Introduction
The complexity and heterogeneity of gastric cancer
(GC) presents substantial difficulties in disease control and
treatment (1). Even with progress
in detection methods and treatment options, GC continues to be a
primary contributor to global cancer-related deaths. In 2020, there
were ~1 million new cases and >769,000 deaths reported,
emphasizing its significant impact on public health (2). The progression and treatment
resistance of GC are closely linked to the tumor microenvironment
(TME) (2). The TME, which
encompasses a diverse array of cellular and acellular components,
plays a pivotal role in shaping the pathogenesis and progression of
GC (3). This dynamic and intricate
ecosystem evolves alongside the tumor, engaging in reciprocal
interactions that profoundly influence cancer cell behavior and
response to therapy (3).
Recent advancements in high-resolution imaging
technologies, single-cell sequencing approaches, and preclinical
modeling have shed light on the intricate organization and
functional heterogeneity within the GC microenvironment (4–6). These
insights have revealed the existence of distinct stromal
compartments, each characterized by unique cellular compositions
and spatial relationships. The plasticity of cancer-associated
fibroblasts (CAFs), immune cells and vascular components is
notable, and they engage in intricate interactions with cancer
cells, influencing their ability to proliferate, invade and
metastasize (7–9). Furthermore, the extracellular matrix
(ECM), an essential element of the TME, experiences dynamic changes
and aids in creating a conducive environment that promotes tumor
expansion and resistance to treatment (10).
In GC, the TME is not a passive observer, but a
dynamic contributor to disease progression. It coordinates an
intricate network of communication routes and released elements
that influence the actions of cancer cells and their reaction to
treatment strategies (11).
Chemotherapy, targeted therapies and immunotherapeutic approaches
often face significant obstacles posed by the TME, leading to
primary or acquired resistance (3).
Understanding the mechanisms by which the GC microenvironment
evolves and adapts to therapeutic pressures is crucial for
developing effective treatment strategies that can overcome
resistance and improve patient outcomes (12–14).
The present review aims to provide a comprehensive
overview of the current understanding of the TME in GC,
highlighting its role in disease pathogenesis and therapeutic
resistance. The cellular and acellular components that constitute
the GC microenvironment, their heterogeneity and the complex
interactions that shape tumor progression will be discussed.
Furthermore, the implications of the TME for metastasis and the
challenges it poses for effective therapeutic interventions will be
explored. By unraveling the intricacies of the GC microenvironment,
the present review aims to identify novel targets and strategies
for improving the management of GC.
Composition and heterogeneity of TME in
GC
The TME in GC is a complex and dynamic ecosystem
that encompasses a diverse array of cellular and acellular
components (12). The complex
environment notably influences the development, advancement, and
treatment outcomes of stomach cancers (3). The cellular composition of the GC
microenvironment is marked by a diverse group of stromal cells,
such as CAFs, immune cells and vascular elements (15) (Fig.
1). These components interact intricately with cancer cells,
aiding in creating a conducive environment for tumor expansion and
metastasis (15). Therefore,
in-depth understanding of the mechanism of TME can bring useful
value to the diagnosis and treatment of GC.
CAFs are a dominant cellular component of the GC
microenvironment and exhibit remarkable plasticity and functional
heterogeneity (16). These cells
are derived from various origins, including resident fibroblasts,
bone marrow-derived mesenchymal stem cells and endothelial cells
that undergo endothelial-to-mesenchymal transition (EndMT)
(10). CAFs secrete a wide range of
growth factors, cytokines and ECM components that modulate the
behavior of cancer cells, immune cells, endothelial cells and
smooth muscle cells (17–19). The activation and differentiation of
CAFs are regulated by complex signaling pathways, such as
transforming growth factor-β (TGF-β) (20), platelet-derived growth factor (PDGF)
(21), fibroblast growth factor
(FGF) signaling (22) and the NF-κB
signaling pathway (23). Therefore,
CAFs of different tumors and individuals have significant
heterogeneity, which also determines the characteristics of tumor
heterogeneity. Recent studies have revealed the existence of
distinct CAF subpopulations with unique molecular signatures and
functional properties (24–26), highlighting the need for a more
nuanced understanding of CAF heterogeneity in GC.
The immune cell component of the GC microenvironment
is highly diverse and plays a critical role in shaping the tumor
immune response (27).
Tumor-associated macrophages (TAMs) are a prominent immune cell
population in gastric tumors and exhibit a spectrum of activation
states, ranging from anti-tumoral M1-like phenotypes to pro-tumoral
M2-like phenotypes (28). TAMs
secrete various cytokines, chemokines and growth factors that
promote tumor growth, angiogenesis and immunosuppression (28–30). T
lymphocytes, including CD8+ cytotoxic T cells and CD4+ helper T
cells, are also present in the GC microenvironment and play a
crucial role in the anti-tumor immune response (31). However, the function of T cells is
often compromised by the immunosuppressive milieu created by cancer
cells and other stromal cells, leading to T cell exhaustion and
impaired anti-tumor immunity (32).
Other immune cell populations, such as regulatory T cells (Tregs),
myeloid-derived suppressor cells (MDSCs) and tumor-associated
neutrophils, contribute to the establishment of an
immunosuppressive microenvironment that promotes tumor progression
and therapeutic resistance (33–36).
Therefore, the human immune system plays an important anti-tumor
role in the early stage of GC, and as the immune system develops
tolerance to the tumor, it loses its protective effect. The
aforementioned findings indicate that the immunosuppressive
microenvironment may be a major factor contributing to the eventual
progression of GC.
In addition, TME provides sufficient nutrients and
oxygen for angiogenesis, which in turn promotes tumor growth
(37). Various drugs targeting
angiogenesis-related molecules, such as apatinib (38), axitinib (39), linifanib (40) and sorafenib (41) are available in clinics and are
effective in treating GC. The vascular structure of the GC
microenvironment is characterized by a complex network of blood
vessels and lymphatic vessels that support tumor growth and
metastasis (42). Tumor
angiogenesis, the formation of new blood vessels from pre-existing
ones, is driven by the production of pro-angiogenic factors, such
as vascular endothelial growth factor (VEGF), by cancer cells and
stromal cells (42). The tumor
vasculature in GC is often abnormal, with irregular branching
patterns, leaky vessel walls and impaired blood flow, leading to
hypoxia and acidosis within the TME (43). These abnormalities contribute to the
establishment of a hostile microenvironment that favors cancer cell
survival, invasion and metastasis (44). Lymphangiogenesis, the formation of
new lymphatic vessels, is also a critical process in GC
progression, facilitating the dissemination of cancer cells to
regional lymph nodes and distant organs (45). In summary, the TME promotes the
progression of GC by regulating the formation of blood vessels and
lymphatics. Therefore, targeting the TME for vascularization plays
an important role in tumor therapy.
The ECM is a key acellular component of the GC
microenvironment and undergoes dynamic remodeling during tumor
progression (46). The ECM is
composed of a complex network of proteins, glycoproteins and
proteoglycans, including collagen, fibronectin, laminin and
hyaluronan (46). Cancer cells and
stromal cells secrete various ECM-modifying enzymes, such as matrix
metalloproteinases (MMPs) and lysyl oxidases (LOXs), which alter
the composition and mechanical properties of the ECM (47). The remodeled ECM provides a
supportive scaffold for cancer cell invasion and migration, and
also serves as a reservoir for growth factors and cytokines that
regulate tumor growth and metastasis (7). The stiffness and porosity of the ECM
also influence the behavior of cancer cells and stromal cells, with
increased matrix stiffness promoting cancer cell proliferation,
survival and invasion (10). The
ECM provides a skeleton environment for the progression of GC,
which is conducive to the progression of tumor cells (25). Therefore, ECM also exerts a crucial
role in the occurrence and development of GC.
The spatial organization of the cellular and
acellular components within the GC microenvironment is highly
heterogeneous and varies across different regions of the tumor
(48). The tumor core, which is
often hypoxic and nutrient-deprived, is characterized by a dense
population of cancer cells and a relatively sparse stromal
compartment (49). By contrast, the
tumor periphery, which is more perfused and oxygenated, exhibits a
more abundant and diverse stromal compartment, with a higher
density of CAFs, immune cells and blood vessels (50). The spatial distribution of immune
cells within the TME also varies, with certain regions exhibiting a
higher density of immunosuppressive cells, such as Tregs and MDSCs,
while other regions may have a more prominent presence of
anti-tumor immune cells, such as CD8+ T cells and M1-like
macrophages (3). The aforementioned
features demonstrate that the treatment of cancer needs to be
individualized.
Recent advances in imaging technologies, such as
multiplex immunohistochemistry and spatial transcriptomics, have
enabled a more comprehensive characterization of the cellular and
spatial heterogeneity within the GC microenvironment (51–53).
These approaches have revealed the existence of distinct
microenvironmental niches, each with unique cellular compositions
and functional properties. For example, the perivascular niche,
which is located in close proximity to blood vessels, is enriched
in CAFs and TAMs that promote angiogenesis and metastasis (50). The invasive front, which is the
interface between the tumor and the adjacent normal tissue, is
characterized by a high density of cancer stem cells (CSCs) and
epithelial-mesenchymal transition (EMT)-undergoing cells that drive
tumor invasion and metastasis (54).
Ultimately, the intricate and ever-changing cellular
composition and framework of the TME in GC significantly influences
tumor development and the effectiveness of treatment (3). The diverse array of cellular and
acellular components within the microenvironment engage in
intricate interactions that support tumor growth, invasion,
metastasis and therapeutic resistance (11). Unraveling the complexity of the GC
microenvironment and its spatial heterogeneity is an ongoing
challenge that requires the integration of advanced imaging
technologies, single-cell analysis and computational modeling
approaches (55). A more profound
comprehension of the TME in GC could lay the groundwork for
creating more efficient, tailored treatment approaches that focus
not just on the cancer cells, but also on the supportive
environment that maintains them.
Influence of TME evolution on GC
progression
The TME provides a conducive setting for the
progression of GC through several mechanisms. Firstly, the
formation of a supportive microenvironment facilitates cancer cell
survival and proliferation (3).
Secondly, the inherent heterogeneity of the TME, characterized by
diverse cellular components and signaling pathways, fosters tumor
adaptability and resilience against therapeutic interventions
(15). Finally, the
microenvironment actively promotes tumor progression and metastasis
by enhancing invasive capabilities and providing necessary growth
factors and nutrients (13).
Collectively, these factors underscore the role of the TME in
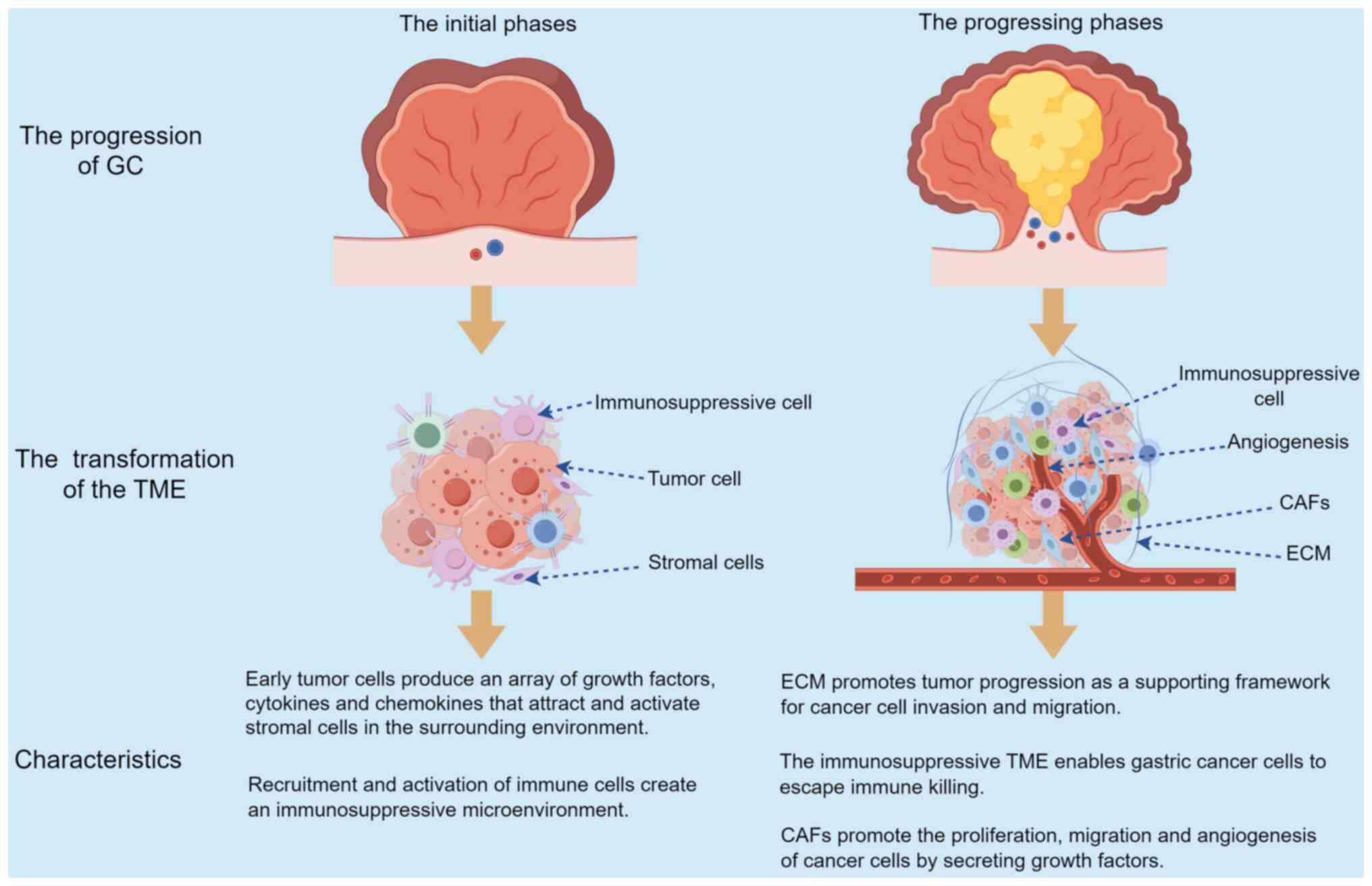
facilitating the aggressive nature and advance of GC (Fig. 2).
TME evolution in GC
The progression and transformation of the TME in GC
is a complex, multi-stage procedure that includes the enlistment
and stimulation of different stromal cells, the restructuring of
the ECM, and the creation of a sophisticated network of signaling
pathways (55). During the initial
phases of stomach cancer development, the conversion of regular
stomach epithelial cells into cancerous ones is frequently
initiated by genetic and epigenetic changes (56). These changes include mutations in
cancer-causing genes (such as Kras and Myc) and tumor inhibiting
genes (such as TP53 and CDH1), along with irregular DNA methylation
and alterations in histone modifications (57–59).
The changes result in the triggering of cancer-causing signal
routes, such as the Wnt/β-catenin (60), PI3K/AKT (61) and MAPK pathways (62), which encourage cell growth,
endurance and infiltration.
As the transformed epithelial cells proliferate and
form early neoplastic lesions, they start producing a range of
growth factors, cytokines and chemokines that attract and activate
stromal cells within the surrounding microenvironment (56). For example, cancer cells secrete
TGF-β, which induces the activation and differentiation of
quiescent fibroblasts into CAFs (63). CAFs, in turn, secrete a wide range
of growth factors, such as FGF, hepatocyte growth factor (HGF) and
VEGF, which promote cancer cell proliferation, migration and
angiogenesis (64–66). The recruitment and activation of
immune cells, such as TAMs and Tregs, is also mediated by cancer
cell-derived factors, such as colony-stimulating factor 1 and
chemokine ligand 2, which create an immunosuppressive
microenvironment that favors tumor growth and escape from immune
surveillance (12).
With the advancement of the tumor, the ECM
experiences active restructuring, marked by the enhanced
accumulation of collagen, fibronectin and laminins, along with the
triggering of matrix-dissolving enzymes such as MMPs and cathepsins
(47). The revamped ECM not only
acts as a supportive framework for the invasion and migration of
cancer cells, but also functions as a storage for growth factors
and cytokines, thereby enhancing the advancement of the tumor
(25). The enhanced rigidity of the
ECM, facilitated by the interconnection of collagen fibers through
LOX enzymes, has been demonstrated to stimulate mechanotransduction
routes in cancer cells, resulting in increased growth, survival and
infiltration (67).
The development of the TME in GC is also affected by
the metabolic restructuring of both cancer cells and stromal cells
(68). The tumor often expands
faster than the formation of new blood vessels, resulting in areas
of low oxygen levels and lack of nutrients (69). Cancer cells respond to hypoxia by
triggering the hypoxia-inducible factor (HIF) pathway (70). This leads to the activation of genes
that play a role in angiogenesis, glycolysis and cell longevity.
The heightened glycolytic activity of cancer cells results in
lactate buildup in the microenvironment (71). This has been demonstrated to
encourage the polarization of TAMs into an immunosuppressive
M2-like phenotype and to trigger the activation of CAFs (72).
The TME in GC also evolves in response to therapy,
such as chemotherapy and radiotherapy (14). While these treatments can
effectively kill cancer cells, they also induce a variety of
cellular and molecular changes in the microenvironment that can
contribute to therapeutic resistance and tumor recurrence (14). For example, chemotherapy has been
shown to induce the activation of CAFs and the recruitment of
MDSCs, which create a protective niche for CSCs and promote tumor
regrowth (13,73). Radiotherapy can also induce the
expression of pro-inflammatory cytokines, such as interleukin-6
(IL-6) and tumor necrosis factor-α (TNF-α), which stimulate the
activation of NF-κB and STAT3 signaling pathways in cancer cells,
leading to enhanced survival and invasion (74).
Comprehending the intricate and ever-changing
characteristics of the TME in GC is vital for creating efficient
treatment plans that target not only cancer cells, but also the
supportive environment that maintains them (3). Discovering the primary cellular and
molecular factors that drive tumor growth and resistance to
treatment within the microenvironment could pave the way for new
treatments, which could interrupt the communication between cancer
and stromal cells, boost the ability of the body to fight tumors,
and conquer resistance to therapy.
Role of TME in GC progression
The TME provides structural support and biochemical
signals that influence tumor behavior (15). Endothelial cells facilitate
angiogenesis, enhancing nutrient supply to the tumor (75). Additionally, the interaction between
tumor cells and surrounding nerves can promote tumor growth and
metastasis (12). Lastly,
lymphocytes exhibit diverse roles, with some aiding tumor
suppression while others contributing to immune evasion (76). These functions play important roles
in the progression of GC (16)
(Table I).
 | Table I.Mechanisms of different components in
the GC microenvironment promoting tumor progression. |
Table I.
Mechanisms of different components in
the GC microenvironment promoting tumor progression.
| Components of the
TME | Mechanism of
different components | (Refs.) |
|---|
| CAFs | CAFs stimulate the
proliferation, migration and angiogenesis of cancer cells by
secreting HGF, VEGF and EGF. | (77–79) |
| ECM | The deposition and
cross-linking of collagen fibers in ECM of GC can activate the
mechanical transduction pathway of cancer cells and promote their
proliferation and invasion. | (80–82) |
|
| ECM also acts as a
reservoir for growth factors and cytokines that regulate cancer
cell behavior and immune cell function, thereby promoting
angiogenesis and tumor growth. |
|
| Endothelial
cells | Tumor endothelial
cells promote cancer cell progression and metastasis by expressing
a variety of surface markers and adhesion molecules, such as IL-8
and MMPs. | (83) |
| Peripheral
nerves | Peripheral nerves
in the GC microenvironment also exhibit functional alterations,
characterized by increased neuronal activity that promotes cancer
cell survival and invasion through the release of neurotransmitters
and neuropeptides. | (84) |
| T cells | The function of T
cells in the GC microenvironment is often inhibited by multiple
immunosuppressive mechanisms, including the expression of
inhibitory receptors such as PD-1 and cytotoxic CTLA-4, as well as
the presence of immunosuppressive cytokines such as TGF-β and
IL-10. | (85) |
| B cells | B cells produce
immunosuppressive factors such as IL-10 and TGF-β to promote the
differentiation of Tregs, thereby inhibiting the function of T
cells and enabling GC cells to escape immune killing. | (86) |
| NKs | The function of NKs
in the GC microenvironment is often impaired by multiple
immunosuppressive mechanisms, including the presence of inhibitory
receptors such as KIRs and immunosuppressive cytokines such as
TGF-β and IL-10. | (87) |
| Macrophages | M2 macrophages
inhibit the function of T cells and NKs by producing
anti-inflammatory cytokines such as IL-10 and TGF-β, thereby
promoting angiogenesis and tissue remodeling to promote tumor
growth and metastasis. | (88) |
| MDSCs | MDSCs inhibit T
cell function through a variety of mechanisms, including the
production of immunosuppressive cytokines such as TGF-β and IL-10
and the expression of inhibitory receptors such as PD-L1. | (89) |
CAFs
CAFs are a prominent cellular component of the GC
microenvironment and exhibit notable functional and phenotypic
heterogeneity (25). Activated
fibroblasts, termed CAFs, originate from a variety of sources such
as resident fibroblasts, mesenchymal stem cells from bone marrow
and endothelial cells undergoing EndMT (90). The activation of CAFs is mediated by
a variety of growth factors and cytokines secreted by cancer cells
and other stromal cells, such as TGF-β, PDGF and FGF (7).
CAFs display a broad spectrum of roles that aid in
tumor expansion, infiltration and spread (91). A range of growth factors, including
HGF (77), VEGF (78) and epidermal growth factor (EGF)
(87), are secreted by CAFs,
promoting the proliferation, migration and angiogenesis of cancer
cells (91). CAFs also discharge a
range of ECM proteins, including collagen, fibronectin and laminin
(73). These proteins offer a
supportive framework for the invasion and migration of cancer cells
(92). The enhanced accumulation
and interconnection of collagen fibers by CAFs result in heightened
matrix rigidity, thereby stimulating mechanotransduction routes in
cancer cells and encouraging their growth and infiltration
(92).
Current research has uncovered the presence of
specific CAF subgroups, each possessing unique molecular
characteristics and functional attributes (93). For example, a subset of CAFs that
express high quantities of α-smooth muscle actin and fibroblast
activation protein (FAP) has been demonstrated to enhance the
invasion and metastasis of cancer cells by secreting MMPs and
activating the TGF-β signaling pathway (93). Another subpopulation of CAFs
expressing high levels of IL-6 and CXCL12 has been shown to create
an immunosuppressive microenvironment by recruiting MDSCs and
inhibiting the function of cytotoxic T cells (94,95).
The arrangement of CAFs in the TME significantly
influences the development of the tumor and the response to
treatment (73). Cancer cells are
frequently located near CAFs, which create a beneficial environment
that encourages their growth and survival (96). The occurrence of CAFs at the tumor
invasive forefront has been linked to a rise in cancer cell
invasion and metastasis, implying that CAFs could serve as a
‘pioneer’ in aiding tumor dissemination (90). The positioning of CAFs in the TME
could also impact the effectiveness of chemotherapy and
immunotherapy (90). This is
because CAFs have been demonstrated to form a physical obstruction
that restricts the penetration of drugs and to release substances
that hinder the activity of immune cells (97). Zhao and Zhu (98) demonstrated that CAF subpopulations
labeled with FAP, CD10 and GPR77 could contribute to resistance to
neoadjuvant chemotherapy in patients suffering from locally
advanced GC. This is achieved by triggering EMT in GC cells and
promoting CSCs.
Previous studies have revealed that CAFs, a major
component of the TME, significantly influence GC development and
response to therapies (99,100). The approach of focusing on CAFs is
being recognized as a potential treatment strategy in managing GC
(50). The latest progress has been
centered around suppressing the pro-cancerous activities of CAFs,
such as restructuring the ECM, fostering tumor development and
aiding in immune system avoidance. For example, the application of
FGFR inhibitors has demonstrated effectiveness in interrupting CAF
signaling pathways, resulting in decreased tumor vascularization
and increased responsiveness to chemotherapy (101). Additionally, blocking the
fibroblast activation protein has been explored to mitigate the
supportive role of CAFs in GC. Experiments are in progress to
assess the simultaneous targeting of CAFs in conjunction with
conventional treatment methods such as chemotherapy and
immunotherapy, with the goal of surmounting the treatment
resistance frequently seen in late stages of GC (102). The results of these studies may
provide new insights into effective multi-modal approaches to
target the TME, ultimately improving the overall prognosis for
patients with GC. As the present understanding of CAF biology
improves, innovative strategies may emerge, paving the way for
novel therapeutic options in GC.
ECM
The ECM is a complex network of proteins,
glycoproteins and proteoglycans that provides a structural and
functional scaffold for cells within the TME (103). The ECM plays a crucial role in
regulating cancer cell behavior, including proliferation, migration
and invasion, as well as in shaping the immune response and
therapeutic efficacy (104). In
GC, the ECM undergoes dynamic remodeling during tumor progression,
characterized by increased deposition of collagen, fibronectin and
laminins, as well as the activation of matrix-degrading enzymes,
such as MMPs and cathepsins (105).
The increased deposition and cross-linking of
collagen fibers in the GC microenvironment leads to increased
matrix stiffness, which has been shown to activate
mechanotransduction pathways in cancer cells and promote their
proliferation and invasion (80).
The enhanced production of LOX enzymes by cancer cells and CAFs is
associated with the cross-linking of collagen fibers and the
emergence of a rigid TME (106).
Studies have demonstrated that suppressing LOX activity can
decrease matrix rigidity and prevent the expansion and spread of
tumors in preclinical GC models (107).
The ECM also serves as a reservoir for growth
factors and cytokines that regulate cancer cell behavior and immune
cell function. For example, the ECM protein fibronectin has been
shown to bind and sequester VEGF, which promotes angiogenesis and
tumor growth (81). The degradation
of the ECM by MMPs and other proteases releases these bound growth
factors and cytokines, making them available for signaling to
cancer cells and immune cells (82). The ECM also regulates the
infiltration and function of immune cells within the TME. For
example, the increased deposition of collagen and other matrix
proteins can create a physical barrier that limits the infiltration
of cytotoxic T cells, NK cells and macrophage cells, while the
presence of certain ECM proteins, such as hyaluronan, has been
shown to promote the recruitment and activation of
immunosuppressive myeloid cells (108).
In the TME of GC, the ECM is crucial, impacting both
the advancement of the tumor and the reaction to treatments
(25). The significance of focusing
on the ECM to improve the effectiveness of treatments has been
underscored in recent research. For example, the restructuring of
the ECM is frequently linked to heightened tumor rigidity, which
can bestow a more hostile phenotype upon GC cells (109). Different tactics for altering or
interfering with the ECM have been investigated by researchers,
such as employing MMPs and substances that focus on particular ECM
elements such as collagen and hyaluronan (110). Moreover, the development of
integrin antagonists has been aimed at disrupting the interactions
between cancer cells and the ECM, with the goal of diminishing
migration and invasion (111). In
summary, improving the comprehension of the involvement of the ECM
in the progression of GC and developing therapeutic strategies to
target it may offer novel approaches for improving patient outcomes
and addressing resistance to current treatments.
Endothelial cells
In the TME of GC, endothelial cells are a vital
element, significantly contributing to angiogenesis and the control
of tumor expansion and metastasis (42). The development of new blood vessels
from those already existing, a process termed angiogenesis, is a
characteristic feature of cancer and is crucial for the advancement
and expansion of tumors (112). In
GC, the prognosis is often poor when there is an increase in
angiogenesis, which is influenced by a range of pro-angiogenic
elements such as VEGF, FGF and PDGF (113). These factors are produced by
cancer cells, CAFs and TAMs present in the TME (42).
Endothelial cells in the TME exhibit distinct
phenotypic and functional characteristics compared with normal
endothelial cells (50).
Tumor-associated endothelial cells (TECs) are often more
proliferative, migratory and permeable than normal endothelial
cells, and express a variety of surface markers and adhesion
molecules that facilitate the extravasation of cancer cells and
their metastatic spread (75). TECs
also secrete a variety of cytokines that promote cancer cell
survival and invasion, such as interleukin-8 (IL-8) and MMPs
(83).
The tumor vasculature in GC is often abnormal and
dysfunctional, characterized by irregular branching patterns, leaky
vessel walls and impaired blood flow (69). These abnormalities contribute to the
development of hypoxia and acidosis within the TME, which can
promote cancer cell survival and therapeutic resistance (70). Hypoxia activates the HIF pathway in
cancer cells, leading to the expression of pro-angiogenic factors
and the activation of survival pathways (70). Acidosis, on the other hand, can
promote the activation of matrix-degrading enzymes and the invasion
of cancer cells (70).
The approach of focusing on angiogenesis has
surfaced as a hopeful treatment strategy for GC. A number of agents
that inhibit angiogenesis have been developed, such as monoclonal
antibodies aimed at VEGF such as bevacizumab (114), and small molecule inhibitors
focused on VEGF receptors, for example, sunitinib and sorafenib
(41,115). The therapeutic effectiveness of
these substances has been restricted, partly because of the
emergence of resistance strategies and the triggering of alternate
pro-angiogenic routes (14). The
arrangement of endothelial cells in the TME is also vital in
influencing the development of tumors and the response to treatment
(116). The existence of
endothelial cells at the invasive edge of the tumor has been linked
to a rise in cancer cell invasion and metastasis, implying that
these cells could aid in the entry of cancer cells into the blood
circulation (116). The spatial
distribution of endothelial cells within the TME may also influence
the efficacy of anti-angiogenic therapies, as the normalization of
the tumor vasculature may require the targeting of specific
subpopulations of endothelial cells with distinct phenotypic and
functional characteristics (117).
Peripheral nerves
The peripheral nervous system significantly
influences the behavior of cancer cells and their response to
treatment in the TME of GC (118).
The stomach is a highly innervated organ, with a complex network of
sensory and motor neurons that regulate gastric motility, secretion
and blood flow (119). In GC, the
TME is characterized by increased nerve density and altered nerve
function, which can promote cancer cell survival, invasion and
metastasis (84).
The increased nerve density in the GC
microenvironment is mediated by the secretion of neurotrophic
factors, such as nerve growth factor (NGF) and brain-derived
neurotrophic factor (BDNF), by cancer cells and other stromal cells
(120). These neurotrophic factors
promote the growth and survival of neurons and the formation of new
nerve fibers within the TME (120). The increased nerve density has
been associated with poor prognosis in patients with GC and has
been shown to promote cancer cell invasion and metastasis in
preclinical models (121).
Furthermore, in the GC microenvironment, the peripheral nerves also
display changes in their function, marked by heightened neuronal
activity and the discharge of neurotransmitters and neuropeptides,
which can enhance the survival and invasion of cancer cells
(84). It has been demonstrated
that the neurotransmitter acetylcholine encourages the growth and
movement of GC cells by activating muscarinic receptors (122). Moreover, it has been demonstrated
that the neuropeptide substance P encourages angiogenesis and the
attraction of immune cells to the TME (123). The aforementioned research
indicated that peripheral nerves and tumors are an interaction
mechanism in the TME, which provides a strong neural regulatory
basis for tumor progression.
In the GC microenvironment, the communication
between cancer cells and peripheral nerves is facilitated through
several signaling routes, such as the neurokinin-1 receptor (NK-1R)
pathway and the tropomyosin receptor kinase (Trk) pathway (121,124). The NK-1R pathway is activated by
substance P and has been shown to promote cancer cell proliferation
and migration, as well as the release of pro-inflammatory cytokines
by immune cells (124). The Trk
pathway, on the other hand, is activated by neurotrophins such as
NGF and BDNF and has been shown to promote cancer cell survival and
invasion, as well as the formation of new nerve fibers within the
TME (121). The development of
effective nerve-targeted therapies is challenged by the complex and
diverse functions of the peripheral nervous system, as well as the
potential off-target effects of inhibiting nerve function in normal
tissues (125). An improved
understanding of the specific nerve-cancer cell interactions that
drive tumor progression and therapeutic resistance in GC is needed
to develop more specific and effective nerve-targeted
therapies.
Leukocytes
An important component of the TME in GC are
leukocytes, which play a key role in shaping the immune response to
the tumor and influencing cancer cell behavior (126). The TME in GC is characterized by a
complex and dynamic infiltration of various leukocyte subsets,
including T cells, B cells, natural killer cells (NKs), macrophages
and MDSCs, each with distinct phenotypic and functional
characteristics (15).
Antitumor immunity in GC is largely mediated by T
cells, which are key components of the adaptive immune response
(127). A cytotoxic CD8+ T cell
recognizes and kills cancer cells, whereas helper CD4+ T cells
provide support for CD8+ T cells to activate and function (127). However, the function of T cells in
the GC microenvironment is often suppressed by immunosuppressive
mechanisms, including inhibitory receptors such as programmed cell
death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated
protein 4, as well as the presence of immunosuppressive cytokines
such as TGF-β and interleukin-10 (IL-10) (85).
A second component of the adaptive immune response,
B cells, play both pro-tumor and anti-tumor roles in GC. They
produce antibodies that bind to antigens associated with tumors and
promote the activation of T cells and NKs (128). B cells also produce
immunosuppressive cytokines such as IL-10 and TGF-β, which inhibit
T cell function and induce Tregs differentiation (86).
Innate lymphoid cells, specifically NKs, are crucial
for the early identification and removal of cancer cells (63). NKs display a range of stimulatory
and suppressive receptors, enabling them to differentiate between
healthy and cancerous cells, and to trigger destructive reactions
against tumor cells (86). In the
GC microenvironment, the function of NKs is often compromised due
to several immunosuppressive tactics. These include the
manifestation of inhibitory receptors such as killer cell
immunoglobulin-like receptors and the existence of
immunosuppressive cytokines such as TGF-β and IL-10 (87).
In the TME of GC, macrophages, a type of innate
immune cell, have a crucial function. Depending on the signals
received from the TME (88),
macrophages may display pro-inflammatory (M1) or anti-inflammatory
(M2) characteristics. M1 macrophages, known for producing
pro-inflammatory cytokines such as TNF-α and IL-12, have
demonstrated the ability to enhance anti-cancer immune reactions.
By contrast, M2 macrophages are identified by their production of
anti-inflammatory cytokines such as IL-10 and TGF-β. They have been
proven to encourage tumor growth and metastasis by inhibiting the
activity of T cells and NKs, and by fostering angiogenesis and
tissue restructuring (88).
A diverse group of immature myeloid cells, known as
MDSCs, accumulate in the TME and are instrumental in inhibiting
anti-cancer immune reactions (35).
There are two primary categories of MDSCs: i) Polymorphonuclear
MDSCs; and ii) monocytic MDSCs, both of which have unique
phenotypic and functional traits (89). MDSCs inhibit T cells function
through a variety of mechanisms including the production of
immunosuppressive cytokines such as TGF-β and IL-10, the
manifestation of inhibitory receptors such as programmed cell
death-ligand 1 (PD-L1) and the reduction of crucial amino acids
such as arginine and tryptophan (89).
The evolution of immunotherapeutic methods such as
checkpoint inhibitors, adoptive cell treatments and cancer vaccines
has made immune system targeting a hopeful treatment strategy for
GC (86,129). Nevertheless, the effectiveness of
these methods is frequently hindered by the immunosuppressive
characteristics of the GC microenvironment, which can damage the
performance of anti-cancer immune cells and encourage the growth of
resistance to immunotherapy (3). To
devise more potent immunotherapeutic approaches that can counteract
immunosuppression and encourage lasting anti-cancer immune
reactions, it is necessary to gain a deeper comprehension of the
intricate interplay among cancer cells, immune cells and other
stromal cells within the GC microenvironment.
Influence of the TME on metastatic
progression
The process of metastasis is complex and involves
several stages, including the spread of cancer cells from the
original tumor location to remote organs, and the formation of
secondary tumors in these organs (130). The TME is crucial at every stage
of the metastatic process, ranging from the first invasion and
movement of cancer cells, to the creation of a conducive
environment in the far-off organ that fosters the survival and
expansion of metastatic cells (131).
The metastatic cascade heavily relies on a crucial
phase where cancer cells invade and migrate from the primary tumor
location, a process significantly affected by the TME (15). The ECM is a key component of the TME
that regulates cancer cell invasion and migration (15). The degradation of the ECM by MMPs,
serine protease and cystinase, secreted by cancer cells and stromal
cells, creates a permissive environment for cancer cell invasion
and migration (131). The
increased deposition and cross-linking of collagen fibers in the
TME also promotes cancer cell invasion and migration by increasing
matrix stiffness and activating mechanotransduction pathways in
cancer cells (10).
The engagement and stimulation of stromal cells,
including CAFs and TAMs, are also crucial in facilitating the
invasion and migration of cancer cells (132). CAFs release a range of growth
factors and cytokines, including TGF-β and HGF, which encourage the
EMT of cancer cells, a procedure that increases their ability to
migrate and invade (133). By
contrast, TAMs produce enzymes such as MMPs and cathepsins that
degrade the matrix, aiding in the invasion and migration of cancer
cells through the ECM (132).
The spread of cancer cells from the main tumor
location to remote organs is facilitated by the blood and lymphatic
vessels (125). The tumor
vasculature in GC is often abnormal and dysfunctional,
characterized by increased permeability and leakiness, which
facilitates the intravasation of cancer cells into the circulation
(134). The interaction of cancer
cells with endothelial cells and platelets in the circulation also
promotes their survival and adhesion to the endothelium of distant
organs (134). The creation of a
pre-metastatic niche in remote organs, facilitated by the release
of exosomes, MMPs, growth factors and cytokines from the primary
tumor, also encourages the outflow and settlement of spread-out
cancer cells (135).
For metastatic cells to survive and grow, it is
crucial to create a supportive environment in the remote organ
(15). The microenvironment of the
distant organ is often hostile to disseminated cancer cells,
characterized by a lack of growth factors and nutrients, and the
presence of immune surveillance mechanisms (15). To overcome these challenges,
disseminated cancer cells must adapt to the new microenvironment
and establish a supportive niche that promotes their survival and
growth (136). The procedure
entails the enlistment and stimulation of stromal cells, including
CAFs and TAMs, that release growth factors and cytokines, fostering
the endurance and multiplication of metastatic cells (136). The restructuring of the ECM in a
remote organ, facilitated by the release of matrix-degrading
enzymes from cancer and stromal cells, establishes a conducive
atmosphere for the proliferation and enlargement of metastatic
tumors (136).
The approach of focusing on the TME has surfaced as
a promising treatment plan for inhibiting and curing metastatic
illness in GC (3). Numerous
strategies have been investigated, such as the application of MMP
inhibitors to hinder the invasion and migration of cancer cells,
the employment of anti-angiogenic substances to stabilize the tumor
vasculature and inhibit the spread of cancer cells, and the
utilization of immunotherapeutic substances to boost anti-tumor
immune reactions and obstruct the formation of a conducive
environment in remote organs (137). The development of efficient
treatments aimed at the metastatic microenvironment is made
difficult by the intricate and ever-changing characteristics of the
metastatic process, along with the diversity of the TME at various
metastatic locations (3). There is
a need to comprehend more deeply the particular microenvironmental
elements that propel metastasis in GC, as well as the variations of
these elements across diverse metastatic locations, in order to
devise more efficient and tailored treatments for metastatic
ailments.
Mechanism of TME promoting GC
resistance
The TME also plays a crucial role in treatment
resistance in GC. Mechanisms such as EMT facilitate cellular
plasticity and promote aggressive tumor behavior (3). Additionally, CSCs contribute to
therapeutic failure through their inherent resistance to
conventional treatments (13). The
physical and chemical properties of the microenvironment can
further enhance resistance by altering drug delivery and efficacy
(138). Moreover, immune evasion
strategies employed by tumor cells allow them to escape immune
surveillance, further complicating treatment outcomes (Fig. 3). Overall, the intricate
interactions within the TME are pivotal in mediating therapeutic
resistance in GC (Table II).
 | Table II.Mechanism of TME on drug resistance
in GC therapy. |
Table II.
Mechanism of TME on drug resistance
in GC therapy.
| Pathways | Mechanisms of
action | (Refs.) |
|---|
| EMT | The EMT process
facilitates the ability of the tumor to invade neighboring tissues
and spread far away. In addition, mesenchymal cells produced by EMT
exhibit metabolic alterations and enhanced survival signaling,
promoting therapeutic resistance. | (139) |
| CSCs | The
microenvironment of GC is characterized by hypoxia, nutrient
deprivation and inflammation, creating an ideal environment for the
growth of CSCs. Factors such as hypoxia-inducible factor-1α are
upregulated under hypoxic conditions, promoting CSC properties and
enhancing resistance to chemotherapy drugs. | (140) |
| Physical and
chemical properties | The ECM of GC is
dense fibrous tissue, which hinders drug delivery, leading to
unsatisfactory drug concentrations in tumor cells. In addition, the
acidity of TME can reduce the effectiveness of the drug. | (141) |
| Immune evasion | GC is often
characterized by a high degree of infiltration of immunosuppressive
cells, such as Tregs and MDSCs, which can alter the local immune
environment. | (143,144) |
|
| Factors released by
tumor cells and stromal cells promote the recruitment and
activation of these immunosuppressive cells, thereby inhibiting
effective anti-tumor immune responses. |
|
EMT
The EMT process is vital in boosting the ability of
GC cells to migrate and invade, thereby playing a significant role
in resistance to treatment (139).
In the process of EMT, cancer cells shed their epithelial features
such as polarity and tight junctions, and gain mesenchymal
properties (54). This
transformation aids their infiltration into nearby tissues and
enhances their capacity to spread to remote locations (54). The shift frequently occurs due to
different elements found in the TME, such as growth factors,
inflammatory cytokines and components of the ECM (25). For example, it has been demonstrated
that TGF-β and FGF induce EMT in GC cells, leading to a more
aggressive phenotype that exhibits greater resistance to standard
treatments such as chemotherapy and targeted agents (139). Additionally, the mesenchymal-like
cells arising from EMT exhibit altered metabolism and enhanced
survival signals, which further contribute to therapy resistance
(54).
CSCs
Another pivotal element within the TME that drives
treatment resistance in GC is the presence of CSCs. These cells
possess self-renewal properties and are considered to be
responsible for tumor initiation and recurrence (13). The microenvironment of gastric
tumors, characterized by hypoxia, nutrient deprivation and
inflammation, creates an ideal niche for CSCs to thrive (13). Factors such as HIF-1α are
upregulated under low oxygen conditions, promoting CSC properties
and enhancing resistance to chemotherapeutic agents (13). Furthermore, the interactions between
CSCs and their microenvironment can lead to the activation of
signaling pathways such as Wnt, Notch and Hedgehog, which provide
the cells with survival advantages and diminish the effects of
therapies (140). Consequently,
the eradication of CSCs may be essential for improving treatment
outcomes in GC.
Physical and chemical properties
The physical and chemical properties of the TME are
instrumental in mediating treatment resistance (25). The ECM in gastric tumors is often
dense and fibrotic, which can physically impede drug delivery,
leading to suboptimal therapeutic concentrations in tumor cells
(25). Additionally, the acidity of
the TME can influence drug efficacy, for instance, certain
chemotherapeutic agents require a neutral pH for optimal activity,
and the acidic milieu can decrease their effectiveness (3). Moreover, the presence of metabolically
active stromal cells can alter the metabolic landscape of the
tumor, leading to a phenomenon termed metabolic cooperation, where
cancer cells adapt to survive by utilizing metabolites produced by
surrounding stromal cells (141).
Such adaptations can help tumor cells resist the cytotoxic effects
of treatment, emphasizing the need to consider the physical and
chemical composition of the microenvironment when developing
therapeutic strategies (141).
Immune evasion
Finally, immune evasion is a significant mechanism
by which the TME contributes to GC treatment resistance. The immune
landscape linked with tumors in GC frequently features a
significant influx of immunosuppressive cells such as Tregs and
MDSCs, altering the local immune setting (142). The secretion of elements by
cancerous and stromal cells can stimulate the attraction and
stimulation of these immune-inhibiting cells, resulting in the
inhibition of potent anti-cancer immune reactions (142). For instance, the expression of
PD-L1 on GC cells can suppress the activation and multiplication of
T-cells, thereby reducing the effectiveness of immune checkpoint
inhibitors (143,144). The development of resistance to
immunotherapy approaches is significantly influenced by this immune
evasion as well (3). Therefore,
tactics focused on counteracting immune inhibition in the TME have
potential to conquer resistance and enhance treatment outcomes in
GC.
In summary, the TME significantly influences
treatment resistance in GC through various mechanisms, including
EMT dynamics, altered physical and chemical properties, and immune
evasion. A comprehensive understanding of these interactions and
their implications in therapy resistance is essential for the
development of effective treatment strategies tailored to
counteract these challenges.
Therapeutic methods of reducing GC
resistance through the TME effect
In previous years, multiple strategies utilizing
the TME hold promise for overcoming treatment resistance in GC.
Combination therapies effectively harness the strengths of various
modalities, while immunotherapy and photodynamic therapy (PDT)
offer innovative angles to tackle tumor resilience. Continued
investigation is warranted to refine these approaches, understand
their limitations, and enhance therapeutic outcomes for patients
with GC (145,146).
Combination therapy
Combination therapy, particularly the use of
chemotherapy with targeted agents or immunotherapy, has
demonstrated efficacy in overcoming TME-induced resistance. For
instance, studies have shown that combining traditional
chemotherapeutics, such as paclitaxel or cisplatin, with
anti-angiogenic agents such as ramucirumab can improve survival
outcomes in patients with GC (147,148). This is largely attributed to the
modulation of the TME, where anti-angiogenics can normalize
aberrant tumor vasculature, thereby improving drug delivery and
efficacy (69). However, the
challenges associated with combination therapy include increased
toxicity and the potential for overlapping side effects, which can
lead to reduced patient compliance and quality of life (149). It is also essential to identify
specific biomarkers to select patients who are more likely to
respond to combination therapies, which remains an area needing
further research (150).
Immunotherapy
Immunotherapy represents a promising avenue for
overcoming resistance in GC by reactivating the host immune
response against tumor cells. Immune checkpoint inhibitors, such as
PD-1 inhibitors, have shown efficacy in microsatellite
instability-high and human epidermal growth factor receptor
2-positive GC (151,152). Additionally, adoptive cell
therapy, including chimeric antigen receptor T-cell therapy, is
currently being explored for solid tumors, including GC, and offers
potential advantages by directly targeting the TME (153). Nevertheless, immunotherapy faces
challenges such as the immunosuppressive nature of the TME, which
often harbors MDSCs and Tregs that can diminish immune responses.
Furthermore, not all patients with GC respond to immunotherapy, and
predictive biomarkers are required to identify suitable candidates
(15).
PDT
PDT is an emerging treatment modality that
harnesses light-sensitive drugs to induce cell death upon light
activation, targeting both tumor cells and the TME (145). PDT can disrupt the TME by altering
the hypoxic environment and inducing immune-mediated responses
(145). Studies have indicated
that PDT combined with immune-modulating agents can potentiate
antitumor immunity in GC models, enhancing the efficacy of the
therapy (145,154). However, limitations of PDT include
its dependence on the precise delivery of light to the tumor site
and potential damage to surrounding healthy tissue. Additionally, a
deeper understanding of the optimal light-dosing and schedule is
crucial for maximizing therapeutic outcomes while minimizing side
effects (155).
Targeting the ECM
The ECM plays a vital role in the TME, affecting
drug delivery and tumor progression (25). Therapies targeting ECM components,
such as hyaluronidase or matrix metalloproteinase inhibitors, are
being investigated for their ability to enhance the permeability of
chemotherapeutic agents and improve treatment efficacy (156,157). By degrading the ECM, these agents
can promote drug diffusion and alleviate mechanical barriers that
contribute to drug resistance (25). Nonetheless, the manipulation of the
ECM may have unintended consequences, such as promoting tumor
metastasis or altering the overall matrix composition, thereby
necessitating a careful assessment of risk vs. benefit in
therapeutic application (105).
Outlook and challenges
The microenvironment of GC is characterized by a
complex and heterogeneous landscape that significantly influences
tumor behavior and therapeutic response. Composed of various cell
types, including CAFs, immune cells, endothelial cells and ECM
components, the GC microenvironment provides both structural
support and dynamic signals that dictate tumor progression
(48). CAFs play a dual role by
promoting tumor growth through the secretion of growth factors and
cytokines while also contributing to the desmoplastic stroma, which
can hinder drug penetration (113). The ECM in GC is often altered,
with changes in composition and stiffness that facilitate cancer
cell migration and invasion (10).
Inflammatory processes, which are mediated by immune and stromal
cells, further compound the complexity of the microenvironment,
with cytokines such as IL-6 and TNF-α creating a pro-tumorigenic
milieu that enhances the aggressiveness of gastric tumors (15).
The GC microenvironment promotes tumor progression
through multiple mechanisms. Studies in previous years have
highlighted the importance of persistent inflammation in the
development of stomach cancer, especially in relation to
Helicobacter pylori infection (158,159). This infection results in the
attraction of immune cells and the generation of inflammatory
cytokines (158). This chronic
inflammatory state contributes to genetic instability and promotes
a conducive environment for malignant transformation (160). Moreover, the existence of
immunosuppressive cells such as Tregs and MDSCs can hinder
efficient anti-cancer immune reactions, enabling cancerous cells to
avoid immune detection and multiply without control (142). Emerging evidence also suggests
that hypoxia, a common feature of solid tumors, shapes the TME,
activating pathways associated with tumor progression and
metastasis (161). By
understanding these interactions, strategies to disrupt the support
systems that the TME provides can be delineated, and thus hinder
tumor advancement.
The influence of the GC microenvironment on
treatment resistance is profound and multifaceted. One of the major
contributions is through cellular plasticity, particularly the
phenomenon of EMT, which endows cancer cells with stem-like
properties that make them more resilient to therapies (162). CSCs, often located within the TME,
can survive aggressive treatments that eliminate differentiated
tumor cells, leading to recurrence and metastasis (163). Additionally, the TME can mediate
drug resistance through altered drug metabolism and transport
mechanisms, often in response to the hypoxic or acidic conditions
that characterize numerous tumors (15). Interactions between cancer cells and
surrounding stromal cells can activate survival signaling pathways,
further shielding cancer cells from the effects of
chemotherapeutics and targeted therapies (25). As a result, understanding the role
of the TME is imperative for developing combination therapies that
can effectively target both tumor cells and their protective
microenvironment.
Comprehending the intricate cellular composition
and spatial arrangement of the GC microenvironment is essential for
devising potent therapeutic approaches aimed at the tumor and its
supporting niche (3). Identifying
crucial cellular and molecular factors that drive tumor growth and
resistance to treatment within the microenvironment could pave the
way for the development of innovative treatments (3). These factors could interrupt the
communication between cancer and stromal cells, boost anti-cancer
immunity and regulate tumor blood vessels (43). Furthermore, the characterization of
distinct microenvironmental niches within gastric tumors may enable
the development of personalized treatment approaches that consider
the unique features of the TME of each patient (27).
The development of therapeutic approaches that
specifically focus on the GC microenvironment is increasingly
attracting attention. Potential approaches include the use of
anti-fibrotic agents aimed at normalizing the ECM to improve drug
delivery, as well as therapies designed to modulate the immune
landscape. For instance, combining immune checkpoint inhibitors
with therapies that deplete immunosuppressive cells or activate the
immune system could improve treatment outcomes (164). Additionally, targeting specific
signaling pathways involved in the tumor-stroma interactions may
mitigate the aggressive behavior of GC (25).
However, several challenges remain. The
heterogeneity of the TME poses a significant hurdle in developing
effective therapies, as different subpopulations of cancer cells
may respond differently to treatments targeting the
microenvironment (25).
Additionally, the dynamic nature of the TME can lead to adaptive
resistance, where cells that survive initial treatments develop new
mechanisms of resilience (15).
Another concern is the potential for off-target effects when
targeting components of the TME, which could impact normal tissue
function and lead to toxicity (180). These factors emphasize the
need for customized therapy strategies designed to suit the
individual features of the TME each patient.
Conclusion
In conclusion, the TME is crucial in the
progression of therapeutic resistance in GC through various methods
such as triggering pro-survival signaling routes, initiating EMT,
promoting CSC survival and growth, generating harsh physical and
chemical surroundings, and inhibiting anti-cancer immune reactions.
Understanding these mechanisms and developing strategies to target
the TME are crucial for overcoming therapeutic resistance and
improving patient outcomes in patients with GC. While several
promising approaches have been identified, including the use of
small molecule inhibitors, immune checkpoint inhibitors,
hypoxia-activated prodrugs, and CAF- and TAM-targeting agents, more
research is needed to fully elucidate the complex interactions
between cancer cells and the TME, and to develop more effective and
personalized therapies for patients with GC.
Acknowledgements
Not applicable.
Funding
This work was supported by The Baiyin Science and Technology
Plan Project (grant no. 2024-0124).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY and YW were responsible for the conception and
writing of the present study. FD and YW were responsible for the
data retrieval and figure production. XW and XY were responsible
for the revision of the paper. RZ and XZ were responsible for the
language editing of the manuscript. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
TME
|
tumor microenvironment
|
|
CAFs
|
cancer-associated fibroblasts
|
|
ECM
|
extracellular matrix
|
|
TGF-β
|
transforming growth factor β
|
|
PDGF
|
platelet-derived growth factor
|
|
FGF
|
fibroblast growth factor
|
|
TAMs
|
tumor-associated macrophages
|
|
Tregs
|
regulatory T cells
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
VEGF
|
vascular endothelial growth
factor
|
|
MMPs
|
matrix metalloproteinases
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HGF
|
hepatocyte growth factor
|
|
IL-6
|
interleukin-6
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LOX
|
lysyl oxidase
|
|
HIF
|
hypoxia-inducible factor
|
|
FAP
|
fibroblast activation protein
|
|
TECs
|
tumor-associated endothelial
cells
|
|
IL-8
|
interleukin-8
|
|
NGF
|
nerve growth factor
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
NK-1R
|
neurokinin-1 receptor
|
|
Trk
|
tropomyosin receptor kinase
|
|
NKs
|
natural killer cells
|
|
PD-1
|
programmed cell death protein 1
|
|
IL-10
|
interleukin-10
|
|
CSCs
|
cancer stem cells
|
References
|
1
|
Kumar V, Ramnarayanan K, Sundar R,
Padmanabhan N, Srivastava S, Koiwa M, Yasuda T, Koh V, Huang KK,
Tay ST, et al: Single-cell atlas of lineage states, tumor
microenvironment, and subtype-specific expression programs in
gastric cancer. Cancer Discov. 12:670–691. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yasuda T and Wang YA: Gastric cancer
immunosuppressive microenvironment heterogeneity: Implications for
therapy development. Trends Cancer. 10:627–642. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Yin J, Zhao L, Zhou G, Dong S,
Zhang Y, Niu P, Ren H, Zheng T, Yan J, et al: Reconstruction of the
gastric cancer microenvironment after neoadjuvant chemotherapy by
longitudinal single-cell sequencing. J Transl Med. 20:5632022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zu M, Hao X, Ning J, Zhou X, Gong Y, Lang
Y, Xu W, Zhang J and Ding S: Patient-derived organoid culture of
gastric cancer for disease modeling and drug sensitivity testing.
Biomed Pharmacother. 163:1147512023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo M, Hu P, Xie J, Tang K, Hu S, Sun J,
He Y, Li J, Lu W, Liu H, et al: Remodeling the immune
microenvironment for gastric cancer therapy through antagonism of
prostaglandin E2 receptor 4. Genes Dis. 11:1011642023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun H, Wang X, Wang X, Xu M and Sheng W:
The role of cancer-associated fibroblasts in tumorigenesis of
gastric cancer. Cell Death Dis. 13:8742022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaopeng Z, Yang Z, Yuan F, Chen H and
Zhengjun Q: Regulation of regulatory T cells and tumor-associated
macrophages in gastric cancer tumor microenvironment. Cancer Med.
13:e69592024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh HL and Tsai MM: Tumor
progression-dependent angiogenesis in gastric cancer and its
potential application. World J Gastrointest Oncol. 11:686–704.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreira AM, Pereira J, Melo S, Fernandes
MS, Carneiro P, Seruca R and Figueiredo J: The extracellular
matrix: An accomplice in gastric cancer development and
progression. Cells. 9:3942020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang F, Zhang T, Li Q, Chen X, Jiang F and
Shen X: The tumor immune-microenvironment in gastric cancer.
Tumori. 108:541–551. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Li C, Lu Y, Liu C and Yang W: Tumor
microenvironment-mediated immune tolerance in development and
treatment of gastric cancer. Front Immunol. 13:10168172022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Meng WJ and Wang ZQ: Cancer stem
cells and the tumor microenvironment in gastric cancer. Front
Oncol. 11:8039742022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Yuan Q, Guo H, Guan H, Hong Z and
Shang D: Deciphering drug resistance in gastric cancer: Potential
mechanisms and future perspectives. Biomed Pharmacother.
173:1163102024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rojas A, Araya P, Gonzalez I and Morales
E: Gastric tumor microenvironment. Adv Exp Med Biol. 1226:23–35.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiori ME, Di Franco S, Villanova L, Bianca
P, Stassi G and De Maria R: Cancer-associated fibroblasts as
abettors of tumor progression at the crossroads of EMT and therapy
resistance. Mol Cancer. 18:702019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi H, Enomoto A, Woods SL, Burt AD,
Takahashi M and Worthley DL: Cancer-associated fibroblasts in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 16:282–295.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helms E, Onate MK and Sherman MH:
Fibroblast heterogeneity in the pancreatic tumor microenvironment.
Cancer Discov. 10:648–656. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang Z, Meng Q, Xu J, Wang W, Zhang B, Liu
J, Liang C, Hua J, Zhao Y, Yu X and Shi S: Signaling pathways in
cancer-associated fibroblasts: Recent advances and future
perspectives. Cancer Commun (Lond). 43:3–41. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan J, Xiao G, Yang C, Liu Q, Lv C, Yu X,
Zhou Z, Lin S, Bai Z, Lin H, et al: Cancer-associated fibroblasts
promote lymphatic metastasis in cholangiocarcinoma via the
PDGF-BB/PDGFR-β mediated paracrine signaling network. Aging Dis.
15:369–389. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita H, Yashiro M, Fukuoka T,
Hasegawa T, Morisaki T, Kasashima H, Masuda G, Noda S and Hirakawa
K: Diffuse-type gastric cancer cells switch their driver pathways
from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor
microenvironments. Carcinogenesis. 36:1511–1520. 2015.PubMed/NCBI
|
|
23
|
Lou M, Iwatsuki M, Wu X, Zhang W,
Matsumoto C and Baba H: Cancer-associated fibroblast-derived IL-8
upregulates PD-L1 expression in gastric cancer through the NF-κB
pathway. Ann Surg Oncol. 31:2983–2995. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galbo PM Jr, Zang X and Zheng D: Molecular
features of cancer-associated fibroblast subtypes and their
implication on cancer pathogenesis, prognosis, and immunotherapy
resistance. Clin Cancer Res. 27:2636–2647. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto Y, Kasashima H, Fukui Y, Tsujio
G, Yashiro M and Maeda K: The heterogeneity of cancer-associated
fibroblast subpopulations: Their origins, biomarkers, and roles in
the tumor microenvironment. Cancer Sci. 114:16–24. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q and Peng C: Cancer-associated
fibroblasts regulate the biological behavior of cancer cells and
stroma in gastric cancer. Oncol Lett. 15:691–698. 2018.PubMed/NCBI
|
|
27
|
Lin Y, Pan X, Zhao L, Yang C, Zhang Z,
Wang B, Gao Z, Jiang K, Ye Y, Wang S and Shen Z: Immune cell
infiltration signatures identified molecular subtypes and
underlying mechanisms in gastric cancer. NPJ Genom Med. 6:832021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gambardella V, Castillo J, Tarazona N,
Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló
S, Roda D, Huerta M, Cervantes A and Fleitas T: The role of
tumor-associated macrophages in gastric cancer development and
their potential as a therapeutic target. Cancer Treat Rev.
86:1020152020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang G, Gao Z, Guo X, Ma R, Wang X, Zhou
P, Li C, Tang Z, Zhao R and Gao P: CAP2 promotes gastric cancer
metastasis by mediating the interaction between tumor cells and
tumor-associated macrophages. J Clin Invest. 133:e1662242023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei
XL, Liu J, Chen J, Zeng ZL, Ju HQ, et al: Targeting the STING
pathway in tumor-associated macrophages regulates innate immune
sensing of gastric cancer cells. Theranostics. 10:498–515. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ivanović T, Božić D, Benzon B, Čapkun V,
Vukojević K and Glavina Durdov M: Histological type, cytotoxic T
cells and macrophages in the tumor microenvironment affect the
PD-L1 status of gastric cancer. Biomedicines. 11:7092023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu HT, Ai X, Lu M, Song Z and Li H:
Characterization of intratumoral and circulating IL-10-producing B
cells in gastric cancer. Exp Cell Res. 384:1116522019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y,
Li B and Li H: The effects of TNF-α/TNFR2 in regulatory T cells on
the microenvironment and progression of gastric cancer. Int J
Cancer. 150:1373–1391. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mesali H, Ajami A, Hussein-Nattaj H,
Rafiei A, Rajabian Z, Asgarian-Omran H, Hosseini V, Taghvaei T and
Tehrani M: Regulatory T cells and myeloid-derived suppressor cells
in patients with peptic ulcer and gastric cancer. Iran J Immunol.
13:167–177. 2016.PubMed/NCBI
|
|
35
|
Tsutsumi C, Ohuchida K, Katayama N, Yamada
Y, Nakamura S, Okuda S, Otsubo Y, Iwamoto C, Torata N, Horioka K,
et al: Tumor-infiltrating monocytic myeloid-derived suppressor
cells contribute to the development of an immunosuppressive tumor
microenvironment in gastric cancer. Gastric Cancer. 27:248–262.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Shi H, Yuan X, Jiang P, Qian H
and Xu W: Tumor-derived exosomes induce N2 polarization of
neutrophils to promote gastric cancer cell migration. Mol Cancer.
17:1462018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weygant N, Ge Y, Westphalen CB, Ma WW and
Vega KJ: Role of the microenvironment in gastrointestinal tumors. J
Oncol. 2019:21534132019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo Q, Dong Z, Xie W, Fu X, Lin L, Zeng Q,
Chen Y, Ye G, Chen M, Hu H, et al: Apatinib remodels the
immunosuppressive tumor ecosystem of gastric cancer enhancing
anti-PD-1 immunotherapy. Cell Rep. 42:1124372023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oh DY, Doi T, Shirao K, Lee KW, Park SR,
Chen Y, Yang L, Valota O and Bang YJ: Phase I study of axitinib in
combination with cisplatin and capecitabine in patients with
previously untreated advanced gastric cancer. Cancer Res Treat.
47:687–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Guo J, Chen Z, Wang J, Liu M and
Pang X: Linifanib (ABT-869) potentiates the efficacy of
chemotherapeutic agents through the suppression of receptor
tyrosine kinase-mediated AKT/mTOR signaling pathways in gastric
cancer. Sci Rep. 6:293822016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ryu MH, Lee KH, Shen L, Yeh KH, Yoo C,
Hong YS, Park YI, Yang SH, Shin DB, Zang DY, et al: Randomized
phase II study of capecitabine plus cisplatin with or without
sorafenib in patients with metastatic gastric cancer (STARGATE).
Cancer Med. 12:7784–7794. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giubelan A, Stancu MI, Honţaru SO,
Mălăescu GD, Badea-Voiculescu O, Firoiu C and Mogoantă SŞ: Tumor
angiogenesis in gastric cancer. Rom J Morphol Embryol. 64:311–318.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng Y, Dai Y, Gong Z, Cheng JN, Zhang L,
Sun C, Zeng X, Jia Q and Zhu B: Association between angiogenesis
and cytotoxic signatures in the tumor microenvironment of gastric
cancer. Onco Targets Ther. 11:2725–2733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brzozowa M, Michalski M, Harabin-Słowińska
M and Wojnicz R: The role of tumour microenvironment in gastric
cancer angiogenesis. Prz Gastroenterol. 9:325–328. 2014.PubMed/NCBI
|
|
45
|
Liu P, Ding P, Sun C, Chen S, Lowe S, Meng
L and Zhao Q: Lymphangiogenesis in gastric cancer: Function and
mechanism. Eur J Med Res. 28:4052023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z, Xue F, Li M, Zhu X, Lu X, Wang C,
Xu E, Wang X, Zhang L, Yu H, et al: Extracellular matrix
characterization in gastric cancer helps to predict prognosis and
chemotherapy response. Front Oncol. 11:7533302021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moreira AM, Ferreira RM, Carneiro P,
Figueiredo J, Osório H, Barbosa J, Preto J, Pinto-do-Ó P, Carneiro
F and Seruca R: Proteomic identification of a gastric tumor ECM
signature associated with cancer progression. Front Mol Biosci.
9:8185522022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi
M, Bin J, Liao Y, Rao J and Liao W: Tumor microenvironment
characterization in gastric cancer identifies prognostic and
immunotherapeutically relevant gene signatures. Cancer Immunol Res.
7:737–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia X, Wang S, Ni B, Xing S, Cao H, Zhang
Z, Yu F, Zhao E and Zhao G: Hypoxic gastric cancer-derived exosomes
promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1α
positive feedback loop. Oncogene. 39:6231–6244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oya Y, Hayakawa Y and Koike K: Tumor
microenvironment in gastric cancers. Cancer Sci. 111:2696–2707.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou Z and Lu ZR: Molecular imaging of the
tumor microenvironment. Adv Drug Deliv Rev. 113:24–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Dam S, Baars MJD and Vercoulen Y:
Multiplex tissue imaging: Spatial revelations in the tumor
microenvironment. Cancers (Basel). 14:31702022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang Y, Wang H, Wu J, Chen C, Yuan Q,
Huang W, Li T, Xi S, Hu Y, Zhou Z, et al: Noninvasive imaging
evaluation of tumor immune microenvironment to predict outcomes in
gastric cancer. Ann Oncol. 31:760–768. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo J, Wang B, Fu Z, Wei J and Lu W:
Hypoxic microenvironment induces EMT and upgrades stem-like
properties of gastric cancer cells. Technol Cancer Res Treat.
15:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang B, Song B, Li Y, Zhao Q and Tan B:
Mapping spatial heterogeneity in gastric cancer microenvironment.
Biomed Pharmacother. 172:1163172024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
57
|
Koda T, Matsushima S, Sasaki A, Danjo Y
and Kakinuma M: c-myc Gene amplification in primary stomach cancer.
Jpn J Cancer Res. 76:551–554. 1985.PubMed/NCBI
|
|
58
|
Cai HQ, Zhang LY, Fu LM, Xu B and Jiao Y:
Mutational landscape of TP53 and CDH1 in gastric cancer. World J
Gastrointest Surg. 16:276–283. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
De Marco K, Sanese P, Simone C and Grossi
V: Histone and DNA methylation as epigenetic regulators of DNA
damage repair in gastric cancer and emerging therapeutic
opportunities. Cancers (Basel). 15:49762023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
61
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Magnelli L, Schiavone N, Staderini F,
Biagioni A and Papucci L: MAP kinases pathways in gastric cancer.
Int J Mol Sci. 21:28932020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zheng L, Xu C, Guan Z, Su X, Xu Z, Cao J
and Teng L: Galectin-1 mediates TGF-β-induced transformation from
normal fibroblasts into carcinoma-associated fibroblasts and
promotes tumor progression in gastric cancer. Am J Transl Res.
8:1641–1658. 2016.PubMed/NCBI
|
|
64
|
Hasegawa T, Yashiro M, Nishii T, Matsuoka
J, Fuyuhiro Y, Morisaki T, Fukuoka T, Shimizu K, Shimizu T, Miwa A
and Hirakawa K: Cancer-associated fibroblasts might sustain the
stemness of scirrhous gastric cancer cells via transforming growth
factor-β signaling. Int J Cancer. 134:1785–1795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi
M, Yu Y, Zhu Z and Zhang J: HGF-mediated crosstalk between
cancer-associated fibroblasts and MET-unamplified gastric cancer
cells activates coordinated tumorigenesis and metastasis. Cell
Death Dis. 9:8672018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hong HN, Won YJ, Shim JH, Kim HJ, Han SH,
Kim BS and Kim HS: Cancer-associated fibroblasts promote gastric
tumorigenesis through EphA2 activation in a ligand-independent
manner. J Cancer Res Clin Oncol. 144:1649–1663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu R, Li B, Zi J, Zhang R, Yu M, Zhou J,
Pu Y and Xiong W: The dual role of LOXL4 in the pathogenesis and
development of human malignant tumors: A narrative review. Transl
Cancer Res. 13:2026–2042. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bin YL, Hu HS, Tian F, Wen ZH, Yang MF, Wu
BH, Wang LS, Yao J and Li DF: Metabolic reprogramming in gastric
cancer: Trojan horse effect. Front Oncol. 11:7452092022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nienhüser H and Schmidt T: Angiogenesis
and anti-angiogenic therapy in gastric cancer. Int J Mol Sci.
19:432017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen J, Zhang M, Ma Z, Yuan D, Zhu J, Tuo
B, Li T and Liu X: Alteration and dysfunction of ion
channels/transporters in a hypoxic microenvironment results in the
development and progression of gastric cancer. Cell Oncol (Dordr).
44:739–749. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hao LS, Liu Q, Tian C, Zhang DX, Wang B,
Zhou DX, Li ZP and Yuan ZX: Correlation and expression analysis of
hypoxia-inducible factor 1α, glucose transporter 1 and lactate
dehydrogenase 5 in human gastric cancer. Oncol Lett. 18:1431–1441.
2019.PubMed/NCBI
|
|
72
|
Shang Z, Ma Z, Wu E, Chen X, Tuo B, Li T
and Liu X: Effect of metabolic reprogramming on the immune
microenvironment in gastric cancer. Biomed Pharmacother.
170:1160302024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ham IH, Lee D and Hur H: Role of
cancer-associated fibroblast in gastric cancer progression and
resistance to treatments. J Oncol. 2019:62707842019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Taeb S, Ashrafizadeh M, Zarrabi A,
Rezapoor S, Musa AE, Farhood B and Najafi M: Role of tumor
microenvironment in cancer stem cells resistance to radiotherapy.
Curr Cancer Drug Targets. 22:18–30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Andreuzzi E, Capuano A, Poletto E, Pivetta
E, Fejza A, Favero A, Doliana R, Cannizzaro R, Spessotto P and
Mongiat M: Role of extracellular matrix in gastrointestinal
cancer-associated angiogenesis. Int J Mol Sci. 21:36862020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang N, Cao M, Duan Y, Bai H, Li X and
Wang Y: Prognostic role of tumor-infiltrating lymphocytes in
gastric cancer: A meta-analysis and experimental validation. Arch
Med Sci. 16:1092–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ding X, Xi W, Ji J, Cai Q, Jiang J, Shi M,
Yu Y, Zhu Z and Zhang J: HGF derived from cancer-associated
fibroblasts promotes vascularization in gastric cancer via PI3K/AKT
and ERK1/2 signaling. Oncol Rep. 40:1185–1195. 2018.PubMed/NCBI
|
|
78
|
Wan X, Guan S, Hou Y, Qin Y, Zeng H, Yang
L, Qiao Y, Liu S, Li Q, Jin T, et al: FOSL2 promotes
VEGF-independent angiogenesis by transcriptionnally activating
Wnt5a in breast cancer-associated fibroblasts. Theranostics.
11:4975–4991. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yoshida CJ: Regulation of heterogeneous
cancer-associated fibroblasts: The molecular pathology of activated
signaling pathways. J Exp Clin Cancer Res. 39:1122020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huang J, Zhang L, Wan D, Zhou L, Zheng S,
Lin S and Qiao Y: Extracellular matrix and its therapeutic
potential for cancer treatment. Signal Transduct Target Ther.
6:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ilan N, Elkin M and Vlodavsky I:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fan HL, Han ZT, Gong XR, Wu YQ, Fu YJ, Zhu
TM and Li H: Macrophages in CRSwNP: Do they deserve more attention?
Int Immunopharmacol. 134:1122362024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu Y, Miller CP, Tykodi SS, Akilesh S and
Warren EH: Signaling crosstalk between tumor endothelial cells and
immune cells in the microenvironment of solid tumors. Front Cell
Dev Biol. 12:13871982024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang K, Zhao XH, Liu J, Zhang R and Li JP:
Nervous system and gastric cancer. Biochim Biophys Acta Rev Cancer.
1873:1883132020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sukri A, Hanafiah A and Kosai NR: The
roles of immune cells in gastric cancer: Anti-cancer or pro-cancer?
Cancers (Basel). 14:39222022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu X, Chen J, Li W, Feng C, Liu Q, Gao W
and He M: Immunology and immunotherapy in gastric cancer. Clin Exp
Med. 23:3189–3204. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tong L, Jiménez-Cortegana C, Tay AHM,
Wickström S, Galluzzi L and Lundqvist A: NK cells and solid tumors:
Therapeutic potential and persisting obstacles. Mol Cancer.
21:2062022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang J, Hu C, Zhang R, Xu J, Zhang Y,
Yuan L, Zhang S, Pan S, Cao M, Qin J, et al: The role of
macrophages in gastric cancer. Front Immunol. 14:12821762023.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu Y, Yi M, Niu M, Mei Q and Wu K:
Myeloid-derived suppressor cells: An emerging target for anticancer
immunotherapy. Mol Cancer. 21:1842022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ozmen E, Demir TD and Ozcan G:
Cancer-associated fibroblasts: Protagonists of the tumor
microenvironment in gastric cancer. Front Mol Biosci.
11:13401242024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang M, Guan WB, Li JL, Li LX, Wang KZ,
Wang RF and Wang LF: Cancer-associated fibroblasts subtypes and
role in invasion and metastasis of gastric cancer. Neoplasma.
69:1277–1288. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Khaloozadeh F, Razmara E,
Asgharpour-Babayian F, Fallah A, Ramezani R, Rouhollah F and
Babashah S: Exosomes derived from colorectal cancer cells take part
in activation of stromal fibroblasts through regulating PHLPP
isoforms. EXCLI J. 23:634–654. 2024.PubMed/NCBI
|
|
94
|
Qin Y, Wang F, Ni H, Liu Y, Yin Y, Zhou X,
Gao G, Li Q, Qi X and Li J: Cancer-associated fibroblasts in
gastric cancer affect malignant progression via the CXCL12-CXCR4
axis. J Cancer. 12:3011–3023. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang Y, Cong X, Li Z and Xue Y: Estrogen
facilitates gastric cancer cell proliferation and invasion through
promoting the secretion of interleukin-6 by cancer-associated
fibroblasts. Int Immunopharmacol. 78:1059372020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yan Y, Wang LF and Wang RF: Role of
cancer-associated fibroblasts in invasion and metastasis of gastric
cancer. World J Gastroenterol. 21:9717–9726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dzobo K, Senthebane DA and Dandara C: The
tumor microenvironment in tumorigenesis and therapy resistance
revisited. Cancers (Basel). 15:3762023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao Z and Zhu Y: FAP, CD10, and
GPR77-labeled CAFs cause neoadjuvant chemotherapy resistance by
inducing EMT and CSC in gastric cancer. BMC Cancer. 23:5072023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhai J, Shen J, Xie G, Wu J, He M, Gao L,
Zhang Y, Yao X and Shen L: Cancer-associated fibroblasts-derived
IL-8 mediates resistance to cisplatin in human gastric cancer.
Cancer Lett. 454:37–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lengyel CG, Hussain S, Seeber A, Jamil
Nidhamalddin S, Trapani D, Habeeb BS, Elfaham E, Mazher SA, Seid F,
Khan SZ, et al: FGFR pathway inhibition in gastric cancer: The
golden era of an old target? Life (Basel). 12:812022.PubMed/NCBI
|
|
102
|
Watabe T and Giesel FL: Fibroblast
activation protein inhibitor PET/CT in gastric cancer. PET Clin.
18:337–344. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gupta R: Epigenetic regulation and
targeting of ECM for cancer therapy. Am J Physiol Cell Physiol.
322:C762–C768. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sleeboom JJF, van Tienderen GS,
Schenke-Layland K, van der Laan LJW, Khalil AA and Verstegen MMA:
The extracellular matrix as hallmark of cancer and metastasis: From
biomechanics to therapeutic targets. Sci Transl Med.
16:eadg38402024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Abbaszadegan MR, Mojarrad M and Moghbeli
M: Role of extra cellular proteins in gastric cancer progression
and metastasis: An update. Genes Environ. 42:182020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li Q, Zhu CC, Ni B, Zhang ZZ, Jiang SH, Hu
LP, Wang X, Zhang XX, Huang PQ, Yang Q, et al: Lysyl oxidase
promotes liver metastasis of gastric cancer via facilitating the
reciprocal interactions between tumor cells and cancer associated
fibroblasts. EBioMedicine. 49:157–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Laczko R and Csiszar K: Lysyl oxidase
(LOX): Functional contributions to signaling pathways.
Biomolecules. 10:10932020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gamradt P, De La Fouchardière C and
Hennino A: Stromal protein-mediated immune regulation in digestive
cancers. Cancers (Basel). 13:1462021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ye L, Li Y, Zhang S, Wang J and Lei B:
Exosomes-regulated lipid metabolism in tumorigenesis and cancer
progression. Cytokine Growth Factor Rev. 73:27–39. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jiang Y, Zhang H, Wang J, Liu Y, Luo T and
Hua H: Targeting extracellular matrix stiffness and
mechanotransducers to improve cancer therapy. J Hematol Oncol.
15:342022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li M, Wang Y, Li M, Wu X, Setrerrahmane S
and Xu H: Integrins as attractive targets for cancer therapeutics.
Acta Pharm Sin B. 11:2726–2737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li P, Zhang H, Chen T, Zhou Y, Yang J and
Zhou J: Cancer-associated fibroblasts promote proliferation,
angiogenesis, metastasis and immunosuppression in gastric cancer.
Matrix Biol. 132:59–71. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Van Cutsem E, de Haas S, Kang YK, Ohtsu A,
Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ
and Shah MA: Bevacizumab in combination with chemotherapy as
first-line therapy in advanced gastric cancer: A biomarker
evaluation from the AVAGAST randomized phase III trial. J Clin
Oncol. 30:2119–2127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bang YJ, Kang YK, Kang WK, Boku N, Chung
HC, Chen JS, Doi T, Sun Y, Shen L, Qin S, et al: Phase II study of
sunitinib as second-line treatment for advanced gastric cancer.
Invest New Drugs. 29:1449–1458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fang J, Lu Y, Zheng J, Jiang X, Shen H,
Shang X, Lu Y and Fu P: Exploring the crosstalk between endothelial
cells, immune cells, and immune checkpoints in the tumor
microenvironment: New insights and therapeutic implications. Cell
Death Dis. 14:5862023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gysler SM and Drapkin R: Tumor
innervation: Peripheral nerves take control of the tumor
microenvironment. J Clin Invest. 131:e1472762021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cifuentes L, Camilleri M and Acosta A:
Gastric sensory and motor functions and energy intake in health and
obesity-therapeutic implications. Nutrients. 13:11582021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Serafim Junior V, Fernandes GMM,
Oliveira-Cucolo JG, Pavarino EC and Goloni-Bertollo EM: Role of
Tropomyosin-related kinase B receptor and brain-derived
neurotrophic factor in cancer. Cytokine. 136:1552702020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hayakawa Y, Sakitani K, Konishi M, Asfaha
S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff
M, et al: Nerve growth factor promotes gastric tumorigenesis
through aberrant cholinergic signaling. Cancer Cell. 31:21–34.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Schledwitz A, Sundel MH, Alizadeh M, Hu S,
Xie G and Raufman JP: Differential actions of muscarinic receptor
subtypes in gastric, pancreatic, and colon cancer. Int J Mol Sci.
22:131532021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Muñoz M and Coveñas R: Involvement of
substance P and the NK-1 receptor in cancer progression. Peptides.
48:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Muñoz M, Rosso M and Coveñas R: The NK-1
receptor antagonist L-732,138 induces apoptosis in human
gastrointestinal cancer cell lines. Pharmacol Rep. 69:696–701.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lan YL, Zou S, Wang W, Chen Q and Zhu Y:
Progress in cancer neuroscience. MedComm (2020). 4:e4312023.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Han X, Liu T, Zhai J, Liu C, Wang W, Nie
C, Wang Q, Zhu X, Zhou H and Tian W: Association between EPHA5
methylation status in peripheral blood leukocytes and the risk and
prognosis of gastric cancer. PeerJ. 10:e137742022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Niu Q, Zhu J, Yu X, Feng T, Ji H, Li Y,
Zhang W and Hu B: Immune response in H. pylori-associated gastritis
and gastric cancer. Gastroenterol Res Pract. 2020:93425632020.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jing Y, Xu F, Liang W, Liu J and Zhang L:
Role of regulatory B cells in gastric cancer: Latest evidence and
therapeutics strategies. Int Immunopharmacol. 96:1075812021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu K, Yuan S, Wang C and Zhu H:
Resistance to immune checkpoint inhibitors in gastric cancer. Front
Pharmacol. 14:12853432023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yeh ES: Special issue: Cancer metastasis
and therapeutic resistance. Biomedicines. 11:13472023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dallavalasa S, Beeraka NM, Basavaraju CG,
Tulimilli SV, Sadhu SP, Rajesh K, Aliev G and Madhunapantula SV:
The role of tumor associated macrophages (TAMs) in cancer
progression, chemoresistance, angiogenesis and metastasis-current
status. Curr Med Chem. 28:8203–8236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liao Z, Tan ZW, Zhu P and Tan NS:
Cancer-associated fibroblasts in tumor microenvironment-Accomplices
in tumor malignancy. Cell Immunol. 343:1037292019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Biagioni A, Peri S, Versienti G, Fiorillo
C, Becatti M, Magnelli L and Papucci L: Gastric cancer
vascularization and the contribution of reactive oxygen species.
Biomolecules. 13:8862023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Liu K, Wang Y, Wang C, Guo C and Zhang D,
Zhong Y, Yin L, Lu Y, Liu F, Zhang Y and Zhang D: Spatial
transcriptomics of gastric cancer brain metastasis reveals atypical
vasculature strategies with supportive immune profiles.
Gastroenterol Rep (Oxf). 12:goae0672024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yuzhalin AE, Lim SY, Kutikhin AG and
Gordon-Weeks AN: Dynamic matrisome: ECM remodeling factors
licensing cancer progression and metastasis. Biochim Biophys Acta
Rev Cancer. 1870:207–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Fields GB: The rebirth of matrix
metalloproteinase inhibitors: Moving beyond the dogma. Cells.
8:9842019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Namee NM and O'Driscoll L: Extracellular
vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev
Cancer. 1870:123–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wang X, Eichhorn PJA and Thiery JP: TGF-β,
EMT, and resistance to anti-cancer treatment. Semin Cancer Biol.
97:1–11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li Y, Wang Z, Ajani JA and Song S: Drug
resistance and cancer stem cells. Cell Commun Signal. 19:192021.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yeldag G, Rice A and Del Río Hernández A:
Chemoresistance and the self-maintaining tumor microenvironment.
Cancers (Basel). 10:4712018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ma ES, Wang ZX, Zhu MQ and Zhao J: Immune
evasion mechanisms and therapeutic strategies in gastric cancer.
World J Gastrointest Oncol. 14:216–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Guo J, Zhao J, Fu W, Xu Q and Huang D:
Immune Evasion and drug resistance mediated by USP22 in cancer:
Novel targets and mechanisms. Front Immunol. 13:9183142022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li J and Stanger BZ: How tumor cell
dedifferentiation drives immune evasion and resistance to
immunotherapy. Cancer Res. 80:4037–4041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang H, Ewetse MP, Ma C, Pu W, Xu B, He P,
Wang Y, Zhu J and Chen H: The ‘light knife’ for gastric cancer:
Photodynamic therapy. Pharmaceutics. 15:1012022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Li K, Zhang A, Li X, Zhang H and Zhao L:
Advances in clinical immunotherapy for gastric cancer. Biochim
Biophys Acta Rev Cancer. 1876:1886152021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shitara K, Kadowaki S, Nishina T, Sakai D,
Yoshikawa R, Piao Y, Ozeki A, Inoue K, Gritli I and Muro K: Safety,
pharmacokinetic, and clinical activity profiles of ramucirumab in
combination with three platinum/fluoropyrimidine doublets in
Japanese patients with chemotherapy-naïve metastatic
gastric/gastroesophageal junction cancer. Gastric Cancer.
21:106–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chen C, Jung A, Yang A, Monroy I, Zhang Z,
Chaurasiya S, Deshpande S, Priceman S, Fong Y, Park AK and Woo Y:
Chimeric antigen receptor-T cell and oncolytic viral therapies for
gastric cancer and peritoneal carcinomatosis of gastric origin:
Path to improving combination strategies. Cancers (Basel).
15:56612023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Patel TH and Cecchini M: Targeted
therapies in advanced gastric cancer. Curr Treat Options Oncol.
21:702020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kwon M, An M, Klempner SJ, Lee H, Kim KM,
Sa JK, Cho HJ, Hong JY, Lee T, Min YW, et al: Determinants of
response and intrinsic resistance to PD-1 blockade in
microsatellite instability-high gastric cancer. Cancer Discov.
11:2168–2185. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Janjigian YY, Kawazoe A, Yañez P, Li N,
Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al: The
KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive
gastric cancer. Nature. 600:727–730. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Entezam M, Sanaei MJ, Mirzaei Y, Mer AH,
Abdollahpour-Alitappeh M, Azadegan-Dehkordi F and Bagheri N:
Current progress and challenges of immunotherapy in gastric cancer:
A focus on CAR-T cells therapeutic approach. Life Sci.
318:1214592023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yu Y, Xu B, Xiang L, Ding T, Wang N, Yu R,
Gu B, Gao L, Maswikiti EP, Wang Y, et al: Photodynamic therapy
improves the outcome of immune checkpoint inhibitors via
remodelling anti-tumour immunity in patients with gastric cancer.
Gastric Cancer. 26:798–813. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu B, He P, Wang Y, Wang H, Zhang J, Zhu
J, Pu W and Chen H: PDT for gastric cancer-the view from China.
Photodiagnosis Photodyn Ther. 42:1033662023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhao R, Cui Y, Zheng Y, Li S, Lv J, Wu Q,
Long Y, Wang S, Yao Y, Wei W, et al: Human hyaluronidase PH20
potentiates the antitumor activities of mesothelin-specific CAR-T
cells against gastric cancer. Front Immunol. 12:6604882021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang X, Liu SS, Ma J and Qu W: Secretory
leukocyte protease inhibitor (SLPI) in cancer pathophysiology:
Mechanisms of action and clinical implications. Pathol Res Pract.
248:1546332023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Lim NR and Chung WC: Helicobacter
pylori-associated chronic atrophic gastritis and progression of
gastric carcinogenesis. Korean J Gastroenterol. 82:171–179. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Graham DY: Helicobacter pylori update:
Gastric cancer, reliable therapy, and possible benefits.
Gastroenterology. 148:719–731.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Deng R, Zheng H, Cai H, Li M, Shi Y and
Ding S: Effects of helicobacter pylori on tumor microenvironment
and immunotherapy responses. Front Immunol. 13:9234772022.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Guo R and Yang B: Hypoxia-induced LXRα
contributes to the migration and invasion of gastric cancer cells.
Folia Biol (Praha). 67:91–101. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li S, Cong X, Gao H, Lan X, Li Z, Wang W,
Song S, Wang Y, Li C, Zhang H, et al: Tumor-associated neutrophils
induce EMT by IL-17a to promote migration and invasion in gastric
cancer cells. J Exp Clin Cancer Res. 38:62019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Fu L, Bu L, Yasuda T, Koiwa M, Akiyama T,
Uchihara T, Baba H and Ishimoto T: Gastric cancer stem cells:
Current insights into the immune microenvironment and therapeutic
targets. Biomedicines. 8:72020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Aghlara-Fotovat S, Nash A, Kim B, Krencik
R and Veiseh O: Targeting the extracellular matrix for
immunomodulation: Applications in drug delivery and cell therapies.
Drug Deliv Transl Res. 11:2394–2413. 2021. View Article : Google Scholar : PubMed/NCBI
|