Introduction
Overexpression of CD44 is observed in various solid
tumors, which are involved in the tumor malignant progression
through the promotion of cellular proliferation, invasiveness and
stemness via specific signaling pathways (1). The diversity of CD44 molecular
function is mediated by the alternative splicing (2). CD44 is encoded in 20 exons. The first
five (1–5) and the last five (16–20)
are constant exons that generate the shortest CD44 standard (CD44s)
isoform. The exons 6–15 are alternatively spliced and inserted into
the CD44s as variant exons (3). The
CD44 splice variants with variant exons are designated CD44 variant
(CD44v) isoforms. The inclusion of CD44v exons in various
combinations is regulated by receptor tyrosine kinase signaling and
splicing activators of pre-mRNA (1).
CD44 possesses an extracellular domain (ectodomain),
a transmembrane domain, and an intracellular cytoplasmic domain
(4). The ectodomain contains a
hyaluronic acid (HA)-binding domain (HABD) that mediates cellular
homing, adhesion, migration and proliferation (4). Both CD44s and CD44v isoforms have the
HABD, and the HA binding causes conformational changes of the CD44,
which results in the promotion of intracellular signaling pathways
to regulate cell migration and proliferation (5). In CD44v isoforms, the variant
exons-encoding sequences form the stem region, which provides a
co-receptor for various growth factors and cytokines. These
functions activate specific signaling pathways to promote invasion
and stemness (6). Therefore,
different isoforms of CD44v exhibit different functions according
to the inserted variant exons in the CD44 ectodomain.
The relationship between CD44 expression and
prognosis in patients with cancer has been evaluated and showed
both poor and favorable outcomes (7). Currently, increased evidence suggests
that overexpression of CD44 and its isoforms is an unfavorable
indicator in patients with cancer (7). In an analysis of multiple studies,
including 583 pancreatic cancer cases, overexpression of CD44 was
predictive of poor overall survival, a more advanced stage and more
lymph node invasion (8).
Furthermore, another meta-analysis investigated the prognostic
significance of cancer stem cell markers, including CD44, from 52
studies of ovarian cancer. The study concluded that CD44
overexpression was predictive of worse disease-free survival and
resistance to chemotherapy (9).
Several anti-CD44 monoclonal antibodies (mAbs) have
been developed for preclinical and clinical research for tumor
therapy. An anti-pan-CD44 mAb (H4C4) reduced tumor growth,
metastasis and post-radiation recurrence in a human pancreatic
tumor xenograft model (10). A
humanized anti-pan-CD44 mAb, RG7356, showed the cytotoxicity for B
cell leukemia but no cytotoxicity on normal B cells. Administration
of RG7356 to immunodeficient mice engrafted with chronic
lymphocytic leukemia cells resulted in complete clearance of
engrafted leukemia cells (11).
Phase I clinical trials with RG7356 were conducted in patients with
acute myeloid leukemia (12) and
advanced CD44-positive solid tumor patients (13). Although RG7356 showed an acceptable
safety profile, the studies were not continued due to the lack of a
clinical and/or pharmacodynamic dose-response relationship with
RG7356 (13).
Therapies using anti-CD44v6 mAbs were considered
since CD44v6 plays critical roles in the malignant progression of
tumors (14). Humanized anti-CD44v6
mAbs, including BIWA-4 and BIWA-8, were developed. These mAbs
labeled with 186Re were evaluated in head and neck
squamous cell carcinoma xenograft-bearing mice and exhibited
therapeutic efficacy (15).
Furthermore, the BIWA-4 was developed into a humanized version-drug
conjugate, bivatuzumab-mertansine (anti-tubulin agent), which was
evaluated in clinical trials (16).
However, the clinical trials were terminated because of the severe
skin toxicity, such as lethal epidermal necrolysis (17). Since CD44v6 is expressed in normal
skin epithelium, the toxicity of mertansine in the skin was most
likely responsible for the high toxicity (17,18).
Previously, a mutated version of BIWA-4, called BIWA-8, was
developed for increasing binding affinity by two amino acid
substitutions of the light chain (15). The BIWA-8 was further developed to
chimeric antigen receptors (CARs). The BIWA-8 CAR-T showed
antitumor activities against multiple myeloma or acute myeloid
leukemia engrafted with immunodeficient mice (19). Furthermore, the BIWA-8 CAR-T
exhibited efficacy in lung and ovarian carcinomas xenograft models
(20), which is expected for an
application toward solid tumors.
In our previous studies, an anti-pan-CD44 mAb,
C44Mab-5 (IgG1, kappa) was developed using
the Cell-Based Immunization and Screening (CBIS) method (21). Another mAb (C44Mab-46)
(22) was created by immunization
of the CD44v3-10 ectodomain. Both C44Mab-5 and
C44Mab-46 have the epitopes within the constant exon 2-
and 5-encoded sequences (23–25)
and could be applied to immunohistochemistry in oral squamous cell
carcinoma (21) and esophageal
squamous cell carcinoma (ESCC) (22), respectively. Furthermore, various
anti-CD44v mAbs have been developed, such as C44Mab-6
(an anti-CD44v3 mAb) (26),
C44Mab-108 (an anti-CD44v4 mAb) (27), C44Mab-3 (an anti-CD44v5
mAb) (28), C44Mab-9 (an
anti-CD44v6 mAb) (29),
C44Mab-34 (an anti-CD44v7/8 mAb) (30), C44Mab-1 (an anti-CD44v9
mAb) (31) and C44Mab-18
(an anti-CD44v10 mAb) (32). The
combinational use of the anti-CD44 mAbs is essential for
comprehensively analyzing human tumors.
In the present study, a mouse IgG2a type
of recombinant C44Mab-5 (5-mG2a) and
C44Mab-46 (C44Mab-46-mG2a) was
produced using fucosyl-transferase 8 (FUT8)-deficient ExpiCHO-S
cells and the antitumor activity in xenograft-bearing mice was
investigated.
Materials and methods
Cell lines
ESCC cell line KYSE770 was obtained from the
Japanese Collection of Research Bioresources. Chinese hamster ovary
(CHO)-K1 was obtained from the American Type Culture Collection.
CHO/CD44s was previously established by transfecting
pCAG-Ble/PA16-CD44s into CHO-K1 cells (22). KYSE770 was cultured in Dulbecco's
Modified Eagle's Medium (DMEM; Nacalai Tesque, Inc.) supplemented
with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.), 0.25 µg/ml amphotericin B, 100 µg/ml
streptomycin, and 100 U/ml penicillin (Nacalai Tesque, Inc.).
CHO-K1 was cultured in Roswell Park Memorial Institute (RPMI)-1640
medium (Nacalai Tesque, Inc.) supplemented with 10% (v/v) FBS, 0.25
µg/ml amphotericin B, 100 µg/ml streptomycin and 100 U/ml
penicillin (RPMI-1640 complete medium). CHO/CD44s were cultured in
RPMI-1640 complete medium containing 0.5 mg/ml Zeocin (InvivoGen).
All cells were cultured in a humidified incubator at 37°C with 5%
CO2.
Antibodies
Anti-pan-CD44 mAbs, C44Mab-5 and
C44Mab-46 were previously established (21,22).
To generate recombinant mAbs, VH cDNAs of
C44Mab-5 or C44Mab-46 and CH of
mouse IgG2a were cloned into the pCAG-Ble vector
(FUJIFILM Wako Pure Chemical Corporation). VL cDNA of
C44Mab-5 or C44Mab-46 and CL cDNA
of mouse kappa light chain were also subcloned into the pCAG-Neo
vector (FUJIFILM Wako Pure Chemical Corporation). The vectors were
transduced into BINDS-09 (FUT8-knockout ExpiCHO-S) cells (33). 5-mG2a and
C44Mab-46-mG2a were purified using Ab-Capcher
(ProteNova Co., Ltd.). 281-mG2a (an anti-hamster
podoplanin mAb, control mouse IgG2a) was previously
described (34).
Flow cytometry
The cells, obtained using 0.25% trypsin and 1 mM
ethylenediamine tetraacetic acid (EDTA; Nacalai Tesque, Inc.), were
treated with 5-mG2a and
C44Mab-46-mG2a, or control blocking buffer
[phosphate-buffered saline (PBS) containing 0.1% bovine serum
albumin (Nacalai Tesque, Inc.)] for 30 min at 4°C. Subsequently,
the cells were treated with anti-mouse IgG conjugated with Alexa
Fluor 488 (1:2,000; Cell Signaling Technology, Inc.) for 30 min at
4°C. Fluorescence data were collected using the SA3800 Cell
Analyzer (Sony Corp.) and analyzed using SA3800 software ver. 2.05
(Sony Corp.).
Antibody-dependent cellular
cytotoxicity (ADCC) reporter bioassay
An ADCC Reporter Bioassay kit (Promega Corporation)
was used for the ADCC reporter bioassay. The human FcγRIIIa
receptor and a nuclear factor of activated T-cells (NFAT) response
element driving firefly luciferase-expressed Jurkat cells were used
as effector cells. The target CHO/CD44s and KYSE770 cells (12,500
cells per well) were seeded into a 96-well white solid plate. The
serially diluted 5-mG2a and
C44Mab-46-mG2a were added to the target
cells. The effector cells (75,000 cells in 25 µl) were then added
and co-cultured with antibody-treated target cells at 37°C for 6 h.
Luminescence was measured with a GloMax luminometer (Promega
Corporation).
Complement-dependent cytotoxicity
(CDC)
The target CHO/CD44s and KYSE770 cells were labeled
using 10 µg/ml Calcein AM (Thermo Fisher Scientific, Inc.). The
target cells (1×104 cells/well) were mixed with 100
µg/ml of control 281-mG2a, 5-mG2a, or
C44Mab-46-mG2a and rabbit complement (final
dilution 1:10, Low-Tox-M Rabbit Complement; Cedarlane
Laboratories). The calcein release to the medium was measured after
a 4-h incubation. The maximum fluorescence of the medium was also
measured after lysing all cells with a buffer containing 0.5%
Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM EDTA. Cytotoxicity
(% of lysis) was calculated as % lysis=(E-S)/(M-S) ×100. E is the
fluorescence of combined target and effector cells. S is the
spontaneous fluorescence of target cells only. M is the maximum
fluorescence measured. Statistical analyses were performed using
GraphPad PRISM 6 software (GraphPad Software, Inc.; Dotmatics).
Antitumor activity of
5-mG2a and C44Mab-46-mG2a in
xenografts of CHO/CD44s and KYSE770
All animal experiments were performed following
regulations and guidelines to minimize animal distress and
suffering in the laboratory by the Institutional Committee for
Experiments of the Institute of Microbial Chemistry (Numazu,
Japan). The animal study protocol was approved (approval nos.
2023-037 and 2023-054) by the Institutional Committee for
Experiments of the Institute of Microbial Chemistry (Numazu,
Japan). BALB/c nude mice (5-week-old female, a total of 72 mice)
were purchased from Jackson Laboratories, Inc. and maintained on an
11-h light/13-h dark cycle at a temperature of 23±2°C and 55±5%
humidity in a specific pathogen-free environment, across the
experimental period. Food and water were supplied ad
libitum. The weight of the mice was monitored twice per week
and their health was monitored three times per week. CHO/CD44s and
KYSE770 cells (5×106 cells) suspended with BD Matrigel
Matrix Growth Factor Reduced (BD Biosciences) were inoculated into
the left flank of the mice subcutaneously. On day 7 after the
inoculation, 100 µg of 5-mG2a (n=8),
C44Mab-46-mG2a (n=8), or control
281-mG2a (n=8) in 100 µl PBS were injected
intraperitoneally. Additional antibody injections were performed on
days 14 and 21. The tumor volume was measured on days 7, 9, 14, 17,
21 and 23. In the KYSE770 ×enograft experiment (500 µg dosage), 500
µg of 5-mG2a (n=8), C44Mab-46-mG2a
(n=8), or control 281-mG2a (n=8) in 100 µl PBS were
injected intraperitoneally on days 8 and 13 after the inoculation.
The tumor volume was measured on days 8, 12, and 19. The tumor
volume was calculated using the formula: Volume=W2 ×
L/2, where W is the short diameter and L is the long diameter. The
loss of original body weight was determined to a point >25%
(35) and/or a maximum tumor size
>3,000 mm3 and/or significant changes in the
appearance of tumors as humane endpoints for euthanasia. Cervical
dislocation was used for euthanasia. Mice death was confirmed by
respiratory arrest and rigor mortis. The xenograft tumors were
carefully removed from the sacrificed mice and weighed
immediately.
Immunohistochemical analysis
The paraffin-embedded xenograft tumors were
autoclaved in citrate buffer (pH 6.0; Nichirei Biosciences, Inc.)
for 20 min. After blocking with SuperBlock T20 (Thermo Fisher
Scientific, Inc.), sections (4 µm) were incubated with 1 µg/ml of
C44Mab-46 and mPMab-1 [a mouse-rat chimeric antibody
against mouse podoplanin (36)] for
1 h at room temperature and then treated with the EnVision+ Kit for
mouse (Agilent Technologies, Inc.) for 30 min. The color was
developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB;
Agilent Technologies, Inc.). Counterstaining was performed with
hematoxylin (Merck KGaA). Hematoxylin and eosin (H&E) staining
was performed using hematoxylin and eosin (FUJIFILM Wako Pure
Chemical Corporation). Leica DMD108 fluorescence microscope (Leica
Microsystems GmbH) was used to examine the sections and obtain
images.
Statistical analyses
All data are expressed as the mean ± standard error
of the mean (SEM). Two-way ANOVA with Tukey's multiple comparisons
test and two-way ANOVA with Sidak's multiple comparisons tests were
conducted in CDC and tumor weight measurement, respectively.
Two-way ANOVA with Sidak's multiple comparisons test was utilized
for tumor volume and mice weight. GraphPad Prism 6 (GraphPad
Software, Inc.; Dotmatics) was used for all calculations. P<0.05
was considered to indicate a statistically significant
difference.
Results
Flow cytometric analysis against
CHO/CD44s and KYSE770 cells using 5-mG2a and
C44Mab-46-mG2a
In our previous study, anti-pan-CD44 mAbs
(C44Mab-5 and C44Mab-46) were established and
were shown to be available for flow cytometry and
immunohistochemistry (21,22). In the present study, a mouse
IgG2a type of C44Mab-5 and
C44Mab-46 (5-mG2a and
C44Mab-46-mG2a) were produced by combining
VH and VL of both mAbs with CH and
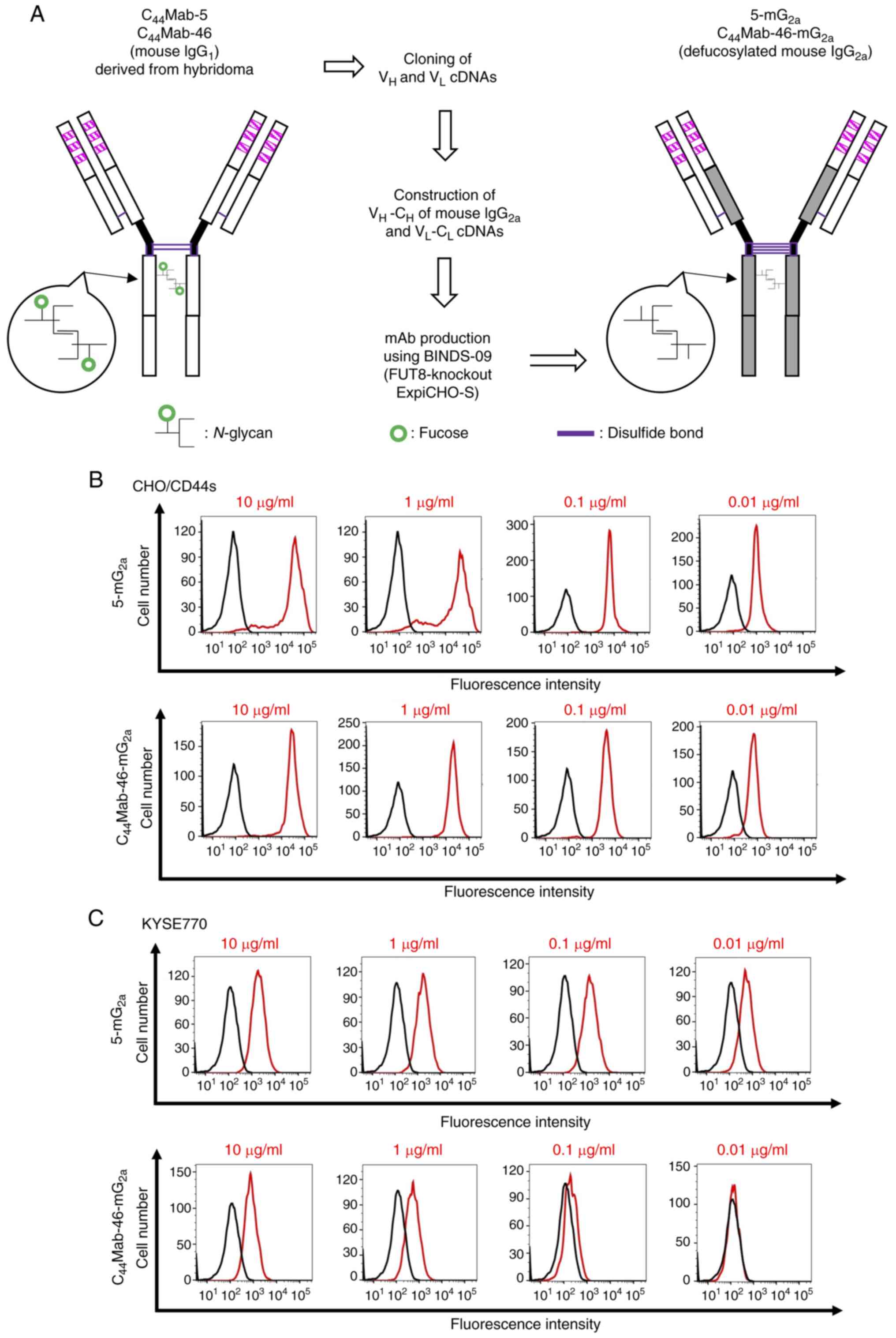
CL of mouse IgG2a, respectively (Fig. 1A). The reactivity of
CD44s-overexpressed CHO-K1 cells (CHO/CD44s) and an endogenous
CD44-expressing esophageal tumor cell line (KYSE770) was first
confirmed. 5-mG2a and
C44Mab-46-mG2a detected CHO/CD44s in a
concentration-dependent manner (Fig.
1B), but did not detect parental CHO-K1 cells (negative
control, Fig. S1).
5-mG2a and C44Mab-46-mG2a also
detected KYSE770 cells in a concentration-dependent manner
(Fig. 1C). KYSE770 cells were also
recognized by anti-CD44v mAbs including C44Mab-6 (an
anti-CD44v3 mAb), C44Mab-3 (an anti-CD44v5 mAb),
C44Mab-9 (an anti-CD44v6 mAb), and C44Mab-18
(an anti-CD44v10 mAb) (Fig. S2).
These results indicated that 5-mG2a and
C44Mab-46-mG2a recognize exogenous and
endogenous CD44.
5-mG2a and
C44Mab-46-mG2a-mediated ADCC pathway
activation in the presence of CHO/CD44s and KYSE770 cells
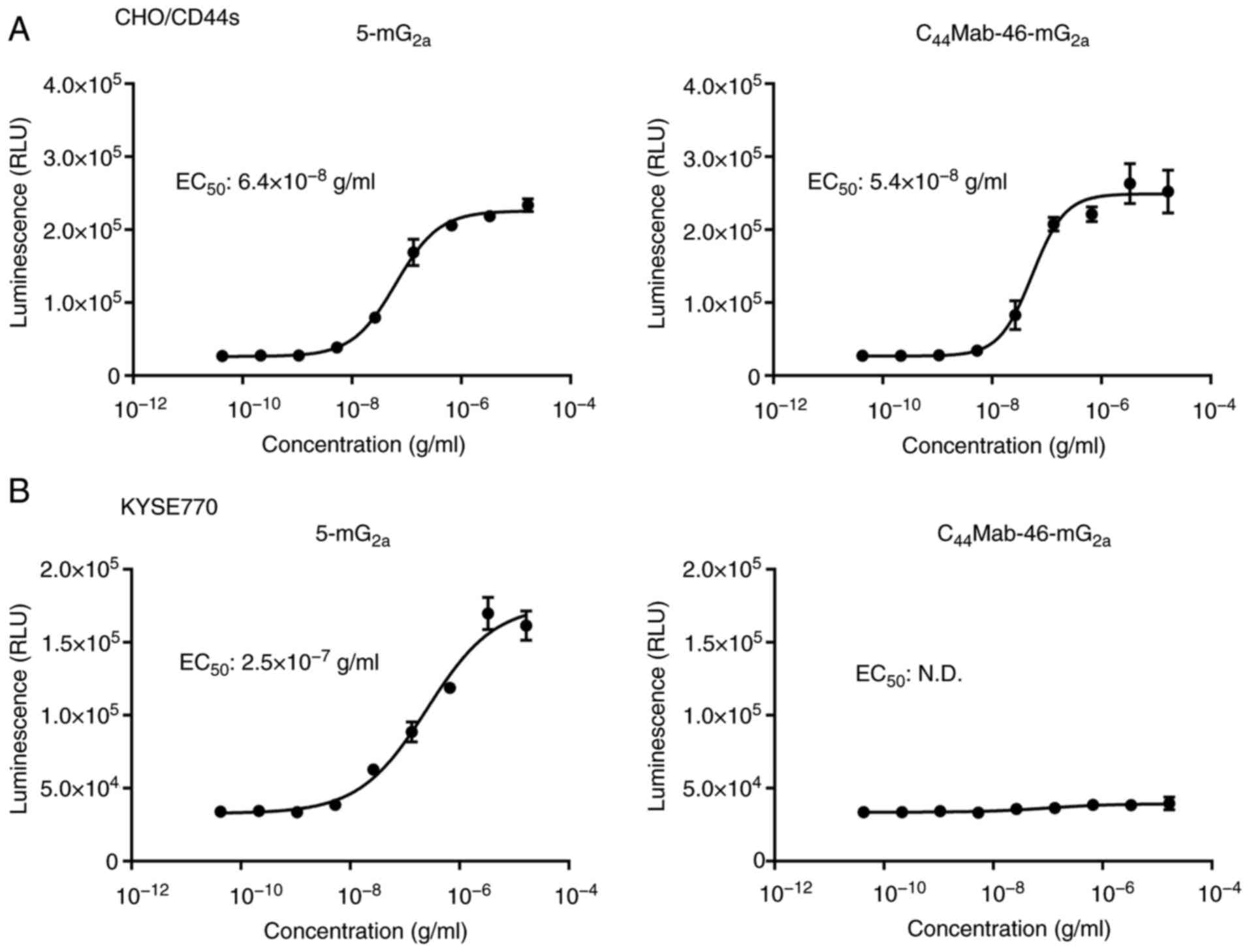
To compare the ADCC pathway activation by
5-mG2a and C44Mab-46-mG2a, the
ADCC reporter bioassay was performed to measure the biological
activity of the FcγRIIIa-mediated pathway activation by mAbs
(37). CHO/CD44s cells were treated
with diluted mAbs and then incubated with effector cells, which
possess the human FcγRIIIa receptor and Firefly luciferase
regulated by an NFAT response element. As demonstrated in Fig. 2A, 5-mG2a and
C44Mab-46-mG2a activated the effector in a
concentration-dependent manner (EC50;
6.4×10−8 g/ml and 5.4×10−8 g/ml,
respectively). In the presence of KYSE770 cells, 5-mG2a
activated the effector (EC50; 2.5×10−7 g/ml,
Fig. 2B). However,
C44Mab-46-mG2a did not (Fig. 2B).
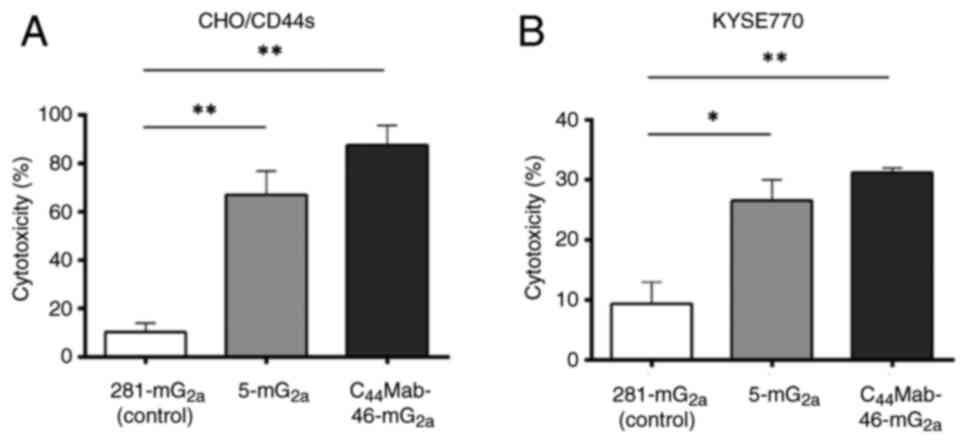
The CDC by 5-mG2a and
C44Mab-46-mG2a against CHO/CD44s and KYSE770
cells
The CDC mediated by 5-mG2a and
C44Mab-46-mG2a against CHO/CD44s and KYSE770
cells was next examined. As shown in Fig. 3, both 5-mG2a and
C44Mab-46-mG2a significantly exerted CDC
against CHO/CD44s cells (67 and 88% cytotoxicity, respectively) and
KYSE770 (27 and 31% cytotoxicity, respectively) compared with those
induced by control mouse IgG2a (281-mG2a).
These results demonstrated that 5-mG2a and
C44Mab-46-mG2a exhibited potent CDC
activities against CHO/CD44s and KYSE770 cells.
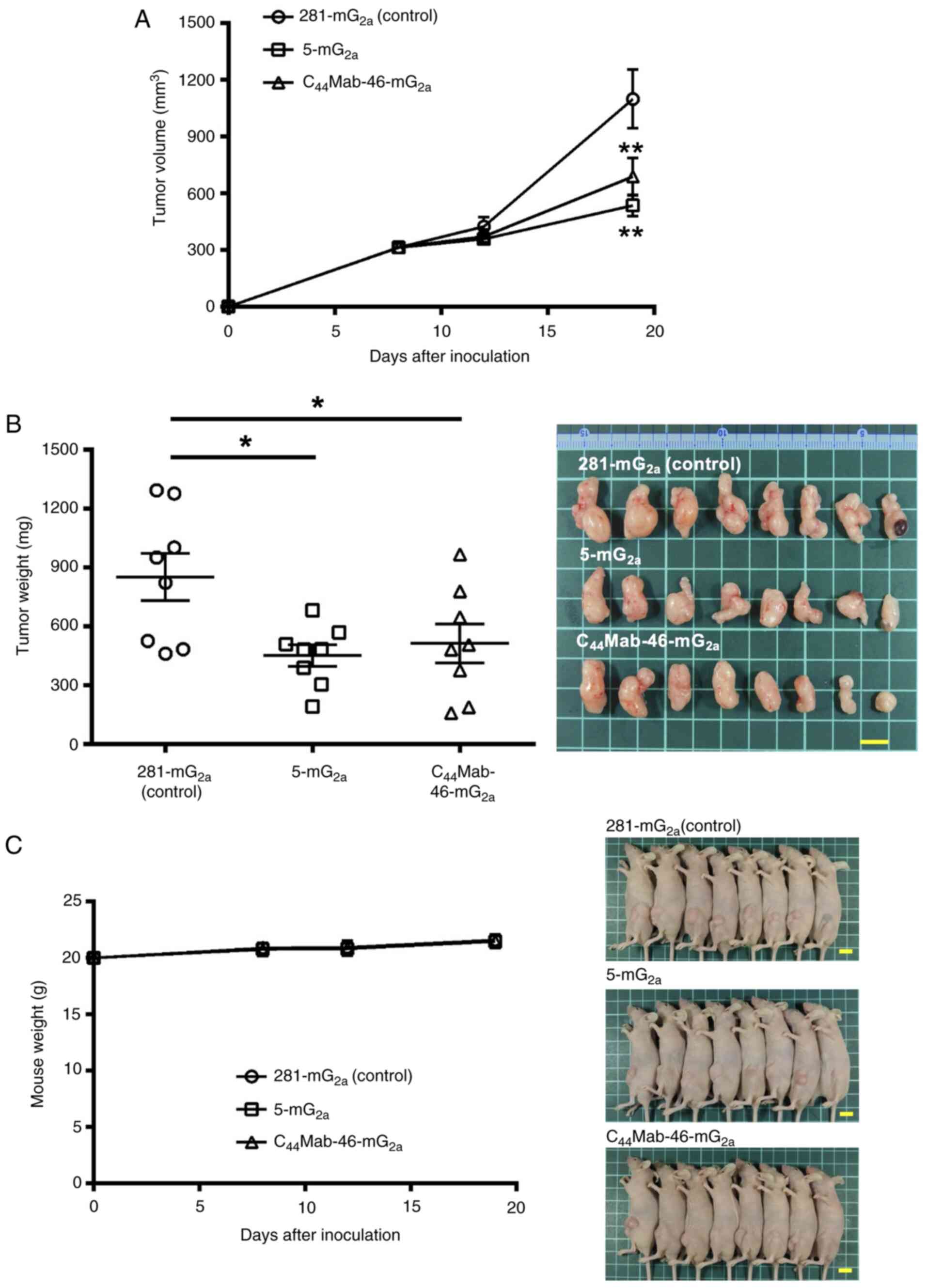
Antitumor effects of 5-mG2a
and C44Mab-46-mG2a against CHO/CD44s
xenograft
In the CHO/CD44s xenograft tumors, 100 µg of
5-mG2a, C44Mab-46-mG2a, or control
mouse IgG2a were injected into mice intraperitoneally on
days 7, 14 and 21, following CHO/CD44s inoculation. On days 7, 9,
14, 17, 21 and 23, the tumor volume was measured. The
5-mG2a and C44Mab-46-mG2a
administration resulted in a significant reduction in tumor volume
on days 17 (P<0.05), 21 (P<0.01) and 23 (P<0.01) compared
with that of the control mouse IgG2a (Fig. 4A). The 5-mG2a and
C44Mab-46-mG2a administration resulted in 46
and 48% reductions in tumor volume compared with that of the
control mouse IgG2a on day 23, respectively. The
histological analyses of tumors are demonstrated in Fig. S3. In the necrotic area of tumors,
leukocytes and erythrocytes were infiltrated. Membranous staining
of CD44 in tumor and podoplanin-positive macrophage were also
detected. However, a significant difference among control,
5-mG2a and C44Mab-46-mG2a-treated
tumors could not be observed.
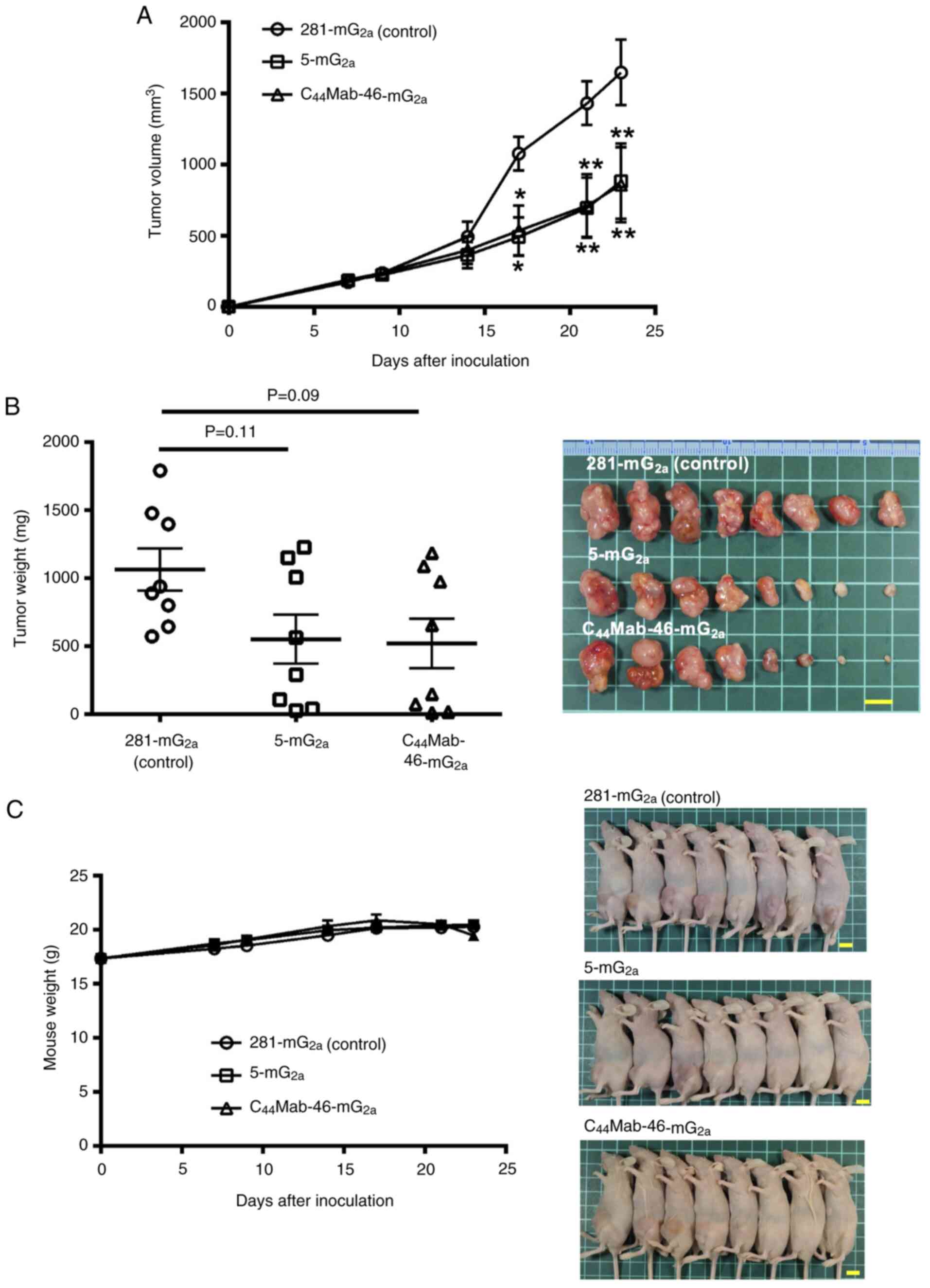 | Figure 4.Antitumor activity of
5-mG2a and C44Mab-46-mG2a against
CHO/CD44s xenograft tumor. (A) Measurement of tumor volume in
CHO/CD44s xenograft. CHO/CD44s cells (5×106 cells) were
injected into mice subcutaneously. On day 7, 100 µg of
5-mG2a and C44Mab-46-mG2a or
control mouse IgG2a (281-mG2a) were injected
into mice intraperitoneally. On days 14 and 21, additional
antibodies were injected. On days 7, 9, 14, 17, 21 and 23 following
the inoculation, the tumor volume was measured. Values are
presented as the mean ± SEM. *P<0.05 and **P<0.01; two-way
ANOVA with Sidak's multiple comparisons test. (B) The weight (left)
and appearance (right) of excised CHO/CD44s xenografts on day 23.
Values are presented as the mean ± SEM; one-way ANOVA with Tukey's
multiple comparisons test. (C) The body weight (left) and
appearance (right) of control mouse IgG2a, 5-mG2a, and
C44Mab-46-mG2a-treated mice. Scale bar, 1 cm. mAb,
monoclonal antibody. |
The weight of CHO/CD44s tumors treated with
5-mG2a and C44Mab-46-mG2a was
lower than that of tumors treated with the control mouse
IgG2a (53 and 55% reduction, respectively; P=0.11 and
P=0.09; Fig. 4B). Loss of body
weight was not observed in the CHO/CD44s tumor-implanted mice
during the treatments (Fig.
4C).
Antitumor effects of 5-mG2a
and C44Mab-46-mG2a on KYSE770 ×enografts
In the KYSE770 ×enograft models, 100 µg of
5-mG2a and C44Mab-46-mG2a and
control mouse IgG2a were injected into mice on days 7,
14 and 21, following KYSE770 inoculation. Although the tendency of
the reduction of tumor volume by the treatment with
5-mG2a and C44Mab-46-mG2a was
observed, significant differences were not obtained (Fig. S4). The dosage (500 µg) was next
increased and the antitumor effects were investigated. As revealed
in Fig. 5, 500 µg of
5-mG2a, C44Mab-46-mG2a and control
mouse IgG2a were injected into mice on days 8 and 13,
following KYSE770 inoculation. On days 8, 12 and 19, the tumor
volume was measured. The 5-mG2a and
C44Mab-46-mG2a administration resulted in a
significant reduction in tumor volume on day 19 (P<0.01)
compared with that of the control mouse IgG2a (Fig. 5A). The 5-mG2a and
C44Mab-46-mG2a administration resulted in 51
and 37% reduction of tumor volume compared with that of the control
mouse IgG2a on day 19, respectively. The histological
analyses of tumors are demonstrated in Fig. S5. Membranous staining of CD44 in
tumors and podoplanin-positive macrophages at the tumor periphery
were observed. Infiltrated podoplanin-positive macrophages into
tumors were not observed compared with KYSE770 ×enograft.
The weight of KYSE770 tumors treated with
5-mG2a and C44Mab-46-mG2a was
significantly lower than that of tumors treated with the control
mouse IgG2a [47% (P<0.05) and 40% (P<0.05)
reduction, respectively; Fig. 5B].
Loss of body weight was not observed in the KYSE770 tumor-implanted
mice during the treatments (Fig.
5C).
Discussion
In the present study, mouse IgG2a type
anti-pan-CD44 mAbs (5-mG2a and
C44Mab-46-mG2a) was produced and antitumor
activity against CHO/CD44s and KYSE770 ×enograft tumors was
evaluated. Although 5-mG2a possesses superior affinity
to CD44-expressing cells compared with
C44Mab-46-mG2a (22,38),
both 5-mG2a and C44Mab-46-mG2a
showed comparable ability of effector activation (Fig. 2A), CDC (Fig. 3A) and antitumor activity against
CHO/CD44s (Fig. 4). Against KYSE770
cells, 5-mG2a exhibited superior reactivity in flow
cytometry (Fig. 1C) and effector
activation (Fig. 2B) compared with
C44Mab-46-mG2a. However, both mAbs showed
similar antitumor activity to KYSE770 ×enograft in high dosage
(Fig. 5).
After the phase I trial of RG7356 in solid tumors,
the developer Roche group reported that the CD44s expression is
associated with HA production and predicts response to treatment
with RG7356 in both xenograft tumor models and clinical response
(39). The group also identified
that overexpression of CD44s stimulates the HA production (39). These results suggest the close
interplay between CD44s and HA and a potential biomarker to enrich
patient responses to anti-CD44 mAb therapy in the clinic. In the
present study, CD44s-overexpressed CHO-K1 cells and KYSE770 cells
were used, which mainly express CD44v (Fig. S2) (22). Due to the lower levels of total CD44
(Fig. 1) and the CD44v expression
in KYSE770 cells, a high dosage (500 µg) of mAbs was needed to
suppress the KYSE770 ×enograft.
As revealed in Fig.
2B, C44Mab-46-mG2a did not activate the
effector cells in the presence of KYSE770 but exerted the antitumor
effect in vivo (Fig. 5). The
CDC is a crucial antitumor mechanism by mAbs (40,41).
Some tendency was observed that
C44Mab-46-mG2a showed a more significant CDC
effect compared with 5-mG2a (Fig. 3). The difference could be sufficient
to exert similar antitumor effects in vivo by
5-mG2a and C44Mab-46-mG2a. There
was less infiltrated podoplanin-positive macrophage into KYSE770
×enograft compared with CHO/CD44 (Figs. S3 and S5). Since the infiltrated effector cells
exert ADCC activity (42), this may
be one of the reasons why ADCC did not contribute markedly in
KYSE770 cells. One more possibility is their epitope.
5-mG2a recognizes the N-terminal part of CD44 [amino
acids 25–36, (25)], and
C44Mab-46-mG2a recognizes the central part of
CD44 [amino acids 174–178, (23,24)].
Furthermore, IgG antibodies can form ordered hexamers upon binding
to their antigen on cell surfaces. These hexamers efficiently bind
the hexavalent complement component C1q, the first step in the
classical pathway of complement activation (43,44).
The structure of C44Mab-46-mG2a-CD44 complex
may provide the adequate access of complements to exert CDC.
Complement has been considered as an adjunctive
component that potentiates the antibody-mediated cytolytic effects.
However, complement is currently considered an essential effector
of tumor cytotoxic responses of antibody-based immunotherapy, which
is guiding new therapeutic options (41). An increasing body of evidence
suggests that complement plays critical roles in not only
mAb-mediated tumor cytolysis but also several immunomodulatory
functions in tumor immunosurveillance and antitumor immunity
(45,46). The complicated crosstalk of
complement effectors with cellular pathways that drive B cell and T
cell responses influences T helper/effector T cell survival,
differentiation and B cell activation. Therefore, the involvement
of complement in our experimental system and/or immunocompetent
mouse models should be investigated in future studies.
Most therapeutic mAbs exhibit adverse effects by
recognizing antigens in normal cells (47). Clinical trials of an anti-CD44v6 mAb
(BIWA-4) bivatuzumab-mertansine drug conjugate to solid tumors
failed because of the skin toxicities (17,18).
Therefore, cancer-selective or specific mAbs would reduce the
adverse effects. Cancer-specific mAbs (CasMabs) against HER2
[H2Mab-214 (48) and
H2Mab-250 (49)] have
been developed by the authors and the reactivity to cancer and
normal cells has been evaluated using flow cytometry. The antitumor
effect in mouse xenograft models has been also reported using a
mouse IgG2a or human IgG1 types recombinant
mAbs (50). Some anti-CD44 mAbs
which exhibit cancer specificity have been reported (51). Among them, the 4C8 mAb recognizes
aberrantly O-glycosylated CD44v6 with Tn
(GalNAca1-O-Ser/Thr) antigen. The 4C8 chimeric antigen
receptor (CAR)-T cells demonstrated target-specific cytotoxicity
in vitro, significant tumor regression, and prolonged
survival in vivo (52). In
our CasMab development against CD44, already established anti-CD44
mAbs were screened by comparing the reactivity against cancer and
normal cells. The CasMabs against CD44 could be applicable for
designing modalities such as antibody-drug conjugates and CAR-T
cells.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Mr Shun-ichi Ohba and Ms Akiko
Harakawa [Institute of Microbial Chemistry (BIKAKEN), Numazu, the
Microbial Chemistry Research Foundation] for technical assistance
with animal experiments.
Funding
The present study was supported in part by Japan Agency for
Medical Research and Development (AMED) (grant nos. JP23ama121008,
JP24am0521010, JP23bm1123027 and JP23ck0106730) and by the Japan
Society for the Promotion of Science (JSPS) Grants-in-Aid for
Scientific Research (KAKENHI) (grant nos. 22K06995, 21K20789,
21K07168 and 22K07224).
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
KI, HS, TO, TN, MY, GL and TT performed the
experiments. MK, MKK and YKato designed the experiments. KI, HS,
AO, YKatori and YKato engaged the analysis and interpretation of
data. KI, HS, and YKato wrote the manuscript. HS and YKato confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Animal experiments were approved (approval nos.
2023-037 and 2023-054) by the Institutional Committee for
Experiments of the Institute of Microbial Chemistry (Numazu,
Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CD44s
|
CD44 standard
|
|
CD44v
|
CD44 variant
|
|
HA
|
hyaluronic acid
|
|
mAb
|
monoclonal antibody
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
CDC
|
complement-dependent cytotoxicity
|
|
FcγR
|
Fcγ receptor
|
|
CAR
|
chimeric antigen receptor
|
|
FUT8
|
fucosyl-transferase 8
|
|
CHO
|
Chinese hamster ovary
|
|
CBIS
|
Cell-Based Immunization and
Screening
|
|
ESCC
|
esophageal squamous cell
carcinoma
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prochazka L, Tesarik R and Turanek J:
Regulation of alternative splicing of CD44 in cancer. Cell Signal.
26:2234–2239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Q, Yang C and Gao F: The state of CD44
activation in cancer progression and therapeutic targeting. FEBS J.
289:7970–7986. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zöller M: CD44, hyaluronan, the
hematopoietic stem cell, and leukemia-initiating cells. Front
Immunol. 6:2352015.PubMed/NCBI
|
|
6
|
Hassn Mesrati M, Syafruddin SE, Mohtar MA
and Syahir A: CD44: A multifunctional mediator of cancer
progression. Biomolecules. 11:18502021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cirillo N: The hyaluronan/CD44 axis: A
double-edged sword in cancer. Int J Mol Sci. 24:158122023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Wu T, Lu D, Zhen J and Zhang L:
CD44 overexpression related to lymph node metastasis and poor
prognosis of pancreatic cancer. Int J Biol Markers. 33:308–313.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao Y, Li H, Huang R, Mo D, Zeng T, Fang M
and Li M: Clinicopathological and prognostic significance of cancer
stem cell markers in ovarian cancer patients: Evidence from 52
studies. Cell Physiol Biochem. 46:1716–1726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Hao X, Qin J, Tang W, He F, Smith A,
Zhang M, Simeone DM, Qiao XT, Chen ZN, et al: Antibody against
CD44s inhibits pancreatic tumor initiation and postradiation
recurrence in mice. Gastroenterology. 146:1108–1118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Wu CC, Fecteau JF, Cui B, Chen L,
Zhang L, Wu R, Rassenti L, Lao F, Weigand S and Kipps TJ: Targeting
chronic lymphocytic leukemia cells with a humanized monoclonal
antibody specific for CD44. Proc Natl Acad Sci USA. 110:6127–6132.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vey N, Delaunay J, Martinelli G, Fiedler
W, Raffoux E, Prebet T, Gomez-Roca C, Papayannidis C, Kebenko M,
Paschka P, et al: Phase I clinical study of RG7356, an anti-CD44
humanized antibody, in patients with acute myeloid leukemia.
Oncotarget. 7:32532–32542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menke-van der Houven van Oordt CW,
Gomez-Roca C, van Herpen C, Coveler AL, Mahalingam D, Verheul HM,
van der Graaf WT, Christen R, Rüttinger D, Weigand S, et al:
First-in-human phase I clinical trial of RG7356, an anti-CD44
humanized antibody, in patients with advanced, CD44-expressing
solid tumors. Oncotarget. 7:80046–80058. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verel I, Heider KH, Siegmund M, Ostermann
E, Patzelt E, Sproll M, Snow GB, Adolf GR and van Dongen GA: Tumor
targeting properties of monoclonal antibodies with different
affinity for target antigen CD44V6 in nude mice bearing
head-and-neck cancer xenografts. Int J Cancer. 99:396–402. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orian-Rousseau V and Ponta H: Perspectives
of CD44 targeting therapies. Arch Toxicol. 89:3–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tijink BM, Buter J, de Bree R, Giaccone G,
Lang MS, Staab A, Leemans CR and van Dongen GA: A phase I dose
escalation study with anti-CD44v6 bivatuzumab mertansine in
patients with incurable squamous cell carcinoma of the head and
neck or esophagus. Clin Cancer Res. 12:6064–6072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riechelmann H, Sauter A, Golze W, Hanft G,
Schroen C, Hoermann K, Erhardt T and Gronau S: Phase I trial with
the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head
and neck squamous cell carcinoma. Oral Oncol. 44:823–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casucci M, Nicolis di Robilant B, Falcone
L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni
M, Bernardi M, et al: CD44v6-targeted T cells mediate potent
antitumor effects against acute myeloid leukemia and multiple
myeloma. Blood. 122:3461–3472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porcellini S, Asperti C, Corna S, Cicoria
E, Valtolina V, Stornaiuolo A, Valentinis B, Bordignon C and
Traversari C: CAR T cells redirected to CD44v6 control tumor growth
in lung and ovary adenocarcinoma bearing mice. Front Immunol.
11:992020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Kaneko MK and Kato Y: Detection of high CD44 expression in oral
cancers using the novel monoclonal antibody, C44Mab-5.
Biochem Biophys Rep. 14:64–68. 2018.PubMed/NCBI
|
|
22
|
Goto N, Suzuki H, Tanaka T, Asano T,
Kaneko MK and Kato Y: Development of a novel Anti-CD44 monoclonal
antibody for multiple applications against esophageal squamous cell
carcinomas. Int J Mol Sci. 23:55352022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takei J, Asano T, Suzuki H, Kaneko MK and
Kato Y: Epitope mapping of the anti-CD44 monoclonal antibody
(C44Mab-46) using alanine-scanning mutagenesis and surface plasmon
resonance. Monoclon Antib Immunodiagn Immunother. 40:219–226. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asano T, Kaneko MK, Takei J, Tateyama N
and Kato Y: Epitope mapping of the anti-CD44 monoclonal antibody
(C44Mab-46) using the REMAP method. Monoclon Antib
Immunodiagn Immunother. 40:156–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asano T, Kaneko MK and Kato Y: Development
of a novel epitope mapping system: RIEDL insertion for epitope
mapping method. Monoclon Antib Immunodiagn Immunother. 40:162–167.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H, Kitamura K, Goto N, Ishikawa K,
Ouchida T, Tanaka T, Kaneko MK and Kato Y: A Novel anti-CD44
variant 3 monoclonal antibody C44Mab-6 was established
for multiple applications. Int J Mol Sci. 24:84112023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki H, Tanaka T, Goto N, Kaneko MK and
Kato Y: Development of a novel anti-CD44 variant 4 monoclonal
antibody C44Mab-108 for immunohistochemistry. Curr
Issues Mol Biol. 45:1875–1888. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo Y, Suzuki H, Tanaka T, Kaneko MK and
Kato Y: Development of a novel Anti-CD44 variant 5 monoclonal
antibody C44Mab-3 for multiple applications against
pancreatic carcinomas. Antibodies (Basel). 12:312023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ejima R, Suzuki H, Tanaka T, Asano T,
Kaneko MK and Kato Y: Development of a novel Anti-CD44 variant 6
monoclonal antibody C44Mab-9 for multiple applications
against colorectal carcinomas. Int J Mol Sci. 24:40072023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki H, Ozawa K, Tanaka T, Kaneko MK and
Kato Y: Development of a novel anti-CD44 variant 7/8 monoclonal
antibody, C44Mab-34, for multiple applications against
oral carcinomas. Biomedicines. 11:10992023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tawara M, Suzuki H, Goto N, Tanaka T,
Kaneko MK and Kato Y: A novel anti-CD44 variant 9 monoclonal
antibody C44Mab-1 was developed for immunohistochemical
analyses against colorectal cancers. Curr Issues Mol Biol.
45:3658–3673. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishikawa K, Suzuki H, Kaneko MK and Kato
Y: Establishment of a novel anti-CD44 variant 10 monoclonal
antibody C44Mab-18 for immunohistochemical analysis
against oral squamous cell carcinomas. Curr Issues Mol Biol.
45:5248–5262. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li G, Suzuki H, Ohishi T, Asano T, Tanaka
T, Yanaka M, Nakamura T, Yoshikawa T, Kawada M, Kaneko MK and Kato
Y: Antitumor activities of a defucosylated anti-EpCAM monoclonal
antibody in colorectal carcinoma xenograft models. Int J Mol Med.
51:182023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nanamiya R, Suzuki H, Takei J, Li G, Goto
N, Harada H, Saito M, Tanaka T, Asano T, Kaneko MK and Kato Y:
Development of monoclonal antibody 281-mG2a-f against
golden hamster podoplanin. Monoclon Antib Immunodiagn Immunother.
41:311–319. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Queiroz AL, Dantas E, Ramsamooj S, Murthy
A, Ahmed M, Zunica ERM, Liang RJ, Murphy J, Holman CD, Bare CJ, et
al: Blocking ActRIIB and restoring appetite reverses cachexia and
improves survival in mice with lung cancer. Nat Commun.
13:46332022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamada S, Kaneko MK, Nakamura T, Ichii O,
Konnai S and Kato Y: Development of mPMab-1, a mouse-rat chimeric
antibody against mouse podoplanin. Monoclon Antib Immunodiagn
Immunother. 36:77–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garvin D, Stecha P, Gilden J, Wang J,
Grailer J, Hartnett J, Fan F, Cong M and Cheng ZJ: Determining ADCC
activity of antibody-based therapeutic molecules using two
bioluminescent reporter-based bioassays. Curr Protoc. 1:e2962021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takei J, Kaneko MK, Ohishi T, Hosono H,
Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al: A
defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts
antitumor effects in mouse xenograft models of oral squamous cell
carcinoma. Oncol Rep. 44:1949–1960. 2020.PubMed/NCBI
|
|
39
|
Birzele F, Voss E, Nopora A, Honold K,
Heil F, Lohmann S, Verheul H, Le Tourneau C, Delord JP, van Herpen
C, et al: CD44 isoform status predicts response to treatment with
anti-CD44 antibody in cancer patients. Clin Cancer Res.
21:2753–2762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Golay J and Taylor RP: The role of
complement in the mechanism of action of therapeutic anti-cancer
mAbs. Antibodies (Basel). 9:582020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reis ES, Mastellos DC, Ricklin D,
Mantovani A and Lambris JD: Complement in cancer: Untangling an
intricate relationship. Nat Rev Immunol. 18:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galon J and Bruni D: Approaches to treat
immune hot, altered and cold tumours with combination
immunotherapies. Nat Rev Drug Discov. 18:197–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hiemstra IH, Santegoets KCM, Janmaat ML,
De Goeij BECG, Ten Hagen W, van Dooremalen S, Boross P, van den
Brakel J, Bosgra S, Andringa G, et al: Preclinical anti-tumour
activity of HexaBody-CD38, a next-generation CD38 antibody with
superior complement-dependent cytotoxic activity. EBioMedicine.
93:1046632023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Jong RN, Beurskens FJ, Verploegen S,
Strumane K, van Kampen MD, Voorhorst M, Horstman W, Engelberts PJ,
Oostindie SC, Wang G, et al: A novel platform for the potentiation
of therapeutic antibodies based on antigen-dependent formation of
IgG hexamers at the cell surface. PLoS Biol. 14:e10023442016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schmudde I, Laumonnier Y and Köhl J:
Anaphylatoxins coordinate innate and adaptive immune responses in
allergic asthma. Semin Immunol. 25:2–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carroll MC and Isenman DE: Regulation of
humoral immunity by complement. Immunity. 37:199–207. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gogia P, Ashraf H, Bhasin S and Xu Y:
Antibody-drug conjugates: A review of approved drugs and their
clinical level of evidence. Cancers (Basel). 15:38862023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arimori T, Mihara E, Suzuki H, Ohishi T,
Tanaka T, Kaneko MK, Takagi J and Kato Y: Locally misfolded HER2
expressed on cancer cells is a promising target for development of
cancer-specific antibodies. Structure. 32:536–549.e5. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaneko MK, Suzuki H and Kato Y:
Establishment of a novel cancer-specific anti-HER2
monoclonal antibody H2Mab-250/H2CasMab-2 for
breast cancers. Monoclon Antib Immunodiagn Immunother. 43:35–43.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaneko MK, Suzuki H, Ohishi T, Nakamura T,
Tanaka T and Kato Y: A cancer-specific monoclonal antibody against
HER2 exerts antitumor activities in human breast cancer xenograft
models. Int J Mol Sci. 25:19412024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lodewijk I, Dueñas M, Paramio JM and Rubio
C: CD44v6, STn & O-GD2: Promising tumor associated antigens
paving the way for new targeted cancer therapies. Front Immunol.
14:12726812023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aasted MKM, Groen AC, Keane JT, Dabelsteen
S, Tan E, Schnabel J, Liu F, Lewis HS, Theodoropulos C, Posey AD
and Wandall HH: Targeting solid cancers with a cancer-specific
monoclonal antibody to surface expressed aberrantly O-glycosylated
proteins. Mol Cancer Ther. 22:1204–1214. 2023. View Article : Google Scholar : PubMed/NCBI
|