Introduction
Ovarian cancer is a type of malignant tumor that is
a serious threat to health of women worldwide (1–3).
Currently, the standard treatment method for ovarian cancer is
cytoreductive surgery and first-line chemotherapy. Patient
sensitivity to initial chemotherapy is ~50–80%, with a recurrence
rate being as high as 80% (4).
Patients with recurrent ovarian cancer are resistant to almost all
chemotherapy drugs. More importantly, the five-year survival rate
of patients is only 30–40% (4,5).
Cisplatin is one of the most widely used chemotherapy drugs, which
has a significant therapeutic effect on ovarian cancer. However,
cisplatin resistance is a major obstacle to achieving satisfactory
ovarian cancer treatment effects (6). Thus, there is an urgent need to
establish strategies for increasing ovarian cancer cisplatin
sensitivity. In recent years, research groups across the globe have
investigated ovarian cancer progression (7), particularly the mechanisms of
resistance to platinum-based drugs in ovarian cancer (8). It has been previously found that
evasion of apoptosis caused by abnormal regulation of this process
plays a crucial role in ovarian cancer cisplatin resistance; in
particular, the high expression of anti-apoptotic proteins is
closely related to drug resistance and recurrence in this disease,
seriously affecting clinical treatment outcomes in patients
(9).
Bcl-2 and Bcl-xL are two crucial anti-apoptotic
proteins of the Bcl-2 family, that are localized in the
mitochondria and regulate mitochondrial outer membrane
permeability. They can inhibit the occurrence of apoptosis. High
expression of these two proteins is related to the occurrence,
development, low survival rate, radiotherapy resistance and
chemotherapy resistance of various tumors, rendering them effective
tumor therapeutic targets (10).
Bcl-2 protein overexpression can significantly reduce the
cisplatin-induced inhibition of ovarian cancer cell proliferation
and apoptosis (11). Additionally,
several studies have demonstrated that inhibiting Bcl-xL expression
can effectively induce ovarian cancer cell death and increase
cisplatin sensitivity of ovarian cancer (12–15).
Therefore, downregulating Bcl-2 and Bcl-xL expression levels can
effectively increase ovarian cancer cisplatin sensitivity, thereby
reversing the clinical drug resistance and improving the patient
five-year survival rate.
ABT-737, a BH3-only protein stimulant, is an
effective small molecule inhibitor of Bcl-xL and Bcl-2.
Mechanistically, ABT-737 can specifically inhibit the binding of
Bcl-2/Bcl-xL to Bak/Bax by competing with the BH3 domain, then
inducing apoptosis through the mitochondrial apoptotic pathway
(16). It has been revealed that
ABT-737 has favorable antitumor activity in a variety of tumor
types (17). ABT-737 not only plays
a synergistic cytotoxic role in different cancers, including
ovarian, lung and bladder cancers, but can also induce significant
levels of apoptosis (18). In
addition, the first author's previous research group found that
ABT-737 could enhance cisplatin-induced apoptosis by regulating
endoplasmic reticulum (ER)-mitochondrial Ca2+ signal
transduction or modulating mitochondrial dynamics in ovarian cancer
cells (18,19). Because of the known roles of Bcl-2
family pro-survival members in mitochondrial metabolism, it was
proposed that ABT-737 possibly affects cisplatin resistance by
modulating reactive oxygen species (ROS) production in human
ovarian cancer cells.
Previous research has uncovered that ABT-737 can
lead to high ROS accumulation in the body and destroy the redox
balance of organisms. This creates oxidative stress in a variety of
tumor cells, including ovarian cancer cells, inducing apoptosis.
These results suggested that ROS and apoptosis levels are
positively correlated (20).
Although numerous studies have revealed that ROS accumulation is
cytotoxic and conducive to cancer treatment, the role of ROS in
addressing cancer cell drug resistance has not been systematically
examined (21). Therefore, it
remains unclear if ABT-737 treatment can increase ovarian cancer
cells sensitivity to cisplatin by regulating ROS generation. These
mechanistic details are a crucial focus of the present study.
ROS are related to the c-Jun N-terminal kinase (JNK)
and play significant roles in various physiological processes, such
as the inflammatory response and apoptosis. JNK and p38
mitogen-activated protein kinase (MAPK) play an important role in
cell apoptosis induced by various types of stress, such as ROS
(22). Moreover, some studies have
confirmed that ABT-737 can enhance activation of the JNK pathway by
inhibiting the effects of Bcl-2/BcL-xL to induce apoptosis
(23–26). Therefore, it was aimed to determine
if ABT-737 can induce JNK pathway activation by inducing ROS
accumulation to promote ovarian cancer cell sensitivity to
cisplatin. Apoptosis signal regulated kinase 1 (ASK1) plays a
bridge and link role in ROS-mediated JNK signaling pathway
activation. The signal pathway activated by ASK1 is one of the
important ways for ROS participation in JNK signal transduction
(22). This led us to hypothesize
that ABT-737 may increase cisplatin sensitivity in ovarian cancer
cells by regulating the ROS-ASK1-JNK signaling pathway.
In the present study, the effects of ABT-737 on
cisplatin sensitivity of A2780/DDP cells and the relevant molecular
mechanisms were examined. It was confirmed that ABT-737 could
significantly increase the sensitivity of A2780/DDP cells to
cisplatin, which is mediated by ROS-dependent activation of the
ASK1-JNK MAPK signaling pathway.
Materials and methods
Reagents and antibodies
RIPA Lysis buffer was purchased from Beyotime
Institute of Biotechnology. ABT-737 (a BH3 mimetic),
N-Acety-L-Cysteine (NAC; ROS inhibitor), U0126 (ERK inhibitor),
SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), LY294002 (Akt
inhibitor), GS-4997 (ASK1 inhibitor) and BAPTA-AM (calcium
chelator) were purchased from Selleck Chemicals. Antibodies against
Bcl-xL (cat. no. 551022; 1:200; mouse) and Bcl-2 (cat. no. 568664;
1:200; mouse) were purchased from BD Biosciences. Antibodies
against Bax (cat. no. ab32503; 1:1,000; rabbit) and Bak (cat. no.
ab32371; 1:1,000; rabbit) were purchased from Abcam. Antibodies
against phosphorylated (p-) Ask1 (cat. no. 28846-1-AP; 1:1,000;
rabbit), Ask1 (cat. no. 67072-1-Ig; 1:1,000; rabbit), p-P38 (cat.
no. 28796-1-AP; 1:500; rabbit), P38 (cat. no. 14064-1-AP; 1:500;
rabbit), p-Akt (cat. no. 66444-1-Ig; 1:2,000; mouse) and Akt (cat.
no. 60203-2-Ig; 1:2,000; mouse) were purchased from Proteintech
Group, Inc. Antibodies against caspase 3 (cat. no. 9662S; 1:2,000;
rabbit), cleaved-caspase 3 (cat. no. 9661S; 1:1,000; rabbit), PARP
(cat. no. 9542S; 1:1,000; rabbit), cleaved-PARP (cat. no. 9541S;
1:1,000; rabbit), p-JNK (cat. no. 4668S; 1:2,000; rabbit), JNK
(cat. no. 9252S; 1:2,000; rabbit), p-ERK (cat. no. 4370S; 1:1,000;
rabbit) and ERK (cat. no. 4695S; 1:1,000; rabbit) were purchased
from Cell Signaling Technology, Inc. The antibody against β-actin
(cat. no. AF0003; 1:2,000; mouse) was purchased from Beyotime
Institute of Biotechnology.
Cell culture
Human cisplatin-resistant A2780/DDP cells were
provided by the Department of Biochemistry and Molecular Biology
(Basic Medical College, Shanxi Medical University). Cells were
cultured in RPMI-1640 culture medium (Gibco; Thermo Fisher
Scientific, Inc.) and supplemented with 10% (v/v) fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. In total, 2 µg/ml cisplatin was used to
maintain the A2780/DDP resistant line alive in cell culture.
Cell viability assays
Human cisplatin-resistant A2780/DDP cells were
plated at 1.2×104 cells/well in 96-well plates (Corning,
Inc.). After incubating the cells for 24 h at 37°C with 5%
CO2, cells were treated as indicated. Next,
3-(4,5-dimethylthizaol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
reagent [10 µl; 5 mg/ml in phosphate-buffered saline (PBS);
Sigma-Aldrich; Merck KGaA] was added to each well, then the plates
were incubated for 4–6 h. Formazan crystals were dissolved in 150
µl DMSO and the wells were shaken for 10 to 15 min using a tablet
oscillation device. Absorbance values were measured at a wavelength
of 490 nm (Bio-Rad Laboratories, Inc.). The mean value of five
replicate wells was calculated for each treatment group.
TUNEL assays
Apoptosis was detected using a TUNEL (TdT-mediated
dUTP Nick-End Labeling) In Situ Cell Death Detection Kit
(Roche Diagnostics) according to the manufacturer's protocol. The
principle of the TUNEL method is that apoptotic cells have DNA
breaks in the genome, with the exposed 3′-OH groups able to be
conjugated with fluorescein (FITC)-labeled dUTP catalyzed by
terminal deoxynucleotidyl transferase (TdT). The apoptotic levels
were detected using TUNEL assays by flow cytometry and confocal
fluorescence microscopy. The detailed operation process was as
follows:
When apoptosis levels were detected using TUNEL
assays by flow cytometry, cells were harvested using trypsin (0.4%,
Beijing Solarbio Science & Technology Co., Ltd.) and washed
with PBS. Cells were fixed with 4% (w/v) paraformaldehyde (Beijing
Solarbio Science & Technology Co., Ltd.)/PBS for 20 min on ice
and washed with PBS. Cells were fixed with 70% (w/v) ethanol for 4
h at −20°C. The cells were centrifuged at 3,000 × g for 5 min at
4°C and washed with PBS, and then incubated with 0.1% Triton X-100
for 5 min at room temperature. After washing with PBS, the cells
were incubated with proteinase K (20 µg/ml) for 8–10 min at room
temperature, washed with PBS, and incubated with 80 µl
equilibration buffer for 5 min at room temperature. The cells were
centrifuged at 3,000 × g for 5 min at 4°C, then pellet was
incubated with 50 µl terminal deoxynucleotidyl transferase (TdT)
mixture (TdT: equilibration buffer, 1:9) for 1 h at 37°C in a
humidified atmosphere. The cells were washed with PBS and
resuspended in 200–300 µl PBS. Finally, the samples were examined
by flow cytometry (Muse Cell Analyzer; MilliporeSigma). The results
are representative of three independent experiments.
When apoptosis levels were detected using TUNEL
assays by confocal fluorescence microscopy, A2780/DDP cells were
plated at 4×104 cells/well in 24-well plates (Corning,
Inc.). After 24 h, the cells were treated as indicated at 37°C with
5% CO2. The cells were washed with 0.1 M PBS three times
and fixed with 4% (w/v) paraformaldehyde/PBS for 20–30 min at room
temperature. After washing with 0.1 M PBS three times, the cells
were incubated with 50 µl reaction solution mixture (TdT:
FITC-12-dUTP labeling mix: equilibration buffer, 1:5:50) for 1 h at
37°C in a humidified atmosphere. The cell nuclei were stained by
FITC-12-dUTP labeling mix, then apoptotic cells with characteristic
nuclear fragmentation (green staining) were counted in six randomly
chosen fields by confocal fluorescence microscopy (scale bar, 10
µm). The experiment was repeated three times.
Detection of cell apoptosis
The Hoechst staining method was used to examine
apoptotic cells by staining the nuclei and observing cell
morphological changes. A2780/DDP cells were cultured in 24-well
plates (Corning, Inc.), then treated with the various indicated
drugs for 24 h. Cells were washed with PBS three times and fixed
with 4% (w/v) paraformaldehyde/PBS for 20–30 min at room
temperature. After washing with PBS three times, the cells were
incubated with Hoechst 33258/H2O (2 µg/ml) for 5 min at
room temperature, then washed with PBS three times. The cells were
examined with a fluorescence microscope (Olympus Corporation). All
samples were run in triplicate.
The Annexin V-FITC/PI Apoptosis Detection Kit
(Dalian Meilun Biotechnology Co., Ltd.) was used to detect cell
apoptosis levels at different stages based on the staining of
Annexin V-FITC and PI. A2780/DDP cells were exposed to the
indicated treatments for various times. The cells were harvested by
trypsin (0.4%; Beijing Solarbio Science & Technology Co., Ltd.)
and washed with PBS for two times. Then, 1X binding buffer was
added to resuspend the cells to a concentration of 1×106
cells/ml and 100 µl cell suspension (total of 1×105
cells) was added into a new tube. Next, 5 µl annexin V-FITC and
5–10 µl PI were added, then the samples were gently mixed and
incubated at room temperature in the dark for 15 min. After this
staining incubation period, 400 µl 1X binding buffer was added to
each tube, was mixed and detected by flow cytometry. The results
were analyzed with FlowJo 10.8.1 software (BD Biosciences). A total
of three replicates were performed for each sample.
Measurement of ROS formation
A2780/DDP cells were cultured in 24-well plates.
After incubation with the indicated treatments for 24 h, DCFH-DA (5
µM; Beyotime Institute of Biotechnology) was added to the cells.
The cells were incubated at 37°C for 20 min, then washed three
times with PBS to sufficiently remove any excess DCFH-DA. A
fluorescence microscope (Olympus Corporation) was used to detect
changes in intracellular ROS production in the A2780/DDP cells. The
experiment was repeated three times.
Western blot analysis
A2780/DDP cells were lysed in 100 µl RIPA lysis
buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS) by sonication. Protein concentrations were
quantified using a protein assay kit (Bio-Rad Laboratories, Inc.).
Equivalent amounts of total proteins (30 to 40 µg) were separated
using 12% SDS-poly-acrylamide gel electrophoresis and transferred
onto PVDF membranes (MilliporeSigma). The membranes were blocked
with 5% non-fat dry milk in PBST buffer (10 mM Tris-HCl pH 7.6, 100
mM NaCl and 0.1% Tween-20) for 90–120 min at room temperature, then
incubated with the relevant primary antibodies overnight at 4°C.
The membranes were washed with PBST buffer three times for 10 min
each, then incubated with a horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (cat. no. A0216; Beyotime Institute
of Biotechnology) or a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (cat. no. A0208; Beyotime Institute
of Biotechnology) at a 1:1,000 dilution for 90 min at room
temperature. The membranes were washed with PBST buffer again three
times for 10 min each. The immunoreactive bands were visualized by
Enhanced Chemiluminescent (ECL) detecting agents (Thermo Fisher
Scientific, Inc.) and measured with an ECL image detection system
(BD Biosciences). The protein levels were quantified by
densitometry using Quantity One 4.6.2 software (Bio-Rad
Laboratories, Inc.). The data are presented as the mean ± standard
deviation (SD) of three independent experiments.
Measurement of oxidative stress
indices
A2780/DDP cells were cultured in 6-well plates and
cultured at 37°C. After the cells were cultured to 80% confluence,
they were incubated with indicated treatments for 24 h. The
intracellular levels of hydrogen peroxide, superoxide anions, and
hydroxyl radicals were detected using Hydrogen Peroxide assay kit
(cat. no. S0038; Beyotime Institute of Biotechnology), Superoxide
Anions assay kit (cat. no. BES-2343BTK; Shanghai Bolsen
Biotechnology Co., Ltd.) and Hydroxyl Radicals assay kit (cat. no.
BES20343BO; Shanghai Bolsen Biotechnology Co., Ltd.), respectively,
according to the manufacturer's protocols. The data were measured
using a microplate reader according to the manufacturer's
protocols. A total of three replicates were performed for each
sample.
Measurement of total antioxidant
capacity (T-AOC)
A2780/DDP cells were cultured in 6-well plates at a
density of 1×105 cells/well, then incubated with the
indicated treatments for 24 h at 4°C. Changes in the T-AOC were
detected using the T-AOC Assay Kit (with ABTS method; cat. no.
S0119; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. A total of three replicates were performed
for each sample.
Statistical analysis
Statistical analysis was performed using SPSS
(version 22.0; IBM Corp.). The data are presented as the mean ± SD.
One-way ANOVA was used for multiple-group comparisons, followed by
Tukey's post hoc test. Differences between two groups were
determined using an unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference. Data
are representative of three independent experiments performed in
triplicate.
Results
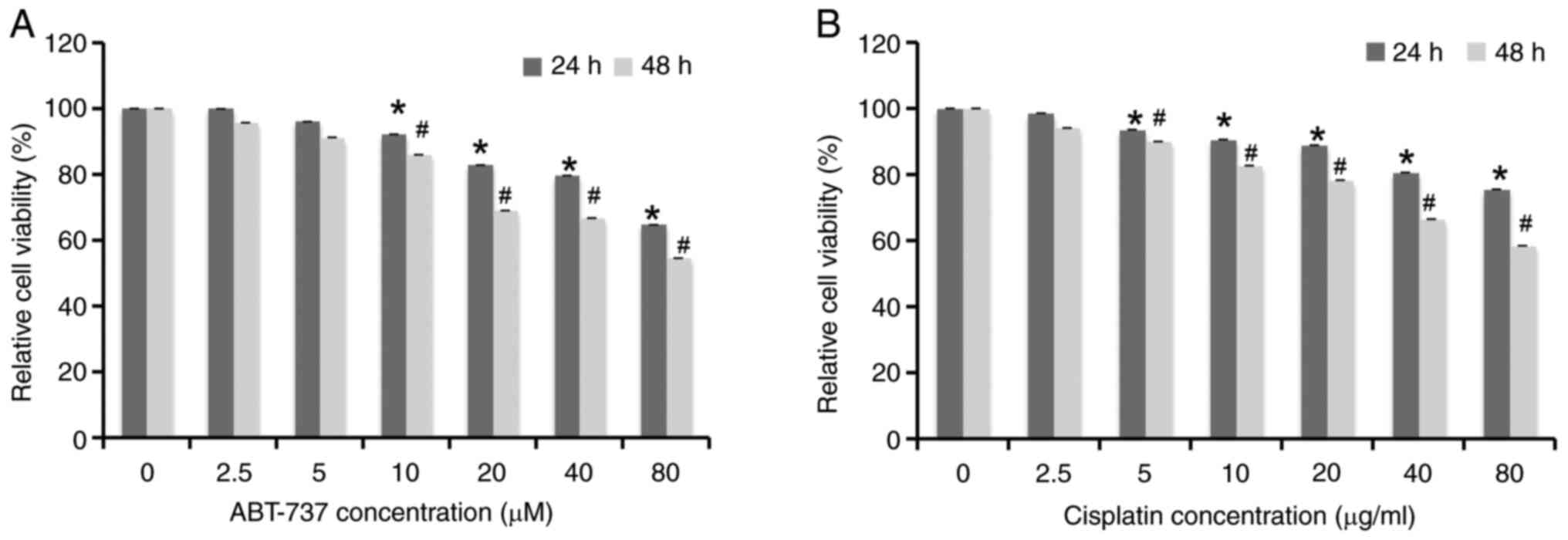
ABT-737 and cisplatin inhibit
A2780/DDP cell growth in a time- and dose-dependent manner
A2780/DDP cells were treated with different
concentrations of ABT-737 (0, 2.5, 5, 10, 20, 40 and 80 µM) and
cisplatin (0, 2.5, 5, 10, 20, 40 and 80 µg/ml) for 24 h or 48 h;
then the cell survival rates were examined by MTT assays. The
results showed that the cell viability decreased in a time- and
dose-dependent manner (Fig. 1A and
B). A literature review suggested that when examining the
combined effect of the two drugs, it is best to choose the dose of
each drug alone when the cell viability inhibition rate was ~10%
(27). From the MTT assay results,
10 µM and 5 µg/ml were selected as the optimal concentrations of
ABT-737 and cisplatin, respectively.
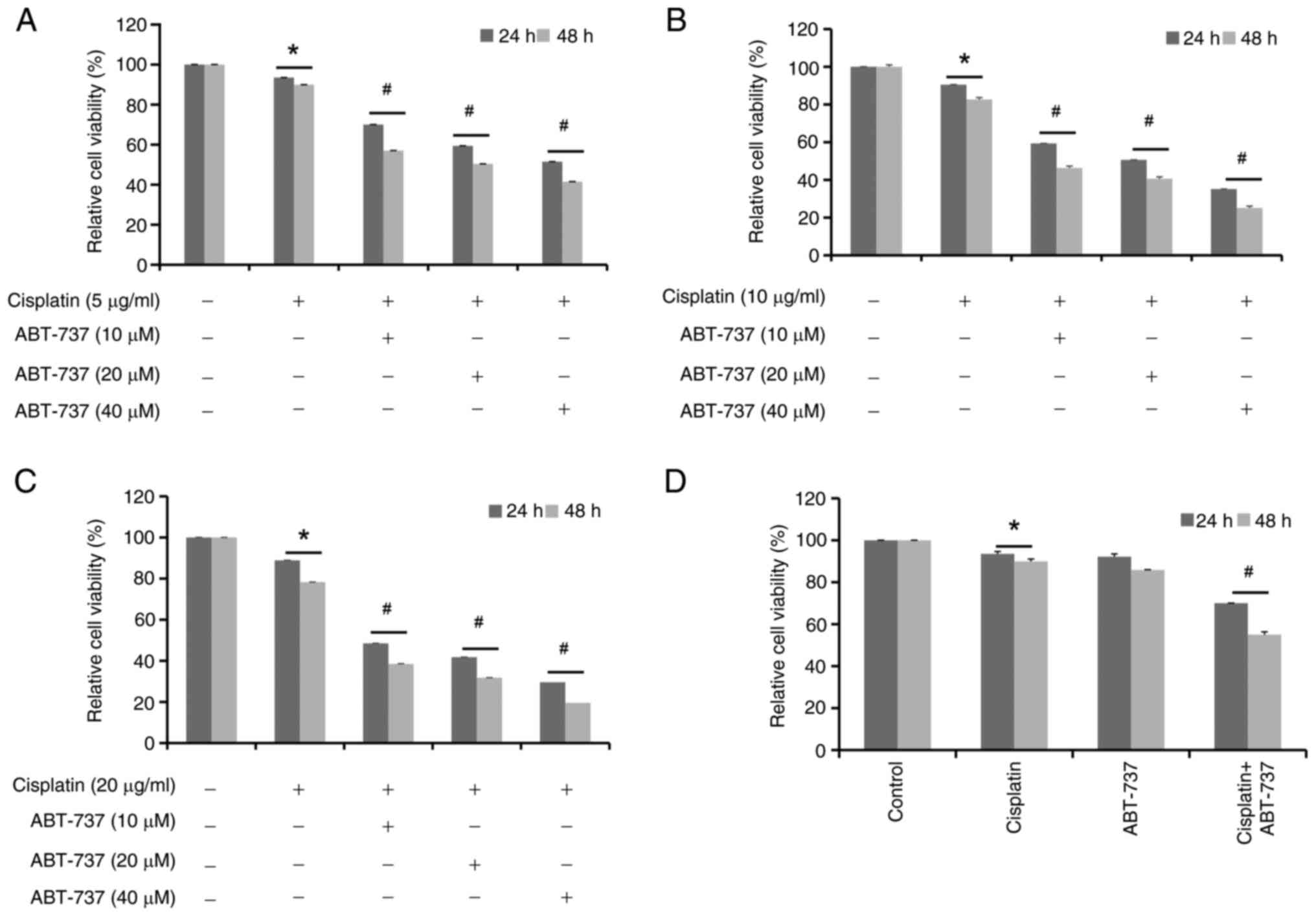
ABT-737 increases A2780/DDP cell
sensitivity to cisplatin
Next, to investigate if ABT-737 can affect the
antitumor effects of cisplatin, A2780/DDP cells were treated with
different concentrations of ABT-737 (10, 20, 40 µM) combined with
cisplatin (5, 10 and 20 µg/ml) for 24 or 48 h, separately (Fig. 2A-C). From the aforementioned MTT
assay results, the optimal treatment combination was selected: 10
µM ABT-737 combined with 5 µg/ml cisplatin for 24 or 48 h in
A2780/DDP cells (Fig. 2D). The MTT
assay results showed that ABT-737 enhanced cisplatin-induced
proliferation inhibition in A2780/DDP cells.
It was then further investigated if ABT-737 could
increase cisplatin-induced apoptosis in these cells. The data shown
in Fig. 2D were analyzed and it was
found that the cell survival rate was ~30% when cisplatin (5 µg/ml)
and ABT-737 (10 µM) were combined for 24 h in A2780/DDP cells.
Next, a literature research was performed and the optimal
experiment condition of cells was considered, finding that 24 h was
more suitable for subsequent experiments (27,28).
Therefore, the cells were treated with cisplatin and/or ABT-737 for
24 h and the expression levels of apoptosis-related proteins Bcl-2,
Bcl-xL, Bak, Bax, total-caspase 3, cleaved-caspase 3, total-PARP
and cleaved-PARP were evaluated. The results revealed that the
cisplatin and ABT-737 combination clearly increased the protein
expression levels of Bax, Bak, cleaved-caspase 3 and cleaved-PARP,
but decreased those of Bcl-2 and Bcl-xL (Fig. 3A-D). In addition, cells were treated
with the cisplatin and ABT-737 combination for 24 h; then apoptotic
levels were evaluated using TUNEL assays, flow cytometry and
confocal microscopy, respectively. The result of the flow cytometry
indicated that dUTP coupling fluorescence signal significantly
increased in the cisplatin and ABT-737 combination group (Fig. 3E), and the data of the confocal
microscopy demonstrated that there was increased DNA fragmentation
in the cisplatin and ABT-737 combination group (Fig. 3F and G), preliminarily suggesting
that ABT-737 could increase cisplatin-induced apoptosis. Based on
these results, cells were treated with cisplatin and/or ABT-737 for
24 h and changes in nuclear morphology were examined using Hoechst
33258 staining. The results indicated that there were chromatin
condensation and nuclear fragmentation in the cisplatin and ABT-737
combination group, suggesting that ABT-737 could increase
cisplatin-induced DNA damage (Fig. 3H
and I). To further confirm the apoptotic levels induced by
cisplatin in combination with ABT-737, caspase 3 enzyme activity
was detected using caspase 3 enzyme activity assay kit. The results
showed a significant increase in caspase 3 enzyme activity in the
cisplatin and ABT-737 combination group, suggesting that ABT-737
could increase cisplatin-induced caspase 3 enzyme activity
(Fig. 3J). Taken together, these
results suggested that ABT-737 treatment could increase
cisplatin-induced apoptosis in A2780/DDP cells.
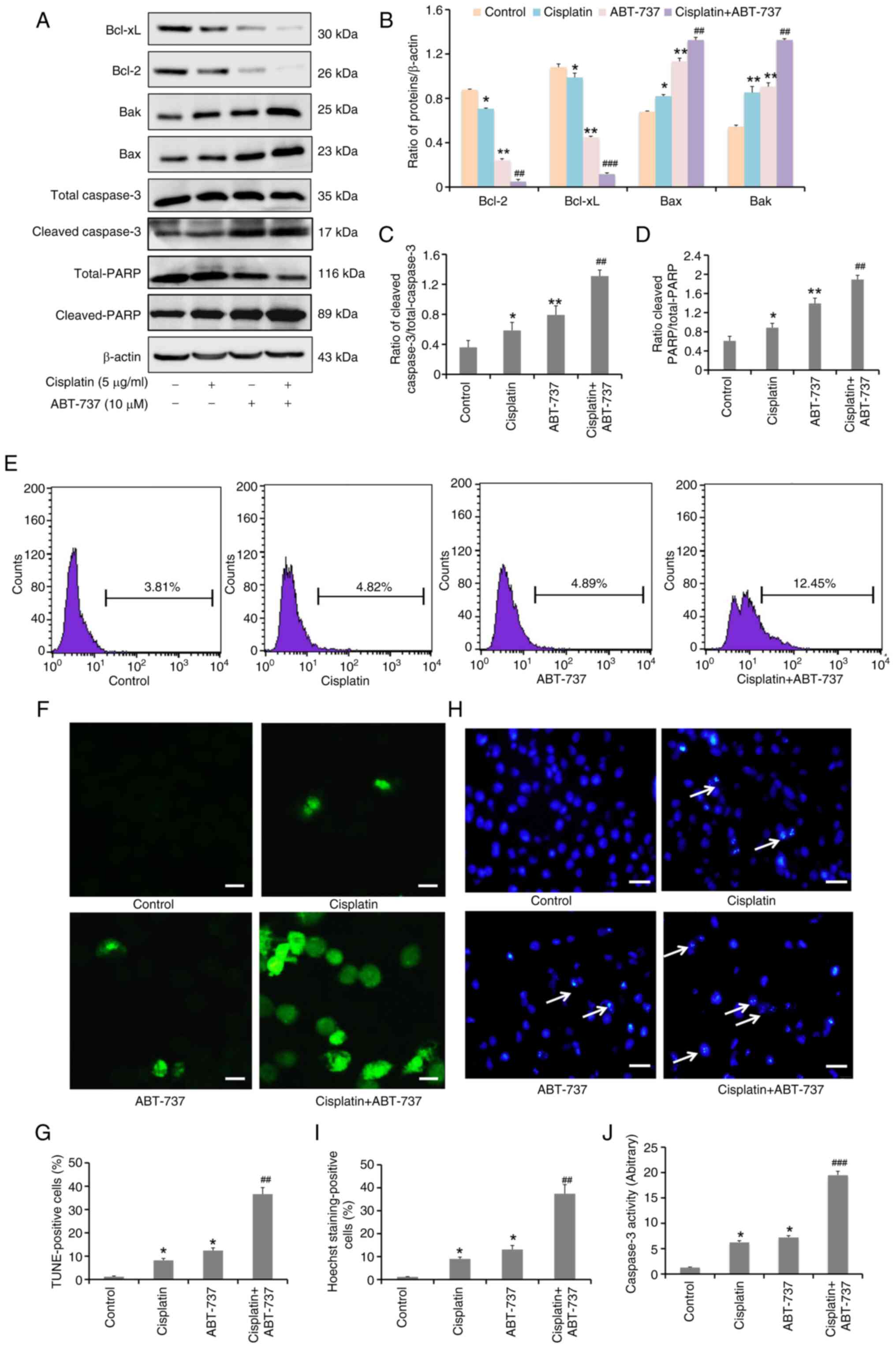 | Figure 3.ABT-737 increases cisplatin-induced
A2780/DDP cell apoptosis. A2780/DDP cells were treated with
cisplatin (5 µg/ml) alone or combined with ABT-737 (10 µM) for 24
h. (A) Western blot analysis was performed to examine Bcl-2,
Bcl-xL, Bak, Bax, total-caspase 3, cleaved-caspase 3 and
cleaved-PARP protein expression. (B) Quantitative analysis of
Bcl-2, Bcl-xL, Bak, Bax and cleaved-PARP protein levels from panel
A. Data are presented as the mean ± SD of three independent
experiments. (C and D) Quantitative analysis of (C) cleaved-caspase
3/total-caspase 3 and (D) cleaved-PARP/total-PARP protein levels
from panel A. Data are presented as the mean ± SD of three
independent experiments. (E) TUNEL assays were performed to detect
apoptosis level in A2780/DDP cells by flow cytometry. (F) TUNEL
assays were performed to detect apoptosis in A2780/DDP cells with
50 µl reaction solution mixture (TdT: FITC-12-dUTP labeling mix:
equilibration buffer=1:5:50) for 1 h at 37°C. Apoptotic cells with
the characteristic nuclear fragmentation (green staining) were
counted in six randomly chosen fields by confocal fluorescence
microscopy (scale bar, 10 µm). (G) Quantitative analysis of
TUNEL-positive cells. Data are presented as the mean ± SD of three
independent experiments. (H) Hoechst staining analysis of nuclear
morphology (scale bar, 10 µm). (I) Quantitative analysis of Hoechst
staining-positive cells. Data are presented as the mean ± SD of
three independent experiments. (J) Caspase 3 enzyme activity assays
were performed in A2780/DDP cells. Data are presented as the mean ±
SD of three independent experiments. *P<0.05 and **P<0.01 vs.
the control group; ##P<0.01 and
###P<0.001 vs. the cisplatin group. |
ABT-737 increases A2780/DDP cell
sensitivity to cisplatin through the JNK pathway
MAPK and PI3K/Akt are the main signaling pathways
that regulate cell proliferation and apoptosis. It has been
previously reported that ABT-737 can regulate different forms of
apoptosis through the MAPK and PI3K/Akt signaling pathways
(29). However, it is unclear
whether ABT-737 can increase the sensitivity of ovarian cancer
cells to cisplatin through these specific signaling pathways.
Therefore, western blot analysis was used to determine the
activation of MAPK and PI3K/Akt signaling pathways activation
status in cells treated with ABT-737 and cisplatin combination for
24 h. The results demonstrated that the combination of ABT-737 and
cisplatin could significantly increase the protein expression
levels of p-JNK, p-ERK, p-p38 and p-Akt (Fig. 4A-E). In addition, specific
inhibitors of the MAPK and PI3K/Akt signaling pathways were used,
finding that 5 µM SP600125 (JNK inhibitor) treatment could
significantly reverse the proliferation inhibition and apoptosis
induced by the combination of ABT-737 and cisplatin. However,
individual treatments with 5 µM U0126 (ERK1/2 inhibitor), 5 µM
SB203580 (p38 inhibitor) and 5 µM LY294002 (Akt inhibitor) had no
inhibitory effects on the slower proliferation and increased
apoptosis induced by the ABT-737 and cisplatin combination
(Fig. 4F-H). These data suggested
that ABT-737 can increase the sensitivity of A2780/DDP to cisplatin
through activation of the JNK pathway.
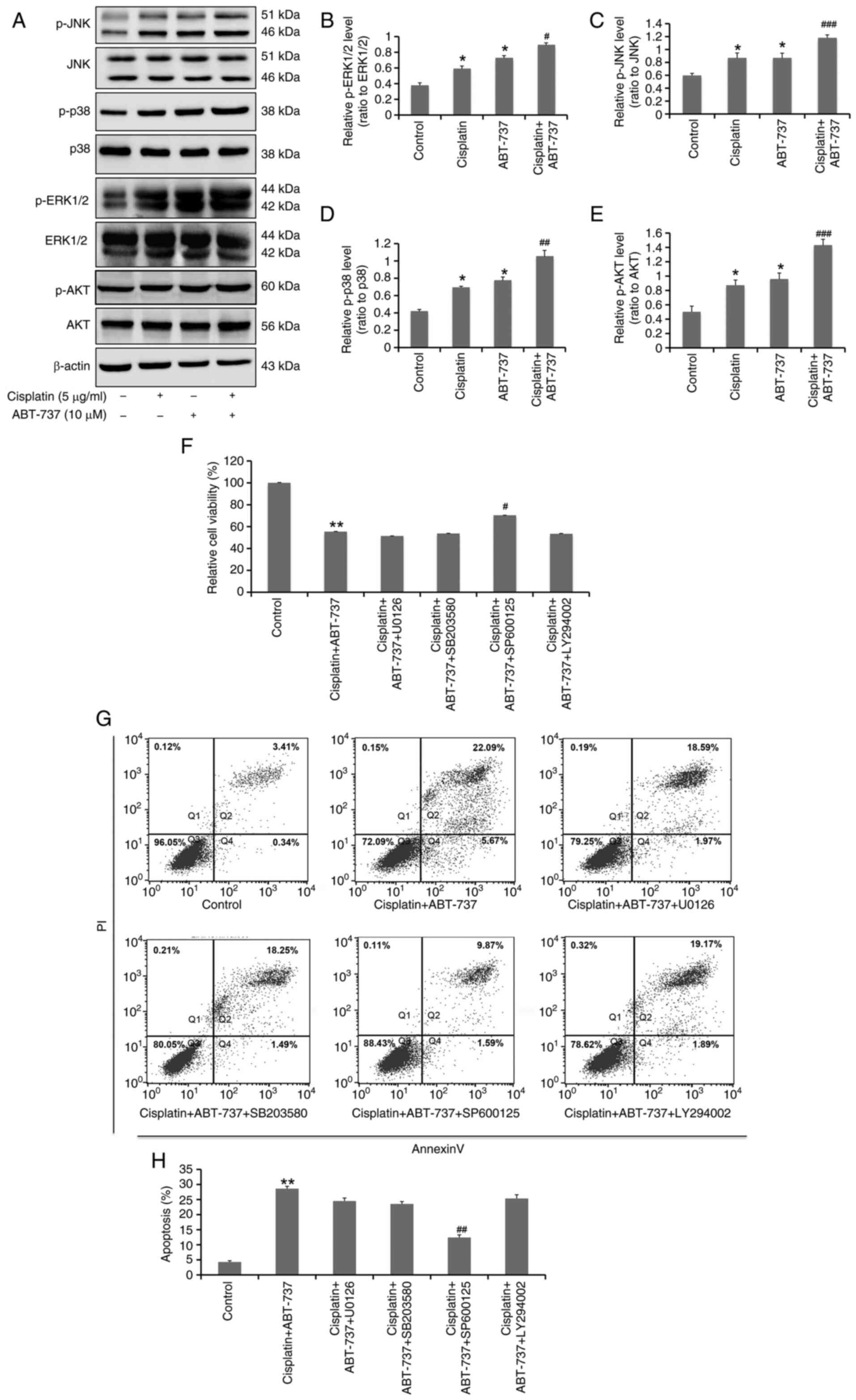 | Figure 4.ABT-737 increases the sensitivity of
A2780/DDP cells to cisplatin through the JNK pathway. (A) A2780/DDP
cells were treated with cisplatin (5 µg/ml) alone or combined with
ABT-737 (10 µM) for 24 h. Western blot analysis of p-JNK, JNK,
p-ERK1/2, ERK1/2, p-p38, p38, p-AKT and AKT protein expression.
(B-E) Quantitative analysis of (B) p-ERK1/2/ERK1/2, (C) p-JNK/JNK,
(D) p-p38/p38 and (E) p-AKT/AKT protein levels from panel A. Data
are presented as the mean ± SD of three independent experiments.
*P<0.05 vs. the control group; #P<0.05,
##P<0.01 and ###P<0.001 vs. the
cisplatin group. (F) A2780/DDP cells were treated with cisplatin (5
µg/ml) combined with ABT-737 (10 µM) or combined with ABT-737 (10
µM) and U0126 (5 µM), SB203580 (5 µM), SP600125 (5 µM) or LY294002
(5 µM) for 24 h; then cell viability was detected by MTT assays.
Data are presented as the mean ± SD of three independent
experiments. **P<0.01 vs. the control group;
#P<0.05 vs. the cisplatin + ABT-737 group. (G)
A2780/DDP cells were treated with cisplatin (5 µg/ml) combined with
ABT-737 (10 µM) or combined with ABT-737 (10 µM) and U0126 (5 µM),
SB203580 (5 µM), SP600125 (5 µM), or LY294002 (5 µM) for 24 h; then
cell apoptosis was determined by flow cytometry. (H) Quantitative
analysis of the flow cytometry results. Data are presented as the
mean ± SD of three independent experiments. **P<0.01 vs. the
control group; ##P<0.01 vs. the cisplatin + ABT-737
group. |
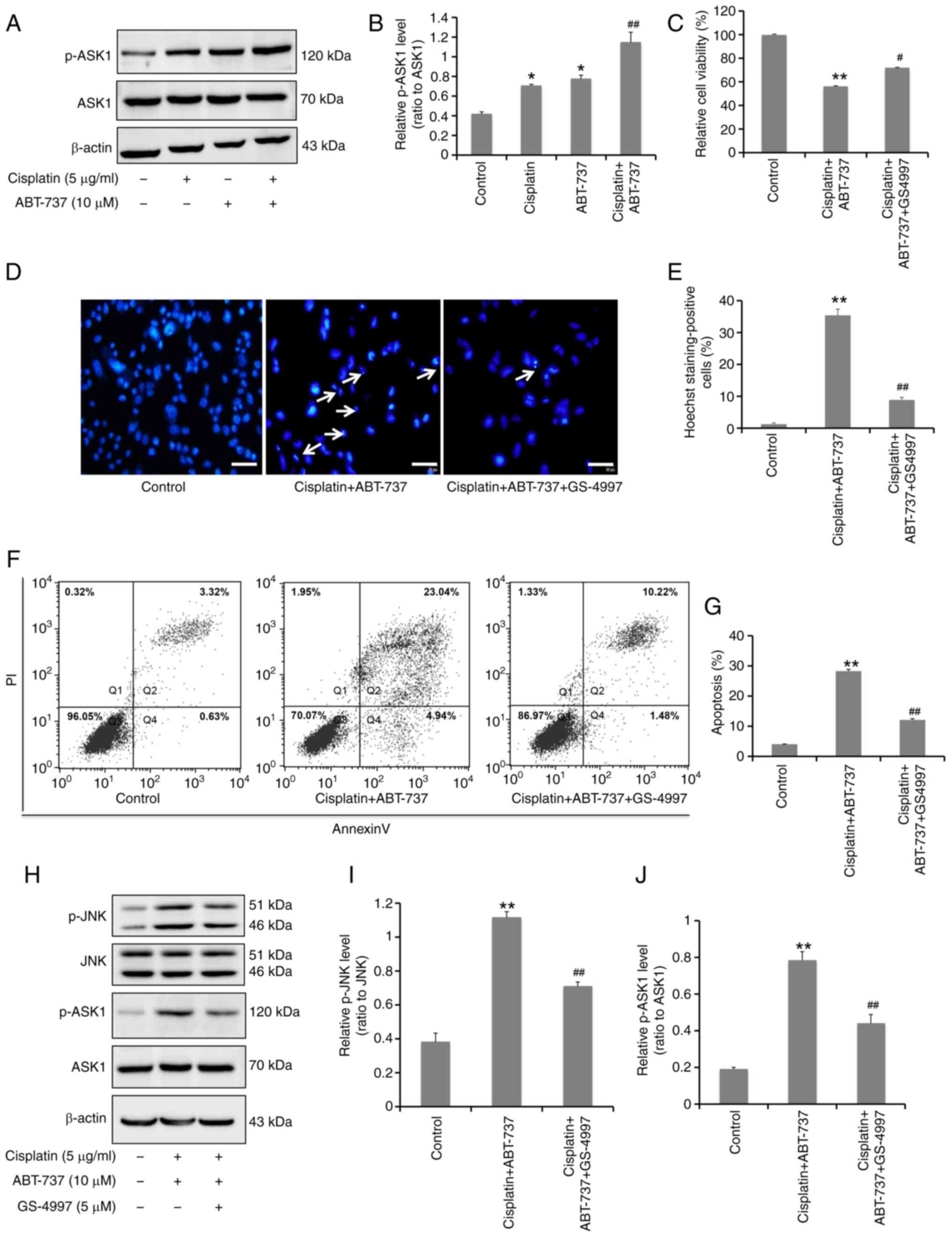
ASK1 is required for ABT-737-induced
JNK activation and apoptosis in A2780/DDP cells
Although it was confirmed that ABT-737 could
increase the sensitivity of A2780/DDP cells to cisplatin by
activating the JNK signaling pathway, the upstream signaling
details remained unclear. In numerous cell types, ASK1 has been
found to play a vital role in activating the p38 and JNK signaling
pathway (30). In the present
study, western blot analysis was used to determine the activation
of ASK1 protein induced by the combination of ABT-737 and cisplatin
for 24 h. It was found that ABT-737 combined with cisplatin could
effectively increase ASK1 protein phosphorylation levels (Fig. 5A and B). After treatment with the 5
µM ASK1 inhibitor GS-4997, MTT assays indicated that the inhibition
of cell survival rate was significantly alleviated (Fig. 5C); Hoechst 33258 nuclear staining
revealed that chromatin pyknosis and nuclear fragmentation were
significantly reduced (Fig. 5D and
E), and the result of Annexin V-FITC/PI double staining data
demonstrated that cell apoptosis levels were significantly reduced
(Fig. 5F and G). Collectively,
these results suggested that ASK1 is important in ovarian cancer
cells apoptosis induced by ABT-737. In addition, western blot
analysis was used to detect the ASK1 and JNK protein
phosphorylation levels after treatment with the ASK1 inhibitor
GS-4997. These results showed that inhibiting ASK1 could
significantly reduce JNK activation in these cells (Fig. 5H-J), indicating that ASK1 is
necessary for ABT-737-induced JNK activation.
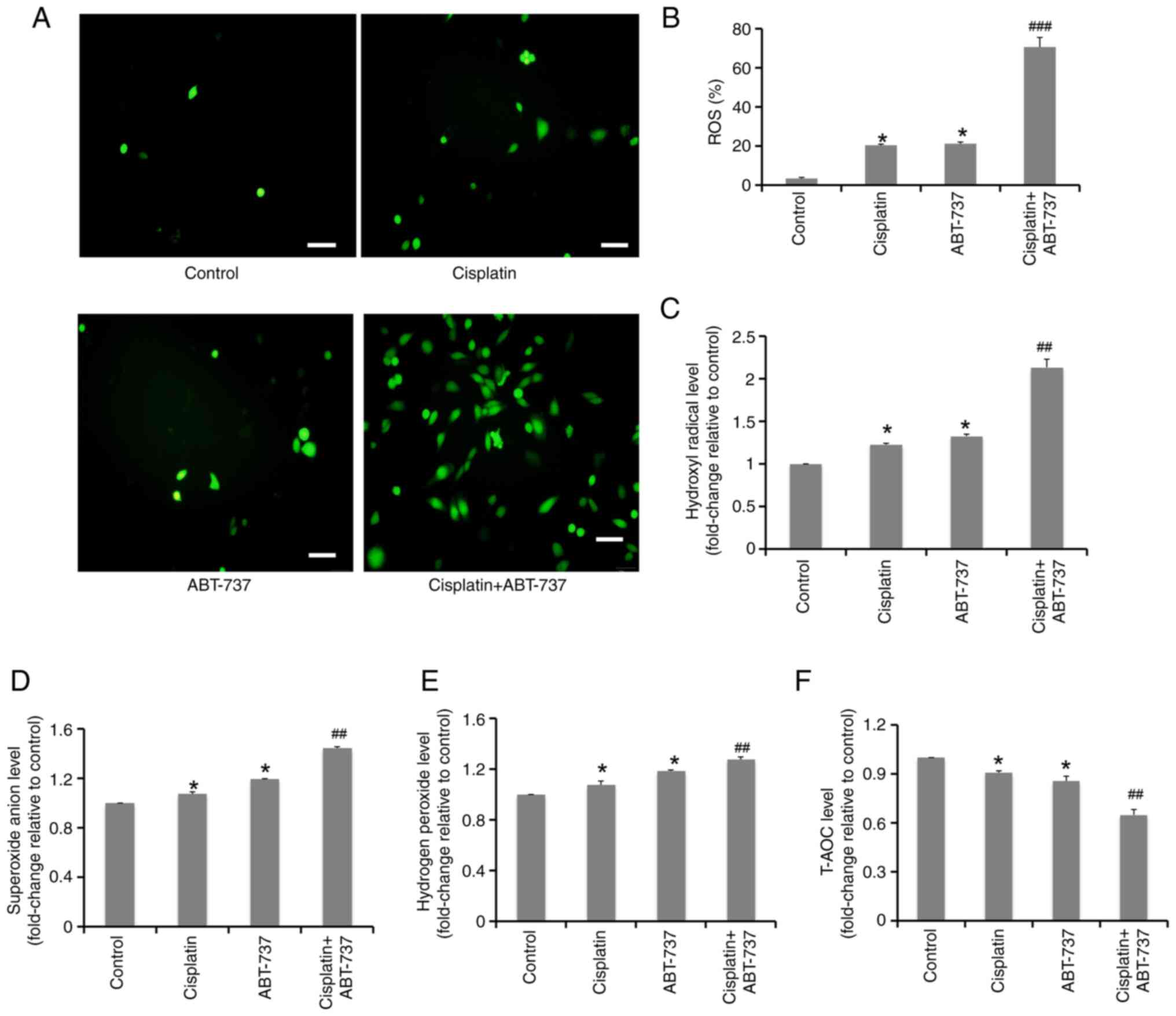
ABT-737 enhances the antioxidant
system imbalance induced by cisplatin in A2780/DDP cells
It has been previously reported that ABT-737 induces
cell apoptosis by increasing oxidative stress, which is one of the
main antitumor mechanisms of certain chemotherapeutic drugs. ROS
play a vital role in maintaining the cell redox balance and can
induce cell apoptosis (31).
Firstly, ROS production in cells was detected after exposure to the
ABT-737 and cisplatin combination for 24 h by fluorescence
microscope. The results showed that ABT-737 combined with cisplatin
could significantly increase ROS production in A2780/DDP cells
(Fig. 6A and B). In addition, the
levels of oxidative stress indices, including hydrogen peroxide,
superoxide anions and hydroxyl radicals, were significantly
increased following exposure to ABT-737 combined with cisplatin for
24 h (Fig. 6C-E), while the T-AOC
levels of A2780/DDP cells were significantly reduced following
combination treatment (Fig. 6F).
These results indicated that ABT-737 could strongly enhance the
imbalance between oxidation and antioxidant status induced by
cisplatin in A2780/DDP cells.
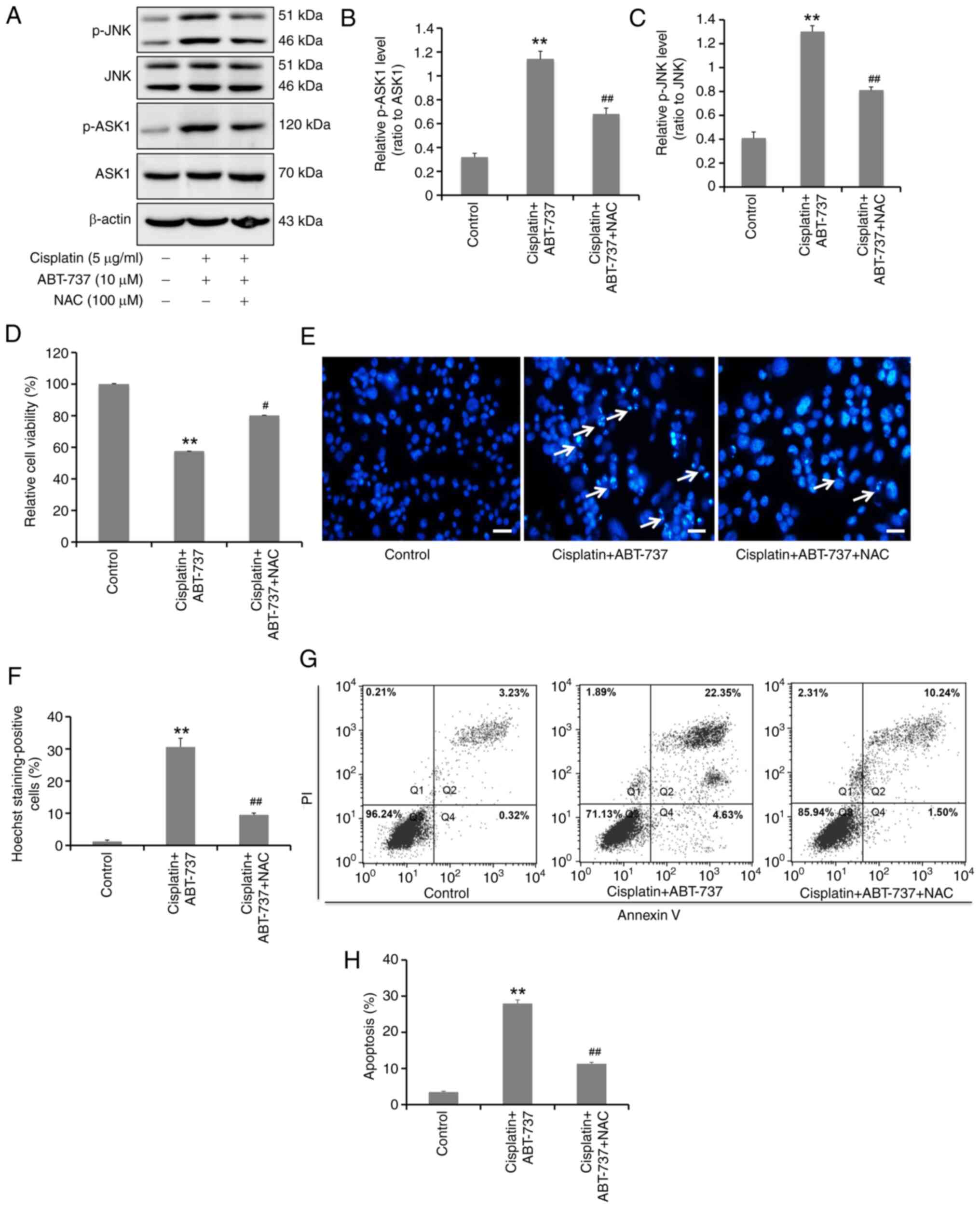
ROS production induced by ABT-737
mediates the activation of the ASK1-JNK signaling pathway and
increases cisplatin-induced apoptosis in A2780/DDP cells
Numerous studies have shown that oxidative stress
can induce activation of the ASK1-JNK signaling pathway in numerous
cell types (32,33). To further clarify the effects of
ABT-737-induced oxidative stress on the ASK1-JNK signaling pathway,
pretreatment NAC (an antioxidant) was first used to detect any ASK1
and JNK phosphorylation changes from the combined action of ABT-737
and cisplatin in A2780/DDP cells. The results identified that NAC
could significantly inhibit the ASK1 and JNK protein
phosphorylation levels (Fig. 7A-C),
suggesting that the ROS formation induced by ABT-737 could mediate
the activation of the ASK1-JNK signaling pathway. In addition, the
effects of ABT-737-induced generation of ROS on cisplatin-induced
apoptosis of A2780/DDP cells were detected. Following NAC
treatment, MTT assays showed that the cell survival inhibition was
significantly alleviated (Fig. 7D),
while Hoechst 33258 nuclear staining indicated that chromatin
pyknosis and nuclear fragmentation were significantly reduced
(Fig. 7E and F). Furthermore,
Annexin V-FITC/PI double staining demonstrated that the cell
apoptotic rate was significantly reduced by NAC (Fig. 7G and H). These results identified
that ROS production induced by ABT-737 could induce
cisplatin-induced apoptosis in A2780/DDP cells. Overall, the data
showed that ABT-737 treatment can induce ROS production in
A2780/DDP cells, which mediates the activation of the ASK1-JNK
signaling pathway and increases cisplatin-induced apoptosis. In
summary, ABT-737 increases cisplatin sensitivity through the
ROS-ASK1-JNK MAPK signaling axis in human ovarian cancer
cisplatin-resistant A2780/DDP cells.
Discussion
Ovarian cancer is one of the three major malignant
tumors of the female reproductive system. Early ovarian cancer is
not obvious, with the main symptoms mostly occurring in late-stage
of the disease. This results in ovarian cancer being difficult to
treat, leading to high mortality rates (34). In most cases, surgical treatment was
performed after the disease was confirmed, with several courses of
paclitaxel combined with platinum chemotherapy administered after
operation to increase patient survival rates. However, the
emergence of chemoresistance has seriously restricted the effect of
this combination therapy. This has even led to treatment failure,
resulting in disease recurrence (35,36).
Therefore, exploring the molecular mechanisms associated with
chemotherapy resistance in ovarian cancer has immeasurable
significance for reversing this obstacle and improving the curative
effect of treatment approaches.
At present, numerous studies have found that high
expression levels of anti-apoptotic proteins Bcl-2 and Bcl-xL are
closely related to chemoresistance in ovarian cancer. Targeting
Bcl-2/Bcl-xL, either using genetic knockdown methods or small
molecule inhibitors, enhanced platinum or paclitaxel sensitivity in
ovarian cancer cell lines (9) and
patient samples (37,38). ABT-737 is a novel and potent
inhibitor of Bcl-2 family proteins, which are critical for cell
survival. These proteins are often overexpressed in numerous tumor
types, with high affinity towards Bcl-xL, Bcl-2 and Bcl-w, but no
affinity towards less homologous proteins, such as Bcl-B, Mcl-1 and
A1. Other research has shown that ABT-737 has single-agent activity
against lymphoma and small cell lung cancer, as well as significant
anti-myeloma activity both in vitro and in vivo
through inhibiting Bcl-2 and Bcl-xL expression (19,39).
The current experimental data also confirmed this view in A2780/DDP
cells. ABT-737 not only inhibits A2780/DDP cell survival in a time-
and dose-dependent manner, but also increases the cisplatin-induced
reduction in cell proliferation in a dose-dependent manner. The
present data suggested that ABT-737 could simultaneously
significantly inhibit the expression levels of anti-apoptotic
proteins Bcl-2 and Bcl-xL, increase the expression levels of
pro-apoptotic proteins cleaved-caspase 3, cleaved-PARP, Bax and
Bak, and enhance caspase 3 activity to collectively increase
cisplatin-induced apoptotic rates in A2780/DDP cells. These results
showed that ABT-737 not only inhibits A2780/DDP cell survival, but
also increases the sensitivity of these cells to cisplatin
treatment. Although it has been confirmed that ABT-737 can increase
ovarian cancer cell cisplatin sensitivity, the mechanistic details
of this resistance reversal require further exploration.
The MAPK family includes a group of evolutionarily
conserved serine-threonine kinases, which can be divided into four
subfamilies: ERK, p38, JNK and ERK5, which represent the four
classical MAPK pathways, respectively (40). MAPK is the critical signaling
pathway that regulates cell promotion, apoptosis and drug
resistance under the stimulation of different types of endogenous
or existing factors (22,40). Inhibiting p38 MAPK has been shown to
synergistically induce apoptosis in melanoma cells in combination
with ABT-737 (23). A previous
study demonstrated that p38 MAPK plays a vital role in A549 and
H1299 cell death induced by ABT-737 (29). However, it is unknown if the MAPK
signaling pathway is involved in ABT-737-mediated reversal of tumor
chemoresistance, including in ovarian cancer. In the present study,
it was found that ABT-737 could significantly increase the
sensitivity of A2780/DDP cells to cisplatin. In addition, the
current results revealed that ABT-737 could significantly increase
the phosphorylation levels of JNK, ERK and p38 in the MAPK
signaling pathway. However, after JNK, ERK and p38 were blocked
using specific inhibitors, only pretreatment with the JNK inhibitor
(SP600125) was found to significantly reduce the A2780/DDP cell
proliferation inhibition induced by ABT-737 combined with
cisplatin. Additionally, SP600125 was the only inhibitor that could
reduce the A2780/DDP cell apoptosis induced by the ABT-737 and
cisplatin combination. These effects were not observed following
pretreatment with the ERK1/2 inhibitor (U0126) or p38 inhibitor
(SB203580) in A2780/DDP cells.
A recent study reported that ABT-737 can also induce
tumor cell apoptosis by regulating the PI3K/AKT signaling pathway
in colon cancer (41). Thus, the
effects of ABT-737 combined with cisplatin were also examined on
the PI3K/AKT signaling pathway, finding that Akt phosphorylation
increased significantly with this combination. However,
pretreatment with LY294002 (Akt inhibitor) did not affect the
proliferation inhibition and apoptosis induced by ABT-737 combined
with cisplatin in A2780/DDP cells. These results indicated that the
ABT-737-mediated increased A2780/DDP cells sensitivity to
cisplatin-induced cytotoxicity may be mediated by the JNK-MAPK
signaling pathway. However, further studies are required to
elucidate the specific molecular mechanism of JNK activation
induced by ABT-737.
ASK1 is also called mitogen activated protein kinase
5 (MAP3K5), which can be activated by several stimuli, including
calcium overloaded, ROS and ER stress. Studies have shown that
activated ASK1 can phosphorylate and activate JNK and p38 in
ovarian cancer (30). Therefore,
ASK1 phosphorylation levels were detected in A2780/DDP cells. The
combination of ABT-737 and cisplatin could significantly increase
ASK1 phosphorylation in A2780/DDP cells. To further confirm if ASK1
is part of the signaling upstream of JNK in the cisplatin
resistance reversal process, A2780/DDP cells were pretreated with
an ASK1 inhibitor (GS-4997). With this treatment, the JNK protein
activation and apoptosis induced by ABT-737 combined with cisplatin
were significantly reversed in A2780/DDP cells. These results
suggested that the ASK1/JNK signaling axis plays a vital role in
ABT-737-mediated reversal of ovarian cancer cisplatin
resistance.
Numerous studies have shown that oxidative stress is
critically involved in ABT-737-induced apoptosis of various tumor
cells (31,42). Dong et al (31) found that ABT-737 reverses the
Warburg effect via the Sirt3-HIF1α axis to promote oxidative
stress-induced apoptosis in ovarian cancer cells. To examine the
involvement of oxidative stress in our experimental system, ROS
levels were detected in A2780/DDP cells using fluorescence
microscopy. The data demonstrated that ABT-737 could induce the
production of ROS, as well as significantly increase the production
of ROS induced by cisplatin alone in A2780/DDP cells. Moreover, the
combined action of ABT-737 and cisplatin in A2780/DDP cells could
significantly reduce intracellular T-AOC. These results indicated
that ABT-737 could clearly increase oxidative stress induced by
cisplatin and reduce the antioxidant capacity in A2780/DDP cells.
In addition, it was found that a ROS inhibitor (NAC) could
significantly reduce the proliferation inhibition and increased
apoptosis induced by ABT-737 combined with cisplatin in A2780/DDP
cells. Taken together, these results suggested that ABT-737 could
increase A2780/DDP cell cisplatin sensitivity by inducing oxidative
stress.
Research has demonstrated that ROS accumulation can
induce activation of the ASK/JNK signaling pathway in different
tumor cell types, which eventually induces apoptosis (22,43).
In the present study, it was found that NAC pretreatment could
significantly reduce the ASK1 and JNK protein phosphorylation
levels induced by the combined action of ABT-737 and cisplatin,
indicating that production of ROS could induce the ASK1-JNK pathway
activation in A2780/DDP cells. Furthermore, compared with the
ABT-737 and cisplatin combination group, the addition of NAC led to
significantly decreased cell apoptotic rates. From these data, it
was hypothesized that oxidative stress-dependent activation of the
ASK1-JNK pathway is necessary for the ABT-737-mediated increase of
ovarian cancer cisplatin sensitivity.
In conclusion, the present study confirmed that
ABT-737 could not only induce A2780/DDP cell apoptosis in a time-
and dose-dependent manner, but also increase the sensitivity of
these cells to cisplatin. In addition, the present study revealed
that the pharmacological inhibition of Bcl-2 could reverse
A2780/DDP cell cisplatin resistance by increasing cisplatin-induced
generation of ROS. ROS accumulation can promote activation of the
ASK1-JNK signaling pathway and induce cell apoptosis, ultimately
increasing sensitivity of A2780/DDP cells to cisplatin-induced
cytotoxicity. Moreover, the present findings are consistent with
the results of the first author's previous research group. However,
this group only explored the ABT-737-mediated enhancement of
cisplatin-induced apoptosis through the regulation of
ER-mitochondrial Ca2+ signal transduction or glycolysis
modulation in ovarian cancer cells (19,44).
The authors did not further investigate the effects of ABT-737 on
ROS production and its downstream signals. Several studies have
shown that mitochondrial calcium overload or glycolysis can induce
significant oxidative stress and intracellular ROS accumulation
(45,46). Therefore, the current study focused
on the effects of ABT-737 on ROS and its downstream signaling
molecules, which effectively complements the previously established
molecular mechanisms of ABT-737-mediated enhancement of ovarian
cancer cisplatin sensitivity through Ca2+ overload or
glycolysis. It is important to note that our research only
investigated the effects of ABT-737 on ovarian cancer cisplatin
sensitivity at the in vitro cellular level. Cellular
experiments do not fully reflect the role of ABT-737 in
vivo. To address this in an improved way, as well as the
effects on the ROS-ASK1-JNK signaling pathway (47), animal experiments should be
conducted in the future to explore the impact of ABT-737 on
cisplatin sensitivity in living animals.
The results of the present study illustrated that
pharmacological inhibition of Bcl-2/Bcl-xL can reverse cisplatin
resistance in A2780/DDP cells by increasing ROS generation,
elucidating the antitumor mechanism of ABT-737 via the ROS-ASK1-JNK
signaling pathway. These data provide an experimental and
theoretical basis for the potentially effective clinical treatment
of ovarian cancer with ABT-737. They also establish a new
therapeutic target and strategy for cisplatin-resistant patients
with high Bcl-2/Bcl-xL expression patterns. From these findings,
screening drugs that target the ROS-ASK1-JNK signalling pathway, in
combination with ABT-737, may effectively increase the clinical
effect of ABT-737 in reversing cisplatin resistance in ovarian
cancer. In clinical practice, ABT-737 is mainly used in diseases
caused by Bcl-2/Bcl-xL overexpression, primarily for tumor
treatment (39). However, the
clinical use of ABT-737 still faces numerous challenges. Although
it has entered clinical trials, this drug does not have a high oral
bioavailability. Additionally, the poor effect of single treatment
hinders its clinical use (48).
Therefore, finding effective ways to increase its bioavailability
and identifying effective drugs that synergize with ABT-737 or in
combination with radiotherapy may effectively improve its clinical
efficacy. Collectively, this could help to effectively address
cisplatin resistance in ovarian cancer.
Acknowledgements
The authors would like to thank Dr J. Iacona for
editing the English text of a draft of this manuscript.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 32300641), the Shanxi Department of
Human Resources and Social Security (grant no. 20230024), the
General project of Shanxi Natural Science Foundation (grant nos.
20210302123322, 202103021224238 and 202103021224240), the Shanxi
Scholarship Council of China (grant no. 2022-118), the Key R&D
program of Shanxi (grant no. 201903D321101), the Key Laboratory of
Cellular Physiology (Shanxi Medical University), Ministry of
Education, China (grant no. CELLPHYSIOL/SXMU-2021-13) and the
Science and Technology Innovation Team of Shanxi (grant no.
202204051002030).
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
XL designed and performed most of the experiments
with assistance from YG, ZX, TG, LY, TY, BC and XW. ZX and TG
sorted the data of the manuscript. LY, TY, BC and XW provided
ABT-737 and other inhibitors and performed the related drug
treatments. BY and RG designed the experiments and supervised the
study. XL wrote the manuscript. XL and RG confirm the authenticity
of all the raw data. All authors reviewed, read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vergote I, González-Martín A, Ray-Coquard
I, Harter P, Colombo N, Pujol P, Lorusso D, Mirza MR, Brasiuniene
B, Madry R, et al: European experts consensus: BRCA/homologous
recombination deficiency testing in first-line ovarian cancer. Ann
Oncol. 33:276–287. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L, Guo H, Chen X, Zhang W, He Q, Ding
L and Yang B: Tackling drug resistance in ovarian cancer with
epigenetic targeted drugs. Eur J Pharmacol. 927:1750712022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marchetti C, De Felice F, Romito A,
Iacobelli V, Sassu CM, Corrado G, Ricci C, Scambia G and Fagotti A:
Chemotherapy resistance in epithelial ovarian cancer: Mechanisms
and emerging treatments. Semin Cancer Biol. 77:144–166. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song M, Cui M and Liu K: Therapeutic
strategies to overcome cisplatin resistance in ovarian cancer. Eur
J Med Chem. 232:1142052022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGlorthan L, Paucarmayta A, Casablanca Y,
Maxwell GL and Syed V: Progesterone induces apoptosis by activation
of caspase-8 and calcitriol via activation of caspase-9 pathways in
ovarian and endometrial cancer cells in vitro. Apoptosis.
26:184–194. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni M, Zhou J, Zhu Z, Xu Q, Yin Z, Wang Y,
Zheng Z and Zhao H: Shikonin and cisplatin synergistically overcome
cisplatin resistance of ovarian cancer by inducing ferroptosis via
upregulation of HMOX1 to promote Fe2+ accumulation.
Phytomedicine. 112:1547012023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara M, Pecorino B, D'Agate MG,
Angelico G, Capoluongo ED, Malapelle U, Pepe F, Scollo P and Mereu
L: Uterine tumours resembling ovarian sex-cord tumors: A case
report and review of the literature. J Clin Med. 12:71312023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ortiz M, Wabel E, Mitchell K and Horibata
S: Mechanisms of chemotherapy resistance in ovarian cancer. Cancer
Drug Resist. 5:304–316. 2022.PubMed/NCBI
|
|
9
|
Stover EH, Baco MB, Cohen O, Li YY,
Christie EL, Bagul M, Goodale A, Lee Y, Pantel S, Rees MG, et al:
Pooled genomic screens identify anti-apoptotic genes as targetable
mediators of chemotherapy resistance in ovarian cancer. Mol Cancer
Res. 17:2281–2293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan J, Lan H, Jiang X, Zeng D and Xiao S:
Bcl-2 family: Novel insight into individualized therapy for ovarian
cancer (review). Int J Mol Med. 46:1255–1265. 2020.PubMed/NCBI
|
|
11
|
Williams J, Lucas PC, Griffith KA, Choi M,
Fogoros S, Hu YY and Liu JR: Expression of Bcl-xL in ovarian
carcinoma is associated with chemoresistance and recurrent disease.
Gynecol Oncol. 96:287–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong M, Tan N, Zha J, Peale FV, Yue P,
Fairbrother WJ and Belmont LD: Navitoclax (ABT-263) reduces
Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol
Cancer Ther. 11:1026–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L, Xie Q, Qi L, Wang C, Xu N, Liu W, Yu
Y, Li S and Xu Y: Bcl-2 overexpression reduces cisplatin
cytotoxicity by decreasing ER-mitochondrial Ca2+ signaling in SKOV3
cells. Oncol Rep. 39:985–992. 2018.PubMed/NCBI
|
|
14
|
Maji S, Panda S, Samal SK, Shriwas O, Rath
R, Pellecchia M, Emdad L, Das SK, Fisher PB and Dash R: Bcl-2
antiapoptotic family proteins and chemoresistance in cancer. Adv
Cancer Res. 137:37–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo T, Gu C, Li B and Xu C: Dual
inhibition of FGFR4 and BCL-xL inhibits multi-resistant ovarian
cancer with BCL2L1 gain. Aging (Albany NY). 13:19750–19759. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whitecross KF, Alsop AE, Cluse LA,
Wiegmans A, Banks KM, Coomans C, Peart MJ, Newbold A, Lindemann RK
and Johnstone RW: Defining the target specificity of ABT-737 and
synergistic antitumor activities in combination with histone
deacetylase inhibitors. Blood. 113:1982–1991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fitzgerald DJ, Moskatel E, Ben-Josef G,
Traini R, Tendler T, Sharma A, Antignani A, Mussai F, Wayne A,
Kreitman RJ and Pastan I: Enhancing immunotoxin cell-killing
activity via combination therapy with ABT-737. Leuk Lymphoma. 52
(Suppl 2):S79–S81. 2011. View Article : Google Scholar
|
|
18
|
Fan Z, Yu H, Cui N, Kong X, Liu X, Chang
Y, Wu Y, Sun L and Wang G: ABT737 enhances cholangiocarcinoma
sensitivity to cisplatin through regulation of mitochondrial
dynamics. Exp Cell Res. 335:68–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X,
Yu C, Jiang X, Xu Y and Su L: ABT737 reverses cisplatin resistance
by regulating ER-mitochondria Ca2+ signal transduction in human
ovarian cancer cells. Int J Oncol. 49:2507–2519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni Z, Wang B, Dai X, Ding W, Yang T, Li X,
Lewin S, Xu L, Lian J and He F: HCC cells with high levels of Bcl-2
are resistant to ABT-737 via activation of the ROS-JNK-autophagy
pathway. Free Radic Biol Med. 70:194–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang M, Li X, Jia S, Liu S, Fu L, Jiang X
and Yang M: Bisphenol AF induces apoptosis via estrogen receptor
beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell
line KGN. Environ Pollut. 270:1160512021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keuling AM, Andrew SE and Tron VA:
Inhibition of p38 MAPK enhances ABT-737-induced cell death in
melanoma cell lines: Novel regulation of PUMA. Pigment Cell
Melanoma Res. 23:430–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Will B, Siddiqi T, Jordà MA, Shimamura T,
Luptakova K, Staber PB, Costa DB, Steidl U, Tenen DG and Kobayashi
S: Apoptosis induced by JAK2 inhibition is mediated by Bim and
enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid
cells. Blood. 115:2901–2909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dasmahapatra G, Lembersky D, Rahmani M,
Kramer L, Friedberg J, Fisher RI, Dent P and Grant S: Bcl-2
antagonists interact synergistically with bortezomib in DLBCL cells
in association with JNK activation and induction of ER stress.
Cancer Biol Ther. 8:808–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konopleva M, Milella M, Ruvolo P, Watts
JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo
P, et al: MEK inhibition enhances ABT-737-induced leukemia cell
apoptosis via prevention of ERK-activated MCL-1 induction and
modulation of MCL-1/BIM complex. Leukemia. 26:778–787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Kong X, Kang J, Su J, Li Y, Zhong
J and Sun L: Oxidative stress induces parallel autophagy and
mitochondria dysfunction in human glioma U251 cells. Toxicol Sci.
110:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Shi J, Mao SY, Xu YS, Zhang D,
Feng LY, Zhang B, Yan YY, Wang SC, Pan JP, et al: Role of p38 MAPK
in enhanced human cancer cells killing by the combination of
aspirin and ABT-737. J Cell Mol Med. 19:408–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong D, Dong Y, Fu J, Lu S, Yuan C, Xia M
and Sun L: Bcl2 inhibitor ABT737 reverses the Warburg effect via
the Sirt3-HIF1α axis to promote oxidative stress-induced apoptosis
in ovarian cancer cells. Life Sci. 255:1178462020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morita K, Saitoh M, Tobiume K, Matsuura H,
Enomoto S, Nishitoh H and Ichijo H: Negative feedback regulation of
ASK1 by protein phosphatase 5 (PP5) in response to oxidative
stress. EMBO J. 20:6028–6036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li H, Yang Q, Huang Z, Liang C, Zhang DH,
Shi HT, Du JQ, Du BB and Zhang YZ: Dual-specificity phosphatase 12
attenuates oxidative stress injury and apoptosis in diabetic
cardiomyopathy via the ASK1-JNK/p38 signaling pathway. Free Radic
Biol Med. 192:13–24. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konstantinopoulos PA and Matulonis UA:
Clinical and translational advances in ovarian cancer therapy. Nat
Cancer. 4:1239–1257. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zuo Y, Zheng W, Liu J, Tang Q, Wang SS and
Yang XS: MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance
of ovarian cancer cells. Neoplasma. 67:93–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moghbeli M: MicroRNAs as the critical
regulators of Cisplatin resistance in ovarian cancer cells. J
Ovarian Res. 14:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Witham J, Valenti MR, De-Haven-Brandon AK,
Vidot S, Eccles SA, Kaye SB and Richardson A: The Bcl-2/Bcl-XL
family inhibitor ABT-737 sensitizes ovarian cancer cells to
carboplatin. Clin Cancer Res. 13:7191–7198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lheureux S, N'Diaye M, Blanc-Fournier C,
Dugué AE, Clarisse B, Dutoit S, Giffard F, Abeilard E, Briand M,
Labiche A, et al: Identification of predictive factors of response
to the BH3-mimetic molecule ABT-737: An ex vivo experiment in human
serous ovarian carcinoma. Int J Cancer. 136:E340–E350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lomovsky A, Baburina Y, Odinokova I,
Kobyakova M, Evstratova Y, Sotnikova L, Krestinin R and Krestinina
O: Melatonin can modulate the effect of navitoclax (ABT-737) in
HL-60 cells. Antioxidants (Basel). 9:11432020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21:11022020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adamová B, Říhová K, Pokludová J, Beneš P,
Šmarda J and Navrátilová J: Synergistic cytotoxicity of perifosine
and ABT-737 to colon cancer cells. J Cell Mol Med. 27:76–88. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng R, You Z, Jia J, Lin S, Han S, Liu
A, Long H and Wang S: Curcumin enhances the antitumor effect of
ABT-737 via activation of the ROS-ASK1-JNK pathway in
hepatocellular carcinoma cells. Mol Med Rep. 13:1570–1576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Hou Y, Li D, Ding Z, Xu X, Hao B,
Xia Q, Li M and Fan L: Berberine induces non-small cell lung cancer
apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann
Transl Med. 10:4852022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu Y, Gao W, Zhang Y, Wu S, Liu Y, Deng X,
Xie L, Yang J, Yu H, Su J and Sun L: ABT737 reverses cisplatin
resistance by targeting glucose metabolism of human ovarian cancer
cells. Int J Oncol. 53:1055–1068. 2018.PubMed/NCBI
|
|
45
|
Sha JF, Xie QM, Chen N, Song SM, Ruan Y,
Zhao CC, Liu Q, Shi RH, Jiang XQ, Fei GH and Wu HM:
TLR2-hif1α-mediated glycolysis contributes to pyroptosis and
oxidative stress in allergic airway inflammation. Free Radic Biol
Med. 200:102–116. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L, Wang Z, Lu T, Meng L, Luo Y, Fu X
and Hou Y: Mitochondrial Ca2+ overload leads to
mitochondrial oxidative stress and delayed meiotic resumption in
mouse oocytes. Front Cell Dev Biol. 8:5808762020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pecorino B, Scibilia G, Ferrara M, Di
Stefano AB, D'Agate MG, Giambanco L and Scollo P: Prognostic
factors and surgical treatment in vulvar carcinoma: Single center
experience. J Obstet Gynaecol Res. 46:1871–1878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li M, Wang D, He J, Chen L and Li H:
Bcl-XL: A multifunctional anti-apoptotic protein.
Pharmacol Res. 151:1045472020. View Article : Google Scholar : PubMed/NCBI
|