Head and neck squamous cell carcinoma (HNSCC) is one
of the most prevalent cancer globally, which arises from stratified
mucosa of the mouth, trachea and larynx. Despite improved
treatments, overall survival remains low, with more than 450,000
deaths in 2018 (1). There is a rise
in annual incidence of oropharynx squamous cell carcinoma, a
subtype of HNSCC, over the past decade, within the non-smokers,
non-alcoholics and aged <50 years white male demographic group.
This occurrence is associated with the human papillomavirus (HPV)
infection, particularly HPV16, with the risk factor being an
increase in sexual partners for oral or vaginal sex at a younger
age (2).
Bromodomain protein 4 (BRD4) is one of the members
of the bromodomain and extra-terminal (BET) family and a dual
bromodomain protein consisting of two N-terminal bromodomains and
an extra-terminal (ET) domain. BRD4 binds and acetylates lysine
residues on target proteins including histones as a transcriptional
and epigenetic regulator (3). BRD4
plays an important role in transcription, replication and DNA
repair. It also binds to non-histone proteins, DNA and RNA,
contributing to development, tissue growth and various
physiological processes (4). BRD4
is a crucial element in the regulation of cell cycle and mitosis
which ensures the integrity of cell differentiation and
development. The protein is also a predominant component of
super-enhancers (SEs) associated with all active promoters and a
significant proportion of active promoters in the genome including
activation of genes involved in cell growth and cell cycle
progression (5,6). The critical roles of BRD4 regarding
transcriptional regulation are growth and cell division, metabolic
processes, immune responses and embryo development regulation. In
normal and transformed cells, BRD4 dysfunction results in
pathogenesis and disease development in humans, such as prolonged
inflammation, as the protein directly regulates the activity of
NF-κB including inflammation, fibrosis, viral infections and
neoplasia (7,8). Several studies have addressed the
involvement of BRD4 in development of various tumors, where BET can
promote aberrant expression of oncogenes such as c-Myc in acute
lymphocytic leukemia and acute myeloid leukemia (AML) (9). The diverse role of BRD4 depends in
several contexts on its interaction partners. It is considered that
interfering with BET activity can reduce cancer cell proliferation
and induce apoptosis (10). The
most significant evidence for involvement of BRD4 in HNSCC
carcinogenesis came from NUT carcinoma. Inhibition of the BRD4-NUT
fusion gene on the BRD4 section using BETi (BET inhibitors) stalled
the growth of NUT carcinoma cells (11). Therefore, targeting BRD4 through
inhibition of BET protein has been explored as a therapeutic option
for various cancers including HNSCC.
BRD4 is an indispensable protein for cellular
development. For example, bone marrow stem cells cannot
differentiate into lymphoid stem cells without the presence of BRD4
(12). Moreover, the full
expression of BRD4 is also essential for embryogenesis. A previous
study conducted in mice reported that one allele of BRD4 was only
enough to allow the embryonic stem cells to differentiate but
insufficient for complete mouse development (13).
Post-translational modification of histone alters
gene expression by regulating the chromatin landscape through
changing the overall charge of the chromatin which recruits
chromatin modifier enzymes (14).
The epigenetic phenomenon is partly operated by BRD4 as the protein
has histone acetyltransferase (HAT) and kinase activities
phosphorylating serine2 residue of the RNA polymerase II
carboxy-terminal domain. The binding of bromodomains to acetylated
histone and lysine residues at the histone H3 site and H4 on
chromatin regulates downstream gene expression. As BRD4 regulates
chromatin remodeling by acetylating histone H3 Lys122, it causes
instability and ejection of nucleosomes from chromatin as well as
chromatin structural detachment; this leads to an increase in
transcription. The resulting chromatin fragmentation permits DNA
accessibility and allows access to transcriptional machinery
(15–17). The perturbed chromatin structure and
nucleosome remodeling at the promoters allow transcription factors
as well as RNA Polymerase II to enter and start the transcription
process (18). Furthermore, BRD4
coupling with RNA Polymerase II complex assists the complex to
elongate through hyperacetylated nucleosomes by interacting with
acetylated histones using bromodomains (19).
The HAT activity of BRD4 is responsible for a smooth
transition from G1 to M phase of the cell cycle as it mediates
transcription and pause-release. Similarly, the G2 to M phase
transition has been known to be under the control of BRD4 via its
interaction with a GAP protein, SPA-1. This again, relieves the
block to cell cycle progression (20). Likewise, BRD4 controls the levels of
Aurora B which is concentrated around the sites of attachment of
chromosomes to spindle microtubules such as the centromeres or
kinetochores and allows for chromosome segregation to occur
appropriately (21). With low
levels of BRD4, mitosis may become abnormal leading to increased
incidence of lagging chromosomes, micronuclei and bridging
chromosomes, eventually resulting in failed cytokinesis and
multilobulated nuclei (16). As
numerous genes regulated by BRD4 are involved in the processes of
cell differentiation and development, dysregulation of BRD4 could
become oncogenic which leads to pathogenesis of a wide variety of
cancers (22).
Aberrant expression or function of BRD4 is
well-connected to oncogenic processes which includes HNSCC
tumorigenesis (23). BRD4 has two
well-structured N-terminal bromodomains (BD1 and BD2); in addition
to BD1 and BD2, the molecular actions of BRD4 depend on the
CK2-phosphorylated region, conserved ET domain and the distinct
C-terminal motif. The regions are the interactive platform for
recruiting chromatin and transcriptional regulators (24). BRD4 has three isoforms of different
lengths but there are two main isoforms, BRD4 long (BRD4-L) and
BRD4 short (BRD4-S). Evidence suggests that a disruption of the
balance between the two BRD4 isoforms occurs in certain cancer
types leading to substantial biological consequences (25). BRD4 and other BET proteins are often
overexpressed in cancer and this leads to abnormal chromatin
remodeling and tumorigenesis-mediated gene transcription.
BRD4-mediated histone modifications regulate gene expression and
maintain normal cellular homeostasis, which are vital for the cells
(26). Studies conducted in human
cancer types have shown that BRD4 overexpression is one of the
reasons for oncogene amplification such as Myc, Notch3 and NRG1
leading to cancer progression (25,27).
The progression of triple-negative breast cancer (TNBC) is also
linked to increased phosphorylation of BRD4 in the acidic region
due to decreased protein phosphatase 2A (PP2A) activity (28). These studies all point to BRD4 as a
central protein for tumor development, specifically by inducing and
maintaining the pool of cancer stem cells in squamous cell
carcinoma including HNSCC (29).
Oncogenic mechanisms resulting from changes in
genome structure may include mutations, copy number changes, or
genome rearrangements. ‘Oncogene addiction’ is a mechanism used by
cancer cells to maintain their unchecked proliferative needs
(30). This is largely due to the
functions of BET proteins, in addition to their role in
transcriptional regulation by forming the Twist/BRD4/P-TEFb/RNA-Pol
II complex which lead to stem cell-like properties and
tumorigenicity (31). BRD4 is a key
protein in numerous cancer hallmarks, it can stimulate cancer cell
proliferation through the functions of Jagged1 and Notch1 in breast
cancer (32). It also controls
oncogenic network gene expression by interacting with acetylated
transcription factors including RELA, ER, p53 and twist (33). BET inhibitors have demonstrated
remarkable anticancer effects for treatment by interfering with
BRD4 expression or activity and effectively inhibit the progression
of the cell cycle and induce apoptosis which reduces tumor cell
proliferation and subsequent cancer development (34–36).
Inhibition of BRD4 has revealed significant effect on sensitizing
various tumor cell types to therapeutic agents including diffuse
large B-cell lymphoma, neuroblastoma, lung cancer, NUT midline
cancer and HNSCC (37–41). BRD4 has been linked with poor
prognosis in a wide range of cancer patients including HNSCC
(23,27). In addition to solid tumors, BRD4
inhibition has also been identified to be effective against
hematological malignancies (42).
The anticancer efficacy of BRD4 has been reported and clinical
trials are ongoing (10,43). Incoming data from these studies will
further validate the use of BRD4 inhibitor in antitumor therapy.
However, it is critical to investigate whether targeting BRD4 is
feasible for HNSCC treatment as there is a currently unsolved
dilemma for the cancer as discussed below.
Genetic mutations resulting from unrepaired DNA
damage may increase the risk of precipitating genetic disorders and
cancers. Although an isoform of BRD4 functions as an internal
inhibitor of DNA damage response by remodeling the chromatin
complex (44), the protein
transcriptionally regulates DNA damage repair-related genes such as
RAD51AP1 and TopBP1 as well as engages in double strand breaks
(DSBs) through both non-homologous end joining (NHEJ) and
homologous recombination (HR) pathways (45–48).
BRD4 particularly involves in HR through direct contact with the
SWI/SNF chromatin remodeling complex (49). It is worth noting that BET proteins
inhibition itself can induce DNA damage potentially through
deposition of R-loops leading to transcription-replication
collision events (46).
Additionally, BRD4 assists in maintaining genome stability through
non-transcriptional functions such as DNA damage repair, checkpoint
activation and telomere homeostasis (27). The use of BET inhibitors, namely JQ1
and AZD5153, has been revealed to prolong DNA DSBs and repress
NHEJ-related genes XRCC4 and SHLD1 (50). JQ1 treatment leads to the
substitution of BET proteins and transcription regulatory complexes
from acetylated chromatin (51).
JQ1 not only increases the damage level of DNA, but also attenuates
DNA damage repair, particularly double strand break repair, which
consequently sensitize the tumor cells to PARP inhibitor Olaparib
(52). Additionally, JQ1 can
inhibit the growth of ARID2-deficient hepatocellular carcinoma
cells as well as induce apoptosis when ARID2-depleted through the
aggravated DNA damage of DSBs (53).
BRD4 amplification has been shown as a prognostic
factor in various cancer types such as ovarian, esophageal,
non-small cell lung cancer and HNSCC (23,25,54,55).
This could be due to numerous pro-survival functions of the protein
including acetylation of histone H4 by DNA damage recruits BRD4 to
stabilize the DNA repair complex (47). The multiple underlying roles of BRD4
in DNA damage repair is conceivably the major contributor in tumor
cell resistance to therapy. For HNSCC, mutations in the DNA repair
genes have enabled HNSCC to become resistant to therapy (56). Additionally, certain DNA damage
repair genes appear to be upregulated including Ku80 and APEX1 and
linked with patient prognosis (57,58).
However, a recent study has indicated that a change in the
expression of individual DNA repair proteins may not necessarily
cause resistance to therapy. Rather, a balanced expression and
coordination within the DNA repair signaling cascade is rather the
actual cause of the resistance (59). Thus, targeting BRD4 protein which is
upstream of DNA damage response may hypothetically benefit cancer
patients, especially those with therapy-resistant HNSCC. The use of
BRD4 inhibitor has been revealed to enhance the radiosensitivity of
HNSCC as well as other tumors in pre-clinical models potentially
through the upregulation of p21 and suppression of RAD51AP1 and
Mcl-1 (41,60–63).
Targeting the protein is also suggested to attenuate YAP, Myc-AP4
and E2F2 signaling which are often upregulated in various tumors
(64–66). Degradation of BRD4 could cause a
genome wide pausing of Pol II as BRD4-PTEFb is the main driving
partner for phosphorylation of Pol II C-terminal domain and Pol II
transcription (67,68). Similarly, BRD4 inhibition has been
shown to sensitize colorectal tumor to doxorubicin as the protein
is the cause of cisplatin resistance in bladder tumor through the
Sonic hedgehog pathway (69,70).
These accumulating data further have supported targeting the
bromodomain protein as a therapeutic option to enhance the efficacy
of current conventional therapies.
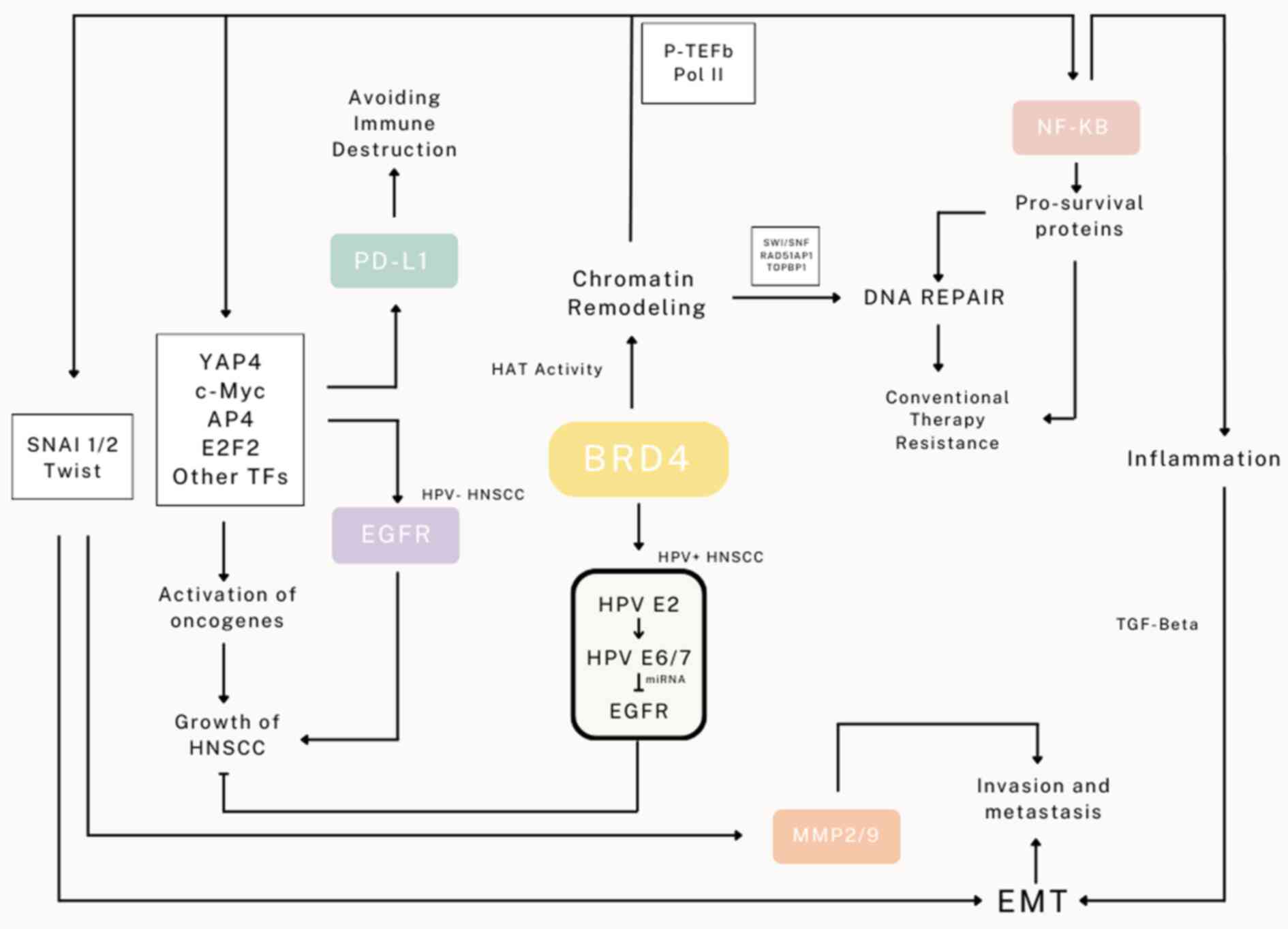
EMT remains a challenge in cancer treatment as the
phenomenon provides not only an escape route with resistant
features for cancer cells under therapeutic stress but also an
opportunity for tumor expansion and metastasis. BRD4 is regarded as
a key regulator of EMT as it governs key transcription factors that
drive EMT particularly through the transcription of snail, both
SNAI1 and SNAI2, as well as involves in TGF-β mediated EMT
(71,72). Coupling between BRD4 and
di-acetylated Twist was also shown to enhance downstream
transcriptional targets of Twist for EMT (31,73).
Overexpression of BRD4 enhances EMT and EMT is inhibited with
reduced expression of BRD4 (74).
There is a controversy whether the other BET proteins are involved
in activation of EMT, namely BRD2 and BRD3; essentially, they are
demonstrated to have a degree of control over EMT activation
(75). Inhibition of BRD4 has
frequently been demonstrated to suppress EMT through various
mechanisms; for example, through activation of the
NF-κB-NLRP3-caspase-1 pyroptosis signaling pathway in renal cell
carcinoma (51), and inhibition of
RelA-initiated TGF-β induced EMT via inflammatory tissue remodeling
(76). BRD4 also regulates Jagged1
expression and Notch1 signaling for cancer cell dissemination
(32). Treatment with BRD4
inhibitors has been identified to effectively suppress
EMT-associated tumor invasion and metastasis through the regulation
of key EMT proteins as well as attenuate the expression of MMP-2
and MMP-9, thus reducing HNSCC metastatic potential (77–80).
Likewise, the activity of BRD4 in transitioning HNSCC cells to
mesenchymal phenotype has equipped the cells to become
cisplatin-resistant (81). These
studies further emphasize the roles of BRD4 in tumor proliferation
and expansion. The multiple functions of BRD4 on the development
and expansion of HNSCC described are demonstrated in Fig. 1.
Research involving BRD4 has evolved greatly as a
result of its role in inflammation, which is associated with cancer
through genomic instability, a cancer hallmark. Various studies
have established that cancer is a disease that develops and
progresses within inflammatory diseases including HNSCC (82). BRD4 enhances acetylation of
RelA-K310ac which activates cyclin-dependent kinase 9 (CDK9),
leading to the phosphorylation of RNA polymerase II to promote
NF-κB gene transcription, thereby initiating the production of
proteins responsible for inflammatory stimuli (83). Additionally, BRD4 regulates the
expression of inflammatory genes through activation of enhancer
RNAs or the Mnk2-eIF4E pathway-dependent translational regulation
of IκBα synthesis in modulating inflammatory gene expression
(84). There is also a direct
association between BRD4 and the acetylated p65 subunit of NF-κB as
well as the transcription factor of Nrf2, a key regulator of
inflammation (85). Studies have
investigated the efficiency of BRD4 inhibition of the inflammatory
process. In primary human umbilical cord-derived vascular
endothelial cells treated with TNF-α, JQ1 lessens the
overexpression of FN1 induced by TNFα and could possibly slow down
the progression of atherosclerosis (86). JQ1 has been shown to effectively
protect colon-tight junctions from endotoxemia-induced inflammatory
injury (87). In rat kidney
triggered by Cadmium for inflammatory response, BRD4 inhibition
reduced NF-κB nuclear translocation and its subsequent
transcriptional activity (83). The
use of I-BET, a BET inhibitor, can effectively inhibit
pro-inflammatory protein production in lipopolysaccharide-activated
macrophages (88). In primary and
human bronchial epithelial cell lines, oxidative stress induced by
IL-1β was significantly reduced by BRD4 inhibition (89). These studies have implicated that
targeting BRD4 can subside inflammation in HNSCC and maybe
beneficial to the patients as various studies have reported that
inflammatory markers are prognostic factors for these patients
(90–93). Thus, alleviating inflammation as a
result of targeting BRD4 could prove to be useful in the treatment
of HNSCC.
Most common in the United States and other
high-income countries, HPV-related HNSCC is becoming far more
common than HPV-associated cervical cancer (94). Oncogenic HPV can be latent and cause
malignant transformation years later; however, infection of
high-risk HPV types can lead to pre-cancerous, in certain tissue,
and cancer (95). A total of ~25%
of all HNSCCs were positive for HPV-DNA with HPV-16 being the most
prevalent subtype (96). There are
15 high-risk types of HPV, but the two most common ones, HPV16 and
HPV18 accounting for ~72% of the total (97). The life cycle of HPV is highly
dependent on the host cellular differentiation program. Although a
receptor for HPV infection has not been recognized, it has been
postulated to be heparan sulfate proteoglycan on the basal membrane
(98). The role of viral protein E1
is unclear, whereas E2 is responsible for the transcription of E6
and E7 viral genes. In addition, the binding of HPV E2 protein to
DNA is involved in viral DNA replication, transcription, genome
maintenance and isolation (99).
HPV E6/E7 expression is required for the binding of viral genome to
DNA in the regions of genomic instability. This is followed by
disruption of the E2 coding region and abnormal regulation of E6/E7
itself. Because of this, HPV can produce persistent infection
(100). Degradation of p53 and
pRb, by E6 and E7, respectively, contribute to cancer induced by
this virus (101).
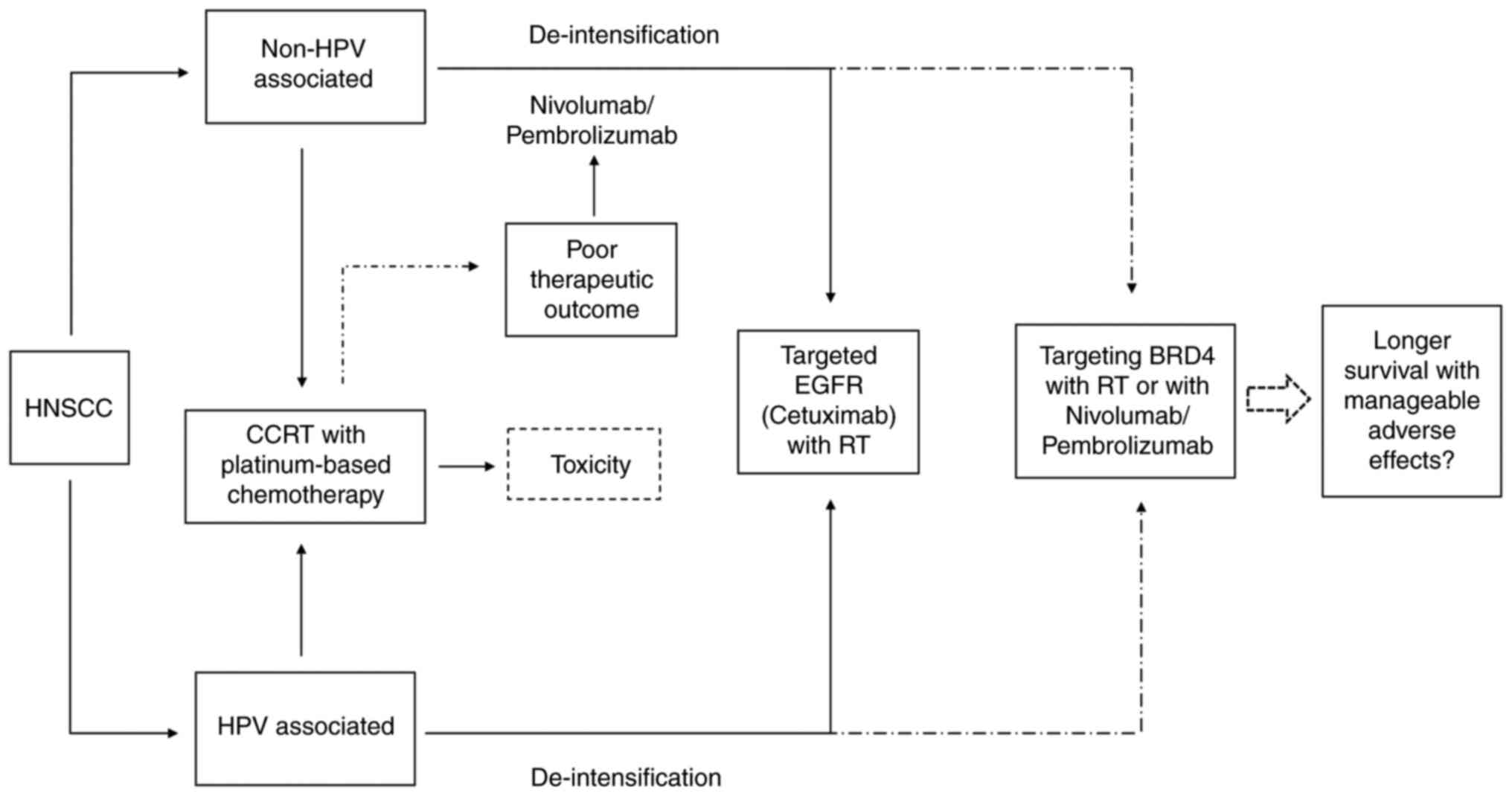
For primary HNSCC, surgical resection of the tumor
and lymph node followed by radiotherapy with or without
platinum-based chemotherapy or definitive concurrent chemoradiation
therapy is the main modality for treating the patients (111). Cisplatin is often the
chemo-reagent for the course; however, significant acute and late
toxicity is often observed (112).
Thus, deintensification approach has been trialed using cetuximab
to target epidermal growth factor receptor (EGFR), replacing
cisplatin for those with HPV-positive HNSCC. However, the patients
receiving cetuximab appear to be at a higher risk of death and
relapse of the disease than those receiving cisplatin (113,114). Despite EGFR upregulation is an
acknowledged biomarker suggesting treatment resistance and
aggressiveness in HNSCC (115),
targeting EGFR has shown significant benefits for HPV-negative
HNSCC (116). However, inhibition
of the receptor in HPV-associated HNSCC leads to lesser therapeutic
outcomes suggesting that EGFR plays opposing roles in the two HNSCC
subtypes (117). Increasing
evidence has demonstrated that cetuximab may not be the best course
for HPV-positive HNSCC therapy (118,119). In HPV-positive HNSCC cells,
overexpression of EGFR suppresses cellular proliferation and
increases radiosensitivity through inhibition of BRD4 via miR-9-5p
and subsequently reduced HPV E6/E7 transcription (37). Therefore, targeting EGFR may not be
the best course of therapy for HPV-positive HNSCC, but targeting
specific signaling pathways such as BRD4 could provide a preferable
new treatment to improve the therapeutic outcome of HNSCC (120).
BRD4 has been clinically linked to several
oncogenes, such as activating Myc in leukemia and lymphoma
(121). It has also been observed
that BRD4 protein and its mRNA levels are abnormally regulated in
HNSCC samples, correlated with tumor features such as size,
proliferation and advanced disease degree (23). A previous study in HNSCC reported
that BRD4 overexpression decreases the mRNA stability of
cyclin-dependent kinase inhibitor 1B (p27), and the protein p27 is
responsible for inhibiting tumor progression (122). The protein can also act as a
pro-oncogene that accelerates tumor growth and metastasis as a
critical part of SEs (123). BRD4
has thus been identified as a prognostic biomarker of HNSCC
(23).
Inhibition of BRD4 using JQ1 has been demonstrated
to induce senescence in head and neck tumor cells through
downregulation of acetylated histone H4 and phosphorylated
SIRT1(ser47) leading to p21 and p16ink4 accumulation (124). Treatment with the inhibitor also
blocks SEs, decreases TP63 expression in HNSCC, and effectively
eliminates both cancer stem cells and lymph node metastasis
(125). Additionally, BRD4 is a
regulator of JOSD1, a protein linked to poor prognosis in patients
with HNSCC. JQ1 treatment has been identified to downregulate both
the JOSD1 protein and mRNA expression. Overexpression of the
protein indicates a poor clinical prognosis for patients with HNSCC
(126). Similarly, cooperation
between YAP1 and BRD4, which can be attenuated by JQ1 treatment,
enhances HNSCC tumorigenesis (127). Treatment with the inhibitor has
been also shown to overcome cetuximab resistance in HPV-negative
subtype of HNSCC (128). These
studies have accumulated evidence in favor of the use of BRD4
inhibitor as a part of HNSCC therapy in the near future to resolve
the dilemma of targeting EGFR in HPV-associated HNSCC, as
demonstrated in Fig. 2.
Tumor cells often modulate the expression of genes
or immune signaling pathways to avoid immune recognition and
promote tumor growth and metastasis (129). Therefore, it is critically
important to recognize their interaction with immunologic cells in
order to stratify toward immunotherapy. Immune cell infiltration
into tumor tissue, particularly for cytotoxic T lymphocytes and
natural killer cells have been closely investigated in recent
years. The roles of BRD4 regarding immune response to tumor have
been investigated with BRD4 expression being associated with levels
of infiltrating monocytes, tumor-associated macrophages, M1/M2
macrophages and T cells (Th1/Th2/Treg) in breast cancer (130). In hepatocellular carcinoma,
expression of BRD4 mRNA is elevated and correlated with immune
infiltrating levels of B cells, CD8+ T cells,
CD4+ T cells, macrophages, neutrophils and dendritic
cells (131). BRD4 expression is
also connected with low infiltration of T-bet+
tumor-infiltrating T lymphocytes leading to poorer prognosis
potentially through activation of Jagged1 signaling pathways
(132). BRD4 regulates programmed
death-ligand 1 (PD-L1) expression through c-Myc implicating that
targeting BRD4 can influence immune system against tumor cells
(133). Suppression of BRD4 leads
to downregulation of PD-L1 in TNBC, thus potentially permitting an
improved outcome with immunotherapy approach (134). BRD4 expression, above the other
BET proteins, is the most negatively correlated with immune
checkpoint as well as abundance of macrophage, neutrophil and
CD8+ T-cell in glioblastoma multiforme (135). These studies have all designated
BRD4 as a prognostic marker for patient survival further
highlighting the bromodomain protein as a therapeutic target.
Furthermore, for HNSCC, the expression of PD-L1 in HNSCC cell lines
could be reduced by JQ1 or MZ1 treatment (136). Likewise, suppression of the BET
protein could enhance antitumor immunity through the induction of
MHC class I expression and consequently improve the efficacy of
anti-PD-1 immunotherapy in an in vivo model (137).
Although the exact mechanism on how BRD4 mediates
tumor microenvironment and immune infiltration needs further
elucidation, BRD4 is involved in the acetylation of lysine-310 of
the RelA NF-κB subunit which activates the transcription factor and
modulates proinflammatory cytokines as well as Th17 immune response
(138). The epigenetic regulator
has been shown to be responsible for the expression of a cohort of
immunosuppressive genes including PD-L1, PD-L2, HVEM, GAL9, IL6,
IL8, CSF2, BIRC3, IDO1 and IL1B (139). BRD4 is also suggested to be the
protein responsible for immunosuppressive M2 macrophage
polarization (140,141). Collectively, these studies have
provided evidence that targeting BRD4 may shift the landscape of
tumor microenvironment for immunotherapy and antitumor immune
response which could be useful for the treatment of HNSCC as well
as other solid tumors. It is enticing to explore the interaction
between BETi and immunotherapeutic agents such as nivolumab and
pembrolizumab which have been approved for HNSCC therapy. The
co-administration between BETi and immunotherapy could lead to an
effective therapy against HNSCC.
Despite the promise of BRD4 inhibition in cancer
therapy mentioned, it has been suggested that the efficacy of BRD4
inhibition as a monotherapy could be transient and moderate
(123). Several studies have
demonstrated that tumor cells may develop resistance after a
prolonged use of JQ1 due to the rewiring of proteins involved in
transcriptional regulation which also affect other
chromatin-targeted therapies. For example, in AML, WNT/β-catenin
signaling pathway is shown as the primary and acquired driver for
resistance to BETi (142,143). In lung adenocarcinoma, BET
inhibition is effective in blocking cell growth through FOS-like 1
(FOSL1) suppression; however, resistance to JQ1 occurs
independently of its effect on FOSL1 or Myc expression.
Phosphorylation of BRD4 by casein kinase 2 (CK2) is suggested as a
cause of BETi resistance (144).
DUB3, which is upregulated by JQ1 treatment, deubiquitinates and
stabilizes BRD4 causing prostate cancer to become resistant to BETi
(145). Similarly, JQ1 resistance
was demonstrated to be due to the loss of BRD4/FOXD3/miR-548d-3p
axis which is compensated by JunD/RSK3 signaling which essentially
builds up BETi resistance in basal-like breast cancer (146). For TNBC, the cells can rapidly
develop resistance due to various mechanisms, including changes in
signaling pathways involving ZNF33A upregulation, deletion of
SNF/SWI complex components as well as ubiquitination-related genes
such as SPOP, UBE2M, CUL3 and USP14 (147,148). In ovarian cancer cell lines,
autophagy (shown by increased expression of ATG5 and Beclin1)
induced by inactivation of Akt (Ser473)/mTOR (Ser2448) pathway, is
linked with resistance to BETi possibly as a way to bypass BET
inhibition (149). Thus, it is
essential to note that resistance to JQ1 has distinct mechanisms
depending on cancer types; however, increase in Myc expression has
been pinpointed as common cause of resistance to BETi (147,150,151). BRD4 stabilization and its
subsequent activation of AKT-mTORC1 activation has also been
described as another route of BETi resistance (152). Therefore, the issue of resistance
to BET inhibition should be closely investigated especially when
BET inhibition is applied particularly as a monotherapy. The use of
BRD4 inhibition as a part of combination therapy could be a more
viable option considering this matter.
Currently, no BETi has been approved by the US FDA
for the use of cancer; however, there are a number of phase I
clinical studies which have provided initial information regarding
the safety of BETi in patients. As shown in Table I, BETi appears to be safe in most
patients with the prevalent treatment emergent adverse events
(TEAEs) including thrombocytopenia, diarrhea, nausea, anorexia,
vomiting, fatigue and anemia. The most significant dose-limiting
toxicities are thrombocytopenia and fatigue. All studies have
concluded that BETi was largely tolerable by the patients; thus,
applying BETi for deintensification of HNSCC therapy could be
viable although more data should be collected from further clinical
studies especially for patients with HNSCC. Regarding the potential
efficacy against HNSCC, it is premature to conclude the antitumor
efficacy of BETi as the majority of these clinical studies have
been conducted in patients with hematologic malignancies. However,
a few studies in solid cancers have mentioned that the patients had
achieved longer progression-free survival with 95% confidence
intervals of [1.8–1.9] and [4.6–12.9] (153,154). Despite the promising safety of
BETi in patients, future clinical studies should proceed with care
and prepare to address the TEAEs which are likely to emerge.
In order to limit the toxicity of BETi, an
alternative approach for precise delivery of BRD4 inhibitors or
other targeting molecules is engineered exosome. The idea of
exploiting exosome as a drug delivery system has become popular as
it can surpass barrier created by tumor microenvironment and can be
equipped with targeting properties. Small molecule drugs such as
paclitaxel and curcumin have been delivered to specific target
cells (156). Delivery of
microRNAs targeting BRD4 could perhaps further alleviate the
adverse effects shown by several BETi as the formation and delivery
of exosomal microRNAs is becoming more practical (157). The precision in drug delivery will
surely offer a more endurable therapy for the patients.
A number of BET inhibitors have shown great
potential to be effective for cancer therapy which could enhance
the efficacy of chemo-, radio- and immunotherapy against HNSCC. As
a chromatin-targeted therapy, BET/BRD4 inhibitor could be a viable
candidate for replacing the EGFR inhibitor knowing that it could be
effective against HNSCC regardless of its HPV association, as
cetuximab may not provide the best outcome for the HPV-associated
subtype of head and neck cancer. Another potential role which
targeting BRD4 may come into play is the de-escalation of the
current HNSCC therapy regimens which are facing a challenge in
terms of the side effects. This could allow lesser adverse effects
to the patients which typically affect the patients' quality of
life. A clinical trial proving the efficacy of BRD4/BET inhibitor
for the treatment of HNSCC is also desirable in order to
demonstrate its clinical application in addition to its potential
shown in vitro and in vivo models. In addition, the
combination between BRD4 and other inhibitors should be considered.
For example, BRD4 inhibitor in combination with suberoylanilide
hydroxamic acid as a histone deacetylase inhibitor have been tested
and exhibited promising results in certain tumors (158,159). This approach could further expand
therapeutic options but may also need to proceed with caution due
to adverse effects of such inhibitions. The investigation
concerning immunological effects of BET inhibition should also be
considered to evaluate the applicability of targeting BRD4 in head
and neck cancer therapy.
Not applicable.
The present study was supported by Chulabhorn Royal Academy
(grant no. ISF06-001/2566).
Not applicable.
NW, WK, NA and TK wrote the first draft of the
manuscript. VY revised the manuscript and generated all figures and
tables. DN conceptualized the study and critically revised the
manuscript. MT provided guidance and edited the manuscript prior to
submission. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare no competing interests.
|
1
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marur S, D'Souza G, Westra WH and
Forastiere AA: HPV-associated head and neck cancer: A virus-related
cancer epidemic. Lancet Oncol. 11:781–789. 2010. View Article : Google Scholar
|
|
3
|
Cipriano A, Milite C, Feoli A, Viviano M,
Pepe G, Campiglia P, Sarno G, Picaud S, Imaide S, Makukhin N, et
al: Discovery of Benzo[d]imidazole-6-sulfonamides as Bromodomain
and Extra-Terminal Domain (BET) Inhibitors with Selectivity for the
First Bromodomain. ChemMedChem. 17:e2022003432022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang Y, Tian J and Wu T: BRD4 in
physiology and pathology: ‘BET’ on its partners. Bioessays.
43:e21001802021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B, Liu X, Han L, Chen X, Wu X, Wu J,
Yan D, Wang Y, Liu S, Shan L, et al: BRD4-directed super-enhancer
organization of transcription repression programs links to
chemotherapeutic efficacy in breast cancer. Proc Natl Acad Sci USA.
119:e21091331192022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakamaki JI, Wilkinson S, Hahn M, Tasdemir
N, O'Prey J, Clark W, Hedley A, Nixon C, Long JS, New M, et al:
Bromodomain protein BRD4 is a transcriptional repressor of
autophagy and lysosomal function. Mol Cell. 66:517–532.e9. 2017.
View Article : Google Scholar
|
|
7
|
Hu J, Pan D, Li G, Chen K and Hu X:
Regulation of programmed cell death by Brd4. Cell Death Dis.
13:10592022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu N, Ling R, Tang X, Yu Y, Zhou Y and
Chen D: Post-Translational modifications of BRD4: Therapeutic
targets for tumor. Front Oncol. 12:8477012022. View Article : Google Scholar
|
|
9
|
Abedin SM, Boddy CS and Munshi HG: BET
inhibitors in the treatment of hematologic malignancies: Current
insights and future prospects. Onco Targets Ther. 9:5943–5953.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu T, Lu W and Luo C: A patent review of
BRD4 inhibitors (2013–2019). Expert Opin Ther Pat. 30:57–81. 2020.
View Article : Google Scholar
|
|
11
|
French CA: NUT Carcinoma:
Clinicopathologic features, pathogenesis, and treatment. Pathol
Int. 68:583–595. 2018. View Article : Google Scholar
|
|
12
|
Dey A, Yang W, Gegonne A, Nishiyama A, Pan
R, Yagi R, Grinberg A, Finkelman FD, Pfeifer K, Zhu J, et al: BRD4
directs hematopoietic stem cell development and modulates
macrophage inflammatory responses. EMBO J. 38:e1002932019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houzelstein D, Bullock SL, Lynch DE,
Grigorieva EF, Wilson VA and Beddington RS: Growth and early
postimplantation defects in mice deficient for the
bromodomain-containing protein Brd4. Mol Cell Biol. 22:3794–3802.
2002. View Article : Google Scholar
|
|
14
|
Gonzales-Cope M, Sidoli S, Bhanu NV, Won
KJ and Garcia BA: Histone H4 acetylation and the epigenetic reader
Brd4 are critical regulators of pluripotency in embryonic stem
cells. BMC Genomics. 17:952016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devaiah BN, Case-Borden C, Gegonne A, Hsu
CH, Chen Q, Meerzaman D, Dey A, Ozato K and Singer DS: BRD4 is a
histone acetyltransferase that evicts nucleosomes from chromatin.
Nat Struct Mol Biol. 23:540–548. 2016. View Article : Google Scholar
|
|
16
|
Devaiah BN, Gegonne A and Singer DS:
Bromodomain 4: A cellular swiss army knife. J Leukoc Biol.
100:679–686. 2016. View Article : Google Scholar
|
|
17
|
Devaiah BN, Lewis BA, Cherman N, Hewitt
MC, Albrecht BK, Robey PG, Ozato K, Sims RJ III and Singer DS: BRD4
is an atypical kinase that phosphorylates serine2 of the RNA
polymerase II carboxy-terminal domain. Proc Natl Acad Sci USA.
109:6927–6932. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jha RK, Levens D and Kouzine F: Mechanical
determinants of chromatin topology and gene expression. Nucleus.
13:94–115. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanno T, Kanno Y, LeRoy G, Campos E, Sun
HW, Brooks SR, Vahedi G, Heightman TD, Garcia BA, Reinberg D, et
al: BRD4 assists elongation of both coding and enhancer RNAs by
interacting with acetylated histones. Nat Struct Mol Biol.
21:1047–1057. 2014. View Article : Google Scholar
|
|
20
|
Farina A, Hattori M, Qin J, Nakatani Y,
Minato N and Ozato K: Bromodomain protein Brd4 binds to
GTPase-activating SPA-1, modulating its activity and subcellular
localization. Mol Cell Biol. 24:9059–9069. 2004. View Article : Google Scholar
|
|
21
|
You J, Li Q, Wu C, Kim J, Ottinger M and
Howley PM: Regulation of aurora B expression by the bromodomain
protein Brd4. Mol Cell Biol. 29:5094–5103. 2009. View Article : Google Scholar
|
|
22
|
Wang R, Cao XJ, Kulej K, Liu W, Ma T,
MacDonald M, Chiang CM, Garcia BA and You J: Uncovering BRD4
hyperphosphorylation associated with cellular transformation in NUT
midline carcinoma. Proc Natl Acad Sci USA. 114:E5352–E5361.
2017.PubMed/NCBI
|
|
23
|
Wu Y, Wang Y, Diao P, Zhang W, Li J, Ge H,
Song Y, Li Z, Wang D, Liu L, et al: Therapeutic targeting of BRD4
in head neck squamous cell carcinoma. Theranostics. 9:1777–1793.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu SY, Lee CF, Lai HT, Yu CT, Lee JE, Zuo
H, Tsai SY, Tsai MJ, Ge K, Wan Y, et al: Opposing functions of BRD4
isoforms in breast cancer. Mol Cell. 78:1114–1132.e10. 2020.
View Article : Google Scholar
|
|
25
|
Drumond-Bock AL and Bieniasz M: The role
of distinct BRD4 isoforms and their contribution to high-grade
serous ovarian carcinoma pathogenesis. Mol Cancer. 20:1452021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
White ME, Fenger JM and Carson WE III:
Emerging roles of and therapeutic strategies targeting BRD4 in
cancer. Cell Immunol. 337:48–53. 2019. View Article : Google Scholar
|
|
27
|
Donati B, Lorenzini E and Ciarrocchi A:
BRD4 and Cancer: Going beyond transcriptional regulation. Mol
Cancer. 17:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shu S, Lin CY, He HH, Witwicki RM,
Tabassum DP, Roberts JM, Janiszewska M, Huh SJ, Liang Y, Ryan J, et
al: Response and resistance to BET bromodomain inhibitors in
triple-negative breast cancer. Nature. 529:413–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamad M, Ali A and Muhammad JS: BRD4
regulates the induction and maintenance of cancer stem cells in
squamous cell carcinoma. Stem Cell Investig. 9:62022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pagliarini R, Shao W and Sellers WR:
Oncogene addiction: Pathways of therapeutic response, resistance,
and road maps toward a cure. EMBO Rep. 16:280–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang
Q, Lin Y, Li J, Kang T, Tao M, et al: Disrupting the interaction of
BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like
breast cancer. Cancer Cell. 25:210–225. 2014. View Article : Google Scholar
|
|
32
|
Andrieu G, Tran AH, Strissel KJ and Denis
GV: BRD4 regulates breast cancer dissemination through
Jagged1/Notch1 signaling. Cancer Res. 76:6555–6567. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samani K, Raj Sharma U, Raj Sharma A, Pm M
and V S: Role of BRD4 in cancer-A review. J Diagnostic Pathol
Oncolo. 5:128–134. 2020. View Article : Google Scholar
|
|
34
|
Shorstova T, Foulkes WD and Witcher M:
Achieving clinical success with BET inhibitors as anti-cancer
agents. Br J Cancer. 124:1478–1490. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan Y, Wang L, Du Y, Liu X, Chen Z, Weng
X, Guo J, Chen H, Wang M and Wang X: Inhibition of BRD4 suppresses
tumor growth in prostate cancer via the enhancement of FOXO1
expression. Int J Oncol. 53:2503–2517. 2018.
|
|
36
|
Wang J, Quan Y, Lv J, Gong S and Dong D:
BRD4 promotes glioma cell stemness via enhancing
miR-142-5p-mediated activation of Wnt/β-catenin signaling. Environ
Toxicol. 35:368–376. 2020. View Article : Google Scholar
|
|
37
|
Nantajit D, Presta L, Sauter T and
Tavassoli M: EGFR-induced suppression of HPV E6/E7 is mediated by
microRNA-9-5p silencing of BRD4 protein in HPV-positive head and
neck squamous cell carcinoma. Cell Death Dis. 13:9212022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmitt A, Grimm M, Kreienkamp N, Junge H,
Labisch J, Schuhknecht L, Schonfeld C, Gorsch ES, Tibello A, Menck
K, et al: BRD4 inhibition sensitizes diffuse large B-cell lymphoma
cells to ferroptosis. Blood. 142:1143–1155. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stathis A, Zucca E, Bekradda M, Gomez-Roca
C, Delord JP, de La Motte Rouge T, Uro-Coste E, de Braud F, Pelosi
G and French CA: Clinical response of carcinomas harboring the
BRD4-NUT oncoprotein to the targeted bromodomain inhibitor
OTX015/MK-8628. Cancer Discov. 6:492–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Guo X, Zhuo R, Tao Y, Liang W,
Yang R, Chen Y, Cao H, Jia S, Yu J, et al: BRD4 inhibitor MZ1
exerts anti-cancer effects by targeting MYCN and MAPK signaling in
neuroblastoma. Biochem Biophys Res Commun. 604:63–69. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zong D, Gu J, Cavalcante GC, Yao W, Zhang
G, Wang S, Owonikoko TK, He X and Sun SY: BRD4 levels determine the
response of human lung cancer cells to BET degraders that potently
induce apoptosis through suppression of Mcl-1. Cancer Res.
80:2380–2393. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bauer K, Berghoff AS, Preusser M, Heller
G, Zielinski CC, Valent P and Grunt TW: Degradation of BRD4-a
promising treatment approach not only for hematologic but also for
solid cancer. Am J Cancer Res. 11:530–545. 2021.PubMed/NCBI
|
|
43
|
Sun Y, Han J, Wang Z, Li X, Sun Y and Hu
Z: Safety and efficacy of bromodomain and Extra-Terminal inhibitors
for the treatment of hematological malignancies and solid tumors: A
systematic study of clinical trials. Front Pharmacol.
11:6210932020. View Article : Google Scholar
|
|
44
|
Floyd SR, Pacold ME, Huang Q, Clarke SM,
Lam FC, Cannell IG, Bryson BD, Rameseder J, Lee MJ, Blake EJ, et
al: The bromodomain protein Brd4 insulates chromatin from DNA
damage signalling. Nature. 498:246–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ni M, Li J, Zhao H, Xu F, Cheng J, Yu M,
Ke G and Wu X: BRD4 inhibition sensitizes cervical cancer to
radiotherapy by attenuating DNA repair. Oncogene. 40:2711–2724.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lam FC, Kong YW, Huang Q, Vu Han TL, Maffa
AD, Kasper EM and Yaffe MB: BRD4 prevents the accumulation of
R-loops and protects against transcription-replication collision
events and DNA damage. Nat Commun. 11:40832020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li X, Baek G, Ramanand SG, Sharp A, Gao Y,
Yuan W, Welti J, Rodrigues DN, Dolling D, Figueiredo I, et al: BRD4
promotes DNA repair and mediates the formation of TMPRSS2-ERG gene
rearrangements in prostate cancer. Cell Rep. 22:796–808. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun C, Yin J, Fang Y, Chen J, Jeong KJ,
Chen X, Vellano CP, Ju Z, Zhao W, Zhang D, et al: BRD4 inhibition
is synthetic lethal with PARP inhibitors through the induction of
homologous recombination deficiency. Cancer Cell. 33:401–416.e8.
2018. View Article : Google Scholar
|
|
49
|
Barrows JK, Lin B, Quaas CE, Fullbright G,
Wallace EN and Long DT: BRD4 promotes resection and
homology-directed repair of DNA double-strand breaks. Nat Commun.
13:30162022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takashima Y, Kikuchi E, Kikuchi J, Suzuki
M, Kikuchi H, Maeda M, Shoji T, Furuta M, Kinoshita I, Dosaka-Akita
H, et al: Bromodomain and extraterminal domain inhibition
synergizes with WEE1-inhibitor AZD1775 effect by impairing
nonhomologous end joining and enhancing DNA damage in nonsmall cell
lung cancer. Int J Cancer. 146:1114–1124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tan YF, Wang M, Chen ZY, Wang L and Liu
XH: Inhibition of BRD4 prevents proliferation and
epithelial-mesenchymal transition in renal cell carcinoma via NLRP3
inflammasome-induced pyroptosis. Cell Death Dis. 11:2392020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller AL, Fehling SC, Garcia PL, Gamblin
TL, Council LN, van Waardenburg R, Yang ES, Bradner JE and Yoon KJ:
The BET inhibitor JQ1 attenuates double-strand break repair and
sensitizes models of pancreatic ductal adenocarcinoma to PARP
inhibitors. EBioMedicine. 44:419–430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
He DD, Shang XY, Wang N, Wang GX, He KY,
Wang L and Han ZG: BRD4 inhibition induces synthetic lethality in
ARID2-deficient hepatocellular carcinoma by increasing DNA damage.
Oncogene. 41:1397–1409. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li L, Gao L, Zhou H, Shi C, Zhang X, Zhang
D and Liu H: High expression level of BRD4 is associated with a
poor prognosis and immune infiltration in esophageal squamous cell
carcinoma. Dig Dis Sci. 68:2997–3008. 2023. View Article : Google Scholar
|
|
55
|
Liao YF, Wu YB, Long X, Zhu SQ, Jin C, Xu
JJ and Ding JY: High level of BRD4 promotes non-small cell lung
cancer progression. Oncotarget. 7:9491–9500. 2016. View Article : Google Scholar
|
|
56
|
Burcher KM, Faucheux AT, Lantz JW, Wilson
HL, Abreu A, Salafian K, Patel MJ, Song AH, Petro RM, Lycan T Jr,
et al: Prevalence of DNA repair gene mutations in blood and tumor
tissue and impact on prognosis and treatment in HNSCC. Cancers
(Basel). 13:31182021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Moeller BJ, Yordy JS, Williams MD, Giri U,
Raju U, Molkentine DP, Byers LA, Heymach JV, Story MD, Lee JJ, et
al: DNA repair biomarker profiling of head and neck cancer: Ku80
expression predicts locoregional failure and death following
radiotherapy. Clin Cancer Res. 17:2035–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mahjabeen I, Ali K, Zhou X and Kayani MA:
Deregulation of base excision repair gene expression and enhanced
proliferation in head and neck squamous cell carcinoma. Tumour
Biol. 35:5971–5983. 2014. View Article : Google Scholar
|
|
59
|
Bold IT, Specht AK, Droste CF, Zielinski
A, Meyer F, Clauditz TS, Munscher A, Werner S, Rothkamm K, Petersen
C, et al: DNA damage response during replication correlates with
CIN70 score and determines survival in HNSCC patients. Cancers
(Basel). 13:11942021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang S, Lai Y, Pan J, Saeed M, Li S, Zhou
H, Jiang X, Gao J, Zhu Y, Yu H, et al: PROTAC Prodrug-Integrated
nanosensitizer for potentiating radiation therapy of cancer. Adv
Mater. e23141322024.doi: 10.1002/adma.202314132 (Epub ahead of
print). View Article : Google Scholar
|
|
61
|
Wang J, Wang Y, Mei H, Yin Z, Geng Y,
Zhang T, Wu G and Lin Z: The BET bromodomain inhibitor JQ1
radiosensitizes non-small cell lung cancer cells by upregulating
p21. Cancer Lett. 391:141–151. 2017. View Article : Google Scholar
|
|
62
|
Garcia PL, Miller AL, Zeng L, van
Waardenburg R, Yang ES and Yoon KJ: The BET inhibitor JQ1
potentiates the anticlonogenic effect of radiation in pancreatic
cancer Cells. Front Oncol. 12:9257182022. View Article : Google Scholar
|
|
63
|
Kim S, Jeon SH, Han MG, Kang MH and Kim
IA: BRD4 inhibition enhances the antitumor effects of radiation
therapy in a murine breast cancer model. Int J Mol Sci.
24:130622023. View Article : Google Scholar
|
|
64
|
Santos-de-Frutos K, Segrelles C and Lorz
C: Hippo pathway and YAP signaling alterations in squamous cancer
of the head and neck. J Clin Med. 8:21312019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Choi SK, Hong SH, Kim HS, Shin CY, Nam SW,
Choi WS, Han JW and You JS: JQ1, an inhibitor of the epigenetic
reader BRD4, suppresses the bidirectional MYC-AP4 axis via multiple
mechanisms. Oncol Rep. 35:1186–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hong SH, Eun JW, Choi SK, Shen Q, Choi WS,
Han JW, Nam SW and You JS: Epigenetic reader BRD4 inhibition as a
therapeutic strategy to suppress E2F2-cell cycle regulation circuit
in liver cancer. Oncotarget. 7:32628–32640. 2016. View Article : Google Scholar
|
|
67
|
Zheng B, Gold S, Iwanaszko M, Howard BC,
Wang L and Shilatifard A: Distinct layers of BRD4-PTEFb reveal
bromodomain-independent function in transcriptional regulation. Mol
Cell. 83:2896–2910.e4. 2023. View Article : Google Scholar
|
|
68
|
Itzen F, Greifenberg AK, Bosken CA and
Geyer M: Brd4 activates P-TEFb for RNA polymerase II CTD
phosphorylation. Nucleic Acids Res. 42:7577–7590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
He Y, Ju Y, Hu Y, Wang B, Che S, Jian Y,
Zhuo W, Fu X, Cheng Y, Zheng S, et al: Brd4 proteolysis-targeting
chimera nanoparticles sensitized colorectal cancer chemotherapy. J
Control Release. 354:155–166. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu T, Zhang Z, Wang C, Huang H and Li Y:
BRD4 promotes the migration and invasion of bladder cancer cells
through the Sonic hedgehog signaling pathway and enhances cisplatin
resistance. Biochem Cell Biol. 100:179–187. 2022. View Article : Google Scholar
|
|
71
|
Lu L, Chen Z, Lin X, Tian L, Su Q, An P,
Li W, Wu Y, Du J, Shan H, et al: Inhibition of BRD4 suppresses the
malignancy of breast cancer cells via regulation of Snail. Cell
Death Differ. 27:255–268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shafran JS, Jafari N, Casey AN, Gyorffy B
and Denis GV: BRD4 regulates key transcription factors that drive
epithelial-mesenchymal transition in castration-resistant prostate
cancer. Prostate Cancer Prostatic Dis. 24:268–277. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shi J, Cao J and Zhou BP: Twist-BRD4
complex: Potential drug target for basal-like breast cancer. Curr
Pharm Des. 21:1256–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang P, Dong Z, Cai J, Zhang C, Shen Z,
Ke A, Gao D, Fan J and Shi G: BRD4 promotes tumor growth and
epithelial-mesenchymal transition in hepatocellular carcinoma. Int
J Immunopathol Pharmacol. 28:36–44. 2015. View Article : Google Scholar
|
|
75
|
Andrieu GP and Denis GV: BET proteins
exhibit transcriptional and functional opposition in the
Epithelial-to-Mesenchymal transition. Mol Cancer Res. 16:580–586.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tian B, Zhao Y, Sun H, Zhang Y, Yang J and
Brasier AR: BRD4 mediates NF-κB-dependent epithelial-mesenchymal
transition and pulmonary fibrosis via transcriptional elongation.
Am J Physiol Lung Cell Mol Physiol. 311:L1183–L1201. 2016.
View Article : Google Scholar
|
|
77
|
Cho HY, Lee SW, Jeon YH, Lee DH, Kim GW,
Yoo J, Kim SY and Kwon SH: Combination of ACY-241 and JQ1
synergistically suppresses metastasis of HNSCC via regulation of
MMP-2 and MMP-9. Int J Mol Sci. 21:68732020. View Article : Google Scholar
|
|
78
|
Hu Y, Zhou J, Ye F, Xiong H, Peng L, Zheng
Z, Xu F, Cui M, Wei C, Wang X, et al: BRD4 inhibitor inhibits
colorectal cancer growth and metastasis. Int J Mol Sci.
16:1928–1948. 2015. View Article : Google Scholar
|
|
79
|
Wang L, Wu X, Wang R, Yang C, Li Z, Wang
C, Zhang F and Yang P: BRD4 inhibition suppresses cell growth,
migration and invasion of salivary adenoid cystic carcinoma. Biol
Res. 50:192017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yamamoto T, Hirosue A, Nakamoto M, Yoshida
R, Sakata J, Matsuoka Y, Kawahara K, Nagao Y, Nagata M, Takahashi
N, et al: BRD4 promotes metastatic potential in oral squamous cell
carcinoma through the epigenetic regulation of the MMP2 gene. Br J
Cancer. 123:580–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Griso AB, Acero-Riaguas L, Castelo B,
Cebrian-Carretero JL and Sastre-Perona A: Mechanisms of cisplatin
resistance in HPV negative head and neck squamous cell carcinomas.
Cells. 11:5612022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bonomi M, Patsias A, Posner M and Sikora
A: The role of inflammation in head and neck cancer. Adv Exp Med
Biol. 816:107–127. 2014. View Article : Google Scholar
|
|
83
|
Gong Z, Liu G, Liu W, Zou H, Song R, Zhao
H, Yuan Y, Gu J, Bian J, Zhu J, et al: The epigenetic regulator
BRD4 is involved in cadmium-triggered inflammatory response in rat
kidney. Ecotoxicol Environ Saf. 224:1126202021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bao Y, Wu X, Chen J, Hu X, Zeng F, Cheng
J, Jin H, Lin X and Chen LF: Brd4 modulates the innate immune
response through Mnk2-eIF4E pathway-dependent translational control
of IκBα. Proc Natl Acad Sci USA. 114:E3993–E4001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu Y and Vakoc CR: Brd4 is on the move
during inflammation. Trends Cell Biol. 24:615–616. 2014. View Article : Google Scholar
|
|
86
|
Jarausch J, Neuenroth L, Andag R, Leha A,
Fischer A, Asif AR, Lenz C and Eidizadeh A: Influence of shear
stress, inflammation and BRD4 inhibition on human endothelial
cells: A holistic proteomic approach. Cells. 11:30862022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen L, Zhong X, Cao W, Mao M, Li W, Yang
H, Li M, Shi M, Zhang Y, Deng Y, et al: JQ1 as a BRD4 inhibitor
blocks inflammatory pyroptosis-related acute colon injury induced
by LPS. Front Immunol. 12:6093192021. View Article : Google Scholar
|
|
88
|
Nicodeme E, Jeffrey KL, Schaefer U, Beinke
S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H,
et al: Suppression of inflammation by a synthetic histone mimic.
Nature. 468:1119–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Khan YM, Kirkham P, Barnes PJ and Adcock
IM: Brd4 is essential for IL-1β-induced inflammation in human
airway epithelial cells. PLoS One. 9:e950512014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jing SL, Afshari K and Guo ZC:
Inflammatory response-related genes predict prognosis in patients
with HNSCC. Immunol Lett. 259:46–60. 2023. View Article : Google Scholar
|
|
91
|
Rassouli A, Saliba J, Castano R, Hier M
and Zeitouni AG: Systemic inflammatory markers as independent
prognosticators of head and neck squamous cell carcinoma. Head
Neck. 37:103–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou S, Yuan H, Wang J, Hu X, Liu F, Zhang
Y, Jiang B and Zhang W: Prognostic value of systemic inflammatory
marker in patients with head and neck squamous cell carcinoma
undergoing surgical resection. Future Oncol. 16:559–571. 2020.
View Article : Google Scholar
|
|
93
|
Charles KA, Harris BD, Haddad CR, Clarke
SJ, Guminski A, Stevens M, Dodds T, Gill AJ, Back M, Veivers D, et
al: Systemic inflammation is an independent predictive marker of
clinical outcomes in mucosal squamous cell carcinoma of the head
and neck in oropharyngeal and non-oropharyngeal patients. BMC
Cancer. 16:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Roman BR and Aragones A: Epidemiology and
incidence of HPV-related cancers of the head and neck. J Surg
Oncol. 124:920–922. 2021. View Article : Google Scholar
|
|
95
|
Serrano B, Brotons M, Bosch FX and Bruni
L: Epidemiology and burden of HPV-related disease. Best Pract Res
Clin Obstet Gynaecol. 47:14–26. 2018. View Article : Google Scholar
|
|
96
|
Betiol J, Villa LL and Sichero L: Impact
of HPV infection on the development of head and neck cancer. Braz J
Med Biol Res. 46:217–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
de Martel C, Georges D, Bray F, Ferlay J
and Clifford GM: Global burden of cancer attributable to infections
in 2018: A worldwide incidence analysis. Lancet Glob Health.
8:e180–e190. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kajitani N, Satsuka A, Kawate A and Sakai
H: Productive lifecycle of human papillomaviruses that depends upon
squamous epithelial differentiation. Front Microbiol. 3:1522012.
View Article : Google Scholar
|
|
99
|
Cricca M, Venturoli S, Leo E, Costa S,
Musiani M and Zerbini M: Disruption of HPV 16 E1 and E2 genes in
precancerous cervical lesions. J Virol Methods. 158:180–183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pal A and Kundu R: Human Papillomavirus E6
and E7: The cervical cancer hallmarks and targets for therapy.
Front Microbiol. 10:31162019. View Article : Google Scholar
|
|
101
|
Yu L, Majerciak V and Zheng ZM: HPV16 and
HPV18 genome structure, expression, and Post-Transcriptional
regulation. Int J Mol Sci. 23:49432022. View Article : Google Scholar
|
|
102
|
Helfer CM, Yan J and You J: The cellular
bromodomain protein Brd4 has multiple functions in E2-mediated
papillomavirus transcription activation. Viruses. 6:3228–3249.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
McBride AA, McPhillips MG and Oliveira JG:
Brd4: Tethering, segregation and beyond. Trends Microbiol.
12:527–529. 2004. View Article : Google Scholar
|
|
104
|
Jang MK, Shen K and McBride AA:
Papillomavirus genomes associate with BRD4 to replicate at fragile
sites in the host genome. PLoS Pathog. 10:e10041172014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
McBride AA and Jang MK: Current
understanding of the role of the Brd4 protein in the papillomavirus
lifecycle. Viruses. 5:1374–1394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
McKinney CC, Kim MJ, Chen D and McBride
AA: Brd4 activates early viral transcription upon human
papillomavirus 18 infection of primary keratinocytes. mBio.
7:e01644–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Iftner T, Haedicke-Jarboui J, Wu SY and
Chiang CM: Involvement of Brd4 in different steps of the
papillomavirus life cycle. Virus Res. 231:76–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Morse MA, Balogh KK, Brendle SA, Campbell
CA, Chen MX, Furze RC, Harada IL, Holyer ID, Kumar U, Lee K, et al:
BET bromodomain inhibitors show anti-papillomavirus activity in
vitro and block CRPV wart growth in vivo. Antiviral Res.
154:158–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang J, Li GL, Ming SL, Wang CF, Shi LJ,
Su BQ, Wu HT, Zeng L, Han YQ, Liu ZH, et al: BRD4 inhibition exerts
anti-viral activity through DNA damage-dependent innate immune
responses. PLoS Pathog. 16:e10084292020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen J, Wang Z, Phuc T, Xu Z, Yang D, Chen
Z, Lin Z, Kendrick S, Dai L, Li HY, et al: Oncolytic strategy using
new bifunctional HDACs/BRD4 inhibitors against virus-associated
lymphomas. PLoS Pathog. 19:e10110892023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cohen EEW, Bell RB, Bifulco CB, Burtness
B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL,
et al: The Society for Immunotherapy of Cancer consensus statement
on immunotherapy for the treatment of squamous cell carcinoma of
the head and neck (HNSCC). J Immunother Cancer. 7:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oosting SF and Haddad RI: Best practice in
systemic therapy for head and neck squamous cell carcinoma. Front
Oncol. 9:8152019. View Article : Google Scholar
|
|
113
|
Rosenberg AJ and Vokes EE: Optimizing
treatment De-Escalation in head and neck cancer: Current and future
perspectives. Oncologist. 26:40–48. 2021. View Article : Google Scholar
|
|
114
|
Swain M, Kannan S, Srinivasan S, Agarwal
JP and Gupta T: Concurrent Cetuximab-based bioradiotherapy versus
Cisplatin-based Chemoradiotherapy in the Definitive Management of
Favourable Biology Human Papillomavirus-associated Oropharyngeal
Squamous Cell Carcinoma: Systematic Review and Meta-analysis. Clin
Oncol (R Coll Radiol). 34:786–795. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lv XX, Zheng XY, Yu JJ, Ma HR, Hua C and
Gao RT: EGFR enhances the stemness and progression of oral cancer
through inhibiting autophagic degradation of SOX2. Cancer Med.
9:1131–1140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Beck TN, Georgopoulos R, Shagisultanova
EI, Sarcu D, Handorf EA, Dubyk C, Lango MN, Ridge JA, Astsaturov I,
Serebriiskii IG, et al: EGFR and RB1 as dual biomarkers in
HPV-Negative head and neck cancer. Mol Cancer Ther. 15:2486–2497.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Alsahafi EN, Thavaraj S, Sarvestani N,
Novoplansky O, Elkabets M, Ayaz B, Tavassoli M and Legends MF: EGFR
overexpression increases radiotherapy response in HPV-positive head
and neck cancer through inhibition of DNA damage repair and HPV E6
downregulation. Cancer Lett. 498:80–97. 2021. View Article : Google Scholar
|
|
118
|
Rieckmann T and Kriegs M: The failure of
cetuximab-based de-intensified regimes for HPV-positive OPSCC: A
radiobiologists perspective. Clin Transl Radiat Oncol. 17:47–50.
2019.
|
|
119
|
Krishnamurthy S, Ahmed I, Bhise R, Mohanti
BK, Sharma A, Rieckmann T, Paterson C and Bonomo P: The dogma of
Cetuximab and Radiotherapy in head and neck cancer-A dawn to dusk
journey. Clin Transl Radiat Oncol. 34:75–81. 2022.
|
|
120
|
Xu K, Chen D, Qian D, Zhang S, Zhang Y,
Guo S, Ma Z and Wang S: AZD5153, a novel BRD4 inhibitor, suppresses
human thyroid carcinoma cell growth in vitro and in vivo. Biochem
Biophys Res Commun. 499:531–537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cortiguera MG, Batlle-López A, Albajar M,
Delgado MD and León J: MYC as therapeutic target in leukemia and
lymphoma. Blood and Lymphatic Cancer: Targets and Therapy. 5:75–91.
2015.
|
|
122
|
Wang C, Zhang Y, Zhou D, Cao G and Wu Y:
miR-204 enhances p27 mRNA stability by targeting Brd4 in head and
neck squamous cell carcinoma. Oncol Lett. 16:4179–4184. 2018.
|
|
123
|
Zhang W, Ge H, Jiang Y, Huang R, Wu Y,
Wang D, Guo S, Li S, Wang Y, Jiang H, et al: Combinational
therapeutic targeting of BRD4 and CDK7 synergistically induces
anticancer effects in head and neck squamous cell carcinoma. Cancer
Lett. 469:510–523. 2020. View Article : Google Scholar
|
|
124
|
Webber LP, Yujra VQ, Vargas PA, Martins
MD, Squarize CH and Castilho RM: Interference with the bromodomain
epigenome readers drives p21 expression and tumor senescence.
Cancer Lett. 461:10–20. 2019. View Article : Google Scholar
|
|
125
|
Dong J, Li J, Li Y, Ma Z, Yu Y and Wang
CY: Transcriptional super-enhancers control cancer stemness and
metastasis genes in squamous cell carcinoma. Nat Commun.
12:39742021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jing C, Liu D, Lai Q, Li L, Zhou M, Ye B,
Wu Y, Li H, Yue K, Wu Y, et al: JOSD1 promotes proliferation and
chemoresistance of head and neck squamous cell carcinoma under the
epigenetic regulation of BRD4. Cancer Cell Int. 21:3752021.
View Article : Google Scholar
|
|
127
|
Chen N, Golczer G, Ghose S, Lin B,
Langenbucher A, Webb J, Bhanot H, Abt NB, Lin D, Varvares M, et al:
YAP1 maintains active chromatin state in head and neck squamous
cell carcinomas that promotes tumorigenesis through cooperation
with BRD4. Cell Rep. 39:1109702022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Leonard B, Brand TM, O'Keefe RA, Lee ED,
Zeng Y, Kemmer JD, Li H, Grandis JR and Bhola NE: BET Inhibition
overcomes receptor tyrosine Kinase-Mediated cetuximab resistance in
HNSCC. Cancer Res. 78:4331–4343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Araujo TG, Mota STS, Ferreira HSV, Ribeiro
MA, Goulart LR and Vecchi L: Annexin A1 as a regulator of immune
response in cancer. Cells. 10:22452021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhong L, Yang Z, Lei D, Li L, Song S, Cao
D and Liu Y: Bromodomain 4 is a potent prognostic marker associated
with immune cell infiltration in breast cancer. Basic Clin
Pharmacol Toxicol. 128:169–182. 2021. View Article : Google Scholar
|
|
131
|
Chen YR, Ouyang SS, Chen YL, Li P, Xu HW
and Zhu SL: BRD4/8/9 are prognostic biomarkers and associated with
immune infiltrates in hepatocellular carcinoma. Aging (Albany NY).
12:17541–17567. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lee M, Tayyari F, Pinnaduwage D, Bayani J,
Bartlett JMS, Mulligan AM, Bull SB and Andrulis IL: Tumoral BRD4
expression in lymph node-negative breast cancer: association with
T-bet+ tumor-infiltrating lymphocytes and disease-free survival.
BMC Cancer. 18:7502018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhao L, Li P, Zhao L, Wang M, Tong D, Meng
Z, Zhang Q, Li Q and Zhang F: Expression and clinical value of
PD-L1 which is regulated by BRD4 in tongue squamous cell carcinoma.
J Cell Biochem. 121:1855–1869. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jing X, Shao S, Zhang Y, Luo A, Zhao L,
Zhang L, Gu S and Zhao X: BRD4 inhibition suppresses PD-L1
expression in triple-negative breast cancer. Exp Cell Res.
392:1120342020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ye Y, Zhong W, Qian J, Zhang J, Xu T, Han
R, Han J, Wang C, Song L, Zeng X, et al: Comprehensive analysis of
the prognosis and immune infiltrates for the BET protein family
reveals the significance of BRD4 in glioblastoma multiforme. Front
Cell Dev Biol. 11:10424902023. View Article : Google Scholar
|
|
136
|
Bhola NE, Njatcha C, Hu L, Lee ED, Shiah
JV, Kim MO, Johnson DE and Grandis JR: PD-L1 is upregulated via
BRD2 in head and neck squamous cell carcinoma models of acquired
cetuximab resistance. Head Neck. 43:3364–3373. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang M, Wang G, Ma Z, Xiong G, Wang W,
Huang Z, Wan Y, Xu X, Hoyle RG, Yi C, et al: BET inhibition
triggers antitumor immunity by enhancing MHC class I expression in
head and neck squamous cell carcinoma. Mol Ther. 30:3394–3413.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Suarez-Alvarez B, Morgado-Pascual JL,
Rayego-Mateos S, Rodriguez RM, Rodrigues-Diez R, Cannata-Ortiz P,
Sanz AB, Egido J, Tharaux PL, Ortiz A, et al: Inhibition of
bromodomain and extraterminal domain family proteins ameliorates
experimental renal damage. J Am Soc Nephrol. 28:504–519. 2017.
View Article : Google Scholar
|
|
139
|
Xia L, Liu JY, Zheng ZZ, Chen YJ, Ding JC,
Hu YH, Hu GS, Xia NS and Liu W: BRD4 inhibition boosts the
therapeutic effects of epidermal growth factor receptor-targeted
chimeric antigen receptor T cells in glioblastoma. Mol Ther.
29:3011–3026. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Joshi S, Singh AR, Liu KX, Pham TV, Zulcic
M, Skola D, Chun HB, Glass CK, Morales GA, Garlich JR, et al:
SF2523: Dual PI3K/BRD4 inhibitor blocks tumor immunosuppression and
promotes adaptive immune responses in cancer. Mol Cancer Ther.
18:1036–1044. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li X, Fu Y, Yang B, Guo E, Wu Y, Huang J,
Zhang X, Xiao R, Li K, Wang B, et al: BRD4 Inhibition by AZD5153
promotes antitumor immunity via depolarizing M2 macrophages. Front
Immunol. 11:892020. View Article : Google Scholar
|
|
142
|
Fong CY, Gilan O, Lam EY, Rubin AF, Ftouni
S, Tyler D, Stanley K, Sinha D, Yeh P, Morison J, et al: BET
inhibitor resistance emerges from leukaemia stem cells. Nature.
525:538–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Rathert P, Roth M, Neumann T, Muerdter F,
Roe JS, Muhar M, Deswal S, Cerny-Reiterer S, Peter B, Jude J, et
al: Transcriptional plasticity promotes primary and acquired
resistance to BET inhibition. Nature. 525:543–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Calder J, Nagelberg A, Luu J, Lu D and
Lockwood WW: Resistance to BET inhibitors in lung adenocarcinoma is
mediated by casein kinase phosphorylation of BRD4. Oncogenesis.
10:272021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jin X, Yan Y, Wang D, Ding D, Ma T, Ye Z,
Jimenez R, Wang L, Wu H and Huang H: DUB3 promotes BET inhibitor
resistance and cancer progression by deubiquitinating BRD4. Mol
Cell. 71:592–605. e5942018. View Article : Google Scholar
|
|
146
|
Tai F, Gong K, Song K, He Y and Shi J:
Enhanced JunD/RSK3 signalling due to loss of BRD4/FOXD3/miR-548d-3p
axis determines BET inhibition resistance. Nat Commun. 11:2582020.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang X, Wei X, Cao Y and Xing P: ZNF33A
promotes tumor progression and BET inhibitor resistance in
Triple-Negative breast cancer. Am J Pathol. 192:1458–1469. 2022.
View Article : Google Scholar
|
|
148
|
Shu S, Wu HJ, Ge JY, Zeid R, Harris IS,
Jovanovic B, Murphy K, Wang B, Qiu X, Endress JE, et al: Synthetic
lethal and resistance interactions with BET bromodomain inhibitors
in Triple-Negative breast cancer. Mol Cell. 78:1096–1113.e8. 2020.
View Article : Google Scholar
|
|
149
|
Luan W, Pang Y, Li R, Wei X, Jiao X, Shi
J, Yu J, Mao H and Liu P: Akt/mTOR-Mediated autophagy confers
resistance to BET inhibitor JQ1 in ovarian cancer. Onco Targets
Ther. 12:8063–8074. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Andrikopoulou A, Liontos M, Koutsoukos K,
Dimopoulos MA and Zagouri F: Clinical perspectives of BET
inhibition in ovarian cancer. Cell Oncol (Dordr). 44:237–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang B, Fan P, Zhao J and Wu H, Jin X and
Wu H: FBP1 loss contributes to BET inhibitors resistance by
undermining c-Myc expression in pancreatic ductal adenocarcinoma. J
Exp Clin Cancer Res. 37:2242018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang P, Wang D, Zhao Y, Ren S, Gao K, Ye
Z, Wang S, Pan CW, Zhu Y, Yan Y, et al: Intrinsic BET inhibitor
resistance in SPOP-mutated prostate cancer is mediated by BET
protein stabilization and AKT-mTORC1 activation. Nat Med.
23:1055–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Piha-Paul SA, Sachdev JC, Barve M, LoRusso
P, Szmulewitz R, Patel SP, Lara PN Jr, Chen X, Hu B, Freise KJ, et
al: First-in-Human study of mivebresib (ABBV-075), an Oral
Pan-Inhibitor of bromodomain and extra terminal proteins, in
patients with Relapsed/Refractory solid tumors. Clin Cancer Res.
25:6309–6319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Aggarwal RR, Schweizer MT, Nanus DM,
Pantuck AJ, Heath EI, Campeau E, Attwell S, Norek K, Snyder M,
Bauman L, et al: A Phase Ib/IIa Study of the Pan-BET Inhibitor
ZEN-3694 in combination with enzalutamide in patients with
metastatic Castration-Resistant prostate cancer. Clin Cancer Res.
26:5338–5347. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Faivre EJ, McDaniel KF, Albert DH, Mantena
SR, Plotnik JP, Wilcox D, Zhang L, Bui MH, Sheppard GS, Wang L, et
al: Selective inhibition of the BD2 bromodomain of BET proteins in
prostate cancer. Nature. 578:306–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ferreira D, Moreira JN and Rodrigues LR:
New advances in exosome-based targeted drug delivery systems. Crit
Rev Oncol Hematol. 172:1036282022. View Article : Google Scholar
|
|
157
|
Bhome R, Del Vecchio F, Lee GH, Bullock
MD, Primrose JN, Sayan AE and Mirnezami AH: Exosomal microRNAs
(exomiRs): Small molecules with a big role in cancer. Cancer Lett.
420:228–235. 2018. View Article : Google Scholar
|
|
158
|
Alcitepe I, Salcin H, Karatekin I and
Kaymaz BT: HDAC inhibitor Vorinostat and BET inhibitor Plx51107
epigenetic agents' combined treatments exert a therapeutic approach
upon acute myeloid leukemia cell model. Med Oncol. 39:2572022.
View Article : Google Scholar
|
|
159
|
Liu S, Li F, Pan L, Yang Z, Shu Y, Lv W,
Dong P and Gong W: BRD4 inhibitor and histone deacetylase inhibitor
synergistically inhibit the proliferation of gallbladder cancer in
vitro and in vivo. Cancer Sci. 110:2493–2506. 2019. View Article : Google Scholar
|
|
160
|
Patel MR, Garcia-Manero G, Paquette R,
Dinner S, Donnellan WB, Grunwald MR, Ribadeneira MD, Schroeder P,
Brevard J, Wilson L, et al: Phase 1 Dose escalation and expansion
study to determine safety, tolerability, pharmacokinetics, and
pharmacodynamics of the BET inhibitor FT-1101 as a single agent in
patients with relapsed or refractory hematologic malignancies.
Blood. 134:3907. 2019. View Article : Google Scholar
|
|
161
|
Piha-Paul SA, Hann CL, French CA, Cousin
S, Brana I, Cassier PA, Moreno V, de Bono JS, Harward SD,
Ferron-Brady G, et al: Phase 1 study of molibresib (GSK525762), a
bromodomain and Extra-Terminal domain protein inhibitor, in NUT
carcinoma and other solid tumors. JNCI Cancer Spectr. 4:pkz0932020.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Dawson M, Stein EM, Huntly BJP,
Karadimitris A, Kamdar M, Fernandez de Larrea C, Dickinson MJ, Yeh
PS-H, Daver N, Chaidos A, et al: A Phase I study of GSK525762, a
selective bromodomain (BRD) and extra terminal protein (BET)
Inhibitor: Results from Part 1 of Phase I/II open label single
agent study in patients with acute myeloid leukemia (AML). Blood.
130:13772017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Falchook G, Rosen S, LoRusso P, Watts J,
Gupta S, Coombs CC, Talpaz M, Kurzrock R, Mita M, Cassaday R, et
al: Development of 2 bromodomain and extraterminal inhibitors with
distinct pharmacokinetic and pharmacodynamic profiles for the
treatment of advanced malignancies. Clin Cancer Res. 26:1247–1257.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Watts JM, Hunter AM, Iurlo A, Xicoy B,
Palandri F, Reeves B, Vannucchi A, Bose P, Ayala Diaz R, Halpern
AB, et al: Bromodomain and extra-terminal (BET) inhibitor
INCB057643 (LIMBER-103) in patients (pts) with relapsed or
refractory myelofibrosis (R/R MF) and other advanced myeloid
neoplasms: A phase 1 study. HemaSphere. 7:e17929062023. View Article : Google Scholar
|
|
165
|
Ameratunga M, Brana I, Bono P,
Postel-Vinay S, Plummer R, Aspegren J, Korjamo T, Snapir A and de
Bono JS: First-in-human Phase 1 open label study of the BET
inhibitor ODM-207 in patients with selected solid tumours. Br J
Cancer. 123:1730–1736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dombret H, Preudhomme C, Berthon C,
Raffoux E, Thomas X, Vey N, Gomez-Roca C, Ethell M, Yee K, Bourdel
F, et al: A Phase 1 Study of the BET-Bromodomain inhibitor OTX015
in patients with advanced acute leukemia. Blood. 124:1172014.
View Article : Google Scholar
|
|
167
|
Doroshow DB, Eder JP and LoRusso PM: BET
inhibitors: A novel epigenetic approach. Ann Oncol. 28:1776–1787.
2017. View Article : Google Scholar
|
|
168
|
Lewin J, Soria JC, Stathis A, Delord JP,
Peters S, Awada A, Aftimos PG, Bekradda M, Rezai K, Zeng Z, et al:
Phase Ib Trial With Birabresib, a Small-Molecule inhibitor of
bromodomain and extraterminal proteins, in patients with selected
advanced solid tumors. J Clin Oncol. 36:3007–3014. 2018. View Article : Google Scholar
|
|
169
|
Moreno V, Sepulveda JM, Vieito M,
Hernandez-Guerrero T, Doger B, Saavedra O, Ferrero O, Sarmiento R,
Arias M, De Alvaro J, et al: Phase I study of CC-90010, a
reversible, oral BET inhibitor in patients with advanced solid
tumors and relapsed/refractory non-Hodgkin's lymphoma. Ann Oncol.
31:780–788. 2020. View Article : Google Scholar
|
|
170
|
Bhattacharya S, Piya S and Borthakur G:
Bromodomain inhibitors: What does the future hold? Clin Adv Hematol
Oncol. 16:504–515. 2018.
|
|
171
|
Mascarenhas J, Kremyanskaya M, Hoffman R,
Bose P, Talpaz M, Harrison CN, Gupta V, Leber B, Sirhan S, Kabir S,
et al: MANIFEST, a Phase 2 Study of CPI-0610, a bromodomain and
extraterminal domain inhibitor (BETi), as monotherapy or ‘Add-on’
to ruxolitinib, in patients with refractory or intolerant advanced
myelofibrosis. Blood. 134:6702019. View Article : Google Scholar
|
|
172
|
Senapati J, Fiskus WC, Daver N, Wilson NR,
Ravandi F, Garcia-Manero G, Kadia T, DiNardo CD, Jabbour E, Burger
J, et al: Phase I results of bromodomain and Extra-terminal
inhibitor PLX51107 in combination with azacitidine in patients with
Relapsed/Refractory myeloid malignancies. Clin Cancer Res.
29:4352–4360. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Patnaik A, Carvajal RD, Komatsubara KM,
Britten CD, Wesolowski R, Michelson G, Alcantar O, Zhang C, Powell
B, Severson P, et al: Phase ib/2a study of PLX51107, a small
molecule BET inhibitor, in subjects with advanced hematological
malignancies and solid tumors. J Clin Oncol. 36:2550. 2018.
View Article : Google Scholar
|
|
174
|
Roboz GJ, Desai P, Lee S, Ritchie EK,
Winer ES, DeMario M, Brennan B, Nuesch E, Chesne E, Brennan L, et
al: A dose escalation study of RO6870810/TEN-10 in patients with
acute myeloid leukemia and myelodysplastic syndrome. Leuk Lymphoma.
62:1740–1748. 2021. View Article : Google Scholar
|
|
175
|
Shapiro GI, LoRusso P, Dowlati A, T Do K,
Jacobson CA, Vaishampayan U, Weise A, Caimi PF, Eder JP, French CA,
et al: A Phase 1 study of RO6870810, a novel bromodomain and
extra-terminal protein inhibitor, in patients with NUT carcinoma,
other solid tumours, or diffuse large B-cell lymphoma. Br J Cancer.
124:744–753. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang JSZ, Vita SD, Karlix JL, Cook C,
Littlewood GM, Hattersley MM, Moorthy G, Edlund H, Fabbri G,
Sachsenmeier KF, et al: First-in-human study of AZD5153, a small
molecule inhibitor of bromodomain protein 4 (BRD4), in patients
(pts) with relapsed/refractory (RR) malignant solid tumor and
lymphoma: Preliminary data. J Clin Oncol. 37:3085. 2019. View Article : Google Scholar
|
|
177
|
Hilton J, Cristea M, Postel-Vinay S,
Baldini C, Voskoboynik M, Edenfield W, Shapiro GI, Cheng ML, Vuky
J, Corr B, et al: BMS-986158, a small molecule inhibitor of the
bromodomain and extraterminal domain proteins, in patients with
selected advanced solid tumors: Results from a Phase 1/2a trial.
Cancers (Basel). 14:40792022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Brown JA, Bal J, Simeoni M, Williams P,
Mander PK, Soden PE, Daga S, Fahy WA, Wong GK, Bloomer JC, et al: A
randomized study of the safety and pharmacokinetics of GSK3358699,
a mononuclear myeloid-targeted bromodomain and extra-terminal
domain inhibitor. Br J Clin Pharmacol. 88:2140–2155. 2022.
View Article : Google Scholar
|
|
179
|
Li Y, Xiang J, Zhang J, Lin J, Wu Y and
Wang X: Inhibition of Brd4 by JQ1 promotes functional recovery from
spinal cord injury by activating autophagy. Front Cell Neurosci.
14:5555912020. View Article : Google Scholar
|
|
180
|
Lee DU, Katavolos P, Palanisamy G, Katewa
A, Sioson C, Corpuz J, Pang J, DeMent K, Choo E, Ghilardi N, et al:
Nonselective inhibition of the epigenetic transcriptional regulator
BET induces marked lymphoid and hematopoietic toxicity in mice.
Toxicol Appl Pharmacol. 300:47–54. 2016. View Article : Google Scholar
|
|
181
|
Bakshi S, McKee C, Walker K, Brown C and
Chaudhry GR: Toxicity of JQ1 in neuronal derivatives of human
umbilical cord mesenchymal stem cells. Oncotarget. 9:33853–33864.
2018. View Article : Google Scholar
|
|
182
|
Leal AS, Williams CR, Royce DB, Pioli PA,
Sporn MB and Liby KT: Bromodomain inhibitors, JQ1 and I-BET 762, as
potential therapies for pancreatic cancer. Cancer Lett. 394:76–87.
2017. View Article : Google Scholar
|
|
183
|
Piquereau J, Boet A, Pechoux C, Antigny F,
Lambert M, Gressette M, Ranchoux B, Gambaryan N, Domergue V, Mumby
S, et al: The BET bromodomain inhibitor I-BET-151 induces
structural and functional alterations of the heart mitochondria in
healthy male mice and rats. Int J Mol Sci. 20:15272019. View Article : Google Scholar
|
|
184
|
Liu CS, Rioja I, Bakr A, Veldwijk MR,
Sperk E, Herskind C, Weichenhan D, Prinjha RK, Plass C, Schmezer P,
et al: Selective inhibitors of bromodomain BD1 and BD2 of BET
proteins modulate radiation-induced profibrotic fibroblast
responses. Int J Cancer. 151:275–286. 2022. View Article : Google Scholar : PubMed/NCBI
|