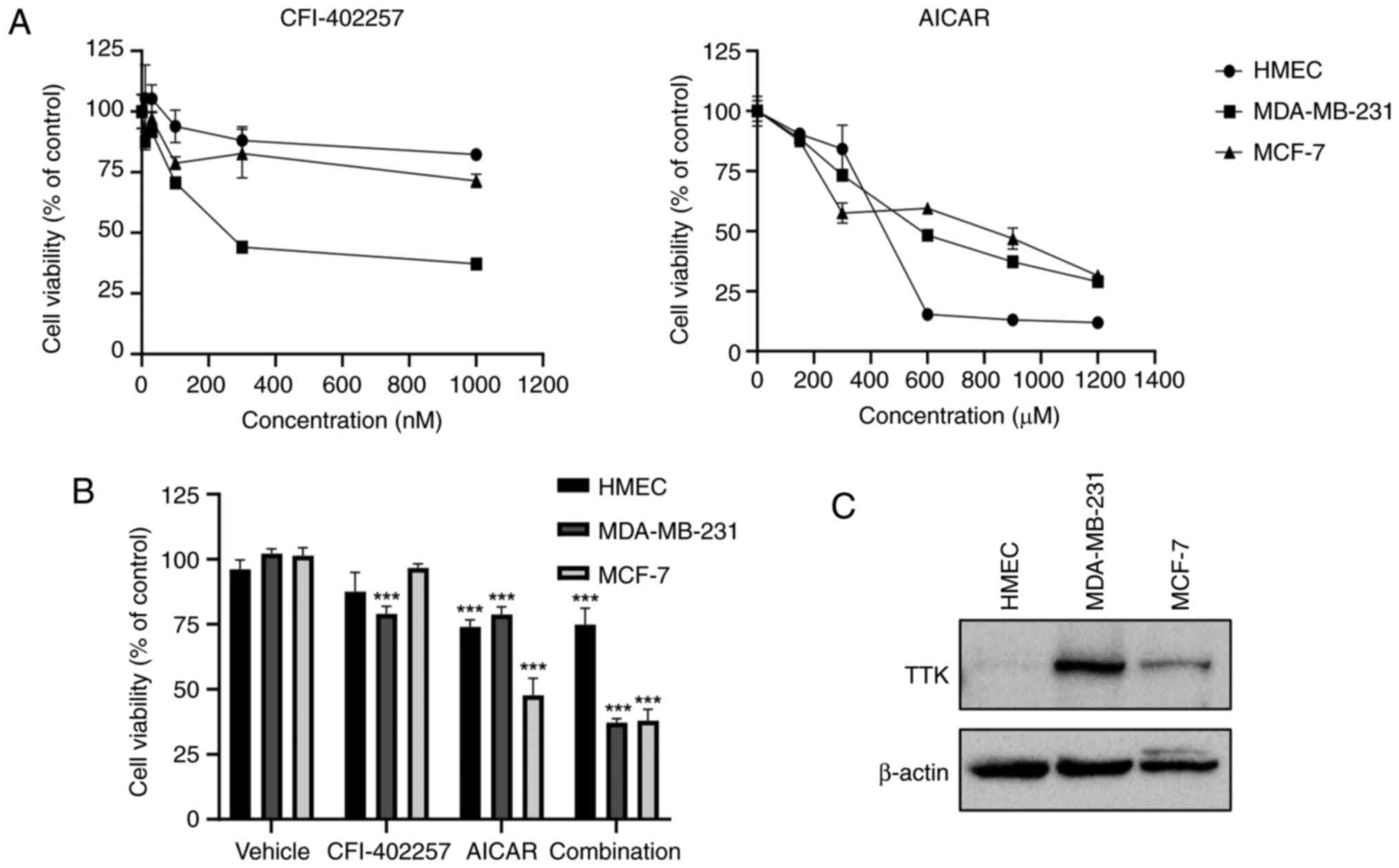
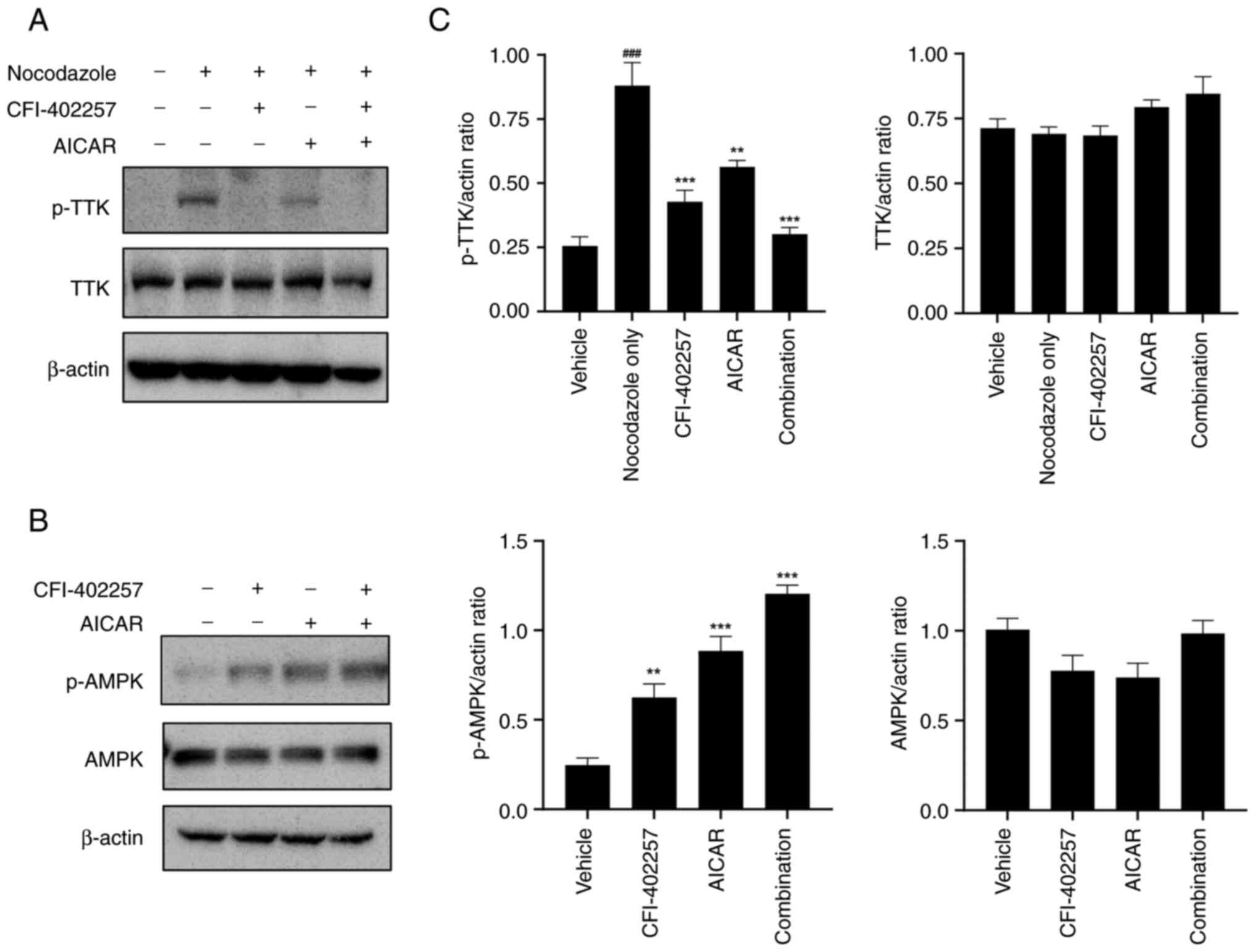
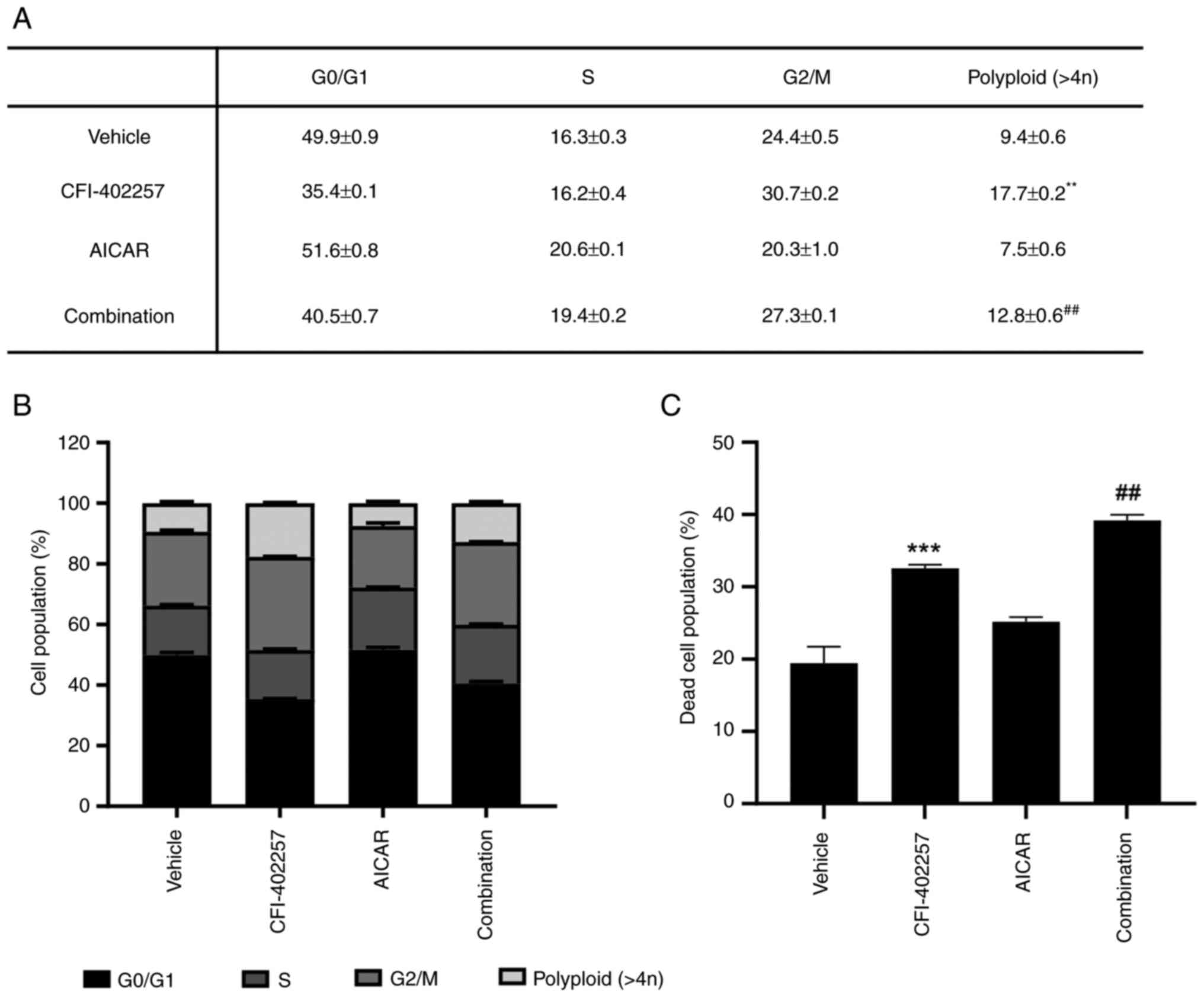
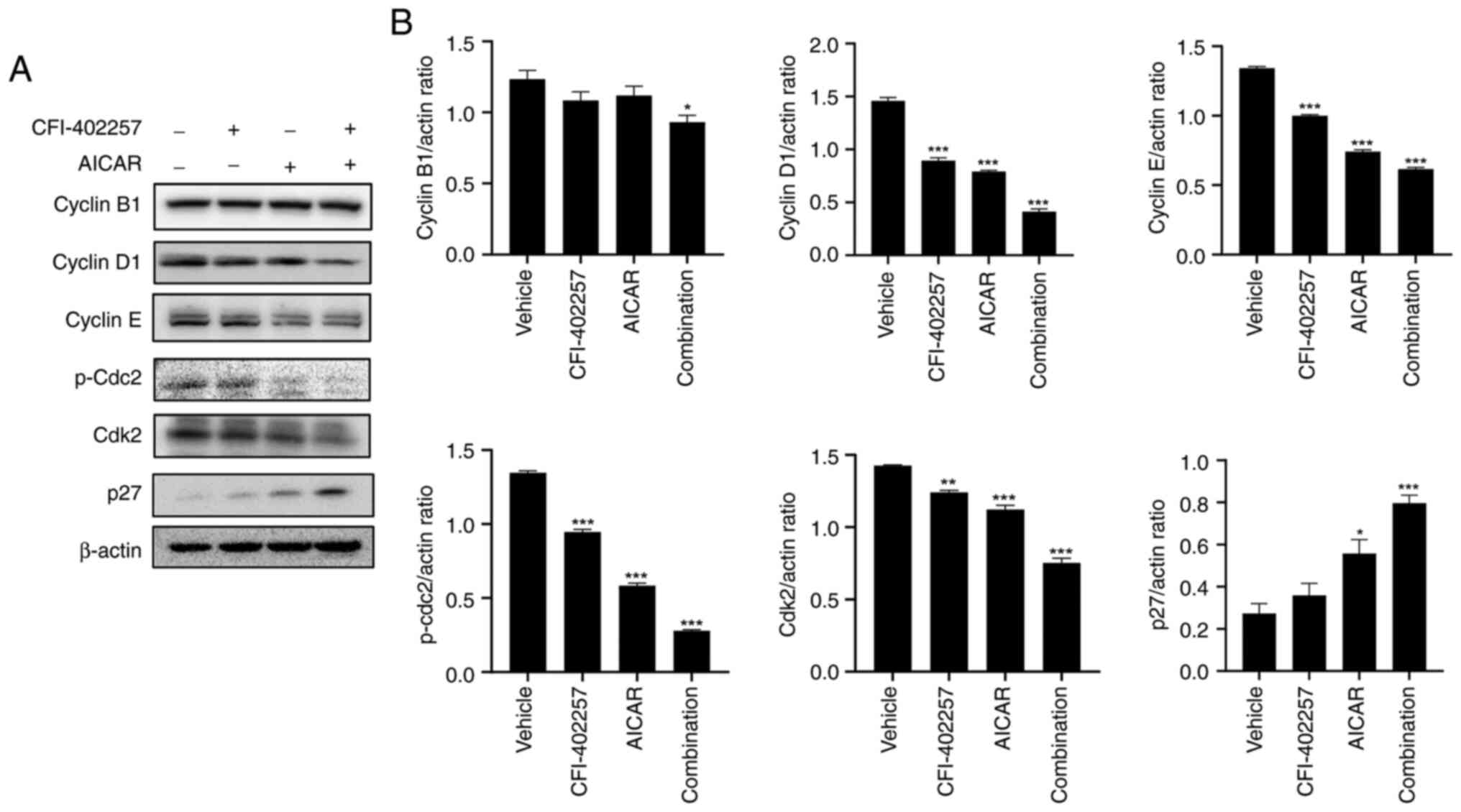
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasmita AO and Wong YP: Organoids as
reliable breast cancer study models: An update. Int J Oncol Res.
1:0082018.
|
|
3
|
Schwentner L, Wolters R, Koretz K,
Wischnewsky MB, Kreienberg R, Rottscholl R and Wöckel A:
Triple-negative breast cancer: The impact of guideline-adherent
adjuvant treatment on survival-a retrospective multi-centre cohort
study. Breast Cancer Res Treat. 132:1073–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai L, Zhou B, Yang CY, Ji J, McEachern D,
Przybranowski S, Jiang H, Hu J, Xu F, Zhao Y, et al: Targeted
degradation of BET proteins in triple-negative breast cancer.
Cancer Res. 77:2476–2487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dominguez-Brauer C, Thu KL, Mason JM,
Blaser H, Bray MR and Mak TW: Targeting mitosis in cancer: Emerging
strategies. Mol Cell. 60:524–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lara-Gonzalez P, Westhorpe FG and Taylor
SS: The spindle assembly checkpoint. Curr Biol. 22:R966–R980. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colombo R, Caldarelli M, Mennecozzi M,
Giorgini ML, Sola F, Cappella P, Perrera C, Depaolini SR, Rusconi
L, Cucchi U, et al: Targeting the mitotic checkpoint for cancer
therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res.
70:10255–10264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tardif KD, Rogers A, Cassiano J, Roth BL,
Cimbora DM, McKinnon R, Peterson A, Douce TB, Robinson R, Dorweiler
I, et al: Characterization of the cellular and antitumor effects of
MPI-0479605, a small-molecule inhibitor of the mitotic kinase Mps1.
Mol Cancer Ther. 10:2267–2275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maire V, Baldeyron C, Richardson M, Tesson
B, Vincent-Salomon A, Gravier E, Marty-Prouvost B, De Koning L,
Rigaill G, Dumont A, et al: TTK/hMPS1 is an attractive therapeutic
target for triple-negative breast cancer. PLoS One. 8:e637122013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salvatore G, Nappi TC, Salerno P, Jiang Y,
Garbi C, Ugolini C, Miccoli P, Basolo F, Castellone MD, Cirafici
AM, et al: A cell proliferation and chromosomal instability
signature in anaplastic thyroid carcinoma. Cancer Res.
67:10148–10158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slee RB, Grimes BR, Bansal R, Gore J,
Blackburn C, Brown L, Gasaway R, Jeong J, Victorino J, March KL, et
al: Selective inhibition of pancreatic ductal adenocarcinoma cell
growth by the mitotic MPS1 kinase inhibitor NMS-P715. Mol Cancer
Ther. 13:307–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan B, Xu Y, Woo JH, Wang Y, Bae YK, Yoon
DS, Wersto RP, Tully E, Wilsbach K and Gabrielson E: Increased
expression of mitotic checkpoint genes in breast cancer cells with
chromosomal instability. Clin Cancer Res. 12:405–410. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fuentes-Antrás J, Bedard PL and Cescon DW:
Seize the engine: Emerging cell cycle targets in breast cancer.
Clin Transl Med. 14:e15442024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez R, Blasina A, Hallin JF, Hu W,
Rymer I, Fan J, Hoffman RL, Murphy S, Marx M, Yanochko G, et al:
Mitotic checkpoint kinase Mps1 has a role in normal physiology
which impacts clinical utility. PLoS One. 10:e01386162015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderhub SJ, Mak GW, Gurden MD, Faisal A,
Drosopoulos K, Walsh K, Woodward HL, Innocenti P, Westwood IM, Naud
S, et al: High proliferation rate and a compromised spindle
assembly checkpoint confers sensitivity to the MPS1 inhibitor
BOS172722 in triple-negative breast cancers. Mol Cancer Ther.
18:1696–1707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wengner AM, Siemeister G, Koppitz M,
Schulze V, Kosemund D, Klar U, Stoeckigt D, Neuhaus R, Lienau P,
Bader B, et al: Novel Mps1 kinase inhibitors with potent antitumor
activity. Mol Cancer Ther. 15:583–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Novais P, Silva PMA, Amorim I and Bousbaa
H: Second-generation antimitotics in cancer clinical trials.
Pharmaceutics. 13:10112021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atrafi F, Boix O, Subbiah V, Diamond JR,
Chawla SP, Tolcher AW, LoRusso PM, Eder JP, Gutierrez M, Sankhala
K, et al: A phase I study of an MPS1 inhibitor (BAY 1217389) in
combination with paclitaxel using a novel randomized continual
reassessment method for dose escalation. Clin Cancer Res.
27:6366–6375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Yang G, Kim Y, Kim J and Ha J: AMPK
activators: Mechanisms of action and physiological activities. Exp
Mol Med. 48:e2242016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang YC, Williams BR, Siegel JJ and Amon
A: Identification of aneuploidy-selective antiproliferation
compounds. Cell. 144:499–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasudevan A, Schukken KM, Sausville EL,
Girish V, Adebambo OA and Sheltzer JM: Aneuploidy as a promoter and
suppressor of malignant growth. Nat Rev Cancer. 21:89–103. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao W, Li J, Hao Q, Vadgama JV and Wu Y:
AMP-activated protein kinase: A potential therapeutic target for
triple-negative breast cancer. Breast Cancer Res. 21:292019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hadad SM, Baker L, Quinlan PR, Robertson
KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG,
et al: Histological evaluation of AMPK signalling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mason JM, Wei X, Fletcher GC, Kiarash R,
Brokx R, Hodgson R, Beletskaya I, Bray MR, Mak TW, et al:
Functional characterization of CFI-402257, a potent and selective
Mps1/TTK kinase inhibitor, for the treatment of cancer. Proc Natl
Acad Sci USA. 114:3127–3132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Ling Y, Guo Y, Bai Y, Shi X, Gong
F, Tan P, Zhang Y, Wei C, He X, et al: Mps1 kinase regulates tumor
cell viability via its novel role in mitochondria. Cell Death Dis.
7:e22922016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Millot C, Millot JM, Morjani H, Desplaces
A and Manfait M: Characterization of acidic vesicles in
multidrug-resistant and sensitive cancer cells by acridine orange
staining and confocal microspectrofluorometry. J Histochem
Cytochem. 45:1255–1264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9:3382018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ippolito MR, Martis V, Hong C, Hong C,
Wardenaar R, Zerbib J, Spierings DCJ, Ben-David U, Foijer F and
Santaguida S: Aneuploidy-driven genome instability triggers
resistance to chemotherapy. Biorxiv. 2020.09.25.313924. 2020.
|
|
29
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo J, Solimini NL and Elledge SJ:
Principles of cancer therapy: Oncogene and non-oncogene addiction.
Cell. 136:823–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manchado E and Malumbres M: Targeting
aneuploidy for cancer therapy. Cell. 144:465–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liberti MV and Locasale JW: The warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song IS, Han J and Lee HK: Metformin as an
anticancer drug: A commentary on the metabolic determinants of
cancer cell sensitivity to glucose limitation and biguanides. J
Diabetes Investig. 6:516–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ly P, Kim SB, Kaisani AA, Marian G, Wright
WE and Shay JW: Aneuploid human colonic epithelial cells are
sensitive to AICAR-induced growth inhibition through EGFR
degradation. Oncogene. 32:3139–3146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L, Li A, Lei Q and Zhang Y:
Tumor-intrinsic signaling pathways: Key roles in the regulation of
the immunosuppressive tumor microenvironment. J Hematol Oncol.
12:1252019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herriage HC, Huang YT and Calvi BR: The
antagonistic relationship between apoptosis and polyploidy in
development and cancer. Semin Cell Dev Biol. 156:35–43. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balko JM, Schwarz LJ, Bhola NE, Kurupi R,
Owens P, Miller TW, Gómez H, Cook RS and Arteaga CL: Activation of
MAPK pathways due to DUSP4 loss promotes cancer stem cell-like
phenotypes in basal-like breast cancer. Cancer Res. 73:6346–6358.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeffrey KL, Camps M, Rommel C and Mackay
CR: Targeting dual-specificity phosphatases: Manipulating MAP
kinase signalling and immune responses. Nat Rev Drug Discov.
6:391–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuo WL, Duke CJ, Abe MK, Kaplan EL, Gomes
S and Rosner MR: ERK7 expression and kinase activity is regulated
by the ubiquitin-proteosome pathway. J Biol Chem. 279:23073–23081.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alkarain A, Jordan R and Slingerland J:
p27 deregulation in breast cancer: Prognostic significance and
implications for therapy. J Mammary Gland Biol Neoplasia. 9:67–80.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blain SW, Scher HI, Cordon-Cardo C and
Koff A: p27 as a target for cancer therapeutics. Cancer Cell.
3:111–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jhaveri TZ, Woo J, Shang X, Park BH and
Gabrielson E: AMP-activated kinase (AMPK) regulates activity of
HER2 and EGFR in breast cancer. Oncotarget. 6:14754–14765. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Theodoropoulou S, Brodowska K, Kayama M,
Morizane Y, Miller JW, Gragoudas ES and Vavvas DG: Aminoimidazole
carboxamide ribonucleotide (AICAR) inhibits the growth of
retinoblastoma in vivo by decreasing angiogenesis and inducing
apoptosis. PLoS One. 8:e528522013. View Article : Google Scholar : PubMed/NCBI
|