Introduction
Prostate cancer (PCa) is the most frequently
diagnosed malignant tumor and a major cause of cancer mortality
(6.8%) in men worldwide (1). A
main problem arising from PCa is bone tumor metastasis. A total of
~80% of patients with advanced PCa develop bone metastases and are
treated with androgen deprivation therapy (2). Androgen receptor (AR) is a leading
factor for the development of bone metastasis, and recent advances
in therapeutic options for PCa highlight the necessity to block AR
signaling (3). However, the role
of AR in osteogenesis in PCa remains controversial and unclear.
Therefore, for future therapeutic developments, it is essential to
determine the underlying mechanism of PCa-driven osteogenesis.
Tumor-induced osteogenesis is a complex process that
involves cell disengagement from the microenvironment in
situ, degradation of the surrounding extracellular matrix,
tumor cell dissemination and final proliferation of distant
secondary bone tumors (4).
Emerging evidence suggests that cytokines involved in the process
mentioned above can also act as chemoattractants on
pre-osteoblastic MC3T3-E1 cells and promote the secretion of
osteogenic factors (5). Almost all
osteogenic factors are activated via two important osteogenic
transcription factors, which are runt-related transcription factor
2 (Runx2) and osteoblast-specific transcription factor
Osterix (6–8). However, the upstream factors and
signaling pathways regulating these two osteogenic factors are
still poorly understood.
As a member of the F-spondin family of secreted
extracellular matrix proteins, spondin 2 is encoded by the
SPON2 gene (9). Initially,
spondin 2 was reported as a diagnostic marker specific for PCa
(10,11). However, previous studies have shown
that spondin 2 is overexpressed in the serum or tissue samples of
malignant tumors, such as colorectal cancer and hepatocellular
carcinoma (12,13). High levels of spondin 2 in
colorectal cancer cells have been indicated to increase cell
motility, thereby resulting in colorectal cancer metastasis in mice
(14). Integrins are transmembrane
heterodimers with α and β subunits that are considered to be major
candidates as receptors for spondin 2 (15). Yang et al (16) analyzed the expression of integrins
in MC3T3-E1 cells by flow cytometry and found high expression of
integrin α5β1. Therefore, it was hypothesized that spondin 2 may
play an important role in osteogenesis caused by PCa through
integrin α5β1.
The aim of the present study was to elucidate the
function as well as the underlying mechanism of PCa cell-derived
spondin 2 during PCa-driven osteogenesis. The detailed mechanisms
of spondin 2 function in PCa-induced bone metastasis need to be
further clarified in future studies.
Materials and methods
Cell culture and treatment
The human RWPE-1 cell line, PCa cell lines (LNCaP
and C4-2 cells) and the osteoblastic cell line MC3T3-E1 were all
purchased from American Type Culture Collection. RWPE-1 cells were
cultured in keratinocyte serum-free medium supplemented with 25
µg/ml bovine pituitary extract, 5 ng/ml human recombinant epidermal
growth factor, 100 U/ml penicillin and 100 µg/ml streptomycin (all
from Invitrogen; Thermo Fisher Scientific, Inc.). LNCaP and C4-2
cells were cultured in DMEM (Invitrogen; Thermo Fisher Scientific,
Inc.), while MC3T3-E1 cells were cultured in α-MEM (Invitrogen;
Thermo Fisher Scientific, Inc.). The culture media were
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin with (or without) 0.5 µg/ml spondin 2 neutralizing
antibody (cat. no. SP2021041B; Wuhan Dian Biotechnology Co., Ltd.),
and cells were maintained in a humidified incubator at 37°C with 5%
CO2. MC3T3-E1 cells were treated with 0.1 or 1.0 µg/ml
spondin 2 recombinant protein (rSpondin 2; cat. no. RPF396Mu01;
Cloud-Clone Corp.) for 24 h in a humidified incubator at 37°C with
5% CO2. Control group cells were treated with 1X PBS for
24 h in a humidified incubator at 37°C with 5% CO2. In
the integrin α5β1 inhibitor assay, MC3T3-E1 cells were treated with
100 µM ATN-161 (MedChemExpress) for 24 h in a humidified incubator
at 37°C with 5% CO2.
Collection of conditioned media
(CM)
LNCaP and C4-2 cells (2×106) were grown
overnight in 100 mm culture dishes. After two washes with PBS, the
cells were cultured in DMEM with 1% FBS for 48 h prior to
collection of CM.
ELISA
The supernatants of normal prostate epithelial cells
(RWPE-1) and PCa cells (LNCaP and C4-2) cultures were centrifuged
at 1,000 × g for 15 min at 4°C before the assay. Proteins were
assessed using a spondin-2 ELISA kit (cat. no. JCSJ2862; Shanghai
Jichun Industrial Co., Ltd.) according to the manufacturer's
instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of MC3T3-E1 cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). according to the protocol provided by the manufacturer. cDNA
was synthesized using Prime Script RT Master Mix Kit (Takara Bio,
Inc.) according to the manufacturer's instructions. RT-qPCR was
performed in duplicate with a SYBR Premix Ex Taq™ kit (Takara Bio,
Inc.) according to the manufacturer's instructions. PCR
amplification conditions were as follows: Pre-denaturation at 95°C
for 15 sec; 45 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 62°C for 30 sec. The following primers were
used in the present study: Osterix forward,
5′-GATGGCGTCCTCTCTGCTTG-3′ and reverse, 5′-TCTTTGTGCCTCCTTTCCCC-3′;
Runx2 forward, 5′-GACGAGGCAAGAGTTTCACC-3′ and reverse,
5′-GGACCGTCCACTGTCACTTT-3′; Gapdh forward,
5′-TCCACCACCCTGTTGCTGTA-3′ and reverse, 5′-ACCACAGTCCATGCCATCAC-3′.
Relative expression of the targeted genes was calculated using the
2ΔΔCq method (17).
Western blotting
MC3T3-E1 cells were collected and lysed using RIPA
buffer (Boster Biological Technology) containing protease inhibitor
cocktail and PMSF (Boster Biological Technology). For determining
the protein concentration, a BCA method was used, and equal amounts
(30 µg) of proteins were separated under 90 V via 10% SDS-PAGE and
subsequently transferred onto PVDF membranes (MilliporeSigma).
After blocking the membranes with 1X TBS-Tween (TBST; 0.05%
Tween-20) containing 5% skimmed milk for 2 h at room temperature,
the membranes were incubated with primary anti-phosphorylated
(p)-PI3K (cat. no. 4228), anti-PI3K (cat. no. 4257), anti-p-AKT
(cat. no. 4060), anti-AKT (cat. no. 9272), anti-p-mTOR (cat. no.
5536), anti-mTOR (cat. no. 2972) (all 1:1,000 dilution; Cell
Signaling Technology, Inc.) and anti-GAPDH (1:1,000 dilution; cat.
no. 60004-1-Ig; ProteinTech Group, Inc.) antibodies overnight at
4°C. GAPDH was used as a normalization control. Membranes were
rinsed in TBST, incubated with secondary anti-mouse IgG, AP-linked
antibody (cat. no. 7056; 1:4,000 dilution; Cell Signaling
Technology, Inc.) and anti-rabbit IgG, HRP-linked antibody (cat.
no. 7074; 1:3,000 dilution; Cell Signaling Technology, Inc.) for 1
h at room temperature and then washed in 1X TBST. After incubation
with the ECL Plus system (Amersham; Cytiva), signals were detected
using the ImageQuant LAS 4000 mini system (GE Healthcare
Bio-Sciences). The signals were detected using Adobe Photoshop CS3
software (Adobe Systems, Inc.).
Public database analysis
Gene expression data (GSE101607 dataset) were
downloaded as raw signals from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) (18), and analyzed using the Geo2R tool
from NCBI (https://www.ncbi.nlm.nih.gov/geo/geo2r). The
differentially expressed genes were filtered by
|log2FoldChange|>1 and FDR<0.05. Accordingly, a heatmap was
generated using the ‘pheatmap’ package in R 3.6.1 (https://mirrors.tuna.tsinghua.edu.cn/CRAN/). Published
gene expression profiles and clinical data of PCa patients were
obtained from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov). The data type was
selected as ‘count’ and transformed into the transcript per million
format. All patients with PCa were divided into two subgroups
according to the median SPON2 expression, namely
SPON2 low group (n=246) and SPON2 high group (n=246).
This part of data was analyzed via Gene Set Enrichment Analysis
(GSEA v3.0; http://www.gsea-msigdb.org/gsea/index.jsp). GSEA is a
computational method that determines whether an a priori defined
set of genes shows statistically significant, concordant
differences between two biological states (19).
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using GraphPad Prism software v6.01 (GraphPad Software,
Inc.). Differences between two groups were assessed using a
two-tailed unpaired Student's t-test. One-way analysis of variance
tests with Bonferroni's post hoc test was used for multiple
comparisons. The association between SPON2 and the survival
rate of patients with PCa was obtained using Kaplan-Meier analysis
(the log-rank test was used to obtain the P-value) in GraphPad
Prism software. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times.
Results
SPON2 expression is increased in
patients with AR-positive PCa
Patients with castration-resistant PCa (GSE101607
dataset) were divided into two subgroups according to AR
positivity, namely the AR-positive group (n=32) and the
non-AR-positive group (n=8). By setting log2 (fold
change) at ±1 and P<0.05, 687 differentially expressed genes
(DEGs) between the AR-positive and the non-AR-positive group were
identified with 188 upregulated DEGs and 499 downregulated DEGs.
According to the heat map of the top five most up- and
downregulated DEGs (Fig. 1A and
Table SI), SPON2 was
indicated to be highly expressed in AR-positive group (Fig. 1B). Consistent with this, ELISA
results also showed that spondin 2 protein was secreted from PCa
cells. Spondin 2 protein in the supernatants of PCa cells was
significantly increased compared with normal prostate epithelial
cells (235.13±61.82 pg/ml in LNCaP and 280.02±90.50 pg/ml in C4-2
cells vs. 34.97±12.60 pg/ml in RWPE-1 cells) (Fig. S1). In the present study, the
association of SPON2 with the survival of patients with PCa
based on TCGA data was also analyzed. The data showed that high
level of SPON2 was associated with poor prognosis, but there
was no significant difference between the two groups (Fig. S2).
PCa cell-derived spondin 2 promotes
osteogenic factor production in osteoblasts
Next, the osteoblastic classification of PCa bone
metastasis was sought to be determined, based on the evidence of
tumor cell-derived osteogenic factors from two AR-positive PCa cell
lines (LNCaP and C4-2), leading to increased bone formation.
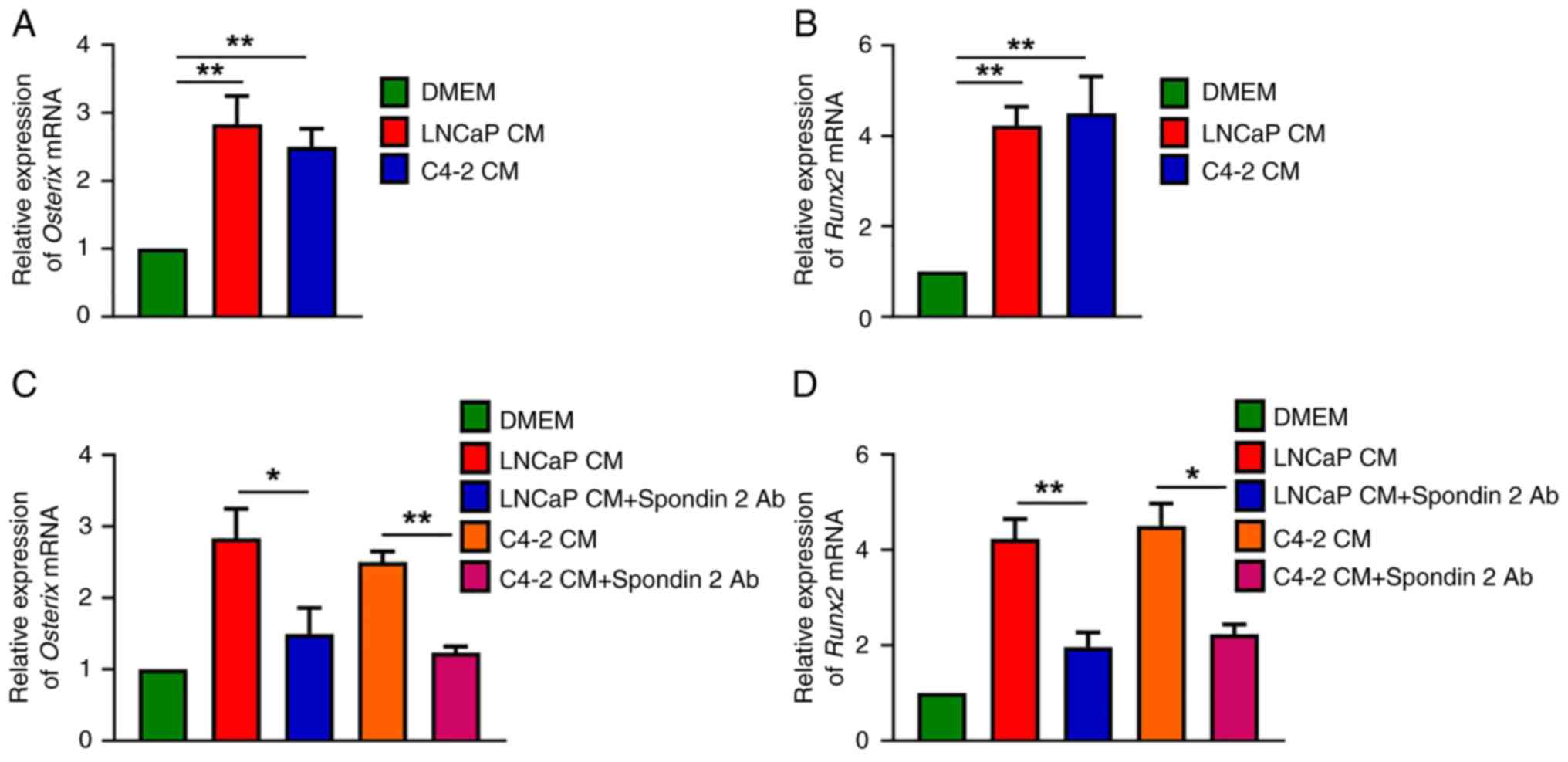
Compared with untreated MC3T3-E1 cells, it was found that CM from
the LNCaP and C4-2 cells enhanced Osterix and Runx2
mRNA expression in osteoblasts (Fig.
2A and B). To examine whether spondin 2 was a critical factor
in PCa cells, CM was treated with spondin 2 antibody. The results
showed that spondin 2 antibody effectively reduced Osterix
and Runx2 mRNA synthesis after treatment with PCa cell CM
(Fig. 2C and D), indicating that
PCa cell-derived spondin 2 promotes the production of osteogenic
factors in osteoblasts.
SPON2 is positively associated with
the PI3K/AKT/mTOR pathway in patients
The expression levels of SPON2 were further
analyzed using TCGA PCa dataset, and it was found that the
expression levels of SPON2 were elevated in tumor compared
with normal tissues (Fig. 3A).
Furthermore, patients with PCa were divided into two subgroups
according to the median SPON2 expression, namely
SPON2 low group (n=246) and SPON2 high group (n=246).
As a previous study indicated that the PI3K/AKT/mTOR pathway could
regulate the migration and invasion of PCa (20,21),
it was then explored whether SPON2 expression was associated
with the PI3K/AKT/mTOR pathway. According to GSEA using TCGA PCa
dataset, high expression of SPON2 was positively associated
with the enrichment of the PI3K/AKT/mTOR signaling pathway
(Fig. 3B).
Spondin 2 activates the PI3K/AKT/mTOR
pathway in osteoblasts
To investigate the specific effect of spondin 2 on
osteogenic factor production in osteoblasts, MC3T3-E1 cells were
treated with rSpondin 2. Different concentrations of rSpondin 2
were used according to a previous study (22), and in the present study it was
observed that two concentrations of rSpondin2 (0.1 and 1.0 µg/ml)
showed the most highly promoting effect on the transcription of
Osterix and Runx2 as well as the phosphorylation of
PI3K, AKT and mTOR. RT-qPCR analysis showed that rSpondin 2-induced
MC3T3-E1 cells had higher transcriptional levels of Osterix
and Runx2 compared with control cells in a
concentration-dependent manner (Fig.
4A and B). To further investigate the underlying mechanism, the
activity of the PI3K/AKT/mTOR pathway was further explored. The
protein levels of PI3K, p-PI3K, AKT, p-AKT, p-mTOR and mTOR were
measured in osteoblasts cultured with various concentrations of
rSpondin 2. According to the results, the phosphorylation of PI3K,
AKT and mTOR were significantly increased after treatment with
rSpondin 2 in a concentration-dependent manner, compared with
control cells. On the other hand, the total PI3K, AKT and mTOR
levels remain unchanged (Fig.
4C).
Inhibition of integrin α5β1 suppresses
the PI3K/AKT/mTOR pathway in osteoblasts
Spondin 2 is known to bind to integrin receptors
(9). Integrins α5 and β1 are known
to be expressed in osteoblasts on the bone surface (23). In order to determine whether
spondin 2 activated the PI3K/AKT/mTOR pathway via integrin α5β1, an
integrin α5β1 inhibitor (ATN-161) was used to determine its effects
on spondin 2-mediated osteogenic factor production. According to
RT-qPCR analysis, ATN-161 significantly inhibited spondin 2-induced
mRNA expression of Osterix and Runx2 (Fig. 5A and B). Furthermore, western blot
analysis indicated that ATN-161 significantly inhibited spondin
2-mediated PI3K, AKT and mTOR phosphorylation (Figs. 5C and S3).
Discussion
A previous study has shown that SPON2 is a
new serum and histological diagnostic biomarker for PCa (11). Similarly, the present study found
that spondin 2 facilitated osteogenic factor production induced by
PCa cells, and SPON2 was upregulated in AR-positive tumors
of patients with PCa. Moreover, it was identified that PCa
cell-derived spondin 2 promoted the osteogenic activity of
osteoblasts by activating the PI3K/AKT/mTOR pathway.
The disruption of homeostasis between osteoblasts is
involved in PCa-induced osteogenesis (2,24).
These processes are regulated by tumor cell-derived cytokines, such
as bone morphogenetic protein, platelet-derived growth factor,
insulin-like growth factor and extracellular calcium (25–28).
As an extracellular matrix protein, increased levels of spondin 2
in serum are correlated with high incidence of osteogenesis induced
by prostate tumor cells (29).
According to previous studies, spondin 2 is a critical regulator of
cancer progression; however, its underlying mechanism in osteoblast
activity remains to be elucidated (11–13).
On the other hand, Runx2 can promote the differentiation of
mesenchymal stem cells towards osteoblasts, while Osterix plays an
important role in osteoblast differentiation (30,31).
On this basis, it was identified that spondin 2 derived from PCa
cells significantly enhanced the expression of the osteogenic genes
Runx2 and Osterix, indicating an increased activity of
osteoblasts.
The PI3K/AKT/mTOR signaling pathway has been
indicated to participate in cell proliferation, inflammation,
immunity and tumorigenesis (32–34).
Inhibition of the PI3K/AKT/mTOR signaling pathway can suppress
tumor growth and tumor-induced osteogenesis (35,36).
Moreover, the PI3K/AKT/mTOR pathway has been reported to be a
potential target in castration resistant PCa (37,38).
However, whether spondin 2 upregulates the PI3K/AKT/mTOR signaling
during osteogenesis driven by PCa progression requires further
investigation. In the present study, it was indicated that spondin
2 secreted by AR-positive PCa cells could promote the
differentiation of osteoblast precursors to mature osteoblasts
(showed by the increased expression of Runx2 and Osterix) through
the PI3K/AKT/mTOR signaling pathway. As a well-known receptor of
spondin 2, integrin α5β1 plays a significant role in bone formation
(23), and the present study
showed that spondin 2 receptor inhibitor ATN-161 could inhibit
spondin 2-mediated PI3K, AKT and mTOR phosphorylation in osteoblast
precursor MC3T3-E1 cells.
The present study explored the role of spondin 2 on
osteogenesis caused by PCa cells in vitro, while the
association between spondin 2 and bone metastasis in animal models
as well as patients with PCa requires further investigation.
In summary, the current study demonstrated that
spondin 2 derived from AR-positive PCa cells could effectively
enhance PCa-induced osteogenesis through activation of the
PI3K/AKT/mTOR signaling cascade (Fig.
6). Furthermore, to the best of our knowledge, the present
study was the first to demonstrate that integrin α5β1 is involved
in spondin 2-regulated osteogenesis in PCa cells in
vitro.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Experiments were conceived and designed by CY and
HW. HW and MZ performed the experiments and data analysis, prepared
the figures and wrote the manuscript. WL helped with the
experimental operation and data analysis. CY participated in
manuscript writing. CY and HW confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guise TA, Mohammad KS, Clines G, Stebbins
EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L
and Chirgwin JM: Basic mechanisms responsible for osteolytic and
osteoblastic bone metastases. Clin Cancer Res. 12:6213s–6216s.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cornford P, van den Bergh RCN, Briers E,
Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N,
Gandaglia G, Gillessen S, et al: EAU-EANM-ESTRO-ESUR-SIOG
guidelines on prostate cancer. Part II-2020 update: Treatment of
relapsing and metastatic prostate cancer. Eur Urol. 79:263–282.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clezardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dayyani F, Varkaris A, Araujo JC, Song JH,
Chatterji T, Trudel GC, Logothetis CJ and Gallick GE: Increased
serum insulin-like growth factor-1 levels are associated with
prolonged response to dasatinib-based regimens in metastatic
prostate cancer. Prostate. 73:979–985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Long F, Wan J, Hu Y and He H:
MicroRNA-205 acts as a tumor suppressor in osteosarcoma via
targeting RUNX2. Oncol Rep. 35:3275–3284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jagga S, Sharma AR, Kim EJ and Nam JS:
Isoflavone-enriched whole soy milk powder stimulates osteoblast
differentiation. J Food Sci Technol. 58:595–603. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YH, Han Y, Jin SW, Lee GH, Kim GS,
Lee DY, Chung YC, Lee KY and Jeong HG: Pseudoshikonin I enhances
osteoblast differentiation by stimulating Runx2 and Osterix. J Cell
Biochem. 119:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Cao C, Jia W, Yu L, Mo M, Wang Q,
Huang Y, Lim JM, Ishihara M, Wells L, et al: Structure of the
F-spondin domain of mindin, an integrin ligand and pattern
recognition molecule. EMBO J. 28:286–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parry R, Schneider D, Hudson D, Parkes D,
Xuan JA, Newton A, Toy P, Lin R, Harkins R, Alicke B, et al:
Identification of a novel prostate tumor target, mindin/RG-1, for
antibody-based radiotherapy of prostate cancer. Cancer Res.
65:8397–8405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian X, Li C, Pang B, Xue M, Wang J and
Zhou J: Spondin-2 (SPON2), a more prostate-cancer-specific
diagnostic biomarker. PLoS One. 7:e372252012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wang XQ, Wang J, Cui SJ, Lou XM,
Yan B, Qiao J, Jiang YH, Zhang LJ, Yang PY and Liu F: Upregulation
of spondin-2 predicts poor survival of colorectal carcinoma
patients. Oncotarget. 6:15095–15110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Y, Hu Y, Mao Q, Guo Y, Liu Y, Xue W
and Cheng S: Upregulation of spondin-2 protein expression
correlates with poor prognosis in hepatocellular carcinoma. J Int
Med Res. 47:569–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmid F, Wang Q, Huska MR,
Andrade-Navarro MA, Lemm M, Fichtner I, Dahlmann M, Kobelt D,
Walther W, Smith J, et al: SPON2, a newly identified target gene of
MACC1, drives colorectal cancer metastasis in mice and is
prognostic for colorectal cancer patient survival. Oncogene.
35:5942–5952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Ou R, Chen X, Zhang Y, Li J,
Liang Y, Zhu X, Liu L, Li M, Lin D, et al: Tumor cell-derived SPON2
promotes M2-polarized tumor-associated macrophage infiltration and
cancer progression by activating PYK2 in CRC. J Exp Clin Cancer
Res. 40:3042021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang RS, Lin WL, Chen YZ, Tang CH, Huang
TH, Lu BY and Fu WM: Regulation by ultrasound treatment on the
integrin expression and differentiation of osteoblasts. Bone.
36:276–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ylitalo EB, Thysell E, Jernberg E,
Lundholm M, Crnalic S, Egevad L, Stattin P, Widmark A, Bergh A and
Wikström P: Subgroups of castration-resistant prostate cancer bone
metastases defined through an inverse relationship between androgen
receptor activity and immune response. Eur Urol. 71:776–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan M, Xu J, Siddiqui J, Feng F and Sun Y:
Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate
tumorigenesis via inactivation of the PI3K/AKT/mTOR axis. Mol
Cancer. 15:812016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YL, Li Q, Yang XM, Fang F, Li J,
Wang YH, Yang Q, Zhu L, Nie HZ, Zhang XL, et al: SPON2 Promotes
M1-like macrophage recruitment and inhibits hepatocellular
carcinoma metastasis by distinct integrin-Rho GTPase-hippo
pathways. Cancer Res. 78:2305–2317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang W, Takeshita N, Maeda T, Sogi C,
Oyanagi T, Kimura S, Yoshida M, Sasaki K, Ito A and Takano-Yamamoto
T: Connective tissue growth factor promotes chemotaxis of
preosteoblasts through integrin α5 and Ras during tensile
force-induced intramembranous osteogenesis. Sci Rep. 11:23682021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai G, Cai Z, Zhai X, Xiong J, Zhang F and
Li H: A new nomogram for the prediction of bone metastasis in
patients with prostate cancer. J Int Med Res.
49:30006052110583642021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee Y, Schwarz E, Davies M, Jo M, Gates J,
Wu J, Zhang X and Lieberman JR: Differences in the cytokine
profiles associated with prostate cancer cell induced osteoblastic
and osteolytic lesions in bone. J Orthop Res. 21:62–72. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boucher J, Balandre AC, Debant M, Vix J,
Harnois T, Bourmeyster N, Péraudeau E, Chépied A, Clarhaut J,
Debiais F, et al: Cx43 present at the leading edge membrane governs
promigratory effects of osteoblast-conditioned medium on human
prostate cancer cells in the context of bone metastasis. Cancers
(Basel). 12:30132020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubin J, Fan X, Rahnert J, Sen B, Hsieh
CL, Murphy TC, Nanes MS, Horton LG, Beamer WG and Rosen CJ: IGF-I
secretion by prostate carcinoma cells does not alter tumor-bone
cell interactions in vitro or in vivo. Prostate. 66:789–800. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tucci M, Mosca A, Lamanna G, Porpiglia F,
Terzolo M, Vana F, Cracco C, Russo L, Gorzegno G, Tampellini M, et
al: Prognostic significance of disordered calcium metabolism in
hormone-refractory prostate cancer patients with metastatic bone
disease. Prostate Cancer Prostatic Dis. 12:94–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marie PJ: Targeting integrins to promote
bone formation and repair. Nat Rev Endocrinol. 9:288–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, Zhang C, Trotter TN, Gowda PS, Lu Y,
Ponnazhagan S, Javed A, Li J and Yang Y: Runx2 deficiency in
osteoblasts promotes myeloma progression by altering the bone
microenvironment at new bone sites. Cancer Res. 80:1036–1048. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Q, Li M, Wang S, Xiao Z, Xiong Y and
Wang G: Recent advances of osterix transcription factor in
osteoblast differentiation and bone formation. Front Cell Dev Biol.
8:6012242020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan G, Lian Z, Liu Q, Lin X, Xie D, Song
F, Wang X, Shao S, Zhou B, Li C, et al: Phosphatidyl inositol
3-kinase (PI3K)-mTOR inhibitor PKI-402 inhibits breast cancer
induced osteolysis. Cancer Lett. 443:135–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu WB, Xiao N and Liu XJ: Dietary
flavonoid tangeretin induces reprogramming of epithelial to
mesenchymal transition in prostate cancer cells by targeting the
PI3K/Akt/mTOR signaling pathway. Oncol Lett. 15:433–440.
2018.PubMed/NCBI
|
|
34
|
Le B, Powers GL, Tam YT, Schumacher N,
Malinowski RL, Steinke L, Kwon G and Marker PC: Multi-drug loaded
micelles delivering chemotherapy and targeted therapies directed
against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer.
PLoS One. 12:e01746582017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
LoRusso PM: Inhibition of the
PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 34:3803–3815.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Li X, Lin J and Lin M: Morroniside
promotes the osteogenesis by activating PI3K/Akt/mTOR signaling.
Biosci Biotechnol Biochem. 85:332–339. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: PI3K/Akt/mTOR pathway inhibitors
enhance radiosensitivity in radioresistant prostate cancer cells
through inducing apoptosis, reducing autophagy, suppressing NHEJ
and HR repair pathways. Cell Death Dis. 5:e14372014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berrak O, Arisan ED, Obakan-Yerlikaya P,
Coker-Gürkan A and Palavan-Unsal N: mTOR is a fine tuning molecule
in CDK inhibitors-induced distinct cell death mechanisms via
PI3K/AKT/mTOR signaling axis in prostate cancer cells. Apoptosis.
21:1158–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|