Proteins, the material basis of life, are essential
components of all cells, tissues and organs in the body.
Intracellular proteins are predominantly degraded through the
lysosomal pathway, cysteine-containing aspartate protease pathway
and ubiquitin-proteasome pathway (1–4). The
ubiquitin-proteasome system is the primary protein degradation
pathway in vivo. More than 80% of proteins in the body are
degraded through this pathway, which is involved in various
metabolic processes in the body (4). The ubiquitin-proteasome pathway can
degrade the cell cycle protein cyclin (5,6),
spindle-related proteins (7), cell
surface receptors such as epidermal growth factor (8), transcription factors (9), the tumor inhibitory factor p53 and
oncogenic products (10). In
addition, abnormal intracellular proteins are degraded by the
ubiquitin-proteasome pathway under stress conditions. Ubiquitin,
ubiquitin-activating (E1) enzymes, ubiquitin-binding (E2) enzymes,
ubiquitin ligases (E3), protein hydrolases and deubiquitinating
enzymes are the main components of the ubiquitin-proteasome pathway
(9,11). In the presence of ATP, the glycine
(Gly) residue at the C-terminus of ubiquitin forms a high-energy
lipid bond with the sulfur group (SH) of the cysteine residue of an
E1 enzyme, and the activated ubiquitin is subsequently transferred
to an E2 enzyme. In the presence of an E3 ubiquitin ligase,
ubiquitin is transferred from the E2 enzyme to the substrate
protein, forming an isopeptide bond with the ε-NH2 group of the Lys
residue of the substrate protein. Subsequently, the C-terminus of
the next ubiquitin molecule is connected to the Lys48 residue of
the previous ubiquitin molecule, thus completing
polyubiquitination. The ubiquitinated substrate proteins are
recognized by cap-shaped regulatory particles of the 19S proteasome
and transported into the cylindrical core of 20S, where they are
hydrolyzed into oligopeptides and amino acids by various enzymes
and are eventually released from the proteasome, thereby completing
ubiquitin-mediated degradation (12–14).
Ubiquitin molecules involved in ubiquitination are dissociated from
substrate proteins and can be reused in the cytoplasm (Fig. 1). E3 ubiquitin ligases, which serve
a key role in ubiquitin-mediated degradation of proteins,
specifically mark the substrate proteins and attach ubiquitin to
them for degradation (9,15). Deubiquitination is an important
mechanism for maintaining intracellular protein stability and is
closely associated with the development of cancer. Deubiquitinating
enzymes (DUBs) hydrolyze the isopeptide bonds in ubiquitinated
substrate proteins, thereby dissociating the ubiquitinated
molecules from the substrate proteins and inhibiting
ubiquitin-mediated protein degradation. A flowchart demonstrating
the mechanism of the ubiquitin-proteasome pathway is shown in
Fig. 1 (16–18).
DUBs are a large family of proteasomes. It is known that ~100 DUBs
are encoded by the human genome, which can be classified as
ubiquitin C-terminal hydrolases (UCHs), ubiquitin-specific
proteases (USPs), ovarian tumor-related proteases (OTUs),
Machado-Joseph disease (MJD) deubiquitinases and metalloproteases
(19–22). Except for the metalloproteinase
family, all other deubiquitinases are cysteine proteases; of which,
USPs are the most structurally diverse class of deubiquitinases
with the largest known membership. USPs inhibit protein degradation
by removing ubiquitin from substrate proteins through interaction
with a catalytic triplet of residues (cysteine, histidine and
aspartate) (23). USPs are involved
in regulation of apoptosis (23),
protein transport (24), regulation
of the cell cycle (25), DNA damage
repair (26), chromatin remodeling
and protein signaling (27,28). In addition to inhibiting the
degradation of ubiquitinated substrate proteins, USPs can regulate
related signaling pathways by affecting protein activity. For
example, in the TGF-β signaling pathway, USP15 and CYLD lysine 63
deubiquitinase (CYLD) affect the stability of the mothers against
decapentaplegic (SMAD) protein in Drosophila by antagonizing
SMAD protein-specific E3 ligase 2, which in turn negatively
regulates the activation of the TGF-β pathway (29). USP is involved in the regulation of
multiple cancer-related pathways, including p53,
Wnt/β-catenin, TGF-β and protein kinase B (Akt) pathways. For
example, the overexpression of USP2a stabilizes murine double
minute 2 (MDM2) through direct deubiquitination, thereby enhancing
the degradation of the tumor suppressor protein p53. The
downregulation of p53 eventually leads to tumor progression
(30). Overexpression of USP10
directly deubiquitinates and stabilizes Krüppel-like factor 4
protein, which directly binds to the promoter region of tissue
inhibitor of metalloproteinase-3 (a tumor suppressor gene) to
promote its transcription and exert positive anti-tumor effects
(31).
Normal cell division, proliferation, differentiation
and ageing maintain the self-stability of the body. Cell cycle
disturbances can lead to abnormal cell proliferation, which is a
common feature of tumor cells (57). The role of USP2 in cell cycle
regulation has been well demonstrated (59,60).
CCND1 is abnormally overexpressed in various tumor cells. Shan
et al (61) screened 76 DUBs
in vitro to assess their catalytic ability to target CCND1.
They identified USP2 as a specific DUB of CCND1, which can directly
interact with CCND1, reduce the polymeric ubiquitination-dependent
degradation of CCND1 and promote tumor cell proliferation. In the
human embryonic kidney cell line 293T, USP2a has been shown to
deubiquitinate CCND1, thereby facilitating cell cycle progression
from the G1 to the S phase (38). A study demonstrated that the protein
expression of CCND1 is significantly higher in human breast cancer
MCF-7 cells and prostate cancer PC3 cells than in normal cells
(38). USP2 knockdown
attenuates CCND1 deubiquitination and stability, promotes
ubiquitin-mediated degradation, reduces CCND1 expression, inhibits
cell progression from the G1 to the S phase and
suppresses cell proliferation (38). In addition, USP2a is a
downstream target of lithocholic acid (LCA) hydroxyamide (LCAHA),
and LCAHA can destabilize CCND1 by inhibiting the expression of the
deubiquitinating enzyme USP2a (62). It induces
G0/G1 phase arrest in colon cancer cells
(HCT116), thus exerting an active anti-tumor effect (62). Leptin and adiponectin are two
hormones secreted by adipose tissue that have contradictory effects
on USP2 expression in tumor cells. Leptin targets USP2 to
upregulate the protein expression of CCND1 to promote cell cycle
progression and tumorigenesis(63).
Adiponectin targets USP2 to promote ubiquitin-mediated
degradation of CCND1 protein, resulting in cell cycle arrest and
inhibition of tumor progression (63). As the intracellular overexpression
of CCND1 is a decisive factor in the development of some tumors,
CCND1 has been used to assess the potential of USP2 inhibitors as
important indicators of the efficacy of antineoplastic drugs
(49,64). USP2 can directly recognize
and deubiquitinate CCND1, thereby preventing its degradation,
stabilizing its expression and promoting tumor development.
Similarly, USP2a can inhibit CCNA1 protein degradation through
deubiquitination, stabilize CCNA1 protein expression and promote
the progression of bladder cancer T24 cells from G1 to S
phase, which in turn promotes bladder cancer T24 cell proliferation
(65).
Aurora-A, a serine/threonine protein kinase, is a
mitotic regulator essential for the replication, maturation and
segregation of centrosomes and the subsequent spindle assembly.
Overexpression of Aurora-A inhibits Hec1 phosphorylation at serine
55 (Hec1-S55) during metaphase and destabilizes the
kinetochore-microtubule attachment, which in turn induces tumor
development (71,72). Shi et al (73) reported that USP2a reverses
ubiquitin-mediated degradation of Aurora-A and promotes mitosis in
pancreatic cancer MIA PaCa-2 cells. They used small interfering
RNAs (siRNAs) to knock down USP2a in MIA PaCa-2 cells, which
enhanced ubiquitin-mediated degradation of Aurora-A and
significantly inhibited the proliferation of tumor cells.
Therefore, USP2a may promote tumor cell proliferation by
stabilizing the Aurora-A protein, and targeting USP2a may
represent an effective strategy for inhibiting the abnormal
proliferation of tumor cells.
EMT is an important biological process in which
epithelial cells acquire the ability to migrate and invade
(74). TGF-β signaling can induce
the transcription of related genes, promote EMT and enhance the
migratory and invasive capabilities of tumor cells (75,76).
TGF-β binds to two types of transmembrane serine/threonine kinase
receptor heterologous complexes to initiate cellular responses
(77). Receptor kinases activate
the intracellular signaling protein SMAD to form heterologous
protein complexes that are transferred to the nucleus, where they
regulate the transcription of EMT-related genes, such as Snail,
Slug (zinc-finger proteins), Twist, N-cadherin and E-cadherin
(78). USP2a promotes the migratory
and invasive capabilities of non-small cell lung cancer A549 cells
by removing the K33-linked polyubiquitin chain from the TGF-β
receptor, thereby promoting the binding of receptor-regulated SMAD
(R-SMAD) to the TGF-β receptor and upregulating the expression of
Snail (79). A study demonstrated
that knockdown of USP2a or treatment with the USP2a-specific
inhibitor ML364 (10 µM) effectively inhibits the migratory and
invasive capabilities of tumor cells. In addition, ML364
significantly prolongs the survival of nude mice injected with
hepatocellular carcinoma (HCC) Hep3B cells (via the tail vein) and
attenuated lung metastasis (79).
Therefore, USP2a may serve as a potential therapeutic target
for cancer.
Drug resistance and metastasis are the major causes
of death among patients with cancer. Developing effective
strategies for reversing drug resistance and elucidating mechanisms
underlying drug resistance constitute the primary focus of modern
medical research. Clinical conventional chemotherapeutic drugs
mainly exert their cytotoxic effects by inducing apoptosis through
the mitochondrial and endoplasmic reticulum stress-mediated
autophagic pathways (93–95). The anti-apoptotic regulator cFILP
serves an important role in death receptor signaling, and its
overexpression is one of the primary mechanisms through which tumor
cells acquire drug resistance (96,97).
On the one hand, cFILP competes with the precursor caspase-8 to
bind to Fas-associated death domain-containing protein (FADD),
which inhibits apoptosis and promotes drug resistance in tumor
cells (98). On the other hand,
cFILP interacts with Akt and enhances the anti-apoptotic function
of Akt by regulating the activity of glycogen synthase kinase-3 β
(GSK3β) to promote drug resistance in tumor cells (99,100).
Previous studies have reported that USP2 stabilizes the
protein expression of cFILP and promotes the proliferation of HCC
Huf7 cells through deubiquitination. Inhibition of USP2 can
reduce cFILP expression in sorafenib-resistant Huf7-SR cells,
promote apoptosis and increase sorafenib sensitivity (98). Additionally, USP2 negatively
regulates the expression of miRNA-1915-3P in oxaliplatin-resistant
colorectal cancer (CRC) cells; inhibits apoptosis and promotes the
proliferative, migratory and invasive capabilities of tumor cells.
Knockdown of USP2 promotes apoptosis and increases the
sensitivity of CRC cells to oxaliplatin (101). In addition, knockdown of
USP2 or treatment with ML363 enhances the sensitivity of
triple-negative breast cancer cells to doxorubicin (102).
In molecularly targeted therapy, specific oncogenes
or gene fragments can be targeted and corresponding targeted drugs
can be developed to act at the cellular level. When these targeted
drugs enter the human body, they specifically target the
cancer-inducing sites, leading to the specific elimination of tumor
cells without damaging normal cells (103–105). Therefore, molecularly targeted
therapy is considered an effective therapeutic strategy for cancer
in modern medicine and is a major focus of cancer research.
USP7, a member of the DUB family, can promote tumor
development by stabilizing the E3 ubiquitin ligase MDM2, promoting
p53 degradation and reducing the expression of downstream proteins
of p53 (106,107). Elevated USP7 expression is
closely associated with the development of several cancers and
USP7 is an important target for the treatment of prostate
cancer (108), malignant melanoma
(109), ovarian cancer (110), multiple myeloma (111) and CRC (112). In addition to USP7, other
USPs such as CYLD, USP1, USP6, USP8, USP9X, USP11, USP15 and
USP28, are considered potential therapeutic targets for
various cancers (23). Studies have
demonstrated that USP2, a multifunctional cysteine protease,
is a key regulator of ubiquitin-mediated degradation of fatty acid
synthase (FAS), MDM2, MDM4, epidermal growth factor receptor
(EGFR), the cell cycle proteins A1 and D1 and other oncogenic
proteins, and is closely associated with the development of a
number of tumors (59,91,106,113). Therefore, targeting USP2 is
a promising strategy for tumor treatment. This section discusses
the current research status of USP2 in cancer therapy and
summarizes the targets and related molecular mechanisms of
USP2 (Table I and Fig. 3).
The Twist protein is a highly conserved basic
helix-loop-helix transcription factor that is repressed in normal
tissue cells but overexpressed in triple-negative breast cancer and
various metastatic tumors (115,116). It serves a key role in the
self-renewal and EMT of tumor stem cells (117,118). USP2 is associated with the
upregulation of Twist protein in clinical tumor specimens.
Inhibition of USP2 expression promotes the
ubiquitin-mediated degradation of Twist, thereby inhibiting tumor
stem cell properties in vitro and tumorigenicity in
vivo (102). The USP2
inhibitor ML364 inhibits tumor growth and enhances the sensitivity
to Adriamycin (102). In addition,
the molecular chaperone function of heat shock protein 90 (HSP90)
serves a critical role in maintaining the stability of various
intracellular proteins and is closely associated with the
development of several tumors (119,120). Clinical trials have demonstrated
the anticancer effects of multiple HSP90 inhibitors, both as
monotherapy and combination therapy with ErbB2-targeting agents
(121,122). A preliminary clinical trial of
tanespimycin (17-AAG) provides additional evidence for the use of
HSP90 inhibitors in the treatment of ErbB2-positive breast cancer
(123). In a recent study, HSP90
inhibitors were found to promote the ubiquitin-mediated degradation
of ErbB2; however, these effects were reversed by USP2.
Additionally, ML364 not only enhanced the degradation of ErbB2 by
HSP90 inhibitors but also inhibited the growth of ErbB2-positive
breast cancer cells and transplanted tumors in mice in vivo
(56). Therefore, USP2 may
serve as a prognostic biomarker and therapeutic target for breast
cancer.
Antisense RNAs refer to RNA molecules that are
complementary to mRNAs. They inhibit the translation of mRNAs and
block gene function by specifically and complementarily binding to
mRNAs (124). Given the medicinal
value of antisense RNAs, their role in cell growth and
differentiation needs to be intensively investigated (125). The expression of
ubiquitin-specific peptidase 2 antisense RNA 1 (lncRNA USP2-AS1), a
USP2-specific antisense RNA, is significantly higher in HCC tissues
than in paraneoplastic tissues. The high expression of USP2-AS1 is
significantly associated with a poorer prognosis. Knockdown of
USP2-AS1 promotes the ubiquitin-mediated degradation of Y-box
binding protein 1-mediated hypoxia-inducible factor 1α; inhibits
the proliferative, migratory and invasive abilities of HCC cells
and reduces the tumorigenicity of HCC cells in mice (126).
Oxaliplatin is widely used as the first-line
chemotherapeutic agent for the treatment of advanced CRC in
clinical settings. The expression of miRNA-1915-3P is reduced in
oxaliplatin-resistant CRC cells and overexpression of miRNA-1915-3P
downregulates the oncogenes
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and
USP2, thus inhibiting tumor proliferation, metastasis and
invasion (101). USP2 is a
negative regulator of miRNA-1915-3P. Overexpression of USP2
restores the proliferative capacity of tumor cells, whereas its
knockdown inhibits tumor cell proliferation (101). Therefore, small-molecule
inhibitors of USP2 may be used to induce oxaliplatin
sensitivity in advanced CRC.
Glioblastoma (GBM) is an astrocytic tumor
characterized by rapid growth, high malignancy and high mortality
rates (132). As a
cancer-promoting factor, TGF-β serves an important role in the
development of GBM (133,134). SMAD7, a key negative regulator of
TGF-β signaling, exerts its inhibitory effects on TGF-β by blocking
receptor activity and inducing receptor degradation (135). USP2 expression is
significantly lower in GBM tissues than in normal human brain
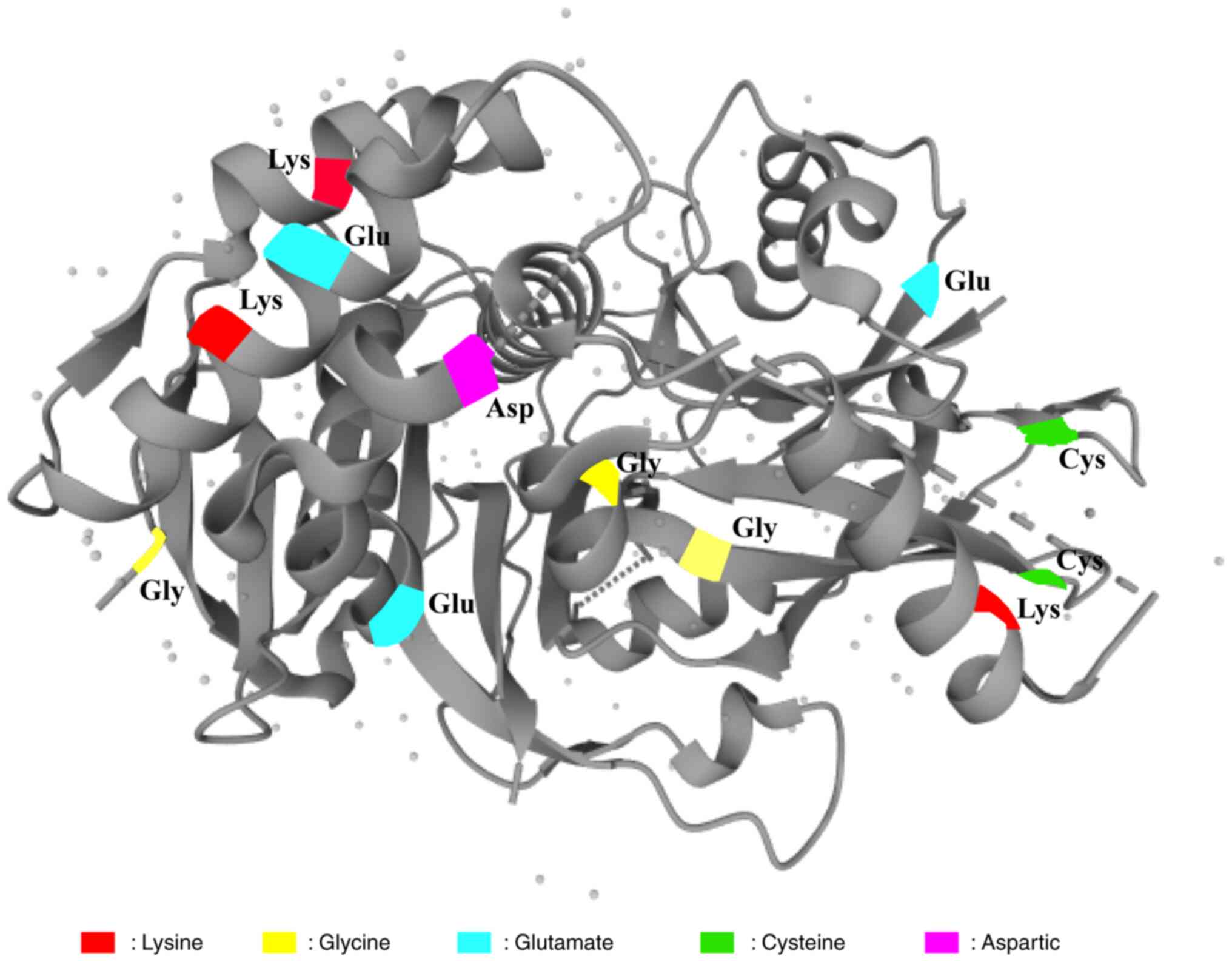
tissues and the prognosis of patients with lower USP2
expression is worse. Overexpression of USP2 can break the
isopeptide bond between ubiquitin and the Lys27 and Lys48 residues
of SMAD7 protein, reduce the recruitment of SMAD7 protein to the E3
ubiquitin ligase HERC3 and inhibit ubiquitin-mediated degradation
of SMAD7, thereby inhibiting the activation of the TGF-β signaling
pathway and the progression of GBM (54). Abnormal DNA methylation transferase
3A (DNMT3A)-mediated methylation of USP2 is the main cause
of low expression of USP2 in GBM tissues, and the DNMT3A
inhibitor GSI-1027 can induce USP2 expression to exert
anti-tumor effects against GBM (54).
Previous studies have reported that MDM4 can
promote endogenous apoptosis by regulating the expression of
oncogene p53 and that USP2a can interact with MDM4 to
inhibit its ubiquitin-mediated degradation (91,136,137). The expression of MDM4 and USP2a is
significantly lower in GBM tissues than in normal brain tissues and
is positively associated with the prognosis of GBM; that is, the
higher the expression, the more improved the prognosis. Knockdown
of USP2a promotes UV irradiation-induced cytochrome c
release, p53 protein expression and apoptosis in U87MG glioma
cells, whereas simultaneous upregulation of MDM4 can reverse these
effects (91). Therefore,
USP2 and MDM4 may serve as effective targets for the
treatment of GBM.
Bladder cancer is the most common life-threatening
tumor of the urinary system. Tight junction protein 1 (TJP1)
interacts with TWIST1 to enhance the invasive ability of tumor
cells and promotes bladder cancer progression by affecting vascular
remodeling (53). TJP1 expression
is significantly higher in clinical bladder cancer tissues compared
with healthy bladder tissues and is associated with tumor
angiogenesis and overall survival of patients (138). In vitro studies have
demonstrated that overexpression of TJP1 promotes the expression of
TWIST1 and chemokine C-C motif ligand 2 (CCL2) in tumor cells,
stimulating tumor cells to recruit more macrophages, which secrete
VEGF under CCL2 stimulation and enhance tumor angiogenesis.
Knockdown of TJP1 inhibits, and overexpression of TWIST1 promotes,
vascular remodeling in bladder cancer. TJP1 promotes vascular
remodeling by reversing ubiquitin-mediated degradation of TWIST1 by
recruiting USP2, whereas knockdown of USP2 promotes
ubiquitin-mediated degradation of TWIST1, reduces tumor
angiogenesis and exerts positive anti-tumor effects (53). In addition, USP2a gene is
highly expressed in bladder cancer cells and there is a physical
interaction between USP2a and CCNA1. USP2a inhibits
ubiquitination degradation of CCNA1 protein through
deubiquitylation, which in turn increases CCNA1 protein expression
and exerts a positive pro-oncogenic effect. Therefore, USP2
and TJP1 may serve as effective therapeutic targets for
bladder cancer.
Cutaneous T-cell lymphoma (CTCL) is caused by the
clonal proliferation of T lymphocytes originating in the skin and
is a type of extranodal non-Hodgkin lymphoma. Psoralen with
ultraviolet A (PUVA) phototherapy is a common treatment strategy
for CTCL in clinical settings (145,146). A study demonstrated that
USP2 is expressed in both quiescent and activated T
lymphocytes, and its expression is significantly reduced in
advanced CTCL. Treatment of MyLa2000 cells with PUVA or the
p53 agonist nutlin3a significantly increases the protein
expression of USP2 and p53 and promoted apoptosis (90). Silencing of USP2, which acts
as a tumor suppressor, reduces the protein expression of MDM2 and
enhances the transcriptional activity of p53, thereby
promoting apoptosis and enhancing the sensitivity of MyLa2000 cells
to PUVA and nutlin3a. In addition, p53 induced USP2
expression and stabilized MDM2 protein via deubiquitination, which
in turn inhibited the pro-apoptotic activity of p53, forming
a negative feedback loop (90).
Therefore, small-molecule inhibitors of USP2 may serve as
sensitizing agents in CTCL.
E2F transcription factor 4 (E2F4), a key factor
regulating cell cycle progression, binds to DNA to promote the
progression of cells from the G0 to the G1
and S phases and is involved in tumor progression (147). E2F4 can directly regulate
the transcription of ATG2A and ULK2 proteins, leading to the
autophagic degradation of metallothionein; it can maintain zinc
homeostasis in tumor cells and promote the proliferative, migratory
and invasive abilities of gastric cancer cells (59). High expression of USP2 and
E2F4 in gastric cancer tissues is associated with a poor
prognosis. Emetine, an autophagy inhibitor, can block the
interaction between USP2 and E2F4 and promote E2F4 degradation for
an oncogenic effect, which can be reversed upon USP2
overexpression (59). Therefore,
USP2 and E2F4 may serve as potential biomarkers for
maintaining zinc homeostasis in the treatment of gastric
cancer.
Previous studies have demonstrated the involvement
of multiple USPs in the tumorigenesis and chemotherapy resistance
of lung cancer (148,149). USPs may serve as therapeutic
targets for lung cancer. For instance, inhibition of USP1
and USP51 can increase cisplatin sensitivity in lung cancer
(150,151); inhibition of USP5 and
USP28 can promote apoptosis of tumor cells (152,153) and promotion of USP52 and USP7 can
inhibit the proliferative, migratory and invasive abilities of lung
cancer cells (107,154). However, the role of USP2 in lung
cancer remains elusive. Zhang et al (155) reported that USP2 expression is
upregulated in the lung cancer cell lines H1229 and H1270.
Knockdown of USP2 promotes ubiquitin-mediated degradation of SKP2
and inhibits the growth of tumor cells. Mechanistically, USP2
interacts with SKP2 and stabilizes its expression to promote lung
cancer progression. Therefore, USP2 and SKP2 may
serve as potential therapeutic targets for lung cancer.
Renal clear cell carcinoma is a common malignant
tumor of the urinary system. The proliferation and apoptosis of
cancer cells cannot be achieved without the participation of USPs
(156,157). The clinical significance of
USP2 in renal clear cell carcinoma has been demonstrated
(158). The mRNA and protein
expression of USP2 is significantly lower in cancer tissues than in
para-cancerous and healthy kidney tissues. Studies have verified
the low protein expression of USP2 in most cancer tissues
via immunohistochemical analysis (56,159,160). Overexpression of USP2
inhibits the proliferative, migratory and invasive abilities of
kidney cancer cells (A498 and CAKi-1) (158). In addition, the abnormal
expression of USP2 is closely related to the clinical stage,
pathological grade and prognosis of patients with renal cancer, and
USP2 has been identified as an independent risk factor for
renal clear cell carcinoma (158).
Therefore, USP2 is a potential target for the diagnosis and
treatment of renal clear cell carcinoma.
With the rapid development of structural biology
and small-molecule drugs, targeted therapy has emerged as the most
promising strategy in clinical tumor treatment. Recent studies have
revealed the role of DUBs in life activities and identified DUBs as
potential therapeutic targets for tumors (170). USP2 is closely associated
with the development of various tumors, such as breast cancer,
liver cancer, CRC, GBM and hematological tumors (49,53,54,56,126).
At present, ML364 is the most common small-molecule inhibitor used
in clinical trials; other inhibitors include Q29, STD1T and LCAHA
(23). The chemical structures of
these small-molecule inhibitors are shown in Fig. 4, and key information regarding their
mechanism of action and targets is summarized in Table II. The use of USP2 as a
target for tumor treatment has received increasing attention from
researchers. Although several USP2-targeted agents have
shown positive anticancer effects in different cancers, the
identified targeting agents are undergoing preclinical
investigation at present. Therefore, these agents should be
evaluated via complex and comprehensive techniques to provide a
theoretical basis for their clinical application.
LCA, a secondary bile acid, serves an important
role in lipid metabolism, and several derivatives of LCA have
anticancer activity (173,174). The most active, LCAHA (Fig. 4C; IC50=5.8 µM), can
directly inhibit the biological activity of USP2a, induce
G0/G1-phase arrest in HCT116 cells and
ubiquitously degrade cell cycle protein D1, thereby exerting
positive anticancer effects (62).
6-TG is a clinical agent for the treatment of AML
and chronic granulocytic leukemia (161,162) (Fig.
4E). Chuang et al (139) used enzyme kinetic and X-ray
crystallographic data to verify that 6-TG is a small-molecule
inhibitor of USP2 that forms covalent bonds with the Cys276
residue of USP2 to inhibit its expression. This finding
provides a rationale for the clinical use of 6-TG in the treatment
of tumors with USP2 upregulation.
Chalcones, members of the flavonoid family, can
regulate the malignant behavior of tumors, such as tumor accretion,
invasion and metastasis, by targeting the ubiquitin-proteasome
system (176,177). Issaenko et al (178) found that the chalcone derivative
RA-9 (Fig. 4G) inhibits the
activity of USP2, USP5 and USP8; downregulates the expression of
CCND1 in breast, ovarian and cervical cancer cells, upregulates the
expression of oncogenes p53, p27 and p16 to promote
apoptosis and exerts positive anticancer effects.
Ubiquitination, one of the post-translational
modifications, serves an important role in the development and
malignant behavior of several cancers and influences protein
expression and signal transduction. DUBs can regulate the stability
of substrate proteins by removing ubiquitin tags, thereby
regulating the cascade responses of the cell cycle, DNA damage
repair, invasion, metastasis and other signaling pathways.
Targeting DUBs represents an effective strategy for the treatment
of cancer. Some USP-targeted drugs are undergoing investigation in
phase II clinical trials. USP2 primarily influences the expression
of CCND1, MDM2, p53 and other proteins by regulating
ubiquitin-mediated protein degradation, which in turn affects tumor
development. However, the following questions remain to be
addressed: i) How do transcription factors recognize USP2
and regulate its transcription? ii) In addition to affecting the
cell cycle and cell death, does USP2 regulate other
biological processes? iii) What are the specific molecular
mechanisms through which USP2 regulates the expression of
related factors?
Not applicable.
The present study was funded by The National Natural Science
Foundation of China (grant no. 82260535) and The 2022 National
Foundation incubation Program of the Affiliated Hospital of Guizhou
Medical University (grant no. gyfynsfc-2022-7).
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
SLZ, YG, and SJZ contributed to the review of data
collection, manuscript writing and revision. SZ was involved in the
consultation process and article revision. YWZ and ZW participated
in the collection and arrangement of materials.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ding Y, Xing D, Fei Y and Lu B: Emerging
degrader technologies engaging lysosomal pathways. Chem Soc Rev.
51:8832–8876. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang TY, Shi YY, Cui XW, Pan YF, Lin YK,
Feng XF, Ding ZW, Yang C, Tan YX, Dong LW and Wang HY: PTEN
deficiency facilitates exosome secretion and metastasis in
cholangiocarcinoma by impairing TFEB-mediated lysosome biogenesis.
Gastroenterology. 164:424–438. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karbowski M, Oshima Y and Verhoeven N:
Mitochondrial proteotoxicity: implications and ubiquitin-dependent
quality control mechanisms. Cell Mol Life Sci. 79:5742022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinam IS, Chanda D, Thoudam T, Kim MJ, Kim
BG, Kang HJ, Lee JY, Baek SH, Kim SY, Shim BJ, et al: Pyruvate
dehydrogenase kinase 4 promotes ubiquitin-proteasome
system-dependent muscle atrophy. J Cachexia Sarcopenia Muscle.
13:3122–3136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien S, Kelso S, Steinhart Z, Orlicky
S, Mis M, Kim Y, Lin S, Sicheri F and Angers S: SCF
FBXW7 regulates G2-M progression through control of
CCNL1 ubiquitination. EMBO Rep. 23:e550442022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Capecchi MR and Pozner A: ASPM regulates
symmetric stem cell division by tuning Cyclin E ubiquitination. Nat
Commun. 6:87632015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Li Q, Yang J, Xu P, Xuan Z, Xu J
and Xu Z: Cytosolic TGM2 promotes malignant progression in gastric
cancer by suppressing the TRIM21-mediated
ubiquitination/degradation of STAT1 in a GTP binding-dependent
modality. Cancer Commun (Lond). 43:123–149. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng X, Jia Y, Zhang Y, Ma F, Zhu Y, Hong
X, Zhou Q, He R, Zhang H, Jin J, et al: Ubiquitination of UVRAG by
SMURF1 promotes autophagosome maturation and inhibits
hepatocellular carcinoma growth. Autophagy. 15:1130–1149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Wang N, Jiang Y, Wang H, Xin Z, An
H, Pan H, Ma W, Zhang T, Wang X and Lin W: E3 ubiquitin ligase
NEDD4L negatively regulates inflammation by promoting
ubiquitination of MEKK2. EMBO Rep. 23:e546032022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nan Y, Luo Q, Wu X, Chang W, Zhao P, Liu S
and Liu Z: HCP5 prevents ubiquitination-mediated UTP3 degradation
to inhibit apoptosis by activating c-Myc transcriptional activity.
Mol Ther. 31:552–568. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao HJ, Jiang H, Ding K, Qiu XS, Ma N,
Zhang FK, Wang YK, Zheng QW, Xia J, Ni QZ, et al: ARID2 mitigates
hepatic steatosis via promoting the ubiquitination of JAK2. Cell
Death Differ. 30:383–396. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mattiroli F and Penengo L: Histone
ubiquitination: An integrative signaling platform in genome
stability. Trends Genet. 37:566–581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roberts JZ, Crawford N and Longley DB: The
role of ubiquitination in apoptosis and necroptosis. Cell Death
Differ. 29:272–284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang K, Liu J, Li YL, Li JP and Zhang R:
Ubiquitination/de-ubiquitination: A promising therapeutic target
for PTEN reactivation in cancer. Biochim Biophys Acta Rev Cancer.
1877:1887232022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Wei L, Hu N, Wang D, Ni J, Zhang S,
Liu H, Lv T, Yin J, Ye M and Song Y: FBW7-mediated ubiquitination
and destruction of PD-1 protein primes sensitivity to anti-PD-1
immunotherapy in non-small cell lung cancer. J Immunother Cancer.
10:e0051162022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Wang T, She Y, Wu K, Gu S, Li L,
Dong C, Chen C and Zhou Y: N6-methyladenosine-modified
circIGF2BP3 inhibits CD8+ T-cell responses to facilitate
tumor immune evasion by promoting the deubiquitination of PD-L1 in
non-small cell lung cancer. Mol Cancer. 20:1052021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Zhao N, Zhou Z, Chen J, Han S, Zhang
X, Bao H, Yuan W and Shu X: PLAGL2 promotes the proliferation and
migration of gastric cancer cells via USP37-mediated
deubiquitination of Snail1. Theranostics. 11:700–714. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie H, Zhou J, Liu X, Xu Y, Hepperla AJ,
Simon JM, Wang T, Yao H, Liao C, Baldwin AS, et al: USP13 promotes
deubiquitination of ZHX2 and tumorigenesis in kidney cancer. Proc
Natl Acad Sci USA. 119:e21198541192022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasaei R, Sarodaya N, Kim KS, Ramakrishna
S and Hong SH: Importance of deubiquitination in
macrophage-mediated viral response and inflammation. Int J Mol Sci.
21:80902020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun T, Liu Z and Yang Q: The role of
ubiquitination and deubiquitination in cancer metabolism. Mol
Cancer. 19:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai J, Culley MK, Zhao Y and Zhao J: The
role of ubiquitination and deubiquitination in the regulation of
cell junctions. Protein Cell. 9:754–769. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Y, Park SH and Chua NH:
UBP12/UBP13-mediated deubiquitination of salicylic acid receptor
NPR3 suppresses plant immunity. Mol Plant. 16:232–244. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Liu Y and Zhou H: Advances in the
development ubiquitin-specific peptidase (USP) inhibitors. Int J
Mol Sci. 22:45462021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato Y, Goto E, Shibata Y, Kubota Y,
Yamagata A, Goto-Ito S, Kubota K, Inoue J, Takekawa M, Tokunaga F
and Fukai S: Structures of CYLD USP with Met1- or Lys63-linked
diubiquitin reveal mechanisms for dual specificity. Nat Struct Mol
Biol. 22:222–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maertens GN, El Messaoudi-Aubert S,
Elderkin S, Hiom K and Peters G: Ubiquitin-specific proteases 7 and
11 modulate Polycomb regulation of the INK4a tumour suppressor.
EMBO J. 29:2553–2565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cruz L, Soares P and Correia M:
Ubiquitin-Specific proteases: Players in cancer cellular processes.
Pharmaceuticals (Basel). 14:8482021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mansilla A, Martin FA, Martin D and Ferrus
A: Ligand-independent requirements of steroid receptors EcR and USP
for cell survival. Cell Death Differ. 23:405–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An Z, Liu Y, Ou Y, Li J, Zhang B, Sun D,
Sun Y and Tang W: Regulation of the stability of RGF1 receptor by
the ubiquitin-specific proteases UBP12/UBP13 is critical for root
meristem maintenance. Proc Natl Acad Sci USA. 115:1123–1128. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim JH, Jono H, Komatsu K, Woo CH, Lee J,
Miyata M, Matsuno T, Xu X, Huang Y, Zhang W, et al: CYLD negatively
regulates transforming growth factor-β-signalling via
deubiquitinating Akt. Nat Commun. 3:7712012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonacci T and Emanuele MJ: Dissenting
degradation: Deubiquitinases in cell cycle and cancer. Semin Cancer
Biol. 67((Pt 2)): 145–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Xia S, Li H, Wang X, Li C, Chao Y,
Zhang L and Han C: The deubiquitinase USP10 regulates KLF4
stability and suppresses lung tumorigenesis. Cell Death Differ.
27:1747–1764. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baek SH, Choi KS, Yoo YJ, Cho JM, Baker
RT, Tanaka K and Chung CH: Molecular cloning of a novel
ubiquitin-specific protease, UBP41, with isopeptidase activity in
chick skeletal muscle. J Biol Chem. 272:25560–25565. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gousseva N and Baker RT: Gene structure,
alternate splicing, tissue distribution, cellular localization, and
developmental expression pattern of mouse deubiquitinating enzyme
isoforms Usp2-45 and Usp2-69. Gene Expr. 11:163–179. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moremen KW, Touster O and Robbins PW:
Novel purification of the catalytic domain of Golgi
alpha-mannosidase II. Characterization and comparison with the
intact enzyme. J Biol Chem. 266:16876–16885. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gerhard DS, Wagner L, Feingold EA, Shenmen
CM, Grouse LH, Schuler G, Klein SL, Old S, Rasooly R, Good P, et
al: The status, quality, and expansion of the NIH full-length cDNA
project: The Mammalian Gene Collection (MGC). Genome Res.
14((10B)): 2121–2127. 2004.PubMed/NCBI
|
|
36
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo H, Ji Y, Gao X, Liu X and Wu Y and Wu
Y: Ubiquitin specific protease 2: Structure, isoforms, cellular
function, relateddiseases and its inhibitors. Oncologie. 24:85–99.
2022. View Article : Google Scholar
|
|
38
|
Zhu HQ and Gao FH: The molecular
mechanisms of regulation on USP2′s alternative splicing and the
significance of its products. Int J Biol Sci. 13:1489–1496. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pouly D, Chenaux S, Martin V, Babis M,
Koch R, Nagoshi E, Katanaev VL, Gachon F and Staub O: USP2-45 is a
circadian clock output effector regulating calcium absorption at
the post-translational level. PLoS One. 11:e01451552016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong X, Buelow K, Guha A, Rausch R and Yin
L: USP2a protein deubiquitinates and stabilizes the circadian
protein CRY1 in response to inflammatory signals. J Biol Chem.
287:25280–25291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Molusky MM, Li S, Ma D, Yu L and Lin JD:
Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and
diurnal glucose metabolism through 11β-hydroxysteroid dehydrogenase
1. Diabetes. 61:1025–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kitamura H, Kimura S, Shimamoto Y, Okabe
J, Ito M, Miyamoto T, Naoe Y, Kikuguchi C, Meek B, Toda C, et al:
Ubiquitin-specific protease 2–69 in macrophages potentially
modulates metainflammation. FASEB J. 27:4940–4953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang S, Wu H, Liu Y, Sun J, Zhao Z, Chen
Q, Guo M, Ma D and Zhang Z: Expression of USP2-69 in mesangial
cells in vivo and in vitro. Pathol Int. 60:184–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haimerl F, Erhardt A, Sass G and Tiegs G:
Down-regulation of the de-ubiquitinating enzyme ubiquitin-specific
protease 2 contributes to tumor necrosis factor-alpha-induced
hepatocyte survival. J Biol Chem. 284:495–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Kim BG, Qian S, Letterio JJ, Fung
JJ, Lu L and Lin F: Hepatic stellate cells inhibit T cells through
active TGF-β1 from a cell surface-bound latent TGF-β1/GARP complex.
J Immunol. 195:2648–2656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mao X, Luo W, Sun J, Yang N, Zhang LW,
Zhao Z, Zhang Z and Wu H: Usp2-69 overexpression slows down the
progression of rat anti-Thy1.1 nephritis. Exp Mol Pathol.
101:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kitamura H, Ishino T, Shimamoto Y, Okabe
J, Miyamoto T, Takahashi E and Miyoshi I: Ubiquitin-Specific
protease 2 modulates the lipopolysaccharide-elicited expression of
proinflammatory cytokines in macrophage-like HL-60 cells. Mediators
Inflamm. 2017:69094152017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mahul-Mellier AL, Datler C, Pazarentzos E,
Lin B, Chaisaklert W, Abuali G and Grimm S: De-ubiquitinating
proteases USP2a and USP2c cause apoptosis by stabilising RIP1.
Biochim Biophys Acta. 1823:1353–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Davis MI, Pragani R, Fox JT, Shen M,
Parmar K, Gaudiano EF, Liu L, Tanega C, McGee L, Hall MD, et al:
Small molecule inhibition of the ubiquitin-specific protease USP2
Accelerates cyclin D1 degradation and leads to cell cycle arrest in
colorectal cancer and mantle cell lymphoma models. J Biol Chem.
291:24628–24640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bedard N, Yang Y, Gregory M, Cyr DG,
Suzuki J, Yu X, Chian RC, Hermo L, O'Flaherty C, Smith CE, et al:
Mice lacking the USP2 deubiquitinating enzyme have severe male
subfertility associated with defects in fertilization and sperm
motility. Biol Reprod. 85:594–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Q, Liu M, Zhang F, Liu X, Ling S, Chen
X, Gu J, Ou W, Liu S and Liu N: Ubiquitin-specific protease 2
regulates Ang II-induced cardiac fibroblasts activation by
up-regulating cyclin D1 and stabilizing β-catenin in vitro. J Cell
Mol Med. 25:1001–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hashimoto M, Fujimoto M, Konno K, Lee ML,
Yamada Y, Yamashita K, Toda C, Tomura M, Watanabe M, Inanami O and
Kitamura H: Ubiquitin-Specific protease 2 in the ventromedial
hypothalamus modifies blood glucose levels by controlling
sympathetic nervous activation. J Neurosci. 42:4607–4618. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu XQ, Shao XR, Liu Y, Dong ZX, Chan SH,
Shi YY, Chen SN, Qi L, Zhong L, Yu Y, et al: Tight junction protein
1 promotes vasculature remodeling via regulating USP2/TWIST1 in
bladder cancer. Oncogene. 41:502–514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tu Y, Xu L, Xu J, Bao Z, Tian W, Ye Y, Sun
G, Miao Z, Chao H, You Y, et al: Loss of deubiquitylase USP2
triggers development of glioblastoma via TGF-β signaling. Oncogene.
41:2597–2608. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nadolny C, Zhang X, Chen Q, Hashmi SF, Ali
W, Hemme C, Ahsan N, Chen Y and Deng R: Dysregulation and
activities of ubiquitin specific peptidase 2b in the pathogenesis
of hepatocellular carcinoma. Am J Cancer Res. 11:4746–4767.
2021.PubMed/NCBI
|
|
56
|
Zhang J, Liu S, Li Q, Shi Y, Wu Y, Liu F,
Wang S, Zaky MY, Yousuf W, Sun Q, et al: The deubiquitylase USP2
maintains ErbB2 abundance via counteracting endocytic degradation
and represents a therapeutic target in ErbB2-positive breast
cancer. Cell Death Differ. 27:2710–2725. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qu Q, Mao Y, Xiao G, Fei X, Wang J, Zhang
Y, Liu J, Cheng G, Chen X, Wang J and Shen K: USP2 promotes cell
migration and invasion in triple negative breast cancer cell lines.
Tumour Biol. 36:5415–5423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang XR, Liu YF, Chen F, Zhou ZX, Zhang
LJ and Lin ZJ: Cell Cycle-Related lncRNAs as innovative targets to
advance cancer management. Cancer Manag Res. 15:547–561. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiao W, Wang J, Wang X, Cai S, Guo Y, Ye
L, Li D, Hu A, Jin S, Yuan B, et al: Therapeutic targeting of the
USP2-E2F4 axis inhibits autophagic machinery essential for zinc
homeostasis in cancer progression. Autophagy. 18:2615–2635. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu M, Wang H, Ding Y, Yang Y, Xu Z, Shi L
and Zhang N: Ribonucleotide reductase holoenzyme inhibitor COH29
interacts with deubiquitinase ubiquitin-specific protease 2 and
downregulates its substrate protein cyclin D1. FASEB J.
36:e223292022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shan J, Zhao W and Gu W: Suppression of
cancer cell growth by promoting cyclin D1 degradation. Mol Cell.
36:469–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Magiera K, Tomala M, Kubica K, De Cesare
V, Trost M, Zieba BJ, Kachamakova-Trojanowska N, Les M, Dubin G,
Holak TA and Skalniak L: Lithocholic acid hydroxyamide destabilizes
cyclin D1 and Induces G (0)/G (1) arrest by inhibiting
deubiquitinase USP2a. Cell Chem Biol. 24:458–470. e182017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nepal S, Shrestha A and Park PH: Ubiquitin
specific protease 2 acts as a key modulator for the regulation of
cell cycle by adiponectin and leptin in cancer cells. Mol Cell
Endocrinol. 412:44–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tomala MD, Magiera-Mularz K, Kubica K,
Krzanik S, Zieba B, Musielak B, Pustula M, Popowicz GM, Sattler M,
Dubin G, et al: Identification of small-molecule inhibitors of
USP2a. Eur J Med Chem. 150:261–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim J, Kim WJ, Liu Z, Loda M and Freeman
MR: The ubiquitin-specific protease USP2a enhances tumor
progression by targeting cyclin A1 in bladder cancer. Cell Cycle.
11:1123–1130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gabay M, Li Y and Felsher DW: MYC
activation is a hallmark of cancer initiation and maintenance. Cold
Spring Harb Perspect Med. 4:a0142412014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, Metabolism, and Cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu CH, van Riggelen J, Yetil A, Fan AC,
Bachireddy P and Felsher DW: Cellular senescence is an important
mechanism of tumor regression upon c-Myc inactivation. Proc Natl
Acad Sci USA. 104:13028–13033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhuang D, Mannava S, Grachtchouk V, Tang
WH, Patil S, Wawrzyniak JA, Berman AE, Giordano TJ, Prochownik EV,
Soengas MS and Nikiforov MA: C-MYC overexpression is required for
continuous suppression of oncogene-induced senescence in melanoma
cells. Oncogene. 27:6623–6634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li B, Zhang G, Wang Z, Yang Y, Wang C,
Fang D, Liu K, Wang F and Mei Y: c-Myc-activated USP2-AS1
suppresses senescence and promotes tumor progression via
stabilization of E2F1 mRNA. Cell Death Dis. 12:10062021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Iemura K, Natsume T, Maehara K, Kanemaki
MT and Tanaka K: Chromosome oscillation promotes Aurora A-dependent
Hec1 phosphorylation and mitotic fidelity. J Cell Biol.
220:e2020061162021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li P, Chen T, Kuang P, Liu F, Li Z, Liu F,
Wang Y, Zhang W and Cai X: Aurora-A/FOXO3A/SKP2 axis promotes tumor
progression in clear cell renal cell carcinoma and dual-targeting
Aurora-A/SKP2 shows synthetic lethality. Cell Death Dis.
13:6062022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shi Y, Solomon LR, Pereda-Lopez A, Giranda
VL, Luo Y, Johnson EF, Shoemaker AR, Leverson J and Liu X:
Ubiquitin-specific cysteine protease 2a (USP2a) regulates the
stability of Aurora-A. J Biol Chem. 286:38960–38968. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gu Y, Zhang Z, Camps MGM, Ossendorp F,
Wijdeven RH and Ten Dijke P: Genome-wide CRISPR screens define
determinants of epithelial-mesenchymal transition mediated immune
evasion by pancreatic cancer cells. Sci Adv. 9:eadf99152023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen J, Ding ZY, Li S, Liu S, Xiao C, Li
Z, Zhang BX, Chen XP and Yang X: Targeting transforming growth
factor-β signaling for enhanced cancer chemotherapy. Theranostics.
11:1345–1363. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
He Q, Cao H, Zhao Y, Chen P, Wang N, Li W,
Cui R, Hou P, Zhang X and Ji M: Dipeptidyl Peptidase-4 Stabilizes
Integrin alpha4β1 complex to promote thyroid cancer cell metastasis
by activating transforming growth factor-beta signaling pathway.
Thyroid. 32:1411–1422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tuersuntuoheti A, Li Q, Teng Y, Li X,
Huang R, Lu Y, Li K, Liang J, Miao S, Wu W and Song W: YWK-II/APLP2
inhibits TGF-β signaling by interfering with the TGFBR2-Hsp90
interaction. Biochim Biophys Acta Mol Cell Res. Jul 19–2023.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Miyazawa K and Miyazono K: Regulation of
TGF-β family signaling by inhibitory smads. Cold Spring Harb
Perspect Biol. 9:a0220952017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao Y, Wang X, Wang Q, Deng Y, Li K,
Zhang M, Zhang Q, Zhou J, Wang HY, Bai P, et al: USP2a supports
metastasis by tuning TGF-β signaling. Cell Rep. 22:2442–2454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Blenman KRM, Marczyk M, Karn T, Qing T, Li
X, Gunasekharan V, Yaghoobi V, Bai Y, Ibrahim EY, Park T, et al:
Predictive markers of response to neoadjuvant durvalumab with
nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in
basal-like triple-negative breast cancer. Clin Cancer Res.
28:2587–2597. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cui Y, Zhao M, Yang Y, Xu R, Tong L, Liang
J, Zhang X, Sun Y and Fan Y: Reversal of epithelial-mesenchymal
transition and inhibition of tumor stemness of breast cancer cells
through advanced combined chemotherapy. Acta Biomater. 152:380–392.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ahangari F, Becker C, Foster DG,
Chioccioli M, Nelson M, Beke K, Wang X, Justet A, Adams T, Readhead
B, et al: Saracatinib, a selective src kinase inhibitor, blocks
fibrotic responses in preclinical models of pulmonary fibrosis. Am
J Respir Crit Care Med. 206:1463–1479. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
van der Wal T and van Amerongen R: Walking
the tight wire between cell adhesion and WNT signalling: A
balancing act for beta-catenin. Open Biol. 10:2002672020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kim J, Alavi Naini F, Sun Y and Ma L:
Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and
stabilizes β-catenin. Am J Cancer Res. 8:1823–1836, eCollection
2018. 2018.PubMed/NCBI
|
|
85
|
Pichiorri F, Suh SS, Rocci A, De Luca L,
Taccioli C, Santhanam R, Zhou W, Benson DM Jr, Hofmainster C, Alder
H, et al: Retraction notice to: Downregulation of p53-inducible
microRNAs 192, 194, and 215 Impairs the p53/MDM2 autoregulatory
loop in multiple myeloma development. Cancer Cell. 40:14412022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wu B and Ellisen LW: Loss of p53 and
genetic evolution in pancreatic cancer: Ordered chaos after the
guardian is gone. Cancer Cell. 40:1276–1278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hassin O and Oren M: Drugging p53 in
cancer: One protein, many targets. Nat Rev Drug Discov. 22:127–144.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dobbelstein M and Levine AJ: Mdm2: Open
questions. Cancer Sci. 111:2203–2211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Stevenson LF, Sparks A, Allende-Vega N,
Xirodimas DP, Lane DP and Saville MK: The deubiquitinating enzyme
USP2a regulates the p53 pathway by targeting Mdm2. EMBO J.
26:976–986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wei T, Biskup E, Gjerdrum LM, Niazi O,
Odum N and Gniadecki R: Ubiquitin-specific protease 2 decreases
p53-dependent apoptosis in cutaneous T-cell lymphoma. Oncotarget.
7:48391–48400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang CL, Wang JY, Liu ZY, Ma XM, Wang XW,
Jin H, Zhang XP, Fu D, Hou LJ and Lu YC: Ubiquitin-specific
protease 2a stabilizes MDM4 and facilitates the p53-mediated
intrinsic apoptotic pathway in glioblastoma. Carcinogenesis.
35:1500–1509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shrestha M and Park PH: p53 signaling is
involved in leptin-induced growth of hepatic and breast cancer
cells. Korean J Physiol Pharmacol. 20:487–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen W, Shi K, Liu J, Yang P, Han R, Pan
M, Yuan L, Fang C, Yu Y and Qian Z: Sustained co-delivery of
5-fluorouracil and cis-platinum via biodegradable thermo-sensitive
hydrogel for intraoperative synergistic combination chemotherapy of
gastric cancer. Bioact Mater. 23:1–15. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang J, Zhang Y, Zhang G, Xiang L, Pang H,
Xiong K, Lu Y, Li J, Dai J, Lin S and Fu S: Radiotherapy-induced
enrichment of EGF-modified doxorubicin nanoparticles enhances the
therapeutic outcome of lung cancer. Drug Deliv. 29:588–599. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Smith ER, Wang JQ, Yang DH and Xu XX:
Paclitaxel resistance related to nuclear envelope structural
sturdiness. Drug Resist Updat. 65:1008812022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jang JH, Lee TJ, Sung EG, Song IH and Kim
JY: Dapagliflozin induces apoptosis by downregulating
cFILPL and increasing cFILPS instability in
Caki-1 cells. Oncol Lett. 24:4012022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Poukkula M, Kaunisto A, Hietakangas V,
Denessiouk K, Katajamaki T, Johnson MS, Sistonen L and Eriksson JE:
Rapid turnover of c-FLIPshort is determined by its unique
C-terminal tail. J Biol Chem. 280:27345–27355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu D, Fan Y, Li J, Cheng B, Lin W, Li X,
Du J and Ling C: Inhibition of cFLIP overcomes acquired resistance
to sorafenib via reducing ER stress-related autophagy in
hepatocellular carcinoma. Oncol Rep. 40:2206–2214. 2018.PubMed/NCBI
|
|
99
|
Iyer AK, Azad N, Talbot S, Stehlik C, Lu
B, Wang L and Rojanasakul Y: Antioxidant c-FLIP inhibits Fas
ligand-induced NF-kappaB activation in a phosphatidylinositol
3-kinase/Akt-dependent manner. J Immunol. 187:3256–3266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Quintavalle C, Incoronato M, Puca L,
Acunzo M, Zanca C, Romano G, Garofalo M, Iaboni M, Croce CM and
Condorelli G: c-FLIPL enhances anti-apoptotic Akt functions by
modulation of Gsk3β activity. Cell Death Differ. 24:11342017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xiao Z, Liu Y, Li Q, Liu Q, Liu Y, Luo Y
and Wei S: EVs delivery of miR-1915-3p improves the
chemotherapeutic efficacy of oxaliplatin in colorectal cancer.
Cancer Chemother Pharmacol. 88:1021–1031. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
He J, Lee HJ, Saha S, Ruan D, Guo H and
Chan CH: Inhibition of USP2 eliminates cancer stem cells and
enhances TNBC responsiveness to chemotherapy. Cell Death Dis.
10:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Min HY and Lee HY: Molecular targeted
therapy for anticancer treatment. Exp Mol Med. 54:1670–1694. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Assoun S, Lemiale V and Azoulay E:
Molecular targeted therapy-related life-threatening toxicity in
patients with malignancies. A systematic review of published cases.
Intensive Care Med. 45:988–997. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rosenberg T, Yeo KK, Mauguen A,
Alexandrescu S, Prabhu SP, Tsai JW, Malinowski S, Joshirao M,
Parikh K, Farouk Sait S, et al: Upfront molecular targeted therapy
for the treatment of BRAF-mutant pediatric high-grade glioma. Neuro
Oncol. 24:1964–1975. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Harakandi C, Nininahazwe L, Xu H, Liu B,
He C, Zheng YC and Zhang H: Recent advances on the intervention
sites targeting USP7-MDM2-p53 in cancer therapy. Bioorg Chem.
116:1052732021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang YT, Cheng AC, Tang HC, Huang GC, Cai
L, Lin TH, Wu KJ, Tseng PH, Wang GG and Chen WY: USP7 facilitates
SMAD3 autoregulation to repress cancer progression in p53-deficient
lung cancer. Cell Death Dis. 12:8802021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Park SH, Fong KW, Kim J, Wang F, Lu X, Lee
Y, Brea LT, Wadosky K, Guo C, Abdulkadir SA, et al:
Posttranslational regulation of FOXA1 by Polycomb and BUB3/USP7
deubiquitin complex in prostate cancer. Sci Adv. 7:eabe22612021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Su D, Wang W, Hou Y, Wang L, Yi X, Cao C,
Wang Y, Gao H, Wang Y, Yang C, et al: Bimodal regulation of the
PRC2 complex by USP7 underlies tumorigenesis. Nucleic Acids Res.
49:4421–4440. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang L, Wang H, Tian L and Li H:
Expression of USP7 and MARCH7 is correlated with poor prognosis in
epithelial ovarian cancer. Tohoku J Exp Med. 239:165–175. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu M, Zhang Y, Wu Y, Jin J, Cao Y, Fang
Z, Geng L, Yang L, Yu M, Bu Z, et al: IKZF1 selectively enhances
homologous recombination repair by interacting with CtIP and USP7
in multiple myeloma. Int J Biol Sci. 18:2515–2526. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhu Y, Gu L, Lin X, Cui K, Liu C, Lu B,
Zhou F, Zhao Q, Shen H and Li Y: LINC00265 promotes colorectal
tumorigenesis via ZMIZ2 and USP7-mediated stabilization of
β-catenin. Cell Death Differ. 27:1316–1327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ullah S, Junaid M, Liu Y, Chen S, Zhao Y
and Wadood A: Validation of catalytic site residues of Ubiquitin
Specific Protease 2 (USP2) by molecular dynamic simulation and
novel kinetics assay for rational drug design. Mol Divers.
27:1323–1332. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Metzig M, Nickles D, Falschlehner C,
Lehmann-Koch J, Straub BK, Roth W and Boutros M: An RNAi screen
identifies USP2 as a factor required for TNF-α-induced NF-κB
signaling. Int J Cancer. 129:607–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang
Q, Lin Y, Li J, Kang T, Tao M, et al: Disrupting the interaction of
BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like
breast cancer. Cancer Cell. 25:210–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Peinado H and Cano A: A hypoxic twist in
metastasis. Nat Cell Biol. 10:253–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mladinich M, Ruan D and Chan CH: Tackling
cancer stem cells via inhibition of EMT transcription factors. Stem
Cells Int. 2016:52858922016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim JY, Cho TM, Park JM, Park S, Park M,
Nam KD, Ko D, Seo J, Kim S, Jung E, et al: A novel HSP90 inhibitor
SL-145 suppresses metastatic triple-negative breast cancer without
triggering the heat shock response. Oncogene. 41:3289–3297. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shih YY, Lin HY, Jan HM, Chen YJ, Ong LL,
Yu AL and Lin CH: S-glutathionylation of Hsp90 enhances its
degradation and correlates with favorable prognosis of breast
cancer. Redox Biol. 57:1025012022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Leow CC, Chesebrough J, Coffman KT,
Fazenbaker CA, Gooya J, Weng D, Coats S, Jackson D, Jallal B and
Chang Y: Antitumor efficacy of IPI-504, a selective heat shock
protein 90 inhibitor against human epidermal growth factor receptor
2-positive human xenograft models as a single agent and in
combination with trastuzumab or lapatinib. Mol Cancer Ther.
8:2131–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Workman P, Burrows F, Neckers L and Rosen
N: Drugging the cancer chaperone HSP90: Combinatorial therapeutic
exploitation of oncogene addiction and tumor stress. Ann N Y Acad
Sci. 1113:202–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Modi S, Stopeck A, Linden H, Solit D,
Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME,
Sugarman S, et al: HSP90 inhibition is effective in breast cancer:
A phase II trial of tanespimycin (17-AAG) plus trastuzumab in
patients with HER2-positive metastatic breast cancer progressing on
trastuzumab. Clin Cancer Res. 17:5132–5139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sesto N, Wurtzel O, Archambaud C, Sorek R
and Cossart P: The excludon: A new concept in bacterial antisense
RNA-mediated gene regulation. Nat Rev Microbiol. 11:75–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Qu X, Alsager S, Zhuo Y and Shan B: HOX
transcript antisense RNA (HOTAIR) in cancer. Cancer Lett.
454:90–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chen SP, Zhu GQ, Xing XX, Wan JL, Cai JL,
Du JX, Song LN, Dai Z and Zhou J: LncRNA USP2-AS1 promotes
hepatocellular carcinoma growth by enhancing YBX1-Mediated HIF1α
protein translation under hypoxia. Front Oncol. 12:8823722022.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pirnia F, Schneider E, Betticher DC and
Borner MM: Mitomycin C induces apoptosis and caspase-8 and −9
processing through a caspase-3 and Fas-independent pathway. Cell
Death Differ. 9:905–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang WD, Shang Y, Wang C, Ni J, Wang AM,
Li GJ, Su L and Chen SZ: c-FLIP promotes drug resistance in
non-small-cell lung cancer cells via upregulating FoxM1 expression.
Acta Pharmacol Sin. 43:2956–2966. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang Y, Hou JQ, Qu LY, Wang GQ, Ju HW,
Zhao ZW, Yu ZH and Yang HJ: Differential expression of USP2, USP14
and UBE4A between ovarian serous cystadenocarcinoma and adjacent
normal tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23:504–506.
2007.(In Chinese). PubMed/NCBI
|
|
130
|
Guo B, Yu L, Sun Y, Yao N and Ma L: Long
Non-Coding RNA USP2-AS1 accelerates cell proliferation and
migration in ovarian cancer by sponging miR-520d-3p and
Up-Regulating KIAA1522. Cancer Manag Res. 12:10541–10550. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li D, Bao J, Yao J and Li J: lncRNA
USP2-AS1 promotes colon cancer progression by modulating Hippo/YAP1
signaling. Am J Transl Res. 12:5670–5682, eCollection 2020.
2020.PubMed/NCBI
|
|
132
|
Tatari N, Khan S, Livingstone J, Zhai K,
McKenna D, Ignatchenko V, Chokshi C, Gwynne WD, Singh M, Revill S,
et al: The proteomic landscape of glioblastoma recurrence reveals
novel and targetable immunoregulatory drivers. Acta Neuropathol.
144:1127–1142. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ji YR, Cheng CC, Lee AL, Shieh JC, Wu HJ,
Huang AP, Hsu YH and Young TH: Poly (allylguanidine)-coated
surfaces regulate TGF-β in glioblastoma cells to induce apoptosis
via NF-κB Pathway Activation. ACS Appl Mater Interfaces.
13:59400–59410. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Joseph JV, Magaut CR, Storevik S, Geraldo
LH, Mathivet T, Latif MA, Rudewicz J, Guyon J, Gambaretti M, Haukas
F, et al: TGF-β promotes microtube formation in glioblastoma
through thrombospondin 1. Neuro Oncol. 24:541–553. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
XH and Chen YG: Smad7 protein interacts with receptor-regulated
smads (R-Smads) to inhibit transforming growth factor-β
(TGF-β)/smad signaling. J Biol Chem. 291:382–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Girish V, Lakhani AA, Thompson SL, Scaduto
CM, Brown LM, Hagenson RA, Sausville EL, Mendelson BE, Kandikuppa
PK, Lukow DA, et al: Oncogene-like addiction to aneuploidy in human
cancers. Science. Jul 6–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mejia-Hernandez JO, Raghu D, Caramia F,
Clemons N, Fujihara K, Riseborough T, Teunisse A, Jochemsen AG,
Abrahmsén L, Blandino G, et al: Targeting MDM4 as a novel
therapeutic approach in prostate cancer independent of p53 status.
Cancers (Basel). 14:39472022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Tsai KW, Kuo WT and Jeng SY: Tight
junction protein 1 dysfunction contributes to cell motility in
bladder cancer. Anticancer Res. 38:4607–4615. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chuang SJ, Cheng SC, Tang HC, Sun CY and
Chou CY: 6-Thioguanine is a noncompetitive and slow binding
inhibitor of human deubiquitinating protease USP2. Sci Rep.
8:31022018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Renatus M, Parrado SG, D'Arcy A, Eidhoff
U, Gerhartz B, Hassiepen U, Pierrat B, Riedl R, Vinzenz D,
Worpenberg S and Kroemer M: Structural basis of ubiquitin
recognition by the deubiquitinating protease USP2. Structure.
14:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kitamura H and Hashimoto M: USP2-Related
cellular signaling and consequent pathophysiological outcomes. Int
J Mol Sci. 22:12092021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Graner E, Tang D, Rossi S, Baron A, Migita
T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti
S and Loda M: The isopeptidase USP2a regulates the stability of
fatty acid synthase in prostate cancer. Cancer Cell. 5:253–261.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Cheng JC, Bai A, Beckham TH, Marrison ST,
Yount CL, Young K, Lu P, Bartlett AM, Wu BX, Keane BJ, et al:
Radiation-induced acid ceramidase confers prostate cancer
resistance and tumor relapse. J Clin Invest. 123:4344–4358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mizutani N, Inoue M, Omori Y, Ito H,
Tamiya-Koizumi K, Takagi A, Kojima T, Nakamura M, Iwaki S,
Nakatochi M, et al: Increased acid ceramidase expression depends on
upregulation of androgen-dependent deubiquitinases, USP2, in a
human prostate cancer cell line, LNCaP. J Biochem. 158:309–319.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Vieyra-Garcia PA and Wolf P: A deep dive
into UV-based phototherapy: Mechanisms of action and emerging
molecular targets in inflammation and cancer. Pharmacol Ther.
222:1077842021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Nakahashi K, Nihira K, Suzuki M, Ishii T,
Masuda K and Mori K: A novel mouse model of cutaneous T-cell
lymphoma revealed the combined effect of mogamulizumab with
psoralen and ultraviolet a therapy. Exp Dermatol. 31:1693–1698.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hsu J and Sage J: Novel functions for the
transcription factor E2F4 in development and disease. Cell Cycle.
15:3183–3190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Moghadami AA, Aboutalebi Vand Beilankouhi
E, Kalantary-Charvadeh A, Hamzavi M, Mosayyebi B, Sedghi H,
Ghorbani Haghjo A and Nazari Soltan Ahmad S: Inhibition of USP14
induces ER stress-mediated autophagy without apoptosis in lung
cancer cell line A549. Cell Stress Chaperones. 25:909–917. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Liu C, Chen Z, Ding X, Qiao Y and Li B:
Ubiquitin-specific protease 35 (USP35) mediates cisplatin-induced
apoptosis by stabilizing BIRC3 in non-small cell lung cancer. Lab
Invest. 102:524–533. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chen J, Dexheimer TS, Ai Y, Liang Q,
Villamil MA, Inglese J, Maloney DJ, Jadhav A, Simeonov A and Zhuang
Z: Selective and cell-active inhibitors of the USP1/UAF1
deubiquitinase complex reverse cisplatin resistance in non-small
cell lung cancer cells. Chem Biol. 18:1390–1400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhou F, Du C, Xu D, Lu J, Zhou L, Wu C, Wu
B and Huang J: Knockdown of ubiquitin-specific protease 51
attenuates cisplatin resistance in lung cancer through
ubiquitination of zinc-finger E-box binding homeobox 1. Mol Med
Rep. 22:1382–1390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang L, Xu B, Qiang Y, Huang H, Wang C,
Li D and Qian J: Overexpression of deubiquitinating enzyme USP28
promoted non-small cell lung cancer growth. J Cell Mol Med.
19:799–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhang Z, Cui Z, Xie Z, Li C, Xu C, Guo X,
Yu J, Chen T, Facchinetti F, Bohnenberger H, et al: Deubiquitinase
USP5 promotes non-small cell lung cancer cell proliferation by
stabilizing cyclin D1. Transl Lung Cancer Res. 10:3995–4011. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhu M, Zhang H, Lu F, Wang Z, Wu Y, Chen
H, Fan X, Yin Z and Liang F: USP52 inhibits cell proliferation by
stabilizing PTEN protein in non-small cell lung cancer. Biosci Rep.
41:BSR202104862021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang F, Zhao Y and Sun Y: USP2 is an SKP2
deubiquitylase that stabilizes both SKP2 and its substrates. J Biol
Chem. 297:1011092021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhou J, Wang T, Qiu T, Chen Z, Ma X, Zhang
L and Zou J: Ubiquitin-specific protease-44 inhibits the
proliferation and migration of cells via inhibition of JNK pathway
in clear cell renal cell carcinoma. BMC Cancer. 20:2142020.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hu W, Su Y, Fei X, Wang X, Zhang G, Su C,
Du T, Yang T, Wang G, Tang Z and Zhang J: Ubiquitin specific
peptidase 19 is a prognostic biomarker and affect the proliferation
and migration of clear cell renal cell carcinoma. Oncol Rep.
43:1964–1974. 2020.PubMed/NCBI
|
|
158
|
Meng X, Xiong Z, Xiao W, Yuan C, Wang C,
Huang Y, Tong J, Shi J, Chen Z, Liu C, et al: Downregulation of
ubiquitin-specific protease 2 possesses prognostic and diagnostic
value and promotes the clear cell renal cell carcinoma progression.
Ann Transl Med. 8:3192020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yi J, Tavana O, Li H, Wang D, Baer RJ and
Gu W: Targeting USP2 regulation of VPRBP-mediated degradation of
p53 and PD-L1 for cancer therapy. Nat Commun. 14:19412023.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhu L, Chen Z, Guo T, Chen W, Zhao L, Guo
L and Pan X: USP2 inhibits lung cancer pathogenesis by reducing
ARID2 protein degradation via ubiquitination. Biomed Res Int.
2022:15252162022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Estlin EJ: Continuing therapy for
childhood acute lymphoblastic leukaemia: Clinical and cellular
pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine.
Cancer Treat Rev. 27:351–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Vora A, Mitchell CD, Lennard L, Eden TO,
Kinsey SE, Lilleyman J and Richards SM; Medical Research Council;
National Cancer Research Network Childhood Leukaemia Working Party,
: Toxicity and efficacy of 6-thioguanine versus 6-mercaptopurine in
childhood lymphoblastic leukaemia: A randomised trial. Lancet.
368:1339–1348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lin HC, Kuan Y, Chu HF, Cheng SC, Pan HC,
Chen WY, Sun CY and Lin TH: Disulfiram and 6-Thioguanine
synergistically inhibit the enzymatic activities of USP2 and USP21.
Int J Biol Macromol. 176:490–497. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tsai HK, Gibson CJ, Murdock HM, Davineni
P, Harris MH, Wang ES, Gondek LP, Kim AS, Nardi V and Lindsley RC:
Allelic complexity of KMT2A partial tandem duplications in acute
myeloid leukemia and myelodysplastic syndromes. Blood Adv.
6:4236–4240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhou X, Zhang P, Aryal S, Zhang L and Lu
R: UTX loss alters therapeutic responses in KMT2A-rearranged acute
myeloid leukemia. Leukemia. 37:226–230. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ikeda J, Shiba N, Tsujimoto SI, Yoshida M,
Nakabayashi K, Ogata-Kawata H, Okamura K, Takeuchi M, Osumi T,
Tomizawa D, et al: Whole transcriptome sequencing reveals a
KMT2A-USP2 fusion in infant acute myeloid leukemia. Genes
Chromosomes Cancer. 58:669–672. 2019.PubMed/NCBI
|
|
167
|
Lopes BA, Poubel CP, Teixeira CE,
Caye-Eude A, Cave H, Meyer C, Marschalek R, Boroni M and
Emerenciano M: Novel Diagnostic and therapeutic options for
KMT2A-Rearranged acute leukemias. Front Pharmacol. 13:7494722022.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Blackburn PR, Smadbeck JB, Znoyko I,
Webley MR, Pitel BA, Vasmatzis G, Xu X, Greipp PT, Hoppman NL,
Ketterling RP, et al: Cryptic and atypical KMT2A-USP2 and
KMT2A-USP8 rearrangements identified by mate pair sequencing in
infant and childhood leukemia. Genes Chromosomes Cancer.
59:422–427. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Meyer C, Lopes BA, Caye-Eude A, Cave H,
Arfeuille C, Cuccuini W, Sutton R, Venn NC, Oh SH, Tsaur G, et al:
Human MLL/KMT2A gene exhibits a second breakpoint cluster region
for recurrent MLL-USP2 fusions. Leukemia. 33:2306–2340. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Liu J, Cheng Y, Zheng M, Yuan B, Wang Z,
Li X, Yin J, Ye M and Song Y: Targeting the
ubiquitination/deubiquitination process to regulate immune
checkpoint pathways. Signal Transduct Target Ther. 6:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Savage RE, Tyler AN, Miao XS and Chan TC:
Identification of a novel glucosylsulfate conjugate as a metabolite
of 3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione (ARQ
501, beta-lapachone) in mammals. Drug Metab Dispos. 36:753–758.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ohayon S, Refua M, Hendler A, Aharoni A
and Brik A: Harnessing the oxidation susceptibility of
deubiquitinases for inhibition with small molecules. Angew Chem Int
Ed Engl. 54:599–603. 2015.PubMed/NCBI
|
|
173
|
Nguyen TT, Ung TT, Li S, Sah DK, Park SY,
Lian S and Jung YD: Lithocholic Acid Induces miR21, Promoting PTEN
Inhibition via STAT3 and ERK-1/2 signaling in colorectal cancer
cells. Int J Mol Sci. 22:102092021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Li W, Wang Z, Lin R, Huang S, Miao H, Zou
L, Liu K, Cui X, Wang Z, Zhang Y, et al: Lithocholic acid inhibits
gallbladder cancer proliferation through interfering
glutaminase-mediated glutamine metabolism. Biochem Pharmacol.
205:1152532022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Altun M, Kramer HB, Willems LI, McDermott
JL, Leach CA, Goldenberg SJ, Kumar KG, Konietzny R, Fischer R,
Kogan E, et al: Activity-based chemical proteomics accelerates
inhibitor development for deubiquitylating enzymes. Chem Biol.
18:1401–1412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Gupta SC, Kim JH, Prasad S and Aggarwal
BB: Regulation of survival, proliferation, invasion, angiogenesis,
and metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Yang H, Landis-Piwowar KR, Chen D, Milacic
V and Dou QP: Natural compounds with proteasome inhibitory activity
for cancer prevention and treatment. Curr Protein Pept Sci.
9:227–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Issaenko OA and Amerik AY: Chalcone-based
small-molecule inhibitors attenuate malignant phenotype via
targeting deubiquitinating enzymes. Cell Cycle. 11:1804–1817. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Aleo E, Henderson CJ, Fontanini A, Solazzo
B and Brancolini C: Identification of new compounds that trigger
apoptosome-independent caspase activation and apoptosis. Cancer
Res. 66:9235–9244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Nicholson B, Leach CA, Goldenberg SJ,
Francis DM, Kodrasov MP, Tian X, Shanks J, Sterner DE, Bernal A,
Mattern MR, et al: Characterization of ubiquitin and
ubiquitin-like-protein isopeptidase activities. Protein Sci.
17:1035–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Vamisetti GB, Meledin R, Gopinath P and
Brik A: Halogen Substituents in the Isoquinoline Scaffold Switches
the Selectivity of Inhibition between USP2 and USP7. Chembiochem.
20:282–286. 2019.PubMed/NCBI
|