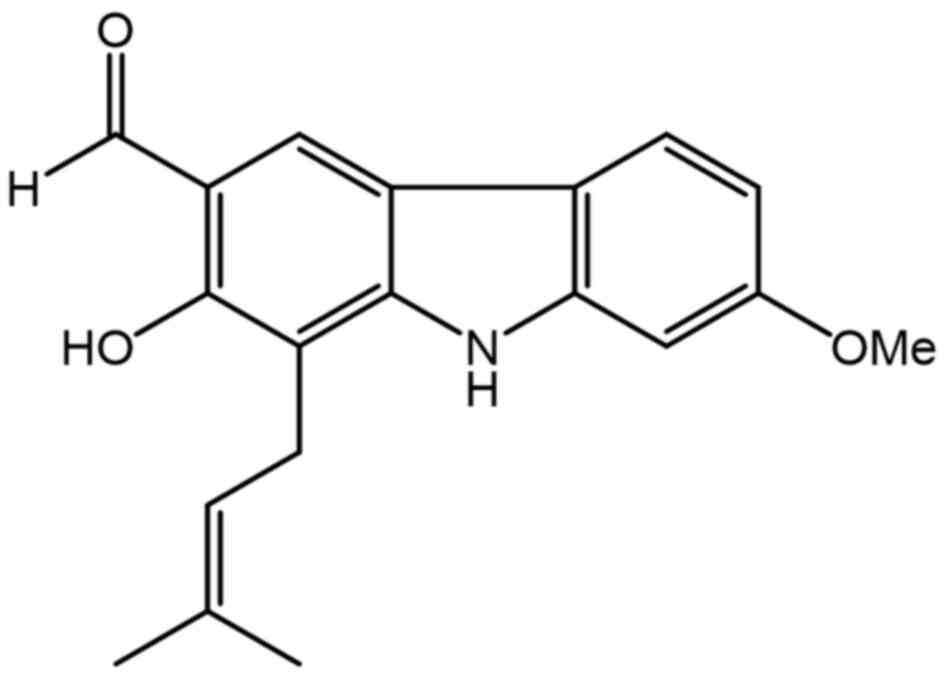
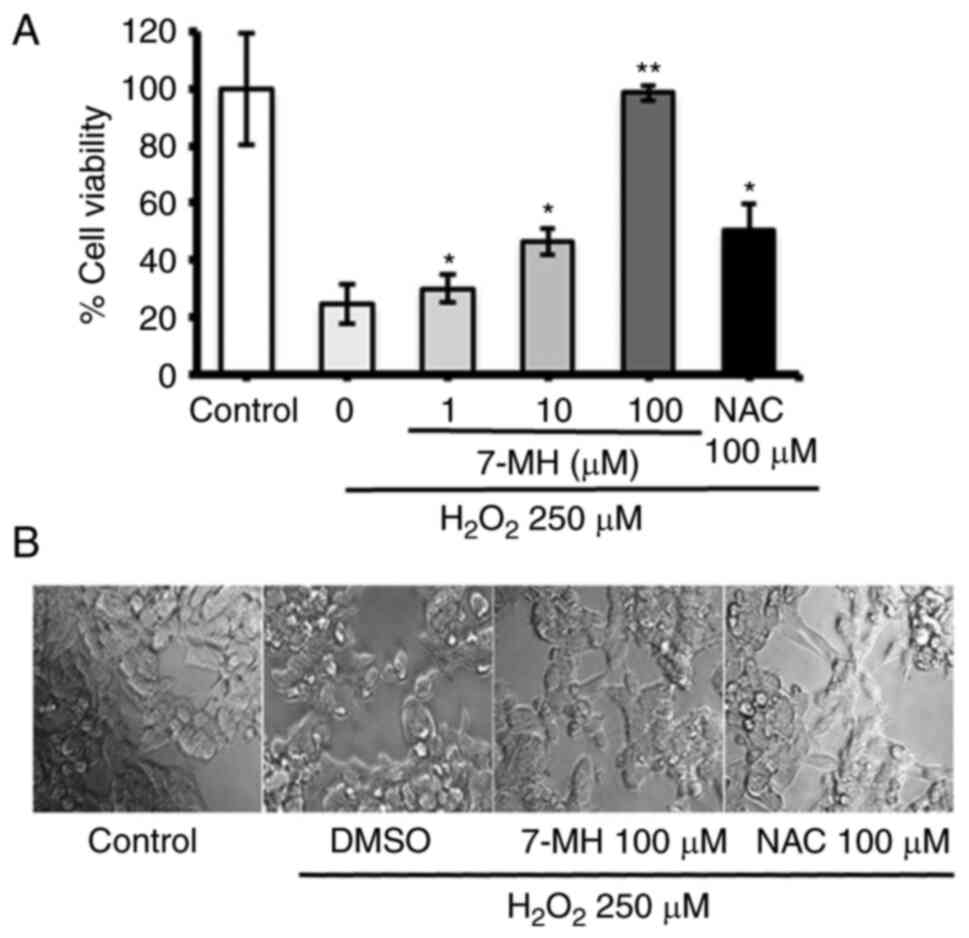
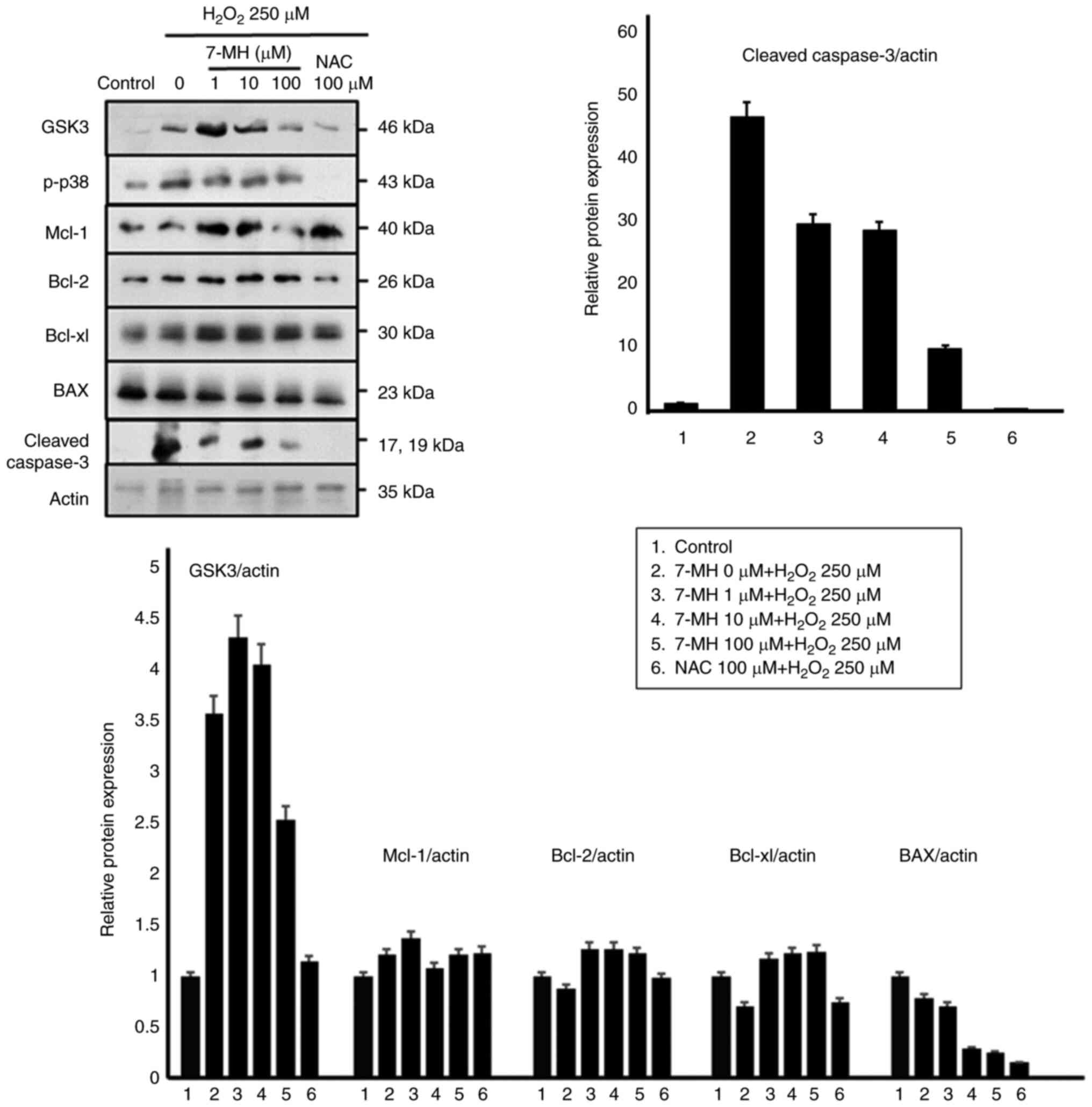
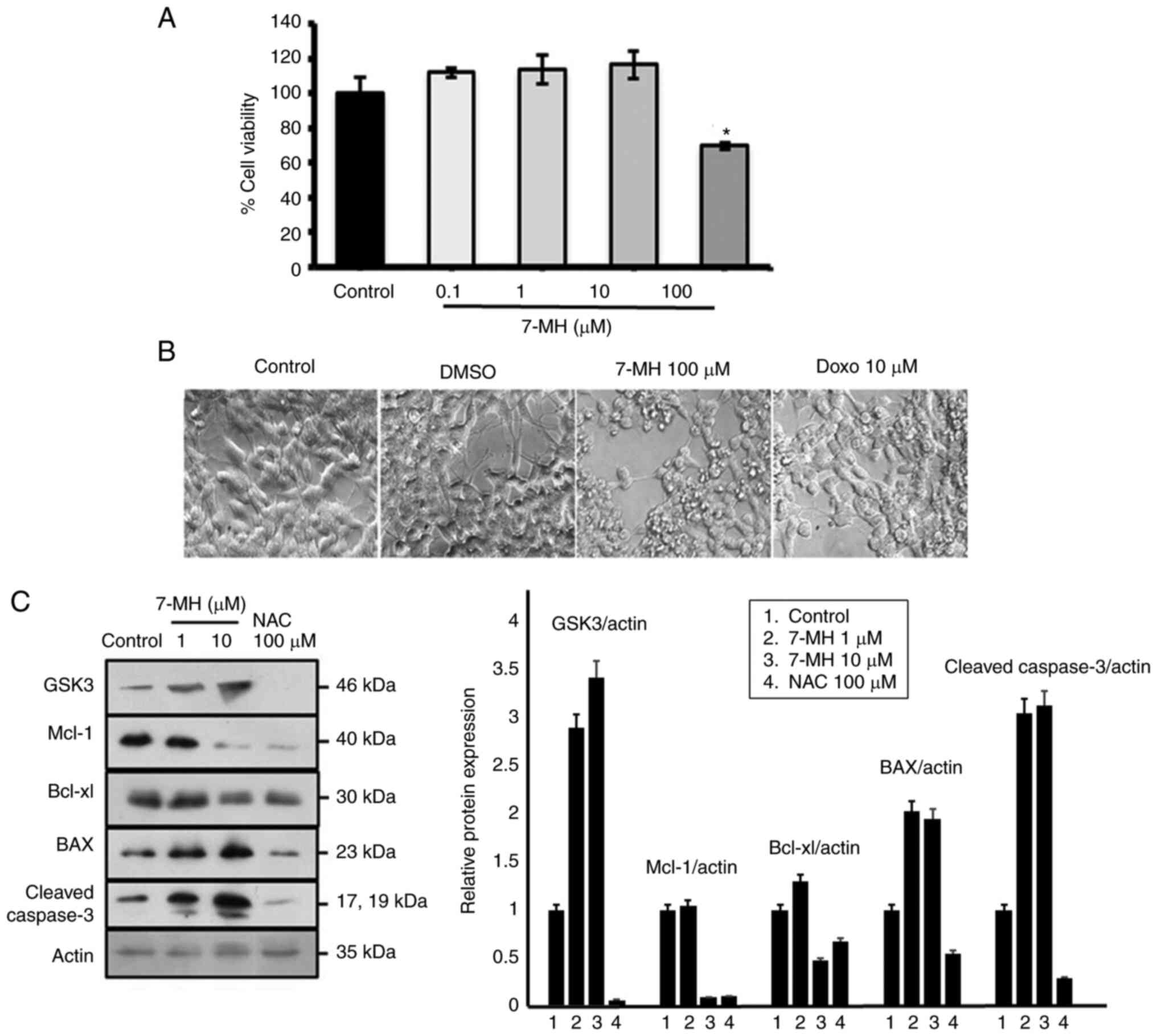
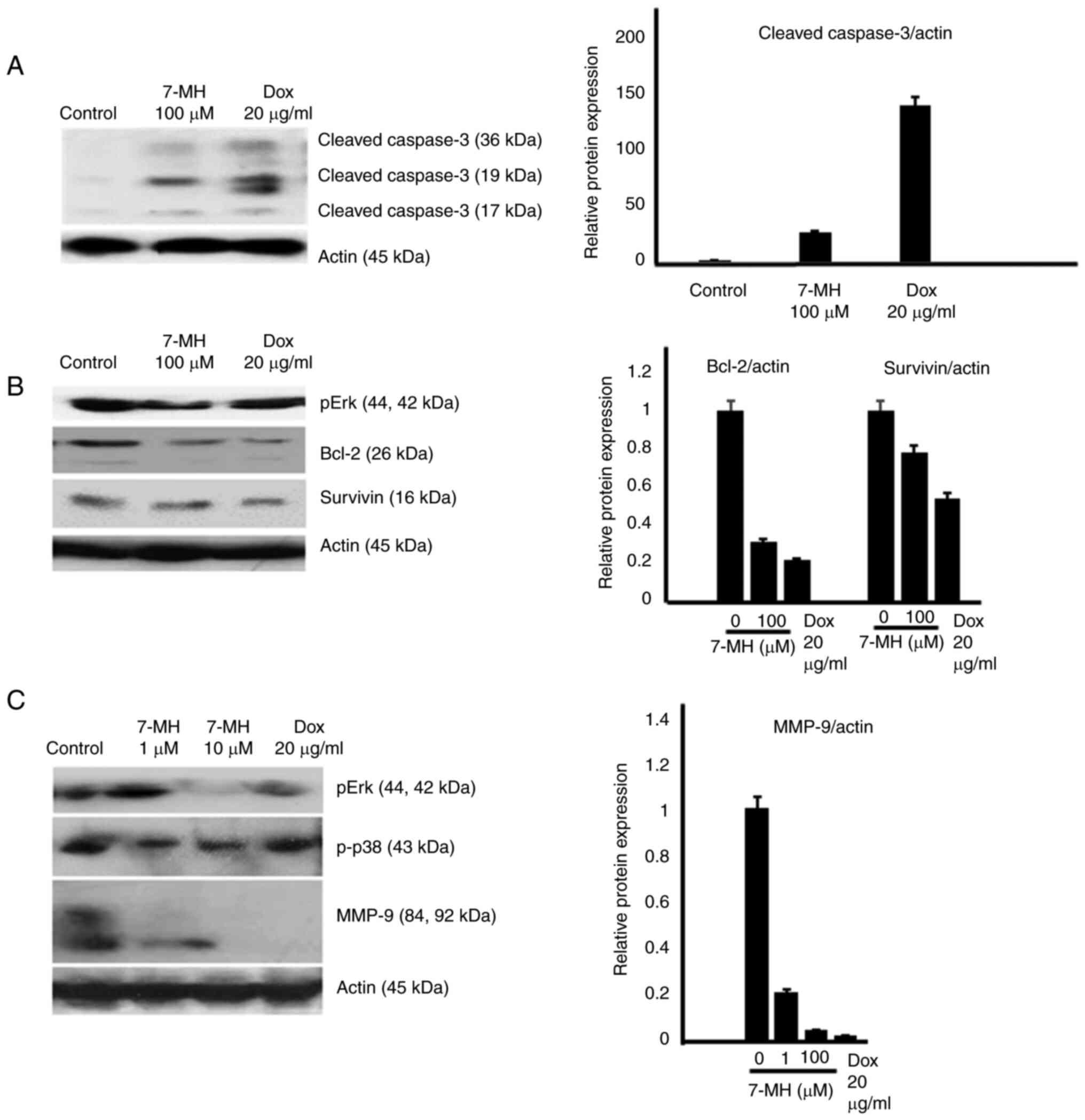
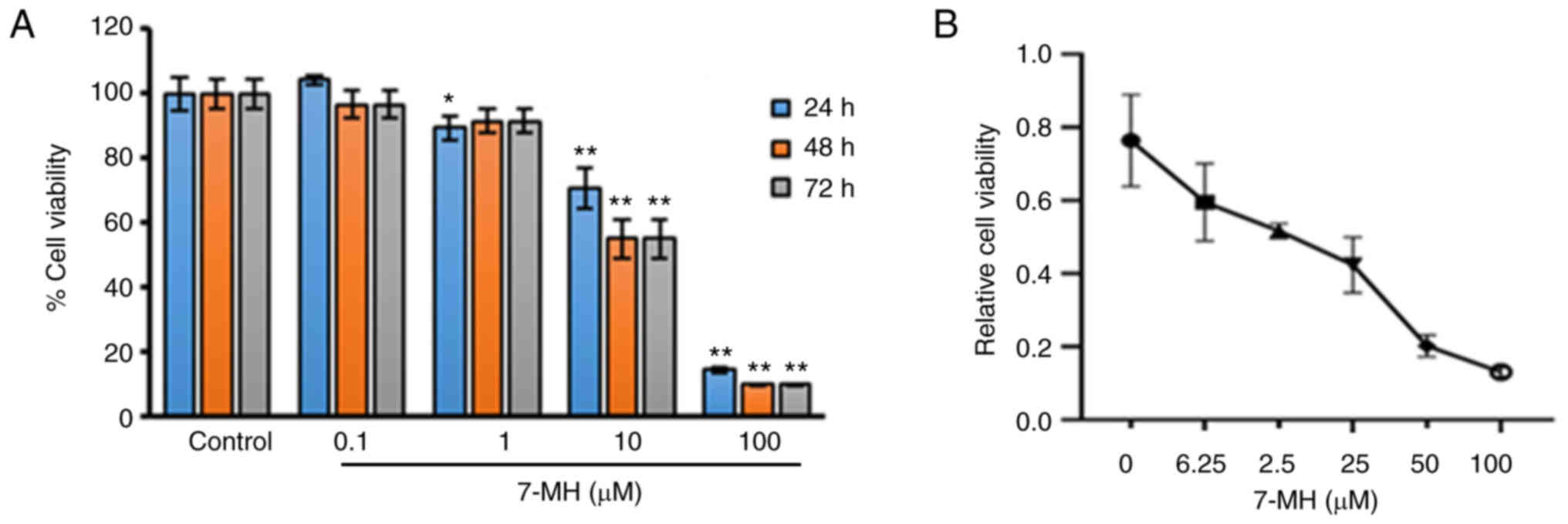
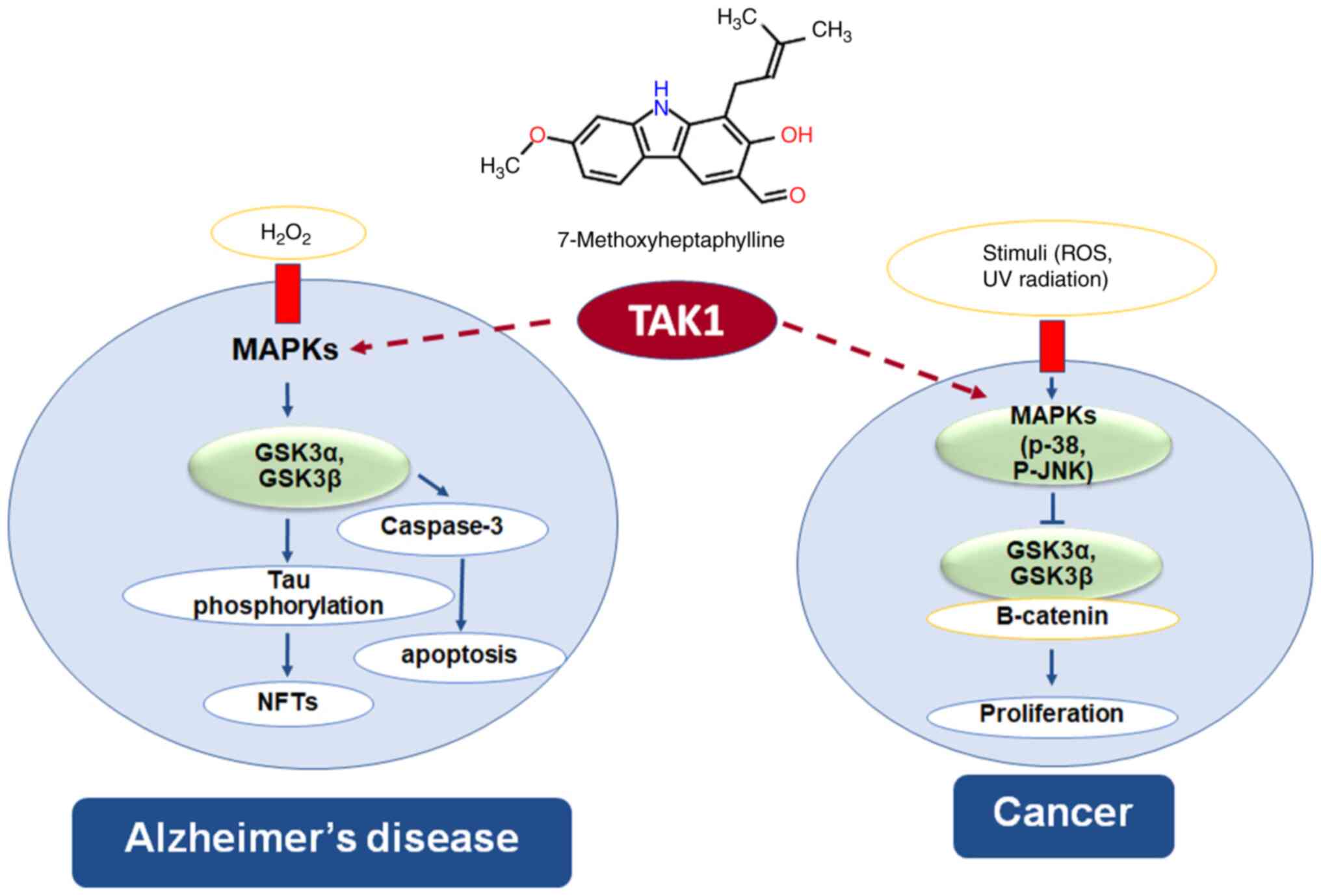
|
1
|
Roe CM, Behrens MI, Xiong C, Miller JP and
Morris JC: Alzheimer disease and cancer. Neurology. 64:895–898.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattson MP: Cellular actions of
beta-amyloid precursor protein and its soluble and fibrillogenic
derivatives. Physiol Rev. 77:1081–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Luca M, Colciaghi F, Pastorino L,
Borroni B, Padovani A and Cattabeni F: Platelets as a peripheral
district where to study pathogenetic mechanisms of alzheimer
disease: The case of amyloid precursor protein. Eur J Pharmacol.
405:277–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoh H, Kataoka H, Koita H, Nabeshima K,
Inoue T, Kangawa K and Koono M: Establishment of a new human cancer
cell line secreting protease nexin-II/amyloid beta protein
precursor derived from squamous-cell carcinoma of lung. Int J
Cancer. 49:436–443. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng JY, Kataoka H, Itoh H and Koono M:
Amyloid beta protein precursor is involved in the growth of human
colon carcinoma cell in vitro and in vivo. Int J Cancer. 92:31–39.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woods NK and Padmanabhan J: Inhibition of
amyloid precursor protein processing enhances gemcitabine-mediated
cytotoxicity in pancreatic cancer cells. J Biol Chem.
288:30114–30124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haven CJ, Howell VM, Eilers PH, Dunne R,
Takahashi M, van Puijenbroek M, Furge K, Kievit J, Tan MH, Fleuren
GJ, et al: Gene expression of parathyroid tumors: Molecular
subclassification and identification of the potential malignant
phenotype. Cancer Res. 64:7405–7411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krause K, Karger S, Sheu SY, Aigner T,
Kursawe R, Gimm O, Schmid KW, Dralle H and Fuhrer D: Evidence for a
role of the amyloid precursor protein in thyroid carcinogenesis. J
Endocrinol. 198:291–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takayama K, Tsutsumi S, Suzuki T,
Horie-Inoue K, Ikeda K, Kaneshiro K, Fujimura T, Kumagai J, Urano
T, Sakaki Y, et al: Amyloid precursor protein is a primary androgen
target gene that promotes prostate cancer growth. Cancer Res.
69:137–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takagi K, Ito S, Miyazaki T, Miki Y,
Shibahara Y, Ishida T, Watanabe M, Inoue S, Sasano H and Suzuki T:
Amyloid precursor protein in human breast cancer: An
androgen-induced gene associated with cell proliferation. Cancer
Sci. 104:1532–1538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyazaki T, Ikeda K, Horie-Inoue K and
Inoue S: Amyloid precursor protein regulates migration and
metalloproteinase gene expression in prostate cancer cells. Biochem
Biophys Res Commun. 452:828–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansel DE, Rahman A, Wehner S, Herzog V,
Yeo CJ and Maitra A: Increased expression and processing of the
Alzheimer amyloid precursor protein in pancreatic cancer may
influence cellular proliferation. Cancer Res. 63:7032–7037.
2003.PubMed/NCBI
|
|
13
|
Shiota M, Yokomizo A and Naito S:
Pro-survival and anti-apoptotic properties of androgen receptor
signaling by oxidative stress promote treatment resistance in
prostate cancer. Endocr Relat Cancer. 19:R243–R253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basu S and Tindall DJ: Androgen action in
prostate cancer. Horm Cancer. 1:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: Current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andersen JK: Oxidative stress in
neurodegeneration: Cause or consequence? Nat Med. 10
(Suppl):S18–S25. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paravicini TM and Touyz RM: Redox
signaling in hypertension. Cardiovasc Res. 71:247–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haigis MC and Yankner BA: The aging stress
response. Mol Cell. 40:333–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bostwick DG, Alexander EE, Singh R, Shan
A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W and Jiang X:
Antioxidant enzyme expression and reactive oxygen species damage in
prostatic intraepithelial neoplasia and cancer. Cancer. 89:123–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharifi N, Hurt EM, Thomas SB and Farrar
WL: Effects of manganese superoxide dismutase silencing on androgen
receptor function and gene regulation: Implications for
castration-resistant prostate cancer. Clin Cancer Res.
14:6073–6080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiota M, Yokomizo A, Tada Y, Inokuchi J,
Kashiwagi E, Masubuchi D, Eto M, Uchiumi T and Naito S: Castration
resistance of prostate cancer cells caused by castration-induced
oxidative stress through Twist1 and androgen receptor
overexpression. Oncogene. 29:237–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiota M, Yokomizo A and Naito S:
Oxidative stress and androgen receptor signaling in the development
and progression of castration-resistant prostate cancer. Free Radic
Biol Med. 51:1320–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santoro R, Zanotto M, Simionato F,
Zecchetto C, Merz V, Cavallini C, Piro G, Sabbadini F, Boschi F,
Scarpa A and Melisi D: Modulating TAK1 expression inhibits YAP and
TAZ oncogenic functions in pancreatic cancer. Mol Cancer Ther.
19:247–257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bang D, Wilson W, Ryan M, Yeh JJ and
Baldwin AS: GSK-3α promotes oncogenic KRAS function in pancreatic
cancer via TAK1-TAB stabilization and regulation of noncanonical
NF-κB. Cancer Discov. 3:690–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santoro R, Carbone C, Piro G, Chiao PJ and
Melisi D: TAK-ing aim at chemoresistance: The emerging role of
MAP3K7 as a target for cancer therapy. Drug Resist Updat. 33–35.
36–42. 2017.PubMed/NCBI
|
|
29
|
Xia S, Ji L, Tao L, Pan Y, Lin Z, Wan Z,
Pan H, Zhao J, Cai L, Xu J and Cai X: TAK1 is a novel target in
hepatocellular carcinoma and contributes to sorafenib resistance.
Cell Mol Gastroenterol Hepatol. 12:1121–1143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Venna VR, Benashski SE, Chauhan A and
McCullough LD: Inhibition of glycogen synthase kinase-3β enhances
cognitive recovery after stroke: The role of TAK1. Learn Mem.
22:336–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aouacheria A, Néel B, Bouaziz Z, Dominique
R, Walchshofer N, Paris J, Fillion H and Gillet G: Carbazolequinone
induction of caspase-dependent cell death in Src-overexpressing
cells. Biochem Pharmacol. 64:1605–1616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thongthoom T, Songsiang U, Phaosiri C and
Yenjai C: Biological activity of chemical constituents from
Clausena harmandiana. Arch Pharm Res. 33:675–680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Songsiang U, Thongthoom T, Boonyarat C and
Yenjai C: Claurailas A-D, cytotoxic carbazole alkaloids from the
roots of Clausena harmandiana. Nat J Prod. 74:208–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiratmatrakul S, Yenjai C, Waiwut P,
Vajragupta O, Reubroycharoen P, Tohda M and Boonyarat C: Synthesis,
biological evaluation and molecular modeling study of novel
tacrine-carbazole hybrids as potential multifunctional agents for
the treatment of Alzheimer's disease. Eur J Med Chem. 75:21–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosini M, Simoni E, Bartolini M, Cavalli
A, Ceccarini L, Pascu N, McClymont DW, Tarozzi A, Bolognesi ML,
Minarini A, et al: Inhibition of acetylcholinesterase, beta-amyloid
aggregation, and NMDA receptors in Alzheimer's disease: A promising
direction for the multi-target-directed ligands gold rush. J Med
Chem. 51:4381–4384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kozurkova M, Hamulakova S, Gazova Z,
Paulikova H and Kristian P: Neuroactive multifunctional tacrine
congeners with cholinesterase, anti-amyloid aggregation and
neuroprotective properties. Pharmaceuticals (Basel). 4:382–418.
2011. View Article : Google Scholar
|
|
37
|
Wangboonskul J and Yenjai C: Antioxidant
activity and cytotoxicity against cholangiocarcinoma of carbazoles
and coumarins from Clausena harmandiana. Sci Asia. 38:75–81.
2012. View Article : Google Scholar
|
|
38
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen Y, Vignali P and Wang R: Rapid
profiling cell cycle by flow cytometry using concurrent staining of
DNA and mitotic markers. Bio Protoc. 7:e25172017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L, Lu Y, Martinez J, Bi Y, Lian G,
Wang T, Milasta S, Wang J, Yang M, Liu G, et al: Proinflammatory
signal suppresses proliferation and shifts macrophage metabolism
from Myc-dependent to HIF1α-dependent. Proc Natl Acad Sci USA.
113:1564–1569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boonyarat C, Yenjai C, Vajragupta O and
Waiwut P: Heptaphylline induces apoptosis in human colon
adenocarcinoma cells through bid and Akt/NF-κB (p65) pathways.
Asian Pac J Cancer Prev. 15:10483–10487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Totzke J, Scarneo SA, Yang KW and Haystead
TAJ: TAK1: A potent tumour necrosis factor inhibitor for the
treatment of inflammatory diseases. Open Biol 10: 200099,
2020.
Brown K, Vial SCM, Dedi N, Long JM, Dunster NJ and Cheetham
GMT: Structural basis for the interaction of TAK1 kinase with its
activating protein TAB1. J Mol Biol. 354:1013–1020. 2005.PubMed/NCBI
|
|
45
|
Mancinelli R, Carpino G, Petrungaro S,
Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E and
Giampietri C: Multifaceted roles of GSK-3 in cancer and
autophagy-related diseases. Oxid Med Cell Longev. 2017:46294952017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rippin I and Eldar-Finkelman H: Mechanisms
and therapeutic implications of GSK-3 in treating
neurodegeneration. Cells. 10:2622021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|