Introduction
Osteosarcoma (OS) represents the predominant form of
primary malignant bone neoplasms, and predominantly affects
children and adolescents, with a second peak in their 60s (1). Advances in combination chemotherapy
and limb-sparing surgery have improved the 5-year survival rates of
patients with OS. However, patients with non-metastatic OS have
recently reached a plateau in survival rates of ~70%, whereas those
with either metastatic or recurrent OS remain <30% (2,3).
Therefore, elucidating the mechanisms underlying disease
progression, chemoresistance and metastasis is crucial (4).
Cancer stem cells (CSCs) are considered to be a
major factor in the treatment failure for malignant tumors,
contributing to issues such as chemotherapy resistance and distant
metastasis (5,6). Therefore, exploring therapeutic
methods against CSCs is essential for improving the outcomes of
patients with malignant tumors. However, CSCs represent only a
small fraction of the cell population, making their collection for
therapeutic research challenging (7). Previously, the authors have succeeded
for the first time in artificially generating CSC-like cells from
the MG-63 OS cell line through transduction with defined gene sets,
including octamer-binding transcription factor 3/4 (OCT3/4),
Krüppel-like factor 4 (KLF4) and SRY-box transcription factor 2
(SOX2) (8). These cells were named
‘MG-OKS’. Notably, MG-OKS cells showed significantly enhanced CSC
properties, including reduced proliferation rates, elevated
chemoresistance, enhanced spheroid formation capacity and increased
migratory potential.
The expression of several specific genes increased
in MG-OKS cells; among the genes whose expression is increased in
MG-OKS cells, the present study focused on small proline-rich
protein 1A (SPRR1A). SPRR1A is present in normal skin and esophagus
(9,10). This protein serves as a structural
component of the cornified envelope with a barrier function, and is
widely acknowledged as a marker for terminal squamous cell
differentiation (11).
Increased SPRR1A expression has been reported in
some types of non-squamous cell carcinomas; however, it is not
usually observed in normal non-squamous tissues. SPRR1A expression
is a possible prognostic factor for colorectal cancer (12), pancreatic ductal adenocarcinoma
(10) and diffuse large B-cell
lymphoma (13). The expression of
molecules that are not expressed in the native cancer lineage is
generally associated with poor prognosis (14–16).
Thus, it was hypothesized that SPRR1A, whose expression is
upregulated in MG-OKS, may play a role in tumor initiation, growth
and poor prognosis of OS. However, the role of SPRR1A in OS remains
unknown. The present study aimed to evaluate the role of SPRR1A in
OS both in vitro and in vivo using our newly
generated artificial MG-OKS cells.
Materials and methods
Cell lines and culture conditions
MG-63, a human OS cell line, was procured from RIKEN
BRC through the National Bio-Resource Project of the MEXT. Cells
were maintained in a medium consisting of Dulbecco's modified
Eagle's medium (DMEM), 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 µg/ml streptomycin (all from MilliporeSigma) at
37°C in a 5% CO2 humidified atmosphere. For retrovirus
production, Plat-A packaging cells (Cosmo Bio Co., Ltd.) were
cultured in the presence of 1 mg/ml puromycin and 10 mg/ml
blasticidin (both from Thermo Fisher Scientific, Inc.).
Generating CSC-like cells from the OS
cell line
A modified retroviral transduction protocol
(17) was employed to generate
CSC-like cells from the MG-63 line. Specifically, the polycistronic
retroviral vector pMXs encoding OCT3/4, KLF4 and SOX2 (pMXs-OKS)
(18) was used for reprogramming,
while pMXs-enhanced green fluorescent protein (EGFP) (19) served as the control vector.
Retroviral vectors (pMXs-OKS or pMXs-EGFP) were transfected into
Plat-A cells (in DMEM containing 10% FBS without antibiotics) to
produce retroviral particles. CSC-like cells were generated by
introducing pMXs-OKS into MG-63 and named ‘MG-OKS’, and those
generated by introducing the control pMXs-EGFP were named ‘MG-GFP’.
The Institutional Review Board of Kobe University approved the
study protocol.
Gene expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The RNeasy Mini Kit (cat. no. 74104; Qiagen, Inc.)
was used to extract total RNA from cultured cells. cDNA synthesis
was performed using the High-capacity cDNA Transcription kit (cat.
no. 4368814; Applied Biosystems; Thermo fisher Scientific, Inc.)
according to the manufacturer's protocol. RT-qPCR was carried out
on an ABI Prism 7500 Sequence Detection System (Applied Biosystems;
Thermo fisher Scientific, Inc.) using SYBR Green Master Mix
(Applied Biosystems; Thermo fisher Scientific, Inc.) under the
following conditions: One cycle at 95°C for 10 min, followed by 40
cycles of amplification at 95°C for 15 sec and 60°C for 1 min. The
relative mRNA expression of the transgenes OCT3/4, KLF4 and SOX2,
along with the established CSCs marker genes CD24, CD26 and CD133,
as previously reported (20,21),
as well as SPRR1A, were evaluated. Pre-designed primers
(Invitrogen; Thermo Fisher Scientific, Inc.) were used to assess
the relative mRNA expression of OCT3/4, KLF4, SOX2, CD24, CD26,
CD133 and SPRR1A. The primer sequences are listed in Table I. The 2−ΔΔCq method
(22) was used for quantification,
with β-actin as the normalization control.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Target gene | Primer sequence
(5′-3′) |
|---|
| hOCT3/4 | S:
CCCCAGGGCCCCATTTTGGTACC |
|
| A/S:
ACCTCAGTTTGAATGCATGGGAGA |
|
| GC |
| hSOX2 | S:
TTCACATGTCCCAGCACTACCAGA |
|
| A/S:
TCACATGTGTGAGAGGGGCAGTG |
|
| TGC |
| hKLF4 | S:
CATGCCAGAGGAGCCCAAGCCAAAG |
|
| AGGGG |
|
| A/S:
CGCAGGTGTGCCTTGAGATGGGA |
|
| ACTCTTT |
| CD24 | S:
TGCTCCTACCCACGCAGATT |
|
| A/S:
GGCCAACCCAGAGTTGGAA |
| CD26 | S:
CAAATTGAAGCAGCCAGACA |
|
| A/S:
CACACTTGAACACGCCACTT |
| CD133 | S:
TGGGGCTGCTGTTTATTATTCT |
|
| A/S:
TGCCACAAAACCATAGAAGATG |
| SPRR1A | S:
CACCCCAAAGTGCCTGAG |
|
| A/S:
TTCTGCTTGGTCTTCTGCTG |
| β-actin | S:
GATCATTGCTCCTCCTGAGC |
|
| A/S:
ACATCTGCTGGAAGGTGGAC |
Knockdown of SPRR1A through siRNA
transfection
MG-OKS were seeded in a 6-well plate 24 h before
transfection to achieve 70–80% confluency on the day of
transfection. Before transfection, 7.2 µl of Lipofectamine™ RNAiMAX
Transfection Reagent (cat. no. 13778075; Invitrogen; Thermo Fisher
Scientific, Inc.) and 1.2 µl of Silencer™ Pre-Designed siRNA SPRR1A
or scramble siRNA (as negative control; Invitrogen; Thermo Fisher
Scientific, Inc.) was diluted in 240 µl of Opti-MEM™ I Reduced
Serum Medium (Gibco; Thermo Fisher Scientific, Inc.). The
transfection complexes were incubated at 25°C for 5 min and then
added dropwise to each well. Following a 24-h incubation at 37°C in
a CO2 incubator, the medium was replaced with a fresh
complete culture medium, and the cells were incubated for an
additional 24 h. The efficiency of SPRR1A knockdown was assessed by
RT-qPCR and immunoblot analyses. Cells with confirmed SPRR1A
inhibition were used for subsequent experiments. MG-OKS cells
transfected with SPRR1A siRNA were named ‘siMG-OKS’, and those
transfected with scramble siRNA were named ‘scMG-OKS’.
Assessment of cell proliferation using
the WST-8 assay
The proliferative capacity of the cells was assessed
using the WST-8 assay. A total of 2 days after siRNA transfection,
cells were seeded into 96-well plates at a density of
5×103 cells/well in a volume of 100 µl culture medium.
Subsequent to a 24-h incubation, 10 µl of the Cell Counting Kit-8
solution (Dojindo Laboratories, Inc.) was introduced into each well
and incubated for an additional 2 h at 37°C in a 5% CO2
environment. The optical density was then quantified at 450 nm
employing a Model 680 Microplate Reader (Bio-Rad Laboratories,
Inc.).
Assessment of cell migration using the
wound healing assay
A total of 2 days after siRNA transfection, cells
were seeded into 6-well culture plates at a density of
2×105 cells per well in 2 ml of culture medium and
cultured until reaching 80% confluence. A linear scratch was
introduced in the cell monolayer using a 200-µl pipette tip to
create an artificial wound. The plates were then washed with
phosphate-buffered saline (PBS; Takara Bio, Inc.) to eliminate
detached cells and maintained in DMEM with 2% FBS (serum-reduced
culture media) for 24 h. Wound healing was monitored at 0 and 24 h,
and images were acquired using a microscopy system (BZ-X710
Microscope and BZ-X Viewer, BZ-X Analyzer imaging system; Keyence
Corporation). The migration distance (MD) for each experimental
group was quantified using the following equation: MD=width 0 at
h-width 24 at h (where width 0 at h represents the width of the
wound at 0 h and width 24 at h represents the width of the wound at
24 h). The MD value of the MG-OKS cell population served as a
reference for calculating the relative cell migration ability,
which was determined using the formula: Relative cell migration
ability=MD (siMG-OKS) or MD (scMG-OKS)/MD (MG-OKS) (23).
Immunoblot analysis
Cell lysis was prepared using the Mammalian Protein
Extraction Reagent (Thermo Fisher Scientific, Inc.). Soluble
proteins were isolated by centrifugation at 20,000 × g for 15 min
at 4°C. Protein concentration was determined using BCA protein
assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.). Equal
quantities of protein (15 µg) were combined with electrophoresis
sample buffer and boiled for 5 min before loading onto a 10.0–20.0%
polyacrylamide gel. The proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred onto polyvinylidene difluoride membranes
(MilliporeSigma). The membranes were blocked with 5% non-fat dry
milk (Bio-Rad Laboratories, Inc.) in Tris-buffered saline with 10%
Tween-20 for 30 min at 25°C and then incubated overnight at 4°C
with primary antibodies diluted in CanGet Signal Solution 1 (Toyobo
Life Science). Following washing steps, the membranes were probed
with horseradish-peroxidase-conjugated secondary antibodies
(1:5,000; cat. no. NA934; GE Healthcare Dharmacon, Inc.) in the
CanGet Signal Solution 2 (Toyobo Life Science) for 30 min at 25°C.
Protein bands were visualized using SuperSignal West Femto enhanced
chemiluminescent substrate (Thermo Fisher Scientific, Inc.), and
chemiluminescence reactions were detected using a Chemilumino
analyzer Las-3000 mini (FUJIFILM Wako Pure Chemical Corporation).
To ensure equal protein loading, the membranes were stripped and
re-probed with a mouse anti-human β-actin antibody (1:5,000; cat
no: A5441; MilliporeSigma). The primary antibody used in the
present study was a rabbit anti-human SPRR1A antibody (1:1,000;
cat. no. NBP2-93464; Novus Biologicals, LLC). Quantification of
protein expression was performed by measuring band intensities
using ImageJ software version 1.53t (National Institutes of
Health).
In vivo xenograft tumor model
Male BALB/c nude mice (5 weeks-old, n=18, body
weight range: 19–22 g) were obtained from CLEA Japan and housed
under specific pathogen-free (SPF) conditions. The mice were
provided with a pathogen-free laboratory diet and allowed to drink
autoclaved water ad libitum. The housing environment was
maintained at a temperature of 25°C with a relative humidity of
50–60% and a 12/12-h light/dark cycle. All animal experiments were
conducted in accordance with the guidelines set forth by the
Japanese Physiological Society for the care and use of laboratory
animals. The study protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Kobe University
(approval no. P230401; date, 2023-02; Kobe, Japan) and adhered to
the university's animal experimentation regulations. For the
xenograft tumor model, a suspension of 2×106 cells in
200 µl of serum-free PBS (Takara Bio, Inc.) was subcutaneously
injected into the dorsal flank region of each mouse (n=6 for each
group). Tumor growth was monitored for 4 weeks
post-transplantation, and tumor volume was calculated using the
formula: 0.5× (length) × (width)2 (8). At the experimental endpoint, all
tumors were surgically excised, and morphometric analyses were
performed by two blinded examiners. Humane endpoints were
established and strictly followed, which included: Xenograft tumor
volume exceeding 10% of the animal's body weight, tumor diameter
surpassing 20 mm, tumor-induced weight loss exceeding 20%, or signs
of immobility, inability to eat, ulceration, infection, or
necrosis. All mice reached the predefined study endpoints and were
humanely euthanized by cervical dislocation under deep anesthesia
induced by an intraperitoneal injection of pentobarbital sodium
(100 mg/kg body weight). Confirmation of death was based on the
absence of heartbeat and the presence of pupil dilation.
Immunofluorescence staining of frozen
tumor sections
A total of 4 weeks post-cell transplantation, the
mice were humanely euthanized, and the tumor tissues were collected
for histological analyses. Frozen tumor sections with a thickness
of 10 µm were prepared using a cryostat. Immunofluorescence
staining was performed to assess the proliferative activity of the
tumor cells. The sections were incubated overnight at 4°C with a
primary rabbit polyclonal antibody targeting human Ki-67 (1:100;
cat. no. NB500-170; Novus Biologicals, LLC) diluted in CanGet
Signal immunostain solution A (cat. no. NKB-501; Toyobo Life
Science). Following washing steps, the sections were incubated with
the secondary antibody, Alexa-Fluor 647 goat anti-rabbit IgG
(2:1,000; cat. no. A-27040; Invitrogen; Thermo Fisher Scientific,
Inc.) diluted in PBS (Takara Bio, Inc.) for 1 h at 25°C. Finally,
nuclear staining was achieved using the 4′,6-diamino-2-phenylindole
solution (1:5,000; MilliporeSigma) diluted in PBS (Takara Bio,
Inc.) for 15 min at 25°C. The proliferative activity of the tumor
tissues was then assessed using a fluorescence microscope (BZ-X710
Microscope and BZ-X Viewer, BZ-X Analyzer Imaging System; Keyence
Corporation). The percentage of Ki-67-positive tumor cells, was
quantified in four randomly selected microscopic fields per section
using ImageJ software version 1.53t (National Institutes of
Health). Two blinded examiners performed all the studies.
Transcriptome profiling by RNA
sequencing
Total RNA was isolated from cultured cells two days
post-siRNA transduction using an RNeasy Mini Kit (cat. no. 74104;
Qiagen, Inc.) according to the manufacturer's protocol. RNA quality
and concentration were assessed using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.) to measure
concentration (MG-OKS, 309.5 ng/µl; siMG-OKS, 296.3 ng/µl;
scMG-OKS, 304.4 ng/µl) and purity (A260/280; MG-OKS, 2.07;
siMG-OKS, 2.1; scMG-OKS, 2.07). Total RNA samples derived from
MG-OKS, siMG-OKS and scMG-OKS cells were submitted to Macrogen,
Inc. Library preparation using the TruSeq Stranded mRNA LT Sample
Prep Kit (cat. no. RS-122-2101; Illumina, Inc.) was performed by
Macrogen, Inc. Paired-end RNA sequencing was subsequently conducted
on an Illumina NovaSeq 6000 System (Illumina, Inc.), with a read
length of 101 bp for both forward and reverse directions (5′-3′).
The obtained reads were aligned to the human transcriptome (hg38)
reference sequences utilizing Strand next-generation sequencing
(NGS) software version 3.1.1. (Strand Life Sciences; http://www.strand-ngs.com/). The aligned reads were
normalized to transcripts per million (TPM), and the resulting
normalized counts were standardized to a value of 1.
Log2-transformed TPM values were utilized to compare gene
expression levels among MG-OKS, siMG-OKS and scMG-OKS cells.
Scatter plots were employed to visualize gene expression data, and
pathway analyses were conducted using WikiPathways database
(https://wikipathways.org/) within the
Strand NGS software platform (https://www.strand-ngs.com/). Z-scores were calculated
to identify differentially expressed genes by subtracting the
overall mean gene intensity from the normalized intensity of each
gene and dividing the result by the standard deviation (SD) of all
measured intensities, as described by the equation:
Z-score=(intensity-mean intensity)/SD (24).
Statistical analyses
Statistical analyses were carried out using the EZR
statistical software version 1.54 (Saitama Medical Centre, Jichi
Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html;
Kanda, 2012). Data were expressed as the mean ± standard error of
the mean. One-way analysis of variance (ANOVA) followed by
Tukey-Kramer test was employed to assess statistical differences
among multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of MG-OKS cells
The properties of MG-OKS were first examined to
identify if they were consistent with previous studies (8). The results revealed that MG-OKS cells
exhibited significantly higher transcript levels of OCT3/4, KLF4
and SOX2 compared with both MG-63 and MG-GFP cells (P<0.05, n=3)
(Fig. S1A). These findings
suggested that the reprogramming process successfully induced the
expression of the desired transcription factors in MG-OKS
cells.
Additionally, the expression of CSC markers
previously reported in various cancers, including CD24, CD26 and
CD133 (20,21), was evaluated. RT-qPCR analysis
demonstrated that MG-OKS cells displayed significantly elevated
mRNA levels of CD24, CD26 and CD133 compared with MG-63 and MG-GFP
cells (P<0.05, n=3) (Fig. S1B).
These results indicated that MG-OKS cells possess a gene expression
profile consistent with CSC-like properties.
Furthermore, morphological assessment of the cells
revealed distinct differences between MG-OKS cells and their MG-63
and MG-GFP counterparts. MG-OKS cells exhibited elongated cell
bodies with protrusions, a feature not found in MG-63 and MG-GFP
cells (Fig. S1C).
Alteration of cell morphology by
knockdown of SPRR1A in MG-OKS cells
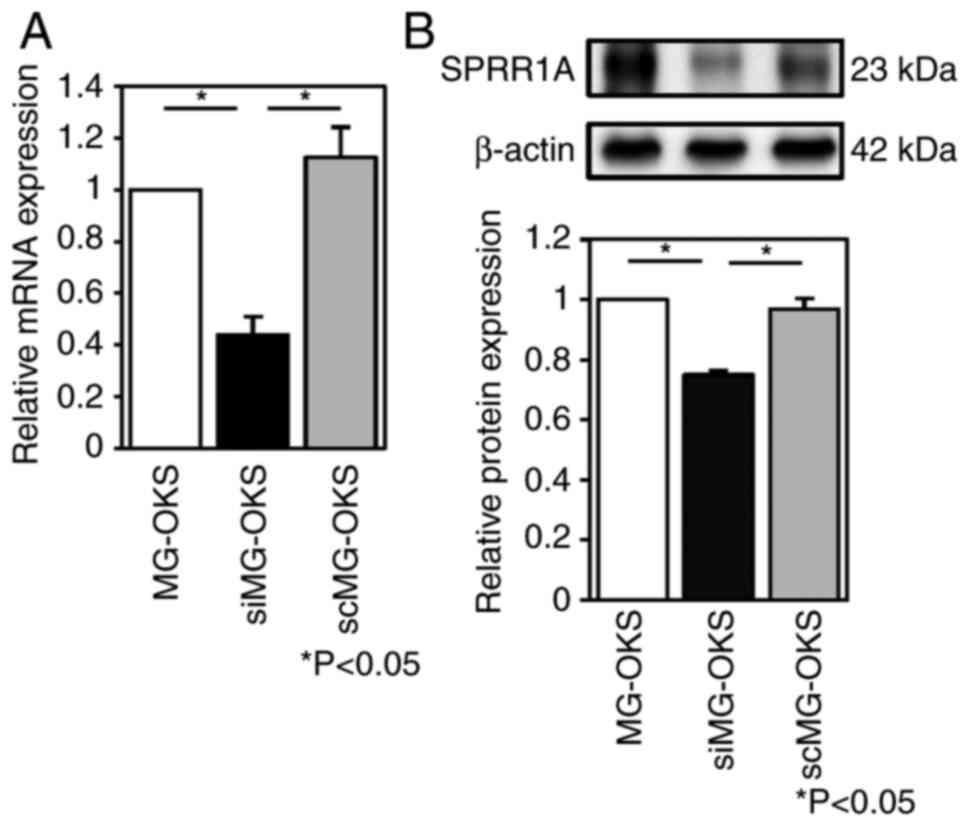
The efficiency of knockdown of SPRR1A by siRNA
transfection was evaluated by performing RT-qPCR and immunoblot
analysis. The expression of the SPRR1A gene was significantly
reduced by 56 and 61% in siMG-OKS cells compared with MG-OKS and
scMG-OKS cells, respectively (P<0.05, n=7) (Fig. 1A). Immunoblot analysis quantified by
band intensity using ImageJ software showed a 25 and 23% reduction
in SPRR1A protein levels compared with MG-OKS and scMG-OKS,
respectively (P<0.05, n=4) (Fig.
1B). No significant differences in SPRR1A gene expression or
protein levels were observed between MG-OKS and scMG-OKS.
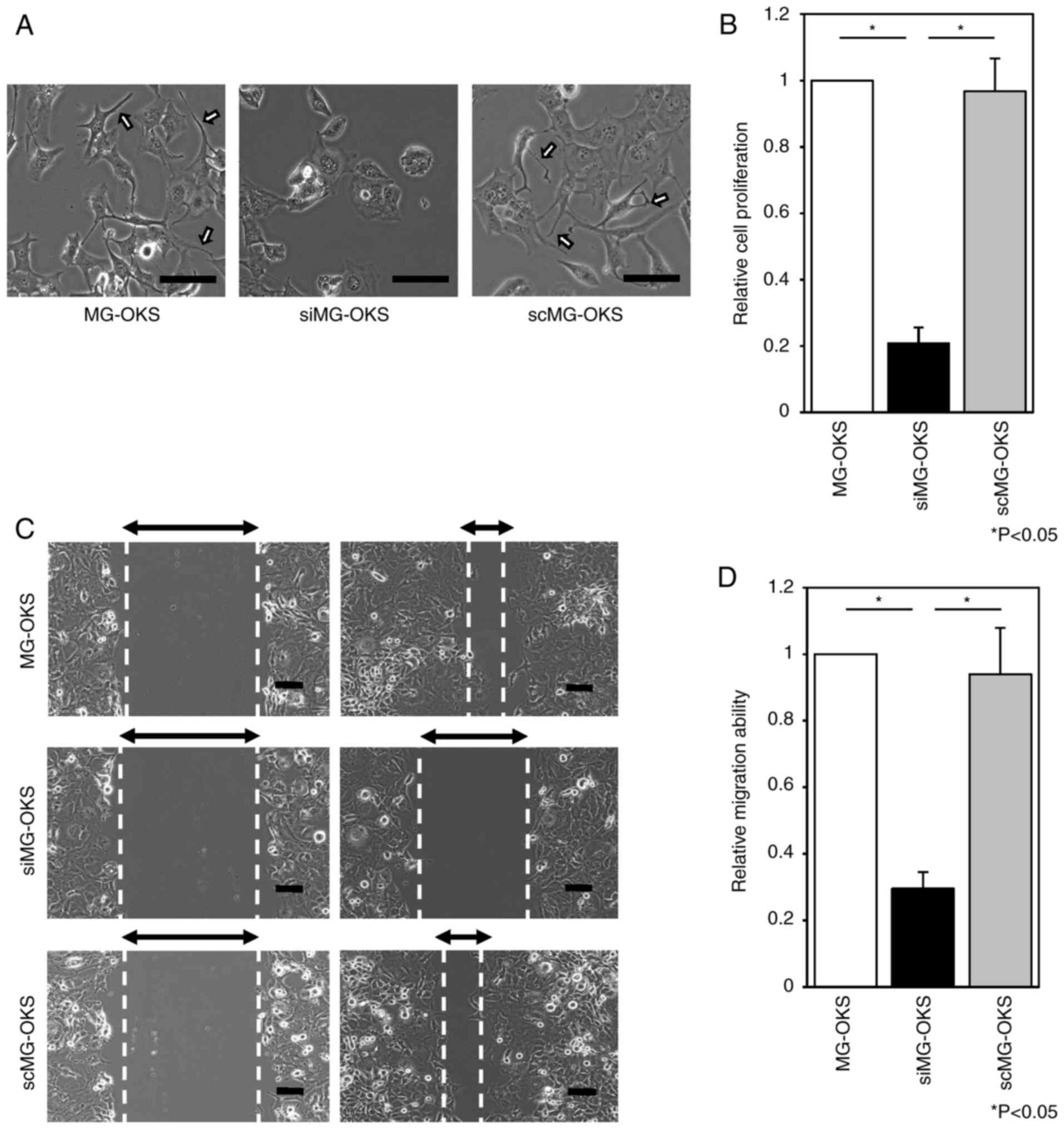
Morphological changes were assessed microscopically,
and the protrusions observed in MG-OKS (8) were decreased in siMG-OKS. By contrast,
they were observed in scMG-OKS as in MG-OKS (Fig. 2A).
SPRR1A knockdown significantly impairs
proliferation and migration of MG-OKS cells
Having established the efficient knockdown of SPRR1A
in MG-OKS cells, it was sought to investigate the functional
consequences of reduced SPRR1A expression on cell proliferation and
migration, two critical processes involved in cancer progression
and metastasis.
To assess the impact of SPRR1A knockdown on cell
proliferation, the WST-8 assay was performed. The siMG-OKS group
revealed a 79 and 78% reduction in cell proliferation at 48 h after
siRNA transfection compared with the MG-OKS and scMG-OKS groups,
respectively (P<0.05, n=6) (Fig.
2B). There were no significant differences in cell
proliferation between the MG-OKS and scMG-OKS groups.
Wound healing assays were performed to assess how
SPRR1A knockdown affects cell migration ability. siMG-OKS group
demonstrated a 70 and 69% reduction in cell migration ability
compared with MG-OKS and scMG-OKS, respectively (P<0.05, n=3)
(Fig. 2C and D). There were no
significant differences in cell migration ability between the
MG-OKS and scMG-OKS groups.
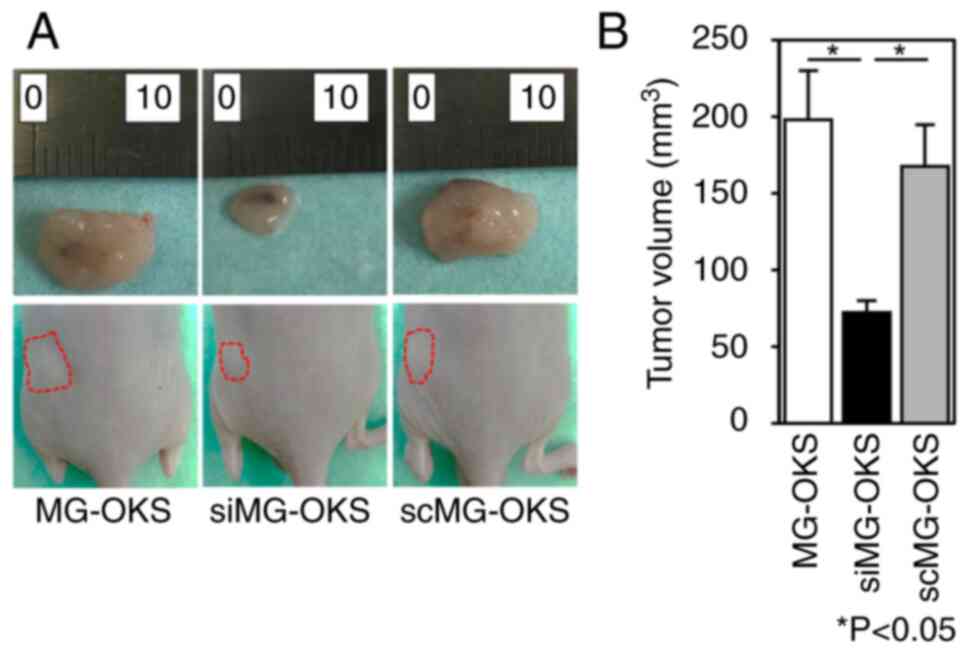
SPRR1A knockdown results in the
suppressed tumorigenicity of MG-OKS cells in vivo
To investigate the impact of SPRR1A knockdown on
tumorigenicity, a xenograft mouse model was employed. MG-OKS,
siMG-OKS and scMG-OKS cells were subcutaneously transplanted into
the dorsal flank region of immunodeficient nude mice. A total of 4
weeks after cell transplantation, tumor volumes were compared, and
immunofluorescence staining was performed. The transplanted
xenografts showed 100% engraftment in all mice. Tumor volume 4
weeks after cell transplantation was significantly smaller in the
siMG-OKS group than in the MG-OKS and scMG-OKS groups (MG-OKS,
0.20±0.032 cm3; siMG-OKS, 0.072±0.007 cm3;
scMG-OKS, 0.17±0.027 cm3) (P<0.05, n=6) (Fig. 3A and B). There was no significant
difference in the tumor volume between the MG-OKS and scMG-OKS
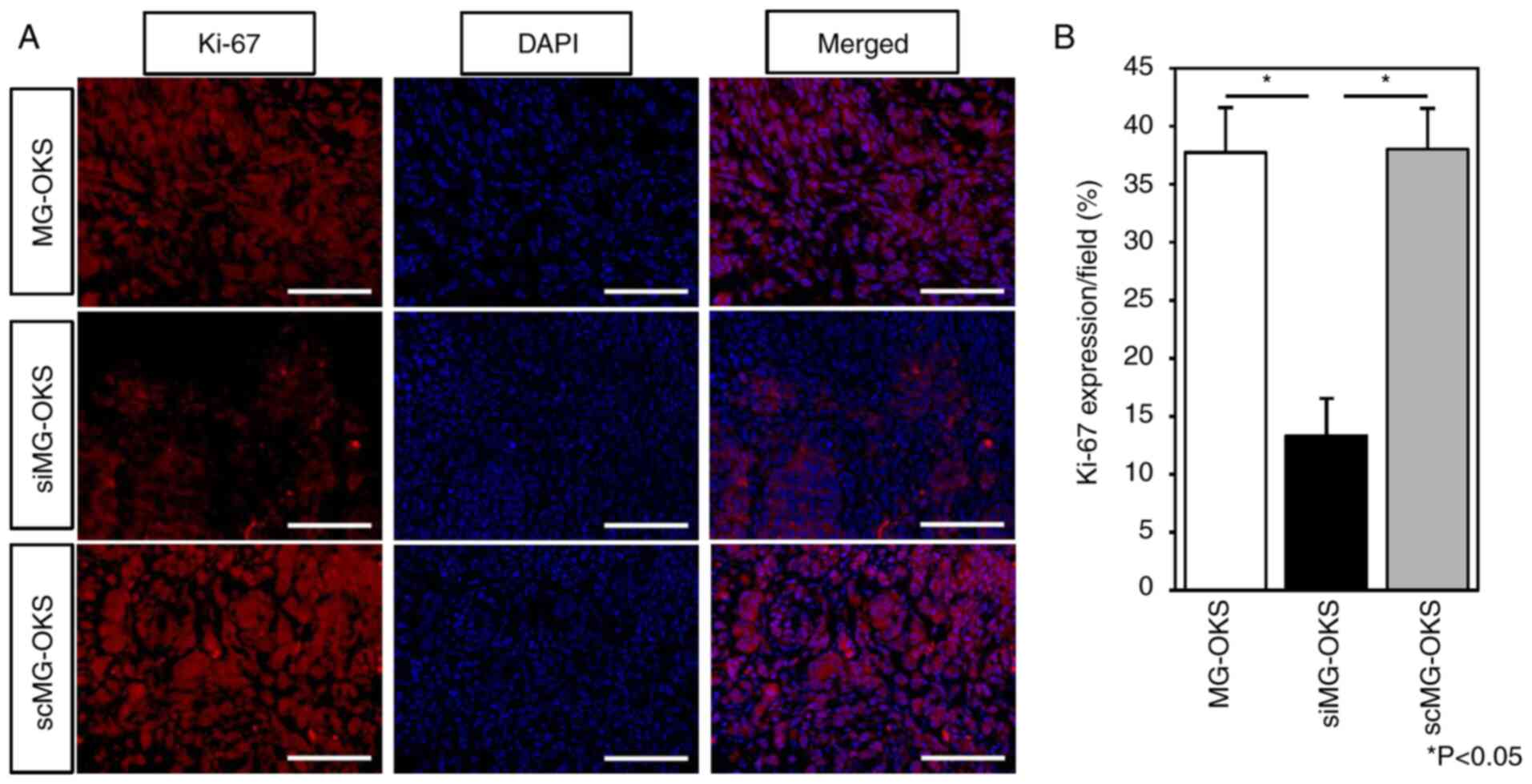
groups. Immunofluorescence staining of implanted tumors revealed
significantly decreased Ki-67 positivity, a proliferation marker
(25,26), in siMG-OKS group compared with
MG-OKS and scMG-OKS groups (MG-OKS, 36±2.7%; siMG-OKS, 11±1.8%;
scMG-OKS, 37±1.7%) (P<0.05, n=6) (Fig. 4A and B). There was no significant
difference in Ki-67 positivity between MG-OKS and scMG-OKS groups.
In addition, RT-qPCR and immunoblot analyses revealed no
significant differences in SPRR1A expression among the three groups
(Fig. S2A and B). Furthermore,
immunostaining also showed similar SPRR1A expression patterns
across all groups (Fig. S2C and
D).
RNA sequencing identifies several
downregulated genes and associated pathways
To analyze the genetic changes induced by the
knockdown of SPRR1A, RNA sequencing was performed. Scatter plots
were used to visualize the comparison of commonly altered genes
between MG-OKS and scMG-OKS cells against siMG-OKS cells. A total
of 961 genes were downregulated <2-fold in siMG-OKS cells
compared with MG-OKS cells (blue dots), whereas 728 genes were
downregulated <2-fold in siMG-OKS cells compared with scMG-OKS
cells (blue dots) (Fig. 5A). Among
these genes, 452 were commonly downregulated in siMG-OKS (Fig. 5B), and 11 pathways were identified
using pathway analysis, including ‘Interleukin-4 and Interleukin-13
signaling’, ‘Extracellular matrix organization, Vitamin D Receptor
Pathway’, and ‘Focal Adhesion PI3K-Akt-mTOR signaling pathway’.
Each of the detected pathways was associated with a
P<10−5 (Fig. 5C). A
heatmap was used to visualize the changes in individual genes. The
results revealed decreased expression of several focal
adhesion-related genes, including focal adhesion kinase (FAK)
(Fig. 5D), and altered expression
levels of several cell cycle-related genes, including S-phase
kinase-associated protein-2 (Skp2), cyclin-dependent kinase 2,4
(CDK2,4), cyclin D (CCND1) and cyclin-dependent kinase inhibitor 1B
(CDKN1B) (Fig. 5E).
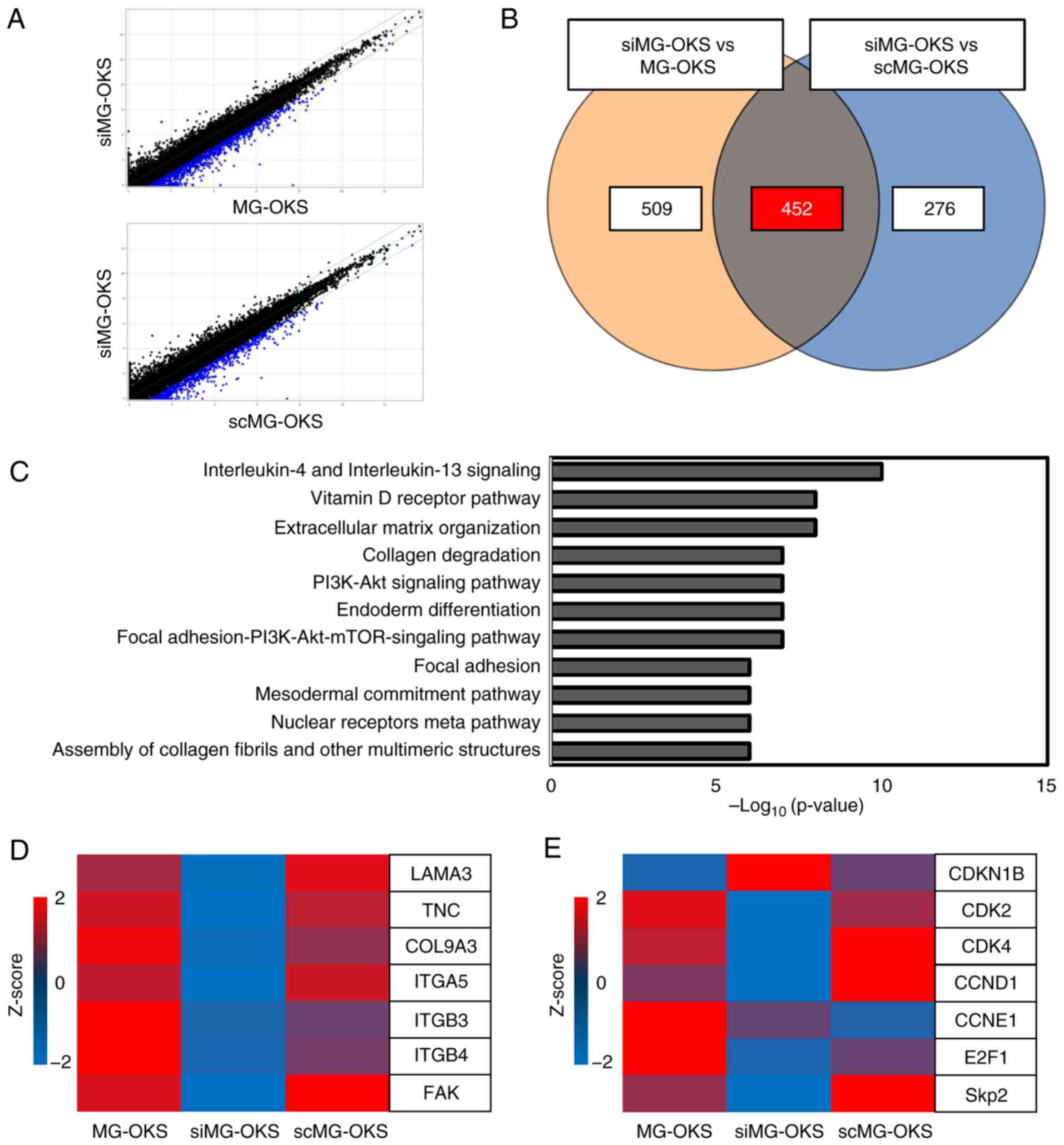 | Figure 5.RNA sequencing of MG-OKS, siMG-OKS,
and scMG-OKS cells. (A) Scatter plots showing ≥2-fold downregulated
genes (blue dots). (B) Venn diagram showing the number of the
2-fold downregulated genes in the comparison between siMG-OKS vs.
MG-OKS (left), siMG-OKS vs. scMG-OKS (right), and the number of
differentially expressed genes in both comparisons (center). (C)
The list of 11 pathways with P<10−5. (D and E)
Expression heat maps of genes associated with (D) cell adhesion and
(E) cycle. Gene signal intensities were normalized and converted to
Z-scores. si-, small interfering; sc-, scrambled; FAK, focal
adhesion kinase; Skp2, S-phase kinase-associated protein-2; CDK2,4,
cyclin-dependent kinase 2,4; CCND1, cyclin D; CDKN1B,
cyclin-dependent kinase inhibitor 1B. |
Discussion
CSCs are postulated to be pivotal contributors to
the unfavorable prognosis observed in patients with refractory OS.
Moreover, these cells are hypothesized to be instrumental in the
etiology of disease recurrence and metastatic progression (5). The authors have previously generated
artificial CSC-like cells from one of the OS cell lines to
elucidate the role of CSCs in OS and it was found that these cells
exhibit upregulation of SPRR1A (8).
Previously, an association between SPRR1A and poor prognosis was
reported in several cancers (10,12),
and although SPRR1A expression levels have been associated with
several cancers, the signaling pathways involved in its induction
and downstream effects on cellular behavior and gene expression are
poorly understood (27). In
particular, no studies have investigated the role of SPRR1A in OS,
and its relevance remains unclear.
In the present study, the knockdown of SPRR1A
expression reduced cellular protrusion formation and gene
expression associated with cell adhesion, including FAK, and
significantly decreased migration ability. Cell adhesion and
migration are intricate, dynamic processes comprising multiple
stages. The process is regulated by FAK, a non-receptor tyrosine
kinase overexpressed in several types of tumors; FAK has been
reported to be related to the engagement of cell adhesion molecules
and assembly of focal adhesions, ultimately controlling cell
migration (28). Maziveyi et
al (29) reported that
inhibition of focal adhesion in breast cancer cells leads to
reduced adhesion to the extracellular matrix, decreased formation
of protrusions, and impaired migration ability (29). Taken together, the results of the
present study suggested that downregulation of genes related to
focal adhesion, such as FAK, induces reduced cellular protrusion
formation and cell migration ability.
On the other hand, the current results revealed that
the knockdown of SPRR1A in MG-OKS significantly reduced cell
proliferation. As aforementioned, SPRR1A knockdown also
exhibited reduced cell adhesion in siMG-OKS. In addition, our
sequencing data showed decreased FAK, Skp2 and CDK2 and increased
CDKN1B in siMG-OKS. Benaud and Dickson (30) reported that decreased cell adhesion
in mammary epithelial cells correlates with G1 phase arrest via
CDK2 inhibition due to increased CDKN1B. It has also been reported
that inactivation of FAK by decreased cell adhesion inhibits the
expression of Skp2, which is responsible for CDKN1B degradation
(31,32). Collectively, the present results
suggested that the increase in CDKN1B in siMG-OKS cells inhibits
CDK2, leading to reduced cell proliferation. Moreover, it has been
reported that the inhibition of the PI3K/AKT/mTOR pathway involves
the inhibition of CCND1 and CDK4 (33), and inhibition of CCND1 and CDK4
leads to cell cycle arrest (34).
Collectively, the sequencing data generated in the present study
suggest that cell cycle inhibition contributes not only to CDK2 but
also to CDK4 inhibition, and that reduced cell adhesion should
induce decreased cell proliferation through multiple pathways
rather than a single pathway.
The results of in vivo studies indicated that
siMG-OKS xenografts had fewer Ki-67 positivity than MG-OKS
xenografts. High expression of Ki-67 has been reported to be
associated with decreased 5-year survival and increased distant
metastases in patients with OS (26,35).
Given the decreased number of Ki-67 positivity, the results
suggested reduced malignant potential in the siMG-OKS cells. The
present results are consistent with previous studies, reporting
that lower SPRR1A expression is associated with lower malignancy in
colorectal and pancreatic ductal carcinomas (10,12).
The present study presents several noteworthy
limitations that warrant consideration in the interpretation of
results and design of future investigations. Firstly, the transient
nature of siRNA-mediated SPRR1A knockdown via lipofection may have
resulted in diminished gene suppression effects by the time of
evaluation, as evidenced by the absence of significant differences
in SPRR1A expression in xenograft samples (Fig. S2A-D). Despite this constraint, the
current findings suggest that SPRR1A knockdown likely exerts its
influence during the early stages of tumor formation. The
observation of significant differences in tumor tissues with
respect to tumorigenicity, despite the temporary nature of SPRR1A
suppression, is particularly intriguing and warrants further
elucidation. Furthermore, the potential for off-target effects
inherent to siRNA-mediated knockdown cannot be overlooked. While
commercially available siRNA sequences designed to minimize
off-target effects were utilized, the possibility of unintended
gene silencing or activation of cellular stress responses cannot be
entirely excluded. In an attempt to address this concern, rescue
experiments were preliminarily performed by utilizing a plasmid
vector to overexpress SPRR1A in siMG-OKS cells. These experiments
showed partial restoration of cell proliferative capacity (data not
shown), suggesting complex interactions with downstream signaling
pathways that warrant further investigation. To address these
technical limitations, future studies should consider employing
more stable knockdown methods, such as shRNA, to investigate the
long-term effects of SPRR1A inhibition, particularly in in
vivo studies.
Secondly, the substantial heterogeneity
characteristic of OS and the limitations of our cell line-based
approach present challenges. The enormous heterogeneity observed in
OS is demonstrated by the identification of multiple pathways (14
driver genes) in exome sequencing studies, which increases the
complexity of effective therapeutic strategies for OS, reflected
clinically as a refractory and relapsing disease (36). The utilization of a single OS cell
line (MG-63) and its derivative MG-OKS cells in the present
investigation potentially constrain the generalizability of our
findings and may not comprehensively encompass the intricate
complexities inherent to the human tumor microenvironment. While
studies employing patient-derived OS samples offer superior
representation of tumor heterogeneity and microenvironment effects
(37), it is pertinent to
acknowledge that such models are constrained by the limited
availability of primary chemo-naive OS specimens (38).
Thirdly, further mechanistic studies are necessary
to fully elucidate SPRR1A's specific regulatory roles in the
identified pathways and genes. Additionally, the absence of
clinical correlation data limits the immediate translational impact
of the present findings.
Fourthly, the current study focused principally on
primary tumor growth and did not address the potential role of
SPRR1A in OS metastasis. Given the clinical significance of
metastatic disease in OS, future research utilizing appropriate
metastasis models is crucial to comprehensively understand SPRR1A's
function in disease progression.
Despite these limitations, the present study is the
first to address the function of SPRR1A in OS using newly generated
artificial OS CSC-like cells, which provides crucial clues for the
development of novel OS treatment strategies that target CSCs.
In conclusion, it is significant that the present
study is the first to provide evidence that SPRR1A is one of the
key cell adhesion-related molecules involved in OS progression, and
further elucidation of the underlying pathophysiology and the
exploration and identification of SPRR1A inhibitors may contribute
to the development of OS therapeutic approaches from a different
perspective.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to express their sincere
gratitude to the exceptional technical support provided by Ms
Minako Nagata, Ms Maya Yasuda and Ms Kyoko Tanaka from the
Department of Orthopaedic Surgery (Kobe University Graduate School
of Medicine, Kobe, Japan).
Funding
The present study was supported by the JSPS KAKENHI (grant no.
21K09250).
Availability of data and materials
The datasets generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the Gene Expression Omnibus under
accession number GSE268670 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE268670.
Authors' contributions
TMi, NF and SF collected and analyzed data and wrote
the manuscript. HH, NF, RS, YN, TK, TT, SY, YM, KK, YH, SH, ToMa
and TaMa collected and/or assembled data. TMi, NF and SF confirm
the authenticity of all the raw data. MK-A performed data analysis
and interpretation. NF, TaA, RK and ToA conceived/designed,
collected and/or assembled data, performed data analysis and
interpretation, and provided final approval of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments involving animals were conducted in
accordance with the Japanese Physiological Society Guidelines for
the care and use of laboratory animals. The Institutional Animal
Care and Use Committee of Kobe University approved the study
protocol (approval no. P230401; date, 2023-02; Kobe, Japan). The
research adhered to the animal experimentation regulations of Kobe
University, Japan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSC
|
cancer stem cell
|
|
EGFP
|
enhanced green fluorescent protein
|
|
MD
|
migration distance
|
|
NGS
|
next-generation sequencing
|
|
OS
|
osteosarcoma
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
scMG-OKS
|
scrambled siRNA-transfected MG-OKS
|
|
siMG-OKS
|
MG-OKS cells transfected with SPRR1A
siRNA
|
|
SE
|
standard error
|
|
TPM
|
transcripts per million
|
|
SPRR1A
|
small proline-rich protein 1A
|
|
FAK
|
focal adhesion kinase
|
|
CDK2
|
cyclin-dependent kinase 2
|
|
CDKN1B
|
cyclin-dependent kinase inhibitor
1B
|
|
Skp2
|
S-phase kinase-associated
protein-2
|
|
CCND1
|
cyclin D
|
References
|
1
|
Wittig JC, Bickels J, Priebat D, Jelinek
J, Kellar-Graney K, Shmookler B and Malawer MM: Osteosarcoma: A
multidisciplinary approach to diagnosis and treatment. Am Fam
Physician. 65:1123–1132. 2002.PubMed/NCBI
|
|
2
|
Odri GA, Tchicaya-Bouanga J, Yoon DJY and
Modrowski D: Metastatic progression of osteosarcomas: A review of
current knowledge of environmental versus oncogenic drivers.
Cancers (Basel). 14:3602022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao X, Wu Q, Gong X, Liu J and Ma Y:
Osteosarcoma: A review of current and future therapeutic
approaches. Biomed Eng Online. 20:242021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiozawa Y, Nie B, Pienta KJ, Morgan TM
and Taichman RS: Cancer stem cells and their role in metastasis.
Pharmacol Ther. 138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abarrategi A, Tornin J, Martinez-Cruzado
L, Hamilton A, Martinez-Campos E, Rodrigo JP, González MV, Baldini
N, Garcia-Castro J and Rodriguez R: Osteosarcoma: Cells-of-origin,
cancer stem cells, and targeted therapies. Stem Cells Int.
2016:36317642016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oshima N, Yamada Y, Nagayama S, Kawada K,
Hasegawa S, Okabe H, Sakai Y and Aoi T: Induction of cancer stem
cell properties in colon cancer cells by defined factors. PLoS One.
9:e1017352014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujiwara S, Kawamoto T, Kawakami Y,
Koterazawa Y, Hara H, Takemori T, Kitayama K, Yahiro S, Kakutani K,
Matsumoto T, et al: Acquisition of cancer stem cell properties in
osteosarcoma cells by defined factors. Stem Cell Res Ther.
11:4292020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinert PM, Candi E, Kartasova T and
Marekov L: Small proline-rich proteins are cross-bridging proteins
in the cornified cell envelopes of stratified squamous epithelia. J
Struct Biol. 122:76–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamakawa K, Koyanagi-Aoi M, Uehara K,
Masuda A, Yanagimoto H, Toyama H, Fukumoto T, Kodama Y and Aoi T:
Increased expression of SPRR1A is associated with a poor prognosis
in pancreatic ductal adenocarcinoma. PLoS One. 17:e02666202022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Candi E, Schmidt R and Melino G: The
cornified envelope: A model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Zheng X, Zhang Y, Xu M, Ye C, Lin
M, Pan J, Xu Z, Lu X and Chi P: High SPRR1A expression is
associated with poor survival in patients with colon cancer. Oncol
Lett. 19:3417–3424. 2020.PubMed/NCBI
|
|
13
|
Zhang H, Gao J, Zhao Z, Li M and Liu C:
Clinical implications of SPRR1A expression in diffuse large B-cell
lymphomas: A prospective, observational study. BMC Cancer.
14:3332014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Tu K, Liu F, Liang M, Yu K, Wang
Y, Luo Y, Yang B, Qin Y, He D, et al: FoxM1 promotes the migration
of ovarian cancer cell through KRT5 and KRT7. Gene. 757:1449472020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J, Zhang LC, Song X, Lu JR and Jin Z:
KRT6 interacting with notch1 contributes to progression of renal
cell carcinoma, and aliskiren inhibits renal carcinoma cell lines
proliferation in vitro. Int J Clin Exp Pathol. 8:9182–9188.
2015.PubMed/NCBI
|
|
16
|
Li Q, Yin L, Jones LW, Chu GCY, Wu JBY,
Huang JM, Li Q, You S, Kim J, Lu YT, et al: Keratin 13 expression
reprograms bone and brain metastases of human prostate cancer
cells. Oncotarget. 7:84645–84657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishida R, Koyanagi-Aoi M, Oshima N, Kakeji
Y and Aoi T: The tissue-reconstructing ability of colon CSCs is
enhanced by FK506 and suppressed by GSK3 inhibition. Mol Cancer
Res. 15:1455–1466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogawa H, Koyanagi-Aoi M, Otani K, Zen Y,
Maniwa Y and Aoi T: Interleukin-6 blockade attenuates lung cancer
tissue construction integrated by cancer stem cells. Sci Rep.
7:123172017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salaria S, Means A, Revetta F, Idrees K,
Liu E and Shi C: Expression of CD24, a stem cell marker, in
pancreatic and small intestinal neuroendocrine tumors. Am J Clin
Pathol. 144:642–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu C, Xie Y, Gao F, Wang Y, Guo Y, Tian H,
Li Y and Fan W: Lgr5 expression as stem cell marker in human
gastric gland and its relatedness with other putative cancer stem
cell markers. Gene. 525:18–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie Y, Sun W, Deng Z, Zhu X, Hu C and Cai
L: MiR-302b suppresses osteosarcoma cell migration and invasion by
targeting Runx2. Sci Rep. 7:133882017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheadle C, Vawter MP, Freed WJ and Becker
KG: Analysis of microarray data using Z score transformation. J Mol
Diagn. 5:73–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cidado J, Wong HY, Rosen DM,
Cimino-Mathews A, Garay JP, Fessler AG, Rasheed ZA, Hicks J,
Cochran RL, Croessmann S, et al: Ki-67 is required for maintenance
of cancer stem cells but not cell proliferation. Oncotarget.
7:6281–6293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng M, Zhou J, Wen L, Zhu Y, Luo Y and
Wang W: The relationship between the expression of Ki-67 and the
prognosis of osteosarcoma. BMC Cancer. 21:2102021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaudhary RK, Patil P, Shetty VV,
Ananthesh L, Kalladka SS, Mateti UV and Raju B: Decoding the role
of SPRR1A and SPRR1B gene in cancer: A comprehensive review. Gene
Rep. 36:1019262024. View Article : Google Scholar
|
|
28
|
Yu H, Gao M, Ma Y, Wang L, Yang S and Liu
X: Inhibition of cell migration by focal adhesion kinase:
Time-dependent difference in integrin-induced signaling between
endothelial and hepatoblastoma cells. Int J Mol Med. 41:2573–2588.
2018.PubMed/NCBI
|
|
29
|
Maziveyi M, Dong S, Baranwal S and Alahari
SK: Nischarin regulates focal adhesion and Invadopodia formation in
breast cancer cells. Mol Cancer. 17:212018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benaud CM and Dickson RB:
Adhesion-regulated G1 cell cycle arrest in epithelial cells
requires the downregulation of c-Myc. Oncogene. 20:4554–4567. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao D, Inuzuka H, Tseng A, Chin RY, Toker
A and Wei W: Phosphorylation by Akt1 promotes cytoplasmic
localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction.
Nat Cell Biol. 11:397–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker JL, Fournier AK and Assoian RK:
Regulation of growth factor signaling and cell cycle progression by
cell adhesion and adhesion-dependent changes in cellular tension.
Cytokine Growth Factor Rev. 16:395–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim GD: Induction of hepatocellular
carcinoma cell cycle arrest and apoptosis by dendropanax morbifera
leveille leaf extract via the PI3K/AKT/mTOR pathwayc. J Cancer
Prev. 28:185–193. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ball KL, Lain S, Fâhraeus R, Smythe C and
Lane DP: Cell-cycle arrest and inhibition of Cdk4 activity by small
peptides based on the carboxy-terminal domain of p21WAF1. Curr
Biol. 7:71–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Liu J, Zhou B, Yang M and Feng H:
The value of Ki-67 as a prognostic biomarker in osteosarcoma: A
systematic review and meta-analysis. Asian J Surg. 45:2978–2980.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kovac M, Blattmann C, Ribi S, Smida J,
Mueller NS, Engert F, Castro-Giner F, Weischenfeldt J, Kovacova M,
Krieg A, et al: Exome sequencing of osteosarcoma reveals mutation
signatures reminiscent of BRCA deficiency. Nat Commun. 6:89402015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rajan S, Franz EM, McAloney CA, Vetter TA,
Cam M, Gross AC, Taslim C, Wang M, Cannon MV, Oles A and Roberts
RD: Osteosarcoma tumors maintain intra-tumoral transcriptional
heterogeneity during bone and lung colonization. BMC Biol.
21:982023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown HK, Tellez-Gabriel M and Heymann D:
Cancer stem cells in osteosarcoma. Cancer Lett. 386:189–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|