Introduction
Breast cancer (BRCA) is the most prevalent malignant
tumor affecting the female population worldwide, and its incidence
rate continues to increase annually. Additionally, the age of onset
is progressively decreasing (1).
BRCA treatment encompasses surgery, radiotherapy, chemotherapy,
targeted therapy and endocrine therapy. While surgery is a common
treatment modality for BRCA, neoadjuvant therapy, comprising of
endocrine therapy, combination therapy and targeted therapy,
provides improved long-term control over the progression of
metastatic BRCA and helps maintain the quality of life of patients
(2). Although significant progress
has been made in BRCA treatment, tumor resistance and metastasis
continue to limit the therapeutic efficacy. Cell signaling pathways
play a crucial role in cell proliferation, differentiation and
apoptosis. Targeted therapies aimed at these pathways can inhibit
the proliferation and differentiation of tumor cells, thereby
restraining their growth. Therefore, developing targeted
therapeutic strategies against cellular pathways is essential.
The Wnt signaling pathway is an evolutionarily
conserved pathway that plays a crucial role in both embryonic
development and neoplastic diseases, comprising three main
branches: The classical Wnt/β-catenin pathway, the Wnt/PCP pathway
(planar cell polarity) and the Wnt/Ca2+ pathway. The Wnt
pathway has been implicated in various types of human cancer and
metastases, including colorectal cancer, hepatocellular carcinoma,
oral squamous carcinoma, BRCA, endometrial cancer and hematologic
disorders (3–5). In primary oral squamous cell
carcinoma, the reduced expression of β-catenin on the cell membrane
is associated with a higher likelihood of nuclear metastasis, which
is significantly associated with invasion and lymph node metastasis
(6). In normal breast tissue,
β-catenin is primarily expressed on the cell membrane. However, in
BRCA, the activation of the Wnt pathway promotes the nuclear
translocation of β-catenin, enhancing signaling. The abnormal
expression of β-catenin is associated with Her2 positivity in the
Wnt/β-catenin pathway, facilitating invasion and lymph node
metastasis (7,8). In summary, the nuclear translocation
of β-catenin enhances tumor invasion and metastasis. Lymphoid
enhancer factor-1 (LEF-1), a member of the LEF/TCF family, belongs
to the high mobility group. As a key transcription factor, LEF-1
serves as a core component of the Wnt signaling pathway. It has
been shown that LEF-1 is highly expressed in BRCA, being associated
with the expression of the G1 cell cycle regulator cyclin D1, thus
regulating the proliferation of BRCA cells (9). LEF-1 can also bind to estrogen
receptor (ER) cis-regulatory elements, inhibiting the binding of ER
to chromatin and contributing to BRCA development (10). Furthermore, the abnormal activation
of the β-catenin/LEF1 pathway can induce the expression of cyclin
D1 and promote tumor transformation in colorectal cancer. The
knockdown of LEF-1 effectively blocks the Wnt/β-catenin signaling
pathway (11,12). In summary, these studies demonstrate
that intervening with the β-catenin/LEF1 signaling pathway may
hinder the development of BRCA, establishing it as a key target for
targeted therapy. Therefore, upstream and downstream factors of
this pathway may represent key targets for intervention therapy and
may aid in the development of novel strategies.
RUVBL1 is a protein exhibiting both DNA-dependent
ATPase and DNA helicase activities. It belongs to the
AAA+ protein family, which is associated with various
cellular activities, including gene regulation, DNA damage repair
and chromatin remodeling (13,14).
Previous studies have revealed that RUVBL1 is closely linked to the
occurrence and progression of cancer. RUVBL1 is overexpressed in
several cancers, including colorectal cancer, hepatocellular
carcinoma, lung adenocarcinoma, oral squamous cell carcinoma, uveal
melanoma, prostate cancer, epithelial ovarian cancer and
osteosarcoma. It is involved in several signaling pathways,
including the β-catenin/LEF1, NF-κB and PLXNA1-CRAF-MAPK pathways,
influencing the occurrence and progression of diseases (15–20). A
previous study indicated that the binding reaction between
RUVBL1-specific antigens and autoantibodies can serve as a
supplement to mammography for diagnosing lymph node-negative
early-stage BRCA (21).
Additionally, astaxanthin inhibits colony formation, spheroid
formation, migration and the invasion of BRCA cells by
downregulating RUVBL1 expression (22). However, the mechanism through which
the inhibition of RUVBL1 expression affects the occurrence and
progression of BRCA remains unclear. Collectively, these findings
suggest that RUVBL1 may serve as a novel therapeutic target for
BRCA.
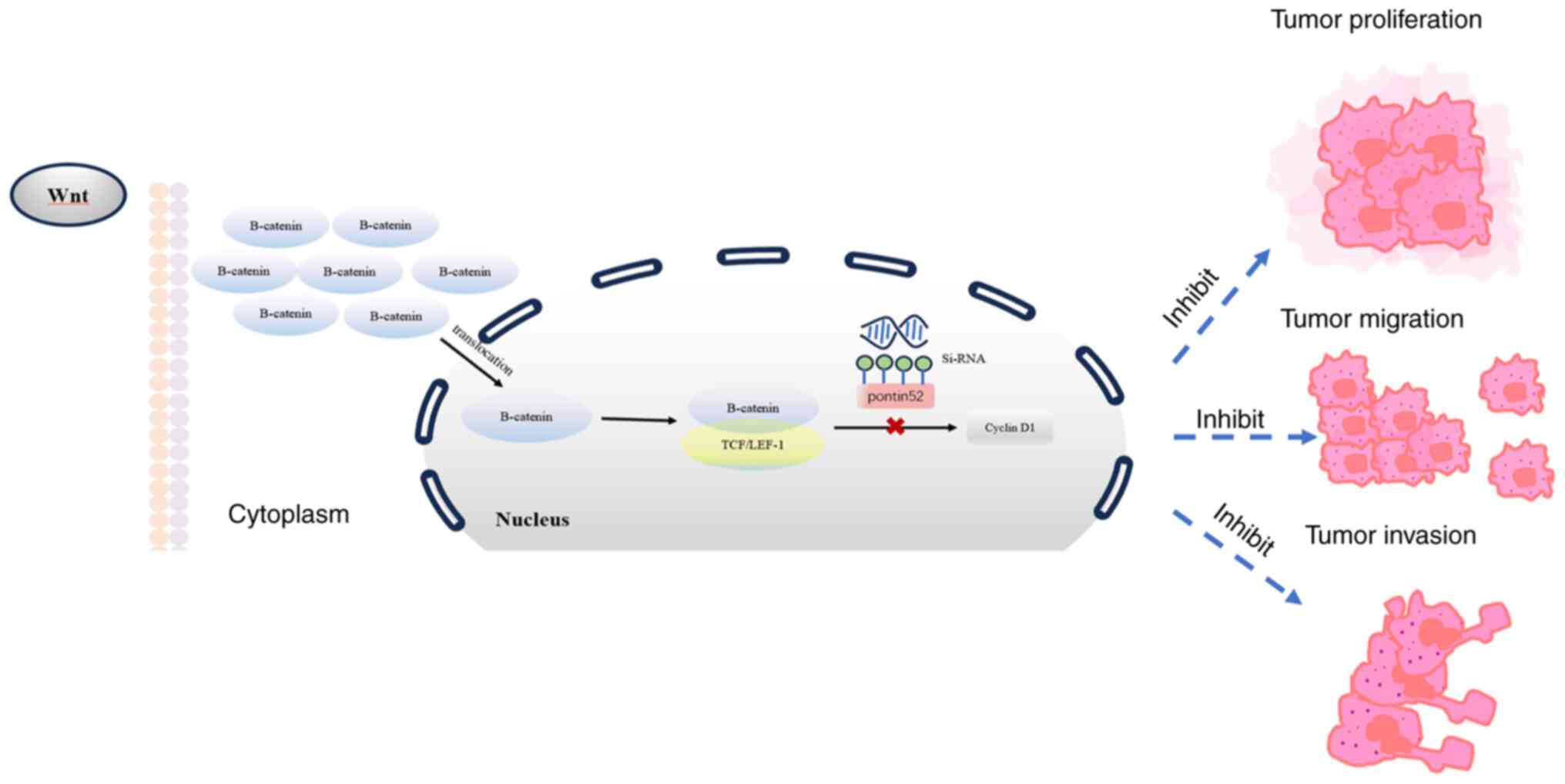
The present study identifies RUVBL1 as being
positioned at the axis of the β-catenin/LEF-1 pathway through
bioinformatics analysis. It is suggested that RUVBL1 may
participate in the signal transduction of β-catenin/LEF-1 and is
associated with the proliferation, invasion and metastasis of BRCA.
Consequently, RUVBL1 may represent a potential therapeutic target
within this signaling pathway.
Materials and methods
Cells and cell culture
The normal breast cell line, MCF-10A, and the BRCA
cell lines, MDA-MB-231 and MCF-7, were obtained from Wuhan Procell
Life Science & Technology Co., Ltd. The MCF-10A cells were
cultured in a specialized medium (CM-0525; Wuhan Procell Life
Science & Technology Co., Ltd.). The MDA-MB-231 and MCF-7 cells
were cultured in DMEM supplemented with 10% fetal bovine serum
(FBS; cat. no. 164210; Wuhan Procell Life Science & Technology
Co., Ltd) and 1% penicillin-streptomycin (cat. no. PB180120; Wuhan
Procell Life Science & Technology Co., Ltd). Following
incubation in a cell incubator with 5% CO2 for 48 h,
cell density reached 80 to 90%, and the cells were passaged at a
ratio of 1:2.
Cell transfection
The MDA-MB-231 and MCF-7 cells were seeded in
six-well plates at a density of 2×105 cells per well.
Once the cell density reached 30%, small interfering RNA
(siRNA)-RUVBL1 (Table I, Guangzhou
RiboBio Co., Ltd.) and siRNA-negative control (NC; siN0000001-1-5;
Guangzhou RiboBio Co., Ltd.) transfection complexes were prepared
according to the instructions provided with the siRNA transfection
reagent (Ribotest; cat. no. R10043.8; Guangzhou RiboBio Co., Ltd.).
siRNA was transfected at a concentration of 50 nM. Subsequent
experiments were performed 24 h after transfection. The knockdown
efficiency was verified by mixing the transfection complex with a
complete medium (without antibiotics) in the six-well plates,
followed by incubation in a CO2 culture environment at
37°C for 48 h.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′-3′) |
|---|
| RUVBL1 | F:
TGGACATTGAGTGCTTCACCTACC |
|
| R:
TGACACAGTTGCCTCGGTTGG |
| β-catenin | F:
ATAGAGGCTCTTGTGCGTACTGTC |
|
| R:
TTGGTGTCGGCTGGTCAGATG |
| lymphoid enhancer
factor-1 | F:
AGTCTTCCTTGGTGAACGAGTCTG |
|
| R:
GTAGGGCTCCTGAGAGGTTTGTG |
| GAPDH | F:
CAGGAGGCATTGCTGATGAT |
|
| R:
GAAGGCTGGGGCTCATTT |
| siRNA-RUVBL1 guide
strand |
TCAAGGTCGAATTCTGTGG |
| siRNA-RUVBL1
passenger strand |
CCACAGAATTCGACCTTGA |
Cell Counting Kit-8 (CCK-8) assay
Cells in the logarithmic growth phase were seeded in
96-well plates at equal densities and cultured for 0, 12, 24, 48
and 96 h. DMEM and CCK-8 solution (cat. no. GK10001; GlpBio) were
added to each well in a 9:1 ratio. Following incubation for 1 h at
37°C, the optical density (OD) of the cells was measured at 450 nm
using a microplate reader (Multiskan SkyHigh; Thermo Fisher
Scientific, Inc.). The percentage of cell proliferation inhibition
was calculated using the following formula: (absorbance of
experimental wells/absorbance of control wells) ×100%.
Scratch assay
A scratch was made on the surface of the culture
plate containing logarithmic phase cells using a sterile 200-µl
pipette tip. The cells were then washed twice with PBS and cultured
in a serum-free medium. Cell migration was recorded at 0, 12 and 24
h using a light microscope (LEICA DM6 B; Leica Microsystems GmbH),
and the scratch area was measured using ImageJ 1.54d software
(National Institutes of Health). The cell migration rate was
calculated using the following formula: (initial scratch area-final
scratch area)/initial scratch area ×100%.
Transwell assay
For proliferation experiments, 200 µl serum-free
cell suspension at a density of 2×104 cells was added to
the upper chamber, and 600 µl medium containing 20% FBS was added
to the lower chamber. For the invasion assay, Matrigel (BD Biocoat;
cat. no. 356234; Corning, Inc.) was thawed overnight at 4°C,
transferred to an ice box before the experiment, and diluted with
serum-free medium at a ratio of 1:8, was applied to the upper
surface of the membrane in the Transwell chamber (8.0-µm pore size;
cat. no. 3422; Corning, Inc.). The cells were incubated at 37°C for
3 h to facilitate membrane formation, after which the cell
suspension and medium were added. After 24 h, the cells were fixed
with 600 µl 4% paraformaldehyde for 30 min. Subsequently,
non-penetrated cells and excess Matrigel were removed using a wet
cotton swab, and the fixed cells were stained with 1% crystal
violet (cat. no. G1063; Beijing Solarbio Science & Technology
Co., Ltd.,) for 20 min at room temperature. After washing and
drying with PBS, the cells were counted and images were captured
under a light microscope (Olympus th4-200; Olympus
Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using the
RNAsimple Total RNA Extraction kit [DP419, RK145, Tiangen Biotech
(Beijing) Co., Ltd.] and reverse transcribed using the PrimeScript™
RT Reagent kit with gDNA Eraser (cat. no. RR047A; Takara Bio, Inc.)
according to the manufacturer's protocol. qPCR reactions were
performed using a Thermal Cycler Dice™ Real-Time System to detect
the fluorescence intensity of TB-Green (TB Green Premix Ex
TaqTM II; cat. no. RR820A; TaKaRa Bio, Inc.) in the
reaction solution and monitor the amplification of PCR products.
The thermal cycling conditions for qPCR were as follows: Initial
denaturation at 1 cycle of 95°C for 30 sec; 40 cycles of 60°C for
30 sec and 95°C for 5 sec. Relative mRNA expression levels were
calculated using the 2−ΔΔCq method (23) with GAPDH as an internal reference.
The primer sequences used are listed in Table I.
Western blot analysis
Whole proteins were extracted by lysing the cells
with RIPA lysis buffer (cat. no. KGP250; KGI Biotechnology Co.,
Ltd.). The total amounts of protein (30 µg) were separated by
electrophoresis on a 10% SDS-PAGE gel and transferred to PVDF
membranes. After blocking with 5% skim milk for 2 h at room
temperature and washing three times with 20% TBST, the PVDF
membrane was incubated at 4°C overnight with primary antibodies
RUVBL1 (IgG; 1:1,000; cat. no. ab133513; Abcam), β-catenin (IgG;
1:5,000, cat. no. 51067-2-AP; Proteintech Group, Inc.), LEF-1 (IgG;
1:1,000; cat. no. 14972-1-AP; Proteintech Group, Inc.) and cyclinD1
(IgG; 1:5,000; cat. no. 26939-1-AP; Proteintech Group, Inc.),
respectively. After re-washing three times with TBST, the membrane
was incubated for 2 h with HRP-labeled secondary sheep anti-rabbit
IgG (1:10,000; cat. no. S0001; Affinity Biosciences; RRID:
AB_2839429.) at room temperature. Following 1 min of incubation
with ECL (ECL Western Blotting Substrate; cat. no. KF8005-100;
Affinity Biosciences; RRID: AB_2846811) at room temperature,
protein bands were visualized using a western blotting exposer.
Densitometric analysis was conducted using Image Lab™ software
(version 6.0.0 Build 25; Bio-Rad Laboratories, Inc.).
Public data collection
RUVBL1 expression in BRCA tissues was obtained from
The Cancer Genome Atlas (TCGA) and genotype-tissue expression
(GTEx) databases via the Gene Expression Profiling Interactive
Analysis (GEPIA2) portal (http://gepia2.cancer-pku.cn/#index). RUVBL1
immunohistochemistry (IHC) data were obtained from the Human
Protein Atlas (HPA) database (https://www.proteinatlas.org/). Prognostic survival
analysis of BRCA sample data was conducted using the Kaplan-Meier
Plotter website (https://kmplot.com/analysis/). The Kyoto Encyclopedia
of Genes and Genomes (KEGG; http://www.genome.jp/kegg/pathway.html) database was
utilized to identify pathways associated with the RUVBL1 gene.
Statistical analysis
GraphPad Prism 9.0 statistical software (Dotmatics)
was employed for statistical analysis. One-way ANOVA with Tukey's
post hoc test was employed to compare the different treatment
groups. Data are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of RUVBL1 is upregulated in
BRCA tissues and is associated with the poor prognosis of
patients
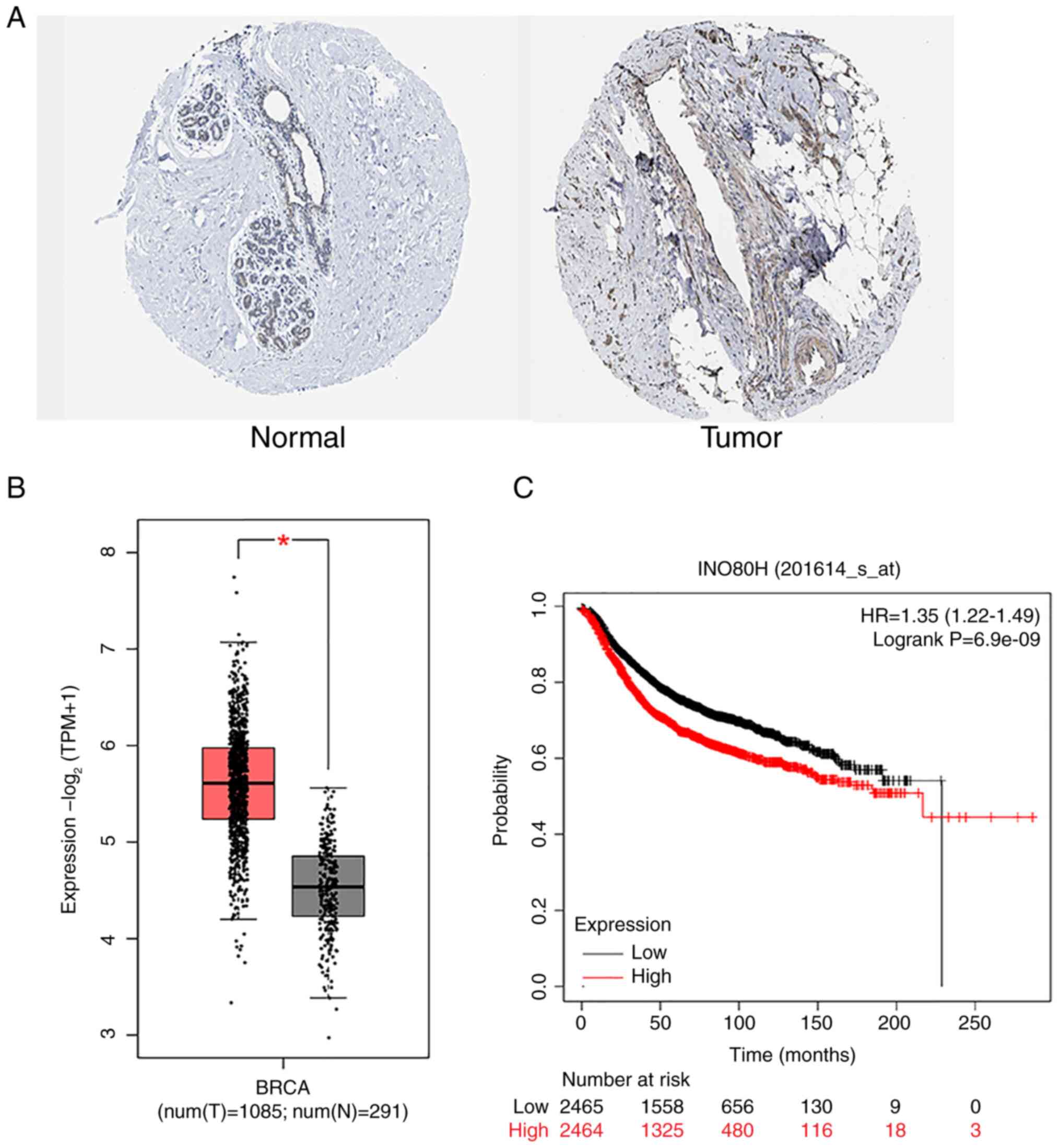
According to the IHC results from the HPA database,
RUVBL1 was expressed at higher levels in BRCA tissues than normal
breast tissues (Fig. 1A). RNA-seq
differential analysis from TCGA and GTEx databases via the GEPIA2
portal revealed that RUVBL1 expression in BRCA tissues was elevated
compared with that in normal breast tissues (Fig. 1B). The overall survival analysis of
patients with BRCA using the Kaplan-Meier Plotter website indicated
that a high mRNA expression of RUVBL1 was associated with a poor
prognosis (Fig. 1C). These results
(Fig. 1) confirmed that RUVBL1
expression is upregulated in BRCA tissues and may be linked to
patient prognosis.
RUVBL1 is highly expressed in human
breast carcinoma cells
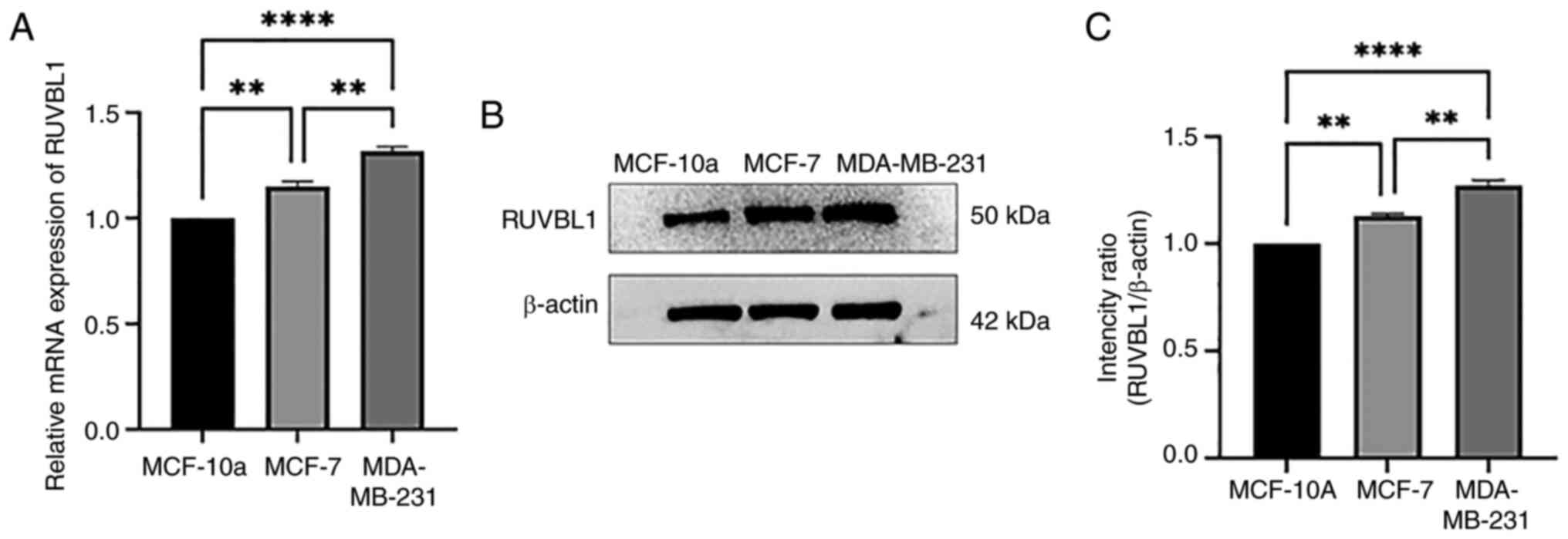
RT-qPCR and western blot analysis were performed on
normal BRCA epithelial cell lines and BRCA cell lines, and the
results are presented in Fig. 2.
Compared with the normal BRCA cell line, MCF-10a, the mRNA and
protein expression of RUVBL1 in the BRCA cell lines (MDA-MB-231 and
MCF7) was upregulated (P<0.01). The mRNA and protein expression
of RUVBL1 in the MDA-MB-231 cell line was higher than that in the
MCF-7 cell line (P<0.01). This may be due to the different
regulation mode of RUVBL1 in invasive and non-invasive BRCA.
Knockdown of RUVBL1 inhibits the
proliferation of BRCA cell lines
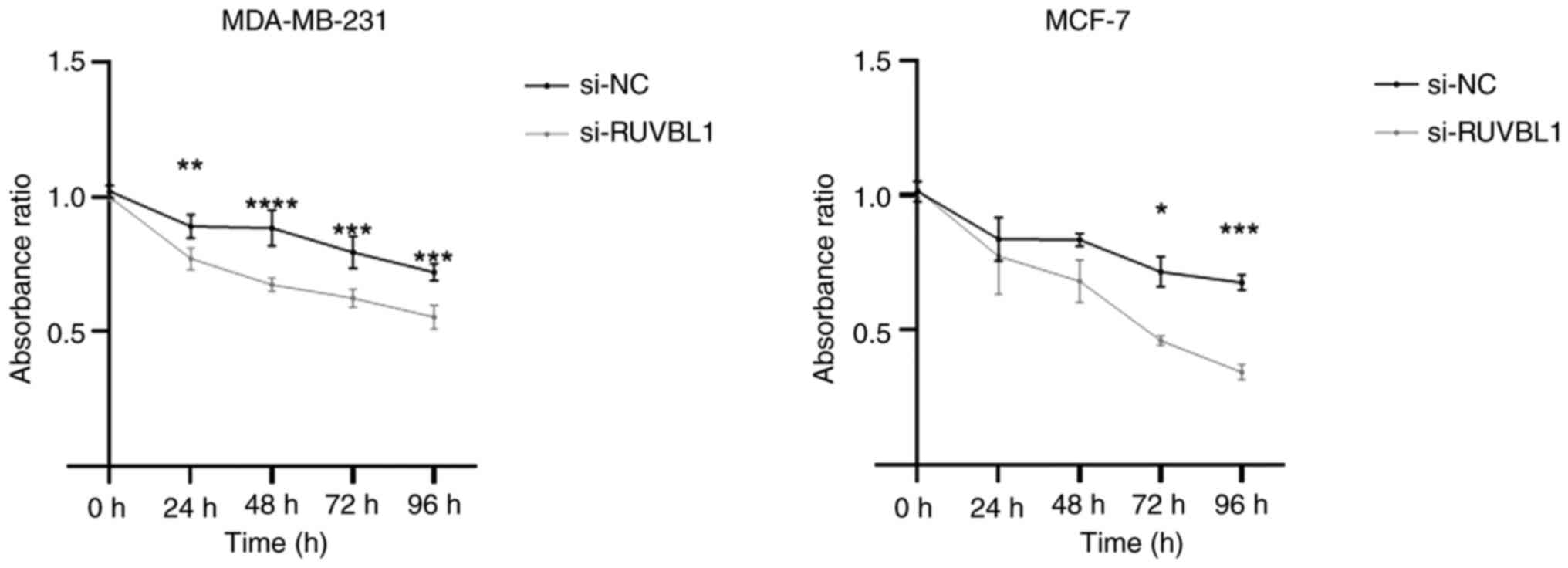
To evaluate whether RUVBL1 affects the proliferation
of BRCA cells, a CCK-8 assay was conducted following the
siRNA-mediated knockdown of RUVBL1. The proliferation of the BRCA
cell lines in the vehicle control, si-NC and si-RUVBL1 groups was
assessed at various time points: 24, 48, 72 and 96 h. Compared with
the si-NC group, the si-RUVBL1 group of BRCA cell lines exhibited a
significant inhibition of proliferation at 48, 72 and 96 h
(Fig. 3).
Knockdown of RUVBL1 inhibits BRCA cell
migration and invasion
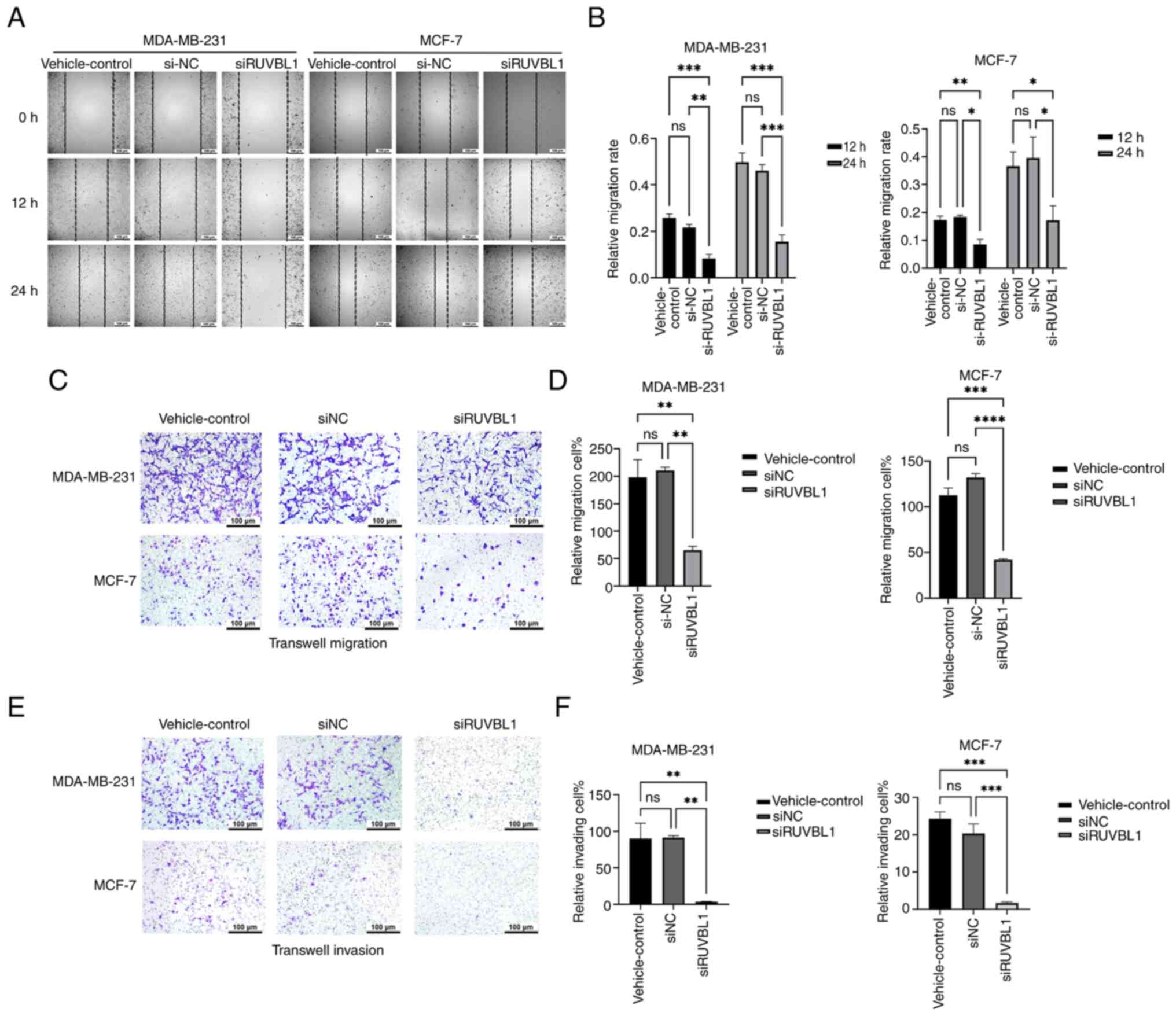
The scratch test was performed to assess the cell
migration ability (Fig. 4A and B).
The results indicated that the migration rate of the BRCA cell
lines, MDA-MB-231 and MCF-7, decreased at 12 and 24 h in the
si-RUVBL1 group compared with the vehicle control and si-NC groups.
Transwell migration (Fig. 4C and D)
and invasion assays (Fig. 4E and F)
revealed that the migration and invasion of the MDA-MB-231 and
MCF-7 cells in the si-RUVBL1 group were reduced compared with the
vehicle control and si-NC groups. In summary, si-RUVBL1
significantly inhibited the migration and invasion of both
MDA-MB-231 and MCF-7 BRCA cell lines.
Knockdown of RUVBL1 downregulates the
mRNA and protein levels of β-catenin and LEF
To investigate whether RUVBL1 regulates the
β-catenin/LEF1 pathway and to identify its downstream genes, a
signaling pathway map of RUVBL1 was accessed using the KEGG
database (Fig. 5A). RT-qPCR
(Fig. 5B and C) and western blot
analysis (Fig. 5D and F) were
utilized to measure the mRNA and protein expression levels in the
MDA-MB-231 and MCF-7 BRCA cell lines following siRNA transfection.
The results indicated that, compared with the vehicle control and
si-NC groups, the mRNA and protein levels of β-catenin and LEF-1
were decreased following RUVBL1 knockdown. Additionally, the
protein level of cyclin D1, a downstream target of LEF-1, was
significantly reduced. These results suggested that the knockdown
of RUVBL1 effectively blocks the β-catenin/LEF1 pathway, reduces
cyclin D1 levels and affects the cell cycle.
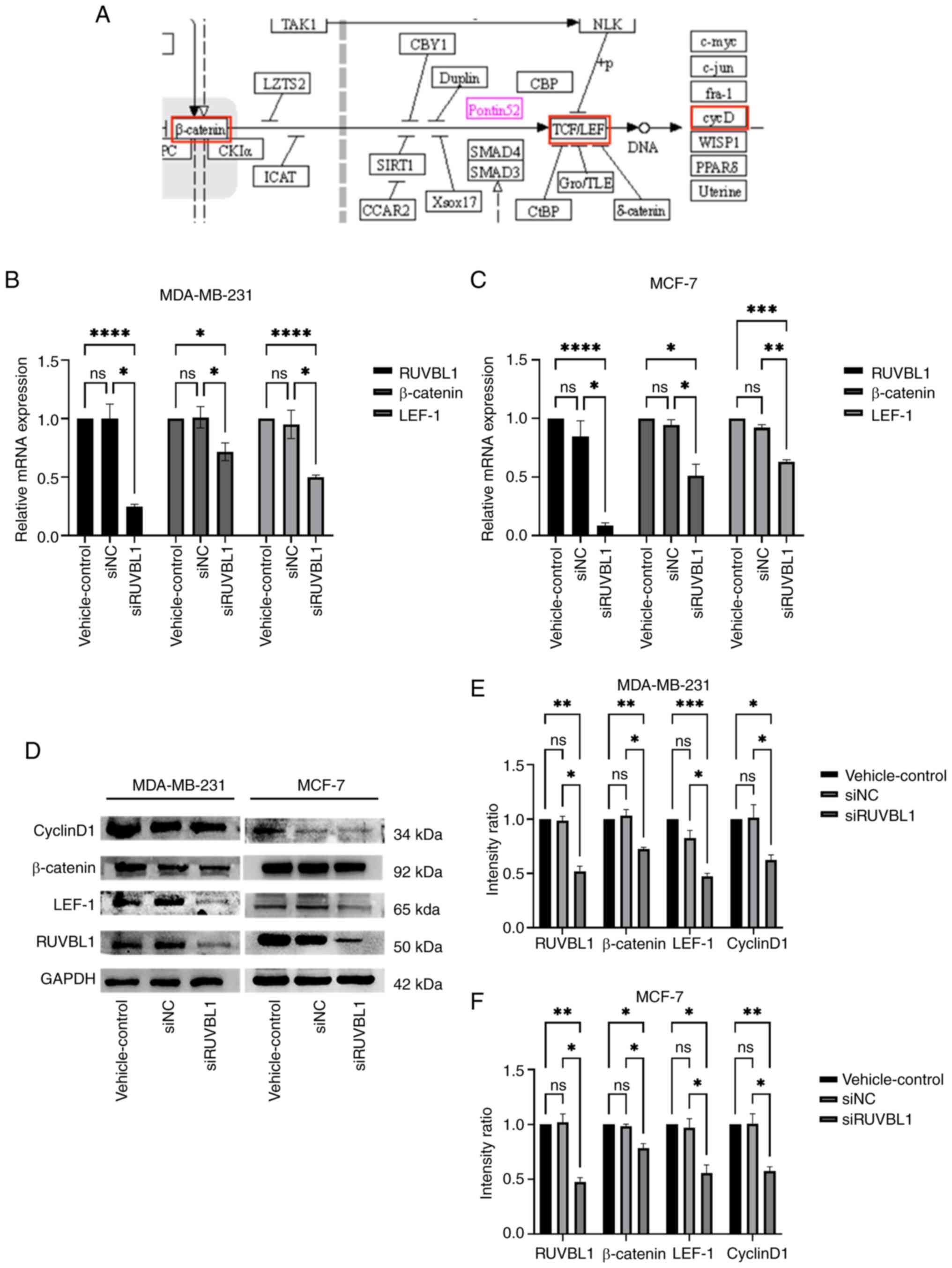 | Figure 5.Expression of β-catenin
pathway-related proteins was detected using western blot analysis.
The intensity of the gray band was calculated using ImageJ software
and shown in the figure. (A) The Kyoto Encyclopedia of Genes and
Genomes database was used to identify the signaling pathway map of
RUVBL1 (Pontin52 is the alias of the RUVBL1 gene; red box indicates
the pathway-related protein). The results revealed that RUVBL1 was
involved in the β-catenin/LEF-1 pathway, and its downstream genes
were related to the cell cycle. (B and C) Reverse
transcription-quantitative PCR and (D-F) western blot analysis were
used to detect the mRNA and protein levels of β-catenin and LEF-1
in breast cancer cells of the three groups of vehicle control,
si-NC and si-RUVBL1, respectively. The expression levels of
β-catenin, LEF-1 and cyclinD1 in the si-RUVBL1 group were decreased
in the MDA-MB-231 and MCF-7 cells. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. si-NC. LEF-1, lymphoid
enhancer factor-1; si-, small interfering; NC, negative control;
ns, not significant. |
Discussion
Previous studies have shown that RUVBL1 promotes
tumorigenesis and progression by regulating chromatin remodeling
and transcriptional activity (15,17,24),
and interacts with the β-catenin pathway to facilitate tumor
progression. However, the molecular mechanisms underlying
RUVBL1-associated proliferation, invasion and metastasis in BRCA
cells remain poorly understood. A previous study of highly
metastatic BRCA cells confirmed that the ectopic expression of
RUVBL1 in the cytoplasm and cell membrane and the formation of
RUVBL1-ITFGI complex jointly promoted the collective invasion of
tumor cells (25). In the present
study, it was found that RUVBL1 knockdown not only significantly
inhibited BRCA cell proliferation, invasion and migration, but was
also associated with the β-catenin/LEF-1 pathway. This pathway can
be regulated by the knockdown of RUVBL1, thereby affecting the
occurrence and development of BRCA cells (Fig. 6). The involvement of RUVBL1 in the
Wnt/β-catenin pathway has also been confirmed in oral squamous cell
carcinoma (18). In the present
study, it was found that RUVBL1 knockdown significantly inhibited
the proliferation, invasion and migration of BRCA cells.
Numerous studies have shown that the dysregulation
of the Wnt signaling pathway is a major contributor to BRCA
development and is implicated in BRCA proliferation (26), metastasis (27), immune microenvironment regulation
(28), stem cell maintenance
(29,30) and treatment resistance (31). Inhibiting the β-catenin/LEF-1
signaling pathway is an emerging therapeutic strategy. Targeted
therapy combined with radiotherapy and chemotherapy has shown a
favorable prospect in the field of cancer treatment. By identifying
specific molecular markers of tumors, precision targeted drugs can
effectively inhibit the proliferation of cancer cells and reduce
side effects. RUVBL1 is expected to become a new therapeutic target
by inhibiting β-catenin/LEF-1 pathway. In the future, if RUVBL1 is
combined with radiotherapy and chemotherapy, it can enhance the
synergistic effect of treatment and improve the cure rate of
tumors, which will promote the progress of precision medicine and
the expansion of clinical application. Thus, investigating specific
molecular targets is crucial for developing novel cancer
therapeutic strategies. In the present study, the knockdown of
RUVBL1 expression in the BRCA cell lines, MDA-MB-231 and MCF-7, not
only decreased β-catenin and LEF-1 expression, but also affected
the regulation of cyclin D1, which is associated with the
proliferation of BRCA cells. The upregulation of RUVBL1 in invasive
BRCA cells is higher than that in non-invasive BRCA cells,
suggesting that RUVBL1 may be closely related to the proliferation,
spread and invasion of invasive BRCA cells. It may also be involved
in mechanisms such as promoting changes in the tumor
microenvironment by participating in signal transduction and
regulating cell cycle. This finding also suggests that RUVBL1 may
be more effective in the treatment of invasive BRCA than
non-invasive BRCA, which will be the direction of our future
research. A high expression of RUVBL1 in BRCA tissues is associated
with the patient survival time. Bioinformatics analysis revealed
that RUVBL1 was involved in the β-catenin/LEF-1 signaling pathway,
which was initially verified by experiments. Therefore, RUVBL1 may
be one of the markers for predicting the survival and prognosis of
patients with BRCA, and its overexpression may become a potential
biological target for the diagnosis, prognosis and treatment of
BRCA. The present study was only a preliminary study on the
mechanism of the RUVBL1 gene in the proliferation, migration and
invasion of BRCA cells at the cellular level. Thus, further studies
are required in the future to determine the drug intervention
effects of the RUVBL1 gene in BRCA cells. In addition, further
studies with a large number of clinical samples are required for
in-depth research and verification to explore the mechanisms
through which RUVBL1 interacts and regulates cellular pathways in
BRCA. This will be the focus of future research.
In conclusion, the present study demonstrates that
RUVBL1 knockdown in BRCA cells regulates the β-catenin/LEF-1
signaling pathway and the expression levels of specific cell
cycle-related genes. These findings suggest that targeting RUVBL1
may serve as a potential therapeutic strategy within the Wnt
pathway, contributing to the development of novel molecular
approaches against BRCA. In further studies, direct binding
experiments such as luciferase assay will be performed to elucidate
the mechanism, and the effect of this factor on the cell cycle will
be examined by flow cytometry. In addition, cell and animal
experiments with combined drugs will be conducted, and a large
number of clinical samples for further research and analysis shall
be collected.
Acknowledgements
The authors would like to thank the Microbiology
Laboratory of Ningxia Medical University and the Surgery Laboratory
of General Hospital of Ningxia Medical University for providing the
experimental platform.
Funding
The present study was supported by the National Science
Foundation of China (grant no. 82260351).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ, DC, WS, GY and WW significantly contributed to
the conception and design of the study, as well as to data
collation and statistical analysis, and data analysis and
interpretation. CM contributed to the design of the study, ensured
the quality and completeness of the data, revised the manuscript
several times to ensure its quality, and provided financial support
for the experiment. XZ and DC confirm the authenticity of all the
raw data. All authors read and the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sancho-Garnier H and Colonna M: Breast
cancer epidemiology. Presse Med. 48:1076–1084. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kal S, Chakraborty S, Karmakar S and Ghosh
MK: Wnt/β-catenin signaling and p68 conjointly regulate CHIP in
colorectal carcinoma. Biochim Biophys Acta Mol Cell Res.
1869:1191852022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mani SKK, Yan B, Cui Z, Sun J, Utturkar S,
Foca A, Fares N, Durantel D, Lanman N, Merle P, et al: Restoration
of RNA helicase DDX5 suppresses hepatitis B virus (HBV)
biosynthesis and Wnt signaling in HBV-related hepatocellular
carcinoma. Theranostics. 10:10957–10972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu C, Wang L, Jiang Q, Zhang J, Zhu L,
Lin L, Jiang H, Lin D, Xiao Y, Fang W and Guo S: Hepatoma-derived
growth factor and DDX5 promote carcinogenesis and progression of
endometrial cancer by activating β-catenin. Front Oncol. 9:2112019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai ZG, Shi XJ, Gao Y, Wei MJ, Wang CY and
Yu GY: Beta-catenin expression pattern in primary oral squamous
cell carcinoma. Chin Med J (Engl). 121:1866–1870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu L, Tian Q, Gao H, Wu K, Wang B, Ge G,
Jiang S, Wang K, Zhou C, He J, et al: PROX1 promotes breast cancer
invasion and metastasis through WNT/β-catenin pathway via
interacting with hnRNPK. Int J Biol Sci. 18:2032–2046. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han S, Hao H, Han H, Xue D, Jiao Y, Xie Y,
Xu Y, Huangfu L, Fu J, Wang S, et al: CUEDC2 drives β-catenin
nuclear translocation and promotes triple-negative breast cancer
tumorigenesis. Cells. 11:30672022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bucan V, Mandel K, Bertram C, Lazaridis A,
Reimers K, Park-Simon TW, Vogt PM and Hass R: LEF-1 regulates
proliferation and MMP-7 transcription in breast cancer cells. Genes
Cells. 17:559–567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes KA, Song JS, Liu XS, Brown M and
Carroll JS: Nkx3-1 and LEF-1 function as transcriptional inhibitors
of estrogen receptor activity. Cancer Res. 68:7380–7385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu S, Liu F, Xie X, Ding M, Wang Z, Xing
X, Xiao T and Sun X: β-Sitosterol blocks the LEF-1-mediated
Wnt/β-catenin pathway to inhibit proliferation of human colon
cancer cells. Cell Signal. 104:1105852023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tucker PA and Sallai L: The AAA+
superfamily-a myriad of motions. Curr Opin Struct Biol. 17:641–652.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu XB, Lin YL, Thome KC, Pian P, Schlegel
BP, Weremowicz S, Parvin JD and Dutta A: An eukaryotic RuvB-like
protein (RUVBL1) essential for growth. J Biol Chem.
273:27786–27793. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z,
Chen S, Yang Y, Wang S, Shen P, et al: CircMYO10 promotes
osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to
enhance the transcriptional activity of β-catenin/LEF1 complex via
effects on chromatin remodeling. Mol Cancer. 18:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mello T, Materozzi M, Zanieri F, Simeone
I, Ceni E, Bereshchenko O, Polvani S, Tarocchi M, Marroncini G,
Nerlov C, et al: Liver haploinsufficiency of RuvBL1 causes hepatic
insulin resistance and enhances hepatocellular carcinoma
progression. Int J Cancer. 146:3410–3422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C and Wu S: RUVBL1-modulated
chromatin remodeling alters the transcriptional activity of
oncogenic CTNNB1 in uveal melanoma. Cell Death Discov. 9:1322023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Y, Kong Y, Liao L and Zhu H:
Involvement of RUVBL1 in WNT/β-catenin signaling in oral squamous
cell carcinoma. Dis Markers. 2022:33984922022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dauden MI, López-Perrote A and Llorca O:
RUVBL1-RUVBL2 AAA-ATPase: A versatile scaffold for multiple
complexes and functions. Curr Opin Struct Biol. 67:78–85. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Li H, Shao MM, Miao J, Fu Y and Hu
B: Downregulation of AHNAK2 inhibits cell cycle of lung
adenocarcinoma cells by interacting with RUVBL1. Thorac Cancer.
14:2093–2104. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lacombe J, Mangé A, Jarlier M,
Bascoul-Mollevi C, Rouanet P, Lamy PJ, Maudelonde T and Solassol J:
Identification and validation of new autoantibodies for the
diagnosis of DCIS and node negative early-stage breast cancers. Int
J Cancer. 132:1105–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn YT, Kim MS, Kim YS and An WG:
Astaxanthin reduces stemness markers in BT20 and T47D breast cancer
stem cells by inhibiting expression of pontin and mutant p53. Mar
Drugs. 18:5772020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li M, Yang L, Chan AKN, Pokharel SP, Liu
Q, Mattson N, Xu X, Chang WH, Miyashita K, Singh P, et al:
Epigenetic control of translation checkpoint and tumor progression
via RUVBL1-EEF1A1 axis. Adv Sci (Weinh). 10:e22065842023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan W, Xie J, Xia J, Zhang Y, Yang M, Wang
H, Pan Y, Zhang M, Han B, Wu B, et al: RUVBL1-ITFG1 Interaction is
required for collective invasion in breast cancer. Biochim Biophys
Acta Gen Subj. 1861:1788–1800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Xie P, Hao N, Zhang M, Liu Y, Liu
P, Semenza GL, He J and Zhang H: HIF-1-regulated expression of
calreticulin promotes breast tumorigenesis and progression through
Wnt/β-catenin pathway activation. Proc Natl Acad Sci USA.
118:e21091441182021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El Ayachi I, Fatima I, Wend P,
Alva-Ornelas JA, Runke S, Kuenzinger WL, Silva J, Silva W, Gray JK,
Lehr S, et al: The WNT10B network is associated with survival and
metastases in chemoresistant triple-negative breast cancer. Cancer
Res. 79:982–993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malladi S, Macalinao DG, Jin X, He L,
Basnet H, Zou Y, de Stanchina E and Massagué J: Metastatic latency
and immune evasion through autocrine inhibition of WNT. Cell.
165:45–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Jung YS, Jun S, Lee S, Wang W,
Schneider A, Sun Oh Y, Lin SH, Park BJ, Chen J, et al: PAF-Wnt
signaling-induced cell plasticity is required for maintenance of
breast cancer cell stemness. Nat Commun. 7:106332016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Won HY, Lee JY, Shin DH, Park JH, Nam JS,
Kim HC and Kong G: Loss of Mel-18 enhances breast cancer stem cell
activity and tumorigenicity through activating Notch signaling
mediated by the Wnt/TCF pathway. FASEB J. 26:5002–5013. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen H, Yan W, Yuan J, Wang Z and Wang C:
Nek2B activates the wnt pathway and promotes triple-negative breast
cancer chemothezrapy-resistance by stabilizing β-catenin. J Exp
Clin Cancer Res. 38:2432019. View Article : Google Scholar : PubMed/NCBI
|