Introduction
Uterine leiomyosarcoma (ULMS) is a lethal
gynecological malignancy. The annual incidence of ULMS is ~0.86 per
100,000 women worldwide (1–3). Surgical resection is the best
treatment option for localized ULMS; however, the majority of cases
eventually result in recurrence (2–4). There
are currently no effective treatment strategies for recurrent and
metastatic ULMS (2). Notably, the
Food and Drug Administration has approved new therapeutic agents,
such as trabectedin and pazopanib, for soft-tissue tumors in the
past decade (3). However, the
prognosis of patients with advanced/recurrent ULMS remains
unsatisfactory (5–7). Several clinical trials have reported
that the median progression-free survival time of
advanced/recurrent ULMS is approximately a few months, and the
median overall survival time is within 2 years (5–7).
Therefore, the development of new therapeutic agents is required in
the clinical setting.
Until recently, the molecular biological
characteristics of soft-tissue tumors, including ULMS, were poorly
understood due to their low incidence. However, the development of
next-generation sequencing may improve understanding of the
characteristics of ULMS and other malignancies. Several genomic
analyses have identified frequently mutated genes in ULMS,
including alterations that affect TP53, RB1, ATRX and
PTEN (8–11). Moreover, transcriptome analyses have
revealed that cell cycle-related kinase activation is a dominant
feature of ULMS (12,13). Notably, the roles of microRNAs
(miRNAs/miRs) in ULMS development remain unclear. miRNAs are small
non-coding RNAs ~22 nucleotides in length, and >2,000 annotated
mature miRNAs are present in the human genome (14–16).
Functionally, miRNAs post-transcriptionally regulate the expression
of their target gene, and a miRNA potentially has multiple target
genes, depending on cell type (17–19).
Thus, miRNAs serve critical roles in cancer development by
modulating fundamental biological processes. ULMS research has
reported the inverse correlation between let-7c and HMGA2 in
clinical samples and has experimentally validated the
tumor-suppressive effect of let-7c (20). Moreover, miR-152 has been reported
to suppress ULMS cell proliferation by regulating MET
expression (21). Therefore,
anomalous miRNA expression may contribute to ULMS development;
however, the significance of the majority of miRNAs remains to be
determined.
The present study performed comprehensive miRNA
sequencing to investigate unique miRNA profiles of ULMS.
Subsequently, the study focused on miR-10b-5p and evaluated its
potential functions in LMS-derived cell lines.
Materials and methods
Patients
Medical records from the National Cancer Center
Hospital (Tokyo, Japan) were retrospectively reviewed. Excluding
patients without written informed consent, all six patients with
ULMS who underwent surgery without neoadjuvant therapy between
January 2011 and September 2020 were included. The archival
fresh-frozen tumor and adjacent normal tissues of these patients,
which were stored at the National Cancer Center Biobank (Tokyo,
Japan), were used in the present study. Moreover, three leiomyoma
tissues from three other patients were used as controls. The case
number corresponds to the case number from our previous report
(13). The clinical information,
such as age, stage, mitotic rate and the presence of necrosis, was
obtained from their clinical records. The International Federation
of Gynecology and Obstetrics staging system was used (2,3). The
study protocol was approved by the ethics committee of the National
Cancer Center (approval no. 2020-160). Written informed consent was
obtained from all patients. Moreover, the study was carried out
according to The Declaration of Helsinki.
Comprehensive miRNA sequencing
Total RNA was extracted using the miRNeasy Mini Kit
(Qiagen GmbH), and small RNA libraries were prepared using the
NEBNext Multiplex Small RNA Library Prep Set for Illumina (cat. no.
E7300L; New England Biolabs, Inc.) according to the manufacturers'
protocol. Subsequently, the small RNA libraries were separated by
electrophoresis (120 V, 60 min) on a 10% TBE gel (cat. no.
EC6265BOX; Thermo Fisher Scientific, Inc.), and DNA fragments
corresponding to 140–160 bp (the lengths of small non-coding RNA
plus the 3′ and 5′ adaptors) were recovered. The cDNA concentration
was then measured using the Qubit dsDNA HS Assay Kit and a Qubit2.0
Fluorometer (Thermo Fisher Scientific, Inc.). Finally, single-end
reads were performed using the MiSeq Reagent Kit v3 (cat. no.
MS-102-3001; Illumina, Inc.) on the Illumina MiSeq (Illumina, Inc.)
and the loading concentration of the final library was 10 pM.
The CLC Genomics Workbench version 9.5.3 program
(Qiagen GmbH) was used for adaptor trimming and mapping to the
miRbase 21 database (https://www.mirbase.org/) without allowing any
mismatch. After normalization using reads per million mapped reads,
low-expressed miRNAs (<10 reads in all samples) were excluded
from further analyses. Subsequently, RStudio (RStudio, Inc.) and R
software (version 4.0.3; http://www.r-project.org/) were used. For the heatmap
analysis, miRNAs with an absolute log2 (fold change) of >0.8
were extracted and utilized. The data were then converted to base
10 logarithms and z-scores, and the heatmap.2 function of the
gplots package (ver. 3.1.0; http://cran.r-project.org/package=gplots) was used.
The P-values for each gene were calculated using the Wald test in
DESeq2 (ver. 1.30.0) for the volcano plots (22).
Cell culture and miRNA mimics
SK-UT-1 (ULMS-derived cell line) and SK-LMS-1
(vulvar LMS-derived cell line) were purchased from the American
Type Culture Collection. The cells were maintained in MEM (Nacalai
Tesque, Inc.) containing 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 1 mM sodium pyruvate (Thermo Fisher Scientific,
Inc.) and penicillin-streptomycin (Thermo Fisher Scientific, Inc.)
in a humidified incubator at 37°C with 5% CO2. The cell
lines tested negative for mycoplasma contamination and were used
between 5 and 40 passages for the experiments.
mirVana miRNA mimics (Thermo Fisher Scientific,
Inc.) were used to induce overexpression of miRNAs in the present
study, and the assay IDs were as follows: miR-10b-5p (MC11108),
miR-29a-3p (MC12499), miR-126-3p (MC12841), miR-186-5p (MC11753)
and Negative Control (NC) #1 (4464058). Cells were transfected with
20 nM miRNA mimics using Lipofectamine® RNAi Max (Thermo
Fisher Scientific, Inc.) at 37°C for ≥24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from clinical samples or
cell lines as aforementioned, and cDNA was synthesized using a
TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific,
Inc.) according to the manufacturers' protocol. Subsequently, qPCR
was performed using TaqMan Fast Advanced Master Mix and TaqMan
Advanced miRNA Assay (Thermo Fisher Scientific, Inc.); the assay
IDs were as follows: miR-10b-5p (478494_miR), miR-29a-3p
(478587_miR), miR-126-3p (477887_miR), miR-186-5p (477940_miR) and
RNU6B (001093). The amplification program was as follows:
Denaturation at 95°C for 10 min, followed by 40 amplification
cycles at 95°C for 15 sec and 60°C for 60 sec. The amplified
product was monitored by measuring the fluorescence intensity of
FAM. U6 was used as a reference gene to normalize the
expression and the 2−ΔΔCq method was used for
quantification (23).
Cell proliferation assay
Cells were seeded into 96-well plates (1,000
cells/well) and transfected with the miR-10b-5p, miR-29a-3p,
miR-126-3p, miR-186-5p or NC mimics. A total of 24, 48 and 72 h
post-transfection, cell proliferation was assessed using the
CellTiter-Glo 2.0 Cell Viability Assay (Promega Corporation), and
luminescence was measured 10 min after adding the reagent using
SpectraMax iD3 (Molecular Devices, LLC) or Infinite 200 PRO (Tecan
Group, Ltd.). Experiments were performed in triplicate and repeated
three times.
Clonogenic assay
Cells were transfected with miR-10b-5p or NC mimic
in 35-mm dishes (50,000 cells/dish). A total of 24 h
post-transfection, the cells were seeded into six-well plates (300
cells/well, six replicates) and incubated for 6 days in a
humidified incubator at 37°C with 5% CO2. Subsequently,
the cells were fixed with 4% paraformaldehyde for 10 min and
stained with 1% crystal violet for 10 min at room temperature, and
the colonies (>50 cells) were manually counted.
Soft agar colony formation assay
For soft agar colony formation assay, 2X MEM was
prepared using 10X MEM (cat. no. M0275; MilliporeSigma), FBS,
sodium hydrogen carbonate (FUJIFILM Wako Pure Chemical
Corporation), GlutaMax (Thermo Fisher Scientific. Inc.), sodium
pyruvate and penicillin-streptomycin (Thermo Fisher Scientific,
Inc.). Cells were transfected with miR-10b-5p or NC mimic in 35-mm
dishes (50,000 cells/dish). Subsequently, 1.6 and 0.6% agar
solutions were prepared using agar powder (FUJIFILM Wako Pure
Chemical Corporation) diluted with PBS. Prewarmed 2X MEM and melted
1.6% agar solution were mixed (1:1 ratio) and transferred into
six-well plates to form the bottom agar layer. Then, a total of 24
h post-transfection, the cells were trypsinized and resuspended in
a prewarmed 2X MEM. The cell suspension and melted 0.6% agar
solution were mixed (1:1 ratio) and placed on the bottom agar layer
(3,000 cells/well). The cells were incubated with culture medium
for 14 days in a humidified incubator at 37°C with 5%
CO2. Then, cells were stained with 0.01% crystal violet
for 1 h at room temperature, and the colonies (>50 cells) were
manually counted. Images were captured using a WRAYCAM-NF300 light
microscope (WRAYMER Inc.).
Cell cycle assay
Cells were transfected with miR-10b-5p or NC mimic
in six-well plates (50,000 cells/well). A total of 48 h
post-transfection, the cells were harvested trypsinization and
washed with 3% FBS in PBS. Then, the cells were fixed in cold 70%
ethanol with gentle vortexing and were placed in 70% ethanol at
−20°C for 24 h. The cells were centrifuged at 500 × g for 15 min at
20°C and resuspended in 3% FBS in PBS. After centrifuging, the cell
pellet was stained with 0.5 ml PI/RNase Staining Buffer (BD
Biosciences) for 15 min at room temperature. The FACSCanto II flow
cytometer (BD Biosciences) was used for cell cycle analysis. The
resulting data were analyzed using FlowJo software (version 10.8.1;
FlowJo LLC). Experiments were performed in triplicate.
RNA sequencing
Cells were transfected with miR-10b-5p or NC mimic
in six-well plates (50,000 cells/dish). A total of 48 h
post-transfection, total RNA was extracted as aforementioned.
Pair-end sequencing was performed by Azenta Life Sciences. Briefly,
total RNA was quantified and qualified using the Qubit RNA HS Assay
Kit (Thermo Fisher Scientific, Inc.) and TapeStation RNA ScreenTape
(Agilent Technologies, Inc.). To enrich poly-A mRNA and to remove
rRNA molecules, the NEBNext Poly(A) mRNA Magnetic Isolation Module
(cat. no. E7490L; New England Biolabs, Inc.) was used.
Subsequently, cDNA synthesis followed by transcriptome library
preparation was conducted using the MGIEasy RNA Directional Library
Prep Kit V2.0 (cat. no. 1000005272; MGI Tech Co., Ltd.). The
resulting sequencing libraries were quantified using the Qubit DNA
HS Assay Kit (Thermo Fisher Scientific, Inc.) and their fragment
size distribution was confirmed by TapeStation D1000 ScreenTape
(Agilent Technologies, Inc.). The double-stranded library fragments
were pooled/multiplexed at an equimolar amount and further
processed into single-stranded circular DNA (sscDNA). The sscDNA
libraries were quantified using the Qubit ssDNA Assay Kit (Thermo
Fisher Scientific), and a 40 fmol sscDNA library pool was used for
generating DNA nanoballs (DNBs) by rolling circle replication
reaction. DNBs were then loaded into a flow cell for sequencing on
the DNBSEQ-G400 platform (MGI Tech Co., Ltd.) with 150 bp
paired-end configuration, according to the manufacturer's
instructions. From the sequencing data, expression levels for each
gene were quantified by Kallisto (ver. 0.46.2) (24). Then, data were summarized using the
tximport package (ver. 1.18.0) of R software, and scaled transcript
per million counts were used for further analyses (25). Genes with low read coverage (maximum
read count, <10 reads) were excluded. Compared with
NC-transfected cells, genes with absolute log2 (fold change)
>0.8 were considered differentially expressed genes (DEGs).
Subsequently, common DEGs in both cell lines were used to generate
the heatmap after converting the data to base 10 logarithms and
z-scores. The heatmap.2 function of the gplots package (ver. 3.1.0;
http://cran.r-project.org/package=gplots) was used.
Pathway analysis was performed using the Ingenuity Pathway Analysis
(IPA) software (ver. 84978992; Qiagen GmbH).
Statistical analysis
Data are presented as the mean ± standard error of
the mean and experiments were performed at least in triplicate and
repeated three times. All statistical analyses were performed using
RStudio and R software (ver. 4.0.3). Welch's t-test was used to
determine the significant differences between the means of two sets
of data, and the paired t-test was used to compare the expression
of miR-10b-5p in paired ULMS and adjacent normal tissues. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comprehensive miRNA sequencing
Small RNA sequencing was performed using archival
fresh-frozen samples from six patients with ULMS and three patients
with myoma. Table I shows the
clinical information of the patients. The heatmap shown in Fig. 1A indicated that the miRNA profiles
of patients with ULMS were usually different from those of patients
with myoma. However, the miRNA profiles were diverse in patients
with ULMS; notably, the miRNA expression pattern in ULMS-3 was more
similar to that of myoma compared with the other types of ULMS. Our
previous study reported that ULMS-3 was characterized by higher
ESR1 expression and a lower mitotic rate than other types of
ULMS, suggesting that ULMS-3 is a gynecological subtype and a
clinically less aggressive subtype of LMS (13,26,27).
Subsequently, a volcano plot that compares ULMS to myoma was
generated to investigate ULMS-associated miRNAs. The volcano plot
revealed that 53 and 11 miRNAs were significantly upregulated or
downregulated, respectively, in ULMS compared with in myoma
(Fig. 1B). The normalized read
counts of the 64 miRNAs are shown in Table SI. miRNAs with abundant expression
were selected according to the baseline expression level. Dot plots
of the top four downregulated miRNAs (miR-10b-5p, miR-29a-3p,
miR-126-3p and miR-186-5p) and the top four upregulated miRNAs
(miR-10a-5p, miR-146a-5p, miR-181a-5p and miR-181b-5p) are shown in
Fig. 1C. In particular, the mean
normalized read count of miR-10b-5p was 93,650 reads in myoma;
however, it was markedly decreased to 27,903 reads in ULMS. Thus,
miR-10b-5p was considered to serve a role in ULMS progression.
RT-qPCR was performed to validate miR-10b-5p downregulation in
ULMS; it was revealed that miR-10b-5p expression was significantly
downregulated in ULMS tissues compared with that in paired normal
tissues (P<0.01; Fig. 1D).
 | Figure 1.miRNA profiles of ULMS and myoma. (A)
Hierarchical clustering and heatmap analysis showing 334
differentially expressed miRNAs between the ULMS and myoma samples.
The differentially expressed miRNAs were defined as an absolute
log2 FC >0.8. (B) Volcano plot between ULMS and myoma samples.
The P-values for each miRNA were calculated using the Wald test in
DESeq2. (C) Normalized reads of miR-10b-5p, miR-29a-3p, miR-126-3p,
miR-186-5p, miR-10a-5p, miR-146a-5p, miR-181a-5p and miR-181b-5p.
(D) Relative expression levels of miR-10b-5p in paired ULMS and
myometrium samples. The relative expression was compared using
paired Student's t-test. Error bars represent standard errors of
the mean. FC, fold change; miR/miRNA, microRNA; RPM, reads per
million; ULMS, uterine leiomyosarcoma. |
 | Table I.Clinical information of patients. |
Table I.
Clinical information of patients.
| Case | Age, years | FIGO stage | Mitotic rate,
cells/10HPF | Necrosis |
|---|
| ULMS-1 | 58 | IB | 38 | + |
| ULMS-2 | 79 | IB | 15 | + |
| ULMS-3 | 74 | IB | 5 | + |
| ULMS-4 | 61 | IIB | 40 | + |
| ULMS-5 | 53 | IB | 70 | + |
| ULMS-6 | 55 | IB | 15 | + |
| Myoma-1 | 54 | - | - | - |
| Myoma-2 | 49 | - | - | - |
| Myoma-3 | 57 | - | - | - |
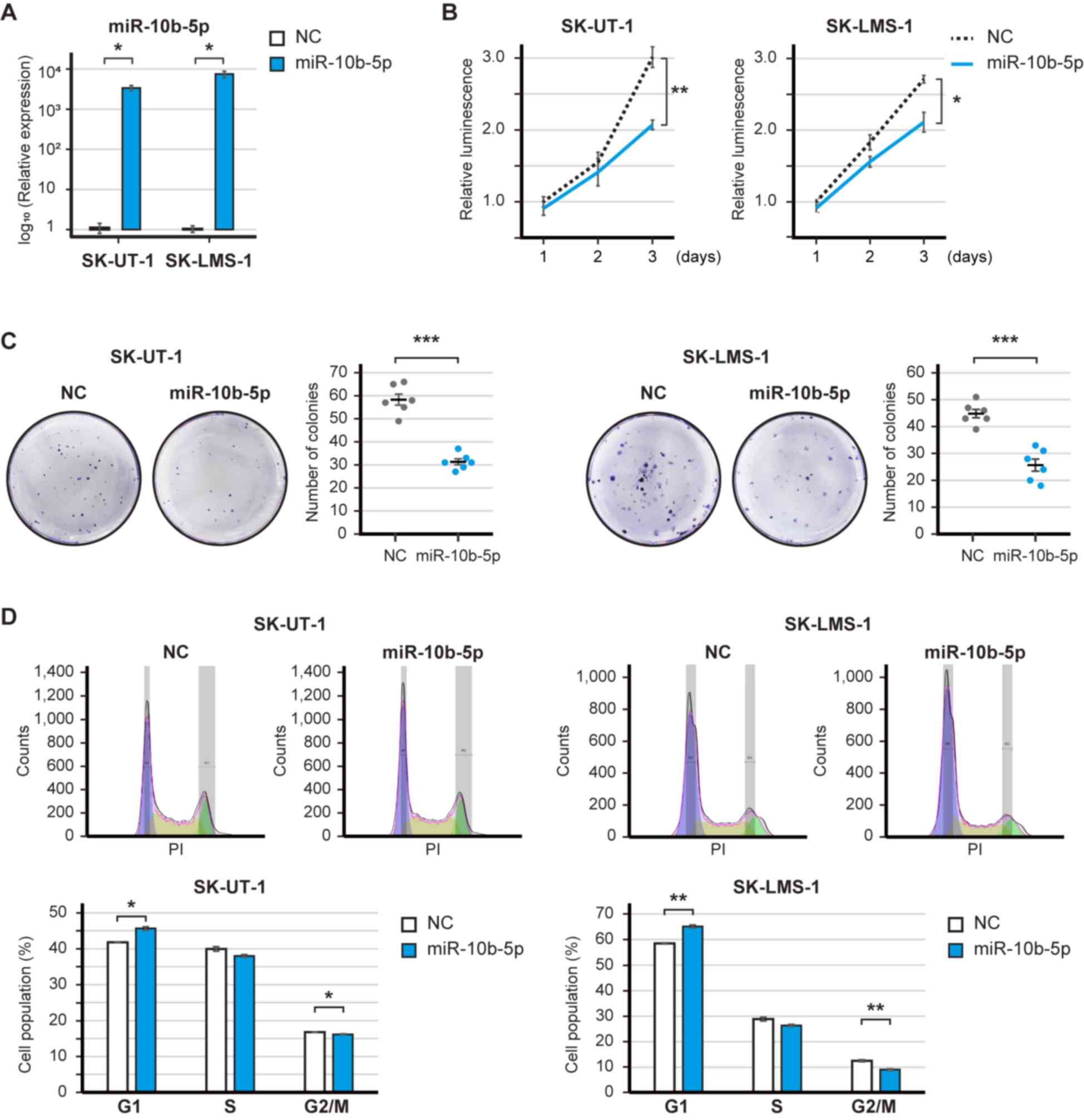
Tumor-suppressive roles of miR-10b-5p
in LMS cells
A gain-of-function analysis was performed to
elucidate the potential roles of the downregulated miRNAs in LMS.
The expression levels of miR-10b-5p were significantly increased
post-transfection with the miR-10b-5p mimic (Fig. 2A). The overexpression of miR-10b-5p
significantly decreased the proliferation of SK-UT-1 and SK-LMS-1
cell (P<0.01 and P<0.05; Fig.
2B). Similarly, miR-29a-3p, miR-126-3p and miR186-5p had
tumor-suppressive roles; in particular, miR-126-3p significantly
decreased the proliferation of SK-UT-1 and SK-LMS-1 cells
(P<0.01 and P<0.001; Fig.
S1A-F). The present study focused on miR-10b-5p because the
baseline expression of miR-10b-5p was ~10-fold higher than that of
miR-126-5p (Fig. 1C). Subsequently,
the clonogenic assay revealed that miR-10b-5p overexpression
significantly reduced the number of SK-UT-1 and SK-LMS-1 cell
colonies (P<0.001 and P<0.001; Fig. 2C). However, soft agar colony
formation assay showed that miR-10b-5p did not suppress the number
of colonies (Fig. S2). In
addition, the overexpression of miR-10b-5p significantly increased
the population of SK-UT-1 and SK-LMS-1 cells in G1 phase
(P<0.05 and P<0.01), and decreased the population of SK-UT-1
and SK-LMS-1 cells in G2/M phase (SK-UT-1 and SK-LMS-1;
P<0.05 and P<0.01) (Fig. 2D).
Therefore, these results may suggest that miR-10b-5p suppressed the
proliferation of LMS cells.
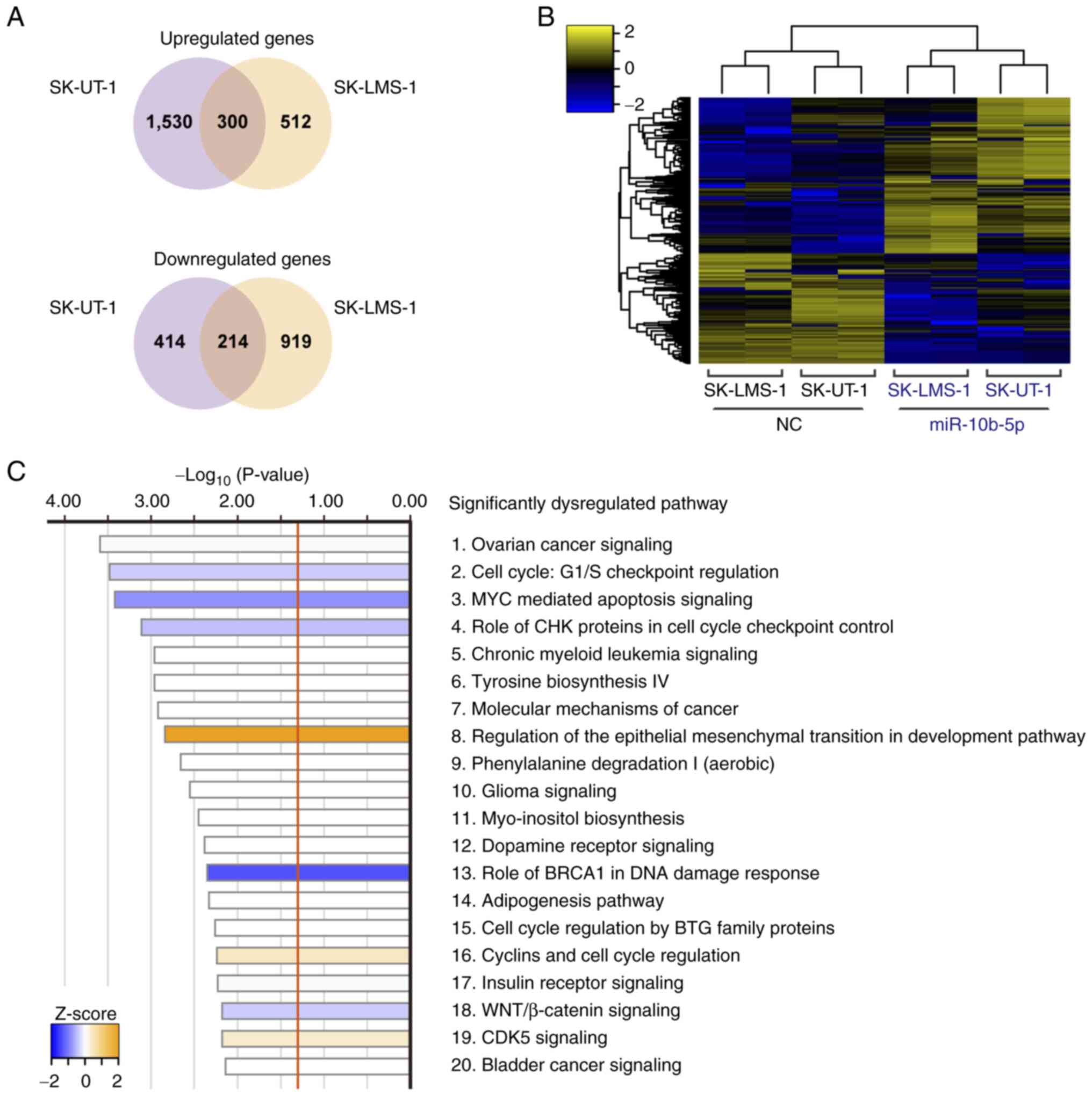
Potential functions of miR-10b-5p in
LMS cells
Transcriptome analysis was performed to investigate
the molecular background of miR-10b-5p-associated tumor
suppression. miR-10b-5p-transfected SK-UT-1 cells had 1,830
upregulated and 628 downregulated genes compared with
NC-transfected SK-UT-1 cells. Similarly, 812 upregulated and 1,133
downregulated genes were observed in miR-10b-5p-transfected
SK-LMS-1 cells. Of these, 300 upregulated and 214 downregulated
genes were identified in both cell lines (Fig. 3A). The heatmap in Fig. 3B shows the levels of 514 common DEGs
in these cells. Subsequently, IPA analysis was performed using the
514 DEGs and 52 significantly dysregulated pathways were revealed
(Fig. 3C; Table SII). For example, ‘Cell Cycle: G1/S
Checkpoint Regulation’ (P=3.31×10−4; z-score=−0.378) and
‘MYC Mediated Apoptosis Signaling’ (P=3.80×10−4;
z-score=−0.816) were significantly inhibited, whereas ‘Regulation
Of The Epithelial-Mesenchymal Transition In Development Pathway’
(P=1.45×10−3; z-score, 2.000) was significantly
activated (Fig. 3C).
Discussion
ULMS is a rare tumor, the molecular biological
features of which are not well understood. Consistent with the
results of the present study, the expression of miR-10b-5p has been
reported to be lower in several uterine sarcomas compared with that
in benign uterine tissues (28).
Moreover, a previous report demonstrated that miR-10b-5p was one of
the downregulated miRNAs in SK-UT-1 cells compared with in myoma
and myometrial cells (THESCs CRL-4003 and PCS-460-011) (29). However, to the best of our
knowledge, no reports have assessed the detailed function of
miR-10b-5p in ULMS cells. It is essential to investigate the
functions of miRNAs in LMS-derived cells because miRNAs can have
oncogenic or tumor-suppressive roles depending on the cell type.
Therefore, the present study provides a novel insight into the
molecular mechanism of ULMS pathogenesis.
Previously, several reports have shown the functions
of miR-10b-5p in other malignancies. According to The Cancer Genome
Atlas data, decreased miR-10b-5p expression is observed in various
malignancies compared with in normal tissues (30). Moreover, miR-10b-5p can suppress
cell proliferation and migration, and increase the rate of
apoptosis by regulating CREB1 expression in renal cancer
(31). Furthermore, the
overexpression of miR-10b-5p can act as a tumor suppressor in
gastric cancer by targeting TIAM1 (32,33).
However, other studies have shown that miR-10b-5p can act as an
oncogene by activating TGFβ signaling in gastric and breast cancer
(34,35). Furthermore, miR-10b-5p has been
reported to promote migration and colony formation by targeting
CDH1 in breast cancer, and the oncogenic miR-10b-5p has been
revealed to target p21 and p53 in colorectal cancer (36,37).
Moreover, miR-10b-5p contributes to glioma progression by targeting
HOXB3 and WWC3 (38,39). Furthermore, miR-10b-5p,
which is delivered by hypoxic glioma-derived extracellular
vesicles, can accelerate macrophage M2 polarization, resulting in
the progression of glioma (40).
These results suggested that miR-10b-5p can have both oncogenic and
tumor-suppressive roles, depending on the organ and cell type.
Therefore, it is essential to investigate the functions of
miR-10b-5p in ULMS. The present study demonstrated that miR-10b-5p
decreased the proliferation and colony formation ability of
LMS-derived cells. Moreover, cell cycle analysis revealed that
overexpression of miR-10b-5p increased the number of cells in
G1 phase. The result of cell cycle analysis may be due
to the prolonged doubling time of the cells, although it is
difficult to determine the cell cycle speed from the present
results.
miRNAs stably exist in body fluids, such as
peripheral blood and urine; therefore, they are potentially
non-invasive biomarkers (41).
Previous reports have indicated that serum miR-10b-5p is elevated
in patients with lung adenocarcinoma or hepatocellular carcinoma
compared with in normal controls (42–44).
In our previous microarray-based study, the serum miRNA profiles of
ULMS were evaluated, and it was revealed that an index calculated
using miR-191-5p and miR-1246 could be an accurate diagnostic
biomarker (45). However, serum
miR-10b-5p did not differ significantly between ULMS and myoma
samples (45). Therefore, the
expression levels of serum miR-10-5p may not be correlated with
those of cellular miR-10b-5p in ULMS and myoma.
The present study has several limitations. First,
the sample size was small, and the individual differences may have
skewed the results of miRNA sequencing. Second, it is still
controversial as to whether myoma is a suitable control for LMS. In
previous reports, the miRNA signature of ULMS was compared with
that of carcinosarcoma or endometrial stromal sarcoma (27,46).
Moreover, miRNA profiles can differ depending on platforms, such as
microarray and next-generation sequencing (47). Therefore, further studies are needed
to conclude the ULMS-associated miRNA profile. Third, the direct
target genes of miR-10b-5p were not assessed. It was hypothesized
that miR-10b-5p may exert tumor-suppressive effects as a result of
the cooperation of the various target genes; however, miR-10b-5p
did not have an effect in soft agar colony formation assay. It was
suggested that transient overexpression may be inappropriate for a
long-term culture protocol. Fourth, the functions of other miRNAs,
such as miR-29a-3p, miR-126-3p and miR-186-5p, were not fully
evaluated. In addition, the in vivo functions of miRNAs were
not assessed using animal models. Therefore, additional experiments
are required to elucidate the molecular background of ULMS and to
develop novel therapeutic strategies targeting miRNA-related
pathways.
In conclusion, the present study identified the
unique miRNA profiles of ULMS through miRNA sequencing, and the
expression of miR-10b-5p was revealed to be significantly
downregulated in ULMS compared with in myoma (Fig. 4). A subsequent in vitro
analysis revealed that the overexpression of miR-10b-5p suppressed
LMS cell proliferation and colony formation, and increased the
number of cells in G1 phase. These findings suggested
that miR-10b-5p may act as a tumor suppressor, and miR-10b-5p and
its target genes could be novel therapeutic targets. Further
elucidation of the molecular background of ULMS may improve patient
prognoses.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by JSPS KAKENHI (grant nos. 21H02721,
21H03075 and 21K16789) and the Fusion Oriented Research for
Disruptive Science and Technology (FOREST) from Japan Science and
Technology Agency (JST). Moreover, this study was supported by the
YOKOYAMA Foundation for Clinical Pharmacology (grant no. YRY-2115),
the Princess Takamatsu Cancer Research Fund (grant no. 20-25237),
the Mochida Memorial Foundation for Medical and Pharmaceutical
Research (grant no. 202102016), Daiichi Sankyo Foundation of Life
Science (grant no. 2021HrCK), the Uehara Memorial Foundation (grant
no. 202110201), the Japan Research Foundation for Clinical
Pharmacology (grant no. 2021A18), and the Foundation for Promotion
of Cancer Research in Japan.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request. Moreover, the raw sequencing data generated
and/or analyzed during the current study are available in the Gene
Expression Omnibus repository [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE200777
and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE201542].
Authors' contributions
KY, AY, TK, HK and YY conceived the present study.
HY and TK made substantial contributions to the acquisition of
data. KY, MK, MS, TY and JN performed and analyzed experiments. KY,
AY and YY confirm the authenticity of all the raw data. AY, HK and
YY supervised the project and were involved in the interpretation
of data. KY, AY and YY acquired funding. KY wrote the original
manuscript, and AY, HK and YY revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee at National Cancer Center (approval no. 2020-160).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skorstad M, Kent A and Lieng M: Uterine
leiomyosarcoma-incidence, treatment, and the impact of
morcellation. A nationwide cohort study. Acta Obstet Gynecol Scand.
95:984–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
George S, Serrano C, Hensley ML and
Ray-Coquard I: Soft tissue and uterine leiomyosarcoma. J Clin
Oncol. 36:144–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roberts ME, Aynardi JT and Chu CS: Uterine
leiomyosarcoma: A review of the literature and update on management
options. Gynecol Oncol. 151:562–572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seagle BL, Sobecki-Rausch J, Strohl AE,
Shilpi A, Grace A and Shahabi S: Prognosis and treatment of uterine
leiomyosarcoma: A national cancer database study. Gynecol Oncol.
145:61–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hensley ML, Patel SR, von Mehren M, Ganjoo
K, Jones RL, Staddon A, Rushing D, Milhem M, Monk B, Wang G, et al:
Efficacy and safety of trabectedin or dacarbazine in patients with
advanced uterine leiomyosarcoma after failure of
anthracycline-based chemotherapy: Subgroup analysis of a phase 3,
randomized clinical trial. Gynecol Oncol. 146:531–537. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blay JY, Schöffski P, Bauer S,
Krarup-Hansen A, Benson C, D'Adamo DR, Jia Y and Maki RG: Eribulin
versus dacarbazine in patients with leiomyosarcoma: Subgroup
analysis from a phase 3, open-label, randomised study. Br J Cancer.
120:1026–1032. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benson C, Ray-Coquard I, Sleijfer S,
Litière S, Blay JY, Le Cesne A, Papai Z, Judson I, Schöffski P,
Chawla S, et al: Outcome of uterine sarcoma patients treated with
pazopanib: A retrospective analysis based on two European
organisation for research and treatment of cancer (EORTC) soft
tissue and bone sarcoma group (STBSG) clinical trials 62043 and
62072. Gynecol Oncol. 142:89–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuppens T, Moisse M, Depreeuw J, Annibali
D, Colas E, Gil-Moreno A, Huvila J, Carpén O, Zikán M, Matias-Guiu
X, et al: Integrated genome analysis of uterine leiomyosarcoma to
identify novel driver genes and targetable pathways. Int J Cancer.
142:1230–1243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hensley ML, Chavan SS, Solit DB, Murali R,
Soslow R, Chiang S, Jungbluth AA, Bandlamudi C, Srinivasan P, Tap
WD, et al: Genomic landscape of uterine sarcomas defined through
prospective clinical sequencing. Clin Cancer Res. 26:3881–3888.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Astolfi A, Nannini M, Indio V, Schipani A,
Rizzo A, Perrone AM, De Iaco P, Pirini MG, De Leo A, Urbini M, et
al: Genomic database analysis of uterine leiomyosarcoma mutational
profile. Cancers (Basel). 12:21262020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi J, Manzano A, Dong W, Bellone S,
Bonazzoli E, Zammataro L, Yao X, Deshpande A, Zaidi S, Guglielmi A,
et al: Integrated mutational landscape analysis of uterine
leiomyosarcomas. Proc Natl Acad Sci USA. 118:e20251821182021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shan W, Akinfenwa PY, Savannah KB,
Kolomeyevskaya N, Laucirica R, Thomas DG, Odunsi K, Creighton CJ,
Lev DC and Anderson ML: A small-molecule inhibitor targeting the
mitotic spindle checkpoint impairs the growth of uterine
leiomyosarcoma. Clin Cancer Res. 18:3352–3365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida K, Yokoi A, Yamamoto T, Hayashi Y,
Nakayama J, Yokoi T, Yoshida H, Kato T, Kajiyama H and Yamamoto Y:
Aberrant activation of cell cycle-related kinases and the potential
therapeutic impact of PLK1 or CHEK1 inhibition in uterine
leiomyosarcoma. Clin Cancer Res. 28:2147–2159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47(D1): D155–D162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida K, Yamamoto Y and Ochiya T: miRNA
signaling networks in cancer stem cells. Regen Ther. 17:1–7. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi G, Perle MA, Mittal K, Chen H, Zou X,
Narita M, Hernando E, Lee P and Wei JJ: Let-7 repression leads to
HMGA2 overexpression in uterine leiomyosarcoma. J Cell Mol Med.
13:3898–3905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pazzaglia L, Novello C, Conti A, Pollino
S, Picci P and Benassi MS: miR-152 down-regulation is associated
with MET up-regulation in leiomyosarcoma and undifferentiated
pleomorphic sarcoma. Cell Oncol (Dordr). 40:77–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray NL, Pimentel H, Melsted P and Pachter
L: Near-optimal probabilistic RNA-seq quantification. Nat
Biotechnol. 34:525–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soneson C, Love MI and Robinson MD:
Differential analyses for RNA-seq: Transcript-level estimates
improve gene-level inferences. F1000Res. 4:15212015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hemming ML, Fan C, Raut CP, Demetri GD,
Armstrong SA, Sicinska E and George S: Oncogenic gene-expression
programs in leiomyosarcoma and characterization of conventional,
inflammatory, and uterogenic subtypes. Mol Cancer Res.
18:1302–1314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leitao MM Jr, Hensley ML, Barakat RR,
Aghajanian C, Gardner GJ, Jewell EL, O'Cearbhaill R and Soslow RA:
Immunohistochemical expression of estrogen and progesterone
receptors and outcomes in patients with newly diagnosed uterine
leiomyosarcoma. Gynecol Oncol. 124:558–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalez Dos Anjos L, de Almeida BC, Gomes
de Almeida T, Mourão Lavorato Rocha A, De Nardo Maffazioli G,
Soares FA, Werneck da Cunha I, Baracat EC and Carvalho KC: Could
miRNA signatures be useful for predicting uterine sarcoma and
carcinosarcoma prognosis and treatment? Cancers (Basel).
10:3152018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Almeida BC, Garcia N, Maffazioli G, dos
Anjos LG, Baracat EC and Carvalho KC: Oncomirs expression profiling
in uterine leiomyosarcoma cells. Int J Mol Sci. 19:522017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Yan Y, Zhang Z and Li Y: Role of
miR-10b-5p in the prognosis of breast cancer. PeerJ. 7:e77282019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Chen D, Li Y, Jin L, Liu J, Su Z, Qi
Z, Shi M, Jiang Z, Ni L, et al: Oncogenic cAMP responsive element
binding protein 1 is overexpressed upon loss of tumor suppressive
miR-10b-5p and miR-363-3p in renal cancer. Oncol Rep. 35:1967–1978.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu G, Shi Y, Zhao X, Gao D, Qu L, Chen L,
Zhao K, Du J and Xu W: CBFβ/RUNX3-miR10b-TIAM1 molecular axis
inhibits proliferation, migration, and invasion of gastric cancer
cells. Int J Clin Exp Pathol. 12:3185–3196. 2019.PubMed/NCBI
|
|
33
|
Liu F, An X, Zhao X, Zhang N, Chen B, Li Z
and Xu W: MiR-10b-5p inhibits tumorigenesis in gastric cancer
xenograft mice model through down-regulating Tiam1. Exp Cell Res.
407:1128102021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan T, Wang X, Wei G, Li H, Hao L, Liu Y,
Yu X, Zhu W, Liu P, Zhu Y and Zhou X: Exosomal miR-10b-5p mediates
cell communication of gastric cancer cells and fibroblasts and
facilitates cell proliferation. J Cancer. 12:2140–2150. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lo PK, Zhang Y, Yao Y, Wolfson B, Yu J,
Han SY, Duru N and Zhou Q: Tumor-associated myoepithelial cells
promote the invasive progression of ductal carcinoma in situ
through activation of TGFβ signaling. J Biol Chem. 292:11466–11484.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang B, Zhang Y, Zhang H, Lin F, Tan Q,
Qin Q, Bao W, Liu Y, Xie J and Zeng Q: Long intergenic non-protein
coding RNA 324 prevents breast cancer progression by modulating
miR-10b-5p. Aging (Albany NY). 12:6680–6699. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu C, Jiang W, Hui B, Rong D, Fu K, Dong
C, Tang W and Cao H: The circ_0021977/miR-10b-5p/P21 and P53
regulatory axis suppresses proliferation, migration, and invasion
in colorectal cancer. J Cell Physiol. 235:2273–2285. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li W, Li C, Xiong Q, Tian X and Ru Q:
MicroRNA-10b-5p downregulation inhibits the invasion of glioma
cells via modulating homeobox B3 expression. Exp Ther Med.
17:4577–4585. 2019.PubMed/NCBI
|
|
39
|
Yang Y, Liu X, Zheng J, Xue Y, Liu L, Ma
J, Wang P, Yang C, Wang D, Shao L, et al: Interaction of BACH2 with
FUS promotes malignant progression of glioma cells via the
TSLNC8-miR-10b-5p-WWC3 pathway. Mol Oncol. 14:2936–2959. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li B, Yang C, Zhu Z, Chen H and Qi B:
Hypoxic glioma-derived extracellular vesicles harboring
MicroRNA-10b-5p enhance M2 polarization of macrophages to promote
the development of glioma. CNS Neurosci Ther. 28:1733–1747. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshida K, Yokoi A, Kato T, Ochiya T and
Yamamoto Y: The clinical impact of intra- and extracellular miRNAs
in ovarian cancer. Cancer Sci. 111:3435–3444. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shan X, Zhang L, Zhu DX, Zhou X, Zhang H,
Liu QX, Tang JW, Wen W, Wang TS, Zhu W and Liu P: Serum microRNA
expression profiling revealing potential diagnostic biomarkers for
lung adenocarcinoma. Chin Med J (Engl). 133:2532–2542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho HJ, Eun JW, Baek GO, Seo CW, Ahn HR,
Kim SS, Cho SW and Cheong JY: Serum exosomal MicroRNA, miR-10b-5p,
as a potential diagnostic biomarker for early-stage hepatocellular
carcinoma. J Clin Med. 9:2812020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghosh S, Bhowmik S, Majumdar S, Goswami A,
Chakraborty J, Gupta S, Aggarwal S, Ray S, Chatterjee R,
Bhattacharyya S, et al: The exosome encapsulated microRNAs as
circulating diagnostic marker for hepatocellular carcinoma with low
alpha-fetoprotein. Int J Cancer. 147:2934–2947. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yokoi A, Matsuzaki J, Yamamoto Y, Tate K,
Yoneoka Y, Shimizu H, Uehara T, Ishikawa M, Takizawa S, Aoki Y, et
al: Serum microRNA profile enables preoperative diagnosis of
uterine leiomyosarcoma. Cancer Sci. 110:3718–3726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ravid Y, Formanski M, Smith Y, Reich R and
Davidson B: Uterine leiomyosarcoma and endometrial stromal sarcoma
have unique miRNA signatures. Gynecol Oncol. 140:512–517. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Git A, Dvinge H, Salmon-Divon M, Osborne
M, Kutter C, Hadfield J, Bertone P and Caldas C: Systematic
comparison of microarray profiling, real-time PCR, and
next-generation sequencing technologies for measuring differential
microRNA expression. RNA. 16:991–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|