Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-related death worldwide with 916,000 deaths in 2020
according to the WHO and studies of CRC markedly attract attention
in both basic and clinical sciences. The discovery of novel
therapeutic targets in cancer cells and increase of anticancer drug
choices have significantly improved CRC treatment strategies in
recent years. Several studies demonstrated that hormonal context of
the patient plays a crucial role in carcinogenesis, progression and
treatment outcome of breast, lung, colon, liver and prostate
cancers (1–4). It was revealed that hyperthyroidism
can increase the risk of cancer transformation and promote tumor
cells proliferation and migration. Concurrently, hypothyroidism was
found to exert opposite effects and may induce apoptosis of cancer
cells (5,6).
Despite the fact that cancer stem cells (CSCs) major
functions include driving the incidence, mortality and metastasis
of cancers (7), the relationship
between thyroid gland function, thyroid hormones activity and CSCs
properties remains controversial and requires further studies.
Since the existence of CSCs was demonstrated for most of cancer
types, including CRC (8), the
analysis of relations between thyroid hormones and CSCs
transformation appears to be crucial in order to improve success
rate of therapeutic strategies and survival rates of CRC
patients.
The aim of the present study was to investigate the
impact of triiodo-L-thyronine (T3) on the features of CRC CSCs
since these aspects of CRC microenvironment are still not fully
understood. Analysis of phenotype, cell cycle, survival and
proliferation abilities of CSCs was conducted in a 3D model of CSCs
cultures. Colonospheres are considered to represent a widely used
reliable surrogate assay to assess CSCs ability as it has been
previously reported by the authors (9,10).
The evaluation of the influence of cancer microenvironment elements
on cancer cells, their proliferative and metastatic capabilities is
a challenge to be addressed.
Materials and methods
Study design
Freshly isolated CRC tissues from CRC patients were
used to isolate and expand CSCs. CSCs were also isolated and
expanded from two commercially available CRC cell lines, HCT116 and
HT29, that were cultured in spherical forms. All subpopulations of
CSCs were treated in vitro with T3 thyroid hormone and
following 3 day-incubation certain biological properties were
determined. All results were compared with untreated control cells.
All human CRC fragments involved into the current study were
obtained from surgically resected specimens from CRC patients.
Written informed consent for receiving samples from the specimens
was obtained from the patients. Ethical approval (approval no.
(NKBBN/203/2020) was obtained from The Independent Bioethics
Committee of Medical University of Gdansk (Gdansk, Poland). The
development of a second neoplastic disease and previous chemo-
and/or radiotherapy were exclusion criteria in the present study.
All included patients underwent cancer staging and treatment
according to EURECCA guidelines (11). Additionally, cancers were staged
according to the tumor, node, metastases (TNM) classification (8th
edition) (12).
For the purpose of this study data were analyzed in
two groups: early (stages I + II) and advanced CRC stage (stages
III + IV). The rationale for this stratification is based on
differing prognosis and treatment guidelines between early
(predominantly surgery) and advanced cancers (surgery with adjuvant
chemotherapy or neoadjuvant chemoradiation in rectum) (11).
CRC primary cell lines isolation and
expansion
A total of 27 CRC patients were enrolled into the
study in the Department of General, Endocrine and Transplant
Surgery, Medical University of Gdansk, Poland. The samples were
collected between July 2020 and April 2021. The solid tumor
fragments were placed into sterile PBS supplemented with
Antibiotic-antimycotic solution and immediately processed in
culture. All chemical supplements and compounds were purchased from
Sigma-Aldrich; Merck KGaA. The growth factors were purchased from
R&D Systems, Inc. CRC fragments were washed in serum-free
Dulbecco's modified Eagle medium (DMEM)-F12 with
antibiotic-antimycotic agent. Afterwards, all specimens were
sectioned into smaller pieces (1–2 mm3) to improve
impregnation with collagenase (20 ng/ml) and hyaluronidase (20
ng/ml) for 90 min. Next, cells were filtrated through a 70-µm cell
strainer. Primary colon spheroid cultures (SC) were obtained in
serum-free stem cell medium (SCM): DMEM-F12 medium supplemented
with ITS Liquid Media Complement (1X), BSA (4 mg/ml), glucose (3
ml/ml), Hepes (5 mM), L-glutamine (2 nM), progesterone (20 nM),
putrescine (9.6 µg/ml), Heparin (4 µg/ml), EGF (20 ng/ml), bFGF (20
ng/ml), and Antibiotic-antimycotic solution (1X). The composition
of SCM was previously established by our group (9,10).
Only early passaged SCs were used for analysis in the current
study.
HCT116 and HT29 cell lines
expansion
Two human CRC cell lines (HT29 and HCT116; (obtained
from American Type culture Collection) were used. Cells were tested
for Mycoplasma contamination. The adherent form of these
cell lines was expanded routinely in McCoy's medium supplemented
with 10% FBS, 1% penicillin-streptomycin and 2 mM L-glutamine. The
cells were passaged with the use of trypsin 2–3 times/week when
they achieved 80% confluency. For the need of the current study,
CRC cell lines were cultured as colonospheres (spherical 3D forms,
tumorospheres) in SCM of the same composition as medium used for
CSCs from CRC patients. HCT116 and HT29 cell lines needed at least
three passages to obtain the appropriate spherical forms to be
included into further analyses.
Cell treatment
HCT116 and HT29 cells (8×105/ml) and
cancer cells isolated from CRC patients were seeded in 24-well
ultra-low attachment plates and maintained in SCM. Spheres were
treated for 3 days with thyroid hormone T3
(3,3′,5-triiodo-L-thyronine; Sigma-Aldrich; Merck KGaA) and
incubated at 37°C under a humidified atmosphere of 5%
CO2. To prepare T3 solutions, 1.0 ml of 1.0 N NaOH to 1
mg of T3 (powder form) was added, gently swirled to dissolve the
powder and 49 ml of sterile medium was then added, according to the
manufacturer's instructions. All T3 solutions were prepared shortly
before use.
Due to limited number of cells (particularly
obtained from CRC patients) certain experiments were conducted with
only chosen T3 concentrations. The cells maintained in the SCM
without T3 were used as control cells. Initially, T3 concentrations
for the present study were established according to literature data
(0.5, 1 or 2 nM) (13–16). Our first analyses indicated that T3
in concentrations higher than 1 nM causes cell death, thus if a
selection had to be made lower T3 concentrations (0.5 or 1 nM) were
used. Specifically, the T3 concentrations used are clearly
presented on each Fig. and in figure legends.
The analysis of CRC cells' phenotype
with flow cytometry
CRC cell lines and cancer cells isolated from CRC
patients were stained with monoclonal antibodies (BD Biosciences)
characteristic for some CSC-specific antigens: anti-CD29-APC (clone
MAR4, IgG1κ; cat. no 559883), anti-CD44-FITC (clone C26, IgG2bκ;
cat. no 555478). Additionally, anti-CD133/2-PE (clone 293C3,
IgG2bκ; cat. no 130-113-186) monoclonal antibody (Miltenyi Biotec,
Inc.) was used. Cells were incubated for 30 min in the dark at room
temperature (RT), washed and resuspended with PBS containing 1 mM
EDTA for FACS analyses which were performed using FACS Calibur flow
cytometer (BD Biosciences) and BD CellQuest Pro 6.0 software. The
frequency of positive cells was compared with untreated control
cells. To set a threshold of positive signal, unstained cells were
used. FACS data are presented as a percentage count of cells with
particular phenotype within population set on FSC/SSC plot with
excluded small and dead cells.
Cell death assay (7AAD)
Following the T3 treatment of HCT116 and HT29 cells
and cancer cells isolated from CRC patients the proportion of dead
cells in our samples was evaluated. For this purpose, 7AAD Via
Probe (BD Biosciences) was used. The cells were incubated for 30
min at RT with 10 µm of a dye, washed with PBS and prepared for
FACS analysis. To set a threshold of positive signal, unstained
cells were used. The frequency of 7AAD− positive cells
was compared with untreated control cells.
Cell cycle analysis
After T3 treatment, 5–10×106 HCT116 and
HT29 cells and cancer cells isolated from CRC patients were washed
twice in PBS (Sigma-Aldrich; Merck KGaA), fixed with 70% ethanol on
ice and stored at −20°C until analysis (up to 7 days). Next, cells
were treated with ribonuclease to remove any contaminating RNA
molecules. Afterwards, the DNA of cells was stained with propidium
iodide (50 µg/ml) (PI; Sigma-Aldrich; Merck KGaA). The fluorescence
of the PI-stained cells was analyzed by flow cytometry
(FACSCalibur™; BD Biosciences) and the internal control was
untreated cells. To set a threshold of positive signal, unstained
cells were used.
Proliferation assay
HCT116 and HT29 cells (1.5×104/ml were
seeded in 96-well low attachment plates in SCM, then newly formed
spheroids were treated with T3 (0.5, 1 or 2 nM) in 3 replicates for
each option. The whole experiment was repeated twice. Non-treated
cells were negative control. After 3 days, cell proliferation was
assessed by colorimetric Cell Tilter 96® Aqueous One
Solution Cell Proliferation Assay (Promega Corporation), according
to the manufacturer's instructions. Briefly, 20 µl of Cell Titer
96® Aqueous One Solution Reagent were added into each
well of the 96-well assay plate containing the samples in 200 µl of
culture medium. After 4 h of incubation at 36°C the absorbance at
490 nm was recorded using a microplate reader (BioTek Instruments,
Inc.).
Viability assay
HCT116 and HT29 cells (1.5×104/ml) were
seeded in 96-well low attachment plates in SCM, then colonospheres
were treated with T3 (0.5, 1 or 2 nM) in 3 replicates for each
option. The whole experiment was repeated twice. Non-treated cells
were used as negative control. After 3 days total levels of
cellular ATP were assessed to analyze the cell viability with the
use of Luminescent ATP Detection Assay kit (Abcam). The luminescent
ATP assay protocol involves lysis of the cell sample, addition of
luciferase enzyme and luciferin, and measurement of the emitted
light using a microplate-based luminometer (BioTek Instruments,
Inc.). This kit irreversibly inactivates ATPases during the lysis
step, ensuring that the luminescent signal obtained truly
corresponds to the endogenous levels of ATP. The procedure was
conducted according to the manufacturer's instructions. Briefly,
detergent solution was added to each well and incubated for 5 min
at RT to lyse and stabilize ATP. Subsequently, substrate solution
was added and after 10 min incubation in dark at RT the
luminescence signal was detected. The concentration of ATP in each
sample was estimated according to the standard curves acquired
after analysis of standard dilutions. Finally, results were
presented in µM units.
Colonosphere formation and
quantification
The colonospheres derived from HCT116 and HT29 cells
or cells isolated from CRC patients were cultured in sphere-forming
media and treated with T3 for 3 days. Then the diameter of at least
50 spheres of each experimental group was measured with an inverted
light microscope (CKX53) coupled with a digital camera Olympus SC50
(Olympus Corporation). Untreated cells were used as internal
control.
Secondary sphere formation
ability
After 3 days of treatment with T3, cells
(5×104/ml) were pooled, suspended and seeded in fresh
SCM in 96-well low attachment plates. Spheres derived from HCT116
and HT29 cells or cells isolated from CRC patients were monitored
by measuring the maximal outgrowth of sphere's diameter after 1
week. Untreated cells were used as internal control. Images were
captured using an inverted microscope (CKX53) coupled with digital
camera Olympus SC50 (Olympus Corporation).
Western blot analysis
Cell lysates were prepared from colonospheres
obtained from both treated and control HCT116 and HT29 cells and
cancer cells isolated from CRC patients. After treatment, cells
were incubated for 30 min on ice in a RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) supplemented with protease and
phosphatase inhibitor cocktail; then centrifuged at 16,000 × g for
10 min at 4°C. Protein concentration in the lysates was measured
with Bradford reagent (Sigma-Aldrich; Merck KGaA). Protein samples
(10 µg) were loaded to 4–20% Mini-PROTEAN® TGX™ Precast
Protein Gels (Bio-Rad Laboratories, Inc.) and electroblotted onto a
PVDF membrane with the use of the Trans-Blot Turbo system (Bio-Rad
Laboratories, Inc.). Membranes were incubated with 5% non-fat milk
in 1% TBST buffer for 1 h at RT. Afterwards, the membranes were
incubated overnight at 4°C with the following primary antibodies
(purchased from Thermo Fisher Scientific, Inc.): rabbit polyclonal
anti-THRα1 antibody (1:500; cat. no BS-6221R), rabbit polyclonal
anti-THRβ1 antibody (1:100; cat. no PA1213A), rabbit polyclonal
anti-DIO2 antibody (1:1,000; cat. no PA549631) and rabbit
polyclonal anti-DIO3 antibody (1:1,000; cat. no PA526537). On the
next day, blots were washed with TBST and incubated for 1.5 h at RT
with HRP-conjugated anti-rabbit IgG antibody (1:10,000; cat. no
1706515; Bio-Rad Laboratories, Inc.). Anti-GAPDH
peroxidase-conjugated IgM antibody (1:50,000; cat. no G9295;
Sigma-Aldrich; Merck KGaA; 1 h at RT) was used as the loading
control. The membranes were washed and subsequently subjected to
luminol reagents (Bio-Rad Laboratories, Inc.). The
chemiluminescence signal was measured with ImageQuant LAS 500
(Cytiva). Changes in protein level were assessed by densitometric
scanning of the bands (ImageQuant™ TL 10.1 analysis software
(Cytiva) and corrected for GAPDH loading control. Each experiment
was performed in triplicate. Proteins with molecular weights 51,
43, 60, 34 and 37 kDa for THRα1, THRβ1, DIO2 in dimeric form, DIO3
and GAPDH, respectively, were analyzed.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (version 6.05; GraphPad Software, Inc.). Data were subjected
to non-parametric Mann-Whitney U test or Kruskal-Wallis test
followed by Dunn's test as a post hoc procedure and Spearman's rank
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference. Data in figures are presented
as the median ± interquartile range or median with min-max
values.
Results
In the present study, early stage CRC consisted of 2
(7.5%) stage I cancer patients and 11 (40.5%) stage II cancer
patients. Group of advanced stage consisted of 12 (44.5%) stage III
cancer patients and 2 (7.5%) stage IV cancer patients. In our study
population, cancer was localized in cecum (11%), ascending colon
(15%), transverse colon (7.5%), descending colon (7.5%), sigmoid
(33%), rectum (22%) and one of the patients (4%) had two CRC in two
different segments of large bowel. In detail 14 patients had lymph
node metastases (stage III or IV) and 2 patients had distant
metastases at the time of diagnosis (stage IV).
Patient-derived spherical cultures contained
variable proportion of CSCs bearing CD133 marker (ranging from 7 to
91%), thus the samples were divided according to CD133 median value
(which was 20%) into 2 groups: with high (43±19%) and low (12±4.4%)
CD133+ cells count. Apparently, the CD133+
cells count was significantly different between these groups
(P<0.0001).
T3 eliminates colorectal CSCs in vitro
culture
Fresh CRC specimens were collected from 27 patients
and it was possible to establish spherical cultures from the cancer
tissue of 21 (77.8%) patients, in the remaining cultures cancer
cells did not survive ex vivo due to bacterial
contamination. All functional tests were conducted with cells
obtained after the first passage since it has been already revealed
that the phenotype of CSCs along the expansion was stable (9). The additional clinicopathological
information concerning patients included into the present study are
presented in Table I.
 | Table I.Clinicopathological characteristics
of CRC patients included into the present study. |
Table I.
Clinicopathological characteristics
of CRC patients included into the present study.
| Cancer stage | I–II | III–IV |
|---|
| Total number | 13 | 14 |
| Sex |
|
|
|
Male | 7 | 8 |
|
Female | 6 | 6 |
| Age, years | 73.4 | 71.4 |
| Mean (min-max) | (56–89) | (59–96) |
| Body mass
index | 27.5 | 25 |
| Mean (min-max) | (21–30) | (20–30) |
| CD133+
(%)a |
|
|
| Mean (min-max) | 31 (7–89) | 28 (7–91) |
Both HCT116 and HT29 CRC cell lines and cancer cells
from patients were cultured in a form of colonospheres which are
highly enriched with CSCs, as previously reported by the authors
(10,17). All cell populations were subjected
to cytometric analysis of phenotype following the treatment with T3
thyroid hormone. The proportion of CSCs with commonly used CSC-like
markers, namely CD133, CD44 and CD29, was evaluated. The number of
CD133+ cells in culture of spheroids obtained from all
cancer cells populations did not change significantly (Figs. 1 and 2 and S1), with two exceptions (Fig. 1A).
The general count of CD133+ CSCs in
colonospheres representing tissue of patients with CRC of stages I
and II did not differ significantly from colonospheres derived from
tissues of advanced cancer patients (stages III and IV), thus these
groups were divided according to the number of CD133+
median value (into Low and High groups). The statistically
significant difference in the percentage of CD133+
expressing cells in colonospheres derived from patients
representing both Low and High groups was confirmed (P<0.05;
Fig. 1). The presented division of
patients' samples (Fig. 1)
demonstrated that both groups with early and advanced cancer
contained samples with high and low count of these primitive cells.
This information is of interest since CSCs are known to be
responsible for the most serious events of carcinogenesis.
Additionally, the analysis of correlation revealed that the amount
of untreated and treated CD133+ cells did not depend
directly on clinical stage of CRC (R=0.249; P=0.25) (Fig. S2). Furthermore, all spherical
populations were analyzed with respect to the presence of CD29 and
CD44 integrins. It was observed that CSCs in colonospheres were
more likely to change the percentage of cells bearing another
analyzed marker in response to T3 treatment. It was noted that CRC
colonospheres treated with T3 had increased count of
CD133+CD44−CD29+ cells
(P<0.05). The alterations in count of
CD133+CD44−CD29+ cancer cells were
a mirror image (Fig. 1C) compared
with CD44+ counterparts (Figs. 1B and C and 2C and D).
T3 in all concentrations caused visible changes in
the morphology of spheres derived from both examined CRC cell lines
and cells of patients with CRC. The sizes of colonospheres were
significantly reduced when the cells were treated (Fig. 3) with T3 (P<0.05) and the number
of dead cells and debris in the medium increased following the
treatment.
Additionally, another assay was carried out to
determine the ability of CRC cells to form colonospheres following
72-h-long incubation of T3-treated cells. Following T3 treatment
cells were transferred into fresh medium without T3 (Fig. 4). After 7 days, the effects of T3
on the ‘secondary sphere’ formation ability were examined,
reflecting the tumor-initiating capacity and susceptibility of the
CSC-like cells to T3. Both CRC cell lines and cells of patients
with CRC presented similar results. Pretreatment with T3 exerted
the constant influence on CRC cells. After one week of incubation
in fresh medium without T3, CRC cells should form colonospheres
with the typical morphology and size, but it was found that the T3
pre-treated colonospheres were evidently smaller (P<0.05) in
comparison to primary spheres. Additionally, numerous freely
floating cells or small aggregates were observed.
T3 decreases the viability of
colorectal CSCs
The percentage of non-viable cells following T3
treatment was evaluated by flow cytometric assay using 7-AAD dye
(Figs. 5 and S3), which is excluded by living cells,
but binds selectively to the DNA of damaged permeabilized cells. It
was revealed that the number of 7AAD-positive cells among cultured
cells increased after incubation with T3 in concentration-dependent
manner (P<0.05). Similar results were observed for colonospheres
derived from both CRC cell lines and cells isolated from patients
with CRC. These results were confirmed by the analysis of cell
cycle using PI (Figs. 6D-F and
S4). The increased proportion of
cells in subG1 phase was noted after the incubation of cells with
T3 in different concentrations in comparison to untreated control.
At both concentrations of T3 the fraction of cells undergoing
apoptosis (in subG1 phase) increased significantly up to 22±4.53%
(median ± SD) for 1 nM T3-treated cells of patients (Fig. 6).
The distribution of cells in various phases of the
cell cycle was determined in colonospheres' cultures of HCT116 and
HT29 cell lines and cancer cells obtained from CRC patients.
Generally, the proportion of cells in active phases (S/G2/M) was
higher in comparison to cells representing quiescence pool (G0/G1)
(Fig. 6A-C) of colonospheres
obtained from all populations included into the present study.
G0/G1 cell cycle growth arrest was observed after the incubation of
spheres with T3, while the number of cells in S and G2-M phases
(active phases of cell cycle) was markedly reduced in comparison to
untreated control.
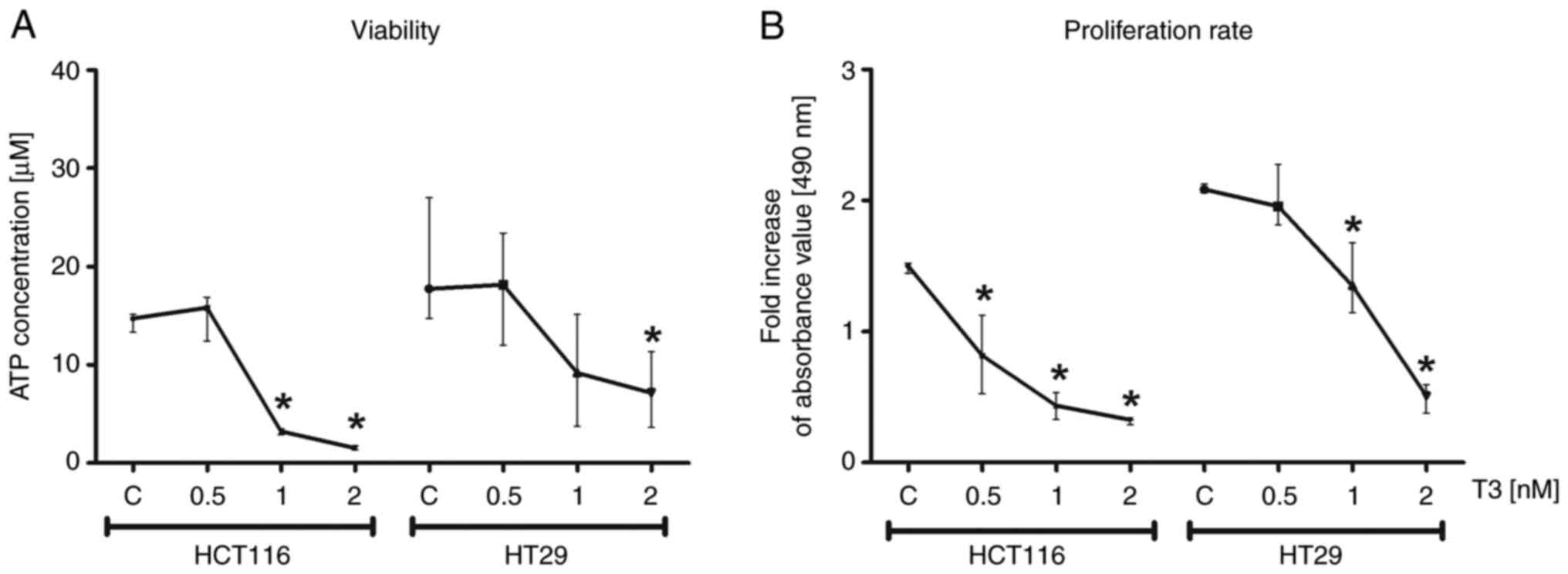
The viability of CRC cells of HCT116 and HT29 cell
lines was analyzed by the assessment of total levels of cellular
ATP. The test assumed the decrease of ATP level in samples as an
indicator of cell death. The results confirmed that the increased
concentration of T3 in culture reduced viability of colonospheres
(P<0.05; Fig. 7A). In addition,
colorimetric test was performed relaying on MTS reagent to
determine the proliferative capacity of HCT116 and HT29-derived
colonospheres treated with T3. It was revealed that the
proliferation rate was significantly decreased following T3
treatment in comparison to control cells (P<0.05; Fig. 7B).
T3 influences the expression of
proteins associated with T3 activity
Since the effects of thyroid hormones depend on the
activity of some proteins engaged in their activation or binding,
the impact of T3 on protein level of two thyroid hormone receptors
(THRα1 and THRβ1) and two deiodinases (DIO2 and DIO3) was evaluated
by western blotting.
As demonstrated in Figs. 8 and 9, the results obtained for CRC cell
lines-derived colonospheres were not fully consistent with changes
observed in cancer cells obtained from patients. Western blotting
of colonospheres originated from HCT116 cells treated with T3
demonstrated increased relative levels of almost all analyzed
proteins. The elevations of THRα1 and DIO3 levels were
statistically significant in comparison to control (P<0.05). The
level of DIO2 remained at the same level after T3 treatment.
Concurrently, the expression of all proteins in colonospheres
obtained from HT29 cells was lower in treated samples than in
control spheres and only DIO3 change did not reach statistical
significance. The western blot analysis of colonospheres from
patients with CRC revealed no substantial alterations in the
expression of proteins assessed in the present study, however, when
samples of patients were divided according to cancer clinical
stages, the expression of DIOs was found lower in cancer cells
obtained from patients with stage III–IV CRC in comparison to
samples collected from patients with stage I–II CRC. Furthermore,
the DIO2 and DIO3 protein relative levels were mostly upregulated
in patients representing group with early cancer (stage I–II).
Discussion
Thyroid hormones play crucial role in the regulation
of multiple physiological activities including differentiation,
growth and metabolism and are required for normal function of
nearly all tissues (18,19). However, thyroid hormones attract
attention since their regulatory functions were found crucial for
both physiological processes in normal cells of healthy tissues and
also have a great impact on the proliferative abilities and cancer
growth of cancer cells. Clinical hypothyroidism was found to be
associated with delayed cancer development. Concurrently,
hyperthyroidism is correlated with increased cancer growth,
including breast, thyroid, lung, brain, liver and CRC (1,18,20–22).
The current study provided novel evidence that T3
can be an important modulator of CRC CSC properties. A model of
expansion of colonospheres derived from CRC cell lines and CRC
cells isolated from cancer tissue was used. These procedures were
previously exercised by the authors (10). The experimental conditions mimicked
the microenvironment with locally increased T3 concentration where
it was monitored how this crucial thyroid hormone altered features
of CSCs or colonospheres. It was found that the proportion of
CD133+CD29+CD44+ stem-like cells
was significantly decreased following the 3-day treatment with T3,
suggesting that treated colonospheres displayed a lower content of
CSCs in comparison to untreated control. Indeed, it was identified
that T3 significantly inhibited the ability of cancer cells to form
colonospheres by increased apoptosis and elevation of G1/G0
silenced pool within cultures. Similarly, the treatment of MCF
breast cancer cell line with T3 resulted in decreased proliferative
and migratory potential of these cells and reduced number of CSCs
(23).
The actions of T3 are initiated by binding to
nuclear thyroid receptors (TRs), encoded by two genes, α and β,
which give rise to different receptor isoforms (24). TRs are widely distributed in
mammalian tissues, but transformed or immortalized cells in general
express very low levels of TRs. In addition, there is increasing
evidence that alterations in TRs are common events in cancer and it
has been suggested that TR genes may function as tumor suppressors
(25–28), although the role of these receptors
in the pathogenesis and progression of neoplastic processes is
currently unclear (28) and
results in different types of cancer are not fully consistent
(20,22,29–32).
The present results are concomitant with this general concept since
lower THRα1 and THRβ1 relative protein levels were observed in
untreated colonospheres derived from HCT116 cell line which
represent CRC with higher progression status in comparison to HT29
cells (TNM3/Dukes' D vs. TNM2/Dukes' C, respectively; P<0.05)
(33). Tissue samples of patients
presented more diverse results which can be partially explained by
heterogeneity of individuals recruited to the present study. CRC
cells appeared to sustain both receptors at low but constant level
for the potential need to activate the expression of T3 target
genes (34). Similar results
concerning expression of thyroid hormone receptors were described
by Wang et al (2), who
reported that thyroid hormones increase cells' self-renewal
capacity and the percentage of CD90+ CSCs and promote
drug resistance of primary human HCC cells. It was suggested that
THRβ1 acts as tumor suppressor in a number of cancers and one of
the proposed mechanism relay on upregulation of the nuclear
receptor co-repressor 1 and suppression of invasion, tumor growth,
and metastasis in human as it was demonstrated for hepatocellular
carcinomas (35) and
neuroblastomas (36). In addition,
breast cancer mammospheres presented reduced tumorigenesis upon
stimulation of THRβ1 (37). The
possible mechanisms are downregulation of cyclin D1 expression and
modulation of the TNFα-NFκB signaling (23,37).
Certain of the present results supported these previous
observations since it was observed that the expression of THRβ1
receptor was significantly lower in HT29 cells following T3
treatment. In addition, an increased level of THRβ1 protein was
demonstrated along with clinical advancement of CRC.
The primary secretory product of the thyroid gland,
3,5,3′,5′ tetra iodothyronine (T4) or thyroxine, must be converted
to T3 for its activation. DIO2 (activating DIO) converts the
prohormone thyroxine to the active thyroid hormone T3, whereas DIO3
(deactivator DIO), by inactivating both T4 and T3, terminates
thyroid hormone action (38). This
pre-receptor process is an essential mechanism that regulates
thyroid hormone function at the intracellular level. In colorectal
adenomas and carcinomas, DIO3 expression is significantly higher
than in normal tissues and negatively correlates with the
histologic grade of the lesions suggesting that DIO3 could be a
marker of early stages of carcinogenesis (38). That appears to be consistent with
the present results conducted with both CRC cell lines and cells of
patients. Higher DIO3 level was observed in samples derived from
patients with lower CRC stage (I–II) in comparison to patients with
III–IV stage. The parallel increased expression of both DIOs in
cancer cells was assumed to rapidly customize the thyroid hormone
signal if necessary what was previously presented for BCC cells as
well (39). The alterations in
protein levels of both enzymes depended on cancer clinical stage
and it was supposed to protect cells from anti-proliferative and
anti-survival effect of T3 in vitro cultures. Similar
conclusions may be drawn from the analysis of levels of DIOs in the
samples of the present study. Higher DIO2 and DIO3 was revealed in
stage I–II CRC samples compared with untreated control and stage
III–IV CRC cells. These results are in accordance with observations
of increased stromal DIO2 level of intestinal polyps of ApcΔ716
mice, a mouse model of familial adenomatous polyposis and early
stage sporadic CRC (40).
Furthermore, it was identified that DIO3 is a direct
transcriptional target of the β-catenin/TCF complex and its
expression was higher in human intestinal adenomas and carcinomas
than in healthy intestinal mucosa. Experimental attenuation of
β-catenin reduced DIO3 levels and induced DIO2 thereby increasing
T3-dependent transcription. In the absence of DIO3, the T3 excess
reduced cell proliferation and promoted differentiation in cultured
cells and in xenograft mouse models (38). Increased level of DIO3 in samples
after treatment with T3 may be an evidence that the overabundance
of active thyroid hormone can compromise the fragile niche
homeostasis. The increasing T3 concentration inhibited
proliferation of treated cells which can be an effect of
insufficient DIO3 level. The decline was more significant for
HCT116 cells with lower initial DIO3 expression. The CSCs tended
toward silencing their activity after treatment with elevated T3
concentration which was revealed with the analysis of phenotype and
cell cycle.
It was also observed that the incubation of
colonospheres with T3 decreased viability, proliferative and
spherogenic potential of cancer cells. In addition, increased
apoptotic rate of CRC cells was revealed. Concurrently, cells of
treated colonospheres were more likely to move into silenced pool
in G0/G1 phase of the cell cycle. Additionally, the smaller sizes
of colonospheres following the treatment with T3 suggested that T3
can lower the proportion of primitive cells actively supplying the
pool which proliferate within spheres. It was hypothesized that
this could be a protective mechanism aimed to avoid the elimination
of all cancer-initiating cells from colonospheres. In this context
it was confirmed that the hormonal constituents of cancer niche
have a crucial role for the fate of CSCs.
The technical limitation is the use of 1.0 N
NaOH-based solvent to prepare T3 solutions. Although our initial
study demonstrated that the buffer did not influence cells
features, the use of clear medium to dissolve T3 powder may have
provided more in vivo-like results.
The authors are conscious that the present study
presents only a part of cancer-related interactions between certain
micro-niche components. The analysis of a narrow fragment of
thyroid hormone homeostasis was conducted, rather than the whole
TSH-T4-T3-rT3 axis. These hormones may be considered biologically
active for healthy cells; however, more efforts are needed to
evaluate the role of T4 and T3 in cancer cells. One of the
limitations of the present study was the insufficient number of
patients to correlate some more clinical parameters with obtained
laboratory results. Individuals with CRC are a heterogeneous group
of patients, for instance, according to the location of the
disease. Cancers localized in rectum and colon are biologically
different. All criteria could not be included because our study
groups would be too small and subsequent analyses of subsets would
be underpowered. Exclusion of patients after neoadjuvant
chemoradiation renders comparisons easier and more homogenous but
it does not analyze the real-life scenario. Particularly that
certain hormones and drugs are known to have a specific interplay
with chemotherapy or radiosensitvity. Nevertheless, the present
study proposed a novel function of thyroid hormone signaling in the
regulation of CRC CSCs fate and the following projects by the
authors may extend the patients group to analyze the effect of
other clinical parameters on thyroid hormone axis in CSCs in
CRC.
In conclusion, considering the present results, it
could be hypothesized that thyroid hormones exert a great impact on
the fate of colorectal CSCs. Since CRC is one of the most prevalent
cancers worldwide the unveiling novel aspects of CRC niche seems to
be extremely important. It was found that the T3 role in cancer
biology depends on clinical stage of CRC and in near future such
data may be taken into consideration in therapeutic decision
making. Proteins involved in thyroid hormone homeostasis by their
concerted actions in cancer niche cooperate in the synchronized
manner with cellular internal elements associated with next steps
of carcinogenesis. However, the signals regulating functions of all
these elements during cancer development are largely unknown. CSCs
which are considered to be responsible for the most serious events
in carcinogenesis were found to be highly sensitive to T3. The
cellular balance of thyroid hormone associated proteins can be
considered as a potential therapeutic target for designing
anticancer drugs. The results of the present study suggested that
thyroid hormone homeostasis may have potential prognostic and
therapeutic value in human CRC. Further research evaluating the
detailed functions of thyroid hormone axis on molecular level and
their exact biological mechanism may enable introduction of
specific chemotherapeutic protocols in the future.
Considering that T3 was found to target CSCs it can
be considered to be supplemented to CRC patients to influence the
content of tumor microenvironment. Previous studies demonstrated
that depletion of endogenous T4 to increase T3 can improve clinical
outcomes of patients with advanced cancer. In patients with
pre-existent primary hypothyroidism, T3 administration was observed
to lower the T4 level and improve survival of patients (4,41).
The adjunctive therapy with thyroid hormones can give a hope to
improve the efficacy of conventional chemotherapy, however,
analyses concerning different type of cancer arises numerous
controversies and further research is needed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science Centre
of Poland (grant no. DEC-2019/03/X/NZ3/00434).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
OR, MS, PS and JK conceived the study. OR, MS, AO-K,
PS and JK developed methodology. OR, MS and JK performed software
analysis. OR, MS and JK validated the data. OR, MS, AO-K and PS
performed formal analysis. MS, AO-K and PS conducted investigation.
MS provided resources. RO, MS and AO-K curated the data. RO and MS
prepared the original draft. AO-K, PS and JK wrote reviewed and
edited the manuscript. MS and JK supervised the study. JK acquired
funding. All authors have read and approved the final version of
the manuscript. OR, AO-K, MS, PS and JK confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki, and was approved
(approval no. NKBBN/203/2020) by The Independent Bioethics
Committee of Medical University of Gdansk (Gdansk, Poland) from
24.04.2020. Informed consent was obtained from all subjects
involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hellevik AI, Asvold BO, Bjøro T,
Romundstad PR, Nilsen TI and Vatten LJ: Thyroid function and cancer
risk: A prospective population study. Cancer Epidemiol Biomarkers
Prev. 18:570–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang T, Xia L, Ma S, Qi X, Li Q, Xia Y,
Tang X, Cui D, Wang Z, Chi J, et al: Hepatocellular carcinoma:
Thyroid hormone promotes tumorigenicity through inducing cancer
stem-like cell self-renewal. Sci Rep. 6:251832016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung IH, Chen CY, Lin YH, Chi HC, Huang
YH, Tai PJ, Liao CJ, Tsai CY, Lin SL, Wu MH, et al: Thyroid
hormone-mediated regulation of lipocalin 2 through the Met/FAK
pathway in liver cancer. Oncotarget. 6:15050–15064. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hercbergs A, Johnson RE, Ashur-Fabian O,
Garfield DH and Davis PJ: Medically induced euthyroid
hypothyroxinemia may extend survival in compassionate need cancer
patients: An observational study. Oncologist. 20:72–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moeller LC and Führer D: Thyroid hormone,
thyroid hormone receptors, and cancer: A clinical perspective.
Endocr Relat Cancer. 20:R19–R29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conde SJ, Luvizotto Rde A, de Síbio MT and
Nogueira CR: Thyroid hormone status interferes with estrogen target
gene expression in breast cancer samples in menopausal women. ISRN
Endocrinol. 2014:3173982014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szaryńska M, Olejniczak A and Kmieć Z: The
role of cancer stem cells in pathogenesis of colorectal cancer.
Postepy Hig Med Dosw (Online). 70:1469–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: Promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szarynska M, Olejniczak A, Wierzbicki P,
Kobiela J, Laski D, Sledzinski Z, Adrych K, Guzek M and Kmiec Z:
FasR and FasL in colorectal cancer. Int J Oncol. 51:975–986. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olejniczak A, Szarynska M and Kmiec Z:
In vitro characterization of spheres derived from colorectal
cancer cell lines. Int J Oncol. 52:599–612. 2018.PubMed/NCBI
|
|
11
|
van de Velde CJ, Boelens PG, Borras JM,
Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek
CB, Brown G, Van Cutsem E, et al: EURECCA colorectal:
Multidisciplinary management: European consensus conference colon
& rectum. Eur J Cancer. 50:1.e1–1.e34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brierley DJ, Gospodarowicz M and Wittekind
C: Colon and Rectum: (ICD-O-3 C18-20). Classification of malignant
tumors digestive system tumours. TNM Online. 73–76. 2017.
View Article : Google Scholar
|
|
13
|
Shinderman-Maman E, Weingarten C,
Moskovich D, Werner H, Hercbergs A, Davis PJ, Ellis M and
Ashur-Fabian O: Molecular insights into the transcriptional
regulatory role of thyroid hormones in ovarian cancer. Mol
Carcinog. 57:97–105. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weingarten C, Jenudi Y, Tshuva RY,
Moskovich D, Alfandari A, Hercbergs A, Davis PJ, Ellis M and
Ashur-Fabian O: The interplay between epithelial-mesenchymal
transition (EMT) and the thyroid hormones-αvβ3 axis in ovarian
cancer. Horm Cancer. 9:22–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen K, Flint N, Shalev S, Erez D,
Baharal T, Davis PJ, Hercbergs A, Ellis M and Ashur-Fabian O:
Thyroid hormone regulates adhesion, migration and matrix
metalloproteinase 9 activity via αvβ3 integrin in myeloma cells.
Oncotarget. 5:6312–6322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh MT, Wang LM, Changou CA, Chin YT,
Yang YSH, Lai HY, Lee SY, Yang YN, Whang-Peng J, Liu LF, et al:
Crosstalk between integrin αvβ3 and ERα contributes to thyroid
hormone-induced proliferation of ovarian cancer cells. Oncotarget.
8:24237–24249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olejniczak-Kęder A, Szaryńska M, Wrońska
A, Siedlecka-Kroplewska K and Kmieć Z: Effects of 5-FU and
anti-EGFR antibody in combination with ASA on the spherical culture
system of HCT116 and HT29 colorectal cancer cell lines. Int J
Oncol. 55:223–242. 2019.PubMed/NCBI
|
|
18
|
Chi HC, Chen CY, Tsai MM, Tsai CY and Lin
KH: Molecular functions of thyroid hormones and their clinical
significance in liver-related diseases. Biomed Res Int.
2013:6013612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oppenheimer JH, Schwartz HL, Mariash CN,
Kinlaw WB, Wong NC and Freake HC: Advances in our understanding of
thyroid hormone action at the cellular level. Endocr Rev.
8:288–308. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamiya Y, Puzianowska-Kuznicka M, McPhie
P, Nauman J, Cheng SY and Nauman A: Expression of mutant thyroid
hormone nuclear receptors is associated with human renal clear cell
carcinoma. Carcinogenesis. 23:25–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JW, Zhao L and Cheng SY: Inhibition
of estrogen-dependent tumorigenesis by the thyroid hormone receptor
β in xenograft models. Am J Cancer Res. 3:302–311. 2013.PubMed/NCBI
|
|
22
|
Heublein S, Mayr D, Meindl A, Angele M,
Gallwas J, Jeschke U and Ditsch N: Thyroid hormone receptors
predict prognosis in BRCA1 associated breast cancer in opposing
ways. PLoS One. 10:e01270722015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
López-Mateo I, Alonso-Merino E,
Suarez-Cabrera C, Park JW, Cheng SY, Alemany S, Paramio JM and
Aranda A: Thyroid hormone receptor β inhibits self-renewal capacity
of breast cancer stem cells. Thyroid. 30:116–132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yen PM: Physiological and molecular basis
of thyroid hormone action. Physiol Rev. 81:1097–1142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drabkin H, Kao FT, Hartz J, Hart I, Gazdar
A, Weinberger C, Evans R and Gerber M: Localization of human ERBA2
to the 3p22-3p24.1 region of chromosome 3 and variable deletion in
small cell lung cancer. Proc Natl Acad Sci USA. 85:9258–9262. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
González-Sancho JM, García V, Bonilla F
and Muñoz A: Thyroid hormone receptors/THR genes in human cancer.
Cancer Lett. 192:121–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin KH, Shieh HY, Chen SL and Hsu HC:
Expression of mutant thyroid hormone nuclear receptors in human
hepatocellular carcinoma cells. Mol Carcinog. 26:53–61. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furuyama K, Kawaguchi Y, Akiyama H,
Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T,
Koizumi M, et al: Continuous cell supply from a Sox9-expressing
progenitor zone in adult liver, exocrine pancreas and intestine.
Nat Genet. 43:34–41. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guigon CJ, Kim DW, Willingham MC and Cheng
SY: Mutation of thyroid hormone receptor-β in mice predisposes to
the development of mammary tumors. Oncogene. 30:3381–3390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
González-Sancho JM, Figueroa A,
López-Barahona M, López E, Beug H and Muñoz A: Inhibition of
proliferation and expression of T1 and cyclin D1 genes by thyroid
hormone in mammary epithelial cells. Mol Carcinog. 34:25–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chi HC, Liao CH, Huang YH, Wu SM, Tsai CY,
Liao CJ, Tseng YH, Lin YH, Chen CY, Chung IH, et al: Thyroid
hormone receptor inhibits hepatoma cell migration through
transcriptional activation of Dickkopf 4. Biochem Biophys Res
Commun. 439:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furuya F, Lu C, Willingham MC and Cheng
SY: Inhibition of phosphatidylinositol 3-kinase delays tumor
progression and blocks metastatic spread in a mouse model of
thyroid cancer. Carcinogenesis. 28:2451–2458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahmed D, Eide PW, Eilertsen IA, Danielsen
SA, Eknæs M, Hektoen M, Lind GE and Lothe RA: Epigenetic and
genetic features of 24 colon cancer cell lines. Oncogenesis.
2:e712013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pascual A and Aranda A: Thyroid hormone
receptors, cell growth and differentiation. Biochim Biophys Acta.
1830:3908–3916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martínez-Iglesias OA, Alonso-Merino E,
Gómez-Rey S, Velasco-Martín JP, Martín Orozco R, Luengo E, García
Martín R, Ibáñez de Cáceres I, Fernández AF, Fraga MF, et al:
Autoregulatory loop of nuclear corepressor 1 expression controls
invasion, tumor growth, and metastasis. Proc Natl Acad Sci USA.
113:E328–E337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
García-Silva S and Aranda A: The thyroid
hormone receptor is a suppressor of ras-mediated transcription,
proliferation, and transformation. Mol Cell Biol. 24:7514–7523.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JW, Zhao L, Willingham MC and Cheng
SY: Loss of tyrosine phosphorylation at Y406 abrogates the tumor
suppressor functions of the thyroid hormone receptor β. Mol
Carcinog. 56:489–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dentice M, Luongo C, Ambrosio R, Sibilio
A, Casillo A, Iaccarino A, Troncone G, Fenzi G, Larsen PR and
Salvatore D: β-Catenin regulates deiodinase levels and thyroid
hormone signaling in colon cancer cells. Gastroenterology.
143:1037–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miro C, Ambrosio R, De Stefano MA, Di
Girolamo D, Di Cicco E, Cicatiello AG, Mancino G, Porcelli T, Raia
M, Del Vecchio L, et al: The concerted action of type 2 and type 3
deiodinases regulates the cell cycle and survival of basal cell
carcinoma cells. Thyroid. 27:567–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kojima Y, Kondo Y, Fujishita T,
Mishiro-Sato E, Kajino-Sakamoto R, Taketo MM and Aoki M: Stromal
iodothyronine deiodinase 2 (DIO2) promotes the growth of intestinal
tumors in ApcΔ716 mutant mice. Cancer Sci.
110:2520–2528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sterle HA, Hildebrandt X, Valenzuela
Álvarez M, Paulazo MA, Gutierrez LM, Klecha AJ, Cayrol F, Díaz
Flaqué MC, Rosemblit C, Barreiro Arcos ML, et al: Thyroid status
regulates the tumor microenvironment delineating breast cancer
fate. Endocr Relat Cancer. 28:403–418. 2021. View Article : Google Scholar : PubMed/NCBI
|