Introduction
Colorectal cancer was the third most commonly
diagnosed cancer worldwide in 2020 and the second most common cause
of cancer-associated death (1,2).
Although advances in diagnosis and surgical techniques have led to
improvement in clinical outcomes, survival rate of patients with
metastatic colorectal cancer remains poor (3). Median overall survival (OS) is 36
months, and a 5-year OS ≤20% (4).
For metastatic colorectal cancer, treatment options include
systemic chemotherapy combined with targeted therapy or
immunotherapy (4). Chemotherapy
remains the standard treatment for metastatic colorectal cancer and
5-fluorouracil (5-FU) is a key component (5). However, prolonged use of 5-FU results
in resistance and tumor recurrence; therefore, mechanisms
underlying 5-FU resistance should be elucidated and alternative or
adjunct therapies that sustain anticancer efficacy must be
developed.
Dysregulation of apoptotic signaling pathways and
evasion of apoptosis are strongly implicated in tumor progression
and chemoresistance (6,7). Cellular apoptosis is induced by an
extrinsic signaling pathway initiated by extracellular factors or
by an intrinsic pathway initiated by intracellular injury and
regulated by antiapoptotic and proapoptotic factors, including
Bcl-2 family proteins (8,9). Antiapoptotic Bcl-2 family proteins are
upregulated in numerous cancer cell types (including prostate,
colorectal, lung, gastric, renal cancer, neuroblastoma,
non-Hodgkin's lymphoma, and both acute and chronic leukaemia)
(10) and associated with tumor
progression or chemoresistance (11), suggesting they can serve as
therapeutic targets (12). Levels
of apoptosis are determined in part by the balance between
proapoptotic and antiapoptotic Bcl-2 family proteins, as these
antagonistic proteins can interact directly via various Bcl-2
homology (BH) protein motifs. BH3 motif acts as a strong cell death
induction signal, and Bcl-2 proteins containing only this motif,
such as BH3-interacting domain death agonist (BID) and Bcl-2
antagonist of cell death (BAD), promote apoptosis by directly
binding antiapoptotic Bcl-2 family protein. Therefore, BH3 motif
may be a useful target for modulating apoptosis signaling.
Recently, small-molecule BH3 mimetics that can
directly inhibit antiapoptotic Bcl-2 family proteins and induce
cancer cell apoptosis have been developed (13). These anticancer effects have been
studied primarily in hematological malignancy and the BH3 mimic
ABT-199 (Venetoclax) has received United States Food and Drug
Administration approval for treatment of primary and relapsed
chronic lymphocytic leukemia/small lymphocytic leukemia, acute
myeloma and several lymphoid malignancies (14). Several studies have reported that
the upregulation of antiapoptotic Bcl-xL and Myeloid cell leukemia
1 (Mcl-1) promote colorectal tumor progression and confer
chemoresistance (15–18), suggesting inhibition of these
antiapoptotic proteins using BH3 mimetics may be a promising
therapeutic strategy. To the best of our knowledge, however, only a
few studies have examined the anticancer effects of Bcl-xL- or
Mcl-1-specific BH3 mimetics on colorectal cancer cells (19–21).
Given the poor prognosis following emergence of chemotherapy
resistance in colorectal cancer, it is key to examine the effects
of Bcl-xL- or Mcl-1-specific BH3 mimetics on 5-FU-resistant (FUR)
colorectal cancer cells.
Wang et al (22) reported the development of A-1331852,
an orally available and highly Bcl-xL-specific BH3 mimetic with
notable efficacy in a xenograft colorectal cancer model. Our
previous study demonstrated that stromal interactions enhance the
expression of Bcl-xL and Mcl-1 in colorectal cancer cells (23), while inhibition of Bcl-xL and Mcl-1
by small interfering (si)RNA transfection or natural flavonoid
treatment effectively induces apoptosis and suppresses
proliferation of colorectal cancer and pancreatic cancer cells
(24,25). Based on these findings, it was
hypothesized that the Bcl-xL-specific BH3 mimetic A-1331852 may
exert suppressive effects on proliferation and survival of 5FUR
colorectal cancer cells.
Materials and methods
Cell culture
The human colorectal cancer cell line HCT116 (cat.
no. CCL-247) was obtained from the American Type Culture
Collection. HCT116 cell line is KRAS mutant; KRAS mutations are
considered a poor prognostic factor (26). HCT116 cells were maintained in DMEM
(FUJIFILM Wako Pure Chemical Corporation) supplemented with 10% FBS
and 1% penicillin-streptomycin solution (both Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2.
Isolation of 5FUR HCT116 (HCT116/5FUR)
colorectal cancer cells
HCT116/5FUR was isolated by continuous passage over
100 passages, starting at 0.2 µM and gradually increasing the
concentration of 5-FU in increments of 0.2–2.0 µM, as previously
reported (5,27). A subline with 270-fold 5-FU
resistance compared with the parental cells was isolated.
HCT116/5FUR cells were cultured in DMEM supplemented with 10% FBS
and 1% penicillin-streptomycin for 2 weeks as aforementioned to
eliminate the acute effects of 5-FU. The subline was authenticated
using short tandem repeat DNA analysis by Japanese Collection of
Research Bioresources Cell Bank.
Reagents
FU was purchased from FUJIFILM Wako Pure Chemical
Corporation; antibodies against Bcl-xL (cat. no. 54H6), Mcl-1 (cat.
no. D35A5), PARP (cat. no. 46D11), cleaved PARP (cat. no. D64E10)
and GAPDH (all 1:1,000, cat. no. 14C10) were purchased from Cell
Signaling Technology, Inc.; horseradish peroxidase (HRP)-conjugated
polyclonal goat antirabbit Ig (1:2,000, cat. no. P0448) was
purchased from Dako (Agilent Technologies, Inc.) and BH3 mimics
A-1331852 and S63845 were purchased from SelleckChem. For in
vitro experiments, 20 mM stock solutions of A-1331852 and
S63845 were prepared in DMSO and stored at −80°C until use.
Transfection of siRNA
Bcl-xL (cat. no. s1922; forward,
5′-GGAACUCUAUGGGAACAAUTT-3′ and reverse,
5′-AUUGUUCCCAUAGAGUUCCAC-3′), Mcl-1 (cat. no. s8583; forward,
5′-CCAGUAUACUUCUUAGAAATT-3′ and reverse,
5′-UUUCUAAGAAGUAUACUGGGA-3′) and negative control siRNA (all 5 nM,
cat. no. 4390843, Silencer Select Negative Control #1) were
purchased from Thermo Fisher Scientific, Inc. For proliferation
assays, cells were seeded on 96-well culture plates at
5×103/well and incubated overnight at 37°C without
antibiotics, followed by transfection. For western blotting and DNA
fragmentation assay, cells were seeded on 6-well culture plates at
a density of 5×105/well and incubated overnight at 37°C
without antibiotics before transfection. In both cases, cells were
transfected with siRNA (final concentration, 100 nM) for 24, 48 or
72 h at 37°C using Lipofectamine RNAiMAX in Opti-MEN medium (both
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Subsequent experiments were performed immediately
following transfection.
Cell proliferation assay
The effects of BH3 mimetics (A-1331852 and S63845)
and Bcl-2 protein knockdown (Bcl-xL or Mcl-1) on proliferation
rates of HCT116 and HCT116/5FUR cells were evaluated using the
Premix WST-1 Cell Proliferation Assay System (Takara Bio, Inc.)
according to the manufacturer's protocol. Cells were seeded in
96-well plates at a density of 5×103/well in 100 µl
complete DMEM (FUJIFILM Wako Pure Chemical Corporation) and allowed
to attach overnight at 37°C. 5FU, A-1331852 and S63845 were added
to each well for 24, 48, or 72 h at 37°C. The concentration of 5-FU
was 0–1,000 µM and the concentration of A-1331852 was 0–1,000 nM,
the concentration of S63845 was 0–10,000 nM. The medium was removed
and 10 µl Premix WST-1 was added to each well, followed by
incubation for 1 h and measurement of absorbance at 450 nm using a
microplate reader (SpectraMax ABC; Molecular Devices, LLC).
IC50 was calculated as follows: 10[log10/(A/B) ×
(50-D)/(C-D) + log10(B)], where A and B are high and low
concentration across 50% inhibition, respectively, and C and D are
inhibition (%) at high and low concentration, respectively.
Western blotting
Cells were harvested and lysate samples were
prepared in RIPA lysis buffer supplemented with Protease Inhibitor
Single Use Cocktail and Phosphatase Inhibitor Cocktail (all Thermo
Fisher Scientific, Inc.). Total lysate protein concentrations were
measured using BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Equal amounts of lysate protein were denatured by boiling at
90°C for 5 min, separated on 10% Mini-PROTEAN TGX gels (20 µg/lane)
and transferred to nitrocellulose membranes (both Bio-Rad
Laboratories, Inc.). Membranes were treated with primary antibodies
against Bcl-xL, Mcl-1, PARP, cleaved PARP and GAPDH and secondary
antibody (HRP-conjugated polyclonal goat antirabbit Ig) using iBind
Flex Western Device (Thermo Fisher Scientific, Inc.) and iBind Flex
Solution Kit (Thermo Fisher Scientific, Inc.) at room temperature
(4 h in total) following the manufacturer's protocol.
Protein-antibody complexes were visualized using SuperSignal West
Pico Chemiluminescent Substrate or SuperSignal West Femto
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Immunoreactive protein bands were detected using the Amersham
Imager 680 (GE Healthcare Life Sciences) and semi-quantified via
densitometry using ImageJ version 1.54 (National Institutes of
Health).
DNA fragmentation assay
Cells were seeded in 6-well culture plates and
incubated at 37°C overnight. DMEM was replenished and cells were
treated with 0, 1, 10, 100 nM of A-1331852 or transfected with
Bcl-xL siRNA or Mcl-1 siRNA for 48 h as aforementioned. DNA
fragmentation was analyzed using Cell Death Detection
ELISAPLUS (Roche Diagnostics) following the
manufacturer's protocol. The rate of apoptosis is presented as
fold-change relative to vehicle-treated or negative control
siRNA-treated cells.
Animal experiments
All experiments were approved by the Animal Care and
Use Committee of the Nagoya City University of Medical Science
(approval no. IDO 23-049; Nagoya, Japan). A total of 10 male BALB/c
nu-nu mice (age, 4 weeks; mean weight, 23.1 g) were purchased from
Japan SLC and housed in standard Plexiglas cages at room
temperature (20–26°C) and humidity (40–60%) under 12/12-h
light/dark cycle with ad libitum access to autoclaved chow
and water. HCT116/5FUR cells were suspended at a density of
5×106 cells in 200 µl PBS and injected subcutaneously
into the right flank of each mouse. When average tumor volume
surpassed ~100 mm3, mice were divided into A-1331852 and
vehicle treatment groups and administered A-1331852 (25 mg/kg in 5%
DMSO, 40% Polyethylene glycol 300, 5% Tween-80 and 50%
ddH2O) or vehicle (5% DMSO, 40% Polyethylene glycol 300,
5% Tween-80 and 50% ddH2O) twice daily through oral
gavage, respectively. The dosage of A-1331852 (25 mg/kg) was based
on previous study (28). Tumor
volume was calculated as follows: Longest tumor diameter × shortest
tumor diameter2/2. The treatment duration was initially
set as 21 consecutive days. Humane endpoints were defined as total
tumor volume >10% of body weight, tumor diameter >20 mm,
weight loss >20%, tumor ulceration, necrosis, gait disturbance
and impaired water and food intake. All mice were euthanized on day
18 due to excessive weight loss via cervical dislocation under
2.0–2.5% isoflurane inhalation anesthesia and tumors were harvested
for analysis.
Immunohistochemistry
Tumors were fixed with 4% paraformaldehyde for 6 h
at 4°C, embedded in paraffin, cut into 3-µm-thick sections, and
mounted on 3-aminopropyltriethoxylsilane-coated slides. Sections
were stained with hematoxylin and eosin. Automated
immunohistochemistry was performed using the Bond RXm system (Leica
Biosystems, Ltd., Newcastle, UK). The Compact Polymer detection
system used BOND Polymer Refine Detection (cat no. DS9800, Leica
Biosystems, Newcastle Upon Tyne, UK), which contains blocking
reagent, polymer reagent (secondary antibody), DAB chromogen and
hematoxylin. Primary antibodies were as follows: Bcl-xL (cat no.
54H6; 1:1,500), cleaved PARP (cat no. D64E10; 1:50; both Cell
Signaling Technology, Inc.) and Ki-67 antibody (clone no. SP6; cat.
no. 418071; 1:2; Nichirei Biosciences, Inc). All steps were
performed according to manufacturer's protocol. Deparaffinization
was performed using Bond Dewax Solution (cat no. AR9222, Leica
Biosystems, Newcastle Upon Tyne, UK) at 72°C, followed by Alcohol
and Bond Wash solution (cat no. AR9590, Leica Biosystems, Newcastle
Upon Tyne, UK). Antigen retrieval was performed using Bond Epitope
Retrieval Solution 1 (cat no. AR9961, Leica Biosystems, Newcastle
Upon Tyne, UK) for 20 min at 100°C. Blocking was performed with
peroxide block reagent for 5 min at ambient temperature. The
primary antibody reaction was performed for 15 min at ambient
temperature. Secondary antibody reaction was performed with Polymer
reagent for 8 min at ambient temperature. Color development was
performed with DAB chromogen for 10 min at ambient temperature.
Hematoxylin was used for counterstaining for 5 min at ambient
temperature. Images were captured using a fluorescence microscope
(BZ-X710; Keyence Corporation, Osaka, Japan). A total of 10
high-magnification (×200) fields of view were acquired for each
tumor to calculate the mean proportion of cleaved PARP- and
Ki-67-positive cells using hybrid cell count software (BZ-X
Analyzer software version 1.4.0.1; Keyence Corporation).
Statistical analysis
Statistical analyses were performed using EZR
software (Easy R) version 4.2.2 (Saitama Medical Center, Jichi
Medical University, Saitama, Japan). In vitro experiments
were performed at least three times. Data are presented as the mean
± standard deviation. Treatment group means were compared using
unpaired Student's t test or one-way ANOVA followed by Dunnett's
post hoc test. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
Emergence of 5-FU resistance is
associated with overexpression of the antiapoptotic protein
Bcl-xL
Long-term culture of HCT116 cells in 5-FU yielded
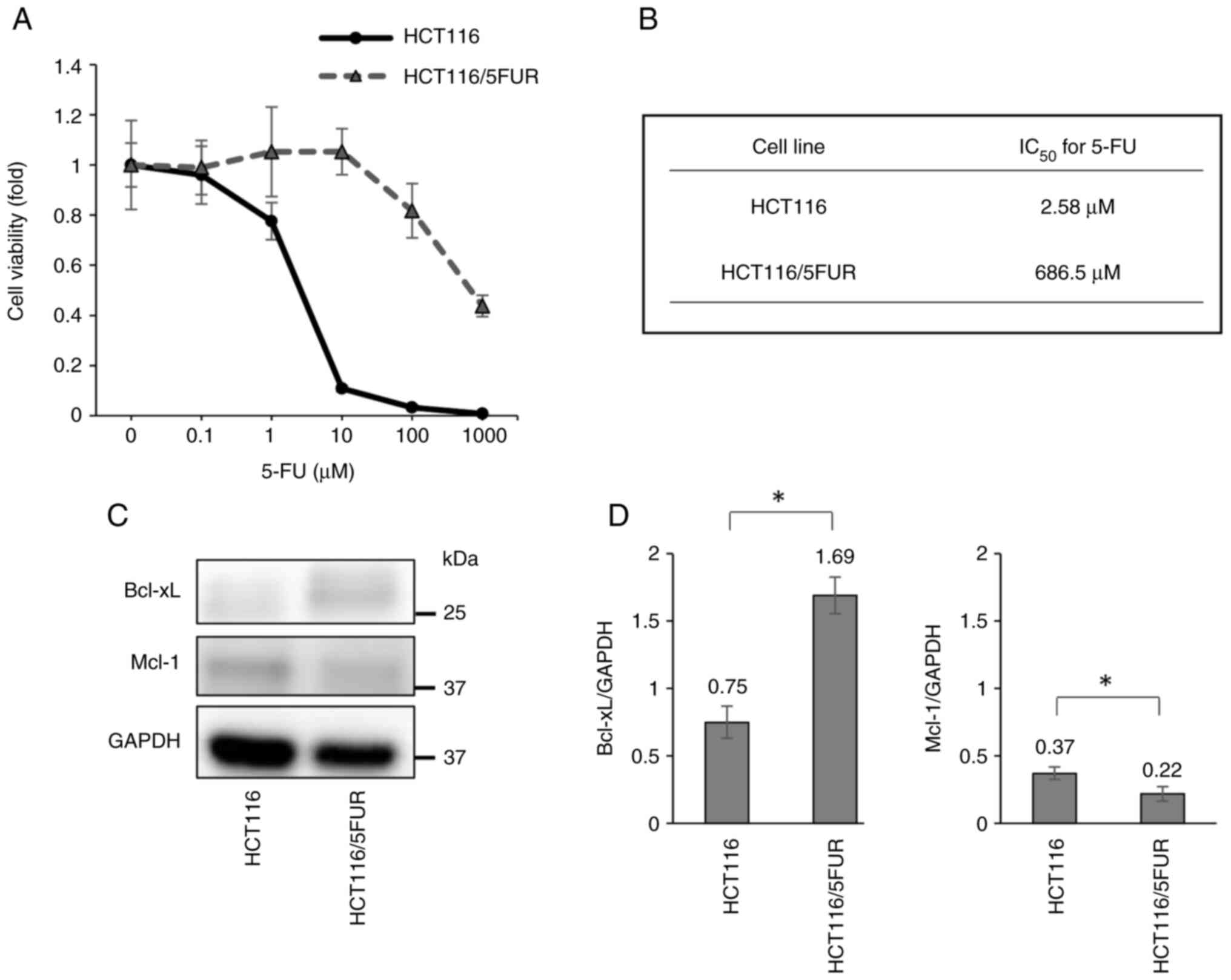
HCT116/5FUR with an IC50 270-fold higher than that of
the parental line (Fig. 1A and B).
To investigate the mechanisms underlying acquired 5-FU resistance,
the present study first compared the expression levels of
antiapoptotic Bcl-2 family proteins Bcl-xL and Mcl-1 via western
blotting and found significantly elevated Bcl-xL and significantly
decreased Mcl-1 expression in HCT116/5FUR line compared with the
parental line (Fig. 1C and 1D).
This result suggested that Bcl-xL upregulation is a contributing
factor to 5-FU resistance.
Inhibition of Bcl-xL via siRNA
transfection suppresses the proliferation of 5FUR colorectal cancer
cells
The present study examined the contribution of
Bcl-xL overexpression to the pro-malignancy characteristics of
colorectal cancer cells, as 5-FU resistance is a key determinant of
poor clinical outcome (29).
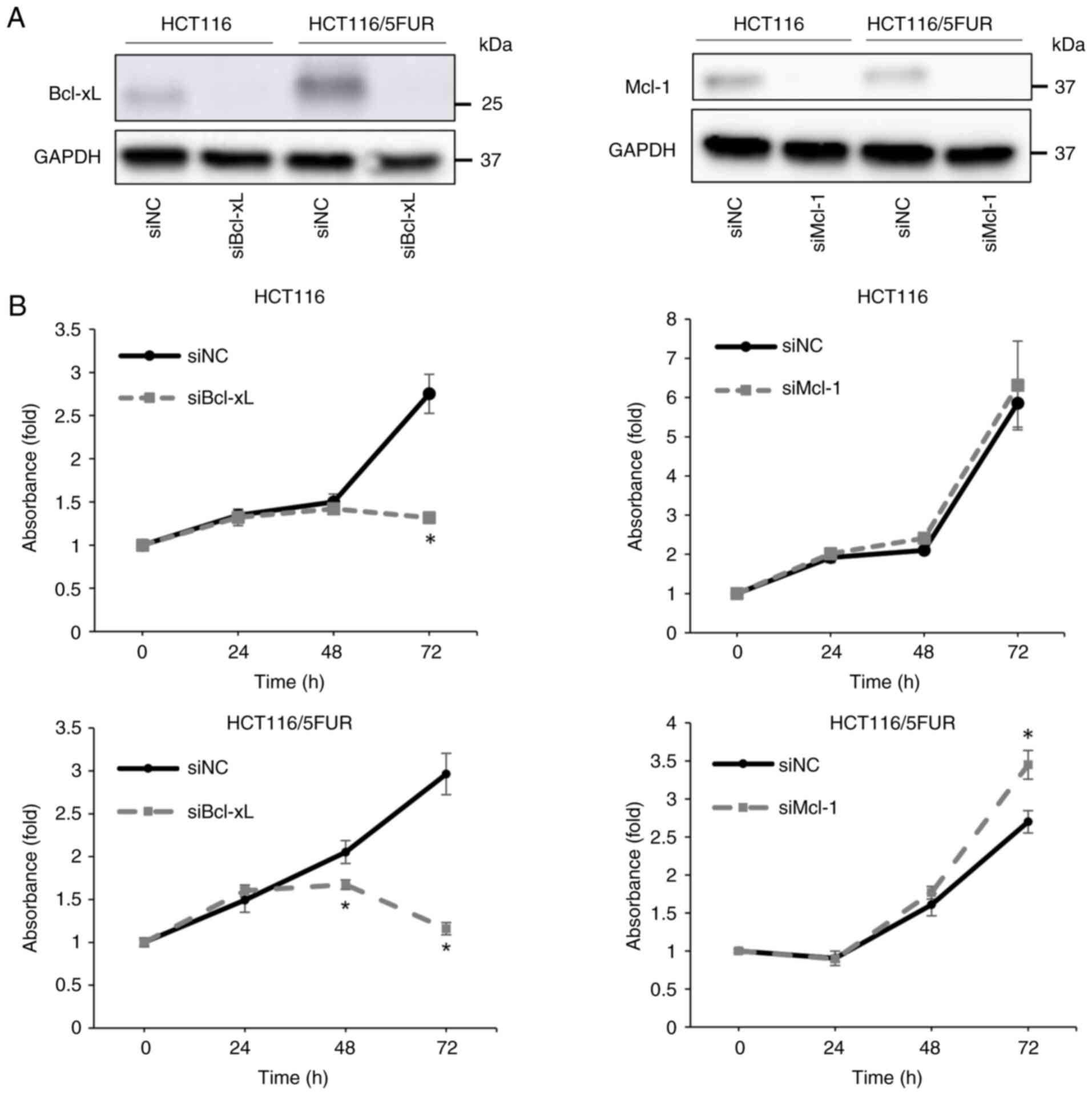
Transfection of HCT116 and HCT116/5FU cells with siRNAs targeting
Bcl-xL or Mcl-1 decreased expression of the corresponding protein
(Fig. 2A). In addition, knockdown
of Bcl-xL significantly inhibited proliferation of HCT116/5FUR and
parental cells. This antiproliferative effect was significantly
greater in the HCT116/5FUR subline than in the parental line after
48 h transfection (Fig. 2B).
Knockdown of Mcl-1 significantly increased proliferation of
HCT116/5FUR cells at 72 h (Fig.
2B).
Bcl-xL knockdown enhances apoptosis
rate of 5FUR colorectal cancer cells
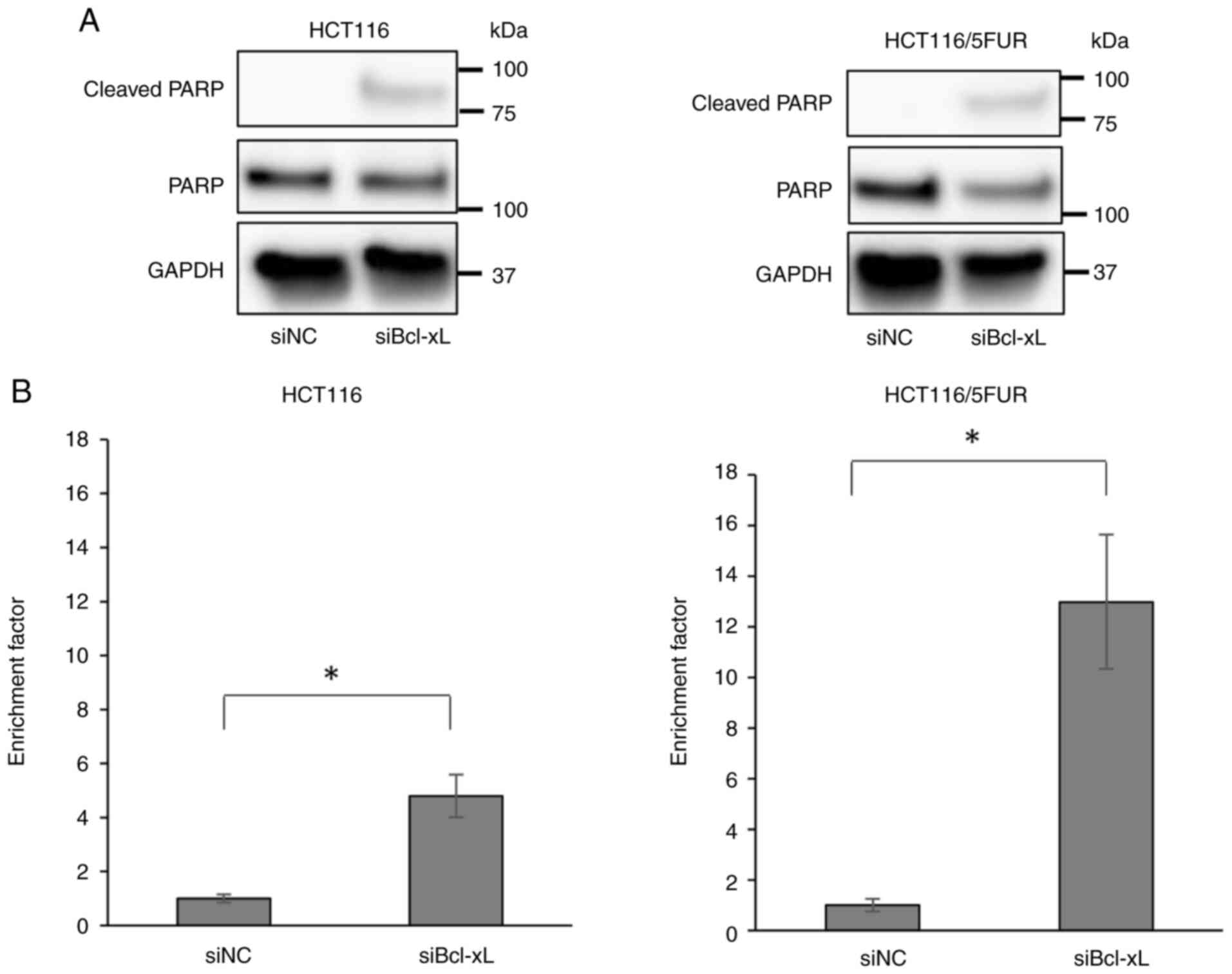
The present study investigated the effect of Bcl-xL
knockdown on apoptosis of 5FUR and parental colorectal cancer cells
using western blot and DNA fragmentation assays. Bcl-xL knockdown
induced apoptosis (Fig. 3A and B).
The effect was greater on HCT116/5FUR than on parental cells, as
revealed via DNA fragmentation assay. This suggested that Bcl-xL
serves an important role in the regulation of apoptosis in both
5FUR and parental colorectal cancer cells.
Bcl-xL-specific BH3 mimetic A-1331852
suppresses proliferation of 5FUR colorectal cancer cells via
induction of apoptosis
As knockdown of Bcl-xL suppresses the proliferation
of HCT116/5FUR cells and induces apoptosis, the present study
evaluated the antiproliferative and proapoptotic effects of the
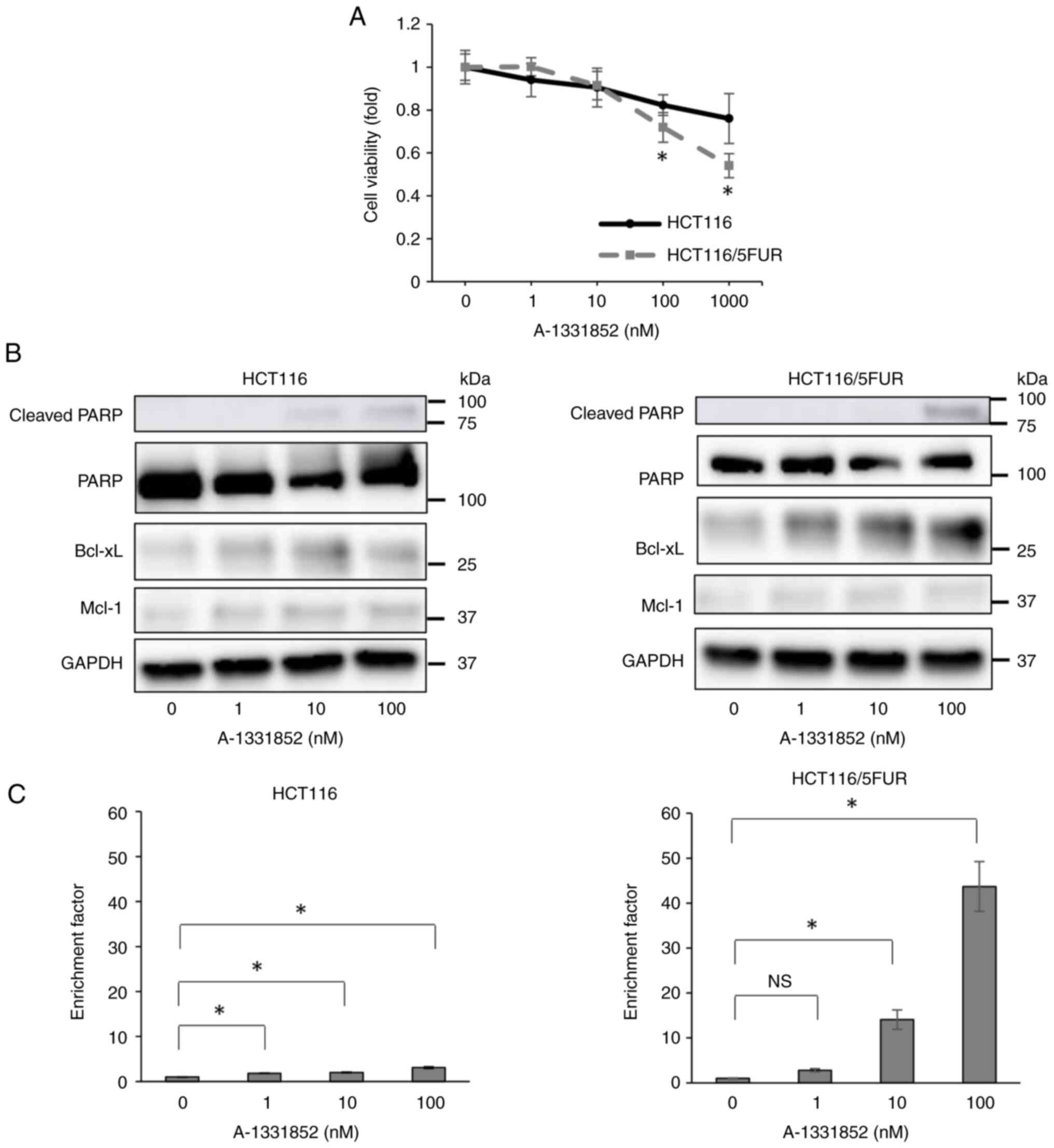
Bcl-xL-specific BH3 mimetic A-1331852 (22). Treatment of HCT116/5FUR and parental
HCT116 cells with A-1331852 for 72 h significantly decreased the
viability of both lines, as evaluated using WST-1 assay (Fig. 4A); this effect was stronger on
HCT116/5FUR than on parental cells. Consistent with Mcl-1
siRNA-mediated knockdown experiments, S63845 did not suppress the
viability of either cell line (Fig.
S1). Moreover, A-1331852 dose-dependently induced apoptosis of
both cell lines and DNA fragmentation assay indicated that the
proapoptotic effect was stronger on HCT116/5FUR than on parental
cells (Fig. 4B and C). Western blot
analysis revealed A-1331852 enhanced Bcl-xL expression in both cell
lines (Fig. 4B). Therefore,
inhibition of Bcl-xL by a specific small-molecule BH3 mimetic
suppressed the proliferation of HCT116/5FUR cells and concomitantly
enhanced apoptosis rate, suggesting that pharmacological Bcl-xL
inhibition may be an effective strategy to prevent 5FUR colorectal
tumor progression.
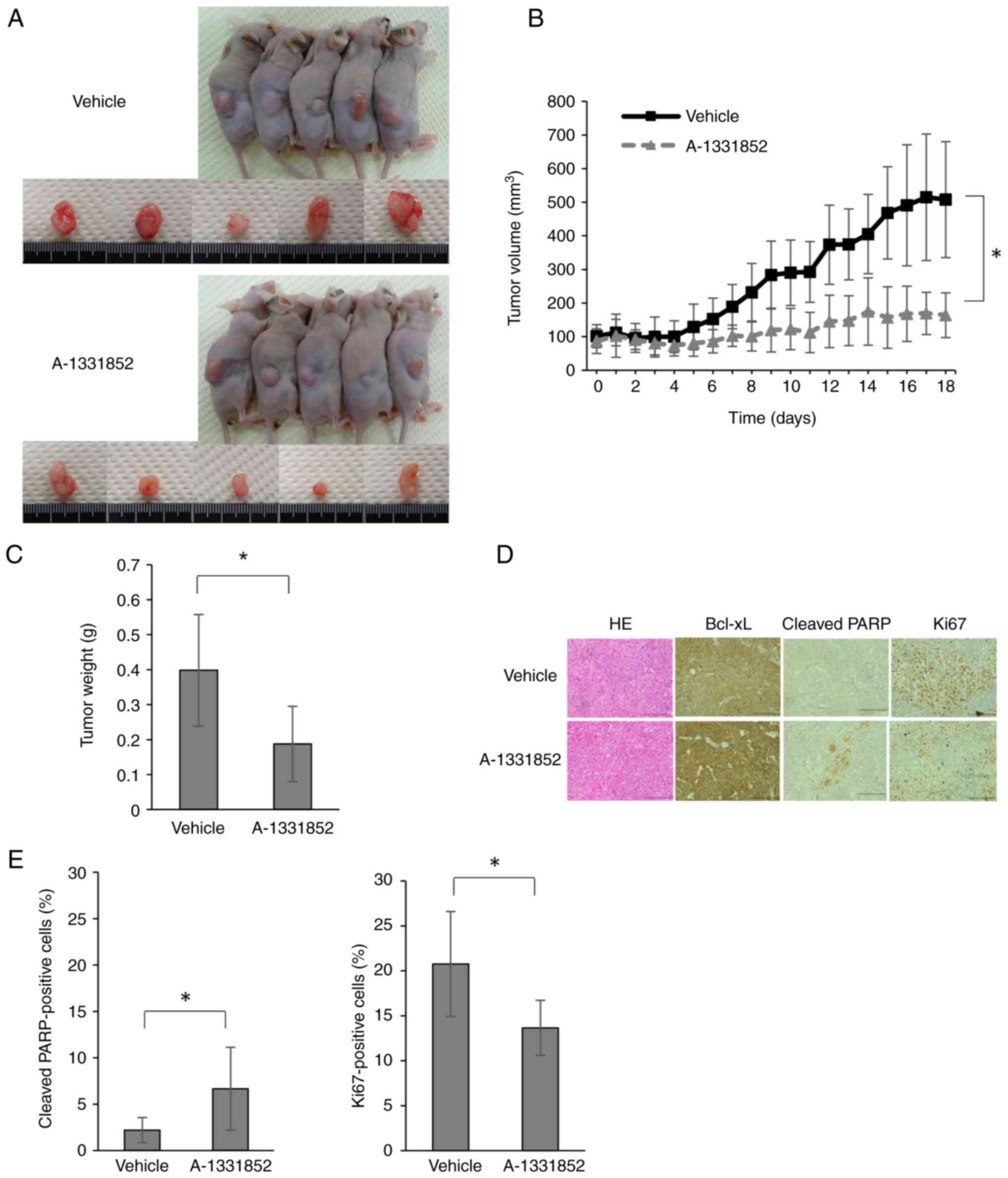
Oral A-1331852 suppresses the growth
of 5FUR colorectal tumors in vivo
To examine if A-1331852 suppresses the growth of
5FUR colorectal tumors in vivo, the present study
established a xenograft mouse model by inoculating HCT116/5FUR
cells. While there was no significant difference in weight loss
between the vehicle and treated groups by day 18 (Fig. S2), tumors were significantly
smaller in the A-1331852 group than those in the vehicle group
(Fig. 5A-C). Moreover, consistent
with cell culture studies, immunohistochemistry for the apoptosis
marker cleaved PARP revealed a significantly increased proportion
of positively stained cells in tumors from mice receiving
A-1331852. Furthermore, expression of Ki-67 was decreased in the
A-1331852 group (Fig. 5D and E).
These results confirmed that the orally available Bcl-xL-specific
BH3 mimetic A-1331852 suppressed the growth of 5FUR colorectal
tumors in mice by promoting apoptosis.
Discussion
The emergence of 5-FU resistance in colorectal
cancer cells was associated with overexpression of antiapoptotic
protein Bcl-xL; inhibition of Bcl-xL activity by either
siRNA-mediated knockdown or Bcl-xL-specific BH3 mimetic (A-1331852)
decreased proliferation of colorectal cancer cells and enhanced
apoptosis. Overall, these results indicated that Bcl-xL
upregulation is an important mechanism conferring resistance to
5-FU and small-molecule Bcl-xL inhibitors may be effective for the
treatment of colorectal cancer with 5-FU resistance. A-1331852
significantly suppressed the growth of tumors derived from 5FUR
colorectal cancer cells in xenograft model mice by inducing
apoptosis.
Multiple processes may contribute to the emergence
of 5-FU resistance in colorectal cancer cells, such as upregulation
of Bcl-xL. Evasion of apoptosis is a key mechanism that allows
cancer cells to survive in the hostile tumor microenvironment and
following treatment with cytotoxic chemotherapeutic agents, such as
5-FU (3). The intrinsic apoptosis
pathway is regulated by interactions between multiple pro- and
antiapoptotic Bcl-2 family proteins and overexpression of
antiapoptotic Bcl-2 family proteins promotes tumor survival and
chemoresistance (30,31). Thus, antiapoptotic Bcl-2 family
proteins may be effective therapeutic targets for cancer treatment.
Bcl-2 is upregulated in adenoma (32), while downregulation of Bcl-xL and
Mcl-1 decrease the chemoresistance of colorectal cancer cells
(15,17). In a previous study examining the
function of antiapoptotic Bcl-2 family proteins in pancreatic
cancer cells, Bcl-xL and Mcl-1, but not Bcl-2, were found to serve
an important role in the regulation of apoptosis (24). Colorectal cancer cells are dependent
on Bcl-xL for survival (19).
Moreover, Bcl-xL is overexpressed in human colorectal cancer
specimens (33) and its
overexpression is associated with poor prognosis (34). These findings suggest Bcl-xL as a
potential therapeutic target for colorectal cancer and the present
study suggested that Bcl-xL overexpression contributes to 5-FU
resistance and that suppression of Bcl-xL can impede progression of
5FUR tumors. The present study did not explore the precise
mechanism of Bcl-xL upregulation in acquiring 5-FU resistance.
Activation of the NF-κB/STAT3 signaling pathway is a key mechanism
for 5-FU resistance and promotes antiapoptotic proteins (35); however, the upstream signaling
pathways that regulate Bcl-xL overexpression in 5FUR cancer cells
remain unknown. Our previous study demonstrated that IL-6
upregulates expression of Bcl-xL and Mcl-1 in colorectal cancer
cells via phosphorylation of STAT3 (25) and interaction of cancer cells and
cancer-associated fibroblasts enhance expression of Bcl-xL and
Mcl-1 via the IL-6/JAK/STAT3 pathway (23); therefore, it was hypothesized that
the IL-6/STAT3 signal pathway may also contribute to 5-FU
resistance. Although the present study was conducted using only
cancer cells and the upstream mechanism of overexpression of Bcl-xL
was not assessed, future studies should investigate the role of the
STAT3 pathway.
Numerous BH3 mimetics have been developed to inhibit
antiapoptotic Bcl-2 family proteins and prevent evasion of
apoptosis by cancer cells under chemotherapy. The first BH3 mimetic
developed, ABT-737, was reported to inhibit BCL-2, Bcl-xL and Bcl-W
(32) and its orally available
analog navitoclax has demonstrated anticancer efficacy against
hematological and solid malignancy both as monotherapy and in
combination with chemotherapy. Overexpression of Bcl-xL is
implicated in the survival and chemoresistance of solid tumors,
including colorectal cancer (13,36);
therefore, Bcl-xL-specific BH3 mimetics may be particularly
effective for colorectal cancer treatment. To the best of our
knowledge, however, only a few studies have directly examined the
efficacy of Bcl-xL-specific BH3 mimetics on colorectal cancer
cells, including 5FUR colorectal cancer cells (19,21).
A-1331852 is the most recently developed Bcl-xL-specific BH3
mimetic (22). Greaves et al
(20) reported that A-1331852
induces apoptosis of colorectal cancer cells in vitro and
Leverson et al (28)
reported that A-1331852 inhibits tumor growth in a xenograft mouse
model established by inoculating colorectal cancer cells. Here,
A-1331852 was also effective against 5FUR tumors. Antiproliferative
and proapoptotic effects of A-1331852 were stronger against 5FUR
than parental cells. To the best of our knowledge, the present
study is the first to report the efficacy of A-1331852 against 5FUR
colorectal cancer in a xenograft mouse model. Bcl-xL serves an
important role in platelet life span and inhibition of Bcl-xL is
reported to be a cause of thrombocytopenia (32). Furthermore, A-1331852 has been
reported to decrease platelet count in rats (28). Therefore, it may be necessary to
decrease the dose of A-1331852 by combination with other
therapeutic agents. Future studies should assess platelet count
following A-1331852 treatment. Inhibition of Mcl-1 by the specific
BH3 mimetic S63845 did not exert an antiproliferative effect on
5FUR and parental cells. Although inhibition of Mcl-1 by siRNA
slightly increased proliferation of 5FUR cells, inhibition of Mcl-1
alone had no inhibitory effect on the proliferation of 5FUR and
parental colorectal cancer cells. However, based on our previous
report that simultaneous inhibition of Bcl-xL and Mcl-1 induces
strong apoptosis in pancreatic (24) and colorectal cancer cells (25), Mcl-1 may serve a key role in the
regulation of apoptosis in cancer cells in concert with Bcl-xL. To
the best of our knowledge, the interaction between Bcl-xL and Mcl-1
during acquisition of 5-FU resistance in colorectal cancer cells
has not been examined and should be assessed in future.
The stronger anticancer effect of A-1331852 against
5FUR colorectal cancer compared with parental cells may result from
a greater dependence on Bcl-xL for survival, in accordance with a
previous study reporting that 5FUR colorectal cancer cells are more
sensitive to siRNA-mediated Bcl-xL knockdown than parental cells
(30). However, the mechanisms by
which 5FUR colorectal cancer cells depend on Bcl-xL for survival
remain unknown. Furthermore, expression of antiapoptotic Bcl-2
proteins do not predict the efficacy of BH3 mimetics (21,37),
suggesting that multiple mechanisms may be involved under different
conditions or in distinct cancer cell types. Colorectal cancer is a
heterogeneous disease, therefore the mechanisms of 5-FU resistance
may differ among cell lines and tumor cell populations. Further
investigation is needed to elucidate mechanisms underlying the
efficacy of BH3 mimetics for apoptosis-mediated destruction of 5FUR
colorectal cancer cells. It is also key to consider that colorectal
cancer cells may acquire resistance to A-1331852. Resistance to BH3
mimetics has been reported in hematological malignancy (38), although the exact mechanism remains
unknown. Elevated levels of antiapoptotic Bcl-2 family proteins,
including Bcl-xL and Mcl-1, are a mechanism for resistance to BH3
mimetics in hematological malignancy (39). In the present study, A-1331852
enhanced Bcl-xL expression in both 5FUR and parental cells, which
may contribute to resistance with long-term use.
The present study had limitations. All experiments
were performed on a single 5FUR cell population. Given the
aforementioned heterogeneity of colorectal cancer, it is necessary
to establish multiple 5FUR cell lines and evaluate the contribution
of Bcl-xL overexpression to identify other potential resistance
mechanisms. In addition, the animal experiments had a small number
of samples. Furthermore, although the present study focused on the
role of Bcl-xL in 5FUR colorectal cancer cells, abnormal apoptosis
is only one factor in the acquisition of 5-FU resistance in
colorectal cancer cells. There are various mechanism of 5-FU
resistance, including the alterations in drug transport, cell
cycle, DNA-damage repair machinery, regulation of autophagy,
epithelial-to-mesenchymal transition, cancer stem cell involvement,
tumor microenvironment interactions, miRNA dysregulations,
epigenetic alterations, redox imbalances (3).
In conclusion, HCT116/5FUR cells exhibited
upregulation of Bcl-xL and Bcl-xL-specific BH3 mimetic A-1331852
suppressed proliferation and promote apoptosis in vitro and
in vivo. Inhibition of Bcl-xL using specific BH3 mimetics
may be an effective treatment strategy for 5FUR colorectal
cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms Seiko Inumaru and
Ms. Miyuki Inoue (Department of Gastroenterological Surgery, Nagoya
City University Graduate School of Medical Sciences, Nagoya, Japan)
for preparing experimental reagents and tumor samples.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the article.
Authors' contributions
AK and HT conceived and designed the study, analyzed
and interpreted data and wrote the manuscript. HA, SU, SH, YF, KW,
TY, TS, HU, KS, YY, RO, AM, YM and ST designed the study. AK, HT,
SU, SH, KW, TS, and KS performed experiments. AK, HT, HA, SU, SH,
YF, KW, TY, TS, HU, KS, YY and YM confirm the authenticity of all
raw data. HT, AM, YM and ST supervised the study. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
In vivo mouse experiments were approved by
the Animal Care and Use Committee of the Nagoya City University
Graduate School of Medical Sciences (approval no. 23-049; Nagoya,
Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azwar S, Seow HF, Abdullah M, Faisal Jabar
M and Mohtarrudin N: Recent updates on mechanisms of resistance to
5-fluorouracil and reversal strategies in colon cancer treatment.
Biology (Basel). 10:8542021.PubMed/NCBI
|
|
4
|
Ríos-Hoyo A, Monzonís X, Vidal J, Linares
J and Montagut C: Unveiling acquired resistance to anti-EGFR
therapies in colorectal cancer: A long and winding road. Front
Pharmacol. 15:13984192024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suetsugu T, Mori R, Futamura M, Fukada M,
Tanaka H, Yasufuku I, Sato Y, Iwata Y, Imai T, Imai H, et al:
Mechanism of acquired 5FU resistance and strategy for overcoming
5FU resistance focusing on 5FU metabolism in colon cancer cell
lines. Oncol Rep. 45:272021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong RSY: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:2018. View Article : Google Scholar
|
|
13
|
Diepstraten ST, Anderson MA, Czabotar PE,
Lessene G, Strasser A and Kelly GL: The manipulation of apoptosis
for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer.
22:45–64. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lew TE and Seymour JF: Clinical
experiences with venetoclax and other pro-apoptotic agents in
lymphoid malignancies: Lessons from monotherapy and chemotherapy
combination. J Hematol Oncol. 15:752022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nita ME, Ono-Nita SK, Tsuno N, Tominaga O,
Takenoue T, Sunami E, Kitayama J, Nakamura Y and Nagawa H: Bcl-X(L)
antisense sensitizes human colon cancer cell line to
5-fluorouracil. Jpn J Cancer Res. 91:825–832. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim
MY, Oak CY, Chung CY, Park HC, Kim JS, et al: Myeloid cell
leukemia-1 is associated with tumor progression by inhibiting
apoptosis and enhancing angiogenesis in colorectal cancer. Am J
Cancer Res. 5:101–113. 2015.PubMed/NCBI
|
|
17
|
Luo MJ, Palmieri M, Riffkin CD,
Sakthianandeswaren A, Djajawi TM, Hirokawa Y, Shuttleworth V, Segal
DH, White CA, Nhu D, et al: Defining the susceptibility of
colorectal cancers to BH3-mimetic compounds. Cell Death Dis.
11:7352020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Xue J, Hessler P, Tahir SK, Chen
J, Jin S, Souers AJ, Leverson JD and Lam LT: Genomic analysis and
selective small molecule inhibition identifies BCL-X(L) as a
critical survival factor in a subset of colorectal cancer. Mol
Cancer. 14:1262015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scherr AL, Mock A, Gdynia G, Schmitt N,
Heilig CE, Korell F, Rhadakrishnan P, Hoffmeister P, Metzeler KH,
Schulze-Osthoff K, et al: Identification of BCL-XL as highly active
survival factor and promising therapeutic target in colorectal
cancer. Cell Death Dis. 11:8752020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greaves G, Milani M, Butterworth M, Carter
RJ, Byrne DP, Eyers PA, Luo X, Cohen GM and Varadarajan S: BH3-only
proteins are dispensable for apoptosis induced by pharmacological
inhibition of both MCL-1 and BCL-XL. Cell Death Differ.
26:1037–1047. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishikawa K, Kawano Y, Arihara Y, Kubo T,
Takada K, Murase K, Miyanishi K, Kobune M and Kato J: BH3 profiling
discriminates the anti-apoptotic status of 5-fluorouracil-resistant
colon cancer cells. Oncol Rep. 42:2416–2425. 2019.PubMed/NCBI
|
|
22
|
Wang L, Doherty GA, Judd AS, Tao ZF,
Hansen TM, Frey RR, Song X, Bruncko M, Kunzer AR, Wang X, et al:
Discovery of A-1331852, a first-in-class, potent, and
orally-bioavailable BCL-XL inhibitor. ACS Med Chem Lett.
11:1829–1836. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maeda A, Takahashi H, Harata S, Watanabe
K, Yanagita T, Suzuki T, Ushigome H, Nakai N, Maeda Y, Hirokawa T,
et al: The interaction between cancer-associated fibroblasts and
cancer cells enhances Bcl-xL and Mcl-1 in colorectal cancer.
Anticancer Res. 42:1277–1288. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi H, Chen MC, Pham H, Matsuo Y,
Ishiguro H, Reber HA, Takeyama H, Hines OJ and Eibl G: Simultaneous
knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax
activation in pancreatic cancer cells. Biochim Biophys Acta.
1833:2980–2987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda Y, Takahashi H, Nakai N, Yanagita T,
Ando N, Okubo T, Saito K, Shiga K, Hirokawa T, Hara M, et al:
Apigenin induces apoptosis by suppressing Bcl-xL and Mcl-1
simultaneously via signal transducer and activator of transcription
3 signaling in colon cancer. Int J Oncol. 52:1661–1673.
2018.PubMed/NCBI
|
|
26
|
Sitthisuk P, Innajak S, Poorahong W,
Samosorn S, Dolsophon K and Watanapokasin R: Effect of Acacia
concinna extract on apoptosis induction associated with
endoplasmic reticulum stress and modulated intracellular signaling
pathway in human colon HCT116 cancer cells. Nutrients. 16:37642024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
28
|
Leverson JD, Phillips DC, Mitten MJ,
Boghaert ER, Diaz D, Tahir SK, Belmont LD, Nimmer P, Xiao Y, Ma XM,
et al: Exploiting selective BCL-2 family inhibitors to dissect cell
survival dependencies and define improved strategies for cancer
therapy. Sci Transl Med. 7:279ra2402015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tirendi S, Marengo B, Domenicotti C, Bassi
AM, Almonti V and Vernazza S: Colorectal cancer and therapy
response: A focus on the main mechanisms involved. Front Oncol.
13:12081402023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu H, Guo W, Zhang L, Davis JJ, Teraishi
F, Wu S, Cao X, Daniel J, Smythe WR and Fang B: Bcl-XL small
interfering RNA suppresses the proliferation of
5-fluorouracil-resistant human colon cancer cells. Mol Cancer Ther.
4:451–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maji S, Panda S, Samal SK, Shriwas O, Rath
R, Pellecchia M, Emdad L, Das SK, Fisher PB and Dash R: Bcl-2
antiapoptotic family proteins and chemoresistance in cancer. Adv
Cancer Res. 137:37–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramesh P and Medema JP: BCL-2 family
deregulation in colorectal cancer: Potential for BH3 mimetics in
therapy. Apoptosis. 25:305–320. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scherr AL, Gdynia G, Salou M,
Radhakrishnan P, Duglova K, Heller A, Keim S, Kautz N, Jassowicz A,
Elssner C, et al: Bcl-xL is an oncogenic driver in colorectal
cancer. Cell Death Dis. 7:e23422016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin-Song Y, Zhao-Xia W, Cheng-Yu L,
Xiao-Di L, Ming S, Yuan-Yuan G and Wei D: Prognostic significance
of Bcl-xL gene expression in human colorectal cancer. Acta
Histochem. 113:810–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Ma L, Xu Y, Liu Y, Li W, Cai J and
Zhang Y: Enalapril overcomes chemoresistance and potentiates
antitumor efficacy of 5-FU in colorectal cancer by suppressing
proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins.
Cell Death Dis. 11:4772020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Townsend PA, Kozhevnikova MV, Cexus ONF,
Zamyatnin AA Jr and Soond SM: BH3-mimetics: Recent developments in
cancer therapy. J Exp Clin Cancer Res. 40:3552021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Ramesh P, Steinmetz M and Medema
JP: BH3 mimetic sensitivity of colorectal cancer cell lines in
correlation with molecular features identifies predictors of
response. Int J Mol Sci. 22:38112021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nwosu GO, Ross DM, Powell JA and Pitson
SM: Venetoclax therapy and emerging resistance mechanisms in acute
myeloid leukaemia. Cell Death Dis. 15:4132024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Al-Zebeeby A, Vogler M, Milani M, Richards
C, Alotibi A, Greaves G, Dyer MJS, Cohen GM and Varadarajan S:
Targeting intermediary metabolism enhances the efficacy of BH3
mimetic therapy in hematologic malignancies. Haematologica.
104:1016–1025. 2019. View Article : Google Scholar : PubMed/NCBI
|