|
1
|
Rindi G, Mete O, Uccella S, Basturk O, La
Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M
and Asa SL: Overview of the 2022 WHO Classification of
Neuroendocrine Neoplasms. Endocr Pathol. 33:115–154. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cives M and Strosberg JR:
Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin.
68:471–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huguet I, Grossman AB and O'Toole D:
Changes in the epidemiology of neuroendocrine tumours.
Neuroendocrinology. 104:105–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norton JA: Surgery for primary pancreatic
neuroendocrine tumors. J Gastrointest Surg. 10:327–331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akirov A, Larouche V, Alshehri S, Asa SA
and Ezzat S: Treatment options for pancreatic neuroendocrine
tumors. Cancers. 11:8282019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das S, Al-Toubah T and Strosberg J:
Chemotherapy in neuroendocrine tumors. Cancers. 13:48722021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai M, Mullins CS, Lu L, Alsfasser G and
Linnebacher M: Recent advances in diagnosis and treatment of
gastroenteropancreatic neuroendocrine neoplasms. World J
Gastrointest Surg. 14:383–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haque A, Brazeau D and Amin AR:
Perspectives on natural compounds in chemoprevention and treatment
of cancer: An update with new promising compounds. Eur J Cancer.
149:165–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel SS, Acharya A, Ray RS, Agrawal R,
Raghuwanshi R and Jain P: Cellular and molecular mechanisms of
curcumin in prevention and treatment of disease. Crit Rev Food Sci
Nutr. 60:887–939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoi V, Galani V, Lianos GD, Voulgaris S,
Kyritsis AP and Alexiou GA: The role of curcumin in cancer
treatment. Biomedicines. 9:10862021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stohs SS, Chen O, Ray SD, Ji J, Bucci LR
and Preuss HG: Highly bioavailable forms of curcumin and promising
avenues for Curcumin-Based research and application. Molecules.
25:13972020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wanninger S, Lorenz V, Subhan A and
Edelmann FT: Metal complexes of curcumin-synthetic strategies,
structures and medicinal applications. Chem Soc Rev. 44:4986–5002.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pagliaricci N, Pettinari R, Marchetti F,
Pettinari C, Cappellacci L, Tombesi A, Cuccioloni M, Hadiji M and
Dyson PJ: Potent and selective anticancer activity of half-sandwich
ruthenium and osmium complexes with modified curcuminoid ligands.
Dalton Trans. 51:13311–13321. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gobbo A, Pereira SAP, Biancalana L,
Zacchini S, Saraiva MLMFS, Dyson PJ and Marchetti F: Anticancer
ruthenium(II) tris(pyrazolyl)methane complexes with bioactive
co-ligands. Dalton Trans. 51:17050–17063. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonfili L, Pettinari R, Cuccioloni M,
Cecarini V, Mozzicafreddo M, Angeletti M, Lupidi G, Marchetti F,
Pettinari C and Eleuteri AM: Arene-RuII complexes of curcumin exert
antitumor activity via proteasome inhibition and apoptosis
induction. Chem Med Chem. 7:2010–2020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pettinari R, Marchetti F, Condello F,
Pettinari C, Lupidi G, Scopelliti R, Mukhopadhyay S, Riedel T and
Dyson PJ: Ruthenium(II)-Arene RAPTA type complexes containing
curcumin and bisdemethoxycurcumin display potent and selective
anticancer activity. Organometallics. 33:3709–3715. 2014.
View Article : Google Scholar
|
|
17
|
Caruso F, Pettinari R, Rossi M, Monti E,
Gariboldi MB, Marchetti F, Pettinari C, Caruso A, Ramani MV and
Subbaraju GVJ: The in vitro antitumor activity of
arene-ruthenium(II) curcuminoid complexes improves when decreasing
curcumin polarity. J Inorg. Biochem. 162:44–51. 2016.
|
|
18
|
Garufi A, Baldari S, Pettinari R,
Gilardini Montani MS, D'Orazi V, Pistritto G, Crispini A, Giorno E,
Toietta G, Marchetti F, et al: A ruthenium(II)-curcumin compound
modulates NRF2 expression balancing the cancer cell death/survival
outcome according to p53 status. J Exp Clin Cancer Res. 39:1222020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garufi A, Pettinari R, Marchetti F, Cirone
M and D'Orazi G: NRF2 and Bip interconnection mediates resistance
to the organometallic ruthenium-cymene bisdemethoxycurcumin complex
cytotoxicity in colon cancer cells. Biomedicines. 11:5932023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Orazi G and Cirone M: Interconnected
adaptive responses: A way out for cancer cells to avoid cellular
demise. Cancers. 14:27802022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garufi A, Pistritto G, D'Orazi V, Cirone M
and D'Orazi G: The impact of NRF2 inhibition on drug-induced colon
cancer cell death and p53 activity: A pilot study. Biomolecules.
2:4612022. View Article : Google Scholar
|
|
22
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jain A, Lamark T, Sjottem E, Bowitz Larsen
K, Awuh JA, Overvatn A, McMahon M, Hayes JD and Johansen T:
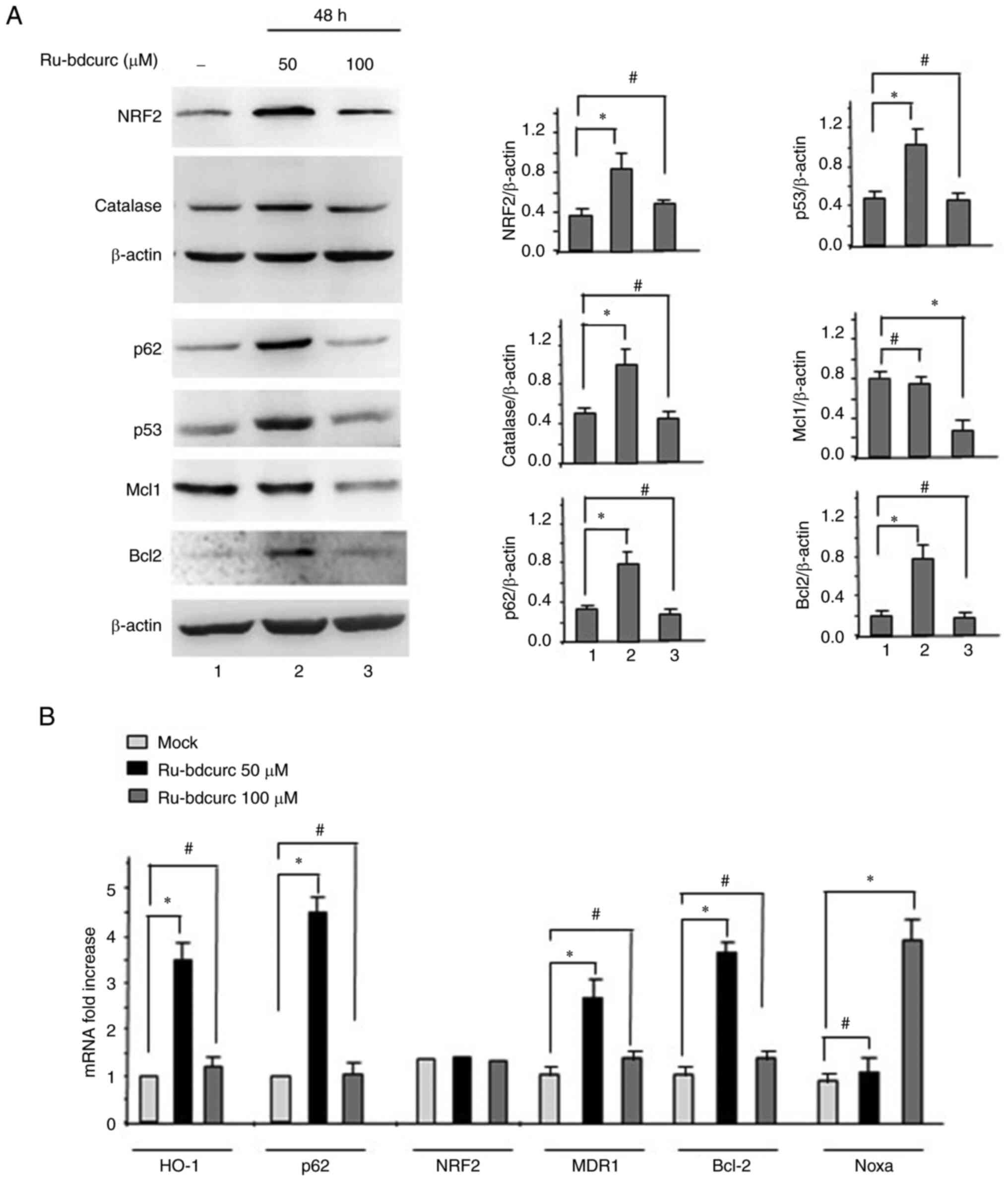
p62/SQSTM1 is a target gene for transcription factor NRF2 and
creates a positive feedback loop by inducing antioxidant response
element-driven gene transcription. J Biol Chem. 285:22576–22591.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komatsu M, Kurokawa H, Waguri S, Taguchi
K, Kobayashi A, Ichmura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et
al: The selective autophagy substrate p62 activates the stress
response transcription factor Nrf2 through inactivation of Keap1.
Nat Cell Biol. 12:213–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang T, Harder B, Rojo de la Vega M, Wong
PK, Chapman E and Zhang DD: p62 links autophagy and Nrf2 signaling.
Free Rad Biol Med. 88:199–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lister A, Nedjadi T, Kitteringham NR,
Campbell F, Costello E, Lloyd B, Copple IM, Williams S, Owen A,
Neoptolemos JP, et al: Nrf2 is overexpressed in pancreatic cancer:
Implications for cell proliferation and therapy. Mol Cancer.
10:372011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evers BM, Ishizuka J, Townsend CM Jr and
Thompson JC: The human carcinoid cell line, BON. A model system for
the study of carcinoid tumors. Ann N Y Acad Sci. 733:393–406. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vandamme T, Peeters M, Dogan F, Pauwels P,
Van Assche E, Beyens M, Mortier G, Vandeweyer G, de Herder W, Van
Camp G, et al: Whole-exome characterization of pancreatic
neuroendocrine tumor cell lines BON-1 and QGP-1. J Mol Endocrinol.
54:137–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luley KB, Biedermann SB, Kunstner A, Busch
H, Franzenburg S, Schrader J, Grabowsi P, Wellner U, Keck T,
Brabant G, et al: A comprehensive molecular characterization of the
pancreatic neuroendocrine tumor cell lines BON-1 and QGP-1. Cancers
(Basel). 12:6912020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
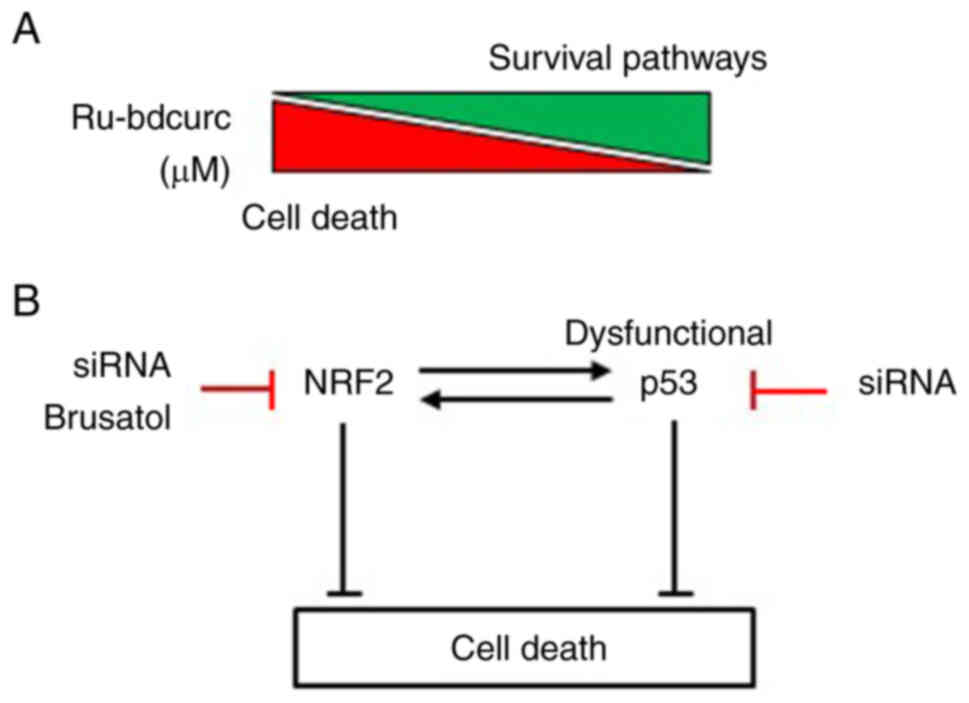
Cirone M and D'Orazi G: NRF2 in cancer:
Cross-talk with oncogenic pathways and involvement in
gammaherpesviruses-driven carcinogenesis. Int J Mol Sci.
24:5952022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vousden HK and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voutsadakis IA: Mutations of p53
associated with pancreatic cancer and therapeutic implications. Ann
Hepatobiliary Pancreat Surg. 25:315–327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vijayvergia N, Boland PM, Handorf E,
Gustafson KS, Gong Y, Cooper HS, Sheriff F, Astsaturov I, Cohen SJ
and Engstrom PF: Molecular profiling of neuroendocrine malignancies
to identify prognostic and therapeutic markers: A Fox Chase Cancer
Center Pilot Study. Br J Cancer. 115:564–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
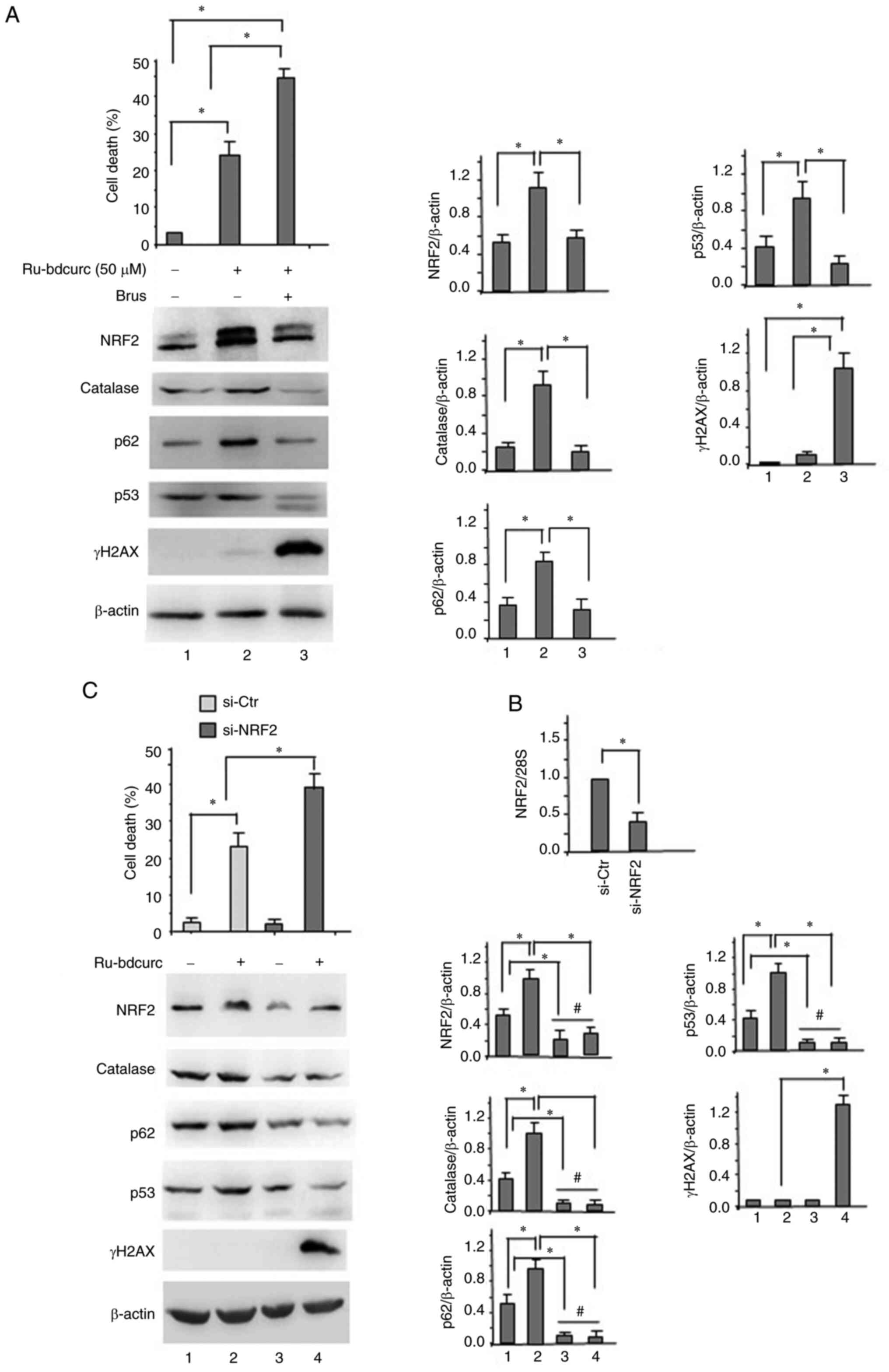
Ren D, Villeneuve NF, Jiang T, Wu T, Lau A
and Toppin HA: Brusatol enhances the efficacy of chemotherapy by
inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci
USA. 108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olayanju A, Copple IM, Bryan HK, Edge GT,
Sison RL, Wong MW, Lai ZQ, Lin ZX, Dunn K, Sanderson CM, et al:
Brusatol provokes a rapid and transient inhibition of Nrf2
signaling and sensitizes mammalian cells to chemical toxicity
implications for therapeutic targeting of Nrf2. Free Rad Biol Med.
78:202–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garufi A, Traversi G, Gilardini Montani
MS, D'Orazi V, Pistritto G, Cirone M and D'Orazi G: Reduced
chemotherapeutic sensitivity in high glucose condition: implication
of antioxidant response. Oncotarget. 10:4691–4702. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cecchinelli B, Lavra L, Rinaldo C,
Iacovelli S, Gurtner A, Gasbarri A, Ulivieri A, Del Prete F,
Trovato M, Piaggio G, et al: Repression of the antiapoptotic
molecule galectin-3 by homeodomain-interacting protein kinase
2-activated p53 is required for p53-induced apoptosis. Mol Cell
Biol. 26:4746–4757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brummelkamp TR, Bernards R and Agami R: A
system stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2020. View Article : Google Scholar
|
|
40
|
Garufi A, Giorno E, Gilardini Montani MS,
Pistritto G, Crispini A, Cirone M and D'Orazi G:
P62/SQSTM1/Keap1/NRF2 axis reduces cancer cells death-sensitivity
in response to Zn(II)-curcumin complex. Biomolecules. 11:3482021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lisek K, Campaner E, Ciani Y, Walerych D
and Del Sal G: Mutant p53 tunes the NRF2-dependent antioxidant
response to support survival of cancer cells. Oncotarget.
9:20508–20523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gong C, Yao H, Liu Q, Chen J, Shi J, Su F
and Song E: Markers of tumor-initiating cells predict
chemoresistance in breast cancer. PLoS One. 5:e156302010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garufi A, Pistritto G, Cirone M and
D'Orazi G: Reactivation of mutant p53 by capsaicin, the major
constituent of peppers. J Exp Clin Cancer Res. 35:1362016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 15:2658–2664. 2003.PubMed/NCBI
|
|
45
|
Nodale C, Sheffer M, Jacob-Hirsch J,
Folgiero V, Falcioni R, Aiello A, Garufi A, Rechavi G, Givol D and
D'Orazi G: HIPK2 downregulates vimentin and inhibits breast cancer
cell invasion. Cancer Biol Ther. 13:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Garufi A, Trisciuoglio D, Porru M,
Leonetti C, Stoppacciaro A, D'Orazi V, AVantaggiati ML, Crispini A,
Pucci D and D'Orazi G: A fluorescent-based Zn(II)-complex
reactivates mutant (R175H and R272H) p53 in cancer cells. J Exp
Clin Cancer Res. 32:722013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garufi A, Pucci D, D'Orazi V, Cirone M,
Bossi G, Avantaggiati ML and D'Orazi G: Degradation of mutant
p53H175 protein by Zn(II) through autophagy. Cell Death Dis.
5:e12712014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bonner WM, Redon CE, Dickey JS, Nakamura
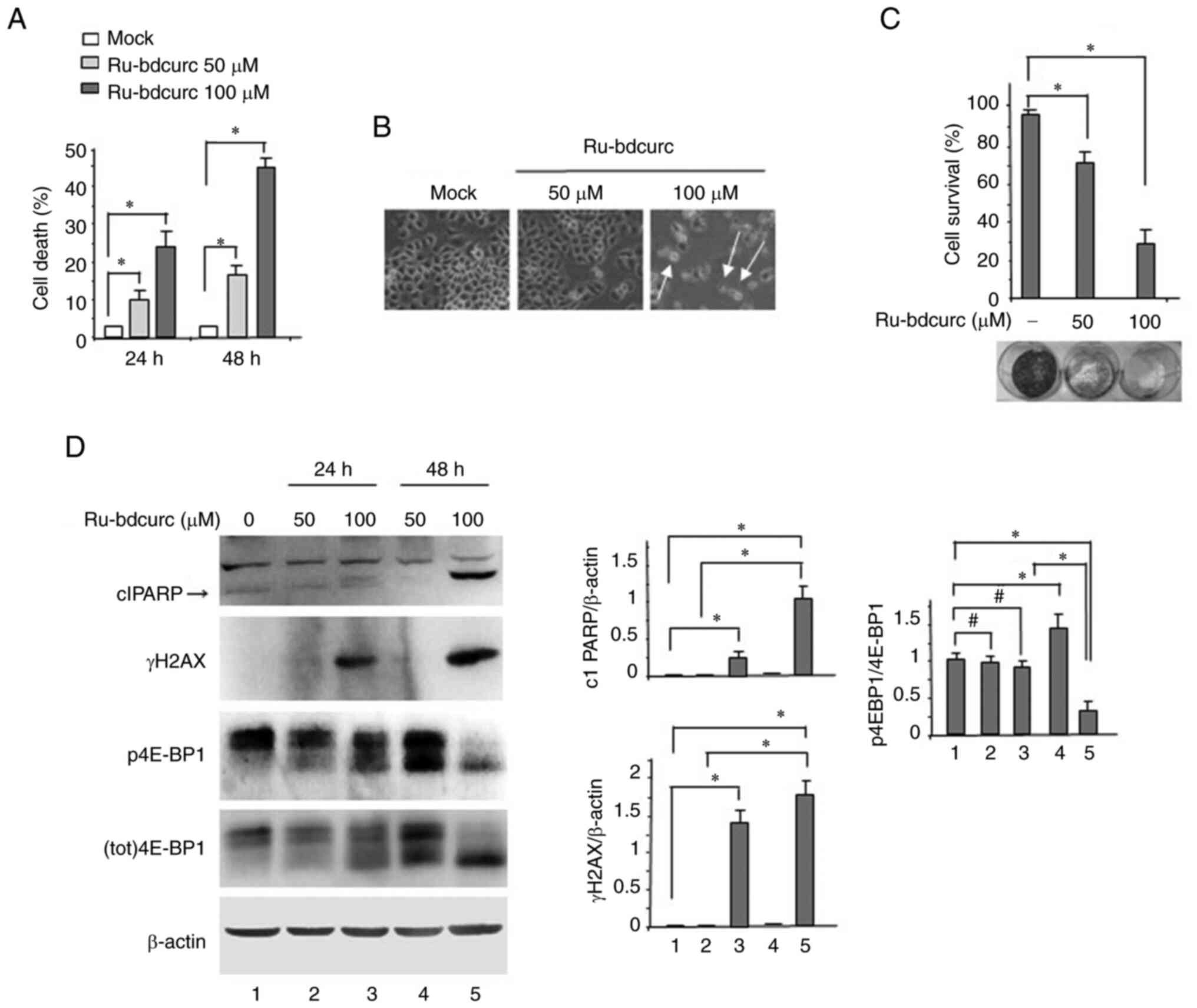
AJ, Sedelnikova OA, Solier S and Pommier Y: Gamma H2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao Y, Chen L, Shi C, Fan R, Chen P, Liu
H, Xia A and Qin H: Increased phosphorylation of 4E-binding protein
1 predicts poor prognosis for patients with colorectal cancer. Mol
Med Rep. 15:3099–3104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonnella R, Zarrella R, Santarelli R,
Germano CA, Gilardini Montani MS and Cirone M: Mechanisms of
sensitivity and resistance of primary effusion lymphoma to Dimethyl
Fumarate (DMF). Int J Mol Sci. 23:67732022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zanini S, Renzi S, Giovinazzo F and
Bermano G: mTOR pathway in gastroenteropancreatic neuroendocrine
tumor (GEP-NETs). Front Endocrinol. 11:5625052020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moscat J, Karin M and Diaz-Meco MT: p62 in
cancer: Signaling adaptor beyond autophagy. Cell. 167:606–609.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Widden H and Placzek WJ: The multiple
mechanisms of Mcl1 in the regulation of cell fate. Commun Biol.
4:10292021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gilardini Montani MS, Cecere N, Granato M,
Romeo MA, Falcinelli L, Ciciarelli U, D'Orazi G, Faggioni A and
Cirone M: Mutant p53, stabilized by its interplay with HSP90,
activates a positive feed-back loop between NRF2 and p62 that
induces chemo-resistance to Apigenin in pancreatic cancer cells.
Cancers. 11:7032019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sampath J, Sun D, Kidd VJ, Grenet J,
Gandhi A, Shapiro LH, Wang Q, Zambetti GP and Schuetz JD: Mutant
p53 cooperates with ETS and selectively up-regulates human MDR1 not
MRP1. J Biol Chem. 276:39359–39367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martin AG, Trama J, Crighton D, Ryan KM
and Fearnhead HO: Activation of p53 and induction of Noxa by DNA
damage requires NG-kappa B. Aging (Albany NY). 1:335–349. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chiou JT, Huang NC, Hiang CH, Wang LJ, Lee
YC, Shi YJ and Chang LS: NOXA-mediated degradation of MCL1 and
BCL2L1 causes apoptosis of daunorubicin-treated human acute myeloid
leukemia cells. J Cell Physiol. 236:7356–7375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park JY, Sohn HY, Koh YH and Jo C:
Curcumin activates Nrf2 through PKCd-mediated p62 phosphorylation
at Ser351. Sci Rep. 11:84302021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kawasaki K, Fujii M and Sato T:
Gastroenteroepatic neuroendocrine neoplasms: Genes, therapies and
models. Dis Model Mech. 11:dmm0295952018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao J, Jia L, Zhou HM, Liu Y and Zhong LF:
Mitochondrial and nuclear DNA damage induced by curcumin in human
hepatoma G2 cells. Toxicol. 91:476–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kunati SR, Yang SM, William BM and Xu Y:
An LC-MS/MS method for simultaneous determination of curcumin,
curcumin glucuronide and curcumin sulfate in a phase II clinical
trial. J Pharm Biomed Anal. 156:189–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Giordano A and Tommonaro G: Curcumin and
Cancer. Nutrients. 11:23762019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Arena A, Romeo MA, Benedetti R, Gilardini
Montani MS, Santarelli R, Gonnella R, D'Orazi G and Cirone M: NRF2
and STAT3: Friends or foes in carcinogenesis? Discov Oncol.
14:372023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sajadimajd S and Khazaei M: Oxidative
stress and cancer: The role of Nrf2. Curr Cancer Drug Targtes.
18:538–557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
No JH, Kim YB and Song YS: Targeting Nrf2
signaling to combat chemoresistance. J Cancer Prev. 19:111–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Torrente L and DeNicola GM: Targeting NRF2
and its downstream processes: Opportunities and challenges. Annu
Rev Pharmacol Toxicol. 62:279–300. 2022. View Article : Google Scholar : PubMed/NCBI
|