Introduction
Advances in targeted therapies have improved the
survival rates of patients with non-small cell lung cancer (NSCLC),
with a 36-month overall survival (OS) time observed among 84% of
patients treated with osimertinib (1). Heart disease is the second leading
cause of morbidity and mortality following cancerous progression
and recurrence among cancer survivors (2). A number of patients with lung cancer
are diagnosed with coexisting heart disease, and some may
experience adverse cardiac events due to anticancer treatment,
including targeted therapies, or from exacerbation of preexisting
cardiac comorbidities. The incidence, presentation and impact of
cardiotoxicity vary depending on the anticancer therapies and
underlying comorbidities (3). A
retrospective, population-based study of 20,689 patients with lung
cancer revealed that 47.4% had heart disease, including myocardial
infarction, congestive heart failure and chronic arrhythmias
(4). In a cohort of 345 consecutive
patients with NSCLC, 32% were diagnosed with heart disease,
presenting a higher risk of mortality and distant metastasis
(5). Data from the Surveillance,
Epidemiology, and End Results (SEER) registry linked to U.S.
Medicare administrative claim files (SEER-Medicare) showed that the
impact of coexisting heart disease on NSCLC survival varies by
cancer stage and treatment. For instance, cardiac comorbidities
significantly increase the risk of death for patients with stage I
and II disease treated with surgery and patients with stage I–IIIB
disease treated with chemotherapy and chemoradiation, but not for
patients with stage-IV (6). The
present study aimed to fully characterize the effects of cardiac
comorbidity on the occurrence of cardiotoxicity in patients with
stage-IV NSCLC.
Cardiotoxicity from anticancer agents can affect the
drug tolerance, quality of life and survival of patients, causing
issues such as arrhythmias, cardiac arrest, coronary artery
disease, heart failure, ischemia, left ventricular dysfunction,
myocardial infarction and tachycardia (7). In recent decades, targeted therapy has
rapidly evolved, improving the management of advanced NSCLC by
targeting mutated driver genes and angiogenesis (8,9). The
National Comprehensive Cancer Network guidelines recommend
appropriate targeted therapy for patients with NSCLC harboring
oncogenic driver mutations (10).
However, different targeted therapies are associated with varying
cardiotoxic effects: Epidermal growth factor receptor (EGFR)
inhibitors can cause coronary artery events, heart failure and
prolonged QT corrected (QTc) interval and anaplastic lymphoma
kinase (ALK) inhibitors most commonly cause atrial
fibrillation and electrocardiogram changes. By contrast,
anti-vascular endothelial growth factor (VEGF) therapy is
more related to vascular complications, including arterial
hypertension, which can lead to cardiac disorders (11).
Studies exploring the relationship between
cardiotoxicity and clinical outcomes in patients with or without
cardiac comorbidities remain limited, particularly advanced disease
studies based on real-world patients. Therefore, the present study
aims to address a critical gap in this knowledge using a 14-year
clinical cohort of patients with stage-IV NSCLC.
Patients and methods
Study population
All patients in the present study were previously
enrolled in an ongoing Mayo Clinic (USA) lung cancer cohort study,
which included ~20,000 patients with newly diagnosed primary lung
cancer from 1997 to 2016 (12–15)
and 144 consecutive cases of stage-IV NSCLC from 2017 to 2019 in
Rochester (USA) (16). The
enrollment and follow-up of patients were conducted with the
approval of the Mayo Clinic Institutional Review Board (Rochester,
USA). The detailed procedures for patient enrollment, diagnosis,
data collection and follow-up have been described in previous
publications (12,17). All patients were staged at the time
of their primary diagnosis according to either the 5th (18) or 7th (19) edition of the TNM staging system. The
enrolled patients met the following inclusion criteria: i) Newly
diagnosed with stage-IV NSCLC from January 1, 2006 to December 31,
2019; and ii) treated with targeted therapies. The exclusion
criteria were as follows: i) Those with unknown toxicity
information; and ii) those lost to follow-up within 1 month after
taking the targeted drugs. All the patients were followed until
April 30, 2022. The targeted therapy mainly consisted of inhibitors
for EGFR, ALK, c-ros oncogene 1 (ROS1), Kirsten rat
sarcoma virus, V-raf murine sarcoma oncogene homolog B1
(BRAF), human epidermal growth factor receptor,
mitogen-activated protein kinase, mesenchymal-epithelial transition
(MET), rearranged during transfection, neurotrophic tyrosine
receptor kinase, mammalian target of rapamycin and
VEGF/VEGFR [including the anti-VEGF antibody,
bevacizumab, and tyrosine kinase inhibitors (TKIs) of the
VEGF receptors].
Data collection
Comprehensive data on each patient were collected
from medical records in two steps: i) At the time of diagnosis
including demographic information, smoking status, cell type, lung
tumor site and prior and concurrent disease; and ii) after
treatment including treatment type, treatment line, targeted
drug-induced toxicity, severity and onset time of cardiotoxicity,
response to drug therapy, recurrence, disease progression and vital
status (20). Patients were
categorized into three groups: Those with cardiac comorbidity,
those with other comorbidities (excluding heart disease) and those
with no comorbidity.
Toxicity, comorbidity and clinical
outcome evaluation
The data were sourced from the electronic health
records at Mayo Clinic. Cardiotoxicity, as well as other targeted
drug-induced toxicities, were identified and graded by the
attending physicians using the Common Terminology Criteria for
Adverse Events version 5.0 (21)
and recorded in the medical records for the patient. Cardiotoxicity
encompassed various cardiac disorders with the terms of ejection
fraction (EF) decreased, tachycardia, heart failure,
electrocardiogram QTc interval prolonged, bradycardia,
pericarditis, atrioventricular block complete, atrial fibrillation,
myocardial infarction and mitral valve disease. Patients who took
>1 targeted drug were analyzed as a whole and separately in
sub-group analysis.
The duration between the initiation of targeted
therapy and the development of cardiotoxicity was defined as the
onset time and identified within 1.5 years. For patients
experiencing cardiotoxicity, detailed information was retrieved and
analyzed, including details on the demographics, gene mutation
status, responsible drugs, symptoms associated with cardiotoxicity,
laboratory values, cardiac investigations, cardiotoxicity
consequences, concurrent with other toxicities, prior therapies,
type of comorbid heart disease, cardiovascular risk factors (such
as hypertension, high cholesterol, stroke, diabetes, obesity and
family history). Electrocardiograms (ECGs) were used for routine
monitoring of heart conditions in patients receiving targeted drugs
(22).
Cardiac comorbidity (such as coronary artery
disease, myocardial infarction, cardiomyopathy, arrhythmia, heart
valve disease and heart failure) and other comorbidities (comorbid
disease beyond heart disease) was defined based on the World Health
Organization (WHO) International Classification of Disease 10th
Revision classifications (23). The
Charlson Comorbidity Index (CCI) score (24,25)
was employed to assess comorbidity. The assigned weights for
scoring were as follows: i) Myocardial infarction, congestive heart
failure, peripheral vascular disease, cerebrovascular disease,
dementia, chronic pulmonary disease, connective tissue disease,
ulcer disease, mild liver disease and diabetes received a score of
1; ii) hemiplegia, moderate or severe renal disease, diabetes with
end organ damage, any tumor, leukemia and lymphoma were scored as
2; iii) moderate or severe liver disease was assigned a score of 3;
and iv) metastatic solid tumor and acquired immunodeficiency
syndrome had a score of 6. Additionally, the diseases in CCI score
list, excluding myocardial infarction and congestive heart failure,
were also evaluated.
Recurrence and progression were assessed during the
targeted therapy. Responses were defined as the best response to
targeted therapy and evaluated by Response Evaluation Criteria in
Solid Tumors version 1.1 (26). The
responses were divided into complete response (CR), partial
response (PR), stable disease and progressive disease. CR was
defined as disappearance of all targeted lesions, and any
pathological lymph nodes (whether target or non-target) must have a
reduction in the short axis to <10 mm; PR was defined as at
least a 30% decrease in the sum of the diameters of target lesions,
taking as reference the baseline sum diameters. CR and PR were
collectively recognized as an objective response and abbreviated as
‘response’.
Statistical analysis
The characteristics and distributions of the
patients are presented as the mean (± standard deviation; SD) or
median for continuous variables, and the count (n) and frequency
(%) for categorical variables. The distribution of the
demographics, smoking status (never vs. ever), cell type
(adenocarcinoma vs. other), tumor site (left vs. right),
recurrence, progression, treatment (drug vs. surgery, drug vs.
surgery with drug, and radiation vs. radiation and drug), treatment
line (palliative first line vs. other), drug-induced toxicity (yes
vs. no) and response to drug therapy (response vs. no response)
within the comorbidity subgroups (cardiac, other or no
comorbidities) were compared using χ2 tests and Fisher's
exact test. Detailed information regarding cardiotoxicity was
descriptively analyzed. The CCI was compared using a two-sample
unpaired t-test. OS was defined from the date of treatment
initiation to the last follow-up date or patient death; notably,
for patients who experienced cardiotoxicity, OS was defined from
the median onset date of all cardiotoxicities to the last follow-up
date or patient death. Patients who were alive or lost to follow-up
were censored in the analysis. Survival analyses of subgroups was
performed using Cox Proportional Hazard models [measured as hazard
ratios (HR) with 95% confidence intervals (95% CI)] and the
log-rank test and graphically illustrated by Kaplan-Meier curves.
Univariable Cox Proportional Hazard models were utilized to analyze
the relationship between known prognostic factors (such as comorbid
diseases, treatment toxicity including cardiotoxicity and any
toxicity, sex, ethnicity, smoking status, side of tumor, cell type,
treatment type, treatment line, treatment response, toxicity
severity, CCI score and age at diagnosis) and to estimate the
5-year survival. Multivariable Cox models were developed using
significant variables (defined as P<0.1) from the univariable
analysis, and the HR with 95% CI was calculated. Two-sided
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were conducted using SAS
version 9.4 (SAS Institute Inc.).
Results
Distribution differences of patients
in the three comorbidity groups
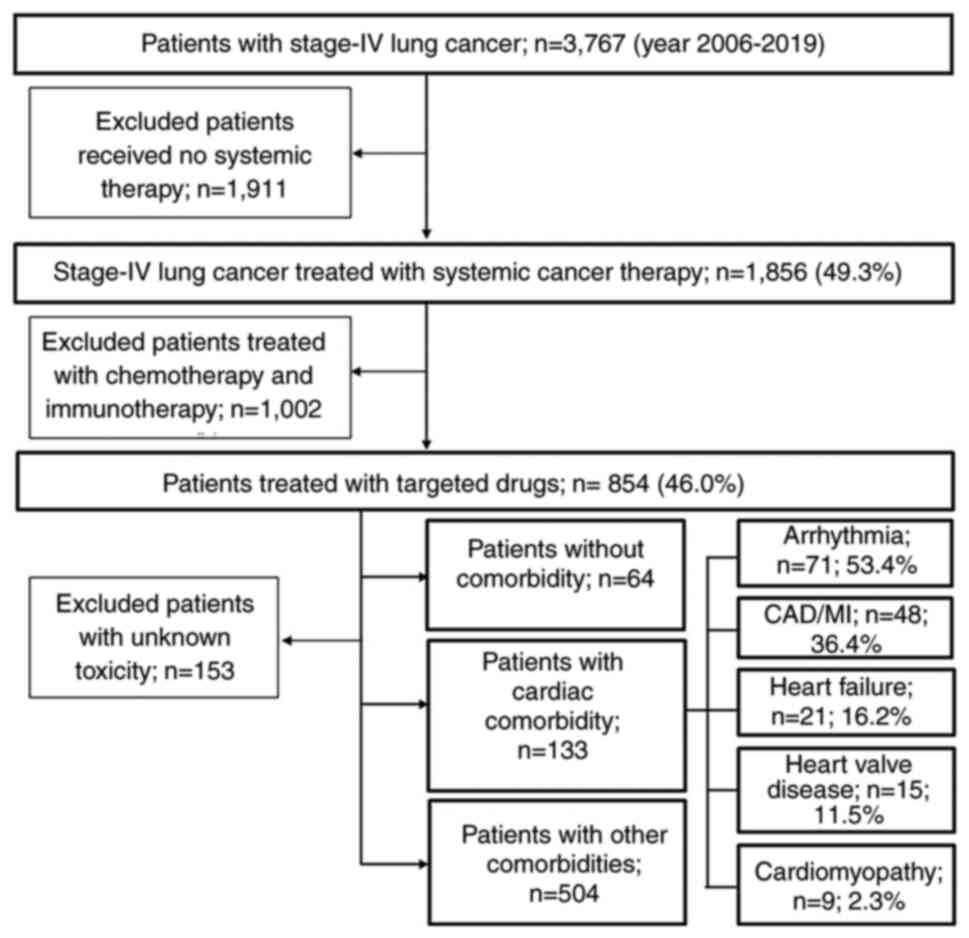
A total of 3,767 patients with newly diagnosed
stage-IV NSCLC were identified. Among them, 1,856 (49.3%) received
systemic therapy. Of the remaining 1,911 (50.7%) patients who did
not receive systemic therapy, 185 underwent surgery and 625
received radiotherapy. All other patients did not receive standard
anticancer therapies at Mayo Clinic. Out of the 1,856 patients
receiving systemic therapy, 1,002 (54.0%) received chemotherapy and
immunotherapy and 854 (46.0%) received targeted therapy. After
excluding 153 patients without information on the targeted
drug-related toxicity, 701 evaluable patients were included in the
analyses (Fig. 1).
Among the included patients, 133 (19.0%) had cardiac
comorbidities, 504 (71.9%) had other comorbidities and 64 (9.1%)
had no comorbidity. Among the 701 patients, the mean (±SD) age at
lung cancer diagnosis was 62 (±12) years with 57.6% being women,
88.6% Caucasian and 42.5% never-smokers (Table I). Patients with heart disease were
older (67±12 years) than those with other comorbidities (62±12
years) and no comorbidity (52±13 years) (P<0.001). There was a
higher frequency of men than women (24.9 vs. 14.6%; P=0.002), more
Caucasian patients than patients of other ethnicities (20.6 vs.
7.6%; P=0.003) and more smokers than never-smokers (22.5 vs. 15.8%;
P=0.020) with heart disease (data not shown). However, no
differences were observed in the treatment type, treatment line or
response to therapy among the three comorbidity groups.
 | Table I.Comorbidities of patients treated
with targeted therapies. |
Table I.
Comorbidities of patients treated
with targeted therapies.
|
| Comorbidity of
targeted drug |
|
|---|
|
|
|
|
|---|
| Variable | None, n=64 | Cardiac, n=133 | Other, n=504 | Total, n=701 | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
| <0.001 |
| Mean
(SD) | 52.1 (12.62) | 66.9 (11.55) | 61.6 (11.65) | 61.7 (12.27) |
|
|
Median | 50.5 | 66 | 63 | 63 |
|
|
Range | 30.0, 79.0 | 38.0, 94.0 | 25.0, 86.0 | 25.0, 94.0 |
|
| Sex, n (%) |
|
|
|
| 0.002 |
|
Female | 35 (54.7) | 59 (44.4) | 310 (61.5) | 404 (57.6) |
|
|
Male | 29 (45.3) | 74 (55.6) | 194 (38.5) | 297 (42.4) |
|
| Ethnicity, n
(%) |
|
|
|
| 0.003 |
|
Caucasian | 50 (79.4) | 127 (95.5) | 439 (88.0) | 616 (88.6) |
|
|
Othera | 13 (20.6) | 6 (4.5) | 60 (12.0) | 79 (11.4) |
|
| NA | 1 | 0 | 5 | 6 |
|
| Smoking status, n
(%) |
|
|
|
| 0.020 |
|
Never | 41 (64.1) | 58 (43.6) | 269 (53.4) | 368 (52.5) |
|
|
Ever | 23 (35.9) | 75 (56.4) | 235 (46.6) | 333 (47.5) |
|
| Cell type, n
(%) |
|
|
|
| 0.321 |
|
Adenocarcinoma | 53 (82.8) | 115 (86.5) | 448 (88.9) | 616 (87.9) |
|
|
Other | 11 (17.2) | 18 (13.5) | 56 (11.1) | 85 (12.1) |
|
| Side of tumor, n
(%) |
|
|
|
| 0.757 |
|
Left | 30 (46.9) | 60 (45.8) | 215 (43.1) | 305 (43.9) |
|
|
Right | 34 (53.1) | 71 (54.2) | 284 (56.9) | 389 (56.1) |
|
| NA | 0 | 2 | 5 | 7 |
|
| Treatment, n
(%) |
|
|
|
| 0.485 |
| Drug
therapy | 50 (78.1) | 103 (77.4) | 369 (73.2) | 522 (74.5) |
|
| Surgery
+ drug | 2 (3.1) | 5 (3.8) | 25 (5.0) | 32 (4.6) |
|
| Surgery
+ radiation + drug | 3 (4.7) | 3 (2.3) | 8 (1.6) | 14 (2.0) |
|
|
Radiation + drug | 9 (14.1) | 22 (16.5) | 102 (20.2) | 133 (19.0) |
|
| Reason for drug
therapy, n (%) |
|
|
|
| 0.520 |
|
Palliative first-line | 59 (95.2) | 122 (93.8) | 472 (96.1) | 653 (95.6) |
|
|
Other | 3 (4.8) | 8 (6.2) | 19 (3.9) | 30 (4.4) |
|
| NA | 2 | 3 | 13 | 18 |
|
| Treatment response,
n (%) |
|
|
|
| 0.554 |
|
Response | 35 (55.6) | 63 (47.7) | 259 (51.8) | 357 (51.4) |
|
| No
response | 28 (44.4) | 69 (52.3) | 241 (48.2) | 338 (48.6) |
|
| NA | 1 | 1 | 4 | 6 |
|
| Toxicity, n
(%) |
|
|
|
| <0.001 |
|
Cardiotoxicity | 0 (0.0) | 10 (7.5) | 5 (1.0) | 15 (2.1) |
|
|
Other | 58 (90.6) | 116 (87.2) | 460 (91.3) | 634 (90.5) |
|
|
None | 6 (9.4) | 7 (5.3) | 39 (7.7) | 52 (7.4) |
|
Characteristics of comorbid
disease
In the three comorbidity groups, the comorbid
disease and CCI burden showed some differences. Among the 133
patients with cardiac comorbidity, 71 (53.4%) had arrhythmia
(predominately 48 with atrial fibrillation, 10 with tachycardia and
3 with bradycardia), 48 (36.4%) had coronary artery disease or
myocardial infarction, 21 (16.2%) had heart failure, 15 (11.5%) had
heart valve disease and 9 (2.3%) had cardiomyopathies (Fig. 1). Some patients had >1 cardiac
comorbidity. Additionally, these patients exhibited conditions such
as hypertension (58.6%), hyperlipidemia (43.2%), stroke (15.8%),
diabetes (18.9%) and obesity (3.0%). In comparison, 504 patients
with other comorbidities showed lower frequencies of hypertension
(33.3%), hyperlipidemia (20.4%), stroke (4%), diabetes (8.1%) and
obesity (0.6%) (P<0.02; data not shown). Furthermore, the
cardiac comorbidity group had a much higher CCI score compared with
the other comorbidity group (2.29 vs. 1.14; P<0.001), as shown
in Table II. A similar difference
in CCI score was observed in the two comorbidity groups when
excluding heart disease (myocardial infarction and congestive heart
failure) (2.02 vs. 1.14; P<0.001; Table II). When focusing on
osimertinib-treated patients, those with cardiac or other
comorbidities showed a significant difference in CCI scores even
when excluding heart diseases (2.16 vs. 0.86; P<0.001), as shown
in Table III. These results
suggested that patients with cardiac comorbidity had a higher risk
of heart-related diseases and a heavier comorbid disease
burden.
 | Table II.Comparison of CCI scores between two
comorbidity groups of targeted drug-treated patients. |
Table II.
Comparison of CCI scores between two
comorbidity groups of targeted drug-treated patients.
| A, CCI scores in
the cardiac and other comorbidity groups |
|---|
|
|---|
| CCI score | Cardiac
comorbidity, n=133 | Other comorbidity,
n=504 | P-value |
|---|
| Mean (± SD) | 2.29 (±2.38) | 1.14 (±1.68) | <0.001 |
| Score distribution,
n (%) |
|
| <0.001 |
| 0 | 29 (21.8) | 218 (43.3) |
|
| 1 | 32 (24.1) | 158 (31.3) |
|
| 2 | 31 (23.3) | 70 (13.9) |
|
| 3+ | 41 (30.8) | 58 (11.5) |
|
|
| B, CCI scores
when excluding for heart diseasea in the cardiac and other
comorbidity groups |
|
| CCI
score | Cardiac
comorbidity, n=133 | Other
comorbidity, n=504 | P-value |
|
| Mean (± SD) | 2.02 (±2.23) | 1.14 (±1.68) | <0.001 |
| Score distribution,
n (%) |
|
| <0.001 |
| 0 | 35 (26.3) | 218 (43.3) |
|
| 1 | 33 (24.8) | 158 (31.3) |
|
| 2 | 28 (21.1) | 70 (13.9) |
|
| 3+ | 37 (27.8) | 58 (11.5) |
|
 | Table III.Comparison of CCI scores between two
comorbidity groups of osimertinib-treated patients. |
Table III.
Comparison of CCI scores between two
comorbidity groups of osimertinib-treated patients.
| A, CCI scores in
the cardiac and other comorbidity groups |
|---|
|
|---|
| CCI score | Cardiac
comorbidity, n=32 | Other comorbidity,
n=132 | P-value |
|---|
| Mean (± SD) | 2.41 (±2.96) | 0.86 (±1.08) | <0.001 |
| Score distribution,
n (%) |
|
|
|
| 0 | 8 (25.0) | 69 (52.3) | 0.005 |
| 1 | 8 (25.0) | 27 (20.5) |
|
| 2 | 7 (21.9) | 25 (18.9) |
|
| 3+ | 9 (28.1) | 11 (8.3) |
|
|
| B, CCI scores
when excluding for heart diseasea in the cardiac and other
comorbidity groups |
|
| CCI | Cardiac
comorbidity, n=32 | Other
comorbidity, n=132 | P-value |
|
| Mean (± SD) | 2.16 (±2.83) | 0.86 (±1.08) | <0.001 |
| Score distribution,
n (%) |
|
| 0.032 |
| 0 | 10 (31.3) | 69 (52.3) |
|
| 1 | 8 (25.0) | 27 (20.5) |
|
| 2 | 6 (18.7) | 25 (18.9) |
|
| 3+ | 8 (25.0) | 11 (8.3) |
|
Cardiotoxicity occurs more frequently
among patients in the cardiac comorbidity group
Out of the 701 included patients, 15 (2.1%)
developed cardiotoxicity, all of which occurred in patients with
comorbidities. Among these, 10 (7.5%) were in the cardiac
comorbidity group and 5 (1.0%) were in the other comorbidities
group. Patients with cardiac comorbidity had a 7.5-fold higher risk
of targeted therapy-related cardiotoxicity compared to those with
other comorbidities (P<0.001; Table
I). Of the 15 patients who experienced cardiotoxicities, the
mean (± SD) age at diagnosis was 64 (±14) years and included 73.3%
men, 93.3% Caucasian individuals and 53.3% smokers. Additionally, 6
of the 15 patients (40%) were diagnosed with left-side lung cancer,
one of whom received a total of 2,000 cGy chest radiation with good
tolerance and response. Small molecule TKIs targeting EGFR
and ALK/ROS1 appeared to cause cardiotoxicities
following 6 months of treatment while anti-VEGF therapy led
to cardiotoxicity following 1.5 months. The most common cardiac
comorbidity was arrhythmia and the predominant cardiovascular risk
factor was hypertension (Table IV,
Table V, Table VI).
 | Table IV.Demographics of patients with
cardiotoxicity (n=15). |
Table IV.
Demographics of patients with
cardiotoxicity (n=15).
| Demographic | Value |
|---|
| Mean age, years
(SD) | 64.2 (13.5) |
| Sex, n (%) |
|
|
Female | 4 (26.7) |
|
Male | 11 (73.3) |
| Ethnicity, n
(%) |
|
|
Caucasian | 14 (93.3) |
|
Other | 1 (6.7) |
| Smoking status, n
(%) |
|
|
Never | 7 (46.7) |
|
Former | 6 (40.0) |
|
Current | 2 (13.3) |
| Tumor site, n
(%) |
|
|
Left | 6 (40.0) |
|
Right | 9 (60.0) |
| Gene mutation, n
(%) |
|
|
EGFR | 6 (40.0) |
|
ALK | 4 (26.7) |
|
ROS1 | 3 (20.0) |
|
Unknown | 2 (13.3) |
 | Table V.Cardiotoxicity characteristics and
outcome of patients with cardiotoxicity (n=15), |
Table V.
Cardiotoxicity characteristics and
outcome of patients with cardiotoxicity (n=15),
| Characteristic | Value |
|---|
| Mean onset time of
cardiotoxicity, months (SD) |
|
|
EGFR inhibitors
(including osimertinib) | 8.1 (7.2) |
|
ALK/ROS1
inhibitors | 4.3 (3.2) |
|
Osimertinib | 11.0 (6.1) |
|
Anti-VEGF therapy | 1 or 2 cycles |
| Median onset time
of cardiotoxicity, months |
|
|
Overall | 3 |
|
Osimertinib | 12 |
|
EGFR inhibitors
(including osimertinib) | 8 |
|
ALK/ROS1
inhibitors | 3 |
|
Anti-VEGF therapy | 1.5 |
| Diagnostic method
of cardiotoxicity, n |
|
|
Symptoms | 6 |
|
Electrocardiogram | 11 |
|
Echocardiogram | 4 |
|
N-terminal pro b-type
natriuretic peptide | 1 |
|
Troponin | 1 |
| Median follow-up
time, months (range) | 52 (3–128) |
 | Table VI.Comorbid disease, toxicity and
treatment of patients with cardiotoxicity (n=15). |
Table VI.
Comorbid disease, toxicity and
treatment of patients with cardiotoxicity (n=15).
| Characteristic | No. of
patients |
|---|
| Cardiac
comorbiditya |
|
|
Arrhythmia | 7 |
| Heart
failure | 5 |
|
Myocardial infarction | 3 |
|
Coronary artery
calcifications | 1 |
| Heart
valve disease | 1 |
|
None | 6 |
| Cardiovascular risk
factorsa |
|
|
Hypertension | 9 |
| High
cholesterol | 5 |
| Family
history | 3 |
|
Stroke | 2 |
|
Diabetes | 1 |
|
None | 3 |
| Other toxicities
(>5 observation)a |
|
|
Nausea | 8 |
|
Fatigue | 7 |
| Skin
toxicity | 5 |
|
Diarrhea | 5 |
|
Hypertension | 5 |
| Prior
antitherapya |
|
| Chest
radiation | 1 |
|
Chemotherapy | 6 |
|
Targeted therapy | 7 |
|
Immunotherapy | 1 |
|
None | 7 |
Frequency of cardiotoxicities varies
by inhibitor (EGFR, ALK/ROS1 and VEGF/VEGFR)
The targeted drugs responsible for cardiotoxicity
involved inhibitors of EGFR, ALK/ROS1 and VEGF/VEGFR
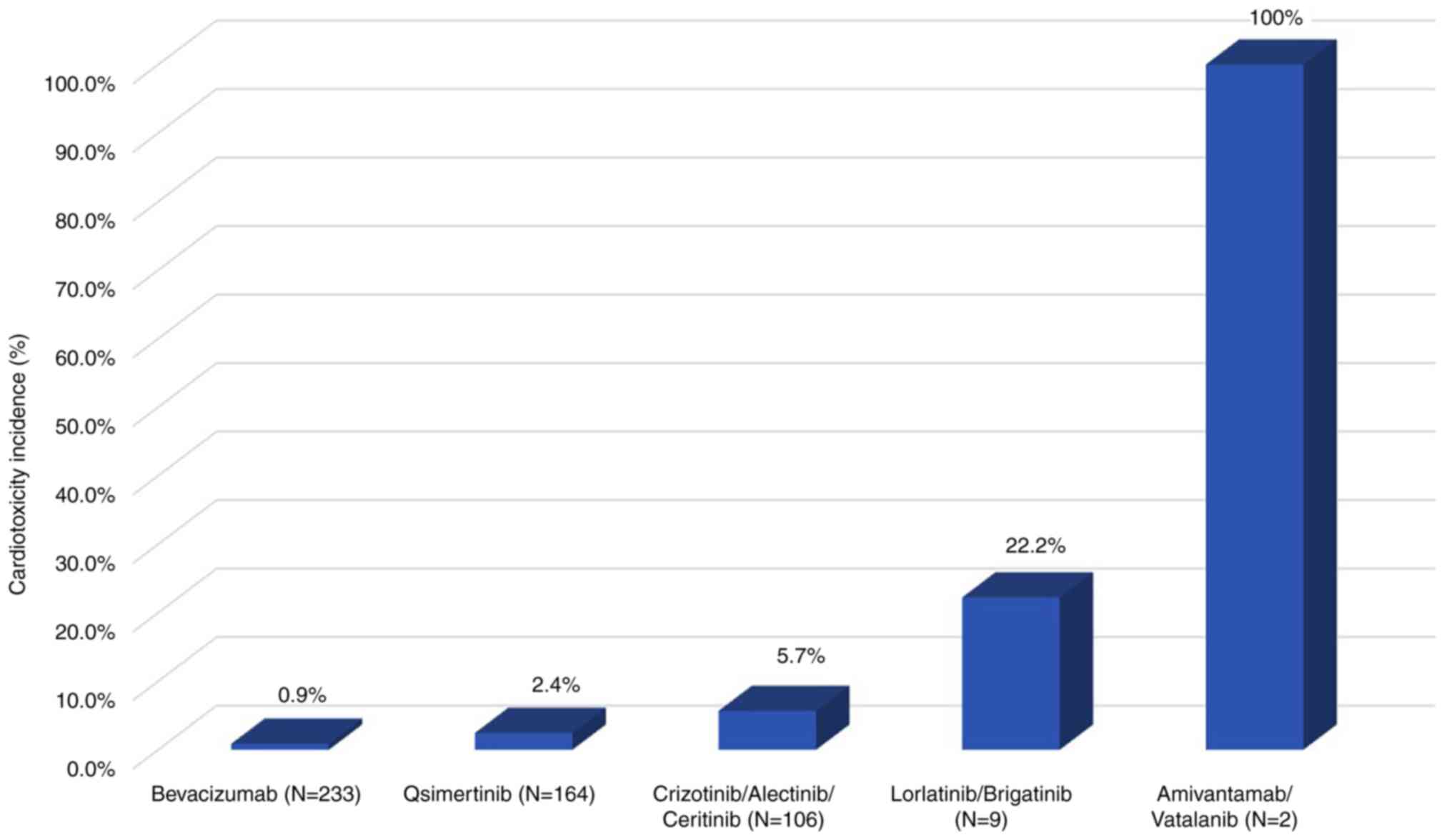
(Fig. 2). The frequency of
cardiotoxicity (n, %) from the lowest to highest were bevacizumab
(2, 0.9%), osimertinib (4, 2.4%), crizotinib (4, 5.9%),
alectinib/ceritinib (1, 5.0–5.6%), lorlatinib/brigatinib (1,
20–25%) and amivantamab/vatalanib (1, 100%).
As shown in Table
SI, the 233 patients who received monoclonal antibodies
(bevacizumab; 233/701, 33.3%) had a similar distribution in terms
of the three comorbidity groups to those patients treated with
small molecule inhibitors, mainly EGFR inhibitors (474/701,
67.6%) and ALK/ROS1 inhibitors (74/701, 10.6%), and
no significant difference was detected between patients treated
with the monoclonal antibody and the small molecule inhibitor
treatment groups (P=0.310). Of the 474 patients treated with
EGFR inhibitors, patients with heart disease had a higher
rate of cardiotoxicities (3.4%) than those with other comorbidities
(0.6%), although the differences did not reach statistical
significance (P=0.054). Notably, within the group treated with
EGFR inhibitors, cardiac comorbidity was significantly
associated with a higher risk of osimertinib-related cardiotoxicity
(9.4 vs. 0.8%; P=0.018). Of the 74 patients treated with
ALK/ROS1 inhibitors, ALK/ROS1 inhibitor-associated
cardiotoxicities were more frequent in patients with heart disease
(30.0%) than in those with other comorbidities (2.1%) (P=0.001). Of
the 251 patients treated with anti-VEGF therapy, bevacizumab
was the most frequent anti-VEGF therapy used (233 patients),
causing cardiac disorders in 2/233 (0.9%) patients. Additionally, 2
of 3 patients with anti-VEGF therapy-associated
cardiotoxicity continued chemotherapy (bevacizumab plus
paclitaxel/carboplatin or vatalanib plus pemetrexed) and tolerated
treatment well when anti-VEGF therapy was stopped. This
suggested that cardiotoxicity was less related to chemotherapy.
Another patient discontinued bevacizumab plus
paclitaxel/carboplatin treatment due to cardiotoxicity, with
bevacizumab considered the primary cause. This patient subsequently
switched to EGFR inhibitors after the detection of a
sensitive mutation and did not report any cardiac problems.
Patient characteristics and the
management of targeted drug-associated cardiotoxicity
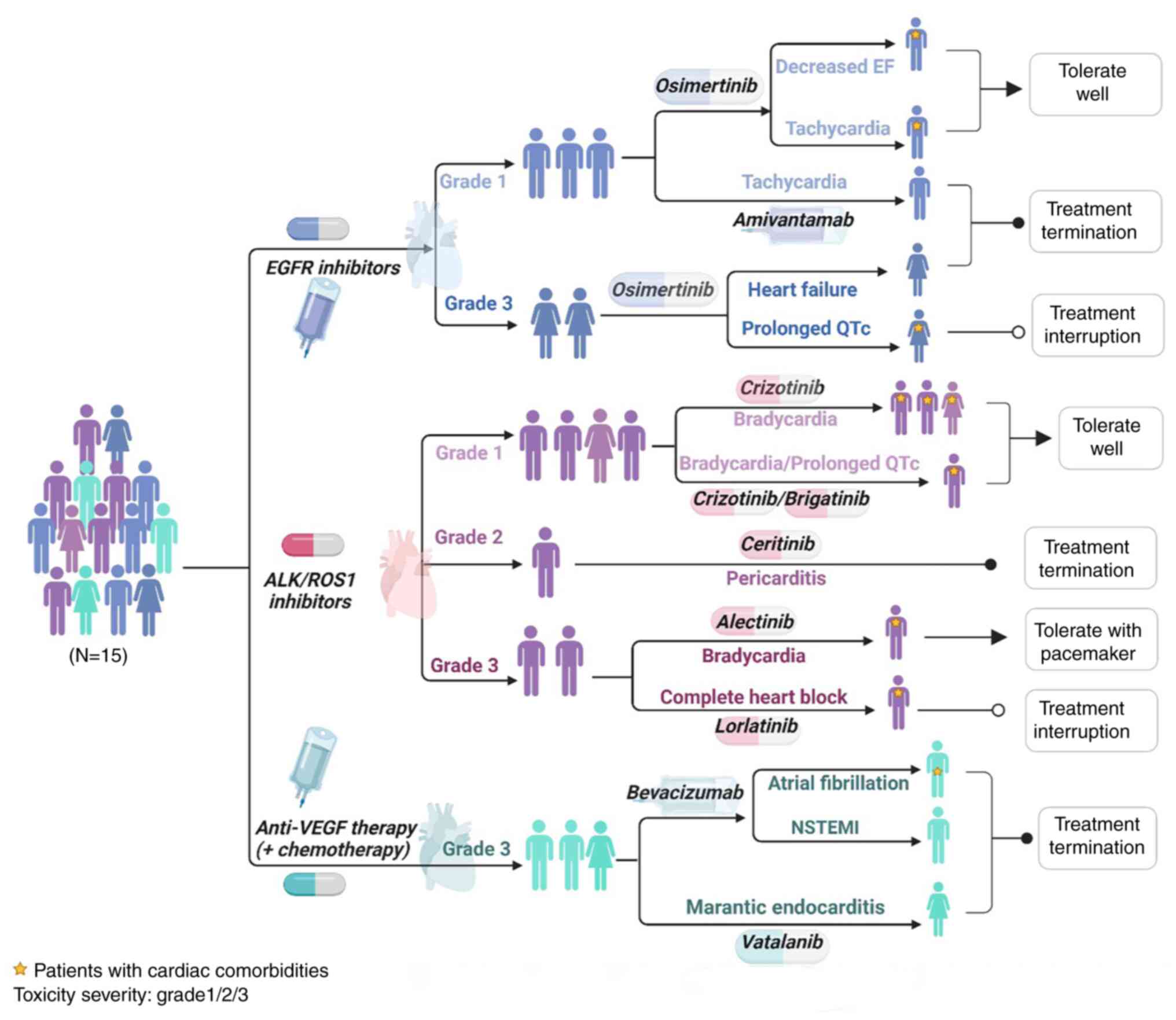
In total, 16 cardiac disorders among the 15 patients
with cardiotoxicity were identified, including 5 with bradycardia,
2 tachycardia, 2 QTc interval prolonged, 1 decreased EF, 1 heart
failure, 1 atrial fibrillation, 1 pericarditis with pericardial
effusion, 1 complete heart block, 1 non-ST-evaluation myocardial
infarction (NSTEMI) and 1 marantic endocarditis of the mitral valve
leading to cardioembolic strokes possibly due to malignancy and
clinical study agent. Additionally, 8 cardiac events presented with
mild symptoms and most could be tolerated well, while grade ≥2
cardiotoxicity frequently led to discontinuation or interruption of
the targeted drugs (Fig. 3). An
asymptomatic patient who had prior bradycardia had a pacemaker
implanted due to lorlatinib-related complete heart block and
restarted treatment with a good tolerance after a 7-day
interruption. Other cardiotoxicities were tolerable under
monitoring and no further intervention was necessary at the time of
detection, including discontinuation or interruption of the
targeted therapy. Furthermore, 10 of the 16 (62.5%)
cardiotoxicities were symptomatic and were identified through
periodic cardiac monitoring. ECGs detected 12 cardiac events,
including prolonged QTc interval (480–501 ms), bradycardia (45–54
bpm), tachycardia (108–134 bpm), atrial fibrillation and heart
block. Echocardiograms were performed to identify 4 cardiac events
of heart failure (EF 28%), decreased EF (48%), pericarditis and
marantic endocarditis. Significantly increased N-terminal pro
b-type natriuretic peptide levels and increasing trends of troponin
were tested for the diagnoses of heart failure and NSTEMI,
respectively.
Survival
The median survival time of patients who displayed
cardiotoxicity was 4.7 years, which was much longer than the 1.9
years observed for those who did not. The 4-year survival curves
showing the survival rate trends following univariable analysis are
shown in Fig. 4A-B. Multivariable
analyses demonstrated cardiac comorbidity was not an independent
prognostic factor among patients with targeted therapy. However,
patients with cardiotoxicities were at a lower risk of death (HR,
0.56; 95% CI, 0.32–0.99) than those without (P=0.003). Smokers (HR,
1.45; 95% CI, 1.23–1.72), non-adenocarcinoma (HR, 1.51; 95% CI,
1.18–1.92), only drug therapy (HR, 1.79; 95% CI, 1.46–2.19),
targeted therapy not used at the first line (HR, 1.21; 95% CI,
1.02–1.44), no response to therapy (HR, 1.61; 95% CI, 1.36–1.91)
and older age (HR, 1.01; 95% CI, 1.00–1.02) predicted a worse
survival (Table VII).
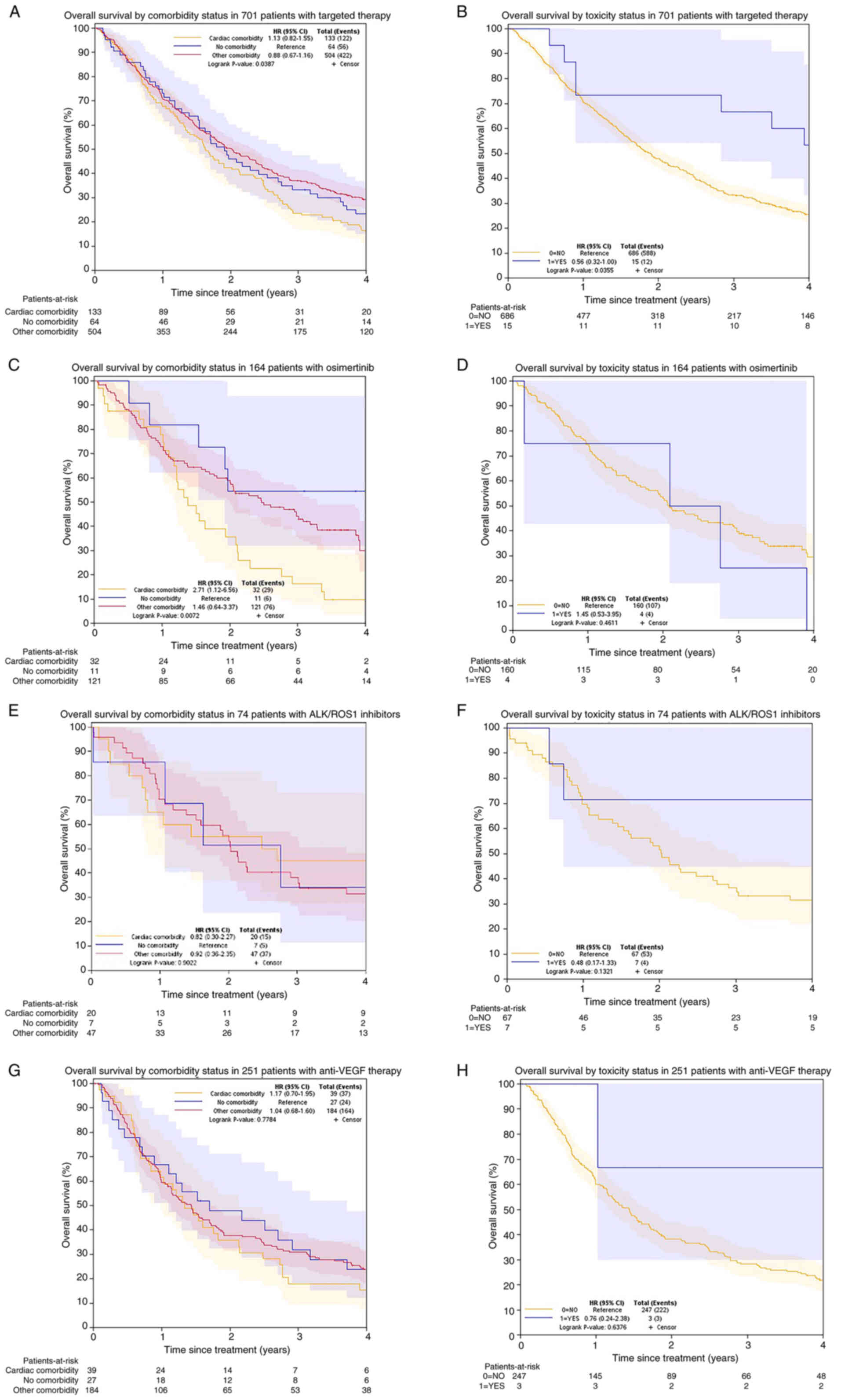 | Figure 4.Kaplan-Meier curves of overall
survival by comorbidity and toxicity status. (A) In 701 patients
receiving targeted therapy, no survival differences were observed
among the three comorbidity groups. (B) In 701 patients receiving
targeted therapy, patients with cardiotoxicity had an improved
survival compared with those without. (C) In 164 patients receiving
osimertinib, patients with cardiac comorbidity had a worse survival
than those with other comorbidities. (D) In 164 patients receiving
osimertinib, no survival difference was observed in patients with
or without cardiotoxicity. (E) In 74 patients with
ALK/ROS1 inhibitors, no survival differences were
observed among three comorbidity groups. (F) In 74 patients with
ALK/ROS1 inhibitors, no survival differences were
observed in patients with or without cardiotoxicity. (G) In 251
patients with anti-VEGF therapy, no survival differences
were observed among the three comorbidity groups. (H) In 251
patients with anti-VEGF therapy, no survival differences
were observed in patients with or without cardiotoxicity. ALK,
anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor;
ROS1, c-ros oncogene 1; VEGFR, vascular endothelial growth factor
receptor; HR, hazard ratio; CI, confidence interval. |
 | Table VII.Univariable and multivariable
survival analysis for 701 patients receiving targeted therapy. |
Table VII.
Univariable and multivariable
survival analysis for 701 patients receiving targeted therapy.
|
|
|
|
|
|
|
| Cox
multivariable |
|---|
|
|
|
|
|
| Cox
univariable |
|
|---|
| Variable | n | Events, n (%) | Median, years | 5-year survival %
(95% CI) |
|
| Likelihood
Ratio |
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value
(n=633) |
|---|
| Comorbidity |
|
|
|
|
| 0.0548 |
| 0.1463 |
| No
comorbidity | 64 | 56 (88) | 1.9 | 14.1
(5.5–22.8) | - |
| - |
|
| Cardiac
comorbidity | 133 | 122 (92) | 1.6 | 13.6
(7.8–19.5) | 1.14
(0.83–1.57) |
| 0.96
(0.68–1.36) |
|
| Other
comorbidity | 504 | 422 (84) | 2 | 23.5
(19.7–27.3) | 0.89
(0.68–1.18) |
| 0.81
(0.60–1.09) |
|
| Sex |
|
|
|
|
| 0.2432 |
|
|
|
Female | 404 | 344 (85) | 2 | 21.3
(17.2–25.5) | - |
|
|
|
|
Male | 297 | 256 (86) | 1.7 | 19.7
(15.1–24.3) | 1.10
(0.94–1.29) |
|
|
|
| Ethnicity |
|
|
|
|
| 0.3592 |
|
|
|
Caucasian | 616 | 535 (87) | 1.9 | 20.7
(17.5–24.0) | - |
|
|
|
|
Other | 79 | 59 (75) | 2.3 | 21.5
(11.5–31.5) | 0.88
(0.67–1.15) |
|
|
|
| Smoking status |
|
|
|
|
| <0.0001 |
| <0.0001 |
|
Never | 368 | 296 (80) | 2.4 | 27.5
(22.8–32.2) | - |
| - |
|
|
Ever | 333 | 304 (91) | 1.3 | 13.2
(9.5–17.0) | 1.60
(1.36–1.88) |
| 1.45
(1.23–1.72) |
|
| Side of tumor |
|
|
|
|
| 0.7769 |
|
|
|
Left | 305 | 262 (86) | 1.9 | 20.6
(15.9–25.2) | - |
|
|
|
|
Right | 389 | 331 (85) | 1.9 | 21.2
(17.0–25.4) | 1.02
(0.87–1.20) |
|
|
|
| Cell type |
|
|
|
|
| <0.0001 |
| 0.0016 |
|
Adenocarcinoma | 616 | 519 (84) | 2.1 | 21.8
(18.4–25.1) | - |
| - |
|
|
Other | 85 | 81 (95) | 1.1 | 12.7
(5.6–19.9) | 1.65
(1.30–2.09) |
| 1.51
(1.18–1.92) |
|
| Treatment type |
|
|
|
|
| <0.0001 |
| <0.0001 |
| Drug
therapy | 522 | 471 (90) | 1.7 | 15.6
(12.4–18.8) | 1.81
(1.49–2.21) |
| 1.79
(1.46–2.19) |
|
| Drug
combined with other therapies | 179 | 129 (72) | 3.3 | 35.5
(28.3–42.7) | - |
| - |
|
| Treatment line |
|
|
|
|
| 0.0026 |
| 0.0266 |
|
Palliative first-line | 425 | 353 (83) | 2.1 | 22.9
(18.8–27.1) | - |
| - |
|
|
Other | 258 | 232 (90) | 1.6 | 16.5
(11.9–21.1) | 1.29
(1.09–1.52) |
| 1.21
(1.02–1.44) |
|
| Treatment
response |
|
|
|
|
| <0.0001 |
| <0.0001 |
|
Response | 357 | 285 (80) | 2.5 | 26.8
(22.1–31.6) | - |
| - |
|
| No
response | 338 | 315 (93) | 1.4 | 13.7
(9.9–17.4) | 1.78
(1.52–2.09) |
| 1.61
(1.36–1.91) |
|
| Any toxicity |
|
|
|
|
| 0.0481 |
|
|
| No | 52 | 46 (88) | 1.4 | 19.2
(8.2–30.3) | - |
|
|
|
|
Yes | 649 | 554 (85) | 1.9 | 20.8
(17.6–24.0) | 0.74
(0.55–1.00) |
|
|
|
| Cardiotoxicity |
|
|
|
|
| 0.0452 |
| 0.0027 |
| No | 686 | 588 (86) | 1.9 | 20.2
(17.1–23.3) | - |
| - |
|
|
Yes | 15 | 12 (80) | 4.7 | 40.0
(15.2–64.8) | 0.56
(0.32–0.99) |
| 0.45
(0.25–0.81) |
|
| Toxicity
severity |
|
|
|
|
| 0.2952 | 0.5331 |
|
|
None | 30 | 28 (93) | 1.6 | 10.0
(0.0–20.7) | - |
|
|
|
| Grade
1 | 407 | 347 (85) | 1.9 | 21.9
(17.8–26.0) | 0.76
(0.52–1.12) |
|
|
|
| Grade
≥2 | 212 | 178 (84) | 2.4 | 21.1
(15.3–26.8) | 0.73
(0.49–1.08) |
|
|
|
| CCI |
|
|
|
|
|
| 0.2031 |
|
| 0 | 312 | 261 (84) | 2 | 22.4
(17.6–27.2) | - |
|
|
|
| 1 | 190 | 166 (87) | 1.9 | 22.0
(16.0–28.0) | 1.10
(0.91–1.34) |
|
|
|
| 2+ | 199 | 173 (87) | 1.6 | 16.8
(11.4–22.2) | 1.19
(0.98–1.44) |
|
|
|
| Age at
diagnosis | 701 | 600 (86) | 1.9 | 20.7
(17.6–23.8) | 1.02
(1.01–1.02) | 0.0001 | 1.01
(1.00–1.02) | 0.0039 |
When focusing on EGFR inhibitors,
osimertinib-treated patients with comorbid heart disease had a
median survival time of 1.4 years, much shorter than those without
comorbidities (4.2 years) and with other comorbidities (2.5 years).
The survival curves showing the survival rate trends following
univariable analysis are shown in Fig.
4C-H. Compared with other comorbidities, the multivariable
analyses showed that cardiac comorbidities predicted a ~1.7-fold
risk of death when adjusted for age, treatment type, response and
cardiotoxicity status; however, the no comorbidity group did not
demonstrate significant preponderance (P=0.069) in multivariable
analysis. Patients treated with ALK/ROS1 inhibitors who
experienced cardiotoxicity had a significantly longer median
survival time (10.8 years) than those who did not experience
cardiotoxicity (2 years), although this was not an independent
prognostic factor in the multivariable analysis (P=0.058). For
patients treated with anti-VEGF therapy, comorbid heart
disease (P=0.737) and cardiotoxicity (P=0.466) showed no
associations with the survival length in the multivariable
analysis.
Discussion
The employment of targeted therapies has led to
paradigm advances in the management of NSCLC and new spectrums in
toxicities. Cardiotoxicity has emerged as a challenge with the
administration of targeted drugs. Data from the WHO
Pharmacovigilance database, VigiBase (https://who-umc.org/vigibase/vigibase-who-s-global-database/),
reported that 1.8% of all arrhythmias and 1.2% of all heart
failures are attributed to targeted drugs among all adverse
reactions for metastatic NSCLC. Additionally,
ALK/ROS1 inhibitors were associated with increased
odds of conduction disorders, while BRAF and EGFR
inhibitors were related to a prolonged QTc interval (27). However, due to the lack of exposure
data of comorbidities in VigiBase, the incidence of cardiotoxicity
and the prevalence of comorbid heart disease could not be
identified. The present study focused on the associations between
comorbid heart disease and targeted therapy-related cardiotoxicity,
along with their influence in clinical outcomes, with long-term
follow-ups among patients with advanced-stage NSCLC.
In the present study, it was found that 19.0% of
patients with stage-IV lung cancer had heart disease, which was
lower than the 32% reported for all-stage patients from a previous
study (5). The correlation between
cardiac disorders and the survival of patients with NSCLC were
investigated through the SEER-Medicare database, which showed
comorbid cardiac arrhythmias (28.6%), heart failure (17.5%) and
myocardial infarction (8.7%) were predictors of a worse survival
(6). In the present study, a
greater comorbidity burden in patients with cardiac disease (2.02)
at a higher CCI score than those with other comorbidities (1.14)
was found even when excluding comorbid conditions. Although it was
not surprising to find that targeted therapy-induced cardiotoxicity
had a greater association with cardiac comorbidity, the results
further validated that baseline cardiovascular disease may
contribute to anticancer agent-associated cardiotoxicity (28). However, the cardiotoxic occurrences
and consequences varied by the type of drugs used under different
comorbidity conditions, and it was noted that cardiotoxicity was
reported with targeted drugs that inhibited EGFR, ALK, ROS1
and VEGF/VEGFR.
EGFR inhibitors have been the standard of
care to treat NSCLC with EGFR-sensitive mutations in 10–16%
Western populations and 40–50% Asian populations who harbor
EGFR-sensitive mutations (29).
Osimertinib, the third-generation irreversible EGFR-TKI, has
been approved for the first-line treatment of advanced-stage NSCLC
based on the improved OS time observed compared with the comparator
in a clinical trial (38.6 vs. 31.8 months; P=0.046) (30), but is also the most related to the
cardiotoxicity risk (4.7–21.6%) profile of EGFR inhibitors
(31,32). Therefore, a subgroup analysis of
patients treated with osimertinib was performed in the present
study and a lower cardiotoxicity frequency (2.4%) was found,
reported as heart failure, prolonged QTc interval, decreased EF and
tachycardia (33). Severe
cardiotoxicity (grade ≥3) was found in 4.9% of (6/123) patients
after osimertinib administration. However, 1 of the 6 patients with
cardiac events had prior heart disease and 4 had cardiovascular
risk factors (hypertension and obesity) (34). These results validated coexisting
heart disease as an independent prognostic factor among patients
treated with osimertinib, predicting a higher risk of death.
Amivantamab, a novel EGFR-MET bispecific antibody
approved for EGFR exon 20 insertion mutations (35), was identified as the cause of
tachycardia in the single patient treated with this drug in the
present study, but this has not been reported in clinical trials
(36). The underlying mechanism of
EGFR-TKI-induced cardiotoxicity might be involved in PI3K signaling
pathway inhibition, ion channel blockade, oxidative stress,
inflammatory response and apoptosis (37).
ALK/ROS1 inhibitors are approved for NSCLC
with ALK and ROS1 fusions observed in 1–10% and
0.9–2.6% of patients, respectively (29). Data on cardiac disorders associated
with ALK inhibitors based on the Food and Drug
Administration Adverse Event Reporting System (FAERS) showed a
median onset time of 33 days and bradycardia as a common associated
event (38). The present study
found potential new cardiotoxicities of ceritinib-initiated
myocarditis and lorlatinib-initiated cardiomyopathy; however,
comorbidity data could not be mined from FAERS. In the present
study, cardiotoxicity was identified at an average of 4.3 months
and validated bradycardia comprised 71% of patients with
ALK/ROS1 inhibitor-induced cardiotoxicity. Prolonged QTc
interval and bradycardia occurred in the clinical setting with
crizotinib (39). There were 2
patients with crizotinib-related bradycardia, which has previously
been found to be related to an impaired autophagy process, causing
cardiomyocyte death and cardiac injury (40). Follow-up of 51 patients with
alectinib showed an incidence of 42% for bradycardia, but no
relationship between bradycardia and prior history of >1
cardiovascular risk factor (including hypertension, diabetes
mellitus, dyslipidemia, familial history and prior cardiovascular
events) was found (P=0.69) (41).
In the present study, 1 patient treated with alectinib experienced
bradycardia at an occurrence rate of 5% and had prior comorbid
sinus bradycardia and hypertension, which were identified as
independent risk factors of alectinib-induced bradycardia (42). Brigatinib-related cardiotoxicity has
scarcely been reported, with only bradycardia reported at 5–8% in
clinical trials (9,43). In the present study, a prolonged QTc
interval was observed in 1 patient treated with brigatinib (25%)
who had previously experienced crizotinib-associated
bradycardia.
Anti-VEGF therapies perform anticancer
efficacy by inhibiting angiogenesis in tumor development and
metastases, among which bevacizumab is the frequently used
anti-VEGF drug for NSCLC (44). Based on the role of VEGF in
the development and functional integrity of the vasculature and the
importance of the coronary artery, it is not surprisingly that the
VEGF antibody universally results in hypertension,
contributing to heart failure in 2–4% of patients receiving
bevacizumab and 3–8% patients receiving all anti-VEGF
therapies; additionally, cardiac ischemia is mechanistically
related to the use of anti-VEGF therapies (45). However, a much lower incidence
(0.9%) of cardiotoxicity among patients treated with bevacizumab
was found in the present study. All 3 patients with
anti-VEGF therapy-associated cardiotoxicity had concurrent
hypertension, supporting that hypertension is a risk factor of
cardiotoxicity (46). Unlike other
studies on cardiovascular toxicities, only cardiotoxicity was
analyzed in the present study and vascular disorders such as
hypertension and venous thrombus were not included, which might
lead to a lower observed incidence of bevacizumab-induced
cardiotoxicity.
Regarding the potential relationship between
cardiotoxicity occurrence and longer survival, in the present
study, the OS time of patients with cardiotoxicity was calculated
from the median cardiotoxicity onset date and compared with those
without cardiotoxicity from the treatment initiation date. Under
this conservative estimated survival, it was delineated that the
presence of cardiotoxicity predicted a longer 5-year survival in
patients treated with targeted drugs. Patients treated with
ALK/ROS1 inhibitors who experienced cardiotoxicity
had a longer median survival time (10.8 years) than those who did
not experience cardiotoxicity (2.0 years) in the multivariable
analysis, although the differences did not reach the threshold for
statistical significance due to limitations in the sample size.
Data from the PROFILE 1001 and PROFILE 1005 clinical trials was
previously analyzed to determine the association between decreased
heart rate and the clinical response to crizotinib. The results
indicated that patients with sinus bradycardia had a significantly
greater overall response rate (62.1 vs. 23.1%; P=0.02) and the
maximum tumor shrinkage (53.0 vs. 21%; P=0.021) compared with those
without (47). In another study,
follow-up of patients with crizotinib-induced asymptomatic sinus
bradycardia also showed excellent tolerance and potential
positivity for clinical response to treatment (48). These results implied cardiotoxicity,
specifically in the form of sinus bradycardia, may be associated
with a more favorable response to therapy and potentially a longer
survival time. However, in a previous study of alectinib, based on
administrative source of data, the results showed no significant
association between bradycardia and clinical efficacy (P=0.687)
(42). Unfortunately, the survival
analyses to evaluate cardiac comorbidity and cardiotoxicity were
unmet synthetically due to the lack of detailed information. The
results of the present study demonstrated that cardiotoxicity may
be a predictor of longer survival in patients treated with targeted
drugs, partly driven by a favorable survival in patients treated
with ALK/ROS1 inhibitors, which necessitates further
studies to validate our hypothesis and elucidate the underlying
mechanism. Additionally, the present study showed that patients
with heart disease were older than those with other comorbidities
or no comorbidity, and age was indeed an independent prognostic
factor in the multivariable analysis. However, age-stratified
analysis could not be performed due to the relatively small number
of events of interest. For instance, all the 15 patients with
cardiotoxicity were in the cardiac and other comorbidity groups,
and their age was relatively concentrated at the mean age of
64.2±13.5 years old. This is another important point for the future
effort.
The present study highlighted the importance of
considering the comorbid disease and risk factors that may
facilitate cardiotoxicity when patients with lung cancer are
administered targeted therapy. Furthermore, the benefit-risk
balance for cardiotoxicity should be individually recognized by the
type of targeted drugs and the severity of toxicity. However, the
findings of the present study remain in their infancy and future
research, including larger, prospective studies are required for
validation.
Although the present study provided a clinical
implication of cardiotoxicity among patients with advanced lung
cancer, limitations should be acknowledged. First, a limitation of
the study stems from its retrospective nature, which may produce
bias with toxicity identification and evaluation. For instance,
cardiotoxicity was identified through the monitoring of heart
condition during treatment and not via a protocol driven
assessment. Second, in a few patients who had no history of cardiac
disease or new cardiac symptoms and did not receive QTc interval
prolongation-related drugs, the risk of cardiotoxicity was likely
underestimated, which have may have produced potential selection
bias. Furthermore, the stratified analysis of cardiotoxicity in
different types of heart diseases was limited by the small sample
size of patients with cardiotoxicity in this single-institution
study. Prospective studies, combined with cardiac evaluation and
surveillance (such as blood pressure, ECG, left ventricular
function, biomarkers and heart medications), are required to
address cardiotoxic susceptibility prior to treatment and for the
appropriate management of cardiotoxicity.
In conclusion, cardiotoxicity was significantly more
prevalent in patients with comorbid heart diseases and was shown to
be a promising predictor of longer survival in patients with
stage-IV NSCLC treated with targeted drugs, indicating an
underlying implication of cardiotoxicity for clinicians. However,
the results were limited by the retrospective nature of the study
and sample size. Future preclinical and clinical studies are needed
to validate the findings, to identify and rank modifiable risk
factors and to investigate the biological and pharmacological
mechanisms of the observed cardiac effects of the targeted
drugs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Institutes of Health
(grant nos. R03 CA77118, R01 CA80127 and R01 CA84354), the National
Institute on Aging (grant nos. R01 AG034676 and R01 AG052425) and
the Mayo Clinic Foundation.
Availability of data and materials
The data generated in the present study are not
publicly available due to patient confidentiality but deidentified
or aggregated data may be requested from the corresponding
author.
Authors' contributions
PY, JAW and YP confirm the authenticity of all the
raw data. PY and YP contributed to conceptualization, design,
methodology and the original draft preparation. DL and JAW
contributed to data collection, visualization, investigation and
analysis using SAS software. YP and YHL contributed to supervision,
data interpretation and validation. AVK, ZG, NK, NYY and ZW
contributed to data collection and assembly. KL and VE contributed
to data interpretation, reviewing and editing. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All participants in the present study consented to
the Mayo Clinic Institutional Review Board approved protocol
(approval no. IRB-225-99) and signed the consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu S, Kato T, Dong X, Ahn MJ, Quang LV,
Soparattanapaisarn N, Inoue T, Wang CL, Huang M, Yang JC, et al:
Osimertinib after chemoradiotherapy in stage III EGFR-mutated
NSCLC. N Engl J Med. 391:585–597. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenneman CG and Sawyer DB:
Cardio-oncology: An update on cardiotoxicity of cancer-related
treatment. Circ Res. 118:1008–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curigliano G, Cardinale D, Dent S,
Criscitiello C, Aseyev O, Lenihan D and Cipolla CM: Cardiotoxicity
of anticancer treatments: Epidemiology, detection, and management.
CA Cancer J Clin. 66:309–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Batra A, Sheka D, Kong S and Cheung WY:
Impact of pre-existing cardiovascular disease on treatment patterns
and survival outcomes in patients with lung cancer. BMC Cancer.
20:10042020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivera DH, Gómez JM, de Bobadilla JCF,
Delgado D, Campo ER, Praena-Fernández JM, Caro RB, Gordillo MJ,
Fernández MC and Guerra JL: Cardiovascular disease and survival in
non-small cell lung cancer: A multicenter prospective assessment.
Clin Transl Oncol. 21:1220–1230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kravchenko J, Berry M, Arbeev K, Lyerly
HK, Yashin A and Akushevich I: Cardiovascular comorbidities and
survival of lung cancer patients: Medicare data based analysis.
Lung Cancer. 88:85–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan SHY, Khatib Y, Webley S, Layton D and
Salek S: Identification of cardiotoxicity related to non-small cell
lung cancer (NSCLC) treatments: A systematic review. Front
Pharmacol. 14:11379832023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dempke WC, Suto T and Reck M: Targeted
therapies for non-small cell lung cancer. Lung Cancer. 67:257–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo H, Zhang J, Qin C, Yan H, Liu T, Hu H,
Tang S, Tang S and Zhou H: Biomarker-targeted therapies in
non-small cell lung cancer: Current status and perspectives. Cells.
11:32002022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, Version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaborowska-Szmit M, Krzakowski M, Kowalski
DM and Szmit S: Cardiovascular complications of systemic therapy in
non-small-cell lung cancer. J Clin Med. 9:12682020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang P, Allen MS, Aubry MC, Wampfler JA,
Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J and Deschamps C:
Clinical features of 5,628 primary lung cancer patients: Experience
at mayo clinic from 1997 to 2003. Chest. 128:452–462. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maneenil K, Xue Z, Liu M, Boland J, Wu F,
Stoddard SM, Molina J and Yang P: Sarcomatoid carcinoma of the
lung: The mayo clinic experience in 127 patients. Clin Lung Cancer.
19:e323–e333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo YH, Luo L, Wampfler JA, Wang Y, Liu D,
Chen YM, Adjei AA, Midthun DE and Yang P: 5-year overall survival
in patients with lung cancer eligible or ineligible for screening
according to US preventive services task force criteria: A
prospective, observational cohort study. Lancet Oncol.
20:1098–1108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ernani V, Du L, Ross HJ, Yi JE, Wampfler
JA, Schild SE, Xie H, Swanson KL, Tazelaar HD and Yang P:
Gastroesophageal reflux disease and paraneoplastic neurological
syndrome associated with long-term survival in limited stage
small-cell lung cancer. Thorac Cancer. 13:925–933. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo YH, Liu H, Wampfler JA, Tazelaar HD,
Li Y, Peikert T, Liu D, Leventakos K, Chen YM, Yang Y, et al:
Real-world efficacy of osimertinib in previously EGFR-TKI treated
NSCLC patients without identification of T790M mutation. J Cancer
Res Clin Oncol. 148:2099–2114. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebbert JO, Williams BA, Sun Z, Aubry MC,
Wampfler JA, Garces YI, Meyer RL and Yang P: Duration of smoking
abstinence as a predictor for non-small-cell lung cancer survival
in women. Lung Cancer. 47:165–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shepherd FA, Crowley J, Van Houtte P,
Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw P;
International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions, :
The International Association for the Study of Lung Cancer lung
cancer staging project: proposals regarding the clinical staging of
small cell lung cancer in the forthcoming (seventh) edition of the
tumor, node, metastasis classification for lung cancer. J Thorac
Oncol. 2:1067–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Z, Aubry MC, Deschamps C, Marks RS,
Okuno SH, Williams BA, Sugimura H, Pankratz VS and Yang P:
Histologic grade is an independent prognostic factor for survival
in non-small cell lung cancer: An analysis of 5018 hospital- and
712 population-based cases. J Thorac Cardiovasc Surg.
131:1014–1020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Institutes of Health NCI, .
Common terminology criteria for adverse events (CTCAE). Version
5.0. 2017.
|
|
22
|
Rao VU, Reeves DJ, Chugh AR, O'Quinn R,
Fradley MG, Raghavendra M, Dent S, Barac A and Lenihan D: Clinical
approach to cardiovascular toxicity of oral antineoplastic agents:
JACC state-of-the-art review. J Am Coll Cardiol. 77:2693–2716.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Centers for Disease Control Prevention, .
International classification of diseases, tenth revision, clinical
modification (ICD-10-CM). Official Guidelines for Coding and
Reporting. 2015.
|
|
24
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Glasheen WP, Cordier T, Gumpina R, Haugh
G, Davis J and Renda A: Charlson comorbidity index: ICD-9 update
and ICD-10 translation. Am Health Drug Benefits. 12:188–197.
2019.PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waliany S, Zhu H, Wakelee H, Padda SK, Das
M, Ramchandran K, Myall NJ, Chen T, Witteles RM and Neal JW:
Pharmacovigilance analysis of cardiac toxicities associated with
targeted therapies for metastatic NSCLC. J Thorac Oncol.
16:2029–2039. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobat H, Elkonaissi I, Foreman E, Davidson
M, Idaikkadar P, O'Brien M and Nabhani-Gebara S: Smoking, diabetes
mellitus, and previous cardiovascular disease as predictors of
anticancer treatment-induced cardiotoxicity in non-small-cell lung
cancer: A real-world study. Clin Lung Cancer. 25:e35–e42. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fois SS, Paliogiannis P, Zinellu A, Fois
AG, Cossu A and Palmieri G: Molecular epidemiology of the main
druggable genetic alterations in non-small cell lung cancer. Int J
Mol Sci. 22:6122021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Deng X, Qiu Q and Wan M: Risk
factors of osimertinib-related cardiotoxicity in non-small cell
lung cancer. Front Oncol. 14:14310232024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bak M, Park H, Lee SH, Lee N, Ahn MJ, Ahn
JS, Jung HA, Park S, Cho J, Kim J, et al: The risk and
reversibility of osimertinib-related cardiotoxicity in a real-world
population. J Thorac Oncol. 10:S1556-0864(24)02377-3. 2024.
|
|
33
|
Chitturi KR, Burns EA, Muhsen IN, Anand K
and Trachtenberg BH: Cardiovascular risks with epidermal growth
factor receptor (EGFR) tyrosine kinase inhibitors and monoclonal
antibody therapy. Curr Oncol Rep. 24:475–491. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kunimasa K, Kamada R, Oka T, Oboshi M,
Kimura M, Inoue T, Tamiya M, Nishikawa T, Yasui T, Shioyama W, et
al: Cardiac adverse events in EGFR-mutated non-small cell lung
cancer treated with osimertinib. JACC CardioOncol. 2:1–10. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Syed YY: Amivantamab: First approval.
Drugs. 81:1349–1353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park K, Haura EB, Leighl NB, Mitchell P,
Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al:
Amivantamab in EGFR Exon 20 insertion-mutated non-small-cell lung
cancer progressing on platinum chemotherapy: Initial results from
the CHRYSALIS phase I study. J Clin Oncol. 39:3391–3402. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Lin Y, Lin S, Huang J and Ruan Z:
Advancements in understanding cardiotoxicity of EGFR- TKIs in
non-small cell lung cancer treatment and beyond. Front Pharmacol.
15:14046922024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Chen C, Rong C, He X and Chen L:
Anaplastic lymphoma kinase tyrosine kinase inhibitor-associated
cardiotoxicity: A recent five-year pharmacovigilance study. Front
Pharmacol. 13:8582792022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tartarone A, Gallucci G, Lazzari C, Lerose
R, Lombardi L and Aieta M: Crizotinib-induced cardiotoxicity: The
importance of a proactive monitoring and management. Future Oncol.
11:2043–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Z, Pan Z, Jin Y, Gao Z, Jiang F, Fu H,
Chen X, Zhang X, Yan H, Yang X, et al: Inhibition of PRKAA/AMPK
(Ser485/491) phosphorylation by crizotinib induces cardiotoxicity
via perturbing autophagosome-lysosome fusion. Autophagy.
20:416–436. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pruis MA, Veerman GDM, Hassing HC, Lanser
DAC, Paats MS, van Schaik RHN, Mathijssen RHJ, Manintveld O and
Dingemans AC: Cardiac toxicity of alectinib in patients with ALK+
lung cancer: Outcomes of cardio-oncology follow-up. JACC
CardioOncol. 5:102–113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan D, Zhu F, Zuo R, Wang Y, Huo G, Cui
J, Yue P and Chen RP: High incidence and reversible bradycardia
events following alectinib initiation. Thorac Cancer. 14:479–488.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han
JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee K, et al: Brigatinib
versus Crizotinib in ALK-Positive non-small-cell lung cancer. N
Engl J Med. 379:2027–2039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cabebe E and Wakelee H: Role of
anti-angiogenesis agents in treating NSCLC: Focus on bevacizumab
and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol.
8:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Touyz RM and Herrmann J: Cardiotoxicity
with vascular endothelial growth factor inhibitor therapy. NPJ
Precis Oncol. 2:132018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Lorenzo G, Autorino R, Bruni G, Cartenì
G, Ricevuto E, Tudini M, Ficorella C, Romano C, Aieta M, Giordano
A, et al: Cardiovascular toxicity following sunitinib therapy in
metastatic renal cell carcinoma: A multicenter analysis. Ann Oncol.
20:1535–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ou SH, Tong WP, Azada M, Siwak-Tapp C, Dy
J and Stiber JA: Heart rate decrease during crizotinib treatment
and potential correlation to clinical response. Cancer.
119:1969–1975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ou SH, Azada M, Dy J and Stiber JA:
Asymptomatic profound sinus bradycardia (heart rate ≤45) in
non-small cell lung cancer patients treated with crizotinib. J
Thorac Oncol. 6:2135–2137. 2011. View Article : Google Scholar : PubMed/NCBI
|