Introduction
The 2020 Global Cancer Statistics report stated that
there were 19.3 million new cancer cases and almost 10 million
cancer-related deaths in 2020 (1).
Previous research has suggested that there will be 22.2 million new
cases among all cancer types in 2030, and cancer will continue to
be the principal cause of increasing morbidity and mortality in all
regions of the world for decades to come (2,3).
However, early screening for cancer is still not widespread,
therefore numerous patients are diagnosed in the late stage, at
which point radical treatments such as surgery are not ideal; thus,
late diagnosis markedly reduces the survival rate of patients.
Recently, an increasing number of new therapeutic methods, such as
natural killer cell-based cancer immunotherapy (4), RAS-targeted cancer therapy (5) and immune checkpoint inhibitor (ICI)
therapy, have been developed (6).
Although the discovery of ICIs represents a major breakthrough in
cancer therapy, there are still some patients who are unresponsive
to treatment or who cannot tolerate the side effects. Therefore,
identifying more effective treatments is crucial for improving
patient prognosis.
In humans, >2,600 mature microRNA (miRNA or miR)
sequences have been identified (7).
MiRNAs are small non-coding RNAs that are ~19-25 nucleotides in
length and negatively modulate gene expression by base pairing to
the 3′-untranslated region (UTR) of target messenger RNAs (mRNAs).
They are usually abnormally expressed in tumor cells and can
modulate the apoptosis, proliferation, survival and metastasis of
cancer cells (8–10). It is considered by numerous scholars
(11–13) that abnormal expression of miR-143-3p
in malignant tumors plays a crucial role in tumor progression and
can also act as a diagnostic and prognostic marker; it has even
been studied as a therapeutic target in the last few years. In the
present review, the functions of miR-143-3p in malignant tumors and
the potential underlying mechanisms involved were systematically
described.
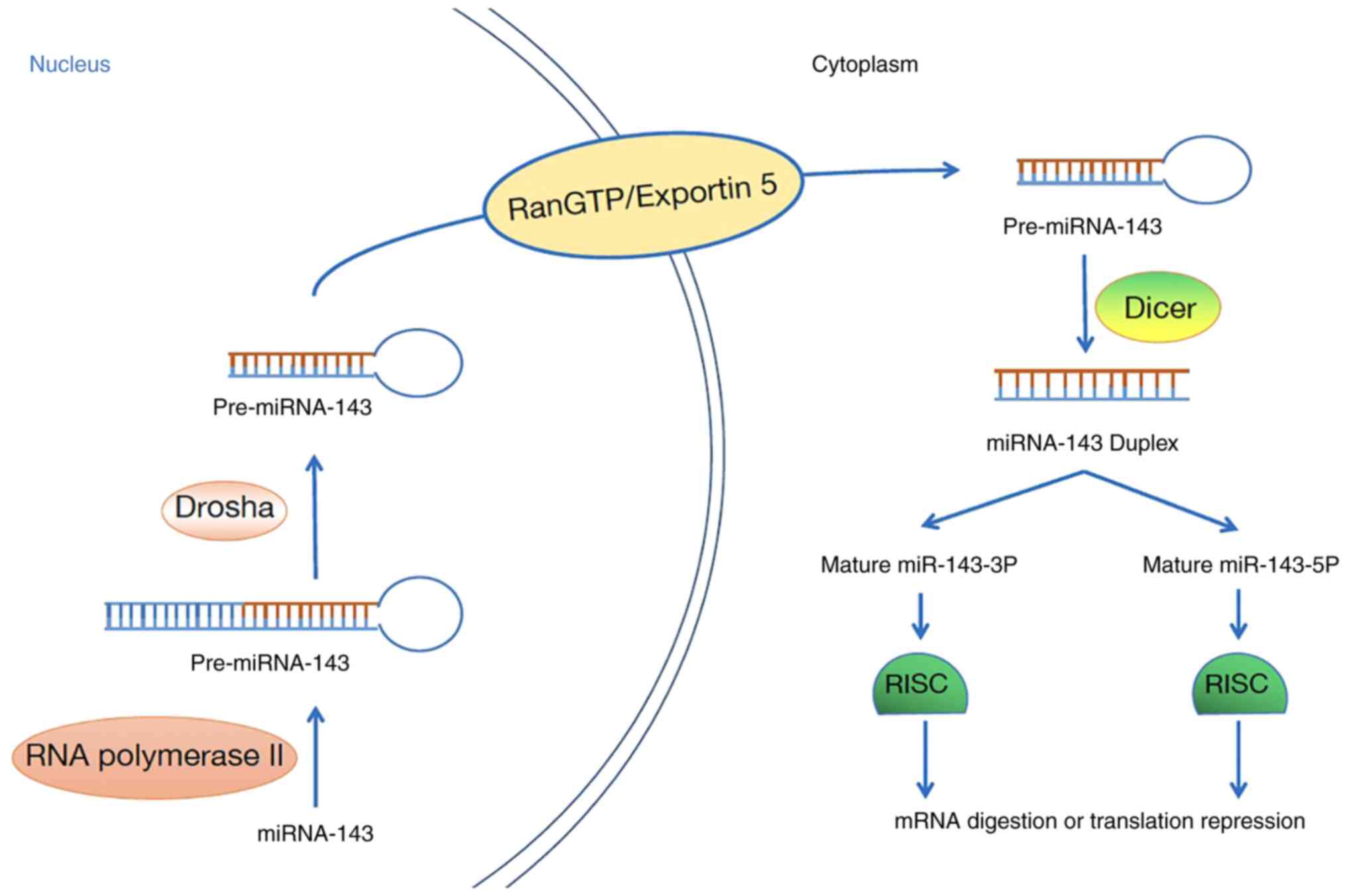
Biogenesis of miR-143-3p
Mature miRNAs are generated by successive
pretreatment events: Pri-miRNAs are processed into 70-nucleotide
pre-miRNAs in the nucleus, after which the pre-miRNAs are processed
into mature miRNAs in the cytoplasm (14).
miR-143-3p is no exception. First, miRNA-143 is
transcribed into pri-miRNA-143 by RNA polymerase II, which
functions in the nucleus (15),
after which the cellular RNase III Drosha processes pri-miRNA-143
into a 60–110 nucleotide structure (16). Then, pre-miRNA-143 is transported
into the cytoplasm via the RanGTP/Exportin 5 pathway (17). Pre-miRNA-143 in the cytoplasm is
cleaved by the RNase III Dicer to generate short miRNA-143
duplexes, which are then rapidly unwound by helicases to produce
mature miRNA-143 (18). Mature
miRNA-143 and proteins bind to form an RNA-induced silencing
complex, which can lead to target mRNA degradation or translation
suppression (17). Interestingly,
under certain conditions, two different mature miRNAs can be
generated from a pre-miRNA (15).
In humans, removal of the opposite arm of pre-miR-143 can produce
two diverse mature miRNAs, namely, Homo sapiens
(has)-miR-143-5p and hsa-miR-143-3p. Although they are generated
from the same primary transcript, their sequences differ widely,
therefore they target different mRNAs to play different roles
(18) (Fig. 1).
Regulation and effect of miR-143-3p
expression
According to previous studies, miR-143-3p is often
downregulated in malignant tumors of various systems, such as lung
cancer (LC) (19), gastric cancer
(GC) (20), bladder cancer (BC)
(21), cervical cancer (CC)
(22), thyroid cancer (TC)
(23) and melanoma (12), and can impact the proliferation,
migration and apoptosis of malignant tumor cells. The regulation
and effect of miR-143-3p expression in various systems have been
investigated in studies mainly focusing on the upstream factors and
target genes that regulate miR-143-3p (Table I).
 | Table I.Expression and role of
microRNA-143-3p in cancers. |
Table I.
Expression and role of
microRNA-143-3p in cancers.
| Cancer types | Expression
level | Upstream
factors | Targets | Functions | (Refs.) |
|---|
| LC | Downregulation |
LncRNA-TMPO-AS1 | CDK1 | Promote survival
and reduce apoptosis | (25) |
| NSCLC | Downregulation | LncRNA UCC | SOX5 | Promote
proliferation, migration and EMT | (24) |
| NSCLC | Downregulation | LINC00667 | RRM2 | Promote growth | (26) |
| NSCLC | Downregulation | CircT UBA1C |
| Promote
proliferation, inhibit apoptosis | (27) |
| LUAD | Downregulation | CircSNK1G3 | HOXA10 | Promotes growth and
metastasis | (19) |
| LUAD | Downregulation | LncRNA PCAT19 |
| Promote migration
and invasion | (28) |
| BM of LC | Upregulation |
| VASH1 | Promote metastasis
and enhance angiogenesis | (29) |
| HCC | Downregulation | Circ0003998 | FOSL2 | Promote EMT | (30) |
| HCC | Downregulation | LncRNA SOX2-OT | MSI2 | Promote malignant
progression | (31) |
| HCC | Downregulation | LncRNA MALAT1 | ZEB1 | Promote
proliferation and invasion | (32) |
| HCC | Downregulation |
| lncRNA
PSMG3-AS1 | Promote
proliferation | (33) |
| HCC | Downregulation |
| FGF1 | Promote
proliferation and invasion | (34) |
| GC | Downregulation | LncRNA
CCDC144NL-AS1 | MAP3K7 | Promotes growth and
metastasis | (35) |
| GC | Downregulation | LINC00200 | SERPINE1 | Promote
proliferation, invasion and migration | (36) |
| GC | Downregulation | Circ_0006089 | IGF1R | Promote
proliferation, invasion and migration | (20) |
| GC | Downregulation | Circ_0006089 | PTBP3 | Promote
proliferation, metastasis and inhibit apoptosis | (37) |
| GC | Downregulation | CircFOXO3 | USP44 | Promote
proliferation and migration | (38) |
| CRC | Downregulation | Circ-ACAP2 | FZD4 | Promote
proliferation, migration, invasion and radiation resistance,
inhibit apoptosis | (41) |
| CRC | Downregulation | LncRNA
TMPO-AS1 |
| Promote
proliferation, migration and invasion | (42) |
| CRC | Downregulation | LINC00908 | KLF5 | Promote
proliferation | (43) |
| CRC | Downregulation | CCDC144NL-AS1 | HMGA2 | Correlated with
tumor stage and size | (44) |
| CRC | Downregulation |
| ITGA6, ASAP3 | Promote invasion
and migration | (45) |
| CRC | Downregulation |
| CTNND1 | Promote
proliferation, migration and invasion | (46) |
| GBC | Downregulation |
| ITGA6 | Promote
proliferation and angiogenesis | (48) |
| GBC | Downregulation | LncRNA
OIP5-AS1 |
| Promote invasion,
proliferation and migration | (49) |
| PDAC | Downregulation |
| KRAS | Promote
proliferation, migration and invasion | (51) |
| Esophageal
cancer | Downregulation | LncRNA CASC7 | HK2 | Promote
proliferation | (52) |
| BC | Downregulation | LINC02470 | SMAD3 | Promote
invasion | (21) |
| BC | Downregulation | LINC00511 | PCMT1 | Promote
proliferation and migration | (53) |
| BC | Downregulation | circ_644 | MSI2 | Promote the
malignant progression | (54) |
| BC | Downregulation | LncRNA SNHG1 | HK2 | Promote
proliferation, migration and invasion | (55) |
| BC | Downregulation | LncRNA
MAFG-AS1 | COX-2 | Promote
proliferation, metastasis and invasion | (56) |
| BC | Downregulation | LncRNA PCAT6 | PCAT6 | Promote
proliferation, migration and invasion | (57) |
| PCa | Downregulation | LncRNA-SChLAP1 | DNMT3A | Promote survival,
migration, and tumorigenicity | (58) |
| PCa | Downregulation | LncRNA-PVT1 | NOP2 | Promote transfer
and progress | (59) |
| PCa | Downregulation | LncRNA PCSEAT | EZH2 | Promote
proliferation | (60) |
| PCa | Downregulation |
| AKT1 | Promote EMT | (61) |
| CRPC | Downregulation | GR | JAG1, NOTCH2 | Promote migration,
invasion, proliferation and inhibit apoptosis | (62) |
| RCC |
| LncRNA SARCC | AKT, MMP-13, K-RAS,
P-ERK | Inhibit invasion,
migration and proliferation | (64) |
| ccRCC | Downregulation | LINC00667 | ZEB1 | Promote progression
of ccRCC and induce chemotherapy resistance | (11) |
| RCC | Downregulation | LncRNA
SLC16A1-AS1 | SLC7A11 | Promote survival,
proliferation and migration | (65) |
| CC | Downregulation | LncRNA
OIP5-AS1 | SMAD3 | Promote migration
and invasion | (66) |
| CC | Downregulation | LncRNA
OIP5-AS1 | ITGA6 | Promote
proliferation and invasion | (67) |
| CC | Down
regulation | LncRNA
OIP5-AS1 | ROCK1 | Promote
proliferation and inhibit apoptosis | (68) |
| CC | Downregulation | LncRNA
MEOX2-AS1 | VDAC1 | Promote
proliferation and metastasis | (22) |
| CC | Downregulation | LncRNA
ACTA2-AS1 | SMAD3 | Promote
proliferation, migration and inhibit apoptosis | (69) |
| CC | Downregulation | LncRNA HOTAIR | BCL2 | Promote
proliferation and inhibit apoptosis | (70) |
| CC | Downregulation | LncRNA
TMPO-AS1 | ZEB1 | Promote
proliferation, migration and invasion | (71) |
| CC | Downregulation |
| CDK1 | Promote
proliferation, migration and invasion | (72) |
| OC | Downregulation |
| RALBP1 | Promote survival
and inhibit apoptosis | (76) |
| OC | Downregulation |
| TAK1 | Promote
proliferation, migration and invasion | (77) |
| OC | Downregulation | LncRNA PCAT6 | TAK1 | Promote
proliferation, migration and invasion | (78) |
| OC | Downregulation | LncRNA MALAT1 | CMPK | Promote migration
and invasion | (79) |
| OC | Downregulation | LncRNA
CDKN2B-AS1 | SMAD3 | Promote growth,
invasion and migration | (80) |
| Endometrial
cancer | Downregulation | LNCRNA UCA1 | FKLF5, RXFP1 | Promote
proliferation, invasion and inhibit apoptosis | (81) |
| TC | Downregulation |
| MSI2 | Promote
proliferation, invasion, migration and inhibit apoptosis | (23) |
| TC | Downregulation | LINC01296 | MSI2 | Promote
proliferation, invasion, migration and inhibit apoptosis | (82) |
| LSCC | Downregulation |
| k-Ras | Promote growth,
migration and invasion | (83) |
| LSCC | Downregulation |
| MAGE-A9 | Promote
proliferation, migration and invasion | (84) |
| NSCC | Downregulation |
| Bcl-2, IGF1R | Promote
proliferation, migration and inhibit apoptosis | (85) |
| BC | Downregulation |
| MYBL2 | Promote
proliferation and inhibit apoptosis | (86) |
| BC | Downregulation |
| MAPK7 | Promote
proliferation and migration | (87) |
| Triple-negative
BC | Downregulation |
| LIMK1 | Promote
proliferation | (88) |
| BC | Downregulation | lncRNA
PSMG3-AS1 | COL1A1 | Promote
proliferation and migration | (89) |
| BC | Downregulation | lncRNA
LOXL1-AS1 |
| Promote
proliferation, migration, invasion and inhibit apoptosis | (90) |
| Neuroblas-toma | Downregulation | CircRNA-ACAP2 | HK2 | Promote migration,
invasion and inhibit apoptosis | (91) |
| AML | Downregulation |
| KAT6A | Promote survival
and proliferation | (92) |
| OS | Downregulation |
| MAPK7 | Promote
proliferation, migration and invasion | (93) |
| OS | Downregulation |
| FOSL2 | Promote
proliferation, metastasis and inhibit apoptosis | (94) |
| OS | Downregulation | lncRNA PCAT6 | ZEB1 | Promote
proliferation, migration and invasion | (95) |
| Melanoma | Downregulation |
| COX-2 | Promote
proliferation and inhibit apoptosis | (12) |
| Melanoma | Downregulation | Circ-FOXM1 | FLOT2 | Promote
proliferation, invasion and inhibit apoptosis | (96) |
| OSCC | Down
regulation | lncRNA UCA1 | MYO6 | Promote growth,
migration and invasion | (97) |
| OSCC | Downregulation | CircPVT1 | SLC7A11 | Promote growth and
metastasis | (98) |
| OSCC | Downregulation | LncRNA MALAT1 | MAGEA9 | Promote
proliferation and migration | (99) |
miR-143-3p in the respiratory system
LC
LC is the second most common cancer and a
significant cause of cancer-related death; LC accounts for ~1/10
cancer cases (11.4%) and 1/5 cancer-related deaths (18.0%)
(1). LC can be divided into two
different types according to histopathological type: Non-small cell
LC (NSCLC) and small cell LC (SCLC), with NSCLC predominating. It
is considered by numerous scholars (19,24,25)
that miR-143-3p acts as an important regulatory gene in NSCLC and
lung adenocarcinoma (LUAD) to affect tumorigenicity and
progression. miR-143-3p is often downregulated in NSCLC and LUAD
and is regulated by its upstream regulators. For instance, the
downregulation of long non-coding RNA (lncRNA)-TMPO-AS1 can result
in high miR-143-3p expression, which leads to a decrease in CDK1
and ultimately participates in accelerating the apoptosis of LC
cells (25).
In NSCLC, the lncRNA UCC is highly expressed and can
act as a competing endogenous RNA (ceRNA) and compete with
miR-143-3p to suppress miR-143-3p expression and subsequently
regulate SOX5 expression. Mechanistically, the lncRNA UCC induces
SOX5 expression through miR-143-3p, induces NSCLC cell
proliferation and migration, and subsequently promotes
epithelial-mesenchymal transition (EMT) in NSCLC (24). Another study revealed that
miR-143-3p suppresses tumor growth, while LINC00667 and RRM2
promote tumor growth. The lncRNA LINC00667 can suppress miR-143-3p
expression and indirectly regulate the expression of RRM2, a target
gene of miR-143-3p. In conclusion, NSCLC growth is modulated by the
LINC00667/miR-143-3p/RRM2 signaling pathway (26). In addition, the circTUBA
1C/miR-143-3p axis plays a significant role in NSCLC. CircTUBA 1C
can negatively modulate miR-143-3p expression. Rescue experiments
illustrated that knockdown of miR-143-3p could reverse the decrease
in tumor growth induced by circTUBA 1C deficiency (27).
In LUAD, circSNK1G3 inhibits miR-143-3p expression.
Downregulation of miR-143-3p induces homeobox (HOX) A10 (HOXA10)
expression, thereby promoting LUAD cell growth and metastasis
(19). On the other hand,
miR-143-3p is targeted by the lncRNA PCAT19, and the expression of
these two genes is positively correlated. LUAD cell proliferation,
invasion and migration can be accelerated by downregulation of
lncRNA PCAT19; nevertheless, the overexpression of miR-143-3p may
attenuate these effects (28).
However, compared with that in patients with LC
without brain metastases (BMs), the expression of miR-143-3p in
patients with LC with BMs is markedly increased, and this increase
in miR-143-3p increases the metastatic potential of LC cells and
promotes angiogenesis (29).
miR-143-3p expression is also upregulated in exosomes derived from
granulocytic myeloid-derived suppressor cells (G-MDSCs) in LC
tissues, and exosomes secreted by G-MDSCs can be transferred to LC
cells to accelerate tumor progression (13). miR-143-3p is clearly associated with
the malignant progression of cancer.
miR-143-3p in the digestive system
Hepatocellular carcinoma (HCC)
Primary liver cancer was the third most common cause
of cancer-related death worldwide according to a 2020 study, and
HCC accounted for ~75–85% of these LC-related deaths (1). It has been suggested that miR-143-3p
plays a major role in HCC progression. The findings of a previous
study suggested that miR-143-3p is expressed at a low level in HCC
and is a potential target of circ0003998. FOS-Like antigen 2
(FOSL2) is a downstream target of the circ0003998/miR-143-3p axis,
and miR-143-3p can downregulate FOSL2 expression and affect the
migration of HCC cells (30).
miR-143-3p is also a target of the lncRNA SOx2-OT, and its
expression levels are negatively correlated. The lncRNA SOx2-OT
competitively binds with miR-143-3p to promote the expression of
Musashi RNA binding protein 2 (MSI2) and consequently promote the
malignant progression of HCC (31).
Another study showed that the lncRNA metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1) is highly expressed in HCC
tissues and binds to miR-143-3p to inhibit miR-143-3p expression;
subsequently, miR-143-3p accelerates the progression of HCC by
regulating zinc finger E-box binding homeobox 1 (ZEB1) expression
(32). On the other hand,
miR-143-3p may suppress HCC cell invasion and proliferation by
directly modulating the target genes lncRNA PSMG3-AS1 and
fibroblast growth factor 1 (FGF1) (33,34).
GC
In 2020, >1 million new cases of GC and 769,000
related deaths were reported worldwide; thus, GC remains an
important cancer worldwide (1). In
GC, miR-143-3p expression is influenced by various genes. As a
ceRNA, the lncRNA CCDC144NL-AS1 interacts with miR-143-3p to
negatively regulate its expression, thereby regulating MAP3K7
expression in GC. Mechanistically, the lncRNA CCDC144NL-AS1 alters
MAP3K7 levels by modulating miR-143-3p expression and subsequently
acts as an oncogene in GC progression (35). LINC00200 also functions as a ceRNA,
negatively regulating miR-143-3p expression, while miR-143-3p
negatively regulates its target gene Serpin Family E Member 1
(SERPINE1). Through the aforementioned process, LINC00200
ultimately affects the proliferation of GC cells (36). Furthermore, miR-143-3p is considered
the downstream target of circ_0006089, and circ_0006089 can
actively modulate insulin-like growth factor 1 receptor (IGF1R)
expression by inhibiting miR-143-3p, thus promoting the malignant
progression of GC cells (20).
Another study demonstrated that circ_0006089 knockdown could
inhibit polypyrimidine tract-binding protein 3 (PTBP3) expression
by increasing miR-143-3p expression, which subsequently inhibited
GC cell proliferation (37).
Similarly, circFOXO3 can directly interact with miR-143-3p and
negatively regulate its expression, consequently upregulating
ubiquitin-specific peptidase 44 (USP44) expression, ultimately
facilitating GC cell proliferation and migration (38).
Colorectal cancer (CRC)
It is estimated that CRC accounted for ~1/10 cancer
cases and related deaths in 2020 (1). There is some evidence suggesting that
miR-143-3p has great potential for predicting the prognosis of
patients with CRC (39,40). Recent research has also suggested
that miR-143-3p influences CRC cell development. In most cases, the
expression level of miR-143-3p is low in CRC. MiR-143-3p can be
targeted by circ-ACAP2, while frizzled class receptor 4 (FZD4) is
the target of miR-143-3p in CRC cells. Circ-ACAP2 induces FZD4
expression through interaction with miR-143-3p, thereby promoting
progression and radioresistance in CRC (41). miR-143-3p is also a target of the
lncRNA TPMO-AS1. By targeting miR-143-3p, CRC cell invasion,
proliferation and migration are influenced (42). In addition, LINC00908 acts as an
oncogenic lncRNA that negatively regulates miR-143-3p. Kruppel-like
factor 5 (KLF5) is a target of miR-143-3p. LINC00908 actively
regulates KLF5 expression by secreting miR-143-3p, thereby
modulating CRC cell apoptosis and proliferation (43). The Coiled-lncRNA Coil Domain
Containing 144 N-Terminal-Like antisense 1 (CCDC144NL-AS1), a novel
carcinogenic lncRNA, is highly expressed in the serum of patients
with CRC. CCDC144NL-AS1 can act as a ‘sponge’ of hsa-miR-143-3p,
thereby upregulating its target protein High Mobility Group AT-hook
2, and this interaction is correlated with tumor stage and size
(44). On the other hand,
miR-143-3p can also directly affect its target genes and
participate in CRC progression. miR-143-3p plays a role as an
anticancer gene by downregulating integrin alpha 6 (ITGA6)/ArfGAP
with the SH3 domain and ankyrin repeat and PH domain 3 protein
expression (45). When
overexpressed, miR-143-3p can also directly target CTNND1 and
suppress its expression, thereby suppressing CRC cell
proliferation, migration and invasion (46).
Gallbladder carcinoma (GBC)
GBC is the most common biliary malignancy and has a
poor prognosis and a 5-year survival rate of ~5% (47). It has been previously reported that
miR-143-3p can downregulate SPLGF expression by targeting ITGA6,
thereby inhibiting tumor angiogenesis and GBC growth (48). In addition, miR-143-3p is a target
of the lncRNA OIP5-AS1, which regulates GBC cell biological
function by mediating miR-143-3p expression (49).
Pancreatic cancer
The prognosis of pancreatic cancer is very poor, and
the 5-year survival rate is low, at 3–5% (50). It is often diagnosed in the advanced
stage and responds poorly to treatment. Therefore, additional
studies are needed to identify effective treatments for pancreatic
cancer. Research has shown that miR-143-3p expression is
downregulated in pancreatic ductal adenocarcinoma (PDAC).
miR-143-3p targets KRAS to inhibit PDAC tumorigenesis (51).
Esophageal cancer
Esophageal cancer is a common tumor with a very poor
prognosis and an extremely high mortality rate. According to 2020
statistics, it ranks sixth overall in terms of the global cancer
mortality rate (1). It has been
recently proposed that miR-143-3p plays a crucial role in
modulating esophageal cancer cell proliferation. miR-143-3p can
interact with the lncRNA CASC7 to regulate hexokinase 2 (HK2)
expression to affect tumor glycolysis and cell proliferation
(52).
miR-143-3p in the urinary system
BC
BC is the 10th most common cancer worldwide and is
more common in men than in women (1). Research has shown that miR-143-3p is
modulated mainly by lncRNAs in BC. Among them, LINC02470 is
upregulated in BC cells to accelerate their invasion. LINC02470
inhibits miR-143-3p to induce SMAD3 expression. Subsequently, SMAD3
activates TGF-β-associated EMT processes, ultimately exacerbating
the malignant behavior of BC cells (21). LINC00511 is also upregulated in BC.
LINC00511 downregulation inhibits tumor growth in vivo, and
LINC00511 competes with miR-143-3p and inhibits its expression.
LINC00511 targets miR-143-3p/PCMT1 in addition to modulating BC
cell migration, apoptosis and proliferation and facilitating BC
cell occurrence and development (53). hsa_circ_0000644 (circ_644) also acts
as a sponge of miR-143-3p in BC, upregulating the expression of
MSI2 and promoting the malignant progression of cancer cells
(54). In addition, the lncRNA
SNHG1 inhibits miR-143-3p expression in BC cells. HK2 is a target
of miR-143-3p. SNHG1 promotes BC cell migration, invasion and
proliferation by repressing miR-143-3p and inducing HK2 expression
(55). Another study showed that
the lncRNA MFG-AS1 targets miR-143-3p to inhibit its expression,
and Cyclooxygenase-2 (COX-2) is a target of miR-143-3p. The lncRNA
MFG-AS1 can promote BC development by regulating the
miR-143-3p/COX-2 axis (56). The
lncRNA prostate cancer-associated transcript 6 (PCAT6) can also
play a carcinogenic role in BC by repressing miR-143-3p expression
and upregulating PDIA6 expression (57).
Prostate cancer (PCa)
PCa is estimated to be the second most common cancer
in men in China and is the most frequently diagnosed cancer in men
in most other countries (1). In
PCa, miR-143-3p is suppressed by lncRNA-Schlap1, and DNMT3a is a
target of miR-143-3p and mediates the oncogenic effect of SChLAP1
in the development of PCa (58). In
addition, the lncRNA Plasmacytoma variant translocation 1 enhances
NOP2 expression by downregulating miR-143-3p, promoting the
metastasis and progression of PCa (59). Similarly, the lncRNA PCSEAT
facilitates PCa cell proliferation by competitively binding to
miR-143-3p and inhibiting its expression, sequentially regulating
enhancer of zeste homolog 2 expression (60). In addition, the overexpression of
miR-143-3p in PCa may inhibit EMT by targeting AKT1 (61). Another study showed that the
glucocorticoid receptor (GR), which downregulates miR-143-3p
expression, is upregulated in castration-resistant PCa (CRPC)
cells. miR-143-3p can target Jagged1 (JAG1) and NOTCH2. GR
accelerates the malignant progression of CRPC through
downregulating miR-143-3p expression and upregulating JAG1/NOTCH2
expression (62).
Renal cell carcinoma (RCC)
RCC is considered to be one of the most aggressive
malignant tumors; it accounts for ~3% of all cancers and is the
main type of renal malignancy (63). In RCC, miR-143-3p is regulated
mainly by lncRNAs. lncRNA-SARCC binds to and induces the
degradation of the androgen receptor (AR) protein, inhibits AR
function, and contributes to the increased expression of
miR-143-3p, which inhibits downstream signaling molecules, such as
K-RAS, P-ERK, AKT and MMP-13; thus, lncRNA-SARCC plays a role in
inhibiting RCC progression (64).
LINC00667 is upregulated in clear cell RCC (ccRCC) and enhances
ZEB1 expression by targeting miR-143-3p, thereby accelerating the
progression of ccRCC and inducing chemotherapy resistance (11). In addition, the lncRNA SLC16A1-AS1
is highly expressed in RCC and can induce ferroptosis, which is a
form of programmed cell death that has antitumor effects on
numerous cancers. SLC16A1-AS1 can also inhibit miR-143-3p
expression. Solute carrier family 7 membrane 11 (SLC7A11) is a
target of miR-143-3p. In brief, silencing of the lncRNA SLC16A1-AS1
induces ferroptosis via miR-143-3p/SLC7A11 signal transduction,
which decreases the viability and suppresses the migration and
proliferation of RCC cells (65).
miR-143-3p in the reproductive
system
CC
CC is the fourth most common cancer in women. While
there are highly effective primary and secondary prevention
measures, including the HPV vaccine and screening, vaccination and
CC screening are unavailable in some low-income countries (1). It is therefore necessary to find more
effective treatments. It has commonly been assumed that miR-143-3p
interacts with lncRNAs to modulate target expression levels to play
a part in CC. In CC, the expression of the lncRNA OIP5-AS1 is
markedly enhanced, which is associated with progression. lncRNA
OIP5-AS1 may induce SMAD3, ITGA6 and ROCK1 expression by
suppressing miR-143-3p expression, subsequently accelerating the
invasion and migration of CC cells (66–68).
In addition, the lncRNA MeOX2-AS1 is highly expressed in CC and can
act as a sponge for miR-143-3p. MeOX2-AS1 knockdown inhibits the
progression of CC by inhibiting voltage-dependent anion channel 1
expression through miR-143-3p (22). The lncRNA ACTA2-AS1 can also
upregulate SMAD3 by acting as a ceRNA of miR-143-3p, thereby
accelerating CC progression (69).
Another study proposed that the lncRNA HOTAIR was upregulated in
CC, while miR-143-3p was downregulated. HOTAIR facilitates the
growth of CC cells by regulating BCL2 through miR-143-3p (70). The lncRNA TMPO-AS1 is also highly
expressed in CC cells. TMPO-AS1 acts as a sponge of miR-143-3p to
suppress its expression to increase ZEB1 expression, ultimately
facilitating the migration, invasion and proliferation of CC cells
(71). On the other hand,
miR-143-3p overexpression can also repress the migration, invasion
and proliferation of CC cells by inhibiting CDK1 (72).
Ovarian cancer (OC)
OC is the most lethal gynecologic malignancy, and
the most common subtype is epithelial OC (EOC). The lack of
specific symptoms prevents early detection of the disease, and most
confirmed cases are diagnosed at an advanced stage. Therefore,
biomarkers for the early diagnosis of OC are urgently needed
(73). In malignant ovarian tumors,
miR-143-3p is downregulated, and cystatin B (CSTB) is
overexpressed. Transforming growth factor-β (TGF-β) is an essential
cytokine that promotes or inhibits tumor growth. TGF-β1 regulates
miR-143-3p so that it can directly bind to the CSTB 3′-UTR,
resulting in a reduction in CSTB expression in OC cells, and
knockdown of CSTB leads to a reduction in OC cell proliferation
(74). In addition, loss of TGF-β
reactivity may lead to downregulation of miR-143-3p, which
consequently leads to upregulation of PNPO and accelerates the
migration and invasion of EOC cells (73). The ribosomal protein L10 (RPL10) is
upregulated in human ovarian malignancies. Although RPL10 is not
targeted by miR-143-3p, its expression is modulated by this miRNA.
miR-143-3p inhibits RPL10 expression, consequently decreasing the
viability, suppressing the invasion and migration, and inducing the
apoptosis of EOC cells (75).
RalA-binding protein 1 (RALBP1) is a target gene of miR-143-3p, and
the downregulation of miR-143-3p leads to increased expression of
RALBP1, thereby promoting the occurrence of OC (76). Other evidence has demonstrated that
the lncRNA PCAT6, as a ceRNA, regulates the TGF-β-activated kinase
1 (TAK1) expression by binding to miR-143-3p. miR-143-3p
downregulates the expression of TAK1 and inhibits invasion,
proliferation and migration in OC cells. Subsequent rescue assays
also confirmed that upregulation of miR-143-3p reduced invasion,
proliferation and migration induced by PCAT6 overexpression
(77,78). Dual-luciferase reporter assays
revealed that the lncRNA MALAT1 interacts with miR-143-3p, and that
a low MALAT1 level leads to increased miR-143-3p expression, which
in turn leads to decreased CMPK protein expression and suppressed
migration and invasion in EOC (79). In addition, the lncRNA
cyclin-dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B-AS1)
is highly expressed in OC and is positively related to the
malignant progression of OC. The lncRNA CDKN2B-AS1 is a molecular
sponge of miR-143-3p; its binding results in the de-repression of
the expression of miR-143-3p, which targets SMAD3 and ultimately
triggers OC progression (80).
Endometrial cancer
Endometrial cancer, the sixth most common cancer in
women, led to 97,000 deaths worldwide in 2020 (1). It has been previously suggested that
miR-143-3p interacts with the lncRNA urothelial carcinoma
associated 1 (UCA1), and that the lncRNA UCA1 is highly expressed
in endometrial cancer. The upregulated expression of UCA1 is
associated with endometrial cancer progression and deterioration of
the disease. UCA1 may upregulate KLF5 and relaxin family peptide
receptor 1 expression by sponging miR-143-3p, thereby promoting the
development of endometrial cancer (81). However, additional studies are
necessary to verify the effect of miR-143-3p in endometrial
cancer.
miR-143-3p in other malignant
tumors
In addition to its role in other cancers, miR-143-3p
is often downregulated in numerous other cancers and affects tumor
progression. miR-143-3p is expressed at low levels in TC and can
interact with LINC01296. Silencing LINC01296 promoted miR-143-3p
expression. MSI2 is targeted by miR-143-3p. Silencing LINC01296 can
repress the development of TC by suppressing its competitive
binding with miR-143-3p and inhibiting MSI2 (82). MiR-143-3p can also directly target
MSI2 in papillary thyroid carcinoma (PTC). Upregulation of
miR-143-3p represses cell growth, invasion and migration by
downregulating MSI2, thereby inhibiting the progression of PTC
(23). miR-143-3p expression is
downregulated in laryngeal squamous cell carcinoma (LSCC). K-Ras is
a target of miR-143-3p in LSCC cells. miR-143-3p can suppress the
growth, invasion and migration of LSCC cells by inhibiting K-Ras
(83). MAGE-A9 is also a target of
miR-143-3p, and downregulation of miR-143-3p contributes to LSCC
progression by upregulating MAGE-A9 (84). In nasal SCC, miR-143-3p has an
obvious tumor suppressive effect via negatively regulating Bcl-2
and IGF1R (85). In breast cancer,
miR-143-3p is downregulated. miR-143-3p targets MYB proto-oncogene
like 2 (MYBL2) and mitogen activated protein kinase 7 (MAPK7) and
inhibits the expression of MYBL2 and MAPK7, sequentially
accelerating proliferation and inhibiting breast cancer cell
apoptosis (86,87). miR-143-3p can also inhibit LIMK1
expression to accelerate triple-negative breast cancer cell
proliferation (88). In addition,
the lncRNA PSMG3-AS1 modulates COL1A1 expression by repressing
miR-143-3p, thereby affecting breast cancer development (89). The lncRNA LOXL1-AS1 affects breast
cancer cell invasion, proliferation and migration by directly
targeting miR-143-3p (90).
In addition to its role in these cancers, miR-143-3p
also affects the progression of neuroblastoma, acute myeloid
leukemia (AML), osteosarcoma (OS), melanoma and oral SCC (12,91–99).
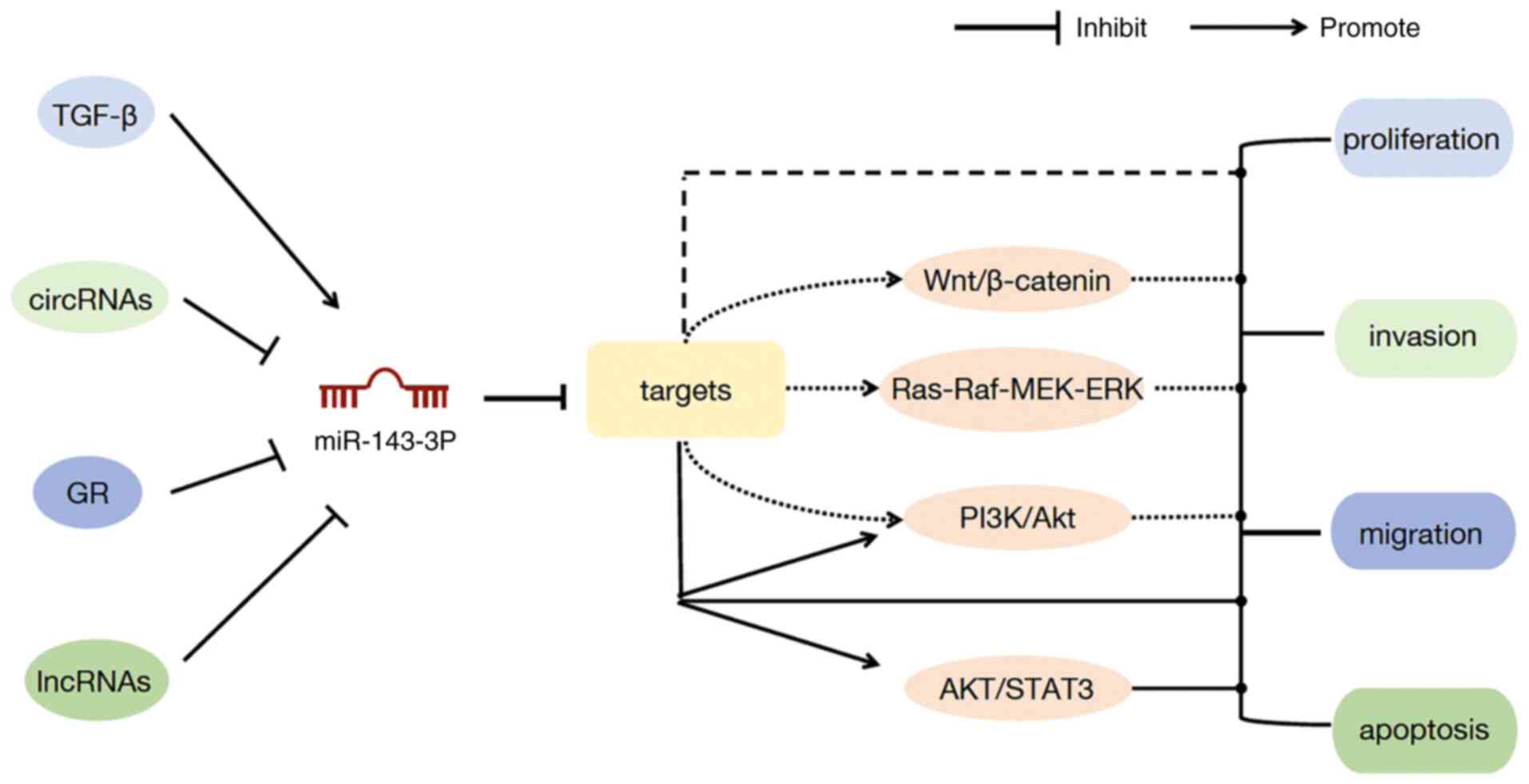
Mechanism of miR-143-3p
According to previous studies (13,21,22,41,45),
it is considered by numerous scholars that the mechanisms of action
of miR-143-3p include the following: i) LncRNAs, circRNAs and
cytokines interact with miR-143-3p to regulate its expression,
thereby affecting its target gene expression; ii) miR-143-3p
directly targets relevant target genes and plays a part in
regulating the expression of target genes; and iii) it regulates
tumor growth by affecting signaling pathways. The first two
mechanisms of action have been aforementioned in various systemic
cancers, and the associated signaling pathways are demonstrated in
Fig. 2.
PI3K/Akt signaling pathway
The PI3K/Akt signaling pathway is a crucial
signaling pathway in a variety of cancer types. It modulates tumor
cell survival, metastasis and metabolism (99). Exosomal miR-143-3p derived from
G-MDSCs in LC tissues promotes cancer progression by targeting
ITM2B to stimulate the PI3K/Akt pathway (13). In addition, circ_0006089 knockdown
suppresses PTBP3 expression and the PI3K/AKT pathway by increasing
miR-143-3p expression, consequently repressing the proliferation of
GC cells (37). In GBC, ITGA6 is
upregulated and negatively associated with miR-143-3p. miR-143-3p
downregulates PLGF expression via the ITGA6/PI3K/AKT/STAT3 pathway,
thereby inhibiting tumor angiogenesis and GBC growth (48).
Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway comprises four
parts: The membrane segment, the extracellular signaling pathway,
the cytoplasmic segment, and the extracellular signaling pathway.
These pathways are regulated mainly by Wnt proteins, which include
Wnt1, Wnt3a and Wnt5a. A variety of serious diseases, including
encompassing cancer and non-cancer diseases, often result from
dysregulation of Wnt/β-catenin signaling (100). It has been suggested that FZD4 can
participate in activating the Wnt/β-catenin pathway. In CRC, FZD4
is a target of miR-143-3p, and the accumulation of FZD4 or
downregulation of miR-143-3p can induce the Wnt/β-catenin pathway
to participate in the modulation of CRC progression and
radioresistance (41).
AKT/STAT3 signaling pathway
The AKT/STAT3 signaling pathway has an essential
function in the development of cancer and can enhance the antitumor
immune response by modulating PD-L1 expression in cancer cells with
abnormal EGFR activity (101). In
addition, MACC1 can influence CC cell invasion, migration,
apoptosis and cancer stemness by modulating the AKT/STAT3 pathway
(102). In TC, LINC01296 can
repress miR-143-3p expression through competitive binding with
miR-143-3p, and downregulation of miR-143-3p can induce MSI2 and
subsequently activate the AKT/STAT3 signaling pathway to promote
the development of TC (82).
Ras-Raf-MEK-ERK signaling pathway
The Ras-Raf-MEK-ERK signaling pathway is the most
well-studied MAPK pathway and has important functions in cell
proliferation, survival, differentiation and development (103). In LSCC, K-Ras is the target of
miR-143-3p. Downregulation of miR-143-3p induces K-ras expression
and subsequently activates the K-ras/Raf/MEK/ERK signaling pathway
to promote cell growth, invasion and migration in LSCC (83).
Potential application of miR-143-3p in
future practice
As a diagnostic marker
Numerous studies (12,13,28,39,40)
have revealed that miRNAs may serve as potential biomarkers in
cancer. Among them, miR-143-3p inhibited human melanoma cell
proliferation and facilitated their apoptosis, demonstrating that
miR-143-3p may be a novel marker for early diagnosis (12). In LUAD, the lncRNA PCAT19 regulates
invasion, proliferation and migration and may be an underlying
diagnostic biomarker of LUAD. miR-143-3p is the target of lncRNA
PCAT19, therefore miR-143-3p may also be a useful diagnostic marker
of LUAD (28). On the other hand,
miR-143-3p has been found to suppress OC cell invasion, migration
and proliferation by targeting TAK1 in vitro and to inhibit
ovarian tumorigenicity in vivo. These findings revealed that
the miR-143-3p-TAK1 pathway has promising application potential in
the clinical diagnosis of OC (77).
As a prognostic marker
Recent research has identified that miR-143-3p may
be useful as a prognostic marker in cancer. In LUAD, the lncRNA
PCAT19 is a self-controlled prognostic factor that can modulate
LUAD cell proliferation, invasion and migration. miR-143-3p is the
target of lncRNA PCAT19, thus miR-143-3p may also predict the
prognosis of patients with LUAD (28). The lncRNA SOx2-OT is highly
expressed in HCC and is positively associated with poor prognosis
in patients with HCC. However, the lncRNA SOx2-OT negatively
modulates miR-143-3p expression. Therefore, miR-143-3p may also be
related to the prognosis of patients with HCC (31). In addition, according to a 2-cohort
study of 254 pretreated patients with rectal adenocarcinoma,
miR-143-3p was overexpressed in tumors and was more highly
expressed in stage II or III tumors than in stage I tumors
(39). Another study proposed that
the miR-143-3p expression level was greater in patients diagnosed
with CRC aged >60 years than in those diagnosed with CRC aged
<60 years (P=0.048), and the miR-143-3p level was greater in
non-mucinous tumor subtypes than in mucinous tumor subtypes
(P=0.025), indicating that miR-143-3p is highly expressed in
patients diagnosed with CRC who are <60 years old (P=0.048).
MiR-143-3p expression is associated with the age at diagnosis,
pathological type and tumor stage of patients with CRC and will be
helpful for early screening of young and elderly patients with CRC
and for more accurate evaluation of patient prognosis (40). Another study reported that
miR-143-3p is significantly downregulated in GBC, and this
downregulation is associated with a poor prognosis in patients with
GBC (48). Low miR-143-3p
expression is also associated with more advanced clinical stages of
LSCC and with a decreased overall survival rate (84). In summary, miR-143-3p can be used as
a prognostic marker in multiple tumors.
As a therapeutic target
In a variety of cancers, miR-143-3p regulates tumor
progression by modulating the expression of target genes. In
melanoma, miR-143-3p targets COX-2 to repress proliferation and
promote apoptosis in human melanoma cells (12). Exosomes of miR-143-3p derived from
G-MDSCs in LC promote the proliferation of LC by targeting ITM2B in
LC (13). In HCC, miR-143-3p
represses HCC cell invasion and proliferation by influencing its
target gene FGF1 (34). In CRC,
miR-143-3p directly targets CTNND1 to affect cell invasion,
proliferation and migration (46).
In OC, miR-143-3p downregulates TAK1 expression and suppresses OC
cell invasion, migration and proliferation in vitro
(77). Moreover, miR-143-3p also
has a similar role in AML, breast cancer and OS. These findings are
sufficient to indicate that miR-143-3p is a potential therapeutic
target in cancer.
Discussion
At present, diagnostic markers and treatment methods
for cancer are still hot topics in the field of cancer research,
and difficult problems are being gradually solved. In recent years,
miRNAs have been found to play important roles in tumorigenesis and
development. Among them, miR-143-3p is upregulated in LC with BMs
and downregulated in numerous tumors; moreover, it can act as an
oncogene to affect tumor progression in most cases. miR-143-3p has
dual effects on RCC through its target genes but plays only
positive roles in other cancers (Fig.
3) Moreover, miR-143-3p represents a favorable biomarker with
great potential for early diagnosis, treatment and prognosis
evaluation in patients with cancer. Moreover, it was discovered
that miR-143-3p expression is modulated by upstream signaling
factors; subsequently miR-143-3p acts on target genes to affect
tumor cell invasion, migration, proliferation and apoptosis.
Mechanistically, miR-143-3p, an upstream signaling factor, can act
on different target genes in the same cancer (20,37).
Different cancers can be regulated by an identical upstream
signaling factor (25,42). After interacting with miR-143-3p,
different upstream signaling factors can also target the same genes
(52,55). The upstream signaling
factor-miR-143-3p-target gene network is complex. Although
miR-143-3p can directly inhibit target genes to affect tumor
progression, it is likely that there are numerous upstream
regulatory factors that have not been discovered, and the discovery
of these upstream regulatory factors will be highly important for
miR-143-3p-targeted cancer treatment. In addition, there are few
studies on the signaling pathways associated with the effect of
miR-143-3p, and further exploration of the potential mechanism is
needed, as this information will lead to additional ideas for
cancer treatments related to miR-143-3p.
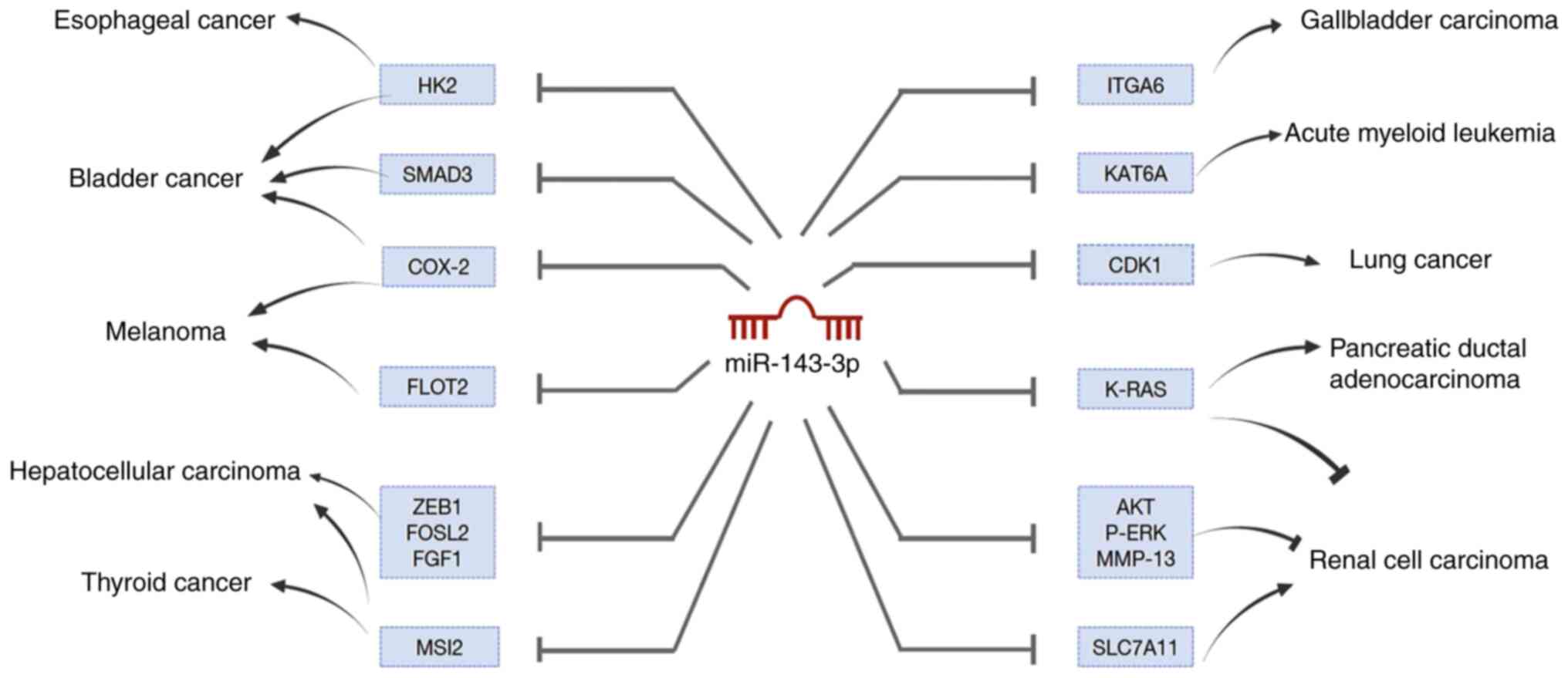 | Figure 3.MiR-143-3p targets a large number of
genes to inhibit or promote cancers' growth, including esophageal
cancer, bladder cancer, melanoma, hepatocellular carcinoma, thyroid
cancer, gallbladder carcinoma, acute myeloid leukemia, lung cancer,
pancreatic ductal adenocarcinoma and renal cell carcinoma. miR,
microRNA; CDK1, cyclin-dependent kinase 1; FGF1, fibroblast growth
factor 1; ITGA6, integrin alpha 6; HK2, hexokinase 2; COX-2,
Cyclooxygenase-2; SLC7A11, solute carrier family 7 membrane 11;
ZEB1, zinc finger E-box binding homeobox 1; MSI2, Musashi RNA
binding protein 2; KAT6A, lysine acetyltransferase 6A; FOSL2,
FOS-Like antigen 2; FLOT2, Flotillin 2. |
Furthermore, in 2015, researchers demonstrated that
the exosomes of bone marrow mesenchymal stem cells (BMSCs)
contained high levels of miR-143-3p (104), and the expression of miR-143-3p in
hMSC exosomes was also significantly increased; these exosomes
could promote the apoptosis of pancreatic cancer cells and suppress
cell growth and invasion (50). In
addition, MSC-derived exosomes are closely related to drug therapy,
molecular targeted therapy, radiotherapy and chemotherapy for
cancer (105). However, there is
little research on miR-143-3p in exosomes at present, and numerous
future studies are urgently needed to prove its importance in
clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81673728 and 82274260).
Availability of data and materials
Not applicable.
Authors' contributions
JW acquired the data and wrote the manuscript. YZ
conceived the study. DL, QC and CB contributed to the revisions.
All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farmer P, Frenk J, Knaul FM, Shulman LN,
Alleyne G, Armstrong L, Atun R, Blayney D, Chen L, Feachem R, et
al: Expansion of cancer care and control in countries of low and
middle income: A call to action. Lancet. 376:1186–1193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maskalenko NA, Zhigarev D and Campbell KS:
Harnessing natural killer cells for cancer immunotherapy:
Dispatching the first responders. Nat Rev Drug Discov. 21:559–577.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Punekar SR, Velcheti V, Neel BG and Wong
KK: The current state of the art and future trends in RAS-targeted
cancer therapies. Nat Rev Clin Oncol. 19:637–655. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zhang H, Liu C, Wang Z, Wu W,
Zhang N, Zhang L, Hu J, Luo P, Zhang J, et al: Immune checkpoint
modulators in cancer immunotherapy: Recent advances and emerging
concepts. J Hematol Oncol. 15:1112022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47(D1): D155–D162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang Y, Lin J and Tsung A: Manipulation
of autophagy by MIR375 generates antitumor effects in liver cancer.
Autophagy. 8:1833–1834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Chen P, Tan C, Cheng X, Zhang W,
Shen C and Zhang D: LncRNA LINC00667 gets involved in clear cell
renal cell carcinoma development and chemoresistance by regulating
the miR-143-3p/ZEB1 axis. Aging (Albany NY). 15:10057–10071. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panza E, Ercolano G, De Cicco P, Armogida
C, Scognamiglio G, Botti G, Cirino G and Ianaro A: MicroRNA-143-3p
inhibits growth and invasiveness of melanoma cells by targeting
cyclooxygenase-2 and inversely correlates with malignant melanoma
progression. Biochem Pharmacol. 156:52–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou JH, Yao ZX, Zheng Z, Yang J, Wang R,
Fu SJ, Pan XF, Liu ZH and Wu K: G-MDSCs-derived exosomal
miRNA-143-3p promotes proliferation via targeting of ITM2B in lung
cancer. Onco Targets Ther. 13:9701–9719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee Y, Jeon K, Lee JT, Kim S and Kim VN:
MicroRNA maturation: Stepwise processing and subcellular
localization. EMBO J. 21:4663–4670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34((Database Issue)):
D140–D144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morales-Martinez M and Vega MI: Role of
MicroRNA-7 (MiR-7) in cancer physiopathology. Int J Mol Sci.
23:90912022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu T, Qiu T, Han B, Wang Y, Sun X, Qin Y,
Liu A, Ge N and Jiao W: Circular RNA circCSNK1G3 induces HOXA10
signaling and promotes the growth and metastasis of lung
adenocarcinoma cells through hsa-miR-143-3p sponging. Cell Oncol
(Dordr). 44:297–310. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Song Z, Meng Q, Xia S, Wang C and
Huang X: Circular RNA circ_0006089 regulates the IGF1R expression
by targeting miR-143-3p to promote gastric cancer proliferation,
migration and invasion. Cell Cycle. May 11;1–14. 2022.(Epub ahead
of print). doi: 10.1080/15384101.2022.2075197. View Article : Google Scholar
|
|
21
|
Huang CS, Tsai CH, Yu CP, Wu YS, Yee MF,
Ho JY and Yu DS: Long Noncoding RNA LINC02470 sponges
MicroRNA-143-3p and enhances SMAD3-mediated
epithelial-to-mesenchymal transition to promote the aggressive
properties of bladder cancer. Cancers (Basel). 14:9682022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XX, Bao QX, Li YM and Zhang YH: The
promotion of cervical cancer progression by signal transducer and
activator of transcription 1-induced up-regulation of lncRNA
MEOX2-AS1 as a competing endogenous RNA through miR-143-3p/VDAC1
pathway. Bioengineered. 12:3322–3335. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZL, Wang C, Liu W and Ai ZL:
Upregulation of microRNA-143-3p induces apoptosis and suppresses
proliferation, invasion, and migration of papillary thyroid
carcinoma cells by targeting MSI2. Exp Mol Pathol. 112:1043422020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen R, Zhang CF, Cheng YD, Wang SQ, Lin H
and Zhang H: LncRNA UCC promotes epithelial-mesenchymal transition
via the miR-143-3p/SOX5 axis in non-small-cell lung cancer. Lab
Invest. 101:1153–1165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Bian Y and Li QL: Down-regulation of
TMPO-AS1 induces apoptosis in lung carcinoma cells by regulating
miR-143-3p/CDK1 axis. Technol Cancer Res Treat.
20:15330338209488802021.PubMed/NCBI
|
|
26
|
Yang Y, Li S, Cao J, Li Y, Hu H and Wu Z:
RRM2 regulated by LINC00667/miR-143-3p signal is responsible for
non-small cell lung cancer cell progression. Onco Targets Ther.
12:9927–9939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Jia Y, Wang B, Yang S, Du K, Luo
Y, Li Y and Zhu B: Circular RNA TUBA1C accelerates the progression
of non-small-cell lung cancer by sponging miR-143-3p. Cell Signal.
74:1096932020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang X, Hua X, Peng X, Pei Y and Chen Z:
Integrated dissection of lncRNA-miRNA-mRNA pairs and potential
regulatory role of lncRNA PCAT19 in lung adenocarcinoma. Front
Genet. 12:7652752022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen
Z, Dinglin X, Ma S, Li D, Wu Y, et al: N6-methyladenosine induced
miR-143-3p promotes the brain metastasis of lung cancer via
regulation of VASH1. Mol Cancer. 18:1812019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song LN, Qiao GL, Yu J, Yang CM, Chen Y,
Deng ZF, Song LH, Ma LJ and Yan HL: Hsa_circ_0003998 promotes
epithelial to mesenchymal transition of hepatocellular carcinoma by
sponging miR-143-3p and PCBP1. J Exp Clin Cancer Res. 39:1142020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Bi M, Lou M, Yang X and Sun L:
Downregulation of SOX2-OT prevents hepatocellular carcinoma
progression through miR-143-3p/MSI2. Front Oncol. 11:6859122021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Yao H, Wang K and Liu X: Long
non-coding RNA MALAT1 regulates ZEB1 expression by sponging
miR-143-3p and promotes hepatocellular carcinoma progression. J
Cell Biochem. 118:4836–4843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Huang J, Chen W, Hu Z and Wang X:
miR-143-3p targets lncRNA PSMG3-AS1 to inhibit the proliferation of
hepatocellular carcinoma cells. Cancer Manag Res. 12:6303–6309.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng J, Wu HJ, Zhang HF, Fang SQ and Zeng
R: miR-143-3p inhibits proliferation and invasion of hepatocellular
carcinoma cells by regulating its target gene FGF1. Clin Transl
Oncol. 23:468–480. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan H, Ge Y, Ma X, Li Z, Shi L, Lin L,
Xiao J, Chen W, Ni P, Yang L and Xu Z: Long non-coding RNA
CCDC144NL-AS1 sponges miR-143-3p and regulates MAP3K7 by acting as
a competing endogenous RNA in gastric cancer. Cell Death Dis.
11:5212020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He W, Zhang D, Li D, Zhu D, Geng Y, Wang
Q, He J and Wu J: Knockdown of long non-coding RNA LINC00200
inhibits gastric cancer progression by regulating
miR-143-3p/SERPINE1 axis. Dig Dis Sci. 66:3404–3414. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin GR, Chen WR, Zheng PH, Chen WS and Cai
GY: Circular RNA circ_0006089 promotes the progression of gastric
cancer by regulating the miR-143-3p/PTBP3 axis and PI3K/AKT
signaling pathway. J Dig Dis. 23:376–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiang T, Jiang HS, Zhang BT and Liu G:
CircFOXO3 functions as a molecular sponge for miR-143-3p to promote
the progression of gastric carcinoma via upregulating USP44. Gene.
753:1447982020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim JK, Qu X, Chen CT, Smith JJ,
Sanchez-Vega F and Garcia-Aguilar J: Identifying diagnostic
MicroRNAs and investigating their biological implications in rectal
cancer. JAMA Netw Open. 4:e21369132021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moreno EC, Pascual A, Prieto-Cuadra D,
Laza VF, Molina-Cerrillo J, Ramos-Muñoz ME, Rodríguez-Serrano EM,
Soto JL, Carrato A, García-Bermejo ML and Guillén-Ponce C: Novel
molecular characterization of colorectal primary tumors based on
miRNAs. Cancers (Basel). 11:3462019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang G, Liu Z, Zhong J and Lin L:
Circ-ACAP2 facilitates the progression of colorectal cancer through
mediating miR-143-3p/FZD4 axis. Eur J Clin Invest. 51:e136072021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao L, Li Y and Song A: Inhibition of
lncRNA TMPO-AS1 suppresses proliferation, migration and invasion of
colorectal cancer cells by targeting miR-143-3p. Mol Med Rep.
22:3245–3254. 2020.PubMed/NCBI
|
|
43
|
Shan TD, Tian ZB, Li Q, Jiang YP, Liu FG,
Sun XG, Han Y, Sun LJ and Chen L: Long intergenic noncoding RNA
00908 promotes proliferation and inhibits apoptosis of colorectal
cancer cells by regulating KLF5 expression. J Cell Physiol.
236:889–899. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abd El Fattah YK, Abulsoud AI, AbdelHamid
SG, AbdelHalim S and Hamdy NM: CCDC144NL-AS1/hsa-miR-143-3p/HMGA2
interaction: In-silico and clinically implicated in CRC
progression, correlated to tumor stage and size in case-controlled
study; step toward ncRNA precision. Int J Biol Macromol.
253:1267392023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo L, Fu J, Sun S, Zhu M, Zhang L, Niu H,
Chen Z, Zhang Y, Guo L and Wang S: MicroRNA-143-3p inhibits
colorectal cancer metastases by targeting ITGA6 and ASAP3. Cancer
Sci. 110:805–816. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding X, Du J, Mao K, Wang X, Ding Y and
Wang F: MicroRNA-143-3p suppresses tumorigenesis by targeting
catenin-delta1 in colorectal cancer. Onco Targets Ther.
12:3255–3265. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shu YJ, Bao RF, Jiang L, Wang Z, Wang XA,
Zhang F, Liang HB, Li HF, Ye YY, Xiang SS, et al: MicroRNA-29c-5p
suppresses gallbladder carcinoma progression by directly targeting
CPEB4 and inhibiting the MAPK pathway. Cell Death Differ.
24:445–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin YP, Hu YP, Wu XS, Wu YS, Ye YY, Li HF,
Liu YC, Jiang L, Liu FT, Zhang YJ, et al: miR-143-3p targeting of
ITGA6 suppresses tumour growth and angiogenesis by downregulating
PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma.
Cell Death Dis. 9:1822018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li J, Zhang H and Luo H: Long non-coding
RNA OIP5-AS1 contributes to gallbladder cancer cell invasion and
migration by miR-143-3p suppression. Cancer Manag Res.
12:12983–12992. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang B, Xu Y, Wei Y, Lv L, Liu N, Lin R,
Wang X and Shi B: Human mesenchymal stem cell-derived exosomal
microRNA-143 promotes apoptosis and suppresses cell growth in
pancreatic cancer via target gene regulation. Front Genet.
12:5816942021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie F, Li C, Zhang X, Peng W and Wen T:
MiR-143-3p suppresses tumorigenesis in pancreatic ductal
adenocarcinoma by targeting KRAS. Biomed Pharmacother.
119:1094242019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun W, Wang D, Zu Y and Deng Y: Long
noncoding RNA CASC7 is a novel regulator of glycolysis in
oesophageal cancer via a miR-143-3p-mediated HK2 signalling
pathway. Cell Death Discov. 8:2312022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dong LM, Zhang XL, Mao MH, Li YP, Zhang
XY, Xue DW and Liu YL: LINC00511/miRNA-143-3p modulates apoptosis
and malignant phenotype of bladder carcinoma cells via PCMT1. Front
Cell Dev Biol. 9:6509992021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou H, Huang J and Wang F: Increased
transcription of hsa_circ_0000644 upon RUNX family transcription
factor 3 downregulation participates in the malignant development
of bladder cancer. Cell Signal. 104:1105902023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiang W, Lyu L, Huang T, Zheng F, Yuan J,
Zhang C and Jiang G: The long non-coding RNA SNHG1 promotes bladder
cancer progression by interacting with miR-143-3p and EZH2. J Cell
Mol Med. 24:11858–11873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li D, Zhong S, Zhu Z, Jiang X, Zhang J, Gu
J and Chen F: LncRNA MAFG-AS1 promotes the progression of bladder
cancer by targeting the miR-143-3p/COX-2 axis. Pathobiology.
87:345–355. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang Y, Chen L and Luo G: Long non-coding
RNA PCAT6 regulates bladder cancer progression via the
microRNA-143-3p/PDIA6 axis. Exp Ther Med. 22:9472021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang K and Tang Y: SChLAP1 promotes
prostate cancer development through interacting with EZH2 to
mediate promoter methylation modification of multiple miRNAs of
chromosome 5 with a DNMT3a-feedback loop. Cell Death Dis.
12:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun F, Wu K, Yao Z, Mu X, Zheng Z, Sun M,
Wang Y, Liu Z and Zhu Y: Long noncoding RNA PVT1 promotes prostate
cancer metastasis by increasing NOP2 expression via targeting tumor
suppressor MicroRNAs. Onco Targets Ther. 13:6755–6765. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang X, Wang L, Li R, Zhao Y, Gu Y, Liu S,
Cheng T, Huang K, Yuan Y, Song D and Gao S: The long non-coding RNA
PCSEAT exhibits an oncogenic property in prostate cancer and
functions as a competing endogenous RNA that associates with EZH2.
Biochem Biophys Res Commun. 502:262–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Armstrong L, Willoughby CE and McKenna DJ:
Targeting of AKT1 by miR-143-3p suppresses
epithelial-to-mesenchymal transition in prostate cancer. Cells.
12:22072023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang L, Jiang H, Zhang Y, Wang C, Xia X
and Sun Y: GR silencing impedes the progression of
castration-resistant prostate cancer through the JAG1/NOTCH2
pathway via up-regulation of microRNA-143-3p. Cancer Biomark.
28:483–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH,
Hofmann F, Hora M, et al: European association of urology
guidelines on renal cell carcinoma: The 2022 update. Eur Urol.
82:399–410. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng
J, Lin W, Huang Q, Li G, Zheng J and Chang C: LncRNA-SARCC
suppresses renal cell carcinoma (RCC) progression via altering the
androgen receptor(AR)/miRNA-143-3p signals. Cell Death Differ.
24:1502–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li YZ, Zhu HC, Du Y, Zhao HC and Wang L:
Silencing lncRNA SLC16A1-AS1 induced ferroptosis in renal cell
carcinoma through miR-143-3p/SLC7A11 signaling. Technol Cancer Res
Treat. 21:153303382210778032022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen X, Xiong D, Yang H, Ye L, Mei S, Wu
J, Chen S, Shang X, Wang K and Huang L: Long noncoding RNA
OPA-interacting protein 5 antisense transcript 1 upregulated SMAD3
expression to contribute to metastasis of cervical cancer by
sponging miR-143-3p. J Cell Physiol. 234:5264–5275. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang J, Jiang B, Hai J, Duan S, Dong X and
Chen C: Long noncoding RNA opa-interacting protein 5 antisense
transcript 1 promotes proliferation and invasion through elevating
integrin α6 expression by sponging miR-143-3p in cervical cancer. J
Cell Biochem. 120:907–916. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Song L, Wang L, Pan X and Yang C: lncRNA
OIP5-AS1 targets ROCK1 to promote cell proliferation and inhibit
cell apoptosis through a mechanism involving miR-143-3p in cervical
cancer. Braz J Med Biol Res. 53:e88832020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Luo L, Wang M, Li X, Luo C, Tan S, Yin S,
Liu L and Zhu X: A novel mechanism by which ACTA2-AS1 promotes
cervical cancer progression: acting as a ceRNA of miR-143-3p to
regulate SMAD3 expression. Cancer Cell Int. 20:3722020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu M, Jia J, Wang X, Liu Y, Wang C and
Fan R: Long non-coding RNA HOTAIR promotes cervical cancer
progression through regulating BCL2 via targeting miR-143-3p.
Cancer Biol Ther. 19:391–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gang X, Yuan M and Zhang J: Long
non-coding RNA TMPO-AS1 promotes cervical cancer cell
proliferation, migration, and invasion by regulating
miR-143-3p/ZEB1 axis. Cancer Manag Res. 12:1587–1599. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tang J, Pan H, Wang W, Qi C, Gu C, Shang A
and Zhu J: MiR-495-3p and miR-143-3p co-target CDK1 to inhibit the
development of cervical cancer. Clin Transl Oncol. 23:2323–2334.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang L, Zhou D, Guan W, Ren W, Sun W, Shi
J, Lin Q, Zhang J, Qiao T, Ye Y, et al: Pyridoxine 5′-phosphate
oxidase is a novel therapeutic target and regulated by the TGF-β
signalling pathway in epithelial ovarian cancer. Cell Death Dis.
8:32142017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Guan W, Wang X, Lin Q, Zhang J, Ren W and
Xu G: Transforming growth factor-β/miR-143-3p/cystatin B axis is a
therapeutic target in human ovarian cancer. Int J Oncol.
55:267–276. 2019.PubMed/NCBI
|
|
75
|
Shi J, Zhang L, Zhou D, Zhang J, Lin Q,
Guan W, Zhang J, Ren W and Xu G: Biological Function of ribosomal
protein L10 on cell behavior in human epithelial ovarian cancer. J
Cancer. 9:745–756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang H and Li W: Dysregulation of
micro-143-3p and BALBP1 contributes to the pathogenesis of the
development of ovarian carcinoma. Oncol Rep. 36:3605–3610. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shi H, Shen H, Xu J, Zhao S, Yao S and
Jiang N: MiR-143-3p suppresses the progression of ovarian cancer.
Am J Transl Res. 10:866–874. 2018.PubMed/NCBI
|
|
78
|
Tan X, Shao Y, Teng Y, Liu S, Li W, Xue L,
Cao Y, Sun C, Zhang J, Han J, et al: The cancer-testis long
non-coding RNA PCAT6 facilitates the malignant phenotype of ovarian
cancer by sponging miR-143-3p. Front Cell Dev Biol. 9:5936772021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lin Q, Guan W, Ren W, Zhang L, Zhang J and
Xu G: MALAT1 affects ovarian cancer cell behavior and patient
survival. Oncol Rep. 39:2644–2652. 2018.PubMed/NCBI
|
|
80
|
Xu C, Zhai J and Fu Y: LncRNA CDKN2B-AS1
promotes the progression of ovarian cancer by miR-143-3p/SMAD3 axis
and predicts a poor prognosis. Neoplasma. 67:782–793. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu T, Wang X, Zhai J, Wang Q and Zhang B:
Long noncoding RNA UCA1 facilitates endometrial cancer development
by regulating KLF5 and RXFP1 gene expressions. Cancer Biother
Radiopharm. 36:521–533. 2021.PubMed/NCBI
|
|
82
|
Wang ZL, Wang C, Liu W and Ai ZL: Emerging
roles of the long non-coding RNA 01296/microRNA-143-3p/MSI2 axis in
development of thyroid cancer. Biosci Rep. 39:BSR201823762019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang F and Cao H: MicroRNA-143-3p
suppresses cell growth and invasion in laryngeal squamous cell
carcinoma via targeting the k-Ras/Raf/MEK/ERK signaling pathway.
Int J Oncol. 54:689–701. 2019.PubMed/NCBI
|
|
84
|
Han L, Tang M, Xu X, Jiang B, Wei Y, Qian
H and Lu X: MiR-143-3p suppresses cell proliferation, migration,
and invasion by targeting melanoma-associated antigen A9 in
laryngeal squamous cell carcinoma. J Cell Biochem. 120:1245–1257.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qian Y, Teng Y, Li Y, Lin X, Guan M, Li Y,
Cao X and Gao Y: MiR-143-3p suppresses the progression of nasal
squamous cell carcinoma by targeting Bcl-2 and IGF1R. Biochem
Biophys Res Commun. 518:492–499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen J and Chen X: MYBL2 is targeted by
miR-143-3p and regulates breast cancer cell proliferation and
apoptosis. Oncol Res. 26:913–922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xia C, Yang Y, Kong F, Kong Q and Shan C:
MiR-143-3p inhibits the proliferation, cell migration and invasion
of human breast cancer cells by modulating the expression of MAPK7.
Biochimie. 147:98–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li D, Hu J, Song H, Xu H, Wu C, Zhao B,
Xie D, Wu T, Zhao J and Fang L: miR-143-3p targeting LIM domain
kinase 1 suppresses the progression of triple-negative breast
cancer cells. Am J Transl Res. 9:2276–2285. 2017.PubMed/NCBI
|
|
89
|
Cui Y, Fan Y, Zhao G, Zhang Q, Bao Y, Cui
Y, Ye Z, Chen G, Piao X, Guo F, et al: Novel lncRNA PSMG3-AS1
functions as a miR-143-3p sponge to increase the proliferation and
migration of breast cancer cells. Oncol Rep. 43:229–239.
2020.PubMed/NCBI
|
|
90
|
Li GH, Yu JH, Yang B, Gong FC and Zhang
KW: LncRNA LOXL1-AS1 inhibited cell proliferation, migration and
invasion as well as induced apoptosis in breast cancer via
regulating miR-143-3p. Eur Rev Med Pharmacol Sci. 23:10400–10409.
2019.PubMed/NCBI
|
|
91
|
Zhu J, Xiang XL, Cai P, Jiang YL, Zhu ZW,
Hu FL and Wang J: CircRNA-ACAP2 contributes to the invasion,
migration, and anti-apoptosis of neuroblastoma cells through
targeting the miRNA-143-3p-hexokinase 2 axis. Transl Pediatr.
10:3237–3247. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xu D, Jiang J, He G, Zhou H and Ji C:
miR-143-3p represses leukemia cell proliferation by inhibiting
KAT6A expression. Anticancer Drugs. 33:e662–e669. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hou Y, Feng H, Jiao J, Qian L, Sun B, Chen
P, Li Q and Liang Z: Mechanism of miR-143-3p inhibiting
proliferation, migration and invasion of osteosarcoma cells by
targeting MAPK7. Artif Cells Nanomed Biotechnol. 47:2065–2071.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun X, Dai G, Yu L, Hu Q, Chen J and Guo
W: miR-143-3p inhibits the proliferation, migration and invasion in
osteosarcoma by targeting FOSL2. Sci Rep. 8:6062018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wu K, Feng Q, Li L, Xiong Y, Liu S, Liu J
and Wu Q: Long-noncoding RNA PCAT6 aggravates osteosarcoma
tumourigenesis via the MiR-143-3p/ZEB1 axis. Onco Targets Ther.
13:8705–8714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tian S, Han G, Lu L and Meng X: Circ-FOXM1
contributes to cell proliferation, invasion, and glycolysis and
represses apoptosis in melanoma by regulating miR-143-3p/FLOT2
axis. World J Surg Oncol. 18:562020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Duan Q, Xu M, Wu M, Zhang X, Gan M and
Jiang H: Long noncoding RNA UCA1 promotes cell growth, migration,
and invasion by targeting miR-143-3p in oral squamous cell
carcinoma. Cancer Med. 9:3115–3129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang S, Li W, Yang L, Yuan J, Wang L, Li N
and Zhao H: CircPVT1 facilitates the progression of oral squamous
cell carcinoma by regulating miR-143-3p/SLC7A11 axis through MAPK
signaling pathway. Funct Integr Genomics. 22:891–903. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yu L, Shao X, Huo L and Zhang T: Long
non-coding RNA (lncRNA) metastasis-associated lung adenocarcinoma
transcript 1 (MALAT1) promotes cell proliferation and migration by
regulating miR-143-3p and MAGE family member A9 (MAGEA9) in oral
squamous cell carcinoma. Med Sci Monit. 26:e9241872020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Abdelhamed S, Ogura K, Yokoyama S, Saiki I
and Hayakawa Y: AKT-STAT3 pathway as a downstream target of EGFR
signaling to regulate PD-L1 expression on NSCLC cells. J Cancer.
7:1579–1586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mei J, Zhu C, Pan L and Li M: MACC1
regulates the AKT/STAT3 signaling pathway to induce migration,
invasion, cancer stemness, and suppress apoptosis in cervical
cancer cells. Bioengineered. 13:61–70. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhao J and Luo Z: Discovery of raf family
is a milestone in deciphering the ras-mediated intracellular
signaling pathway. Int J Mol Sci. 23:51582022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Baglio SR, Rooijers K, Koppers-Lalic D,
Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen
HW, Baldini N and Pegtel DM: Human bone marrow- and
adipose-mesenchymal stem cells secrete exosomes enriched in
distinctive miRNA and tRNA species. Stem Cell Res Ther. 6:1272015.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lin Z, Wu Y, Xu Y, Li G, Li Z and Liu T:
Mesenchymal stem cell-derived exosomes in cancer therapy
resistance: Recent advances and therapeutic potential. Mol Cancer.
21:1792022. View Article : Google Scholar : PubMed/NCBI
|