Introduction
Uterine leiomyosarcoma (Ut-LMS) is a malignant tumor
of the smooth muscles of the uterus (myometrium). It is an uncommon
disease that accounts for <1% of uterine malignancies and 25–36%
of uterine sarcomas (1,2), but can spread to the surrounding
tissues and organs (3). The 5-year
disease-specific survival rate for patients with stage 3 or 4
Ut-LMS is 29–45% (4). Disease
symptoms include abdominal pain and vaginal bleeding. A surgical
biopsy should be performed to differentiate between Ut-leiomyoma
and Ut-LMS because of the largely indistinguishable tissue
appearance (5). Although
Ut-leiomyomas rarely progress to Ut-LMS, Ut-leiomyomas may be a
precursor form of Ut-LMS. If the tumor grows quickly or Ut-LMS is
suspected after an ultrasound scan, it is necessary to periodically
monitor whether it progresses to a malignant tumor, and additional
examinations, such as MRI, are required.
Early diagnosis of Ut-LMS, based on a few methods
before curative surgery, is difficult and studies on new biomarkers
and the molecular mechanisms of Ut-LMS that could help overcome
these challenges are scarce. For instance, activation of the
Akt-mTOR pathway plays a key role in Ut-LMS development (6), and inhibitors of the PI3K and mTOR
pathways suppress Ut-LMS growth both in vitro and in
vivo (7). In addition, PTK787,
a VEGFR/PDGFR inhibitor, can influence Ut-LMS tumor survival
(8). LMP2 deficiency in mice leads
to spontaneous development of Ut-LMS, and aberrant expression of
LMP2 may be a strong risk factor for Ut-LMS (9). A previous study on Ut-LMS involving
The Cancer Genome Atlas network identified mutations and deletions
corresponding to cancer genes such as RB1, tumor protein p53
and PTEN (10). In addition,
other similar studies using whole-exome sequencing of Ut-LMS
demonstrated frequent mutations in the tumor protein p53, RB1,
ATRX and MED12 genes (11,12).
Sustained cellular proliferation is a key hallmark
of malignant tumors, and leiomyosarcomas are characterized by
notable levels of proliferative activity (13). Several studies have been published
regarding leiomyosarcoma and the presence of Ki67, which is a
marker of cell proliferation. Ki67 is recognized as a predictive
and prognostic indicator of various cancers, including breast
(14,15), prostate (16,17)
and adrenocortical carcinoma (18).
A number of studies proposed that Ki67 holds prognostic
significance in leiomyosarcoma as well (19,20).
2′,3′,4′-trihydroxyflavone (2-D08) is a synthetic
flavone that mechanistically has been identified as a specific
inhibitor of protein SUMOylation (21). Previous studies have demonstrated
that 2-D08 has various biological functions, including anticancer
activity. For example, 2-D08 has novel antiaggregatory and
neuroprotective effects against amyloid-beta protein neurotoxicity
in Alzheimer's disease (22) and
inhibits the migration of pancreatic cancer cells by inducing K-Ras
deSUMOylation (23). In addition,
2-D08 promotes apoptosis by inducing the accumulation of reactive
oxygen species (ROS) in acute myeloid leukemia cells, probably via
NOX2 deSUMOylation (24). Lastly,
2-D08 treatment negatively influences C2C12 myoblast cell
proliferation and differentiation (25).
The effect of 2-D08 on the growth of Ut-LMS is not
fully understood. In the present study, the anticancer effects
induced by 2-D08 in human LMS cell lines were explored and the
potential underlying molecular mechanisms were investigated.
Materials and methods
Cell lines and reagents
The human Ut-LMS cancer cell lines SK-LMS-1 (cat.
no. HTB-88) and SK-UT-1B (cat. no. HTB-115) were purchased from the
American Type Culture Collection. The cells were cultured in a
humidified incubator maintained in Minimum Essential Medium (cat.
no. LM007-07; Welgene, Inc.) containing 10% fetal bovine serum
(cat. no. SH30919.03; Hyclone; Cytiva) and 1%
streptomycin-penicillin (cat. no. 15140122; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. The human uterine
smooth muscle cells (Ut-SMCs; cat. no. C-12575) were purchased from
PromoCell GmbH and were cultured in smooth muscle cell growth
medium 2 (cat. no. C-39267; PromoCell GmbH) supplemented with 1%
streptomycin-penicillin. 2-D08 was obtained from MilliporeSigma
(cat. no. SML1052-5MG). Dimethyl sulfoxide (cat. no. DMS555.500;
BioShop Canada Inc.) was used as a control. Cycloheximide (CHX)
solution (cat. no. C4859-1ML), N-acetyl-L-cysteine (NAC; cat. no.
A7250), and catalase (CAT; cat. no. C1345) were purchased from
Sigma-Aldrich; Merck KGaA.
MTT assays
The cells (5×103 cells per well) were
seeded in 96-well plates for 24 h and then treated with different
concentrations of 2-D08 for 24 or 48 h. MTT stock solution (cat.
no. M2128; Sigma-Aldrich) was added to each well, before incubation
at 37°C for 2 h 30 min and addition of dimethyl sulfoxide (150 µl
per well) to all wells after removing the medium. Cell viability
was measured at 570 nm using an INNO microplate spectrophotometer
(LTEK Co., Ltd.) and normalized to that of the control group.
Colony formation assay
SK-LMS-1 and SK-UT-1B cells were seeded in 6-well
plates (1×103 cells per well) for 24 h. After removing
the medium, 2-D08 was added to a fresh medium containing different
concentrations (10, 20, 50, and 100 µM). The cells were cultured at
37°C for 7 days until colonies were visible. To observe colony
growth, a crystal violet assay kit (cat. no. ab232855; Abcam) was
employed following the manufacturer's protocol. In brief, the cells
were fixed with 100% ice-cold methanol for 20 min at −20°C, stained
with 2% crystal violet solution for 20 min at room temperature, and
colonies containing ≥50 cells were counted. Bright-field microscopy
images of the formed colonies were acquired using the EVOS FL Cell
Imaging System (Thermo Fisher Scientific, Inc.). The surviving
fraction was calculated as the mean number of colonies/[number of
inoculated cells × (plating efficiency/100)]. Plating efficiency
was defined as 100× (mean number of colonies/number of inoculated
cells for control). Survival curves were determined using a
linear-quadratic model with the GraphPad Prism 6.0 software
(GraphPad; Dotmatics).
Lactate dehydrogenase (LDH) assay
LDH was quantified using the Dyne LDH PLUS
Cytotoxicity Assay Kit (cat. no. GBL-P500; Dyne Bio Inc.) according
to the manufacturer's instructions. Briefly, the cells were treated
with 2-D08 at 37°C for 24 or 48 h, as indicated. Subsequently, 100
µl of supernatant was transferred from each well to a new 96-well
plate, followed by the addition of 100 µl of LDH PLUS Reaction
Mixture to each well and a gentle mix. The plates were incubated at
room temperature, protected from light, for 30 min before adding 10
µl of stop solution to each well and being gently mixed. The LDH
concentration was measured at 490 nm and the treated groups were
compared with the untreated.
ROS quantification using flow
cytometry
Flow cytometric measurement of ROS production was
performed using a Muse Oxidative Stress Kit (cat. no. MCH100111;
Luminex) according to the manufacturer's instructions. Briefly,
SK-LMS-1 cells were seeded in 6-well plates (5×104
cells/well) and allowed to grow at 37°C for 24 h. The cells were
then pretreated with 0, 20, 50, or 100 µM 2-D08, diluted in fresh
medium at 37°C, for 24 and 48 h. Next, the cells were detached and
resuspended in 1X Assay Buffer, and Muse Oxidative Stress Reagent
working solution was added. The samples were incubated for 30 min
at 37°C before analysis using the Guava Muse Cell Analyzer (cat.
no. 0500-3115; Luminex), and further analyzed using Muse analysis
software (version 1.5; Luminex).
Flow cytometric analysis of
apoptosis
The apoptotic profiles were studied using the Muse
Annexin V & Dead Cell Kit (cat. no. MCH100105; Luminex).
Annexin V-PE detects phosphatidylserine on the external membrane of
apoptotic cells, while 7-AAD permeates late-stage apoptotic and
dead cells by being excluded from live and healthy cells. Ut-LMS
cells were treated with 2-D08 at different concentrations (20, 50,
or 100 µM) for 24 or 48 h in 6-well plates at 5×104
cells per well. The cells were trypsinized, harvested, and
resuspended in a fresh medium. Then, 100 µl of Muse Annexin V &
Dead Cell reagent was added. After staining for 20 min at room
temperature in the dark, the apoptotic profiles of the cells were
recorded using the Guava Muse Cell Analyzer following the
manufacturer's instructions and further analyzed using Muse
analysis software (version 1.5; Luminex).
Ki67 immunostaining
SK-LMS-1 cells were fixed with IC Fixation Buffer
(cat. no. 00-8222-49; Invitrogen; Thermo Fisher Scientific, Inc.)
for 20 min at room temperature and permeabilized with 0.2% Triton
X-100 (cat. no. T8787-50ML; Sigma-Aldrich) in 1X phosphate-buffered
saline (cat. no. LB 001–02; Welgene, Inc.) for 5 min at room
temperature. The cells were then blocked with 2% normal goat serum
(cat. no. 5425; Cell Signaling Technology, Inc.) in
phosphate-buffered saline and incubated with the anti-Ki67 antibody
(1:250; cat. no. MA5-14520; Invitrogen; Thermo Fisher Scientific,
Inc.) at 4°C overnight. The anti-Ki67 antibody was detected by
incubating the cells with an anti-rabbit IgG antibody (Fab2 Alexa
Fluor 594 Conjugate; 1:500; cat. no. 8889S; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The slides were
mounted using ProLong Gold Antifade Mountant with DAPI (cat. no.
P36935; Invitrogen; Thermo Fisher Scientific, Inc.). Fluorescence
images were obtained using the EVOS FL Cell Imaging System.
Flow cytometric assessment of Ki67
expression
To determine the percentage of proliferating cells
based on Ki67 expression, the Muse Ki67 Proliferation Kit (cat. no.
MCH10014; Luminex) was used. Briefly, cells expressing Ki67 were
harvested, fixed with 1X fixation solution for 15 min at room
temperature, and washed with 1X assay buffer. After resuspension,
the cells were treated with permeabilization solution for 15 min at
room temperature and washed again. Next, the cells were incubated
with either Muse Hu IgG1-PE or Muse Hu Ki67-PE antibody for 30 min
at room temperature and analyzed using the Guava Muse Cell
Analyzer, followed by further analysis using Muse analysis software
(version 1.5; Luminex).
Bromodeoxyuridine (BrdU) cell
proliferation assay
The BrdU Cell Proliferation Assay Kit (cat. no.
6831S; Cell Signaling Technology, Inc.) was used to study the
proliferating cells. In brief, cells (5×103 cells/well)
were seeded into 96-well plates and treated with 2-D08 at the
indicated concentrations for 24 or 48 h. The cells were then
exposed to BrdU at 37 °C for 24 h, fixed at room temperature for 30
min, and incubated with mouse anti-BrdU antibody at room
temperature for 1 h, and treated according to the manufacturer's
protocol. Lastly, the absorbance was measured at 450 nm using an
INNO microplate spectrophotometer.
Flow cytometric evaluation of cell
cycle distribution
The Muse Cell Cycle Kit (cat. no. MCH100106;
Luminex) was used to determine the cell cycle phases of the
samples. The cells were centrifuged and fixed for 3 h in 70%
ethanol at −20°C. After fixation, the cells were stained with the
Muse Cell Cycle Reagent and incubated in the dark at room
temperature for 30 min. The samples were analyzed using the Guava
Muse Cell Analyzer, followed by further analysis using Muse
analysis software (version 1.5; Luminex).
Protein preparation and western blot
analysis
Ut-LMS cells were exposed to various concentrations
of 2-D08 (0, 20, or 50 µM) for 48 h. The cells were then lysed in
NP-40 lysis buffer (cat. no. MBS355472; MyBiosource, Inc.)
containing a protease inhibitor cocktail (cat. no. P8340;
Sigma-Aldrich; Merck KGaA). The Pierce BCA Protein Assay kit (cat.
no. 23225; Thermo Fisher Scientific, Inc) was used to measure the
protein concentration of lysate. Equal amounts of protein (10–50 µg
from cell lysate), denatured by heating, were subjected to 12–15%
SDS-PAGE. After electrophoresis, the separated proteins were
transferred from the gel to PVDF membranes (cat. no. IPVH00010;
Merck KGaA), which were blocked with 5% skimmed milk (cat. no.
262100; BD Biosciences) for 1 h at room temperature, and
subsequently incubated with the primary antibodies overnight at 4°C
with gentle shaking. The primary antibodies used in the present
study were RIP (1:1,000; cat. no. 3493T), LC3B (1:1,000; cat. no.
2775S), p21 (1:1,000; cat. no. 2947S), p27 (1:1,000; cat. no.
3686T), p53 (1:1,000; cat. no. 2527S), Caspase-3 (1:1,000; cat. no.
9665S), cleaved Caspase-3 (1:1,000; cat. no. 9664S), Caspase-9
(1:1,000; cat. no. 9502S), PARP (1:1,000; cat. no. 9542S) and
β-Actin (1:1,000; cat. no. 4967S) (all from Cell Signaling
Technology, Inc.); and α-smooth muscle (SM) actin (1:1,000; cat.
no. ab5694), TAGLN (1:5,000; cat. no. ab14106) and Calponin 1
(1:1,000; cat. no. ab46794) (all from Abcam). After washing with
the TBST (Tris-buffered saline, 0.05% Tween 20) solution, the
membranes were incubated with anti-rabbit (1:5,000; cat. no. 7074S;
Cell Signaling Technology, Inc.) and anti-mouse horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
7076S; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The signals were detected using the Immobilon ECL
Ultra Western HRP Substrate (cat. no. WBKLS0100; Merck KGaA) and
quantified using the Azure c280 chemiluminescent image system.
Densitometric analyses of the resulting images were performed using
ImageJ 1.53a software (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from SK-LMS-1 cells using
NucleoZOL (cat. no. 740404.200; Macherey-Nagel GmbH & Co. KG).
RNA samples were reverse transcribed into cDNA using a ReverTra Ace
qPCR RT Kit (cat. no. FSQ-101; Toyobo Life Science). In brief, RNA
was denatured at 65°C for 5 min, then placed on ice. The reaction
solution was prepared with nuclease-free water, 5X RT buffer, RT
enzyme mix, primer mix, and RNA (0.5 pg-1 µg) to a total volume of
10 µl. The mixture was incubated at 37°C for 15 min, heated at 98°C
for 5 min, and then stored at 4°C or −20°C. RT-qPCR was performed
using a QuantiTect SYBR Green RT-PCR Kit (cat. no. 204243; Qiagen
GmbH) and the primer pairs listed in Table SI. The cycling conditions included
an initial denaturation step at 95°C for 10 min. Amplification was
carried out for 40 cycles with denaturation at 95°C 15 sec,
followed by annealing at 50°C for 20 sec to achieve optimal
annealing temperatures, and extension at 72°C for 20 sec. The RNA
level was expressed as a relative result via the 2−ΔΔCq
method (26) against the endogenous
standard control β-actin.
RNA-sequencing (RNA-seq)
Total RNA was extracted from SK-LMS-1 cells using
NucleoSpin RNA/Protein (cat. no. 740933.50; Macherey-Nagel GmbH
& Co. KG) according to the manufacturer's instructions. RNA
quality was assessed with a 4200 TapeStation System with an RNA
Screen Tape (Agilent Technologies, Inc.), and RNA was quantified
using a Qubit fluorometer (Thermo Fisher Scientific, Inc.).
Libraries with total RNAs were constructed using the KAPA RNA
HyperPrep Kit with RiboErase (cat. no. 08098140702; Roche
Sequencing Solution, Inc.) according to the manufacturer's
instructions. The loading concentration of the final library was
measured using the TapeStation 4200 System (Agilent Technologies,
Inc.) and qPCR was performed on the LightCycler 480 System (Roche
Diagnostics) with the Kapa Library Quantification Kit (cat. no.
07960298001; Roche Sequencing Solution, Inc.), resulting in the
final library being loaded at 2 nM. High-throughput sequencing was
performed in paired-end 150 sequencing runs using a NovaSeq 6000
(Illumina, Inc.) at Genome Insight, Inc. Raw reads were assembled
and low-quality reads were filtered using Cutadapt (v.3.4; National
Bioinformatics Infrastructure Sweden). Filtered reads were aligned
on a reference genome downloaded from Ensembl (GRCh38; Homo
sapiens; http://www.ensembl.org) using STAR
(v.2.7.8a) (27) and the gene-level
expression for each sample was calculated using RSEM (v.1.3.1)
(28). Differentially expressed
genes were analyzed using a two-slided Wald test under each
condition with the DESeq2 (version 1.26.0) (29) and edgeR (version 3.28.1) (30) packages.
Statistical analysis
The GraphPad Prism software (v.6.0; Graphpad;
Dotmatics) was used for the statistical analyses. The results are
expressed as the mean ± standard error of the mean (SEM), and the
data were analyzed using one-way analysis of variance, followed by
Tukey's post hoc test for comparison among multiple groups.
*P<0.05 was considered to indicate a statistically significant
difference.
Results
2-D08 inhibits the viability of Ut-LMS
cells
The cytotoxic effect of 2-D08 treatment (Fig. 1A) on Ut-LMS cells was first
investigated using the well-established SK-LMS-1 and SK-UT-1B cell
models. The viability of Ut-LMS cells was determined using MTT
assays followed by treatment with 2-D08 at different concentrations
(0–100 µM) for 24 or 48 h. As revealed in Fig. 1B, the viability of SK-LMS-1 cells
significantly decreased to 21% in the 100-µM 2-D08-treated group at
24 h, although different doses did not lead to statistically
significant changes (Fig. 1B, left
graph). After 48 h of treatment, SK-LMS-1 cell viability decreased
significantly to 80 and 17% at 50 and 100 µM 2-D08 concentrations,
respectively (Fig. 1B, right
graph). Treatment of SK-UT-1B cells with 2-D08 at concentrations
>10 µM for 24 and 48 h significantly inhibited cell viability
(Fig. 1C). By contrast, treatment
of normal Ut-SMCs with 2-D08 for 24 and 48 h had a slight
inhibitory effect on viability (Fig.
1D). These results indicated that the viability of Ut-LMS cells
was significantly affected by 2-D08 treatment.
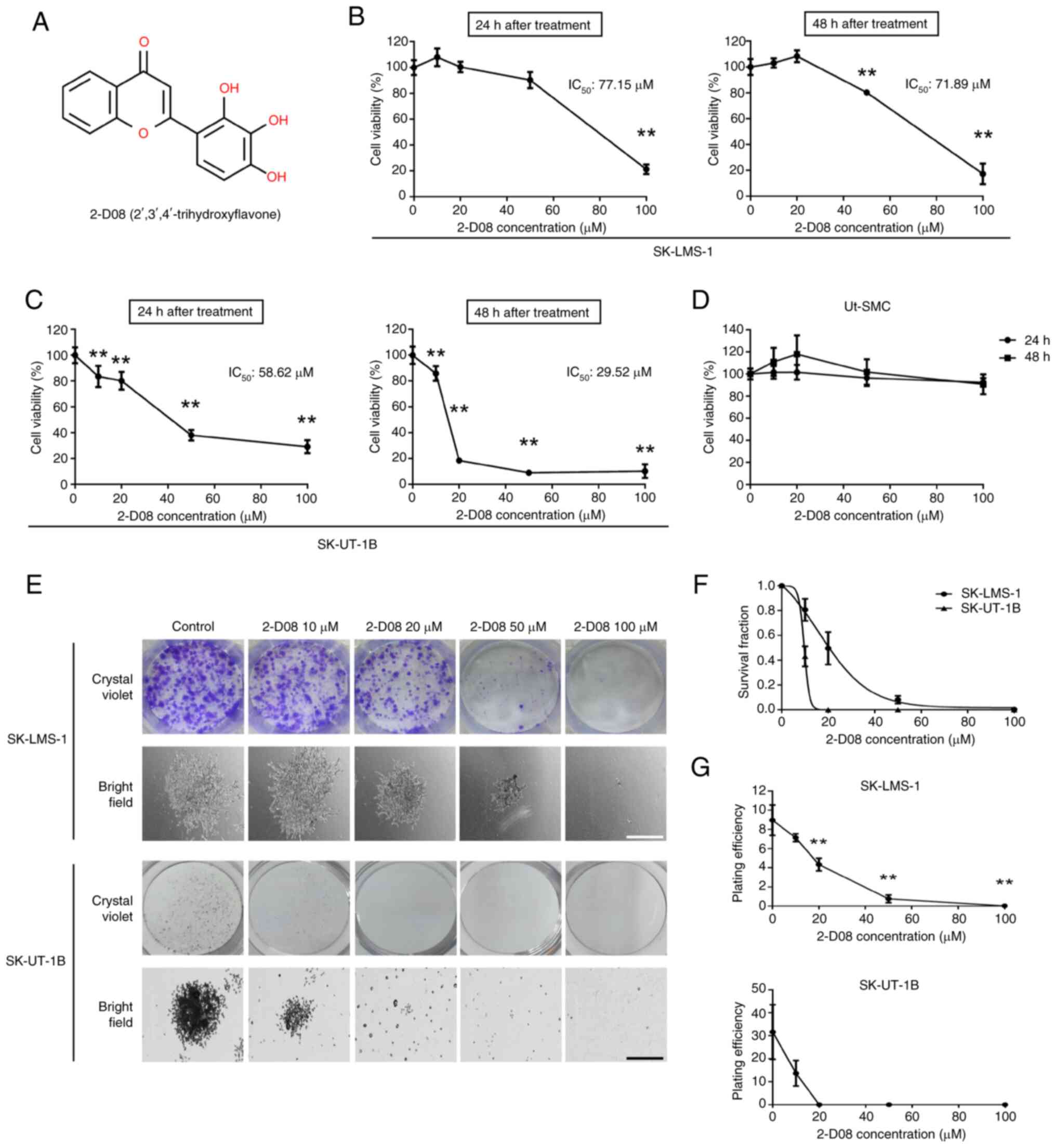 | Figure 1.2-D08 decreases viability and colony
formation ability in the Ut-LMS cells. (A) Chemical structure of
2-D08. (B-D) Ut-LMS cells and Ut-SMCs were treated with various
concentrations (0–100 µM) of 2-D08 for 24 or 48 h. After the end of
incubation, cell viability was measured using MTT assays. Graphs of
viability versus 2-D08 concentrations were used to calculate
IC50 values based on the trendline equation for Ut-LMS
cells. (E) Ut-LMS cells were treated with 2-D08 at the indicated
concentration (0–100 µM) for 7 days. Representative microscopic
images of the colonies stained using crystal violet (white scale
bar, 1,000 µm; black scale bar, 400 µm). (F and G) After 7 days,
dose-survival curves derived from a clonogenic survival assay and
comparisons of plating efficiency were performed. Surviving
fractions were calculated based on colony counts and plating
efficiency. The data are reported as the mean ± SEM. All
experiments were repeated thrice. **P<0.01 compared with the
control group. 2-D08, 2′,3′,4′-trihydroxyflavone; Ut-LMS, uterine
leiomyosarcoma; Ut-SMCs, uterine smooth muscle cells; SEM, standard
error of the mean. |
2-D08 inhibits colony formation of
Ut-LMS cells
It was investigated whether 2-D08 affects the
proliferation of Ut-LMS cells using a colony formation assay. As
demonstrated in the crystal violet-stained (upper panels) and
bright-field images (lower panels) in Fig. 1E, the size of a single colony in the
2-D08-treated groups (20, 50 and 100 µM) after 7 days was smaller
than that in the control group. Compared with control cells,
treatment of Ut-LMS cells with 2-D08 (10, 20, 50, and 100 µM) for 7
days induced a dose-dependent decline in survival fraction
(Fig. 1F). Furthermore, plating
efficiency was measured to assess the colony-forming ability of
Ut-LMS cells compared with that of untreated cells. The mean
plating efficiency of Ut-LMS cells decreased significantly
(Fig. 1G). These findings indicated
that 2-D08 plays a key role in inhibiting colony formation and is a
potential drug for clinical applications in Ut-LMS cells.
2-D08-induced apoptotic and necrotic
cell death is dependent on RIP1 in SK-UT-1B cells
To determine whether the aforementioned reductions
in cell viability induced by 2-D08 in SK-UT-1B cells were
associated with apoptotic cell death and cell cycle arrest, a flow
cytometric analysis was performed. Apoptotic cells were identified
using Annexin V. As revealed in Fig.
2A, the percentage of apoptotic cells slightly increased in
2-D08-treated SK-UT-1B cells compared with that in untreated cells.
Additionally, SK-UT-1B cells were stained with propidium iodide,
and the cell cycle profiles were evaluated using the Guava Muse
Cell Analyzer. The results indicated that treatment with 2-D08 did
not affect the progression of the cell cycle in SK-UT-1B cells
(Fig. 2B). To investigate whether
2-D08 regulates necrosis or autophagy in SK-UT-1B cells, the
expression of RIP (a necrosis marker) and LC3B (an autophagy
marker) were analyzed. Cells were treated with 2-D08 at the
indicated concentrations, and the expression of RIP1 and LC3B was
assessed using western blot analysis. The level of RIP1 was
significantly higher in 2-D08-treated cells than in untreated
cells, while that of LC3B remained unchanged (Fig. 2C). Furthermore, 2-D08-treated
SK-UT-1B cells were evaluated using LDH assays to confirm necrosis.
As shown in Fig. 2D, 2-D08
significantly increased the levels of LDH release in SK-UT-1B
cells. These results suggested that 2-D08 significantly induces
apoptosis and necrosis in SK-UT-1B cells.
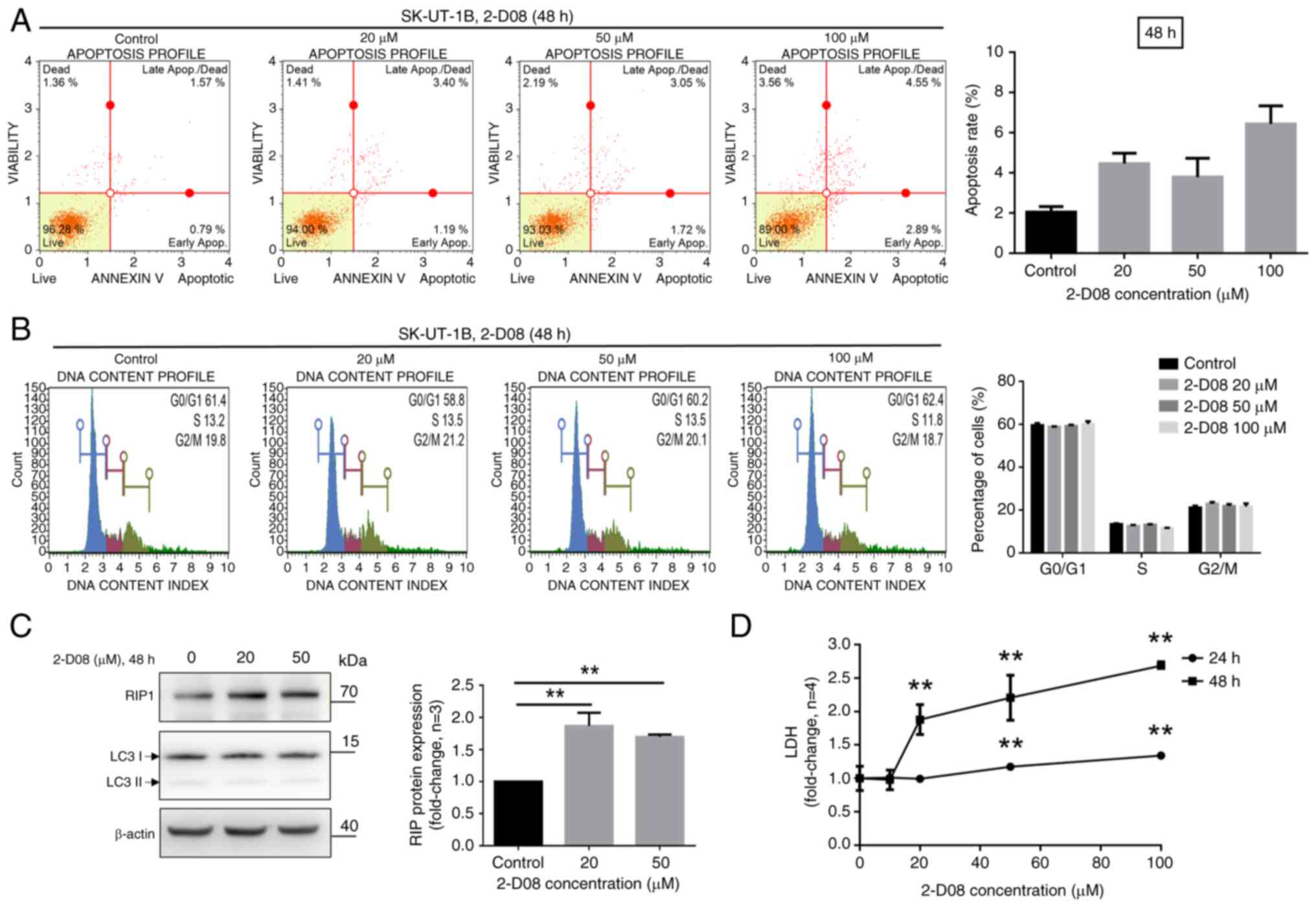 | Figure 2.2-D08-induced apoptotic and necrotic
cell death is dependent on RIP1. (A and B) After treatment with
2-D08 at the indicated concentrations, SK-UT-1B cells were stained
with Annexin V and propidium iodide to analyze the apoptotic rates
and cell cycle distribution, respectively, using flow cytometry.
The quantitative data are shown in the right panel. (C)
Representative western blots of the necrosis marker RIP1 and
autophagy maker LC3B. SK-UT-1B cells were treated with 2-D08 at 20
and 50 µM and the indicated proteins were analyzed 48 h after
treatment. (D) In vitro LDH assay of SK-UT-1B cells. The
cells were treated for 24 or 48 h with 2-D08 at indicated
concentrations (10, 20, 50, or 100 µM) and the supernatants were
analyzed for LDH content, as described in the materials and
methods. Cell lysis solution served as a positive control for 100%
cell lysis and LDH release. Three experiments were performed that
showed similar results. The data are presented as the mean ± SEM.
**P<0.01. 2-D08, 2′,3′,4′-trihydroxyflavone; LDH, lactate
dehydrogenase; SEM, standard error of the mean. |
2-D08 triggers intracellular ROS level
increment
Excessive accumulation of ROS in cells can lead to
oxidative stress, resulting in damage to nucleic acids, lipids,
proteins, membranes and mitochondria (31). To assess whether 2-D08 increases ROS
levels in SK-LMS-1 cells, the cells were cultured with different
concentrations of 2-D08 (0, 20, 50 and 100 µM) for 24 and 48 h,
followed by analysis with the Guava Muse Cell Analyzer. ROS levels
were at 33.0, 43.0, 50.5 and 77.6% in control and 2-D08 treated
SK-LMS-1 cells at the indicated concentration for 24 h,
respectively (Fig. 3A, upper
panels). After 48 h of 2-D08 treatment, the respective results were
43.7, 61.3, 82.55 and 88.48% (Fig.
3A, bottom panels). Consequently, these results indicated that
2-D08 induces ROS generation in SK-LMS-1 cells.
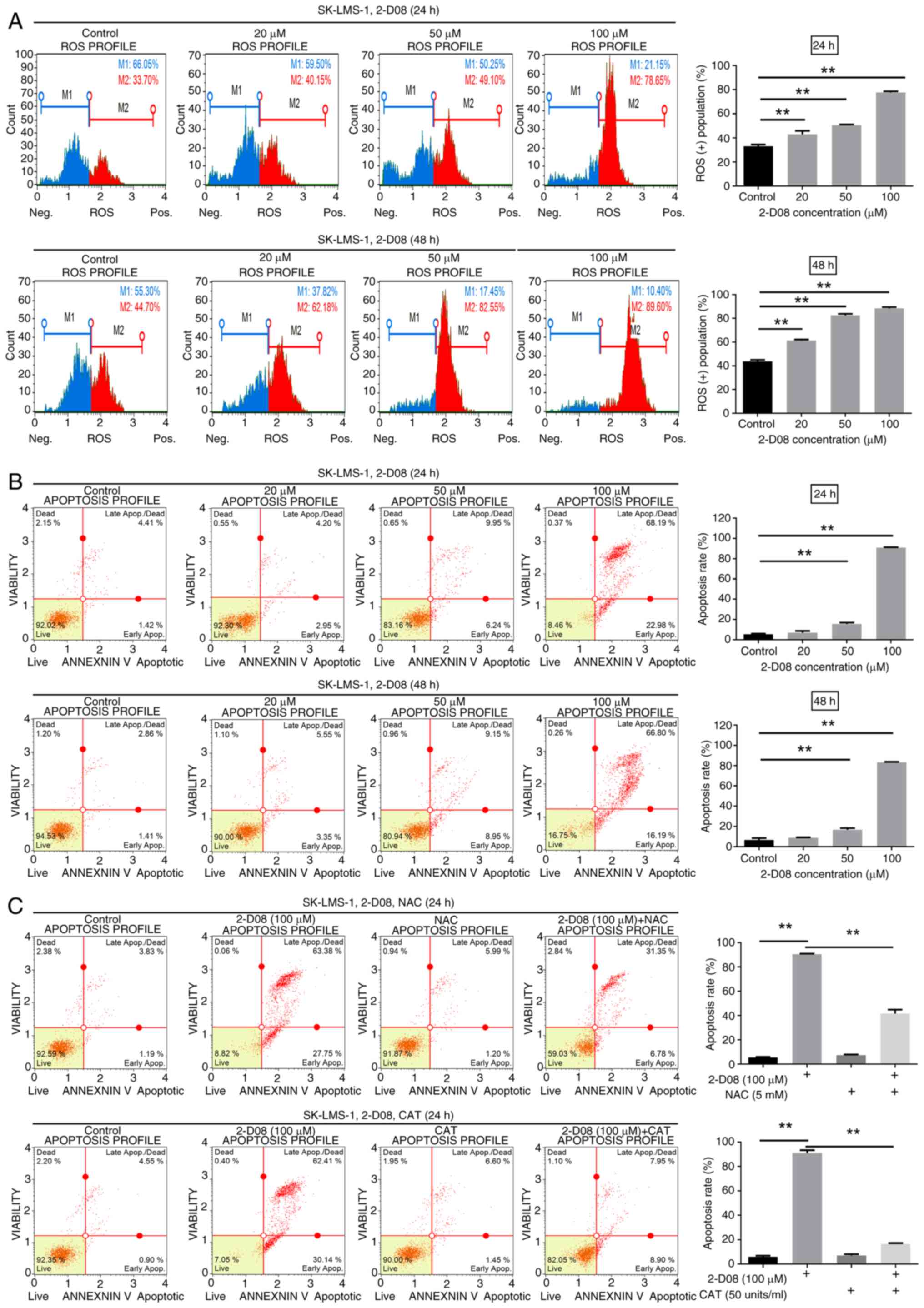 | Figure 3.2-D08 induces ROS production and
apoptosis in SK-LMS-1 cells. (A) SK-LMS-1 cells were exposed to
2-D08 (0, 20, 50, or 100 µM) for 24 and 48 h. Intracellular ROS
fluorescence signals were detected using the Muse Oxidative Stress
kit, and typical ROS profile plots are shown. A similar pattern was
observed in three independent experiments. The percentage of
ROS-positive cells is revealed in the histogram graph (right). (B)
SK-LMS-1 cells were treated with 0, 20, 50, or 100 µM of 2-D08 for
24 and 48 h. Apoptosis was decided using Annexin V staining and
flow cytometry. Representative results are demonstrated in the left
panel, and the statistical analysis is represented in the right
panel. (C) Apoptosis was measured in SK-LMS-1 cells treated with
2-D08 (100 µM) in the presence or absence NAC (5 mM) and CAT (50
units/ml) for 24 h. NAC and CAT, acting as ROS scavengers, partly
rescued the 2-D08-induced apoptosis in SK-LMS-1 cells. The data are
expressed as the mean ± SEM; n=3. **P<0.01. 2-D08,
2′,3′,4′-trihydroxyflavone; ROS, reactive oxygen species; NAC,
N-acetyl-L-cysteine; CAT, catalase; SEM, standard error of the
mean. |
2-D08 promotes apoptosis in SK-LMS-1
cells
To examine whether cells undergo apoptosis, SK-LMS-1
cells were treated with different concentrations (20, 50, or 100
µM) of 2-D08 for 24 and 48 h. Apoptotic cells were determined in
flow cytometry using Annexin V. As demonstrated in Fig. 3B, the percentage of apoptotic cells
significantly increased in a dose-dependent manner. After 24 h of
treatment, the percentages of apoptotic cells were 7.01% at 20 µM,
15.66% at 50 µM and 90.93% at 100 µM, compared with 5.56% for the
control cell culture (Fig. 3B,
upper panels). After 48 h of treatment, the percentages of
apoptotic cells were 8.81% at 20 µM, 16.77% at 50 µM, and 83.39% at
100 µM, compared with 6.53% for the control cell culture (Fig. 3B, bottom panels). To further
identify whether ROS production is involved in 2-D08-induced
apoptosis, SK-LMS-1 cells were treated with 2-D08 (100 µM) with or
without NAC and CAT. Both NAC and CAT, known as ROS scavengers
(32,33), effectively reduced 2-D08-induced
apoptosis in SK-LMS-1 cells (Fig.
3C). These results indicated that 2-D08 induces ROS-mediated
apoptosis in SK-LMS-1 cells. However, antioxidants, acting as
ROS-scavengers, block the anticancer effect of 2-D08 on SK-LMS-1
cells.
2-D08 suppresses the proliferation of
SK-LMS-1 cells in vitro
The aforementioned experiments demonstrated that a
consistent proliferation inhibitory effect occurs with 20–50 µM
2-D08 and that high doses (100 µM) have the potential to induce
non-specific cytotoxicity. Based on these findings, subsequent
experiments were conducted using 20- and 50-µM doses at 48 h. Cell
proliferation was assessed by evaluating Ki67 expression and using
a BrdU assay. The expression of the proliferation marker Ki67 is
demonstrated in Fig. 4A. To
accurately quantify the expression of Ki67 in SK-LMS-1 cells, flow
cytometry was utilized. The proportion of Ki67-positive cells
decreased from 93.48 to 67.79 (20 µM) and 65.03% (50 µM) after a
48-h treatment with 2-D08 (Fig.
4B). BrdU incorporation in proliferating cells significantly
decreased in 50-µM 2-D08-treated cells at 48 h compared with that
in the control (n=10) (Fig. 4C).
These results indicated that 2-D08 treatment induces
antiproliferative effects in SK-LMS-1 cells.
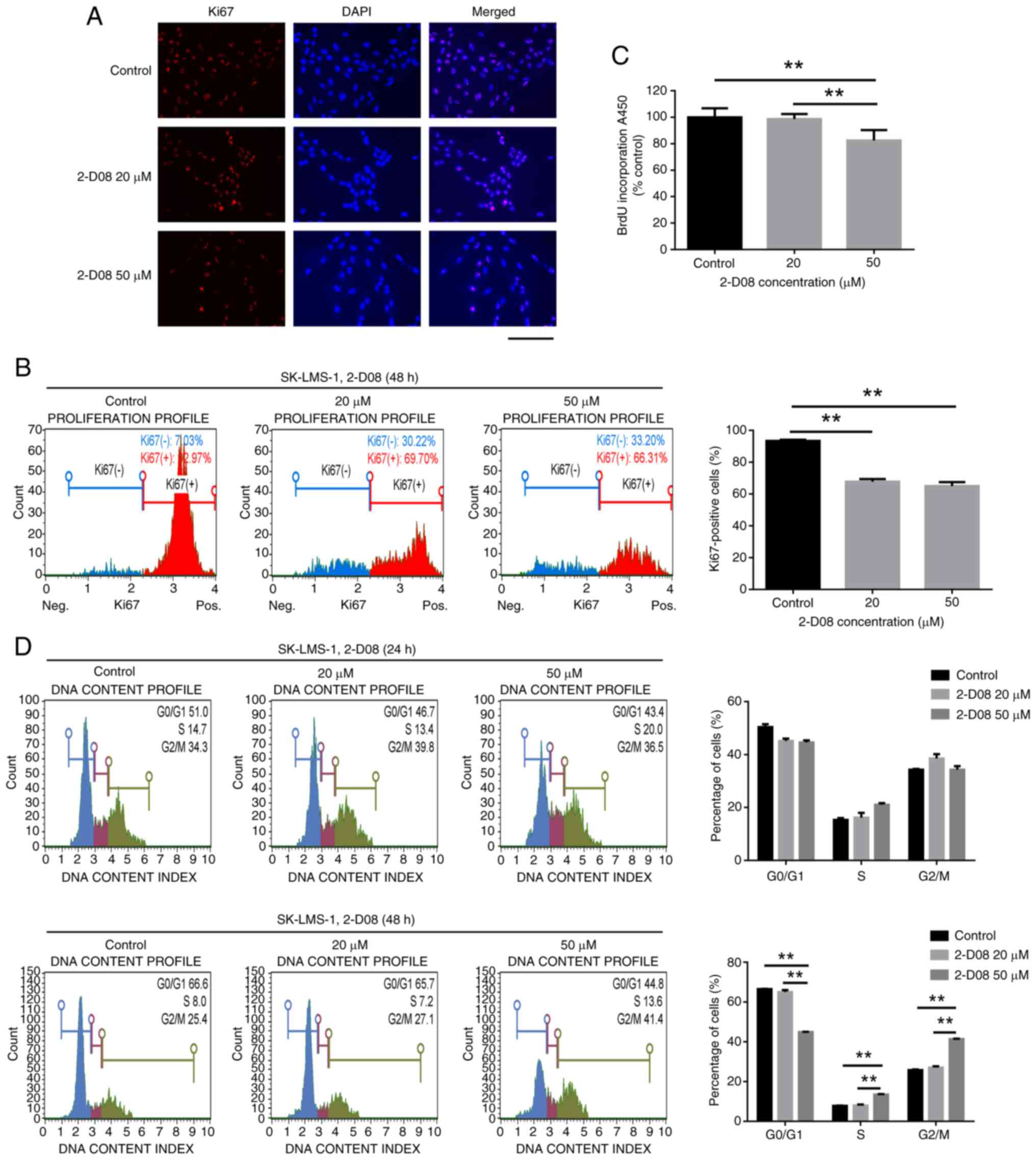 | Figure 4.Inhibition of SK-LMS-1 cell
proliferation and cell cycle by 2-D08. (A) SK-LMS-1 cells were
treated with 20 and 50 µM of 2-D08 for 48 h, and immunofluorescence
assays were used to analyze the expression of the cell
proliferation marker Ki67 in the treated cells. Red, Ki67; blue,
nuclear DNA. Scale bar, 200 µm. (B) The percentage of Ki67-positive
cells at the indicated concentrations at 48 h. The Muse Ki67
Proliferation kit allowed for the quantification of the percentages
of proliferating and non-proliferating cells based on Ki67
expression. (C) A BrdU assay was performed after 48 h of treatment
of SK-LMS-1 cells with 2-D08 (20 or 50 µM). Absorbance was measured
at 450 nm using a microplate reader. (D) SK-LMS-1 cells were
treated with 2-D08 (0, 20, or 50 µM) for 24 and 48 h and then
subjected to a DNA content analysis using flow cytometry. The left
panel shows a representative histogram of the cell cycle
distribution, while the right panel quantifies the cell cycle
distributions. The data are presented as the mean ± SEM of three
independent experiments. **P<0.01 compared with each group.
2-D08, 2′,3′,4′-trihydroxyflavone; SEM, standard error of the
mean. |
2-D08-mediated S and G2/M arrest
contributes to growth inhibition in SK-LMS-1 cells
To investigate whether 2-D08-mediated inhibition of
cell proliferation is related to cell cycle arrest, SK-LMS-1 cells
were treated with varying concentrations of 2-D08 for 24 and 48 h.
The cell cycle profiles were assessed using the Guava Muse Cell
Analyzer. Interestingly, 2-D08 significantly increased the cell
population at the S and G2/M phases, while simultaneously reducing
it in the G0/G1 phase after 48 h of treatment (Fig. 4D). This experiment confirmed that
2-D08-mediated cell cycle arrest contributes to inhibition of
proliferation in SK-LMS-1 cells.
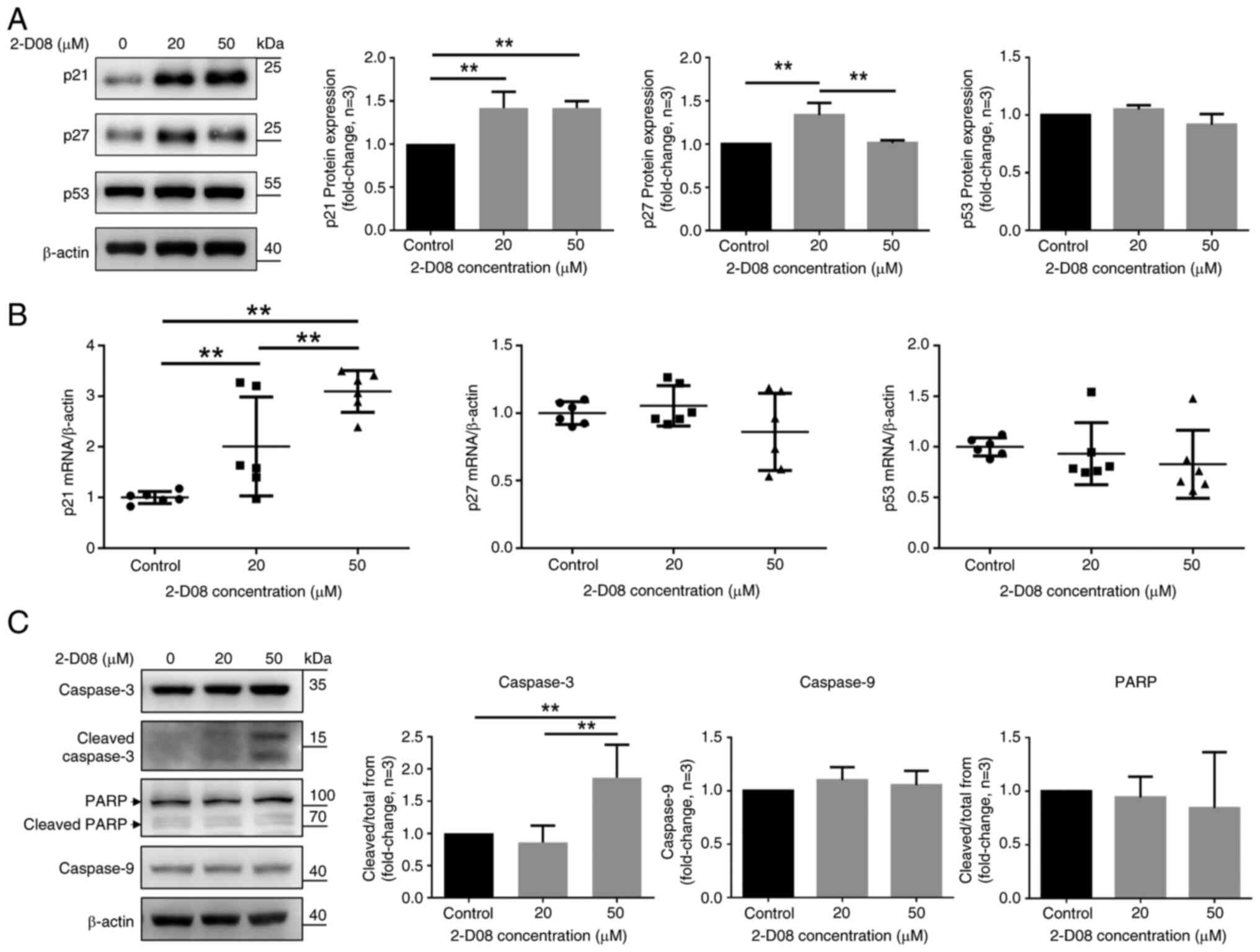
Effect of 2-D08 on the expression of
proteins related to cell proliferation and apoptosis in SK-LMS-1
cells
To study the detailed mechanism(s) of action of
2-D08, western blotting was employed to detect the protein levels
of the cell cycle-regulatory proteins p21, p27 and p53. Application
of 2-D08 in the SK-LMS-1 cells significantly increased p21 protein
levels at 20 and 50 µM, but not p53 levels. Treatment with the low
dose of 2-D08 (20 µM) increased p27 levels, while, at a high dose
(50 µM), no significant increase was observed (Fig. 5A). CHX assay was also conducted to
inhibit protein synthesis in SK-LMS-1 and SK-UT-1B cells to
evaluate the expression of p53. When treated with CHX for the
indicated time, the expression of the p53 protein remained
unchanged in SK-LMS-1 cells but decreased in SK-UT-1B cells
(Fig. S1). The mRNA levels of p21,
but not of p27 and p53, significantly increased compared with the
group treated with 2-D08 (Fig. 5B).
To improve understanding of how 2-D08 triggers apoptosis in
SK-LMS-1 cells, western blot analysis was conducted by
investigating the expression of Caspase-3, the pro-apoptotic
protein cleaved Caspase-3, Caspase-9 and PARP antibodies with cell
lysates. Caspase-3 cleavage was detected under these conditions,
indicating that 2-D08 activated Caspase-3 at a concentration of 50
µM (Fig. 5C). By contrast, there
was no change in the expression of Caspase-9 or PARP (Fig. 5C). Together, these findings
suggested that the modulation of cell cycle- and apoptosis-related
proteins may contribute to the 2-D08-induced suppression of
proliferation and apoptosis in SK-LMS-1 cells.
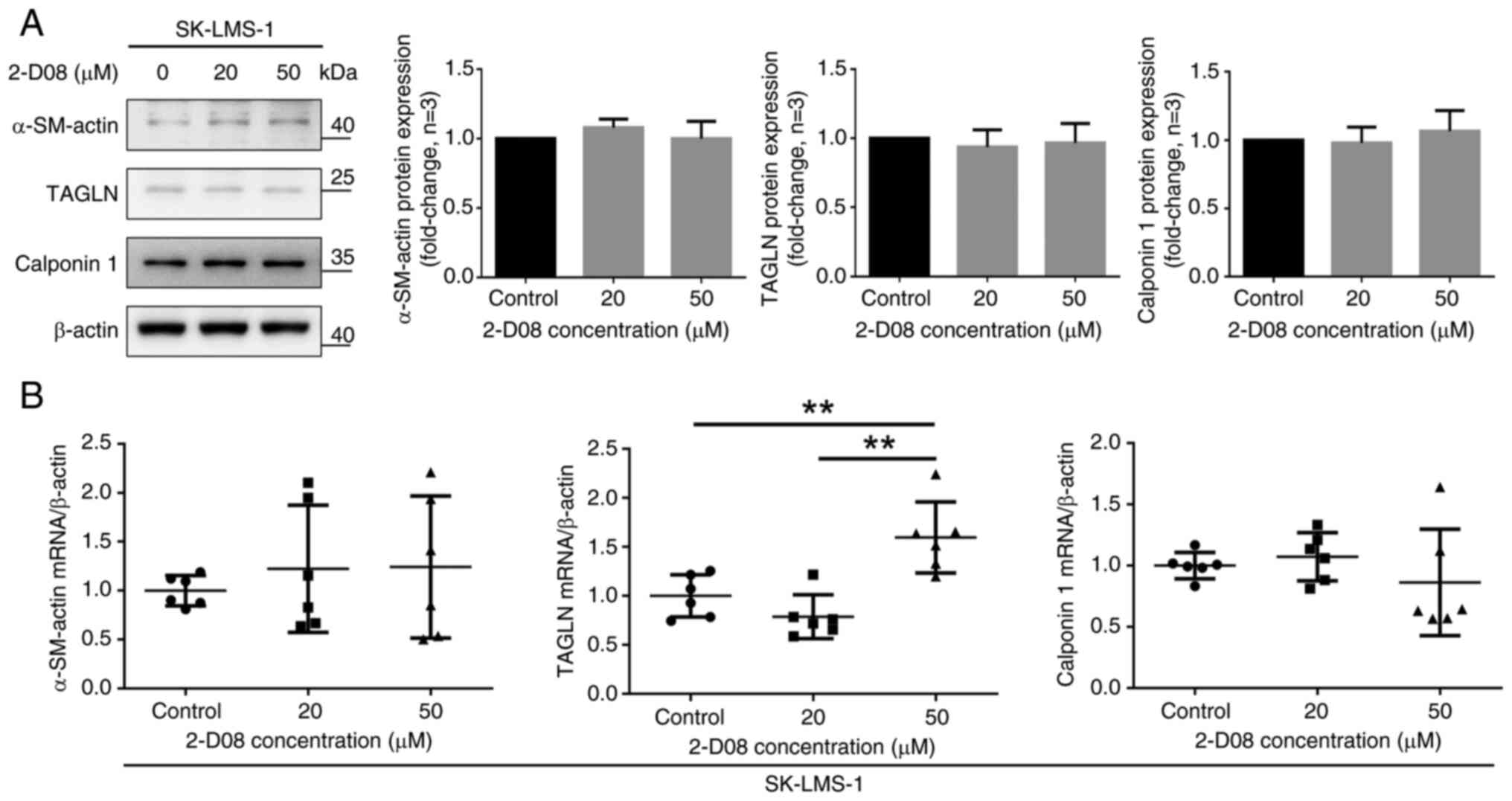
Effect of 2-D08 on the expression of
differentiation marker proteins in SK-LMS-1 cells
The contractile apparatus of SMCs is characterized
by the presence of high levels of α-SM-actin, calponin 1, TAGLN
(SM22α) and SM-MHC, which are specific markers of the stage of cell
differentiation (34,35). Various SMC malignancies can modulate
the differentiated-to-differentiated phenotype in response to
changes in the surrounding environment (36–40).
For example, the expression of SM-specific markers in human Ut-LMS
cell lines, including SK-LMS-1, is low because these cancerous
cells have a dedifferentiated SMC phenotype (40). It was investigated whether 2-D08
affects the expression of SM-specific markers. As demonstrated in
Fig. 6A, compared with the control
group, treatment of SK-LMS-1 cells with 2-D08 did not significantly
affect the expression of α-SM-actin, TAGLN, or calponin 1.
Furthermore, the mRNA levels of SM-specific markers, excluding
TAGLN, did not change significantly compared with those in the
group treated with 2-D08 (Fig. 6B).
Taken together, these findings demonstrated that 2-D08 does not
directly regulate the phenotypic modulation of SK-LMS-1 cells.
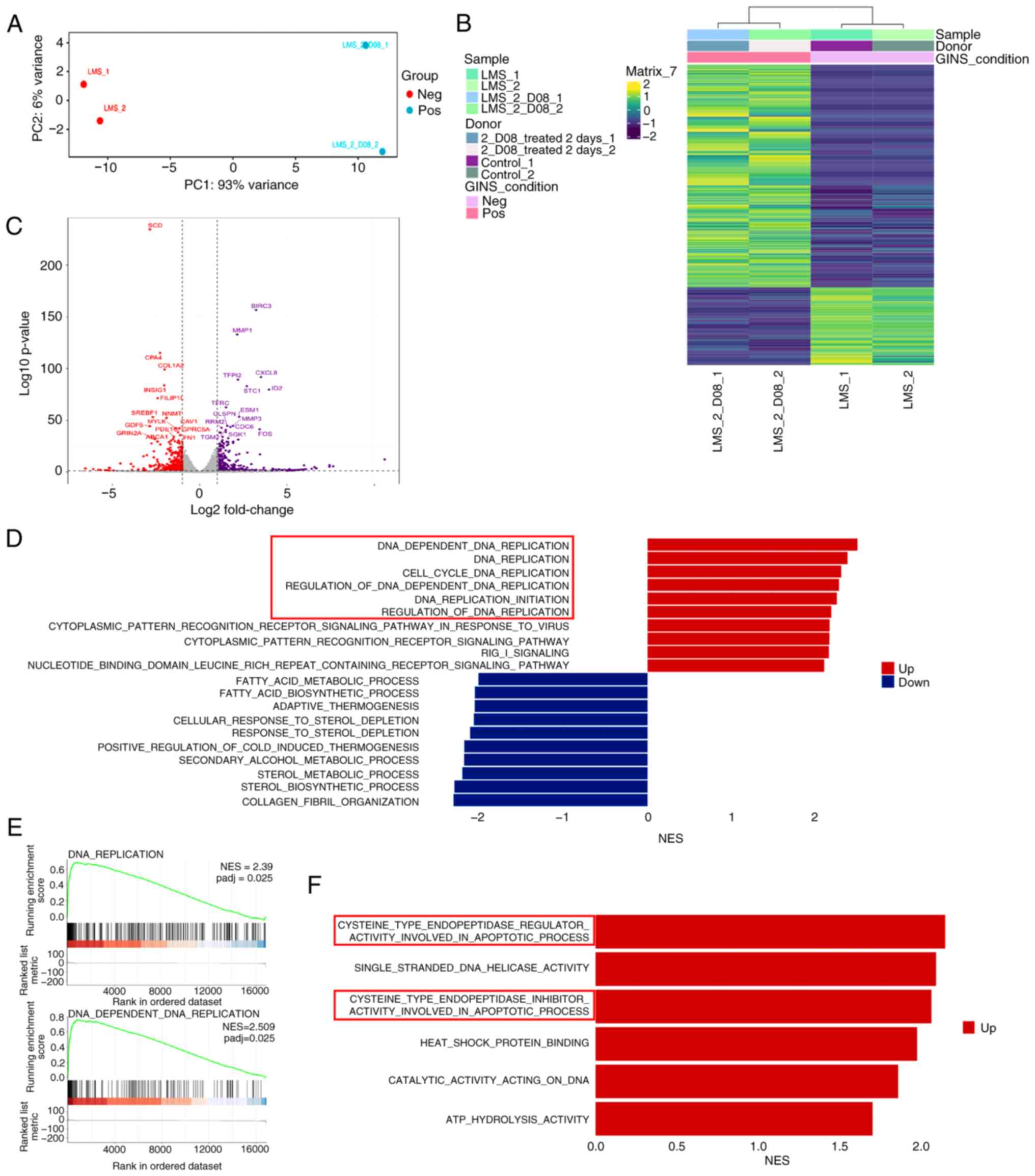
Transcriptome analysis in
2-D08-treated SK-LMS-1 cells
It was aimed to obtain additional insights into the
effects of 2-D08 and conducted transcriptome analyses with RNA-seq
in both control and SK-LMS-1 cells treated with 50 µM 2-D08 for 48
h. Principal component analysis revealed clear clustering per
treatment, indicating significant differences in the transcriptome
profiles between the two groups (Fig.
7A). The hierarchical clustering heatmap showed that 259 genes
were differentially expressed between these two groups (Fig. 7B, adjusted P-value <1.3 and |log2
fold change (FC)|>2). Of the 259 genes, the expression of 192
was upregulated and that of 67 was downregulated in 2-D08-treated
SK-LMS-1 cells. The genes with high FCs are listed in Table I and all genes with significantly
altered expression are included in Table SII. To obtain information on
2-D08-specific target genes in SK-LMS-1 cells, DEseq2 was utilized
to identify differentially expressed genes (DEGs). This analysis
revealed that the expression of 1,031 genes was downregulated and
that of 2,907 genes was upregulated in 2-D08-treated SK-LMS-1 cells
compared with those in the control (adjusted P-value <0.05 and
|log2FC|>1). The distribution of DEGs is illustrated in a
volcano plot (Fig. 7C). DEGs
between the two groups were also examined using edgeR. As revealed
in Fig. S2, 3,827 DEGs were
identified, with 2,834 showing upregulated expression and 993
showing downregulated expression in two groups. Using gene ontology
(GO) functional analysis, statistically significant enrichment was
identified for numerous GO biological processes related to DNA
replication (cell cycle) between the two groups (Fig. 7D). Gene set enrichment analysis
observed that DNA replication (normalized enrichment score=2.35)
and DNA-dependent DNA replication (normalized enrichment
score=2.509) were positively regulated in the 2-D08-treated group
(Fig. 7E). Additionally, various GO
molecular functions related to the apoptotic process were
significantly enriched (Fig. 7F).
Taken together, these findings suggested that 2-D08 modulates
pathways related to the cell cycle and apoptosis in SK-LMS-1
cells.
 | Table I.High fold-change genes among the
significantly up- and downregulated genes. |
Table I.
High fold-change genes among the
significantly up- and downregulated genes.
| Upregulated genes
top 10-fold change |
|---|
|
|---|
| Gene symbol | Description | log2FoldChange | P-value |
|---|
| HSPA6 | Heat shock protein
family A (Hsp70) member 6 | 10.56313299 | 12.14147123 |
| TINCR | TINCR ubiquitin
domain containing | 7.622131682 | 5.477771463 |
| RASD1 | Ras related
dexamethasone induced 1 | 7.409021026 | 6.643901651 |
| FRG2B | FSHD region gene 2
family member B | 7.403884047 | 5.03755407 |
| RIMBP3C | RIMS binding
protein 3C | 6.660468995 | 3.55968452 |
| AFF2 | ALF transcription
elongation factor 2 | 6.633655223 | 3.527869717 |
| ARC | Activity regulated
cytoskeleton associated protein | 6.546234801 | 4.829959694 |
| ASXL3 | ASXL
transcriptional regulator 3 | 6.386994619 | 3.064184081 |
| FCRL3 | Fc receptor like
3 | 6.070912873 | 2.678081617 |
| CCDC73 | Coiled-coil domain
containing 73 | 6.057297181 | 2.670620262 |
|
| Downregulated
genes top 10-fold change |
|
| LCN12 | Lipocalin 12 | −4.939386897 | 1.359228814 |
| CCN5 | Cellular
communication network factor 5 | −5.149869976 | 3.976541161 |
|
TNFSF12-TNFSF13 | TNFSF12-TNFSF13
readthrough | −5.177935809 | 1.336744493 |
| ABCG1 | ATP binding
cassette subfamily G member 1 | −5.202302215 | 4.051255441 |
| MUSTN1 | Musculoskeletal,
embryonic nuclear protein 1 | −5.277064096 | 1.669719597 |
| tspan32 | tetraspanin 32 | −5.420116874 | 1.814259284 |
| CPAMD8 | C3 and PZP like
alpha-2-macroglobulin domain containing 8 | −5.683960725 | 2.050450132 |
| TBC1D3C | TBC1 domain family
member 3C | −5.804635788 | 2.100940952 |
| URGCP-MRPS24 | URGCP-MRPS24
readthrough | −6.100406013 | 2.762892929 |
| ATF7-NPFF | ATF7-NPFF
readthrough | −6.546939056 | 3.447437941 |
Discussion
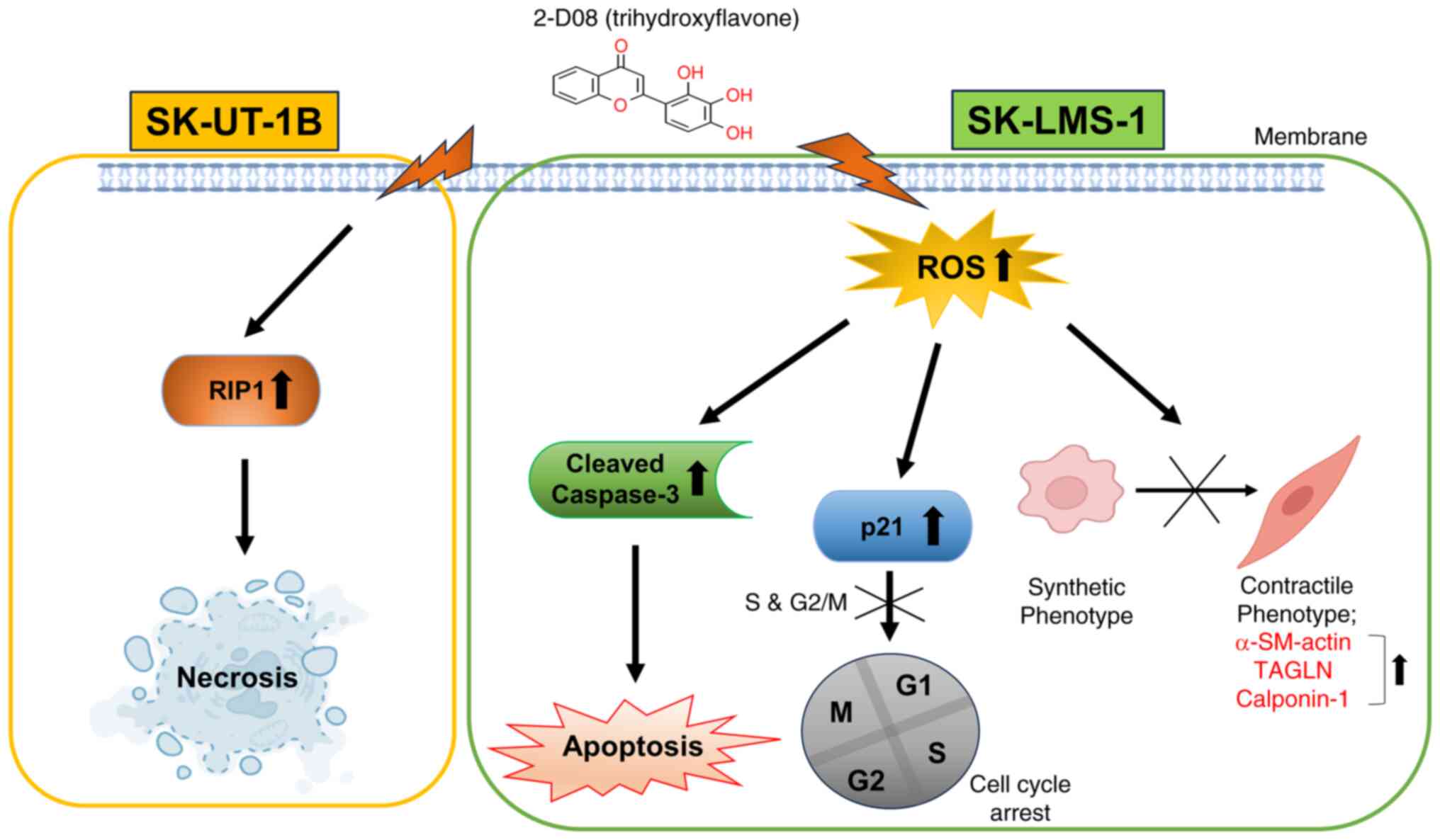
In the present study, a new function of 2-D08 in
inducing multiple signaling pathways in Ut-LMS cells was
identified, including ROS generation, which serve as key mediators
of its anticancer effects (Fig. 8).
Although 2-D08 is currently known as a SUMO inhibitor, several
studies have shown that it participates in numerous biological
functions in different cells (22–24).
Recently, 2-D08 was shown to suppress the proliferation and
differentiation of C2C12 myoblast cells (25). In the current study, the hypothesis
that 2-D08 can affect the proliferation of Ut-LMS in vitro
was tested. The data revealed that 2-D08 suppressed cell viability
and enhanced anticancer effects in Ut-LMS cells (Fig. 1B and C). In particular, both
low-dose (10–20 µM) and high-dose (50–100 µM) treatment with 2-D08
significantly inhibited long-term colony formation compared with
that in the control (Fig. 1E).
Furthermore, the data revealed that inhibition of proliferation
mediated by 2-D08 was accompanied by induction in the rate of
apoptosis in Ut-LMS cells (Figs. 2A
and 3B). Interestingly, after 48 h
of treatment with 20 µM 2-D08, there was a significant decrease in
Ki67 expression (Fig. 4A),
indicating reduced cell proliferative activity. However, there was
no change in cell viability as measured by the MTT assay (Fig. 1B), suggesting that this decrease in
Ki67 expression did not affect the survival ability of the cells.
ROS induces lipid peroxidation, which is used as a cell death
signal to induce programmed cell death (41). According to a previous study, 2-D08
induces ROS accumulation and mediates apoptosis in acute myeloid
leukemia cells (24). Consistent
with the results of previous studies (24), the results of the present study
revealed that induction of apoptosis by 2-D08 was significantly
influenced by ROS accumulation in SK-LMS-1 cells (Fig. 3A). Furthermore, these results
indicated the presence of basal ROS levels in SK-LMS-1 cells and
supported the notion that cancer cells exhibit higher basal levels
of ROS compared with normal cells due to an imbalance between
oxidants and antioxidants (42).
According to previous studies, p21, p27 and p53 are
direct or indirect suppressors of tumor development and play
different roles in human biological systems (43). Uterine leiomyosarcomas are
characterized by increased p53 expression compared with normal
leiomyomas and SM tumors of uncertain malignant potential (44,45).
Additionally, upregulation of p21 expression contributes to cell
cycle arrest by activating p53 (46). Consistent with these studies, it was
hypothesized that 2-D08 affects the expression of p21, p27 and p53
in SK-LMS-1 cells. The antiproliferative effects of low-dose (20
µM) 2-D08 treatment for 48 h and the significant increase in the
expression levels of p21 and p27 proteins were demonstrated in
Fig. 5A. The expression of p21
protein only increased when 2-D08 was supplied at a high dose (50
µM) through the induction of antiproliferative activity and arrest
at the S and G2/M phases (Fig. 4D,
bottom panels). This is an important strategy in cancer treatment,
as it inhibits the proliferation and division of cancer cells by
interfering with the cell cycle that regulates cell division.
Therefore, these results indicated that p21 and p27 can act
individually under each experimental condition and that p21 is more
strongly associated with the 2-D08-induced effects against cell
proliferation than p27. However, as shown in Fig. 1E, p27 may also be involved in the
inhibition of Ut-LMS cell proliferation at low doses of long-term
2-D08 treatment. Interestingly, the protein level of p53 was not
significantly related to either dose (Fig. 5A), and 2-D08 triggered apoptosis in
these cells possibly through enhanced expression of cleaved
Caspase-3 at a high dose (50 µM) (Figs.
3B and 5C). Additional
experiments are required to verify these differences and further
confirm the mechanism involved.
Unlike cardiac or skeletal muscle cells, SMCs are
unique in their capacity to switch phenotypes (39,47,48).
SMC phenotypic conversion has been extensively investigated in
vascular diseases such as atherosclerosis (49). Although vascular SMC conversion has
been intensively studied, phenotypic modulation appears to strongly
correlate with the development of Ut-LMS. In a study published in
2010, a less-differentiated SMC phenotype was observed in human
Ut-LMS cell lines, whereas the exogenous expression of myocardin in
Ut-LMS cells induced increased expression of SM-specific markers
and stabilization of actin fibers (40). Thus, despite the assumption that
2-D08 is associated with an altered phenotype of SK-LMS-1 cells,
the expression of SMC marker genes did not change significantly in
2-D08-treated-SK-LMS-1 cells (Fig.
6A). These results suggested that 2-D08 represents an
antiproliferative and apoptotic effector rather than a phenotypic
modulator in SK-LMS-1 cells. Although the mechanisms underlying
2-D08-mediated phenotypic conversion remain unclear, other
candidate genes or drugs may play pivotal roles in the phenotypic
conversion of Ut-LMS.
Transcriptome analysis of 2-D08-treated SK-LMS-1
cells revealed significant changes in gene expression, with 259
genes differentially expressed and distinct clustering observed for
each treatment group (Fig. 7B). The
analysis identified 2-D08-specific target genes and showed
downregulated expression for 1,031 genes and upregulated expression
for 2,907 genes in 2-D08-treated cells (Fig. 7C; Table
SII). GO functional analysis revealed significant enrichment
for biological processes related to DNA replication (cell cycle)
and various molecular functions related to the apoptotic process
(Fig 7D-F). These findings
suggested that 2-D08 modulates pathways related to the cell cycle
and apoptosis in SK-LMS-1 cells, and potentially offers a new
therapeutic strategy against cancer. The exact molecular mechanisms
through which 2-D08 modulates the cell cycle and apoptotic pathways
in SK-LMS-1 cells are not fully understood and require further
research. It is also important to note that, while this analysis
provides valuable insights into the potential effects of 2-D08 on
Ut-LMS cells, additional studies are needed to validate these
findings and explore their broader implications.
Analysis of RNA-seq data has revealed that treatment
with 2-D08 enhances the expression of dexamethasone-induced
Ras-related protein 1 (RASD1), a key estrogen signal transducer in
female reproductive organs (50,51).
Rapid upregulation of the expression of RASD1 induced by estrogen
occurs via the p38-MAPK and ERK1/2 pathways in ovariectomized and
prepubertal mice. Reduced expression of RASD1 in patients with
repeated implantation failure correlates with lower estrogen levels
during the menstrual cycle, suggesting impaired implantation
(50). Under normal conditions,
RASD1 facilitates uterine modulation for successful implantation,
while decreased levels in patients with repeated implantation
failure result in abnormal uterine regulation, hindering
implantation (51). Additionally,
RASD1 overexpression suppresses cell proliferation in various cell
types, including NIH-3T3 fibroblast cells, MCF-7 breast cancer, and
A549 lung adenocarcinoma cell lines (52). Further studies are needed to improve
understanding of the relationship between 2-D08 and RASD1 and to
gain clinical insights into uterine leiomyosarcoma.
A significant decrease in viability and colony
formation capacity was observed in SK-UT-1B cells, demonstrating an
anticancer effect (Fig. 1C and E).
However, the experiments related to the cell cycle using flow
cytometry did not demonstrate any effect of 2-D08 on SK-UT-1B cells
compared with the results with SK-LMS-1 cells (Fig. 2B). Even though SK-LMS-1 and SK-UT-1B
are both LMS cell lines, differences in gene expression due to cell
origin appear to have led to these results. As depicted in Fig. S1, a CHX assay was conducted to
inhibit protein synthesis and no difference was observed in the
expression of p53 in SK-LMS-1 cells, although, a decrease was
observed in SK-UT-1B cells, in the indicated timepoints. These
results suggested that the stability of p53 may differ between the
two cell lines. The difference in p53 protein stability between the
two cell lines is likely attributed to variations in mutation sites
within the p53 gene (53,54), but further research is needed for
confirmation. Additionally, SK-UT-1B is slightly faster in doubling
time compared with SK-LMS-1, as indicated by a previous study
(55). Despite conducting a
comprehensive cell cycle analysis, no clear relationship was found
between the doubling times of cell lines with similar histology or
those with similar doubling times. Furthermore, 2-D08 is involved
in various signaling pathways such as ROS, apoptosis and
proliferation in SK-LMS-1 cells compared with SK-UT-1B cells.
Studying the changes in gene expression related to these signaling
pathways could lead to the development of substances targeting
them, maximizing their anticancer effects. To investigate which
genes are involved in these signaling pathways and contribute to
the anticancer effect of 2-D08, RNA-seq analysis was performed on
SK-LMS-1 cells treated with 2-D08 (Fig.
7). Therefore, further studies on the mechanism in Ut-LMS cell
lines can help to more clearly define the direction of LMS
treatment, considering that the response to cancer therapy can vary
greatly depending on individual genetic differences. Such research
contributes to the personalization of cancer management and plays
an important role in determining the most effective treatment for
specific patient groups.
Among the limitations of this study, the authors
acknowledge that extensive research using, for example, animal
models is warranted to determine the biological effects of 2-D08
and the mechanisms underlying its roles. In addition, most in
vitro experiments were conducted using two short-term (48 h)
doses (20 and 50 µM) of 2-D08. Selection of the optimal dose of
2-D08 is required for treating human Ut-LMS. Lastly, using
established Ut-LMS treatment drugs such as trabectedin, pazopanib
and dacarbazine as positive controls in the present study could
enhance reliability. However, it remains unclear which signaling
pathways mediate the effects of these drugs in Ut-LMS cell
lines.
In summary, the present study demonstrated that
2-D08 elicits anticancer effects via multiple signaling pathways in
Ut-LMS cells. Although additional research is needed to fully
understand the underlying biological mechanisms, these results
suggested that treatment with 2-D08 can repress proliferation,
induce apoptosis and potentially affect tumor development in
Ut-LMS.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chosun University (grant
no. K208554001), the National Research Foundation of Korea (NRF)
funded by the Korea government (MSIT) (grant no.
NRF-2020R1C1C1003272), the Basic Science Research Program through
NRF funded by the Ministry of Education (grant no.
NRF-2022R1I1A1A01053069) and the NRF funded by the Korea government
(MSIT) (grant no. RS-2022-00166501).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the GEO database (accession no.
GSE264332).
Authors' contributions
HJ designed the experiments and commented on the
manuscript. HL and HJ conducted the experiments and wrote the
manuscript. HL and HJ participated in the data analyses and confirm
the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
2-D08
|
2′,3′,4′-trihydroxyflavone
|
|
BrdU
|
bromodeoxyuridine
|
|
CHX
|
cycloheximide
|
|
DEGs
|
differentially expressed genes
|
|
FC
|
fold change
|
|
GO
|
gene ontology
|
|
LDH
|
lactate dehydrogenase
|
|
NAC
|
N-acetyl-L-cysteine
|
|
RASD1
|
dexamethasone-induced Ras-related
protein 1
|
|
RNA-seq
|
RNA-sequencing
|
|
ROS
|
reactive oxygen species
|
|
SEM
|
standard error of the mean
|
|
SM
|
smooth muscle
|
|
Ut-LMS
|
uterine leiomyosarcoma
|
|
Ut-SMCs
|
uterine smooth muscle cells
|
References
|
1
|
Echt G, Jepson J, Steel J, Langholz B,
Luxton G, Hernandez W, Astrahan M and Petrovich Z: Treatment of
uterine sarcomas. Cancer. 66:35–39. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim WY, Chang SJ, Chang KH, Yoon JH, Kim
JH, Kim BG, Bae DS and Ryu HS: Uterine leiomyosarcoma: 14-year
two-center experience of 31 cases. Cancer Res Treat. 41:24–28.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tirumani SH, Deaver P, Shinagare AB,
Tirumani H, Hornick JL, George S and Ramaiya NH: Metastatic pattern
of uterine leiomyosarcoma: Retrospective analysis of the predictors
and outcome in 113 patients. J Gynecol Oncol. 25:306–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chern JY, Boyd LR and Blank SV: Uterine
sarcomas: The latest approaches for these rare but potentially
deadly tumors. Oncology (Williston Park). 31:229–236.
2017.PubMed/NCBI
|
|
5
|
Murakami M, Ichimura T, Kasai M, Matsuda
M, Kawamura N, Fukuda T and Sumi T: Examination of the use of
needle biopsy to perform laparoscopic surgery safely on uterine
smooth muscle tumors. Oncol Lett. 15:8647–8651. 2018.PubMed/NCBI
|
|
6
|
Hernando E, Charytonowicz E, Dudas ME,
Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L,
Maki RG, et al: The AKT-mTOR pathway plays a critical role in the
development of leiomyosarcomas. Nat Med. 13:748–753. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babichev Y, Kabaroff L, Datti A, Uehling
D, Isaac M, Al-Awar R, Prakesch M, Sun RX, Boutros PC, Venier R, et
al: PI3K/AKT/mTOR inhibition in combination with doxorubicin is an
effective therapy for leiomyosarcoma. J Transl Med. 14:672016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaumann AK, Drexler HC, Lang SA,
Stoeltzing O, Diermeier-Daucher S, Buchdunger E, Wood J, Bold G and
Breier G: The inhibition of tyrosine kinase receptor signalling in
leiomyosarcoma cells using the small molecule kinase inhibitor
PTK787/ZK222584 (Vatalanib®). Int J Oncol. 45:2267–2277.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi T, Horiuchi A, Sano K, Hiraoka N,
Kanai Y, Shiozawa T, Tonegawa S and Konishi I: Molecular approach
to uterine leiomyosarcoma: LMP2-deficient mice as an animal model
of spontaneous uterine leiomyosarcoma. Sarcoma. 2011:4764982011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cancer Genome Atlas Research Network.
Electronic address, . simpleelizabeth.demicco@sinaihealthsystem.ca
and Cancer Genome Atlas Research Network: Comprehensive and
integrated genomic characterization of adult soft tissue sarcomas.
Cell. 171:950–965.e28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mäkinen N, Aavikko M, Heikkinen T, Taipale
M, Taipale J, Koivisto-Korander R, Bützow R and Vahteristo P: Exome
sequencing of uterine leiomyosarcomas identifies frequent mutations
in TP53, ATRX, and MED12. PLoS Genet. 12:e10058502016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuyoshi H and Yoshida Y: Molecular
biomarkers for uterine leiomyosarcoma and endometrial stromal
sarcoma. Cancer Sci. 109:1743–1752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeCensi A, Guerrieri-Gonzaga A, Gandini S,
Serrano D, Cazzaniga M, Mora S, Johansson H, Lien EA, Pruneri G,
Viale G and Bonanni B: Prognostic significance of Ki-67 labeling
index after short-term presurgical tamoxifen in women with
ER-positive breast cancer. Ann Oncol. 22:582–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davey MG, Hynes SO, Kerin MJ, Miller N and
Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast
cancer. Cancers (Basel). 13:44552021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tollefson MK, Karnes RJ, Kwon ED, Lohse
CM, Rangel LJ, Mynderse LA, Cheville JC and Sebo TJ: Prostate
cancer Ki-67 (MIB-1) expression, perineural invasion, and gleason
score as biopsy-based predictors of prostate cancer mortality: The
mayo model. Mayo Clin Proc. 89:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maia R, Santos GAD, Reis S, Viana NI,
Pimenta R, Guimarães VR, Recuero S, Romão P, Leite KRM, Srougi M
and Passerotti CC: Can we use Ki67 expression to predict prostate
cancer aggressiveness? Rev Col Bras Cir. 49:e20223200. 2022.(In
English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Zhang F, Liu Z, Wu K, Zhu Y and
Lu Y: Prognostic role of Ki-67 in adrenocortical carcinoma after
primary resection: A retrospective mono-institutional study. Adv
Ther. 36:2756–2768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akhan SE, Yavuz E, Tecer A, Iyibozkurt CA,
Topuz S, Tuzlali S, Bengisu E and Berkman S: The expression of
Ki-67, p53, estrogen and progesterone receptors affecting survival
in uterine leiomyosarcomas. A clinicopathologic study. Gynecol
Oncol. 99:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travaglino A, Raffone A, Catena U, De Luca
M, Toscano P, Del Prete E, Vecchione ML, Lionetti R, Zullo F and
Insabato L: Ki67 as a prognostic marker in uterine leiomyosarcoma:
A quantitative systematic review. Eur J Obstet Gynecol Reprod Biol.
266:119–124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YS, Keyser SG and Schneekloth JS Jr:
Synthesis of 2′,3′,4′-trihydroxyflavone (2-D08), an inhibitor of
protein sumoylation. Bioorg Med Chem Lett. 24:1094–1097. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marsh DT, Das S, Ridell J and Smid SD:
Structure-activity relationships for flavone interactions with
amyloid beta reveal a novel anti-aggregatory and neuroprotective
effect of 2′,3′,4′-trihydroxyflavone (2-D08). Bioorg Med Chem.
25:3827–3834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi BH, Philips MR, Chen Y, Lu L and Dai
W: K-Ras Lys-42 is crucial for its signaling, cell migration, and
invasion. J Biol Chem. 293:17574–17581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou P, Chen X, Li M, Tan J, Zhang Y, Yuan
W, Zhou J and Wang G: 2-D08 as a SUMOylation inhibitor induced ROS
accumulation mediates apoptosis of acute myeloid leukemia cells
possibly through the deSUMOylation of NOX2. Biochem Biophys Res
Commun. 513:1063–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Lee SM and Joung H: 2-D08 treatment
regulates C2C12 myoblast proliferation and differentiation via the
Erk1/2 and proteasome signaling pathways. J Muscle Res Cell Motil.
42:193–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo C, Sun L, Chen X and Zhang D:
Oxidative stress, mitochondrial damage and neurodegenerative
diseases. Neural Regen Res. 8:2003–2014. 2013.PubMed/NCBI
|
|
32
|
Nishikawa M, Hashida M and Takakura Y:
Catalase delivery for inhibiting ROS-mediated tissue injury and
tumor metastasis. Adv Drug Deliv Rev. 61:319–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhitkovich A: N-Acetylcysteine:
Antioxidant, aldehyde scavenger, and more. Chem Res Toxicol.
32:1318–1319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beamish JA, He P, Kottke-Marchant K and
Marchant RE: Molecular regulation of contractile smooth muscle cell
phenotype: Implications for vascular tissue engineering. Tissue Eng
Part B Rev. 16:467–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie C, Ritchie RP, Huang H, Zhang J and
Chen YE: Smooth muscle cell differentiation in vitro: Models and
underlying molecular mechanisms. Arterioscler Thromb Vasc Biol.
31:1485–1494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amada S, Nakano H and Tsuneyoshi M:
Leiomyosarcoma versus bizarre and cellular leiomyomas of the
uterus: A comparative study based on the MIB-1 and proliferating
cell nuclear antigen indices, p53 expression, DNA flow cytometry,
and muscle specific actins. Int J Gynecol Pathol. 14:134–142. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sprogøe-Jakobsen S and Hølund B:
Immunohistochemistry (Ki-67 and p53) as a tool in determining
malignancy in smooth muscle neoplasms (exemplified by a myxoid
leiomyosarcoma of the uterus). APMIS. 104:705–708. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Horiuchi A, Nikaido T, Ito K, Zhai Y, Orii
A, Taniguchi S, Toki T and Fujii S: Reduced expression of calponin
h1 in leiomyosarcoma of the uterus. Lab Invest. 78:839–846.
1998.PubMed/NCBI
|
|
39
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kimura Y, Morita T, Hayashi K, Miki T and
Sobue K: Myocardin functions as an effective inducer of growth
arrest and differentiation in human uterine leiomyosarcoma cells.
Cancer Res. 70:501–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakamura H and Takada K: Reactive oxygen
species in cancer: Current findings and future directions. Cancer
Sci. 112:3945–3952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Philipp-Staheli J, Kim KH, Liggitt D,
Gurley KE, Longton G and Kemp CJ: Distinct roles for p53, p27Kip1,
and p21Cip1 during tumor development. Oncogene. 23:905–913. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Neill CJ, McBride HA, Connolly LE and
McCluggage WG: Uterine leiomyosarcomas are characterized by high
p16, p53 and MIB1 expression in comparison with usual leiomyomas,
leiomyoma variants and smooth muscle tumours of uncertain malignant
potential. Histopathology. 50:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schaefer IM, Hornick JL, Sholl LM, Quade
BJ, Nucci MR and Parra-Herran C: Abnormal p53 and p16 staining
patterns distinguish uterine leiomyosarcoma from inflammatory
myofibroblastic tumour. Histopathology. 70:1138–1146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bunz F, Dutriaux A, Lengauer C, Waldman T,
Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Le Guen L, Marchal S, Faure S and de Santa
Barbara P: Mesenchymal-epithelial interactions during digestive
tract development and epithelial stem cell regeneration. Cell Mol
Life Sci. 72:3883–3896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scirocco A, Matarrese P, Carabotti M,
Ascione B, Malorni W and Severi C: Cellular and molecular
mechanisms of phenotypic switch in gastrointestinal smooth muscle.
J Cell Physiol. 231:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chakraborty R, Chatterjee P, Dave JM,
Ostriker AC, Greif DM, Rzucidlo EM and Martin KA: Targeting smooth
muscle cell phenotypic switching in vascular disease. JVS Vasc Sci.
2:79–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim HR, Cho KS, Kim E, Lee OH, Yoon H, Lee
S, Moon S, Park M, Hong K, Na Y, et al: Rapid expression of RASD1
is regulated by estrogen receptor-dependent intracellular signaling
pathway in the mouse uterus. Mol Cell Endocrinol. 446:32–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong K and Choi Y: Role of estrogen and
RAS signaling in repeated implantation failure. BMB Rep.
51:225–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vaidyanathan G, Cismowski MJ, Wang G,
Vincent TS, Brown KD and Lanier SM: The Ras-related protein
AGS1/RASD1 suppresses cell growth. Oncogene. 23:5858–5863. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park DJ, Nakamura H, Chumakov AM, Said JW,
Miller CW, Chen DL and Koeffler HP: Transactivational and DNA
binding abilities of endogenous p53 in p53 mutant cell lines.
Oncogene. 9:1899–1906. 1994.PubMed/NCBI
|
|
54
|
Smardová J, Pavlová S, Svitáková M,
Grochová D and Ravcuková B: Analysis of p53 status in human cell
lines using a functional assay in yeast: detection of new non-sense
p53 mutation in codon 124. Oncol Rep. 14:901–907. 2005.PubMed/NCBI
|
|
55
|
Mills J, Matos T, Charytonowicz E, Hricik
T, Castillo-Martin M, Remotti F, Lee FY and Matushansky I:
Characterization and comparison of the properties of sarcoma cell
lines in vitro and in vivo. Hum Cell. 22:85–93. 2009. View Article : Google Scholar : PubMed/NCBI
|