeted drug- and gene therapy are emerging as
the treatment breakthroughs for cancer therapy. These modalities
aim to target the patient's own immune system or genes to eliminate
cancer cells. With few exceptions, these interventions are not yet
part of standard therapy despite their advantages and the progress
made in the field (6).
Though cancer is a genetic disorder, cancer cells
hold genetic and phenotypic heterogeneity between individuals and
from one cell to another within the same tumor. In the line of this
within-tumor heterogeneity, combination therapy is now a plausible
therapeutic approach where cancer management relies on more than
one method. Improved clinical outcomes of standard cancer control
interventions and increased investment in translatable
immunotherapy and targeted therapy research would augment progress
against cancer. Consequently, treatment advancements would help
managing treatment-related adverse effects and hurdles, allowing
for an improved quality of life for cancer patient survivals. In
the present review, both conventional and new cancer therapy
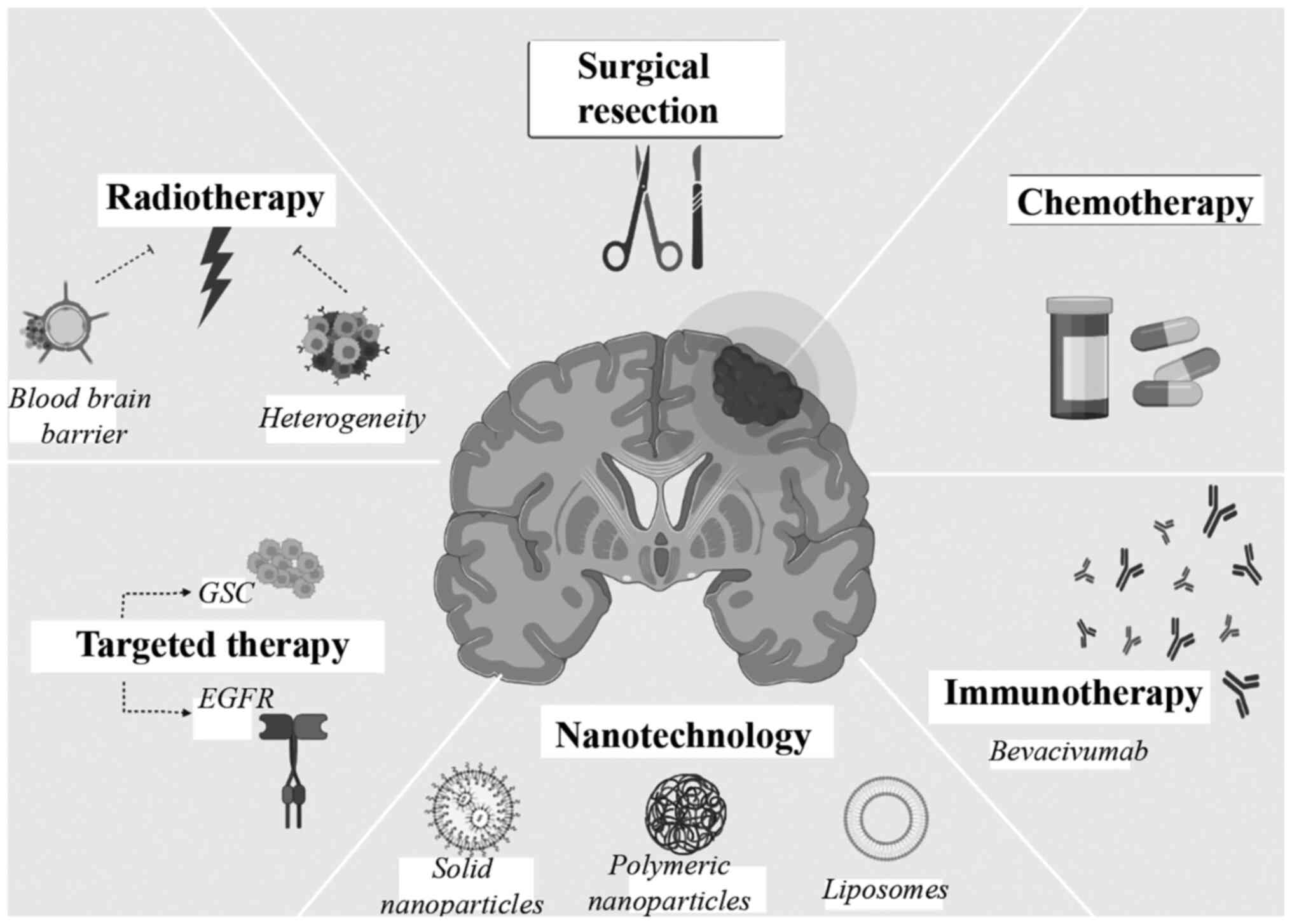
strategies were reviewed within the context of GBM (Fig. 1), and recent treatment
breakthroughs were illustrated in the light of the corresponding
potentials.
Radiotherapy (RT)
Since the discovery of X-rays in 1896, RT has
emerged as a main source for cancer treatment, either alone or in
combination with surgery and other medical treatments (5). RT aims to deliver the optimal isodose
of radiation to the target tumor volume with almost no radiation on
organs at risk (OAR). Radiation acts mainly as a tumor initiating
agent at low doses resulting in DNA modifications, whereas
radiation of higher doses relevant to RT is considered a tumor
promoter (7). For almost a
century, several technological innovations were implemented in RT
to achieve high delivery doses in tumors without exceeding OAR
tolerance dose and thus translated into medical benefit. In
addition, randomized trials aided in improving the use of RT and
altering clinical practice (8).
Starting in the 1990s, technological research benefited from the
computer assisted technology and CT-based simulations to deliver
radiation to a 3D conformal approach allowing for an improved dose
distribution (9). Later,
high-precision modern era of RT was made possible with stereotactic
RT used to target mobile tumor using image-guided technology
(10) in addition to an
image-guided RT approach which accounts for changes in patient
anatomy and tumor shape (11,12).
It is worth noting that, in certain cases, patients with GBM having
a highly immunosuppressive tumor environment do not efficiently
respond to traditional RT. For this reason, other viable therapies,
such as Proton Beam Treatment (PBT), have emerged to substitute for
traditional treatments. After the invention of particle
accelerators, proton therapy was made possible with remarkable
developments reported in the past 20 years (13). This radiation aims to reduce the
volume of OAR and irradiated normal tissues thus avoiding
radiation-induced secondary cancers (8). In a study conducted by Lee et al
(14) in 2021, combining PBT and
Tumor Treating Fields (TTFields), which uses anti-mitotic
alternating low and intermediate electric field frequency, showed
that PTB sensitizes GBM cells to TTFields. The study provided
evidence that this combined therapy could limit cell migration and
metastasis by downregulating the NFkB, MAPK and PI3K/AKT signaling
pathways (14). These technologies
paved the way for personalized therapies where different RT
machines are used according to the special types of tumors and/or
patients conditions (15). Given
technological advancements, and owing to its cost effective
(16) and curative treatment
(17), RT is considered a
conservative cancer therapy that yields improved patient care over
the past decades.
RT has long been the standard adjuvant approach for
GBM. Among the multimodal therapeutic strategies used to treat GBM,
RT remains the primary treatment approach for patients with
unresectable GBM (18). Randomized
trials by Walker et al (19)
showed a dose-dependent relationship with improved survival rates
in patients with proven malignant gliomas. The median survival of
these patients increased from 18 to 28 weeks upon the
administration of no radiation vs. 50 Gy, respectively.
Additionally, the median life span of these patients doubled when
employed with 55 Gy and increased by 2.3 times at 60 Gy when
compared with those who did not receive RT. Doses of 60 Gy or 60
Gy, plus a booster dose of 10 Gy, to whole brain were associated
with survival rates of ~35 and 31 weeks respectively in patients
with GBM (20). While the
administration of doses below 60 Gy had relatively poorer outcome,
increased radiation doses through standard fractionation increased
the risk of normal brain injury (21). In this regard, the European
Organization for Research and Treatment of Cancer recommends a
total dose of 60 Gy in 30 fractions delivered to patients with GBM
in a single-phase technique with a unique gross tumor volume. This
comprises the T1 contrast enhancement region plus a margin of 20 to
30 mm (22).
In general, and for most cancer types, second cancer
is one of the most serious sequelae of RT. RT-associated second
cancer can be also influenced by environmental factors, lifestyle
and genetic susceptibility of the host (23). Second neoplasms may develop at
various organs depending on the sites of first neoplasms and the
degree of risk differs depending on the organ (24). Additionally, one of the hallmarks
of GBM is tumor heterogeneity and its capability for local relapse
(25). Its intertumor and/or
intratumor heterogeneity feature renders it to resist standard
treatments including RT. Therefore, to improve the overall survival
rate of patients with GBM and decrease the treatment toxicity,
radiation oncology should improve the mode of delivery, define the
target in an improved way and optimize the dose of radiation
(26). Consequently, there is an
urge for mechanism-based studies of radiation carcinogenesis, in
addition to epidemiological studies (lifestyle and physiological
and heritable factors), to jointly allow for long-lasting
cancer-preventing measures.
Chemotherapy
Chemotherapy is considered the primary treatment for
cancer therapy where drugs are used to destroy cancer cells.
Chemotherapeutic drugs target growing, and dividing cells thus they
have a greater effect on tumor cells. However, they can still
damage healthy cells. Planning for chemotherapy course treatment
depends on the age and health of a patient, in addition to the
type, size and location of a tumor, and whether it has spread.
Accordingly, chemotherapy is used in different ways: i) it could be
the primary and the only treatment as in the case of cancers of the
blood or lymphatic system, ii) it could be used to shrink tumors by
slowing cancer growth prior to other cancer treatments or eliminate
any remaining cancer cells after surgery or RT (27,28).
There are numerous drugs used in chemotherapy to
treat cancer. Chemotherapeutic drugs can either damage the genetic
material of a cell affecting its growth or inhibit certain
chemicals or enzymes that the cell needs in order to divide.
Depending on their mode of action, chemotherapy drugs are
classified into four main groups; i) alkylating agents that are the
most common chemotherapeutic drugs used today (damage the DNA of
tumor cells inhibiting cell division), ii) plant alkaloids
(topoisomerase inhibitors and mitotic inhibitors that inhibit cell
division by interfering with topoisomerases and inhibiting enzymes
the cells for replication), iii) antimetabolites (mimic structures
in the DNA of tumor cells incorporating into the cellular
metabolism and change the function of enzymes disrupting cell
division), and iv) antitumor antibiotics (uncoil DNA strands of
cancer cells and prevent cell replication) (6,29,30).
Among the chemotherapeutic drugs used to treat GBM
is Temozolomide (TMZ). TMZ, a DNA alkylating agent known to induce
cell cycle arrest at G2/M, is approved by the US Food and Drug
Administration (FDA) for use in the treatment of newly diagnosed
adult GBM patients in 2005 (31).
This lipophilic agent is the current gold standard treatment for
GBM due to its ability to easily penetrate the blood brain barrier
(BBB) and to effectively target cancerous cells (32). In a study conducted by Malmström
et al (33), TMZ
chemotherapy was found to be a potential alternative to RT in
elderly and frail patients with GBM. The overall survival rate was
significantly improved in elderly patients who received TMZ alone
(32 weeks in overall patients) compared with those who received 60
Gy standard RT (24 weeks in overall patients). A Surveillance,
Epidemiology, and End Results-based study evaluated the efficacy of
chemotherapy for 25,698 patients diagnosed with GBM between 2004
and 2015 (34). In this study, the
median overall survival of the chemotherapy cohort was ~4.3 times
higher than the cohort with no chemotherapy. The survival of
elderly patients improved by chemotherapy, however, younger
patients benefited more. Tumor recurrence in patients with GBM
confers a dismal prognosis associated with low progression free
survival rate despite the treatment with conventional methods
(35). However, chemotherapy
remains the most common treatment option for GBM. Akin to TMZ,
carmustine is another alkylating agent used to treat patients with
GBM. A meta-analysis evaluating the efficacy of carmustine in newly
diagnosed and recurrent patients with GBM showed that this
nitrosourea derivative contributes favorably to both groups
(36).
There are several reasons that limit the efficacy of
chemotherapeutic treatments. These include mutational accumulations
leading to tumor heterogeneity, drug resistance, BBB, and low
selectivity, in addition to adverse and side effects (37). Chemotherapy may be associated with
numerous severe side effects that include immediate signs of
toxicity and late signs of chronic toxicity. Their intensity can be
mild (grade 1), moderate (grade 2), severe (grade 3), or
life-threatening or disabling (grade 4), according to WHO
classification. For instance, high doses of alkylating agents can
damage the bone marrow and increase the risk of developing
leukemia. High doses of antitumor antibiotics on the other hand can
damage the heart. Plant alkaloids are also accompanied with side
effects where topoisomerase inhibitors increase the chances of
developing a second cancer, while mitotic inhibitors can cause
nerve damage when delivered at high doses. Common adverse events
associated with TMZ treatment include nervous system, hematological
and vascular disorder (38).
Carmustine-based chemotherapy causes nausea, vomiting and
hematotoxicity in patients with GBM and more dreaded side effects
such as pulmonary fibrosis and severe bone marrow suppression
(35,36). Consequently, chemotherapeutic
strategies are now combined with RT or targeted modalities to
increase its efficacy or lessen its side effects.
Chemoradiation: combined conventional
treatments
Treatment of primary tumors with conventional
fractionated RT in numerous cancer sites is associated with local
failure rate, while chemotherapeutic treatment of several common
solid tumors shows a relatively poor efficacy. Therefore, the
combination of RT with chemotherapeutic cytotoxic agents started to
gain attention and the first combined chemoradiation therapy took
place in the seventies (39).
Since then, chemoradiotherapy has been considered a standard
modality for the treatment of locally advanced solid tumors in a
plenty of clinical examples. This combined therapy aims to achieve
spatial cooperation and to enhance the local response, thus
improving the survival rate.
Chemotherapy can be administered prior to
(neoadjuvant chemotherapy) or during (concurrent chemoradiation) or
after (adjuvant chemotherapy) RT. Neoadjuvant chemotherapy aims to
increase tumor shrinkage reducing the tumor volume to be irradiated
and thus sparing normal tissues (40,41).
Neoadjuvant chemotherapy has shown, clinically, meaningful survival
benefits to several cancer types. In this context, it improves the
survival rate and decreases distant metastasis in non-small cell
lung cancer (NSCLC) (42). This
method is particularly efficient in non-rapid distant metastatic
tumors such as advanced pancreatic tumor (43). It also offers an advantage in organ
preservation specifically in laryngeal-hypopharyngeal tumor
(44). In addition to its role in
improving the pathological response, neoadjuvant chemotherapy
increases chances of breast conservation in earlier stage breast
cancer patients (45). However,
this was not the case with GBM. In a randomized trial, Malmström
et al (46) in 2017 showed
that neoadjuvant TMZ treatment prior to standard RT did not have
any significant survival benefit in GBM patients ≥60 years of age,
since the median overall survival was 15.3 months for neoadjuvant
TMZ vs. 18.1 months for standard RT. Notably, in the same study, a
substantial survival benefit was observed for the anaplastic
astrocytoma subgroup.
Concurrent chemotherapy has a major role in treating
solid tumors and is usually administered with fractionated RT. It
offers enhancement in local control rate and organ preservation as
well as suppression of distant micro-metastasis (47). Adjuvant chemotherapy on the other
hand aims to improve drug delivery after decreasing the tumor
burden and can be efficient against occult metastasis, as in the
case of locally advanced nasopharyngeal carcinoma where patients
are at risk of distant metastasis (47). Both concurrent and adjuvant
treatments demonstrated effectiveness in patients newly diagnosed
with GBM. In a previous randomized, multicenter phase III trial in
patients with GBM younger than 70 years, the risk of death
decreased by 37% upon receiving concurrent treatment of TMZ with
RT, followed by adjuvant therapy with TMZ four weeks after the
initial treatment. The survival benefit of the cohort who received
chemotherapy and RT was 2.5 months with a progression free survival
rate of 10.7% compared with RT alone (1.5%) (48). Poor prognosis, tolerance to
treatment, existing conditions and the toxic effects of
conventional therapies have made it difficult to manage GBM in
elderly patients (49). In this
regard, the impact of short course RT with TMZ was assessed in
newly diagnosed elderly patients with GBM. This new chemoradiation
strategy conferred survival advantage with a 37.8% overall survival
rate at 12 months as compared with 22.2% with short course RT alone
(50). Proton beam therapy (PBT)
concurrent with chemotherapy was also evaluated for effective
treatment against GBM. In a study conducted by Mizumoto et
al (51) in 2016, the safety
and efficacy of PBT administrated with concurrent chemotherapy,
using either TMZ or nimustine hydrochloride (ACNU), was examined on
carefully selected GBM patients with similar characteristics. The
study reported 21.1 months of median survival with no significant
difference between the TMZ and ACNU groups, suggesting that the
proton therapy concurrent with TMZ or ACNU is tolerable and
promising (51). Chemoradiation
has shown potential survival benefits in patients with GBM.
However, the challenge remains to optimize the clinical outcomes by
reducing the toxic effects, minimizing death rates, and finding the
optimal combination of RT with the cytotoxic agents.
Targeted therapy
Targeted cancer therapies have been developed to
specifically target cancer cells, leaving normal cells unaffected.
Similar to chemotherapy, the targeted approach utilizes compounds
that inhibit cancer metastasis and growth. Nonetheless, targeted
treatment focuses on specific tumorigenic proteins rather than a
broad range of targets (52–54).
The two major players in targeted therapy are the small molecule
inhibitors and monoclonal antibodies. The former works by
penetrating cells, inactivating enzymes, and thus interfering with
the tumor cell growth. Therapeutic monoclonal antibodies, however,
recognize targets outside the cell and control downstream cellular
processes such as cell cycle progression and cell death (55).
Cancer cells need to overcome low nutrient- and
oxygen-availability to grow, divide, and infiltrate into
surrounding tissues. Tumors are thus dependent on angiogenesis by
which they form new blood vessels from a pre-existing vasculature
(neovascularization), or undergo vasculogenesis; de novo
formation of vessels from hematopoietic precursor cells (56,57).
Since the process of angiogenesis is regulated by vascular
endothelial growth factor (VEGF) signaling, and considering the
defining feature of GBM in neovascularization, anti-angiogenic
agents that target VEGF, or its receptors are considered as
potential therapeutic options (56,58).
Expression of VEGF and VEGF receptors (VEGFR) predicts the
aggressiveness of gliomas and is robustly expressed in malignant
gliomas such as GBM (58). The US
FDA and the National Medical Products Administration of China
approved 89 small molecule targeted antitumor drugs such as
kinases, proteasomes, epigenetic regulatory proteins and DNA damage
repair enzymes by December of 2020 (59). Cediranib (AZD2171) and sunitinib
are two receptor tyrosine kinase inhibitors actively targeting all
three VEGFRs (VEGFR-1, VEGFR-2 and VEGFR-3). Sorafenib and
cabozantinib (XL184) target only the VEGFR-2 from the VEGFR family
(59). These drugs are evaluated
on patients with recurrent GBM. Phase II clinical trials of
sunitinib and sorafenib demonstrated limited activity for patients
with recurrent GBM (NCT00923117 and NCT00597493). Cediranib on the
other hand had an encouraging phase II clinical trial (NCT00305656)
with six-month progression-free survival of 25.8% (60) which led to phase III trial
(NCT00777153) and was evaluated in combination with
chemotherapeutic lomustine.
Bevacizumab (BEV), also called Avastin, is a highly
studied humanized therapeutic monoclonal antibody. The FDA approved
the use of BEV to treat multiple cancers such as colon, lung,
kidney and cervix. In May 2009, the FDA approved the use of BEV as
a single agent treatment for patients with recurrent GBM and had
failed to respond to other treatment options (61). Since then, it has become the
standard of care for this group of patients. This recombinant
immunoglobulin (Ig) G1 monoclonal antibody works by neutralizing
VEGF-A from the circulation. Receptor signaling thus decreases
within endothelial cells leading to tumor vasculature regression
(62,63). Another anti-VEGF candidate is
aflibercept. This recombinant fusion protein has the second Ig
domain of VEGFR-1 and the third Ig domain of VEGFR-2, fused to the
constant region (Fc) of human IgG1. Aflibercept targets not only
VEGF-A but also VEGF-B and placental growth factor (64). Despite having broader targets than
BEV, a single-arm phase II clinical study (NCT00369590) revealed
that a single agent aflibercept had minimal activity in patients
with recurrent GBM with a considerably low six month
progression-free survival rate (7.7%) mainly due to toxicity issues
(65).
It is well known that ionizing radiation eradicates
tumors, mainly, through DNA damage and that tumor radiosensitivity
depends on the DNA damage repair mechanisms of tumor cells.
Elements of DNA damage repair are thus targets for cancer therapy
in attempts to intensify the therapeutic effects of RT (66). Olaparib is a small molecule
inhibitor of poly(ADP-ribose) polymerase which is an important
protein in DNA repair pathways. Olaparib was revealed to be an
effective radio-sensitizing agent in GBM and other cancer cell
lines and preclinical glioma models (66,67).
In a recent phase I trial OPARATIC (NCT01390571), Olaparib was
shown to penetrate core and margin regions of GBM at
radio-sensitizing concentrations and was reported to be safely
administered with continuous low-dose of chemotherapeutic TMZ
(68).
Targeted gene therapy
In the last three decades, gene therapy has emerged
as a promising tool of genome editing that can potentially aid in
the treatment of various diseases such as heart failure,
neurodegeneration and cancer (69). Gene therapy implicates the
correction of a defective gene by introducing a functional and
healthy version thus compensating for the abnormal/missing gene
(70). Advancements in this field
paved the way for considering gene therapy as an adjuvant treatment
to conventional chemo- and RT, allowing for a reduced dose of
standard modalities in combinatorial approaches.
Several different strategies are currently employed
for targeting tumors using gene therapy. One of the common
strategies is the delivery of different gene types expressing tumor
suppressor genes to compensate for the defective or missing gene,
or expressing suicide genes, tumor antigens and growth factors in
order to induce apoptosis or improve tumor sensitivity to
conventional therapies. Plenty of tumor suppressor genes have been
evaluated for gene therapy such as Rb and PTEN
(71), and p53 was
introduced to treat non-small cell lung carcinoma (72). TNF-related apoptosis inducing
ligand and Interleukin-24 (IL-24) are examples of apoptosis
inducers that induce apoptosis in various tumor cells with minimal
effect on normal cells (73,74).
Another strategy is the gene interference using RNA
interference (RNAi) approach to block the expression of an oncogene
and, in numerous cases, sensitize tumor cells to RT (75). In vivo and in vitro
studies showed that tumor cells treated with antisense RNA
targeting c-myc gene and K-ras suppress tumor growth
in melanoma and pancreatic cancer cells respectively (76,77).
A platform for the treatment of GBM has also been developed using
RNAi technology including small interfering (si)RNA, microRNA
(miRNA), short hairpin (sh)RNA and long non-coding (lnc)RNAs. siRNA
is used in several studies to silence the target proteins that are
overexpressed in the GBM (78).
Danhier et al (79) in 2015
targeted galectin-1 (gal-1) which is involved in the development
and progression of chemoresistance in GBM. Using anti-gal-1 and
anti-EGFR siRNA administrated via nanocapsules with TMZ,
researchers reported that the median survival rate of mice bearing
glioma cell increases (79).
Another study using anti-CD73 siRNA reported tumor volume
suppression by 60% in C6 glioma rats (80). A first-in-human early phase I
clinical trial (NCT03020017) examined the safety and efficiency of
NU-0129 drug, which is an RNAi-based spherical nucleic acids
(SNAs), in patients with recurrent glioblastoma. SNAs consist of
gold nanoparticle cores covalently conjugated with siRNA
oligonucleotides specific for the GBM oncogene Bcl2Like12
(siBcl2L12). Results showed successful enrichment of gold particle
in tumor-associated endothelium, macrophages and tumor cells, along
with decreased expression of tumor-associated Bcl2L12. The study
demonstrated the potential of SNA nanoconjugates as a
brain-penetrant precision medicine strategy for the systemic
management of GBM (81).
GBM cells display abnormal pattern of miRNAs that
may result in increased expression of oncogenes or decreased
expression of tumor suppressor genes, thus affecting various
downstream signaling pathways (78) [reviewed in (82)]. The glioma angiogenesis, for
instance, is significantly influenced by miR-26a, which is found in
glioma tissues. Additionally, serum exosomes of patients with GBM
showed overexpressed miR-21 (83).
According to a previous study conducted by Møller et al
(84), the microenvironment of
GBMs was found to overexpress 256 miRNAs while underexpressing 95
miRNAs when compared with the normal brain. Numerous miRNAs are
thus considered as possible biomarker for early diagnosis and
prognosis of GBM, and investigated for gene therapy (83,85).
Transfection of miR-34a, which is a miRNA normally downregulated in
GBM tumor microenvironment (TME), restores normal physiological
conditions by inhibiting cell proliferation and invasion, and
suppressing tumoral survival in glioma cell lines and glioma
xenograft model (86). miR-21 and
miR-10b, however, are usually upregulated in GBM cells and exhibit
oncogenic properties. In an in vitro model, the inhibition
of these miRNAs with synthetic anti-miRNA counterparts prevents
cell cycle progression (87).
shRNAs are artificially generated RNA molecules and
contain hair-pin-like structures intended to silence target mRNAs.
Viel et al (88) in 2013
used shRNA to target
O6-Methylguanine-DNA-methyltransferase (MGMT), a DNA
repair enzyme. Treatment of GBM xenografts with anti-MGMT shRNA and
TMZ results in a significant decrease in tumor size (88). Another study showed that knocking
down cyclin D1 by shRNA results in the inhibition of cell
proliferation and migration and induces apoptosis in GBM cell lines
(89). Moreover, Song et al
(90) in 2016 used Sox2-shRNA to
downregulate Sox2, a gene associated with stemness of CD133
positive GBM cells. The aforementioned study reported that
Sox2-shRNA abrogates tumor initiation and drug resistance of these
cells, thus, suggesting that RNAi technology could help decreasing
GBM cell resistant to anti-GBM treatments.
GBM cells also express aberrant expressions of
lncRNAs associated with pluripotency and tumorigenesis. Akin to
miRNAs, several lncRNAs are downregulated while others are
upregulated in GBM cells compared with normal human brain tissue
(78). Thus, numerous lncRNAs are
suggested as biomarkers for GBM tumorigenesis and chemoresistance.
For instance, Zhang et al (91) in 2019 provided evidence that
knockdown of lncSBF2-AS1 decreases chemoresistance of GBM cell
lines to TMZ. These expression patterns of lncRNA revealed a
relationship between tumor histopathological differentiation and
malignancy grade that could be effectively used to treat GBM
(91).
Achieving an effective delivery to the
intra-cerebral tumors is the main difficulty in treating GBM. Due
to the degradation of therapeutic active molecules, the development
and marketing of various nucleic acid-based therapies into clinical
trials have been hampered by the uncertainty of effective delivery,
therapeutic efficacy and toxicity profile. As enormous research is
conducted to investigate the safety and efficacy of gene therapy,
delivery tools are thus acquiring a great research focus for more
efficient GBM treatment (discussed in ‘Novel Approaches’
section).
Targeting glioblastoma stem cells
In addition to the highly invasive nature of
glioblastomas and the presence of BBB, the presence of cancer stem
cells is another factor that limits clinical options and treatments
of GBMs (92). Glioblastoma stem
cells (GSCs), which are self-autonomous units, play a significant
role in tumor initiation and growth as well as therapeutic
resistance (93). Their prolonged
proliferation and ability to metastasize and suppress
anti-inflammatory responses are among the most significant
mechanisms by which GSCs contribute to tumor malignancy (94). Enriched understanding of GCS
biology paved the way for numerous studies that are now
being conducted to target GSC. In the present review, major aspects
of GSCs recognized as hot target for improved GBM treatment
were discussed.
GSCs are affected by differentiated glioblastoma
cells (DGC) due to a reciprocal signaling pathway. DGCs express
brain-derived neurotrophic factor (BDNF), whereas GSCs express the
BDNF receptor NTRK2 which accelerates GSC tumor growth (94). It is thus important to understand
the reciprocal signaling between GSCs and DGCs for improved
targeted cancer treatment. BDNF induces nerve growth factor (VGF)
expression in GSCs through the NTRK2-PI3K-AKT signaling pathway.
VGF promotes GSC growth and self-renewal and further secretion of
BDNF by DGC for its survival. Thus, BDNF-NTRK2-VGF paracrine
signaling pathway enhances the cooperation between DGC and GSC in
promoting tumor growth (94).
Several studies have thus proposed NTRK and VGF as potential
therapeutic targets for GBM treatment (94,95).
CD133 positive GSCs play a significant role in
maintaining and proliferating GBM (96). Eliminating CD133 positive stem
cells is a method that has proven to increase the survival rate in
patients with GBM. Depleting CD133 can be achieved by numerous
methods (97). Knocking down BMI1
gene, an oncogene that is involved in the regulation of
self-renewal and differentiation of stem cells, may destroy CD133
stem cells (98). Targeting these
stem cells is thus another promising target for GBM therapy.
Another GCS target is the mitochondria that plays an important role
in tumorigenesis and tumor progression, particularly through
oxidative phosphorylation (OXPHOS), which is the major source of
ATP in numerous types of cancers (99). Among GBM cells, GSCs depend on
OXPHOS which requires mitochondrial translation (100). Blocking mitochondrial translation
by a bacterial antibiotic quinupristin/dalfopristin (Q/D) does not
only suppress the growth of the GSCs, but also dysregulates cell
cycle and promotes apoptosis (100). These findings propose that
inhibiting mitochondrial translation may be investigated to
therapeutically inhibit GSC growth and that Q/D may be considered
for the treatment of GBM.
One promising investigational drug is salinomycin
that preferentially eliminates GSCs and other varieties of CSCs.
Salinomycin and its derivatives are currently extensively
investigated as an anti-CSCs treatment for numerous types of
malignancies, however, clinical trials evaluating the potential
efficacy of salinomycin in glioblastoma have not yet been published
(101). Collectively, these
findings suggested that targeting GSCs represents a promising
strategy for improved treatment of GBM.
To ensure targeted therapy success, the following
key steps should be taken into consideration: identification of
targets, identification and development of target-specific small
molecules and antibodies followed by pre-clinical studies and
clinical trials. Current major challenges in this field include the
development of drug resistance and low efficiency to drugs. To
date, except for BEV, targeted therapies in GBM patients have not
provided significant survival benefits (59,102).
Immunotherapy
Considered the ‘fifth pillar’ of cancer therapy
following surgery, chemotherapy, RT, and targeted therapy,
immunotherapy revolutionized the field of oncology (103). Immune cells of the adaptive and
innate immune systems can modulate tumor progression and are thus
the cellular underpinnings of immunotherapy. Therefore, cancer
immunotherapy employs the immune system by boosting natural
defenses to eliminate malignant cells (104). Since 1868, several categories of
immunotherapies were developed, including cancer vaccines, cytokine
therapies, adoptive cell therapies, and immune checkpoint
inhibitors (104). These forms
showed promising clinical responses in numerous aggressive tumors
such as NSCLC, melanoma, renal cancer, and several hematological
malignancies. Nevertheless, to date, no immunotherapies have been
approved by the FDA for GBM, bringing this type of tumor to the
forefront of immunotherapy research (105). Immunotherapy strategies in
clinical phases II and III are summarized in Table I.
 | Table I.Summary of immunotherapy strategies
in clinical phases II and III. |
Table I.
Summary of immunotherapy strategies
in clinical phases II and III.
| Therapeutic
agent | Therapeutic
strategy | Recruitment
status | Status |
|---|
| ADCTA | ADCTA vaccine with
Bevacizumab as a standard therapy vs. standard therapy alone | Recruiting | Phase III
NCT04277221 |
| Dendritic cell
immunization | Immunization with
DCs after finalizing radiotherapy and concomitant temozolomide | Recruiting | Phase II and III
NCT03548571 |
| Ipilimumab and
Nivolumab | Ipilimumab and
Nivolumab vs. Temozolomide | Recruiting | Phase II and III
NCT04396860 |
| Temozolomide | Temozolomide Plus
Radiation Therapy Combined with Nivolumab or Placebo | Active, not
recruiting | Phase III
NCT02667587 |
Cancer vaccines
To prevent tumor growth and eradicate tumor cells in
patients with cancer, cancer vaccines are intensively studied as a
promising therapeutic approach. This method activates the humoral
and cellular immunity of patients with cancer (106). To achieve a favorable clinical
efficacy, a critical step of cancer vaccine design is antigen
selection. An ideal antigen for a cancer vaccine should be
specifically expressed and present on all cancer cells-but not in
normal cells-, highly immunogenic and essential for cancer cell
survival (107). Consequently,
the two major classes of antigens employed in cancer vaccine design
include tumor-associated antigens (TAA) and tumor-specific antigens
(TSA). TAAs are abnormally overexpressed self-antigens derived from
tumor cells. However, they may be expressed as well in a subset of
normal cells at certain level (108). Differentiation antigens, such as
Melan A and CD19, and overexpressed antigens such as HER2 and TROP2
are examples of TAAs (109).
Utilizing these antigens may potentially induce autoimmunity
against normal tissues and possibly skip T cell recognition, since
these antigens are typically deleted from the immune repertoire by
central and peripheral tolerance mechanisms (107). TSAs, on the other hand, are
exclusively expressed by cancer cells and are not present in normal
host cells. This reduces the risk of autoimmune destruction and
strongly activates high-affinity antibodies and T cells against the
non-self-antigens (110). TSAs
include onco-viral antigens, such as E6 and E7 of the human
papillomavirus, private neoantigens, and shared neoantigens such as
KRAS and p53 (109). To destroy
tumor cells, cancer vaccines employ the following mechanism of
action. After antigen delivery, tumor antigens are taken up and
processed by antigen-presenting cells (APCs) such as dendritic
cells (DC). DCs present the antigens to major histocompatibility
complex (MHC) class I and II molecules (104). Next, these cells migrate to the
vaccine draining lymph nodes (primary site for T cell priming), to
recruit and activate immune cells by presenting relevant antigens
on MHC I and MHC II to CD8+ and CD4+ T cells,
respectively (106). Activated T
lymphocytes differentiate into memory T cells and effector T cells.
Effector T cells migrate to the TME, recognize tumor cells, and
destroy them through exploiting multiple mechanisms. Cytotoxic T
cells can eliminate cancer cells by releasing cytotoxic particles
such as perforin and granzymes. Furthermore, they can induce cancer
cell apoptosis through direct cell to cell-mediated interactions.
Additionally, mediators such as interferon-γ (IFN-γ) and tumor
necrosis factor-α (TNF-α) induce cytotoxicity (111). Besides T cells, B lymphocytes and
cells of the innate immune system such as natural killer (NK) cells
and macrophages promote tumor eradication (111). Peptide-based, DC-based and
personalized vaccines are being explored as potential vaccine
therapies in GBM (112,113).
Multiple DC based vaccines are under investigation
for the treatment of GBM. One notable DC vaccine is the
DCVax®-L developed by Northwest Biotherapeutics. Newly
diagnosed patients with GBM in phase I and II clinical trials who
received the vaccine and the standard of care treatment had an
overall survival of 36 months and almost 24 months of progression
free survival with no major side effects (114). An ongoing randomized,
placebo-controlled phase III clinical trial (NCT00045968) involves
348 eligible newly diagnosed GBM participants. The primary
objective of the study is to compare the overall survival between
patients receiving the vaccine and the control group receiving the
standard of care treatment. The treatment cohort will receive two
intradermal injections per treatment of DCVax®-L vaccine
at days 0, 10, 20 and at weeks 8, 16, 32, 48, 72, 96 and 120.
Another cancer vaccine that represents a silver
lining for GBM immunotherapy is ICT-107, an autologous DC vaccine.
Immune cells are pulsed with six synthetic peptide epitopes that
recognize and target glioma TAA and stem cell-associated antigens
such as HER-2, MAGE-1, AIM-2, TRP2, gp100, and IL13Rα2 expressed in
83% of tumors (115). In an
open-label, single-institution, single-arm phase I clinical trial,
newly diagnosed patients with GBM who previously received a
conventional treatment prior to vaccination had promising responses
to ICT-107 (116). The trial
achieved its primary endpoint of immunogenicity. The vaccine was
well tolerated and efficacious with a median overall survival of
38.4 months in newly diagnosed patients with GBM (116). The aforementioned study was
followed by a randomized, double blinded, placebo-controlled phase
II trial (NCT01280552). Similarly, the vaccine was well tolerated,
however, there was no significant difference in the overall
survival when compared with the control group (115). DC vaccines thus offer a favorable
immunotherapeutic strategy to treat patients with GBM.
The concept of personalized vaccination in
glioblastoma gained interest after two phase I clinical trials that
used multi-epitope-based personalized vaccine to target neoantigens
only (NCT02287428) (117) or both
neoantigens and unmutated tumor-specific antigens (NCT02149225)
(118). Keskin et al
(117) in 2019 compared
whole-exome sequencing data from the surgically removed
glioblastoma and matched normal cells in order to discover
neoantigens. By identifying the coding mutations for each patient,
they chose a pool of 7–20 peptides comprising actionable
neoepitopes anticipated to bind to the HLA class I molecules with
high affinity. Results reported neoantigen-specific CD4+
and CD8+ T cell responses migrating into an intracranial
glioblastoma tumor with increased number of infiltrating T cells
(117). On the other hand, and in
order to maximize the amount of actionable epitopes, Hilf et
al (118) in 2019 targeted
unmutated tumor-specific antigens in addition to neoantigens. For
each patient, unmutated antigens were chosen from a common pool of
HLA-bound peptides that were specific to glioblastoma and based on
unique HLA immunopeptidome data and pre-vaccination T cell
reactivity of patients. Vaccination plan involved an actively
personalized vaccine of 9 unmutated peptides (APVAC1) followed by a
20-peptide pool that targeted neoantigens (APVAC2). Researchers
reported results similar to those aforementioned by Keskin et
al, with 29 month median OS (118). Another phase II clinical trial is
testing personalized cancer vaccine AV-GBM-1 (developed by AIVITA
Biomedical Inc.) based on autologous DCs full of autologous tumor
neoantigens (NCT03400917). These neoantigens are derived from
tumor-initiating cells following standard surgical resection. The
aforementioned study reported significantly improved
progression-free survival (PFS) in 57 participants (119). These studies along with others
demonstrated the positive effect of immunization using vaccines in
patients with GBM.
Cytokine therapies
Cytokine therapy is considered an important
immunotherapeutic approach to activate the immune system of cancer
patients, recognize cancer cells and destroy them (120). As molecular messengers of the
innate and adaptive immunity, these regulators enable cells of the
immune system to communicate over short distances in paracrine and
autocrine manners (120). To
date, only two cytokines are approved by the FDA to treat selected
malignancies (121). Interleukin
2 (IL-2) is approved for metastatic renal cell carcinoma and
metastatic melanoma treatment, whereas Interferon-α (IFN-α) is
approved to treat hairy cell leukemia, follicular melanoma,
non-Hodgkin lymphoma and AIDS-related Kaposi's sarcoma (122). Cytokine therapies stimulate the
function, survival and proliferation of NK and T cells that mediate
immune responses against tumors. Despite their antitumor activity
in murine models and clinical treatment of selected human cancers,
several issues are associated with this therapeutic approach
(120,123). These limitations include the
short half-life of most cytokines in vivo, narrow
therapeutic windows, severe toxicity at therapeutic doses and lack
of efficacy (122,123). While certain cytokines support
the growth and invasiveness of glioma cells, others may decrease or
inhibit their growth (124).
Among the cytokines studied, IL-2, 12, 15 and 21, as well as type 1
interferons, IFN-γ and granulocyte macrophage colony stimulating
factor affect various populations of the immune cells with
antitumor properties (125). In
GBM, cytokines such as transforming growth factor-β (TGF-β), IFN-α,
IFN-γ and IL-12 have an antitumor activity decreasing the size of
the tumor, inhibiting GBM cell growth and tumorigenesis at the
early stages (124).
In a multicenter phase I dose escalation clinical
trial (NCT02026271), 31 patients with recurrent high grade glioma
were treated with Ad-RTS-hIL-12, an inducible adenoviral vector
that encodes the hIL-12 p70 transgene put under the control of the
RTS gene switch (126). The oral
administration of 20 mg of the hIL-12 activator veledimex (VDX)
showed an improved safety and tolerability profile, less severe
cytokine severe syndrome, increased but tolerable IFN-γ and tumor
infiltrating lymphocyte generation and improved median overall
survival of 12.7 months (126).
The efficacy and safety of a single tumoral injection of
Ad-RTS-hIL-12 with VDX in combination with the FDA-approved
antibody cemiplimab completed its phase II trial targeting patients
with recurrent or progressive GBM (NCT04006119). Preliminary data
revealed a tolerated outcome of the combined treatment with
elevated levels of serum cytokine and a significant increase in
circulating cytotoxic T cells (127).
The immunomodulating effects of interferons offer
another possible therapeutic potential in treating malignant
gliomas. IFN-γ, for instance, has an array of antitumor properties
such as inducing programmed cell death, inhibiting glioma
proliferation and angiogenesis, and enhancing tumor immunogenicity
(128). Thus, researchers
consider it as an immunotherapeutic option. Furthermore, a phase I
open-label trial evaluated the combination of INF-β and
conventional therapy with TMZ. Out of the 23 enrolled patients, 16
were newly diagnosed with high-grade glioma and 7 with recurrent
high-grade glioma. Newly diagnosed patients received RT,
intravenous INF-β and TMZ, and had a median overall survival time
of 17.1 months. The study outcome emphasized that the combination
therapy caused minimal toxicity (129).
The role of multiple cytokines remians under study
in regards to their clinical effectiveness to treat gliomas. The
safest and most effective dosages of the cytokines are being
delineated with some considered as a promising adjunct to other
therapies (130).
Adoptive cell therapy (ACT). ACT is an
immunotherapeutic approach that developed rapidly in the recent
years and is considered a promising and effective anticancer
treatment. This treatment method involves the isolation of
autologous T lymphocytes with antitumor activity of a cancer
patient, ex vivo expansion, and the adoptive transfer of the
amplified tumor-resident or engineered T cells back to patients
(131,132). There are three categories of ACT
used to modulate the immune system and target cancer cells: i) ACT
with tumor infiltrating lymphocytes (TIL), ii) ACT with genetically
modified peripheral blood T cells such as T cell receptor (TCR),
and iii) chimeric antigen receptor (CAR) gene therapies (133). TIL are genetically unmodified
killer lymphocytes that infiltrate the TME (134). These cells, however, can lose
their tumor eliminating ability due to immunosuppressive factors of
the TME. Accordingly, the number of specific TIL should be abundant
to enhance the efficacy of TIL therapy before intravenous adoptive
transfer into the patient (134).
T cells equipped with a TCR can recognize peptides presented on MHC
molecules and target cancer cells. Nevertheless, and to overcome
the limitation of antigen presentation, CAR-modified T cells
recognize various types of antigens regardless of their
presentation on MHC molecules (135). The aforementioned categories have
different mechanisms of action to eliminate cancer cells.
Among the three aforementioned ACT approaches, CAR-T
cell gene therapy gained ample interest in eradicating cancer
cells. These modified T cells can recognize and bind surface
expressed antigens through the single-chain variable fragment
recognition domain. After recognition, T cells mediate tumor
elimination via three different mechanisms: the perforin and
granzyme axis, cytokine secretion and Fas and Fas ligand axis
(136). Currently, 24 clinical
trials are studying the effectiveness of CAR-T cell therapy on GBM
(Accessed through Clinicaltrials.gov on July 1, 2022). Amongst these,
one is in the early phase I study, 21 in phase I study and 2
studies in phase II clinical trial. Since its discovery, multiple
CARs were developed to target GBM antigens including, but not
limited to, IL13Rα2, EGFRvIII, HER2 and CD70 (137).
To assess the feasibility, safety, anti-glioblastoma
activity and the persistence of T cells in patients, an open-label
phase I dose-escalation study tested the activity of HER2-specific
CAR-modified virus-specific T cells (VSTs) (NCT01109095). 17 HER2
positive patients with progressive recurrent GBM received the
treatment. The infusion of VSTs was safe and well tolerated with no
dose-limiting effects and the median overall survival was 24.5
months from diagnosis. It is worth mentioning that although T cells
were present in the peripheral blood, HER2-CAR VSTs did not expand
(138). Another clinical study
utilized a retroviral vector containing the CAR that recognizes
EGFRvIII tumor antigen (NCT01454596). A total of 18 patients with
malignant gliomas expressing EGFRvIII received immunotherapy to
evaluate the safety and feasibility of the T cells expressing
anti-EGFRvIII CAR. One more study evaluated the clinical benefit of
CAR-engineered autologous primary human CD8+ T
lymphocytes against IL13 receptor α2 (IL-13Rα2) in 3 patients with
recurrent GBM (NCT00730613). IL-13Rα2 is reported to be
overexpressed in more than 50% of patients with GBM. In this first
human pilot study, the intracranial administration of IL-13Rα2
specific CAR-T cells exhibited anti-glioma activity which was
promising for CAR-T cell immunotherapy (139).
The outcomes of CAR-T cell immunotherapy is
promising as it displays efficacy with minimal toxicity.
Nonetheless, limitations such as tumor heterogeneity and the
heterogonous expression of antigens as well as the function of T
lymphocytes at the sites of the tumor render it difficult to
eradicate the tumor (137).
Immune checkpoint-blocking
antibodies
Using monoclonal antibodies, researchers are able to
target cancer cells by utilizing immune checkpoint inhibitors.
These monoclonal antibodies are involved mainly to free T
lymphocytes from the negative regulation of several immune
checkpoint proteins, which are in turn involved in helping cancer
cells evade the immune system (140,141). Cytotoxic T lymphocyte-associated
protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and
programmed cell death ligand 1 (PD-L1) are highly studied targets
for inhibition (104). CTLA-4 and
PD-1 are proteins expressed on cytotoxic T cells. The former
interacts with cluster differential 80 (CD80) and induces T cell
immunosuppression. Cluster differential 28 (CD28) protein, however,
can also interact with CD80 and induce T cell activation. Similar
to CTLA-4, once PD-1 binds to PD-L1, a protein expressed on tumor
cells and APCs, it negatively regulates the T cell activation
(142). Accordingly, and to
surpass the negative regulation, immune checkpoint inhibitors are
developed. The FDA approved several immune checkpoint inhibitor
antibodies for several cancer types including, but not limited to,
melanoma, renal cell carcinoma, NSCLC, small cell lung cancer and
head and neck squamous cell cancer (143). Ipilimumab is used to target
CTLA-4, pembrolizumab, nivolumab and cemiplimab for PD-1, and
atezolizumab, avelumab and durvalumab for PD-L1 (143).
In patients with GBM, immune checkpoint inhibitors
have so far demonstrated limited activity in regard to efficacy,
safety and toxicity. In a randomized, open-label multi-institution
pilot study, the immunotherapeutic activity of pembrolizumab, an
anti-PD-1 monoclonal antibody, was examined on 35 patients with
recurrent surgically respectable GBM (NCT02337686). The
aforementioned study included 16 patients for neoadjuvant therapy
and 19 in the adjuvant group to receive only the monoclonal
antibody. Overall, pembrolizumab was well tolerated in the
neoadjuvant group with improved overall and progression-free
survival. The neoadjuvant arm had a median overall survival of 13.7
months, whereas the adjuvant arm had 7.5 months of median overall
survival. Notably, PD-1 blockade alters the gene expression profile
of the patients with a transcriptional increase in INF-γ genes,
increased T cell expression and enhanced T cell clonal expansion
(144).
Following the evaluation of nivolumab safety and
tolerability in phase I cohorts of CheckMate 143 with recurrent GBM
(145), a phase III clinical
trial was conducted. In this open-label, multicenter, randomized
phase III trial, the PD-1 blocking monoclonal antibody, nivolumab,
was compared with bevacizumab to determine its survival benefit. A
total of 369 patients were randomized to receive nivolumab or
bevacizumab after standard radiation and TMZ therapy. The CheckMate
143 phase III clinical trial (NCT02017717) did not meet its primary
endpoint. The median overall survival was almost similar with
nivolumab and bevacizumab, 9.8 months vs. 10.0 months respectively.
Likewise, the median progression free survival and
treatment-related grade 3/4 adverse events were similar among both
groups (146).
The activity of a CTLA-4 blocker, ipilimumab, was
investigated alone or in combination with other immune checkpoint
inhibitors such as nivolumab. In NCT02311920 phase I trial,
patients with newly diagnosed GBM received ipilimumab, nivolumab,
or the combination after standard chemoradiotherapy with adjuvant
TMZ. The aforementioned study reported that the treatments were
safe and tolerated, with no grade 5 adverse events (147). In patients with recurrent GBM, a
recent study showed that the intratumoral and intracavitary
administration of ipilimumab and nivolumab combination is under
phase I trial (NCT03233152) with median overall survival of 71
weeks (148).
Glioma cells have often different mechanisms to
escape immune surveillance. This is achieved by activating immune
checkpoint ligands. Accordingly, ligand inhibition is considered a
promising immunotherapeutic approach to treating GBM (137).
Novel approaches
Due to their high proliferation rate and remarkable
neovascularization, GBMs can infiltrate the basic structures of the
brain. Clinical options and drug delivery are limited due to the
presence of BBB and certain highly integrated physiological systems
in the brain (149,150). Thus, targeted delivery of gene
therapy acquired a great awareness since safety and efficiency are
two major factors of this treatment modality. Viral and non-viral
vectors have been designed to allow for optimized gene delivery and
expression. Retrovirus, adenovirus, herpes simplex virus and
adeno-associated virus are common viral vectors used in cancer gene
therapy. Despite the gene transfer efficiency supported by these
vectors, toxicity and immunogenicity of viral proteins are two big
concerns for this delivery mode (71). Thus, vector development is giving
increased focus on non-viral vectors for targeted gene delivery.
This includes the use of molecular conjugates or liposomes that can
evade clearance by the immune system. However, these delivery
methods are limited with short term gene expression for molecular
conjugates and the lack cell-specific targeting in the case of
liposomes (71,151). Targeted gene therapy was also
evaluated using nano-based carriers and their applications are
currently in development stage Phases I–III for various types of
cancer (151). In the present
review, nanotechnology and oncolytic viruses as therapeutic
delivery tools to limit the toxicity driven by combined
chemoradiotherapy were discussed.
Nanoparticles can be classified based on the type
of colloidal drug carriers from which they are constructed. These
colloidal drug carriers include liposomes, polymeric nanoparticles,
solid lipid nanoparticles (92)
(nanotechnology used in GBM clinical trials summarized in Table II). Specifically, liposomes are
similar in structure to cell membrane. This lipophilic
characteristic enables such molecules to cross the BBB.
Nanotechnology can be used in two main ways: i) Combining RT with
nanoparticles loaded with cytotoxic drugs or ii) developing
nanoparticles that can be co-loaded with cytotoxic drugs and
therapeutic radioisotopes (64Cu, 131I and 177Lu). It is thus
considered that the chemoradiotherapy nanomedicine will be
progressively applied in clinic in the following years (152). This breakthrough enables for
future studies combining nanoparticles and monoclonal antibodies to
improve the efficiency of immunotherapy by designing a new novel
combined treatment nano-immunotarget therapy (92).
 | Table II.Summary of nanotechnology used in
glioblastoma multiforme clinical trials. |
Table II.
Summary of nanotechnology used in
glioblastoma multiforme clinical trials.
| Therapeutic
agent | Therapeutic
strategy | Recruitment
status | Status |
|---|
| Polysiloxane
Gd-Chelates based | AGuIX Nanoparticles
with Radiotherapy Plus | Recruiting | Phase I and II |
| nanoparticles
(AGuIX) | Concomitant
Temozolomide |
| (randomized) |
|
|
|
| NCT04881032 |
| NU-0129 based on
Spherical | NU-0129 IV plus
standard of care tumor | Completed | Early Phase I |
| Nucleic Acid (SNA)
platform | resection within
8–48 h. |
| NCT03020017 |
| ABI-009
(nab-Rapamycin) | ABI-009
(alone) | Active, not | Phase II |
|
|
| recruiting | NCT03463265 |
|
| ABI-009 +
bevacizumab |
|
|
|
| ABI-009 +
temozolomide |
|
|
|
| ABI-009 +
lomustine |
|
|
|
| ABI-009 +
temozolomide + radiotherapy |
|
|
|
| ABI-009 +
marizomib |
|
|
| Nanoliposomal
CPT-11 | NL CPT-11 in
patients with recurrent high-grade | Completed | Phase I |
| (NL CPT-11) | gliomas:
glioblastoma multiforme (GBM), gliosarcoma (GS), anaplastic
astrocytoma (AA), anaplastic oligodendroglioma (AO), anaplastic
mixed oligoastrocytoma (AMO), or malignant astrocytoma NOS (not
otherwise specified |
| NCT00734682 |
On the other hand, oncolytic viruses are vectors of
targeted gene therapy and could be considered as a promising
strategy to treat GBM due to their ability to overcome BBB
(oncolytic viruses used in GBM clinical trials summarized in
Table III). Research conducted
by Staquicini et al (3)
examined the delivery of two different genes: Cytotoxic TNF and
theranostic Herpes simplex virus thymidine kinase (HSVtk),
delivered by an adeno-associated virus and phage (AAVP) to treat
GBM. Both AAVP constructs exhibited tumor-associated neovasculature
and induced cell death after treatment (3). Despite its promising outcomes in
treating GBM-targeted therapy, oncolytic viruses possess numerous
limitations such as poor transduction efficiency, poor penetrance
after intertumoral injections and lack of viral receptors for viral
vectors. Thus, further studies should be performed to enhance the
transduction efficiency of the transgene by vectors into tumor
areas.
 | Table III.Summary of oncolytic viruses used in
glioblastoma multiforme clinical trials. |
Table III.
Summary of oncolytic viruses used in
glioblastoma multiforme clinical trials.
| Therapeutic
agent | Therapeutic
strategy | Recruitment
status | Status |
|---|
| DNX-2440 virus | Injected during
stereotactic biopsy | Recruiting | Phase I
NCT03714334 |
| DNX-2401 virus | Single intratumoral
injection of DNX-2401 associated with Interferon-gamma (IFN-γ) | Completed | Phase I
NCT02197169 |
| H-1 parvovirus
(H-1PV) | Administered either
intratumoral or intravenously, with tumor resection after 10 days
with a subsequent administration of H-1PV into the walls of the
resection cavity | Completed | Phase I and II
NCT01301430 |
| rQNestin (a virus
modified from herpes simplex virus) | Treatment with
rQNestin34.5v.2 and Immunomodulation With Cyclophosphamide | Recruiting | Phase I
NCT03152318 |
| DNX-2401 (a
conditionally Replicative Adenovirus) | DNX-2401 with
Pembrolizumab (KEYTRUDA®) | Completed | Phase II
NCT02798406 |
| DNX2401 | Combination of
DNX-2401 with a short course of Temozolomide | Completed | Phase I
NCT01956734 |
| DNX-2401 | DNX-2401 with
therapeutic conventional surgery | Recruiting | Phase I
NCT03896568 |
| G207 (a virus
modified from herpes simplex virus | Combination G207
with a single low dose of radiation | Recruiting | Phase I
NCT03911388 |
| reovirus
(REOLYSIN®) | Injected
intralesionally | Completed | Phase I
NCT00528684 |
Despite the significant pre-clinical progress of
enhanced targeting and expression of a transgene, the
translatability of gene therapy still faces several challenges; the
safety of vectors and their specificity, regulation of transgene
expression and long-term integration in the host. Improved
understanding of vector biology for optimized gene delivery is a
key element for the implementation of this technique. The US FDA
applies regulatory considerations for the development of gene
therapy products, while clinical trial designs are restricted with
unique features of gene therapy products and safety measures
[reviewed in (153)]. Until
today, the FDA approved 20 cellular and gene therapy products and
only recently, the first cell-based gene therapy for patients with
multiple myeloma (ABECMA) has been approved (154). Future research is focusing on
optimizing vector design to minimize toxicity and exploit gene
targeting in GBM treatment. This paves the way not only for the
development of new cancer therapies, but also for the inception of
gene therapy-based vaccines and permanent cures.
Discussion
Cancer remains one of the most dreaded diseases of
the 21th century and is still spreading with increasing incidences
affecting all ages and populations. Owing to the improvements in
lifestyle, early detection and cancer treatment, cancer death rate
scored 31% drop in overall mortality in the US following a peak in
1991 through 2018 (155). Cancer
patients profited from continuous therapeutic innovations over the
past few decades, endowing increased survival and improved quality
of life. In the present review, cancer therapies between
conventional strategies and novel concepts were presented, with
particular focus on GBM cancer treatment (Fig. 1).
With the accelerated progress in designing new
methods for cancer treatments, chemotherapy and RT continue to be
the main cancer weapons despite cytotoxicity of normal tissues.
Improved survival and quality of life provided by these standard
modalities could possibly outweigh their adverse effects. For
instance, chemotherapy shows a favorable success rate treating
locally advanced and irresectable pancreatic cancer thus offering a
chance for secondary resection (156). Moreover, combined chemotherapy
and RT noticeably provides an improved control of loco-regional
tumors, thus improving the outcome after treatment and prolonging
survival in patients with locally advanced solid tumors (157). The evolution of chemoradiotherapy
relies on evidence-based medicine grounded on randomized trials to
optimize the use of chemoradiation and direct clinical practices.
Thus, medical oncology societies are urged to re-evaluate the use
of cytotoxic drugs with severe side effects, and update clinical
practices to achieve spatial cooperation and to enhance the local
response. Currently, surgical resection of the tumor, followed by
RT and TMZ is the typical treatment used for GBM (158). Cancer management relies nowadays
on more than one therapeutic modality and combination therapy in
providing improved anticancer effects and improved after-treatment
outcome. Multimodal tactics provide the advantage of targeting
multiple pathways, minimizing non-specificity and MDR caused by
chemotherapy, and improving the therapeutic ratio of radiation
using RT.
Improved comprehension of the biology of cancer
facilitated the development of novel therapeutic concepts derived
from biological types of procedures and thus opened new horizons to
enhance the standard treatment of patients with advanced cancers.
Increment advancements in this field introduced immunotherapy and
targeted drug therapy as mild approaches for cancer treatment with
the advantage of mild and well-tolerated side effects. The aim of
these approaches is to provide a specific method of treatment that
is made-to-measure the molecular properties of a tumor and the
integrity of the immune system of the patient. Well understanding
of basic tumor immunology helped designing monoclonal antibodies,
checkpoint inhibitors and cancer vaccines or cancer immunotherapy.
While numerous targeted therapies are being investigated and early
phase clinical vaccine studies show promising results, no
ground-breaking outcomes are achieved yet for GBM treatment. It is
thus considered that immune diagnosis, monitoring and follow-up
will be the new doors opened for cancer immunotherapy (159).
Animal models of glioblastoma
The enhanced understanding of the GBM biology
discussed, was in part, possible due to an expansion of murine
preclinical GBM models. Current models include glioblastoma
cell-line xenografts, patient-derived xenografts (PDX) and
genetically engineered mouse (GEM) models [reviewed in (160)]. PDX are anticipated to serve as
effective preclinical models in translational research, compared
with glioblastoma cell-line xenografts, since they retain the
genetic and histological characteristics of the parent tumor.
However, they do not entirely reflect the antitumor immunity of the
host. On the other hand, GBM GEM models allow for the
identification of specific genomic changes involved in tumor
initiation and development and can provide information about the
consequences brought on by certain mutations. GEM models can be
used to examine how the microenvironment affects tumor biology and
are thus helpful for evaluating therapeutic plans as well (160,161). Although mice have historically
been the best organism for simulating the genetic and physiological
characteristics of cancer, its translational potential has been
constrained by the substantial differences between mice and humans.
Therefore, and in order to bridge the translational gap between
murine animal studies and human clinical trials, investigations in
large-animal models of several cancer types have evolved. It is
reasoned that anatomy and physiology of large animals has an
improved applicability to human disease and could thus reliably
recapitulate human GBM. For instance, GBM can develop spontaneously
in canines, however, this event is relatively rare and it is thus
difficult to reproduce experiments (162,163). On the other hand, several
reproducible models of glioma have been successfully developed in
pigs, evolving as the most promising preclinical large-animal
models (164–167). Xenograft and genetically
engineered porcine models are thus being studied as prospective
research tools as a promising transitional step between murine
models and human clinical trials (163).
Challenges
Drug resistance and normal tissue damage are not
the only factors limiting GBM cancer treatment. The BBB and the
plasticity and heterogeneity of both GBM and TME are challenges
hindering successful GBM treatment. In GBM, the integrity and
function of the BBB is impaired resulting in a resistant barrier
known as Blood Brain Tumor Barrier (BBTB). BBTB is characterized by
overexpression of efflux transporters thus restricting drug
delivery to the brain (168–171). Additionally, the multiple
cellular states and the existence of ‘cancer stem cell likes’ add
to the complexity of the heterogeneous cancer cell populations
within the same tumor (172,173). To overcome these challenges, the
genetic, epigenetic and molecular profile of each patient should be
evaluated.
In conclusion, despite its adverse side effects,
chemotherapy remains the first line therapy for cancer, solely or
in combination with RT and surgery. However, late sequelae of these
modalities are of a big concern particularly in regard of young
cancer patients who are expected to live longer. Thus, improved
survival health and quality of life for cancer survivors are a
public health priority. Improved understanding of the molecular
aspects of a tumor initiated the emergence of biology-driven
therapeutic means and made personalized-cancer therapy possible.
Doctors can customize cancer treatment to a tumor of a patient
thanks to personalized or precision medicine. With the use of
precision medicine, clinicians can select the treatment that is
most likely to be effective based on the exact genetics of that
patient's particular cancer rather than using a ‘passe-partout’
strategy. Precision medicine is beginning to be utilized more
frequently to treat cancer patients, thanks to developments
resulting in quicker and less expensive gene sequencing. It is also
advised that physicians examine the structure of BBB to evaluate
the ability of a drug to cross the BBB before enrolling patients in
any clinical trials. Along with advanced technologies in
conventional methods, immunotherapy and targeted therapy provide a
hope for improved survival and quality of life for the patients.
Finally, the effectiveness of the GBM treatment may be improved by
using a combination therapy approach that targets both tumor cells
and cells in the surrounding TME. While healthcare is a huge
market, it is worth to note that changing the routine of cancer
therapy is not easy. It is therefore more challenging now for an
oncologist to navigate through different therapeutic options (both
single and combinatorial) to select the optimal treatment whilst
ethics are to be respected.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Department of Natural
science at the Lebanese American University.
Availability of data and materials
Not applicable.
Authors' contributions
MES conceived the review, provided resources,
finalized the writing and edited the manuscript. RN and HD co-wrote
the first draft of the manuscript. HB and AB wrote large sections
of the review and helped with the research.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taylor OG, Brzozowski JS and Skelding KA:
Glioblastoma multiforme: An overview of emerging therapeutic
targets. Front Oncol. 9:9632019. View Article : Google Scholar
|
|
2
|
Ostrom QT, Cioffi G, Waite K, Kruchko C
and Barnholtz-Sloan JS: CBTRUS statistical report: Primary brain
and other central nervous system tumors diagnosed in the United
States in 2014–2018. Neuro Oncol. 23 (Suppl 2):iii1–iii105. 2021.
View Article : Google Scholar
|
|
3
|
Staquicini FI, Smith TL, Tang FHF,
Gelovani JG, Giordano RJ, Libutti SK, Sidman RL, Cavenee WK, Arap W
and Pasqualini R: Targeted AAVP-based therapy in a mouse model of
human glioblastoma: A comparison of cytotoxic versus suicide gene
delivery strategies. Cancer Gene Ther. 27:301–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vigneswaran K, Neill S and Hadjipanayis
CG: Beyond the World Health Organization grading of infiltrating
gliomas: Advances in the molecular genetics of glioma
classification. Ann Transl Med. 3:952015.PubMed/NCBI
|
|
5
|
Roentgen WC: On a new kind of ray (first
report). Munch Med Wochenschr. 101:1237–1239. 1959.(In German).
PubMed/NCBI
|
|
6
|
Schirrmacher V: From chemotherapy to
biological therapy: A review of novel concepts to reduce the side
effects of systemic cancer treatment (Review). Int J Oncol.
54:407–419. 2019.
|
|
7
|
Newhauser WD and Durante M: Assessing the
risk of second malignancies after modern radiotherapy. Nat Rev
Cancer. 11:438–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thariat J, Hannoun-Levi JM, Sun Myint A,
Vuong T and Gérard JP: Past, present, and future of radiotherapy
for the benefit of patients. Nat Rev Clin Oncol. 10:52–60. 2013.
View Article : Google Scholar
|
|
9
|
Mohan R: Field shaping for
three-dimensional conformal radiation therapy and multileaf
collimation. Semin Radiat Oncol. 5:86–99. 1995. View Article : Google Scholar
|
|
10
|
Milano MT, Katz AW, Zhang H and Okunieff
P: Oligometastases treated with stereotactic body radiotherapy:
Long-term follow-up of prospective study. Int J Radiat Oncol Biol
Phys. 83:878–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bucci MK, Bevan A and Roach M III:
Advances in radiation therapy: Conventional to 3D, to IMRT, to 4D,
and beyond. CA Cancer J Clin. 55:117–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling CC, Yorke E and Fuks Z: From IMRT to
IGRT: Frontierland or Neverland? Radiother Oncol. 78:119–122. 2006.
View Article : Google Scholar
|
|
13
|
De Ruysscher D, Mark Lodge M, Jones B,
Brada M, Munro A, Jefferson T and Pijls-Johannesma M: Charged
particles in radiotherapy: A 5-year update of a systematic review.
Radiother Oncol. 103:5–7. 2012. View Article : Google Scholar
|
|
14
|
Lee WS, Seo SJ, Chung HK, Park JW, Kim JK
and Kim EH: Tumor-treating fields as a proton beam-sensitizer for
glioblastoma therapy. Am J Cancer Res. 11:4582–4594.
2021.PubMed/NCBI
|
|
15
|
Thwaites DI and Malicki J: Physics and
technology in ESTRO and in Radiotherapy and Oncology: Past, present
and into the 4th dimension. Radiother Oncol. 100:327–332. 2011.
View Article : Google Scholar
|
|
16
|
Borella L, Finkel S, Crapeau N, Peuvrel P,
Sauvage M, Perrier L, Lepage E, Villeminot J and Garrigues B:
Volume and costs of the hospital management of cancer in France in
1999. Bull Cancer. 89:809–821. 2002.(In French). PubMed/NCBI
|
|
17
|
Halperin EC, Wazer DE, Perez CA and Brady
LW: Perez and Brady's: Principles and practice of radiation
oncology. 6e2018.
|
|
18
|
Barani IJ and Larson DA: Radiation therapy
of glioblastoma. Current Understanding and Treatment of Gliomas.
Raizer J and Parsa A: Springer International Publishing; Cham: pp.
49–73. 2015
|
|
19
|
Walker MD, Strike TA and Sheline GE: An
analysis of dose-effect relationship in the radiotherapy of
malignant gliomas. Int J Radiat Oncol Biol Phys. 5:1725–1731. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang CH, Horton J, Schoenfeld D, Salazer
O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson JS and Tsukada Y:
Comparison of postoperative radiotherapy and combined postoperative
radiotherapy and chemotherapy in the multidisciplinary management
of malignant gliomas. A joint radiation therapy oncology group and
eastern cooperative oncology group study. Cancer. 52:997–1007.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grossman SA and Batara JF: Current
management of glioblastoma multiforme. Semin Oncol. 31:635–644.
2004. View Article : Google Scholar
|
|
22
|
Dhermain F: Radiotherapy of high-grade
gliomas: Current standards and new concepts, innovations in imaging
and radiotherapy, and new therapeutic approaches. Chin J Cancer.
33:16–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Travis LB, Demark Wahnefried W, Allan JM,
Wood ME and Ng AK: Aetiology, genetics and prevention of secondary
neoplasms in adult cancer survivors. Nat Rev Clin Oncol.
10:289–301. 2013. View Article : Google Scholar
|
|
24
|
Imaoka T, Ishii N, Kawaguchi I,
Homma-Takeda S, Doi K, Daino K, Nakanishi I, Tagami K, Kokubo T,
Morioka T, et al: Biological measures to minimize the risk of
radiotherapy-associated second cancer: A research perspective. Int
J Radiat Biol. 92:289–301. 2016. View Article : Google Scholar
|
|
25
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gzell C, Back M, Wheeler H, Bailey D and
Foote M: Radiotherapy in glioblastoma: The past, the present and
the future. Clin Oncol (R Coll Radiol). 29:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galmarini D, Galmarini CM and Galmarini
FC: Cancer chemotherapy: A critical analysis of its 60 years of
history. Crit Rev Oncol Hematol. 84:181–199. 2012. View Article : Google Scholar
|
|
28
|
DeVita VT and Chu E: A history of cancer
chemotherapy. Cancer Res. 68:8643–8653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schütte J and Seeber S:
Tumordefinitionen/remissionskriterien. Therapiekonzepte Onkologie.
Seeber S and Schütte J: Springer; Berlin: pp. 3–12. 1993,
View Article : Google Scholar
|
|
30
|
Dax SL: Antibacterial Chemotherapeutic
Agents. Springer Science & Business Media. 31–416. 1996.
|
|
31
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomar MS, Kumar A, Srivastava C and
Shrivastava A: Elucidating the mechanisms of Temozolomide
resistance in gliomas and the strategies to overcome the
resistance. Biochim Biophys Acta Rev Cancer. 1876:1886162021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malmström A, Grønberg BH, Marosi C, Stupp
R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi
ME, et al: Temozolomide versus standard 6-week radiotherapy versus
hypofractionated radiotherapy in patients older than 60 years with
glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol.
13:916–926. 2012. View Article : Google Scholar
|
|
34
|
Wen J, Chen W, Zhu Y and Zhang P: Clinical
features associated with the efficacy of chemotherapy in patients
with glioblastoma (GBM): A surveillance, epidemiology, and end
results (SEER) analysis. BMC Cancer. 21:812021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reithmeier T, Graf E, Piroth T, Trippel M,
Pinsker MO and Nikkhah G: BCNU for recurrent glioblastoma
multiforme: Efficacy, toxicity and prognostic factors. BMC Cancer.
10:302010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao ZZ, Wang ZF, Lan T, Huang WH, Zhao
YH, Ma C and Li ZQ: Carmustine as a supplementary therapeutic
option for glioblastoma: A systematic review and meta-analysis.
Front Neurol. 11:10362020. View Article : Google Scholar
|
|
37
|
Mooney J, Bernstock JD, Ilyas A, Ibrahim
A, Yamashita D, Markert JM and Nakano I: Current approaches and
challenges in the molecular therapeutic targeting of glioblastoma.
World Neurosurg. 129:90–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: A randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jaffe N, Paed D, Traggis D, Salian S and
Cassady JR: Improved outlook for Ewing's sarcoma with combination
chemotherapy (vincristine, actinomycin D and cyclophosphamide) and
radiation therapy. Cancer. 38:1925–1930. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishimura Y: Rationale for
chemoradiotherapy. Int J Clin Oncol. 9:414–420. 2004. View Article : Google Scholar
|
|
41
|
Bernier J and Bentzen SM: Altered
fractionation and combined radio-chemotherapy approaches:
Pioneering new opportunities in head and neck oncology. Eur J
Cancer. 39:560–571. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Le Chevalier T, Arriagada R, Quoix E,
Ruffie P, Martin M, Tarayre M, Lacombe-Terrier MJ, Douillard JY and
Laplanche A: Radiotherapy alone versus combined chemotherapy and
radiotherapy in nonresectable non-small-cell lung cancer: First
analysis of a randomized trial in 353 patients. J Natl Cancer Inst.
83:417–423. 1991. View Article : Google Scholar
|
|
43
|
Mukherjee S, Hurt CN, Bridgewater J, Falk
S, Cummins S, Wasan H, Crosby T, Jephcott C, Roy R, Radhakrishna G,
et al: Gemcitabine-based or capecitabine-based chemoradiotherapy
for locally advanced pancreatic cancer (SCALOP): A multicentre,
randomised, phase 2 trial. Lancet Oncol. 14:317–326. 2013.
View Article : Google Scholar
|
|
44
|
Lefebvre JL, Chevalier D, Luboinski B,
Kirkpatrick A, Collette L and Sahmoud T: Larynx preservation in
pyriform sinus cancer: Preliminary results of a European
organization for research and treatment of cancer phase III trial.
EORTC head and neck cancer cooperative group. J Natl Cancer Inst.
88:890–899. 1996. View Article : Google Scholar
|
|
45
|
Redden MH and Fuhrman GM: Neoadjuvant
chemotherapy in the treatment of breast cancer. Surg Clin North Am.
93:493–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malmström A, Poulsen HS, Grønberg BH,
Stragliotto G, Hansen S, Asklund T, Holmlund B, Łysiak M, Dowsett
J, Kristensen BW, et al: Postoperative neoadjuvant temozolomide
before radiotherapy versus standard radiotherapy in patients 60
years or younger with anaplastic astrocytoma or glioblastoma: A
randomized trial. Acta Oncol. 56:1776–1785. 2017. View Article : Google Scholar
|
|
47
|
Brunner TB: The rationale of combined
radiotherapy and chemotherapy-joint action of castor and pollux.
Best Pract Res Clin Gastroenterol. 30:515–528. 2016. View Article : Google Scholar
|
|
48
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brandes AA, Franceschi E, Tosoni A,
Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F
and Ermani M: Temozolomide concomitant and adjuvant to radiotherapy
in elderly patients with glioblastoma: Correlation with MGMT
promoter methylation status. Cancer. 115:3512–3518. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Perry JR, Laperriere N, O'Callaghan CJ,
Brandes AA, Menten J, Phillips C, Fay M, Nishikawa R, Cairncross
JG, Roa W, et al: Short-course radiation plus temozolomide in
elderly patients with glioblastoma. N Engl J Med. 376:1027–1037.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mizumoto M, Yamamoto T, Ishikawa E,
Matsuda M, Takano S, Ishikawa H, Okumura T, Sakurai H, Matsumura A
and Tsuboi K: Proton beam therapy with concurrent chemotherapy for
glioblastoma multiforme: Comparison of nimustine hydrochloride and
temozolomide. J Neurooncol. 130:165–170. 2016. View Article : Google Scholar
|
|
52
|
Al-Dimassi S, Salloum G, Saykali B, Khoury
O, Liu S, Leppla SH, Abi-Habib R and El-Sibai M: Targeting the MAP
kinase pathway in astrocytoma cells using a recombinant anthrax
lethal toxin as a way to inhibit cell motility and invasion. Int J
Oncol. 48:1913–1920. 2016. View Article : Google Scholar
|
|
53
|
Bao X, Zeng J, Huang H, Ma C, Wang L, Wang
F, Liao X and Song X: Cancer-targeted PEDF-DNA therapy for
metastatic colorectal cancer. Int J Pharm. 576:1189992020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Branford S, Hughes T, Milner A, Koelmeyer
R, Schwarer A, Arthur C, Filshie R, Moreton S, Lynch K and Taylor
K: Efficacy and safety of imatinib in patients with chronic myeloid
leukemia and complete or near-complete cytogenetic response to
interferon-alpha. Cancer. 110:801–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie YH, Chen YX and Fang JY: Comprehensive
review of targeted therapy for colorectal cancer. Signal Transduct
Target Ther. 5:222020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Robles Irizarry L, Hambardzumyan D, Nakano
I, Gladson CL and Ahluwalia MS: Therapeutic targeting of VEGF in
the treatment of glioblastoma. Expert Opin Ther Targets.
16:973–984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guarnaccia L, Navone SE, Trombetta E,
Cordiglieri C, Cherubini A, Crisà FM, Rampini P, Miozzo M, Fontana
L, Caroli M, et al: Angiogenesis in human brain tumors: Screening
of drug response through a patient-specific cell platform for
personalized therapy. Sci Rep. 8:87482018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Reardon DA, Wen PY, Desjardins A,
Batchelor TT and Vredenburgh JJ: Glioblastoma multiforme: An
emerging paradigm of anti-VEGF therapy. Expert Opin Biol Ther.
8:541–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan
T, Yang W, Tian C, Miao Z, Wang T and Yang S: Small molecules in
targeted cancer therapy: Advances, challenges, and future
perspectives. Signal Transduct Target Ther. 6:2012021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Batchelor TT, Duda DG, di Tomaso E,
Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J,
Hochberg FH, Benner T, et al: Phase II study of cediranib, an oral
pan-vascular endothelial growth factor receptor tyrosine kinase
inhibitor, in patients with recurrent glioblastoma. J Clin Oncol.
28:2817–2823. 2010. View Article : Google Scholar
|
|
61
|
Kim MM, Umemura Y and Leung D: Bevacizumab
and glioblastoma: Past, present, and future directions. Cancer J.
24:180–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Diaz RJ, Ali S, Qadir MG, De La Fuente MI,
Ivan ME and Komotar RJ: The role of bevacizumab in the treatment of
glioblastoma. J Neurooncol. 133:455–467. 2017. View Article : Google Scholar
|
|
63
|
Wong ET, Gautam S, Malchow C, Lun M, Pan E
and Brem S: Bevacizumab for recurrent glioblastoma multiforme: A
meta-analysis. J Natl Compr Canc Netw. 9:403–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ciombor KK and Berlin J: Aflibercept-a
decoy VEGF receptor. Curr Oncol Rep. 16:3682014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
de Groot JF, Lamborn KR, Chang SM, Gilbert
MR, Cloughesy TF, Aldape K, Yao J, Jackson EF, Lieberman F, Robins
HI, et al: Phase II study of aflibercept in recurrent malignant
glioma: A North American brain tumor consortium study. J Clin
Oncol. 29:2689–2695. 2011. View Article : Google Scholar
|
|
66
|
Carruthers R and Chalmers AJ: Improving
the therapeutic ratio of radiotherapy by targeting the DNA damage
response. Increasing the Therapeutic Ratio of Radiotherapy. Tofilon
PJ and Camphausen K: Springer International Publishing; Cham: pp.
1–34. 2017, View Article : Google Scholar
|
|
67
|
Chalmers AJ: Overcoming resistance of
glioblastoma to conventional cytotoxic therapies by the addition of
PARP inhibitors. Anticancer Agents Med Chem. 10:520–533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hanna C, Kurian KM, Williams K, Watts C,
Jackson A, Carruthers R, Strathdee K, Cruickshank G, Dunn L,
Erridge S, et al: Pharmacokinetics, safety, and tolerability of
olaparib and temozolomide for recurrent glioblastoma: Results of
the phase I OPARATIC trial. Neuro Oncol. 22:1840–1850. 2020.
View Article : Google Scholar
|
|
69
|
Wirth T, Parker N and Ylä-Herttuala S:
History of gene therapy. Gene. 525:162–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Friedmann T: A brief history of gene
therapy. Nat Genet. 2:93–98. 1992. View Article : Google Scholar
|
|
71
|
Das SK, Menezes ME, Bhatia S, Wang XY,
Emdad L, Sarkar D and Fisher PB: Gene therapies for cancer:
Strategies, challenges and successes. J Cell Physiol. 230:259–271.
2015. View Article : Google Scholar
|
|
72
|
Roth JA, Nguyen D, Lawrence DD, Kemp BL,
Carrasco CH, Ferson DZ, Hong WK, Komaki R, Lee JJ, Nesbitt JC, et
al: Retrovirus-mediated wild-type p53 gene transfer to tumors of
patients with lung cancer. Nat Med. 2:985–991. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Griffith TS, Stokes B, Kucaba TA, Earel JK
Jr, VanOosten RL, Brincks EL and Norian LA: TRAIL gene therapy:
From preclinical development to clinical application. Curr Gene
Ther. 9:9–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dai W, Wu J, Wang D and Wang J: Cancer
gene therapy by NF-κB-activated cancer cell-specific expression of
CRISPR/Cas9 targeting telomeres. Gene Ther. 27:266–280. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Putney SD, Brown J, Cucco C, Lee R,
Skorski T, Leonetti C, Geiser T, Calabretta B, Zupi G and Zon G:
Enhanced anti-tumor effects with microencapsulated c-myc antisense
oligonucleotide. Antisense Nucleic Acid Drug Dev. 9:451–458. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fleming JB, Shen GL, Holloway SE, Davis M
and Brekken RA: Molecular consequences of silencing mutant K-ras in
pancreatic cancer cells: Justification for K-ras-directed therapy.
Mol Cancer Res. 3:413–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Singh P, Singh A, Shah S, Vataliya J,
Mittal A and Chitkara D: RNA interference nanotherapeutics for
treatment of glioblastoma multiforme. Mol Pharm. 17:4040–4066.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Danhier F, Messaoudi K, Lemaire L, Benoit
JP and Lagarce F: Combined anti-Galectin-1 and anti-EGFR
siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide
resistance in glioblastoma: In vivo evaluation. Int J Pharm.
481:154–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Azambuja JH, Schuh RS, Michels LR,
Gelsleichter NE, Beckenkamp LR, Iser IC, Lenz GS, de Oliveira FH,
Venturin G, Greggio S, et al: Nasal administration of cationic
nanoemulsions as CD73-siRNA delivery system for glioblastoma
treatment: A new therapeutical approach. Mol Neurobiol. 57:635–649.
2020. View Article : Google Scholar
|
|
81
|
Kumthekar P, Ko CH, Paunesku T, Dixit K,
Sonabend AM, Bloch O, Tate M, Schwartz M, Zuckerman L, Lezon R, et
al: A first-in-human phase 0 clinical study of RNA
interference-based spherical nucleic acids in patients with
recurrent glioblastoma. Sci Transl Med. 13:eabb39452021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mercatelli N, Galardi S and Ciafrè SA:
MicroRNAs as multifaceted players in glioblastoma multiforme. Int
Rev Cell Mol Biol. 333:269–323. 2017. View Article : Google Scholar
|
|
83
|
Choppavarapu L and Kandi SM: Circulating
MicroRNAs as potential biomarkers in glioma: A mini-review. Endocr
Metab Immune Disord Drug Targets. 21:195–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Møller HG, Rasmussen AP, Andersen HH,
Johnsen KB, Henriksen M and Duroux M: A systematic review of
microRNA in glioblastoma multiforme: Micro-modulators in the
mesenchymal mode of migration and invasion. Mol Neurobiol.
47:131–144. 2013. View Article : Google Scholar
|
|
85
|
Sugio K, Sakurai F, Katayama K, Tashiro K,
Matsui H, Kawabata K, Kawase A, Iwaki M, Hayakawa T, Fujiwara T and
Mizuguchi H: Enhanced safety profiles of the telomerase-specific
replication-competent adenovirus by incorporation of normal
cell-specific microRNA-targeted sequences. Clin Cancer Res.
17:2807–2818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Guessous F, Zhang Y, Kofman A, Catania A,
Li Y, Schiff D, Purow B and Abounader R: microRNA-34a is tumor
suppressive in brain tumors and glioma stem cells. Cell Cycle.
9:1031–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ananta JS, Paulmurugan R and Massoud TF:
Tailored nanoparticle codelivery of antimiR-21 and antimiR-10b
augments glioblastoma cell kill by temozolomide: Toward a
‘personalized’ anti-microRNA therapy. Mol Pharm. 13:3164–3175.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Viel T, Monfared P, Schelhaas S, Fricke
IB, Kuhlmann MT, Fraefel C and Jacobs AH: Optimizing glioblastoma
temozolomide chemotherapy employing lentiviral-based anti-MGMT
shRNA technology. Mol Ther. 21:570–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang D, Dai D, Zhou M, Li Z, Wang C, Lu
Y, Li Y and Wang J: Inhibition of cyclin D1 expression in human
glioblastoma cells is associated with increased temozolomide
chemosensitivity. Cell Physiol Biochem. 51:2496–2508. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Song WS, Yang YP, Huang CS, Lu KH, Liu WH,
Wu WW, Lee YY, Lo WL, Lee SD, Chen YW, et al: Sox2, a stemness
gene, regulates tumor-initiating and drug-resistant properties in
CD133-positive glioblastoma stem cells. J Chin Med Assoc.
79:538–545. 2016. View Article : Google Scholar
|
|
91
|
Zhang Z, Yin J, Lu C, Wei Y, Zeng A and
You Y: Exosomal transfer of long non-coding RNA SBF2-AS1 enhances
chemoresistance to temozolomide in glioblastoma. J Exp Clin Cancer
Res. 38:1662019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hsu JF, Chu SM, Liao CC, Wang CJ, Wang YS,
Lai MY, Wang HC, Huang HR and Tsai MH: Nanotechnology and
nanocarrier-based drug delivery as the potential therapeutic
strategy for glioblastoma multiforme: An update. Cancers (Basel).
13:1952021. View Article : Google Scholar
|
|
93
|
Lathia JD, Heddleston JM, Venere M and
Rich JN: Deadly teamwork: Neural cancer stem cells and the tumor
microenvironment. Cell Stem Cell. 8:482–485. 2011. View Article : Google Scholar
|
|
94
|
Wang X, Prager BC, Wu Q, Kim LJY, Gimple
RC, Shi Y, Yang K, Morton AR, Zhou W, Zhu Z, et al: Reciprocal
signaling between glioblastoma stem cells and differentiated tumor
cells promotes malignant progression. Cell Stem Cell.
22:514–528.e5. 2018. View Article : Google Scholar
|
|
95
|
Ni J, Xie S, Ramkissoon SH, Luu V, Sun Y,
Bandopadhayay P, Beroukhim R, Roberts TM, Stiles CD, Segal RA, et
al: Tyrosine receptor kinase B is a drug target in astrocytomas.
Neuro Oncol. 19:22–30. 2017. View Article : Google Scholar
|
|
96
|
Yuan X, Curtin J, Xiong Y, Liu G,
Waschsmann-Hogiu S, Farkas DL, Black KL and Yu JS: Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene.
23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Brescia P, Ortensi B, Fornasari L, Levi D,
Broggi G and Pelicci G: CD133 is essential for glioblastoma stem
cell maintenance. Stem Cells. 31:857–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Carlsson SK, Brothers SP and Wahlestedt C:
Emerging treatment strategies for glioblastoma multiforme. EMBO Mol
Med. 6:1359–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Porporato PE, Filigheddu N, Pedro JMB,
Kroemer G and Galluzzi L: Mitochondrial metabolism and cancer. Cell
Res. 28:265–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sighel D, Notarangelo M, Aibara S, Re A,
Ricci G, Guida M, Soldano A, Adami V, Ambrosini C, Broso F, et al:
Inhibition of mitochondrial translation suppresses glioblastoma
stem cell growth. Cell Rep. 35:1090242021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sharifzad F, Ghavami S, Verdi J, Mardpour
S, Mollapour Sisakht M, Azizi Z, Taghikhani A, Łos MJ, Fakharian E,
Ebrahimi M and Hamidieh AA: Glioblastoma cancer stem cell biology:
Potential theranostic targets. Drug Resist Updat. 42:35–45. 2019.
View Article : Google Scholar
|
|
102
|
Bai RY, Staedtke V and Riggins GJ:
Molecular targeting of glioblastoma: Drug discovery and therapies.
Trends Mol Med. 17:301–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Oiseth SJ and Aziz MS: Cancer
immunotherapy: A brief review of the history, possibilities, and
challenges ahead. J Cancer Metastasis Treat. 3:250–261. 2017.
View Article : Google Scholar
|
|
104
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar
|
|
105
|
Yu MW and Quail DF: Immunotherapy for
glioblastoma: Current progress and challenges. Front Immunol.
12:6763012021. View Article : Google Scholar
|
|
106
|
Liu J, Fu M, Wang M, Wan D, Wei Y and Wei
X: Cancer vaccines as promising immuno-therapeutics: Platforms and
current progress. J Hematol Oncol. 15:282022. View Article : Google Scholar
|
|
107
|
Hollingsworth RE and Jansen K: Turning the
corner on therapeutic cancer vaccines. NPJ Vaccines. 4:72019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Buonaguro L and Tagliamonte M: Selecting
target antigens for cancer vaccine development. Vaccines (Basel).
8:6152020. View Article : Google Scholar
|
|
109
|
Kim CG, Sang YB, Lee JH and Chon HJ:
Combining cancer vaccines with immunotherapy: Establishing a new
immunological approach. Int J Mol Sci. 22:80352021. View Article : Google Scholar
|
|
110
|
Schietinger A, Philip M and Schreiber H:
Specificity in cancer immunotherapy. Semin Immunol. 20:276–285.
2008. View Article : Google Scholar
|
|
111
|
Rüttinger D, Winter H, van den Engel NK,
Hatz R, Jauch KW, Fox BA and Weber JS: Immunotherapy of cancer: Key
findings and commentary on the third Tegernsee conference.
Oncologist. 15:112–118. 2010. View Article : Google Scholar
|
|
112
|
Oh T, Sayegh ET, Fakurnejad S, Oyon D,
Lamano JB, DiDomenico JD, Bloch O and Parsa AT: Vaccine therapies
in malignant glioma. Curr Neurol Neurosci Rep. 15:5082015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Baratta MG: Glioblastoma is ‘hot’ for
personalized vaccines. Nat Rev Cancer. 19:1292019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Polyzoidis S and Ashkan K:
DCVax®-L-developed by northwest biotherapeutics. Hum
Vaccines Immunother. 10:3139–3145. 2014. View Article : Google Scholar
|
|
115
|
Wen PY, Reardon DA, Armstrong TS,
Phuphanich S, Aiken RD, Landolfi JC, Curry WT, Zhu JJ, Glantz M,
Peereboom DM, et al: A randomized double-blind placebo-controlled
phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed
patients with glioblastoma. Clin Cancer Res. 25:5799–5807. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Phuphanich S, Wheeler CJ, Rudnick JD,
Mazer M, Wang H, Nuño MA, Richardson JE, Fan X, Ji J, Chu RM, et
al: Phase I trial of a multi-epitope-pulsed dendritic cell vaccine
for patients with newly diagnosed glioblastoma. Cancer Immunol
Immunother. 62:125–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur
V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V,
van der Burg SH, et al: Actively personalized vaccination trial for
newly diagnosed glioblastoma. Nature. 565:240–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
AIVITA Biomedical, Inc., . AIVITA
biomedical's phase 2 glioblastoma trial shows improved progression
free survival. 2021.
|
|
120
|
Conlon KC, Miljkovic MD and Waldmann TA:
Cytokines in the treatment of cancer. J Interferon Cytokine Res.
39:6–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Rallis KS, Corrigan AE, Dadah H, George
AM, Keshwara SM, Sideris M and Szabados B: Cytokine-based cancer
immunotherapy: Challenges and opportunities for IL-10. Anticancer
Res. 41:3247–3252. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Berraondo P, Sanmamed MF, Ochoa MC,
Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME,
Ponz-Sarvise M, Castañón E and Melero I: Cytokines in clinical
cancer immunotherapy. Br J Cancer. 120:6–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xue D, Hsu E, Fu YX and Peng H:
Next-generation cytokines for cancer immunotherapy. Antib Ther.
4:123–133. 2021.PubMed/NCBI
|
|
124
|
Zhu VF, Yang J, Lebrun DG and Li M:
Understanding the role of cytokines in glioblastoma multiforme
pathogenesis. Cancer Lett. 316:139–150. 2012. View Article : Google Scholar
|
|
125
|
Chulpanova DS, Kitaeva KV, Green AR,
Rizvanov AA and Solovyeva VV: Molecular aspects and future
perspectives of cytokine-based anti-cancer immunotherapy. Front
Cell Dev Biol. 8:4022020. View Article : Google Scholar
|
|
126
|
Chiocca EA, Yu JS, Lukas RV, Solomon IH,
Ligon KL, Nakashima H, Triggs DA, Reardon DA, Wen P, Stopa BM, et
al: Regulatable interleukin-12 gene therapy in patients with
recurrent high-grade glioma: Results of a phase 1 trial. Sci Transl
Med. 11:eaaw56802019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Garcia D: Encouraging clinical data for
controlled IL-12 for the treatment of glioblastoma and DIPG.
Onco'Zine. 2020.
|
|
128
|
Kane A and Yang I: Interferon-gamma in
brain tumor immunotherapy. Neurosurg Clin N Am. 21:77–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wakabayashi T, Kayama T, Nishikawa R,
Takahashi H, Hashimoto N, Takahashi J, Aoki T, Sugiyama K, Ogura M,
Natsume A and Yoshida J: A multicenter phase I trial of combination
therapy with interferon-β and temozolomide for high-grade gliomas
(INTEGRA study): The final report. J Neurooncol. 104:573–577. 2011.
View Article : Google Scholar
|
|
130
|
Iwami K, Natsume A and Wakabayashi T:
Cytokine therapy of gliomas. Intracranial Gliomas Part III–Innov
Treat Modalities. 32:79–89. 2018. View Article : Google Scholar
|
|
131
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Redeker A and Arens R: Improving adoptive
T cell therapy: The particular role of T cell costimulation,
cytokines, and post-transfer vaccination. Front Immunol. 7:3452016.
View Article : Google Scholar
|
|
133
|
Rohaan MW, Wilgenhof S and Haanen JBAG:
Adoptive cellular therapies: The current landscape. Virchows Arch.
474:449–461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lin B, Du L, Li H, Zhu X, Cui L and Li X:
Tumor-infiltrating lymphocytes: Warriors fight against tumors
powerfully. Biomed Pharmacother. 132:1108732020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chruściel E, Urban-Wójciuk Z, Arcimowicz
Ł, Kurkowiak M, Kowalski J, Gliwiński M, Marjański T, Rzyman W,
Biernat W, Dziadziuszko R, et al: Adoptive cell therapy-harnessing
antigen-specific T cells to target solid tumours. Cancers (Basel).
12:6832020. View Article : Google Scholar
|
|
136
|
Benmebarek MR, Karches CH, Cadilha BL,
Lesch S, Endres S and Kobold S: Killing mechanisms of chimeric
antigen receptor (CAR) T cells. Int J Mol Sci. 20:12832019.
View Article : Google Scholar
|
|
137
|
Xu S, Tang L, Li X, Fan F and Liu Z:
Immunotherapy for glioma: Current management and future
application. Cancer Lett. 476:1–12. 2020. View Article : Google Scholar
|
|
138
|
Ahmed N, Brawley V, Hegde M, Bielamowicz
K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et
al: HER2-specific chimeric antigen receptor-modified virus-specific
T cells for progressive glioblastoma: A phase 1 dose-escalation
trial. JAMA Oncol. 3:1094–1101. 2017. View Article : Google Scholar
|
|
139
|
Brown CE, Badie B, Barish ME, Weng L,
Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et
al: Bioactivity and safety of IL13Rα2-redirected chimeric antigen
receptor CD8+ T cells in patients with recurrent glioblastoma. Clin
Cancer Res. 21:4062–4072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
de Mello RA, Veloso AF, Esrom Catarina P,
Nadine S and Antoniou G: Potential role of immunotherapy in
advanced non-small-cell lung cancer. OncoTargets Ther. 10:21–30.
2016. View Article : Google Scholar
|
|
141
|
Dine J, Gordon R, Shames Y, Kasler MK and
Barton-Burke M: Immune checkpoint inhibitors: An innovation in
immunotherapy for the treatment and management of patients with
cancer. Asia Pac J Oncol Nurs. 4:127–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wilky BA: Immune checkpoint inhibitors:
The linchpins of modern immunotherapy. Immunol Rev. 290:6–23. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Mpakali A and Stratikos E: The role of
antigen processing and presentation in cancer and the efficacy of
immune checkpoint inhibitor immunotherapy. Cancers (Basel).
13:1342021. View Article : Google Scholar
|
|
144
|
Cloughesy TF, Mochizuki AY, Orpilla JR,
Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA,
Sanders CM, et al: Neoadjuvant anti-PD-1 immunotherapy promotes a
survival benefit with intratumoral and systemic immune responses in
recurrent glioblastoma. Nat Med. 25:477–486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Omuro A, Vlahovic G, Lim M, Sahebjam S,
Baehring J, Cloughesy T, Voloschin A, Ramkissoon SH, Ligon KL,
Latek R, et al: Nivolumab with or without ipilimumab in patients
with recurrent glioblastoma: Results from exploratory phase I
cohorts of CheckMate 143. Neuro Oncol. 20:674–686. 2018. View Article : Google Scholar
|
|
146
|
Reardon DA, Brandes AA, Omuro A,
Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr
O, et al: Effect of nivolumab vs bevacizumab in patients with
recurrent glioblastoma: The CheckMate 143 phase 3 randomized
clinical trial. JAMA Oncol. 6:1003–1010. 2020. View Article : Google Scholar
|
|
147
|
Sloan AE, Gilbert MR, Zhang P, Aldape KD,
Wu J, Rogers LR, Wen PY, Barani IJ, Iwamoto FM, Raval RR, et al:
NRG BN002: Phase I study of checkpoint inhibitors anti-CTLA-4,
anti-PD-1, the combination in patients with newly diagnosed
glioblastoma. J Clin Oncol. 36 (15 Suppl):S20532018. View Article : Google Scholar
|
|
148
|
Schwarze JK, Duerinck J, Dufait I, Awada
G, Klein S, Fischbuch L, Seynaeve L, Vaeyens E, Rogiers A, Everaert
H, et al: A phase I clinical trial on intratumoral and
intracavitary administration of ipilimumab and nivolumab in
patients with recurrent glioblastoma. J Clin Oncol. 38 (15
Suppl):S25342020. View Article : Google Scholar
|
|
149
|
Batich KA and Sampson JH: Standard of care
and future pharmacological treatment options for malignant glioma:
An urgent need for screening and identification of novel
tumor-specific antigens. Expert Opin Pharmacother. 15:2047–2061.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Jessen NA, Munk AS, Lundgaard I and
Nedergaard M: The glymphatic system: A beginner's guide. Neurochem
Res. 40:2583–2599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zaimy MA, Saffarzadeh N, Mohammadi A,
Pourghadamyari H, Izadi P, Sarli A, Moghaddam LK, Paschepari SR,
Azizi H, Torkamandi S and Tavakkoly-Bazzaz J: New methods in the
diagnosis of cancer and gene therapy of cancer based on
nanoparticles. Cancer Gene Ther. 24:233–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhao CY, Cheng R, Yang Z and Tian ZM:
Nanotechnology for cancer therapy based on chemotherapy. Molecules.
23:8262018. View Article : Google Scholar
|
|
153
|
Husain SR, Han J, Au P, Shannon K and Puri
RK: Gene therapy for cancer: Regulatory considerations for
approval. Cancer Gene Ther. 22:554–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Research C for BE and ABECMA (idecabtagene
vicleucel). FDA; 2021
|
|
155
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Springfeld C, Jäger D, Büchler MW, Strobel
O, Hackert T, Palmer DH and Neoptolemos JP: Chemotherapy for
pancreatic cancer. Presse Med. 48:e159–e174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wilson GD, Bentzen SM and Harari PM:
Biologic basis for combining drugs with radiation. Semin Radiat
Oncol. 16:2–9. 2006. View Article : Google Scholar
|
|
158
|
Karachi A, Dastmalchi F, Mitchell DA and
Rahman M: Temozolomide for immunomodulation in the treatment of
glioblastoma. Neuro Oncol. 20:1566–1572. 2018. View Article : Google Scholar
|
|
159
|
Liu EK, Sulman EP, Wen PY and Kurz SC:
Novel therapies for glioblastoma. Curr Neurol Neurosci Rep.
20:192020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kijima N and Kanemura Y: Mouse models of
glioblastoma. In: Glioblastoma. De Vleeschouwer S: Codon
Publications; Brisbane, AU: 2017
|
|
161
|
Charles NA and Holland EC: The
perivascular niche microenvironment in brain tumor progression.
Cell Cycle. 9:3084–3093. 2010. View Article : Google Scholar
|
|
162
|
LeBlanc AK and Mazcko CN: Improving human
cancer therapy through the evaluation of pet dogs. Nat Rev Cancer.
20:727–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Hicks WH, Bird CE, Pernik MN, Haider AS,
Dobariya A, Abdullah KG, Aoun SG, Bentley RT, Cohen-Gadol AA,
Bachoo RM, et al: Large animal models of glioma: Current status and
future prospects. Anticancer Res. 41:5343–5353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tora MS, Texakalidis P, Neill S, Wetzel J,
Rindler RS, Hardcastle N, Nagarajan PP, Krasnopeyev A, Roach C,
James R, et al: Lentiviral vector induced modeling of high-grade
spinal cord glioma in minipigs. Sci Rep. 10:52912020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Selek L, Seigneuret E, Nugue G, Wion D,
Nissou MF, Salon C, Seurin MJ, Carozzo C, Ponce F, Roger T and
Berger F: Imaging and histological characterization of a human
brain xenograft in pig: The first induced glioma model in a large
animal. J Neurosci Methods. 221:159–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Khoshnevis M, Carozzo C, Bonnefont-Rebeix
C, Belluco S, Leveneur O, Chuzel T, Pillet-Michelland E, Dreyfus M,
Roger T, Berger F and Ponce F: Development of induced glioblastoma
by implantation of a human xenograft in Yucatan minipig as a large
animal model. J Neurosci Methods. 282:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Khoshnevis M, Carozzo C, Brown R, Bardiès
M, Bonnefont-Rebeix C, Belluco S, Nennig C, Marcon L, Tillement O,
Gehan H, et al: Feasibility of intratumoral 165Holmium siloxane
delivery to induced U87 glioblastoma in a large animal model, the
Yucatan minipig. PLoS One. 15:e02347722020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Rama AR, Alvarez PJ, Madeddu R and Aranega
A: ABC transporters as differentiation markers in glioblastoma
cells. Mol Biol Rep. 41:4847–4851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Ishihara H, Kubota H, Lindberg RL, Leppert
D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y and Frei
K: Endothelial cell barrier impairment induced by glioblastomas and
transforming growth factor beta2 involves matrix metalloproteinases
and tight junction proteins. J Neuropathol Exp Neurol. 67:435–448.
2008. View Article : Google Scholar
|
|
170
|
Liebner S, Fischmann A, Rascher G, Duffner
F, Grote EH, Kalbacher H and Wolburg H: Claudin-1 and claudin-5
expression and tight junction morphology are altered in blood
vessels of human glioblastoma multiforme. Acta Neuropathol.
100:323–331. 2000. View Article : Google Scholar
|
|
171
|
Wolburg H, Wolburg-Buchholz K, Kraus J,
Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W
and Engelhardt B: Localization of claudin-3 in tight junctions of
the blood-brain barrier is selectively lost during experimental
autoimmune encephalomyelitis and human glioblastoma multiforme.
Acta Neuropathol. 105:586–592. 2003. View Article : Google Scholar
|
|
172
|
Neftel C, Laffy J, Filbin MG, Hara T,
Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM,
et al: An integrative model of cellular states, plasticity, and
genetics for glioblastoma. Cell. 178:835–849.e21. 2019. View Article : Google Scholar
|
|
173
|
Bhaduri A, Di Lullo E, Jung D, Müller S,
Crouch EE, Espinosa CS, Ozawa T, Alvarado B, Spatazza J, Cadwell
CR, et al: Outer radial glia-like cancer stem cells contribute to
heterogeneity of glioblastoma. Cell Stem Cell. 26:48–63.e6. 2020.
View Article : Google Scholar
|