Introduction
Globally, colorectal cancer (CRC) is one of the most
common malignant tumors of the digestive tract. In recent years,
the incidence and mortality of CRC have increased, ranking third
and second relative to other carcinomas, respectively. In 2020
alone, it was estimated that there were >1.9 million new cases
of CRC and ~935,000 CRC-associated deaths worldwide (1). Thus, the exploration of novel early
screening methods, as well as the development of early prevention
and treatment strategies is of utmost importance. Modern microbial
information technology has allowed in-depth research into microbial
species, biological characteristics and disease. Furthermore, each
microbial habitat exhibits distinct microbial populations that may
have varying effects on physiological homeostasis. In humans, the
gut and oral microbiomes are the two major microbial ecosystems
that play a significant role in microbiome-related diseases
(2). Recently, Park et al
(3) demonstrated that both the
oral and gut microbiomes interdependently regulate physiological
functions and pathological processes, and that the oral-to-gut and
gut-to-oral microbial transmission can shape and/or reshape
microbial ecosystems in both habitats. Such transmissions modulate
disease pathogenesis, suggesting the existence of an ‘oral-gut’
microbiome axis.
The present review aims to comprehensively discuss
the oral and gut microbes of the oral-gut axis and their roles in
the occurrence, early screening and prevention of CRC. In addition,
novel prevention and treatment strategies drawn from the literature
are suggested.
Theoretical basis for the oral-gut axis
In recent years, with advancements being made in the
development of microbiology and bioinformatic strategies, research
into microbial species, biological characteristics and diseases has
become increasingly extensive. Microbial dysbiosis is associated
with multiple diseases in humans, where each microbial habitat
exhibits distinct patterns of microbial populations. Modern
medicine has proposed the concept of several organ microbial axes
related to gut microbes, such as the ‘lung-gut’ microbial axis
(4) and the ‘brain-gut’ microbial
axis (5,6), amongst others. It is hypothesized
that the mutual influence and interaction of gut microbes with
other organs results in the occurrence and development of diseases.
In addition to the gut, the oral compartment is the other largest
microbial habitat in the human body. The oral cavity is the first
component of the digestive tract. It is well understood that this
region and the intestinal tract are continuous areas connected by
the gastrointestinal tract. Due to the oral-gut barrier, the
distribution of microbiota between the oral cavity and the gut
tract is well separated. In the following sections, the question of
whether there is also a micro-ecological oral-gut axis that plays a
major role in microbiome-related diseases will be discussed.
Microecology oral-gut axis from an
anatomical perspective
The human digestive system consists of the digestive
tract and accessory digestive organs, including the liver and
pancreas. The digestive tract begins in the oral cavity and ends in
the gut, or more precisely, the anus. Thus, the oral cavity and gut
are anatomically contiguous regions connected by the
gastrointestinal tract. There is also a chemical link as saliva and
digested food pass through the gastrointestinal tract (7). Importantly, the oral cavity is the
initial site of the digestive tract, which provides several
different binding sites for the adhesion and colonization of
microorganisms. Therefore, there is a plausible association between
oral microbes, and the induction and development of
gastrointestinal tumors. The pathogenesis of a disease is a
comprehensive reflection of the interaction between microorganisms
and the host immune system. Under normal circumstances, the
microorganisms in the oral biofilm are in a dynamic balance that
can resist the interference of the external environment,
participate in the natural immune defense mechanism of the host,
and play an important role in maintaining the health of the host.
However, when the disturbance exceeds the regulatory capacity of
the bacterial biofilm, the oral microbial community structure will
become dysregulated, thereby affecting oral and, potentially,
systemic health (8,9). Relevant studies have indicated that
the imbalance of oral microbes can cause periodontitis and
periodontitis-associated systemic diseases, including digestive
tract diseases. Furthermore, periodontitis may influence the
occurrence and development of digestive tract tumors (10,11).
Oral-gut axis from a microecological
perspective
The basic level of the human micro-ecosystem can be
divided into micro-ecological subsystems, micro-ecological regions
and micro-ecological niches. The oral cavity and gut tract belong
to the microbial ecosystem, and changes to its local microbial
ecology can affect the changes of the microecology elsewhere or
elicit whole-body changes. Therefore, the oral microbial ecosystem
and gut microbial ecosystem are organically linked as a whole, the
interrelationship of which influences structure, function and
pathological changes. Normally, the oral and gut microbiomes are
well segregated due to the presence of the gut-gut barrier,
physical distance and chemical barriers, such as gastric acid and
bile (12,13). However, when the oral-gut barrier
is damaged, it can lead to the displacement and communication
between organs, which can lead to the occurrence of disease.
Effect of oral microorganisms on the
gut microbiome (oral-gut translocation)
Bacteria in the oral cavity are not strictly
localized to the oral cavity. Oral microorganisms can migrate to
the gut and other parts of the body, and affect their physiological
functions. Typical oral-resident species have been detected in
pathological conditions of the gastrointestinal tract (14,15),
such as inflammatory bowel disease (IBD), where the gut mucosa of
patients is heavily enriched with Haemophilus and
Veillonella bacteria, which are known oral commensal
microorganisms (16). Atarashi
et al (17) confirmed that
Klebsiella bacteria in saliva that have been transplanted
into the gut can induce chronic enteritis. Additionally, Kong et
al (18) found that the
intestinal Firmicutes and salivary Chloroflexi
bacteria were strongly associated with autism spectrum disorder
(ASD). Furthermore, it was found that intestinal
Bifidobacterium, Escherichia coli and Clostridium
species levels were positively associated with bacterial genera in
the oral cavity and/or with ASD pathophysiology (19–21).
Sasaki et al (22) found
that actinomycetes and fecal atrophic bacteria colonized the gut,
and only selectively colonized the oral cavity. This observation
may explain the pathogenicity of endocarditis-related disease.
Finally, the gut microbiome in patients with colon cancer contains
several oral taxa, including Clostridium (23), indicating that the oral microbiota
can disrupt and colonize the intestinal mucosa to become
opportunistic pathogens.
Influence of the gut microbiota on
oral microbes (gut-oral translocation)
In general, gut microbes rarely colonize the oral
cavity; however, poor hygiene and/or immunocompromising conditions
may promote the translocation of microbes from the gut to the oral
cavity. For example, in patients with IBD, gut microbes can
directly or indirectly affect the composition of oral flora by
affecting the immune function of the host (24). Bifidobacterium in the gut
has been found in the saliva of newborns (25), and similarly, the detection rate of
oral bacteria, such as Porphyromonas, Fusobacterium and
Pseudoramibacter species in the gut of elderly individuals
is higher than that of healthy adults (26,27).
Furthermore, apart from in vivo transmission, the fecal-oral
axis is also considered an important mechanism in the
human-to-human transmission of pathogens. The microbiome of the
human hand significantly overlaps with oral and gut microbiota
patterns, suggesting that the hand is a vehicle for fecal-to-oral
microbial transmission (28). Gut
microbes can be transmitted by direct contact via the fecal-oral
route or indirect contact with contaminated fluids and food
(29). Enteroviruses, such as
hepatitis A virus and hepatitis E virus are known to be transmitted
by the fecal-oral axis and are therefore easily transmitted by
person-to-person contact. In addition to enteroviruses,
Helicobacter pylori, the major pathogen of severe
gastroduodenal disease, can also be transmitted by the fecal-oral
route (30). In other systemic
diseases, oral and gut microbial flora have common effects at the
same time. For example, Bertolini and Dongari-Bagtzoglou (31) found that oral inoculation of
Candida albicans in mice caused serious oral and gut
microbial flora composition disorders.
In conclusion, oral microbes and gut microbiota are
intrinsically linked. Furthermore, the bidirectional interaction of
microbes from the oral-gut and the gut-oral axes can mutually shape
and/or reshape the microbial ecosystems of the two habitats to
ultimately regulate the physiological and pathological processes of
the intestinal system. The microecological oral-gut axis formed by
bidirectional crosstalk between the oral and gut microbiota plays a
key role in the occurrence and development of CRC and warrants
further investigation.
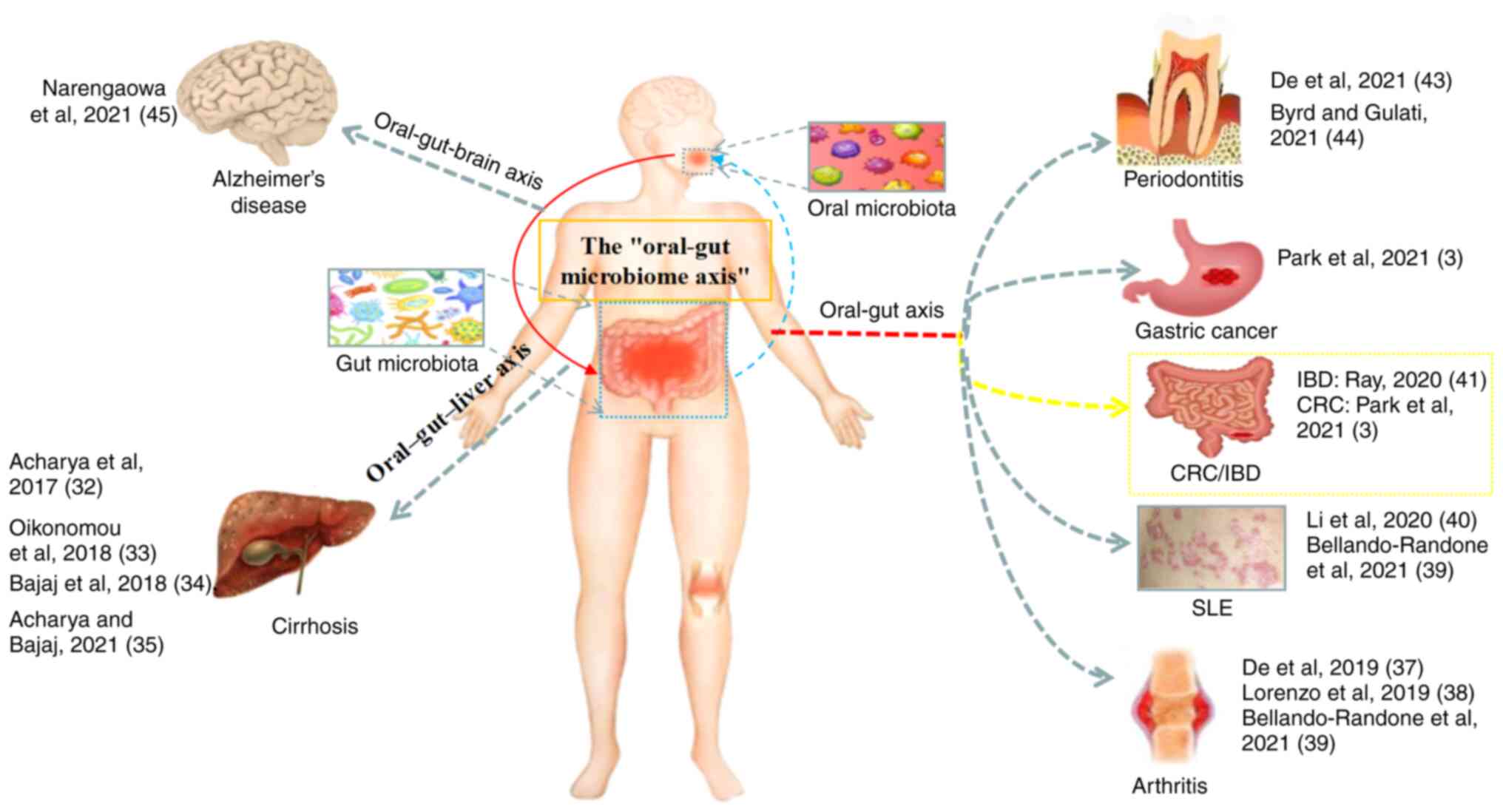
Relevant research on the oral-gut axis
In 2017, Acharya et al (32) proposed the oral-gut-liver axis
theory on the basis of the gut-liver axis, and mentioned the
oral-gut axis for the first time. Oikonomou et al (33) further elaborated that the microbial
link between the liver and the oral cavity may lead to liver
cirrhosis by impairing the permeability of the gut, allowing the
direct translocation of bacteria from the oral cavity to the
systemic circulation. Bajaj et al (34) found that periodontal therapy
exerted a favorable modulating effect on the oral-gut-liver axis in
patients with liver cirrhosis, while Acharya and Bajaj (2021)
(35) provided further evidence
supporting the oral-gut-liver axis. Imai et al (2021)
(36) demonstrated that the
ectopic colonization of oral bacteria in the gut can serve as a
biomarker for gastrointestinal and liver diseases. Du Teil Espina
et al (37) proposed the
association between the oral-gut microbial axis and the
pathogenesis of rheumatoid arthritis, and proposed the oral-gut
microbial axis for the first time, but this did not involve the
association with CRC. Lorenzo et al (38) suggested that changes in the oral
and gut microbiota appear to play a crucial role in the
pathogenesis of rheumatoid arthritis and osteoarthritis, although
further research is required. The study by Bellando-Randone et
al (39) suggested the oral
microbiome as a promising diagnostic biomarker for rheumatic
disorders. Li et al (40)
found that the imbalance of oral microflora and abnormal metabolic
pathways were associated with the pathogenesis of systemic lupus
erythematosus. A study by Ray (41) found that oral bacteria can promote
colitis in mice through intestinal colonization, and the induction
and migration of bacterial-reactive T cells. Xiang et al
(42) presented a conceptual
framework for the potential impact of SARS-CoV-2 oral infection on
the local and distant microbiota of the respiratory and
gastrointestinal tract (oral-lung axis). De Oliveira et al
(43) suggested that the oral-gut
axis may be a pathway connecting periodontal and systemic diseases,
while Byrd and Gulati (44)
proposed the concept of the ‘gingival-gut’ axis and noted the
importance of collaborative treatment and research programs between
physicians and gastrointestinal physicians. Narengaowa et al
(45) comprehensively discussed
the possible mechanisms of the oral-gut-brain association related
to the pathogenesis of Alzheimer's disease, and proposed an
oral-gut-brain axis. Park et al (3) described the association between the
oral-gut axis and gastrointestinal-related cancer, and first
proposed the association between the oral-gut microbiota axis and
CRC. However, a comprehensive and in-depth analysis has not yet
been performed, at least to the best of our knowledge.
To better explain the close association between the
oral-gut axis of the microbiota and the occurrence of diseases, the
present study reviewed recent research on diseases related to the
oral-gut axis of the microbiome (Fig.
1). The aim of this review section was to provide a robust
basis for the proposal of the oral-gut axis. At the same time, it
is also aimed to help better understand the importance of the
microbial oral-gut axis in the pathogenesis of CRC, which may prove
to be beneficial for the accurate screening/diagnosis and effective
prevention and treatment of the disease.
Oral microbes and CRC
The oral cavity is the initial part of the digestive
tract and is also the region with the most abundant species of
flora in the whole body. At the species level, there are >700
different species of oral microorganisms (46). Oral microorganisms have a direct
association with oral health, and can also affect the systemic
health status. Changing the oral microbiota can further regulate
systemic diseases, such as cardiovascular disease and
gastrointestinal tumors, among others (47–50).
Under normal conditions, the oral and gut microbiomes are well
segregated due to the presence of the gut-gut barrier, physical
distance and chemical barriers (such as gastric acid and bile)
(7). When the oral-gut barrier is
damaged, it will lead to the translocation and communication of
microorganisms between organs, and oral microorganisms can migrate
to the gut and other organs, which is considered to be another
mechanism of oral microbial dysbiosis causing systemic diseases
(51,52). The study by Atarashi et al
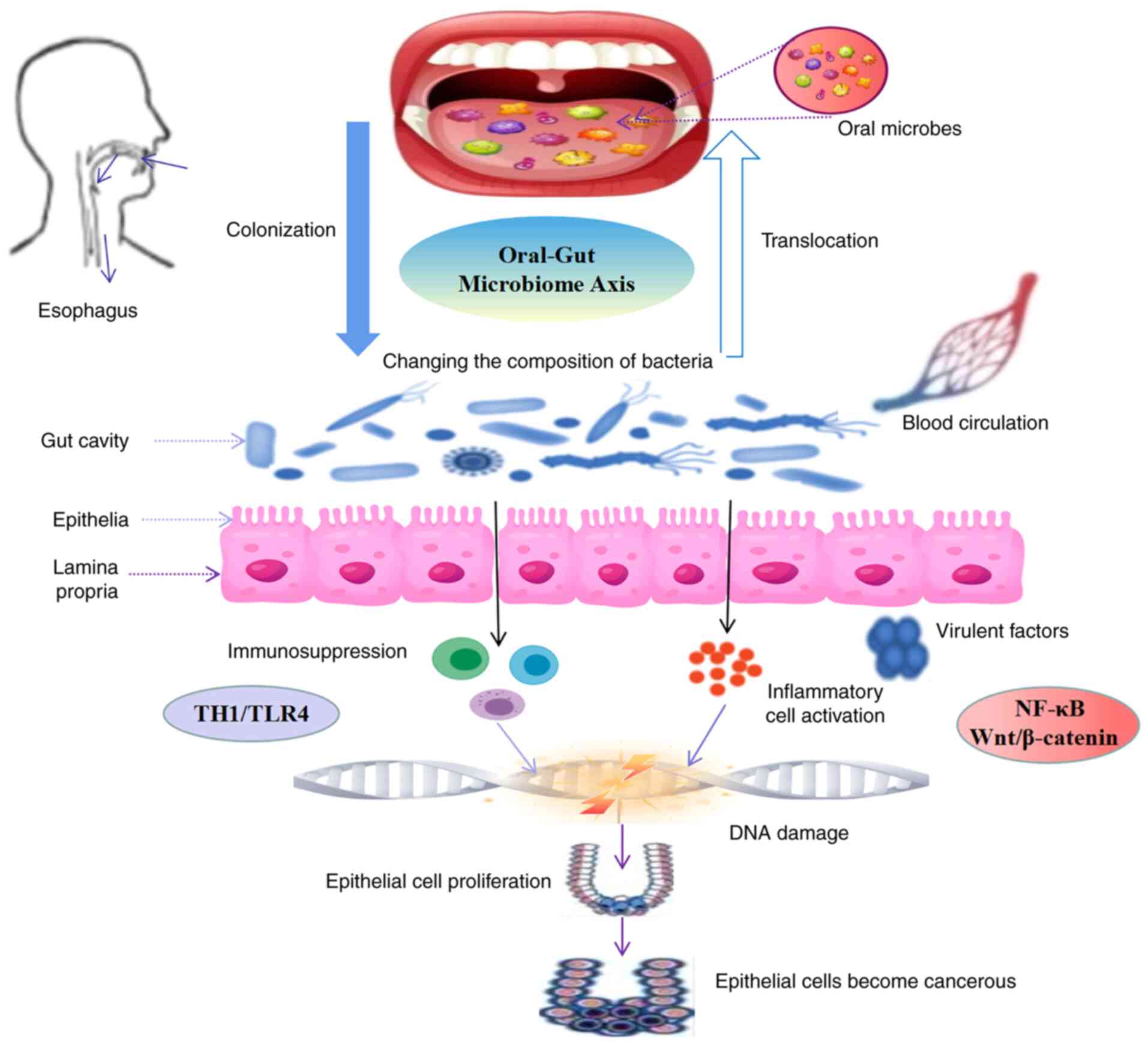
(17) demonstrated that the
rooting of Klebsiella pneumoniae in the oral cavity in the
gut can trigger the excessive activation of T-helper 1 (Th1) cells,
leading to the occurrence of IBD, thereby initiating the
inflammatory-cancer transformation of the intestine. The study by
Kitamoto et al (53) found
that the oral microbial disorder caused by periodontitis can induce
the production of Th17 cells, and that Th17 cell migration to the
intestine is amplified under the action of bacterial antigens,
which promotes the occurrence of gut tumors.
In addition, Fusobacterium nucleatum (Fn) is
a Gram-negative anaerobic bacterium colonized in the human oral
cavity; it plays a critical role in the occurrence, metastasis and
disease outcomes of CRC (54–56).
An imbalance in the oral and gut microecology can lead to the
occurrence of gut inflammation, and thus the development of CRC
(57). Studies have found that the
main mechanisms of Fn and CRC occurrence are as follows: Fn can
promote the production of TNF-α, activate NF-κB signaling and the
STAT3 signaling pathway, and enhance the migration and invasion
ability of tumor cells (58).
FadA, as a toxic protein secreted by Fn, binds to E-cadherin in
intestinal epithelial cells, activates the β-catenin signaling
pathway, enhances Wnt transcriptional activity, and promotes
inflammatory factors and cancer cell proliferation (59). Fn in CRC tissues can increase the
content of reactive oxygen species in the gut, leading to the
occurrence of microsatellite instability, which in turn promotes
the occurrence of CRC (60).
Gut microbes and CRC
The gut is the largest and most characteristic
microbial ecosystem in the human body. The gut microbes are
dominated by anaerobic bacteria, which are composed of five main
phyla: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria
and Verrucomicrobiota. Of these, Bacteroidetes and Firmicutes
account for >90% of all bacteria present (61). When the balance of the microbial
ecosystem is affected, the number of probiotic species decreases
and pathogenic bacteria have the opportunity to multiply, causing
an imbalance in the intestinal flora. The mucosal barrier is
transferred to tissues and organs other than the gut and even the
blood, which further leads to gut flora imbalances, which has a
marked effect on the host's immune system and body stability
(62). Intestinal dysbiosis is
closely associated with several diseases, such as obesity, IBD,
adenoma and CRC (63). The
pathogenesis of CRC is attributed to a variety of factors. Two
studies successively reported that E. coli and Fn can induce
CRC in humans (59,64); these were the first studies to
explore the mechanism of CRC from the perspective of intestinal
bacterial infection.
Inflammation is a recognized risk factor for CRC,
and the balance of the gut flora in patients with IBD who have an
increased risk of CRC is affected (65); the gut flora can regulate the
mucosal and systemic immune systems, which in turn affects the
progression of CRC. Cytolethal distending toxin, as one of the
components of bacterial toxins (cyclomodulins) that regulates the
eukaryotic cell cycle, can promote intestinal colonization and
induce the pro-inflammatory molecules, NF-κB, TNF, IL-6 and
cyclooxygenase 2 (COX-2), which are involved in carcinogenesis.
E. coli is one of the characteristic bacteria associated
with IBD; its colonization in the intestinal mucosa of patients
with IBD is abnormal, and it can induce the expression of COX-2 in
macrophages. Intestinal inflammation plays a role in the
development of CRC (66). With
regard to bacteria closely associated with CRC, in addition to Fn,
several studies have found that enterotoxigenic Bacteroides
fragilis can also promote the release of epithelial
pro-inflammatory factors and further stimulate the activation of
the NF-κB signaling pathway, which is involved in CRC pathogenesis;
Bacteroides fragilis can also play an indirect role by
secreting Bacteroides fragilis toxin, a zinc-dependent
metalloproteinase toxin that can cleave the tumor suppressor
protein-E-cadherin, resulting in enhanced Wnt/β-catenin signaling
that ultimately leads to CRC cell proliferation (67–69).
Association between the oral-gut axis and
the occurrence of CRC
Deleterious alterations in the oral and gut
microbiomes have been associated with the occurrence and
development of tumors. Previous research has indicated that the
risk of cancer due to microbial infection is ~16.1% (70). The microecological oral-gut axis
formed by the bidirectional displacement and interaction between
the oral and gut microbiomes is rarely studied in the occurrence
and development of CRC. Under normal circumstances, due to the
existence of the gut-gut barrier, the oral and gut microbiomes are
well segregated and in a dynamic balance. When host immunity is
reduced or affected by external factors, a microecological
imbalance can occur. The dysregulation of the microecological
oral-gut axis can then facilitate the onset of disease. When the
oral microbial flora is in a state of imbalance, lesions may appear
within the oral cavity. This phenomenon is commonly observed in
periodontitis, glossitis, gingivitis, oral ulcers and oral cancer.
Furthermore, the translocation/colonization of oral microbial flora
to the gut may induce or even exacerbate gut diseases (3,51,52).
For example, Porphyromonas gingivalis is the main pathogen
of periodontitis, and its colonization in the gut tract can cause
the disorder of the microbial community structure and increase
serum endotoxin levels, which induce gut inflammation (71). Subsequently, excessive inflammation
initiates the inflammation-cancer pathway, leading to the
occurrence and development of CRC. Patients with IBD are a
high-risk group for colon cancer development and often exhibit
extraintestinal manifestations of disease, such as chronic
periodontitis and oral ulcers, suggesting that the process of
colitis-cancer evolution is not an isolated event (72,73).
Despite these data, further studies are required to elucidate the
association between the microbial oral-gut axis and CRC. However,
it is well understood that microbial alterations, whether in the
oral-gut pathway or the gut-oral pathway, affect the NF-κB and/or
Wnt/β-catenin signaling pathway, and are involved in the occurrence
and development of CRC (59,74)
(Fig. 2).
In conclusion, the multi-factor and multi-pathway
occurrence of CRC has been demonstrated by a number of previous
studies, and the association between the microecological oral-gut
axis and the occurrence of CRC has become one of the hot spots in
CRC research. To clarify the association between the
microecological oral-gut axis and the occurrence and development of
CRC, the precise screening and prevention of CRC may be realized by
manipulating the microecological oral-intestinal axis. This will
provide further insight into the clinical diagnosis and
treatment.
Clinical significance of oral-gut axis in
CRC prevention and treatment
Early screening of CRC: Exploring
oral-specific microbial markers
Early screening can effectively reduce the morbidity
and mortality rates in patients with CRC, and is essential for CRC
prevention and treatment. Microbes play a crucial role in the
body's metabolism, immune regulation and inflammatory response, and
as aforementioned, are closely associated with the development of
CRC. It has been shown that there are key differences in the oral
microbial structure between patients with CRC and healthy
individuals. Using oral microorganisms and their metabolites,
scientists and clinicians can construct new CRC screening tools to
improve screening efficiency. Yamaoka et al (75) demonstrated that the abundance of Fn
was significantly increased in patients with stage IV CRC.
Furthermore, the study demonstrated that patients with CRC with a
higher abundance of Fn had larger tumors and a shorter survival
time, suggesting that Fn abundance could predict the prognosis of
CRC. Therefore, Klebsiella pneumoniae and Fn in the oral
cavity may be useful oral-specific microbial biomarkers for CRC,
which may be useful for early screening.
A novel model of CRC prevention: The
importance of oral hygiene and the early intervention of oral
disease
At present, the most effective strategy with which
to improve CRC outcomes is prevention and early detection. CRC
prevention guidelines recommend a multi-fiber diet, avoiding the
intake of carcinogens, and emphasize the importance of early
treatment of colorectal adenomas. Owing to advancements being made
in scientific tools and protocols, the understanding of CRC
pathogenesis is being increasingly improved upon and a large
variety of microorganisms have been associated with the occurrence
and development of the disease. According to oral microbiota
analysis (76),
Fusobacterium levels are higher in patients with oral
squamous cell carcinoma than in normal healthy individuals.
Therefore, the early intervention and treatment of oral hygiene and
oral diseases, respectively, are two important strategies with
which to prevent the occurrence and development of CRC. Considering
these data, a new model of CRC prevention is proposed, namely the
early intervention and early treatment of oral hygiene and oral
diseases.
Proposing a novel concept:
Simultaneous oral and gut treatment for management of CRC
The treatment of CRC primarily includes surgery,
radiotherapy, chemotherapy, biologically targeted therapy and
adjuvant traditional Chinese medicine (TCM) therapy, among others;
however, these strategies are focused on the treatment of
colorectal intestinal lesions. To date, only a few studies have
assessed the treatment of CRC combined with oral disease
intervention. The present review discussed the micro-ecological
oral-gut axis and its role in the screening, incidence and
treatment of CRC. The novel concept breaks away from the
traditional concept of symptomatic treatment and rather focuses on
the ‘holistic’ perspective for disease management. A new treatment
concept for CRC is proposed, namely the oral-gut simultaneous
treatment strategy. This new concept of oral-gut co-treatment not
only conforms to the requirements of modern evidence-based
medicine, but also reflects the ‘holistic view’. Whilst several
studies support this strategy, large-scale experiments and clinical
trials are urgently required to substantiate this
hypothesis/treatment strategy.
Conclusions
In conclusion, the occurrence of CRC is closely
associated with microbial dysbiosis in the oral and gut
microbiomes. It has proposed that the oral-gut axis is a novel
system that may be of interest in the early screening, prevention
and treatment of CRC. However, whether it is possible to correct
the imbalances in the oral-gut axis through novel oral-gut
treatment strategies warrants further investigation.
Acknowledgements
Not applicable.
Funding
The research was supported by the National Key Clinical
Discipline [no. grant no (2012) 649].
Availability of data and materials
Not applicable.
Authors' contributions
FL, DS, HZ, HCL, QZ, BC and DLR helped write and
review the manuscript. FL wrote the original draft. DS and HZ
created the figures. HCL and QZ performed the formal analysis. BC
and DLR corrected the final version. All authors read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
NIH HMP Working Group, . Peterson J,
Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V,
McEwen JE, Wetterstrand KA, et al: The NIH human microbiome
project. Genome Res. 19:2317–2323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SY, Hwang BO, Lim M, Ok SH, Lee SK,
Chun KS, Park KK, Hu Y, Chung WY and Song NY: Oral-gut microbiome
axis in gastrointestinal disease and cancer. Cancers (Basel).
13:21242021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi C, Lin L, Xie T, et al: Based on the
‘lung-gut’ axis to explore the influence of lung and gut
microecology on lung diseases. J Nanjing Univ Tradit Chin Med.
02:168–173. 2020.https://t.cnki.net/kcms/detail?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7mYRGNWDareZlYKpvhaXcgDMecYtweZkXRiOLhIZAUMCDDjKrapSPMVkJLBTMn0eV&uniplatform=NZKPT&uid=WEEvREdxOWJmbC9oM1NjYkcyTjdROWp3THN6dy9lNCtTTG4zd1MwcFFNeDg=$R1yZ0H6jyaa0en3RxVUd8df-oHi7XMMDo7mtKT6mSmEvTuk11l2gFA!!
|
|
5
|
Cerdó T, Ruíz A, Suárez A and Campoy C:
Probiotic, prebiotic, and brain development. Nutrients. 9:12472017.
View Article : Google Scholar
|
|
6
|
Muller PA, Schneeberger M, Matheis F, Wang
P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro TBR,
Furuichi M, et al: Microbiota modulate sympathetic neurons via a
gut-brain circuit. Nature. 583:441–446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt TS, Hayward MR, Coelho LP, Li SS,
Costea PI, Voigt AY, Wirbel J, Maistrenko OM, Alves RJ, Bergsten E,
et al: Extensive transmission of microbes along the
gastrointestinal tract. Elife. 8:e426932019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamont RJ, Koo H and Hajishengallis G: The
oral microbiota: Dynamic communities and host interactions. Nat Rev
Microbiol. 16:745–759. 2018. View Article : Google Scholar
|
|
9
|
Mark Welch JL, Ramírez-Puebla ST and
Borisy GG: Oral microbiome geography: Micron-scale habitat and
niche. Cell Host Microbe. 28:160–168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y: Comparison and analysis of oral
flora structure in patients with gingival cancer and periodontitis:
Commonalities and differences. China Med Univ. 2020.https://t.cnki.net/kcms/detail?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkJrOyyi_z7N8gBvuHP4X-Bqg6NwePhrlvgbwoWSsn9prYQqxr4×Vg8WYkOENyNYHk&uniplatform=NZKPT&uid=WEEvREdxOWJmbC9oM1NjYkcyTjdROWp3THN6dy9lNCtTTG4zd1MwcFFNeDg=$R1yZ0H6jyaa0en3RxVUd8df-oHi7XMMDo7mtKT6mSmEvTuk11l2gFA!!
|
|
11
|
Chukkapalli SS, Easwaran M, Rivera-Kweh
MF, Velsko IM, Ambadapadi S, Dai J, Larjava H, Lucas AR and
Kesavalu L: Sequential colonization of periodontal pathogens in
induction of periodontal disease and atherosclerosis in LDLRnull
mice. Pathog Dis. 75:ftx0032017. View Article : Google Scholar
|
|
12
|
Segata N, Haake SK, Mannon P, Lemon KP,
Waldron L, Gevers D, Huttenhower C and Izard J: Composition of the
adult digestive tract bacterial microbiome based on seven mouth
surfaces, tonsils, throat and stool samples. Genome Biol.
13:R422012. View Article : Google Scholar
|
|
13
|
Ridlon JM, Kang DJ, Hylemon PB and Bajaj
JS: Bile acids and the gut microbiome. Curr Opin Gastroenterol.
30:332–338. 2014. View Article : Google Scholar
|
|
14
|
Huh JW and Roh TY: Opportunistic detection
of Fusobacterium nucleatum as a marker for the early gut
microbial dysbiosis. BMC Microbiol. 20:2082020. View Article : Google Scholar
|
|
15
|
Del Castillo E, Meier R, Chung M, Koestler
DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J and
Michaud DS: The microbiomes of pancreatic and duodenum tissue
overlap and are highly subject specific but differ between
pancreatic cancer and noncancer subjects. Cancer Epidemiol
Biomarkers Prev. 28:370–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gevers D, Kugathasan S, Denson LA,
Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song
SJ, Yassour M, et al: The treatment-naive microbiome in new-onset
Crohn's disease. Cell Host Microbe. 15:382–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atarashi K, Suda W, Luo C, Kawaguchi T,
Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et
al: Ectopic colonization of oral bacteria in the intestine drives
TH1 cell induction and inflammation. Science.
358:359–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong X, Liu J, Cetinbas M, Sadreyev R, Koh
M, Huang H, Adeseye A, He P, Zhu J, Russell H, et al: New and
preliminary evidence on altered oral and gut microbiota in
individuals with autism spectrum disorder (ASD): Implications for
ASD diagnosis and subtyping based on microbial biomarkers.
Nutrients. 11:21282019. View Article : Google Scholar
|
|
19
|
Ding HT, Taur Y and Walkup JT: Gut
microbiota and autism: Key concepts and findings. J Autism Dev
Disord. 47:480–489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luna RA, Oezguen N, Balderas M,
Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J,
Anderson GM, Savidge T and Williams KC: Distinct
microbiome-neuroimmune signatures correlate with functional
abdominal pain in children with autism spectrum disorder. Cell Mol
Gastroenterol Hepatol. 3:218–230. 2016. View Article : Google Scholar
|
|
21
|
Gargari G, Taverniti V, Gardana C, Cremon
C, Canducci F, Pagano I, Barbaro MR, Bellacosa L, Castellazzi AM,
Valsecchi C, et al: Fecal clostridiales distribution and
short-chain fatty acids reflect bowel habits in irritable bowel
syndrome. Environ Microbiol. 20:3201–3213. 2018. View Article : Google Scholar
|
|
22
|
Sasaki M, Shimoyama Y, Ishikawa T, Kodama
Y, Tajika S and Kimura S: Contribution of different adherent
properties of Granulicatella adiacens and Abiotrophia defectiva to
their associations with oral colonization and the risk of infective
endocarditis. J Oral Sci. 62:36–39. 2020. View Article : Google Scholar
|
|
23
|
Nakatsu G, Li X, Zhou H, Sheng J, Wong SH,
Wu WK, Ng SC, Tsoi H, Dong Y, Zhang N, et al: Gut mucosal
microbiome across stages of colorectal carcinogenesis. Nat Commun.
6:87272015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia M, Jin Z, Zheng C, et al: Intervention
of traditional Chinese medicine on colitis-cancer transformation
based on oral micro-ecology. China J Tradit Chin Med Pharm.
06:2566–2570. 2019.https://t.cnki.net/kcms/detail?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7CoKB_BvAJUQwzoapsKG3EAOyzzGLBX35huQLjuJ1cPcmeJlWp_hGquH37rR5bVDu&uniplatform=NZKPT&uid=WEEvREdxOWJmbC9oM1NjYkcyTjdROWp3THN6dy9lNCtTTG4zd1MwcFFNeDg=$R1yZ0H6jyaa0en3RxVUd8df-oHi7XMMDo7mtKT6mSmEvTuk11l2gFA!!
|
|
25
|
Toda K, Hisata K, Satoh T, Katsumata N,
Odamaki T, Mitsuyama E, Katayama T, Kuhara T, Aisaka K, Shimizu T
and Xiao JZ: Neonatal oral fluid as a transmission route for
bifidobacteria to the infant gut immediately after birth. Sci Rep.
9:86922019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwauchi M, Horigome A, Ishikawa K, Mikuni
A, Nakano M, Xiao JZ, Odamaki T and Hironaka S: Relationship
between oral and gut microbiota in elderly people. Immun Inflamm
Dis. 7:229–236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Odamaki T, Kato K, Sugahara H, Hashikura
N, Takahashi S, Xiao JZ, Abe F and Osawa R: Age-related changes in
gut microbiota composition from newborn to centenarian: A
cross-sectional study. BMC Microbiol. 16:902016. View Article : Google Scholar
|
|
28
|
Shaffer M and Lozupone C: Prevalence and
source of fecal and oral bacteria on infant, child, and adult
hands. mSystems. 3:e00192–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Graaf M, Beck R, Caccio SM, Duim B,
Fraaij P, Le Guyader FS, Lecuit M, Le Pendu J, de Wit E and
Schultsz C: Sustained fecal-oral human-to-human transmission
following a zoonotic event. Curr Opin Virol. 22:1–6. 2017.
View Article : Google Scholar
|
|
30
|
Bui D, Brown HE, Harris RB and Oren E:
Serologic evidence for fecal-oral transmission of Helicobacter
pylori. Am J Trop Med Hyg. 94:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bertolini M and Dongari-Bagtzoglou A: The
relationship of Candida albicans with the oral bacterial
microbiome in health and disease. Adv Exp Med Biol. 1197:69–78.
2019. View Article : Google Scholar
|
|
32
|
Acharya C, Sahingur SE and Bajaj JS:
Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI
Insight. 2:e944162017. View Article : Google Scholar
|
|
33
|
Oikonomou T, Papatheodoridis GV, Samarkos
M, Goulis I and Cholongitas E: Clinical impact of microbiome in
patients with decompensated cirrhosis. World J Gastroenterol.
24:3813–3820. 2018. View Article : Google Scholar
|
|
34
|
Bajaj JS, Matin P, White MB, Fagan A, Deeb
JG, Acharya C, Dalmet SS, Sikaroodi M, Gillevet PM and Sahingur SE:
Periodontal therapy favorably modulates the oral-gut-hepatic axis
in cirrhosis. Am J Physiol Gastrointest Liver Physiol.
315:G824–G837. 2018. View Article : Google Scholar
|
|
35
|
Acharya C and Bajaj JS: Is it time to
spit? More evidence for the oral-gut-liver axis in liver disease.
Hepatol Int. 15:4–5. 2021. View Article : Google Scholar
|
|
36
|
Imai J, Kitamoto S and Kamada N: The
pathogenic oral-gut-liver axis: New understandings and clinical
implications. Expert Rev Clin Immunol. 17:727–736. 2021. View Article : Google Scholar
|
|
37
|
Du Teil Espina M, Gabarrini G, Harmsen
HJM, Westra J, van Winkelhoff AJ and van Dijl JM: Talk to your gut:
The oral-gut microbiome axis and its immunomodulatory role in the
etiology of rheumatoid arthritis. FEMS Microbiol Rev. 43:1–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lorenzo D, GianVincenzo Z, Carlo Luca R,
Karan G, Jorge V, Roberto M and Javad P: Oral-gut microbiota and
arthritis: Is there an evidence-based axis? J Clin Med. 8:2019.
|
|
39
|
Bellando-Randone S, Russo E, Venerito V,
Matucci-Cerinic M, Iannone F, Tangaro S and Amedei A: Exploring the
oral microbiome in rheumatic diseases, state of art and future
prospective in personalized medicine with an AI approach. J Pers
Med. 11:6252021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li BZ, Zhou HY, Guo B, Chen WJ, Tao JH,
Cao NW, Chu XJ and Meng X: Dysbiosis of oral microbiota is
associated with systemic lupus erythematosus. Arch Oral Biol.
113:1047082020. View Article : Google Scholar
|
|
41
|
Ray K: The oral-gut axis in IBD. Nat Rev
Gastroenterol Hepatol. 17:5322020. View Article : Google Scholar
|
|
42
|
Xiang Z, Koo H, Chen Q, Zhou X, Liu Y and
Simon-Soro A: Potential implications of SARS-CoV-2 oral infection
in the host microbiota. J Oral Microbiol. 13:18534512020.
View Article : Google Scholar
|
|
43
|
De Oliveira AM, Lourenço TGB and Colombo
APV: Impact of systemic probiotics as adjuncts to subgingival
instrumentation on the oral-gut microbiota associated with
periodontitis: A randomized controlled clinical trial. J
Periodontol. 93:31–44. 2022. View Article : Google Scholar
|
|
44
|
Byrd KM and Gulati AS: The ‘Gum-Gut’ axis
in inflammatory bowel diseases: A hypothesis-driven review of
associations and advances. Front Immunol. 12:6201242021. View Article : Google Scholar
|
|
45
|
Narengaowa, Kong W, Lan F, Awan UF, Qing H
and Ni J: The oral-gut-brain axis: The influence of microbes in
Alzheimer's disease. Front Cell Neurosci. 15:6337352021. View Article : Google Scholar
|
|
46
|
Yamashita Y and Takeshita T: The oral
microbiome and human health. J Oral Sci. 59:201–206. 2017.
View Article : Google Scholar
|
|
47
|
Kilian M, Chapple IL, Hannig M, Marsh PD,
Meuric V, Pedersen AM, Tonetti MS, Wade WG and Zaura E: The oral
microbiome-an update for oral healthcare professionals. Br Dent J.
221:657–666. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zarco MF, Vess TJ and Ginsburg GS: The
oral microbiome in health and disease and the potential impact on
personalized dental medicine. Oral Dis. 18:109–120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao L, Xu T, Huang G, Jiang S, Gu Y and
Chen F: Oral microbiomes: More and more importance in oral cavity
and whole body. Protein Cell. 9:488–500. 2018. View Article : Google Scholar
|
|
50
|
Wade WG: The oral microbiome in health and
disease. Pharmacol Res. 69:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Flemer B, Warren RD, Barrett MP, Cisek K,
Das A, Jeffery IB, Hurley E, O'Riordain M, Shanahan F and O'Toole
PW: The oral microbiota in colorectal cancer is distinctive and
predictive. Gut. 67:1454–1463. 2018. View Article : Google Scholar
|
|
52
|
Gaiser RA, Halimi A, Alkharaan H, Lu L,
Davanian H, Healy K, Hugerth LW, Ateeb Z, Valente R, Fernández Moro
C, et al: Enrichment of oral microbiota in early cystic precursors
to invasive pancreatic cancer. Gut. 68:2186–2194. 2019. View Article : Google Scholar
|
|
53
|
Kitamoto S, Nagao-Kitamoto H, Jiao Y,
Gillilland MG III, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil
JC, Kuffa P, et al: The intermucosal connection between the mouth
and gut in commensal pathobiont-driven colitis. Cell.
182:447–462.e14. 2020. View Article : Google Scholar
|
|
54
|
Bullman S, Pedamallu CS, Sicinska E,
Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T,
et al: Analysis of Fusobacterium persistence and antibiotic
response in colorectal cancer. Science. 358:1443–1448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Casasanta MA, Yoo CC, Udayasuryan B,
Sanders BE, Umaña A, Zhang Y, Peng H, Duncan AJ, Wang Y, Li L, et
al: Fusobacterium nucleatum host-cell binding and invasion
induces IL-8 and CXCL1 secretion that drives colorectal cancer cell
migration. Sci Signal. 13:eaba91572020. View Article : Google Scholar
|
|
56
|
Brennan CA and Garrett WS:
Fusobacterium nucleatum-symbiont, opportunist and
oncobacterium. Nat Rev Microbiol. 17:156–166. 2019. View Article : Google Scholar
|
|
57
|
Gopalakrishnan V, Helmink BA, Spencer CN,
Reuben A and Wargo JA: The influence of the gut microbiome on
cancer, immunity, and cancer immunotherapy. Cancer Cell.
33:570–580. 2018. View Article : Google Scholar
|
|
58
|
Wei Z, Cao S, Liu S, Yao Z, Sun T, Li Y,
Li J, Zhang D and Zhou Y: Could gut microbiota serve as prognostic
biomarker associated with colorectal cancer patients' survival? A
pilot study on relevant mechanism. Oncotarget. 7:46158–46172. 2016.
View Article : Google Scholar
|
|
59
|
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G
and Han YW: Fusobacterium nucleatum promotes colorectal
carcinogenesis by modulating E-cadherin/β-catenin signaling via its
FadA adhesin. Cell Host Microbe. 14:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Arumugam M, Raes J, Pelletier E, Le
Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto
JM, et al: Enterotypes of the human gut microbiome. Nature.
473:174–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chong ES: A potential role of probiotics
in colorectal cancer prevention: Review of possible mechanisms of
action. World J Microbiol Biotechnol. 30:351–374. 2014. View Article : Google Scholar
|
|
63
|
Biedermann L and Rogler G: The intestinal
microbiota: Its role in health and disease. Eur J Pediatr.
174:151–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arthur JC, Perez-Chanona E, Mühlbauer M,
Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B,
Rogers AB, et al: Intestinal inflammation targets cancer-inducing
activity of the microbiota. Science. 338:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ray K: IBD. Gut microbiota in IBD goes
viral. Nat Rev Gastroenterol Hepatol. 12:1222015. View Article : Google Scholar
|
|
66
|
Raisch J, Rolhion N, Dubois A,
Darfeuille-Michaud A and Bringer MA: Intracellular colon
cancer-associated Escherichia coli promote protumoral
activities of human macrophages by inducing sustained COX-2
expression. Lab Invest. 95:296–307. 2015. View Article : Google Scholar
|
|
67
|
Gagnière J, Raisch J, Veziant J, Barnich
N, Bonnet R, Buc E, Bringer MA, Pezet D and Bonnet M: Gut
microbiota imbalance and colorectal cancer. World J Gastroenterol.
22:501–518. 2016. View Article : Google Scholar
|
|
68
|
Rossi M, Mirbagheri SEYEDS, Keshavarzian A
and Bishehsari F: Nutraceuticals in colorectal cancer: A
mechanistic approach. Eur J Pharmacol. 833:396–402. 2018.
View Article : Google Scholar
|
|
69
|
Li Q, Ding C, Meng T, Lu W, Liu W, Hao H
and Cao L: Butyrate suppresses motility of colorectal cancer cells
via deactivating Akt/ERK signaling in histone deacetylase dependent
manner. J Pharmacol Sci. 135:148–155. 2017. View Article : Google Scholar
|
|
70
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar
|
|
71
|
Yu L, Ge S, Feng X, et al: Research
progress of Porphyromonas gingivalis. J Prev Treat Stomatol
Dis. 05:314–317. 2016.https://t.cnki.net/kcms/detail?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7bFPagIuZu8mSNNqTzqA10SmLmWs9SCrXF-1zbj6qv97WC8n64gYCrTahio_5NDeO&uniplatform=NZKPT&uid=WEEvREdxOWJmbC9oM1NjYkcyTjdROWp3THN6dy9lNCtTTG4zd1MwcFFNeDg=$R1yZ0H6jyaa0en3RxVUd8df-oHi7XMMDo7mtKT6mSmEvTuk11l2gFA!!
|
|
72
|
Zhao L: Discussion on the treatment of
extraintestinal symptoms and complications in patients with
inflammatory bowel disease. Clin Res Pract. 2:142–143. 2017.
|
|
73
|
Barton MK: Evidence accumulates indicating
periodontal disease as a risk factor for colorectal cancer or
lymphoma. CA Cancer J Clin. 67:173–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang Y, Weng W, Peng J, Hong L, Yang L,
Toiyama Y, Gao R, Liu M, Yin M, Pan C, et al: Fusobacterium
nucleatum increases proliferation of colorectal cancer cells
and tumor development in mice by activating Toll-like receptor 4
signaling to nuclear factor-κB, and up-regulating expression of
MicroRNA-21. Gastroenterology. 152:851–866.e24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida
T, Fujimoto M, Watanabe M, Imanaga D, Sakai K, Matsumoto T,
Nishioka M, et al: Fusobacterium nucleatum as a prognostic
marker of colorectal cancer in a Japanese population. J
Gastroenterol. 53:517–524. 2018. View Article : Google Scholar
|
|
76
|
Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW,
Liu H, Huang PJ, Hu SN, Liao CT, Chang KP and Chang YL: Oral
microbiota community dynamics associated with oral squamous cell
carcinoma staging. Front Microbiol. 9:8622018. View Article : Google Scholar
|