Introduction
As is well-established, tumors constantly affect
human health (1). Numerous studies
have demonstrated that tumor development and resistance to
radiotherapy and chemotherapy are closely related to abnormal
autophagic activities, and a comprehensive understanding of the
molecular mechanisms of autophagy on tumors, can help develop
targeted therapeutic strategies and provide novel insights into
tumor treatment (2–5).
Autophagy is a highly conserved metabolic pathway by
which cells regulate autophagy-related genes to remove abnormal
proteins, damaged organelles and pathogenic microorganisms, in
order to maintain cellular homeostasis (6–8). Under
normal physiological conditions, autophagy allows cells to degrade
damaged intracellular proteins, circulate nutrients, and generate
energy in a timely manner to maintain cell viability in most
tissues and under unfavorable conditions such as hypoxia or
ischemia, as a cellular protective mechanism (9). However, persistent abnormalities in
cellular autophagic activity can lead to various diseases (10–12),
such as cardiovascular (13),
neurodegenerative (14) and
infectious diseases (15), as well
as malignant tumors (16).
During the onset of autophagy, there are various
autophagy-associated proteins (ATGs) that regulate and control the
different stages of autophagosome formation and are key regulators
of autophagosome formation (17,18).
To date, scientists have identified >40 genes encoding ATG
proteins in yeast, and most of them are highly conserved in yeast
and mammals (19–21). In mammalian cells,
starvation-induced autophagy is regulated by ~20 core ATG genes,
and the more studied ATGs include ATG5, ATG6 (Beclin1), ATG7, and
ATG8 (LC3-PE), among which ATG5 and ATG7 are decisive regulators of
the pre-autophagic ubiquitination (22–24).
ATG5 is dysregulated in numerous tumors and its role varies greatly
among tumors, making it a double-edged sword with both promoting
and inhibiting effects on tumors. This property also provides two
concepts for tumor therapy: Inhibition of ATG5 to improve the
effect of anticancer therapy or activation of ATG5 to induce
autophagic death of tumor cells. The aim of the present study was
to provide an overview of the basic functions of ATG5 and its
mechanism of action in tumor development and treatment.
Methods
A biomedical literature retrieval website
(http://www.ncbi.nlm.nih.gov/pubmed)
was used to search for topic keywords related to this review.
Subsequently, ‘advanced’ was selected on the PubMed homepage to
enter the advanced search page, the corresponding search term was
placed in the search box, and ‘search’ was selected to enter the
search results interface.
In terms of ‘Introduction’, the keyword was ‘ATG5’,
and initially 4,627 articles were gathered. Further screening was
conducted, and 24 articles were ultimately selected.
In terms of ‘Structure of ATG5’, the key word was
‘ATG5, structure’, and initially 390 articles were collected.
Further screening was conducted, and three articles were finally
cited.
In terms of ‘Biological functions of ATG5’, this was
divided it into the following aspects: In terms of ‘DNA level
regulation’, the key words were ‘ATG5, gene’, and 2,262 articles
were initially obtained, which were further analyzed and finally
three articles were cited; in terms of ‘Post-transcriptional level
regulation’, the key words were ‘ATG5, mRNA’, and 548 articles were
initially selected, which were further screened and two articles
were ultimately mentioned; in terms of ‘Post-translational
modifications’, the key words were ‘(ATG5) AND (Phosphorylation) OR
(Ubiquitination) OR (acetylation)’. Initially, 1,179 articles were
amassed and further screening was performed, and finally seven
articles were cited.
In terms of ‘ATG5 is involved in tumorigenesis
development’, the key words were ‘(ATG5) AND (cancer)’. Initially,
1,648 articles were obtained and after further assessment, these
were divided into the following aspects: In terms of ‘ATG5 is
involved in the autophagic process of tumors’, after further
analysis, nine articles were selected; in terms of ‘ATG5 promotes
tumor cell apoptosis’, after further study, five articles were
cited; in terms of ‘ATG5 is involved in tumorigenesis development
in other ways’, after further screening, ultimately four articles
were selected.
In terms of ‘Dual effects of ATG5 on tumors’, the
key words were ‘(ATG5) AND (cancer)’. Initially 1,648 articles were
obtained and further investigated, and divided into the following
aspects: In terms of ‘Upregulation of ATG5 expression’, after
further assessment, four articles were selected; in terms of
‘Downregulation of ATG5 expression’, after further screening,
ultimately 30 articles were cited.
In terms of ‘Role of ATG5 in tumor treatment’, the
key words were ‘(ATG5) AND (cancer)’. Initially 1,648 articles were
obtained and further examined, and divided into the following
aspects: In terms of ‘Therapeutic strategies for downregulation of
ATG5’, after further screening, 13 articles were cited; in terms of
‘Therapeutic strategies for upregulation of ATG5’, following
further assessment, five articles were selected.
Structure of ATG5
The ATG5 protein consists of three main structural
domains (Fig. 1): Two
ubiquitin-like structural domains at the N-terminal and C-terminal
ends are connected by two junctional regions (L1 and L2) on either
side of the multi-helix structural domain, respectively. Thr-193,
located in the L2 junctional area, is the site where calpains
cleave full-length 33 kDa ATG5 into 24 kDa truncated-ATG5 (tATG5),
that promotes apoptosis by targeting mitochondria (25). Both ubiquitin-like structures
consist of five-stranded β-pleated sheets and two α-helices. The
multi-helix structure consists of one short α-helix and two long
α-helices, and the conjugation site Lys-149 of ATG5 and ATG12 is
located in this structural domain. The ATG5-ATG12 complex binds
ATG16 to generate the autophagy elongation complex to promote
autophagy. There is also an α-helix structural domain in addition
to the N-terminal ubiquitin-like structural domain (26,27).
Biological functions of ATG5
DNA level regulation
The c allele of the rs2245214 ATG5 gene polymorphism
is associated with increased susceptibility to non-small cell lung
cancer (NSCLC), while the (guanine/cytosine) GC genotype of this
polymorphism is associated with reduced risk and therefore may have
a protective role in the development of NSCLC (28). SMARCB1 is a tumor suppressor gene
that inhibits the malignant proliferation of chordoma cells both
in vitro and in vivo. The molecular mechanism of
tumor suppression directly binds to the ATG5 promoter (+8 to +263
bp) and epigenetically suppresses its transcription, which
decreases ATG5 expression and downregulates autophagy (29). Nuclear respiratory factor 1 (NRF1)
can act as a transcription factor that binds to the ATG5 promoter,
promoting ATG5 transcription and subsequently upregulating
autophagy levels, while reduced autophagic activity contributes to
the development of melanoma (30).
Post-transcriptional level
regulation
MicroRNAs (miRNAs/miRs) can negatively regulate ATG5
at the post-transcriptional level by binding to the 3′UTR of ATG5
mRNA (31). LncRNA IDH1-AS1
regulates the stability of ATG5 mRNA by interacting with the
selective splicing regulatory protein PTBP3 to upregulate
expression, which in turn promotes autophagy and prostate cancer
cell proliferation (32).
Post-translational modifications
Phosphorylation
In hypoxia-induced glioblastoma (GBM) cells,
hypoxia-induced ELP3-mediated acetylation of PAK1 inhibits PAK1
dimerization and enhances its activity, thus leading to
PAK1-mediated phosphorylation of ATG5 at residue T101, which
protects ATG5 from ubiquitin-dependent degradation and increases
the affinity between the ATG12-ATG5 complex and ATG16L1, that
promotes the formation of autophagosomes (33).
Ubiquitination
Ubiquitination-proteasome is a major intracellular
protein degradation pathway in eukaryotes, and the immunoproteasome
subunit β5i in cardiac myocytes promotes ubiquitination and
degradation of ATG5 protein, thereby inhibiting autophagy that
leads to myocardial hypertrophy (34). Ubiquitin-specific peptidase 22
(USP22) stabilizes ATG5 by reducing the ubiquitination of ATG5 at
the K27- and K48-linkages Lys118 site, promoting autophagosome
formation, inhibiting NLRP3 inflammatory vesicle activation, and
preventing excessive inflammation (35).
Acetylation
Acetylation which is an important post-translational
modification of proteins in mammalian cells, is involved in the
regulation of numerous biological processes (36–38).
Histone acetyltransferase p300 inhibits autophagy by acetylating
other autophagy-related proteins of ATG5, regulating cell growth
and proliferation (39).
ATG5 is involved in tumorigenesis
development
ATG5 is involved in the autophagic
process of tumors
Cellular autophagy can be divided into five phases:
Autophagy induction phase, nucleation process, extension phase of
the autophagosome, maturation phase of the autophagosome, and lysis
phase of the autophagosome (40).
ATGs are continuously recruited near the vesicles and assembled to
form autophagic precursors, whose maturation requires the
continuous extension of autophagosomal membranes (41,42).
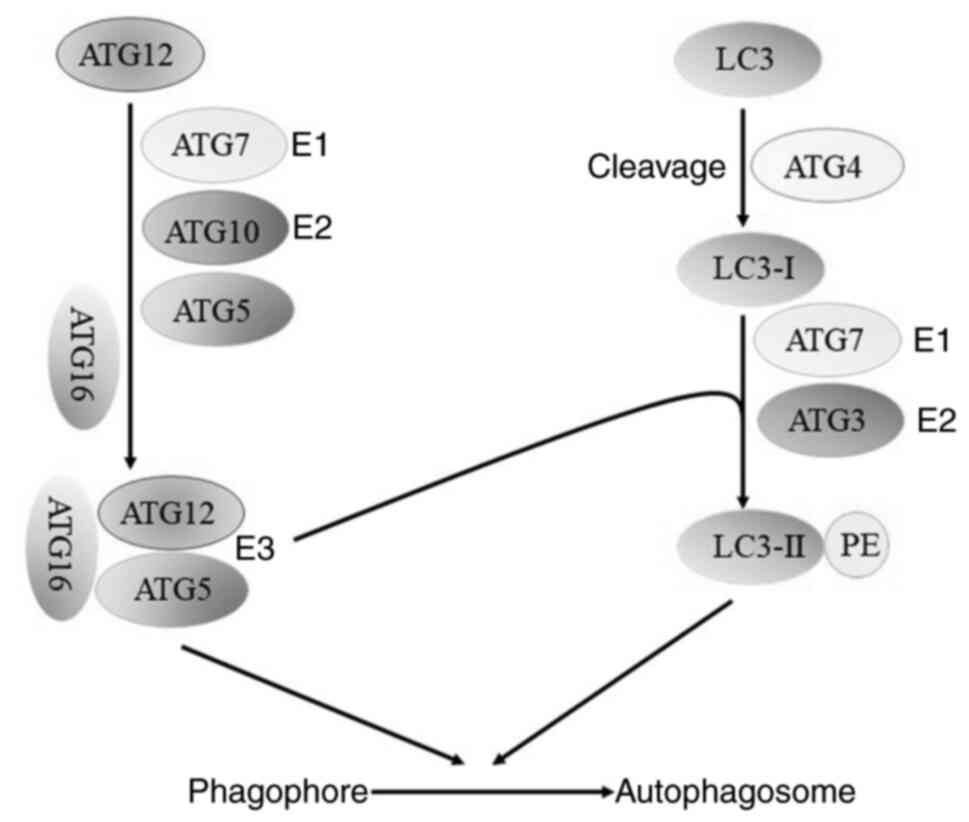
ATG5, as the main regulator of the pre-autophagic ubiquitination
process, plays a decisive role in the development of autophagy.
ATG12 covalently modifies ATG5 by the E1-like enzyme, ATG7, and the
E2-like enzyme, ATG10, and binds to ATG5 to form the ATG12-ATG5
conjugate, which eventually binds to ATG16 to form the
ATG12-ATG5-ATG16 ubiquitin-like protein conjugation system that
participates in the membrane elongation process in two ways:
Directly bound to the membrane or as an E3-like enzyme involved in
LC3III-PE splicing for subsequent activation of autophagy (43–45)
(Fig. 2).
It has been shown that in HBx-associated
hepatocellular carcinoma (HCC) cells, where autophagy levels are
upregulated, downregulation of autophagy levels by inhibition of
ATG5 expression attenuates HBx-induced cell cycle acceleration and
G1/S block-induced proliferative responses, thereby inhibiting HCC
proliferation (46). Wang et
al (47) revealed that
inhibition of ATG5 expression in human colon cancer cells (HCT116)
followed by induction of EMT through the SQSTM1-NFKB pathway could
promote cell migration and induce invasion. Liang et al
(48) reported that cadmium, a
carcinogenic heavy metal, inhibited cellular autophagy and promoted
the proliferation and migration of breast cancer cells through the
downregulation of ATG5 expression, suggesting that ATG5 expression
can inhibit the metastasis of certain cancer cells.
ATG5 promotes tumor cell
apoptosis
In addition to regulating autophagosome formation of
tumor cells, ATG5 also has an important role in tumor cell
apoptosis, with direct or indirect pro-apoptotic effects. In breast
cancer cells, ATG5 is cleaved by calpain and the cleavage product
truncated-ATG5 (1–193) (tATG5),
targets mitochondria with pro-apoptotic activity (25). Cyathin Q is a diterpene compound
extracted from a fungus that can inhibit the growth and
proliferation of HCT116. Upon compound action on cells,
mitochondria produce reactive oxygen species (ROS), which on the
one hand induces apoptosis by directly downregulating the
apoptosis-inhibiting protein Bcl-2 and upregulating the
pro-apoptotic protein Bim, and on the other hand, promotes cell
death by cleaving the ATG5 protein to convert autophagy into
apoptosis (49). Cinobufagin, a
butyrolactone steroid with anticancer activity, can reduce the
expression of autophagy-related proteins, such as ATG5, to
downregulate autophagy levels and enhance apoptosis levels, thus
inhibiting gastric cancer cell proliferation (50). In NSCLC, low expression of TECPR1
downregulates ATG5 expression, downregulates Bax and LC3-II/LC3-I,
upregulates p62 and Bcl-2, thus inhibiting apoptosis and enhancing
cell viability (51). Zheng et
al (52) reported that in
rectal colon cancer cells, high expression of lncRNA HAGLROS
targeted upregulation of ATG5 through competitive binding with
miR-100, which in turn activated the PI3K/AKT/mTOR pathway and
inhibited HCT116 cell apoptosis.
ATG5 is involved in tumorigenesis
development in other ways
ATG5 mediates the cell cycle distribution of acute
myeloid leukemia mesenchymal stem cells (AML MSC) and silencing of
the ATG5 gene increases the proportion of the G0/G1 phase and
decreases the proportion of the G2 phase, inhibiting the
proliferation of AML MSC (53).
ATG5 also has an important role in the tumor immune
microenvironment, and it was determined that ATG5 is an essential
protein for the presentation of tumor antigens by dendritic cells
to activate CD4+ T cells to produce cytokines such as
IL-2 and IFN-γ, thus initiating an immune response to inhibit tumor
growth and proliferation (54).
ATG5 also plays a role in DNA damage repair. Demirbag-Sarikaya
et al (55) revealed that
when 293T and HeLa cells were subjected to genotoxic compounds such
as etoposide, cisplatin and adriamycin, ATG5 interacted directly
with the non-homologous end joining (NHEJ) repair mechanism protein
Ku70 in the nucleus to repair damaged DNA, rendering the cells
resistant to the drug, and it was also reported the ATG5-Ku70
interaction was required for DNA damage repair. In addition,
nuclear translocation of ATG5 can cause drug resistance in tumor
cells. By analyzing clinical rectal colon cancer specimens, Sun
et al (56) revealed that
ATG5 exhibited nuclear translocation in rectal colon cancer cells,
nuclear ATG5 bound to Mis18α to form ATG5-Mis18α interaction, and
ATG5-Mis18α overexpression induced MLH1 deletion by promoting MLH1
promoter CpG island hypermethylation, thus leading to drug
resistance in rectal colon cancer cells. Therefore, ATG5 or
ATG5-Mis18α may be used as a therapeutic target for rectal colon
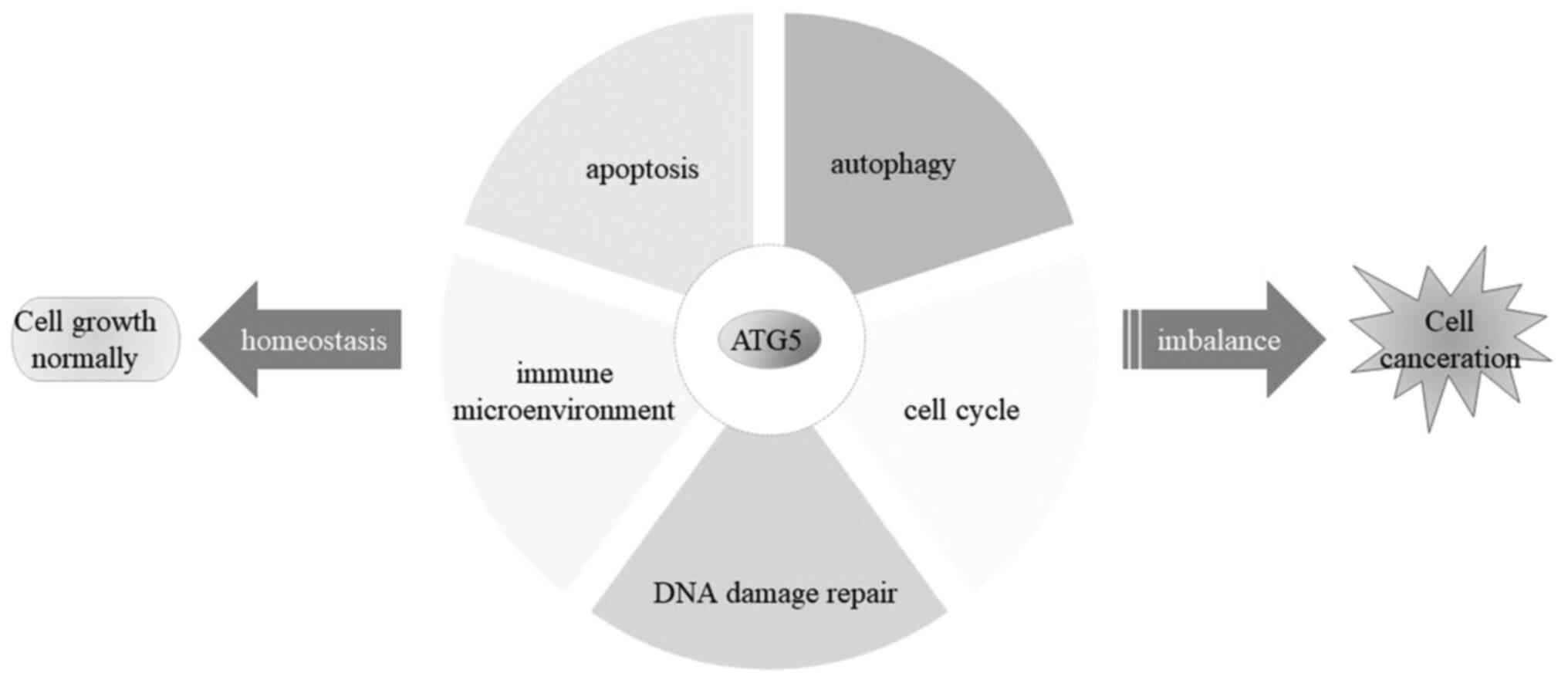
cancer cells. The pattern of ATG5 involvement in tumorigenesis
development is shown in Fig. 3.
Dual effects of ATG5 on tumors
Upregulation of ATG5 expression
Yu et al (57) determined that hypoxia-inducible
factor HIF1α could directly bind to the ATG5 promoter of human
prostate cancer cells (PC-3) to upregulate the expression of ATG5,
thereby increasing the level of autophagy and promoting the
proliferation and migration of PC-3 cells. Wang et al
(58) revealed that miR-20a
increased the level of autophagy by targeting ATG5 and upregulating
its expression to promote the proliferation of osteosarcoma cells.
Zhou et al (59) through KM
analysis demonstrated that among numerous ATGs, ATG5 was the most
detrimental factor affecting the prognosis of patients with
cervical cancer, and the survival of patients with cervical cancer
with high ATG5 expression was shorter regardless of clinical stage
and pathological grading. Analysis also revealed that ATG5 was
involved in ERK/NFκBp65/mTOR pathway-induced epithelial-mesenchymal
transition (EMT) promoting migration and invasion of cervical
cancer cell lines. This suggests that ATG5 may be a potentially
powerful therapeutic target for cervical cancer. In prostate cancer
cells (PCa) (60), CHRM1 was highly
expressed and targeted ATG5 through the AMPK/mTOR signaling pathway
to regulate cellular autophagy and promote cancer cell infiltration
and metastasis.
Downregulation of ATG5 expression
ATG5 expression was revealed to be significantly
reduced in melanoma tissues, and associated with poor patient
prognosis, as a consequence of reduced nuclear respiratory factor 1
(NRF1) activity (30). In papillary
thyroid carcinoma, RBM47 expression was reduced and decreased ATG5
expression through the SNHG5/FOXO3/ATG5 axis to decrease autophagy
levels, thus promoting cancer cell proliferation (61). ANXA1 was demonstrated to promote
nasopharyngeal carcinoma cell migration and invasive metastasis by
activating the PI3K/AKT signaling pathway, downregulating ATG5
expression, and decreasing autophagy levels (62).
Long non-coding RNAs (lncRNAs) are a group of
non-coding RNAs that are >200 nucleotides in length, typically
accounting for >80% of a whole-genome transcript (63–65).
Circular RNAs (circRNAs) are a class of non-coding RNAs that do not
have a 5′ end cap and a 3′ end poly(A) tail and are covalently
bonded to form a circular structure (66). LncRNAs and circRNAs can both compete
as endogenous competing RNAs (ceRNAs) to bind miRNAs (67–69).
MiRNAs are a group of small non-coding RNAs of ~22–24 nucleotides
in length that negatively regulate target genes at the
post-transcriptional level by binding to the 3′UTR of target mRNAs,
and the competitive binding of ceRNAs to miRNAs can reverse this
negative regulation. An increasing number of studies (70–78)
have shown that the regulatory pattern of ceRNA/miRNAs/ATG5 plays
an important role in tumorigenesis development. The
ceRNA/miRNAs/ATG5 regulatory patterns in different types of tumors
are listed in Table I.
 | Table I.ceRNA/miRNAs/ATG5 regulatory
signaling pathways in various types of tumors. |
Table I.
ceRNA/miRNAs/ATG5 regulatory
signaling pathways in various types of tumors.
| Types of
tumors | Tumor marker | Signaling
pathway | ATG5
expression | Autophagy
level | Effects on
tumors | (Refs.) |
|---|
| Gastric cancer | lncRNA XIST ↑ | miR-30c/ATG5 | ↑ | ↑ | Promotes | (70) |
| Gastric cancer | lncRNA CCT1 ↑ |
miR-140-3p/ATG5 | ↑ | ↑ | Promotes | (71) |
| Lung cancer | lncRNA PVT1 ↑ |
miR-140-3p/ATG5 | ↑ | ↑ |
Chemoresistance | (72) |
| Papillary
thyroid | lncRNA |
miR-187-3p/ATG5 | ↓ | ↓ | Promotes | (73) |
| Carcinoma | GAS8-AS1 ↓ |
|
|
|
|
|
| Non-small cell lung
cancer | circ-FOXM1 ↓ |
miR-149-5p/ATG5 | ↓ | ↓ | Promotes | (74) |
| Colorectal
cancer | miR-183-3p ↑ |
miR-183-3p/ATG5 | ↓ | ↓ |
Radioresistance | (75) |
| Colorectal
cancer | miR-20a ↓ |
miR-20a/ATG5/FI200 | ↑ | ↑ | Promotes | (76) |
| Bladder cancer | miR-30a-3p ↓ |
miR-30a-3p/ATG5 | ↑ | ↑ |
Chemoresistance | (77) |
| Renal cell
carcinoma | miR-30d-5p ↓ |
miR-30d-5p/ATG5 | ↑ | ↑ | Promotes | (78) |
Therefore, ATG5 has a dual role in tumors through
the activation of autophagic activity. This dual role is reflected
in different stages of tumor development. On the one hand, usually,
autophagy plays an oncogenic role in the initiation stage of
tumorigenesis, and in the early stage of tumorigenesis, autophagy
can reduce tumorigenesis by inhibiting the continuous growth of
precancerous cells. Tumor cells can survive by using the autophagy
mechanism to fight against nutrient deficiency and hypoxia in the
intermediate and advanced stages of tumor development (43,79,80),
which indicates that autophagy inhibition may be an effective
anticancer therapy for intermediate and advanced cancers. On the
other hand, cellular autophagy also plays a dual role in tumor
migration, infiltration, and differentiation of tumor stem cells,
being involved in both inhibition of tumor growth to promote cancer
cell death (cytotoxic/non-protective autophagy) and possibly
providing nutrients to tumor cells to promote cancer cell survival
(protective autophagy) (81–83).
Moreover, ATG5 has different dual roles for various types of cancer
cells, with high ATG5 expression associated with poor prognosis in
CESC (59), early esophageal
squamous cell carcinoma (84), and
neuroblastoma (85). By contrast,
high ATG5 expression predicts a favorable prognosis for patients
with breast cancer (86) and
osteosarcoma (87). This property
also provides two concepts for tumor treatment: Inhibition of ATG5
to improve anticancer therapy or activation of ATG5 to induce
autophagic death of tumor cells.
Role of ATG5 in tumor treatment
Studies have revealed that inhibition of
non-protective autophagy in tumor cells has little effect on the
sensitivity of cancer cells to chemotherapy and radiotherapy
(88), but inhibition of protective
autophagy can increase chemotherapeutic drug-induced apoptosis in
cancer cells (89), and inhibition
of protective autophagy in tumor cells can suppress STAT3 signaling
pathway-mediated DNA damage repair (90), thus increasing the sensitivity of
tumor cells to radiotherapy. Therefore, protective autophagy
inhibitors combined with conventional treatment of tumors provide
novel therapeutic strategies for cancer treatment (91–95).
ATG5 plays a pivotal role in regulating cancer resistance to
radiation and drug resistance through the activation of autophagy,
and blocking or activating autophagy through ATG5 may be used to
develop a promising tumor treatment strategy.
Therapeutic strategies for
downregulation of ATG5
Chen et al (96) revealed that the cisplatin-induced
apoptosis of A549 lung cancer cells could be promoted by inhibiting
the expression of ATG5, suggesting that ATG5 can be used as a drug
target for tumor treatment, thus providing theoretical support for
the precise treatment of tumors. SMARCB1, an oncogene, is a core
component of the SWI/SNF complex that binds directly to the
promoter region of ATG5 in the nucleus to epigenetically repress
ATG5 transcription, thereby downregulating autophagy and inhibiting
cell carcinogenesis (29). Mo et
al (97) revealed that
inhibition of the expression of the autophagy-related gene ATG5,
decreased Rad51 mRNA expression and increased DNA damage levels and
induced apoptosis in tumor cells, enhancing the radiosensitivity of
nasopharyngeal carcinoma cells. It was reported that the
radiosensitivity of head and neck squamous cell carcinoma (HNSCC)
could be activated by inhibiting ATG5 expression, and ATG5
inhibitor combined with solute carrier family 3 member 2 targeting
(SLC3A2) may be an effective strategy for radiosensitization of
HNSCC (98). Bellare et al
(99) revealed that autophagy in
breast cancer cells could cause the development of PARP inhibitor
(PARPi) talazoparib resistance, and that drug-induced DNA damage
repair could be converted to NHEJ by inhibiting ATG5 expression,
ultimately leading to genomic instability and cell death. Han et
al (100) reported that MCF10A
cells secreting exosomes delivering miR-567 could be taken up by
trastuzumab-resistant cells, thus reversing trastuzumab resistance
in breast cancer cells by targeting the downregulation of ATG5.
Decreased miR-137 expression in adriamycin-activated pancreatic
cancer cells, rendered pancreatic cancer cells resistant to
chemotherapy. In this study, miR-137 could also enhance cellular
chemosensitivity by directly inhibiting ATG5 and downregulating
autophagy levels (101). In
ADR-resistant liver carcinoma (HepG2/ADR) cells, the downregulation
of miR-155-5p expression and the upregulation of ATG5 expression
rendered HCC cells resistant to the drug (102). In NSCLC, miRNA-153-3p suppressed
autophagy by directly binding to the ATG5 gene to downregulate ATG5
expression. However, in gefitinib-resistant NSCLC, miRNA-153-3p
expression was reduced, leading to the upregulation of autophagy
levels, thus rendering the cells resistant to the drug (103). Polypyrimidine tract-binding
protein 1 (PTBP1) is a common RNA-binding protein whose main
function is to selectively splice exons or introns to produce
different mRNA isoforms by binding to specific sequences of target
gene precursor mRNAs as a splicing factor. Compared to sensitive
cells, IncRNA ZNF649-AS1 was revealed to be more highly expressed
in trastuzumab-resistant breast cancer cells, where it increased
ATG5 transcription by binding to PTBP1 in the cytoplasm, thereby
increasing the level of ATG5 expression, which in turn upregulated
the level of autophagy and rendered the cells resistant to the drug
(104). Curcumin was demonstrated
to upregulate miR-181a expression in triple-negative breast cancer
cells (TNBC). miR-181a downregulated ATG5 levels by binding
directly to ATG5, thus downregulating autophagy levels in TNBC
cells, maintaining cell stemness, and inhibiting tumor cell growth
(31). Lomeguatrib inhibited the
proliferation, invasion, migration, and autophagy of PanC-1/GEM
cells by inhibiting MGMT, downregulating the expression of the
apoptosis inhibitory proteins Bcl-2, Beclin1, and autophagy-related
protein ATG5. Upregulation of the expression of pro-apoptotic
proteins caspase-3 and Bax could promote apoptosis in PanC-1/GEM
cells (105). Icariin could
significantly downregulate the expression of autophagy-related
proteins such as ATG5, inhibit cellular autophagy, and induce G0/G1
phase cell cycle arrest and apoptosis, thereby inhibiting MCF-7/TAM
cell proliferation (106).
Therefore, combining ATG5 inhibitors with traditional antitumor
treatment modalities can provide novel insights into tumor
treatment.
Therapeutic strategies for
upregulation of ATG5
Quinoline derivatives are a new class of antitumor
drugs with the potential for development. A series of
4,7-disubstituted quinoline derivatives were designed, synthesized,
and evaluated for their anti-proliferative activity. The results
revealed that compounds 10c, 10g, 10i, 10j, and 10k had strong
anti-proliferative activity against human tumor cells, with
compound 10k being the most active, and could inhibit colorectal
cancer cell growth by targeting and stabilizing ATG5 to induce
autophagy (107).
It has also been recently revealed that ATG5 is
required for the presentation of tumor antigens by dendritic cells
to initiate an anti-tumor CD4+ T cell immune response,
acting as an antitumor agent. Therefore, upregulation of ATG5
expression in certain tumors can exploit the immune response of the
tumor tissue to act as an antitumor agent (54). Procyanidin B2 (PB2) is a natural
flavonoid compound with antitumor effects that can inhibit gastric
cancer cell proliferation by inhibiting the PI3K/Akt/mTOR pathway,
promoting the protein expression of ATG5 and Beclin-1, and
upregulating autophagy levels (108). Muyin extract (MSE), a 1:1 mixture
of Muyin seed and Epimedium extract, has been revealed to
regulate apoptosis-related protein expression by blocking the
Akt/mTOR pathway, regulate apoptosis-related proteins to promote
apoptosis, upregulate the expression levels of autophagy-related
genes ATG5 and Beclin-1, induce autophagy, and exhibits favorable
antitumor activity in NSCLC (109). Cinnamaldehyde (CA) is the active
component in cinnamon with inhibitory effects on tumor growth,
migration, and invasion, which can induce the expressions of
Beclin-1, ATG5, and LC3B and inhibit the expression of p62 through
the PERK/ATF4/CHOP pathway to lead to autophagic cell death
(110).
ATG5 is a double-edged sword in cancer progression,
as autophagy induced by ATG5 provides essential nutrients to cancer
cells, maintains their metabolism, and allow cells to survive after
increased stress. However, after excessive induction of autophagy
degradation, cancer cell death may also be induced. During tumor
progression, ATG5 expression is also regulated by other pathways,
not by the autophagy pathway. Therefore, the tumor suppressive
function of ATG5 in cancer may exist independently of autophagy. In
addition, ATG5 expression levels may vary in different types of
tumor cells and at different stages of tumor development. These
observations suggest that inhibiting or activating ATG5 may be a
favorable strategy to inhibit cancer progression. On the one hand,
in some cancers, such as colorectal (107), gastric (108,110) and non-small cell lung cancer
(109), autophagy can be induced
by ATG5 activator to promote the apoptosis of tumor cells, thus
aiding tumor treatment. On the other hand, in some cancers, such as
nasopharyngeal (97), breast
(100) and pancreatic cancer
(101), autophagy can be inhibited
by ATG5 inhibitors to achieve successful tumor treatment (Fig. 4).
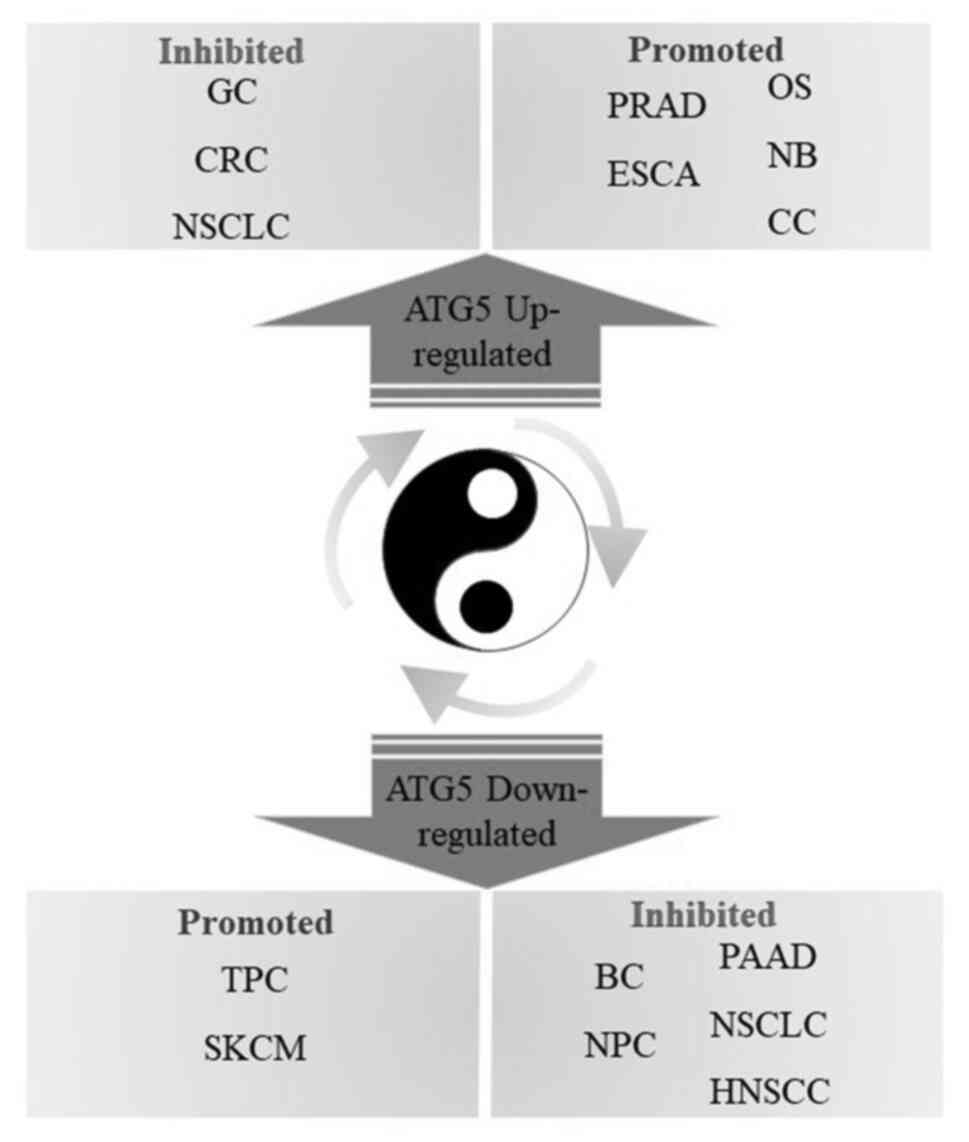 | Figure 4.Double-edged sword role of ATG5 in
tumor treatment. ATG5, autophagy-related gene 5; GC, gastric
cancer; NSCLC, non-small cell lung cancer; BC, breast cancer; CRC,
colorectal cancer; NB, neuroblastoma; OS, osteosarcoma; PRAD,
prostate cancer; CC, cervical cancer; ESCA, esophageal squamous
cell carcinoma; PAAD, pancreatic adenocarcinoma; NPC,
nasopharyngeal carcinoma; HNSCC, head and neck squamous cell
carcinoma; SKCM, skin cutaneous melanoma; TPC, thyroid papillary
carcinoma. |
Conclusion
ATG5 is an important member of numerous
autophagy-related genes. In addition to participating in the
classical autophagy pathway, ATG5 also plays an important role in
the occurrence and development of tumors, such as apoptosis
(51), cell cycle regulation
(53), maintaining genomic
stability (55) and immune
inflammatory signaling pathways (54).
In conclusion, the study of the molecular mechanism
of the effect of ATG5 on tumors can help develop targeted
therapeutic strategies and provide novel insights into tumor
treatment (4). In the future,
activators or inhibitors of ATG5 could be used as drug candidates
for cancer treatment. ATG5 may also be used as a tumor marker for
diagnosis (59), and has a
reference value for predicting patient prognosis. Although the
interplay between its involvement in the autophagy activation
mechanism and apoptosis, as well as the association between
autophagy and clinical drug resistance still requires further
investigation, it is considered that more drugs selectively
targeting ATG5 will be used in the future, which may aid in
overcoming cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YZ conceived and designed the review. PZ and ZZ
wrote the review. PZ, ZZ, ML and PL completed all the figures and
the table. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xue C, Chu Q, Zheng Q, Jiang S, Bao Z, Su
Y, Lu J and Li L: Role of main RNA modifications in cancer:
N6-methyladenosine, 5-methylcytosine, and pseudouridine.
Signal Transduct Target Ther. 7:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kocaturk NM, Akkoc Y, Kig C, Bayraktar O,
Gozuacik D and Kutlu O: Autophagy as a molecular target for cancer
treatment. Eur J Pharm Sci. 134:116–137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onorati AV, Dyczynski M, Ojha R and
Amaravadi RK: Targeting autophagy in cancer. Cancer. 124:3307–3318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferro F, Servais S, Besson P, Roger S,
Dumas JF and Brisson L: Autophagy and mitophagy in cancer metabolic
remodelling. Semin Cell Dev Biol. 98:129–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amaravadi RK, Kimmelman AC and Debnath J:
Targeting autophagy in cancer: Recent advances and future
directions. Cancer Discov. 9:1167–1181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin PW, Chu ML and Liu HS: Autophagy and
metabolism. Kaohsiung J Med Sci. 37:12–19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gómez-Virgilio L, Silva-Lucero MD,
Flores-Morelos DS, Gallardo-Nieto J, Lopez-Toledo G,
Abarca-Fernandez AM, Zacapala-Gómez AE, Luna-Muñoz J, Montiel-Sosa
F, Soto-Rojas LO, et al: Autophagy: A key regulator of homeostasis
and disease: An overview of molecular mechanisms and modulators.
Cells. 11:22622022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen X, Yang Y and Klionsky DJ: Moments in
autophagy and disease: Past and present. Mol Aspects Med.
82:1009662021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao W, Li J, Yang K and Cao D: An overview
of autophagy: Mechanism, regulation and research progress. Bull
Cancer. 108:304–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klionsky DJ, Petroni G, Amaravadi RK,
Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K,
Cecconi F, Choi AMK, et al: Autophagy in major human diseases. EMBO
J. 40:e1088632021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Behera J, Ison J, Tyagi A, Mbalaviele G
and Tyagi N: Mechanisms of autophagy and mitophagy in skeletal
development, diseases and therapeutics. Life Sci. 301:1205952022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mameli E, Martello A and Caporali A:
Autophagy at the interface of endothelial cell homeostasis and
vascular disease. FEBS J. 289:2976–2991. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu QY, Ye LQ and Li HL: Molecular
interaction of stress granules with Tau and autophagy in
Alzheimer's disease. Neurochem Int. 157:1053422022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carinci M, Palumbo L, Pellielo G, Agyapong
ED, Morciano G, Patergnani S, Giorgi C, Pinton P and Rimessi A: The
multifaceted roles of autophagy in infectious, obstructive, and
malignant airway diseases. Biomedicines. 10:19442022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernandez GA and Perera RM: Autophagy in
cancer cell remodeling and quality control. Mol Cell. 82:1514–1527.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levine B and Kroemer G: Biological
functions of autophagy genes: A disease perspective. Cell.
176:11–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Q, Liu H, Zhen H and Zhao B:
Autophagy and its role in regeneration and remodeling within
invertebrate. Cell Biosci. 10:1112020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pradel B, Robert-Hebmann V and Espert L:
Regulation of Innate Immune Responses by Autophagy: A Goldmine for
Viruses. Front Immunol. 11:5780382020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao Y, Luo Y, Zou J, Ouyang J, Cai Z, Zeng
X, Ling H and Zeng T: Autophagy and its role in gastric cancer.
Clin Chim Acta. 489:10–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shu F, Xiao H, Li QN, Ren XS, Liu ZG, Hu
BW, Wang HS, Wang H and Jiang GM: Epigenetic and post-translational
modifications in autophagy: Biological functions and therapeutic
targets. Signal Transduct Target Ther. 8:322023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng W, Xie W, Yin D, Luo R, Liu M and
Guo F: ATG5 and ATG7 induced autophagy interplays with UPR via PERK
signaling. Cell Commun Signal. 17:422019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He M, Li M, Guan Y, Wan Z, Tian J, Xu F,
Zhou H, Gao M, Bi H and Chong T: A New prognostic risk score: Based
on the analysis of autophagy-related genes and renal cell
carcinoma. Front Genet. 12:8201542021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yousefi S, Perozzo R, Schmid I, Ziemiecki
A, Schaffner T, Scapozza L, Brunner T and Simon HU:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsushita M, Suzuki NN, Obara K, Fujioka
Y, Ohsumi Y and Inagaki F: Structure of Atg5.Atg16, a complex
essential for autophagy. J Biol Chem. 282:6763–6772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noda NN, Fujioka Y, Hanada T, Ohsumi Y and
Inagaki F: Structure of the Atg12-Atg5 conjugate reveals a platform
for stimulating Atg8-PE conjugation. EMBO Rep. 14:206–211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikseresht M, Shahverdi M, Dehghani M,
Abidi H, Mahmoudi R, Ghalamfarsa G, Manzouri L and Ghavami S:
Association of single nucleotide autophagy-related protein 5 gene
polymorphism rs2245214 with susceptibility to non-small cell lung
cancer. J Cell Biochem. 120:1924–1931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Shen Y, Xiong Y, Wang S, Li C, Bai J
and Zhang Y: Loss of SMARCB1 promotes autophagy and facilitates
tumour progression in chordoma by transcriptionally activating
ATG5. Cell Prolif. 54:e131362021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frangež Ž, Gérard D, He Z, Gavriil M,
Fernández-Marrero Y, Seyed Jafari SM, Hunger RE, Lucarelli P,
Yousefi S, Sauter T, et al: ATG5 and ATG7 expression levels are
reduced in cutaneous melanoma and regulated by NRF1. Front Oncol.
11:7216242021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JW, Kim Y, Lee SB, Oh CW, Lee EJ, Ko
JY and Park JH: Autophagy inhibits cancer stemness in
triple-negative breast cancer via miR-181a-mediated regulation of
ATG5 and/or ATG2B. Mol Oncol. 16:1857–1875. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang N, Li Z, Bai F and Zhang S:
PAX5-induced upregulation of IDH1-AS1 promotes tumor growth in
prostate cancer by regulating ATG5-mediated autophagy. Cell Death
Dis. 10:7342019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng X, Zhang H, Meng L, Song H, Zhou Q,
Qu C, Zhao P, Li Q, Zou C, Liu X and Zhang Z: Hypoxia-induced
acetylation of PAK1 enhances autophagy and promotes brain
tumorigenesis via phosphorylating ATG5. Autophagy. 17:723–742.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie X, Bi HL, Lai S, Zhang YL, Li N, Cao
HJ, Han L, Wang HX and Li HH: The immunoproteasome catalytic β5i
subunit regulates cardiac hypertrophy by targeting the autophagy
protein ATG5 for degradation. Sci Adv. 5:eaau04952019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Q, Zhao X, Tang H, Li X, Xiao Y, Wu H,
Wu Z, Quan J and Chen W: USP22 suppresses the NLRP3 inflammasome by
degrading NLRP3 via ATG5-dependent autophagy. Autophagy.
19:873–885. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Liu S and Tao Y: Regulating tumor
suppressor genes: Post-translational modifications. Signal
Transduct Target Ther. 5:902020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou L, Ng DS, Yam JC, Tham CC, Pang CP
and Chu WK: Post-translational modifications on the retinoblastoma
protein. J Biomed Sci. 29:332022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Song C and Zhan X: The role of
protein acetylation in carcinogenesis and targeted drug discovery.
Front Endocrinol (Lausanne). 13:9723122022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan W, You Z, Xu Y, Zhou L, Guan Z, Peng
C, Wong CCL, Su H, Zhou T, Xia H and Liu W: mTORC1 Phosphorylates
Acetyltransferase p300 to Regulate Autophagy and Lipogenesis. Mol
Cell. 68:323–335.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin TY, Chan HH, Chen SH, Sarvagalla S,
Chen PS, Coumar MS, Cheng SM, Chang YC, Lin CH, Leung E and Cheung
CHA: BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and
an autophagy-induced DNA damage suppressor in human cancer and
mouse embryonic fibroblast cells. Autophagy. 16:1296–1313. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Otto FB and Thumm M: Mechanistic
dissection of macro- and micronucleophagy. Autophagy. 17:626–639.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cicchini M, Karantza V and Xia B:
Molecular pathways: Autophagy in cancer-a matter of timing and
context. Clin Cancer Res. 21:498–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Don Wai Luu L, Kaakoush NO and
Castaño-Rodríguez N: The role of ATG16L2 in autophagy and disease.
Autophagy. 18:2537–2546. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Changotra H, Kaur S, Yadav SS, Gupta GL,
Parkash J and Duseja A: ATG5: A central autophagy regulator
implicated in various human diseases. Cell Biochem Funct.
40:650–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lei Y, Xu X, Liu H, Chen L, Zhou H, Jiang
J, Yang Y and Wu B: HBx induces hepatocellular carcinogenesis
through ARRB1-mediated autophagy to drive the G1/S
cycle. Autophagy. 17:4423–4441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Xiong H, Liu D, Hill C, Ertay A,
Li J, Zou Y, Miller P, White E, Downward J, et al: Autophagy
inhibition specifically promotes epithelial-mesenchymal transition
and invasion in RAS-mutated cancer cells. Autophagy. 15:886–899.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang Y, Pi H, Liao L, Tan M, Deng P, Yue
Y, Xi Y, Tian L, Xie J, Chen M, et al: Cadmium promotes breast
cancer cell proliferation, migration and invasion by inhibiting
ACSS2/ATG5-mediated autophagy. Environ Pollut. 273:1165042021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He L, Han J, Li B, Huang L, Ma K, Chen Q,
Liu X, Bao L and Liu H: Identification of a new cyathane diterpene
that induces mitochondrial and autophagy-dependent apoptosis and
shows a potent in vivo anti-colorectal cancer activity. Eur J Med
Chem. 111:183–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiong X, Lu B, Tian Q, Zhang H, Wu M, Guo
H, Zhang Q, Li X, Zhou T and Wang Y: Inhibition of autophagy
enhances cinobufagin-induced apoptosis in gastric cancer. Oncol
Rep. 41:492–500. 2019.PubMed/NCBI
|
|
51
|
Cao L and Lin F: TECPR1 Induces apoptosis
in non-small cell lung carcinoma via ATG5 Upregulation-Induced
autophagy promotion. Ann Clin Lab Sci. 52:580–592. 2022.PubMed/NCBI
|
|
52
|
Zheng Y, Tan K and Huang H: Long noncoding
RNA HAGLROS regulates apoptosis and autophagy in colorectal cancer
cells via sponging miR-100 to target ATG5 expression. J Cell
Biochem. 120:3922–3933. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Jiang Y, Cheng J, Ma J, Li Q and
Pang T: ATG5 regulates mesenchymal stem cells differentiation and
mediates chemosensitivity in acute myeloid leukemia. Biochem
Biophys Res Commun. 525:398–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oh DS and Lee HK: Autophagy protein ATG5
regulates CD36 expression and anti-tumor MHC class II antigen
presentation in dendritic cells. Autophagy. 15:2091–2106. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Demirbag-Sarikaya S, Akkoc Y, Turgut S,
Erbil-Bilir S, Kocaturk NM, Dengjel J and Gozuacik D: A novel ATG5
interaction with Ku70 potentiates DNA repair upon genotoxic stress.
Sci Rep. 12:81342022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun SY, Hu XT, Yu XF, Zhang YY, Liu XH,
Liu YH, Wu SH, Li YY, Cui SX and Qu XJ: Nuclear translocation of
ATG5 induces DNA mismatch repair deficiency (MMR-D)/microsatellite
instability (MSI) via interacting with Mis18α in colorectal cancer.
Br J Pharmacol. 178:2351–2369. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu K, Xiang L, Li S, Wang S, Chen C and Mu
H: HIF1α promotes prostate cancer progression by increasing ATG5
expression. Anim Cells Syst (Seoul). 23:326–334. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang H, Yin J, Huang J, Liu Z and Pei S:
miR-20a-enhanced cell migration and invasion via ATg5 in
osteosarcoma. Minerva Endocrinol. 44:415–417. 2019.PubMed/NCBI
|
|
59
|
Zhou S, Wang X, Ding J, Yang H and Xie Y:
Increased ATG5 expression predicts poor prognosis and promotes EMT
in cervical carcinoma. Front Cell Dev Biol. 9:7571842021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Q, Chen J, Zhang M, Wang H, Zeng Y,
Huang Y and Xu C: Autophagy induced by muscarinic acetylcholine
receptor 1 mediates migration and invasion targeting Atg5 via
AMPK/mTOR pathway in prostate cancer. J Oncol.
2022:65231952022.PubMed/NCBI
|
|
61
|
Qin Y, Sun W, Wang Z, Dong W, He L, Zhang
T, Lv C and Zhang H: RBM47/SNHG5/FOXO3 axis activates autophagy and
inhibits cell proliferation in papillary thyroid carcinoma. Cell
Death Dis. 13:2702022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu JF, Huang W, Yi HM, Xiao T, Li JY,
Feng J, Yi H, Lu SS, Li XH, Lu RH, et al: Annexin A1-suppressed
autophagy promotes nasopharyngeal carcinoma cell invasion and
metastasis by PI3K/AKT signaling activation. Cell Death Dis.
9:11542018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He J, Huang B, Zhang K, Liu M and Xu T:
Long non-coding RNA in cervical cancer: From biology to therapeutic
opportunity. Biomed Pharmacother. 127:1102092020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yan H and Bu P: Non-coding RNA in cancer.
Essays Biochem. 65:625–639. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang L, Cho KB, Li Y, Tao G, Xie Z and Guo
B: Long Noncoding RNA (lncRNA)-mediated competing endogenous RNA
networks provide novel potential biomarkers and therapeutic targets
for colorectal cancer. Int J Mol Sci. 20:57582019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang Q, Li F, He AT and Yang BB: Circular
RNAs: Expression, localization, and therapeutic potentials. Mol
Ther. 29:1683–1702. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Volovat SR, Volovat C, Hordila I, Hordila
DA, Mirestean CC, Miron OT, Lungulescu C, Scripcariu DV,
Stolniceanu CR, Konsoulova-Kirova AA, et al: MiRNA and LncRNA as
potential biomarkers in Triple-negative breast cancer: A review.
Front Oncol. 10:5268502020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang H and Lu B: The roles of
ceRNAs-mediated autophagy in cancer chemoresistance and metastasis.
Cancers (Basel). 12:29262020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huang J, Huang B, Kong Y, Yang Y, Tian C,
Chen L, Liao Y and Ma L: Polycystic ovary syndrome: Identification
of novel and hub biomarkers in the autophagy-associated
mRNA-miRNA-lncRNA network. Front Endocrinol (Lausanne).
13:10320642022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fisher L: Retraction: Long non-coding RNA
XIST promotes proliferation, autophagy and inhibits apoptosis by
regulating microRNA-30c/ATG5 axis in gastric cancer. RSC Adv.
11:42332021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang F, Peng ZX, Ji WD, Yu JD, Qian C, Liu
JD and Fang GE: LncRNA CCAT1 upregulates ATG5 to enhance autophagy
and promote gastric cancer development by absorbing miR-140-3p. Dig
Dis Sci. 67:3725–3741. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang J, Dong Z, Sheng Z and Cai Y:
Hypoxia-induced PVT1 promotes lung cancer chemoresistance to
cisplatin by autophagy via PVT1/miR-140-3p/ATG5 axis. Cell Death
Discov. 8:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qin Y, Sun W, Wang Z, Dong W, He L, Zhang
T, Shao L and Zhang H: ATF2-Induced lncRNA GAS8-AS1 promotes
autophagy of thyroid cancer cells by targeting the miR-187-3p/ATG5
and miR-1343-3p/ATG7 Axes. Mol Ther Nucleic Acids. 22:584–600.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wei H, Li L, Zhang H, Xu F, Chen L, Che G
and Wang Y: Circ-FOXM1 knockdown suppresses non-small cell lung
cancer development by regulating the miR-149-5p/ATG5 axis. Cell
Cycle. 20:166–178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zheng S, Zhong YF, Tan DM, Xu Y, Chen HX
and Wang D: miR-183-5p enhances the radioresistance of colorectal
cancer by directly targeting ATG5. J Biosci. 44:922019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Che J, Wang W, Huang Y, Zhang L, Zhao J,
Zhang P and Yuan X: miR-20a inhibits hypoxia-induced autophagy by
targeting ATG5/FIP200 in colorectal cancer. Mol Carcinog.
58:1234–1247. 2019.PubMed/NCBI
|
|
77
|
Hwang TI, Chen PC, Tsai TF, Lin JF, Chou
KY, Ho CY, Chen HE and Chang AC: Hsa-miR-30a-3p overcomes the
acquired protective autophagy of bladder cancer in chemotherapy and
suppresses tumor growth and muscle invasion. Cell Death Dis.
13:3902022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liang L, Yang Z, Deng Q, Jiang Y, Cheng Y,
Sun Y and Li LL: miR-30d-5p suppresses proliferation and autophagy
by targeting ATG5 in renal cell carcinoma. FEBS Open Bio.
11:529–540. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Das S, Shukla N, Singh SS, Kushwaha S and
Shrivastava R: Mechanism of interaction between autophagy and
apoptosis in cancer. Apoptosis. 26:512–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Russo M and Russo GL: Autophagy inducers
in cancer. Biochem Pharmacol. 153:51–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Seo W, Silwal P, Song IC and Jo EK: The
dual role of autophagy in acute myeloid leukemia. J Hematol Oncol.
15:512022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; The three main axes of metastasis.
Biomed Pharmacother. 133:1109092021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang PW, Hsieh MS, Chang YH, Huang PM and
Lee JM: Genetic polymorphisms of ATG5 predict survival and
recurrence in patients with early-stage esophageal squamous cell
carcinoma. Oncotarget. 8:91494–91504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheng X, Xu Q, Zhang Y, Shen M, Zhang S,
Mao F, Li B, Yan X, Shi Z, Wang L, et al: miR-34a inhibits
progression of neuroblastoma by targeting autophagy-related gene 5.
Eur J Pharmacol. 850:53–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang L, Yao L, Zheng YZ, Xu Q, Liu XP, Hu
X, Wang P and Shao ZM: Expression of autophagy-related proteins
ATG5 and FIP200 predicts favorable disease-free survival in
patients with breast cancer. Biochem Biophys Res Commun.
458:816–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM and Guo QN: TSSC3
promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR
pathway to suppress tumorigenesis and metastasis in osteosarcoma,
and predicts a favorable prognosis. J Exp Clin Cancer Res.
37:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dong M, Ye T, Bi Y, Wang Q, Kuerban K, Li
J, Feng M, Wang K, Chen Y and Ye L: A novel hybrid of 3-benzyl
coumarin seco-B-ring derivative and phenylsulfonylfuroxan induces
apoptosis and autophagy in non-small-cell lung cancer.
Phytomedicine. 52:79–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu Q, Zhang H, Liu H, Han Y, Qiu W and Li
Z: Inhibiting autophagy flux and DNA repair of tumor cells to boost
radiotherapy of orthotopic glioblastoma. Biomaterials.
280:1212872022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang X, Zhao M, Wu Z, Chen C, Zhang Y,
Wang L, Guo Q, Wang Q, Liang S, Hu S, et al: Nano-ultrasonic
contrast agent for chemoimmunotherapy of breast cancer by immune
metabolism reprogramming and tumor autophagy. ACS Nano.
16:3417–3431. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mi W, Wang C, Luo G, Li J, Zhang Y, Jiang
M, Zhang C, Liu N, Jiang X, Yang G, et al: Targeting ERK induced
cell death and p53/ROS-dependent protective autophagy in colorectal
cancer. Cell Death Discov. 7:3752021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kinsey CG, Camolotto SA, Boespflug AM,
Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap
JT, et al: Protective autophagy elicited by RAF→MEK→ERK inhibition
suggests a treatment strategy for RAS-driven cancers. Nat Med.
25:620–627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu J, Liu Y, Li H, Wei C, Mao A, Liu W
and Pan G: Polyphyllin D induces apoptosis and protective autophagy
in breast cancer cells through JNK1-Bcl-2 pathway. J
Ethnopharmacol. 282:1145912022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ho CY, Chang AC, Hsu CH, Tsai TF, Lin YC,
Chou KY, Chen HE, Lin JF, Chen PC and Hwang TI: Miconazole induces
protective autophagy in bladder cancer cells. Environ Toxicol.
36:185–193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen J, Zhang L, Zhou H, Wang W, Luo Y,
Yang H and Yi H: Inhibition of autophagy promotes cisplatin-induced
apoptotic cell death through Atg5 and Beclin 1 in A549 human lung
cancer cells. Mol Med Rep. 17:6859–6865. 2018.PubMed/NCBI
|
|
97
|
Mo N, Lu YK, Xie WM, Liu Y, Zhou WX, Wang
HX, Nong L, Jia YX, Tan AH, Chen Y, et al: Inhibition of autophagy
enhances the radiosensitivity of nasopharyngeal carcinoma by
reducing Rad51 expression. Oncol Rep. 32:1905–1912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Digomann D, Linge A and Dubrovska A:
SLC3A2/CD98hc, autophagy and tumor radioresistance: A link
confirmed. Autophagy. 15:1850–1851. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pai Bellare G, Saha B and Patro BS:
Targeting autophagy reverses de novo resistance in homologous
recombination repair proficient breast cancers to PARP inhibition.
Br J Cancer. 124:1260–1274. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Han M, Hu J, Lu P, Cao H, Yu C, Li X, Qian
X, Yang X, Yang Y, Han N, et al: Exosome-transmitted miR-567
reverses trastuzumab resistance by inhibiting ATG5 in breast
cancer. Cell Death Dis. 11:432020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang ZC, Huang FZ, Xu HB, Sun JC and Wang
CF: MicroRNA-137 inhibits autophagy and chemosensitizes pancreatic
cancer cells by targeting ATG5. Int J Biochem Cell Biol. 111:63–71.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu Q, Xu XP, Yin XM and Peng XQ:
miR-155-5p increases the sensitivity of liver cancer cells to
adriamycin by regulating ATG5-mediated autophagy. Neoplasma.
68:87–95. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang W, Dong YZ, Du X, Peng XN and Shen
QM: MiRNA-153-3p promotes gefitinib-sensitivity in non-small cell
lung cancer by inhibiting ATG5 expression and autophagy. Eur Rev
Med Pharmacol Sci. 23:2444–2452. 2019.PubMed/NCBI
|
|
104
|
Han M, Qian X, Cao H, Wang F, Li X, Han N,
Yang X, Yang Y, Dou D, Hu J, et al: lncRNA ZNF649-AS1 induces
trastuzumab resistance by promoting ATG5 expression and autophagy.
Mol Ther. 28:2488–2502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shi Y, Wang Y, Qian J, Yan X, Han Y, Yao N
and Ma J: MGMT expression affects the gemcitabine resistance of
pancreatic cancer cells. Life Sci. 259:1181482020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cheng X, Tan S, Duan F, Yuan Q, Li Q and
Deng G: Icariin induces apoptosis by suppressing autophagy in
tamoxifen-resistant breast cancer cell line MCF-7/TAM. Breast
Cancer. 26:766–775. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li X, Chen Q, Ao J, Lin W, Qiu L and Cao
R: Synthesis of novel 4,7-disubstituted quinoline derivatives as
autophagy inducing agents via targeting stabilization of ATG5.
Bioorg Chem. 127:1059982022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li Y, Lu X, Tian P, Wang K and Shi J:
Procyanidin B2 induces apoptosis and autophagy in gastric cancer
cells by inhibiting Akt/mTOR signaling pathway. BMC Complement Med
Ther. 21:762021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kan Y, Song M, Cui X, Yang Q, Zang Y, Li
Q, Li Y, Cai W, Chen Y, Weng X, et al: Muyin extract inhibits
non-small-cell lung cancer growth by inducing autophagy and
apoptosis in vitro and in vivo. Phytomedicine. 96:1538342022.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim TW: Cinnamaldehyde induces
autophagy-mediated cell death through ER stress and epigenetic
modification in gastric cancer cells. Acta Pharmacol Sin.
43:712–723. 2022. View Article : Google Scholar : PubMed/NCBI
|