Sirtuins (SIRTs) are members of a highly conserved
protein family characterized by adenosine monophosphate
(AMP)-transferring enzyme activity and histone deacetylase (HDAC)
function, both of which are dependent on NAD+ (1). As of now, seven SIRTs (SIRT1 to SIRT7)
have been identified in human cells, each located in different
subcellular compartments, collectively comprising the class III
HDACs (2). SIRT1, SIRT6 and SIRT7
are situated within the nucleus, while SIRT3, SIRT4 and SIRT5 are
found in the mitochondria and SIRT2 is localized in the cytoplasm
but can translocate to the nucleus to participate in certain
functions when needed (3,4). Mammalian SIRTs rely on their enzymatic
activities and post-translationally modify a variety of proteins,
including both histones and non-histone proteins. The modification
can either initiate or inhibit the expression of downstream target
proteins, which are involved in a variety of physiological
mechanisms, including adenosine triphosphate synthesis, DNA repair,
fatty acid metabolism, glucose metabolism, insulin secretion and
regulation of the cell cycle. When an organism is subjected to
endogenous or exogenous stimuli, SIRTs participate in a variety of
pathological processes including apoptosis, cellular autophagy,
oxidative stress and inflammatory responses (5). Lately, multiple studies have
recognized the association of the SIRT family with the
pathophysiology of various diseases, encompassing Alzheimer's
disease, cancer, diabetes and obesity, as well as cardiovascular,
inflammatory and neurodegenerative diseases. Lung cancer stands as
the most prevalent cancer worldwide and it is the leading cause of
cancer-related death. Lung cancer is categorized into small cell
lung cancer (SCLC) and non-SCLC (NSCLC) on the basis of clinical
and histopathologic features. Among them, NSCLC is a common type of
lung cancer, encompassing lung adenocarcinoma, large-cell lung
cancer and squamous cell carcinoma, collectively accounting for
~85% of lung cancer cases. Due to its high invasiveness and the
lack of effective early screening markers, 70% of patients with
NSCLC are diagnosed at advanced stages (6). The current study revealed
differentially expressed SIRTs among patients of different sexes
and lung cancer types. These SIRTs exhibit dual roles as either
tumor promoters or tumor suppressors, influencing various
pathophysiological processes within tumor cells, such as autophagy,
apoptosis and energy metabolism. Consequently, they play a crucial
role in regulating NSCLC, thus emerging as prognostic biomarkers
and potential therapeutic targets for NSCLC (7,8).
SIRT1 is the most comprehensively studied member of
the SIRT family and exhibits dual roles by acting both as a
promoter and an inhibitor of NSCLC progression (9). The findings of a meta-analysis
indicated a notable association between SIRT1 overexpression and
survival in patients with lung cancer, suggesting that elevated
SIRT1 levels predict an unfavorable prognosis for individuals with
solid cancers (10). Chen et
al (9) and Li and Zhong
(11) observed a substantial
upregulation of SIRT1 expression in A549 and H1299 cells compared
to non-cancerous cells. Furthermore, they demonstrated that
inhibition of SIRT1 attenuates the invasion and proliferation of
NSCLC cells while inducing apoptosis in tumor cells. Ahmad et
al (12) identified that SIRT1
directly interacts with cytoplasm through the activation of IGF-1
to increase cell migration, invasion and metastasis, thereby
promoting tumor progression. Xie et al (13) observed that SIRT1 promotes
endothelial cell branching and proliferation, increases vascular
density and facilitates lung tumor growth by delta-like canonical
Notch ligand 4/NOTCH signaling pathway downregulation and N1IC
deacetylation. Lung adenocarcinoma ranks among the most prevalent
and lethal types of cancer. Elevated expression of SIRT1 protein is
associated with recurrence and an unfavorable prognosis in patients
diagnosed with lung adenocarcinoma (14). Han et al (15) found that SIRT1 exhibits high
expression levels in NSCLC brain metastatic tissues compared to
NSCLC tissues, and knockdown of SIRT1 significantly and
specifically inhibits A549 migration. However, another study
reported that SIRT1 expression is significantly reduced in NSCLC
tissues or cells, and SIRT1 overexpression inhibits NSCLC
progression (16). For instance,
Hosseninia et al (17)
discovered that serum SIRT1 levels were lower in patients with lung
cancer, with significantly lower levels observed in patients with
adenocarcinoma relative to those with SCLC and squamous carcinoma.
Costa-Machado et al (18)
found that oncogenic K-RAS in human lung adenocarcinoma cell lines
downregulates SIRT1 in a manner dependent on MEK and PI3K.
Furthermore, overexpression of SIRT1 delayed the appearance of lung
adenocarcinoma driven by K-Ras (18). Li et al (19) discovered that SIRT1 overexpression
protects NSCLC cells from NF-κB acetylation and
epithelial-mesenchymal transition induced by osteoblasts. In
addition, it attenuates cell proliferation, migration and invasion,
thereby inhibiting NSCLC progression (19). Circular RNA (Circ)-SIRT1 has been
found to be downregulated in NSCLC. Furthermore, the expression of
circ-SIRT1 is associated with the staging of tumor size and tumor
lymph node metastasis in patients with NSCLC. Elevated circ-SIRT1
inhibits NSCLC cellular activity and glycolysis, and knockdown of
circ-SIRT1 promotes the malignant behavior of NSCLC cells.
Circ-SIRT1 inhibits the malignant progression of NSCLC cells by
targeting SMAD7 via microRNA (miR)-510-5p, suggesting its potential
as a therapeutic target for NSCLC therapy (20). Grbesa et al (21) revealed that SIRT1 protein expression
is notably elevated in NSCLC cell lines and primary lung tumors
compared to normal cells and tissues. They also found that this
expression is more pronounced in adenocarcinoma. Of note, high
SIRT1 expression levels in patients with NSCLC are associated with
short recurrence-free survival (21).
SIRT1 is regulated by different factors and
deacetylates regulatory histones and non-histone proteins, thereby
influencing tumor progression. MiR-124 and miR-142 inhibit
autophagy by directly targeting SIRT1 and enhancing cisplatin
sensitivity in NSCLC cells (22).
MiR-133a-3p promotes tumor growth and metastasis after incomplete
microwave ablation by decreasing SIRT1 expression and increasing
viability, migratory invasiveness and proliferation of NSCLC cells
(23). MiR-22 directly targets
fibroblast growth factor receptor 1 and SIRT1 in endothelial cells
and inhibits all key angiogenic activities in endothelial cells.
This inhibition leads to inactivation of the AKT/mammalian target
of rapamycin (mTOR) pathway, thus suppressing NSCLC growth
(24). MiR-217 inhibits NSCLC cell
invasion and proliferation while inducing tumor cell apoptosis by
targeting SIRT1 and inhibiting the SIRT1-mediated AMP-activated
protein kinase (AMPK)/mTOR signaling pathway (11). MiR-326 inhibits SIRT1 by suppressing
hypoxia-inducible factor (HIF)-1α expression, thereby inhibiting
chemoresistance and proliferation and promoting apoptosis in NSCLC
cells (25). Low expression levels
of small nucleolar RNA host gene 10 (SNHG10) predict poor survival
in patients with NSCLC. Overexpression of SNHG10 leads to
upregulation of SIRT1, a downstream target of miR-543, and
overexpression of SIRT1 results in reduced proliferation of NSCLC
cells. Conversely, elevated expression of miR-543 reduces the
effects of SNHG10 and SIRT1 overexpression, suggesting that SNHG10
inhibits tumor cell proliferation by regulating miR-543
upregulation of tumor-suppressive SIRT1 (26). CircRNA hsa_circ_0001946 facilitates
the growth of lung adenocarcinoma cells by modulating
miR-135a-5p/SIRT1 consequently inhibiting NSCLC growth (27). Hsa-miR-217 along with its target
gene SIRT1 serve as a metastasis suppressor and initiator genes,
respectively, in NSCLC. The hsa-miR-217/SIRT1/P53/CD82 metastasis
regulatory pathway exhibits a key influence in NSCLC brain
metastasis (28). Deacetylation of
K-Ras by SIRT1 increases the conversion of Ras-GTP to Ras-GDP and
promotes ERK1/2 downstream activation. In turn, the Ras/ERK pathway
regulates SIRT1 transcription (29). Inhibition of SIRT1 results in the
acetylation of heat shock protein family A (Hsp70) member 5
(HSPA5), leading to the activation of activating transcription
factor 4 (ATF4) and DNA damage inducible transcript 4 (DDIT4),
which induces autophagy. This process ultimately suppresses the
mTOR signaling pathway in NSCLC cells (30). SIRT1 may induce pro-apoptotic
effects by transcription factor deacetylation in A549/CADD cells
(31). In the NSCLC
microenvironment, SIRT1 participates in the molecular metabolic
mechanism underlying hypoxia-induced chemoresistance by regulating
the peroxisome proliferator activated receptor γ (PPARG)
coactivator 1 (PGC-1α)/PPAR-γ signaling pathway (32).
A cross-sectional analysis by the Cancer Genome
Atlas Program demonstrated that the SIRT2 gene is amplified in ~4%
of patients with NSCLC, suggesting that it has a bifacial role in
NSCLC (33). For instance, Li et
al (34) observed significant
downregulation of SIRT2 expression in tumor tissues. Furthermore,
they found that SIRT2 overexpression inhibits cell proliferation
and induction of apoptosis, leading to cell cycle arrest and
increased sensitivity to cisplatin treatment. Further investigation
revealed that overexpression of SIRT2 increases the production of
reactive oxygen species (ROS) and p27 levels (34). In addition, research uncovered that
SIRT2 levels are notably reduced in lung cancer cell lines and
SIRT2 overexpression promotes deacetylation and degradation of
S-phase kinase-associated protein 2 (Skp2), consequently increasing
p27 levels and inhibiting NSCLC cell growth. By contrast, SIRT2
knockdown restrains deacetylation and degradation of Skp2, leading
to decreased p27 levels and elevated NSCLC cell growth (35). Xu et al (36) discovered that SIRT2 could suppress
KDM4A expression by binding to the promoter region of the KDM4A
gene. This action inhibits the clone formation, proliferation and
tumor growth of NSCLC cells (36).
However, research demonstrated that SIRT2 has an oncogenic role in
NSCLC. For instance, Gao et al (37) identified that the median survival
time of patients exhibiting high SIRT2 expression was substantially
lower compared to those with low SIRT2 expression. They proposed
that SIRT2 may serve as a distinct prognostic biomarker for
non-metastatic lung adenocarcinoma (37). Hoffmann et al (38) found that AEM1 and AEM2, the
selective inhibitors of SIRT2, sensitize NSCLC cell lines to
etoposide-induced apoptosis by increased p53 activation resulting
from decreased SIRT2-dependent p53 deacetylation. Xu et al
(39) demonstrated that SIRT2
deacetylates and promotes the activation of the K100 residue of
phosphoglycerate-converting enzyme, which enhances NADPH production
and accelerates tumor growth. Tang et al (40) depicted the involvement of SIRT2 in
the induction of a protective autophagy mechanism in HL60/A cells,
which is closely related to drug resistance in patients. However,
conflicting studies indicate that SIRT2 expression is downregulated
in NSCLC and that SIRT2 inhibits tumor growth (41).
SIRT2 contributes to NSCLC progression by modulating
acetylated protein levels. Inhibition of SIRT2 in cancer cells
increases forkhead box (FOX)O1 acetylation, promotes interactions
of FOXO1 related to autophagy and ultimately induces autophagy. Of
note, autophagic processes are negatively correlated with tumor
progression (42). Deacetylation of
aldo-keto reductase family 1 member C1 (AKR1C1) by SIRT2 inhibits
the binding of AKR1C1 to STAT3, thereby reducing the
transcriptional activity of STAT3 and suppressing the migration of
NSCLC cells (43). Inhibition of
SIRT2 triggers autophagy in human NSCLC cells by preventing the
mTOR signaling pathway through increased acetylation of HSPA5 and
upregulation of the expression levels of ATF4 and DDIT4. SIRT2
binds directly to the transcription factor EB and regulates
apoptosis induced by acute shear stress through autophagy
modulation and exosome release, thereby inhibiting NSCLC onset and
metastasis (30). SIRT2
overexpression induces the deacetylation and rapid degradation of
Skp2, eliminating the impact of Skp2 on p27, leading to an increase
in the p27 expression level and subsequent inhibition of NSCLC cell
growth (35). ATP citrate lyase
(ACLY) displays oncogenic functions in NSCLC, and SIRT2
deacetylates and promotes the degradation of ACLY, leading to a
reduction in fatty acid synthesis and delaying tumor growth in
NSCLC cells. SIRT2 acts as a primary HDAC for ACLY in NSCLC cells,
thereby attenuating the oncogenic activity of ACLY (44). SIRT2 deacetylates AKR1C1 and
inhibits the transactivation of STAT3 target genes, thereby
inhibiting cell migration. The acetylation on Lysines 185 and 201
of AKR1C1 determines its potential to promote metastasis both in
vitro and in vivo. The restoration of SIRT2 acetylation
offers a potential therapeutic target for the treatment of patients
with metastatic NSCLC who exhibit elevated AKR1C1 expression
(43). SIRT2 regulates histone and
non-histone activities by acetylation and is regulated by upstream
factors. For instance, miR-150 impacts NSCLC cell viability and
mobility by regulating the SIRT2/JMJD2A pathway (36,45);
synoviolin 1 (SYVN1) stimulates pathological processes such as
tumor cell growth, ontogeny and migration by regulating SIRT2
(41). The level of speckle type
BTB/POZ protein (SPOP) is significantly reduced and the level of
SIRT2 is significantly increased in NSCLC cell lines; mutations in
NSCLC suppress the ability of SPOP to degrade SIRT2 and inhibit
NSCLC cell growth (46). In
addition, some somatic mutations within SIRT2 have been identified
in NSCLC that alter SIRT2 protein levels (47).
The involvement of SIRT2 in cancer is complex and
controversial, with evidence supporting both tumor-suppressive and
oncogenic roles. Initially, SIRT2 was proposed as a tumor
suppressor due to its regulatory effects on the mitotic checkpoint
and its deacetylase activity on histone H3K56, a modification
frequently observed in cancer cells (48,49).
Genetic experiments further supported this role, showing that mice
deficient in SIRT2 had a higher incidence of tumors (50). Conversely, SIRT2 has been shown to
promote tumor growth by deacetylating p53, leading to decreased p53
transcriptional activity (51). In
addition, SIRT2 deacetylates lactate dehydrogenase A at K5,
increasing its activity and protein levels, thereby accelerating
glycolysis and lactate production, which enhances cancer cell
proliferation and migration (52).
Furthermore, the broad anticancer activity observed with SIRT2
inhibitors (53,54) suggests their potential as
therapeutic agents in cancer treatment. These findings underscore
the dual nature of SIRT2 in cancer, highlighting its complex and
multifaceted role in tumor biology.
SIRT3, the most important mitochondrial SIRT, is
widely studied and exhibits a dual role in NSCLC, promoting both
tumor progression and exerting antitumor effects. The findings of
meta-analysis and systematic review indicate that SIRT3 is linked
with poor prognosis in NSCLC (55).
Yang et al (56) discovered
that SIRT3 expression is notably elevated in NSCLC tissues.
Furthermore, the levels of SIRT3 were identified as an independent
factor in the prognosis of patients with NSCLC (56). The findings of Ahmed et al
(57) reveal that SIRT3 exhibits
oncogenic properties in high-fat diet (HFD)-induced tumorigenesis,
and inhibition of SIRT3 may attenuate the pro-carcinogenic effects
of HFD. Radiotherapy is an indispensable therapeutic tool for the
treatment of NSCLC; however, the resistance of tumor cells against
ionizing radiation often leads to treatment failure. Cao et
al (58) found that expression
of SIRT3 is upregulated in lung cancer tissues and NSCLC cell
lines. They noted that SIRT3 knockdown significantly increases cell
cycle arrest and radiation-induced apoptosis. Conversely, SIRT3
overexpression promotes radioresistance in lung cancer cells, which
is linked to the activation of the ATM-checkpoint kinase 2 pathway
following irradiation (58).
However, Tao et al (59)
found that SIRT3 mRNA and protein levels are significantly reduced
in lung cancer tissues and serum samples. They observed a negative
association between SIRT3 expression and tumor size, tumor lymph
node metastasis stage and metastasis. Furthermore, they revealed
that the serum levels of SIRT3 are able to differentiate between
patients with lung cancer and healthy individuals with high
sensitivity and specificity. These findings suggest that SIRT3 may
serve as a biomarker for early diagnosis of lung cancer (59). Xiao et al (60) revealed downregulated expression of
SIRT3 in human lung adenocarcinoma tissues. Furthermore, they
observed that overexpression of SIRT3 substantially suppresses the
proliferation of the A549 lung adenocarcinoma cell line, increases
the ratio of Bax/Bcl-2 and Bad/Bcl-xL, as well as upregulates the
protein levels of p21 and p53, ultimately inducing cell apoptosis
(60). Cisplatin resistance poses a
significant challenge in chemotherapy treatment for patients with
lung cancer. Cao et al (61)
revealed that the expression of chromatin licensing and DNA
replication factor 1 (CDT1), FOXO3 and SIRT3 is inhibited in both
cells and tissues of lung cancer. They found that FOXO3 positively
regulates the expression of CDT1. In addition, they observed that
elevated levels of SIRT3 suppress FOXO3 through acetylation,
consequently enhancing FOXO3 expression. Elevated levels of CDT1,
FOXO3 or SIRT3 inhibit cisplatin resistance and diminish in
vitro survival, invasion, and proliferation of lung cancer
cells. It was further demonstrated that SIRT3 deletion elevates
Ki-67 and VEGFA levels, while the overexpression of SIRT3 increases
the expression of the FOXO3a/CDT1 axis, thereby enhancing the
sensitivity of lung cancer cells (61). Geoghegan et al (62) found that decreased SIRT3 expression
reduces oxidative metabolism, decreases mitochondrial abundance and
increases glycolytic flux and ROS production in H1299 lung large
cell carcinoma cells with acquired cisplatin-resistance.
SIRT3 is regulated by different factors and
deacetylates regulatory histones and non-histone proteins, thereby
affecting tumor progression. For instance, Zhang et al
(63) found that cancer-associated
fibroblasts (CAF) induce endothelial cell angiogenesis and enhance
the malignant phenotype of NSCLC cells through the establishment of
a CAF-NSCLC co-culture model. MiR-224 targets the 3′-untranslated
region of SIRT3 mRNA, consequently suppressing SIRT3/AMPK, while
activating mTOR/HIF-1α. Forced overexpression of SIRT3 leads to the
upregulation of AMPK and inactivation of the mTOR/HIF-1α signaling
pathway. Conversely, suppression of HIF-1α notably enhances
SIRT3/AMPK levels and decreases mTOR phosphorylation. It is
noteworthy that both the overexpression of SIRT3 and the inhibition
of HIF-1α result in a reduction of miR-224 levels and NSCLC
promotion facilitated by miR-224. This observation suggests the
existence of a positive feedback loop involving the
miR-224-SIRT3/AMPK/mTOR/HIF-1α axis in the regulation of NSCLC
carcinogenesis induced by CAF (63). Xiong et al (64) identified that circRNA Rac GTPase
activating protein 1 (circRACGAP1) exhibits elevated expression in
NSCLC. Deficiency of circRACGAP1 led to the inhibition of
epithelial-mesenchymal transition, metastasis and stemness of stem
cells. CircRACGAP1 enhances SIRT3 stability and expression by
recruiting associated proteins, which subsequently leads to
deacetylation of replication timing regulatory factor 1 (RIF1) and
activates the Wnt/β-catenin pathway. Overexpression of circRACGAP1
counteracts SIRT3 or RIF1 knockdown-mediated inhibition of NSCLC
cell stemness and metastasis. The absence of circRACGAP1 impedes
tumor development and metastasis in vivo, suggesting its
role in promoting SIRT3-mediated deacetylation of RIF1 through the
recruitment of related proteins, thereby promoting differentiation
and metastasis of NSCLC cells (64). Xiong et al (65) found that SIRT3 expression is
significantly increased and P53 expression is almost negative in
clinical samples of phosphatase and tensin homologue-deficient
NSCLC. Furthermore, they demonstrated that SIRT3 promotes P53
degradation by deacetylating P53 at lysines 320 and 382.
SIRT5 exhibits a dual role in cancer, functioning as
a tumor suppressor in certain cancers while acting as an oncogene
in others. Of note, the expression of SIRT5 is not specific but
rather significantly dependent on the cellular environment. SIRT5,
as a tumor suppressor, prevents the Warburg effect, enhances
protection against ROS, and decreases cell proliferation and
metastasis. However, as an oncogene, it exhibits opposing effects
by increasing resistance to chemotherapeutic agents and/or
radiotherapy (66,67). Mendelian randomization analysis
reveals that SIRT5 acts as a protective factor and is causally
associated with lung squamous cell carcinoma (64). However, most studies concluded that
SIRT5 has an oncogenic role in NSCLC. For instance, one study
reported upregulated SIRT5 expression in tumor tissues from certain
patients with NSCLC, with the higher SIRT5 levels correlating with
poorer clinical prognosis (68).
Deng et al (69) identified
that hsa_circ_0081664 enhances the expression of SIRT5 by adsorbing
miR-507, which promotes cisplatin resistance in NSCLC cells,
silences SIRT5 to inhibit tumor cell proliferation, reduces cell
invasion and migration, and promotes cell apoptosis. Lu et
al (70) were the first to find
in A549 lung cancer cells that SIRT5 binds to pyruvate kinase
isoform M2 (PKM2) and desuccinates its K498 residue, which inhibits
its activity, reduces ROS production, and promotes tumor growth and
cell proliferation. In addition, Ye et al (68) found that PKM2 desuccinylation
mediated by SIRT5 favors tumor cell growth and promotes cell
survival and proliferation, and that suppression of SIRT5 inhibits
tumor cell proliferation by PKM2 K498 desuccinylation. Li et
al (71) found that SIRT5
facilitates the onset and progression of NSCLC by decreasing the
acetylation level of fatty acid binding protein 4. Wu et al
(72) discovered that in HCC827 and
PC9 lung adenocarcinoma cells, APC down-regulated 1 like-antisense
1, a long non-coding RNA, positively regulates SIRT5 expression
with the sponge miR-1322/miR-1972/miR-324-3pin. This regulation
suppresses autophagic degradation of epidermal growth factor
receptor, thereby inducing resistance to imatinib (72).
SIRT6 demonstrates both pro- and anti-tumor effects
in NSCLC in multiple ways. For instance, Azuma et al
(73) identified that enhanced
SIRT6 expression is associated with T and N staging in tumors of
patients with NSCLC. Furthermore, its overexpression facilitates
metastasis and chemoresistance of NSCLC cells, leading to poor
prognosis. Bai et al (74)
were the first to demonstrate that SIRT6 is upregulated in NSCLC.
Furthermore, they found that its overexpression enhances
extracellular signal-regulated kinase 1/2 phosphorylation,
activates matrix metalloproteinase 9, and promotes tumor cell
invasion and migration (74).
Subramani et al (75) found
that SIRT6 functions as an oncogene in NSCLC, and SIRT6 silencing
suppresses NSCLC cell proliferation and induces apoptosis. The
NOTCH signaling pathway plays a crucial role in cell survival and
modulates both cell proliferation and differentiation. SIRT6
silencing substantially facilitates and stabilizes the acetylation
state of DNA methyltransferase 1, which then translocates into the
nucleus and methylates the Notch receptor 1 (NOTCH1) promoter
region, leading to the blockage of NOTCH1-mediated NOTCH signaling
(75). Krishnamoorthy et al
(76) found that SIRT6 expression
is significantly increased in A549, NCI-H460 and NCI-H520. The
small interfering RNA-mediated silencing of SIRT6 activates
p53/p21-induced suppression of cell proliferation, resulting in
apoptosis induction and cell cycle arrest. This suggests that SIRT6
is a tumor promoter in the ontogeny, progression and regulation of
NSCLC, and thus, SIRT6 silencing is expected to be a new approach
for lung cancer treatment (76).
Furthermore, Kim et al (77)
identified that decreased expression of SIRT6 mediates the
enhancement of apoptosis induced by radiation through cAMP
signaling in lung cancer cells. However, SIRT6 functions as a tumor
suppressor involved in NSCLC progression (78). SIRT6 increases the radiosensitivity
of NSCLC and protects against lung injury induced by radiation.
Wang et al (79) found that
the alveolar wall of the radiotherapy group was significantly
thickened and a large amount of proliferative fibrous tissue could
be observed in the alveolar interstitium, while the thickness of
the alveolar wall and interstitial fibrosis were substantially
decreased in the SIRT6 overexpression group, and the level of
inflammatory factors was significantly reduced so that SIRT6 could
inhibit inflammation and reduce radiation pneumonitis and lung
injury (79). SIRT6 expression
inhibits HIF-1α and VEGF expression and promotes egl-9 family HIF-1
expression, thereby inhibiting angiogenesis and tumor growth
(80).
SIRT4 is a mitochondrial SIRT and research on its
functions related to NSCLC is currently scarce. Fu et al
(81) found reduced expression of
SIRT4 in 70 out of 133 NSCLC cases through immunohistochemistry.
They noted that a low expression level of SIRT4 was associated with
the metastatic stage of tumor lymph nodes, the histological type of
the tumor, lymph node status, Ki-67 and poor overall survival. A
further study revealed that SIRT4 suppresses lung cancer cell
proliferation by regulating mitochondrial dynamics through the
ERK-dynaminrelated protein 1 pathway. Furthermore, SIRT4 blocks the
cell cycle, and suppresses cell invasion and migration, suggesting
its crucial role as an important anti-tumor protein in NSCLC
(81). Autophagy is one of the
processes leading to the increasingly prevalent resistance to
cytotoxic chemotherapy. Jiang et al (82) discovered that SIRT7 protein levels
are significantly elevated after treatment with gemcitabine, an
antimetabolite drug. Furthermore, they observed that SIRT7
deficiency promotes gemcitabine-induced cell death and enhances the
antitumor activity of gemcitabine. These findings suggest that
SIRT7 inhibition can enhance the effectiveness of antimetabolite
therapy in NSCLC cells (82).
SIRT-targeted therapies in NSCLC involve
manipulating the functions of SIRT proteins to inhibit tumor growth
and enhance the effectiveness of existing cancer treatments. The
mechanisms through which SIRTs influence NSCLC can be broadly
categorized based on their roles in cellular metabolism, DNA repair
and regulation of gene expression.
SIRT6 plays a crucial role in DNA repair and cells
lacking SIRT6 (knockout) exhibit genomic instability and heightened
sensitivity to DNA damage. In response to DNA double-strand breaks
(DSBs), SIRT6 dynamically associates with chromatin and
significantly reduces overall H3K9 acetylation in cells. This
action facilitates the formation of a macromolecular complex with
the DNA DSB repair factor DNA-dependent protein kinase, enhancing
the repair of DNA DSBs. Furthermore, SIRT6 recruits the chromatin
remodeling factor SWI/SNF related, matrix associated, actin
dependent regulator of chromatin, subfamily a, member 5 homolog to
the DSB sites and is essential for deacetylating H3K56, which opens
up the chromatin-an early step in the DNA damage response (83). Recent studies have also observed
that c-Jun N-terminal kinase phosphorylates SIRT6 to stimulate DNA
DSB repair in response to oxidative stress (84). Following this post-translational
modification, SIRT6 recruits poly(ADP-ribose) polymerase 1 to DNA
breaks and activates it through mono-ADP-ribosylation, thereby
promoting homologous recombination and non-homologous end joining,
and enhancing the repair of DNA breaks. By boosting SIRT6 activity,
the sensitivity of cancer cells to DNA-damaging agents used in
chemotherapy can be increased, thereby improving therapeutic
outcomes.
SIRT1 is an NAD-dependent deacetylase that plays a
crucial role in transcription, DNA replication and repair. p53 was
identified as the first non-histone substrate of SIRT1, with its
acetylation levels being regulated by the activity of SIRT1
(85,86). SIRT1 induces deacetylation of p53 at
its C-terminal residues in an NAD-dependent manner, which
suppresses its transcriptional activation ability and ultimately
inhibits p53-mediated transcription-dependent apoptosis (86). Studies have shown that dysfunction
of the SIRT1-p53 axis is closely associated with various cancers
(87–89). In preclinical studies, compounds
targeting the SIRT1-p53 axis have proven to be effective cancer
therapies. These findings suggest that the SIRT1-p53 pathway may be
a promising therapeutic target for cancer treatment.
In addition, SIRT3 deacetylates and activates
superoxide dismutase 2 (SOD2); S-glutathionylation-induced
inactivation of SIRT3 leads to hyperacetylation of SOD2 and
exacerbates hypertension in angiotensin II-induced SIRT3 knockout
and SOD2-depleted mice (90). The
activity of SIRT3 is also regulated by mitochondrial function and
matrix pH. A reduction in membrane potential decreases SIRT3
activity, shifting substrate utilization from carbohydrate
oxidation to lactate production. This shift increases the NADH/NAD+
ratio and raises the levels of acetyl-CoA, thereby reducing SIRT3
activity (91). As a mitochondrial
SIRT, SIRT3 primarily promotes apoptosis in cancer cells by
enhancing mitochondrial function and increasing oxidative stress.
Enhancing SIRT3 activity could exploit mitochondrial
vulnerabilities in cancer cells, leading to their death.
In ~20% of patients with NSCLC, an association
between SIRT2 and resistance mutations in EGFR has been observed.
EGFR tyrosine kinase inhibitors are currently the standard
treatment for patients with NSCLC with EGFR mutations. However,
resistance remains a major factor impacting the efficacy of cancer
treatments. Bajpe et al (97) demonstrated through extensive
screening that the absence of SIRT2 confers resistance to EGFR
inhibitors in NSCLC and colorectal cancer. SIRT2 deacetylates MEK1
and inhibits its activation. The absence of SIRT2 leads to
increased acetylation and phosphorylation levels of MEK1,
potentially causing cancer relapse due to enhanced activation of
MEK1 and subsequent phosphorylation of downstream ERK. Similarly,
the lack of SIRT2 also results in resistance to B-Raf
proto-oncogene, serine/threonine kinase (BRAF) and MEK inhibitors
in melanoma with BRAF mutations and colorectal cancer with Kras
mutations (98). Conversely,
increased levels of SIRT2 expression may induce multidrug
resistance in acute myeloid leukemia by activating the ERK1/2
signaling pathway (99).
Furthermore, SIRT2 exerts a protective role against
chemotherapy-induced peripheral neuropathy in a subcutaneous lung
cancer mouse model, a common reason for the reduction and
discontinuation of chemotherapy dosages. Cisplatin induces the
nuclear accumulation of SIRT2 in dorsal root ganglion neurons,
allowing SIRT2 to participate in the repair of DNA damage caused by
cisplatin (100). Multiple studies
suggest that SIRT2 contributes to the stemness of cancer stem
cells, providing further links between SIRT2 and chemoresistance in
NSCLC (101).
In summary, SIRT proteins, particularly SIRT1, SIRT3
and SIRT6, exhibit complex and context-dependent roles in NSCLC,
functioning both as promoters and suppressors of tumor growth based
on specific conditions and interactions within the cellular and
tumor environments. SIRT1 can promote NSCLC progression by
inhibiting the tumor suppressor p53, reducing apoptosis and
allowing cancer cell proliferation. However, under different
conditions, SIRT1 also enhances the action of chemotherapeutic
drugs by acetylating p53, thereby promoting its tumor-suppressive
functions. Similarly, while SIRT3 generally acts as a tumor
suppressor by improving mitochondrial function and increasing
oxidative stress that can lead to cancer cell death, it may also
support cancer cell survival under certain metabolic conditions by
enhancing energy production. SIRT6 mainly exhibits
tumor-suppressive properties in NSCLC by maintaining genomic
stability through DNA repair and chromatin remodeling, and by
suppressing glycolysis, a key metabolic pathway heavily relied upon
by cancer cells. SIRT6′s ability to repress the expression of
glycolytic genes diminishes a critical energy source for tumor
cells, thereby inhibiting their growth and survival. These SIRT
family members' roles are significantly influenced by the tumor
microenvironment, genetic variations and differential expression
levels, which can shift their function from tumor-promoting to
tumor-suppressing. This dual functionality makes SIRTs valuable
targets for NSCLC therapy, highlighting the need for precise
targeting in therapeutic strategies to exploit their
cancer-regulating capabilities effectively. Clinical trials and
preclinical studies suggest that modulating SIRT activity, either
through small molecule inhibitors or gene therapy, could enhance
the response to standard chemotherapies and provide a broader
strategy for managing resistance. So far, the exploration of SIRT
proteins in NSCLC has highlighted their potential as both
biomarkers and therapeutic targets, reflecting their complex roles
in tumor biology. Continued research is essential to fully
understand and harness these proteins' capabilities to improve
NSCLC treatment outcomes.
SIRTinol is a specific inhibitor of SIRT1 and SIRT2
and a novel anticancer drug. SIRTinol chelates iron in NSCLC cells,
leading to a substantial reduction in unstable iron and a cell
lineage-specific adaptive response (66). SIRTinol potentially suppresses the
proliferation of NSCLC cells and induces apoptosis by modulating
the AKT-β-catenin-FOXO3a axis (102). The combination of SIRTinol and
AGK2 with sodium dichloroacetate leads to elevated lysine
acetylation and reduced serine phosphorylation of pyruvate
dehydrogenase α1, resulting in synergistic therapeutic effects
(103). Inauhzin is an inhibitor
of SIRT1 and a P53 activator. It prevents MDM2-mediated p53
degradation, induces p53 activation and inhibits the activity of
SIRT1 to indirectly interrupt the negative feedback loop of
MDM2-p53. Subsequently, this action enhances p53 acetylation, thus
making Inauhzin a promising new strategy for anticancer therapy
(104). Doxorubicin and Trifolium
pratense L. (Red clover) extract, when combined, have been shown to
reduce serum levels of inflammatory cytokines, increase SIRT1
expression, and inhibit tumor growth and distant metastasis
(105). MDL-800, a constitutive
activator of SIRT6, dose-dependently induces histone H3
deacetylation in NSCLC cell lines and inhibits NSCLC cell
proliferation. Its antiproliferative effect is significantly
attenuated after the knockdown of SIRT6. MDL-800 enhances the
antiproliferative effect of EGFR tyrosine kinase inhibitor and
inhibits the MAPK pathway in osimertinib-resistant cells and
patient-derived primary tumor cells. Furthermore, intraperitoneal
injection of MDL-800 in nude mice xenografted from HCC827 cells
significantly inhibits tumor growth, enhances SIRT6-dependent
histone H3 deacetylation and reduces phosphorylated (p-)ERK and
p-MEK in tumor tissues, suggesting that MDL-800 may be a potential
compound for the treatment of NSCLC (106).
Melatonin is an endogenous molecule produced by the
pineal gland, affecting circadian rhythms and cellular redox
status. In addition, melatonin serves as an important
immunomodulatory molecule, exhibiting inhibitory effects on the
growth of certain tumors. Initially, melatonin was found to inhibit
lung cancer growth and suppress NSCLC cell proliferation in a Lewis
lung carcinoma mouse model. This inhibition was associated with
metabolic reprogramming of cancer cells, characterized by a shift
from aerobic glycolysis in the cytoplasm to oxidative
phosphorylation. These metabolic changes were accompanied by higher
ATP production, elevated coupled oxygen consumption by ATP
production, higher levels of ROS, higher mitochondrial ROS levels
and lower lactate secretion. A further study revealed that
melatonin significantly enhances mitochondrial energy metabolism by
stimulating SIRT3 to increase the recombination activity of
pyruvate dehydrogenase, thereby reversing the Warburg effect
(112). It has also been found
that melatonin combined with local radiofrequency ablation
suppresses the progression of multiple lung nodules in the
unablated region, thereby reducing patient trauma and tumor
recurrence (113).
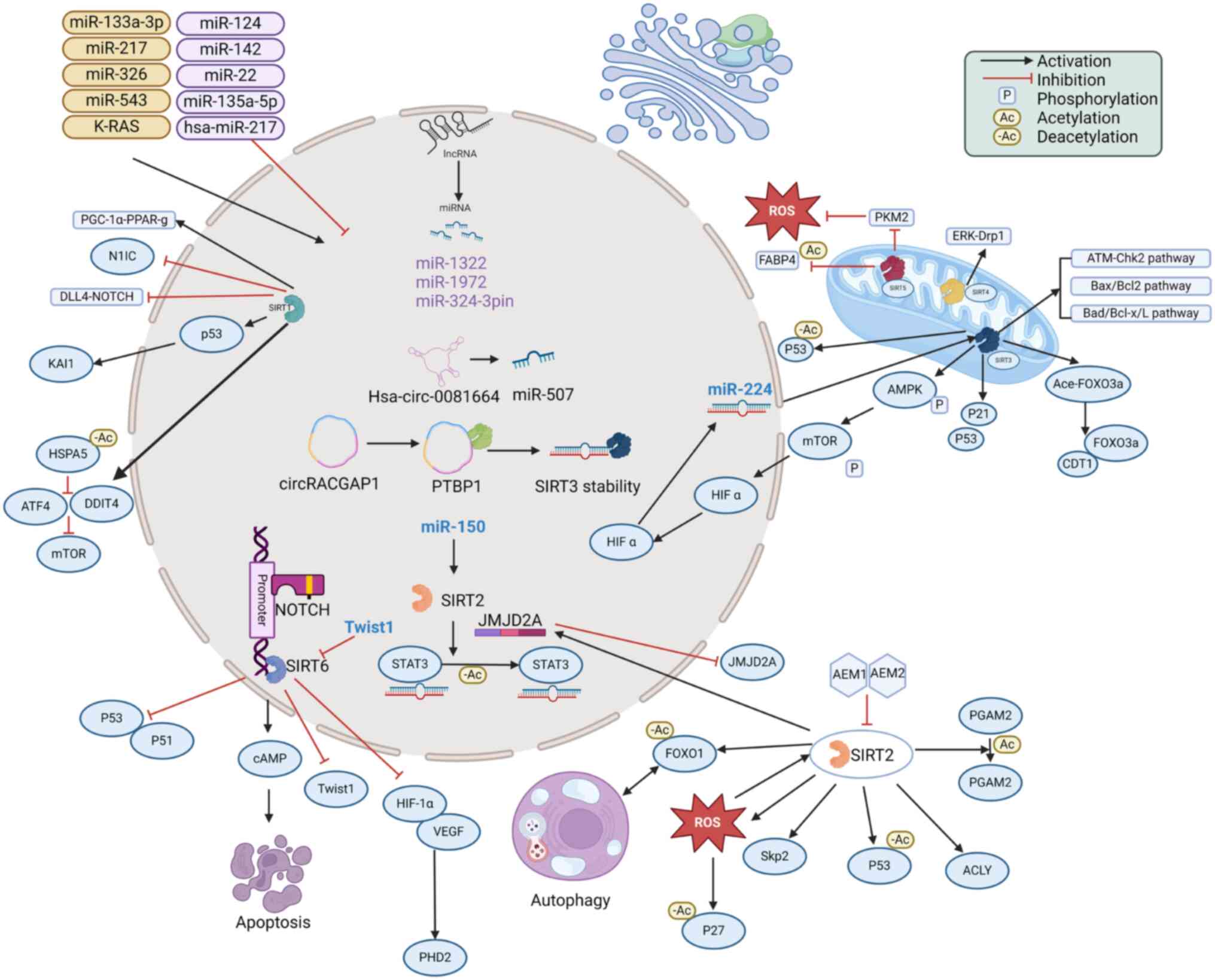
SIRTs exert dual roles as either tumor promoters or
suppressors in NSCLC, influencing a variety of pathophysiological
processes such as autophagy, apoptosis and energy metabolism within
tumor cells (Fig. 1). Their
involvement contributes to the regulation of various biological
properties of NSCLC cells, such as growth, invasion, migration and
proliferation. SIRTs regulate the activity of related proteins at
the acetylation level and are regulated by multiple upstream
factors, thus affecting lung cancer progression. Although SIRT1,
SIRT3 and SIRT6 have been studied in NSCLC, investigations on
SIRT2, SIRT4 and SIRT7 are still scarce and SIRTs are understudied
in SCLC. Therefore, further exploration is needed to explore the
potential mechanisms of SIRTs in lung cancer and to identify novel
antitumor agents.
Not applicable.
Funding: No funding was received.
Not applicable.
RFL and MZ conceived and designed the study and
wrote and revised the manuscript. MZ and LW performed the initial
literature search and prepared the manuscript. RFL and LW reviewed
and revised the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
North BJ and Verdin E: Sirtuins:
Sir2-related NAD-dependent protein deacetylases. Genome Biol.
5:2242004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajabi N, Galleano I, Madsen AS and Olsen
CA: Targeting sirtuins: Substrate specificity and inhibitor design.
Prog Mol Biol Transl Sci. 154:25–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
North BJ, Marshall BL, Borra MT, Denu JM
and Verdin E: The human Sir2 ortholog, SIRT2, is an NAD+-dependent
tubulin deacetylase. Mol Cell. 11:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL,
Liu YY, Liu YS, Zheng G, Zhao JQ, Wei YF, et al: The sirtuin family
in health and disease. Signal Transduct Target Ther. 7:4022022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abe T, Ohga Y, Tabayashi N, Kobayashi S,
Sakata S, Misawa H, Tsuji T, Kohzuki H, Suga H, Taniguchi S and
Takaki M: Left ventricular diastolic dysfunction in type 2 diabetes
mellitus model rats. Am J Physiol Heart Circ Physiol.
282:H138–H148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Zhou D, Feng Y, Li B, Cui Y, Chen
G and Li N: Association of sirtuins (SIRT1-7) with lung and
intestinal diseases. Mol Cell Biochem. 477:2539–2552. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong J, Wang H, Lou W, Wang G, Tao H, Wen
H, Liu Y and Xie Q: Associations of sirtuins with
clinicopathological parameters and prognosis in non-small cell lung
cancer. Cancer Manag Res. 10:3341–3356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Hokka D, Maniwa Y, Ohbayashi C,
Itoh T and Hayashi Y: Sirt1 is a tumor promoter in lung
adenocarcinoma. Oncol Lett. 8:387–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Yang W, Dong F, Guo Y, Tan J, Ruan
S and Huang T: The prognostic role of Sirt1 expression in solid
malignancies: A meta-analysis. Oncotarget. 8:66343–66351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G and Zhong S: MicroRNA-217 inhibits
the proliferation and invasion, and promotes apoptosis of non-small
cell lung cancer cells by targeting sirtuin 1. Oncol Lett.
21:3862021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmad SMS, Al-Mansoob M and Ouhtit A:
SIRT1, a novel transcriptional downstream target of CD44, linking
its deacetylase activity to tumor cell invasion/metastasis. Front
Oncol. 12:10381212022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie M, Liu M and He CS: Sirt1 regulates
endothelial Notch signaling in lung cancer. PLoS One. 7:e453312012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun J, Li G, Liu Y, Ma M, Song K, Li H,
Zhu D, Tang X, Kong J and Yuan X: Targeting histone deacetylase
SIRT1 selectively eradicates EGFR TKI-resistant cancer stem cells
via regulation of mitochondrial oxidative phosphorylation in lung
adenocarcinoma. Neoplasia. 22:33–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han L, Liang XH, Chen LX, Bao SM and Yan
ZQ: SIRT1 is highly expressed in brain metastasis tissues of
non-small cell lung cancer (NSCLC) and in positive regulation of
NSCLC cell migration. Int J Clin Exp Pathol. 11:2357–2365.
2013.PubMed/NCBI
|
|
16
|
Yang F: The expression and mechanism of
Sirt1 and Ampk in nonsmall cell lung cancer. J BUON. 23:106–110.
2018.PubMed/NCBI
|
|
17
|
Hosseninia S, Ameli A, Aslani MR,
Pourfarzi F and Ghobadi H: Serum levels of sirtuin-1 in patients
with lung cancer and its association with karnofsky performance
status. Acta Biomed. 92:e20210122021.PubMed/NCBI
|
|
18
|
Costa-Machado LF, Martín-Hernández R,
Sanchez-Luengo MÁ, Hess K, Vales-Villamarin C, Barradas M, Lynch C,
de la Nava D, Diaz-Ruiz A, de Cabo R, et al: Sirt1 protects from
K-Ras-driven lung carcinogenesis. EMBO Rep. 19:e438792018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Jiang Z, Li X and Zhang X: SIRT1
overexpression protects non-small cell lung cancer cells against
osteopontin-induced epithelial-mesenchymal transition by
suppressing Nf-kb signaling. Onco Targets Ther. 11:1157–1171. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Z, Zhang H, Zhang F, Ji Y, Peng Y,
Wang F and Zhao L: Circular RNA sirtuin-1 restrains the malignant
phenotype of non-small cell lung cancer cells via the
microRNA-510-5p/Smad family member 7 axis. Acta Biochim Pol.
70:855–863. 2023.PubMed/NCBI
|
|
21
|
Grbesa I, Pajares MJ, Martínez-Terroba E,
Agorreta J, Mikecin AM, Larráyoz M, Idoate MA, Gall-Troselj K, Pio
R and Montuenga LM: Expression of sirtuin 1 and 2 is associated
with poor prognosis in non-small cell lung cancer patients. PLoS
One. 10:e01246702015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song X, Kong F, Zong Z, Ren M, Meng Q, Li
Y and Sun Z: miR-124 and miR-142 enhance cisplatin sensitivity of
non-small cell lung cancer cells through repressing autophagy via
directly targeting SIRT1. RSC Adv. 9:5234–5243. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Yu F, Huang G, Ni Y, Zhang T, Zou
Z and Meng M: Exosomal miR-133a-3p promotes the growth and
metastasis of lung cancer cells following incomplete microwave
ablation. Int J Hyperthermia. 40:21900652023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu Y, Pais G, Becker V, Körbel C, Ampofo
E, Ebert E, Hohneck J, Ludwig N, Meese E, Bohle RM, et al:
Suppression of endothelial miR-22 mediates non-small cell lung
cancer cell-induced angiogenesis. Mol Ther Nucleic Acids.
26:849–864. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei J, Meng G, Wu J, Wang Y, Zhang Q, Dong
T, Bao J, Wang C and Zhang J: MicroRNA-326 impairs chemotherapy
resistance in non small cell lung cancer by suppressing histone
deacetylase SIRT1-mediated HIF1α and elevating VEGFA.
Bioengineered. 13:5685–5699. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Nong L, Chen ML, Gu XL, Zhao WW,
Liu MH and Cheng WW: Long noncoding RNA SNHG10 sponges miR-543 to
upregulate tumor suppressive SIRT1 in nonsmall cell lung cancer.
Cancer Biother Radiopharm. 35:771–775. 2020.PubMed/NCBI
|
|
27
|
Yao Y, Hua Q, Zhou Y and Shen H: CircRNA
has_circ_0001946 promotes cell growth in lung adenocarcinoma by
regulating miR-135a-5p/SIRT1 axis and activating Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 111:1367–1375. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang W, Hou L, Wei J, Du Y, Zhao Y, Deng
X and Lin X: Hsa-miR-217 inhibits the proliferation, migration, and
invasion in non-small cell lung cancer cells via targeting SIRT1
and P53/KAI1 signaling. Balkan Med J. 37:208–214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng D, Zhao L, Xu Y, Ou R, Li G, Yang H
and Li W: K-Ras promotes the non-small lung cancer cells survival
by cooperating with sirtuin 1 and p27 under ROS stimulation. Tumour
Biol. 36:7221–7232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu N, Lei Y, Wang Y, Wang Y, Duan Q, Ma G,
Liu X and Su L: Inhibition of SIRT1/2 upregulates HSPA5 acetylation
and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in
human lung cancer cells. Apoptosis. 24:798–811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu H, Kim YM and Cho M:
Cytoplasm-localized SIRT1 downregulation attenuates apoptosis and
cell cycle arrest in cisplatin-resistant lung cancer A549 cells. J
Cancer. 11:4495–4509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu R, Luo X, Ye X, Li H, Liu H, Du Q and
Zhai Q: SIRT1/PGC-1α/PPAR-γ correlate with hypoxia-induced
chemoresistance in non-small cell lung cancer. Front Oncol.
11:6827622021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng M, Hu C, Wu M and Chin YE: Emerging
role of SIRT2 in non-small cell lung cancer. Oncol Lett.
22:7312021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Xie QR, Chen Z, Lu S and Xia W:
Regulation of SIRT2 levels for human non-small cell lung cancer
therapy. Lung Cancer. 82:9–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Huang J, Yuan H, Chen Z, Luo Q and
Lu S: SIRT2 inhibits non-small cell lung cancer cell growth through
impairing Skp2-mediated p27 degradation. Oncotarget. 7:18927–18939.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu W, Jiang K, Shen M, Qian Y and Peng Y:
SIRT2 suppresses non-small cell lung cancer growth by targeting
JMJD2A. Biol Chem. 396:929–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao CX, Chen B, Xie HK, Han CN and Luo J:
Immunohistochemistry and clinical value of sirtuin 2 in
non-metastasized non-small cell lung cancer. J Thorac Dis.
11:3973–3979. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoffmann G, Breitenbücher F, Schuler M and
Ehrenhofer-Murray AE: A novel sirtuin 2 (SIRT2) inhibitor with
p53-dependent pro-apoptotic activity in non-small cell lung cancer.
J Biol Chem. 289:5208–5216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu Y, Li F, Lv L, Li T, Zhou X, Deng CX,
Guan KL, Lei QY and Xiong Y: Oxidative stress activates SIRT2 to
deacetylate and stimulate phosphoglycerate mutase. Cancer Res.
74:3630–3642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang HX, Wang MY, Xiao W and Wen JW:
SIRT2-reverses drug-resistance of HL-60/A through autophagy
mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 27:409–414. 2019.(In
Chinese). PubMed/NCBI
|
|
41
|
Liu L, Yu L, Zeng C, Long H, Duan G, Yin
G, Dai X and Lin Z: E3 ubiquitin ligase HRD1 promotes lung
tumorigenesis by promoting sirtuin 2 ubiquitination and
degradation. Mol Cell Biol. 40:e00257–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao Y, Yang J, Liao W, Liu X, Zhang H,
Wang S, Wang D, Feng J, Yu L and Zhu WG: Cytosolic FoxO1 is
essential for the induction of autophagy and tumour suppressor
activity. Nat Cell Biol. 12:665–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu H, Hu Y, Zeng C, Chang L, Ge F, Wang
W, Yan F, Zhao Q, Cao J, Ying M, et al: The SIRT2-mediated
deacetylation of AKR1C1 is required for suppressing its
pro-metastasis function in non-small cell lung cancer.
Theranostics. 10:2188–2200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin R, Tao R, Gao X, Li T, Zhou X, Guan
KL, Xiong Y and Lei QY: Acetylation stabilizes ATP-citrate lyase to
promote lipid biosynthesis and tumor growth. Mol Cell. 51:506–518.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang K, Shen M, Chen Y and Xu W: miR-150
promotes the proliferation and migration of non-small cell lung
cancer cells by regulating the SIRT2/JMJD2A signaling pathway.
Oncol Rep. 40:943–951. 2018.PubMed/NCBI
|
|
46
|
Luo J, Bao YC, Ji XX, Chen B, Deng QF and
Zhou SW: SPOP promotes SIRT2 degradation and suppresses non-small
cell lung cancer cell growth. Biochem Biophys Res Commun.
483:880–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Head PE, Zhang H, Bastien AJ, Koyen AE,
Withers AE, Daddacha WB, Cheng X and Yu DS: Sirtuin 2 mutations in
human cancers impair its function in genome maintenance. J Biol
Chem. 292:9919–9931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Inoue K, Mallakin A and Frazier DP: Dmp1
and tumor suppression. Oncogene. 26:4329–4335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Das C, Lucia MS, Hansen KC and Tyler JK:
CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature.
459:113–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Serrano L, Martínez-Redondo P,
Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB,
Kane-Goldsmith N, Tong Q, Rabanal RM, et al: The tumor suppressor
SirT2 regulates cell cycle progression and genome stability by
modulating the mitotic deposition of H4K20 methylation. Genes Dev.
27:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin YH, Kim YJ, Kim DW, Baek KH, Kang BY,
Yeo CY and Lee KY: Sirt2 interacts with 14-3-3 beta/gamma and
down-regulates the activity of p53. Biochem Biophys Res Commun.
368:690–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang
P, Xu YH, Dong B, Xiong Y, Lei QY and Guan KL: Lysine-5 acetylation
negatively regulates lactate dehydrogenase A and is decreased in
pancreatic cancer. Cancer Cell. 23:464–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hamaidi I, Zhang L, Kim N, Wang MH,
Iclozan C, Fang B, Liu M, Koomen JM, Berglund AE, Yoder SJ, et al:
Sirt2 inhibition enhances metabolic fitness and effector functions
of tumor-reactive T cells. Cell Metab. 32:420–436.e12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jing H, Hu J, He B, Negrón Abril YL,
Stupinski J, Weiser K, Carbonaro M, Chiang YL, Southard T,
Giannakakou P, et al: A SIRT2-selective inhibitor promotes c-Myc
oncoprotein degradation and exhibits broad anticancer activity.
Cancer Cell. 29:6072016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou Y, Cheng S, Chen S and Zhao Y:
Prognostic and clinicopathological value of SIRT3 expression in
various cancers: A systematic review and meta-analysis. Onco
Targets Ther. 11:2157–2167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang GC, Fu BC, Zhang DY, Sun L, Chen W,
Bai L, Gao T, Lu HG, Wang ZY, Kong QQ, et al: The expression and
related clinical significance of SIRT3 in non-small-cell lung
cancer. Dis Markers. 2017:82419532017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ahmed MA, O'Callaghan C, Chang ED, Jiang H
and Vassilopoulos A: Context-dependent roles for SIRT2 and SIRT3 in
tumor development upon calorie restriction or high fat diet. Front
Oncol. 9:14622020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao K, Chen Y, Zhao S, Huang Y, Liu T, Liu
H, Li B, Cui J, Cai J, Bai C, et al: Sirt3 promoted DNA damage
repair and radioresistance through ATM-Chk2 in non-small cell lung
cancer cells. J Cancer. 12:5464–5472. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tao F, Gu C, Li N, Ying Y, Feng Y, Ni D,
Zhang Q and Xiao Q: SIRT3 acts as a novel biomarker for the
diagnosis of lung cancer: A retrospective study. Medicine
(Baltimore). 100:e265802021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xiao K, Jiang J, Wang W, Cao S, Zhu L,
Zeng H, Ouyang R, Zhou R and Chen P: Sirt3 is a tumor suppressor in
lung adenocarcinoma cells. Oncol Rep. 30:1323–1328. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cao Y, Li P, Wang H, Li L and Li Q: SIRT3
promotion reduces resistance to cisplatin in lung cancer by
modulating the FOXO3/CDT1 axis. Cancer Med. 10:1394–1404. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Geoghegan F, Buckland RJ, Rogers ET,
Khalifa K, O'Connor EB, Rooney MF, Behnam-Motlagh P, Nilsson TK,
Grankvist K and Porter RK: Bioenergetics of acquired cisplatin
resistant H1299 non-small cell lung cancer and P31 mesothelioma
cells. Oncotarget. 8:94711–94725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang J, Han L, Yu J, Li H and Li Q:
miR-224 aggravates cancer-associated fibroblast-induced progression
of non-small cell lung cancer by modulating a positive loop of the
SIRT3/AMPK/mTOR/HIF-1α axis. Aging (Albany NY). 13:10431–10449.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xiong H, Liu B, Liu XY, Xia ZK, Lu M, Hu
CH and Liu P: circ_rac GTPase-activating protein 1 facilitates
stemness and metastasis of non-small cell lung cancer via
polypyrimidine tract-binding protein 1 recruitment to promote
sirtuin-3-mediated replication timing regulatory factor 1
deacetylation. Lab Invest. 103:1000102023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiong Y, Wang L, Wang S, Wang M, Zhao J,
Zhang Z, Li X, Jia L and Han Y: SIRT3 deacetylates and promotes
degradation of P53 in PTEN-defective non-small cell lung cancer. J
Cancer Res Clin Oncol. 144:189–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Petronek MS, Bayanbold K, Amegble K,
Tomanek-Chalkley AM, Allen BG, Spitz DR and Bailey CK: Evaluating
the iron chelator function of sirtinol in non-small cell lung
cancer. Front Oncol. 13:11857152023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lagunas-Rangel FA: Role of SIRT5 in
cancer. Friend or Foe? Biochimie. 209:131–141. 2023.PubMed/NCBI
|
|
68
|
Xiangyun Y, Xiaomin N, Linping G, Yunhua
X, Ziming L, Yongfeng Y, Zhiwei C and Shun L: Desuccinylation of
pyruvate kinase M2 by SIRT5 contributes to antioxidant response and
tumor growth. Oncotarget. 8:6984–6993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deng Z, Tu Q, Hu G and Xing M: Knockdown
of circLRWD1 weakens DDP resistance via reduction of SIRT5
expression through releasing miR-507 in non-small cell lung cancer.
Anticancer Drugs. 33:861–870. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lu W, Zuo Y, Feng Y and Zhang M: SIRT5
facilitates cancer cell growth and drug resistance in non-small
cell lung cancer. Tumour Biol. 35:10699–10705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Z, Yu DP, Wang N, Tao T, Luo W and Chen
H: SIRT5 promotes non-small cell lung cancer progression by
reducing FABP4 acetylation level. Neoplasma. 69:909–917. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wu J, Zheng C, Wang Y, Yang Z, Li C, Fang
W, Jin Y, Hou K, Cheng Y, Qi J, et al: Correction: LncRNA
APCDD1L-AS1 induces icotinib resistance by inhibition of EGFR
autophagic degradation via the miR-1322/miR-1972/miR-324-3p-SIRT5
axis in lung adenocarcinoma. Biomark Res. 11:512023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Azuma Y, Yokobori T, Mogi A, Altan B,
Yajima T, Kosaka T, Onozato R, Yamaki E, Asao T, Nishiyama M and
Kuwano H: SIRT6 expression is associated with poor prognosis and
chemosensitivity in patients with non-small cell lung cancer. J
Surg Oncol. 112:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bai L, Lin G, Sun L, Liu Y, Huang X, Cao
C, Guo Y and Xie C: Upregulation of SIRT6 predicts poor prognosis
and promotes metastasis of non-small cell lung cancer via the
ERK1/2/MMP9 pathway. Oncotarget. 7:40377–40386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Subramani P, Nagarajan N, Mariaraj S and
Vilwanathan R: Knockdown of sirtuin6 positively regulates
acetylation of DNMT1 to inhibit NOTCH signaling pathway in
non-small cell lung cancer cell lines. Cell Signal. 105:1106292023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Krishnamoorthy V and Vilwanathan R:
Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in
non-small cell lung cancer cell lines. Genomics. 112:3703–3712.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim EJ and Juhnn YS: Cyclic AMP signaling
reduces sirtuin 6 expression in non-small cell lung cancer cells by
promoting ubiquitin-proteasomal degradation via inhibition of the
Raf-Mek-Erk (Raf/mitogen-activated extracellular signal-regulated
kinase/extracellular signal-regulated kinase) pathway. J Biol Chem.
290:9604–9613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu B, Yan Y, Shao B, Tian L and Zhou W:
Downregulation of SIRT6 is associated with poor prognosis in
patients with non-small cell lung cancer. J Int Med Res.
46:1517–1527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang J, Cai Y and Sheng Z: Experimental
studies on the protective effects of the overexpression of
lentivirus-mediated sirtuin 6 on radiation-induced lung injury. Adv
Clin Exp Med. 29:873–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang J, Sheng Z and Cai Y: SIRT6
overexpression inhibits HIF1α expression and its impact on tumor
angiogenesis in lung cancer. Int J Clin Exp Pathol. 11:2940–2947.
2018.PubMed/NCBI
|
|
81
|
Fu L, Dong Q, He J, Wang X, Xing J, Wang
E, Qiu X and Li Q: SIRT4 inhibits malignancy progression of NSCLCs,
through mitochondrial dynamics mediated by the ERK-Drp1 pathway.
Oncogene. 36:2724–2736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang Y, Han Z, Wang Y and Hao W:
Depletion of SIRT7 sensitizes human non-small cell lung cancer
cells to gemcitabine therapy by inhibiting autophagy. Biochem
Biophys Res Commun. 506:266–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Toiber D, Erdel F, Bouazoune K, Silberman
DM, Zhong L, Mulligan P, Sebastian C, Cosentino C, Martinez-Pastor
B, Giacosa S, et al: SIRT6 recruits SNF2H to DNA break sites,
preventing genomic instability through chromatin remodeling. Mol
Cell. 51:454–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
McCord RA, Michishita E, Hong T, Berber E,
Boxer LD, Kusumoto R, Guan S, Shi X, Gozani O, Burlingame AL, et
al: SIRT6 stabilizes DNA-dependent protein kinase at chromatin for
DNA double-strand break repair. Aging (Albany NY). 1:109–121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gonfloni S, Iannizzotto V, Maiani E,
Bellusci G, Ciccone S and Diederich M: P53 and Sirt1: Routes of
metabolism and genome stability. Biochem Pharmacol. 92:149–156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
van Leeuwen I and Lain S: Sirtuins and
p53. Adv Cancer Res. 102:171–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang S, Yang Y, Huang S, Deng C, Zhou S,
Yang J, Cao Y, Xu L, Yuan Y, Yang J, et al: SIRT1 inhibits gastric
cancer proliferation and metastasis via STAT3/MMP-13 signaling. J
Cell Physiol. 234:15395–15406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Leng S, Huang W, Chen Y, Yang Y, Feng D,
Liu W, Gao T, Ren Y, Huo M, Zhang J, et al: SIRT1 coordinates with
the CRL4B complex to regulate pancreatic cancer stem cells to
promote tumorigenesis. Cell Death Differ. 28:3329–3343. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liarte S, Alonso-Romero JL and Nicolás FJ:
SIRT1 and estrogen signaling cooperation for breast cancer onset
and progression. Front Endocrinol (Lausanne). 9:5522018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dikalova AE, Itani HA, Nazarewicz RR,
McMaster WG, Flynn CR, Uzhachenko R, Fessel JP, Gamboa JL, Harrison
DG and Dikalov SI: Sirt3 impairment and SOD2 hyperacetylation in
vascular oxidative stress and hypertension. Circ Res. 121:564–574.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dikalov SI and Dikalova AE: Crosstalk
between mitochondrial hyperacetylation and oxidative stress in
vascular dysfunction and hypertension. Antioxid Redox Signal.
31:710–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Han Z, Liu L, Liu Y and Li S: Sirtuin
SIRT6 suppresses cell proliferation through inhibition of Twist1
expression in non-small cell lung cancer. Int J Clin Exp Pathol.
7:4774–4781. 2014.PubMed/NCBI
|
|
93
|
Xiong X, Tao R, DePinho RA and Dong XC:
Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts
on glucose metabolism through downregulation of gluconeogenesis and
upregulation of glycolysis. PLoS One. 8:e743402013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ohtake F, Takeyama K, Matsumoto T,
Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J,
Chambon P, et al: Modulation of oestrogen receptor signalling by
association with the activated dioxin receptor. Nature.
423:545–550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang P, Tu B, Wang H, Cao Z, Tang M,
Zhang C, Gu B, Li Z, Wang L, Yang Y, et al: Tumor suppressor p53
cooperates with SIRT6 to regulate gluconeogenesis by promoting
FoxO1 nuclear exclusion. Proc Natl Acad Sci USA. 111:10684–10689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Song MY, Wang J, Ka SO, Bae EJ and Park
BH: Insulin secretion impairment in Sirt6 knockout pancreatic β
cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway.
Sci Rep. 6:303212016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bajpe PK, Prahallad A, Horlings H,
Nagtegaal I, Beijersbergen R and Bernards R: A chromatin modifier
genetic screen identifies SIRT2 as a modulator of response to
targeted therapies through the regulation of MEK kinase activity.
Oncogene. 34:531–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xu H, Li Y, Chen L, Wang Q, Zhang H, Lin
Y, Li Q and Pang T: SIRT2 mediates multidrug resistance in acute
myelogenous leukemia cells via ERK1/2 signaling pathway. Int J
Oncol. 48:613–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang M, Du W, Acklin S, Jin S and Xia F:
SIRT2 protects peripheral neurons from cisplatin-induced injury by
enhancing nucleotide excision repair. J Clin Invest. 130:2953–2965.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao D, Mo Y, Li MT, Zou SW, Cheng ZL, Sun
YP, Xiong Y, Guan KL and Lei QY: NOTCH-induced aldehyde
dehydrogenase 1A1 deacetylation promotes breast cancer stem cells.
J Clin Invest. 124:5453–5465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wei R, He D and Zhang X: Role of SIRT2 in
regulation of stemness of cancer stem-like cells in renal cell
carcinoma. Cell Physiol Biochem. 49:2348–2357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fong Y, Lin YC, Wu CY, Wang HM, Lin LL,
Chou HL, Teng YN, Yuan SS and Chiu CC: The antiproliferative and
apoptotic effects of sirtinol, a sirtuin inhibitor on human lung
cancer cells by modulating Akt/β-catenin-Foxo3a axis.
ScientificWorldJournal. 2014:9370512014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ma W, Zhao X, Wang K, Liu J and Huang G:
Dichloroacetic acid (DCA) synergizes with the SIRT2 inhibitor
Sirtinol and AGK2 to enhance anti-tumor efficacy in non-small cell
lung cancer. Cancer Biol Ther. 19:835–846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang Y, Zhang Q, Zeng SX, Zhang Y, Mayo
LD and Lu H: Inauhzin and Nutlin3 synergistically activate p53 and
suppress tumor growth. Cancer Biol Ther. 13:915–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Akbaribazm M, Khazaei MR, Khazaei F and
Khazaei M: Doxorubicin and Trifolium pratense L. (Red clover)
extract synergistically inhibits brain and lung metastases in 4T1
tumor-bearing BALB/c mice. Food Sci Nutr. 8:5557–5570. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shang JL, Ning SB, Chen YY, Chen TX and
Zhang J: MDL-800, an allosteric activator of SIRT6, suppresses
proliferation and enhances EGFR-TKIs therapy in non-small cell lung
cancer. Acta Pharmacol Sin. 42:120–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Feng S, Li Y, Huang H, Huang H, Duan Y,
Yuan Z, Zhu W, Mei Z, Luo L and Yan P: Isoorientin reverses lung
cancer drug resistance by promoting ferroptosis via the
SIRT6/Nrf2/GPX4 signaling pathway. Eur J Pharmacol. 954:1758532023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fang C, Liu Y, Chen L, Luo Y, Cui Y, Zhang
N, Liu P, Zhou M and Xie Y: α-Hederin inhibits the growth of lung
cancer A549 cells in vitro and in vivo by decreasing SIRT6
dependent glycolysis. Pharm Biol. 59:11–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Iskandar AR, Liu C, Smith DE, Hu KQ, Choi
SW, Ausman LM and Wang XD: β-cryptoxanthin restores
nicotine-reduced lung SIRT1 to normal levels and inhibits
nicotine-promoted lung tumorigenesis and emphysema in A/J mice.
Cancer Prev Res (Phila). 6:309–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
You J, Cheng J, Yu B, Duan C and Peng J:
Baicalin, a Chinese herbal medicine, inhibits the proliferation and
migration of human non-small cell lung carcinoma (NSCLC) Cells,
A549 and H1299, by activating the Sirt1/Ampk signaling pathway. Med
Sci Monit. 24:2126–2133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen X, Hao B, Li D, Reiter RJ, Bai Y,
Abay B, Chen G, Lin S, Zheng T, Ren Y, et al: Melatonin inhibits
lung cancer development by reversing the Warburg effect via
stimulating the SIRT3/PDH axis. J Pineal Res. 71:e127552021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li M, Hao B, Zhang M, Reiter RJ, Lin S,
Zheng T, Chen X, Ren Y, Yue L, Abay B, et al: Melatonin enhances
radiofrequency-induced NK antitumor immunity, causing cancer
metabolism reprogramming and inhibition of multiple pulmonary tumor
development. Signal Transduct Target Ther. 6:3302021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lee BB, Kim Y, Kim D, Cho EY, Han J, Kim
HK, Shim YM and Kim DH: Metformin and tenovin-6 synergistically
induces apoptosis through LKB1-independent SIRT1 down-regulation in
non-small cell lung cancer cells. J Cell Mol Med. 23:2872–2889.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cha BK, Kim YS, Hwang KE, Cho KH, Oh SH,
Kim BR, Jun HY, Yoon KH, Jeong ET and Kim HR: Celecoxib and
sulindac inhibit TGF-β1-induced epithelial-mesenchymal transition
and suppress lung cancer migration and invasion via downregulation
of sirtuin 1. Oncotarget. 7:57213–57227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hwang KE, Kim YS, Hwang YR, Kwon SJ, Park
DS, Cha BK, Kim BR, Yoon KH, Jeong ET and Kim HR: Pemetrexed
induces apoptosis in malignant mesothelioma and lung cancer cells
through activation of reactive oxygen species and inhibition of
sirtuin 1. Oncol Rep. 33:2411–2419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lai TC, Lee YL, Lee WJ, Hung WY, Cheng GZ,
Chen JQ, Hsiao M, Chien MH and Chang JH: Synergistic tumor
inhibition via energy elimination by repurposing penfluridol and
2-deoxy-D-glucose in lung cancer. Cancers (Basel). 14:27502022.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Slanovc J, Mikulčić M, Jahn N, Wizsy NGT,
Sattler W, Malle E and Hrzenjak A: Prostaglandin
15d-PGJ2 inhibits proliferation of lung adenocarcinoma
cells by inducing ROS production and activation of apoptosis via
sirtuin-1. Biochim Biophys Acta Mol Basis Dis. 1870:1669242024.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tae H, Park EY, Dey P, Son JY, Lee S-Y,
Jung JH, Saloni S, Kim M-H and Kim HS: Novel SIRT1 inhibitor
15-deoxy-Δ12,14-prostaglandin J2 and its derivatives exhibit
anticancer activity through apoptotic or autophagic cell death
pathways in SKOV3 cells. Int J Oncol. 53:2518–2530. 2018.PubMed/NCBI
|
|
120
|
Hwang KE, Kim HJ, Song IS, Park C, Jung
JW, Park DS, Oh SH, Kim YS and Kim HR: Salinomycin suppresses
TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the
AMPK/SIRT1 pathway in non-small cell lung cancer. Int J Med Sci.
18:715–726. 2021. View Article : Google Scholar : PubMed/NCBI
|