Introduction
Biliary tract cancers include intrahepatic
cholangiocarcinoma (ICC), perihilar, distal cholangiocarcinoma and
gall bladder carcinoma based on their location of origin. Notably,
ICC stands out as a type of cancer associated with increased
mortality. It ranks as the second most prevalent primary liver
cancer, constituting ≤20% of liver cancers and 3% of all
gastrointestinal cancers (1,2). The
prognosis for patients with ICC undergoing surgery with the intent
to cure is poor, with a 5-year overall survival (OS) range of 5–17%
(3). Patients with unresectable ICC
have a median survival of 11.7 months with palliative chemotherapy,
primarily gemcitabine and cisplatin (4). Despite the potential of emerging
treatments such as triplet regimens, immunotherapy and targeted
therapies for specific genetic alterations (5), the persistent challenges of tumor
resistance and low OS rates underscore the limited efficacy of
current clinical treatments for ICC. Therefore, the development of
more effective anti-ICC drugs is critical and pressing issue in
medical oncology research to improve treatment outcomes and patient
survival.
Natural products continue to be a vital and
promising reservoir for chemotherapeutic agent discovery,
exemplified by drugs such as artemisinin, an antimalarial agent
(6). Between 1981 and 2014, 49% of
the anticancer drugs approved by the FDA originated either directly
from natural sources or as derivatives. These include notable drugs
such as vinblastine and colchicine, highlighting the significant
role of natural resources in cancer drug development (7,8).
Digitoxin (DT), a cardiac glycoside naturally sourced from species
of the genus Digitalis (9),
has served as an effective Na+/K+-ATPase
inhibitor. For >4 decades, it has been used in the clinical
management of congestive heart failure (10). Numerous studies have investigated
the anticancer effects of DT, revealing its potent antitumor
efficacy in a diverse range of cancer types (11–14).
However, the ability and underlying mechanism of DT as an
anticancer reagent against ICC, a lethal cancer without
satisfactory chemotherapy, remains unknown.
In the present study, a screening of a TCM library
of 2,538 unique natural compounds was conducted to discover
potential lead compounds exhibiting anti-ICC activity. Among the
noteworthy compounds discovered, DT emerged as a significant
anti-ICC chemotherapeutic agent. The effects of DT on ICC cell
proliferation and metastasis were further evaluated through cell
functional assays, and the key signaling pathways involved were
explored. ST6 β-galactoside α-2,6-sialyltransferase 1 (ST6GAL1),
which facilitates the addition of sialic acid to N-glycosylated
proteins, is substantially upregulated in various types of
malignancies, playing a crucial role in the progression of cancer.
It was revealed that the mRNA and protein ST6GAL1 expression in ICC
cells was suppressed by DT. Overexpression of ST6GAL1 could reverse
the inhibitory effect of DT on ICC. It was shown that DT inhibited
ICC cell proliferation and metastasis by targeting the
NF-κB/ST6GAL1 signaling pathway. The findings of the present study
indicated the therapeutic potential of DT in the treatment of
ICC.
Materials and methods
Cell culture
HCCC-9810, RBE and 293T cell lines were obtained
from the Chinese Academy of Sciences, Shanghai Branch Cell Bank.
HuCCT1 were purchased from Cyagen, and normal human intrahepatic
biliary epithelial cells (HiBEpiCs) were obtained from Qingqi
(Shanghai) Biotechnology development Co., Ltd. Cells were cultured
in RPMI-1640 medium (cat. no. 11875119; Gibco, Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (cat. no. 10099141C;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (cat. no. 15140122; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator (HERA cell
VIOS; Themo Fisher Scientific, Inc.) with 5% CO2. 293T
cells were cultured in DMEM (cat. no. 11995065; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin. Dimethyl sulfoxide (DMSO) was obtained
from Sigma-Aldrich; Merck KGaA, and DT (cat. no. HY-B1357) was
purchased from MedChemExpress.
Cell viability assay
The cell suspension (100 µl/well) was seeded in
96-well culture plates (cat. no. 3599; Corning, Inc.) at a density
range of 1–2×105 cells/ml. The cells were treated and
incubated with DT at serial concentrations for 24, 48 and 72 h. A
total of 10 µl CCK-8 (cat. no. HY-K0301; MedChemExpress) solution
was then added to each well of the plate. The plate was incubated
for 3 h at 37°C and absorbance was measured at 450 nm using the
Molecular Devices Spectra Max iD3 Microplate Detection System. The
cell viability rate was calculated as experimental optical density
(OD) value/control OD value.
High-throughput screening of TCM
library
A total of 2,538 compounds in the TCM Active
Compound Library (cat. no. HY-L065; MedChemExpress) were used for
the screening experiment. Compounds were initially screened at a
concentration of 10 µM. A further screening was conducted with the
criterion that ICC cell viability should be decreased by 70%
compared with the control (DMSO). Subsequently, compounds that
maintained a viability rate >80% in HiBEpiCs were selected as
candidates for anti-ICC drugs.
Overexpression of ST6GAL1 in ICC
cells
The overexpression of ST6GAL1 plasmids and control
plasmids (pHBLV-CMV-MCS-3FLAG-EF1-ZsGreen-T2A-PURO) was carried out
by Hanbio Biotechnology Co., Ltd. For the construction of the
ST6GAL1-overexpression cell line, transfer plasmid (10 µg;
A260/A280=1.94), envelope plasmid (pMD2G, 5 µg; A260/A280=1.93) and
packaging plasmids (pSPAX2, 10 µg; A260/A280=1.92) were
co-transfected into 293T packaging cells using Lipofectamine 3000
(cat. np. L3000015; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions at 37°C. The culture medium was
replenished 16 h later, and the culture supernatant was collected
at 2 and 3 days. The virus supernatant was centrifuged in a 50-ml
centrifuge tube at 4°C and 2,000 × g for 10 min to remove cell
debris. Then, the clarified viral supernatant was collected and
placed into an ultracentrifuge tube to be centrifuged at 4°C and
82,700 × g for 120 min. The viral pellet was resuspended in 400 µl
complete medium and finally the resuspended solution was aliquoted
into sterilized viral tubes. A total of 2×105 HCCC-9810
or HuCCT1 cells were seeded in 12-well plates overnight and
subsequently infected with 50 µl lentivirus expressing the ST6GAL1
gene or a control lentivirus. The transfected cells were screened
using puromycin (1 µg/ml) 1 week later.
Overexpression of P65/NF-κB in 293T
cells
The overexpression of P65/NF-κB plasmids and control
plasmids pcDNA3.1 was carried out by Hanbio Biotechnology Co., Ltd.
The P65/NF-κB overexpression plasmids (4 µg; A260/A280=1.93) were
transfected into 293T cells at 37°C using Lipofectamine 3000 (cat.
np. L3000015; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The medium was refreshed 6 h after
transfection, 50 nM DT was added 24 h post-transfection, and WB and
RT-qPCR were performed at 48 h.
Colony formation and
5-ethynyl-2′-deoxyuridine (EdU) assays
A total of 1,000 ICC cells/well treated with
different concentrations of DT and the ST6GAL1-overexpressed ICC
cells treated with DT were plated in 12-well plates (cat. no. 3513;
Corning, Inc.) and cultured at 37°C for 7 days. Subsequently, they
were fixed in 4% paraformaldehyde (w/v) for 10 min at RT. After
washing three times with PBS, staining was performed using 0.1%
crystal violet staining solution (cat. no. C0121; Beyotime
Institute of Biotechnology) for 10 min at room temperature (RT).
Images were captured using a BZ-X800 fluorescence microscope
(Keyence Corporation), and the colonies with aggregates of ≥50
cells were counted.
The ICC cells treated with different concentrations
of DT and the ST6GAL1-overexpressed ICC cells treated with DT were
seeded into a 24-well plate (cat. no. 3524; Corning, Inc.). Cell
proliferation was assessed by incorporating EdU (cat. no. C0071S;
Beyotime Institute of Biotechnology) following the manufacturer's
instructions. The percentage of EdU+ cells was
calculated by three random images captured with the BZ-X800
microscope.
Wound healing assay
The ICC cells treated with different concentrations
of DT and the ST6GAL1-overexpressing ICC cells treated with DT were
seeded in 12-well plates and allowed to incubate for 1 day. When
the cells reached ~90% confluence, the bottom of each well was
scratched with a 10-µl pipette tip. After washing with PBS, the
culture medium was replenished with serum-free medium for culture.
Images were captured under an inverted light microscope (cat. no.
DMi8; Leica Microsystems) at 0 and 24 h, and the migration area was
computed using ImageJ software (version 1.53a; National Institutes
of Health).
Cell migration
The ICC cells treated with different concentrations
of DT and the ST6GAL1-overexpressing ICC cells treated with DT were
collected and diluted with serum-free DMEM to a concentration of
1×105 cells/ml. The upper chamber (8 µm; cat. no.
353097; Falcon; Corning, Inc.) was filled with 200 µl cell
suspension in order to measure the capacity for migration. The
migratory cells were subsequently stained with 0.1% crystal violet
staining solution for 10 min at RT and observed using a BZ-X800
microscope. The number of migratory cells was counted using three
randomly selected images of selected fields.
RNA sequencing (RNA-seq)
RNA-seq and analysis were conducted by Shanghai
Biotechnology Co., Ltd. RNA extraction was performed using the
MJzol Animal RNA Isolation Kit (Majorivd; http://www.majorbio.com/shop/goods/71), followed by
purification with the RNAClean XP Kit (Beckman Coulter, Inc.) and
RNase-Free DNase Set (Qiagen, Inc.). RNA integrity and quality
(260/280 nm ratio >1.8) were assessed using the Agilent 2100
Bioanalyzer/Agilent 4200 TapeStation and quantified with the Qubit
2.0® Fluorometer (Thermo Fisher Scientific, Inc.) and
NanoDrop ND-2000 (Thermo Fisher Scientific, Inc.). The mRNA was
isolated from the purified RNA, fragmented and synthesized into
cDNA, with subsequent library construction steps including end
repair, adenylation, adapter ligation and enrichment. Purified
libraries were quantified by Qubit® 2.0 Fluorometer
(Thermo Fisher Scientific, Inc.) and validated by Agilent 2100
bioanalyzer (Agilent Technologies, Inc.) to confirm the insert size
and calculate the mole concentration. Cluster was generated by cBot
with the library diluted to 10 pM and then were sequenced on the
Illumina NovaSeq6000 platform (Illumina, Inc.) with the PE150
(Pair-end 150 bp) mode. Following data analysis at the transcript
level and differential gene screening, differentially expressed
genes were identified. Subsequent analyses were carried out to
determine the Gene Ontology (GO) functional significance and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway significance.
Reverse transcription-quantitative PCR
(RT-qPCR)
The EZ-press RNA purification kit (cat. no. B0004D;
EZBioscience) was used to extracted total RNA from cells. The
concentration of the extracted RNA was measured by NanoDrop (Thermo
Fisher Scientific, Inc.) at 260 nm. PrimeScript™ RT Reagent Kit
(cat. no. RR037A; Takara Bio, Inc.) was applied to reverse
transcribe RNA into cDNA according to the manufacturer's protocol.
Each RT reaction contained 500 ng RNA. TB Green™ Premix Ex Taq™
(cat. no. RR420A; Takara Bio, Inc.) was used for qPCR, and data
collection and analysis were carried out using an Applied
Biosystems PCR System (7500 Real-Time PCR System; Thermo Fisher
Scientific, Inc.). The PCR thermocycling conditions were as
follows: i) Stage 1, 95°C for 30 sec; ii) Stage 2, 95°C for 5 sec,
60°C for 34 sec, 40 cycles; iii) Stage 3, 95°C for 30 sec, 60°C for
1 min, 95°C for 15 sec. The 2−ΔΔCq method (15) was used to ascertain the relative
expression levels of each gene, with GAPDH serving as the internal
reference. Primer sequences were as follows: ST6GAL1 forward,
5′-CCCCAATCAGCCCTTTTACATCCTC-3′ and reverse,
5′-CCTGGTCACACAGCGTCATCATG-3′; and GAPDH forward,
5′-CCAGCAAGAGCACAAGAGGAAGAG-3′ and reverse,
5′-GGTCTACATGGCAACTGTGAGGAG-3′.
Western blotting and lectin
blotting
Cells treated with different concentrations of DT
were lysed using cell lysis buffer (cat. no. 9803s; Cell Signaling
Technology, Inc.) containing ProtLytic Protease and Phosphatase
Inhibitor Cocktail (cat. no. P002; NCM Biotech). Nuclear proteins
were extracted using the Nuclear and Cytoplasmic Protein Extraction
Kit (cat. no. P0028; Beyotime Institute of Biotechnology). The
protein concentrations were quantified by the BCA protein assay kit
(cat. no. P0011; Beyotime Institute of Biotechnology). A total of
30 µg proteins were separated by 7.5 or 10% SDS-PAGE, and the
resulting proteins were then moved from the gel onto PVDF membranes
(cat. no. ISEQ00010; MilliporeSigma).
After 1 h of incubation with 5% skimmed milk at RT,
membranes were incubated with primary antibodies against Bcl-2
(1:1,000; cat. no. 12789-1-AP; Proteintech Group, Inc.), ST6GAL1 (1
µg/ml; cat. no. AF5924; R&D Systems, Inc.), BAX (1:1,000; cat.
no. 50599-2-Ig; Proteintech Group, Inc.), phosphorylated (p)-NF-κB
p65 (1:1,000; cat. no. 3033; Cell Signaling Technology, Inc.),
NF-κB p65 (1:1,000; cat. no. 8242; Cell Signaling Technology,
Inc.), p-p38 MAPK (1:1,000; cat. no. 4511; Cell Signaling
Technology, Inc.), p38 MAPK (1:1,000; cat. no. 8690; Cell Signaling
Technology, Inc.), GAPDH (1:5,000; cat. no. 10494-1-AP; Proteintech
Group, Inc.) and biotinylated Sambucus nigra (SNA) lectin
recognizing α2,6-linked sialic acid (1:2,000; cat. no. B-1305;
Vector Laboratories) at 4°C overnight. The membranes were then
incubated with secondary antibodies at RT, goat anti-rabbit
(1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.), goat
anti-mouse (1:5,000; cat. no. 7076; Cell Signaling Technology,
Inc.), or streptavidin-HRP (1:5,000; cat. no. 3999; Cell Signaling
Technology, Inc.). Protein visualization was carried out using a
chemiluminescence ECL Detection kit (NcmECL Ultra; cat. no. P10300;
NCM). The software used for densitometric analysis was ImageJ
software (version 1.53a; National Institutes of Health).
In situ fluorescence detection of
α2,6-linked sialic acid
The ICC cells treated with varying concentrations of
DT and the DT-treated ST6GAL1-overexpressing ICC cells were fixed
for 10 min with 4% paraformaldehyde at RT. Following fixation,
cells were blocked for 1 h using blocking buffer (cat. no. P0260;
Beyotime Institute of Biotechnology) for immunological staining at
RT. Next, the cells were incubated with Cy5 labeled SNA lectin
(cat. no. CL-1305-1; Vector Laboratories) overnight at 4°C. Each
step involved washing three times with PBS for 5 min. Finally, the
slides were sealed using an antifade mounting medium containing 1
µg/ml DAPI (cat. no. A4084; UElandy; http://www.uelandy.com/productDe_235.html); then
images were captured by a BZ-X800 microscope.
Flow cytometry
After harvesting ICC cells with ST6GAL1
overexpression treated with DT, the cells were blocked for 20 min
on ice using a carbo-free blocking solution (cat. no. SP-5040;
Vector Laboratories) at a volume of 0.2 ml for 1×106
cells. Subsequently, these cells were incubated with Cy5-labeled
SNA lectin (1 µl per 1×106 cells). Cells were analyzed
with a BD FACSCanto II Flow Cytometer (BD Biosciences). FlowJo
software (version 10.8.1; Becton Dickinson and Company) was used
for analysis.
Statistical analysis
GraphPad Prism (version 9; Dotmatics) was used to
conduct statistical analyses. Error bars in the experiments
represent SD. The legends provide information about the number of
events and independent experiments. Statistical comparisons between
two experimental conditions were conducted using the Mann-Whitney
test or unpaired Student's t-test, and the Wilcoxon rank-sum test
was used for all paired samples. A one-way ANOVA was used to
compare multiple experimental groups, followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Screening of monomeric compounds with
anti-ICC activity
High-throughput drug screening was performed to
evaluate the antitumor properties of TCM monomeric compounds in a
TCM Active Compound Library containing 2,538 compounds. The
screening aimed to assess the inhibitory effects of these compounds
on the viability of ICC. In the initial screening (Fig. S1A), 83 monomers were identified
that exhibited a growth inhibition rate >70% in HCCC9810 and RBE
cells (Fig. S1B). Through a
secondary screening, 64 monomers with consistent inhibitory
activity >70% were selected (Fig.
S1C). By assessing the viability of HiBEpiCs, 15 active
monomers were identified that showed inhibitory effects on ICC
without adverse effects on HiBEpiCs (Fig. S1D). DT emerged as a standout among
the 15 active compounds tested, showcasing potent anti-ICC cell
activity and promising therapeutic potential against ICC.
DT reduces the proliferation of ICC
cells
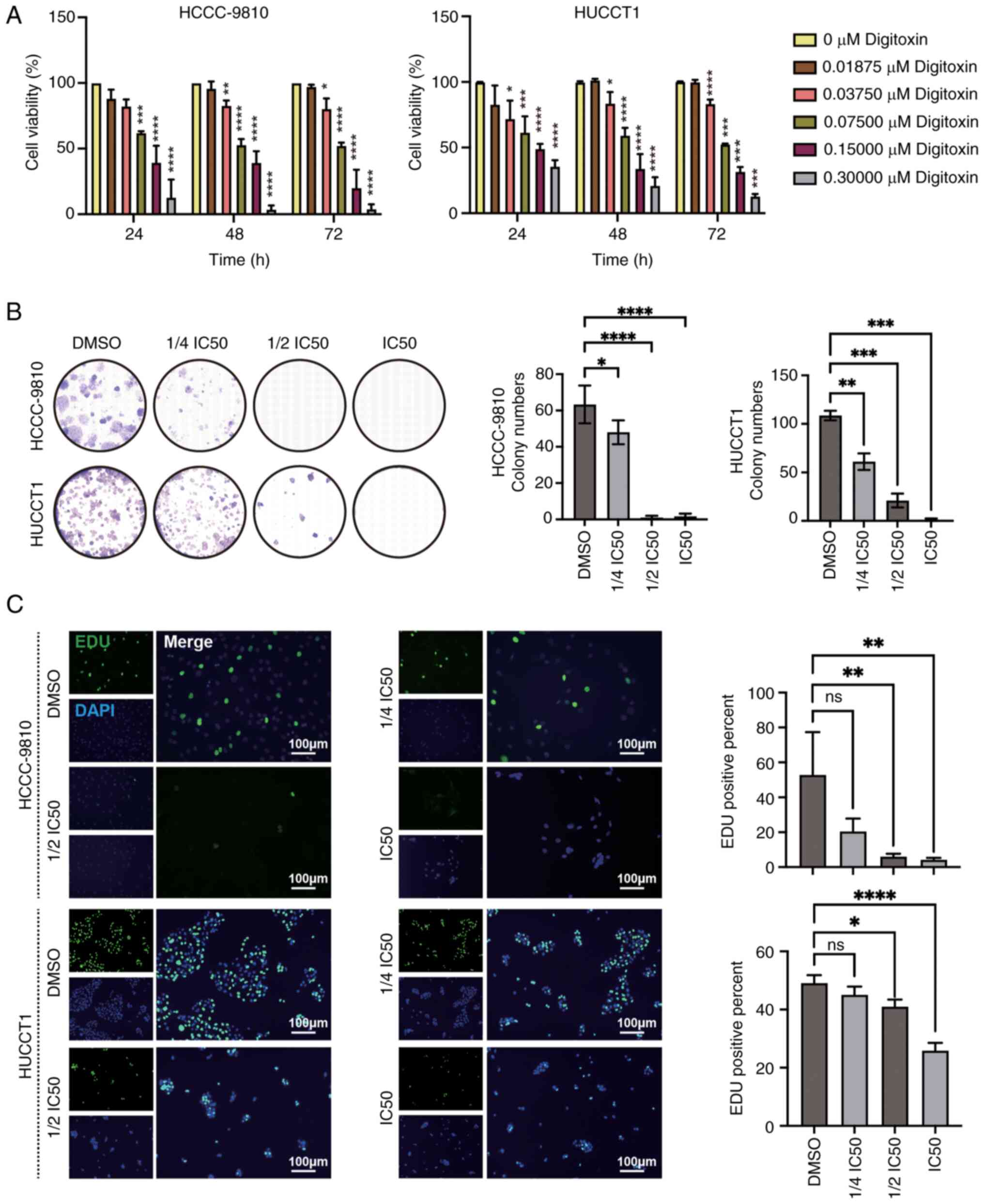
To assess the inhibitory effects of DT on ICC cells,
a CCK-8 assay was conducted which allowed to evaluate the impact of
varying concentrations and treatment durations of DT on cell
viability. The findings revealed a significant dose-dependent
reduction in the viability of both HuCCT1 and HCCC9810 cell lines
(Fig. 1A). More specifically, the
IC50 values for HCCC9810 cells after 24, 48 and 72 h of
DT treatment were 106.5±23.2, 92.4±12.1 and 77.9±10.9 nM,
respectively. For HuCCT1 cells, the corresponding IC50
values were 132.3±33.8, 105.1±26.6 and 98.5±4.2 nM, respectively,
indicating a progressive decrease in IC50 values, which
suggests an increase in drug sensitivity over time. Subsequently,
to further probe the effects of DT on cell proliferation, a series
of concentration gradients were applied: 0, 1/4 IC50,
1/2 IC50, IC50 of DT at 48 h (Table SI); and the proliferation capacity
of ICC cells was assessed at 24 h. Through the analysis of the
colony formation and EdU incorporation assays, it was observed that
DT significantly decreased the colony formation and proliferation
ability of HuCCT1 and HCCC9810 cells, demonstrating a
dose-dependent inhibition (Fig. 1B and
C). Taken together, these findings strongly suggest that DT
possesses the ability to hinder both the viability and
proliferation of ICC cells.
DT suppresses the migration of ICC
cells
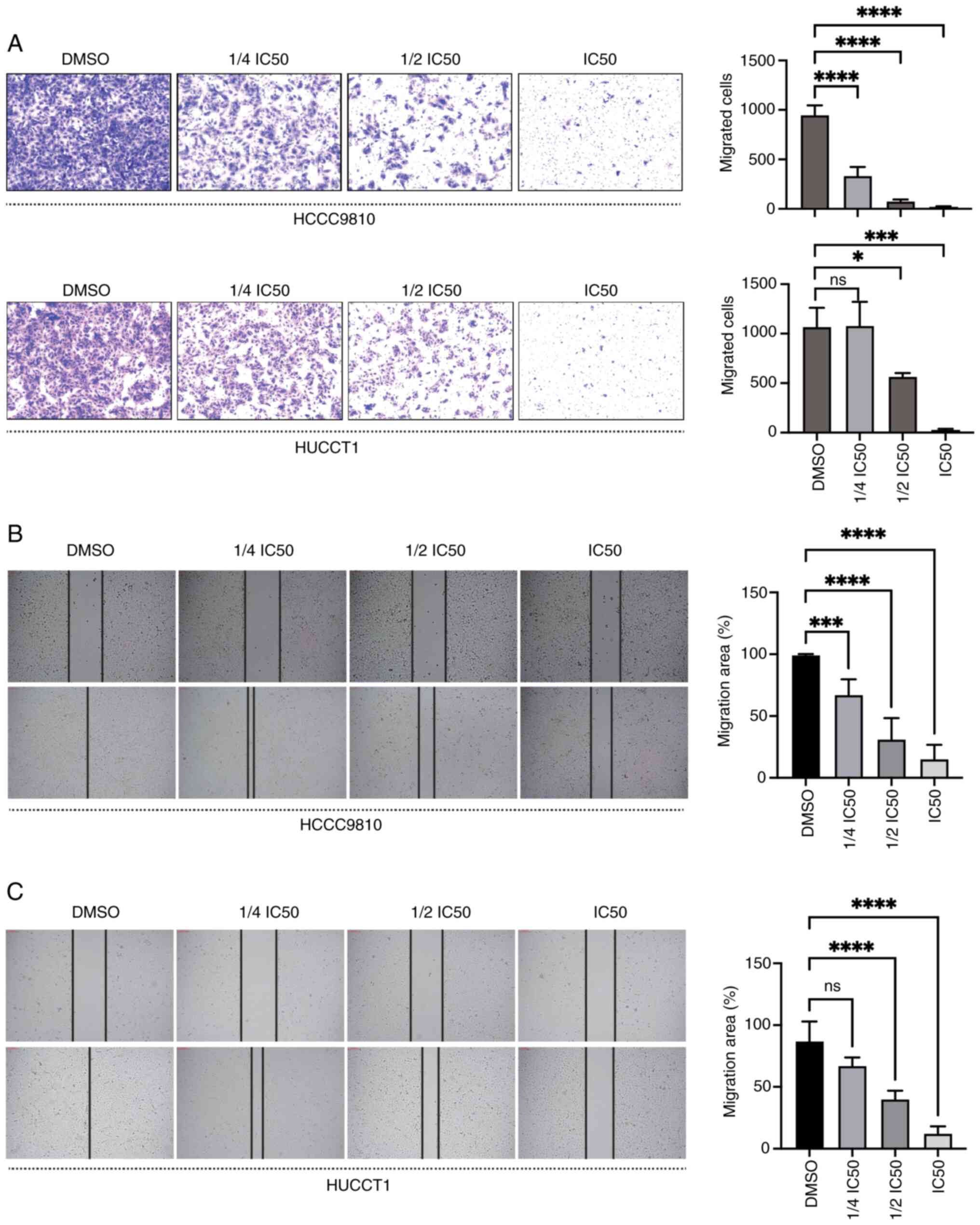
To explore the potential suppressive impact of DT on
the migration capabilities of ICC cells, the cells were exposed to
varying concentrations of DT, and wound healing and Transwell
assays were carried out. The Transwell assay results showed that DT
significantly decreased the migration of HuCCT1 and HCCC9810 cells
in a dose-dependent manner (P<0.05; Fig. 2A). Additionally, an increase in DT
concentration led to a decrease in the migration areas for both
HCCC9810 (Fig. 2B) and HuCCT1
(Fig. 2C) cells, underscoring a
progressive impediment to cell movement. Collectively, these
findings demonstrated the ability of DT to inhibit the migration of
ICC cells.
The NF-κB/ST6GAL1 pathway plays a
crucial role in the inhibitory effects of DT on ICC cells
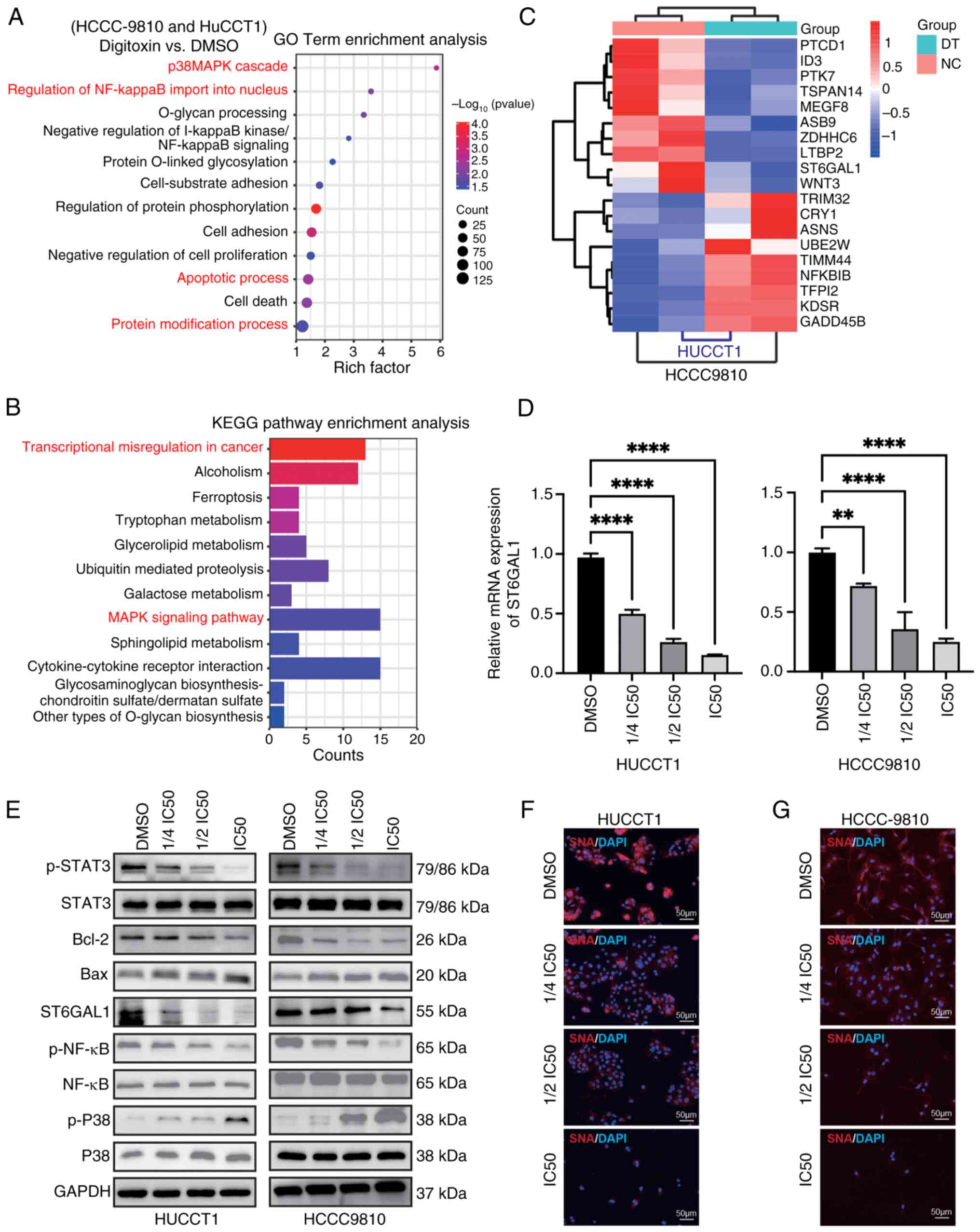
Subsequently, the primary signaling pathways through
which DT exerted its inhibitory effects on ICC cell functions were
investigated. The aim was to delineate the in vitro
molecular mechanisms underlying the suppression of ICC cell
activities with DT. Firstly, the intersection of differentially
expressed genes in HCCC9810 and HUCCT1 cells after treatment with
DT was selected, including both upregulated and downregulated
genes. Subsequently, GO and KEGG functional enrichment analyses
were conducted. RNA-seq analysis unveiled significant alterations
in pathways related to glycosylation, regulation of NF-κB imported
into the nucleus and transcriptional mis-regulation in ICC cells
following treatment with DT (Fig. 3A
and B). The heatmap of differentially expressed genes suggested
that DT could downregulate the transcriptional levels of ST6GAL1
(Fig. 3C). Furthermore, the RT-qPCR
results confirmed that DT could effectively inhibit the mRNA
expression of ST6GAL1 (Fig. 3D).
Next, the effects of DT on the NF-κB, P38 and STAT3 signaling
pathways, as well as ST6GAL1 expression were investigated. DT
treatment resulted in inhibition of phosphorylation of p-NF-κB and
p-STAT3, while it upregulated the phosphorylation of P38 and
reduced the expression of the anti-apoptotic protein BCL-2
(Fig. 3E; statistical charts,
Fig. S2). It was found that NF-κB
interacted with the promoter region of the ST6GAL1 gene, initiating
its transcription (16). To confirm
the effect of NF-κB on ST6GAL1, a P65/NF-κB overexpression plasmid
was constructed, transfected into 293T cells and followed by DT
treatment (Fig. S3). The results
indicated that overexpression of NF-κB promoted the expression of
ST6GAL1, and also demonstrated the inhibitory effect of DT on
ST6GAL1 in 293T cells. Finally, lectin blotting with SNA was used
which selectively adheres to α2,6-sialic acid structures to
evaluate the α2,6-sialylation levels in situ. A
dose-dependent reduction in α2,6-sialylation, predominantly
triggered by DT, was observed (Fig. 3F
and G). These findings collectively revealed the involvement of
the NF-κB/ST6GAL1 pathway in mediating the effects of DT in ICC
cells, underscoring its significance in the modulation of ICC cell
abilities.
ST6GAL1 counteracts the inhibitory
effects of DT on cell proliferation and migration
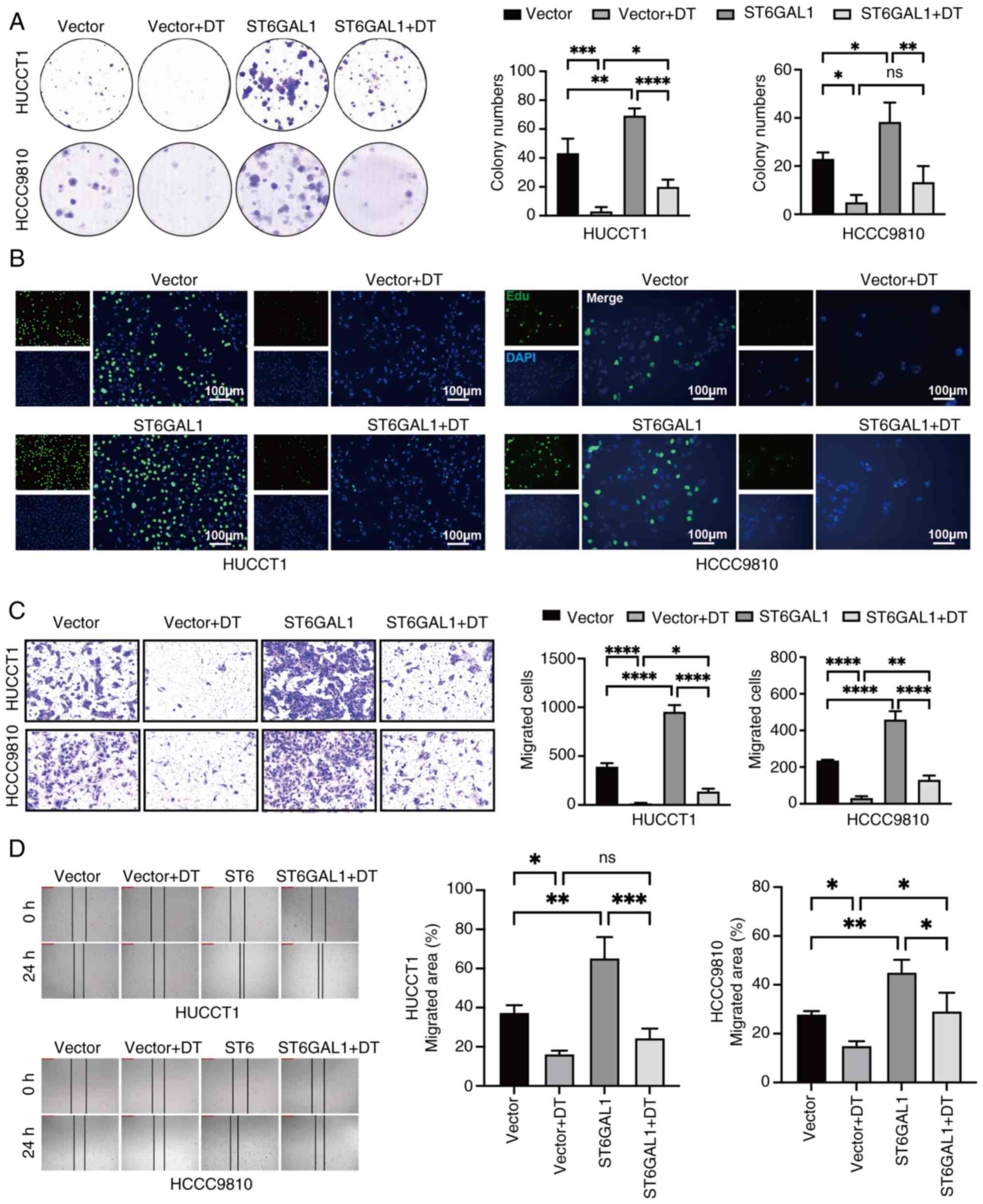
The aforementioned results revealed that DT reduced
the expression of ST6GAL1 in a dose-dependent manner. Consequently,
the ability of overexpressing ST6GAL1 to reverse the inhibitory
effects of DT on ICC cells was investigated.
ST6GAL1-overexpressing-ICC cells were generated by a lentivirus
vector system. The increased levels of ST6GAL1 in
ST6GAL1-overexpressing HCCC9810 and HuCCT1 cells were confirmed by
RT-qPCR and western blotting (Fig. S4A
and B). The colony formation and EdU assays provided compelling
evidence that overexpression of ST6GAL1 significantly promotes the
proliferation of ICC cells. These assays highlighted that ST6GAL1
could not only enhance cell proliferation, but also counteract the
suppression caused by DT (Fig. 4A and
B). Further investigation into the impact of ST6GAL1 on cell
migration was conducted using wound healing and Transwell migration
assays. The outcomes from these assays confirmed that ST6GAL1
overexpression not only augmented the migratory capabilities of ICC
cells, but also effectively neutralized the migration-inhibiting
influence of DT (Fig. 4C and D).
Collectively, these findings revealed that ST6GAL1 acted as a
potent promoter of both proliferation and migration of ICC cells,
effectively reversing the inhibitory effects imposed by DT
treatment.
DT inhibits ICC progression through
the NF-κB/ST6GAL1 signaling pathway
To ascertain the critical role of ST6GAL1 in the
signaling pathway through which DT inhibited ICC cells,
ST6GAL1-overexpressing HCCC9810 and HuCCT1 cells were treated with
DT at IC50 value. Western blotting results revealed that
DT inhibited the expression of the anti-apoptotic protein Bcl-2,
while overexpression of ST6GAL1 enhanced Bcl-2 expression and
partially reversed the inhibitory effect of DT. Moreover, DT was
shown to suppress ST6GAL1 expression in both control vector cells
and ST6GAL-overexpressing cells (Fig.
5A and B). The aforementioned results indicated that DT could
inhibit the phosphorylation of NF-κB, STAT3 and activate p-P38.
Consequently, nuclear proteins were next extracted to examine the
changes in nuclear p-NF-κB, p-STAT3 and p-P38 in ICC cells treated
with DT at the IC50 concentration. Western blotting
results suggested that DT could activate p-P38, and inhibit p-NF-κB
and p-STAT3 in whole cells (Fig. 5A and
B; statistical charts, Fig. S5A
and B), and further nuclear protein detection revealed an
increased expression of nuclear p-P38, and a decrease in p-NF-κB
and p-STAT3 (Fig. 5C and D;
statistical charts, Fig. S5C and
D). In addition, SNA lectin flow cytometry was employed to
evaluate the α2,6 sialylation levels in cells, with the aim of
exploring the enzymatic activity of ST6GAL1. The experimental
findings revealed that DT could reduce the α2,6 sialylation on the
cell membrane, and the overexpression of ST6GAL1 was able to
counteract the reduction effect induced by DT (Fig. 5E and F). These findings further
elucidated that DT can modulate the expression of ST6GAL1 by
impeding NF-κB activation, diminishing the nuclear presence of
p-NF-κB and ultimately suppressing ICC cell progression through the
downregulation of the effector protein Bcl-2.
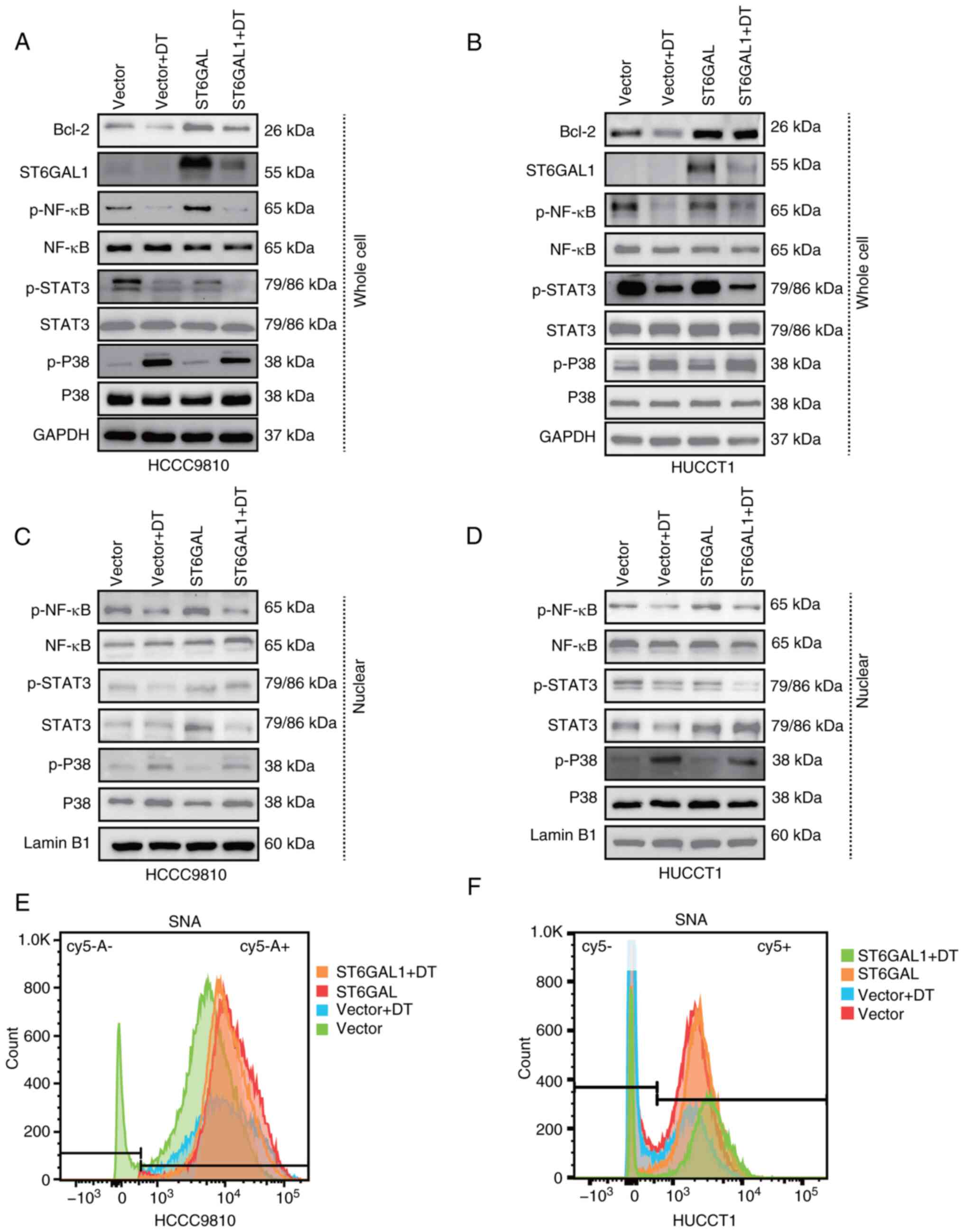 | Figure 5.ST6GAL1 overexpression counteracts
the signaling pathway involved in the DT-mediated effects in
intrahepatic cholangiocarcinoma cells. (A and B) Western blots
showing the protein profiles of ST6GAL1, Bcl-2, total STAT3,
p-STAT3, total P38, p-P38, total NF-κB and p-NF-κB in HCCC9810
cells treated with DT. (C and D) Western blots showing the protein
profiles of ST6GAL1, Bcl-2, total Akt, p-Akt, total P38, p-P38,
total NF-κB and p-NF-κB in HuCCT1 cells treated with DT. (E and F)
Flow cytometry was used to assess the levels of α2,6-sialylation in
the different treatment groups. ST6GAL1, ST6 β-galactoside
α-2,6-sialyltransferase 1; DT, digitoxin; p, phosphorylated. |
Discussion
TCM plays an important role in cancer prevention and
interception (17–19). TCM has long been used to maintain
health and treat ailments. Growing evidence supports the use of
TCM, alone or combined with other treatments, in reducing adverse
effects associated with chemotherapy, improving the quality of life
of patients, lowering the risk of recurrence and prolonging OS
(20). In recent years, researchers
have been increasingly interested in exploring potential anticancer
compounds from TCM. Nobiletin, a major component of Pericarpium
Citri Reticulatae, could inhibit cholangiocarcinoma (CCA)
proliferation by direct binding to GSK3β (21). β-elemene, a potent component found
in turmeric from the Chinese herbal medicine Rhizoma
Zedoariae, has the potential to halt the advancement of CCA
through the reactivation of PCDH9 expression (22). The screening results of the present
study showed that DT, bufalin, neriifolin and telocinobufagin
strongly suppressed ICC cell viability. DT, bufalin, neriifolin and
telocinobufagin share common characteristics as cardiotonic
steroids. They inhibit the plasma membrane
Na+/K+-ATPase pumps leading to increased
intracellular levels of Na+ and Ca+,
decreased K+ levels and enhanced cardiac contractile
force (23). DT was isolated from
the Digitalis species and features a steroid ring with a
five-carbon unsaturated butyrolactone moiety (24,25).
Bufadienolides such as bufalin, cinobufagin, telocinobufagin and
others, found in Venenum Bufonis from toad species, possess
a six-carbon unsaturated pyrone ring attached to the steroid ring
(26,27). Among them, only DT has been approved
by the FDA for specific cardiac conditions, particularly heart
failure and certain heart arrhythmias. Consequently, the focus of
the present study was on the possible therapeutic effects of DT on
ICC.
The current study presented evidence from cellular
functional assays indicating that DT effectively inhibited the
proliferation and migration of ICC cells in a dose-dependent
manner. Through GO and KEGG enrichment analyses, confirmed by
RT-qPCR and western blotting validation, it was established that DT
notably dampened the activation of the NF-κB signaling pathway,
including its nuclear transcription activities, impeded the
activation of p-STAT3 and activated the phosphorylation of P38.
This was consistent with prior studies highlighting the ability of
DT to inhibit p-STAT3 activation (28) and NF-κB (29). Additionally, while DT has been
revealed to inhibit IL-1β-induced activation of p44/42-MAPK and
NF-κB but not that of p38-MAPK in endothelial cells (30), the findings of the current study
revealed that DT could activate p38-MAPK in ICC cells. During
cellular stress, active p38 had been identified to phosphorylate
and induce the degradation of BCL2 (31). Taken together, these findings
revealed the role of DT as a comprehensive inhibitor of cancer
progression by modulating critical signaling pathways pivotal to
cancer cell growth and dissemination.
Sialylation is crucial in deciding cell destiny
throughout development, reprogramming and cancer progression
(32). The enzyme ST6GAL1, which
catalyzes the attachment of α2,6-linked sialic acid to specific
N-glycosylated proteins, either present on the cell membrane or
secreted into the bloodstream, has been found to be upregulated in
various cancers, where it promotes growth and invasiveness
(33). Elevated levels of ST6GAL1
have also been linked to increased resistance to chemoradiotherapy
in cancer models by inhibiting cell death (34). Furthermore, ST6GAL1 sourced from
external environments can mimic the effects of endogenous proteins,
fostering aggressive tumor proliferation and invasion (35). The present study demonstrated that
ST6GAL1 overexpression could enhance ICC cell proliferation and
migration, effectively countering the inhibition of proliferation
and migration of DT. The engagement of NF-κB with the ST6GAL1 gene
promoter initiates its transcription (16), a process inhibited by DT through the
inhibition of NF-κB phosphorylation and nuclear translocation,
leading to reduced ST6GAL1 mRNA and protein levels. Furthermore, it
was demonstrated that overexpressing P65/NF-κB in 293T cells
enhanced the expression of ST6GAL1. These findings indicated that
DT impeded the progression of ICC by curtailing ST6GAL1 expression
via the NF-κB pathway inhibition.
Despite DT being recognized as an essential drug by
the World Health Organization, its potent activity and narrow
therapeutic window pose significant challenges for clinical use
(36). Adults should typically have
therapeutic plasma DT value range of 10–30 ng/m (13.1–39.2 nM)
(37). However, within the
therapeutic concentration range of 25–40 nM, DT demonstrates no
marked adverse effects on normal cells, highlighting its potential
for safe application (38,39). It is crucial to monitor for possible
cardiac complications associated with DT, especially at serum
concentrations indicative of toxicity. Evidence showed that a serum
concentration range of 108–205 ng/ml (140–270 nM) might lead to DT
poisoning (10). However, the
current study found that lower concentrations, specifically at half
the IC50 value, were within a therapeutically effective
range that demonstrated a significant inhibitory impact on ICC
cells. Furthermore, previous studies have reported that DT can
produce anticancer effects at concentration range of 20–33 nM
without manifesting notable toxicity in patients with cardiac
issues (40,41). Notably, there is a lack of research
on the effects of DT on ICC. The findings of the current study
indicated that a low concentration of DT, specifically at 1/4
IC50 value (23 nM for HCCC9810 and 26 nM for HuCCT1) at
48 h, fell within an effective range for exerting significant
inhibitory effects on ICC cells. This observation was consistent
with the previous studies of the anticancer efficacy of DT at
concentrations that are not toxic. Furthermore, with a half-life
range of 5–8 days in the human serum, DT provides a foundation for
sustained tumor suppression (42).
In conclusion, DT, an effective anti-ICC compound,
was identified from a TCM library. It was demonstrated that DT
inhibited the progression of ICC by targeting ST6GAL1, a promoter
of ICC growth. The findings of the current study revealed that DT
downregulated ST6GAL1 at both the mRNA and protein levels in ICC
cells and impeded NF-κB activation, which was crucial for the
transcriptional activation of ST6GAL1. Therefore, the mechanism of
DT in inhibiting ICC cell proliferation and metastasis was mediated
through its interaction with the NF-κB/ST6GAL1 signaling pathway.
These results not only emphasized the potential role of DT as a
therapeutic agent for ICC, but also highlighted the critical
function of ST6GAL1 in cancer progression. Nonetheless, a
significant limitation of the present study was the lack of in
vivo experiments to confirm the effectiveness of DT in treating
ICC. Further investigation is necessary to comprehensively evaluate
the in vivo effect.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science
Foundation of China (grant no. 82372321), the Shanghai Shenkang
Hospital Development Center Project (grant no. SHDC2023CRT015) and
the Shanghai ‘Rising Stars of Medical Talent’ Youth Development
Program (grant no. 2024-70).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The RNA-seq data
generated in the present study may be found in the SRA database
under accession number PRJNA1112815 or at the following URL:
https://www.ncbi.nlm.nih.gov/sra/PRJNA1112815.
Authors' contributions
CG conceived and directed the project, and designed
the experiments. YZ, RW, CH, XXu, XXi, LW, JW and TL carried out
the experiments. YZ and RW conducted the data analysis and
interpreted the results. YZ and CG wrote and edited the manuscript,
and confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The requirement for the ethics approval of purchased
primary human cells was waived by the Ethics Committee of Yueyang
Integrated Traditional Chinese and Western Medicine Affiliated with
Shanghai University of Traditional Chinese Medicine in accordance
with Article 32 of the Chinese Measures for the Ethical Review of
Life Science and Medical Research Using Humans.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
TCM
|
Traditional Chinese Medicine
|
|
ST6GAL1
|
ST6 β-galactoside
α-2,6-sialyltransferase 1
|
|
DT
|
digitoxin
|
|
DMSO
|
dimethyl sulfoxide
|
|
OD
|
optical density
|
|
HiBEpiCs
|
intrahepatic bile duct epithelial
cells
|
|
EdU
|
5-ethynyl-2′-deoxyuridine
|
|
RNA-seq
|
RNA-sequencing
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
SNA
|
Sambucus nigra
|
|
CCK-8
|
Cell Counting Kit-8
|
|
OS
|
overall survival
|
|
CCA
|
cholangiocarcinoma
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beal EW, Tumin D, Moris D, Zhang XF,
Chakedis J, Dilhoff M, Schmidt CM and Pawlik TM: Cohort
contributions to trends in the incidence and mortality of
intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr.
7:270–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YT, Wang JJ, Luu M, Noureddin M,
Nissen NN, Patel TC, Roberts LR, Singal AG, Gores GJ and Yang JD:
Comparison of clinical features and outcomes between intrahepatic
cholangiocarcinoma and hepatocellular carcinoma in the United
States. Hepatology. 74:2622–2632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdel-Rahman O, Elsayed Z and Elhalawani
H: Gemcitabine-based chemotherapy for advanced biliary tract
carcinomas. Cochrane Database Syst Rev. 4:CD0117462018.PubMed/NCBI
|
|
5
|
Pellino A, Loupakis F, Cadamuro M,
Dadduzio V, Fassan M, Guido M, Cillo U, Indraccolo S and Fabris L:
Precision medicine in cholangiocarcinoma. Transl Gastroenterol
Hepatol. 3:402018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harvey AL, Edrada-Ebel R and Quinn RJ: The
re-emergence of natural products for drug discovery in the genomics
era. Nat Rev Drug Discov. 14:111–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trenti A, Zulato E, Pasqualini L,
Indraccolo S, Bolego C and Trevisi L: Therapeutic concentrations of
digitoxin inhibit endothelial focal adhesion kinase and
angiogenesis induced by different growth factors. Br J Pharmacol.
174:3094–3106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel S: Plant-derived cardiac glycosides:
Role in heart ailments and cancer management. Biomed Pharmacother.
84:1036–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YZ, Chen X, Fan XX, He JX, Huang J,
Xiao DK, Zhou YL, Zheng SY, Xu JH, Yao XJ, et al: Compound library
screening identified cardiac glycoside digitoxin as an effective
growth inhibitor of gefitinib-resistant non-small cell lung cancer
via downregulation of alpha-tubulin and inhibition of microtubule
formation. Molecules. 21:3742016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DH, Lee CS, Kim DW, Ae JE and Lee TH:
Digitoxin sensitizes glioma cells to TRAIL-mediated apoptosis by
upregulation of death receptor 5 and downregulation of survivin.
Anticancer Drugs. 25:44–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Feng LX, Sun P, Liu W, Mi T, Lei M,
Wu W, Jiang B, Yang M, Hu L, et al: Knockdown of Apolipoprotein E
Enhanced sensitivity of Hep3B cells to cardiac steroids via
regulating Na+/K+-ATPase signalosome. Mol Cancer Ther.
15:2955–2965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pollard BS, Suckow MA, Wolter WR, Starr
JM, Eidelman O, Dalgard CL, Kumar P, Battacharyya S, Srivastava M,
Biswas R, et al: Digitoxin inhibits
Epithelial-to-Mesenchymal-Transition in hereditary castration
resistant prostate cancer. Front Oncol. 9:6302019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan Q, Li M, Zhao W, Zhang K, Li M and Li
W: Hyper alpha2,6-sialylation promotes CD4(+) T-cell activation and
induces the occurrence of ulcerative colitis. Adv Sci (Weinh).
10:e23026072023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye L, Jia Y, Ji KE, Sanders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in the
prevention and treatment of cancer and cancer metastasis. Oncol
Lett. 10:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan Z, Lai Z and Lin J: Anticancer
properties of traditional Chinese medicine. Comb Chem High
Throughput Screen. 20:423–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu HA, Chen CH, Hsieh MH, Wu YC, Chiu JP,
Huang CJ and Hsu CH: The Benefit of Enhanced daycare of traditional
Chinese medicine for cancer treatment related adverse events: A
retrospective study of medical records. Integr Cancer Ther.
20:153473542110256342021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You L, Lin J, Yu Z, Qian Y, Bi Y, Wang F,
Zhang L, Zheng C, Zhang J, Li W, et al: Nobiletin suppresses
cholangiocarcinoma proliferation via inhibiting GSK3β. Int J Biol
Sci. 18:5698–5712. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Shi X, Pan Y, Liao X, Xu J, Gu X, Yu
W, Chen Y and Yu G: The chemopreventive role of β-Elemene in
cholangiocarcinoma by restoring PCDH9 expression. Front Oncol.
12:8744572022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JKT, Portbury S, Thomas MB, Barney S,
Ricca DJ, Morris DL, Warner DS and Lo DC: Cardiac glycosides
provide neuroprotection against ischemic stroke: Discovery by a
brain slice-based compound screening platform. Proc Natl Acad Sci
USA. 103:10461–10466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prassas I and Diamandis EP: Novel
therapeutic applications of cardiac glycosides. Nat Rev Drug
Discov. 7:926–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Botelho AFM, Pierezan F, Soto-Blanco B and
Melo MM: A review of cardiac glycosides: Structure, toxicokinetics,
clinical signs, diagnosis and antineoplastic potential. Toxicon.
158:63–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei WL, An YL, Zhang YZ, Li ZW, Zhou Y,
Lei M, Zhang JQ, Qu H, Da J, Wu WY and Guo DA: Quantitative
analysis of fourteen bufadienolides in Venenum bufonis crude
drug and its Chinese patent medicines by ultra-high performance
liquid chromatography coupled with tandem mass spectrometry. J
Ethnopharmacol. 251:1124902020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumavath R, Paul S, Pavithran H, Paul MK,
Ghosh P, Barh D and Azevedo V: Emergence of cardiac glycosides as
potential drugs: Current and future scope for cancer therapeutics.
Biomolecules. 11:12752021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mi C, Cao X, Ma K, Wei M, Xu W, Lin Y,
Zhang J and Wang TY: Digitoxin promotes apoptosis and inhibits
proliferation and migration by reducing HIF-1α and STAT3 in KRAS
mutant human colon cancer cells. Chem Biol Interact.
351:1097292022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eldawud R, Wagner A, Dong C, Gupta N,
Rojanasakul Y, O'Doherty G, Stueckle TA and Dinu CZ: Potential
antitumor activity of digitoxin and user-designed analog
administered to human lung cancer cells. Biochim Biophys Acta Gen
Subj. 1864:1296832020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jagielska J, Salguero G, Schieffer B and
Bavendiek U: Digitoxin elicits anti-inflammatory and vasoprotective
properties in endothelial cells: Therapeutic implications for the
treatment of atherosclerosis? Atherosclerosis. 206:390–396. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Whitaker RH and Cook JG: Stress relief
techniques: p38 MAPK determines the balance of cell cycle and
apoptosis pathways. Biomolecules. 11:14442021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li F and Ding J: Sialylation is involved
in cell fate decision during development, reprogramming and cancer
progression. Protein Cell. 10:550–565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scott E, Archer Goode E, Garnham R,
Hodgson K, Orozco-Moreno M, Turner H, Livermore K, Putri Nangkana
K, Frame FM, Bermudez A, et al: ST6GAL1-mediated aberrant
sialylation promotes prostate cancer progression. J Pathol.
261:71–84. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smithson M, Irwin R, Williams G, Alexander
KL, Smythies LE, Nearing M, McLeod MC, Al Diffalha S, Bellis SL and
Hardiman KM: Sialyltransferase ST6GAL-1 mediates resistance to
chemoradiation in rectal cancer. J Biol Chem. 298:1015942022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hait NC, Maiti A, Wu R, Andersen VL, Hsu
CC, Wu Y, Chapla DG, Takabe K, Rusiniak ME, Bshara W, et al:
Extracellular sialyltransferase st6gal1 in breast tumor cell growth
and invasiveness. Cancer Gene Ther. 29:1662–1675. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ambrosy AP and Gheorghiade M: Targeting
digoxin dosing to serum concentration: Is the bullseye too small?
Eur J Heart Fail. 18:1082–1084. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Belz GG, Breithaupt-Grogler K and Osowski
U: Treatment of congestive heart failure-current status of use of
digitoxin. Eur J Clin Invest. 31 (Suppl 2):S10–S17. 2001.
View Article : Google Scholar
|
|
38
|
Lopez-Lazaro M, Pastor N, Azrak SS, Ayuso
MJ, Cortes F and Austin CA: Digitoxin, at concentrations commonly
found in the plasma of cardiac patients, antagonizes etoposide and
idarubicin activity in K562 leukemia cells. Leuk Res. 30:895–898.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bavendiek U, Berliner D, Dávila LA, Schwab
J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber
K, et al: Rationale and design of the DIGIT-HF trial (DIGitoxin to
Improve ouTcomes in patients with advanced chronic Heart Failure):
A randomized, double-blind, placebo-controlled study. Eur J Heart
Fail. 21:676–684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lopez-Lazaro M, Pastor N, Azrak SS, Ayuso
MJ, Austin CA and Cortes F: Digitoxin inhibits the growth of cancer
cell lines at concentrations commonly found in cardiac patients. J
Nat Prod. 68:1642–1645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daniel D, Susal C, Kopp B, Opelz G and
Terness P: Apoptosis-mediated selective killing of malignant cells
by cardiac steroids: Maintenance of cytotoxicity and loss of
cardiac activity of chemically modified derivatives. Int
Immunopharmacol. 3:1791–1801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bohmer T and Roseth A: Prolonged digitoxin
half-life in very elderly patients. Age Ageing. 27:222–224. 1998.
View Article : Google Scholar : PubMed/NCBI
|