The primary reason for cancer deaths is metastasis,
which occurs when tumor cells from the originating site infiltrate
into lymphatic veins, blood vessels or other passageways and are
transported to other places for continued growth, resulting in
tumors of the same type as the primary-site tumors (1–3) and
the original tumors transform into metastatic ones. Metastasis is
one of the defining characteristics of types of cancer (4). Lymphatic, vascular and implant
metastases are some of the main transmission mechanisms (5). The majority of cancer cells enter the
local lymph nodes (LNs) through lymphatic vessels and form
intra-lymphatic metastasis to cause primary cell deaths (6). Once invading lymphatic vessels, the
cancer cells may shed from tumors to form an embolus or proliferate
in the vessels to form a continuous mass. As a result,
cancer-related morbidity and mortality are primarily caused by
metastatic diseases (7). Lungs,
livers, bones and the brain are frequent sites for tumor metastasis
(8).
The following is a summary of what is known of the
characteristics of metastatic types of cancer: i) Metastatic cells
are less stable and have a higher rate of spontaneous mutations
than non-metastatic cells of the same origin. ii) Metastases can
grow into invasive tumors even in the absence of a substantial
initial tumor mass (9). iii) A
number of primary solid tumors contain either localized or distant
metastases and are physiologically diverse prior to detection
(3,10). iv) The cells of a tumor are
physiologically diverse (10). v)
The primary factor for the treatment failure and deaths of patients
with malignant malignancies is metastasis (11). vi) The phases of interacting with
the microenvironment, invasion, migration, resistance to apoptosis
and angiogenesis generation should be completed by tumor cells
(12).
A number of studies have verified that early-onset
metastases can occur in a number of types of cancer such as
gallbladder cancer (21), lung
cancer (18), breast cancer
(5,19), urothelial carcinoma of the bladder
(22), esophageal cancer (23) and colorectal cancer (CRC) (24,25),
which are often biologically aggressive. In particular, in
gallbladder cancer, early distant metastases have been demonstrated
in 16% of resected T2 lesions (26). Similarly, the rate of LN metastases
among all patients with T1-2 CRC ranges from 2–8.4% (24). In addition, compared with a 5-year
survival rate of >90% among T1-2 patients without Stage I LN
metastases (LNMs) in CRC, the survival rate of T1-2 patients with
positive Stage III LNMs is <70% (24), suggesting that the high incidence of
Stage I LNMs, including T1 and T2, leads to a higher TNM mortality
and staging (25,27). Bone metastases in the fallopian
tubes, peritoneum and ovary with advanced bone diagnosis have
little prognostic effect, whereas early bone metastases have a
significant impact. These findings suggest that distant metastases
play an active role in the progression of early types of cancer and
it can be concluded that if detected early, cancerous patients can
show good survival rates. However, some type of cancer, such as
esophageal squamous cell carcinoma, despite having been detected
early and resected completely, the 5-year survival rate remains low
with the prognosis remaining poor (28). Therefore, predicting the status of
LN metastases of patients with early-stage types of cancer (T1-2)
is essential for observing the clinicopathological characteristics
and prognosis of patients while determining the type of treatment
they should receive, which will be discussed later.
Various types of cancer exhibit distinctive
characteristics concerning early metastases. For instance, in
breast cancer, patients with primary tumors located in the caudal
axilla or invasive ductal carcinoma are more likely to test
positive for LNMs (19).
Conversely, in terms of colon cancer, the propensity is often
towards the left side (24).
Furthermore, the TNM staging of early tumor metastases differs. In
breast cancer, LN positivity tends to be higher among T1 patients
compared with T2 patients (19). In
summary, early-onset metastatic types of cancer typically manifest
multifactorial clinicopathologic features. For early-onset gastric
cancer (GC), bowel type, T1b stage and tumor size emerge as the
risk factors of LNM development, with T1b and LNMs positivity
serving as risk factors for their survival (29). These findings suggest a systematic
and distinctive distribution of early-onset metastatic types of
cancer across both time and space.
Numerous studies have underscored that early-onset
metastatic types of cancer are subject to a myriad of factors, with
their demographic distribution exhibiting distinct characteristics.
Younger patients exhibit a higher propensity for developing LNMs in
comparison with their older counterparts (18,19,30).
This observation implies that an early detection at the initial
stage may enhance the survival outcomes of patients (31,32).
Additionally, there is a noteworthy disparity based on race
(33). Furthermore, the primary
sites of different metastatic types of cancer vary due to
differences in the sites of metastases. For instance, a predominant
site of liver metastases among patients with pancreatic cancer is
the tail of the pancreas (30),
underscoring the substantial influence of tumor location on
metastases. Moreover, individuals with detrimental lifestyle
habits, such as smoking and alcohol abuse, exhibit a heightened
susceptibility to developing early-onset metastatic types of cancer
(34). In conclusion, the
occurrence of early-onset metastatic types of cancer is intricately
linked to tumor characteristics, demographic factors and lifestyle
habits.
Early-onset metastatic types of cancer are usually
associated with abnormalities in signal transduction pathways.
Elevated rates of P53 mutations among individuals with an
early-onset breast cancer impede the expression of the
growth-arrest-specific 7 (GAS7) gene, which has notably been
identified as a potent inhibitor of breast cancer metastases,
exerting its effect on the cytoplasmic FMRP-interacting protein
(CYFIP1) and WASP-family verprolin-homologous 2 (WAVE2) complex to
obstruct CYFIP1 and Rac1 protein interactions, actin polymerization
as well as the β1-integrin/FAK/Src signaling pathway. Rac1, an
activated GTP form, stimulates actin polymerization by binding to a
WAVE2 subunit. However, the interaction of GAS7 isoform b (GAS7b)
with CYFIP1 thwarts this process, concurrently inhibiting the
β1-integrin/FAK/Src signaling pathway, ultimately impeding breast
cancer metastases (35).
Similarly, the metastases of early-onset prostate
cancer, a specific molecular subtype, are primarily governed by the
transmembrane protease, serine 2, a gene with the erythroblast
transformation-specific-related gene (TMPRSS2-ERG fusion gene). To
a lesser extent, alterations in the androgen receptor, speckle-type
POZ protein and additional sex comb-like 1 also contribute to the
regulatory landscape of this process (36). Meanwhile, the BRCA1 gene assumes a
pivotal role in the metastases of early-onset colon cancer.
Functioning as an antioncogene involved in diverse biological
processes, variations in the BRCA1 gene have been associated with a
five-fold increase in the risk of CRC development (37). Furthermore, the early expression of
BRCA1 gene mutations is closely linked to a poor prognosis of CRC
(37). These findings underscore
the significance of biochemical characteristic alterations as
contributory factors for the initiation of early metastatic types
of cancer.
The clinical features of various types of cancer are
important prognostic indicators of patients with cancer. The
survival rate of patients with distant metastases is very low
(38,39). It has been established that the
degree of vascular invasion and differentiation is an independent
prognostic indicator of the overall survival after 5 years
(40). In oral tongue cancer, the
large tumor volume (≥20 cm3) is significantly associated
with the 5-year disease-specific survival (41). In addition, the sequence of
insurances, radiotherapy, surgeries and chemotherapy compared with
surgeries is another important independent prognostic factor
(23).
The evidence that a number of molecular
characteristics can influence the metastases of types of cancer has
been explored in numerous studies and some indicators are usually
taken into account, such as age, race, tumor size, tumor location,
tumor number, histological grade, pathological grade and T-status
(19,21–23,41–43).
In most cases, these factors, which have a strong effect on types
of cancer, usually consist of predictive models. For example, in
gallbladder cancer, histologic grade has the highest discrimination
(44) and a poor grade is the
strongest indicator of distant metastases (45). In squamous cell carcinoma, age has
been found to be significantly associated with distant metastases
(46). In different types of
cancer, each tumor has its own specific criteria for detection. In
addition to the aforementioned indicators, the nerve terminal
invasion and clinical assessment of LNMs (cLNMs) are two other
biomarkers of colorectal tumor metastases to LNs (24). Similarly, lymphovascular invasion
(LVI) can help diagnose uroepithelial carcinoma of the bladder
(22), as well as axillary node
metastases in breast cancer (47)
and gastric cancer (42,48,49).
Similarly, in gastric cancer, the exclusive features predicted
compared with other types of cancer are ulcerative findings, and
the LN status is reported through computed tomography (48–50).
The phenomenon of metastases in early-onset types of
cancer is closely related to genetic factors. A previous study on
the early-onset metastatic CRC indicate that younger patients
(<50 years old) have a significantly shorter progression-free
and overall survival compared with older patients, showing a
disparity that can be attributed to distinct genomic profiles
influencing treatment-related adverse events (51). At the same time, the precision
provided by the next-generation sequencing (NGS) technology and the
knowledge of circulating tumor DNA (ctDNA) offer new insights as
well as possibilities for the diagnosis and treatment of types of
cancer.
NGS technology has emerged as an indispensable tool
for research on types of cancer, providing unprecedented insights
into the genetic factors that may contribute to the phenomenon of
early-onset metastases. A previous study (52) highlights the use of NGS in
identifying mutations within the SF3B1 gene associated with an
increased risk of early metastases among patients with uveal
melanoma. This groundbreaking work illustrates the ability of NGS
to uncover specific genetic alterations that could serve as
predictive biomarkers for metastases, offering a more nuanced
approach to patient stratification and prognosis.
By integrating the results of these studies, it has
been found that NGS has become critical for identifying genetic
factors associated with early-onset metastases. Each study brings
to light the promise of NGS in enabling the detection of genetic
markers that can predict the course of types of cancer more
accurately than ever before, marking a significant advancement
towards personalized oncology with improved patient outcomes.
The understanding of ctDNA is rapidly evolving in
research on modern oncology. A previous study has shown its great
potential for early cancer diagnosis, treatment monitoring and
minimal residual disease assessment (58). Particularly for CRC treatment, ctDNA
analysis assists in accurately categorizing the prognoses of
patients and guiding personalized adjuvant chemotherapy. However,
challenges such as the handling of liquid biopsy samples, the
variability of assay sensitivities and specificities as well as
technological limitations remain in the clinical application of
ctDNA analysis.
Further advancements in oncology encompass various
cancer types, with significant developments in treating blood and
solid malignancies, groundbreaking immunotherapies for rectal
cancer, novel engineered cell therapies as well as clinical trials
for pancreatic cancer and other solid tumors. The progresses in
targeting the tumor microenvironment as well as developing drugs
and cancer vaccines, along with ctDNA research, are revolutionizing
the situation of cancer diagnosis and treatment, offering new hopes
and strategies for combating this complex disease.
In some cases, cytokines are an important factor in
tumor progression. First, blood counts are a routine part of the
preoperative examination. Studies have indicated that
inflammation-related factors and hematological parameters are also
responsible for LN metastases and tumor progression in different
types of cancer (59,60). Previous studies have shown that the
neutrophil-to-lymphocyte ratio, the platelet-to-lymphocyte ratio
(PLR) and fibrinogen are important hematological predictors of LNMs
(61,62). For example, neurospecific enolase,
PLR, carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH)
and cytokeratin 19 fragment are independent hematological
parameters associated with distant metastases in lung
adenocarcinoma. Similarly, CEA is a biomarker of distant metastases
in colorectal tumor (25), while
the pre-CEA level is a biomarker of predict LNs (24). In addition, the statuses of human
epidermal growth factor receptor 2, progesterone receptor and
estrogen receptor are other important predictors of breast cancer
(19). Similarly, the statuses of
tumor LDH and serum LDH are two hematological parameters of
triple-negative breast cancer (63), implying that different clinical
factors have an important impact on early-onset metastatic types of
cancer.
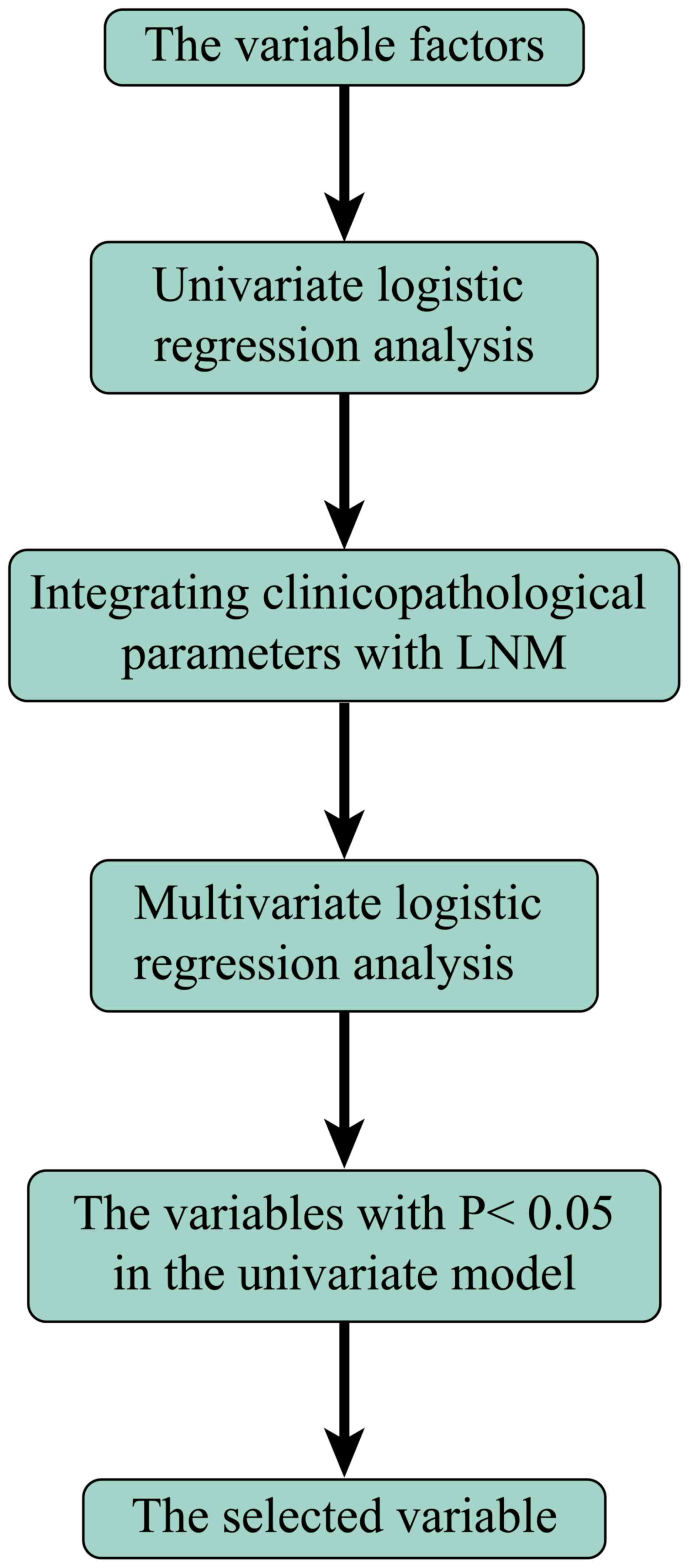
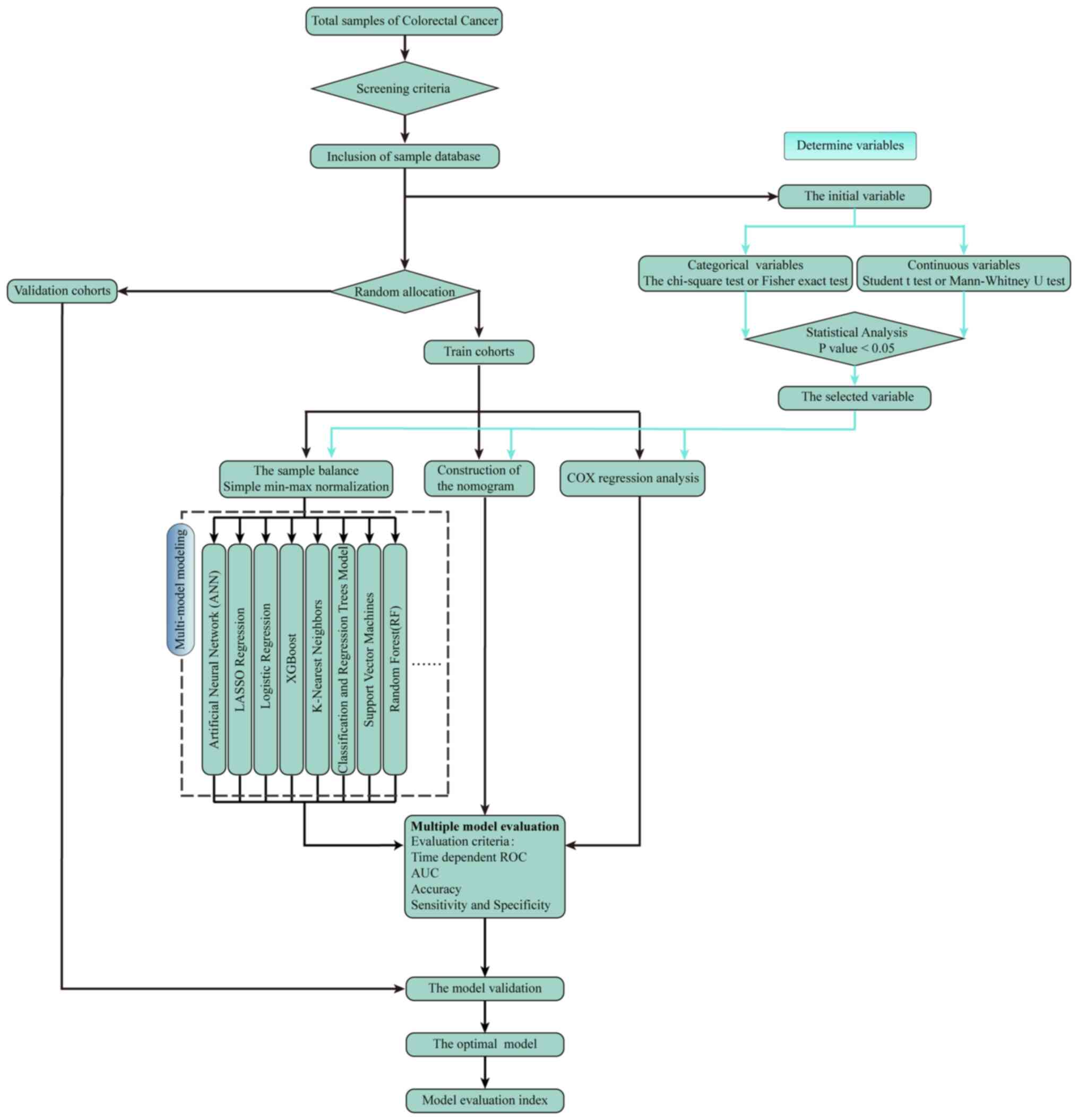
Accordingly, univariate and multivariate logistic
regression analysis and identification were used to screen out
influential factors (64–67). After the exclusion of unknown data,
the remaining factors were selected to build a prediction model to
detect distant metastases (Fig. 1).
After building an appropriate model, in order to assess the impact
of each factor, it was easy to calculate the total score by summing
up each particular score, and by processing the total score to a
lower criterion, it is possible to predict the probability of
LNMs.
At present, several methods including imaging
techniques such as magnetic resonance imaging (MRI), computed
tomography (CT) (68,69), quantitative comparative proteomics
and histological analysis (70)
have been used to identify factors influencing the prediction of
distant metastases. In urological tumors, positron emission
computed tomography/CT using radionuclides such as 11C-choline has
become one of the routine imaging tools (71,72),
whose advantage is that it allows the assessment of the prostate
bed and reduces the urinary excretion of patients (73).
A series of experiments have demonstrated that
conventional MRI diagnostic models based on shape and size do not
reflect the true state of distant metastases (74,75),
which, even with the most advanced imaging techniques, are still
difficult and expensive to be accurately predicted (69,76,77).
Therefore, we should explore a more accurate model for clinical
diagnosis based on combining analytical factors such as
epidemiological features, pathological features and inflammatory
indicators to accurately identify the metastases of cancer.
Nomograms, developed in the multivariate logistic
regression mode, are popular visual graphs used to show the
predicted probability of an event for decision support while
achieving greater clinical benefits (78). This model also allows clinicians to
screen patients at a high risk of distant metastases for closer
follow-ups and adjuvant therapies (Fig.
2).
Machine learning (ML) is a model of artificial
intelligence in which various probabilistic, optimization and
statistical techniques are used, allowing computers to learn
summarized information from historical data and make predictions
from new data (79,80). Several studies have shown that ML
can surpass human judgments in a number of aspects in predicting
patient outcomes or cancer risks (81–84).
In contrast to traditional statistical methods that rely on
predetermined models such as logistic regression (LR), ML can be
used to detect deeply the interactions among variables and update
algorithms by learning from iterations on the data. In addition,
the ML technique can help clinicians to provide new ideas for more
personalized patient care (Fig.
3).
Radiomics, as another detection system, can also
help identify patients with LN metastases. In combination with
patient/tumor characteristics, radiomic features can be utilized
through clinical decision support systems to make medical decisions
and ensure diagnostic accuracy. For example, in terms of cervical
cancer, a radiomics model has been developed, which incorporates
the squamous cell carcinoma antigen level and has shown good
predictive results (85).
Notably, there are some methodological indications
of the established models. Based on a receiver operating
characteristic curve (ROC) analysis, calibration curves and the
C-index, these models have improved performance compared with
traditional methods such as CT and MRI. Therefore, these modeling
techniques will play an important role in the analysis of medical
datasets. In addition, decision curves are used to assess clinical
utility, such as in esophageal squamous cell carcinoma (23). In addition, the Cox univariate
regression analysis is a method to assess predictable independent
prognostic factors (23), which
means that it offers a novel approach to assess the clinical value
of various testing models.
Cancer metastasis refers to the spread of diseases
from one part of the body to another that is not directly related
to it. With the development of extensive data analysis and
retrospective studies, it has been found that cancer metastases can
also occur in the early stages of types of cancer, and the
definition of ‘early metastatic cancer’ was refined by Hüsemann
et al (14) A study
demonstrated that cells from early low-density lesions express more
stem cells, which have more migratory and metastatic functions than
cells from advanced large-density tumors (15), implying that early-onset metastatic
types of cancer may play an important role in cancer progression,
causing great harm to the human body. In order to grasp the distant
metastases and characteristic distribution of various types of
cancer such as breast, gallbladder, bladder urothelial, colorectal
and gastric cancer, the present review systematically evaluated and
discussed the clinicopathological features of different early-onset
metastatic types of cancer while summarizing their epidemiological
characteristics. In detail, the early onset of metastases was
associated with a large number of clinicopathological features.
Predictors vary from tumor to tumor, but tumor size, tumor
location, tumor number, histologic grade, pathologic grade and
T-status are usually the most common indicators. In addition, some
biochemical features can be other important predictors. In
different types of cancer, the predictors are specific. It has been
found that early-onset metastatic types of cancer are associated
with the poor prognosis of cancerous patients. Depending on
different factors, a number of studies have validated that a number
of new models can be developed to effectively predict whether
early-onset metastatic types of cancer occur (86,87).
The area under curve (AUC) associated with ROC represents the
accuracy of detection and decision curve analysis can be used to
assess the clinical utility and ensure the reliability of model
prediction significantly. Nomograms and ML have become common
models compared with traditional imaging techniques, which are
relatively advanced and effective. A few studies (88–91)
have also been conducted using new approaches, such as radiomics,
through which some accuracy can also be achieved. Due to fewer
studies, these models cannot be widely used. Taken together, the
development of these models suggests that it may become an
important detectable prognostic factor for patients (41). However, the present review had a
number of shortcomings. First of all, the sample size of all
reference studies was small, which was associated with information
biases and unavoidable selection biases and the present review was
unable to extract more representative conclusions. Second, the
validation cohorts of some predictable models had low AUCs, which
might affect the accuracy of the models. Finally, all the data was
from delineated patient subgroups; an external validation of the
models remains necessary. Most importantly, various studies have
shown that early-onset metastatic types of cancer play an important
role in cancer development. Therefore, it is hoped to build models
to predict it as soon as possible, so as to take clinical
treatments and therapies for cancerous patients.
Not applicable.
Funding: No funding was received.
Not Applicable.
XLT and WO conceived and designed this review. LY,
ZX, XFT, ZZ and ZX contributed in the writing of the manuscript.
LY, ZH and ZX was involved in article revision. LY and ZH surveyed
the literature and provided suggestions. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A, Kalluri R and Kshitiz: Systems biology of cancer
metastasis. Cell Syst. 9:109–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Targeting metastasis. Nat Rev
Cancer. 16:201–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harper KL, Sosa MS, Entenberg D, Hosseini
H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis
RJ, et al: Mechanism of early dissemination and metastasis in
Her2+ mammary cancer. Nature. 540:588–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turajlic S and Swanton C: Metastasis as an
evolutionary process. Science. 352:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linde N, Casanova-Acebes M, Sosa MS,
Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I,
Jones J, et al: Macrophages orchestrate breast cancer early
dissemination and metastasis. Nat Commun. 9:212018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sosa MS, Bragado P and Aguirre-Ghiso JA:
Mechanisms of disseminated cancer cell dormancy: An awakening
field. Nat Rev Cancer. 14:611–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlidis N, Khaled H and Gaafar R: A mini
review on cancer of unknown primary site: A clinical puzzle for the
oncologists. J Adv Res. 6:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klein CA, Blankenstein TJ, Schmidt-Kittler
O, Petronio M, Polzer B, Stoecklein NH and Riethmüller G: Genetic
heterogeneity of single disseminated tumour cells in minimal
residual cancer. Lancet. 360:683–689. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gianni L, Dafni U, Gelber RD, Azambuja E,
Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch
C, et al: Treatment with trastuzumab for 1 year after adjuvant
chemotherapy in patients with HER2-positive early breast cancer: A
4-year follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bacac M and Stamenkovic I: Metastatic
cancer cell. Annu Rev Pathol. 3:221–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanger N, Effenberger KE, Riethdorf S, Van
Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M and
Pantel K: Disseminated tumor cells in the bone marrow of patients
with ductal carcinoma in situ. Int J Cancer. 129:2522–2526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Husemann Y, Geigl JB, Schubert F, Musiani
P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G and
Klein CA: Systemic spread is an early step in breast cancer. Cancer
Cell. 13:58–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosseini H, Obradovic MMS, Hoffmann M,
Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C,
Maneck M, et al: Early dissemination seeds metastasis in breast
cancer. Nature. 540:552–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aguirre-Ghiso JA, Bragado P and Sosa MS:
Metastasis awakening: Targeting dormant cancer. Nat Med.
19:276–277. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polzer B and Klein CA: Metastasis
awakening: The challenges of targeting minimal residual cancer. Nat
Med. 19:274–275. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu W, Hu M, Wang W, Shi C and Mei J:
Development and validation of a novel nomogram for predicting
tumor-distant-metastasis in patients with Early T1-2 stage lung
adenocarcinoma. Ther Clin Risk Manag. 16:1213–1225. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao YX, Liu YR, Xie S, Jiang YZ and Shao
ZM: A nomogram predicting lymph node metastasis in T1 breast cancer
based on the surveillance, epidemiology, and end results program. J
Cancer. 10:2443–2449. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu J, Zheng J, Li L, Huang R, Ren H, Wang
D, Dai Z and Su X: Application of machine learning algorithms to
predict central lymph node metastasis in T1-T2, Non-invasive, and
clinically node negative papillary thyroid carcinoma. Front Med
(Lausanne). 8:6357712021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai YL, Lin YX, Jiang LS, Ye H, Li FY and
Cheng NS: A Novel nomogram predicting distant metastasis in T1 and
T2 gallbladder cancer: A SEER-based study. Int J Med Sci.
17:1704–1712. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ou N, Song Y, Liu M, Zhu J, Yang Y and Liu
X: Development and validation of a nomogram to predict lymph node
metastasis in patients with T1 High-grade urothelial carcinoma of
the bladder. Front Oncol. 10:5329242020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Hu W, Yao N, Sun M, Li X, Wang L,
Yang Y and Li B: Development and validation of a nomogram to
predict overall survival of T1 esophageal squamous cell carcinoma
patients with lymph node metastasis. Transl Oncol. 14:1011272021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mo S, Zhou Z, Dai W, Xiang W, Han L, Zhang
L, Wang R, Cai S, Li Q and Cai G: Development and external
validation of a predictive scoring system associated with
metastasis of T1-2 colorectal tumors to lymph nodes. Clin Transl
Med. 10:275–287. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo K, Feng Y, Yuan L, Wasan HS, Sun L,
Shen M and Ruan S: Risk factors and predictors of lymph nodes
metastasis and distant metastasis in newly diagnosed T1 colorectal
cancer. Cancer Med. 9:5095–5113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fong Y, Jarnagin W and Blumgart LH:
Gallbladder cancer: Comparison of patients presenting initially for
definitive operation with those presenting after prior noncurative
intervention. Ann Surg. 232:557–569. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu DY, Cao B, Li SH, Li P and Zhang ST:
Incidence, risk factors, and a predictive model for lymph node
metastasis of submucosal (T1) colon cancer: A population-based
study. J Dig Dis. 20:288–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Yang Y, Shafiulla Shaik M, Hu J,
Wang K, Gao C, Shan T and Yin D: Three-Field versus Two-field
lymphadenectomy for esophageal squamous cell carcinoma: A
Meta-analysis. J Surg Res. 255:195–204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang CT and Chen SH: Higher lymph node
metastasis rate and poorer prognosis of intestinal-type gastric
cancer compared to diffuse-type gastric cancer in early-onset
early-stage gastric cancer: A retrospective study. Front Med.
8:7589772021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He C, Zhong L, Zhang Y, Cai Z and Lin X:
Development and validation of a nomogram to predict liver
metastasis in patients with pancreatic ductal adenocarcinoma: A
large cohort study. Cancer Manag Res. 11:3981–3991. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7:1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joyner AB and Runowicz CD: Ovarian cancer
screening and early detection. Womens Health (Lond). 5:693–699.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou QP, Ge YH and Liu CY: Comparison of
metastasis between early-onset and late-onset gastric signet ring
cell carcinoma. BMC Gastroenterol. 20:1–12. 2020. View Article : Google Scholar
|
|
34
|
Rohlfing ML, Mays AC, Isom S and Waltonen
JD: Insurance status as a predictor of mortality in patients
undergoing head and neck cancer surgery. Laryngoscope.
127:2784–2789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang JW, Kuo WH, Lin CM, Chen WL, Chan
SH, Chiu MF, Chang IS, Jiang SS, Tsai FY, Chen CH, et al: Wild-type
p53 upregulates an early onset breast cancer-associated gene GAS7
to suppress metastasis via GAS7-CYFIP1-mediated signaling pathway.
Oncogene. 37:4137–4150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chalmers ZR, Burns MC, Ebot EM, Frampton
GM, Ross JS, Hussain MHA and Abdulkadir SA: Early-onset metastatic
and clinically advanced prostate cancer is a distinct clinical and
molecular entity characterized by increased TMPRSS2-ERG fusions.
Prostate Cancer Prostatic Dis. 24:558–566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Freire MV, Martin M, Thissen R, Van Marcke
C, Segers K, Sépulchre E, Leroi N, Lété C, Fasquelle C, Radermacher
J, et al: Case report series: Aggressive HR deficient colorectal
cancers related to BRCA1 pathogenic ger1. Suhail Y, Cain MP, Vanaja
K, Kurywchak PA, Levchenko A, Kalluri R and Kshitiz: Systems
biology of cancer metastasis. Cell Syst. 9:109–127. 2019.
View Article : Google Scholar
|
|
38
|
Ashour Badawy A, Khedr G, Omar A, Bae S,
Arafat W and Grant S: Site of metastases as prognostic factors in
unselected population of Stage IV Non-small cell lung cancer. Asian
Pac J Cancer Prev. 19:1907–1910. 2018.PubMed/NCBI
|
|
39
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun ZQ, Ma S, Zhou QB, Yang SX, Chang Y,
Zeng XY, Ren WG, Han FH, Xie X, Zeng FY, et al: Prognostic value of
lymph node metastasis in patients with T1-stage colorectal cancer
from multiple centers in China. World J Gastroenterol.
23:8582–8590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joo YH, Hwang SH, Sun DI, Cho KJ, Park JO
and Kim MS: Relationships between tumor volume and lymphatic
metastasis and prognosis in early oral tongue cancer. Clin Exp
Otorhinolaryngol. 6:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mu J, Jia Z, Yao W, Song J, Cao X, Jiang J
and Wang Q: Predicting lymph node metastasis in early gastric
cancer patients: Development and validation of a model. Future
Oncol. 15:3609–3617. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan Y, Liu H, Mao K, Zhang M, Zhou Q, Yu
W, Shi B, Wang J and Xiao Z: Novel nomograms to predict lymph node
metastasis and liver metastasis in patients with early colon
carcinoma. J Transl Med. 17:1932019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu K, Yang X, Li L, Ruan M, Liu W, Lu W,
Zhang C and Li S: Neurovascular invasion and histological grade
serve as the risk factors of cervical lymph node metastases in
early tongue squamous cell carcinoma. Mol Neurobiol. 53:2920–2926.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Butte JM, Gönen M, Allen PJ, D'Angelica
MI, Kingham TP, Fong Y, Dematteo RP, Blumgart L and Jarnagin WR:
The role of laparoscopic staging in patients with incidental
gallbladder cancer. HPB (Oxford). 13:463–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuperman DI, Auethavekiat V, Adkins DR,
Nussenbaum B, Collins S, Boonchalermvichian C, Trinkaus K, Chen L
and Morgensztern D: Squamous cell cancer of the head and neck with
distant metastasis at presentation. Head Neck. 33:714–718. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lyman GH, Giuliano AE, Somerfield MR,
Benson AB III, Bodurka DC, Burstein HJ, Cochran AJ, Cody HS III,
Edge SB, Galper S, et al: American Society of Clinical Oncology
guideline recommendations for sentinel lymph node biopsy in
early-stage breast cancer. J Clin Oncol. 23:7703–7720. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sekiguchi M, Oda I, Taniguchi H, Suzuki H,
Morita S, Fukagawa T, Sekine S, Kushima R and Katai H: Risk
stratification and predictive risk-scoring model for lymph node
metastasis in early gastric cancer. J Gastroenterol. 51:961–970.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SM, Lee H, Min BH, Kim JJ, An JY, Choi
MG, Bae JM, Kim S, Sohn TS and Lee JH: A prediction model for lymph
node metastasis in early-stage gastric cancer: Toward tailored
lymphadenectomy. J Surg Oncol. 120:670–675. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yin XY, Pang T, Liu Y, Cui HT, Luo TH, Lu
ZM, Xue XC and Fang GE: Development and validation of a nomogram
for preoperative prediction of lymph node metastasis in early
gastric cancer. World J Surg Oncol. 18:22020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Meng L, Thapa R, Delgado MG, Gomez MF, Ji
R, Knepper TC, Hubbard JM, Wang X, Permuth JB, Kim RD, et al:
Association of age with treatment-related adverse events and
survival in patients with metastatic colorectal cancer. JAMA Netw
Open. 6:e2320035. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Drabarek W, van Riet J, Nguyen JQ, Smit
KN, van Poppelen NM, Jansen R, Medico-Salsench E, Vaarwater J,
Magielsen FJ, Brands T, et al: Identification of early-onset
metastasis in SF3B1 mutated uveal melanoma. Cancers. 14:8462022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Penney ME, Parfrey PS, Savas S and Yilmaz
YE: A genome-wide association study identifies single nucleotide
polymorphisms associated with time-to-metastasis in colorectal
cancer. BMC Cancer. 19:1–12. 2019. View Article : Google Scholar
|
|
54
|
Kishida Y, Oishi T, Sugino T, Shiomi A,
Urakami K, Kusuhara M, Yamaguchi K, Kitagawa Y and Ono H:
Associations between loss of ARID1A expression and
clinicopathologic and genetic variables in T1 early colorectal
cancer. Am J Clin Pathol. 152:463–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kyrochristos ID, Ziogas DE, Goussia A,
Glantzounis GK and Roukos DH: Bulk and single-cell next-generation
sequencing: Individualizing treatment for colorectal cancer.
Cancers (Basel). 11:18092019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wen T, Ehivet F, Stanislaw C, Mao R and
Hegde M: Hereditary colorectal cancer diagnosis by next-generation
sequencing. Curr Protoc. 3:e9412023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Poliani L, Greco L, Barile M, Dal Buono A,
Bianchi P, Basso G, Giatti V, Genuardi M, Malesci A and Laghi L;
Alliance Against Cancer, : Canonical and uncanonical pathogenic
germline variants in colorectal cancer patients by next-generation
sequencing in a European referral center. ESMO Open. 7:1006072022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Y, Yang L, Bao H, Fan X, Xia F, Wan
J, Shen L, Guan Y, Bao H, Wu X, et al: Utility of ctDNA in
predicting response to neoadjuvant chemoradiotherapy and prognosis
assessment in locally advanced rectal cancer: A prospective cohort
study. PLoS Med. 18:e10037412021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen YH, Chen YF, Chen CY, Shih JY and Yu
CJ: Clinical factors associated with treatment outcomes in EGFR
mutant non-small cell lung cancer patients with brain metastases: A
case-control observational study. BMC Cancer. 19:10062019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang Q, Zhang P, Wu R, Lu K and Zhou H:
Identifying the best marker combination in CEA, CA125, CY211, NSE,
and SCC for lung cancer screening by combining ROC curve and
logistic regression analyses: Is it feasible? Dis Markers.
2018:20828402018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu
G and Shen X: Combination of preoperative platelet/lymphocyte and
neutrophil/lymphocyte rates and tumor-related factors to predict
lymph node metastasis in patients with gastric cancer. Eur J
Gastroenterol Hepatol. 28:493–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiang J, Zhou L, Li X, Bao W, Chen T, Xi
X, He Y and Wan X: Preoperative Monocyte-to-Lymphocyte ratio in
peripheral blood predicts stages, metastasis, and histological
grades in patients with ovarian cancer. Transl Oncol. 10:33–39.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dong T, Liu Z, Xuan Q, Wang Z, Ma W and
Zhang Q: Tumor LDH-A expression and serum LDH status are two
metabolic predictors for triple negative breast cancer brain
metastasis. Sci Rep. 7:60692017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ouyang W, Jiang Y, Bu S, Tang T, Huang L,
Chen M, Tan Y, Ou Q, Mao L, Mai Y, et al: A prognostic risk score
based on Hypoxia-, Immunity-, and Epithelialto-mesenchymal
transition-related genes for the prognosis and immunotherapy
response of lung adenocarcinoma. Front Cell Dev Biol. 9:7587772021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng S, Chen L, Wang J, Wang H, Hu Z, Li
W, Xu C, Ma M, Wang B, Huang Y, et al: A clinical prediction model
for lung metastasis risk in osteosarcoma: A multicenter
retrospective study. Front Oncol. 13:10012192023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu XY, Li B, Zhang J, Duan LL, Hu BX and
Gao YJ: Analysis of the clinical factors affecting excellent
response of Iodine-131 treatment for pulmonary metastases from
differentiated thyroid cancer. Heliyon. 9:e208532023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu Q, Jiang S, Cheng T, Xu M and Lu B: A
novel pyroptosis-related prognostic model for hepatocellular
carcinoma. Front Cell Dev Biol. 9:7703012021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kiss B, Thoeny HC and Studer UE: Current
status of lymph node imaging in bladder and prostate cancer.
Urology. 96:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brunocilla E, Ceci F, Schiavina R,
Castellucci P, Maffione AM, Cevenini M, Bianchi L, Borghesi M,
Giunchi F, Fiorentino M, et al: Diagnostic accuracy of
(11)C-choline PET/CT in preoperative lymph node staging of bladder
cancer: A systematic comparison with contrast-enhanced CT and
histologic findings. Clin Nucl Med. 39:e308–e312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ghafoor S, Burger IA and Vargas AH:
Multimodality imaging of prostate cancer. J Nucl Med. 60:1350–1358.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nanni C, Schiavina R, Boschi S, Ambrosini
V, Pettinato C, Brunocilla E, Martorana G and Fanti S: Comparison
of 18F-FACBC and 11C-choline PET/CT in patients with radically
treated prostate cancer and biochemical relapse: Preliminary
results. Eur J Nucl Med Mol Imaging. 40 (Suppl 1):S11–S17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Garcia Garzon JR, de Arcocha Torres M,
Delgado-Bolton R, Ceci F, Alvarez Ruiz S, Orcajo Rincón J, Caresia
Aróztegui AP, García Velloso MJ, García Vicente AM; Oncology Task
Force of Spanish Society of Nuclear Medicine, ; Molecular Imaging:
68Ga-PSMA PET/CT in prostate cancer. Rev Esp Med Nucl
Imagen Mol (Engl Ed). 37:130–138. 2018.PubMed/NCBI
|
|
73
|
Hodolic M: Role of (18)F-choline PET/CT in
evaluation of patients with prostate carcinoma. Radiol Oncol.
45:17–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kumar V, Gu Y, Basu S, Berglund A,
Eschrich SA, Schabath MB, Forster K, Aerts HJ, Dekker A,
Fenstermacher D, et al: Radiomics: The process and the challenges.
Magn Reson Imaging. 30:1234–1248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gillies RJ, Kinahan PE and Hricak H:
Radiomics: Images are more than pictures, they are data. Radiology.
278:563–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Birkhauser FD, Studer UE, Froehlich JM,
Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A
and Thoeny HC: Combined ultrasmall superparamagnetic particles of
iron oxide-enhanced and diffusion-weighted magnetic resonance
imaging facilitates detection of metastases in normal-sized pelvic
lymph nodes of patients with bladder and prostate cancer. Eur Urol.
64:953–960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhu C, You Y, Liu S, Ji Y and Yu J: A
nomogram to predict distant metastasis for patients with esophageal
cancer. Oncol Res Treat. 43:2–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Eastham JA, Kattan MW and Scardino PT:
Nomograms as predictive models. Semin Urol Oncol. 20:108–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kourou K, Exarchos TP, Exarchos KP,
Karamouzis MV and Fotiadis DI: Machine learning applications in
cancer prognosis and prediction. Comput Struct Biotechnol J.
13:8–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bur AM, Shew M and New J: Artificial
intelligence for the otolaryngologist: A state of the art review.
Otolaryngol Head Neck Surg. 160:603–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Elfiky AA, Pany MJ, Parikh RB and
Obermeyer Z: Development and application of a machine learning
approach to assess Short-term mortality risk among patients with
cancer starting chemotherapy. JAMA Netw Open. 1:e1809262018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bur AM, Holcomb A, Goodwin S, Woodroof J,
Karadaghy O, Shnayder Y, Kakarala K, Brant J and Shew M: Machine
learning to predict occult nodal metastasis in early oral squamous
cell carcinoma. Oral Oncol. 92:20–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Obermeyer Z and Emanuel EJ: Predicting the
Future-Big data, machine learning, and clinical medicine. N Engl J
Med. 375:1216–1219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sidey-Gibbons JAM and Sidey-Gibbons CJ:
Machine learning in medicine: A practical introduction. BMC Med Res
Methodol. 19:642019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yan L, Yao H, Long R, Wu L, Xia H, Li J,
Liu Z and Liang C: A preoperative radiomics model for the
identification of lymph node metastasis in patients with
early-stage cervical squamous cell carcinoma. Br J Radiol.
93:202003582020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang SC, Liew PL, Ansar M, Lin SY, Wang
SC, Hung CS, Chen JY, Jain S and Lin RK: Hypermethylation and
decreased expression of TMEM240 are potential early-onset
biomarkers for colorectal cancer detection, poor prognosis, and
early recurrence prediction. Clin Epigenetics. 12:672020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wei W, Zhao W and Zhang Y: CBX4 provides
an alternate mode of colon cancer development via potential
influences on circadian rhythm and immune infiltration. Front Cell
Dev Biol. 9:6692542021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Woo S, Suh CH, Kim SY, Cho JY and Kim SH:
The diagnostic performance of MRI for detection of lymph node
metastasis in bladder and prostate cancer: An updated systematic
review and diagnostic meta-analysis. AJR Am J Roentgenol.
210:W95–W109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang X, Liu M, Ren W, Sun J, Wang K, Xi X
and Zhang G: Predicting of axillary lymph node metastasis in
invasive breast cancer using multiparametric MRI dataset based on
CNN model. Front Oncol. 12:10697332022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dong X, Ren G, Chen Y, Yong H, Zhang T,
Yin Q, Zhang Z, Yuan S, Ge Y, Duan S, et al: Effects of MRI
radiomics combined with clinical data in evaluating lymph node
metastasis in mrT1-3a staging rectal cancer. Front Oncol.
13:11941202023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lv B, Cheng X, Cheng Y, Kong X and Jin E:
Predictive value of MRI-detected tumor deposits in locally advanced
rectal cancer. Front Oncol. 13:11535662023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li H, Tang L, Chen Y, Mao L, Xie H, Wang S
and Guan X: Development and validation of a nomogram for prediction
of lymph node metastasis in early-stage breast cancer. Gland Surg.
10:901–913. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wo JY, Chen K, Neville BA, Lin NU and
Punglia RS: Effect of very small tumor size on cancer-specific
mortality in node-positive breast cancer. J Clin Oncol.
29:2619–2627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang X, Yu GY, Chen M, Wei R, Chen J and
Wang Z: Pattern of distant metastases in primary extrahepatic
bile-duct cancer: A SEER-based study. Cancer Med. 7:5006–5014.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rahman R, Simoes EJ, Schmaltz C, Jackson
CS and Ibdah JA: Trend analysis and survival of primary gallbladder
cancer in the United States: A 1973–2009 population-based study.
Cancer Med. 6:874–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ahn JH, Kwak MS, Lee HH, Cha JM, Shin HP,
Jeon JW and Yoon JY: Development of a novel prognostic model for
predicting lymph node metastasis in early colorectal cancer:
Analysis based on the surveillance, epidemiology, and end results
database. Front Oncol. 11:6143982021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fang X, Wei J, He X, An P, Wang H, Jiang
L, Shao D, Liang H, Li Y, Wang F and Min J: Landscape of dietary
factors associated with risk of gastric cancer: A systematic review
and dose-response meta-analysis of prospective cohort studies. Eur
J Cancer. 51:2820–2832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sakaguchi T, Watanabe A, Sawada H, Yamada
Y, Tatsumi M, Fujimoto H, Emoto K and Nakano H: Characteristics and
clinical outcome of proximal-third gastric cancer. J Am Coll Surg.
187:352–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu J, Chen QX, Shen DJ and Zhao Q: A
prediction model for lymph node metastasis in T1 esophageal
squamous cell carcinoma. J Thorac Cardiovasc Surg. 155:1902–1908.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tian D, Jiang KY, Huang H, Jian SH, Zheng
YB, Guo XG, Li HY, Zhang JQ, Guo KX and Wen HY: Clinical nomogram
for lymph node metastasis in pathological T1 esophageal squamous
cell carcinoma: A multicenter retrospective study. Ann Transl Med.
8:2922020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
D'Journo XB: Clinical implication of the
innovations of the 8th edition of the TNM classification
for esophageal and esophago-gastric cancer. J Thorac Dis. 10 (Suppl
22):S2671–S2681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu SG, Zhang WW, Sun JY, Li FY, Lin Q and
He ZY: Patterns of distant metastasis between histological types in
esophageal cancer. Front Oncol. 8:3022018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu Y, Liu J, Han C, Chong Y, Wang Z, Gong
L, Zhang J, Gao X, Guo C, Liang N and Li S: Preoperative prediction
of lymph node metastasis in patients with Early-T-Stage Non-small
cell lung cancer by machine learning algorithms. Front Oncol.
10:7432020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Krag DN, Anderson SJ, Julian TB, Brown AM,
Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier
TG, et al: Technical outcomes of sentinel-lymph-node resection and
conventional axillary-lymph-node dissection in patients with
clinically node-negative breast cancer: Results from the NSABP B-32
randomised phase III trial. Lancet Oncol. 8:881–888. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lyman GH, Temin S, Edge SB, Newman LA,
Turner RR, Weaver DL, Benson AB III, Bosserman LD, Burstein HJ,
Cody H III, et al: Sentinel lymph node biopsy for patients with
early-stage breast cancer: American Society of Clinical Oncology
clinical practice guideline update. J Clin Oncol. 32:1365–1383.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kang J, Choi YJ, Kim IK, Lee HS, Kim H,
Baik SH, Kim NK and Lee KY: LASSO-based machine learning algorithm
for prediction of lymph node metastasis in T1 colorectal cancer.
Cancer Res Treat. 53:773–783. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kudo SE, Ichimasa K, Villard B, Mori Y,
Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, et al:
Artificial intelligence system to determine risk of T1 colorectal
cancer metastasis to lymph node. Gastroenterology.
160:1075–1084.e2. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
De Herdt MJ, van der Steen B, van der Toom
QM, Aaboubout Y, Willems SM, Wieringa MH, Baatenburg de Jong RJ,
Looijenga LHJ, Koljenović S and Hardillo JA: The potential of MET
immunoreactivity for prediction of lymph node metastasis in early
oral tongue squamous cell carcinoma. Front Oncol. 11:6380482021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Shan J, Jiang R, Chen X, Zhong Y, Zhang W,
Xie L, Cheng J and Jiang H: Machine learning predicts lymph node
metastasis in Early-stage oral tongue squamous cell carcinoma. J
Oral Maxillofac Surg. 78:2208–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lee SM, Tsui SK, Chan KK, Garcia-Barcelo
M, Waye MM, Fung KP, Liew CC and Lee CY: Chromosomal mapping,
tissue distribution and cDNA sequence of four-and-a-half LIM domain
protein 1 (FHL1). Gene. 216:163–170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tian Y, He Y, Li X and Liu X: Novel
nomograms to predict lymph node metastasis and distant metastasis
in resected patients with early-stage non-small cell lung cancer.
Ann Palliat Med. 10:2548–2566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang B, Tian J, Pei S, Chen Y, He X, Dong
Y, Zhang L, Mo X, Huang W, Cong S and Zhang S: Machine
Learning-assisted system for thyroid nodule diagnosis. Thyroid.
29:858–867. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhao HN, Liu JY, Lin QZ, He YS, Luo HH,
Peng YL and Ma BY: Partially cystic thyroid cancer on conventional
and elastographic ultrasound: A retrospective study and a machine
learning-assisted system. Ann Transl Med. 8:4952020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kang S, Kang WD, Chung HH, Jeong DH, Seo
SS, Lee JM, Lee JK, Kim JW, Kim SM, Park SY and Kim KT:
Preoperative identification of a low-risk group for lymph node
metastasis in endometrial cancer: A Korean gynecologic oncology
group study. J Clin Oncol. 30:1329–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, et al:
Uterine neoplasms, version 1.2018, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 16:170–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Huang CY, Liao KW, Chou CH, Shrestha S,
Yang CD, Chiew MY, Huang HT, Hong HC, Huang SH, Chang TH and Huang
HD: Pilot study to establish a novel Five-gene biomarker panel for
predicting lymph node metastasis in patients with early stage
endometrial cancer. Front Oncol. 9:15082019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kan Y, Dong D, Zhang Y, Jiang W, Zhao N,
Han L, Fang M, Zang Y, Hu C, Tian J, et al: Radiomic signature as a
predictive factor for lymph node metastasis in early-stage cervical
cancer. J Magn Reson Imaging. 49:304–310. 2019. View Article : Google Scholar : PubMed/NCBI
|