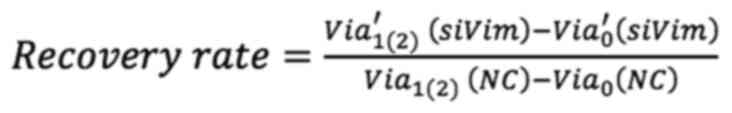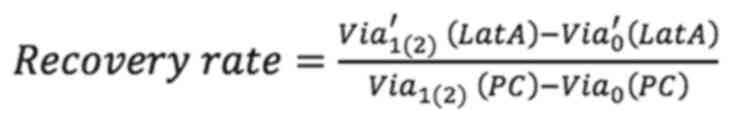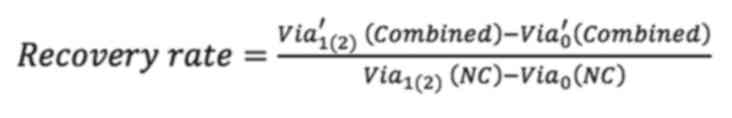Introduction
The cytoskeleton is a dynamic network of filamentous
proteins that controls cell shape, maintains intracellular
organization and is involved in cell motility (1,2).
Alterations in cytoskeletal structures serve a pivotal role in
controlling cancer cell behavior, including cell adhesion,
migration and invasion (3,4).
The cytoskeleton comprises three proteins: Actin
filaments (F-actin), intermediate filaments, microtubules and
various filament-associated proteins such as molecular motors
(2). Among the protein filaments,
vimentin and F-actin undergo pronounced changes during
tumorigenesis. Vimentin, a type III intermediate filament, is
highly expressed in mesenchymal cells compared with that in
epithelial cancer cells. In addition, vimentin expression is
upregulated during the epithelial-mesenchymal transition (EMT)
(5,6). Cancer cells lose their junctions
during EMT and undergo morphological changes from epithelial cells
with an apical-basal polarity to a spindle-shaped mesenchymal
phenotype (7). Overexpression of
vimentin in cancer cells is correlated with tumor growth,
metastasis and poor prognosis (8,9).
Unlike vimentin, F-actin is downregulated in most
cancer cells. Therefore, the relative content of F-actin to
vimentin in cancer cells is lower than in normal cells (10). In normal cells, F-actin content is
higher than vimentin. F-actin has a highly dynamic structure that
undergoes continuous polymerization and degradation. A monomer of
actin (G-actin) polymerizes to F-actin under physiological
conditions and F-actin dissociates to G-actin to maintain
equilibrium (11). Dynamic
organization of F-actin is a prerequisite for cancer cell migration
and invasion. Reduced F-actin results in changes in the mechanical
properties of cancer cells; specifically, cancer cells are less
elastic than normal cells (10).
Cytoskeletal proteins are emerging as attractive
therapeutic targets for inhibiting cancer metastasis because they
serve important roles in cancer cell migration and invasion.
Several compounds, such as ajoene, ginsenosides, withaferin A (WFA)
and arylquin 1 have been introduced as vimentin-targeting drugs
(12). They downregulate vimentin
expression, induce disassembly of vimentin filaments and induce
apoptosis. Dynamic actin assembly can be disrupted by
pharmacological inhibitors, such as latrunculin A (LatA) and
cytochalasin, which inhibit cancer cell migration and invasion
(13,14). LatA blocks the new F-actin by
sequestering G-actin, whereas cytochalasin blocks the fast-growing
barbed ends of F-actin. The disassembly and rearrangement of the
F-actin can also be inhibited by these drugs. Pharmacologically
perturbing the motility of cancer cells by altering vimentin and
F-actin can alter their organotropism for growth and metastasis
(15).
Therefore, compounds targeting cytoskeletal dynamics
are of increasing interest in cancer therapeutics (16,17).
However, only a handful of drugs have been proven effective and are
in clinical trials and even those need further validation. For
example, recent phase I safety and pharmacokinetic trials of WFA
have confirmed the importance of using the minimum effective dose
due to potential side effects (18,19).
In the case of arylquin 1, it has been demonstrated that apoptosis
can be induced in cancer cells by regulating the activity of Par-4
function, but additional analysis of the molecular mechanism is
required (20,21). In addition, the clinical application
of cytoskeleton-targeted therapeutics remains limited owing to
several challenges. First, because of their ubiquitous role,
cytoskeleton-targeted drugs can affect various processes in cancer
and normal cells, including cell migration, proliferation and
exocytosis (22). Second, it is
challenging to sustain or irreversibly interrupt F-actin assembly.
F-actin can be disassembled by drugs or external stress but rapidly
reassembles and reorganizes to maintain homeostasis (23). Third, the importance of vimentin in
metastasis has made it an attractive drug target; however, no drugs
that specifically target intermediate filaments are currently in
use (24). However, more specific
vimentin-targeting drugs with low toxicity are still needed.
Fourth, the complementary actions of F-actin and vimentin in
response to forced inhibition are poorly understood. As vimentin
and F-actin have similar mechanisms of action, particularly in cell
motility and structure formation, it is important to understand the
relationship and interactions between the two proteins.
To overcome the shortcomings of the current
cytoskeleton-targeting drugs and develop effective cancer
therapeutics via cytoskeletal protein modulation, the present study
investigated the effects of vimentin and actin deficiency on
metastatic cancer cells. It is well known that the content of
F-actin is reduced, and that of vimentin is increased, in cancer
cells compared with normal cells. Therefore, the present study
focused on the complementary interactions between vimentin and
F-actin. Vimentin and F-actin levels were reduced by ~50% in both
cancer (MDA-MB-231) and normal (MCF10A) cells. The effect of
F-actin downregulation on vimentin expression in cancer and normal
cells was analyzed. Changes in F-actin levels caused by vimentin
reduction were also analyzed as were the effects of altering the
expression of vimentin and F-actin on cell viability, morphology
and motility.
Materials and methods
Cell culture
The metastatic breast cancer cell line (MDA-MB-231)
was purchased from the Korean Cell Line Bank (KCLB; Seoul, Korea)
and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium
(Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1%
antibiotics/antimycotics, 300 mg/l L-glutamine, 25 mM hydroxyethyl
piperazine ethanesulfonic acid and 25 mM sodium bicarbonate. Normal
epithelial cells (MCF10A) were purchased from the American Type
Culture Collection. MCF10A cells were cultured using Dulbecco's
modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) (Thermo
Fisher Scientific, Inc.) with 5% horse serum, 20 ng/ml epidermal
growth factor (EGF), 0.5 mg/ml hydrocortisone, 100 ng/ml cholera
toxin, 10 µg/ml insulin and 1% antibiotics/antimycotics.
Cell viability
Cell viability was analyzed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MilliporeSigma) assay to determine the effects of vimentin
knockdown and F-actin depolymerization. MCF10A and MDA-MB-231 cells
were seeded into 35-mm diameter culture dishes at a density of
4.5×105 cells/well. After two days of small interfering
(si) RNA transfection and 2 min of LatA treatment, the cells were
incubated for 12 and 24 h. EZ-Cytox solution (DoGen Bio, Seoul,
Korea) was added to each cell dish (10 ml) and incubated at 37°C
for 1 h. A Multiskan EX ELISA microplate reader (Thermo Fisher
Scientific, Inc.) was used to measure the absorbance at 450 nm.
siRNA knockdown of vimentin
Cells were seeded at ~70% confluence for siRNA
transfection and cultured for 24 h. For MDA-MB-231 cells,
transfection reagents Opti-MEM I (300 µl; Thermo Fisher Scientific,
Inc.), Lipofectamine (9 µl; Invitrogen; Thermo Fisher Scientific,
Inc.) and 21-mer siRNA (Thermo Fisher Scientific, Inc.; cat. no.
4390824; 5 µl) were used. The sequence of siRNA is 5′
GGUUGAUACCCACUCAAAATT-3′ and 3′-TTAAAACUCACCCAUCGUUGG-5′. For
MCF10A cell transfection, 20 µl of siRNA was added. The negative
control (NC) siRNA (Thermo Fisher Scientific, Inc.; cat. no.
4390843; 3 µl) was added to Opti-MEM (300 µl) and Lipofectamine (9
µl). After 5 min of incubation at room temperature, transfection
solutions were added to the cells. After two days of incubation,
the transfected cells were visualized by fluorescence microscopy
and analyzed by western blotting.
F-actin depolymerization
Cells were seeded at a density of 0.5×105
cells/well in 35-mm diameter culture dishes. MDA-MB-231 cells were
treated with LatA (Invitrogen; Thermo Fisher Scientific, Inc.) at a
concentration of 0.3 µM for 2 min (37°C) to depolymerize the
F-actin content to ~50%. MCF10A cells were treated with LatA at a
concentration of 0.5 µM for 2 min (37°C). After 2 min of LatA
treatment, the medium was replaced with fresh medium at 37°C.
Analysis of cell recovery
To assess the effect of F-actin and vimentin
deletion on cell growth, the present study compared how much the
cells grew over time after actin and vimentin loss. Cell viability
was measured by MTT assay at 0, 24 and 48 h following LatA
treatment and siRNA knockdown of vimentin, respectively. To
quantitatively analyze the effect of vimentin knockdown, cell
viability after 24 and 48 h of siRNA transfection was normalized to
the viability day zero. The values were then divided by the
viability of the (NC) group and referred to as the recovery rate.
Non-targeting siRNA was used as a NC to provide a baseline for
target gene silencing by controlling for non-specific effects
associated with siRNA delivery. The untreated cells were designated
as the positivecontrol (PC). The recovery rate is defined as
follows:
where Via1(2)(NC) and
Via1(2)(PC) are the cell viability of the
NC and PC groups after one (or two days) of culture, respectively.
Via0′(siVim) is the cell
viability of transfected cells on day 1 of culture and
Via′1(2) (siVim) is the
viability after 1 (or 2) days of culture.
Via′0 (LatA) is the cell
viability of LatA-treated cells on day 0 of culture and
Via′1(2) (siVim) is the
viability after 1 (or 2) days of LatA treatment.
Via′0 (Combined) is the
viability of cells simultaneously depleted of vimentin and F-actin
on day 0 of culture and Via′1(2)
(siVim) is the viability 1 (or 2) days after the
depletion.
Western blotting
Cells were washed several times with
phosphate-buffered saline (PBS) and scraped into a
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing a protease inhibitor cocktail. To separate
intracellular proteins (vimentin and F-actin) and cell debris, the
cells were centrifuged at 374 × g for 5 min at 4°C. The supernatant
was centrifuged at 15,000 × g for 5 min (4°C) to separate F-actin
in the form of pellets and the remaining solution contained
G-actin. For determination of total protein concentration in each
sample was used BCA Protein Assay Kit (Pierce; Thermo Fisher
Scientific, Inc.). After protein quantification each sample (50 µg
of protein) was prepared for SDS-PAGE by heating it in 5XSDS-PAGE
loading buffer for 5 min at 95°C. For western blot analysis,
vimentin (10 µl), G-actin and F-actin proteins were separately
loaded onto 12.5% polyacrylamide gels. The resolved proteins were
transferred onto nitrocellulose membranes. The membrane was blocked
with 5% fat-free milk in PBS (pH 7.4) for 30 min at room
temperature and then incubated with primary anti-vimentin (Cell
Signaling Technology Inc.; cat. no. 5741) or anti-actin
(Cytoskeleton Inc.). Anti-vimentin and anti-actin antibodies were
mixed with Tris-buffered saline with 0.01% Tween 20 (TBS-T) at
1:1,000 and 1:500 dilution, respectively, overnight at 4°C.
Finally, the membranes were treated with anti-rabbit secondary
antibodies (GenDEPOT Inc.; cat. no. SA202-500)/fat-free milk at
1:6,500 dilution and incubated for 1 h at room temperature.
Integrin was analyzed using a similar process as follows. The
lysates were incubated for 30 min at 4°C and then centrifuged at
14,207 × g for 20 min (4°C). The supernatant was mixed with an
equal amount of loading buffer (2XLaemmli sample buffer with 5%
β-mercaptoethanol) and boiled for 5 min. The size marker (6 µl) and
protein (40 µl) were separately loaded in 8.0% polyacrylamide gels.
The resolved proteins were transferred to nitrocellulose membranes,
blocked with 5% BSA/TBS-T for 1 h at room temperature and then
incubated with a primary antibody [anti-integrin β1 antibody
(P5D2); Abcam; cat. no. ab24693] at a 1:1,000 dilution overnight at
4°C. The secondary antibody [Alexa Fluor 555 goat anti-mouse IgG
(H+L); Abcam; cat. no. ab150114] was incubated with blocking buffer
at a 1:5,000 dilution for 1 h at room temperature. Finally, the
membranes were subjected to enhanced chemiluminescence (Pierce;
Thermo Fisher Scientific, Inc.) and autoradiography using the
ChemiDoc XRS+Imaging System (Bio-Rad Laboratories, Inc.).
Atomic force microscopy (AFM) and
force distance (FD) curve measurement
The morphology and cellular elasticity were measured
using AFM (Nano N8 Neos; Bruker Corporation) under liquid
conditions. An Au-coated contact-mode probe (ContGD; BudgetSensors;
Innovative Solutions Bulgaria Ltd.) was used for imaging and FD
curve measurements. The detailed information of the contact mode
probe was: Resonance frequency of 13 kHz (± 4 kHz), force constant
of 0.2 N/m (0.07–0.4 N/m), cantilever length of 450 µm (± 10 µm),
cantilever width of 50 µm (± 5 µm), cantilever thickness of 2 µm (±
1 µm), tip height of 17 µm (± 2 µm) and tip radius of <10 nm.
The loading force and loading rate of the probe were set to <10
nN and <1 µm/s, respectively. FD-curve was analyzed using the
Sneddon model (25).
Two-dimensional (2D) optical tracking
assay
Cell migration in 2D was tracked in real-time at a
low cell density of 0.5×105 cells. Without fluorescent
staining, the cells were placed in a portable incubator and the
movement of the cells was measured at 1-min intervals for 4 h based
on the position of the nucleus under an optical microscope
(magnification, ×20). The migration distance of a single cell was
analyzed as a scalar value by using a video analysis and modeling
program (Tracker, Video Analysis and Modeling Tool ver. 5.0.2;
http://physlets.org/tracker/).
Analysis of protrusion dynamics
Single-cell movements were captured in real-time at
10 sec intervals for 30 min. Based on the change in light contrast
in the optical image, the periodic motion of the protrusions on the
leading edge of the cells was analyzed. Light contrast ranged from
0 luma (black) to 250 luma (white). The period and amplitude of the
periodic function were extracted using an analysis program
(Tracker, Video Analysis and Modeling Tool ver. 5.0.2; http://physlets.org/tracker/).
Immunofluorescence staining
Cells were fixed with 3.7% formaldehyde for 15 min
at room temperature and then washed with PBS for 30 sec. Cells were
treated with rhodamine-phalloidin (100 nM; Alexa Fluor 488
phalloidin; Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
A12379) for F-actin fluorescence staining for 30 min at room
temperature, washed several times with PBS and then stored in the
dark at 4°C. For vimentin fluorescence staining, cells were
permeabilized in 0.5% TritonX/PBS for 5 min and blocked with bovine
serum albumin (BSA; GenDEPOT Inc.) for 30 min at 21°C. Cells were
then incubated with primary antibodies [Vimentin (D21H3) XP Rabbit
mAb, 1:200, Cell Signaling Technology Inc.; cat. no. 5741] for 1 h
at 21°C. A secondary antibody of Alexa Fluor 555 goat anti-rabbit
IgG (H+L; Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
SA202-500) was used at a 1:500 dilution for 1 h at 21°C. A
fluorescence optical microscope (Nikon Ti-E; Nikon Corporation) was
used to detect rhodamine-phalloidin (excitation: ~495 nm; emission:
~518 nm) and Alexa Fluor 555 (excitation: ~555 nm; emission: ~565
nm).
Statistical analysis
All data were statistically analyzed and expressed
as mean ± standard deviation (SD). Analyses were performed using
one-way ANOVA and post hoc analysis of Tukey's test. The software
used for the statistical analysis was R program (2020-year; version
4.0.0; http://www.R–project.org/). P<0.05 was considered
to indicate a statistically significant difference.
Results
Knockdown of vimentin differentially
affects the growth of normal and cancer cells
Vimentin is a major intermediate filament protein
that serves an essential role in the stabilization of cellular
structures and migration. For vimentin knockdown, MCF10A and
MDA-MB-231 cells were transfected with siRNA for two days. Western
blotting confirmed partial knockdown of vimentin expression in both
cell lines (Figs. 1A and S1). Non-targeting siRNA was used as NC
group to provide a baseline for target gene silencing by
controlling for the non-specific effects associated with siRNA
delivery. Vimentin expression in siRNA-transfected (siVim) MCF10A
cells was reduced by ~50% compared with that in the NC group. In
MDA-MB-231 cells, vimentin expression was decreased by ~57%
following siRNA transfection.
The two cell types contracted slightly immediately
following vimentin deficiency. However, there was no significant
change in adhesion to the matrix after 4 h (Fig. 1B). To investigate whether the loss
of vimentin affects cell viability or proliferation, siVim cells
were cultured for two days and their viability was compared with
that of the NC group. Vimentin knockdown reduced the viability of
both cell lines (Fig. 1C and D).
There was no significant difference in cell viability after one day
of transfection in either cell type; however, the difference
increased over time. The viability of the NC group increased over
time in MCF10A cells, whereas the siVim group showed no significant
change (Fig. 1C). The viability of
MDA-MB-231 cells increased over time in both NC and siVim groups
(Fig. 1D). The difference in cell
viability between the NC and siVim groups was statistically
significant after 24 h for both MCF10A and MDA-MB-231 cells. These
results demonstrated that vimentin serves a role in cell
survival.
To quantitatively analyze the effect of vimentin
knockdown, cell viability after one and two days of siRNA
transfection was normalized to the viability on day zero. For
MCF10A cells, the recovery rate was 29 and 16% after 1 and 2 days,
respectively. In MDA-MB-231 cells, the recovery rate reached 83% on
the 1st day of transfection and decreased slightly on the 2nd day
(75%; Fig. 1E). These results
indicated that the proliferation of MCF10A cells was significantly
inhibited by vimentin deficiency, whereas the growth of MDA-MB-231
cells recovered over time.
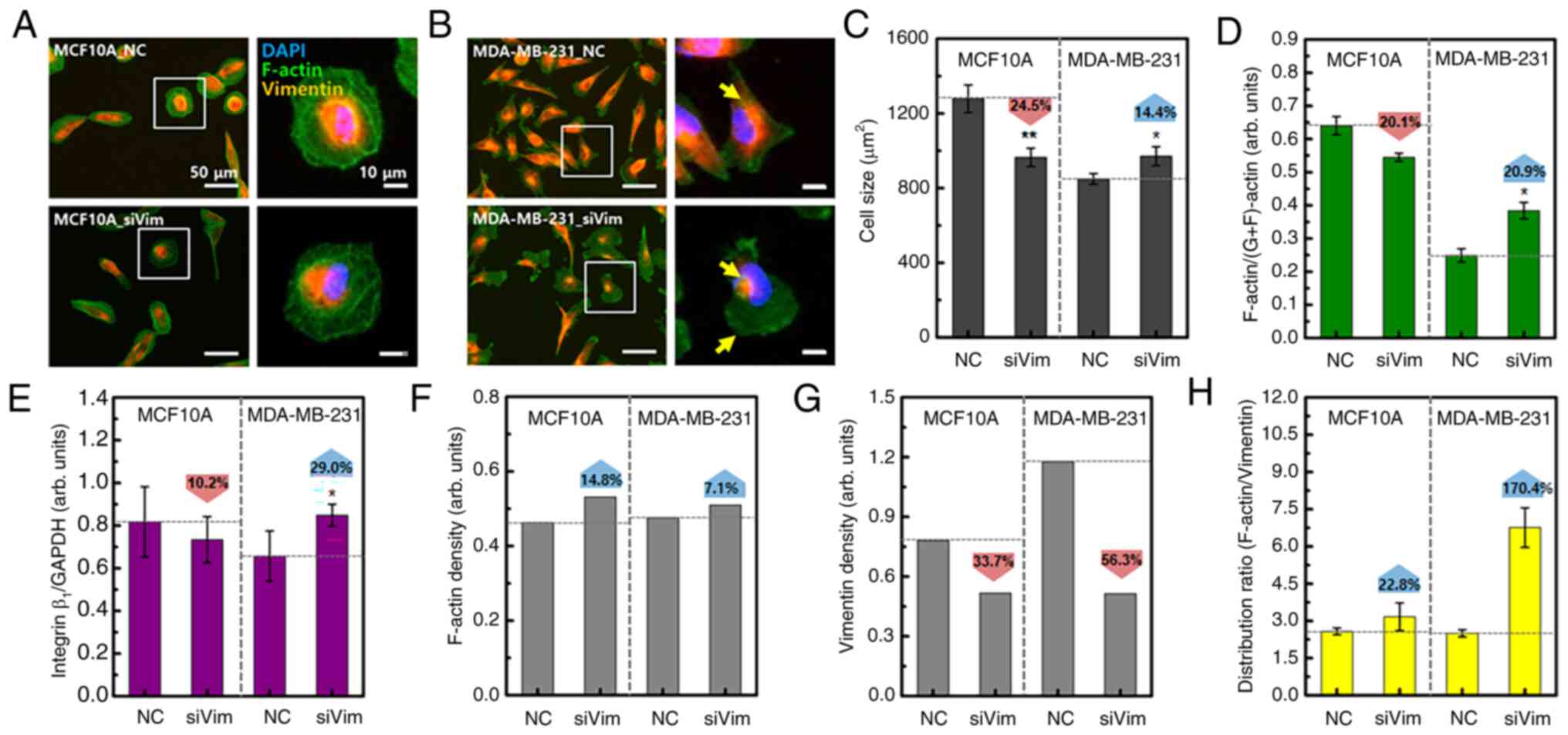
Rearrangement and content change of
F-actin to compensate for vimentin deficiency
Since F-actin serves an important role in the growth
and migration of cancer cells, together with vimentin, the effect
of vimentin deficiency on the distribution and content of F-actin
was analyzed. In MCF10A cells, there were no notable changes in the
cell shape or F-actin distribution due to vimentin deficiency
(Fig. 2A). By contrast, cells
exhibited rounded membrane edges were frequently observed in
MDA-MB-231_siVim cells (Fig. 2B).
Vimentin was mostly concentrated around the nucleus and F-actin was
widely distributed in MDA-MB-231_siVim membranes.
Vimentin knockdown decreased the cell size by 24.5%
in MCF10A cells and increased the cell size by 14.4% in MDA-MB-231
cells (Fig. 2C). MCF10A and
MDA-MB-231 cells also showed differences in the content changes of
F-actin and integrin β1. F-actin and integrin
β1 are directly correlated with respect to adhesion to
substrates required for cell migration. One side of the integrin
attaches to the ECM forming focal adhesions and the other side
connects to F-actin. As cells migrate, integrins repeatedly bind to
and dissociate from the ECM. Depletion of integrin β1
induces disruption of focal adhesions, resulting in inhibition of
cell migration (26). For this
reason, a quantitative experiment on integrin was performed.
Vimentin knockdown reduced the levels of F-actin and integrin
β1 in MCF10A cells and increased their levels in
MDA-MB-231 cells (Figs. 2D and E
and S2). Consequently, the F-actin
content relative to cell size increased after vimentin knockdown in
both cell types (Fig. 2F). Compared
with cell size, the vimentin content was reduced by 33.7% in MCF10A
cells and 56.3% in MDA-MB-231 cells (Fig. 2G). As the average F-actin or
vimentin content of the entire group was normalized to the average
cell size, statistical significance was not analyzed (Fig. 2F and G). After vimentin knockdown,
the intracellular distribution range of F-actin in MCF10A cells
increased by 22.8 and 170.4% in MDA-MB-231 cells, compared with the
vimentin distribution area (Fig.
2H). These results showed that the response to vimentin
deficiency was different in MCF10A and MDA-MB-231 cell models.
Normal cells appear to reduce in size to minimize the changes in
cytoskeletal proteins caused by vimentin knockdown. By contrast,
cancer cells appear to have an increased F-actin content to
compensate for vimentin deficiency.
Vimentin knockdown affects cellular
elasticity and motility
High-resolution AFM images were obtained for both
MCF10A and MDA-MB-231 cells to obtain the FD-curve under liquid
conditions. The image on the left in Fig. 3A depicts the topography of the
MDA-MB-231 cells and the image on the right shows the error image
of the topography. The error image is the output of the
differential amplifier, which is measured by tracking the probe on
the surface using optimal proportional-integral-differential (PID)
parameters. Instantaneous phase differences appear where the height
of the sample changes, allowing the nucleus and the cell body to be
more clearly distinguished. The error image is, therefore, helpful
in determining where to measure the FD-curve. The FD-curve was
obtained from the body parts of the cells to avoid the influence of
the nucleus and matrix. The measurement location of the FD curve
was determined from the error image. FD-curve was obtained at 10
different locations on each cell and ~20 different cells in each
group were measured.
The FD-curve for individual cells were extracted
using the magnitude of the load applied to the cell based on the
indentation depth of the AFM tip. The indentation depth was ~1,000
nm at a loading force of 5 nN. The FD-curve was analyzed using the
Sneddon model because a pyramid-shaped tip was used for the
measurements (27). Fig. 3B shows representative FD-curve
obtained from MCF10A_NC and MCF10A_siVim cells. The slope of the
FD-curve for MCF10A_siVim was larger than that for MCF10A_NC. A
large slope indicated high elasticity. By contrast, the slope of
the curve for the MDA-MB-231 cells decreased after siRNA
transfection (Fig. 3C). The Young's
modulus of MCF10A_NC (15.4±2.1 kPa) was higher than that of
MDA-MB-231_NC (12.6±2.9 kPa; Fig. 3D
and E). Notably, after vimentin knockdown, the elasticity was
significantly increased in MCF10A_siVim (30.0±4.1 kPa) but
decreased in MDA-MB-231_siVim (8.1±1.4 kPa). Opposite changes in
Young's modulus were observed with a decrease in F-actin.
Since vimentin is a cytoskeletal protein involved in
cell motility, the effect of vimentin loss on cell migration was
investigated. In MCF10A_NC cells, the leading edge of the cell
developed in one direction and the cell exhibited directional
movement (Fig. 3F; Video S1). There were no significant
changes in the motility of MCF10A cells following vimentin
deficiency. In MDA-MB-231_NC cells, the leading edge developed
uniaxially; therefore, the cell did not move in one direction.
Following vimentin deficiency, the leading edge developed in all
directions, making directional movements more difficult (Fig. 3F). Cell movement was tracked in 2D
space and traveling path was measured by scalar values
(xi, yi) at 1.0 min intervals based on the
nucleus (Fig. 3G). The directional
movement of MCF10A cells was clearly visible in the traveling path
observed for 4 h. MCF10A_NC and MCF10A_siVim cells both migrated
mainly in the y-direction, as shown in Fig. 3H. MDA-MB-231 cells did not move in a
specific direction and hovered at the same location. As expected,
the total migration distance of the MCF10A cells was greater than
that of the MDA-MB-231 cells (Fig.
3I). The MCF10A_NC migrated 38.8±11.8 µm and MDA-MB-231_NC
migrated 24.9±9.4 µm for 4 h, respectively. Migration distance was
decreased by vimentin knockdown in both cell lines and the rate of
decrease was slightly greater in MCF10A cells.
Effects of vimentin knockdown on
protrusion dynamics
Cell migration is governed by spatially and
temporally coordinated changes in the F-actin cytoskeleton. The
first step in cell migration is the generation of cell protrusions,
which are plasma membranes that expand in the direction of
migration. There are four types of protrusions: Lamellipodia,
filopodia, blebs and invadopodia (28). Each of these protrusions uniquely
contributes to migration, depending on specific circumstances, such
as the cell type and microenvironment.
The present study quantitatively analyzed the
effects of vimentin knockdown on protrusion dynamics using an
optical microscope. First, it was confirmed that the change in
brightness of the protrusion observed with a real-time optical
microscope was consistent with the fluorescence distribution of
F-actin (Fig. 4A). F-actin was
concentrated in the dark region of the continuously moving
protrusions. Therefore, moving protrusions along the membrane edge
were selected for the real-time analysis (Fig. 4B). The protrusions were dynamic. For
example, cells expand their lamellipodia for 100–400 sec after
external stimulation and then contract (29). Cells were imaged at 10 sec intervals
for 30 min and a total of 180 frames were recorded. Using an image
analysis program, the brightness change in the optical image was
converted into a numerical value (luma) (Fig. 4C). Luma was defined as the relative
luminance and showed the level of brightness in an image. Black in
the image corresponded to 0 luma and white corresponded to 250
luma. Areas with high accumulation of cytoskeletal and cytoplasmic
proteins appear dark and areas with low accumulation appear bright.
The F-actin appeared dark because it was relatively dense compared
with that of G-actin. Thus, a luma close to 0 and 250 indicated
more F- and G-actin, respectively. If the polymerization and
depolymerization of F-actin occurred periodically, the change in
image brightness could be expressed as a periodic function
(Fig. S3). In the periodic
function, the period represents the rate of actin
polymerization/depolymerization and the amplitude indicates the
content of F-actin or G-actin. Fig.
4D illustrates the variations in protrusion brightness as a
function of time in MCF10A cells. This was not perfect however; the
brightness varied periodically (Fig.
S3). The brightness changes in MDA-MB-231 cells showed more
periodic behavior with a larger amplitude (Fig. 4E). The period of MCF10A cells was
longer than that of MDA-MB-231 cells and was slightly increased by
vimentin knockdown in both cell lines (Fig. 4F). The amplitude of MDA-MB-231_NC
was 2.5 times larger than that of MCF10A_NC and was decreased by
vimentin knockdown (Fig. 4G).
These results indicated that the reduction in
vimentin affected the formation of protrusions and their mobility
in MDA-MB-231 cells. Protrusions are regulated by the relationship
between the intracellular F-actin and matrix (30). If the F-actin is coupled to the
matrix, newly polymerized F-actin can be converted into a
protrusion. However, even if F-actin was polymerized at the leading
edge of the cells, protrusions could not form if the F-actin was
not attached to the matrix. As shown in Fig. 3F, protrusions developed in all
directions in the MDA-MB-231_siVim cells, indicating that vimentin
knockdown accelerated the formation of protrusions. This change
could be related to the promotion of actin polymerization and
integrin β1 expression after vimentin knockdown, as
shown in Fig. 2D and E. However,
the formation rate and size of each protrusion decreased in
MDA-MB-231_siVim cells, which was consistent with the reduced
mobility shown in Fig. 3I.
Different responses to F-actin
depolymerization between cancer and normal cells
Based on the results of the vimentin-deficiency
experiment, it was hypothesized that the response to F-actin
reduction could differ between cancer cells and normal cells. To
test these hypotheses, F-actin content was reduced by 53% following
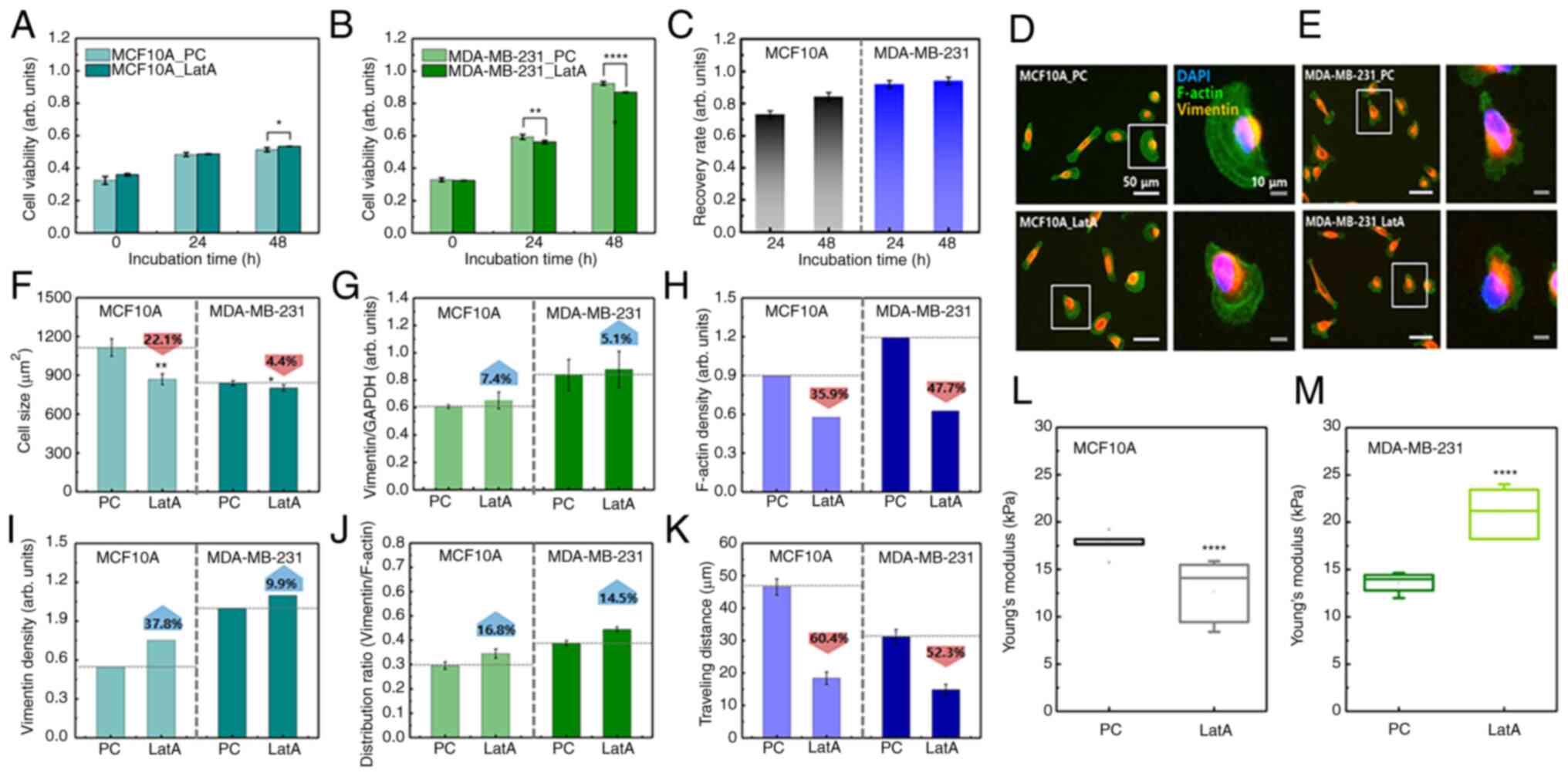
LatA treatment in both cell lines (Fig. S4) LatA-treated MCF10A cells did not
show a significant change in viability compared with the positive
control (PC) during long-term culture (48 h), whereas the viability
of LatA-treated MDA-MB-231 cells was lower than that of the control
group (Fig. 5A and B). Notably, PC
indicated untreated cells. After 48 h of incubation, the recovery
rates of MCF10A and MDA-MB-231 were 0.84 and 0.94, respectively
(Fig. 5C). This was a relatively
high recovery rate compared with that of vimentin-deficient cells,
indicating that the decrease in F-actin did not significantly
affect the survival of either cell. No significant change was
observed in the distribution of vimentin in LatA-treated cells
(Fig. 5D and E). MCF10A and
MDA-MB-231 cells both shrank rapidly after LatA treatment but
recovered within 4 h. Compared with the control, the cell size of
MCF10A_LatA decreased by 22.1%, whereas that of MDA-MB-231_LatA
slightly decreased by 4.4% (Fig.
5F). Vimentin content increased slightly in both LatA-treated
cell lines, but the increase was not statistically significant
(Fig. 5G). Following the LatA
treatment, the average F-actin content was divided by the mean cell
size. The F-actin density decreased in both cell lines. However,
this reduction was more pronounced in the MDA-MB-231 cells
(Fig. 5H). This notable decrease in
the number of MDA-MB-231 cells could be related to a small change
in cell size, as shown in Fig. 5F.
The density of vimentin increased by 37.8 and 9.9% in the
LatA-treated MCF10A and MDA-MB-231 cells, respectively (Fig. 5I). The intracellular distribution
range of vimentin, compared with that of F-actin in both cell types
after F-actin depolymerization, increased by 16.8 and 14.5% in
LatA-treated MCF10A and MDA-MB-231 cells, respectively (Fig. 5J). These results indicated that
there was no compensation for F-actin reduction in either cell
type. The migration distance was reduced by F-actin
depolymerization in both cell lines (Fig. 5K). In MCF10A, the distance traveled
over 4 h decreased from 46.5±2.5 µm to 18.4±1.9 µm due to reduced
F-actin. The traveling distance was decreased from 31.1±2.2 µm to
14.8±1.6 µm in MDA-MB-231. As F-actin was the most essential
component of cell migration, this result was predictable. The
change in cellular elasticity due to the decrease in F-actin showed
opposite results to those caused by vimentin deficiency. The
Young's modulus of MCF10A cells decreased by 34.6% owing to F-actin
reduction, whereas that of MDA-MB-231 cells increased by 36.9%
(Fig. 5L and M and S5).
Decrease in the recovery rate and
motility due to simultaneous deficiency of F-actin and
vimentin
The simultaneous depletion of vimentin and F-actin
significantly impaired cell growth and motility. Upon simultaneous
depletion of both cytoskeletal proteins, the viability of MCF10A
cells remained similar until 24 h and then decreased at 48 h
(Fig. 6A). MDA-MB-231 cells
proliferated slightly even after simultaneous depletion of vimentin
and F-actin (Fig. 6B). At 24 and 48
h, the viability of the MDA-MB-231 cells was higher than that at 0
h. The recovery rates of both normal and cancer cells according to
the depletion conditions of cytoskeletal proteins were interesting.
The viability of normal cells was not significantly affected by
F-actin reduction and gradually restored over time; however,
viability did not recover when vimentin was disrupted (Fig. 6C). Cell death occurred 48 h after
the disruption of both vimentin and F-actin. In the case of
MDA-MB-231 cells, when F-actin or vimentin was partially depleted
separately, the survival rate was hardly affected and the cells
grew to a level similar to that of the control group (Fig. 6D). Although MDA-MA-213 cells grew
even after simultaneous depletion of the two proteins, cell growth
was significantly inhibited compared with that in the PC and NC
groups. To evaluate which condition had the most significant effect
on cell migration, traveling distance was measured in cells treated
with different conditions of PC, LatA treatment and combined
treatment (siVim+LatA), separately. For comparison in all cases,
the traveling distances of NC and siVim cells were taken from
Fig. 3I. The migratory ability of
both MCF10A and MDA-MB-231 cells was significantly reduced by the
simultaneous deletion of vimentin and F-actin (Fig. 6E and F). F-actin depletion also had
a significant effect on the mobility of both cell types. The effect
of vimentin depletion on cell mobility was greater in MCF10A cells
than in MDA-MB-213 cells (Table
SI).
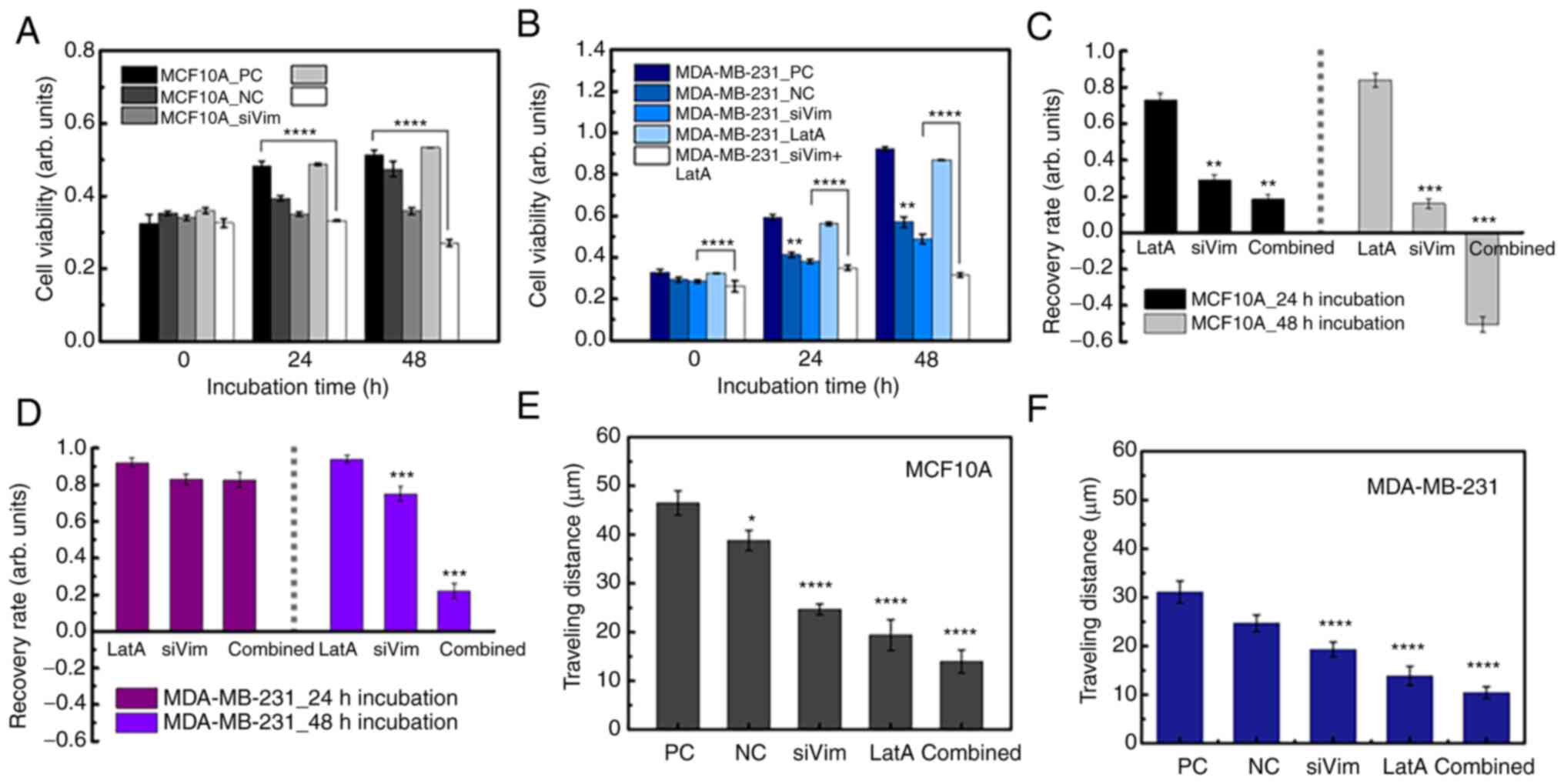 | Figure 6.Comparing the effects of vimentin and
F-actin deficiency on MCF10A and MDA-MB-231. Effects of
simultaneous depletion of vimentin and F-actin on cell viability in
(A) MCF10A and (B) MDA-MB-231 cells. Comparison of cell viability
recovery following the treatment conditions of (C) MCF10A and (D)
MDA-MB-231 cells. The recovery of MCF10A and MDA-MB-231 cells was
calculated from Fig. 6A and B,
respectively. Comparison of migration ability following the
treatment conditions of (E) MCF10A and (F) MDA-MB-231 cells. The
one-way ANOVA was performed on all groups and if significant
differences were observed (P<0.05), post hoc analysis using
Tukey's test was performed. The significant differences were
indicated based on the reference groups, PC (A, B, E and F) or Lat
A (C and D), highlighting the groups that exhibited significant
variations. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
vs. PC, positive control; NC, negative control; si, small
interfering. |
Discussion
Metastasis and therapeutic resistance are major
problems in breast cancer (25–29).
The present study investigated the effect of vimentin and F-actin
deficiency on the survival and function of metastatic breast cancer
cells and discovered some intriguing results (Fig. 7A).
In breast cancer cells, F-actin compensates for
vimentin deficiency. Vimentin is highly expressed in breast,
prostate, lung and malignant melanoma cancers (12). The upregulation of vimentin is
associated with poor prognosis (8,9).
Vimentin serves an important role in cancer metastasis by mediating
the maturation of focal adhesions and the reorganization of
cytoskeletal structures to maintain mechanical integrity during EMT
(31). When the amount of vimentin
in breast cancer cells was decreased by 57%, the amount of F-actin
increased by ~21%. The amount of integrin β1 and the
cell size also increased by 29 and 14%, respectively. This
indicated that actin polymerization is promoted to compensate for
the decrease in vimentin expression, leading to the expression of
integrins that link intracellular F-actin to the substrate,
resulting in improved cell adhesion to the substrate. In normal
cells, the expression of F-actin and integrin β1
decreases with a decrease in vimentin expression, leading to a
decrease in cell size. It is possible that the simultaneous
decrease in vimentin and F-actin levels impairs the ability of the
cell to maintain its structure and attach to the substrate.
Integrins are transmembrane proteins that connect
the extracellular matrix to the cytoskeleton. Integrins bind to
ligands in the extracellular matrix (ECM), such as fibronectin,
vitronectin, collagen and laminin and the ligands to which an
integrin can bind are determined by which α (integrin α) and β
(integrin β) subunits the integrin is composed of (32). Integrin β is primarily responsible
for forming focal adhesions on adherent cells and positioning
integrin heterodimers in the proper location on the cell.
Primarily, integrin β1 subunit predominantly bind to
tumor-upregulated ECM ligands, such as fibronectin, collagen I and
tenascin-C (33). Thus, integrin
β1 contributes to the stability of adhesion between
cells and the ECM of the tumor and facilitates cell migration via a
reciprocal signaling system with F-actin. The cytoplasmic domain of
integrin β1 binds to F-actin. In breast cancer cells,
the multiple downstream signaling pathways of β1,
including FAK, PI3K and ERK/MAPK, coordinating signaling through
receptor tyrosine kinases, are involved in the modulation of tumor
initiation, progression and ultimately metastasis (26). FAK acts as a scaffolding protein by
interacting with SH3 domain-containing proteins, including p130Cas
and endophilin A2 (34). In
particular, its interaction with p130Cas has been demonstrated to
be important in regulating cell migration and breast cancer
progression (35). FAK
autophosphorylation at tyrosine 397 and PI3K activation inhibits
apoptosis of cells by activating Akt kinase (36,37).
FAK is also involved in cell cycle regulation by forming FAK/Src
complexes at focal contacts, which activate ERK and cyclin D1 but
inhibit p21 expression (38). In
the present study, F-actin content was manipulated, so to
understand the altered motility of cancer cells, changes in the
content of integrins were identified based on F-actin content.
Thus, it appears that the increased expression of integrins due to
a decrease in vimentin contributes to stable cell adhesion, which
quickly restores cell viability in breast cancer cells.
When the F-actin content was reduced by 57% in both
normal and cancer cells, the amount of vimentin increased slightly
to 7.4 and 5.1% in MCF10A and MDA-MB-213 cells, respectively. The
reduction in F-actin levels did not significantly impair the
survival of either cell type. The size of the normal cells
decreased by 27%, but the size of the cancer cells did not change
significantly. As aforementioned, the relative proportion of
F-actin is lower in cancer cells than in normal cells because the
amount of vimentin increases during malignant transformation,
whereas the amount of F-actin decreases (39,40).
Therefore, a reduction in F-actin levels in cancer cells is not
thought to have a significant effect on cell morphology (Fig. 7B). However, the migration distance
was substantially reduced in both cell types by >50%. As
aforementioned, cells migrate through actin successive
polymerization and depolymerization. Therefore, the decrease in
migration distance can be easily understood as the inhibition of
mobility due to F-actin reduction. These results suggested that
actin-targeted therapies are not very effective at killing cancer
cells but are very effective at inhibiting the motility of
metastatic cancer cells. Thus, combining toxic drugs with high
selectivity for cancer cells with actin-targeted therapies may
synergistically affect metastatic cancer cells.
While the elasticity of normal cells depends on
F-actin density, the elasticity of cancer cells appears to depend
primarily on vimentin density. Cancer cells are less elastic than
normal cells. This was associated with reduced F-actin levels
during malignant transformation. Some studies have shown that low
elasticity is advantageous for cancer migration (41–45).
However, conflicting results have been reported in which cells with
high elasticity migrate more readily (46). The correlation between cellular
elasticity and migration remains controversial; however, it is
clear that lower elasticity favors invasion. To metastasize, cancer
cells change their elasticity through morphological and phenotypic
conversions and then detach from the primary tissues (47). Cells that have acquired motility
enter lymphatic vessels or bloodstream and are seeded into distant
organs. If the cellular elasticity is low, the cell can easily
deform. Therefore, the low elasticity of cancer cells is
advantageous for invasion. Cellular elasticity is regulated by
F-actin. When the content of F-actin is high or its network is
dense and well-organized, the elasticity of the cells increases.
The elasticity of MCF10A and MDA-MB-231 cells is altered by
vimentin knockdown and F-actin depolymerization. However, this
change was not related to F-actin content. As the cell size also
changed due to the decrease in vimentin and F-actin content, the
elasticity was compared with the density of the two filaments.
Notably, the elastic change in MCF10A cells coincided with the
change in F-actin density, whereas the elastic change in MDA-MB-231
cells followed a change in vimentin density. Based on these
results, it was hypothesized that F-actin could serve a major role
in the elasticity of normal cells and that vimentin could serve a
key role in cancer cells.
In summary, reduction in vimentin or F-actin levels
did not substantially affect the growth or migration of breast
cancer cells. However, the simultaneous depletion of F-actin and
vimentin had a more notable effect on the survival and motility of
breast cancer cells. The cytoskeleton is considered the backbone of
a cell, as it provides the cell with its shape and structure and
facilitates cellular movements. The three cytoskeletal
proteins-actin filaments, intermediate filaments and
microtubules-have unique roles and share functions as skeletal
organelles. To date, most cytoskeleton-targeting drugs developed
typically target a single protein. The results of the present study
showed that drugs targeting metastatic cancer cells are effective
in simultaneously reducing vimentin and F-actin levels.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (grant no. 2021R1I1A1A01055772) funded by the
Korean government (MSIT).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SK and KK conceived the study and prepared the
first draft of the manuscript. SK and SH conducted the experiments
and analyzed the data. SK and SH critically reviewed and revised
the manuscript. SK and KK confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin Z, Han Y, Wu B and Fang W: Altered
cytoskeletal structures in transformed cells exhibiting obviously
metastatic capabilities. Cell Res. 1:141–51. 1990. View Article : Google Scholar
|
|
2
|
Fletcher DA and Mullins RD: Cell mechanics
and the cytoskeleton. Nature. 463:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang DD and Gerlach BD: The roles and
regulation of the actin cytoskeleton, intermediate filaments and
microtubules in smooth muscle cell migration. Respir Res.
18:542017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun B, Fang Y, Li Z, Chen Z and Xiang J:
Role of cellular cytoskeleton in epithelial-mesenchymal transition
process during cancer progression. Biomed Rep. 3:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Battaglia RA, Delic S, Herrmann H and
Snider NT: Vimentin on the move: New developments in cell
migration. F1000Res. 7:17962018. View Article : Google Scholar
|
|
8
|
Wu S, Du Y, Beckford J and Alachkar H:
Upregulation of the EMT marker vimentin is associated with poor
clinical outcome in acute myeloid leukemia. J Transl Med.
16:1702108. View Article : Google Scholar
|
|
9
|
Tadokoro A, Kanaji N, Liu D, Yokomise H,
Haba R, Ishii T, Takagi T, Watanabe N, Kita N, Kadowaki N and
Bandoh S: Vimentin regulates invasiveness and is a poor prognostic
marker in non-small cell lung cancer. Anticancer Res. 36:1545–1551.
2106.PubMed/NCBI
|
|
10
|
Kwon S, Yang W, Moon D and Kim KS:
Comparison of cancer cell elasticity by cell type. J Cancer.
11:5403–5412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Kierfeld J and Lipowsky R: Actin
polymerization and depolymerization coupled to cooperative
hydrolysis. Phys Rev Lett. 103:0481022009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng GE, Wilson SR and Weiner OD: A
pharmacological cocktail for arresting actin dynamics in living
cells. Mol Biol Cell. 22:3986–3994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stehn JR, Haass NK, Bonello T, Desouza M,
Kottyan G, Treutlein H, Zeng J, Nascimento PRBB, Sequeira VB,
Butler TL, et al: A novel class of anticancer compounds targets the
actin cytoskeleton in tumor cells. Cancer Res. 73:5169–5182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang K, Xin Y, Li K, Chen X and Tan Y:
Cell cytoskeleton and stiffness are mechanical indicators of
organotropism in breast cancer. Biology (Basel).
10:2592021.PubMed/NCBI
|
|
16
|
Gandalovičová A, Rosel D, Fernandes M,
Veselý P, Heneberg P, Čermák V, Petruželka L, Kumar S, Sanz-Moreno
V and Brábek J: Migrastatics-anti-metastatic and anti-invasion
drugs: Promises and challenges. Trends Cancer. 3:391–406. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Brit J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atteeq M: Evaluating anticancer properties
of withaferin A-a potent phytochemical. Front Pharmacol.
13:9753202022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagy Z, Cheung BB, Tsang W, Tan O, Herath
M, Ciampa OC, Shadma F, Carter DR and Marshall GM: Withaferin A
activates TRIM16 for its anti-cancer activity in melanoma. Sci Rep.
10:197242020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burikhanov R, Sviripa VM, Hebbar N, Zhang
W, Layton WJ, Hamza A, Zhan CG, Watt DS, Liu C and Rangnekar VM:
Arylquins target vimentin to trigger Par-4 secretion for tumor cell
apoptosis. Nat Chem Biol. 10:924–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheratta AR, Thayyullathil F,
Pallichankandy S, Subburayan K, Alakkal A and Galadari S: Prostate
apoptosis response-4 and tumor suppression: It's not just about
apoptosis anymore. Cell Death Dis. 12:472021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giganti A and Friederich E: The actin
cytoskeleton as a therapeutic target: State of the art and future
directions. Prog Cell Cycle Res. 5:511–525. 2003.PubMed/NCBI
|
|
23
|
Cramer LP: Role of actin-filament
disassembly in lamellipodium protrusion in motile cells revealed
using the drug jasplakinolide. Curr Biol. 9:1095–1105. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strouhalova K, Přechová M, Gandalovičová
A, Brábek J, Gregor M and Rosel D: Vimentin intermediate filaments
as potential target for cancer treatment. Cancers (Basel).
12:1842020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han R and Chen J: A modified Sneddon model
for the contact between conical indenters and spherical samples. J
Mater Res. 36:1762–1771. 2021. View Article : Google Scholar
|
|
26
|
Hou S, Isaji T, Hang Q, Im S, Fukada T and
Gu J: Distinct effects of integrin β1 on cell
proliferation and cellular signaling in MDA-MB-231 breast cancer
cells. Sci Rep. 6:184302016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vinckier A and Semenza G: Measuring
elasticity of biological materials by atomic force microscopy. FEBS
Lett. 430:12–16. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gagliardi PA, Puliafito A, Blasio L,
Chianale F, Somale D, Seano G, Bussolino F and Primo L: Real-time
monitoring of cell protrusion dynamics by impedance responses. Sci
Rep. 5:102062015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilson K, Lewalle A, Fritzsche M,
Thorogate R, Duke T and Charras G: Mechanisms of leading-edge
protrusion in interstitial migration. Nat Commun. 4:28962013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duarte S, Viedma-Poyatos Á,
Navarro-Carrasco E, Martínez AE, Pajares MA and Pérez-Sala D:
Vimentin filaments interact with the actin cortex in mitosis
allowing normal cell division. Nat Commun. 10:42002019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mezu-Ndubuisi OJ and Maheshwari A: The
role of integrins in inflammation and angiogenesis. Pediatr Res.
89:1619–1626. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Molecular biology of the cell. 4th edition.
New York: Garland Science; 2002
|
|
34
|
Luo M and Guan JL: Focal adhesion kinase:
A prominent determinant in breast cancer initiation, progression
and metastasis. Cancer Lett. 289:127–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gemperle J, Dibus M, Koudelková L, Rosel D
and Brábek J: The interaction of p130Cas with PKN3 promotes
malignant growth. Mol Oncol. 13:264–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Madan R, Smolkin MB, Cocker R, Fayyad R
and Oktay MH: Focal adhesion proteins as markers of malignant
transformation and prognostic indicators in breast carcinoma. Hum
Pathol. 37:9–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guan JL and Shalloway D: Regulation of
focal adhesion-associated protein tyrosine kinase by both cellular
adhesion and oncogenic transformation. Nature. 358:690–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan JL: Integrin signaling through FAK in
the regulation of mammary stem cells and breast cancer. IUBMB Life.
62:268–276. 2010.PubMed/NCBI
|
|
39
|
Hahm ER, Mathan SV, Singh RP and Singh SV:
Breast cancer selective disruption of actin cytoskeleton by diallyl
trisulfide. J Cancer Prev. 27:101–111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng Z, Li Z, Xie M, Yu H, Jiang L and Yao
X: TM9SF4 is an F-actin disassembly factor that promotes tumor
progression and metastasis. Nat Commun. 13:57282022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mierke CT: Mechanical cues affect
migration and invasion of cells from three different directions.
Front Cell Dev Biol. 8:5832262020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jonietz E: Mechanics: The forces of
cancer. Nature. 491:S56–S57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alibert C, Goud B and Manneville JB: Are
cancer cells really softer than normal cells? Biol Cell.
109:167–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu W, Mezencev R, Kim B, Wang L, McDonald
J and Sulchek T: Cell stiffness is a biomarker of the metastatic
potential of ovarian cancer cells. PLoS One. 7:e466092012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim TH, Gill NK, Nyberg KD, Nguyen AV,
Hohlbauch SV, Geisse NA, Nowell CJ, Sloan EK and Rowat AC: Cancer
cells become less deformable and more invasive with activation of
β-adrenergic signaling. J Cell Sci. 129:4563–4575. 2016.PubMed/NCBI
|
|
46
|
Sahai E: Illuminating the metastatic
process. Nat Rev Cancer. 7:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis-molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|