Introduction
Obesity, defined by an excessive accumulation of
body fat, has escalated to epidemic levels globally. Since 1980,
its prevalence has more than doubled, affecting >1.9 billion
adults worldwide, with 650 million categorized as living with
obesity (1). Over a third of the
global population is currently classed as overweight or obese, with
projections suggesting that by 2030, 38% of adults will be
overweight and 20% will suffer from obesity (2). In the US, obesity affects ~35% of
adults and 30% of children, with even higher rates in specific
subpopulations, such as Hispanic and non-Hispanic Black adults,
where obesity prevalence reaches 43 and 48%, respectively (2). In Europe, obesity prevalence in adults
increased from 13 to 17% between 1992 and 2005, with projections
indicating it could reach 30% by 2015 (2). The economic burden is notable, with
obesity-related healthcare costs in the US alone estimated at $190
billion annually, and the risk of type 2 diabetes increasing
threefold for overweight individuals and sevenfold for those with
obesity (2). Multiple factors,
including sedentary lifestyles, unhealthy dietary patterns, genetic
predispositions and environmental conditions, influence this surge
in obesity rates (2).
The rising prevalence of obesity has far-reaching
implications for public health, as it is associated with an
increased risk of various chronic diseases, including
cardiovascular disease, diabetes and several types of cancer
(2). Among the cancers linked to
obesity, lung cancer is particularly concerning given its status as
the leading cause of cancer-related mortality globally (3). Lung cancer comprises a diverse group
of malignancies originating from the epithelial cells of the lungs,
with the primary subtypes being non-small cell lung cancer (NSCLC)
and small cell lung cancer (SCLC) (4). Despite advances in early detection and
therapeutic strategies, lung cancer prognosis remains poor, largely
due to late-stage diagnoses and limited treatment options (5).
In the context of lung cancer, the association
between obesity and survival rates has produced conflicting
findings. While obesity is a well-established risk factor for the
development of lung cancer, some studies suggest that patients with
obesity who are diagnosed with lung cancer may have improved
survival rates, a phenomenon known as the obesity paradox (6–8). The
obesity paradox is a term used in medical research to describe the
unexpected observation that, in certain populations, being
overweight or having obesity is associated with a lower risk of
mortality compared with individuals of a normal weight (9). This paradoxical phenomenon challenges
the traditional understanding that obesity universally leads to
worse health outcomes (9). The
concept first gained attention in patients with chronic diseases,
particularly cardiovascular disease and chronic kidney disease
(9). It has been noticed that
overweight or individuals with mild obesity occasionally had
improved survival rates compared with those of normal weight within
these specific groups (9). The
paradox has also been observed in conditions such as heart failure,
stroke and diabetes (9).
Several hypotheses have been proposed to explain the
mechanisms behind the obesity paradox in cancer. One theory
suggests that excess adipose tissue in individuals with obesity may
have protective effects through the secretion of adipokines, which
possess potential anti-inflammatory and immunomodulatory properties
(10). Additionally, patients with
obesity may have greater metabolic reserves, allowing them to
better tolerate aggressive cancer treatments and withstand the
physiological stresses associated with cancer progression (11). Moreover, certain genetic and
molecular alterations present in individuals with obesity, such as
dysregulation in insulin-like growth factor (IGF) signaling,
chronic inflammation, adipokine imbalance, alterations in the
PI3K/AKT/mTOR pathway and changes in estrogen metabolism, may
influence tumor biology and response to therapy, potentially
contributing to observed survival benefits. Additionally, metabolic
reprogramming, hypoxia-driven angiogenesis, and obesity-associated
epigenetic modifications may further affect the therapeutic
outcomes in obese individuals (12,13).
However, the intricate interplay between obesity, cancer biology
and treatment outcomes is complex and multifaceted, necessitating
further research to elucidate the underlying mechanisms.
The aim of the present review is to examine the
association between obesity and lung cancer. First, the
epidemiological evidence will be presented and the impact of
adiposity and related pathophysiological mechanisms on lung cancer
development, progression and treatment outcomes will be explored.
The mechanisms by which obesity influences treatment modalities and
survival will be analyzed, the potential protective mechanisms
underlying the obesity paradox will be assessed and the
implications for clinical management will be evaluated. Finally,
novel directions for future research to improve understanding and
address the complexities of the obesity-lung cancer association
will be proposed.
Impact of obesity on lung cancer
Epidemiological evidence
While the link between obesity and cancer is
well-established for several malignancies, such as breast and
colorectal cancer, the association with lung cancer is more complex
and remains a topic of ongoing research (14).
Body mass index (BMI), a measure of obesity, and the
risk of developing lung cancer have both been associated favorably
in numerous meta-analyses and cohort studies (15–18).
For instance, a meta-analysis found a significant positive
association between excess body weight and the risk of lung cancer,
particularly among non-smokers and women (15). Furthermore, evidence for an
association of BMI with lung cancer was provided by a re-analysis
of dose-response meta-analyses of observational studies (16).
Additionally, it appears that factors such as
smoking status, sex and histological subtypes have an impact on the
association between obesity and lung cancer risk. While smoking
remains the primary risk factor for lung cancer, obesity may exert
independent effects on lung carcinogenesis, particularly among
non-smokers (17). Female
ever-smokers seem to have worse outcomes at extreme BMI levels,
both underweight and with obesity, compared with male ever-smokers
regarding lung cancer (17).
Moreover, evidence suggests that obesity may confer a higher risk
of developing specific histological subtypes of lung cancer, such
as adenocarcinoma, while its impact on other subtypes remains less
clear (18).
By contrast, recent studies and meta-analyses have
shown that a high BMI is an independent predictor of lower lung
cancer risk, improved treatment outcomes and longer overall
survival (OS) (17,19,20).
While BMI is the most commonly used metric in the studies described
in the present review, it does not differentiate between distinct
types of adipose tissue with respect to metabolic activity and
distribution in different anatomic locations. This is important
because different adiposity patterns are associated with different
biological effects. For example, visceral fat is more biologically
active and is associated with poorer outcomes when compared with
subcutaneous fat (21).
Additionally, BMI is known to overestimate obesity when there is
excess muscle mass and underestimate obesity in patients with
cancer (22).
Potential pathophysiological
mechanisms
The biological mechanisms underlying the association
between obesity and lung cancer risk are multifaceted and
presumably involve complex interactions between adipose tissue,
systemic inflammation, hormonal dysregulation and metabolic
alterations.
Adipose tissue is a dynamic endocrine and metabolic
organ secreting various pro-inflammatory cytokines and other
adipokines, such as tumor necrosis factor-α (TNF-α), interleukin-6
(IL-6) and leptin (23). Chronic
low-grade inflammation linked to obesity and dysfunctional adipose
tissue leads to the promotion of tumorigenesis by creating a
pro-inflammatory microenvironment, conducive to cancer initiation
and progression (24). Insulin
resistance, which includes impaired insulin signaling and
hyperinsulinemia, are frequently associated with obesity (25). Insulin and IGF-1 play critical roles
in cell proliferation, apoptosis and tumor growth, thereby
promoting carcinogenesis in various tissues, including the lungs
(26). Obesity impacts sex hormone
metabolism, leading to alterations in estrogen and androgen levels,
which may contribute to the development of hormone-related cancers,
including lung cancer (27).
Estrogen, in particular, has been implicated in lung cancer
pathogenesis, with evidence suggesting a higher prevalence of
estrogen receptor expression in lung tumors among female patients
(28). Obesity-induced oxidative
stress and associated DNA damage represent additional mechanisms
linked to lung cancer development as excess adiposity is associated
with increased production of reactive oxygen species and reduced
antioxidant defenses, resulting in oxidative damage to DNA and
genomic instability, predisposing to malignant transformation
(29,30).
Obesity and survival with lung cancer
While obesity is commonly associated with an
increased risk of developing lung cancer, its impact on patient
survival following lung cancer diagnosis is less clear.
Survival disparities in lung cancer
and obesity
Survival disparities in lung cancer among
individuals with normal weight and obesity represent a complex
intersection of biological, behavioral and socioeconomic
factors.
One of the key mechanisms linking obesity to poor
lung cancer outcomes is chronic inflammation and adipose tissue,
which secretes pro-inflammatory cytokines and adipokines, creating
a tumor microenvironment (TME) conducive to cancer progression and
metastasis (31). This inflammatory
milieu can promote tumor growth, angiogenesis and resistance to
chemotherapy and immunotherapy, ultimately compromising treatment
efficacy and patient survival (31). Moreover, obesity-related
comorbidities, such as diabetes, cardiovascular disease and
obstructive sleep apnea, further complicate lung cancer management.
These conditions increase the risk of surgical complications and
treatment toxicities but also contribute to poorer overall health
outcomes and decreased survival rates among patients with obesity
and lung cancer (32,33).
In addition to biological factors, behavioral and
lifestyle factors associated with obesity may also influence lung
cancer survival. Individuals with obesity are more likely to have
unhealthy habits such as sedentary lifestyles, poor nutrition and
smoke, all of which can exacerbate cancer-related complications and
hinder treatment adherence and effectiveness (34). Furthermore, social determinants of
health, including access to healthcare, socioeconomic status and
healthcare disparities, may disproportionately affect individuals
with obesity, limiting their ability to receive timely and
appropriate cancer care (35).
Different histological subtypes and
survival from lung cancer in patients with obesity
NSCLC
Barbi et al (12) analyzed 513 patients with stage I and
II NSCLC undergoing lobectomy, revealing that a high visceral fat
index (VFI) was associated with decreased recurrence-free survival
(RFS) and OS. Specifically, patients in the top VFI tertile
exhibited significantly worse outcomes compared with those in the
bottom tertile, with hazard ratios (HR) of 1.79 for RFS and 1.84
for OS. The study also explored the TME in 159 patients with
advanced-stage NSCLC, finding that high VFI was associated with a
non-inflamed TME, characterized by reduced expression of
immune-related genes, which likely contributed to poorer
survival.
In a study by Xiong et al (36) involving 554 patients with advanced
NSCLC from the ALTER-0302 and ALTER-0303 trials, researchers
investigated the impact of obesity on outcomes in patients
receiving anlotinib, a novel anti-angiogenesis drug. The patients
were categorized into non-obesity (BMI <28 kg/m2) and
obesity (BMI ≥28 kg/m2) subgroups. The results indicated
a U-shaped relationship between BMI and the risk of death in
patients treated with anlotinib. Specifically, patients with
obesity had a trend toward worse OS compared with patients without
obesity, with a HR of 2.33 (95% CI, 0.77-7.06; P=0.136). This
finding suggests that obesity may predict poorer outcomes in
patients receiving anlotinib for advanced NSCLC.
In a study by Nitsche et al (37) of 994 patients with NSCLC treated
between 2008 and 2020, visceral obesity was measured using
cross-sectional abdominal fat areas from computerized tomography
(CT) scans. The VFI, defined as the ratio of visceral fat area to
total fat area, was used to assess visceral obesity. The study
found that male patients had significantly higher VFI compared with
female patients, and VFI was modestly correlated with age but not
with BMI. Furthermore, a subset of 175 patients had their tumors
profiled for the expression of 397 cancer- and immunity-related
genes, revealing that higher VFI was associated with a lower tumor
immunogenicity signature score, which correlates with reduced
immune response in tumors. This suggests that visceral obesity may
attenuate the tumor immune environment, potentially leading to
poorer outcomes in patients with NSCLC.
SCLC
In a study by Lee et al (38) conducted on 173 patients with SCLC,
researchers investigated the impact of BMI on OS. The patients were
divided into two groups based on their BMI: i) individuals with a
BMI ≥23; and ii) individuals with a BMI <23. The study found
that patients with a BMI ≥23 had significantly improved OS compared
with those with BMI <23, with a median OS of 620.0 days vs.
311.7 days, respectively (P<0.001). Multivariate Cox analysis
further indicated that a BMI ≥23 was an independent prognostic
factor for OS, with a HR of 0.45 (95% CI, 0.31-0.79; P=0.004).
Additionally, patients with an improved performance status (PS; ≤2)
and a BMI ≥23 had the longest median OS of 17.3 months, suggesting
a potential protective effect of higher BMI in SCLC.
In a retrospective study by Kwon et al
(39) involving 1,146 Asian
patients with SCLC who underwent platinum-etoposide chemotherapy,
researchers investigated the prognostic significance of BMI and its
association with skeletal muscle status. The study found that being
underweight (BMI <18.5 kg/m2) was associated with
significantly shorter OS in both limited-disease (LD) and
extensive-disease (ED) groups, with HRs of 1.77 (95% CI, 1.01-3.09)
and 1.71 (95% CI, 1.18-2.48), respectively. The negative impact of
being underweight remained significant even after adjusting for
skeletal muscle index and skeletal muscle attenuation, with
underweight patients in the LD group having a HR of 1.96 (95% CI,
1.09-3.51) and in the ED group having a HR of 1.75 (95% CI,
1.17-2.61). This study highlights that being underweight is an
independent poor prognostic factor in SCLC, irrespective of
skeletal muscle status.
Obesity paradox: An intriguing
phenomenon
Obesity has traditionally been viewed as a risk
factor for poor prognosis in cancer, including lung cancer
(40). However, emerging evidence
suggests that patients with obesity may exhibit improved survival
rates following a lung cancer diagnosis, a phenomenon known as the
obesity paradox (9). This
unexpected observation has fueled debate and led to further
research into the mechanisms behind this paradox in cancer.
The mechanisms underlying the obesity paradox in
lung cancer survival are likely driven by complex interactions
between adipose tissue biology, systemic inflammation, metabolic
alterations, treatment responses and tumor biology (41). One potential explanation is that
patients with obesity often have greater metabolic reserves and
nutritional stores than their normal-weight counterparts (41). These reserves may provide a survival
advantage during cancer treatment (41). Specifically, the enhanced energy
stores and nutrients can help patients with obesity endure the
physiological stresses associated with cancer progression and
aggressive treatments such as surgery, chemotherapy and radiation
(431). This ability to withstand such challenges may contribute to
the observed improved survival outcomes in patients with obesity
(41).
Adipose tissue also plays a crucial role in
modulating the TME and immune responses through the secretion of
various adipokines and inflammatory mediators. For instance,
adiponectin, an adipokine, exhibits anti-inflammatory and
anti-tumor properties, whereas leptin, another adipokine, may
promote tumor growth and metastasis (42). The regulation of these functions
depends on multiple factors, including the type of adipokines
secreted, the local tissue environment and systemic metabolic
conditions (43). Leptin promotes
cancer by enhancing cell proliferation, migration and angiogenesis,
and regulation involves controlling leptin levels and signaling
pathways (43). High levels of
leptin can activate oncogenic pathways such as JAK/STAT, PI3K/AKT
and MAPK (43). Adiponectin usually
suppresses cancer by inhibiting cell proliferation and inducing
apoptosis. Increasing adiponectin levels or mimicking its activity
through drugs can enhance its cancer-suppressive effects (43). It works through pathways such as
AMPK and PPARα, which inhibit cancer cell growth and promote
apoptosis (43).
Chronic inflammation associated with obesity can
promote cancer (44). Reducing
systemic inflammation through lifestyle changes (such as diet and
exercise) or pharmacological interventions (such as
anti-inflammatory drugs) can help tilt the balance towards cancer
suppression (44). In addition,
obesity alters immune cell function. For example, macrophages in
obese adipose tissue often exhibit a pro-inflammatory phenotype
(M1) that promotes cancer (44).
Promoting the anti-inflammatory (M2) phenotype of macrophages can
help suppress cancer (44).
The balance between pro-inflammatory and
anti-inflammatory signals within the TME may significantly impact
disease progression and treatment responses, ultimately influencing
survival outcomes (45).
Obesity-related metabolic alterations and molecular pathways also
play a role in tumor biology and treatment responses. For instance,
obesity is linked to changes in insulin signaling, sex hormone
metabolism and oxidative stress pathways, all of which may modulate
tumor growth, angiogenesis and metastasis (45). Furthermore, obesity-related
molecular alterations, such as mutations in oncogenes or tumor
suppressor genes, may impart distinct biological characteristics to
tumors, affecting their response to therapy and overall prognosis
(46).
Insulin resistance and hyperinsulinemia, which are
common in patients with obesity, are linked to cancer development
through multiple mechanisms. Elevated insulin levels can directly
promote cell proliferation by activating insulin and IGF receptors,
leading to enhanced signaling through pathways such as PI3K/AKT and
MAPK, which are critical for cancer cell growth and survival
(47). Additionally,
hyperinsulinemia can lower sex hormone-binding globulin (SHBG)
levels, increasing free sex hormones that drive hormone-sensitive
cancers such as breast and prostate cancer (47). The chronic low-grade inflammation
often associated with insulin resistance further contributes to a
pro-tumorigenic environment, enhancing the risk of cancer
development in individuals with obesity (47).
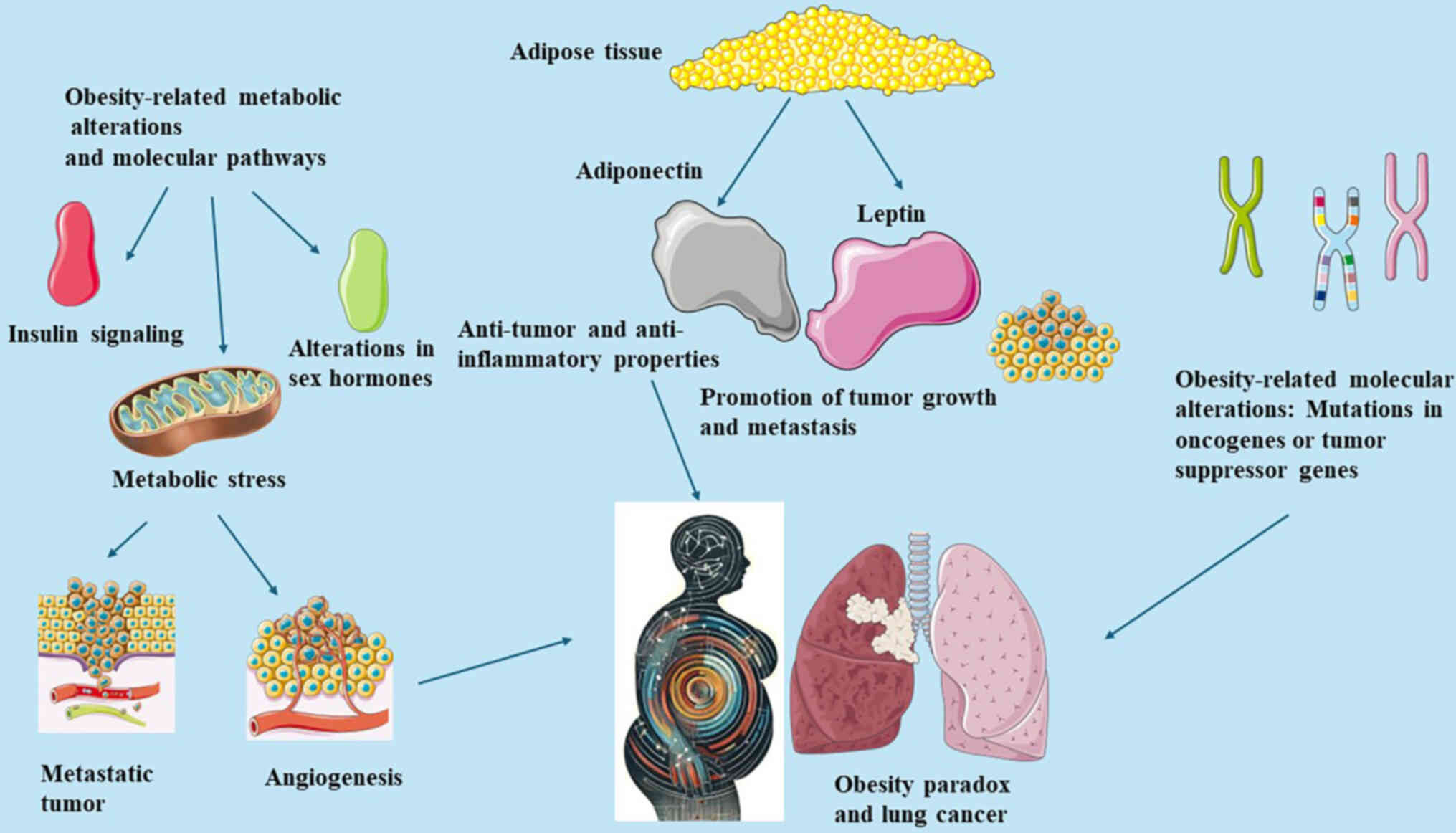
The mechanisms underlying the obesity paradox in
lung cancer are briefly illustrated in Fig. 1. Evidence from several studies
indicate the existence of the obesity paradox in lung cancer. In
the study by Lee et al (38)
involving 820 Asian patients with advanced NSCLC undergoing immune
checkpoint inhibitor (ICI) therapy, it was found that those with a
BMI ≥25 kg/m2 had significantly improved OS compared
with those with a normal BMI. Specifically, the obesity BMI group
had a HR of 0.64 for OS, indicating a 36% lower risk of death
compared with those with a normal BMI, independent of clinical
covariates such as skeletal muscle and visceral fat indices
(38).
The study conducted by Lam et al (46) investigated the obesity paradox in
patients with locally advanced NSCLC who were treated with
definitive chemoradiotherapy. The analysis included 291 patients,
stratified by BMI into underweight, normal weight, overweight and
obese categories. The findings demonstrated that a higher BMI was
associated with improved OS. Specifically, patients with obesity
had a 34% lower risk of mortality compared with those of a normal
weight, even after adjusting for multiple variables such as age,
stage, smoking history and PS. The study also observed that statin
use, which was more common among patients with obesity, was
independently associated with improved survival (46).
Addressing potential confounding factors and biases
is crucial in studies examining the obesity paradox, a phenomenon
where overweight or individuals with obesity appear to have
improved outcomes in certain diseases compared with those with a
normal weight, as it challenges conventional understanding of the
health impacts of obesity. Confounding factors such as age, smoking
status, pre-existing conditions and physical fitness play
significant roles in influencing outcomes. For instance, older
adults may exhibit the paradox due to survival bias, while smokers,
who often have lower body weight but higher mortality rates, may
skew results if not properly adjusted for (15). Additionally, chronic diseases can
confound the association between obesity and outcomes, and physical
fitness may independently affect health outcomes regardless of
weight (15). Biases, including
survivor bias, reverse causation, measurement bias and selection
bias, also need to be carefully managed. Survivor bias may occur if
studies only include those who have lived long enough to be part of
the study, potentially excluding those who died earlier due to
obesity-related complications (15). Reverse causation can mislead results
when weight loss from illness is mistaken for a naturally lower
weight (38). Measurement bias
arises from the method by which obesity is assessed, such as using
BMI, which does not distinguish between fat and muscle mass
(38). Finally, selection bias can
occur if studies disproportionately include certain subgroups, such
as hospitalized patients, who may not represent the general
population (38,48). Managing these confounders and biases
is essential for accurately interpreting the obesity paradox and
its implications for health outcomes (15,38,48).
There is evidence suggesting an obesity paradox in
patients with renal cell carcinoma (RCC), the most prevalent type
of kidney cancer, accounting for 2.4% of all adult cancers
(49). While adiposity is a notable
risk factor for RCC, it may also enhance prognosis (49). The increased incidence of RCC is
considered to result from insulin resistance and elevated levels of
IGF-1, which suppresses Bcl-2 to reduce apoptosis while promoting
proliferative and angiogenic factors (50). Improved prognosis in patients with
obesity may be due to similar factors as in lung cancer, such as
BMI categorization and changes in the tumor immune
microenvironment, with higher levels of IL-6, TNF-α and c-peptide
observed in patients with obesity (51). Several studies have indicated
improved OS and progression-free survival in RCC patients with
obesity (52), including those with
metastatic disease (53) and those
who underwent nephrectomy (54,55).
Patients with obesity also tend to have improved outcomes and
longer OS when treated with anti-VEGF therapies such as sunitinib,
sorafenib, bevacizumab and axitinib (56,57).
This suggests a clear obesity paradox in RCC when patients receive
immunotherapy.
Similar to NSCLC and RCC, patients with melanoma and
obesity treated with immunotherapy generally experience increased
survival and an improved response to checkpoint inhibitors compared
with their normal-weight counterparts (58). Patients with obesity show more
robust responses to ICIs and have higher OS (59). They also exhibit significantly
improved responses to treatments such as dabrafenib, ipilimumab,
trametinib, the BRAF inhibitor vemurafenib and PD-1 therapies
(60,61). In an experimental study, mice with
obesity and melanoma treated with PD-1 therapy showed improved
responses than normal-weight mice (62). Thus, an obesity paradox is evident
in patients with melanoma treated with immunotherapy.
Impact of timing of obesity
It has been shown that pre-diagnostic obesity can
offer a metabolic reserve during cancer treatment, potentially
improving outcomes (63). For
instance, an early study involving 262 patients with SCLC found
that obesity at the start of treatment was not associated with
increased toxicity or shortened survival, suggesting that
pre-diagnostic obesity may not negatively impact treatment outcomes
(63). This metabolic reserve can
help patients tolerate aggressive cancer treatments better than
their lean counterparts (63).
A previous retrospective study, which included 200
patients with lung cancer, found that a decrease in BMI during
chemotherapy cycles was associated with poor survival (64). This indicates that weight loss and
the onset of cachexia after diagnosis can significantly worsen
outcomes (64).
Another study analyzed data from 25,430 patients
with NSCLC and 2,787 patients with SCLC to explore the impact of
BMI on lung cancer outcomes. The results revealed a U-shaped
relationship between BMI and NSCLC survival, whereby underweight
and morbidly obese patients had worse prognosis, while overweight
and patients with obesity had improved survival. A similar but
non-significant pattern was observed in SCLC. Additionally, a
decrease in BMI from young adulthood to diagnosis was linked to
poorer outcomes in both NSCLC and SCLC. Thus, being underweight or
morbidly obese at diagnosis and a decreasing BMI from early
adulthood are associated with lower survival in patients with lung
cancer (65).
In addition, another study investigated the impact
of pre-surgery BMI and muscle mass on survival after major lung
resection for NSCLC. In a retrospective analysis of 304 patients,
7.6% were underweight, 51.6% were normal weight, 28.6% were
overweight and 12.6% had obesity. Weight loss and gain were
observed in 5 and 44.4% of patients, respectively. Low muscle mass
was more common in patients with BMI <25 kg/m2.
Higher pre-disease and pre-surgery BMI were associated with
improved OS, especially with weight gain. Low muscle mass and
weight loss negatively affected outcomes. Multivariable models
confirmed the prognostic value of higher pre-disease and
pre-surgery BMI and the absence of low muscle mass (66).
Impact of demographics
The study by Jiang et al (67) evaluated the impact of sex, smoking
and ethnicity on the association between BMI and OS in NSCLC using
pooled data from 16 ILCCO studies. Among 20,937 patients with
NSCLC, the effect of BMI on survival varied by ethnicity.
Specifically, underweight white patients had poorer survival
compared with black patients, and overweight/obese black patients
had improved OS compared with white patients. BMI was least
associated with survival in Asian patients and never-smokers.
Female ever-smokers had worse outcomes at extreme BMI levels, both
underweight and with obesity, compared with male ever-smokers.
Thus, the association of BMI with NSCLC prognosis differs by sex,
smoking status and ethnicity, with black patients having more
favorable outcomes at extreme BMI levels compared with white
patients, and these associations were not observed in Asian
patients and never-smokers (67).
The study by Bethea et al (68) analyzed data from the Black Women's
Health Study to investigate the association between BMI and lung
cancer risk in African American women. Among 59,000 participants,
323 lung cancer cases were identified from 1995 to 2011. Higher BMI
(≥30) was associated with a lower risk of lung cancer compared with
normal weight individuals (BMI, 18.5-24.9), with a HR of 0.69. This
inverse relationship was particularly notable among current smokers
(HR, 0.62). Adjusting for smoking and other factors did not
significantly alter the results. Waist circumference and waist/hip
ratio were not associated with lung cancer risk. The findings
suggest that high BMI may lower lung cancer risk in African
American women, especially among smokers (68).
The study by Kim et al (69) evaluated the associations between
sex-specific incidence of EGFR mutations in lung adenocarcinoma and
factors such as age and obesity, using data from 1,378 cases.
Obesity was categorized by BMI. In men, EGFR mutation incidence was
inversely associated with age [adjusted odds ratio (OR), 0.76;
P-trend=0.003] and positively associated with obesity (adjusted OR,
1.23; P-trend=0.04). In women, EGFR mutation incidence was
positively associated with age (adjusted OR, 1.19; P-trend=0.02)
but not significantly associated with obesity (adjusted OR, 1.03;
P-trend=0.76). These findings suggest that age and obesity may
influence the sex-specific incidence of EGFR mutations in lung
adenocarcinoma differently (69).
Obesity and the immune system
The persistent low-grade inflammation associated
with obesity and the diverse effects of adipokines on the immune
system have been linked to the development of various inflammatory
conditions, including rheumatic autoimmune and inflammatory
diseases (70). Obesity disrupts
multiple aspects of the immune system, including the integrity of
lymphoid tissues, the development and function of leukocytes, the
activation of the complement system, and the coordination of innate
and adaptive immune responses (71). Consequently, obesity leads to a less
effective immune response to infectious agents (71).
Targeting adipose tissue-tumor
crosstalk mediators
Therapy resistance in tumor cells is often linked to
a metabolic switch from glycolytic to lipid metabolism,
highlighting the clinical importance of enzymes regulating these
metabolic pathways (72–79). These enzymes are being explored as
targets for novel antitumor therapies, either as standalone
treatments or as chemotherapy adjuncts for therapy-resistant
patients (72–79).
Fatty acid synthase (FASN) is a key focus in
antitumor therapy. First-generation FASN-targeting drugs such as
cerulenin, C75 and Orlistat showed promising preclinical results by
reducing tumor xenograft growth but faced clinical challenges due
to side effects such as anorexia and weight loss (80). Newer FASN inhibitors, such as
TVB-3166 and TVB-2640, have shown effective antitumoral potential
in preclinical colorectal and breast cancer models and improved
tolerability in clinical trials (81). Given the link between FASN
expression and HER-2 signaling, FASN-targeting therapies could help
stratify patients based on their response to standard chemotherapy
(82). Additionally, combining FASN
inhibition with standard chemotherapy has been successful in
treating therapy-resistant ovarian cancer in vitro and in
vivo (82–84).
Cancer-associated adipocytes (CAAs) play an
immunomodulatory role during cancer progression and are a promising
therapeutic target to enhance immunotherapy (85). Strategies include directly
interfering with adipocytes or blocking signals derived from CAAs
(85). However, targeting CAAs is
challenging due to the loss of adipocyte-specific markers and the
risk of affecting healthy organs (85). Indirect targeting, such as
inhibiting CD36 fatty acid transport proteins, has shown promise.
In breast cancer, CD36 inhibition decreased intratumoral Tregs and
increased antitumoral T cells, and combining the CD36 monoclonal
antibody with anti-PD-1 therapy enhanced antitumor activity
(86). CD36, a transmembrane
glycoprotein involved in fatty acid uptake and angiogenesis, is
highly expressed in ovarian cancer cells co-cultured with
adipocytes, leading to increased fatty acid uptake (87). CD36 inhibition reduced tumor growth
in vivo, suggesting its potential as a therapeutic target to
limit tumor aggressiveness (87).
PPARγ is a key regulator of glucose homeostasis and
can upregulate tumor suppressor genes such as BRCA1 and PTEN
(88,89). PPARγ antagonists, such as GW9662,
have been proposed to target the crosstalk between adipocytes and
cancer stem cells (88,89). GW9662 sensitized ER-responsive
tumors to fulvestrant therapy in mice and inhibited bladder cell
proliferation and tumor growth (88,89).
Exosomes delivering miRNA offer a promising
therapeutic strategy for cancer treatment. miRNAs are stable and
can regulate cell proliferation, differentiation and
chemosensitivity (90). In
hepatocarcinoma, downregulation of miRNA-122 is associated with
poor prognosis, and miRNA-122-enriched exosomes from adipocytes can
sensitize hepatocarcinoma to sorafenib treatment (90). In ovarian cancer, miRNA-121 in
exosomes from CAAs induced chemoresistance to paclitaxel by
downregulating APAF1. Therefore, targeting miRNA-121 or
upregulating APAF-1 could reduce chemoresistance (91).
Lactate, a metabolite released in the TME,
represents a therapeutic target for inhibiting CAA-mediated
immunosuppression (92,93). Inhibiting lactate dehydrogenase,
which converts pyruvate to lactate, has also been shown to regress
NSCLC tumors and activate the immune system (92,93).
Overall, targeting adipocytes, CAAs, and their adipokines and
metabolites presents a promising strategy for cancer treatment.
Obesity and paraneoplastic syndromes
of lung cancer
Acanthosis nigricans (AN) is a rare paraneoplastic
syndrome associated with lung cancer (94). In the majority of cases, AN reflects
metabolic disturbances associated with obesity, metabolic syndrome,
diabetes or medications such as insulin, glucocorticoids, oral
contraceptives and antipsychotics (94). The most common histologic cancer
type associated with AN is adenocarcinoma, generally involving the
gastrointestinal system, and less commonly, paraneoplastic AN is
associated with NSCLC (94). AN is
characterized by gray-brown hyperpigmented, velvety plaques that
often affect the neck, flexor area, and anogenital regions
(94).
Impact of obesity on anti-tumor
therapies
Obesity is a complex metabolic condition associated
with various physiological and molecular alterations that can
influence the efficacy and safety of anti-tumor therapies.
Surgery
Surgery plays a critical role in the management of
early-stage and operable cancers, offering the potential for
curative treatment and long-term survival (95). However, obesity-related
physiological changes and technical challenges can complicate
surgical procedures and impact postoperative outcomes in patients
with cancer and obesity (96).
Obesity is associated with increased surgical complexity, longer
operative times and higher rates of intraoperative complications,
such as wound infections, bleeding and anastomotic leaks (97). Surgical teams may encounter
technical challenges related to patient positioning, access to
surgical sites and tissue manipulation, requiring specialized
equipment and expertise to ensure safe and effective surgical
outcomes (97). Furthermore,
patients with obesity undergoing cancer surgery are at a higher
risk of postoperative complications, including surgical site
infections, pulmonary complications (such as atelectasis and
pneumonia), venous thromboembolism (VTE) and delayed wound healing
(89–100). Obesity-related factors, such as
impaired tissue perfusion, compromised wound healing and reduced
respiratory function, contribute to increased morbidity and
mortality rates in surgical patients with obesity (98–100).
Preoperative optimization of patients with obesity
undergoing cancer surgery is essential to mitigate postoperative
complications, with current protocols emphasizing weight reduction,
glycemic control and tailored nutritional interventions (101). Enhanced Recovery After Surgery
protocols, which include strategies such as multimodal pain
management, early mobilization and specific anesthetic techniques,
have been particularly effective in reducing complications and
shortening recovery times in this population (101). Additionally, VTE prophylaxis,
incorporating both mechanical and pharmacological methods, is
critical given the heightened risk in patients with obesity
(101).
While obesity is generally associated with poorer
long-term survival in patients with cancer, the impact of obesity
on surgical outcomes and survival following cancer surgery varies
across tumor types and patient populations. Studies investigating
the association between obesity and long-term survival in
surgically treated patients with cancer have produced conflicting
results, highlighting the need for further research to clarify the
association between obesity, surgery and cancer outcomes (102,103).
A study by Tong et al (104) examined the association between
obesity and perioperative outcomes in elderly patients undergoing
thoracoscopic anatomic lung cancer surgery at Shanghai Chest
Hospital (Shanghai, China). Among 4,164 patients aged 65 or older,
those categorized as having obesity showed higher rates of
intraoperative hypoxemia (3.9 vs. 1.2%; P=0.001) and new-onset
arrhythmia (4.3 vs. 2.3%; P=0.034) compared with nonobese patients.
However, other perioperative outcomes, such as pulmonary
complications and hospital stay, were not significantly different
between the groups (P>0.05). The study supports the obesity
paradox, suggesting that obesity should not preclude elderly
patients from undergoing thoracoscopic anatomic lung cancer surgery
(104).
The study by Guerrera et al (105) assessed the impact of morbid
obesity on perioperative clinical and oncological outcomes
following video-assisted thoracic surgery (VATS) lobectomy using
data from the Italian VATS lobectomy Registry, which included 4,412
patients from 55 institutions between 2016 and 2019. Among the
patients, 74 (1.7%) had morbid obesity. Multivariable-adjusted
analysis indicated that morbid obesity was associated with a higher
rate of complications (32.8 vs. 20.3%), but not with increased
conversion to thoracotomy, surgical margin positivity, surgical
time, lymph-node retrieval, intraoperative blood loss, hospital
postoperative length of stay or chest tube duration. The most
frequent postoperative complications in morbidly obese patients
were pulmonary-related (35%). The study concluded that VATS
lobectomy can be safely and effectively performed in morbidly obese
patients, maintaining equivalent short-term oncological outcomes
(105).
The study by Lee et al (106) assessed the prognostic value of
obesity and its link to skeletal muscle mass in patients with lung
adenocarcinoma. Data from 636 patients (2011–2015) were analyzed.
Obese patients had longer OS than non-obese patients (110.2 vs.
98.7 months; P=0.015). Multivariable Cox regression analysis showed
that obesity was associated with longer survival (HR, 0.59; 95% CI,
0.40-0.86; P=0.007), even after adjusting for skeletal muscle mass
(HR, 0.57; 95% CI, 0.36-0.89; P=0.014). No significant interaction
was found between skeletal muscle mass and BMI on survival. Thus,
obesity was linked to improved OS, independent of skeletal muscle
mass (106).
The study by Tulinský et al (107) evaluated the effect of BMI on
short-term outcomes following lung lobectomy. A retrospective
comparison was made between obese and non-obese patients who
underwent anatomical lung resection for cancer, ensuring both
groups had similar risk factors and surgical approaches
(thoracoscopy vs. thoracotomy). Among 144 patients (48 obese, 96
non-obese), the frequency of perioperative and postoperative
complications did not significantly differ between groups.
Non-obese patients had higher postoperative morbidity (34.4% vs.
27.1%), but this was not statistically significant (P=0.053).
Hospital stay and postoperative mortality were similar in both
groups, while surgery time was slightly longer for patients with
obesity (P=0.133). The findings suggest that obesity does not
increase the risk of complications after lung lobectomy and may
even offer some protective benefits (107).
The study by Seder et al (108) compared robotic surgery (RTS) and
video-assisted thoracoscopic surgery (VATS) for patients with
obesity undergoing lung resection. Data from 8,108 patients
revealed that those who underwent VATS were more than five times as
likely to convert to thoracotomy than those who underwent RTS (OR,
5.33; 95% CI, 4.14-6.81; P<0.001). Patients that underwent VATS
also had longer hospital stays, higher rates of respiratory failure
and were less likely to be discharged home. RTS is associated with
fewer conversions to thoracotomy and improved perioperative
outcomes in patients with obesity (108).
The study by Leonardi et al (109) assessed the feasibility and safety
of one-lung ventilation in patients with obesity undergoing
thoracoscopic lobectomy. Among 111 patients (26 obese, 85 nonobese)
treated between October 2019 and February 2022, patients with
obesity more frequently used a single-lumen tube with bronchial
blocker (81 vs. 19%; P=0.001). Intubation time was longer for
patients with obesity (94.0 vs. 85.0 s; P=0.0004), with a higher
failure rate on the first attempt (23 vs. 5%; P=0.01). Furthermore,
obesity did not increase complications or mortality. The study
concludes that one-lung ventilation is safe and feasible in
patients with obesity, with no negative impact on outcomes
(109).
Chemotherapy
Obesity can lead to changes in drug
pharmacokinetics, including alterations in drug absorption,
distribution, metabolism and elimination (110). These changes may affect
chemotherapy dosing, drug exposure and toxicity profiles,
potentially influencing treatment efficacy and tolerability
(110). For instance, lipophilic
chemotherapeutic agents, such as cisplatin and paclitaxel, may
exhibit altered distribution and clearance in individuals with
obesity due to changes in adipose tissue composition and blood flow
(72). Patients with obesity may
require individualized dosing strategies to achieve therapeutic
drug levels while minimizing toxicity (111). However, determining optimal dosing
regimens for patients with obesity can be challenging due to
limited data and variability in pharmacokinetic parameters
(111). Clinicians should consider
factors such as ideal body weight, actual body weight, body surface
area and renal function when calculating chemotherapy doses for
patients with obesity, aiming to achieve a balance between efficacy
and safety (112).
Obesity-related comorbidities, such as diabetes,
hypertension, and cardiovascular disease, may exacerbate
chemotherapy-related toxicities and complications, leading to
treatment interruptions, dose reductions and poorer outcomes
(113). Close monitoring and
proactive management of treatment-related toxicities, including
hematologic, gastrointestinal and neurotoxic effects, are essential
for optimizing treatment adherence and quality of life in patients
with obesity undergoing chemotherapy (113).
In the study by Kicken et al (114) overweight patients experienced
significantly improved OS and PFS compared with normal weight
patients, with adjusted HRs for OS at 0.72 (95% CI, 0.59-0.89) and
for PFS at 0.74 (95% CI, 0.61-0.90). By contrast, patients with
obesity did not demonstrate significant differences in OS or PFS
relative to normal weight individuals. However, obesity was linked
to a notably higher incidence of severe thrombocytopenia (grade
≥3), with an adjusted OR of 3.47 (95% CI, 1.75-6.90) and more
frequent dose reductions due to toxicity, as evidenced by a lower
relative dose intensity (RDI); 35% of patients with obesity had an
RDI <80% in cycle 1 compared with 17% of normal weight patients.
Despite these increased toxicity risks, higher BMI was not
significantly associated with greater rates of toxicity-related
hospitalization (114).
In the study by Kashiwabara et al (115) overweight women with lung cancer
and obesity who received carboplatin-paclitaxel doublet
chemotherapy were analyzed for overdosing-related toxicity and
prognosis. The study found no significant difference in OS or PFS
between overweight/obese patients and those with a normal BMI.
Specifically, the median OS was 285 days for the BMI >25 group
compared with 282 days for the BMI ≤25 group (P=0.820). However,
overweight/obese patients experienced a higher incidence of Grade 4
neutropenia during the second cycle (39% in the BMI >25 group
vs. 13% in the BMI ≤25 group; P=0.003), leading to more frequent
dose reductions. Despite these toxicity concerns, there was no
increased risk of hospitalization or other severe toxicities,
suggesting that appropriate dose adjustments can mitigate the risks
of overdosing in this population (115).
Immunotherapy
Immunotherapy, particularly ICIs, has
revolutionized cancer treatment by harnessing the immune system to
target and eliminate cancer cells (116). However, obesity-related
alterations in immune function and the TME may impact the efficacy
and safety of immunotherapy in patients with cancer and obesity
(117).
Obesity is associated with chronic low-grade
inflammation, immune dysregulation and alterations in immune cell
populations, which may impair antitumor immune responses and
compromise the efficacy of immunotherapy (118). Additionally, adipose
tissue-derived cytokines and adipokines, such as leptin and IL-6,
can modulate immune cell function and promote tumor immune evasion,
potentially reducing the effectiveness of immunotherapy (118). Clinical studies investigating the
impact of obesity on immunotherapy outcomes have yielded
conflicting results, with some studies suggesting reduced response
rates and shorter survival in patients with obesity, while others
report comparable or improved outcomes (119,120).
Obesity-related comorbidities, such as metabolic
syndrome and insulin resistance, may increase the risk of
immune-related adverse events (irAEs) in patients with obesity
receiving immunotherapy. Common irAEs, including dermatitis,
colitis, pneumonitis and endocrinopathies, may be more frequent or
severe in individuals with obesity, requiring close monitoring and
timely intervention to prevent treatment complications and ensure
patient safety (121,122).
In the study by Zhang et al (123) the impact of BMI on survival
outcomes in patients with NSCLC treated with ICIs was investigated
through a meta-analysis of nine studies encompassing 4,602
patients. The study found no significant difference in PFS (HR,
0.885; 95% CI, 0.777-1.009; P=0.068) or OS (HR, 0.947; 95% CI,
0.789-1.137; P=0.560) between patients with low BMI and those with
high BMI. However, subgroup analysis revealed that overweight or
obese patients had significantly prolonged PFS (HR, 0.862; 95% CI,
0.760-0.978; P=0.021) and OS (HR, 0.818; 95% CI, 0.741-0.902;
P<0.0001) compared with normal-weight patients (123).
Radiotherapy
Radiotherapy plays a crucial role in the management
of localized and locally advanced cancers, including lung cancer,
by delivering targeted radiation doses to cancerous tissues while
sparing surrounding healthy tissues (124).
Obesity-related anatomical and physiological
changes can pose challenges for radiotherapy planning and delivery
in patients with obesity (125,126). Obesity can alter patient anatomy
and body contour, affecting target delineation, organ-at-risk (OAR)
sparing and radiation dose distribution (125,126). Larger body size and increased
adipose tissue thickness may result in greater tissue heterogeneity
and attenuate radiation beams, potentially compromising treatment
accuracy and efficacy (125,126). Advanced radiotherapy techniques,
such as intensity-modulated radiotherapy and volumetric modulated
arc therapy, may help optimize dose conformality and minimize
radiation exposure to OARs in patients with obesity (125,126).
Obesity-related comorbidities, such as diabetes
mellitus, arterial hypertension and obstructive sleep apnea, may
exacerbate radiotherapy-related toxicities and complications in
patients with cancer and obesity (127). Common radiation-induced
toxicities, including fatigue, skin reactions, mucositis and
radiation pneumonitis, may be more pronounced or occur at higher
frequencies in individuals with obesity, necessitating proactive
symptom management and supportive care interventions (128).
Patient-specific factors, such as tumor
characteristics, treatment compliance and overall health status,
may influence treatment outcomes and should be considered when
assessing the impact of obesity on radiotherapy efficacy (8).
In the study by Welsh et al (129) the impact of obesity on the
development of chest wall pain and skin toxicity following thoracic
stereotactic body radiation therapy (SBRT) was examined. The study
included 265 patients treated with SBRT for lung tumors located
within 2.5 cm of the chest wall. It was found that patients with a
BMI of 29 or higher had almost twice the risk of developing chronic
chest wall pain compared with those with a lower BMI, with an OR of
2.45 (95% CI, 1.24-4.84; P=0.01). Additionally, diabetes mellitus
in patients with obesity further increased the risk of severe chest
wall pain (129).
Deciphering potential pathophysiological
mechanisms connecting obesity and lung cancer
Adipose tissue dysfunction in
obesity
Obesity is characterized by excess adiposity and
dysregulated adipose tissue function, marked by adipocyte
hypertrophy, hypoxia, inflammation and altered adipokine secretion
(130–139). Adipose tissue dysfunction
contributes to systemic metabolic disturbances, insulin resistance,
dyslipidemia and chronic low-grade inflammation, which promote the
development and progression of obesity-related diseases, including
cancer (140).
Due to their presence in white adipose tissue,
adipose stromal cells (ASCs) are likely significant contributors to
the obesity-cancer link (141). In
fact, syngeneic mouse models of melanoma, breast, prostate and lung
cancer demonstrated that selectively depleting ASCs with
pro-apoptotic peptides inhibits tumor vascularization and
proliferation, leading to necrosis (141). Notably, this effect was more
pronounced in obese mice compared with lean mice, where ASCs are
more abundant (141).
Adipocyte hypertrophy
In obesity, adipocytes undergo hypertrophy and
hyperplasia to accommodate excess lipid storage, leading to adipose
tissue expansion (142). Enlarged
adipocytes experience metabolic stress, increased lipolysis and
altered adipokine secretion, contributing to adipose tissue
dysfunction and systemic inflammation (142). Moreover, the increased lipolysis
observed in enlarged adipocytes contributes to elevated circulating
levels of free fatty acids, which can further exacerbate metabolic
dysfunction and inflammation. Excess free fatty acids can impair
insulin signaling pathways in various tissues, including liver,
muscle and adipose tissue, promoting insulin resistance (143). Insulin resistance, a hallmark of
obesity and metabolic syndrome, is associated with increased
insulin and IGF levels, which can stimulate tumor cell
proliferation and survival through activation of the PI3K/Akt/mTOR
signaling pathway (143).
Additionally, these fatty acids serve as ligands
for Toll-like receptors (TLRs) on immune cells, triggering
inflammatory signaling cascades and promoting the secretion of
pro-inflammatory cytokines (144).
This chronic low-grade inflammation not only perpetuates adipose
tissue dysfunction but also contributes to the development of
insulin resistance and metabolic syndrome, linking obesity to
systemic metabolic complications (144,145). In lung cancer, inflammation plays
a critical role in tumor initiation and promotion (144,145). Chronic inflammation of the lungs,
often induced by factors such as smoking or environmental
pollutants, can lead to the activation of pro-inflammatory pathways
and the recruitment of immune cells to the TME (144,145). TLR activation by fatty acids
released from adipose tissue in obesity further amplifies this
inflammatory response, fueling tumor progression (144,145).
Hypoxia and inflammation
In the context of lung cancer, the inadequate
vascularization and resultant tissue hypoxia associated with
adipose tissue expansion in obesity can contribute to the
development and progression of the disease (146). Adipose tissue hypoxia is apparent
in rodent models of obesity but is still debatable in humans as
extensively reviewed elsewhere (146,147). It potentially triggers a cascade
of inflammatory responses involving the secretion of
pro-inflammatory cytokines and chemokines by hypoxic adipocytes and
infiltrating immune cells (147,148). These pro-inflammatory mediators,
including TNF-α, IL-6 and monocyte chemoattractant protein-1
(MCP-1), create a microenvironment conducive to tumor growth,
invasion and metastasis (147,148). TNF-α and IL-6, for example, are
known to promote cancer cell proliferation, survival and
angiogenesis, while MCP-1 facilitates the recruitment of
pro-tumorigenic immune cells to the tumor site (147,148). The potential pathophysiological
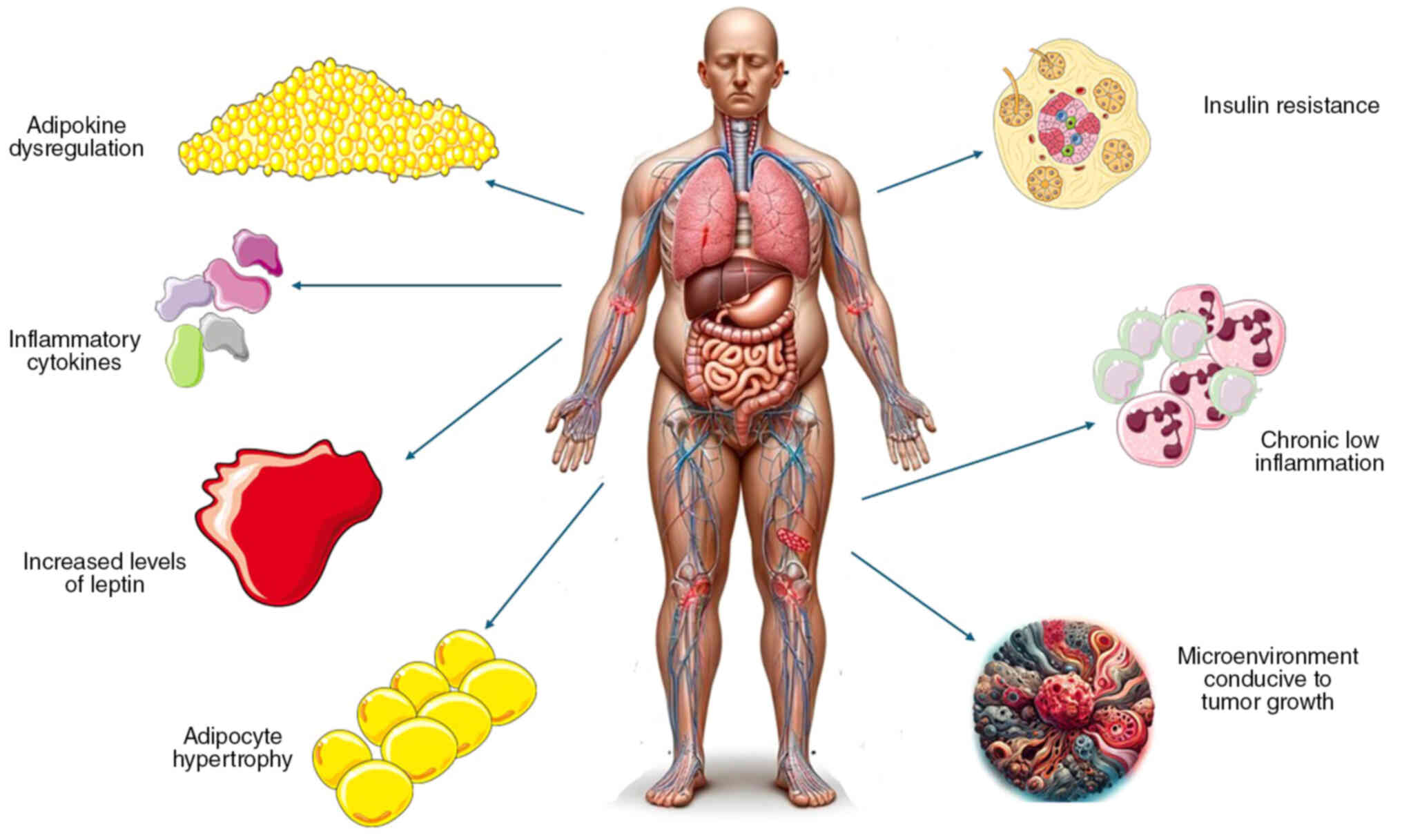
mechanisms connecting obesity and lung cancer are briefly
illustrated in Fig. 2.
Adipokine dysregulation
Adipose tissue secretes a diverse array of
adipokines, including adiponectin, leptin, resistin, visfatin and
inflammatory cytokines, which regulate energy metabolism, appetite,
insulin sensitivity and inflammation (148). Dysregulated adipokine secretion in
obesity disrupts metabolic homeostasis, promotes insulin resistance
and contributes to systemic inflammation, creating a
pro-tumorigenic microenvironment conducive to cancer development
and progression (31,149). Emerging evidence suggests that
obesity-related alterations in adipokine secretion and chronic
low-grade inflammation contribute to lung cancer development,
progression and treatment outcomes (150).
Adiponectin, an adipokine with anti-inflammatory,
anti-tumorigenic and insulin-sensitizing properties, is inversely
associated with obesity and insulin resistance (149). Adiponectin exerts
anti-proliferative and pro-apoptotic effects on lung cancer cells
through AMPK activation and inhibition of the PI3K/Akt/mTOR
signaling pathway, suggesting a protective role against lung
tumorigenesis (149–161). Reduced circulating levels of
adiponectin in obesity may contribute to increased cancer risk and
poorer outcomes in patients with lung cancer (160).
Leptin, a hormone that adipocytes primarily
secrete, controls inflammation, appetite and energy balance
(154). Elevated circulating
levels of leptin in obesity are associated with leptin resistance,
chronic inflammation and increased cancer risk (154–157). Leptin promotes lung cancer cell
proliferation, migration and invasion through the activation of the
JAK/STAT, MAPK/ERK and PI3K/Akt signaling pathways, fostering tumor
growth and metastasis (154–157).
Metabolically healthy obesity, sarcopenia,
cachexia, unhealthy lean, body fat distribution and lung
cancer
Beyond body weight alone, there are a number of
other factors that affect the complex association between obesity
and lung cancer.
Metabolically healthy obesity
Not all individuals with obesity are metabolically
unhealthy (158). Metabolically
healthy obesity (MHO) is distinguished by the lack of typical
metabolic irregularities linked with obesity, including insulin
resistance, dyslipidemia and hypertension (158). Despite excess adiposity,
individuals with MHO have preserved insulin sensitivity, favorable
lipid profiles and lower cardiovascular risk compared with
metabolically unhealthy obese individuals (158,159). The presence of individuals with
MHO in the obesity population adds complexity to understanding the
association between obesity and lung cancer, as MHO individuals may
have different cancer risk profiles and treatment responses
compared with their metabolically unhealthy counterparts (160,161).
The study by Shao et al (160) on MHO within a cohort of 450,482 UK
Biobank participants reveals that individuals with MHO have a
significantly lower risk of developing lung cancer compared with
metabolically unhealthy individuals. Specifically, MHO individuals
were found to have a 24% reduced risk of lung cancer compared with
metabolically healthy normal-weight individuals. This suggests that
despite the excess body weight, metabolic health plays a protective
role in cancer risk (160).
A systematic review and meta-analysis found that
while obesity typically correlates with higher cancer risks and
adverse outcomes, individuals with MHO exhibit a distinct risk
profile. Specifically, the study observed that individuals with MHO
tend to have a lower risk of metastasis and reduced rates of
surgical complications compared with their metabolically unhealthy
counterparts (161).
Sarcopenia
Sarcopenia, characterized by the loss of skeletal
muscle mass and function, is increasingly recognized as a
significant prognostic factor in patients with lung cancer
(162). This condition, often
associated with aging and chronic illness, can profoundly impact
treatment tolerance, response to therapy and OS rates (162). Sarcopenia is frequently present in
patients with lung cancer at the time of diagnosis, and
cancer-related cachexia, a multifactorial syndrome characterized by
involuntary weight loss, muscle wasting and systemic inflammation,
exacerbates it (163–165). The presence of sarcopenia in
patients with lung cancer has been linked to several adverse
outcomes, including increased treatment-related toxicities, higher
rates of treatment interruptions or dose reductions and poorer
surgical outcomes (163–165).
Furthermore, sarcopenia can impact treatment
response and tolerance in patients with lung cancer. Chemotherapy,
radiation therapy and surgical interventions place significant
physiological stress on the body, exacerbating muscle wasting and
compromising functional status (166,167). Reduced muscle mass and strength
may limit the ability of patients to tolerate aggressive treatment
regimens, leading to treatment delays, dose reductions or early
treatment discontinuation, which can ultimately impact survival
outcomes (166,167).
Cachexia
Cachexia is a multifactorial syndrome characterized
by unintentional weight loss, muscle wasting, anorexia and
metabolic abnormalities commonly observed in patients with advanced
cancer (168). Cachexia is
associated with increased morbidity, treatment complications and
reduced quality of life, contributing to poor treatment outcomes
and shortened survival in patients with cancer (168,169). The presence of cachexia
complicates the management of lung cancer, as it may limit
treatment options, compromise treatment efficacy and exacerbate
treatment-related toxicities, underscoring the need for early
recognition and intervention in cachectic patients with cancer
(169).
Unhealthy lean phenotype
While obesity is traditionally associated with
increased cancer risk and a poor prognosis, the unhealthy lean
phenotype, characterized by low muscle mass, a high body fat
percentage and metabolic abnormalities, may also confer elevated
cancer risk and inferior outcomes (170). Unhealthy lean individuals,
particularly those with visceral adiposity and metabolic
dysfunction, may exhibit similar cancer risk profiles and treatment
responses to individuals with obesity, highlighting the importance
of considering both adiposity and muscle mass when evaluating
cancer risk and prognosis (170).
Body fat distribution
Body fat distribution, particularly abdominal
obesity, plays a critical role in cancer development and
progression (171). Abdominal
obesity is associated with visceral adiposity and metabolic
dysfunction, characterized by increased levels of pro-inflammatory
cytokines, adipokines and insulin resistance (171). Visceral adipose tissue secretes
bioactive molecules that promote tumor growth, angiogenesis and
metastasis, contributing to the development of aggressive cancer
phenotypes, including lung cancer (171). Abdominal obesity may also
influence treatment responses and survival outcomes in patients
with lung cancer, highlighting the importance of assessing body fat
distribution in addition to overall adiposity when evaluating
cancer risk and prognosis (172).
It should be noted that BMI is not an ideal
indicator of obesity, as it does not accurately represent body
composition (173). Consequently,
studies have sought to identify body composition parameters that
more accurately reflect obesity. Previous studies have investigated
the association between treatment outcomes and body composition
parameters obtained through CT or positron emission tomography CT
in patients with NSCLC treated with ICIs (174,175). One study, for example, examined
the association between measures of skeletal muscle mass and
adiposity (including intramuscular, visceral and subcutaneous
adipose tissue) and changes during treatment, focusing on disease
progression and OS in patients with advanced lung cancer receiving
immunotherapy (176). This study
investigated the association between body composition, specifically
skeletal muscle mass and various adipose tissue measures, with
disease progression and OS in patients with advanced lung cancer
receiving ICIs. The results showed that increases in intramuscular
and subcutaneous adipose tissue by 10% were significantly
associated with improved disease-free survival and OS, while
skeletal muscle mass and visceral adipose tissue showed no such
associations. These findings suggest that changes in specific fat
deposits, rather than muscle mass, may predict improved outcomes in
patients with lung cancer undergoing immunotherapy. This unexpected
result regarding intramuscular fat highlights the need for further
research to understand its role in cancer treatment. The study
emphasizes the potential for personalized treatment strategies
based on body composition changes (176).
Other risk factors related to obesity
predisposing to lung cancer
Despite the fact that obesity is a recognized major
risk factor for numerous cancers, including lung cancer, there are
other factors besides adiposity that play a role in this
association.
Hormonal dysregulation
Obesity is associated with alterations in sex
hormone metabolism, particularly increased estrogen levels and
decreased levels of SHBG, leading to estrogen dominance and
hormonal imbalances (177). The
pivotal role of estrogen in lung cancer pathogenesis cannot be
understated, as evidenced by the higher prevalence of estrogen
receptor expression in lung tumors among women (178,179). The binding of excess estrogen to
these receptors can activate signaling pathways that promote tumor
growth and inhibit cell death, creating a conducive environment for
lung cancer development (178,179). This effect is magnified by the
pro-inflammatory state associated with obesity, further enhancing
cancer risk (178,179). This observation underscores the
significance of dysregulated sex hormone signaling in promoting the
development and progression of lung cancer, especially in
hormone-responsive tumors (178,179). Consequently, it is imperative to
recognize and account for hormonal factors when assessing the
obesity-related cancer risk (178,179).
Lifestyle factors
Obesity often coincides with unhealthy lifestyle
factors such as poor diet, physical inactivity and smoking, each of
which independently increases lung cancer risk but become
particularly dangerous when combined (180). Diets high in calories, processed
foods and sugary drinks contribute to obesity by promoting fat
accumulation and metabolic dysfunction, leading to insulin
resistance and chronic inflammation, which create a favorable
environment for cancer development (180). Physical inactivity exacerbates
these issues by reducing energy expenditure and worsening
inflammation and oxidative stress, further damaging cellular
structures and increasing the likelihood of mutations (180). Smoking, a well-established lung
cancer risk factor, interacts with the inflammatory and metabolic
disturbances caused by obesity, intensifying the effects of
carcinogens in tobacco smoke and overwhelming the ability of the
body to repair DNA damage (181).
Together, these factors create a synergistic effect that
significantly amplifies the risk of lung cancer, as the combined
impact of metabolic disruption, oxidative stress and direct DNA
damage from smoking creates a highly carcinogenic environment
(181).
Environmental exposures
Obesity may interact with environmental exposures
such as air pollution, occupational hazards and environmental
toxins to significantly heighten lung cancer risk (182,183). Airborne pollutants such as
particulate matter and polycyclic aromatic hydrocarbons, which are
established carcinogens, exert more harmful effects in individuals
with obesity because obesity is associated with chronic
inflammation and impaired detoxification processes (184,185). The persistent inflammation in
individuals with obesity creates a pro-carcinogenic environment,
where these pollutants can more easily induce DNA damage and
cellular mutations (184,185). Additionally, occupational
exposures to carcinogens such as asbestos and diesel exhaust pose
an even greater risk for individuals with obesity, as their excess
fat tissue can store fat-soluble toxins, prolonging their presence
in the body and extending their harmful effects (184,185). This prolonged exposure increases
the likelihood of sustained cellular damage, ultimately fostering
an environment that is highly conducive to lung cancer development
(184,185).
Conclusions and future perspectives
Precision medicine
Advances in molecular profiling and genomic
sequencing technologies offer unprecedented opportunities for
personalized cancer treatment. Integrating obesity-related
biomarkers, such as adipokine profiles, metabolic signatures and
genetic variants, into molecular diagnostic and prognostic models
may improve risk stratification and treatment selection for
patients with lung cancer (186).
Immunotherapy and targeted
therapies
Immunotherapy and targeted therapies like EGFR
inhibitors (such as Erlotinib), ALK inhibitors (such as Crizotinib)
and BRAF inhibitors (such as Dabrafenib) have transformed lung
cancer treatment (116).
Understanding the interplay between obesity, immune dysregulation
and tumor immunogenicity is crucial for optimizing the efficacy and
safety of immunotherapeutic approaches in patients with cancer and
obesity (187).
Lifestyle interventions
Promoting healthy lifestyle behaviors, including
weight management, physical activity and balanced nutrition, is
essential for cancer prevention and control (188). Public health initiatives targeting
obesity prevention and tobacco cessation can reduce modifiable
cancer risk factors and improve overall health outcomes in at-risk
populations (188).
Multidisciplinary care
Adopting a multidisciplinary approach to cancer
care, involving oncologists, surgeons, dietitians, exercise
physiologists and mental health professionals, is essential for
addressing the complex needs of patients with cancer and obesity
(189). Comprehensive supportive
care interventions, including nutritional counseling, physical
rehabilitation and psychosocial support, can optimize treatment
outcomes and enhance quality of life (189,190).
Environmental health
Mitigating environmental risk factors, such as air
pollution, occupational hazards and environmental toxins, is
crucial for reducing the burden of cancer in high-risk populations
(191). Collaborative efforts
between policymakers, healthcare providers and environmental
agencies are needed to implement evidence-based interventions and
regulations aimed at protecting public health and minimizing cancer
risk (191).
Existing public health initiatives aim at reducing
obesity and promoting healthy lifestyles, such as sugar-sweetened
beverage taxes, school-based nutrition programs, and front-of-pack
labeling, have shown varying degrees of effectiveness. For
instance, beverage taxes have led to reduced consumption of sugary
drinks, which could positively impact obesity rates over time
(192). Similarly, school-based
programs have improved the dietary habits and physical activity
levels of children, although their long-term impact on obesity
remains uncertain (193).
Front-of-pack labeling has helped consumers make healthier choices,
but its overall influence on reducing obesity prevalence depends on
broader dietary changes (192,193),
Future perspectives
Future research should focus on identifying
biomarkers that can predict how patients with lung cancer and
obesity will respond to various treatments, including chemotherapy,
immunotherapy and targeted therapies. These biomarkers could help
in tailoring personalized treatment plans and improving outcomes.
Another critical area is the development of therapeutic strategies
that consider the unique pathophysiological characteristics of
patients with lung cancer and obesity. This could include
optimizing drug dosing to account for altered pharmacokinetics in
obesity, as well as investigating how obesity-induced changes in
the TME affect treatment efficacy.
Long-term studies could explore how different
trajectories of obesity (such as weight loss vs. weight gain during
cancer treatment) influence lung cancer progression and patient
survival. Understanding these dynamics could lead to improved
management strategies for patients with lung cancer and obesity.
Further research could also assess the direct impact of public
health interventions on reducing obesity-related lung cancer
incidence and mortality. This would provide evidence for scaling up
successful interventions and inform future public health policies.
These aforementioned enhancements would provide a more detailed and
actionable outlook on both prevention and the future direction of
research in the association between obesity and lung cancer.
In conclusion, obesity represents a significant
risk factor for lung cancer, profoundly impacting various aspects
of the disease. It influences disease biology by contributing to
tumor development and progression through mechanisms such as
chronic inflammation, hormonal imbalances and metabolic
dysregulation. Additionally, obesity affects treatment responses,
potentially altering the efficacy and tolerability of therapies
such as chemotherapy, immunotherapy and radiotherapy. Furthermore,
the presence of obesity complicates patient outcomes, often leading
to poorer prognoses, increased treatment-related complications and
a higher likelihood of comorbid conditions. Understanding and
addressing these multifaceted impacts are essential for improving
the management and outcomes of lung cancer in populations with
obesity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DAS and VEG conceptualized the study; IGL, VEG, PS,
NT, YH and DAS made a substantial contribution to data
interpretation and analysis and wrote and prepared the draft of the
manuscript. VEG and DAS analyzed the data and provided critical
revisions. All authors contributed to manuscript revision. All
authors read approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, the artificial
intelligence tool Chat GPT was used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence tool
as necessary, taking full responsibility for the ultimate content
of the present manuscript.
References
|
1
|
Chooi YC, Ding C and Magkos F: The
epidemiology of obesity. Metabolism. 92:6–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hruby A and Hu FB: The epidemiology of
obesity: A big picture. Pharmacoeconomics. 33:673–689. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji X, Chen J, Ye J, Xu S, Lin B and Hou K:
Epidemiological analysis of global and regional lung cancer
mortality: Based on 30-year data analysis of global burden disease
database. Healthcare (Basel). 11:29202023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
6
|
Khaddour K, Gomez-Perez SL, Jain N, Patel
JD and Boumber Y: Obesity, sarcopenia, and outcomes in non-small
cell lung cancer patients treated with immune checkpoint inhibitors
and tyrosine kinase inhibitors. Front Oncol. 10:5763142020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrelli F, Cortellini A, Indini A,
Tomasello G, Ghidini M, Nigro O, Salati M, Dottorini L, Iaculli A,
Varricchio A, et al: Association of obesity with survival outcomes
in patients with cancer: A systematic review and meta-analysis.
JAMA Netw Open. 4:e2135202021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Liu Y, Shao H and Zheng X:
Obesity paradox in lung cancer prognosis: Evolving biological
insights and clinical implications. J Thorac Oncol. 12:1478–1488.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lennon H, Sperrin M, Badrick E and Renehan
AG: The obesity paradox in cancer: A review. Curr Oncol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawai T, Autieri MV and Scalia R: Adipose
tissue inflammation and metabolic dysfunction in obesity. Am J
Physiol Cell Physiol. 320:C375–C391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assumpção JAF, Pasquarelli-do-Nascimento
G, Duarte MSV, Bonamino MH and Magalhães KG: The ambiguous role of
obesity in oncology by promoting cancer but boosting antitumor
immunotherapy. J Biomed Sci. 29:122022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barbi J, Patnaik SK, Pabla S, Zollo R,
Smith RJ Jr, Sass SN, Srinivasan A, Petrucci C, Seager R, Conroy J,
et al: Visceral obesity promotes lung cancer progression-toward
resolution of the obesity paradox in lung cancer. J Thorac Oncol.
16:1333–1348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen HL, Geukens T, Maetens M, Aparicio
S, Bassez A, Borg A, Brock J, Broeks A, Caldas C, Cardoso F, et al:
Obesity-associated changes in molecular biology of primary breast
cancer. Nat Commun. 14:44182023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pati S, Irfan W, Jameel A, Ahmed S and
Shahid RK: Obesity and cancer: A current overview of epidemiology,
pathogenesis, outcomes, and management. Cancers (Basel).
15:4852023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Dong J, Sun K, Zhao L, Zhao F,
Wang L and Jiao Y: Obesity and incidence of lung cancer: A
meta-analysis. Int J Cancer. 132:1162–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi EK, Park HB, Lee KH, Park JH,
Eisenhut M, van der Vliet HJ, Kim G and Shin JI: Body mass index
and 20 specific cancers: Re-analyses of dose-response meta-analyses
of observational studies. Ann Oncol. 29:749–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Z, Xie S, Wang F, Chen S, Su K, Li F,
Cui H, Cao W, Yu Y, Qin C, et al: BMI changes and the risk of lung
cancer in male never-smokers: A prospective cohort study. Cancer
Med. 11:1336–1346. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu D, Zheng W, Johansson M, Lan Q, Park Y,
White E, Matthews CE, Sawada N, Gao YT, Robien K, et al: Overall
and central obesity and risk of lung cancer: A pooled analysis. J
Natl Cancer Inst. 110:831–842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu H and Zhang S: Body mass index and
lung cancer risk in never smokers: A meta-analysis. BMC Cancer.
18:6352018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Xu H, Zhou S, Wang D, Zhu L, Hou
J, Tang J, Zhao J and Zhong S: Body mass index and mortality in
lung cancer patients: A systematic review and meta-analysis. Eur J
Clin Nutr. 72:4–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neeland IJ, Poirier P and Després JP:
Cardiovascular and metabolic heterogeneity of obesity: Clinical
challenges and implications for management. Circulation.
137:1391–1406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caan BJ, Cespedes Feliciano EM and Kroenke
CH: The importance of body composition in explaining the overweight
paradox in cancer-counterpoint. Cancer Res. 78:1906–1912. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan M and Joseph F: Adipose tissue and
adipokines: The association with and application of adipokines in
obesity. Scientifica (Cairo). 2014:3285922014.PubMed/NCBI
|
|
24
|
Liu X, Yin L, Shen S and Hou Y:
Inflammation and cancer: Paradoxical roles in tumorigenesis and
implications in immunotherapies. Genes Dis. 10:151–164. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kahn BB and Flier JS: Obesity and insulin
resistance. J Clin Invest. 106:473–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leroith D, Scheinman EJ and Bitton-Worms
K: The role of insulin and insulin-like growth factors in the
increased risk of cancer in diabetes. Rambam Maimonides Med J.
2:e00432011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong W, Wang X, Wang Y, Sun G, Zhang J
and Li Z: Obesity and endocrine-related cancer: The important role
of IGF-1. Front Endocrinol (Lausanne). 14:10932572023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rodriguez-Lara V and Avila-Costa MR: An
overview of lung cancer in women and the impact of estrogen in lung
carcinogenesis and lung cancer treatment. Front Med (Lausanne).
8:6001212021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Savini I, Catani MV, Evangelista D,
Gasperi V and Avigliano L: Obesity-associated oxidative stress:
Strategies finalized to improve redox state. Int J Mol Sci.
14:10497–10538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Usman M and Volpi EV: DNA damage in
obesity: Initiator, promoter and predictor of cancer. Mutat Res Rev
Mutat Res. 778:23–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JW, Kim JH and Lee YJ: The role of
adipokines in tumor progression and its association with obesity.
Biomedicines. 12:972024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarfati D, Koczwara B and Jackson C: The
impact of comorbidity on cancer and its treatment. CA Cancer J
Clin. 66:337–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Søgaard M, Thomsen RW, Bossen KS, Sørensen
HT and Nørgaard M: The impact of comorbidity on cancer survival: A
review. Clin Epidemiol. 5 (Suppl 1):S3–S29. 2013. View Article : Google Scholar
|
|
34
|
Al-Jawaldeh A and Abbass MMS: Unhealthy
dietary habits and obesity: The major risk factors beyond
non-communicable diseases in the eastern mediterranean region.
Front Nutr. 9:8178082022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Washington TB, Johnson VR, Kendrick K,
Ibrahim AA, Tu L, Sun K and Stanford FC: Disparities in access and
quality of obesity care. Gastroenterol Clin North Am. 52:429–441.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong A, Nie W, Cheng L, Zhong H, Chu T,
Zhong R, Lu J, Wang S, Xu J, Shen Y, et al: Association between
obesity and poor prognosis in patients receiving anlotinib for
advanced non-small cell lung cancer. Front Pharmacol.
13:8125552022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nitsche L, Vedire Y, Kannisto E, Wang X,
Seager RJ, Pabla S, Patnaik SK and Yendamuri S: Visceral Obesity in
non-small cell lung cancer. Cancers (Basel). 14:34502022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CH, Lin C, Wang CY, Huang TC, Wu YY,
Chien WC and Chen JH: Premorbid BMI as a prognostic factor in
small-cell lung cancer-a single institute experience. Oncotarget.
9:24642–24652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kwon YJ, Yoon YC, Kim HS, Cha MJ, Park S
and Lee JH: Prognostic significance of body mass index in
small-cell lung cancer: Exploring the relationship with skeletal
muscle status. J Cachexia Sarcopenia Muscle. 14:2939–2947. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sutandyo N, Hanafi AR, Jayusman AM,
Kurniawati SA and Hanif MA: Overweight and Obesity are associated
with poorer survival among patients with advanced non-small cell
lung cancer receiving platinum-based chemotherapy. Int J Gen Med.
16:85–93. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tu H, McQuade JL, Davies MA, Huang M, Xie
K, Ye Y, Chow WH, Rodriguez A and Wu X: Body mass index and
survival after cancer diagnosis: A pan-cancer cohort study of 114
430 patients with cancer. Innovation (Camb).
3:1003442022.PubMed/NCBI
|
|
42
|
Booth A, Magnuson A, Fouts J and Foster M:
Adipose tissue, obesity and adipokines: Role in cancer promotion.
Horm Mol Biol Clin Investig. 21:57–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bocian-Jastrzębska A, Malczewska-Herman A
and Kos-Kudła B: Role of leptin and adiponectin in carcinogenesis.
Cancers (Basel). 15:42502023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ramos-Nino ME: The role of chronic
inflammation in obesity-associated cancers. ISRN Oncol.
2013:6975212013.PubMed/NCBI
|
|
45
|
Wen X, Zhang B, Wu B, Xiao H, Li Z, Li R,
Xu X and Li T: Signaling pathways in obesity: Mechanisms and
therapeutic interventions. Signal Transduct Target Ther. 7:2982022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lam VK, Bentzen SM, Mohindra P, Nichols
EM, Bhooshan N, Vyfhuis M, Scilla KA, Feigenberg SJ, Edelman MJ and
Feliciano JL: Obesity is associated with long-term improved
survival in definitively treated locally advanced non-small cell
lung cancer (NSCLC). Lung Cancer. 104:52–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gallagher EJ and LeRoith D: Insulin,
insulin resistance, obesity, and cancer. Curr Diab Rep. 10:93–100.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukumoto K, Mori S, Shintani Y, Okami J,
Ito H, Ohtsuka T, Toyooka S, Mori T, Watanabe SI, Asamura H, et al:
Impact of the preoperative body mass index on the postoperative
outcomes in patients with completely resected non-small cell lung
cancer: A retrospective analysis of 16,503 cases in a Japanese lung
cancer registry study. Lung Cancer. 149:120–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Rourke RW: Obesity and cancer: At the
crossroads of cellular metabolism and proliferation. Surg Obes
Relat Dis. 10:1208–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li M and Bu R: Biological support to
obesity paradox in renal cell carcinoma: A review. Urol Int.
104:837–848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Björndahl M, Cao R, Nissen LJ, Clasper S,
Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ and Cao Y:
Insulin-like growth factors 1 and 2 induce lymphangiogenesis in
vivo. Proc Natl Acad Sci USA. 102:15593–15598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Q, Tu H, Zhu M, Liang D, Ye Y, Chang
DW, Long Y and Wu X: Circulating obesity-driven biomarkers are
associated with risk of clear cell renal cell carcinoma: A
two-stage, case-control study. Carcinogenesis. 40:1191–1197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kamat AM, Shock RP, Naya Y, Rosser CJ,
Slaton JW and Pisters LL: Prognostic value of body mass index in
patients undergoing nephrectomy for localized renal tumors.
Urology. 63:46–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Albiges L, Hakimi AA, Xie W, McKay RR,
Simantov R, Lin X, Lee JL, Rini BI, Srinivas S, Bjarnason GA, et
al: Body mass index and metastatic renal cell carcinoma: Clinical
and biological correlations. J Clin Oncol. 34:3655–3663. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi Y, Park B, Jeong BC, Seo SI, Jeon SS,
Choi HY, Adami HO, Lee JE and Lee HM: Body mass index and survival
in patients with renal cell carcinoma: A clinical-based cohort and
meta-analysis. Int J Cancer. 132:625–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jeon HG, Jeong IG, Lee JH, Lee CJ, Kwak C,
Kim HH, Lee SE and Lee E: Prognostic value of body mass index in
Korean patients with renal cell carcinoma. J Urol. 183:448–454.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choueiri TK, Xie W, Kollmannsberger CK,
Rini BI, McDermott DF, Knox JJ and Heng DY: The impact of body mass
index (BMI) and body surface area (BSA) on treatment outcome to
vascular endothelial growth factor (VEGF)-targeted therapy in
metastatic renal cell carcinoma: Results from a large international
collaboration. J Clin Oncol. 28 (15 Suppl):S45242010. View Article : Google Scholar
|
|
58
|
Naik GS, Waikar SS, Johnson AEW,
Buchbinder EI, Haq R, Hodi FS, Schoenfeld JD and Ott PA: Complex
inter-relationship of body mass index, gender and serum creatinine
on survival: Exploring the obesity paradox in melanoma patients
treated with checkpoint inhibition. J Immunother Cancer. 7:892019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Donnelly D, Bajaj S, Yu J, Hsu M, Balar A,
Pavlick A, Weber J, Osman I and Zhong J: The complex relationship
between body mass index and response to immune checkpoint
inhibition in metastatic melanoma patients. J Immunother Cancer.
7:2222019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pencheva N, Buss C, Posada J, Merghoub T
and Tavazoie SF: Broad-spectrum therapeutic suppression of
metastatic melanoma through nuclear hormone receptor activation.
Cell. 156:986–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
McQuade JL, Daniel CR, Hess KR, Mak C,
Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al:
Association of body-mass index and outcomes in patients with
metastatic melanoma treated with targeted therapy, immunotherapy,
or chemotherapy: A retrospective, multicohort analysis. Lancet
Oncol. 19:310–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Z, Aguilar EG, Luna JI, Dunai C,
Khuat LT, Le C, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et
al: Paradoxical effects of obesity on T cell function during tumor
progression and PD-1 checkpoint blockade. Nat Med. 25:141–151.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Georgiadis MS, Steinberg SM, Hankins LA,
Ihde DC and Johnson BE: Obesity and therapy-related toxicity in
patients treated for small-cell lung cancer. J Natl Cancer Inst.
87:361–366. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nattenmüller J, Wochner R, Muley T, Steins
M, Hummler S, Teucher B, Wiskemann J, Kauczor HU, Wielpütz MO and
Heussel CP: Prognostic impact of CT-quantified muscle and fat
distribution before and after first-line-chemotherapy in lung
cancer patients. PLoS One. 12:e01691362017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shepshelovich D, Xu W, Lu L, Fares A, Yang
P, Christiani D, Zhang J, Shiraishi K, Ryan BM, Chen C, et al: Body
mass index (BMI), BMI change, and overall survival in patients with
SCLC and NSCLC: A pooled analysis of the international lung cancer
consortium. J Thorac Oncol. 14:1594–1607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Icard P, Schussler O, Loi M, Bobbio A,
Lupo AM, Wislez M, Iannelli A, Fournel L, Damotte D and Alifano M:
Pre-disease and pre-surgery BMI, weight loss and sarcopenia impact
survival of resected lung cancer independently of tumor stage.
Cancers (Basel). 12:2662020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang M, Fares AF, Shepshelovich D, Yang
P, Christiani D, Zhang J, Shiraishi K, Ryan BM, Chen C, Schwartz
AG, et al: The relationship between body-mass index and overall
survival in non-small cell lung cancer by sex, smoking status, and
race: A pooled analysis of 20,937 international lung cancer
consortium (ILCCO) patients. Lung Cancer. 152:58–65. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bethea TN, Rosenberg L, Charlot M,
O'Connor GT, Adams-Campbell LL and Palmer JR: Obesity in relation
to lung cancer incidence in African American women. Cancer Causes
Control. 24:1695–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim HR, Kim SY, Kim CH, Yang SH, Lee JC,
Choi CM and Na II: Sex-specific incidence of EGFR mutation and its
association with age and obesity in lung adenocarcinomas: A
retrospective analysis. J Cancer Res Clin Oncol. 143:2283–2290.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gremese E, Tolusso B, Gigante MR and
Ferraccioli G: Obesity as a risk and severity factor in rheumatic
diseases (autoimmune chronic inflammatory diseases). Front Immunol.
5:5762014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Siopis G: Obesity: A comorbidity-acquired
immunodeficiency syndrome (CAIDS). Int Rev Immunol. 42:415–429.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cao Y: Adipocyte and lipid metabolism in
cancer drug resistance. J Clin Invest. 129:3006–3017. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Havas KM, Milchevskaya V, Radic K, Alladin
A, Kafkia E, Garcia M, Stolte J, Klaus B, Rotmensz N, Gibson TJ, et
al: Metabolic shifts in residual breast cancer drive tumor
recurrence. J Clin Invest. 127:2091–2105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Iwamoto H, Abe M, Yang Y, Cui D, Seki T,
Nakamura M, Hosaka K, Lim S, Wu J, He X, et al: Cancer lipid
metabolism confers antiangiogenic drug resistance. Cell Metab.
28:104–117.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hoy AJ, Nagarajan SR and Butler LM: Tumour
fatty acid metabolism in the context of therapy resistance and
obesity. Nat Rev Cancer. 21:753–766. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Camarda R, Zhou AY, Kohnz RA, Balakrishnan
S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G, et
al: Inhibition of fatty acid oxidation as a therapy for
MYC-overexpressing triple-negative breast cancer. Nat Med.
22:427–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang Q, Wang Q, Li D, Wei X, Jia Y, Zhang
Z, Ai B, Cao X, Guo T and Liao Y: Co-administration of
20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI
resistance by decreasing SCD1 induced lipid accumulation in
non-small cell lung cancer. J Exp Clin Cancer Res. 38:1292019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Watt MJ, Clark AK, Selth LA, Haynes VR,
Lister N, Rebello R, Porter LH, Niranjan B, Whitby ST, Lo J, et al:
Suppressing fatty acid uptake has therapeutic effects in
preclinical models of prostate cancer. Sci Transl Med.
11:eaau57582019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pascual G, Avgustinova A, Mejetta S,
Martin M, Castellanos A, Attolini CSO, Berenguer A, Prats N, Toll
A, Hueto JA, et al: Targeting metastasis-initiating cells through
the fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Buckley D, Duke G, Heuer TS, O'Farrell M,
Wagman AS, McCulloch W and Kemble G: Fatty acid synthase-Modern
tumor cell biology insights into a classical oncology target.
Pharmacol Ther. 177:23–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zaytseva YY, Rychahou PG, Le AT, Scott TL,
Flight RM, Kim JT, Harris J, Liu J, Wang C, Morris AJ, et al:
Preclinical evaluation of novel fatty acid synthase inhibitors in
primary colorectal cancer cells and a patient-derived xenograft
model of colorectal cancer. Oncotarget. 9:24787–24800. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sheng X, Parmentier JH, Tucci J, Pei H,
Cortez-Toledo O, Dieli-Conwright CM, Oberley MJ, Neely M, Orgel E,
Louie SG and Mittelman SD: Adipocytes sequester and metabolize the
chemotherapeutic daunorubicin. Mol Cancer Res. 15:1704–1713. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bauerschlag DO, Maass N, Leonhardt P,
Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba
R, Meinhold-Heerlein I and Bräutigam K: Fatty acid synthase
overexpression: Target for therapy and reversal of chemoresistance
in ovarian cancer. J Transl Med. 13:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Papaevangelou E, Almeida GS, Box C,
deSouza NM and Chung YL: The effect of FASN inhibition on the
growth and metabolism of a cisplatin-resistant ovarian carcinoma
model. Int J Cancer. 143:992–1002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Altieri DC: Mitochondria on the move:
Emerging paradigms of organelle trafficking in tumour plasticity
and metastasis. Br J Cancer. 117:301–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang H, Franco F, Tsui YC, Xie X, Trefny
MP, Zappasodi R, Mohmood SR, Fernández-García J, Tsai CH, Schulze
I, et al: CD36-mediated metabolic adaptation supports regulatory T
cell survival and function in tumors. Nat Immunol. 21:298–308.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ladanyi A, Mukherjee A, Kenny HA, Johnson
A, Mitra AK, Sundaresan S, Nieman KM, Pascual G, Benitah SA, Montag
A, et al: Adipocyte-induced CD36 expression drives ovarian cancer
progression and metastasis. Oncogene. 37:2285–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yuan H, Kopelovich L, Yin Y, Lu J and
Glazer RI: Drug-targeted inhibition of peroxisome
proliferator-activated receptor-gamma enhances the chemopreventive
effect of anti-estrogen therapy. Oncotarget. 3:345–356. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cheng S, Qian K, Wang Y, Wang G, Liu X,
Xiao Y and Wang X: PPARγ inhibition regulates the cell cycle,
proliferation and motility of bladder cancer cells. J Cell Mol Med.
23:3724–3736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lou G, Song X, Yang F, Wu S, Wang J, Chen
Z and Lu Y: Exosomes derived from miR-122-modified adipose
tissue-derived MSCs increase chemosensitivity of hepatocellular
carcinoma. J Hematol Oncol. 8:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Au Yeung CL, Co NN, Tsuruga T, Yeung TL,
Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al: Exosomal
transfer of stroma-derived miR21 confers paclitaxel resistance in
ovarian cancer cells through targeting APAF1. Nat Commun.
7:111502016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Polanski R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xie H, Hanai J, Ren JG, Kats L, Burgess K,
Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, et al:
Targeting lactate dehydrogenase-a inhibits tumorigenesis and tumor
progression in mouse models of lung cancer and impacts
tumor-initiating cells. Cell Metab. 19:795–809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Karakas Y, Esin E, Lacin S, Ceyhan K,
Heper AO and Yalcin S: A case of acanthosis nigricans as a
paraneoplastic syndrome with squamous cell lung cancer. Onco
Targets Ther. 9:4815–4820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lawrenson R, Lao C, Brown L, Moosa L,
Chepulis L, Keenan R, Kidd J, Middleton K, Conaglen P, de Groot C,
et al: Management of patients with early stage lung cancer-why do
some patients not receive treatment with curative intent? BMC
Cancer. 20:1092020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Plassmeier L, Hankir MK and Seyfried F:
Impact of excess body weight on postsurgical complications. Visc
Med. 37:287–297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tjeertes EKM, Hoeks SE, Beks SBJ,
Valentijn TM, Hoofwijk AGM and Stolker RJ: Obesity-a risk factor
for postoperative complications in general surgery? BMC
Anesthesiol. 15:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hodgson LE, Murphy PB and Hart N:
Respiratory management of the obese patient undergoing surgery. J
Thorac Dis. 7:943–952. 2015.PubMed/NCBI
|
|
99
|
Pierpont YN, Dinh TP, Salas RE, Johnson
EL, Wright TG, Robson MC and Payne WG: Obesity and surgical wound
healing: A current review. ISRN Obes. 2014:6389362014.PubMed/NCBI
|
|
100
|
De Camilli AR, Cadwell JB, Weiss H,
Tollinche LE, McFarlane D, Broach V, Leitao MM, Kitzler R and
Afonso AM: Perioperative considerations for cancer patients with
obesity: A narrative review. Trends Anaesth Crit Care. 46:33–41.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lang LH, Parekh K, Tsui BYK and Maze M:
Perioperative management of the obese surgical patient. Br Med
Bull. 124:135–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vedire Y, Kalvapudi S and Yendamuri S:
Obesity and lung cancer-a narrative review. J Thorac Dis.
15:2806–2823. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Alifano M, Daffré E, Iannelli A, Brouchet
L, Falcoz PE, Le Pimpec Barthes F, Bernard A, Pages PB, Thomas PA,
Dahan M and Porcher R: The reality of lung cancer paradox: The
impact of body mass index on long-term survival of resected lung
cancer. A French nationwide analysis from the epithor database.
Cancers (Basel). 13:45742021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tong C, Li T, Shen Y, Zhu H, Zheng J and
Wu J: Obesity does not increase perioperative outcomes in older
patients undergoing thoracoscopic anatomic lung cancer surgery.
Front Oncol. 12:8814672022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Guerrera F, Lyberis P, Lausi PO,
Cristofori RC, Giobbe R, Molinatti M, Filosso PL, Curcio C, Crisci
R and Ruffini E; on the behalf of the Italian VATS Group, : Does
morbid obesity influence perioperative outcomes after
video-assisted thoracic surgery (VATS) lobectomy for non-small cell
lung cancer? Analysis of the Italian VATS group registry. Surg
Endosc. 36:3567–3573. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee JH, Yoon YC and Kim HS, Cha MJ, Kim
JH, Kim K and Kim HS: Obesity is associated with improved
postoperative overall survival, independent of skeletal muscle mass
in lung adenocarcinoma. J Cachexia Sarcopenia Muscle. 13:1076–1086.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tulinský L, Sengul I, Ihnát P, Ostruszka
P, Toman D, Guňková P, Pelikán A and Sengul D: Obesity in cases
undergoing the surgical procedure of lung lobectomy: Risk or
benefit? Rev Assoc Med Bras (1992). 68:1090–1095. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Seder CW, Farrokhyar F, Nayak R, Baste JM,
Patel Y, Agzarian J, Finley CJ, Shargall Y, Thomas PA, Dahan M, et
al: Robotic vs thoracoscopic anatomic lung resection in obese
patients: A propensity-adjusted analysis. Ann Thorac Surg.
114:1879–1885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Leonardi B, Forte S, Natale G, Messina G,
Rainone A, Opromolla G, Puca MA, Grande M, Martone M, Leone F, et
al: One-lung ventilation in obese patients undergoing thoracoscopic
lobectomy for lung cancer. Thorac Cancer. 14:281–288. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Silvestris N, Argentiero A, Natalicchio A,
D'Oronzo S, Beretta GD, Acquati S, Adinolfi V, Di Bartolo P, Danesi
R, Faggiano A, et al: Antineoplastic dosing in overweight and obese
cancer patients: an associazione italiana oncologia medica
(AIOM)/associazione medici diabetologi (AMD)/Società Italiana
Endocrinologia (SIE)/Società Italiana Farmacologia (SIF)
multidisciplinary consensus position paper. ESMO Open.
6:1001532021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Barras M and Legg A: Drug dosing in obese
adults. Aust Prescr. 40:189–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Griggs JJ, Bohlke K, Balaban EP, Dignam
JJ, Hall ET, Harvey RD, Hecht DP, Klute KA, Morrison VA, Pini TM,
et al: Appropriate systemic therapy dosing for obese adult patients
with cancer: ASCO guideline update. J Clin Oncol. 39:2037–2048.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dent SF, Kikuchi R, Kondapalli L,
Ismail-Khan R, Brezden-Masley C, Barac A and Fradley M: Optimizing
cardiovascular health in patients with cancer: A practical review
of risk assessment, monitoring, and prevention of cancer
treatment-related cardiovascular toxicity. Am Soc Clin Oncol Educ
Book. 40:1–15. 2020.PubMed/NCBI
|
|
114
|
Kicken MP, Kilinc HD, Cramer-van der Welle
CM, Houterman S, van den Borne BEEM, Smit AAJ, van de Garde EMW and
Deenen MJ; Santeon NSCLC study group, : The association of body
mass index with safety and effectiveness of first-line
carboplatin-based chemotherapy in patients with metastatic
non-small cell lung cancer. Cancer Treat Res Commun. 34:1006762023.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kashiwabara K, Yamane H and Tanaka H:
Toxicity and prognosis in overweight and obese women with lung
cancer receiving carboplatin-paclitaxel doublet chemotherapy.
Cancer Invest. 31:251–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Georgakopoulou VE, Garmpis N, Mermigkis D,
Damaskos C, Chlapoutakis S, Mantzouranis K, Gkoufa A, Papageorgiou
C, Garmpi A, Makrodimitri S, et al: Pulmonary adverse events due to
immune checkpoint inhibitors: A literature review. Monaldi Arch
Chest Dis. 92:2021.PubMed/NCBI
|
|
117
|
Vick LV, Canter RJ, Monjazeb AM and Murphy
WJ: Multifaceted effects of obesity on cancer immunotherapies:
Bridging preclinical models and clinical data. Semin Cancer Biol.
95:88–102. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Woodall MJ, Neumann S, Campbell K,
Pattison ST and Young SL: The effects of obesity on anti-cancer
immunity and cancer immunotherapy. Cancers (Basel). 12:12302020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sanhueza S, Simón L, Cifuentes M and Quest
AFG: The adipocyte-macrophage relationship in cancer: A potential
target for antioxidant therapy. Antioxidants (Basel). 12:1262023.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hahn AW, Venkatesh N, Msaouel P and
McQuade JL: The influence of obesity on outcomes with immune
checkpoint blockade: Clinical evidence and potential biological
mechanisms. Cells. 12:25512023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Guo H, Lin XY, Feng S, Wang C, Yuan LQ,
Sheng XG and Li DP: Prognostic value of obesity in patients with
cancer treated with immune checkpoint inhibitors: An updated
meta-analysis and systematic review. Mol Clin Oncol. 20:52023.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Leiter A, Carroll E, De Alwis S, Brooks D,
Shimol JB, Eisenberg E, Wisnivesky JP, Galsky MD and Gallagher EJ:
Metabolic disease and adverse events from immune checkpoint
inhibitors. Eur J Endocrinol. 184:857–865. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang T, Li S, Chang J, Qin Y and Li C:
Impact of BMI on the survival outcomes of non-small cell lung
cancer patients treated with immune checkpoint inhibitors: A
meta-analysis. BMC Cancer. 23:10232023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Winters E and Poole C: Challenges and
impact of patient obesity in radiation therapy practice.
Radiography (Lond). 26:e158–e163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mercieca S, Belderbos JSA and van Herk M:
Challenges in the target volume definition of lung cancer
radiotherapy. Transl Lung Cancer Res. 10:1983–1998. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ross KH, Gogineni K, Subhedar PD, Lin JY
and McCullough LE: Obesity and cancer treatment efficacy: Existing
challenges and opportunities. Cance. 125:1588–1592. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang K and Tepper JE: Radiation
therapy-associated toxicity: Etiology, management, and prevention.
CA Cancer J Clin. 71:437–454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Welsh J, Thomas J, Shah D, Allen PK, Wei
X, Mitchell K, Gao S, Balter P, Komaki R and Chang JY: Obesity
increases the risk of chest wall pain from thoracic stereotactic
body radiation therapy. Int J Radiat Oncol Biol Phys. 81:91–96.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lempesis IG, Karlafti E, Papalexis P,
Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas
E and Georgakopoulou VE: COVID-19 and liver injury in individuals
with obesity. World J Gastroenterol. 29:908–916. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lempesis IG and Georgakopoulou VE:
Implications of obesity and adiposopathy on respiratory infections;
focus on emerging challenges. World J Clin Cases. 11:2925–2933.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lempesis IG, Varrias D, Sagris M, Attaran
RR, Altin ES, Bakoyiannis C, Palaiodimos L, Dalamaga M and
Kokkinidis DG: Obesity and peripheral artery disease: Current
evidence and controversies. Curr Obes Rep. 12:264–279. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lempesis IG, Georgakopoulou VE, Papalexis
P, Chrousos GP and Spandidos DA: Role of stress in the pathogenesis
of cancer (Review). Int J Oncol. 63:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lempesis IG, Hoebers N, Essers Y, Jocken
JWE, Dineen R, Blaak EE, Manolopoulos KN and Goossens GH: Distinct
inflammatory signatures of upper and lower body adipose tissue and
adipocytes in women with normal weight or obesity. Front Endocrinol
(Lausanne). 14:12057992023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lempesis IG, van Meijel RLJ, Manolopoulos
KN and Goossens GH: Oxygenation of adipose tissue: A human
perspective. Acta Physiol (Oxf). 228:e132982020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lempesis IG and Georgakopoulou VE:
Physiopathological mechanisms related to inflammation in obesity
and type 2 diabetes mellitus. World J Exp Med. 13:7–16. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lempesis IG, Liu J and Dalamaga M: The
catcher in the gut: Tirzepatide, a dual incretin analog for the
treatment of type 2 diabetes mellitus and obesity. Metabol Open.
16:1002202022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lempesis IG, Tsilingiris D, Liu J and
Dalamaga M: Of mice and men: Considerations on adipose tissue
physiology in animal models of obesity and human studies. Metabol
Open. 15:1002082022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Georgakopoulou VE, Lempesis IG and
Spandidos DA: The parallel lives of pandemics: COVID-19 and
obesity. Exp Ther Med. 27:1842024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Longo M, Zatterale F, Naderi J, Parrillo
L, Formisano P, Raciti GA, Beguinot F and Miele C: Adipose tissue
dysfunction as determinant of obesity-associated metabolic
complications. Int J Mol Sci. 20:23582019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Daquinag AC, Tseng C, Zhang Y,
Amaya-Manzanares F, Florez F, Dadbin A, Zhang T and Kolonin MG:
Targeted proapoptotic peptides depleting adipose stromal cells
inhibit tumor growth. Mol Ther. 24:34–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jin X, Qiu T, Li L, Yu R, Chen X, Li C,
Proud CG and Jiang T: Pathophysiology of obesity and its associated
diseases. Acta Pharm Sin B. 13:2403–2424. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Capurso C and Capurso A: From excess
adiposity to insulin resistance: The role of free fatty acids.
Vascul Pharmacol. 57:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Jung UJ and Choi MS: Obesity and its
metabolic complications: The role of adipokines and the
relationship between obesity, inflammation, insulin resistance,
dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci.
15:6184–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cavaliere G, Cimmino F, Trinchese G,
Catapano A, Petrella L, D'Angelo M, Lucchin L and Mollica MP: From
obesity-induced low-grade inflammation to lipotoxicity and
mitochondrial dysfunction: Altered multi-crosstalk between adipose
tissue and metabolically active organs. Antioxidants (Basel).
12:11722023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Divella R, De Luca R, Abbate I, Naglieri E
and Daniele A: Obesity and cancer: The role of adipose tissue and
adipo-cytokines-induced chronic inflammation. J Cancer.
7:2346–2359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Himbert C, Delphan M, Scherer D, Bowers
LW, Hursting S and Ulrich CM: Signals from the adipose
microenvironment and the obesity-cancer link-a systematic review.
Cancer Prev Res (Phila). 10:494–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fantuzzi G: Adipose tissue, adipokines,
and inflammation. J Allergy Clin Immunol. 115:911–920. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Louie SM, Roberts LS and Nomura DK:
Mechanisms linking obesity and cancer. Biochim Biophys Acta.
1831:1499–1508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dalamaga M, Diakopoulos KN and Mantzoros
CS: The role of adiponectin in cancer: A review of current
evidence. Endocr Rev. 33:547–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Nigro E, Scudiero O, Monaco ML, Palmieri
A, Mazzarella G, Costagliola C, Bianco A and Daniele A: New insight
into adiponectin role in obesity and obesity-related diseases.
Biomed Res Int. 2014:6589132014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Parida S, Siddharth S and Sharma D:
Adiponectin, obesity, and cancer: Clash of the bigwigs in health
and disease. Int J Mol Sci. 20:25192019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Dutta D, Ghosh S, Pandit K, Mukhopadhyay P
and Chowdhury S: Leptin and cancer: Pathogenesis and modulation.
Indian J Endocrinol Metab. 16 (Suppl 3):S596–S600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lang K and Ratke J: Leptin and
Adiponectin: New players in the field of tumor cell and leukocyte
migration. Cell Commun Signal. 7:272009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Iikuni N, Lam QLK, Lu L, Matarese G and La
Cava A: Leptin and inflammation. Curr Immunol Rev. 4:70–79. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mullen M and Gonzalez-Perez RR:
Leptin-induced JAK/STAT signaling and cancer growth. Vaccines
(Basel). 4:262016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Cho YK, Lee Y and Jung CH: Pathogenesis,
murine models, and clinical implications of metabolically healthy
obesity. Int J Mol Sci. 23:96142022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Blüher M: Metabolically healthy obesity.
Endocr Rev. 41:bnaa0042020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lin TY, Chen YF, Wu WT, Han DS, Tsai IC,
Chang KV and Özçakar L: Impact of sarcopenia on the prognosis and
treatment of lung cancer: An umbrella review. Discov Oncol.
13:1152022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Shao F, Chen Y, Xu H, Chen X, Zhou J, Wu
Y, Tang Y, Wang Z, Zhang R, Lange T, et al: Metabolic obesity
phenotypes and risk of lung cancer: A prospective cohort study of
450,482 UK Biobank participants. Nutrients. 14:33702022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Malki A, Shaik RA and Sami W: Association
between metabolically healthy obesity and metastasis in lung cancer
patients-a systematic review and meta-analysis. Front Endocrinol
(Lausanne). 14:12384592023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Sørensen J: Lung cancer cachexia: Can
molecular understanding guide clinical management? Integr Cancer
Ther. 17:1000–1008. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Anjanappa M, Corden M, Green A, Roberts D,
Hoskin P, McWilliam A and Choudhury A: Sarcopenia in cancer:
Risking more than muscle loss. Tech Innov Patient Support Radiat
Oncol. 16:50–57. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Peixoto da Silva S, Santos JMO, Costa E
Silva MP, Gil da Costa RM and Medeiros R: Cancer cachexia and its
pathophysiology: Links with sarcopenia, anorexia and asthenia. J
Cachexia Sarcopenia Muscle. 11:619–635. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kim EY, Lee HY, Kim KW, Lee JI, Kim YS,
Choi WJ and Kim JH: Preoperative computed tomography-determined
sarcopenia and postoperative outcome after surgery for non-small
cell lung cancer. Scand J Surg. 107:244–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bozzetti F: Forcing the vicious circle:
Sarcopenia increases toxicity, decreases response to chemotherapy
and worsens with chemotherapy. Ann Oncol. 28:2107–2118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Law ML: Cancer cachexia: Pathophysiology
and association with cancer-related pain. Front Pain Res
(Lausanne). 3:9712952022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Vaughan VC, Martin P and Lewandowski PA:
Cancer cachexia: Impact, mechanisms and emerging treatments. J
Cachexia Sarcopenia Muscle. 4:95–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Kidd AC, Skrzypski M, Jamal-Hanjani M and
Blyth KG: Cancer cachexia in thoracic malignancy: A narrative
review. Curr Opin Support Palliat Care. 13:316–322. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ding C, Chan Z and Magkos F: Lean, but not
healthy: The ‘metabolically obese, normal-weight’ phenotype. Curr
Opin Clin Nutr Metab Care. 19:408–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Donohoe CL, Doyle SL and Reynolds JV:
Visceral adiposity, insulin resistance and cancer risk. Diabetol
Metab Syndr. 3:122011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Crudele L, Piccinin E and Moschetta A:
Visceral adiposity and cancer: Role in pathogenesis and prognosis.
Nutrients. 13:21012021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Park JE, Jo J, Youk J, Kim M, Yoon SH,
Keam B, Kim TM and Kim DW: Prognostic utility of body composition
parameters based on computed tomography analysis of advanced
non-small cell lung cancer treated with immune checkpoint
inhibitors. Insights Imaging. 14:1822023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Popinat G, Cousse S, Goldfarb L, Becker S,
Gardin I, Salaün M, Thureau S, Vera P, Guisier F and Decazes P:
Sub-cutaneous Fat Mass measured on multislice computed tomography
of pretreatment PET/CT is a prognostic factor of stage IV non-small
cell lung cancer treated by nivolumab. Oncoimmunology.
8:e15801282019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Magri V, Gottfried T, Di Segni M, Urban D,
Peled M, Daher S, Stoff R, Bar J and Onn A: Correlation of body
composition by computerized tomography and metabolic parameters
with survival of nivolumab-treated lung cancer patients. Cancer
Manag Res. 11:8201–8207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Khan A, Welman CJ, Abed A, O'Hanlon S,
Redfern A, Azim S, Lopez P, Singh F and Khattak A: Association of
computed tomography measures of muscle and adipose tissue and
progressive changes throughout treatment with clinical endpoints in
patients with advanced lung cancer treated with immune checkpoint
inhibitors. Cancers (Basel). 15:13822023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Park G, Song K, Choi Y, Oh JS, Choi HS,
Suh J, Kwon A, Kim HS and Chae HW: Sex Hormone-binding globulin is
associated with obesity and dyslipidemia in prepubertal children.
Children (Basel). 7:2722020.PubMed/NCBI
|
|
178
|
Musial C, Zaucha R, Kuban-Jankowska A,
Konieczna L, Belka M, Marino Gammazza A, Baczek T, Cappello F,
Wozniak M and Gorska-Ponikowska M: Plausible role of estrogens in
pathogenesis, progression and therapy of lung cancer. Int J Environ
Res Public Health. 18:6482021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Stabile LP, Davis AL, Gubish CT, Hopkins
TM, Luketich JD, Christie N, Finkelstein S and Siegfried JM: Human
non-small cell lung tumors and cells derived from normal lung
express both estrogen receptor alpha and beta and show biological
responses to estrogen. Cancer Res. 62:2141–2150. 2002.PubMed/NCBI
|
|
180
|
McKenzie F, Biessy C, Ferrari P, Freisling
H, Rinaldi S, Chajès V, Dahm CC, Overvad K, Dossus L, Lagiou P, et
al: Healthy lifestyle and risk of cancer in the European
prospective investigation into cancer and nutrition cohort study.
Medicine (Baltimore). 95:e28502016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Rock CL, Thomson C, Gansler T, Gapstur SM,
McCullough ML, Patel AV, Andrews KS, Bandera EV, Spees CK, Robien
K, et al: American Cancer Society guideline for diet and physical
activity for cancer prevention. CA Cancer J Clin. 70:245–271. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Khalil WJ, Akeblersane M, Khan AS, Moin
ASM and Butler AE: Environmental pollution and the risk of
developing metabolic disorders: obesity and diabetes. Int J Mol
Sci. 24:88702023. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Shi X, Zheng Y, Cui H, Zhang Y and Jiang
M: Exposure to outdoor and indoor air pollution and risk of
overweight and obesity across different life periods: A review.
Ecotoxicol Environ Saf. 242:1138932022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Holme JA, Vondráček J, Machala M,
Lagadic-Gossmann D, Vogel CFA, Le Ferrec E, Sparfel L and Øvrevik
J: Lung cancer associated with combustion particles and fine
particulate matter (PM2.5)-The roles of polycyclic
aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor
(AhR). Biochem Pharmacol. 216:1158012023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Bhattacharjee P, Paul S and Bhattacharjee
P: Risk of occupational exposure to asbestos, silicon and arsenic
on pulmonary disorders: Understanding the genetic-epigenetic
interplay and future prospects. Environ Res. 147:425–434. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Malone ER, Oliva M, Sabatini PJB, Stockley
TL and Siu LL: Molecular profiling for precision cancer therapies.
Genome Med. 12:82020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Li MSC, Mok KKS and Mok TSK: Developments
in targeted therapy & immunotherapy-how non-small cell lung
cancer management will change in the next decade: A narrative
review. Ann Transl Med. 11:3582023. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Rippe JM: Lifestyle medicine: The health
promoting power of daily habits and practices. Am J Lifestyle Med.
12:499–512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Ko C and Chaudhry S: The need for a
multidisciplinary approach to cancer care. J Surg Res. 105:53–57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Berardi R, Morgese F, Rinaldi S, Torniai
M, Mentrasti G, Scortichini L and Giampieri R: Benefits and
limitations of a multidisciplinary approach in cancer patient
management. Cancer Manag Res. 12:9363–9374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Manisalidis I, Stavropoulou E,
Stavropoulos A and Bezirtzoglou E: Environmental and health impacts
of air pollution: A review. Front Public Health. 8:142020.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Sánchez-Romero LM, Penko J, Coxson PG,
Fernández A, Mason A, Moran AE, Ávila-Burgos L, Odden M, Barquera S
and Bibbins-Domingo K: Projected impact of mexico's sugar-sweetened
beverage tax policy on diabetes and cardiovascular disease: A
modeling study. PLoS Med. 13:e10021582016. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Cecchini M and Warin L: Impact of food
labelling systems on food choices and eating behaviours: A
systematic review and meta-analysis of randomized studies. Obes
Rev. 17:201–210. 2016. View Article : Google Scholar : PubMed/NCBI
|