Breast cancer (BC) is the most common malignancy
among women worldwide, with an heterogeneous nature resulting from
various risk factors, such as endogenous and exogenous estrogen
exposure, lifestyle choices, dietary habits and exposure to toxic
environmental elements, such as heavy metals and chemicals
(1,2). In total, ~15% of BC cases can be
attributed to genetic susceptibility and genetic factors (3). Notably, research has revealed a
substantial discrepancy in the 5-year survival rates between
patients with advanced invasive BC (representing 24% of cases with
distant metastasis) and those with early-stage BC, with a 99%
survival rate (4). In addition,
some patients with cancer may benefit from monotherapies, such as
hormone therapy, immunotherapy or chemotherapy; however, the
effectiveness of these therapies may diminish over time, and some
patients may become resistant (5).
Thus, the development of novel therapeutic targets is crucial for
the treatment of BC.
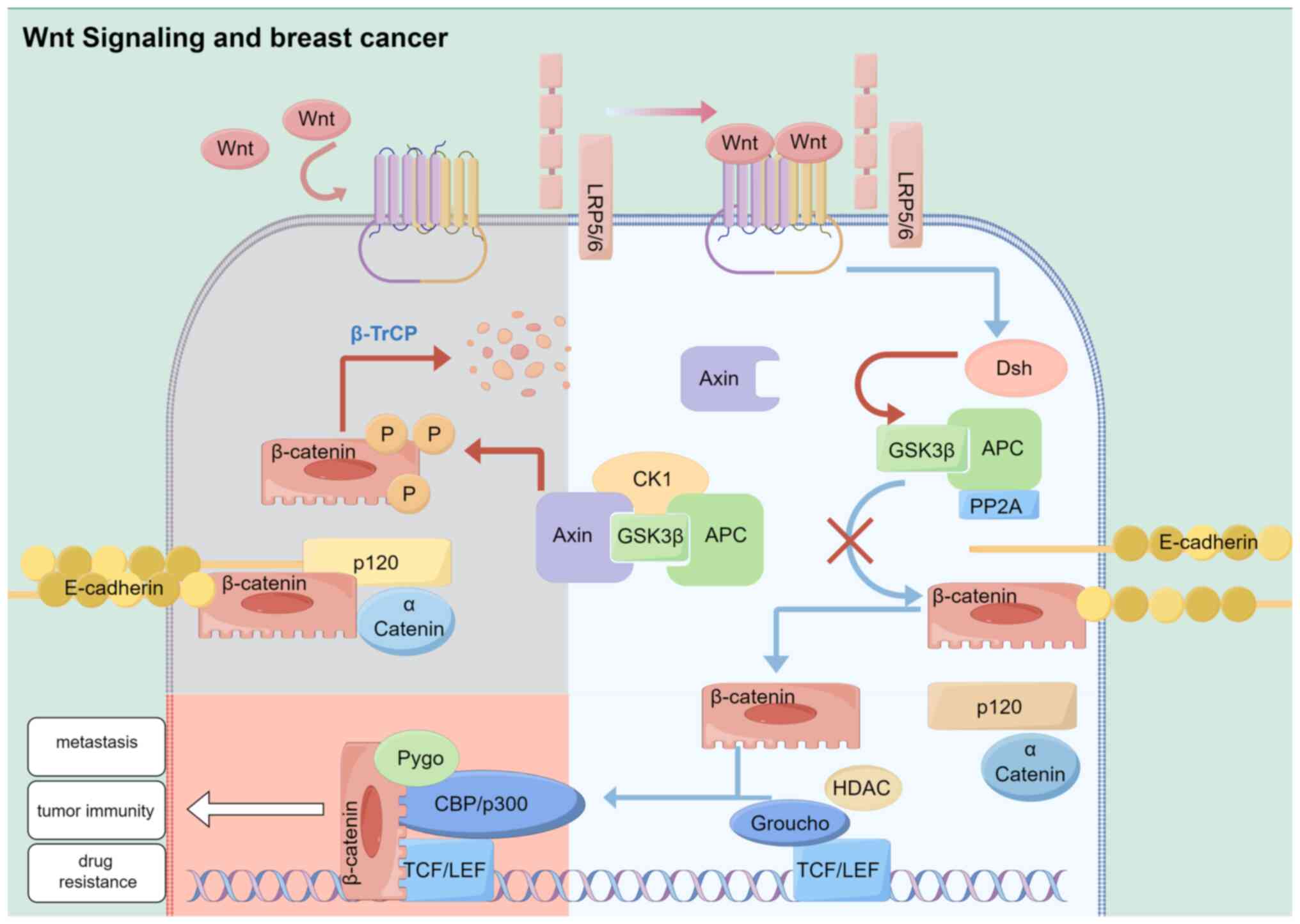
In addition to regulating cell proliferation and
cell fate during embryonic development and tissue homeostasis, Wnt
signaling determines cell polarity (6,7).
Notably, Wnt is a secreted glycoprotein within this pathway
(8). Diverse intracellular
signaling pathways may be activated by these interactions, which
may intersect or function independently (9). Collectively, an integrin gene, Int-1,
and a segmental polarity gene, Wingless, form the term ‘Wnt’
(10,11). Wnt-1, formerly known as the Int-1
oncogene, has been recognized as the integration site for the mouse
mammary tumor virus (MMTV) (10).
In addition, BC was the first tumor to be associated with Wnt
signaling. Results of previous studies demonstrated that Wnt
signaling is involved in multiple aspects of cancer, including
proliferation (12,13), metastasis (14,15),
immune regulation (16,17), therapeutic resistance (18) and phenotype shaping (19). Moreover, a number of inhibitors
targeting components of the Wnt signaling pathway exhibit potential
in the treatment of cancer (20).
Thus, the present review demonstrated the interaction between
exosomal microRNAs (miRNAs) and proteins in signaling cascades,
demonstrating their role in BC and in mechanisms of the Wnt
signaling pathway.
Exosomes are nano-sized vesicle structures ranging
from 50–150 nm. These are derived from endosomes and are present in
diverse tumor cells (21,22). Numerous proteins and lipids are
present in these vesicles, in addition to miRNA (23). A variety of model systems have been
used to demonstrate that cancer cells secrete extracellular
vesicles, and these subsequently metastasize from primary tumors to
the circulatory system (24).
Research focused on exosomal miRNAs have demonstrated their role in
cellular and molecular biology (25). Notably, miRNAs are endogenous small
RNAs that are 20–24 nucleotides in length. In tumor cells, miRNAs
play a crucial regulatory role in various signaling pathways within
exosomes (26). Cancer-specific
exosomal miRNAs exhibit distinct expression patterns and play a
significant role in the progression of the disease, highlighting
their potential as biomarkers of cancer (27). Notably, BC tissue expresses altered
exosomal miRNAs both before and after invasion (28,29).
Thus, through studying the functional role of exosomal miRNA in BC,
a novel theoretical basis may be developed to understand its
etiology.
Results of previous studies demonstrated that
activation or silencing of the Wnt signaling pathway impact
epithelial-mesenchymal transition (EMT)-dependent metastasis, the
immune microenvironment and the resistance of BC (52–54).
Notably, cells undergo EMT during the differentiation of epithelial
cells into mesenchymal cells (55).
EMT is an important feature of BC, and plays a key role in triple
negative BC (56). Thus, research
is focused on regulating EMT for the treatment of BC (57). Li et al (58) demonstrated that activation of Wnt
signaling enhanced the invasion and metastasis of BC (58). Notably, a number of intrinsic
EMT-transcription factors (TFs) are mechanically activated by
Wnt/catenin signaling, epidermal growth factor (EGF)/fibroblast
growth factor (FGF)-receptor tyrosine kinase signaling and Notch
signaling. These pathways also initiate changes in the expression
of genes, including E-cadherin and ZO-1. In addition, the
aforementioned pathways may activate N-Cadherin, MMPs, integrins
and fibronectin (59). EMT-TFs
expressed by Wnt regulate BC morphogenesis, including lamellipodia
formation, and directly secrete MMPs, resulting in their migration
and invasion (60). Results of a
previous study revealed that Wnt/β-catenin signaling suppresses
antitumor immunity (61). A
cancerous breast cell that expresses Wnt signaling may develop
strategies to avoid being recognized and destroyed by the immune
system (62). To prevent
phagocytosis by macrophages, BC cells express CD24 and CD47 through
interactions with Siglec-10 and SIRP-α, respectively (63,64).
Notably, CD24 directly targets Wnt1, while CD47 indirectly targets
SNAI1 and ZEB1 through Wnt signaling in BC (65,66).
Therefore, Wnt signaling plays a key role in the immune
microenvironment of BC. Metastatic BC is characterized by frequent
changes in the TP53 gene (67). The
loss of TP53 in BC cells trigger the secretion of Wnt1, Wnt6 and
Wnt7a (31). These proteins bind to
Fzd7 and Fzd9 on the surface of TAM, stimulating the production of
IL-1 by TAMβ. Results of a previous study revealed that mutations
in TP53 may lead to drug resistance in BC (68). For example, TP53 mutations are
associated with endocrine therapeutic resistance in early luminal
BC (69). Wnt-driven systemic
inflammation and immunosuppression niches are associated with BC
multidrug resistance. Cancer resistance is considered a
multifaceted issue, involving tumor heterogeneity, drug
efflux/inactivation and activation of survival pathways (70). Results of a previous study
demonstrated that inactivation of Wnt signaling leads to BC
entering a drug insensitive resting state (71), leading to multidrug resistance.
Thus, Wnt signaling is a dynamic and multifaceted process in BC,
and Wnt signaling may exhibit potential as a target in the
treatment of BC.
The non-canonical Wnt pathways include the Wnt-PCP
signaling pathway and the Wnt-Ca2+ signaling pathway.
Genetically engineered mice exhibit increased distant metastasis
and collective cell migration following Vangl-dependent Wnt-PCP
signaling (72). In basal BC,
overexpression of the Wnt/PCP protein, VANGL2, is associated with a
poor prognosis and an increase in tumor size (73). In addition, results of a previous
study revealed that exosomes released by fibroblasts activate
Wnt-PCP signaling to drive BC cell invasion (14). Secreted fried-associated protein 2
(SFRP2) is overexpressed in the blood vessels of 85% of human
breast tumors, and SFRP2 promotes tumor angiogenesis through the
Wnt-Ca2+ pathway (74).
At present, research is focused on the non-canonical Wnt pathway in
BC, and this pathway exhibits potential in further understanding
the pathogenesis of BC.
Exosomes are membrane-bound microvesicles that range
from 30–150 nanometers in size, and these are secreted into the
extracellular environment by all cells, including prokaryotes and
eukaryotes. Exosomes contain a diverse array of miRNAs, mRNA,
proteins, lipids and other substances (75). They play a crucial role in
facilitating intercellular material exchange and information
transmission. miRNAs accelerate mRNA degradation or inhibit mRNA
translation through interactions with the 3′-untranslated region of
target mRNAs. This regulation of post-transcriptional gene
expression in recipient cells has been observed in various models
(23). Abnormal expression or
mutations in miRNAs are associated with a range of tumors,
including BC, where they function as oncogenes or tumor suppressors
(76,77). However, miRNAs are inherently
unstable. Exosomes, with a phospholipid bilayer membrane structure,
provide stability to miRNAs via protection from enzymatic
degradation (78). Therefore,
exosomal miRNAs are valuable for understanding the pathogenesis of
malignant tumors. Extensive research has demonstrated that exosomal
miRNAs exhibit potential as biomarkers and therapeutic targets in
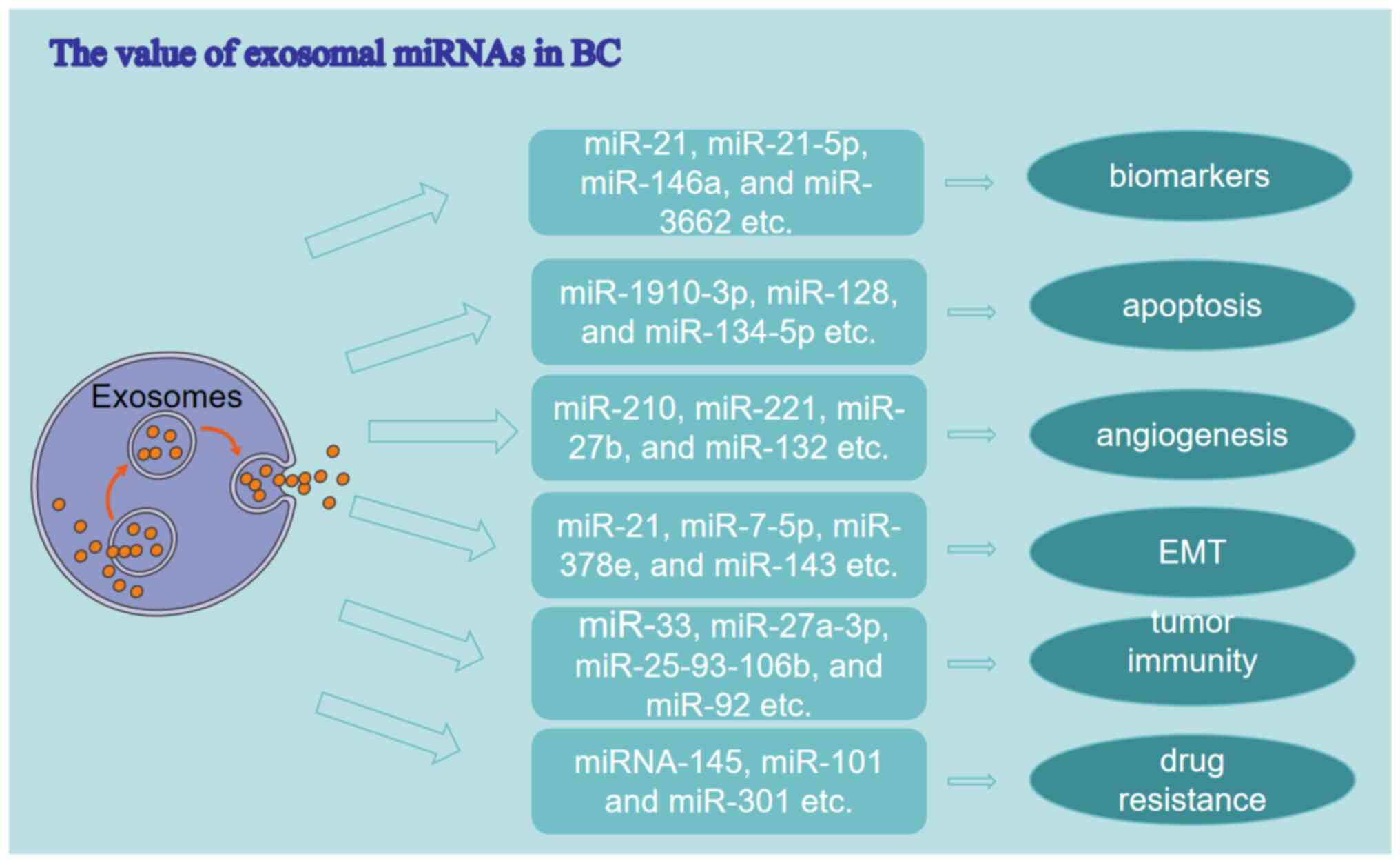
BC (Fig. 2).
A biomarker quantifies a normal biological or
pathological process, or an impact of a therapeutic intervention
(79). Exosomal miRNAs exhibit
potential as biomarkers in the prediction, diagnosis and prognosis
of BC (80). The present study
demonstrated that serum exosomal miR-21 may be used as a biomarker
for the early detection and diagnosis of BC (81). Moreover, the plasma exosomal
miR-21-5p has also been identified as a potential biomarker for the
diagnosis of BC (82). Li et
al (83) demonstrated that
exosomes miR-3662, miR-146a and miR-1290 exhibit potential in
predicting disease, and these may be used as biomarkers for
diagnosis and treatment. Through monitoring exosomal miRNAs, BC
occurrence, recurrence, prognosis and responses to common therapies
can be predicted.
Apoptosis plays a key role in promoting tumor
occurrence. Exosomal miRNAs play a significant role in the
apoptosis of BC cells, exhibiting abnormal expression patterns in
patients (84). Notably, BC cells
release exosomes containing miR-1910-3p, which inhibits apoptosis
and facilitates tumor development through the transfer of
miR-1910-3p to target cells (85).
In addition, Wei et al (86)
revealed that miR-128 is specifically sorted into exosomes and
enhances the proliferation of MCF-7 cells through targeting the Bax
gene. Bax plays a key role in the inhibition of apoptosis. A
previous study identified exosomal miR-134-5p as a potential
therapeutic target for BC, as it promotes apoptosis through
inhibiting the PI3K/AKT pathway (86).
In hypoxic environments, the secretion of exosomal
miRNAs by cancer cells is upregulated to modulate tumor
angiogenesis, a critical and dynamic process in the progression of
tumorigenesis (87). Exosomal
miR-210 enhances angiogenesis through the modulation of vascular
remodeling-associated genes, Ephrin A3 and PTP1B, consequently
influencing the development of BC and the dissemination of hypoxic
BC cells to adjacent tissues (88).
Results of previous studies demonstrated that exosomal miRNAs play
a significant role in promoting vascular survival through the
regulation of FGF, vascular endothelial growth factor (VEGF), EGF
and angiopoietin-1, ultimately contributing to the progression and
metastasis of BC (89,90). In patients with BC, miR-221, miR-27b
and miR-132 enhance angiogenesis through modulating the angiogenic
properties of VEGF (91,92). However, several exosomal miRNAs have
been identified as inhibitors of tumor angiogenesis. Notably,
exosomal miR-16 inhibits angiogenesis in BC cells through the
direct regulation of VEGF expression (93). Overall, exosomal miRNAs play a role
in modulating the progression of BC via angiogenesis.
EMT facilitates the acquisition of metastatic
capabilities by epithelial cells, serving as a crucial prerequisite
for metastasis. Results of a previous study demonstrated that
exosomes released by CAFs transfer specific miRNAs, including
exosomal miR-21, miR-378e and miR-143 to BC cells, promoting EMT
characteristics (94). Yan et
al (95) further elucidated
that CAF-derived exosomal miR-18b induces EMT, invasion and
metastasis in BC through targeting TCEAL7 to activate the NF-κB
signaling pathway. Wang et al (96) demonstrated that exosomal miR-181d-5p
derived from CAFs promotes the proliferation, invasion, migration
and EMT of BC cells through regulating CDX2 and HOXA5 genes
(96). Thus, targeting CAF-derived
exosomal miR-18b and miR-181d-5p may exhibit potential in the
treatment of BC. In addition, miR-103-107 may inhibit miRNA
biosynthesis through targeting the Dicer gene in BC, resulting in
enhanced EMT and metastatic characteristics in epithelial tumor
cells (97).
Moreover, exosomal miRNAs exhibit anticancer
properties in BC through the inhibition of EMT. For example,
miR-34a, a transcriptional target of p53, suppresses the
aggressiveness of BC cells through targeting EMT and the zinc
finger transcriptional inhibitor, Snail (98). In addition, exosomal miR-16-5p
attenuates EMT via downregulation of EPHA1 and NF-κB signaling
pathways, ultimately impeding the proliferation, invasion and
migration of BC cells (99).
Moreover, miR-7-5p exhibits differential expression levels in
various invasive BC cell lines. miR-7-5p inhibits EMT through
targeting RYK and decreasing the phosphorylation of JNK, thereby
reducing the metastasis of BC (100).
Exosomal miRNAs play a significant role in mediating
the communication between BC cells and immune cells, thereby
influencing immune regulation. Results of previous studies
demonstrated the involvement of exosomal miRNAs in modulating the
polarization of macrophages and the secretion of proinflammatory
cytokines (101,102). Specifically, M1 macrophages
exhibit a pro-inflammatory phenotype, characterized by the
expression of cytokines, such as IL-12. This may contribute to the
destruction of cancer cells. On the other hand, M2 macrophages
produce anti-inflammatory cytokines, such as IL-10, thus promoting
tumor progression (103). BC is
characterized by the presence of tumor-associated macrophages
(TAMs), predominantly displaying the M2 phenotype (104). Results of previous studies
demonstrated that BC-derived exosomal miR-16 and miR-33 inhibit the
M1 polarization of TAMs, through suppressing the expression of
epigenetic factors, while simultaneously stimulating M2
polarization (102,105). This mechanism ultimately
facilitates the advancement of metastatic BC. Previous research has
focused on the miRNA-mediated inhibition of mRNA translation in the
regulation of endoplasmic reticulum stress and immune evasion in
human tumors. Specifically, in the context of endoplasmic reticulum
stress, BC exosomes containing miR-27a-3p, miR-25-93-106b and
miR-92 enhance the expression of PD-L1 in macrophages, thereby
facilitating immune evasion (106,107). These findings suggest that
targeting exosomal miRNAs may exhibit potential in the treatment of
BC.
Primary breast tumors often respond well to initial
treatment, and develop drug resistance after a few months (70). At present, research is focused on
exosomal miRNA-mediated drug resistance mechanisms in BC cells
(108). Results of a previous
study highlighted that modulation of exosome miRNA expression may
impact the responsiveness of BC cells to hormonal treatments,
targeted therapies and chemotherapeutic agents via diverse
signaling pathways (109). BC is
categorized according to hormone receptor expression, with ~70% of
BC cases being labelled as estrogen receptor-positive. These cases
are treated with anti-estrogen drugs, such as tamoxifen or
fluoxetine; however, cells may develop resistance. Notably,
exosomal miR-101 and miR-301 may cause BC cells to become resistant
to tamoxifen, through the inhibition of PTEN (110). Exosomal miR-221 and miR-222
increase the therapeutic resistance of sensitive MCF7 BC cells to
tamoxifen through downregulation of p27 and ERα targets. Exosomal
miR-221/222 also promote BC cell resistance to fluoxetine through
dysfunction of TGF-β and β-catenin signaling networks; thus,
impacting the survival of patients with ER-positive BC (111,112). Results of a previous study
revealed a high correlation between changes in exosomal miRNA
expression and adriamycin resistance. For example, upregulation of
miR-145 may sensitize BC to doxorubicin chemotherapy (113). In conclusion, exosomal miRNAs may
exhibit potential as targets for increasing the chemosensitivity of
BC.
Exosomal miRNAs may impact various aspects of tumor
progression through regulation of Wnt signaling. Patients with
recurring BC exhibited significantly lower levels of exosomal
miR-18a-5p (114). Results of a
recent study demonstrated that exosomal miR-18a-5p promotes the EMT
and metastasis of nasopharyngeal carcinoma cells through activating
the Wnt/β-catenin signaling pathway (115). In addition, the migration and
invasion of BC cells are promoted via the downregulation of
exosomal miR-7-5p through WNT signaling (100). In addition, exosomal miR-1260b
promotes cell invasion through the Wnt/β-catenin signaling pathway
(116). miRNA-1260b also plays a
role in promoting tumor invasion in BC (117). Exosomal miR-10527-5p inhibits
migration, invasion, lymphangiogenesis and lymphatic metastasis via
Wnt/β-catenin signaling (118). In
cancer cells, exosome-derived miR-375 targets DIP2C and regulates
Wnt signaling, thus promoting osteoblastic metastasis (119). Li et al (120) revealed that exosomal miR-92a
promotes cytarabine resistance through activating the Wnt/β-catenin
signaling pathway (120). In
conclusion, exosomal miRNAs may influence tumor progression through
regulating Wnt signaling.
Results of previous studies highlighted that
activation of Wnt/β-catenin signaling may impact the expression of
exosomal miR-301a and promote resistance to radiation (121,122). In addition, Wnt signaling impacts
the release of exosomal miR-454 to maintain the biological
properties of cancer stem cells through BC cells (123). Exosomal miR-1323 is involved in
cervical cancer progression and resistance to radiation, which may
exhibit potential in the treatment of cervical cancer (124). In addition, the chemosensitivity
of bladder cancer cells was increased via Wnt/β-catenin
pathway-mediated downregulation of exosomal miR-148b-3p in CAFs
(125). In conclusion, the release
of miRNA in exosomes may be influenced by the activation or
deactivation of Wnt signaling. Thus, regulation of Wnt signaling
may regulate the expression of exosomal miRNAs in BC.
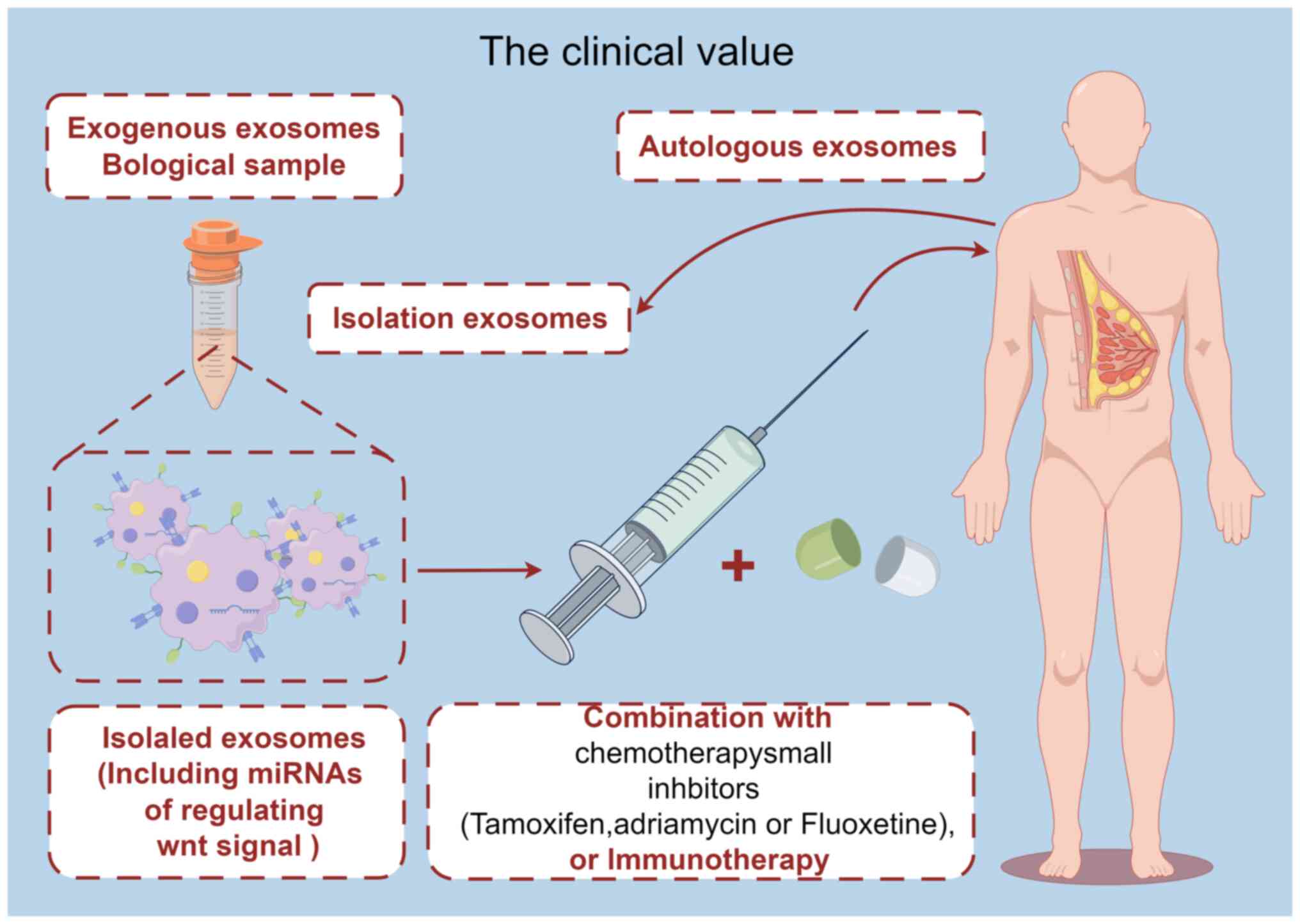
At present, research is focused on the role of
exosomal miRNAs in BC. Results of previous studies highlighted the
involvement of multiple exosomal miRNAs in various aspects of BC
progression, including apoptosis regulation, cell metastasis, tumor
immunity, drug resistance and modulation of Wnt signaling pathways.
Thus, targeting multiple biological processes of exosomal miRNAs
may exhibit potential in the treatment of BC (Fig. 3). At present, the use of exosomal
miRNAs is limited due to difficulties in batch isolation and the
extraction of exosomes. However, further investigations into the
role of exosomal miRNAs and Wnt signaling pathways in BC may
enhance the current understanding of the pathogenesis of BC, and
aid in the development of exosomal miRNA-based therapies. In
conclusion, exosomal miRNAs and Wnt signaling exhibit potential as
effective diagnostic and therapeutic targets for BC. Further
investigations into the regulatory mechanisms of exosomal miRNAs in
Wnt signaling are required for the development of novel diagnostic
and therapeutic targets in BC.
Not applicable.
Funding: No funding was received.
Not applicable.
HL organized the manuscript and produced the
figures. XL completed the exosome section of the manuscript. WD
provided the outline of the present review and completed the
‘Conclusions’ section of the manuscript. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hachey SJ, Hatch CJ, Gaebler D, Mocherla
A, Nee K, Kessenbrock K and Hughes CCW: Targeting tumor-stromal
interactions in triple-negative breast cancer using a human
vascularized micro-tumor model. Breast Cancer Res. 26:52024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abeni E, Grossi I, Marchina E, Coniglio A,
Incardona P, Cavalli P, Zorzi F, Chiodera PL, Paties CT, Crosatti
M, et al: DNA methylation variations in familial female and male
breast cancer. Oncol Lett. 21:4682021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDonald ES, Clark AS, Tchou J, Zhang P
and Freedman GM: Clinical diagnosis and management of breast
cancer. J Nucl Med. 57 (Suppl 1):9S–16S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rim EY, Clevers H and Nusse R: The wnt
pathway: From signaling mechanisms to synthetic modulators. Annu
Rev Biochem. 91:571–598. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Lin X, Wei L, Wu Y, Xu L, Wu L,
Wei X, Zhao S, Zhu X and Xu F: A framework for Frizzled-G protein
coupling and implications to the PCP signaling pathways. Cell
Discov. 10:32024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Ma F, Arai S, Wang Y, Varkaris A,
Poluben L, Voznesensky O, Xie F, Zhang X, Yuan X and Balk SP: WNT5a
signaling through ROR2 activates the hippo pathway to suppress YAP1
activity and tumor growth. Cancer Res. 83:1016–1030. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neiheisel A, Kaur M, Ma N, Havard P and
Shenoy AK: Wnt pathway modulators in cancer therapeutics: An update
on completed and ongoing clinical trials. Int J Cancer.
150:727–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Ooyen A and Nusse R: Structure and
nucleotide sequence of the putative mammary oncogene int-1;
proviral insertions leave the protein-encoding domain intact. Cell.
39:233–240. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wend P, Runke S, Wend K, Anchondo B,
Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, LesniakM
S, et al: WNT10B/β-catenin signalling induces HMGA2 and
proliferation in metastatic triple-negative breast cancer. EMBO Mol
Med. 5:264–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katkat E, Demirci Y, Heger G, Karagulle D,
Papatheodorou I, Brazma A and Ozhan G: Canonical Wnt and TGF-β/BMP
signaling enhance melanocyte regeneration but suppress
invasiveness, migration, and proliferation of melanoma cells. Front
Cell Dev Biol. 11:12979102023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harper KL, Sosa MS, Entenberg D, Hosseini
H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis
RJ, et al: Mechanism of early dissemination and metastasis in
Her2(+) mammary cancer. Nature. 540:588–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malladi S, Macalinao DG, Jin X, He L,
Basnet H, Zou Y, de Stanchina E and Massagué J: Metastatic latency
and immune evasion through autocrine inhibition of WNT. Cell.
165:45–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung CON, Yang Y, Leung RWH, So KKH, Guo
HJ, Lei MML, Muliawan GK, Gao Y, Yu QQ, Yun JP, et al:
Broad-spectrum kinome profiling identifies CDK6 upregulation as a
driver of lenvatinib resistance in hepatocellular carcinoma. Nat
Commun. 14:66992023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piva M, Domenici G, Iriondo O, Rábano M,
Simões BM, Comaills V, Barredo I, López-Ruiz JA, Zabalza I, Kypta R
and Vivanco M: Sox2 promotes tamoxifen resistance in breast cancer
cells. EMBO Mol Med. 6:66–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang
Q, Lin Y, Li J, Kang T, Tao M, et al: Disrupting the interaction of
BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like
breast cancer. Cancer Cell. 25:210–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen XJ, Guo CH, Wang ZC, Yang Y, Pan YH,
Liang JY, Sun MG, Fan LS, Liang L and Wang W: Hypoxia-induced ZEB1
promotes cervical cancer immune evasion by strengthening the
CD47-SIRPα axis. Cell Commun Signal. 22:152024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu X, Odenthal M and Fries JW: Exosomes as
miRNA carriers: Formation-function-future. Int J Mol Sci.
17:20282016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Sun HT, Wang S, Huang SL, Zheng Y,
Wang CQ, Hu BY, Qin W, Zou TT, Fu Y, et al: Isolation and
characterization of exosomes for cancer research. J Hematol Oncol.
13:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Han Y, Meng Y and Yin L: Small
RNA-big impact: Exosomal miRNAs in mitochondrial dysfunction in
various disease. RNA Biol. 21:1–20. 2024. View Article : Google Scholar
|
|
26
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lakshmi S, Hughes TA and Priya S: Exosomes
and exosomal RNAs in breast cancer: A status update. Eur J Cancer.
144:252–268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Jin LJ and Zhang XY: Exosomal
miRNA-205 promotes breast cancer chemoresistance and tumorigenesis
through E2F1. Aging (Albany NY). 13:18498–18514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scognamiglio I, Cocca L, Puoti I, Palma F,
Ingenito F, Quintavalle C, Affinito A, Roscigno G, Nuzzo S,
Chianese RV, et al: Exosomal microRNAs synergistically trigger
stromal fibroblasts in breast cancer. Mol Ther Nucleic Acids.
28:17–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wellenstein MD, Coffelt SB, Duits DEM, van
Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic
S, Hau CS, et al: Loss of p53 triggers WNT-dependent systemic
inflammation to drive breast cancer metastasis. Nature.
572:538–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Staal FJ and Clevers HC: WNT signalling
and haematopoiesis: A WNT-WNT situation. Nat Rev Immunol. 5:21–30.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sidaway P: Prostate cancer: Wnt signalling
induces resistance. Nat Rev Urol. 12:5972015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Q and Chen Z, Jin X, Mao R and Chen
Z: The many postures of noncanonical Wnt signaling in development
and diseases. Biomed Pharmacother. 93:359–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ozawa M, Baribault H and Kemler R: The
cytoplasmic domain of the cell adhesion molecule uvomorulin
associates with three independent proteins structurally related in
different species. EMBO J. 8:1711–1717. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCrea PD and Gumbiner BM: Purification of
a 92-kDa cytoplasmic protein tightly associated with the cell-cell
adhesion molecule E-cadherin (uvomorulin). Characterization and
extractability of the protein complex from the cell cytostructure.
J Biol Chem. 266:4514–4520. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhan T, Chen M, Liu W, Han Z, Zhu Q, Liu
M, Tan J, Liu J, Chen X, Tian X and Huang X: MiR-455-3p inhibits
gastric cancer progression by repressing Wnt/β-catenin signaling
through binding to ARMC8. BMC Med Genomics. 16:1552023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y and Mlodzik M: Wnt-Frizzled/planar
cell polarity signaling: Cellular orientation by facing the wind
(Wnt). Annu Rev Cell Dev Biol. 31:623–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katoh M: WNT/PCP signaling pathway and
human cancer (review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
41
|
Saneyoshi T, Kume S, Amasaki Y and
Mikoshiba K: The Wnt/calcium pathway activates NF-AT and promotes
ventral cell fate in Xenopus embryos. Nature. 417:295–299. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang H, Chen Q, Coles AH, Anderson SJ,
Pihan G, Bradley A, Gerstein R, Jurecic R and Jones SN: Wnt5a
inhibits B cell proliferation and functions as a tumor suppressor
in hematopoietic tissue. Cancer Cell. 4:349–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhuang X, Zhang H, Li X, Li X, Cong M,
Peng F, Yu J, Zhang X, Yang Q and Hu G: Differential effects on
lung and bone metastasis of breast cancer by Wnt signalling
inhibitor DKK1. Nat Cell Biol. 19:1274–1285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mahdi T, Hänzelmann S, Salehi A, Muhammed
SJ, Reinbothe TM, Tang Y, Axelsson AS, Zhou Y, Jing X, Almgren P,
et al: Secreted frizzled-related protein 4 reduces insulin
secretion and is overexpressed in type 2 diabetes. Cell Metab.
16:625–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Slusarski DC, Corces VG and Moon RT:
Interaction of wnt and a frizzled homologue triggers
g-protein-linked phosphatidylinositol signalling. Nature.
390:410–413. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang Y, Xiao X, Wang J, Dasari S, Pepin D,
Nephew KP, Zamarin D and Mitra AK: Cancer associated fibroblasts
serve as an ovarian cancer stem cell niche through noncanonical
Wnt5a signaling. NPJ Precis Oncol. 8:72024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ge J, Yu YJ, Li JY, Li MY, Xia SM, Xue K,
WangS Y and Yang C: Activating Wnt/β-catenin signaling by
autophagic degradation of APC contributes to the osteoblast
differentiation effect of soy isoflavone on osteoporotic
mesenchymal stem cells. Acta Pharmacol Sin. 44:1841–1855. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Y, Zhang E, Gao H, Shang C, Yin M, Ma
M, Liu Y, Zhang X and Li X: Resistomycin inhibits Wnt/β-catenin
signaling to induce the apoptotic death of human colorectal cancer
cells. Mar Drugs. 21:6222023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rui Q, Dong S, Jiang W and Wang D:
Response of canonical Wnt/β-catenin signaling pathway in the
intestine to microgravity stress in Caenorhabditis elegans.
Ecotoxicol Environ Saf. 186:1097822019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Šopin T, Liška F, Kučera T, Cmarko D and
Vacík T: Lysine demethylase KDM2A promotes proteasomal degradation
of TCF/LEF transcription factors in a neddylation-dependent manner.
Cells. 12:26202023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Y, Yang Z, Yuan H, Li Z, Li Y, Liu Q
and Chen J: PCDH10 inhibits cell proliferation of multiple myeloma
via the negative regulation of the Wnt/β-catenin/BCL-9 signaling
pathway. Oncol Rep. 34:747–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang C, Zhang R, Wang X, Zheng Y, Jia H,
Li H, Wang J, Wang N, Xiang F and Li Y: Silencing of KIF3B
suppresses breast cancer progression by regulating EMT and
Wnt/β-catenin signaling. Front Oncol. 10:5974642020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Malla RR and Kiran P: Tumor
microenvironment pathways: Cross regulation in breast cancer
metastasis. Genes Dis. 9:310–324. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang L, Jin Z, Master RP, Maharjan CK,
Carelock ME, Reccoppa TBA, Kim MC, Kolb R and Zhang W: Breast
cancer stem cells: Signaling pathways, cellular interactions, and
therapeutic implications. Cancers (Basel). 14:32872022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dri A, Arpino G, Bianchini G, Curigliano
G, Danesi R, De Laurentiis M, Del Mastro L, Fabi A, Generali D,
Gennari A, et al: Puglisi, Breaking barriers in triple negative
breast cancer (TNBC)-Unleashing the power of antibody-drug
conjugates (ADCs). Cancer Treat Rev. 123:1026722024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park M, Kim D, Ko S, Kim A, Mo K and Yoon
H: Breast cancer metastasis: Mechanisms and therapeutic
implications. Int J Mol Sci. 23:68062022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Dijke PT, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Q, Chen F, Yang N, Xu L, Yu X, Wu M
and Zhou Y: DEPDC1B-mediated USP5 deubiquitination of β-catenin
promotes breast cancer metastasis by activating the wnt/β-catenin
pathway. Am J Physiol Cell Physiol. 325:C833–C848. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Barkal AA, Brewer RE, Markovic M, Kowarsky
M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal
LJ and Weissman IL: CD24 signalling through macrophage Siglec-10 is
a target for cancer immunotherapy. Nature. 572:392–396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shulewitz M, Soloviev I, Wu T, Koeppen H,
Polakis P and Sakanaka C: Repressor roles for TCF-4 and Sfrp1 in
Wnt signaling in breast cancer. Oncogene. 25:4361–4369. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Noman MZ, Van Moer K, Marani V, Gemmill
RM, Tranchevent LC, Azuaje F, Muller A, Chouaib S, Thiery JP,
Berchem G and Janji B: CD47 is a direct target of SNAI1 and ZEB1
and its blockade activates the phagocytosis of breast cancer cells
undergoing EMT. Oncoimmunology. 7:e13454152018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Blondeaux E, Arecco L, Punie K, Graffeo R,
Toss A, De Angelis C, Trevisan L, Buzzatti G, Linn SC, Dubsky P, et
al: Germline TP53 pathogenic variants and breast cancer: A
narrative review. Cancer Treat Rev. 114:1025222023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang X, Shi D, Zou X, Wu X, Huang S, Kong
L, Yang M, Xiao Y, Chen B, Chen X, et al: BAG2 drives
chemoresistance of breast cancer by exacerbating mutant p53
aggregate. Theranostics. 13:339–354. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grote I, Bartels S, Kandt L, Bollmann L,
Christgen H, Gronewold M, Raap M, Lehmann U, Gluz O, Nitz U, et al:
TP53 mutations are associated with primary endocrine resistance in
luminal early breast cancer. Cancer Med. 10:8581–8594. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bai X, Ni J, Beretov J, Graham PA and Li
Y: Cancer stem cell in breast cancer therapeutic resistance. Cancer
Treat Rev. 69:152–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
VanderVorst K, Dreyer CA, Hatakeyama J,
Bell GRR, Learn JA, Berg AL, Hernandez M, Lee H, Collins SR and
Carraway KL III: Vangl-dependent Wnt/planar cell polarity signaling
mediates collective breast carcinoma motility and distant
metastasis. Breast Cancer Res. 25:522023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Puvirajesinghe TM, Bertucci F, Jain A,
Scerbo P, Belotti E, Audebert S, Sebbagh M, Lopez M, Brech A,
Finetti P, et al: Identification of p62/SQSTM1 as a component of
non-canonical Wnt VANGL2-JNK signalling in breast cancer. Nat
Commun. 7:103182016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Courtwright A, Siamakpour-Reihani S,
Arbiser JL, Banet N, Hilliard E, Fried L, Livasy C, Ketelsen D,
Nepal DB, Perou CM, et al: Secreted frizzle-related protein 2
stimulates angiogenesis via a calcineurin/NFAT signaling pathway.
Cancer Res. 69:4621–4628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li BL, Lu W, Qu JJ, Ye L, Du GQ and Wan
XP: Loss of exosomal miR-148b from cancer-associated fibroblasts
promotes endometrial cancer cell invasion and cancer metastasis. J
Cell Physiol. 234:2943–2953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kim CK and Pak TR: miRNA degradation in
the mammalian brain. Am J Physiol Cell Physiol. 319:C624–C629.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Califf RM: Biomarker definitions and their
applications. Exp Biol Med (Maywood). 243:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Petroušková P, Hudáková N, Maloveská M,
Humeník F and Cizkova D: Non-Exosomal and exosome-derived miRNAs as
promising biomarkers in canine mammary cancer. Life (Basel).
12:5242022.PubMed/NCBI
|
|
81
|
Li H and Tie XJ: Exploring research
progress in studying serum exosomal miRNA-21 as a molecular
diagnostic marker for breast cancer. Clin Transl Oncol.
11:10.1007/s12094–024-03454-z. 2024.
|
|
82
|
Liu M, Mo F, Song X, He Y, Yuan Y, Yan J,
Yang Y, Huang J and Zhang S: Exosomal hsa-miR-21-5p is a biomarker
for breast cancer diagnosis. PeerJ. 9:e121472021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li S, Zhang M, Xu F, Wang Y and Leng D:
Detection significance of miR-3662, miR-146a, and miR-1290 in serum
exosomes of breast cancer patients. J Cancer Res Ther. 17:749–755.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang W and Luo YP: MicroRNAs in breast
cancer: Oncogene and tumor suppressors with clinical potential. J
Zhejiang Univ Sci B. 16:18–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang B, Mao JH, Wang BY, Wang LX, Wen HY,
Xu LJ, Fu JX and Yang H: Exosomal miR-1910-3p promotes
proliferation, metastasis, and autophagy of breast cancer cells by
targeting MTMR3 and activating the NF-κB signaling pathway. Cancer
Lett. 489:87–99. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen
K and Li L: Shikonin inhibits the proliferation of human breast
cancer cells by reducing tumor-derived exosomes. Molecules.
21:7772016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jung KO, Youn H, Lee CH, Kang KW and Chung
JK: Visualization of exosome-mediated miR-210 transfer from hypoxic
tumor cells. Oncotarget. 8:9899–9910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG and Iorio MV: Exosome-mediated delivery of miR-9
induces cancer-associated fibroblast-like properties in human
breast fibroblasts. Cell Death Dis. 7:e23122016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kong W, He L, Richards EJ, Challa S, Xu
CX, Permuth-Wey J, Lancaster JM, Coppola D, Sellers TA, Djeu JY and
Cheng JQ: Upregulation of miRNA-155 promotes tumour angiogenesis by
targeting VHL and is associated with poor prognosis and
triple-negative breast cancer. Oncogene. 33:679–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kontomanolis E, Mitrakas A, Giatromanolaki
A, Kareli D, Panteliadou M, Pouliliou S and Koukourakis MI: A pilot
study on plasma levels of micro-RNAs involved in angiogenesis and
vascular maturation in patients with breast cancer. Med Oncol.
34:202017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Luengo-Gil G, Gonzalez-Billalabeitia E,
Perez-Henarejos SA, Manzano EN, Chaves-Benito A, Garcia-Martinez E,
Garcia-Garre E, Vicente V and Ayala de la Peña F: Angiogenic role
of miR-20a in breast cancer. PLoS One. 13:e01946382018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Donnarumma E, Fiore D, Nappa M, Roscigno
G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C,
et al: Cancer-associated fibroblasts release exosomal microRNAs
that dictate an aggressive phenotype in breast cancer. Oncotarget.
8:19592–19608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q,
Shi L, Li H, Yin C, Luo H, Hao C, et al: Cancer-associated
fibroblast-derived exosomal miR-18b promotes breast cancer invasion
and metastasis by regulating TCEAL7. Cell Death Dis. 12:11202021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang H, Wei H, Wang J, Li L, Chen A and Li
Z: MicroRNA-181d-5p-containing exosomes derived from CAFs promote
EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic
Acids. 19:654–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Martello G, Rosato A, Ferrari F, Manfrin
A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T,
et al: A MicroRNA targeting dicer for metastasis control. Cell.
141:1195–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Weng YS, Tseng HY, Chen YA, Shen PC, Al
Haq AT, Chen LM, Tung YC and Hsu HL: MCT-1/miR-34a/IL-6/IL-6R
signaling axis promotes EMT progression, cancer stemness and M2
macrophage polarization in triple-negative breast cancer. Mol
Cancer. 18:422019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang Y, Lai X, Yue Q, Cao F, Zhang Y, Sun
Y, Tian J, Lu Y, He L, Bai J and Wei Y: Bone marrow mesenchymal
stem cells-derived exosomal microRNA-16-5p restrains
epithelial-mesenchymal transition in breast cancer cells via
EPHA1/NF-κB signaling axis. Genomics. 114:1103412022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liang Z, Liu L, Gao R, Che C and Yang G:
Downregulation of exosomal miR-7-5p promotes breast cancer
migration and invasion by targeting RYK and participating in the
atypical WNT signalling pathway. Cell Mol Biol Lett. 27:882022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang X, Luo G, Zhang K, Cao J, Huang C,
Jiang T, Liu B, Su L and Qiu Z: Correction: Hypoxic tumor-derived
exosomal miR-301a mediates M2 macrophage polarization via
PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res.
80:9222020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen WX, Wang DD, Zhu B, Zhu YZ, Zheng L,
Feng ZQ and Qin XH: Exosomal miR-222 from adriamycin-resistant
MCF-7 breast cancer cells promote macrophages M2 polarization via
PTEN/Akt to induce tumor progression. Aging (Albany NY).
13:10415–10430. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pakravan K, Mossahebi-Mohammadi M,
Ghazimoradi MH, Cho WC, Sadeghizadeh M and Babashah S: Monocytes
educated by cancer-associated fibroblasts secrete exosomal miR-181a
to activate AKT signaling in breast cancer cells. J Transl Med.
20:5592022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hao C, Sheng Z, Wang W, Feng R, Zheng Y,
Xiao Q and Zhang B: Tumor-derived exosomal miR-148b-3p mediates M2
macrophage polarization via TSC2/mTORC1 to promote breast cancer
migration and invasion. Thorac Cancer. 14:1477–1491. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yao X, Tu Y, Xu Y, Guo Y, Yao F and Zhang
X: Endoplasmic reticulum stress-induced exosomal miR-27a-3p
promotes immune escape in breast cancer via regulating PD-L1
expression in macrophages. J Cell Mol Med. 24:9560–9573. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu
W, Guo X and Yu J: Cancer exosome-derived miR-9 and miR-181a
promote the development of early-stage MDSCs via interfering with
SOCS3 and PIAS3 respectively in breast cancer. Oncogene.
39:4681–4694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Salehi M, Vafadar A, Khatami SH,
Taheri-Anganeh M, Vakili O, Savardashtaki A, Negahdari B, Naeli P,
Behrouj H, Ghasemi H and Movahedpour A: Gastrointestinal cancer
drug resistance: the role of exosomal miRNAs. Mol Biol Rep.
49:2421–2432. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hu W, Tan C, He Y, Zhang G, Xu Y and Tang
J: Functional miRNAs in breast cancer drug resistance. Onco Targets
Ther. 11:1529–1541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sachdeva M, Wu H, Ru P, Hwang L, Trieu V
and Mo YY: MicroRNA-101-mediated Akt activation and
estrogen-independent growth. Oncogene. 30:822–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Miller TE, Ghoshal K, Ramaswamy B, Roy S,
Datta J, Shapiro CL, Jacob S and Majumder S: MicroRNA-221/222
confers tamoxifen resistance in breast cancer by targeting p27Kip1.
J Biol Chem. 283:29897–29903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y,
Li H, Zhu X, Yao L and Zhang J: Exosomal miR-221/222 enhances
tamoxifen resistance in recipient ER-positive breast cancer cells.
Breast Cancer Res Treat. 147:423–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gao M, Miao L, Liu M, Li C, Yu C, Yan H,
Yin Y, Wang Y, Qi X and Ren J: miR-145 sensitizes breast cancer to
doxorubicin by targeting multidrug resistance-associated protein-1.
Oncotarget. 7:59714–59726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sueta A, Yamamoto Y, Tomiguchi M,
Takeshita T, Yamamoto-Ibusuki M and Iwase H: Differential
expression of exosomal miRNAs between breast cancer patients with
and without recurrence. Oncotarget. 8:69934–69944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhong Q, Nie Q, Wu R and Huang Y: Exosomal
miR-18a-5p promotes EMT and metastasis of NPC cells via targeting
BTG3 and activating the Wnt/β-catenin signaling pathway. Cell
Cycle. 22:1544–1562. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Xia Y, Wei K, Hu LQ, Zhou CE, Lu ZB, Zhan
GS, Pan XL, Pan CF, Wang J, Wen W, et al: Exosome-mediated transfer
of miR-1260b promotes cell invasion through Wnt/β-catenin signaling
pathway in lung adenocarcinoma. J Cell Physiol. 235:6843–6853.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Huang Z, Zhen S, Jin L, Chen J, Han Y, Lei
W and Zhang F: miRNA-1260b promotes breast cancer cell migration
and invasion by downregulating CCDC134. Curr Gene Ther. 23:60–71.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Xiao Z, Feng X, Zhou Y, Li P, Luo J, Zhang
W, Zhou J, Zhao J, Wang D, Wang Y, et al: Exosomal miR-10527-5p
inhibits migration, invasion, lymphangiogenesis and lymphatic
metastasis by affecting Wnt/β-catenin signaling via Rab10 in
esophageal squamous cell carcinoma. Int J Nanomedicine. 18:95–114.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu Y, Yang C, Chen S, Liu W, Liang J, He
S and Hui J: Cancer-derived exosomal miR-375 targets DIP2C and
promotes osteoblastic metastasis and prostate cancer progression by
regulating the Wnt signaling pathway. Cancer Gene Ther. 30:437–449.
2023.PubMed/NCBI
|
|
120
|
Li H, Xie C, Lu Y, Chang K, Guan F and Li
X: Exosomal mir92a promotes cytarabine resistance in
myelodysplastic syndromes by activating Wnt/β-catenin signal
pathway. Biomolecules. 12:14482022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yue X, Lan F and Xia T: Hypoxic glioma
cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling
and promotes radiation resistance by targeting TCEAL7. Mol Ther.
27:1939–1949. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yue X, Cao D, Lan F, Pan Q, Xia T and Yu
H: MiR-301a is activated by the Wnt/β-catenin pathway and promotes
glioma cell invasion by suppressing SEPT7. Neuro Oncol.
18:1288–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang L, He M, Fu L and Jin Y: Exosomal
release of microRNA-454 by breast cancer cells sustains biological
properties of cancer stem cells via the PRRT2/Wnt axis in ovarian
cancer. Life Sci. 257:1180242020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Fang F, Guo C, Zheng W, Wang Q and Zhou L:
Exosome-mediated transfer of miR-1323 from cancer-associated
fibroblasts confers radioresistance of c33a cells by targeting
PABPN1 and activating Wnt/β-catenin signaling pathway in cervical
cancer. Reprod Sci. 29:1809–1821. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shan G, Zhou X, Gu J, Zhou D, Cheng W, Wu
H, Wang Y, Tang T and Wang X: Downregulated exosomal
microRNA-148b-3p in cancer associated fibroblasts enhance
chemosensitivity of bladder cancer cells by downregulating the
Wnt/β-catenin pathway and upregulating PTEN. Cell Oncol (Dordr).
44:45–59. 2021. View Article : Google Scholar : PubMed/NCBI
|