Introduction
Gastrointestinal (GI) cancers represent more than
25% of all cancer incidence and 35% of cancer-related mortality
globally (1). These malignancies
include colorectal cancer (CRC) (2), gastric (3), esophageal (4) and pancreatic cancer (5), each contributing notably to morbidity
and mortality worldwide. Despite advancements in diagnosis and
treatment, prognosis for patients with GI cancer remains poor,
primarily due to late-stage detection and the aggressive nature of
these tumors. Understanding the molecular mechanisms underlying GI
cancer progression is key for developing more effective therapeutic
strategies.
A hallmark of cancer metabolism is the Warburg
effect, characterized by increased glycolysis and lactate
production even in the presence of oxygen (6,7). This
metabolic reprogramming supports rapid cell proliferation and
creates an acidic tumor microenvironment (TME) that promotes
invasion, metastasis and immune evasion. Lactate, traditionally
viewed as a metabolic waste product, has emerged as a key player in
cancer biology, serving as both a signaling molecule and an energy
source (7). Lactate influences
various aspects of tumor progression by modifying the TME,
promoting immune evasion and enhancing cancer cell survival and
metastasis (8–10).
Recent advances have identified lactylation, a
post-translational modification driven by lactate, as a key
regulatory mechanism linking cellular metabolism with gene
expression (8,11). Histone lactylation, in particular,
has emerged as a key process influencing chromatin structure and
function, thereby affecting gene transcription (8). This modification underscores the
intricate interplay between metabolism and epigenetics,
highlighting the potential for novel therapeutic targets in cancer
treatment (11).
Lactate and lactylation modulate various cellular
processes, including inflammation, immune response and
proliferation (12). In GI cancers,
these modifications are hypothesized to contribute to tumor
development and progression (13).
Elucidating the mechanisms underlying lactate production and
lactylation may identify new biomarkers for early detection and
prognosis, as well as novel therapeutic strategies to disrupt these
metabolic and epigenetic pathways.
The present review aimed to provide a comprehensive
overview of current progress in understanding lactate and
lactylation in GI cancers, the biochemical basis of these
processes, impact on the TME and their clinical relevance. The
present review highlights the potential of targeting lactate
metabolism and lactylation as innovative approaches for treating GI
cancer.
Lactate metabolism in GI cancer
Glycolysis and the Warburg effect
Cancer cells possess unique metabolic traits
distinct from normal cells, notably the Warburg effect (14,15).
This phenomenon involves cancer cells preferring energy production
via glycolysis followed by lactate fermentation in cytosol, despite
sufficient oxygen for oxidative phosphorylation. This metabolic
shift facilitates rapid proliferation of tumor cells and notably
alters the TME. The excess lactate produced is exported, leading to
acidification of the TME, which degrades the extracellular matrix
and promotes tumor invasion and metastasis. Additionally, the
acidic environment suppresses immune cell function, helping cancer
cells evade the immune system. This shift also supports
biosynthetic processes essential for rapid cancer cell
proliferation (7). Typically, cells
generate ATP through oxidative phosphorylation in mitochondria,
producing up to 36 ATP/glucose molecule. Conversely, glycolysis in
cytoplasm yields only 2 ATP/glucose molecule but occurs at a much
faster rate, enabling rapid ATP production. Cancer cells enhance
glycolysis through various mechanisms. Tumor cells often show
elevated levels of glycolytic enzymes such as hexokinase,
phosphofructokinase and pyruvate kinase, facilitating glucose to
pyruvate conversion and promoting glycolytic flux (8). Oncogenes such as MYC and RAS
upregulate glycolysis, while tumor suppressors such as p53, which
typically inhibit glycolysis, are frequently mutated or inactivated
in cancer. Hypoxic conditions within tumors stabilize
hypoxia-inducible factor (HIF)-1, which activates genes involved in
glycolysis, including glucose transporter 1 and glycolytic enzymes.
HIF-1 also induces lactate dehydrogenase (LDH) expression,
converting pyruvate to lactate (12). The Warburg effect notably influences
tumor progression. Elevated glycolytic activity leads to high
lactate production, exported via monocarboxylate transporters
(MCTs), contributing to acidification of the TME (10). The acidic environment promotes tumor
invasion and metastasis by degrading extracellular matrix and
inhibiting immune cell function while selecting aggressive,
apoptosis-resistant cancer cells. Additionally, glycolysis
intermediaries support biosynthetic pathways essential for the
synthesis of nucleotides, amino acids and lipids required by
rapidly proliferating cancer cells (16). Non-malignant cells, such as
tumor-associated macrophages (TAMs) and cancer-associated
fibroblasts, also contribute to lactate accumulation in the TME
through the reverse Warburg effect (16). Hypoxic tumor cells secrete lactate,
which normoxic tumor cells take up, facilitating glucose diffusion
towards hypoxic cells. This lactate-based metabolic symbiosis
supports both hypoxic and normoxic cancer cells (11). Studies indicate lactate
concentration is associated with cancer grade and prognosis
(17,18). Isotope tracer measurements reveal
rapid lactate exchange between tumors and circulation, with tumors
converting pyruvate to lactate faster than adjacent benign tissue
(17,18).
In GI cancers, including CRC, gastric, esophageal
and pancreatic cancers, the Warburg effect is prominent. These
tumors exhibit high glycolytic rates and elevated lactate levels,
which are associated with poor prognosis and therapy resistance
(6). Targeting glycolysis and its
regulatory pathways offers a promising therapeutic strategy for GI
cancer. Inhibitors of key glycolytic enzymes, lactate transporters
and HIF-1 are under investigation for their potential to disrupt
metabolic adaptability of cancer cells and enhance treatment
outcomes (8). Recent studies have
also underscored the role of glycolysis in modulating the immune
response within the TME (12,19).
For example, lactate inhibits cytolytic functions of cytotoxic T
cells and natural killer (NK) cells by reducing the production of
key molecules such as perforin, granzyme and IFN-γ, and by
downregulating signaling pathways such as nuclear factor of
activated T cells and Peroxisome proliferator-activated receptor
(PPARγ), ultimately facilitating tumor immune evasion (19). Understanding the interaction between
cancer metabolism and immune regulation is key for developing
effective immunotherapies. In summary, the Warburg effect is a key
aspect of cancer metabolism that promotes tumor cell proliferation,
survival and invasiveness. Elucidating the molecular mechanisms of
this metabolic reprogramming may identify novel therapeutic targets
for combating GI cancers.
Lactate transport and
accumulation
Lactagenesis, or augmented lactate production, has
been proposed as an explanation for the Warburg effect that drives
carcinogenesis. This process encompasses five steps: Enhanced
glucose uptake, increased expression and activity of glycolytic
enzymes, decreased mitochondrial function, elevated lactate
generation, accumulation and release and upregulation of MCT1/4 for
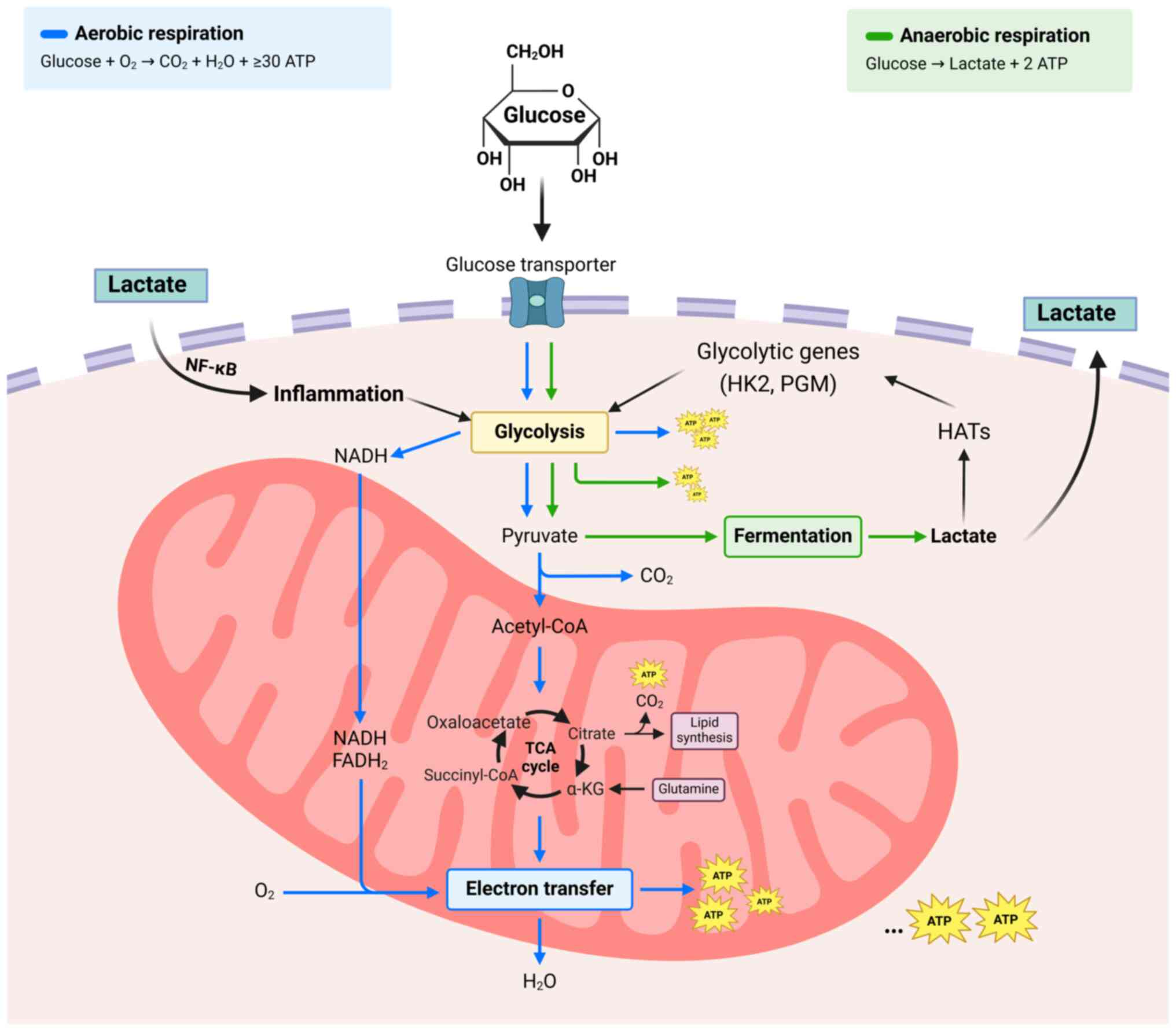
accelerated lactate shuttling (Fig.
1) (20).
Lactate, a byproduct of the Warburg effect, serves a
pivotal role in the metabolic reprogramming of cancer cells. Its
accumulation in the TME, facilitated by specialized transporters,
notably impacts tumor progression, immune evasion and metastasis.
Understanding lactate transport and accumulation mechanisms may
provide potential therapeutic targets for GI cancer (19). Lactate crosses the cell membrane via
MCTs, a family of proton-linked transporters mediating
bidirectional transfer of lactate and other MCs, such as pyruvate
and ketone bodies. The primary MCTs involved in cancer cell lactate
transport are MCT1 and MCT4. MCT1 is widely expressed by various
tissues, including skeletal muscle, heart, brain, liver, and
endothelial cells (21). It plays a
crucial role in facilitating lactate uptake and export depending on
the metabolic needs of the cell and the tissue environment. In
tumor cells, MCT1 imports lactate to support oxidative metabolism
and prevent toxic intracellular lactate accumulation (21). MCT4 primarily exports lactate and is
upregulated in highly glycolytic cells, such as those in the
hypoxic tumor core (21). This
transporter maintains intracellular pH by removing excess lactate,
thereby supporting continuous glycolytic activity. Several factors,
including HIF-1 and c-Myc, regulate MCT1 and MCT4 expression.
HIF-1, stabilized under hypoxic conditions, upregulates glycolytic
enzymes and lactate transporters to adapt to low-oxygen
environments (22).
Elevated glycolytic activity in cancer cells leads
to excessive lactate production, which is exported into the TME via
MCTs. This lactate export, coupled with proton co-transport,
acidifies the extracellular space, creating a low-pH environment
that promotes tumor invasion and metastasis (21). Acidification activates proteolytic
enzymes that degrade the extracellular matrix and select for more
aggressive cancer cell phenotypes (23). Lactate accumulation in the TME also
inhibits immune cells, including cytotoxic T and NK cells, by
suppressing cytokine production and decreasing their cytolytic
activity, thus enabling tumor cells to evade immune surveillance
(24). Additionally, lactate acts
as a signaling molecule promoting angiogenesis by inducing vascular
endothelial growth factor (VEGF) expression, facilitating novel
blood vessel formation and providing the tumor with increased
oxygen and nutrient supply, thereby supporting continued tumor
growth (8).
Mechanisms and roles of lactylation in
cancer
Discovery and mechanism of
lactylation
Lactylation, first identified as a
post-translational modification by Zhang et al (25), involves addition of lactate
molecules to lysine residues on histones, termed histone
lactylation. This followed an investigation into the non-metabolic
functions of lactate, revealing its role beyond traditional
metabolic contexts (25). Advanced
mass spectrometry techniques have facilitated the precise detection
and characterization of histone lactylation, showing its occurrence
under both normoxic and hypoxic conditions, indicating its
potential relevance in numerous physiological and pathological
contexts, including cancer (26).
Protein lactylation, especially histone lactylation, involves
covalent attachment of lactate to the ε-amino group of lysine
residues. This process is similar to other acylation modifications
such as acetylation and methylation, but uniquely uses lactate as
the modifying group. The lactylation begins with lactate
production, where the Warburg effect in cancer cells increases
lactate production via glycolysis, with pyruvate reduced to lactate
by LDH (27). For lactylation to
occur, lactate must be activated, potentially due to the formation
of lactyl-CoA, a high-energy intermediary that donates the lactate
moiety to target proteins. The exact enzymatic pathways and
cofactors involved in this activation remain under investigation
(8). The transfer of lactate to
lysine residues on histones and other proteins is hypothesized to
be facilitated by specific enzymes, though definitive
lactyltransferases have not yet been identified. It is hypothesized
that enzymes with acyltransferase activity may catalyze this
process (8). Potential candidates
include histone acetyltransferases (HATs), which catalyze addition
of acetyl groups to histones, although direct evidence linking HATs
to lactylation is lacking. Sirtuins, a family of
NAD+-dependent deacetylases, may serve a role in
reversing lactylation, similar to their role in deacetylation;
understanding the interplay between lactylation and deacetylation
may reveal more about the regulatory mechanisms underlying
lactylation (28,29). The involvement of cofactors such as
NAD+ and acetyl-CoA analogs such as lactyl-CoA suggests
a complex regulatory network, potentially influencing the
availability and activity of lactylation enzymes. In GI cancers,
lactylation represents a crucial regulatory mechanism linking
metabolic reprogramming to gene expression (30,31).
Similar to other epigenetic modifications such as
methylation and acetylation, lactylation is regulated by writers
(enzymes that add lactyl groups), erasers (enzymes that remove
lactyl groups) and readers (proteins that recognize lactylation and
mediate its effects) (32).
Lactyl-CoA, detected in mammalian cells by liquid chromatography
mass spectrometry, serves as the substrate for enzymatic
lactylation, while lactyl-glutathione participates in non-enzymatic
lactylation (33). The enzyme p300
has been identified as a lactylation writer and may share functions
with other epigenetic modifiers, indicating potential overlaps in
enzymatic regulation of different epigenetic markers (34).
Histone lactylation and gene
expression
Histone lactylation, a key post-translational
modification, affects chromatin structure and function by loosening
chromatin, thereby facilitating transcription of genes involved in
tumor progression, invasion, and immune evasion (35). This modification links cellular
metabolism to gene regulation by adding lactate molecules to lysine
residues on histones, influencing chromatin dynamics and gene
expression (36). Metabolic shifts
in cancer cells directly impact the epigenetic landscape and drive
tumor progression. By altering the chemical properties of histone
proteins, histone lactylation modulates the interactions between
histones and DNA, as well as the binding of regulatory proteins to
chromatin (37). Understanding the
precise molecular mechanisms of lactylation is key for developing
targeted therapies aimed at disrupting this modification to hinder
cancer progression. Neutralizing the positive charge of histones
via lactylation reduces their affinity for negatively charged DNA,
resulting in a more relaxed chromatin structure that facilitates
transcriptional activation (25).
Histone lactylation activates transcription of genes
involved in metabolic processes, immune responses and cell stress
pathways (38–40). Research has shown that lactylation
induces transcriptional activation in response to metabolic changes
within cells (41). In cancer
cells, histone lactylation upregulates genes associated with
glycolysis and other metabolic pathways, ensuring metabolic needs
of rapidly proliferating tumor cells are met, thereby promoting
sustained proliferation and survival. Additionally, lactylation
regulates immune response genes (42,43).
For example, in macrophages, histone lactylation activates genes
involved in the anti-inflammatory response, highlighting its
potential role in modulating the tumor immune landscape (40).
Under cellular stress conditions such as hypoxia,
lactylation activates stress response genes, aiding cells in
adapting to adverse conditions, which is key in the TME where
cancer cells are subject to fluctuating oxygen and nutrient levels.
Identifying specific genes and pathways regulated by histone
lactylation is an area of active research, such as mapping
lactylation sites across the genome and associating them with
changes in gene expression (44,45).
Techniques such as chromatin immunoprecipitation followed by
sequencing have identified that lactylation is enriched at
promoters and enhancers of actively transcribed genes (46,47).
Functional studies have demonstrated that genes
regulated by lactylation participate in key cellular processes,
including cell cycle regulation, apoptosis and DNA repair (48,49).
For example, lactylation of histone H3 at lysine 18 (H3K18la)
activates genes associated with cell proliferation and survival
(48,49). Understanding the mechanisms and
consequences of histone lactylation provides insights into the
metabolic-epigenetic interplay in cancer and may reveal novel
therapeutic targets. By modulating chromatin structure and
function, lactylation activates genes that drive tumor progression,
immune evasion and adaptation to stress.
Impact on the TME
Cancer cells export lactate and protons via MCTs,
mainly MCT1 and MCT4, thereby lowering the extracellular pH and
creating an acidic environment (50–52).
This acidification activates proteolytic enzymes such as MMPs,
which degrade the extracellular matrix and facilitate tumor cell
invasion. Additionally, acidic pH enhances cancer cell migration
and invasion by inducing epithelial-mesenchymal transition (EMT),
increasing cell motility (53,54).
Reprogrammed metabolism, a hallmark of cancer, links
lactylation and cell metabolism (55,56).
Histone lactylation connects metabolic reprogramming with abnormal
gene expression in cancer cells. Metabolic changes alter lactate
levels, reshaping the histone lactylation landscape and
transcriptomic profile to adapt to metabolic reprogramming
(57). In non-small cell lung
cancer, lactate modulates metabolism by altering the expression of
glycolytic and tricarboxylic acid cycle enzymes through promoter
histone lactylation. Histone lactylation is associated with
arginase (Arg)1 expression in TAMs and enhances VEGFA transcription
during TAM polarization (55).
Immunosuppressive effects of
lactylation
In addition to serving as an intermediate metabolite
in energy and biosynthetic pathways, lactate accumulates during
local inflammation, linking it to tumor-associated inflammation, a
hallmark of cancer (19).
Macrophages, key for regulating immune response and maintaining
tissue homeostasis, exhibit plasticity modulated by epigenetic
dynamics during inflammation (58).
Macrophages exist in two types: Proinflammatory M1 and immune
regulatory M2. The transition from M1 to M2 is vital for restoring
immune homeostasis. B cell adapter for PI3K facilitates this
transition by elevating lactate production, enhancing histone
lactylation and upregulating genes such as Forkhead box protein O1
and glycogen synthase kinase-3β (GSK3β) (59). Lactate-derived histone lactylation
induces expression of homeostatic genes such as Arg1, which is
highly expressed by immunosuppressive myeloid-derived suppressor
cells and TAMs during the macrophage transition from M1 to M2
(60). Lysine lactylation is
considered a consequence rather than a cause of macrophage
activation, consistent with Arg1-dependent metabolic rewiring
during inflammation (60). TAMs
exhibit an M1 phenotype with anti-tumor activity at tumor
initiation but shift to an M2 phenotype during cancer progression
(61). Lactate mediates
immunosuppressive effects of efferocytosis under hypoxia by
inducing anti-inflammatory genes (62). While high lactate and low pH in
inflamed tissues under hypoxia can aid pathogen clearance by
confining T cells to the inflammatory site, this is detrimental
during tumor-associated inflammation, suppressing cytolytic
function of CD8+ T cells and inducing the T helper 17
phenotype in CD4+ T cells (63). Under hypoxia, lactate mediates
immunosuppressive effects by inducing expression of
anti-inflammatory genes (58,63).
Lactate accumulation in the TME impairs the immune
response, facilitating tumor immune evasion. Elevated lactate
levels inhibit cytotoxic T and NK cells (36,64) by
impairing cytokine production, such as IFN-γ, essential for
anti-tumor activity. Additionally, lactate decreases the
proliferation and activity of these immune cells, weakening their
ability to target and eliminate cancer cells. The acidic
environment created by lactate further suppresses immune function
by inducing mitochondrial stress and apoptosis in NK cells,
particularly in liver-resident NK cells in colorectal liver
metastasis (65,66).
Lactate promotes differentiation and activity of
regulatory T cells (Tregs), which suppress the immune response
against tumors (67,68). Tregs in the TME inhibit cytotoxic
immune cells, aiding tumor immune evasion. Lactate-induced Tregs
produce anti-inflammatory cytokines and inhibit effector T and NK
cells, creating an immunosuppressive environment that fosters tumor
growth and progression (69).
In summary, lactate in the TME modulates the immune
response by inhibiting key immune cells and promoting
immunosuppressive Tregs. These effects enhance the immune evasion
of tumors, underscoring the potential of targeting lactate
metabolism as a therapeutic strategy in GI cancers.
Lactate and lactylation in GI cancer
Lactate accumulation in GI tumors
The accumulation of lactate in GI tumors is a
hallmark of the altered metabolic state of cancer cells. There are
elevated lactate levels in various types of GI cancer, including
CRC (70), gastric (71–73)
and pancreatic (74) cancers. This
elevation is primarily due to the Warburg effect (13).
Advanced techniques have quantified lactate levels
in tumors, enhancing the understanding of lactate metabolism in
cancer. Proton magnetic resonance spectroscopy is a non-invasive
imaging technique widely used to measure lactate concentration
in vivo (16,75), allowing real-time monitoring of
metabolic changes in tumors. LC-MS (76) precisely quantifies lactate levels in
tumor tissue and blood samples with high sensitivity and
specificity.
Clinical studies consistently show significantly
elevated lactate levels in GI tumors compared with normal tissues
(70,71,71).
For example, patients with CRC have lactate concentrations in tumor
tissue several fold higher than in adjacent non-tumorous tissues.
Similar findings in gastric and pancreatic cancer show an
association between high lactate levels with advanced disease stage
and poor prognosis (71,74), underscoring lactate as a biomarker
for tumor aggressiveness and potential therapeutic target.
Lactate accumulation in GI tumors influences tumor
progression, invasion and therapy resistance. Further research into
the mechanisms of lactate accumulation and its effects on the TME
may advance the understanding of GI cancer biology and improve
clinical outcomes.
Lactylation in GI cancer
progression
Lactylation connects cellular metabolism to gene
expression, affecting tumor development, invasion and
metastasis.
Lactylation notably impacts tumor growth by
activating genes involved in cell proliferation and survival. For
example, H3K18la upregulates genes that enhance glycolysis and
other metabolic pathways essential for rapidly dividing cancer
cells. By boosting expression of these genes, lactylation meets the
metabolic demands of tumor cells, supporting sustained tumor growth
and expansion. Aberrant lactate production facilitates cancer
hallmarks such as uncontrolled growth and resistance to cell death.
For example, eliminating tumor-produced lactate by deleting MCT1 in
lung cancer cells halts tumor growth (77). Similarly, inhibiting MCT1 or MCT4
stops proliferation of leukemia cells, and selective inhibition of
MCT4 impedes invasive bladder cancer cell proliferation (78–80).
Additionally, cancer-produced lactate activates G-protein-coupled
receptor 81, with its deletion arresting breast cancer tumor growth
in vitro and in vivo, underscoring the role of
lactate in cancer proliferation (81,82).
Lactylation also enhances the invasive and
metastatic potential of GI cancers. Histone lactylation activates
genes encoding MMPs and other enzymes that degrade the
extracellular matrix, facilitating tumor cell invasion into
surrounding tissue and the establishment of metastases in distant
organs (83). Additionally,
lactylation-induced gene expression changes promote EMT, enabling
cancer cells to acquire migratory and invasive capability (84–86).
By modulating the expression of genes involved in
immune regulation, lactylation helps cancer cells evade immune
surveillance (36). For example,
lactylation suppresses pro-inflammatory cytokines and enhances
anti-inflammatory factors, creating an immunosuppressive TME
(84). This environment inhibits
the activity of immune cells, such as cytotoxic T and NK cells,
allowing tumor cells to proliferate unchecked (86).
Key proteins and pathways modified by lactylation in
gastrointestinal GI cancers impact tumor behavior and progression
(87,88). Lactylation enhances HIF-1 stability
and activity, promoting angiogenesis, glycolysis and cell survival
under hypoxic conditions common in tumors (89). This stabilization activates genes
that support tumor growth and adaptation to low-oxygen environments
(90). Similarly, lactylation
modifies NF-κB, a transcription factor key for in inflammation and
cell survival pathways. Lactylation increases NF-κB transcriptional
activity, upregulating genes involved in inflammation, cell
proliferation and resistance to apoptosis, thus aiding tumor
progression (91,92). Histone lactylation, particularly at
markers such as H3K18la, influences cancer progression by
activating genes that drive EMT, immune evasion and tumor
aggressiveness (92). By modulating
these critical pathways, lactylation facilitates the complex
network of gene regulation enabling GI tumors to grow, invade and
resist therapeutic intervention.
Understanding lactylation mechanisms and effects in
GI cancer is essential for developing targeted therapy to disrupt
this modification and improve patient outcomes (Table I).
 | Table I.Mechanisms of lactylation in
gastrointestinal tumors. |
Table I.
Mechanisms of lactylation in
gastrointestinal tumors.
| Cancer | Proteins modified
by lactylation | Affected
genes/pathways | Role of
lactylation | (Refs.) |
|---|
| Colorectal | β-catenin | Wnt/β-catenin | Promotes cell
proliferation and stemness | (121) |
|
| H3K18 | Autophagy | Promotes resistance
to bevacizumab by enhancing autophagy. | (70) |
| Gastric | METTL16 | FDX1 | Enhances
cuproptosis sensitivity | (71) |
|
| - | GLUT3 | Promotes
lactylation modification by regulating LDHA | (83) |
| Esophageal | H3K9 | LAMC2 | Promotes LAMC2
expression, enhancing cell proliferation under hypoxia | (122) |
| Pancreatic | H3K18 | METTL3 | Promotes
immunosuppression of tumor-infiltrating myeloid cells | (42) |
Differential mechanisms of lactylation
in GI tumor subtypes
Lactylation serves distinct regulatory roles in
various types of GI cancer, including gastric, CRC and pancreatic
cancers, through different molecular pathways. In gastric cancer,
lactylation primarily affects glycolysis and mitochondrial function
by enhancing activity of key metabolic enzymes such as LDH-A, which
increases glycolytic flux. This metabolic reprogramming leads to
rapid cellular proliferation and migration, as well as upregulation
of oncogenic and cell cycle-related genes, contributing to tumor
aggressiveness and resistance to apoptosis (83). Additionally, lactylation can
regulate mitochondrial dynamics and energy production, supporting
the highly proliferative nature of gastric cancer cells (93). In CRC, lactylation plays a critical
role in shaping the TME by modulating immune cell function. High
levels of lactylation are associated with increased recruitment of
Tregs and suppression of cytotoxic T cells, leading to immune
evasion and poor prognosis (94,95).
Furthermore, lactylation has been shown to promote chemoresistance
in CRC by regulating genes involved in drug metabolism and efflux
pathways (93). Recent studies have
identified a panel of lactylation-associated genes associated with
poor survival outcomes that may serve as potential prognostic
markers (95,96). Targeting these lactylation-related
pathways may overcome therapeutic resistance and improve patient
outcomes. In pancreatic cancer, lactylation impacts stromal
interactions and extracellular matrix remodeling. By activating
cancer-associated fibroblasts, lactylation enhances production of
extracellular matrix proteins, leading to a dense, fibrotic stroma
that is characteristic of pancreatic tumors. This stromal barrier
not only impedes drug delivery but also creates an
immunosuppressive environment that shields the tumor from immune
surveillance (36,96). Additionally, lactylation modulates
key signaling pathways such as HIF-1α and TGF-β, further promoting
fibrosis, tumor growth and metastasis (96).
Therapeutic implications
Targeting lactate metabolism
Targeting lactate metabolism offers a promising
therapeutic strategy for GI cancer due to its role in tumor
progression and immune evasion. Various approaches aim to disrupt
lactate production and transport, thereby altering the TME and
enhancing the efficacy of existing treatments.
Inhibitors of glycolytic enzymes
One approach involves inhibiting key glycolytic
enzymes to reduce lactate production. Agents targeting enzymes such
as hexokinase, phosphofructokinase and LDH have shown potential in
preclinical studies (97–100). For example, LDH inhibitors
decrease lactate levels, leading to reduced tumor growth and
improved chemotherapy response. Inhibitors such as oxamate
(97,98), which targets LDH-A, have
demonstrated effectiveness in preclinical models by decreasing
lactate production and promoting immune activation within the TME
(99). Additionally, targeting
pyruvate dehydrogenase kinase, which regulates conversion of
pyruvate to lactate, has shown efficacy in reducing lactate
production and tumor cell proliferation (100).
MCT inhibitors
Another strategy involves blocking lactate export
from cancer cells by inhibiting MCTs, particularly MCT1 and MCT4
(101). MCT inhibitors prevent the
acidification of the TME, enhancing immune cell function and
reducing tumor invasion. AZD3965, an MCT1 inhibitor, has shown
promise in clinical trials, demonstrating potential to disrupt
lactate transport and improve treatment outcomes in various types
of cancer (102–104). MCT4 facilitates efflux of lactic
acid from glycolytic cancer cells (105). Knocking down or silencing MCT4
leads to cytoplasmic acidification and subsequent tumor cell death.
MCT4 is predominantly expressed in hypoxic regions of rapidly
proliferating tumors, making it a promising therapeutic target.
Although existing drugs aimed at MCT4 lack specificity, further
investigation into their potential effectiveness in cancer therapy
is needed (106,107). Additionally, localization and
stability of MCT1 and MCT4 at the plasma membrane are regulated by
CD147. Targeting CD147 could be a novel strategy to inhibit both
transporters. AC-73, a dimerized and humanized anti-CD147 antibody,
has demonstrated antitumor activity in preclinical study (108). In summary, inhibiting lactic acid
transporters MCT1 and MCT4, along with their chaperones, may have
antitumor potential. However, clinical data supporting these
findings are currently lacking (108). Blocking MCT1 and MCT4 normalizes
the pH of the TME, thereby enhancing immune responses and reducing
metastatic potential.
Combination therapy
Combining lactate metabolism inhibitors with other
therapeutic modalities, such as immunotherapy and conventional
chemotherapy, has been researched (109–111). For example, combining LDH
inhibitors with immune checkpoint inhibitors has shown synergistic
effects, enhancing anti-tumor immune responses by reversing
lactate-induced immunosuppression. This multi-faceted approach
targets various aspects of tumor biology, potentially improving
patient outcomes (112–114). Combination therapy can enhance the
efficacy of therapies such as chimeric antigen receptor T cell
therapy by decreasing lactate-induced immunosuppression (108,115). In summary, targeting lactate
metabolism using glycolytic enzyme and MCT inhibitors and
combination therapies shows promise for treating GI cancers. These
strategies aim to disrupt the lactate-driven metabolic
reprogramming and immune evasion mechanisms that tumors exploit,
enhancing the overall effectiveness of cancer therapy. Further
research and clinical trials are essential to validate these
approaches and translate them into effective treatments. Inhibitors
of LDH and MCTs are still in early-phase clinical trials, with
mixed results regarding efficacy and safety (108,109). Challenges include potential
off-target effects, limited tumor specificity and TME complexity,
which may reduce treatment effectiveness. Furthermore, the
metabolic flexibility of cancer cells may enable resistance to
these therapies. Addressing these issues requires comprehensive
clinical studies, better biomarkers for patient selection and
combination therapies to overcome resistance and enhance
therapeutic outcomes.
Inhibiting lactylation
Inhibiting lactylation is a therapeutic approach in
GI cancers due to its role in regulating gene expression and
promoting tumor progression (116–120).
Histone deacetylase (HDAC)
inhibitors
HDAC inhibitors, which can also affect histone
lactylation, have been explored for their potential to modulate
epigenetic markers in cancer cells (116). By decreasing lactylation levels,
these inhibitors alter gene expression involved in tumor growth and
immune evasion. For example, vorinostat and panobinostat, two
prominent HDAC inhibitors, have efficacy in various types of
cancers by disrupting epigenetic modifications and enhancing tumor
suppressor gene expression (116,117). Vorinostat and panobinostat prevent
removal of acetyl and, potentially, lactyl groups from histones,
maintaining a more open chromatin structure conducive to gene
expression that counteracts tumor growth (116,117).
Direct inhibitors of lactylation
enzymes
Identifying and targeting specific enzymes
responsible for lactylation, such as potential lactyltransferases,
represents a promising research direction. Although these enzymes
have not been definitively identified, studies have investigated
small molecules that specifically inhibit lactylation, thereby
disrupting its oncogenic effects (118,119). General control non-depressible 5
is a possible lactyltransferase for ERK in the MAPK signaling
pathway (118,119). Inhibitors targeting such
lactyltransferases could reduce tumor progression and metastasis by
altering key signaling pathways involved in cancer cell survival
and proliferation.
Combining lactylation inhibitors with
other therapy
Combining lactylation inhibitors with other
treatment modalities may enhance therapeutic efficacy (41). Integrating lactylation inhibition
with immunotherapy or targeted therapy may provide a comprehensive
approach to disrupting cancer progression and overcoming resistance
to single-agent treatments. Studies have shown that combining
lactylation inhibitors with immune checkpoint inhibitors or
chemotherapeutic agents produces synergistic effects, enhancing
overall anti-tumor response by reversing immunosuppression and
decreasing tumor growth (41,120).
Targeting lactylation presents a potential
therapeutic approach for GI cancer. HDAC and direct lactylation
enzyme inhibitors and combination therapies offer multiple avenues
to disrupt lactylation-driven oncogenic processes. Further research
and clinical trials are key to translate these strategies into
effective treatments for patients with cancer.
Future research directions
Identifying and characterizing enzymes directly
responsible for protein lactylation is essential. Understanding
specific mechanisms and regulatory pathways of lactylation will aid
in developing targeted inhibitors. Therefore, research should
prioritize discovering and validating lactyl transferases, which
serve a key role in catalyzing lactylation. Identifying these
enzymes may offer novel therapeutic targets to inhibit lactylation
oncogenic effects and improve cancer treatment outcomes.
Developing reliable biomarkers for lactate
metabolism and lactylation may enhance patient stratification and
treatment monitoring. Biomarkers can identify patients likely to
benefit from therapy targeting these pathways, thereby improving
personalized treatment approaches. Efforts should prioritize
discovering biomarkers that reflect lactate metabolism and
lactylation activity, such as specific lactylation markers on
histones or lactate levels in the TME.
Expanding clinical trials to assess the safety and
efficacy of lactate metabolism and lactylation inhibitors in GI
cancer is crucial. These trials should explore various combinations
of inhibitors with existing therapy to identify the most effective
regimens. Clinical research should also investigate potential side
effects and resistance mechanisms, ensuring safe and effective
integration into cancer treatment protocols.
Elucidating the complex interactions between
lactate, lactylation, and other metabolic and epigenetic processes
in cancer cells requires in-depth mechanistic studies to reveal the
broader implications of targeting these pathways and potential
resistance mechanisms. Research should focus on understanding how
lactate and lactylation affect gene expression, tumor metabolism
and the immune microenvironment to identify new therapeutic targets
and strategies, ultimately improving clinical outcomes for patients
with GI cancer and potentially leading to more personalized and
effective cancer treatment.
Conclusion
Lactate and lactylation serve a key role in the
progression of GI cancers by modulating the TME, gene expression
and immune response. Elevated lactate levels promote tumor growth,
invasion and immune evasion through acidification and immune
suppression. Lactylation regulates gene transcription and further
supports tumor progression. Targeting lactate metabolism and
lactylation presents potential therapeutic strategies, including
glycolytic enzyme and MCT inhibitors and combination therapies.
Future research should identify specific lactylation enzymes,
develop reliable biomarkers, expand clinical trials and elucidate
the underlying mechanisms to enhance personalized treatments and
improve clinical outcomes for patients with GI cancer.
Additionally, exploring the interplay between metabolic
reprogramming and epigenetic modifications may reveal novel
therapeutic targets and strategies, ultimately increasing the
effectiveness of cancer therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YH, YH, LR and QY wrote the manuscript. YH and YH
created figures and tables. PP, LR and QY revised the manuscript.
LR and QY supervised the research. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang J, Lucero-Prisno DE III, Zhang L, Xu
W, Wong SH, Ng SC and Wong MCS: Updated epidemiology of
gastrointestinal cancers in East Asia. Nat Rev Gastroenterol
Hepatol. 20:271–287. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah SC and Itzkowitz SH: Colorectal
cancer in inflammatory bowel disease: Mechanisms and management.
Gastroenterology. 162:715–730.e3. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joshi SS and Badgwell BD: Current
treatment and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu H, Ma X, Ye T, Wang H, Wang Z, Liu Q
and Zhao K: Esophageal cancer in China: Practice and research in
the new era. Int J Cancer. 152:1741–1751. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y and Patti GJ: The Warburg effect: A
signature of mitochondrial overload. Trends Cell Biol.
33:1014–1020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv X, Lv Y and Dai X: Lactate, histone
lactylation and cancer hallmarks. Expert Rev Mol Med. 25:e72023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan H, Yang F, Xiao Z, Luo H, Chen H, Chen
Z, Liu Q and Xiao Y: Lactylation: Novel epigenetic regulatory and
therapeutic opportunities. Am J Physiol Endocrinol Metab.
324:E330–E338. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu
MQ, Zhuo QF, Ji SR, Yu XJ, Xu XW and Qin Y: Lactate-induced protein
lactylation: A bridge between epigenetics and metabolic
reprogramming in cancer. Cell Prolif. 56:e134782023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Cao X, Li B, Zhao Z, Chen S, Lai
SWT, Muend SA, Nossa GK, Wang L, Guo W, et al: Warburg effect is a
cancer immune evasion mechanism against macrophage
immunosurveillance. Front Immunol. 11:6217572021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kes MMG, Van den Bossche J, Griffioen AW
and Huijbers EJM: Oncometabolites lactate and succinate drive
pro-angiogenic macrophage response in tumors. Biochim Biophys Acta
Rev Cancer. 1874:1884272020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheung SM, Husain E, Masannat Y, Miller
ID, Wahle K, Heys SD and He J: Lactate concentration in breast
cancer using advanced magnetic resonance spectroscopy. Br J Cancer.
123:261–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Zhan L, Yanxiang Guo J, et al:
Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nelson SJ, Kurhanewicz J, Vigneron DB,
Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok
R, Park I, et al: Metabolic imaging of patients with prostate
cancer using hyperpolarized [1-¹3C]pyruvate. Sci Transl
Med. 5:198ra082013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mortazavi Farsani SS and Verma V: Lactate
mediated metabolic crosstalk between cancer and immune cells and
its therapeutic implications. Front Oncol. 13:11755322023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
San-Millán I and Brooks GA: Reexamining
cancer metabolism: Lactate production for carcinogenesis could be
the purpose and explanation of the Warburg effect. Carcinogenesis.
38:119–133. 2017.PubMed/NCBI
|
|
21
|
Petersen C, Nielsen MD, Andersen ES, Basse
AL, Isidor MS, Markussen LK, Viuff BM, Lambert IH, Hansen JB and
Pedersen SF: MCT1 and MCT4 expression and lactate flux activity
increase during white and brown adipogenesis and impact adipocyte
metabolism. Sci Rep. 7:131012017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Xin C, Wang S, Zhuo S, Zhu J, Li
Z, Liu Y, Yang L and Chen Y: Lactate transported by MCT1 plays an
active role in promoting mitochondrial biogenesis and enhancing TCA
flux in skeletal muscle. Sci Adv. 10:eadn45082024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mai Z, Lin Y, Lin P, Zhao X and Cui L:
Modulating extracellular matrix stiffness: A strategic approach to
boost cancer immunotherapy. Cell Death Dis. 15:3072024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caslin HL, Abebayehu D, Pinette JA and
Ryan JJ: Lactate is a metabolic mediator that shapes immune cell
fate and function. Front Physiol. 12:6884852021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu Y, He Z, Li Z, Wang Y, Wu N, Sun H,
Zhou Z, Hu Q and Cong X: Lactylation: The novel histone
modification influence on gene expression, protein function, and
disease. Clin Epigenetics. 16:722024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun P, Ma L and Lu Z: Lactylation: Linking
the Warburg effect to DNA damage repair. Cell Metab. 36:1637–1639.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liebner T, Kilic S, Walter J, Aibara H,
Narita T and Choudhary C: Acetylation of histones and non-histone
proteins is not a mere consequence of ongoing transcription. Nat
Commun. 15:49622024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shvedunova M and Akhtar A: Modulation of
cellular processes by histone and non-histone protein acetylation.
Nat Rev Mol Cell Biol. 23:329–349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie N, Zhang L, Gao W, Huang C, Huber PE,
Zhou X, Li C, Shen G and Zou B: NAD+ metabolism:
Pathophysiologic mechanisms and therapeutic potential. Signal
Transduct Target Ther. 5:2272020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Navas LE and Carnero A: NAD+
metabolism, stemness, the immune response, and cancer. Signal
Transduct Target Ther. 6:22021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen AN, Luo Y, Yang YH, Fu JT, Geng XM,
Shi JP and Yang J: Lactylation, a novel metabolic reprogramming
code: Current status and prospects. Front Immunol. 12:6889102021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varner EL, Trefely S, Bartee D, von
Krusenstiern E, Izzo L, Bekeova C, O'Connor RS, Seifert EL, Wellen
KE, Meier JL and Snyder NW: Quantification of lactoyl-CoA
(lactyl-CoA) by liquid chromatography mass spectrometry in
mammalian cells and tissues. Open Biol. 10:2001872020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang T, Chen K, Yao W, Zheng R, He Q, Xia
J, Li J, Shao Y, Zhang L, Huang L, et al: Acetylation of lactate
dehydrogenase B drives NAFLD progression by impairing lactate
clearance. J Hepatol. 74:1038–1052. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang N, Wang W, Wang X, Mang G, Chen J,
Yan X, Tong Z, Yang Q, Wang M, Chen L, et al: Histone lactylation
boosts reparative gene activation post-myocardial infarction. Circ
Res. 131:893–908. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Huang L, Gu Y, Cang W, Sun P and
Xiang Y: Lactate-lactylation hands between metabolic reprogramming
and immunosuppression. Int J Mol Sci. 23:119432022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X
and Jia R: Histone lactylation drives oncogenesis by facilitating
m6A reader protein YTHDF2 expression in ocular melanoma.
Genome Biol. 22:852021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao
H, Zhao F, Wang Z, Yang X, Jin M, et al: Metabolic regulation of
homologous recombination repair by MRE11 lactylation. Cell.
187:294–311.e21. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang N, Zhang Y, Xu J, Wang P, Wu B, Lu
S, Lu X, You S, Huang X, Li M, et al: α-myosin heavy chain
lactylation maintains sarcomeric structure and function and
alleviates the development of heart failure. Cell Res. 33:679–698.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Yang P, Yu T, Gao M, Liu D, Zhang
J, Lu C, Chen X, Zhang X and Liu Y: Lactylation of PKM2 suppresses
inflammatory metabolic adaptation in pro-inflammatory macrophages.
Int J Biol Sci. 18:6210–6225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Song H, Li M and Lu P: Histone
lactylation bridges metabolic reprogramming and epigenetic rewiring
in driving carcinogenesis: Oncometabolite fuels oncogenic
transcription. Clin Transl Med. 14:e16142024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H,
Wang H, Song Y, Du Y, Cui B, et al: Lactylation-driven
METTL3-mediated RNA m6A modification promotes
immunosuppression of tumor-infiltrating myeloid cells. Mol Cell.
82:1660–1677.e10. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S,
Shen X, Wu Y, Zhang S, Wang X, et al: Lactylome analysis suggests
lactylation-dependent mechanisms of metabolic adaptation in
hepatocellular carcinoma. Nat Metab. 5:61–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C
and Zhang J: Lactylation: A passing fad or the future of
posttranslational modification. Inflammation. 45:1419–1429. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Fan W, Li N, Ma Y, Yao M, Wang G,
He S, Li W, Tan J, Lu Q and Hou S: YY1 lactylation in microglia
promotes angiogenesis through transcription activation-mediated
upregulation of FGF2. Genome Biol. 24:872023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan
M, Xiang J, He N, Hu Z and Wang F: CircXRN2 suppresses tumor
progression driven by histone lactylation through activating the
Hippo pathway in human bladder cancer. Mol Cancer. 22:1512023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen J, Zhang M, Liu Y, Zhao S, Wang Y,
Wang M, Niu W, Jin F and Li Z: Histone lactylation driven by
mROS-mediated glycolytic shift promotes hypoxic pulmonary
hypertension. J Mol Cell Biol. 14:mjac0732023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang P, Xie D, Xiao T, Cheng C, Wang D,
Sun J, Wu M, Yang Y, Zhang A and Liu Q: H3K18 lactylation promotes
the progression of arsenite-related idiopathic pulmonary fibrosis
via YTHDF1/m6A/NREP. J Hazard Mater. 461:1325822024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galle E, Wong CW, Ghosh A, Desgeorges T,
Melrose K, Hinte LC, Castellano-Castillo D, Engl M, de Sousa JA,
Ruiz-Ojeda FJ, et al: H3K18 lactylation marks tissue-specific
active enhancers. Genome Biol. 23:2072022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Qin L, Chen W, Chen Q, Sun J and
Wang G: Novel strategies to improve tumour therapy by targeting the
proteins MCT1, MCT4 and LAT1. Eur J Med Chem. 226:1138062021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
She X, Wu Q, Rao Z, Song D, Huang C, Feng
S, Liu A, Liu L, Wan K, Li X, et al: SETDB1 methylates MCT1
promoting tumor progression by enhancing the lactate shuttle. Adv
Sci (Weinh). 10:e23018712023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hadjihambi A, Konstantinou C, Klohs J,
Monsorno K, Le Guennec A, Donnelly C, Cox IJ, Kusumbe A, Hosford
PS, Soffientini U, et al: Partial MCT1 invalidation protects
against diet-induced non-alcoholic fatty liver disease and the
associated brain dysfunction. J Hepatol. 78:180–190. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang F, Yi J, Chen Y, Bai X, Lu C, Feng S
and Zhou X: PRSS2 regulates EMT and metastasis via MMP-9 in gastric
cancer. Acta Histochem. 125:1520712023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hwang KE, Kim HJ, Song IS, Park C, Jung
JW, Park DS, Oh SH, Kim YS and Kim HR: Salinomycin suppresses
TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the
AMPK/SIRT1 pathway in non-small cell lung cancer. Int J Med Sci.
18:715–726. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang J, Huang D, Jiang Y, Hou J, Tian M,
Li J, Sun L, Zhang Y, Zhang T, Li Z, et al: Lactate modulates
cellular metabolism through histone lactylation-mediated gene
expression in non-small cell lung cancer. Front Oncol.
11:6475592021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Izzo LT and Wellen KE: Histone lactylation
links metabolism and gene regulation. Nature. 574:492–493. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Irizarry-Caro RA, Mcdaniel MM, Overcast
GR, Jain VG, Troutman TD and Pasare C: TLR signaling adapter BCAP
regulates inflammatory to reparatory macrophage transition by
promoting histone lactylation. Proc Natl Acad Sci USA.
117:30628–30638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ivashkiv LB: The hypoxia-lactate axis
tempers inflammation. Nat Rev Immunol. 20:85–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang F, Parayath NN, Ene CI, Stephan SB,
Koehne AL, Coon ME, Holland EC and Stephan MT: Genetic programming
of macrophages to perform anti-tumor functions using targeted mRNA
nanocarriers. Nat Commun. 10:39742019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dichtl S, Lindenthal L, Zeitler L, Behnke
K, Schlösser D, Strobl B, Scheller J, El Kasmi KC and Murray PJ:
Lactate and IL6 define separable paths of inflammatory metabolic
adaptation. Sci Adv. 7:eabg35052021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Siska PJ, Singer K, Evert K, Renner K and
Kreutz M: The immunological Warburg effect: Can a
metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy?
Immunol Rev. 295:187–202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang ZH, Peng WB, Zhang P, Yang XP and
Zhou Q: Lactate in the tumour microenvironment: From immune
modulation to therapy. EBioMedicine. 73:1036272021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
de la Cruz-López KG, Castro-Muñoz LJ,
Reyes-Hernández DO, García-Carrancá A and Manzo-Merino J: Lactate
in the regulation of tumor microenvironment and therapeutic
approaches. Front Oncol. 9:11432019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tay C, Tanaka A and Sakaguchi S:
Tumor-infiltrating regulatory T cells as targets of cancer
immunotherapy. Cancer Cell. 41:450–465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luo Y, Li L, Chen X, Gou H, Yan K and Xu
Y: Effects of lactate in immunosuppression and inflammation:
Progress and prospects. Int Rev Immunol. 41:19–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lopez Krol A, Nehring HP, Krause FF, Wempe
A, Raifer H, Nist A, Stiewe T, Bertrams W, Schmeck B, Luu M, et al:
Lactate induces metabolic and epigenetic reprogramming of
pro-inflammatory Th17 cells. EMBO Rep. 23:e546852022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m6A-modification on FDX1 mRNA
in gastric cancer. Nat Commun. 14:65232023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z,
Geng D, Yue J, Tang Y, Tian L, et al: The alanyl-tRNA synthetase
AARS1 moonlights as a lactyltransferase to promote YAP signaling in
gastric cancer. J Clin Invest. 134:e1745872024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang H, Zou X, Yang S, Zhang A, Li N and
Ma Z: Identification of lactylation related model to predict
prognostic, tumor infiltrating immunocytes and response of
immunotherapy in gastric cancer. Front Immunol. 14:11499892023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang J, Ren B, Yang G, Wang H, Chen G, You
L, Zhang T and Zhao Y: The enhancement of glycolysis regulates
pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shirbandi K, Rikhtegar R, Khalafi M, Mirza
Aghazadeh Attari M, Rahmani F, Javanmardi P, Iraji S, Babaei Aghdam
Z and Rezaei Rashnoudi AM: Functional magnetic resonance
spectroscopy of lactate in Alzheimer disease: A comprehensive
review of Alzheimer disease pathology and the role of lactate. Top
Magn Reson Imaging. 32:15–26. 2023.PubMed/NCBI
|
|
76
|
Afshar M and van Hall G: LC-MS/MS method
for quantitative profiling of ketone bodies, α-keto acids, lactate,
pyruvate and their stable isotopically labelled tracers in human
plasma: An analytical panel for clinical metabolic kinetics and
interactions. J Chromatogr B Analyt Technol Biomed Life Sci.
1230:1239062023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pivovarova AI and Macgregor GG:
Glucose-dependent growth arrest of leukemia cells by MCT1
inhibition: Feeding Warburg's sweet tooth and blocking acid export
as an anticancer strategy. Biomed Pharmacother. 98:173–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Saulle E, Spinello I, Quaranta MT,
Pasquini L, Pelosi E, Iorio E, Castelli G, Chirico M, Pisanu ME,
Ottone T, et al: Targeting lactate metabolism by inhibiting MCT1 or
MCT4 impairs leukemic cell proliferation, induces two different
related death-pathways and increases chemotherapeutic sensitivity
of acute myeloid leukemia cells. Front Oncol. 10:6214582021.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Todenhöfer T, Seiler R, Stewart C,
Moskalev I, Gao J, Ladhar S, Kamjabi A, Al Nakouzi N, Hayashi T,
Choi S, et al: Selective inhibition of the lactate transporter MCT4
reduces growth of invasive bladder cancer. Mol Cancer Ther.
17:2746–2755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xie Q, Zhu Z, He Y, Zhang Z, Zhang Y, Wang
Y, Luo J, Peng T, Cheng F, Gao J, et al: A lactate-induced
Snail/STAT3 pathway drives GPR81 expression in lung cancer cells.
Biochim Biophys Acta Mol Basis Dis. 1866:1655762020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brown TP, Bhattacharjee P, Ramachandran S,
Sivaprakasam S, Ristic B, Sikder MOF and Ganapathy V: The lactate
receptor GPR81 promotes breast cancer growth via a paracrine
mechanism involving antigen-presenting cells in the tumor
microenvironment. Oncogene. 39:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang H, Yang S, He J, Li W, Zhang A, Li N,
Zhou G and Sun B: Glucose transporter 3 (GLUT3) promotes
lactylation modifications by regulating lactate dehydrogenase A
(LDHA) in gastric cancer. Cancer Cell Int. 23:3032023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Huang YF, Wang G, Ding L, Bai ZR, Leng Y,
Tian JW, Zhang JZ, Li YQ, Ahmad Qin YH, et al: Lactate-upregulated
NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes
to ROS-induced osteoarthritis chondrocyte damage. Redox Biol.
67:1028672023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nareika A, He L, Game BA, Slate EH,
Sanders JJ, London SD, Lopes-Virella MF and Huang Y: Sodium lactate
increases LPS-stimulated MMP and cytokine expression in U937
histiocytes by enhancing AP-1 and NF-kappaB transcriptional
activities. Am J Physiol Endocrinol Metab. 289:E534–E542. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Long L, Xiong W, Lin F, Hou J, Chen G,
Peng T, He Y, Wang R, Xu Q and Huang Y: Regulating lactate-related
immunometabolism and EMT reversal for colorectal cancer liver
metastases using shikonin targeted delivery. J Exp Clin Cancer Res.
42:1172023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F,
Gill PS, Ha T, Liu L, Williams DL and Li C: Lactate promotes
macrophage HMGB1 lactylation, acetylation, and exosomal release in
polymicrobial sepsis. Cell Death Differ. 29:133–146. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X,
Shao Q, Zhou B, Zhou H, Wei S, et al: Tumor metabolite lactate
promotes tumorigenesis by modulating MOESIN lactylation and
enhancing TGF-β signaling in regulatory T cells. Cell Rep.
39:1109862022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Albogami SM, Al-Kuraishy HM, Al-Maiahy TJ,
Al-Buhadily AK, Al-Gareeb AI, Alorabi M, Alotaibi SS, De Waard M,
Sabatier JM, Saad HM and Batiha GE: Hypoxia-inducible factor 1 and
preeclampsia: A new perspective. Curr Hypertens Rep. 24:687–692.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
You L, Wu W, Wang X, Fang L, Adam V,
Nepovimova E, Wu Q and Kuca K: The role of hypoxia-inducible factor
1 in tumor immune evasion. Med Res Rev. 41:1622–1643. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T,
Williams DL and Li C: Lactate suppresses macrophage
pro-inflammatory response to LPS stimulation by inhibition of YAP
and NF-κB activation via GPR81-mediated signaling. Front Immunol.
11:5879132020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wei L, Yang X, Wang J, Wang Z, Wang Q,
Ding Y and Yu A: H3K18 lactylation of senescent microglia
potentiates brain aging and Alzheimer's disease through the NFκB
signaling pathway. J Neuroinflammation. 20:2082023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang G, Zou X, Chen Q, Nong W, Miao W, Luo
H and Qu S: The relationship and clinical significance of
lactylation modification in digestive system tumors. Cancer Cell
Int. 24:2462024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang H, Chen K, Zhu Y, Hu Z, Wang Y, Chen
J, Li Y, Li D and Wei P: A multi-dimensional approach to unravel
the intricacies of lactylation related signature for prognostic and
therapeutic insight in colorectal cancer. J Transl Med. 22:2112024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zha J, Zhang J, Lu J, Zhang G, Hua M, Guo
W, Yang J and Fan G: A review of lactate-lactylation in malignancy:
Its potential in immunotherapy. Front Immunol. 15:13849482024.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Su J, Zheng Z, Bian C, Chang S, Bao J, Yu
H, Xin Y and Jiang X: Functions and mechanisms of lactylation in
carcinogenesis and immunosuppression. Front Immunol.
14:12530642023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li X, Lu W, Hu Y, Wen S, Qian C, Wu W and
Huang P: Effective inhibition of nasopharyngeal carcinoma in
vitro and in vivo by targeting glycolysis with oxamate.
Int J Oncol. 43:1710–1718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Manerba M, Di Ianni L, Govoni M, Roberti
M, Recanatini M and Di Stefano G: Lactate dehydrogenase inhibitors
can reverse inflammation induced changes in colon cancer cells. Eur
J Pharm Sci. 96:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Moreno-Sánchez R, Marín-Hernández Á, Del
Mazo-Monsalvo I, Saavedra E and Rodríguez-Enríquez S: Assessment of
the low inhibitory specificity of oxamate, aminooxyacetate and
dichloroacetate on cancer energy metabolism. Biochim Biophys Acta
Gen Subj. 1861:3221–3236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wigfield SM, Winter SC, Giatromanolaki A,
Taylor J, Koukourakis ML and Harris AL: PDK-1 regulates lactate
production in hypoxia and is associated with poor prognosis in head
and neck squamous cancer. Br J Cancer. 98:1975–1984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Quanz M, Bender E, Kopitz C, Grünewald S,
Schlicker A, Schwede W, Eheim A, Toschi L, Neuhaus R, Richter C, et
al: Preclinical efficacy of the novel monocarboxylate transporter 1
inhibitor BAY-8002 and associated markers of resistance. Mol Cancer
Ther. 17:2285–2296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Curtis NJ, Mooney L, Hopcroft L,
Michopoulos F, Whalley N, Zhong H, Murray C, Logie A, Revill M,
Byth KF, et al: Pre-clinical pharmacology of AZD3965, a selective
inhibitor of MCT1: DLBCL, NHL and Burkitt's lymphoma anti-tumor
activity. Oncotarget. 8:69219–69236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Polański R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Colen CB, Shen Y, Ghoddoussi F, Yu P,
Francis TB, Koch BJ, Monterey MD, Galloway MP, Sloan AE and
Mathupala SP: Metabolic targeting of lactate efflux by malignant
glioma inhibits invasiveness and induces necrosis: An in vivo
study. Neoplasia. 13:620–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ullah MS, Davies AJ and Halestrap AP: The
plasma membrane lactate transporter MCT4, but not MCT1, is
up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J
Biol Chem. 281:9030–9037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Grasa L, Chueca E, Arechavaleta S,
García-González MA, Sáenz MÁ, Valero A, Hördnler C, Lanas Á and
Piazuelo E: Antitumor effects of lactate transport inhibition on
esophageal adenocarcinoma cells. J Physiol Biochem. 79:147–161.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Spinello I, Saulle E, Quaranta MT,
Pasquini L, Pelosi E, Castelli G, Ottone T, Voso MT, Testa U and
Labbaye C: The small-molecule compound AC-73 targeting CD147
inhibits leukemic cell proliferation, induces autophagy and
increases the chemotherapeutic sensitivity of acute myeloid
leukemia cells. Haematologica. 104:973–985. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhai X, Yang Y, Wan J, Zhu R and Wu Y:
Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and
increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol
Rep. 30:2983–2991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Schwab M, Thunborg K, Azimzadeh O, von
Toerne C, Werner C, Shevtsov M, Di Genio T, Zdralevic M, Pouyssegur
J, Renner K, et al: Targeting cancer metabolism breaks
radioresistance by impairing the stress response. Cancers (Basel).
13:37622021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lin YL, Yuksel Durmaz Y, Nör JE and
ElSayed MEH: Synergistic combination of small molecule inhibitor
and RNA interference against antiapoptotic Bcl-2 protein in head
and neck cancer cells. Mol Pharm. 10:2730–2738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
García-Castillo V, López-Urrutia E,
Villanueva-Sánchez O, Ávila-Rodríguez MÁ, Zentella-Dehesa A,
Cortés-González C, López-Camarillo C, Jacobo-Herrera NJ and
Pérez-Plasencia C: Targeting metabolic remodeling in triple
negative breast cancer in a murine model. J Cancer. 8:178–189.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Muramatsu H, Sumitomo M, Morinaga S,
Kajikawa K, Kobayashi I, Nishikawa G, Kato Y, Watanabe M, Zennami
K, Kanao K, et al: Targeting lactate dehydrogenase-A promotes
docetaxel-induced cytotoxicity predominantly in
castration-resistant prostate cancer cells. Oncol Rep. 42:224–230.
2019.PubMed/NCBI
|
|
114
|
Manerba M, Di Ianni L, Fiume L, Roberti M,
Recanatini M and Di Stefano G: Lactate dehydrogenase inhibitors
sensitize lymphoma cells to cisplatin without enhancing the drug
effects on immortalized normal lymphocytes. Eur J Pharm Sci.
74:95–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Daei Sorkhabi A, Mohamed Khosroshahi L,
Sarkesh A, Mardi A, Aghebati-Maleki A, Aghebati-Maleki L and
Baradaran B: The current landscape of CAR T-cell therapy for solid
tumors: Mechanisms, research progress, challenges, and
counterstrategies. Front Immunol. 14:11138822023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ogura M, Ando K, Suzuki T, Ishizawa K, Oh
SY, Itoh K, Yamamoto K, Au WY, Tien HF, Matsuno Y, et al: A
multicentre phase II study of vorinostat in patients with relapsed
or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell
lymphoma. Br J Haematol. 165:768–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kaufman JL, Mina R, Jakubowiak AJ,
Zimmerman TL, Wolf JJ, Lewis C, Gleason C, Sharp C, Martin T,
Heffner LT, et al: Combining carfilzomib and panobinostat to treat
relapsed/refractory multiple myeloma: Results of a multiple myeloma
research consortium phase I study. Blood Cancer J. 9:32019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lu W, Zhang L, Ji K, Ding L and Wu G:
Regulatory mechanisms of GCN5 in osteogenic differentiation of MSCs
in periodontitis. Clin Exp Dent Res. 9:464–471. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shao G, Liu Y, Ma T, Zhang L, Yuan M and
Zhao S: GCN5 inhibition prevents IL-6-induced prostate cancer
metastases through PI3K/PTEN/Akt signaling by inactivating Egr-1.
Biosci Rep. 38:BSR201808162018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Qu J, Li P and Sun Z: Histone lactylation
regulates cancer progression by reshaping the tumor
microenvironment. Front Immunol. 14:12843442023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Miao Z, Zhao X and Liu X: Hypoxia induced
β-catenin lactylation promotes the cell proliferation and stemness
of colorectal cancer through the wnt signaling pathway. Exp Cell
Res. 422:1134392023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zang Y, Wang A, Zhang J, Xia M, Jiang Z,
Jia B, Lu C, Chen C, Wang S, Zhang Y, et al: Hypoxia promotes
histone H3K9 lactylation to enhance LAMC2 transcription in
esophageal squamous cell carcinoma. iScience. 27:1101882024.
View Article : Google Scholar : PubMed/NCBI
|