Introduction: Epidemiology, clinical
presentation, diagnosis and treatment
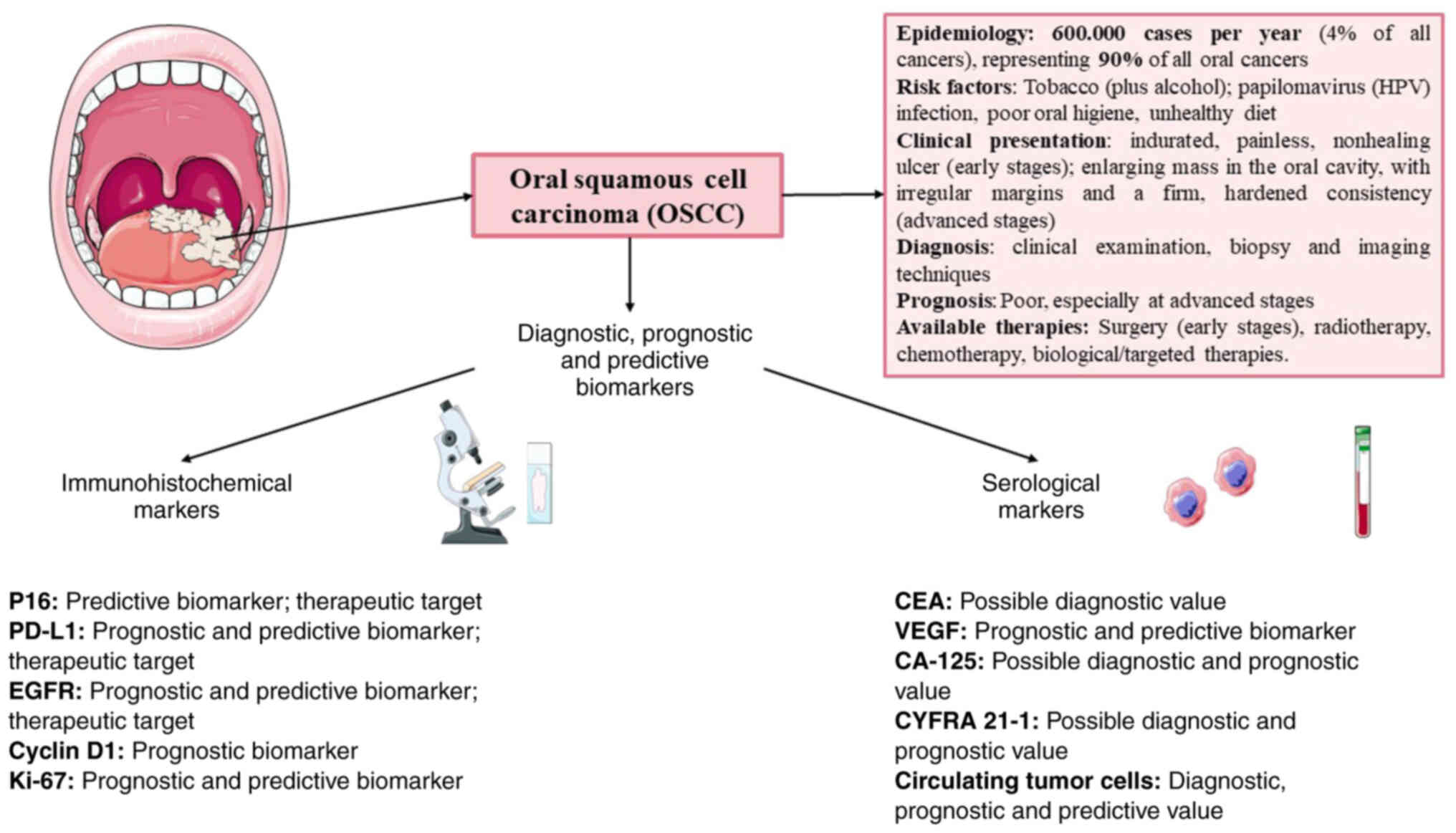
Oral squamous cell carcinoma (OSCC) affects ~600,000
patients per year, accounting for ~4% of all cancer cases, and this
disease has high cancer-related morbidity and mortality rates. The
overall 5-year survival rate for OSCC remains as low as ~50–60%,
with early-stage cancers having a considerably better prognosis
than advanced-stage tumours. The presence of regional lymph node
metastasis is a key predictor of survival, with 5-year survival
rates dropping to ~30–40% in such cases (1,2). OSCC
is the most common type of oral cancer, accounting for >90% of
all oral malignancies. It should be noted that the global incidence
and mortality rates of OSCC vary significantly across geographic
regions, with higher rates observed in South Asia, Southeast Asia
and Europe than in other regions. OSCC affects men more frequently
than women and its incidence increases with age (3). These variations in OSCC burden across
populations may be attributed to differences in exposure to risk
factors, genetic predisposition and health care access.
It should be highlighted that several risk factors
have been identified for OSCC, including smoking or smokeless
(chewing) tobacco use, which is the leading risk factor for OSCC,
contributing to ~75% of all cases (4). Other risk factors that may be
highlighted are excessive alcohol intake, which is an independent
risk factor for OSCC. The combined effect of tobacco and alcohol
use exponentially increases the risk of dysplasia and cancer, and
it should be noted that in recent years, the importance of human
papillomavirus (HPV) infection in the oral cavity, which has been
recognized as an aetiological factor for squamous neoplasia,
primarily affecting the oropharynx, has been observed. While most
HPV infections are asymptomatic and transient, there are high-risk
HPV types, such as HPV-16 and HPV-18, that have been linked with
the development of various malignancies, including cervical,
anogenital and oropharyngeal cancers, and have an important role in
OSCC (5,6). It should be noted that the risk
factors for HPV-positive OSCC are different from those of
HPV-negative OSCC; the former is more frequently associated with
younger age, fewer lifetime sexual partners, and a lower prevalence
of tobacco and alcohol use (7)
Other risk factors, such as poor oral hygiene and diets low in
fruits and vegetables, which have been linked to an increased risk
of chronic inflammation and cancer, are not as important as
previous factors, but have an important role in OSCC, increasing
the risk of squamous dysplasia in the oral cavity (8).
The clinical presentation of OSCC may vary among
patients. Early-stage OSCC may present with nonspecific signs and
symptoms, such as an indurated, painless, nonhealing ulcer, which
is one of the most common early signs of OSCC (9). The ulcer may be accompanied by raised,
rolled or everted edges and may have irregular margins. Other early
signs include the presence of leukoplakia and erythroplakia (the
latter with a higher risk of malignant transformation), which may
be precursors of OSCC or represent early-stage lesions (10). Patients may also report oral pain,
discomfort or a burning sensation that may be persistent or
intermittent and associated with unexplained tooth mobility or
tooth changes. In cases involving the base of the tongue or
oropharynx, this may present as persistent sore throat or
hoarseness (11).
On the other hand, patients with advanced OSCC may
have an enlarging mass or growth in the oral cavity, which is often
firm with irregular margins. This is a characteristic finding in
advanced OSCC associated with dysphagia, odynophagia or dysarthria
due to tumour infiltration or obstruction of the oral cavity or
oropharynx. It may also present as facial swelling or asymmetry
with involvement of cervical lymphadenopathy (12).
It is important to note that the diagnosis of OSCC
is based on clinical examination, biopsy and imaging techniques to
determine the extent of the disease and histologic confirmation of
malignancy. The first step is clinical examination with visual
inspection of the oral cavity and oropharynx, which may identify
lesions suggestive of OSCC, such as leukoplakia and erythroplakia,
palpation of the oral cavity, and evaluation of cranial nerves to
identify any neurologic deficits that may indicate tumour
infiltration or compression of adjacent nerves. It is important to
emphasize that a biopsy is required to confirm the diagnosis of
OSCC by histopathologic analysis (13).
Of note, symptom-based panendoscopy (laryngoscopy,
bronchoscopy and oesophagoscopy) may be performed in the first
assessment and the incidence rate of second primary upper
aerodigestive tract tumours with this method is 2.4 to 4.5%; this
method may also provide information about synchronous lesions. On
the other hand, fine-needle aspiration biopsy (FNAB) combined with
ultrasound has an important role in the evaluation of cervical
lymph nodes and subsequent cytological evaluation is performed to
assess suspicious lymph nodes (14). FNAB has proven to be an invaluable
diagnostic tool in the management of OSCC, particularly in the
evaluation of suspicious cervical lymph nodes, with a diagnostic
accuracy of 89–98%, and may be used to evaluate potential
recurrence or residual disease in patients with OSCC after
treatment, particularly when imaging results are inconclusive
(15). It may also be performed
with excisional biopsy of the lesion with a surrounding margin of
healthy tissue in easily accessible lesions and may be used both
diagnostically and therapeutically in selected patients.
Imaging studies have a critical role in the
diagnosis and staging of OSCC by providing detailed information
about the size of the tumour, its location and possible invasion of
adjacent structures. This usually includes computed tomography
(CT), which is useful for assessing bone invasion, regional
lymphadenopathy and distant metastases. Contrast-enhanced CT scans
may improve the visualization of soft tissue involvement and
vascular structures and positron emission tomography (PET) is
frequently combined with CT (PET-CT) to perform OSCC staging,
detect regional lymph node involvement and identify distant
metastases or synchronous primary tumours (16). In this context, it should be
emphasized that magnetic resonance imaging (MRI) provides a better
soft-tissue resolution than CT, making it the tool of choice for
assessing tumour extension, perineural spread and involvement of
vital structures, such as nerves and blood vessels. MRI is also
useful for distinguishing tumour recurrence from posttreatment
changes such as fibrosis (17).
With regard to metastatic disease, it is important to assess
distant invasion in OSCC. The incidence of metastatic disease
ranges from 2 to 26% and varies according to locoregional extent,
lymphatic involvement and histologic grade (18). Typically, distant metastases are
asymptomatic and the most common sites are the lungs, liver and
bones, with the CT scan being the most sensitive method for
screening metastases in patients with OSCC, revealing malignant
findings in 4–19% of newly diagnosed cases (19).
In terms of treatment, advances in diagnostic and
therapeutic approaches over the years have improved patient
outcomes, but the prognosis remains poor, particularly in
advanced-stage cases. Current treatment strategies for OSCC are
based on locoregional and distant disease classification according
to the TNM staging classification system, which was updated in 2018
by the American Joint Committee on Cancer and the Union for
International Cancer Control (20).
The primary treatment for early-stage OSCC remains surgery, which
includes wide local excision of the tumour and lymphadenectomy with
reconstructive surgery when appropriate. It is effective, but
potential complications include difficulty swallowing, speech
problems and aesthetic issues that may affect patients' quality of
life (21). In certain cases,
radiation therapy may be used as a complementary treatment after
surgery or as the only treatment for patients who are not
candidates for surgery, and it includes intensity-modulated
radiotherapy and stereotactic body radiotherapy, which aim to
deliver high doses of radiation to the tumour while sparing
surrounding normal tissue but are usually associated with acute
toxicities, such as mucositis, xerostomia and dysphagia (22,23).
Chemotherapies such as cisplatin, carboplatin and 5-fluorouracil
are often used in conjunction with radiotherapy and immunotherapy
for locally advanced cases and are preferred for symptomatic
patients (24). In recent years,
the importance of various biomarkers has been highlighted, which
has enabled the development of targeted agents and immunotherapies.
For instance, agents targeting the epidermal growth factor receptor
(EGFR), such as cetuximab, have shown promise in improving survival
when combined with radiotherapy and recently, immune checkpoint
inhibitors (ICIs) have also shown promise. ICIs such as
pembrolizumab and nivolumab have been approved for
relapsed/metastatic OSCC and represent the first-line treatment for
locoregional and metastatic disease in patients with OSCC (25–27).
In conclusion, OSCC is a complex disease that
requires a multifaceted approach to its prevention, diagnosis and
treatment. The introduction of new diagnostic tools and continuing
advances in treatment modalities require ongoing research and
clinical trials, with the aim of improving early detection,
optimizing treatment and increasing survival rates and quality of
life for patients with OSCC.
Role of immunohistochemical biomarkers
Currently, one of the most promising approaches to
improve OSCC treatment is the identification and application of
histological biomarkers. These biomarkers may provide information
regarding prognosis, predict the response to treatment and monitor
disease progression or recurrence. In the context of OSCC,
histological biomarkers may provide a deeper understanding of the
biological behaviour of the tumour, which is important for
understanding the underlying pathophysiology of the tumour and
facilitates clinical decision making and personalized treatment.
All of these biomarkers are summarised in Table I and Fig. 1.
 | Table I.Summary of different biomarkers in
oral squamous cell carcinoma. |
Table I.
Summary of different biomarkers in
oral squamous cell carcinoma.
| Biomarker | Type of
analysis | Utility | (Refs.) |
|---|
| p16 |
Immunohistochemical | Prognosis | (31–35) |
| PD-L1 |
Immunohistochemical | Treatment,
prognosis | (36–42) |
| EGFR |
Immunohistochemical | Treatment,
prognosis | (43–47) |
| Cyclin D1 |
Immunohistochemical | Treatment,
prognosis | (50–56) |
| Ki-67 |
Immunohistochemical | Prognosis | (58–65) |
| CEA | Serological | Diagnosis,
prognosis | (66–68) |
| VEGF | Serological,
immunohistochemical | Diagnosis,
prognosis | (70–73) |
| CA-125 | Serological | Diagnosis,
prognosis | (75–78) |
| CYFRA 21-1 | Serological | Diagnosis,
prognosis | (81–83) |
| Circulating tumor
cells | Serological | Diagnosis,
prognosis, treatment | (85–91) |
| Salivary
biomarkers | Multimodal use of
cytokines and proteins. | Diagnosis,
prognosis | (84–99) |
Among the various histological biomarkers available
in OSCC are a number of molecular alterations in proteins or genes
related to cell proliferation, apoptosis, angiogenesis, immune
response and metastatic invasion. In recent years, several
potential histological biomarkers of p16INK4A have been
identified and their functions in OSCC have been studied in detail.
These include markers of cell cycle regulation
(p16INK4A, cyclin D1), EGFR, cell proliferation (Ki-67),
immune checkpoint proteins [programmed cell death 1 (PD-1) ligand 1
(PD-L1)] and others involved in tissue invasion and metastasis,
such as metalloproteinases (28).
p16
The protein p16INK4A or p16, encoded by
the cyclin-dependent kinase (CDK) inhibitor 2A gene, has a critical
role in the regulation of the cell cycle by inhibiting CDK4 and
CDK6, which are essential for the transition from the G1 phase to
the S phase of the cell cycle. Dysregulation of p16INK4A
is frequently observed in numerous types of cancer, including OSCC
(29).
HPV expresses the oncoproteins E6 and E7, which
deactivate tumour suppressor proteins. The E6 protein induces the
degradation of P53, whose function is to halt cell division at the
G1 level and induce apoptosis through ubiquitin-mediated
proteolysis, inactivating its activity (30–33).
Cells expressing E6 are unable to respond to DNA damage signaling
mediated by P53 and are thus susceptible to genomic instability.
The E7 protein binds and inactivates the retinoblastoma tumour
suppressor gene product (pRB), causing the cell to enter S phase,
leading to cell cycle disruption, proliferation and malignant
transformation. Inactivation of pRB leads to upregulation of
p16INK4a as a cellular response to uncontrolled cell
cycle progression (30).
Its importance as a prognostic marker is related to
HPV infection. The prevalence of HPV in patients with OSCC has been
examined in various research studies. According to Vergori et
al (30), HIV-positive
individuals are more likely to harbour HPV in the oral cavity than
HIV-negative individuals.
A relevant study on an HIV-infected population have
indicated an overall prevalence of HPV DNA in the oral cavity
ranging from 20 to 45%, with the oncogenic type HPV16 found in 12
to 26% of cases (31). A study by
Castro and Filho (32) found that
HPV is an independent risk factor for OSCC. The prevalence of HPV
in oral cancer, particularly SCC, was observed to be higher for
HPV16 (32). One of the most
significant studies regarding the relevance of p16 in OSCC is a
meta-analysis of 31 studies including ~5,000 patients conducted by
Katirachi et al (33). The
overall prevalence of HPV+ OSCC in this meta-analysis was ~6% (95%
CI; 3–10%), and only one study identified a significant correlation
between HPV and OSCC (33).
From a morphological perspective, it has been
observed that HPV+ OSCC originates more frequently in the tonsillar
crypts and presents more often in advanced stages (III or IV) than
HPV-OSCC. On the other hand, the prognostic utility of p16 in OSCC
differs among studies given its low prevalence, although according
to studies such as that by Doll et al (34), P16 expression is connected to a
better survival rate in individuals undergoing primary surgery with
adjuvant radio(chemo)therapy (34).
However, meta-analyses such as that of Christianto et al
(35), which evaluated 22 articles
comprising 3,065 patients with OSCC, observed that HPV-positive
OSCC is associated with significantly decreased survival.
Therefore, dysregulation of p16INK4A is
associated with various types of malignancies, including OSCC,
where its importance is directly associated with HPV infection. As
mentioned above, several studies have examined the prevalence of
HPV in OSCC and found considerably high rates of p16 positivity.
While certain reports suggest better outcomes for patients with
P16-expressing OSCC undergoing specific therapies, meta-analyses
offer a more nuanced perspective and indicate decreased outcomes in
HPV-positive cases. This intricate interplay underscores the need
for specialized research and approaches. Understanding these
processes has the potential to offer more effective defences
against OSCC, reflecting the evolving nature of cancer
treatment.
PD-L1
PD-L1, also known as CD274 or B7-H1, is a protein
that has an important role in oncology, particularly in the context
of immunotherapy. In OSCC, the role of PD-L1 is increasingly
recognized as crucial, not only in terms of understanding tumour
biology but also for determining prognosis and establishing
therapeutic strategies. PD-L1 is typically expressed on the surface
of certain cells, including cancer cells, where it interacts with
its receptor, PD-1, found on T cells (36). This interaction inhibits the immune
response and allows cancer cells to evade the immune system. This
mechanism of immune evasion is particularly important in OSCC, as
there is growing evidence that PD-L1 expression is associated with
more aggressive disease features, such as advanced tumour stage,
nodal metastases and unfavourable overall survival. In recent
years, immune checkpoint inhibitors that block this interaction
have shown promising results in various cancer types, including
OSCC (37,38). This is because PD-L1 expression
appears to be regulated by multiple signalling pathways, including
MAPK, PI3K and Akt/PKB, which are commonly altered in head and neck
carcinoma. As a consequence of these molecular interactions, it is
a dynamic biomarker that is subject to variations (39).
In the last decade, several immune checkpoint
inhibitors targeting PD-1 or PD-L1 have been approved for the
treatment of recurrent or metastatic OSCC. Among these drugs are
pembrolizumab and nivolumab (targeting PD-1) and atezolizumab and
durvalumab (targeting PD-L1). These inhibitors have shown promising
results in terms of response rates and survival, particularly in
patients with PD-L1-positive tumours, changing the treatment
paradigm for this patient population. For instance, pembrolizumab
was approved as a first-line treatment for relapsed or metastatic
OSCC with a PD-L1 combined positive score of 1 or higher according
to the KEYNOTE-048 trial (40,41).
This study demonstrated that pembrolizumab improved overall
survival compared to that with the standard therapy EXTREME
(cetuximab with platinum-based chemotherapy) (42).
Therefore, PD-L1+ tumours tend to exhibit better
response rates to anti-PD-1/PD-L1 therapies compared to
PD-L1-tumours (40). It is
important to note that, albeit to a lesser extent, PD-L1-tumours
also benefit from these treatments (41), indicating the need to consider
additional factors, such as HPV status or tumour mutational burden,
when initiating these treatments.
However, studies such as CHECKMATE-141 failed to
show a significant association between PD-L1 expression and tumour
response or survival when evaluating nivolumab in
platinum-resistant patients (42).
The lack of concordance in these results obtained from different
studies may be explained by the heterogeneity in biomarker
expression, as well as the lack of uniformity in assays and the
variability in thresholds used to define PD-L1 positivity, leading
to the establishment of harmonization projects for PD-L1 assays by
the scientific community and regulatory bodies (41,42).
Anti-PD-L1 therapies, such as atezolizumab and
durvalumab, are also being studied at various stages of clinical
trials for OSCC and are showing promising results. Despite these
advances, overall response rates to monotherapies against
PD-1/PD-L1 remain relatively modest, underscoring the need for
strategies to improve their efficacy (43). The combination of immune checkpoint
inhibitors with other therapies, such as cytotoxic chemotherapy,
radiotherapy or other immunotherapeutic agents, is a promising
approach under active investigation (44).
EGFR
EGFR is a transmembrane receptor that belongs to the
ErbB family of receptor tyrosine kinases. This receptor has a
critical role in the pathogenesis and progression of several
cancers, including OSCC. EGFR is frequently overexpressed in OSCC
and its upregulation is associated with aggressive tumour
behaviour, increased invasiveness and poor prognosis (45). In this sense, it correlates with
advanced tumour stage, lymph node metastasis and lower overall
survival rates. Therefore, the determination of EGFR expression
levels in OSCC may serve as a valuable prognostic indicator to aid
in treatment planning and patient management (46).
EGFR is not only a prognostic marker but also an
emerging therapeutic target in OSCC. Several strategies, including
monoclonal antibodies (such as cetuximab) and small molecule
tyrosine kinase inhibitors (such as erlotinib), have been developed
to inhibit EGFR signalling. Cetuximab has been shown to be
clinically effective in combination with radiotherapy or
chemotherapy for locally advanced OSCC, leading to improved
survival rates. It is important to highlight that in recent years,
EGFR therapies have been shown to have the potential to sensitize
tumours to other treatments, improve the efficacy of chemotherapy
and radiotherapy, and overcome treatment resistance (47). Related to treatment resistance,
several molecular alterations, such as EGFR gene amplification,
mutations and activation of downstream signalling pathways, remain
a major challenge in these patients and represent one of the main
limitations of these therapies (48).
EGFR has a critical role in the pathogenesis and
progression of OSCC. Its overexpression is associated with
aggressive tumour behaviour and poor prognosis. Targeting EGFR
represents a promising therapeutic approach for OSCC that may
improve treatment outcomes. However, further research is needed to
refine patient selection criteria, overcome resistance mechanisms
and optimize treatment strategies to fully realize the therapeutic
potential of EGFR inhibition in OSCC.
Cyclin D1
Cyclin D1 is an important regulatory protein
involved in cell-cycle progression and has been extensively studied
in various cancers, including OSCC. Cyclin D1 has a critical role
in regulating the transition from G1 to S phase of the cell cycle
by forming complexes with CDKs. Overexpression of cyclin D1 has
been observed in OSCC, which is associated with impaired cell cycle
control, increased proliferation and tumour progression (49). Multiple molecular alterations,
including gene amplifications, mutations and dysregulated
signalling pathways, contribute to the upregulation of cyclin D1 in
OSCC (50).
Numerous studies have investigated the prognostic
significance of cyclin D1 expression in OSCC. Increased expression
of cyclin D1 has been associated with unfavourable
clinicopathological features, including advanced tumour stage,
lymph node metastasis and poor overall survival. Its overexpression
serves as an independent prognostic marker for aggressive tumour
behaviour and may aid in risk stratification and treatment planning
(51–53).
Cyclin D1 is closely involved in the interaction
with various oncogenic signalling pathways in OSCC, such as p53, RB
and components of the Wnt/β-catenin pathway, thus influencing
cell-cycle progression, apoptosis and tumour growth (54). Despite the growing evidence for the
link between cyclin D1 and OSCC, several challenges remain to be
overcome. These include standardization of cyclin D1 determination
methods, identification of predictive biomarkers for treatment
response, and understanding the complex network of interactions
involving cyclin D1 in OSCC (55,56).
In conclusion, cyclin D1 has a critical role in the
pathogenesis of OSCC and serves as a prognostic marker. Its
dysregulation contributes to tumour progression and poor clinical
outcomes. Further exploration of the molecular mechanisms
underlying cyclin D1 involvement in OSCC and clinical validation of
therapeutic approaches targeting cyclin D1 will pave the way for
more effective treatment strategies and improved outcomes for
OSCC.
Ki-67
Ki-67 is a nuclear protein associated with cell
proliferation and has been shown to be a valuable biomarker in
cancer research, including OSCC. Ki-67 is commonly used as a marker
of cell proliferation, as it is present in the active phases of the
cell cycle (G1, S, G2 and mitosis) (57). OSCC is characterized by dysregulated
cell proliferation and Ki-67 expression levels reflect
proliferation activity in the tumour. High Ki-67 expression in OSCC
is associated with aggressive clinicopathological features in
various cancers, such as breast cancer (58,59).
Numerous studies have investigated the prognostic
value of Ki-67 expression in head and neck cancer. Increased Ki-67
expression has been associated with decreased overall survival and
disease-free survival, and increased risk of recurrence (60,61).
Its evaluation may be helpful in risk stratification, treatment
planning and posttreatment surveillance. Ki-67 expression has been
shown to be particularly valuable in identifying high-risk
subgroups among patients with early-stage OSCC, for whom accurate
prognosis prediction is difficult. Ki-67 expression has also been
studied as a predictive marker of treatment response in OSCC
(62). Low Ki-67 expression has
been associated with a better response to therapy, including
surgery, radiotherapy and chemotherapy (63). Conversely, high Ki-67 expression may
indicate treatment resistance and the need for more aggressive
therapeutic approaches. Incorporating Ki-67 assessment into
treatment decision-making may help personalize treatment strategies
and optimize outcomes (64).
Combining Ki-67 assessment with other molecular
biomarkers has the potential to improve risk stratification and
treatment planning in OSCC. Integration of Ki-67 with factors such
as those previously discussed (PD-L1, cyclin D1, EGFR and p16) may
lead to a more comprehensive understanding of tumour behaviour and
enable personalized therapeutic approaches (65).
In summary, the evaluation of histologic biomarkers
in OSCC, including p16, EGFR, PD-L1, cyclin D1 and Ki-67, provides
valuable insight into tumour behaviour, prognosis and response to
treatment. These biomarkers have the potential to aid in risk
stratification, treatment planning and the development of targeted
therapies for OSCC.
Serological markers
Serological markers are biomolecules present in the
blood that can indicate cancer development and progression and
response to treatment. The potential of combining multiple markers
to improve diagnostic accuracy and specificity is also a key point,
and the integration of diverse markers into routine clinical
practice may aid in early detection. In this sense, a diverse group
of molecules has been described in OSCC. All of these biomarkers
are summarised in Table I.
Carcinoma-specific carcinoembryonic
antigen (CEA)
CEA is a high-molecular-weight glycoprotein,
isolated from a tumour cell extract, and is found in the
cytoplasmic membrane of numerous glandular cells. The term
carcinoembryonic is due to its presence in foetal colonic mucosa
(66). Its physiological function
is unknown. Due to its structural similarity with members of the
immunoglobulin family, it may be involved in cellular recognition
mechanisms or cell adhesion mechanisms.
It is usually present at very low concentrations in
human serum, generally below 5 ng/ml. However, of note, higher
levels (5–10 ng/ml) may be found in healthy smokers. Initially, CEA
was thought to be a specific marker for digestive tract tumours;
later studies revealed that it is elevated in various types of
tumour, including OSCC (67). It
may also be elevated in nontumor diseases, such as renal
insufficiency, liver cirrhosis, pancreatitis and inflammatory bowel
disease.
Several studies have evaluated the diagnostic value
of CEA in OSCC. For instance, a study investigated the diagnostic
value of serum tumour markers, including CEA, in patients with
OSCC, and the results showed that CEA levels were significantly
higher in these patients than in patients with benign oral tumours
and healthy controls (68). This
suggests that CEA may be a useful biomarker for its detection, as
its overexpression in these types of cancer may be related to the
malignant transformation of epithelial cells (67). However, due to the variability in
CEA levels among patients and the possibility of elevations in
other types of diseases, CEA is not used as a sole marker for the
diagnosis of head and neck cancer but rather as part of a more
comprehensive diagnostic approach (68).
In addition to its diagnostic value, CEA levels may
also be useful for monitoring treatment response in patients with
head and neck cancer. A decrease in CEA levels during or after
treatment may indicate a favourable treatment response (67,68).
Apart from CEA, other tumour markers have also been
studied in OSCC. For instance, a study identified serum
autoantibodies against sideroflexin 3 as a potential tumour marker
for OSCC (67). Furthermore,
another study revealed that CEA levels in saliva increase in the
presence of oral cavity malignancies, including OSCC (69). These findings suggest that CEA,
along with other tumour markers, may be of diagnostic value.
It is important to note that the diagnostic accuracy
of CEA and other tumour markers in OSCC may vary depending on the
specific marker and the studied population. For instance, the
diagnostic accuracy of progastrin-releasing peptide (ProGRP), CEA,
cytokeratin 19 fragment antigen (CYFRA 21-1) and neuron-specific
enolase in the differential diagnosis of small cell lung cancer
demonstrated that ProGRP exhibited higher detection accuracy than
CEA in lung cancer (68).
Therefore, further research is needed to determine the specific
diagnostic value of CEA in OSCC.
Vascular endothelial growth factor
(VEGF)
VEGF has a crucial role in OSCC by promoting
angiogenesis, invasiveness, aggressiveness and proliferation of
cancer cells (70). The
overexpression of VEGF has been associated with tumour progression,
lymph node metastasis and poor prognosis in OSCC (71,72).
Several studies have investigated the expression and role of VEGF
in OSCC.
The expression of VEGF-A and its receptor VEGFR-2
has been evaluated in patients with OSCC. The results showed that
VEGF-A gene expression and serum levels were significantly higher
in patients with OSCC than in controls. In addition, VEGFR-2
expression was observed in tumour tissues. These findings suggest
that VEGF-A and VEGFR-2 may have a role in the pathophysiology of
OSCC (71).
Furthermore, the correlation between
clinicopathological factors and VEGF expression in OSCC was
examined. Immunohistochemical staining and reverse
transcription-quantitative (RT-q)PCR were performed to assess VEGF
expression. The results showed that high-level staining of VEGF was
observed in poorly differentiated and invasive OSCC. There were
significant correlations between VEGF expression and histologic
differentiation and tumour size. These findings suggest that VEGF
expression may be associated with tumour aggressiveness in OSCC
(72).
In addition to its role in angiogenesis, VEGF has
been implicated in the epithelial-to-mesenchymal transition (EMT)
process in OSCC. Neuropilin-1 (NRP1), a coreceptor of VEGF, has
been shown to promote EMT by activating the NF-κB pathway (72). In this sense, NRP1 overexpression
promotes EMT in OSCC. Inhibition of the NF-κB pathway reverses the
NRP1-mediated EMT process. These findings suggest that VEGF-NRP1
signalling may contribute to the invasive and metastatic potential
of OSCC (73).
Furthermore, Alsafadi and Manadili (74) investigated the expression of VEGF in
OSCC after inducing it in hamsters and subjecting them to
radiotherapy. The results showed that VEGF expression was not
significantly different between the group that received
radiotherapy and the group that did not. In addition, tumour cells
that were resistant to radiotherapy showed positive expression of
VEGF. These findings suggest that VEGF expression may be associated
with radioresistance in OSCC (74).
In conclusion, VEGF has a significant role in the pathogenesis and
progression of OSCC. Its overexpression has been associated with
tumour aggressiveness, lymph node metastasis and poor prognosis in
OSCC.
Cancer antigen 125 (CA-125)
CA-125 is a glycoprotein initially isolated from
ovarian tumours and produced under normal conditions by structures
derived from Müller's ducts (fallopian tube, endocervix and vaginal
fundus), as well as in the pleural, pericardial and peritoneal
mesothelia. Concentrations <35–40 U/ml are considered normal.
CA-125 is the marker of choice for epithelial ovarian tumours
(except mucinous ovarian tumours), but several studies have
investigated the expression of CA-125 in patients with OSCC and its
potential as a biomarker with diagnostic, predictive and prognostic
utility (75,76).
The serum concentration of CA-125 accurately
reflects the malignant degree of the tumour mass, and thus,
pretreatment serum values are directly related to the tumour stage
in patients (75). However, higher
levels of CA-125 may be detected in benign conditions, such as
endometriosis, benign ovarian tumours, cysts or tuberculosis.
Saliva has also been investigated as a diagnostic
medium to detect biomarkers in patients with OSCC. Zhang et
al (75) reported that an
increase in CA-125 levels in saliva may be considered a salivary
biomarker for cancers of the oral cavity. Similarly, Roi et
al (76) found that CA-125
levels were significantly elevated in the saliva of patients with
oral cancer. These findings suggest that CA-125 may be a potential
salivary biomarker for OSCC (75,76).
Furthermore, Goldoni et al (77) found that CA-125 levels were
significantly elevated in saliva samples from patients with OSCC
analysed using the immunoblot technique. They also reported that
elevated levels of soluble CD44 in saliva were present in the
majority of OSCC cases and could distinguish cancer from benign
tumours with high specificity (77).
Furthermore, a review mentioned that CA-125 has been
studied as a diagnostic marker in head and neck SCC, including
OSCC. They emphasized the importance of protein markers, such as
CA-125 in the diagnosis and progression of various cancer types
(78).
In this context, the available literature suggests
that CA-125 may be a potential biomarker for OSCC, but further
research is needed to validate its diagnostic and prognostic value
in patients with OSCC. Saliva-based diagnostic methods, including
the analysis of salivary biomarkers such as CA-125, show promise
for the early detection of OSCC.
CYFRA 21-1
CYFRA 21-1 is a soluble form of cytokeratin 19, a
structural protein found in epithelial cells. Research has been
conducted to determine the clinical value of CYFRA 21-1 in the
diagnosis, prognosis and follow-up of head and neck cancer.
Rajkumar et al (79) investigated the levels of salivary
and serum CYFRA 21-1 in patients with oral precancer and OSCC. The
study found that CYFRA 21-1 levels were increased in both salivary
and serum samples of patients with OSCC compared to healthy
controls (79). This suggests that
CYFRA 21-1 may serve as a potential biomarker for the detection of
OSCC. In reference to its prognostic utility, Liu et al
(80) highlighted that high levels
of CYFRA 21-1 were significantly associated with shorter overall
survival.
CYFRA 21-1 is not specific to OSCC and has been
studied in other types of cancer as well. However, the studies
mentioned above provide evidence for the potential utility of CYFRA
21-1 as a biomarker in OSCC.
SCC antigen (SCCA)
SCCA is a member of the serine protease inhibitor
family located in the serine protease inhibitor (serpin) group on
chromosome 18q21.3. It is a glycoprotein with a molecular weight of
~48 kDa, a molecule initially described from cervical tissue.
Molecular studies demonstrate that SCCA is transcribed by two
nearly identical genes (SCCA1 and SCCA2), and it has been observed
that both SCCA1 and SCCA2 are expressed in moderately and
well-differentiated tumours. It is thought that SCCA has a role in
protecting tissue against enzymatic degradation. However, its exact
function remains to be fully elucidated. It has been shown that
SCCA2 inhibits chymotrypsin-like proteinases, such as cathepsin G
and mast cell chymase, while SCCA1 inhibits cysteine proteinases
such as cathepsins K, L and S (81,82).
In terms of its clinical utility, its use as a
marker for the diagnosis, prognosis, monitoring and histological
differentiation of certain squamous lineage cancers, such as lung
SCC, cervical cancer, OSCC and anal cancer, has been explored
(81). This is particularly useful,
since ~90% of head and neck cancer cases are of a squamous
lineage.
Regarding its diagnostic utility, in certain cases,
elevated levels of SCCA in the blood may suggest the presence of
SCC. Furthermore, the presence of SCCA has been associated with
unfavourable prognosis in certain cases, serving as a guide for
clinical decision making (82).
This antigen may be physiologically found in saliva,
hair and skin particles; thus, there is a certain risk of
contamination that may lead to inaccurate SCCA values in current
assays. Furthermore, it may be present in other benign conditions
and its levels may vary based on the method of blood sample
collection for subsequent analysis (83).
In OSCC, SCCA is useful for aiding in the diagnosis
in certain cases, as elevated levels of SCCA in the blood may
suggest the presence of SCC. In addition, the presence of SCCA has
been associated with a poorer prognosis in certain cases. Due to
its short half-life, which is <24 h, it may assist in
postoperative monitoring; in this way, SCCA levels in the blood
generally decrease after tumour resection. It also aids in
monitoring the response to a specific therapy, as it may decrease
in responsive tumours and increase with recurrence (82,83).
The increased SCCA2/SCCA1 mRNA ratio in a primary tumour is also
associated with cancer recurrence; hence, it is interesting to
detect both SCCA1 and SCCA2 to determine total SCCA expression
(82). However, currently available
assay systems cannot differentiate between the two isoforms in this
manner, as the assays were not developed for this specific purpose
(81).
It is worth noting that currently, there is no firm
evidence or reports on the interaction between HPV status and SCCA
in relation to OSCC prognosis (83).
Research on SCCA remains active, with the aim of
better understanding its function, clinical utility and
limitations. In addition, more sensitive and specific assay methods
for its detection and quantification are being developed and
evaluated (82).
Circulating tumour cells (CTCs)
CTCs are those cells that break away from a primary
tumour and enter the blood or lymphatic circulation, giving them
the potential to spread to other parts of the body. Techniques used
to detect and analyse CTCs include immunocapture, which uses
specific antibodies to identify and isolate CTCs, and techniques
based on nucleic acid amplification, such as PCR and RT-qPCR, to
amplify and detect specific markers of CTCs. Currently, the
screening test approved by the Food and Drug Administration is
based on the detection of epithelial cell adhesion
molecule)/cytokeratin-expressing cells in the blood using
antibodies through the CellSearch platform (84,85).
Molecular analysis of CTCs provides information on
the genetic and molecular characteristics of the primary tumour; in
this way, specific genetic mutations may be examined, such as
mutations in the TP53 gene, which is common in head and neck
cancer. In addition, gene expression and chromosomal alterations in
CTCs may be analysed to gain a more detailed understanding of the
specific characteristics of the tumour (85,86).
In OSCC, the detection and characterization of CTCs
have been studied to gain insight into tumour biology and to
develop novel therapeutic strategies. Studies have indicated that
the presence of CTCs is associated with poor prognosis and an
increased risk of metastasis in patients with OSCC. The
quantification and analysis of CTCs may provide valuable
information about tumour progression, treatment response and the
development of resistance (86,87).
Certain specific markers have been identified in
CTCs, such as increased expression of Ki-67 (a cell proliferation
marker) or genes associated with invasion and metastasis, such as
Twist1 or Snail, which have been associated with unfavourable
prognosis and a higher probability of relapse in this tumour type
(88,89).
Another aspect to consider is that CTCs may acquire
characteristics that confer resistance to conventional cancer
treatments. This may be due to genetic and molecular changes that
occur in tumour cells during the metastasis process. For instance,
CTCs may develop mutations in genes related to drug response, such
as genes involved in DNA repair or in the EGFR signalling pathway.
One of the characteristics that confers these resistances to CTCs
is their heterogeneity; certain CTCs may be inherently resistant to
drugs, while others may develop resistance during treatment or may
alter the expression of genes that are involved in the response to
these therapies. Therefore, the study of CTCs and their resistance
to treatments may provide key information to develop therapeutic
strategies aimed at overcoming these resistances. Drug combinations
may also be evaluated to address the heterogeneity of CTCs and
target multiple survival pathways (90,91).
Another useful application of CTC analysis is the
evaluation of the efficacy of treatments and the monitoring of
tumour response over time. Changes in the number and
characteristics of CTCs may indicate a positive or negative
response to treatment. For instance, a decrease in the number of
CTCs or changes in their molecular profile may suggest a favourable
response. Conversely, persistent or increased CTCs during or after
treatment may indicate a suboptimal response and poor prognosis.
This may be particularly useful in the early detection of tumour
progression and in therapeutic decision-making; for instance, the
persistence of CTCs after treatment has been associated with an
increased risk of relapse (92,93).
Salivary biomarkers
Although biopsies or serological markers have been
considered the ‘gold standard’ for oral cancer diagnosis, other
noninvasive techniques are being implemented to predict its
development. Body fluids, such as saliva, have a specific
structural composition for each condition or disease. Studies have
been conducted on predictive, diagnostic or prognostic biomarkers
found in saliva, with positive results for carcinomatous,
inflammatory and genetic oral diseases. Furthermore, it has been
observed that saliva may be useful for assessing certain systemic
diseases with varying sensitivity and specificity (94).
One of the possible sample types is gingival
crevicular fluid (GCF), a biological fluid found in the space
between the tooth and the gum, known as the gingival sulcus. GCF is
a clear, watery liquid that originates from periodontal tissues and
contains a variety of components, such as proteins, enzymes,
hormones, immune cells and tissue breakdown products. This fluid
has an important role in maintaining periodontal health and may
vary in composition based on oral conditions, such as gingival
health, inflammation and periodontal disease. The analysis of GCF
may provide valuable information about the health status of the
gums and periodontal tissues. Another fluid of interest is dentinal
tubular fluid (DTF), which has a fundamental role in protecting the
pulp from microbial invasion when the pulp-dentin complex is
injured. With the onset of the inflammatory cascade, the exudate of
dentinal fluid mainly contains polymorphonuclear leukocytes,
migrating macrophages, B cells and T cells. Therefore, the
characterization of DTF may provide estimates of the extent of
dentopulpal injury, the degree of pulpal inflammation or the
efficacy of dental restoration (94,95).
Salivary biomarkers are proteomic or genomic
macromolecules; >100 salivary biomarkers have been identified;
these include DNA, RNA, mRNA, defensin-1, P53, Cyfra 21-1,
tissue-specific polypeptide antigen, dual-specificity phosphatase,
spermidine/spermine N1-acetyltransferase, profilin, cofilin-1 and
transferrin. However, to date, no single marker has been agreed
upon due to a lack of research and consensus among researchers.
However, there are promising results regarding cytokines (96).
Cytokines are a group of low-molecular-weight
glycoproteins produced by immune and nonimmune cells that have a
crucial role in the regulation, signalling, maintenance and
induction of most cellular interactions. They have roles in
phenotypes and processes such as pleiotropy, inflammation and
cellular apoptosis. Oral conditions, both benign (such as aphthous
ulcers or periodontitis) and malignant (such as dysplastic lesions
and oral and pharyngeal carcinomas), contribute to elevated
cytokine levels. Among the most studied cytokines are interleukin 8
(IL8) and IL6, as well as tumour necrosis factor α (TNF-α)
(97).
Among the cytokine analysis techniques for saliva
samples, enzyme-linked immunosorbent assays are used for
quantitative evaluation, and PCR is used for qualitative
evaluation. Additional techniques include western blotting,
migration assays, immunohistochemical staining, liquid
chromatography and commercial colorimetric methods. Luminex
bead-based multiplex assays are used to discoverdiagnostic
biomarkers in human saliva and plasma responsible for tumour
progression in patients with OSCC, reporting that IL-1β, macrophage
inflammatory protein 1β, IFN-γ, TNF-α, IL-6, IL-8 and eotaxin are
plausible salivary biomarkers (97,98).
Certain studies determined the presence of IL-6 and
IL-8 cytokines in patients with OSCC, potentially malignant lesions
(PML), and a control group. A significant increase in IL-6 and IL-8
values was observed in saliva samples from patients with OSCC
compared to the control group. However, in the PML group, only IL-8
was found to be elevated. Furthermore, in the OSCC patient group,
the ratio of these cytokines in serum and blood was compared,
revealing that the levels of IL-8 were similar in serum and saliva
samples, while the levels of IL-6 were higher in serum than in
saliva samples from patients diagnosed with OSCC (98). However, in other studies, although
these biomarkers were elevated in the OSCC group compared to the
control group, no statistically significant differences were found
(99).
While IL-8 levels may be altered due to lifestyle,
geographical distribution, ethnic differences, genetic differences,
gingivitis or periodontitis, none of these causes have led to
levels as high as those observed in cases of OSCC. This is because
IL-8 possesses angiogenic properties and contributes to tumour
progression. Other cytokines, such as IL-6 and TNF-α, are also
elevated in patients with oral leukoplakia, oral lichen planus and
oral submucosal fibrosis, making them possible diagnostic markers
for precancerous oral lesions (100).
Patients with OSCC are commonly diagnosed at
advanced stages of malignancy and therefore have an unfavourable
prognosis. Thus, identified salivary biomarkers may have a valuable
role as a complement in the early detection and management of
OSCC.
Conclusions
The study of histological and serological biomarkers
in OSCC has shown great promise in improving the diagnosis,
prognosis and management of this deadly disease. Histological
examination remains the gold standard for diagnosing OSCC,
providing valuable insight into tumour characteristics, such as
tumour size, grade, invasion and lymph node involvement. However,
the limitations of histology, including subjectivity and
intraobserver variability, have prompted researchers to explore
alternative approaches, such as serological biomarkers. Serological
biomarkers, which may be easily measured in blood samples, offer a
noninvasive and cost-effective means of detecting and monitoring
OSCC. Various biomarkers, including CTCs, circulating tumour DNA
and tumour-specific antigens, have been investigated. These
biomarkers hold tremendous potential in aiding in early detection,
prediction of the treatment response, monitoring of disease
progression or identification of patients at high risk of
recurrence; furthermore, the integration of histological and
serological biomarkers has the potential to revolutionize OSCC
management. Combining the morphological information obtained from
histological analysis with the molecular insight offered by
serological biomarkers may enhance diagnostic accuracy, enable
personalized treatment and guide clinicians in making informed
decisions about patient care. This multimodal approach may also
facilitate the identification of novel therapeutic targets and the
development of targeted therapies, leading to improved patient
outcomes. The use of saliva for early cancer detection in the quest
for new clinical markers is a promising approach since sample
collection is simple and noninvasive.
On the other hand, it is essential to acknowledge
the existing challenges and limitations in the field of
histological and serological biomarkers in OSCC. Standardization of
sample collection and processing and analysis methods is crucial to
ensure reliable and reproducible results. In addition, large-scale
multicentre studies are necessary to validate the clinical utility
and establish the optimal cut-off values for different
biomarkers.
To summarize, these biomarkers have the potential to
transform the landscape of OSCC diagnosis, prognosis and treatment
by providing valuable information on tumour characteristics and
patient outcomes, but further research and collaboration are needed
to refine these biomarkers and establish their clinical utility in
the management of OSCC.
Acknowledgements
Not applicable.
Funding
This work was partially supported by grants from the Mutua
Madrileña, Programa de Actividades de I+D de la Comunidad de Madrid
en Biomedicina (grant no. P2022/BMD-7321), Halekulani S.L.,
ProACapital and MJR.
Availability of data and materials
Not applicable.
Authors' contributions
LP, MJGG, ASC, JC, TP, JA, RDP and MAO were involved
in the conceptualization of the study. MAO, MAM and JA were
involved in funding acquisition. MAO and JA were involved in
project administration. LP, MJGG, ASC, JC, TP, OFM, CGM, LLG, ARP,
MAM, JA, RDP and MAO were involved in the investigative aspects of
the study. All authors have read and agreed to the published
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cadoni G, Giraldi L, Petrelli L,
Pandolfini M, Giuliani M, Paludetti G, Pastorino R, Leoncini E,
Arzani D, Almadori G and Boccia S: Prognostic factors in head and
neck cancer: A 10-year retrospective analysis in a
single-institution in Italy. Acta Otorhinolaryngol Ital.
37:458–466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reyes-Gibby CC, Anderson KO, Merriman KW,
Todd KH, Shete SS and Hanna EY: Survival patterns in squamous cell
carcinoma of the head and neck: Pain as an independent prognostic
factor for survival. J Pain. 15:1015–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barsouk A, Aluru JS, Rawla P, Saginala K
and Barsouk A: Epidemiology, risk factors, and prevention of head
and neck squamous cell carcinoma. Med Sci (Basel).
11:422023.PubMed/NCBI
|
|
4
|
Gormley M, Creaney G, Schache A,
Ingarfield K and Conway DI: Reviewing the epidemiology of head and
neck cancer: Definitions, trends and risk factors. Br Dent J.
233:780–786. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SM: Human papilloma virus in oral
cancer. J Korean Assoc Oral Maxillofac Surg. 42:327–336. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sathish N, Wang X and Yuan Y: Human
papillomavirus (HPV)-associated oral cancers and treatment
strategies. J Dent Res. 93 (Suppl 7):29S–36S. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oliveira AC, Cavalcanti de Lima IC, Frez
Marques VM, Alves de Araújo WH and De Campos Ferreira C: Human
papillomavirus prevalence in oral and oropharyngeal squamous cell
carcinoma in South America: A systematic review and meta-analysis.
Oncol Rev. 16:5522022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petti S: Lifestyle risk factors for oral
cancer. Oral Oncol. 45:340–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Candotto V, Lauritano D, Nardone M, Baggi
L, Arcuri C, Gatto R, Gaudio RM, Spadari F and Carinci F: HPV
infection in the oral cavity: Epidemiology, clinical manifestations
and relationship with oral cancer. Oral Implantol (Rome).
10:209–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gileva OS, Libik TV and Danilov KV: Oral
precancerous lesions: Problems of early detection and oral cancer
prevention. AIP Conf Proc. 1760:0200192016. View Article : Google Scholar
|
|
12
|
Güneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baykul T, Yilmaz HH, Aydin U, Aydin MA,
Aksoy M and Yildirim D: Early diagnosis of oral cancer. J Int Med
Res. 38:737–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGuirt WF: Panendoscopy as a screening
examination for simultaneous primary tumors in head and neck
cancer: A prospective sequential study and review of the
literature. Laryngoscope. 92:569–576. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fulciniti F, Califano L, Zupi A and
Vetrani A: Accuracy of fine needle aspiration biopsy in head and
neck tumors. J Oral Maxillofac Surg. 55:1094–1097. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burkill GJC, Evans RM, Raman VV and Connor
SEJ: Modern radiology in the management of head and neck cancer.
Clin Oncol (R Coll Radiol). 28:440–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai YL and King AD: State of the art MRI
in head and neck cancer. Clin Radiol. 73:45–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irani S: Distant metastasis from oral
cancer: A review and molecular biologic aspects. J Int Soc Prev
Community Dent. 6:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Naaj IA, Leiser Y, Shveis M, Sabo E and
Peled M: Incidence of oral cancer occult metastasis and survival of
T1-T2N0 oral cancer patients. J Oral Maxillofac Surg. 69:2674–2679.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zanoni DK, Patel SG and Shah JP: Changes
in the 8th edition of the American joint committee on cancer (AJCC)
staging of head and neck cancer: Rationale and implications. Curr
Oncol Rep. 21:522019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah JP and Gil Z: Current concepts in
management of oral cancer-surgery. Oral Oncol. 45:394–401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang SH and O'Sullivan B: Oral cancer:
Current role of radiotherapy and chemotherapy. Med Oral Patol Oral
Cir Bucal. 18:e233–e240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lang K, Baur M, Held T, Shafie RE, Moratin
J, Freudlsperger C, Zaoui K, Bougatf N, Hoffmann J, Plinkert PK, et
al: Definitive radiotherapy for squamous cell carcinoma of the oral
cavity: A single-institution experience. Radiol Oncol. 55:467–473.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartner L: Chemotherapy for oral cancer.
Dent Clin North Am. 62:87–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura I, Kitahara H, Ooi K, Kato K,
Noguchi N, Yoshizawa K, Nakamura H and Kawashiri S: Loss of
epidermal growth factor receptor expression in oral squamous cell
carcinoma is associated with invasiveness and
epithelial-mesenchymal transition. Oncol Lett. 11:201–207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang T, Sun S, Zeng X and Li J: ICI-based
therapies: A new strategy for oral potentially malignant disorders.
Oral Oncol. 140:1063882023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doumas S, Foukas PG, Economopoulou P,
Kotsantis I and Psyrri A: Atypical patterns of responses in the era
of immune checkpoint inhibitors in head and neck cancer. Oral
Oncol. 100:1044772020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohanty V, Subbannayya Y, Patil S,
Puttamallesh VN, Najar MA, Datta KK, Pinto SM, Begum S, Mohanty N,
Routray S, et al: Molecular alterations in oral cancer using
high-throughput proteomic analysis of formalin-fixed
paraffin-embedded tissue. J Cell Commun Signal. 15:447–459. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tokuzen N, Nakashiro KI, Tojo S, Goda H,
Kuribayashi N and Uchida D: Human papillomavirus-16 infection and
p16 expression in oral squamous cell carcinoma. Oncol Lett.
22:5282021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vergori A, Garbuglia AR, Piselli P, Del
Nonno F, Sias C, Lupi F, Lapa D, Baiocchini A, Cimaglia C, Gentile
M, et al: Oral human papillomavirus DNA detection in HIV-positive
men: Prevalence, predictors, and co-occurrence at anal site. BMC
Infect Dis. 18:252018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sritippho T, Chotjumlong P and Iamaroon A:
Roles of human papillomaviruses and p16 in oral cancer. Asian Pac J
Cancer Prev. 16:6193–6200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castro TPPG and Bussoloti Filho I:
Prevalence of human papillomavirus (HPV) in oral cavity and
oropharynx. Braz J Otorhinolaryngol. 72:272–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katirachi SK, Grønlund MP, Jakobsen KK,
Grønhøj C and von Buchwald C: The prevalence of HPV in oral cavity
squamous cell carcinoma. Viruses. 15:4512023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Doll C, Steffen C, Beck-Broichsitter B,
Richter M, Neumann K, Pohrt A, Lohneis P, Lehmann A, Heiland M,
Stromberger C, et al: The prognostic significance of p16 and its
role as a surrogate marker for human papilloma virus in oral
squamous cell carcinoma: An analysis of 281 cases. Anticancer Res.
42:2405–2413. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Christianto S, Li KY, Huang TH and Su Y:
The prognostic value of human papilloma virus infection in oral
cavity squamous cell carcinoma: A meta-analysis. Laryngoscope.
132:1760–1770. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miranda-Galvis M, Rumayor Piña A, Sales de
Sá R, Almeida Leite A, Agustin Vargas P, Calsavara VF, Lópes Pinto
CA, Teng Y and Kowalski LP: PD-L1 expression patterns in oral
cancer as an integrated approach for further prognostic
classification. Oral Dis. 27:1699–1710. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenouvel D, González-Moles MÁ, Ruiz-Ávila
I, Chamorro-Santos C, González-Ruiz L, González-Ruiz I and
Ramos-García P: Clinicopathological and prognostic significance of
PD-L1 in oral cancer: A preliminary retrospective
immunohistochemistry study. Oral Dis. 27:173–182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dave K, Ali A and Magalhaes M: Increased
expression of PD-1 and PD-L1 in oral lesions progressing to oral
squamous cell carcinoma: A pilot study. Sci Rep. 10:97052020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu T, Tang C, Tao R, Yong X, Jiang Q and
Feng C: PD-L1-mediated immunosuppression in oral squamous cell
carcinoma: Relationship with macrophage infiltration and epithelial
to mesenchymal transition markers. Front Immunol. 12:6938812021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ries J, Agaimy A, Wehrhan F, Baran C,
Bolze S, Danzer E, Frey S, Jantsch J, Möst T, Büttner-Herold M, et
al: Importance of the PD-1/PD-L1 axis for malignant transformation
and risk assessment of oral leukoplakia. Biomedicines. 9:1942021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Botticelli A, Cirillo A, Strigari L,
Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, Zizzari IG, Rocca
CD, D'Amati G, et al: Anti-PD-1 and anti-PD-L1 in head and neck
cancer: A network meta-analysis. Front Immunol. 12:7050962021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fasano M, Corte CMD, Liello RD, Viscardi
G, Sparano F, Iacovino ML, Paragliola F, Piccolo A, Napolitano S,
Martini G, et al: Immunotherapy for head and neck cancer: Present
and future. Crit Rev Oncol Hematol. 174:1036792022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wongpattaraworakul W, Gibson-Corley KN,
Choi A, Buchakjian MR, Lanzel EA, Rajan Kd A and Simons AL:
Prognostic role of combined EGFR and tumor-infiltrating lymphocytes
in oral squamous cell carcinoma. Front Oncol. 12:8852362022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen IH, Chang JT, Liao CT, Wang HM, Hsieh
LL and Cheng AJ: Prognostic significance of EGFR and Her-2 in oral
cavity cancer in betel quid prevalent area cancer prognosis. Br J
Cancer. 89:681–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kang JJ, Ko A, Kil SH, Mallen-St Clair J,
Shin DS, Wang MB and Srivatsan ES: EGFR pathway targeting drugs in
head and neck cancer in the era of immunotherapy. Biochim Biophys
Acta Rev Cancer. 1878:1888272023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moharil R, Khandekar S, Dive A and Bodhade
A: Cyclin D1 in oral premalignant lesions and oral squamous cell
carcinoma: An immunohistochemical study. J Oral Maxillofac Pathol.
24:3972020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lam KY, Ng IO, Yuen AP, Kwong DL and Wei
W: Cyclin D1 expression in oral squamous cell carcinomas:
Clinicopathological relevance and correlation with p53 expression.
J Oral Pathol Med. 29:167–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ahmed MAS, Omer N, Suliman AM and Ellaithi
M: Expression of cyclin D1 in oral squamous cell carcinoma. Sudan J
Med Sci. 16:558–566. 2021.
|
|
52
|
Saawarn S, Astekar M, Saawarn N, Dhakar N
and Gomateshwar Sagari S: Cyclin D1 expression and its correlation
with histopathological differentiation in oral squamous cell
carcinoma. ScientificWorldJournal. 2012:9783272012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Acero-Mondragón E, Rodríguez-Farías R,
Salazar-Hernández M and Figueroa-Avendaño J: Histological
correlation of Ki-67 and D1 cycline markers expression in squamous
cell carcinoma in the oral cavity. Rev Odontol Mex. 20:e225–e229.
2016. View Article : Google Scholar
|
|
54
|
Ramos-García P, González-Moles MÁ,
González-Ruiz L, Ayén Á, Ruiz-Ávila I, Bravo M and Gil-Montoya JA:
Clinicopathological significance of tumor cyclin D1 expression in
oral cancer. Arch Oral Biol. 99:177–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang SF, Cheng SD, Chuang WY, Chen IH,
Liao CT, Wang HM and Hsieh LL: Cyclin D1 overexpression and poor
clinical outcomes in Taiwanese oral cavity squamous cell carcinoma.
World J Surg Oncol. 10:402012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Awawdeh MA, Sasikumar R, Aboalela AA,
Siddeeqh S, Gopinathan PA, Sawair F and Khanagar SB: Evaluation of
prognostic significance of the expression of p53, cyclin D1, EGFR
in advanced oral squamous cell carcinoma after chemoradiation-A
systematic review. Appl Sci. 13:52922023. View Article : Google Scholar
|
|
57
|
Takkem A, Barakat C, Zakarea S, Zaid K,
Najmeh J, Ayoub M and Seirawan MY: Ki-67 prognostic value in
different histological grades of oral epithelial dysplasia and oral
squamous cell carcinoma. Asian Pac J Cancer Prev. 19:3279–3286.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Davey MG, Hynes SO, Kerin MJ, Miller N and
Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast
cancer. Cancers (Basel). 13:44552021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xie S, Liu Y, Qiao X, Hua RX, Wang K, Shan
XF and Cai ZG: What is the prognostic significance of Ki-67
positivity in oral squamous cell carcinoma? J Cancer. 7:758–767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meyer HJ, Gundermann P and Surov A:
Associations between FDG-PET and Ki 67-index in head and neck
cancer: A meta-analysis. Medicine (Baltimore). 98:e174722019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lopes VKM, de Jesus AS, de Souza LL,
Miyahara LAN, Guimarães DM, Pontes HAR, Pontes FSC and Carvalho PL:
Ki-67 protein predicts survival in oral squamous carcinoma cells:
An immunohistochemical study. Braz Oral Res. 31:e662017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mohamed AA, EL-Shall OS and EL-Kilani NS:
Role of expression Ki-67 in diagnosis of oral cancer. Al-Azhar Dent
J Girls. 7:441–445. 2020. View Article : Google Scholar
|
|
64
|
Govindaraj PK, Kallarakkal TG, Mohd Zain
R, Tilakaratne WM and Lew HL: Expression of Ki-67, Cornulin and
ISG15 in non-involved mucosal surgical margins as predictive
markers for relapse in oral squamous cell carcinoma (OSCC). PLoS
One. 16:e02615752021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pan W, Zhang C, Chen M, Min S, Xu L and
Chi Z: Expression of Ki-67 and P16 are related with HPV in squamous
cell carcinoma of the external auditory canal. J Otolaryngol Head
Neck Surg. 51:402022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jun W, Shaobo O, Xianhua Z, Siyu Z,
Mingyang C, Xin F, Ying C and Lan L: Deregulation of
hsa_circ_0001971/miR-186 and hsa_circ_0001874/miR-296 signaling
pathways promotes the proliferation of oral squamous carcinoma
cells by synergistically activating SHP2/PLK1 signals. Sci Rep.
11:205612021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murase R, Abe Y, Takeuchi T, Nabeta M,
Imai Y, Kamei Y, Kagawa-Miki L, Ueda N, Sumida T, Hamakawa H and
Kito K: Serum autoantibody to sideroflexin 3 as a novel tumor
marker for oral squamous cell carcinoma. Proteomics Clin Appl.
2:517–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mauro C, Passerini R, Spaggiari L, Galetta
D, Radice D, Lentati P and Sandri MT: New and old biomarkers in the
differential diagnosis of lung cancer: Pro-gastrin-releasing
peptide in comparison with neuron-specific enolase,
carcinoembryonic antigen, and CYFRA 21-1. Int J Biol Markers.
34:163–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deepa T and Thirrunavukkarasu N: Saliva as
a potential diagnostic tool. Indian J Med Sci. 64:293–306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Johnstone S and Logan RM: The role of
vascular endothelial growth factor (VEGF) in oral dysplasia and
oral squamous cell carcinoma. Oral Oncol. 42:337–342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Edirisinghe ST, Weerasekera M, De Silva
DK, Devmini MT, Pathmaperuma S, Wijesinghe GK, Nisansala T,
Maddumage A, Huzaini H, Rich AM, et al: Vascular endothelial growth
factor A (VEGF-A) and vascular endothelial growth factor receptor 2
(VEGFR-2) as potential biomarkers for oral squamous cell carcinoma:
A Sri Lankan study. Asian Pac J Cancer Prev. 24:267–274. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim SK, Park SG and Kim KW: Expression of
vascular endothelial growth factor in oral squamous cell carcinoma.
J Korean Assoc Oral Maxillofac Surg. 41:11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8:11182019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Alsafadi R and Manadili A: The correlation
between CD44 and angiogenesis in oral squamous cell carcinoma
induced in buccal pouch in syrian hamster that underwent
radiotherapy. Asian Pac J Cancer Prev. 23:3571–3576. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P,
Xu X and Zhou XD: Saliva in the diagnosis of diseases. Int J Oral
Sci. 8:133–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Roi A, Rusu LC, Roi CI, Luca RE, Boia S
and Munteanu RI: A new approach for the diagnosis of systemic and
oral diseases based on salivary biomolecules. Dis Markers.
2019:87618602019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Goldoni R, Scolaro A, Boccalari E, Dolci
C, Scarano A, Inchingolo F, Ravazzani P, Muti P and Tartaglia G:
Malignancies and biosensors: A focus on oral cancer detection
through salivary biomarkers. Biosensors (Basel). 11:3962021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Konings H, Stappers S, Geens M, De Winter
BY, Lamote K, van Meerbeeck JP, Specenier P, Vanderveken OM and
Ledeganck KJ: A literature review of the potential diagnostic
biomarkers of head and neck neoplasms. Front Oncol. 10:10202020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rajkumar K, Ramya R, Nandhini G, Rajashree
P, Ramesh Kumar A and Nirmala Anandan S: Salivary and serum level
of CYFRA 21-1 in oral precancer and oral squamous cell carcinoma.
Oral Dis. 21:90–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu L, Xie W, Xue P, Wei Z, Liang X and
Chen N: Diagnostic accuracy and prognostic applications of CYFRA
21-1 in head and neck cancer: A systematic review and
meta-analysis. PLoS One. 14:e02165612019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu Y, Cao Y, He S and Cai W: Technical
and clinical performance of two methods to detect squamous cell
carcinoma antigen levels for comparing pathological diagnosis
coincidence rates in lung, cervical, and head and neck cancers.
Clin Lab. 66:2020. View Article : Google Scholar
|
|
82
|
Yasumatsu R, Nakano T, Hashimoto K, Kogo
R, Wakasaki T and Nakagawa T: The clinical value of serum squamous
cell carcinoma antigens 1 and 2 in head and neck squamous cell
carcinoma. Auris Nasus Larynx. 46:135–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Obata K, Yutori H, Yoshida K, Sakamoto Y,
Ono K and Ibaragi S: Relationships between squamous cell carcinoma
antigen and cytokeratin 19 fragment values and renal function in
oral cancer patients. Int J Oral Maxillofac Surg. 52:417–422. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Signal Transduct Target
Ther. 6:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Deng Z, Wu S, Wang Y and Shi D:
Circulating tumor cell isolation for cancer diagnosis and
prognosis. EBioMedicine. 83:1042372022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kulasinghe A, Hughes BGM, Kenny L and
Punyadeera C: An update: Circulating tumor cells in head and neck
cancer. Expert Rev Mol Diagn. 19:1109–1115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Perumal V, Corica T, Dharmarajan A, Sun Z,
Dhaliwal SS, Dass CR and Dass J: Circulating tumour cells (CTC),
head and neck cancer and radiotherapy; future perspectives. Cancers
(Basel). 11:3672019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liskova A, Samec M, Koklesova L, Giordano
FA, Kubatka P and Golubnitschaja O: Liquid biopsy is instrumental
for 3PM dimensional solutions in cancer management. J Clin Med.
9:27492020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Anjum A, Shree R.VB, Jayaram Thambiah L
and Rodrigues C: A comparitive and prognostic study of circulating
tumor cells and vegfr-2 in different histological grades of oral
squamous cell carcinoma. Int J Adv Res. 10:745–754. 2022.
View Article : Google Scholar
|
|
90
|
Qayyumi B, Bharde A, Aland G, D'Souza A,
Jayant S, Singh N, Tripathi S, Badave R, Kale N, Singh B, et al:
Circulating tumor cells as a predictor for poor prognostic factors
and overall survival in treatment naïve oral squamous cell
carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol.
134:73–83. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gauthier A, Philouze P, Lauret A, Alphonse
G, Malesys C, Ardail D, Payen L, Céruse P, Wozny AS and
Rodriguez-Lafrasse C: Circulating tumor cell detection during
neoadjuvant chemotherapy to predict early response in locally
advanced oropharyngeal cancers: A prospective pilot study. J Pers
Med. 12:4452022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Curtin J, Thomson P, Wong G, Lam A and
Choi SW: The impact of surgery on circulating malignant tumour
cells in oral squamous cell carcinoma. Cancers (Basel). 15:5842023.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang X, Ekanayake Weeramange C, Hughes
BGM, Vasani S, Liu ZY, Warkiani ME, Hartel G, Ladwa R, Thiery JP,
Kenny L and Punyadeera C: Application of circulating tumour cells
to predict response to treatment in head and neck cancer. Cell
Oncol (Dordr). 45:543–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sahibzada HA, Khurshid Z, Khan RS, Naseem
M, Siddique KM, Mali M and Zafar MS: Salivary IL-8, IL-6 and TNF-α
as potential diagnostic biomarkers for oral cancer. Diagnostics
(Basel). 7:212017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Khurshid Z, Zafar MS, Khan RS, Najeeb S,
Slowey PD and Rehman IU: Role of salivary biomarkers in oral cancer
detection. Advances in Clinical Chemistry. Vol. 86. Elsevier; pp.
pp23–70. 2018, http://dx.doi.org/10.1016/bs.acc.2018.05.002
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Khurshid Z, Warsi I, Moin SF, Slowe PD,
Latif M, Zohaib S and Zafar MS: Biochemical analysis of oral fluids
for disease detection. Advances in Clinical Chemistry. Vol. 100.
Elsevier; pp. pp205–253. 2021, http://dx.doi.org/10.1016/bs.acc.2020.04.005
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chu HW, Chang KP, Hsu CW, Chang IY, Liu
HP, Chen YT and Wu CC: Identification of salivary biomarkers for
oral cancer detection with untargeted and targeted quantitative
proteomics approaches. Mol Cell Proteomics. 18:1796–1806. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chiamulera MMA, Zancan CB, Remor AP,
Cordeiro MF, Gleber-Netto FO and Baptistella AR: Salivary cytokines
as biomarkers of oral cancer: A systematic review and
meta-analysis. BMC Cancer. 21:2052021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
St John MA, Li Y, Zhou X, Denny P, Ho CM,
Montemagno C, Shi W, Qi F, Wu B, Sinha U, et al: Interleukin 6 and
interleukin 8 as potential biomarkers for oral cavity and
oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck
Surg. 130:929–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cheng YSL, Rees T and Wright J: A review
of research on salivary biomarkers for oral cancer detection. Clin
Transl Med. 3:32014. View Article : Google Scholar : PubMed/NCBI
|