Vulvar cancer is the fourth most prevalent
gynecological cancer globally, representing 5% of lower genital
tract tumors and ranking below uterine corpus, ovarian and cervical
cancer (1–3). Key contributors to vulvar cancer
development include age, human papillomavirus (HPV) infection,
smoking, inflammatory vulvar conditions, prior pelvic radiation,
immunodeficiency and anogenital warts (4–6). Among
the different histological types of vulvar cancer, vulvar squamous
cell carcinoma (VSCC) is the most common type (95%), followed by
melanoma, sarcoma and basal cell carcinoma (2,3). VSCC
has traditionally been regarded as a disease of postmenopausal
women, although the mean age of incidence has fallen in recent
years worldwide owing to the increase in HPV infections (1). Nonetheless, the age-specific incidence
ranges from 0.4 per 100,000 in younger women to 20 per 100,000 in
women >70 years old (7).
VSCC manifests in two types with different pathways:
The basaloid and/or warty type often associated with HPV (HPV
subtypes 16, 18, 31 and 33) which is predisposed from usual vulvar
intraepithelial neoplasia (uVIN), and the second type linked to
chronic vulvar dermatoses and differentiated vulvar intraepithelial
neoplasia (dVIN) (8). Common
TP53 mutations are observed in the second type and are
mostly independent of HPV (8).
Although there are survival differences in these two types of VSCC,
treatment is predominantly surgery with or without
radio-chemotherapy. Immunotherapy has reformed the therapeutic
paradigms of multiple malignancies, but its impact is limited in
VSCC (1). In addition, treatment of
unresectable/metastatic disease often leads to frequent
comorbidities, particularly in elderly and frail women,
highlighting the need for innovative and more effective treatment
approaches.
Carcinomas comprise both malignant and non-malignant
cells, including fibroblasts, immune cells, vascular cells and
neuronal cells (9). Non-malignant
cells actively shape the tumor microenvironment (TME) by secreting
cytokines, chemokines, growth factors and extracellular matrix
(ECM) proteins (9). Under normal
physiological conditions, resident cells maintain tissue balance
through ECM deposition, degradation and remodeling (10). However, carcinogenesis disrupts ECM
remodeling, creating a dysfunctional ECM conducive to a supportive
TME that promotes cancer growth, invasion and metastasis (11). While specific research on ECM
proteins in VSCC is limited, studies are starting to explore the
role of ECM remodeling in VSCC invasion and metastasis.
The present review aims to provide an overview on
the role of ECM during VSCC metastasis and the current
understanding of the role of ECM in regulating VSCC dissemination.
The present study also explored the therapeutic potential of
targeting ECM in other types of squamous-epithelial cancers, and
the potential prognostic and predictive biomarkers, discussing
their impact on developing more efficacious antitumor
therapies.
The ECM is commonly defined as the non-malignant,
non-cellular component of tissue that provides essential
biochemical and structural support to its cellular constituents
(12,13). Emerging research suggests that the
ECM is not merely an intercellular filler but a physiologically
active component of living tissue, playing crucial roles in
cell-cell communication, adhesion and proliferation (14,15).
Resident fibroblasts are responsible for creating and arranging ECM
components according to the specific needs of the tissue (14,16).
Major components of the ECM, including collagen, laminin, elastin
and proteoglycans, exhibit distinct physical and biochemical
properties. A detailed overview of ECM components is shown in
Table I.
Dysregulation of the ECM is a critical factor in
cancer development and progression, influencing various key
mechanisms. One such mechanism is cellular signaling, where
abnormalities in the ECM contribute to uncontrolled cell growth,
survival and proliferation, all fundamental cancer hallmarks
(14). Moreover, the ECM plays a
critical role in cell adhesion and migration, facilitated by
proteins such as integrins and cadherins. Disruption of these
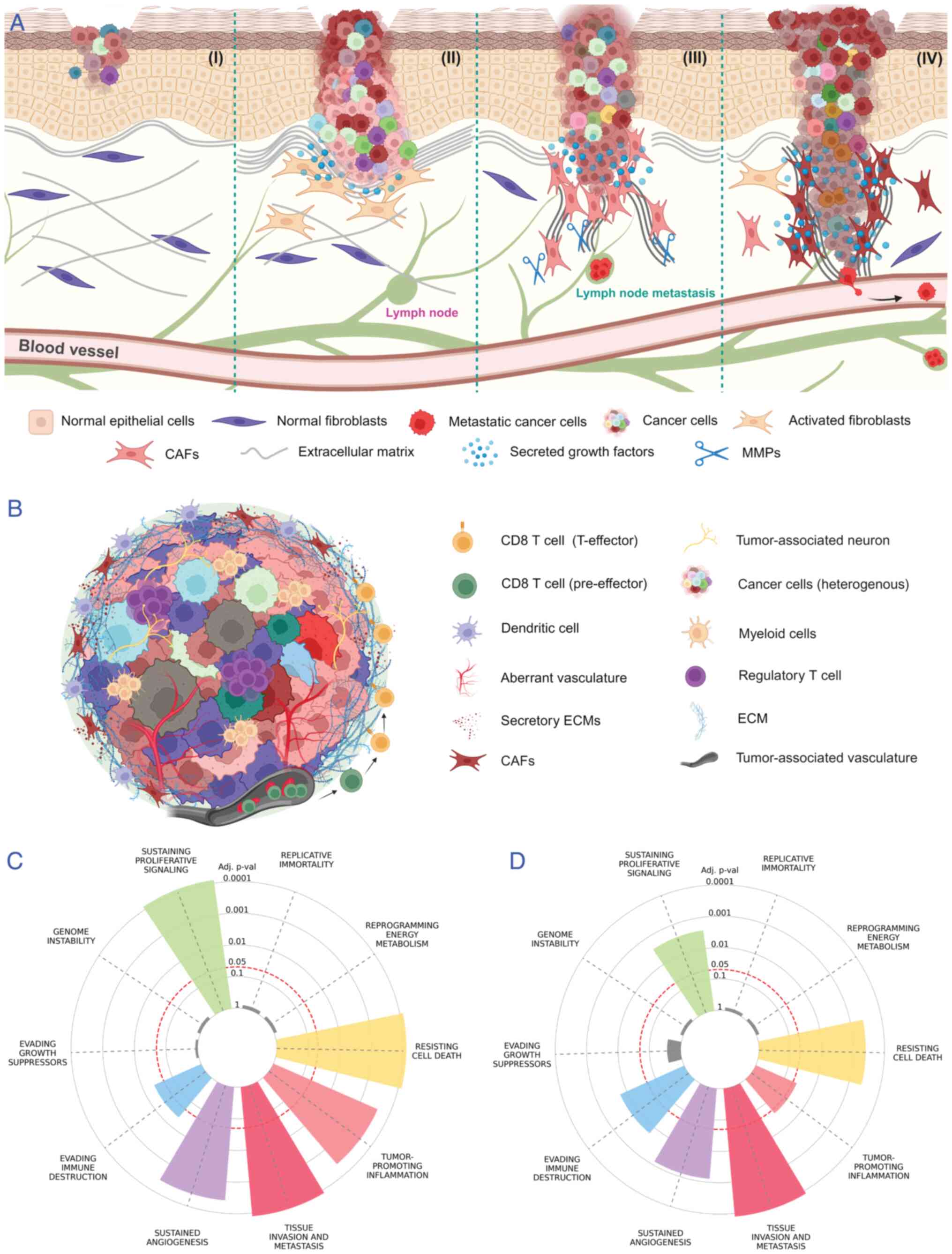
processes promotes the invasion of cancer cells into surrounding
tissues (Fig. 1A) (10). The ECM also affects the immune
response within TME, contributing to immune evasion by cancer cells
(14). It has been shown that ECM
components impede and educate immune cell types such as natural
killer cells, macrophages and tumor-infiltrating lymphocytes,
specifically CD8+ T cells, within the TME and evade the
antitumor immune response (14,17,18).
Components such as cross-linked collagen, fibronectin, laminin,
periostin, osteopontin, integrins and matrix metalloproteinases
(MMPs) can erect physical barriers, hindering immune cell movement
and impairing the ability of immune cells such as cytotoxic
CD8+ T-cells to target and eliminate cancer cells
(Fig. 1B) (19,20).
Understanding the intricate interplay between
tumorigenesis and the ECM is essential for developing targeted
therapeutic approaches. Fig. 1C
illustrates the significant involvement of ECM components in
various cancer hallmarks. Over time, intensive research has been
dedicated to exploring interventions targeting ECM components or
disrupting ECM-associated signaling pathways in tumors originating
from squamous epithelia. These endeavors have yielded valuable
scientific insights, which are outlined in Table II, and offer promising directions
for advancing SCCs treatment (21–102).
In order to decipher key ECM proteins involved in
VSCC progression, a thorough literature search was performed in the
present study. The following MeSH terms were used in PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Scopus (https://www.scopus.com/home.uri) and Web of Science
(https://mjl.clarivate.com/home) to
select literature describing ECM and VSCC tumor progression and
development: (‘genital neoplasms, female’[MeSH Terms] OR ‘female
genital neoplasm*’[All Fields] OR ‘Gynaecologic neoplasm*’[All
Fields] OR ‘Vulvar Neoplasms’[MeSH Terms] OR ‘vulvar neoplasm*’[All
Fields] OR ‘vulva neoplasm*’[All Fields] OR ‘cancer of vagina*’[All
Fields] OR ‘cancer of vulva*’[All Fields] OR ‘vulva squamous cell
carcinoma*’[All Fields] OR ‘vaginal neoplasm*’[All Fields] OR
‘vagina cancer*’[All Fields]) AND (‘Extracellular Matrix’[MeSH
Terms] OR ‘extracellular matrix*’[All Fields] OR ‘extracellular
matrix protein*’[All Fields]) AND 1980/01/01:2023/12/31
[date-publication]. In the following section, each of the ECM
proteins implicated in VSCC progression are described in detail.
Table III (103–115) summarizes the key findings
regarding ECM proteins in VSCC. This table provides a concise
overview of the significant associations, functional implications
and clinical relevance of ECM proteins identified in VSCC.
Collagens, which are the predominant component of
the ECM, are widely distributed across various types of tissue.
With 28 different types, the collagen superfamily forms fibers,
networks and filaments within the ECM, interacting with
mesenchymal-origin cells through various receptor families to
regulate their proliferation, migration and differentiation
(116). While studies have
extensively investigated the role of collagens in driving cancer
invasion and metastasis, their specific involvement in VSCC remains
understudied. Recent research utilizing Second Harmonic Generation
imaging has analyzed collagen parameters (quantity, uniformity and
organization) in VSCC, revealing associations with lymph node
metastasis (103). Additionally,
based on collagen organization, two morphologic variants have been
identified in VSCC: An indolent type growing as ‘sheets of cells’
with a pushing border in lymphoplasmacytic stroma, and an
aggressive variant growing as ‘single tumor cells’ with a
finger-like border in fibromyxoid stroma (104). Proteomic analyses have further
demonstrated that the aggressive variants are associated with
higher rates of lymph node metastasis and tumor recurrences.
Consistent with these findings, previous studies have shown a
significant correlation between the ‘sheet of cells’ morphology,
HPV infection and improved survival rate (8). Collectively, these findings suggest
that morphological variants of collagen fibers in the TME may serve
as prognostic indicators for aggressive VSCC. This highlights the
potential significance of collagen parameters in understanding VSCC
aggressiveness and may offer insights into therapeutic strategies
targeting the tumor microenvironment.
The laminin family, comprising of ~20 glycoproteins,
forms a cross-linked web intertwined with the type IV collagen
network in basement membranes. Laminins are heterotrimers composed
of three polypeptide chains (α, β, γ) and play crucial roles in
early embryonic development, organogenesis and various cell
type-specific functions such as adhesion, differentiation,
migration, phenotype maintenance and resistance to apoptosis
(15). However, in tumors of the
lower female genital tract, laminin expression becomes dysregulated
(117). Prior research has
emphasized that elevated expression of the γ2 chain of laminin-5
(LAMC2) is linked to patient survival in VSCC. Notably,
intracytoplasmic expression of the γ2 chain along the invasive
tumor front correlates with short-term survival. Larger tumors tend
to exhibit increased γ2 chain expression, although no significant
correlation has been observed with tumor staging. These findings
suggest that heightened expression of the γ2 chain may be involved
in the initiation of VSCC tumorigenesis rather than progression
(115). However, further
investigations are warranted to elucidate the dynamics of LAMC2 in
VSCC tumorigenesis.
Matricellular proteins form a diverse family of
non-structural matrix glycoproteins, including thrombospondins,
secreted acidic protein and rich in cysteine, tenascins, fibulins,
osteopontin, cartilage oligomeric matrix protein and CNN family
proteins such as periostin and R-spondins (116). Among these, osteopontin (OPN),
initially identified as a bone matrix protein, is now recognized as
a cytokine produced by activated T cells and transformed cells. It
is highly inducible as it is expressed and secreted by both tumor
cells and cells in the stroma (105). In VSCC, OPN expression was
investigated across various stages of vulvar lesions, including
VSCC, VIN, vulvar lichen sclerosus (VLS) and normal vulvar tissue
samples. Proteomic analysis revealed a gradual increase in OPN
expression from VIN to VLS, with the highest expression observed in
VSCC tumor tissue samples. Additionally, OPN expression was found
to be associated with the pathological stage, suggesting its
potential role in VSCC tumor progression through neoplastic
transformation (105).
Collectively, this observation suggested that OPN could be a
predictive biomarker for the early detection of VSCC, and further
studies are required to understand VSCC pathogenesis through
OPN.
Dystroglycan, a transmembrane glycoprotein, serves
as a crucial link between the ECM and the intracellular
cytoskeleton, thereby providing structural integrity. Comprising α
and β components, dystroglycan facilitates cell adhesion to the ECM
and plays a pivotal role in regulating cytoskeletal organization
(118,119). Dysregulation of dystroglycan is a
common occurrence observed in various human epithelial cancers,
suggesting its potential involvement in tumor development (118,119). Notably, previous research has
revealed disrupted expression levels of α-dystroglycan in
conditions such as VLS, squamous cell hyperplasia, VIN and invasive
VSCC. Specifically, decreased expression of α-dystroglycan has been
observed across preneoplastic lesions of VSCC, and this
downregulation is associated with advanced stages of VSCC. These
findings suggest that α-dystroglycan may play a significant role in
maintaining cytoskeletal dynamics, and its reduced expression could
promote VSCC progression (106).
Integrins, heterodimeric receptors comprising α and
β subunits, are frequently dysregulated in skin cancers (107). Serving as bridging molecules,
integrins connect ECMs with the cell cytoskeleton, governing cell
adhesion and motility. The intracellular tail of integrin β1
associates with proteins such as talin, α-actinin and vinculin,
linking it to the actin cytoskeleton and regulating cell motility,
keratinocyte wound healing and the collective movement of tumor
cells (115). Increased expression
of various integrin (ITG) family proteins, including α2, α3, α5, α6
and β1 has been observed in VSCC. Among these, β1 (ITGB1) plays a
pivotal role in mediating cell adhesion, migration and invasion
(107). Knockdown experiments
targeting β1 result in significant alterations in VSCC tumor
morphology compared with control tumors. Specifically, β1 knockdown
leads to a more encapsulated and less invasive tumor phenotype,
indicating the crucial involvement of integrin β1 in VSCC
invasiveness and disease progression (107). The present study underscores the
significance of β1 integrin in VSCC tumor advancement and suggests
potential therapeutic avenues for intervention.
Hyaluronic acid receptor CD44 is a surface-expressed
glycoprotein that facilitates interactions with a spectrum of
molecules, including collagen, fibronectin, OPN, MMPs and growth
factors (70,71). CD44 plays a pivotal role in cell
adhesion, interactions, migration and metastasis. CD44 isoforms
bolster malignant cell affinity to ECM ligands, thereby fostering
tumor dissemination (70,71). A previous study has demonstrated a
significant association between CD44 variants, particularly CD44v3
and CD44v6 and VSCC tumor progression, as well as adverse patient
outcomes (108). Elevated CD44
expression correlates with poor tumor differentiation, positive
lymph node involvement, advanced-stage VSCC and diminished survival
rates, indicating its potential as a prognostic marker (108).
MMPs are a family of calcium-dependent,
zinc-containing endopeptidases that target various molecules,
including matrix components, growth factors, cytokines and
signaling molecules. Synthesized as zymogens, MMPs are secreted
after cleavage of their propeptide form. Invasion and metastasis of
malignant cells involve the degradation of the stromal matrix,
mediated by specific MMPs (120).
MMP2 plays a pivotal role in degrading crucial
components of basement membranes such as type IV collagen and
fibronectin, facilitating the invasion of tumor cells into stromal
and vascular regions (25,110). Overexpression of MMP2 has been
observed across various disease stages of VSCC, including VIN
(grades-I, II, III) and VLS and this heightened expression is
significantly associated with the invasiveness of VSCC (110). Morphologically, MMP2 manifests as
cytoplasmic granular or diffuse staining in stromal cells. However,
in cases of invasive VSCC, MMP2-positive cells are notably observed
in the stroma adjacent to neoplastic islands or infiltrating groups
of tumor cells (110). These
highly MMP2-expressing tumor cells secrete factors that contribute
to the aggressiveness of VSCC, including invasion and metastasis,
suggesting potential therapeutic approaches targeting MMP2.
MMP12, recognized for its ability to degrade elastin
and various substrates such as type IV collagen, fibronectin and
laminin, plays a multifaceted role in cancer progression,
particularly in VSCC. It contributes to limiting tumor growth by
converting plasminogen into angiostatin, which inhibits endothelial
cell proliferation and angiogenesis, essential processes for tumor
vascularization (111). While
typically associated with macrophages, MMP12 is also expressed by
transformed epithelial cells in VSCC, with its expression level
correlating with tumor dedifferentiation and histological
aggressiveness (112). In a study
involving 33 VSCC samples, MMP12 mRNA was prevalent, and higher
expression in cancer cells was associated with more aggressive and
poorly differentiated tumors (112). Notably, macrophage-derived MMP12
was more abundant in well-differentiated grade I tumors compared
with grade III tumors (112).
These findings suggest MMP12 potential role in modulating VSCC
histology and influencing treatment strategies. However, further
research is warranted to fully understand MMP12 implications in
VSCC and its therapeutic relevance.
MMP13, recognized for its ability to cleave
fibrillar collagens and various stromal matrix components, stands
out as a versatile proteolytic tool crucial for tumor cell invasion
(16,116,120,121). A previous study has highlighted
the abundant expression of MMP13 in VSCC tumor tissues, often
correlating with lymph node metastasis (109). Moreover, in vitro
investigations have revealed a significant increase in MMP13 levels
in VSCC cell lines compared with control epithelial cells (109). These findings underscore MMP13
specific expression by malignantly transformed squamous epithelial
cells, including VSCC cells, suggesting its potential as a marker
for their invasive potential (109).
Fibroblasts are the main architects of the ECM,
orchestrating changes that play a major role in tumor progression,
inflammation, therapy resistance and immunosuppression (9,122).
CAFs are perhaps the cells most proficient at remodeling the ECM as
they deposit collagens and release various growth factors,
chemokines, cytokines and MMPs (Fig.
1A). Significantly altered gene pathways in CAFs include
regulation of substrate adhesion, tissue remodeling, cell
migration, secretion, growth regulation and angiogenesis (123). Although, at present, VSCC-specific
subpopulations of CAFs have not been reported, single-cell RNA
sequencing data suggests that various CAF subpopulations exist in
the TME of other types of SCCs (124,125).
The most commonly identified CAF subpopulations are
those involved in ECM remodeling [myofibroblast CAFs (myCAFs)] and
immunomodulatory cancer-associated fibroblasts] (126). MyCAFs have been reported to be
involved in tissue remodeling and often express ACTA2 gene
(126). Inflammatory CAFs often
exhibit increased IL-6 signaling in various tumor types (126–129). This makes them potential targets
for anti-IL-6 therapies, such as siltuximab and tocilizumab
(130). Moreover, research has
shown that immunoregulatory CAFs can activate JAK-STAT signaling in
cancer cells (129), suggesting
that inhibition of this pathway may be a promising therapeutic
strategy.
Mounting evidence suggests that CAFs also play a key
role in therapy-resistance in SCCs, particularly in head and neck
SCC (HNSCC) and esophageal SCC (131–133). It has been previously reported
that chemotherapy promotes CAF survival and alters exosome
biogenesis, which leads to malignant characteristics in HNSCC
(134). Notably, CAFs also respond
to radiotherapy by upregulating transforming growth factor β1
(TGFβ1) expression via IL-8/NF-κB signaling in HNSCC, which
increases the rate of invasive growth. This signaling can be
inhibited by using Tranilast, a known TGFβ1 inhibitor (135). A subset of CAFs have also been
reported to be involved in TGFβ1 dependent
PD-1+/TIM-3+ exhaustion of CD8 T cells in
HNSCC, leading to exclusion of T cells, thereby negatively
impacting immunotherapy response (124,136). Previously, we have shown that CAFs
play a decisive role in VSCC invasion and that VSCC cells exhibit a
fibroblast-dependent tumorigenic potential (137). However, there exists a significant
heterogeneity with CAFs showing both tumor-promoting and
tumor-inhibiting CAFs (9,122). Gaining a more profound insight
into the molecular and phenotypic variations among CAF populations
in VSCC may contribute to overcoming challenges related to therapy
resistance and the targeting of cancer cells.
The therapeutic potential of ECM proteins in
treating VSCC holds promise for pioneering treatment strategies.
Identifying specific ECM proteins involved in VSCC tumorigenesis
will unveil potential therapeutic targets. While the pathways
linked with ECM proteins in VSCC are not fully understood, emerging
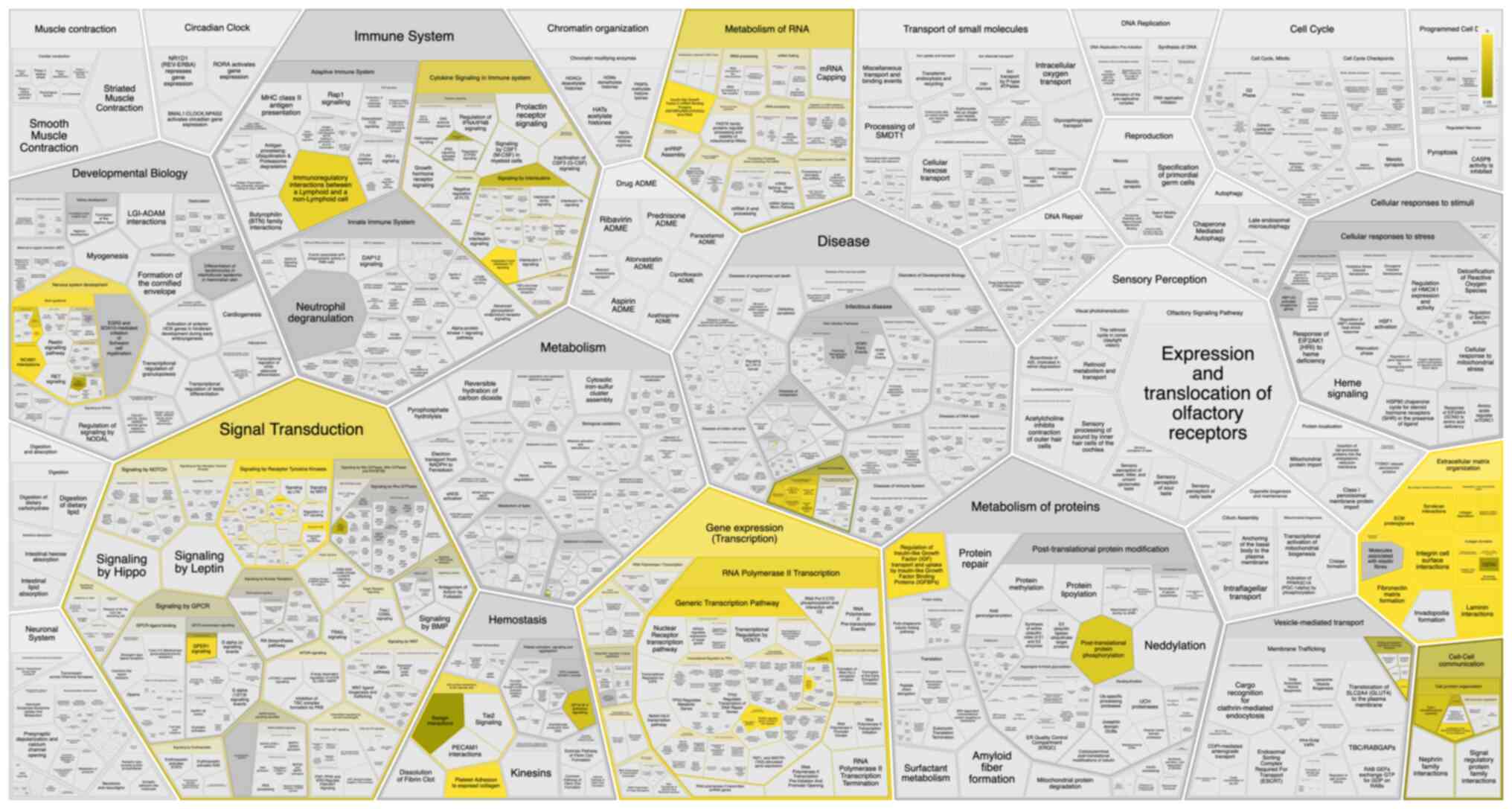
bioinformatic platforms (138)
provide opportunities to pinpoint key pathways and potential
targeted therapies for this relatively underexplored cancer.
Notably, these proteins, irrespective of ECM organization, are
implicated in modulating immune systems, as depicted in Fig. 2.
Despite the absence of reports on ECM proteins
regulating immune compartments in VSCC, insights from other
epithelial-origin tumors in The Cancer Genome Atlas suggests that
investigating ECM in VSCC may be beneficial. Bioinformatics
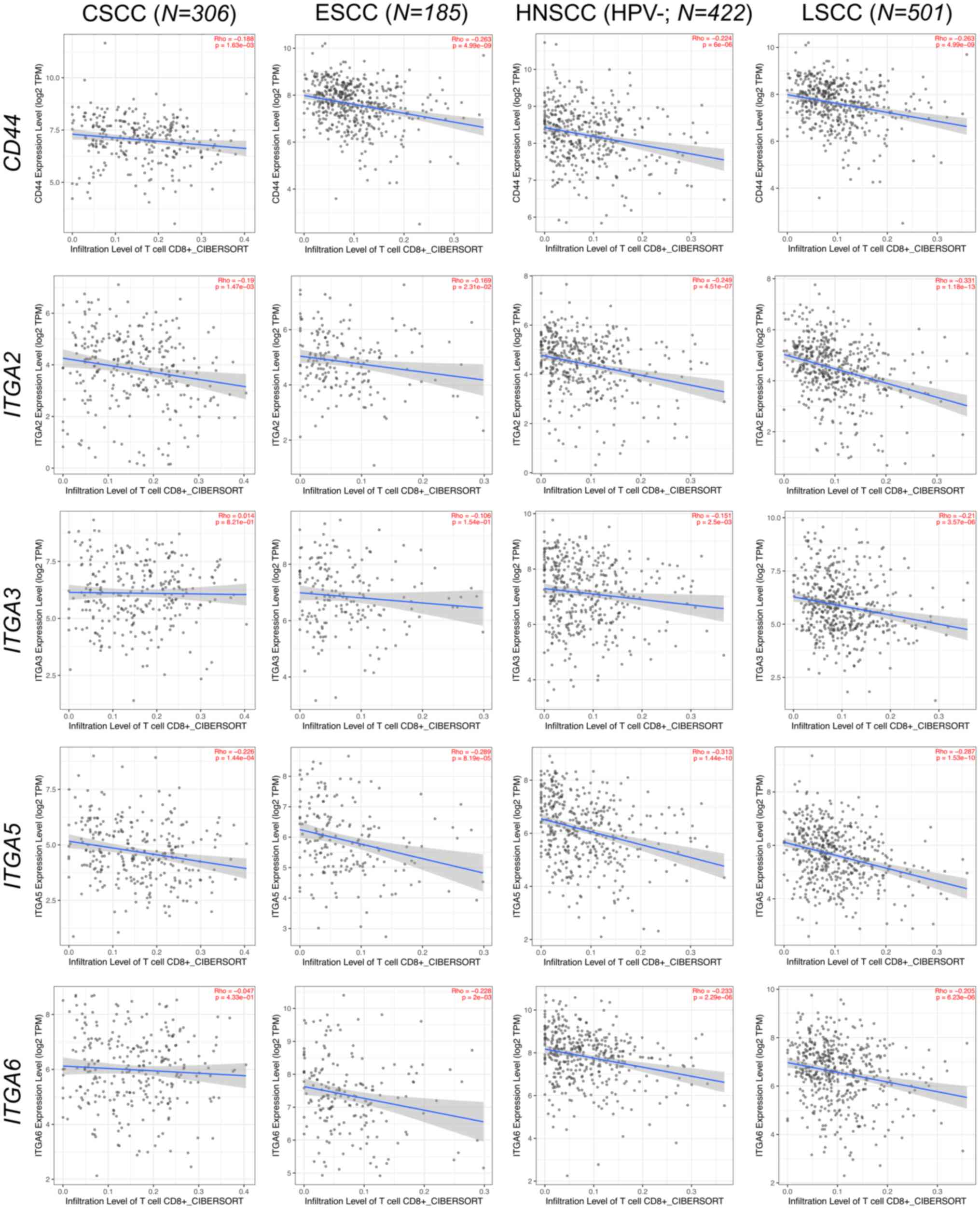
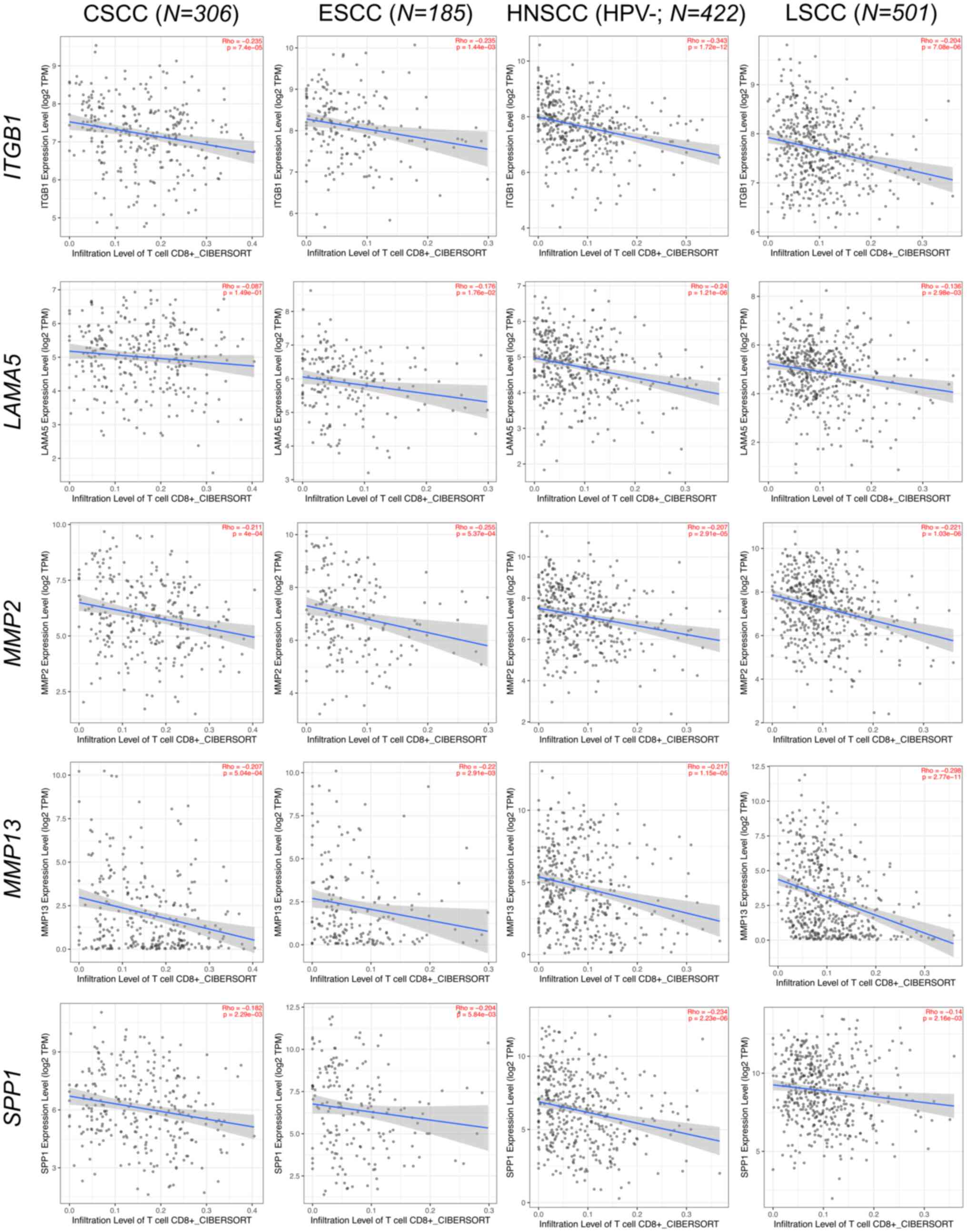
platforms such as TIMER2 (http://timer.cistrome.org/) (139) confirm that higher expression
levels of ECM-related genes are associated with reduced
CD8+ T cell infiltration within the TME, fostering
immune evasion and tumor progression across various epithelial
cancers (140–143) (Figs.
3 and 4). Consequently, over
the last few decades, several ECM inhibitors have been developed
and successfully employed across different SCCs (Table IV) (144–158). Therefore, understanding the
intricate relationship between ECM components in the context of
VSCC is essential for devising innovative therapeutic strategies
tailored to this patient population. Such endeavors hold the
potential to pave the way for the emergence of novel
therapeutics.
The present study thoroughly investigated the
intricate landscape of the ECM in VSCC, examining the roles of
various components such as collagens, laminin, osteopontin,
dystroglycan, integrin, CD44 and MMPs in VSCC progression.
Furthermore, the present study highlights the evolving
understanding of ECM, going beyond its structural role to actively
influence adhesion, proliferation and cell communication. Attention
is provided to collagen types, emphasizing their structural
significance and their correlation with changes in collagen levels
and lymph node metastasis in VSCC.
The present study acknowledges the rising incidence
of vulvar cancer and the limitations of current treatment
approaches, particularly in incurable or metastatic cases. It
advocates for the exploration of novel therapeutic pathways and
targets based on the detailed analysis of ECM components in VSCC.
Recognizing the complex interactions within the TME underscores the
necessity for innovative and targeted therapeutic approaches to
enhance outcomes for individuals affected by VSCC. Further research
and clinical exploration of these ECM targets could pave the way
for more effective therapeutic strategies in the management of
VSCC.
The results shown here are in part based upon data
generated by the TCGA Research Network: https://www.cancer.gov/tcga.
This work was supported by the Research Council of Norway
through its Centers of Excellence funding scheme (grant no.
22325).
Not applicable.
EI and KCD conducted the literature review and
drafted the manuscript. RM, KO, SI and HND contributed to the
writing of the manuscript and made corrections. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Olawaiye AB, Cuello MA and Rogers LJ:
Cancer of the vulva: 2021 update. Int J Gynaecol Obstet. 155 (Suppl
1):7–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogers LJ and Cuello MA: Cancer of the
vulva. Int J Gynaecol Obstet. 143 (Suppl 2):S4–S13. 2018.
View Article : Google Scholar
|
|
3
|
Alkatout I, Schubert M, Garbrecht N,
Weigel MT, Jonat W, Mundhenke C and Günther V: Vulvar cancer:
Epidemiology, clinical presentation, and management options. Int J
Womens Health. 7:305–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capria A, Tahir N and Fatehi M: Vulva
Cancer. StatPearls. StatPearls Publishing; Treasure Island, FL:
2024, PubMed/NCBI
|
|
5
|
Bucchi L, Pizzato M, Rosso S and Ferretti
S: New insights into the epidemiology of vulvar cancer: Systematic
literature review for an update of incidence and risk factors.
Cancers (Basel). 14:3892022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brinton LA, Thistle JE, Liao LM and
Trabert B: Epidemiology of vulvar neoplasia in the NIH-AARP Study.
Gynecol Oncol. 145:298–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gadducci A, Tana R, Barsotti C, Guerrieri
ME and Genazzani AR: Clinico-pathological and biological prognostic
variables in squamous cell carcinoma of the vulva. Crit Rev Oncol
Hematol. 83:71–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dongre HN, Elnour R, Tornaas S, Fromreide
S, Thomsen LCV, Kolseth IBM, Nginamau ES, Johannessen AC, Vintermyr
OK, Costea DE and Bjørge L: TP53 mutation and human papilloma virus
status as independent prognostic factors in a Norwegian cohort of
vulva squamous cell carcinoma. Acta Obstet Gynecol Scand.
103:165–175. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dongre H and Costea DE: Tumor-Fibroblast
Interactions in CarcinomasBiomarkers of the Tumor Microenvironment.
Springer; New York, NY: pp. 109–124. 2022, View Article : Google Scholar
|
|
10
|
Dzobo K and Dandara C: The Extracellular
Matrix: Its composition, function, remodeling, and role in
tumorigenesis. Biomimetics (Basel). 8:1462023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pappa KI, Jacob-Hirsch J, Vlachos GD,
Christodoulou I, Partsinevelos G, Amariglio N, Markaki S, Antsaklis
A and Anagnou NP: Expression profiling of vulvar carcinoma: clues
for deranged extracellular matrix remodeling and effects on
multiple signaling pathways combined with discrete patient subsets.
Transl Oncol. 4:301–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sleeboom JJF, van Tienderen GS,
Schenke-Layland K, van der Laan LJW, Khalil AA and Verstegen MMA:
The extracellular matrix as hallmark of cancer and metastasis: From
biomechanics to therapeutic targets. Sci Transl Med.
16:eadg38402024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mukherjee A and Bravo-Cordero JJ:
Regulation of dormancy during tumor dissemination: the role of the
ECM. Cancer Metastasis Rev. 42:99–112. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popova NV and Jücker M: The functional
role of extracellular matrix proteins in cancer. Cancers (Basel).
14:2382022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walker C, Mojares E and Del Río Hernández
A: Role of extracellular matrix in development and cancer
progression. Int J Mol Sci. 19:30282018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue B: Biology of the extracellular
matrix: An overview. J Glaucoma. 23 (8 Suppl 1):S20–S23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Condic M, Rohr A, Riemann S, Staerk C,
Ayub TH, Doeser A, Zillinger T, Merkelbach-Bruse S, Buettner R,
Barchet W, et al: Immune profiling of vulvar squamous cell cancer
discovers a macrophage-rich subtype associated with poor prognosis.
Cancer Res Commun. 4:861–875. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Esch EM, van Poelgeest MI, Trimbos JB,
Fleuren GJ, Jordanova ES and van der Burg SH: Intraepithelial
macrophage infiltration is related to a high number of regulatory T
cells and promotes a progressive course of HPV-induced vulvar
neoplasia. Int J Cancer. 136:E85–E94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruni S, Mercogliano MF, Mauro FL, Cordo
Russo RI and Schillaci R: Cancer immune exclusion: breaking the
barricade for a successful immunotherapy. Front Oncol.
13:11354562023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Wei JR, Dong J, Lin QG, Tang H, Jia
YX, Tan W, Chen QY, Zeng TT, Xing S, et al: Laminin γ2-mediating T
cell exclusion attenuates response to anti-PD-1 therapy. Sci Adv.
7:eabc83462021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Huang X, Huang R, Zhu H, Ye P,
Lin X, Zhang S, Wu M and Jiang F: MMP1 overexpression promotes
cancer progression and associates with poor outcome in head and
neck carcinoma. Comput Math Methods Med.
2022:30583422022.PubMed/NCBI
|
|
22
|
Liu M, Hu Y, Zhang MF, Luo KJ, Xie XY, Wen
J, Fu JH and Yang H: MMP1 promotes tumor growth and metastasis in
esophageal squamous cell carcinoma. Cancer Lett. 377:97–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurnia I, Rauf S, Hatta M, Arifuddin S,
Hidayat YM, Natzir R, Kaelan C, Bukhari A, Pelupessy NU and
Patelonggi IJ: Molecular Patho-mechanisms of cervical cancer
(MMP1). Ann Med Surg (Lond). 77:1034152022.PubMed/NCBI
|
|
24
|
Han L, Sheng B, Zeng Q, Yao W and Jiang Q:
Correlation between MMP2 expression in lung cancer tissues and
clinical parameters: A retrospective clinical analysis. BMC Pulm
Med. 20:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samantaray S, Sharma R, Chattopadhyaya TK,
Gupta SD and Ralhan R: Increased expression of MMP-2 and MMP-9 in
esophageal squamous cell carcinoma. J Cancer Res Clin Oncol.
130:37–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azevedo Martins JM, Rabelo-Santos SH, do
Amaral Westin MC and Zeferino LC: Tumoral and stromal expression of
MMP-2, MMP-9, MMP-14, TIMP-1, TIMP-2, and VEGF-A in cervical cancer
patient survival: A competing risk analysis. BMC Cancer.
20:6602020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Yang F, Chai L, Zhang L, Li S, Xu Z
and Kong L: CCAAT/Enhancer Binding Protein β-Mediated MMP3
upregulation promotes esophageal squamous cell cancer invasion in
vitro and is associated with metastasis in human patients. Genet
Test Mol Biomarkers. 23:304–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gkouveris I, Nikitakis NG, Aseervatham J,
Rao N and Ogbureke KUE: Matrix metalloproteinases in head and neck
cancer: Current perspectives. Metalloproteinases Med. 4:47–61.
2017. View Article : Google Scholar
|
|
29
|
Shao L, Wang X, Liu W, Zhang C, Ma W, Yu X
and Han J: The role and function of secretory protein MMP3 in
cervical cancer. researchsquare. https://doi.org/10.21203/rs.3.rs-2449297/v1
|
|
30
|
Liu D, Nakano J, Ishikawa S, Yokomise H,
Ueno M, Kadota K, Urushihara M and Huang CL: Overexpression of
matrix metalloproteinase-7 (MMP-7) correlates with tumor
proliferation, and a poor prognosis in non-small cell lung cancer.
Lung Cancer. 58:384–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chuang HC, Su CY, Huang HY, Huang CC,
Chien CY, Du YY and Chuang JH: Active matrix metalloproteinase-7 is
associated with invasion in buccal squamous cell carcinoma. Mod
Pathol. 21:1444–1450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu L, Zheng X, Du Y, Xing Y, Xu K and Cui
L: Matrix metalloproteinase-7 may serve as a novel biomarker for
cervical cancer. Onco Targets Ther. 11:4207–4220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Ma J, Guo Q, Duan F, Tang F, Zheng
P, Zhao Z and Lu G: Overexpression of MMP-2 and MMP-9 in esophageal
squamous cell carcinoma. Dis Esophagus. 22:664–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsukamoto S, Koma YI, Kitamura Y, Tanigawa
K, Azumi Y, Miyako S, Urakami S, Hosono M, Kodama T, Nishio M, et
al: Matrix metalloproteinase 9 induced in esophageal squamous cell
carcinoma cells via close contact with tumor-associated macrophages
contributes to cancer progression and poor prognosis. Cancers
(Basel). 15:29872023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deraz EM, Kudo Y, Yoshida M, Obayashi M,
Tsunematsu T, Tani H, Siriwardena SB, Keikhaee MR, Qi G, Iizuka S,
et al: MMP-10/stromelysin-2 promotes invasion of head and neck
cancer. PLoS One. 6:e254382011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Qin YR, Bi J, Guo A, Fu L and Guan
XY: Overexpression of matrix metalloproteinase 10 is associated
with poor survival in patients with early stage of esophageal
squamous cell carcinoma. Dis Esophagus. 25:656–663. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang G, Miyake M, Lawton A, Goodison S
and Rosser CJ: Matrix metalloproteinase-10 promotes tumor
progression through regulation of angiogenic and apoptotic pathways
in cervical tumors. BMC Cancer. 14:3102014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma B, Ran R, Liao HY and Zhang HH: The
paradoxical role of matrix metalloproteinase-11 in cancer. Biomed
Pharmacother. 141:1118992021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsin CH, Chou YE, Yang SF, Su SC, Chuang
YT, Lin SH and Lin CW: MMP-11 promoted the oral cancer migration
and Fak/Src activation. Oncotarget. 8:32783–32793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv FZ, Wang JL, Wu Y, Chen HF and Shen XY:
Knockdown of MMP12 inhibits the growth and invasion of lung
adenocarcinoma cells. Int J Immunopathol Pharmacol. 28:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JM, Kim HJ, Koo BS, Rha KS and Yoon
YH: Expression of matrix metalloproteinase-12 is correlated with
extracapsular spread of tumor from nodes with metastasis in head
and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
270:1137–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han F, Zhang S, Zhang L and Hao Q: The
overexpression and predictive significance of MMP-12 in esophageal
squamous cell carcinoma. Pathol Res Pract. 213:1519–1522. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kudo Y, Iizuka S, Yoshida M, Tsunematsu T,
Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H,
Ishimaru N, et al: Matrix metalloproteinase-13 (MMP-13) directly
and indirectly promotes tumor angiogenesis. J Biol Chem.
287:38716–38728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiao XL, Chen D, Wang JG and Zhang KJ:
Clinical significance of serum matrix metalloproteinase-13 levels
in patients with esophageal squamous cell carcinoma (ESCC). Eur Rev
Med Pharmacol Sci. 18:509–515. 2014.PubMed/NCBI
|
|
45
|
Zheng Cl, Lu Q, Zhang N, Jing PY, Zhang
JP, Wang WP and Li GZ: Comprehensive analysis of the immune and
prognostic implication of MMP14 in lung cancer. Dis Markers.
2021:59175062021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kurahara S, Shinohara M, Ikebe T, Nakamura
S, Beppu M, Hiraki A, Takeuchi H and Shirasuna K: Expression of
MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral
cavity: Correlations with tumor invasion and metastasis. Head Neck.
21:627–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Zhang Y, Zhang Y, Liu W and Wang
J: Cryptotanshinone inhibits lung cancer invasion via
microRNA-133a/matrix metalloproteinase 14 regulation. Oncol Lett.
18:2554–2559. 2019.PubMed/NCBI
|
|
48
|
Chen N, Zhang G, Fu J and Wu Q: Matrix
metalloproteinase-14 (MMP-14) downregulation inhibits esophageal
squamous cell carcinoma cell migration, invasion, and
proliferation. Thorac Cancer. 11:3168–3174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li M, Li S, Zhou L, Yang L, Wu X, Tang B,
Xie S, Fang L, Zheng S and Hong T: Immune Infiltration of MMP14 in
pan cancer and its prognostic effect on tumors. Front Oncol.
11:7176062021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fan QC, Tian H, Wang Y and Liu XB:
Integrin-α5 promoted the progression of oral squamous cell
carcinoma and modulated PI3K/AKT signaling pathway. Arch Oral Biol.
101:85–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park SJ, Min HJ, Yoon C, Kim SH, Kim JH
and Lee SY: Integrin β1 regulates the perineural invasion and
radioresistance of oral squamous carcinoma cells by modulating
cancer cell stemness. Cell Signal. 110:1108082023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramos DM, But M, Regezi J, Schmidt BL,
Atakilit A, Dang D, Ellis D, Jordan R and Li X: Expression of
integrin beta 6 enhances invasive behavior in oral squamous cell
carcinoma. Matrix Biol. 21:297–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishida Y, Shintani T, Nobumoto T, Sakurai
S, Hamana T, Yanamoto S and Hayashido Y: Interaction of Integrin
αvβ8 With Type I collagen promotes squamous cell carcinoma cell
motility via RAC1 activation. Anticancer Res. 43:4833–4841. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thomas GJ, Jones J and Speight PM:
Integrins and oral cancer. Oral Oncol. 33:381–388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hou S, Hao X, Li J, Weng S, Wang J, Zhao
T, Li W, Hu X, Deng B, Gu J and Hang Q: TM4SF1 promotes esophageal
squamous cell carcinoma metastasis by interacting with integrin α6.
Cell Death Dis. 13:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xie YH, Ran LQ, Wu ZY, Sun C, Xu XE, Zou
HY, Fang WK and Xie JJ: Role of Integrin β1 in the progression and
chemo-resistance of esophageal squamous cell carcinoma. J Cancer.
13:2074–2085. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie JJ, Guo JC, Wu ZY, Xu XE, Wu JY, Chen
B, Ran LQ, Liao LD, Li EM and Xu LY: Integrin α5 promotes tumor
progression and is an independent unfavorable prognostic factor in
esophageal squamous cell carcinoma. Hum Pathol. 48:69–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dmello C, Sawant S, Alam H, Gangadaran P,
Tiwari R, Dongre H, Rana N, Barve S, Costea DE, Chaukar D, et al:
Vimentin-mediated regulation of cell motility through modulation of
beta4 integrin protein levels in oral tumor derived cells. Int J
Biochem Cell Biol. 70:161–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu S, Liao G and Li G: Regulatory effects
of COL1A1 on apoptosis induced by radiation in cervical cancer
cells. Cancer Cell Int. 17:732017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Geng Q, Shen Z, Li L and Zhao J: COL1A1 is
a prognostic biomarker and correlated with immune infiltrates in
lung cancer. PeerJ. 9:e111452021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin P, Tian P, Pang J, Lai L, He G, Song Y
and Zheng Y: Clinical significance of COL1A1 and COL1A2 expression
levels in hypopharyngeal squamous cell carcinoma. Oncol Lett.
20:803–809. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li G, Jiang W, Kang Y, Yu X, Zhang C and
Feng Y: High expression of collagen 1A2 promotes the proliferation
and metastasis of esophageal cancer cells. Ann Transl Med.
8:16722020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shen Y, Li X, Wang D, Zhang L, Li X, Su L,
Fan X and Yang X: COL3A1: Potential prognostic predictor for head
and neck cancer based on immune-microenvironment alternative
splicing. Cancer Med. 12:4882–4894. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tian X, Sun J, Li C and Zhang K: COL4A1
promotes the proliferation and migration of oral squamous cell
carcinoma cells by binding to NID1. Exp Ther Med. 25:1762023.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Di YB, Bao Y, Guo J, Liu W, Zhang SX,
Zhang GH and Li TK: COL11A1 as a potential prognostic target for
oral squamous cell carcinoma. Medicine (Baltimore). 101:e309892022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sok JC, Lee JA, Dasari S, Joyce S,
Contrucci SC, Egloff AM, Trevelline BK, Joshi R, Kumari N, Grandis
JR and Thomas SM: Collagen type XI α1 facilitates head and neck
squamous cell cancer growth and invasion. Br J Cancer.
109:3049–3056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Galiger C, Löffek S, Stemmler MP, Kroeger
JK, Mittapalli VR, Fauth L, Esser PR, Kern JS, Meiss F, Laßmann S,
et al: Targeting of cell surface proteolysis of collagen XVII
impedes squamous cell carcinoma progression. Mol Ther. 26:17–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu L, Jung SN, Oh C, Lee K, Won HR, Chang
JW, Kim JM and Koo BS: LAMB3 is associated with disease progression
and cisplatin cytotoxic sensitivity in head and neck squamous cell
carcinoma. Eur J Surg Oncol. 45:359–365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Marangon Junior H, Rocha VN, Leite CF, de
Aguiar MC, Souza PE and Horta MC: Laminin-5 gamma 2 chain
expression is associated with intensity of tumor budding and
density of stromal myofibroblasts in oral squamous cell carcinoma.
J Oral Pathol Med. 43:199–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen J, Zhou J, Lu J, Xiong H, Shi X and
Gong L: Significance of CD44 expression in head and neck cancer: A
systemic review and meta-analysis. BMC Cancer. 14:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mirhashemi M, Sadeghi M, Ghazi N,
Saghravanian N, Dehghani M and Aminian A: Prognostic value of CD44
expression in oral squamous cell carcinoma: A meta-analysis. Ann
Diagn Pathol. 67:1522132023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sawant S, Ahire C, Dongre H, Joshi S,
Jamghare S, Rane P, Kane S and Chaukar D: Prognostic significance
of elevated serum CD44 levels in patients with oral squamous cell
carcinoma. J Oral Pathol Med. 47:665–673. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Maleš J, Mihalj H, Šestak A, Kralik K and
Smolić M: Osteopontin levels in patients with squamous metastatic
head and neck cancer. Medicina (Kaunas). 57:1852021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li J, Wang X, Zheng K, Liu Y, Li J and
Wang S, Liu K, Song X, Li N, Xie S and Wang S: The clinical
significance of collagen family gene expression in esophageal
squamous cell carcinoma. PeerJ. 7:e77052019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhou J, Yang Y, Zhang H, Luan S, Xiao X,
Li X, Fang P, Shang Q, Chen L, Zeng X and Yuan Y: Overexpressed
COL3A1 has prognostic value in human esophageal squamous cell
carcinoma and promotes the aggressiveness of esophageal squamous
cell carcinoma by activating the NF-κB pathway. Biochem Biophys Res
Commun. 613:193–200. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang B, Zhang C, Yang X, Chen Y, Zhang H,
Liu J and Wu Q: Cytoplasmic collagen XIαI as a prognostic biomarker
in esophageal squamous cell carcinoma. Cancer Biol Ther.
19:364–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Meng X, Chen X, Lu P, Ma W, Yue D, Song L
and Fan Q: MicroRNA-202 inhibits tumor progression by targeting
LAMA1 in esophageal squamous cell carcinoma. Biochem Biophys Res
Commun. 473:821–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shen XM, Wu YP, Feng YB, Luo ML, Du XL,
Zhang Y, Cai Y, Xu X, Han YL, Zhang X, et al: Interaction of
MT1-MMP and laminin-5gamma2 chain correlates with metastasis and
invasiveness in human esophageal squamous cell carcinoma. Clin Exp
Metastasis. 24:541–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang
K, Guan X, Yang K and Bai Y: LncRNA CASC9 promotes esophageal
squamous cell carcinoma metastasis through upregulating LAMC2
expression by interacting with the CREB-binding protein. Cell Death
Differ. 25:1980–1995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fang L, Che Y, Zhang C, Huang J, Lei Y, Lu
Z, Sun N and He J: LAMC1 upregulation via TGFβ induces inflammatory
cancer-associated fibroblasts in esophageal squamous cell carcinoma
via NF-κB-CXCL1-STAT3. Mol Oncol. 15:3125–3146. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kamil Mohammed Al-Mosawi A, Cheshomi H,
Hosseinzadeh A and M Matin M: Prognostic and Clinical Value of CD44
and CD133 in Esophageal Cancer: A Systematic Review and
Meta-analysis. Iran J Allergy Asthma Immunol. 19:105–116.
2020.PubMed/NCBI
|
|
83
|
Miyako S, Koma YI, Nakanishi T, Tsukamoto
S, Yamanaka K, Ishihara N, Azumi Y, Urakami S, Shimizu M, Kodama T,
et al: Periostin in cancer-associated fibroblasts promotes
esophageal squamous cell carcinoma progression by enhancing cancer
and stromal cell migration. Am J Pathol. 194:828–848. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen B, Liang S, Guo H, Xu L, Li J and
Peng J: OPN promotes cell proliferation and invasion through NF-κB
in human esophageal squamous cell carcinoma. Genet Res (Camb).
2022:31548272022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chiu TJ, Lu HI, Chen CH, Huang WT, Wang
YM, Lin WC and Li SH: Osteopontin expression is associated with the
poor prognosis in patients with locally advanced esophageal
squamous cell carcinoma receiving preoperative chemoradiotherapy.
Biomed Res Int. 2018:90982152018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Duan Y, Liu G, Sun Y, Wu J, Xiong Z, Jin T
and Chen M: Collagen type VI α5 gene variations may predict the
risk of lung cancer development in Chinese Han population. Sci Rep.
10:50102020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang L, Sun Y, Guo Z and Liu H: COL3A1
overexpression associates with poor prognosis and cisplatin
resistance in lung cancer. Balkan Med J. 39:393–400. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Voiles L, Lewis DE, Han L, Lupov IP, Lin
TL, Robertson MJ, Petrache I and Chang HC: Overexpression of type
VI collagen in neoplastic lung tissues. Oncol Rep. 32:1897–1904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee CS, Siprashvili Z, Mah A, Bencomo T,
Elcavage LE, Che Y, Shenoy RM, Aasi SZ and Khavari PA: Mutant
collagen COL11A1 enhances cancerous invasion. Oncogene.
40:6299–6307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu M, Cai R, Wang T, Yang X, Wang M,
Kuang Z, Xie Y, Zhang J and Zheng Y: LAMC2 promotes the
proliferation of cancer cells and induce infiltration of
macrophages in non-small cell lung cancer. Ann Transl Med.
9:13922021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Akashi T, Ito E, Eishi Y, Koike M,
Nakamura K and Burgeson RE: Reduced Expression of Laminin alpha3
and alpha5 Chains in Non-small Cell Lung Cancers. Jpn J Cancer Res.
92:293–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rousselle P and Scoazec JY: Laminin 332 in
cancer: When the extracellular matrix turns signals from cell
anchorage to cell movement. Semin Cancer Biol. 62:149–165. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jiang H, Zhao W and Shao W: Prognostic
value of CD44 and CD44v6 expression in patients with non-small cell
lung cancer: meta-analysis. Tumour Biol. 35:7383–7389. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xu CH, Wang W, Lin Y, Qian LH, Zhang XW,
Wang QB and Yu LK: Diagnostic and prognostic value of serum
periostin in patients with non-small cell lung cancer. Oncotarget.
8:18746–18753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang W, Wang S and Zhang M: Evaluation of
kininogen 1, osteopontin and α-1-antitrypsin in plasma,
bronchoalveolar lavage fluid and urine for lung squamous cell
carcinoma diagnosis. Oncol Lett. 19:2785–2792. 2020.PubMed/NCBI
|
|
96
|
Hou T, Tong C, Kazobinka G, Zhang W, Huang
X, Huang Y and Zhang Y: Expression of COL6A1 predicts prognosis in
cervical cancer patients. Am J Transl Res. 8:2838–2844.
2016.PubMed/NCBI
|
|
97
|
Skyldberg B, Salo S, Eriksson E, Aspenblad
U, Moberger B, Tryggvason K and Auer G: Laminin-5 as a Marker of
Invasiveness in Cervical Lesions. J Natl Cancer Inst. 91:1882–1887.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Noel JC, Fernandez-Aguilar S, Fayt I,
Buxant F, Ansion MH, Simon P and Anaf V: Laminin-5γ2 chain
expression in cervical intraepithelial neoplasia and invasive
cervical carcinoma. Acta Obstet Gynecol Scand. 84:1119–1123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kainz C, Kohlberger P, Tempfer C, Sliutz
G, Gitsch G, Reinthaller A and Breitenecker G: Prognostic value of
CD44 splice variants in human stage III cervical cancer. Eur J
Cancer. 31:1706–1709. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wei WF, Chen XJ, Liang LJ, Yu L, Wu XG,
Zhou CF, Wang ZC, Fan LS, Hu Z, Liang L and Wang W:
Periostin+cancer-associated fibroblasts promote lymph node
metastasis by impairing the lymphatic endothelial barriers in
cervical squamous cell carcinoma. Mol Oncol. 15:210–227. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gillot L, Lebeau A, Baudin L, Pottier C,
Louis T, Durré T, Longuespée R, Mazzucchelli G, Nizet C, Blacher S,
et al: Periostin in lymph node pre-metastatic niches governs
lymphatic endothelial cell functions and metastatic colonization.
Cell Mol Life Sci. 79:2952022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Qin S, Yi L, Liang Y, Chen Y, Wang W, Liao
Y, Zhang C, Huang H, Huang J and Yao S: Biological and
Clinicopathological Characteristics of OPN in Cervical Cancers.
Front Genet. 13:8365092022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Castor MDGFC, Torres LC, Mello RJV, Natal
RA and Vassallo J: Study on collagen parameters in vulvar cancer
and preneoplastic lesions by Second Harmonic Generation microscopy.
Sci Rep. 10:55682020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Holthoff ER, Byrum SD, Mackintosh SG,
Kelly T, Tackett AJ, Quick CM and Post SR: Vulvar squamous cell
carcinoma aggressiveness is associated with differential expression
of collagen and STAT1. Clin Proteomics. 14:402017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu Z, Shen Y, Gong K, Wu Z, Zhang T, Zhang
X and Li S: Increased osteopontin expression is associated with
progression from vulvar precancerous lesions to vulvar squamous
cell carcinoma. Arch Gynecol Obstet. 289:637–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sgambato A, Tarquini E, Resci F, De Paola
B, Faraglia B, Camerini A, Rettino A, Migaldi M, Cittadini A and
Zannoni GF: Aberrant expression of alpha-dystroglycan in cervical
and vulvar cancer. Gynecol Oncol. 103:397–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Brockbank EC, Bridges J, Marshall CJ and
Sahai E: Integrin beta1 is required for the invasive behaviour but
not proliferation of squamous cell carcinoma cells in vivo. Br J
Cancer. 92:102–112. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hefler LA, Concin N, Mincham D, Thompson
J, Swarte NB, van Eijkeren MA, Sie-Go DM, Hammond I, McCartney AJ,
Tempfer CB and Speiser P: The prognostic value of
immunohistochemically detected CD44v3 and CD44v6 expression in
patients with surgically staged vulvar carcinoma: A multicenter
study. Cancer. 94:125–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Johansson N, Vaalamo M, Grénman S,
Hietanen S, Klemi P, Saarialho-Kere U and Kähäri VM: Collagenase-3
(MMP-13) is expressed by tumor cells in invasive vulvar squamous
cell carcinomas. Am J Pathol. 154:469–480. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bovo AC, da Silva ID, Takita LC, Fochi J,
Stávale JN, Marks G and de Lima GR: A comparative study of MMP-2 in
vulvar neoplasms. Gynecol Oncol. 93:454–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: An evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kerkelä E, Ala-aho R, Klemi P, Grénman S,
Shapiro SD, Kähäri VM and Saarialho-Kere U: Metalloelastase
(MMP-12) expression by tumour cells in squamous cell carcinoma of
the vulva correlates with invasiveness, while that by macrophages
predicts better outcome. J Pathol. 198:258–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Goepel C, Stoerer S and Koelbl H: Tenascin
in preinvasive lesions of the vulva and vulvar cancer. Anticancer
Res. 23:4587–4591. 2003.PubMed/NCBI
|
|
114
|
Surico N, Priori L, Savoia P, Cremona O
and Marchisio PC: Distribution of integrins and extracellular
matrix proteins in vulvar squamous cell carcinomas. Eur J Gynaecol
Oncol. 16:147–154. 1995.PubMed/NCBI
|
|
115
|
Hellman K, Hellström AC, Silfverswärd C,
Salo S, Aspenblad U, Nilsson B, Frankendal B, Tryggvasson K and
Auer G: Cancer of the vagina: Laminin-5gamma2 chain expression and
prognosis. Int J Gynecol Cancer. 10:391–396. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Theocharis AD, Manou D and Karamanos NK:
The extracellular matrix as a multitasking player in disease. FEBS
J. 286:2830–2869. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Diao B and Yang P: Comprehensive analysis
of the expression and prognosis for laminin genes in ovarian
cancer. Pathol Oncol Res. 27:16098552021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jing J, Lien CF, Sharma S, Rice J, Brennan
PA and Górecki DC: Aberrant expression, processing and degradation
of dystroglycan in squamous cell carcinomas. Eur J Cancer.
40:2143–2151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sgambato A and Brancaccio A: The
dystroglycan complex: From biology to cancer. J Cell Physiol.
205:163–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: the
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Costea DE, Hills A, Osman AH, Thurlow J,
Kalna G, Huang X, Pena Murillo C, Parajuli H, Suliman S, Kulasekara
KK, et al: Identification of two distinct carcinoma-associated
fibroblast subtypes with differential tumor-promoting abilities in
oral squamous cell carcinoma. Cancer Res. 73:3888–3901. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Obradovic A, Graves D, Korrer M, Wang Y,
Roy S, Naveed A, Xu Y, Luginbuhl A, Curry J, Gibson M, et al:
Immunostimulatory cancer-associated fibroblast subpopulations can
predict immunotherapy response in head and neck cancer. Clin Cancer
Res. 28:2094–2109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Puram SV, Tirosh I, Parikh AS, Yizhak K,
Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, Deschler DG, et
al: Single-Cell transcriptomic analysis of primary and metastatic
tumor ecosystems in head and neck cancer. Cell. 171:1611–1624.e24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hu C, Zhang Y, Wu C and Huang Q:
Heterogeneity of cancer-associated fibroblasts in head and neck
squamous cell carcinoma: Opportunities and challenges. Cell Death
Discov. 9:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Mirkeshavarz M, Ganjibakhsh M, Aminishakib
P, Farzaneh P, Mahdavi N, Vakhshiteh F, Karimi A, Gohari NS, Kamali
F, Kharazifard MJ, et al: Interleukin-6 secreted by oral
cancer-associated fibroblast accelerated VEGF expression in tumor
and stroma cells. Cell Mol Biol (Noisy-le-grand). 63:131–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bae JY, Kim EK, Yang DH, Zhang X, Park YJ,
Lee DY, Che CM and Kim J: Reciprocal interaction between
carcinoma-associated fibroblasts and squamous carcinoma cells
through interleukin-1α induces cancer progression. Neoplasia.
16:928–938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Öhlund D, Handly-Santana A, Biffi G,
Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA,
Lee EJ, et al: Distinct populations of inflammatory fibroblasts and
myofibroblasts in pancreatic cancer. J Exp Med. 214:579–596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lavie D, Ben-Shmuel A, Erez N and
Scherz-Shouval R: Cancer-associated fibroblasts in the single-cell
era. Nat Cancer. 3:793–807. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
El Herch I, Tornaas S, Dongre HN and
Costea DE: Heterogeneity of cancer-associated fibroblasts and
tumor-promoting roles in head and neck squamous cell carcinoma.
Front Mol Biosci. 11:13400242024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chen J, Zhang L, Zhu Y, Zhao D, Zhang J,
Zhu Y, Pang J, Xiao Y, Wu Q, Wang Y and Zhan Q:
AKT2(S128)/CCTα(S315/319/323)-positive cancer-associated
fibroblasts (CAFs) mediate focal adhesion kinase (FAK) inhibitors
resistance via secreting phosphatidylcholines (PCs). Signal
Transduct Target Ther. 9:212024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li W, Xu T, Jin H, Li M and Jia Q:
Emerging role of cancer-associated fibroblasts in esophageal
squamous cell carcinoma. Pathol Res Pract. 253:1550022024.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Qin X, Guo H, Wang X, Zhu X, Yan M, Wang
X, Xu Q, Shi J, Lu E, Chen W and Zhang J: Exosomal miR-196a derived
from cancer-associated fibroblasts confers cisplatin resistance in
head and neck cancer through targeting CDKN1B and ING5. Genome
Biol. 20:122019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Huang W, Zhang L, Yang M, Wu X, Wang X,
Huang W, Yuan L, Pan H, Wang Y, Wang Z, et al: Cancer-associated
fibroblasts promote the survival of irradiated nasopharyngeal
carcinoma cells via the NF-κB pathway. J Exp Clin Cancer Res.
40:872021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li X, González-Maroto C and Tavassoli M:
Crosstalk between CAFs and tumour cells in head and neck cancer.
Cell Death Discov. 10:3032024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Dongre H, Rana N, Fromreide S, Rajthala S,
Bøe Engelsen I, Paradis J, Gutkind JS, Vintermyr OK, Johannessen
AC, Bjørge L and Costea DE: Establishment of a novel cancer cell
line derived from vulvar carcinoma associated with lichen sclerosus
exhibiting a fibroblast-dependent tumorigenic potential. Exp Cell
Res. 386:1116842020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Milacic M, Beavers D, Conley P, Gong C,
Gillespie M, Griss J, Haw R, Jassal B, Matthews L, May B, et al:
The reactome pathway knowledgebase 2024. Nucleic Acids Res.
52((D1)): D672–D678. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48:W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cancer Genome Atlas Network, .
Comprehensive genomic characterization of head and neck squamous
cell carcinomas. Nature. 517:576–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cancer Genome Atlas Research Network and
Albert Einstein College of Medicine; Analytical Biological
Services; Barretos Cancer Hospital; Baylor College of Medicine;
Beckman Research Institute of City of Hope; Buck Institute for
Research on Aging; Canada's Michael Smith Genome Sciences Centre;
Harvard Medical School; Helen F. Graham Cancer Center &Research
Institute at Christiana Care Health Services et al., . Integrated
genomic and molecular characterization of cervical cancer. Nature.
543:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cancer Genome Atlas Research Network;
Analysis Working Group; Asan University; BC Cancer Agency; Brigham
and Women's Hospital; Broad Institute; Brown University; Case
Western Reserve University; Dana-Farber Cancer Institute; Duke
University et al., . Integrated genomic characterization of
oesophageal carcinoma. Nature. 541:169–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bergonzini C, Kroese K, Zweemer AJM and
Danen EHJ: Targeting integrins for cancer therapy-disappointments
and opportunities. Front Cell Dev Biol. 10:8638502022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yan Z, Hu X, Tang B and Deng F: Role of
osteopontin in cancer development and treatment. Heliyon.
9:e210552023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zagani R, Hamzaoui N, Cacheux W, de
Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C and
Lamarque D: Cyclooxygenase-2 inhibitors down-regulate osteopontin
and Nr4A2-new therapeutic targets for colorectal cancers.
Gastroenterology. 37:1358–1366.e1-e3. 2009. View Article : Google Scholar
|
|
148
|
Sroka TC, Pennington ME and Cress AE:
Synthetic D-amino acid peptide inhibits tumor cell motility on
laminin-5. Carcinogenesis. 27:1748–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Tran M, Rousselle P, Nokelainen P,
Tallapragada S, Nguyen NT, Fincher EF and Marinkovich MP: Targeting
a tumor-specific laminin domain critical for human carcinogenesis.
Cancer Res. 68:2885–2894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Heider KH, Sproll M, Susani S, Patzelt E,
Beaumier P, Ostermann E, Ahorn H and Adolf GR: Characterization of
a high-affinity monoclonal antibody specific for CD44v6 as
candidate for immunotherapy of squamous cell carcinomas. Cancer
Immunol Immunother. 43:245–253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Sandström K, Nestor M, Ekberg T, Engström
M, Anniko M and Lundqvist H: Targeting CD44v6 expressed in head and
neck squamous cell carcinoma: Preclinical characterization of an
111In-labeled monoclonal antibody. Tumour Biol. 29:137–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Song K, Yu Z, Zu X, Li G, Hu Z and Xue Y:
Collagen remodeling along cancer progression providing a novel
opportunity for cancer diagnosis and treatment. Int J Mol Sci.
23:105092022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Singh B, Sims H, Trueheart I, Simpson K,
Wang KC, Patzkowsky K, Wegman T, Soma JM, Dixon R, Jayes F, et al:
A Phase I clinical trial to assess safety and tolerability of
injectable collagenase in women with symptomatic uterine fibroids.
Reprod Sci. 28:2699–2709. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Oo KK, Kamolhan T, Soni A, Thongchot S,
Mitrpant C, O-Charoenrat P, Thuwajit C and Thuwajit P: Development
of an engineered peptide antagonist against periostin to overcome
doxorubicin resistance in breast cancer. BMC Cancer. 21:652021.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Nakazawa Y, Taniyama Y, Sanada F,
Morishita R, Nakamori S, Morimoto K, Yeung KT and Yang J: Periostin
blockade overcomes chemoresistance via restricting the expansion of
mesenchymal tumor subpopulations in breast cancer. Sci Rep.
8:40132018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhu M, Saxton RE, Ramos L, Chang DD,
Karlan BY, Gasson JC and Slamon DJ: Neutralizing monoclonal
antibody to periostin inhibits ovarian tumor growth and metastasis.
Mol Cancer Ther. 10:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lee YJ, Kim IS, Park SA, Kim Y, Lee JE,
Noh DY, Kim KT, Ryu SH and Suh PG: Periostin-binding DNA aptamer
inhibits breast cancer growth and metastasis. Mol Ther.
21:1004–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu GX, Xi HQ, Sun XY and Wei B: Role of
periostin and its antagonist PNDA-3 in gastric cancer metastasis.
World J Gastroenterol. 21:2605–2613. 2015. View Article : Google Scholar : PubMed/NCBI
|