Introduction
Endometrial cancer is a common gynecological
malignancy of the inner epithelial lining of the uterus (1,2). It is
the sixth most common cancer in females worldwide, with an
increasing incidence and mortality (3,4).
Although most patients with early stage and low-grade endometrial
cancer can achieve favorable survival results through surgery
alone, patients with advanced and metastatic disease progress
rapidly within one year (5,6) and the five-year overall survival rate
for patients with stage IV endometrial cancer is ~15% (7). Endometrial cancer cell invasion
through the myometrium and metastasis to nearby lymph nodes are key
factors affecting the prognosis (8). Therefore, elucidating the mechanisms
underlying endometrial cancer progression is of great significance
for disease prevention and treatment.
Normal cells rely primarily on the tricarboxylic
acid cycle and oxidative phosphorylation as their main sources of
adenosine triphosphate when oxygen is sufficient. By contrast,
cancer cells rely on glycolysis as their primary energy source,
even under oxygen-rich conditions when it is called aerobic
glycolysis (9–11). This continuous use of aerobic
glycolysis, known as the Warburg effect, is considered a hallmark
of numerous cancers (12,13).
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4)
belongs to a family of bifocal enzymes that regulate the balance
between fructose-6-phosphate and fructose-2, 6-bisphosphatase, and
is a key enzyme in glycolysis. PFKFB4 has both kinase and
phosphatase activities and can maintain the intracellular level of
fructose-2, 6-diphosphate, the main allosteric activator of the
glycolytic rate-rater 3-phosphoinositide dependent protein
kinase-1, through the dual action of phosphorylation and
dephosphorylation. Therefore, PFKFB4 controls glycolysis by
regulating the level of fructose-2,6-diphosphate (14). Importantly, PFKFB4 has been
demonstrated to be involved in numerous cancer-related processes
such as proliferation, metastasis and invasion, and PFKFB4
overexpression has been identified in gastric, bladder, lung and
breast cancers (15–18). PFKFB4 affects carcinogenesis and
cancer metabolism by participating in glucose metabolism regulation
by enhancing glycolysis and the pentose phosphate pathway (19). Enhanced glycolysis increases the
cancer cell survival rate in a microenvironment with limited oxygen
supply, thus promoting metastatic development (20). It has been reported that PFKFB4
mediated CD44-driven proliferation in prostate cancer (21). Lu et al (22) reported that PFKFB4 is abnormally
highly expressed in endometriosis, and that phosphorylation of
PFKFB4 results in endometriosis, glycolysis, and development via
proviral insertion in murine lymphomas 2. However, the molecular
mechanisms of PFKFB4 in endometrial cancer have not yet been fully
elucidated.
Steroid receptor coactivator-3 (SRC-3), is a member
of the SRC family that regulates the transcriptional activity of
steroid hormone receptors and other transcription factors (23). It also acts as a key modulator of
regulatory T cell-mediated tumor evasion to promote cancer
progression (24) and inhibition of
SRC-3 has been shown to be a potential therapeutic strategy for
cancers such as aggressive mantle cell lymphoma and breast cancer
(25,26). PFKFB4 was shown to phosphorylate
oncogenic SRC-3 at serine 857 and enhance its transcriptional
activity in breast cancer cells, resulting in a pro-proliferative
effect (15). However, the role of
PFKFB4 on SRC-3 phosphorylation and function in endometrial cancer
remains unclear. Thus, exploring the mechanism of action of PFKFB4
in endometrial cancer will facilitate the development of new drugs
for endometrial cancer treatment.
The objective of the present study was to
investigate the role and molecular mechanism of PFKFB4 in
endometrial cancer from the perspective of glucose metabolism. It
was hypothesized that PFKFB4 promotes the endometrial cancer
progression by regulating SRC-3. The results of the present study
may provide new methods for studying endometrial cancer
pathogenesis and treatment.
Materials and methods
Patients
The present study included two parts of tissue
specimens: one part of specimens were 180 paraffin-embedded
endometrial cancer tissues (median age: 55 years old, age range:
35–81) and 60 paraffin-embedded normal endometrial tissues. The
cancer tissues were collected from patients with endometrial cancer
who underwent surgical treatment in strict accordance with surgical
indications between January 1, 2017 and March 31, 2021 in our
hospital. The normal endometrial tissues were collected from
participants who underwent hysterectomy for uterine fibroids in the
same period. The other part of tissue specimens were frozen cancer
and para-carcinoma tissues collected from ten patients with
endometrial cancer (median age: 56 years old; age range: 37–80) who
underwent surgical treatment in our hospital from March 1, 2022 to
July 31, 2022. All the patients included in the study were first
diagnosed, without obvious distant metastasis lesions. They were
treated with surgery and had no radiotherapy or preoperative
chemotherapy, and the malignancy was evaluated according to the
international TNM clinical staging standard.
The present study was approved by the Ethics
Committee of the People's Hospital of Shanxi (approval no. 13;
Taiyuan, China), and all participants provided written informed
consent in accordance with the Declaration of Helsinki.
Cell culture
Endometrial cancer cell lines HEC-1A, Ishikawa, and
AN3CA, as well as the human normal endometrial epithelial cell line
HEEC, were obtained from the Chinese Academy of Sciences Cell Bank
(Shanghai, China) and cultured in RPMI-1640 (Corning, Inc.)
containing 10% fetal bovine serum (Ausbian, http://aipunuobio.com/mxproduct/1440.html) and 100
U/ml penicillin/100 µg/ml streptomycin (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. When the cells reached
90–100% confluence, pancreatin was added for subculture.
Cell transfection
Three small interfering RNAs (siRNAs) targeting
PFKFB4, including si-PFKFB4-1 (GACGTGGTCAAGACCTACAAA), si-PFKFB4-2
(CCGCATCGTATATTACCTCAT), and si-PFKFB4-3 (GCTGGCCTACTTCCTCGACAA)
and the corresponding negative control (NC) (si-NC,
TCTCCGAACGTGTCACGT) were designed by Guangzhou RiboBio Co., Ltd.
The siRNAs (50 nM), SRC-3 overexpression plasmid (SRC-3-OE, 1
µg/µl), and corresponding NC were transfected into HEC-1A cells
using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) at a
temperature of 37°C for a duration of 6 h. Cells were collected for
subsequent experimentation after 48 h. Transfection efficiency was
determined using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
RT-qPCR
Total RNA was extracted using TRIzol
(MilliporeSigma) and reverse transcription was conducted using
HiScript QRT Super Mix (Vazyme Biotech Co., Ltd.). The quantitative
PCR was conducted using Power SYBR Green PCR Master Mix (Thermo
Fisher Scientific, Inc.). The qPCR thermal cycling conditions were
as follows: Initial denaturation at 95°C for 60 sec; followed by 45
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 30
sec and extension at 72°C for 30 sec. Primers used are listed in
Table SI. GAPDH was used as a
reference gene. Relative changes in the transcriptional levels of
all genes were analyzed using the 2−∆∆Cq method
(27).
Western blotting
Tissues or cells were homogenized in RIPA solution
containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS and sodium orthovanadate (Beyotime Institute
of Biotechnology) and centrifuged at 15,000 × g and 4°C for 10 min,
and the total protein content was determined using the BCA method.
Proteins were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to the
polyvinylidene fluoride membrane. After blocking in 5% skim milk 1
h at room temperature (around 25°C), the membrane was incubated
with primary antibodies, including anti-PFKFB4 (1:1,000; cat. no.
A17915; Abclonal Biotech Co., Ltd.), anti-hexokinase 2 (HK2;
1:3,000; cat. no. 66974-1-Ig; Proteintech Group, Inc.), anti-SRC3
(1:1,000; cat. no. ab133611; Abcam), anti-phosphorylated (p)-SRC3
(1:1,000; cat. no. PA5-64836; Thermo Fisher Scientific, Inc.),
anti-pyruvate kinase M2 (PKM2; 1:5,000; cat. no. 15822-1-AP;
Proteintech Group, Inc.), anti-lactate dehydrogenase A (LDHA;
1:1,000; cat. no. 66287-1-Ig; Proteintech Group, Inc.),
anti-glucose transporter 1 (GLUT1; 1:750; cat. no. bs-4855R;
BIOSS), anti-hypoxia-inducible factor 1α (1:2,000; cat. no.
20960-1-AP; Proteintech Group, Inc.), anti-cleaved caspase 3
(1:500; cat. no. AF7022; Affinity Biosciences), anti-caspase 3
(1:500; cat. no. AF6311; Affinity Biosciences), anti-Bax (1:500;
cat. no. AF0120; Affinity Biosciences), anti-β-actin (1:5,000; cat.
no. 200068-8F10; Chengdu Zhengneng Biotechnology Co., Ltd.) and
anti-GAPDH (1:30,000; cat. no. 60004-1-lg; Proteintech Group, Inc.)
overnight at 4°C and then with the HRP-conjugated secondary
antibody (1:3,000; cat. no. A0208/A0216; Beyotime Institute of
Biotechnology,) at 37°C for 1 h. Finally, specific binding sites
were analyzed using enhanced chemiluminescence detection solutions
(MilliporeSigma). ImageJ software (version 1.53t, National
Institutes of Health) was used for densitometric analysis.
Cell Counting Kit-8 (CCK-8) assay
HEC-1A cells were seeded into a 96-well plate at a
density of 5×103 cells/well and treated with 10 µl of
CCK-8 reagent (cat. no. 96992; MilliporeSigma) for 1–4 h.
Absorption was measured at 450 nm on a multi-mode microplate reader
(M2009PR; Tecan Group, Ltd.).
5-ethynyl-2′-deoxyuridine (EdU)
assay
Cells seeded in a 96-well plate at a density of
6×103 cells/well were labeled with EdU (Guangzhou
RiboBio Co., Ltd.), fixed with 4% paraformaldehyde at room
temperature (~25°C) for 20 min, and images were captured using a
fluorescence microscope (Olympus Corporation).
Transwell migration assay
Transwell chambers (8.0 µm, Corning, Inc.) were
placed in 24-well plates. Serum-free medium (100 µl) was added to
the chamber for 1–2 h and then removed. Next, 600 µl of medium with
30% fetal bovine serum was added to the lower chamber, and
1×105 cells/well were added to the upper chamber. A
total of 24 h later, the cells that remained on the upper surface
were removed, and the migratory cells on the lower surface were
stained with 0.1% crystal violet at room temperature (~25°C) for 15
min. After staining, five images of the migratory cells were
randomly captured using an inverted microscope (CKX31; Olympus
Corporation).
Cell apoptosis assay
Cell supernatants from different groups were
collected in centrifuge tubes. Then, the cells in the culture
plates were washed and digested with trypsin. Complete culture
medium was then added to terminate the digestion. The cells were
collected in the same centrifuge tube as their supernatant. After
centrifugation at 400 × g at 4°C for 10 min, the cells were
collected and resuspended in 200 µl of 1X apoptosis buffer,
followed by staining with 5 µl annexin V-APC and 5 µl propidium
iodide for 15 min. Then, 800 µl of 1X apoptosis buffer was added to
resuspend the cells in 1 ml. The cell suspension was added to a
96-well plate and analyzed by FlowJo (version 10.8.1; BD
Biosciences) using a flow cytometer (cat. no. ABC250-22INT;
MilliporeSigma).
Glucose and lactic acid content
assays
Glucose or lactic acid contents of fresh tissues or
HEC-1A cells were detected using a glucose colorimetric assay kit
(cat. no. E-BC-K234-M) or a D-lactic acid colorimetric assay kit
(cat. no. E-BC-K002; both from Elabscience Biotechnology, Inc.),
respectively.
Animals
Animal experiments were conducted according to the
ARRIVE guidelines (28) and
approved by the Ethics Committee of the People's Hospital of Shanxi
(approval no. 9; Taiyuan, China). Eighteen Female, 4-week-old
specific pathogen free BALB/c nude mice, weighted 16–18 g (HFK
Bioscience Co. Ltd.) were housed in an 12/12-h photoperiods at
21–23°C and 40–60% humidity environment with free access to food
and water. They were divided into three groups: blank, si-NC, and
si-PFKFB4 (n=6) after 1-week of acclimation, and were implanted
subcutaneously with HEC-1A cells, HEC-1A transfected with si-NC,
and HEC-1A transfected with si-PFKFB4 (5×106 cells for
each mouse), respectively. The maximum length and width of the
tumors were measured every 2–3 days after one-week of cell
implantation. Tumor volume was calculated by length ×
width2/2. All mice were anesthetized with 2.5%
isoflurane inhalation and euthanized by cervical dislocation at the
end of the experiment (day 15). The tumors were excised, weighed,
and fixed in 4% paraformaldehyde at 4°C for 12 h for terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
staining and immunohistochemistry (IHC) or stored at −80°C for
western blotting.
TUNEL staining
The tumor tissues fixed in 4% paraformaldehyde were
embedded in paraffin and cut into 4-µm slides. The slides were then
deparaffinized in xylene, rehydrated with gradient ethanol, and
stained with TUNEL (Beyotime Institute of Biotechnology). After
washing with phosphate-buffered saline (PBS) and stained with 5
µg/ml DAPI at room temperature (~25°C) for 10 min to visualize
nuclei, the slides were sealed with anti-fluorescence quenching
mounting medium and observed under a fluorescence microscope
(Olympus Corporation).
IHC staining
Formalin-fixed and paraffin-embedded tissue sections
(4 µm) were subjected to IHC (29).
After dewaxing and rehydration, the endogenous peroxidase activity
was blocked with 0.3% H2O2 in methanol. The
sections were then incubated with rabbit polyclonal antibodies
against PFKFB4 (1:100; cat. no. AB137785; Abcam) and Ki-67 (1:100;
cat. no. AF0198; Affinity Biosciences) at 4°C overnight. Then,
enzyme-labeled anti-rabbit IgG was added and incubated for 30 min.
A NC was obtained by replacing the antibodies with normal PBS.
Staining was developed by 3,3′-diaminobenzidine, followed by
counterstaining with hematoxylin, dehydration in gradients of
ethanol, and clearing in xylene.
The expression level of PFKFB4 or Ki-67 was
calculated by multiplying the staining intensity by the percentage
of stained cells in ten random fields of view. Staining intensity
was scored as 0 for negative staining, 1 for weakly positive
staining, 2 for moderately positive staining, and 3 for strongly
positive staining (30). The
percentage of positively stained cells was scored as 1 for 1–25%
positive staining, 2 for 26–50% positive staining, 3 for 51–75%
positive staining, or 4 for 76–100% staining. A final score of 0–1
indicated negative expression, 2–5 low expression, and 6–12 high
expression.
Statistical analysis
SPSS 25.0 (IBM Corp.) was used for data analyses.
For measurement data, the Shapiro-Wilk test was first performed to
determine whether the data followed a normal distribution. Data
that conformed to a normal distribution were expressed as the mean
± standard deviation. The differences between groups were
calculated using a unpaired Student's t-test or one-way analysis of
variance followed by a Bonferroni post-hoc test. Data that did not
conform to a normal distribution were expressed as median
(interquartile range), and the differences between groups were
calculated using the Mann-Whitney U-test or Kruskal-Wallis test.
Categorical data were expressed as number (percentage), and the
differences between two groups were calculated using the Chi-square
test or Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PFKFB4 expression and glycolysis
increase in endometrial cancer tissues
Data from 180 patients with human endometrial cancer
and 60 patients with a normal endometrium were analyzed. The
average age of patients with cancer and normal endometrium was 55.1
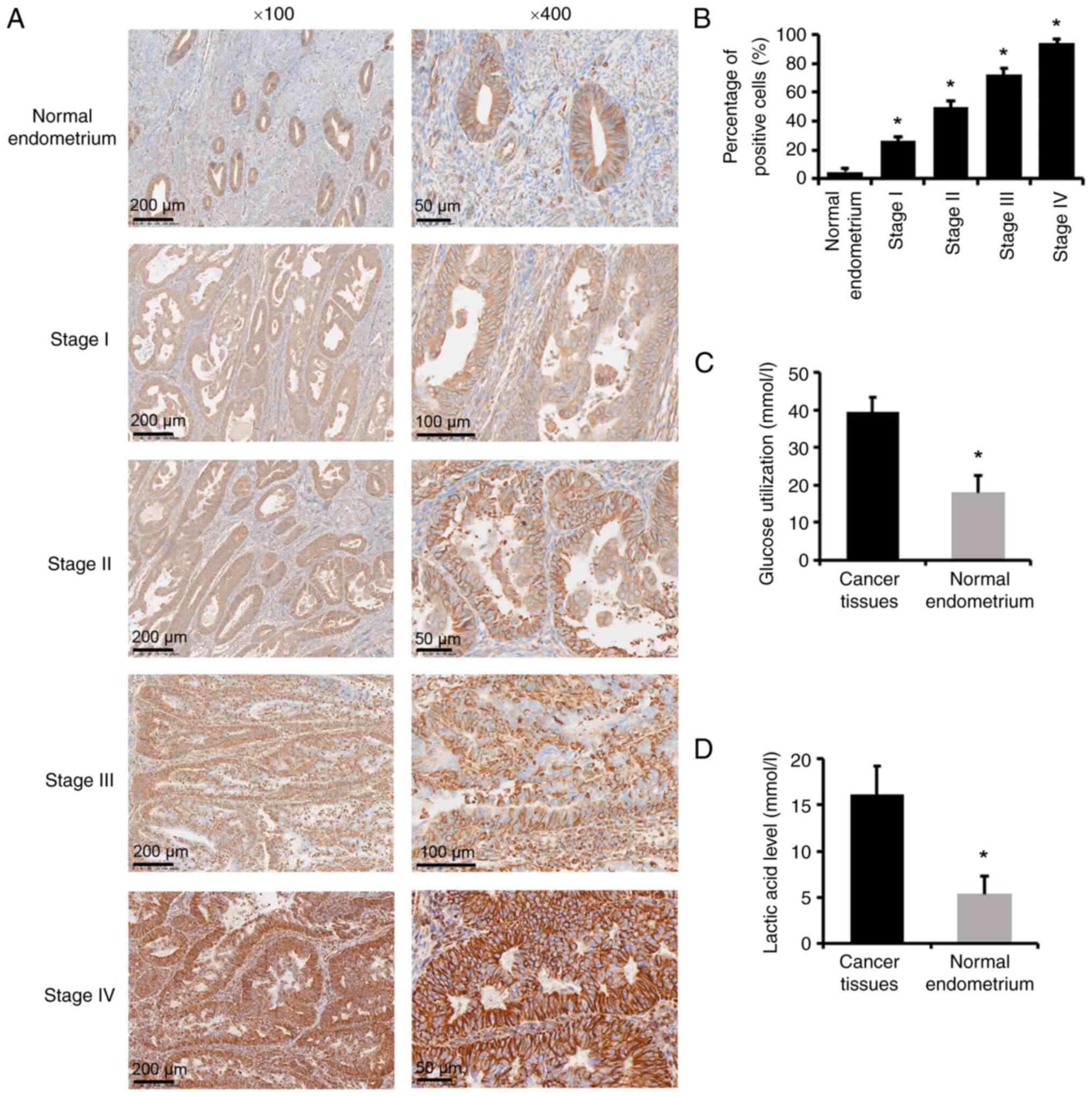
and 50.8 years, respectively. Tumor samples underwent IHC staining
for PFKFB4. PFKFB4-positive staining was primarily observed in the
cytoplasm of the endometrial stroma and glandular epithelium, and
most intra- or extra-tumoral stromal cells were PFKFB4 negative
(Fig. 1A). In the normal
endometrium, most endometrial glands showed negative expression and
a few exhibited low expression of PFKFB4. The expression of PFKFB4
increased with increasing stage (P<0.05; Fig. 1B). In addition, glucose and lactic
acid levels were significantly increased in cancer tissues compared
with those in normal endometrium (P<0.05; Fig. 1C and D).
Among the 180 cases of endometrial cancer, 29 had
high PFKFB4 expression and 151 had low expression, with a high
expression rate of 16.11% (29/180). In the control group of 60
cases, 3 cases had high PFKFB4 expression and 57 cases had low
expression, with a high expression rate of 5% (3/60), which was
significantly lower than that in the endometrial cancer group
(P=0.028; Table I).
 | Table I.Comparison of the PFKFB4 expression
in endometrial cancer and control groups. |
Table I.
Comparison of the PFKFB4 expression
in endometrial cancer and control groups.
|
| Expression level of
PFKFB4 |
|
|
|---|
|
|
|
|
|
|---|
| Group | Low, n (%) | High, n (%) | χ2 | P-value |
|---|
| Endometrial
cancer | 151 (83.9) | 29 (16.1) | 4.808 | 0.028 |
| Control | 57 (95) | 3 (5) |
|
|
| Total | 208 | 32 |
|
|
In addition, both glucose consumption and lactic
acid production increased significantly in endometrial cancer
tissues compared with those in normal endometrium (P<0.05).
PFKFB4 expression is associated with
clinicopathological parameters of endometrial cancer
Subsequently, the relationship between PFKFB4
expression and clinicopathological parameters in endometrial cancer
tissues was analyzed. PFKFB4 upregulation was positively correlated
with the depth of myometrial invasion, vascular invasion, surgical
pathological stage, and lymph node metastasis (Table II).
 | Table II.Correlation of PFKFB4 expression with
clinicopathological parameters. |
Table II.
Correlation of PFKFB4 expression with
clinicopathological parameters.
|
| Expression level of
PFKFB4 |
|
|
|---|
|
|
|
|
|
|---|
| Parameters | Low, n (%) | High, n (%) | χ2 | P-value |
|---|
| Age, years |
|
| 1.833 | 0.176 |
|
<55 | 78 (87.6) | 11 (12.4) |
|
|
|
≥55 | 73 (80.2) | 18 (19.8) |
|
|
| Menopausal or
not |
|
| 1.608 | 0.205 |
| No | 66 (88) | 9 (12.1) |
|
|
|
Yes | 85 (81) | 20 (19) |
|
|
| Histological
subtype |
|
|
| 0.23 |
|
Endometrioid
adenocarcinoma | 142 (85) | 25 (15) |
|
|
|
Non-endometrioid
adenocarcinoma | 9 (69.2) | 4 (30.8) |
|
|
| Histopathological
grade |
|
|
| 0.583 |
|
High | 28 (90.3) | 3 (9.7) |
|
|
|
Median | 95 (81.9) | 21 (18.1) |
|
|
|
Low | 28 (84.8) | 5 (15.2) |
|
|
| Depth of myometrium
invasion |
|
| 20.262 | <0.001 |
|
<1/2 | 119 (91.5) | 11 (8.5) |
|
|
|
≥1/2 | 32 (64) | 18 (36) |
|
|
| Lymph node
metastasis |
|
|
| <0.001 |
| No | 146 (88.5) | 19 (11.5) |
|
|
|
Yes | 5 (33.3) | 10 (66.7) |
|
|
| Surgical
pathological stage |
|
|
| <0.001 |
| I +
II | 149 (94.3) | 9 (5.7) |
|
|
| III +
IV | 2 (9.1) | 20 (90.9) |
|
|
| Vascular
invasion |
|
|
| 0.002 |
| No | 135 (87.7) | 19 (12.3) |
|
|
|
Yes | 16 (61.5) | 10 (38.5) |
|
|
Among the 180 endometrial cancer patients, 130 had a
muscle layer invasion depth of <1/2, among which 11 had high
PFKFB4 expression (11/130; 8.46%), 119 had low PFKFB4 expression.
Fifty of the endometrial cancer patients had a muscle layer
invasion depth of ≥1/2, among which 18 had high PFKFB4 expression
(18/50; 36%), and 32 cases had low expression. The high PFKFB4
expression rate at a muscle layer invasion depth of ≥1/2 was
significantly lower than that at a muscle layer invasion depth of
<1/2 (P<0.001). There were 154 patients without vascular
invasion, including 19 with high PFKFB4 expression (19/154,
12.34%). Among the 26 patients with vascular invasion, 10 had high
PFKFB4 expression, with a high expression rate of 38.46% (10/26),
which was significantly higher than in patients without vascular
invasion (P=0.002). According to the surgical pathological stages
of endometrial cancer (FIGO, 2009), 158 patients were in stages
I–II, and 22 were in stages III–IV. The high PFKFB4 expression
rates were 5.70% (9/158) for stages I–II and 90.90% (20/22) for
stages III–IV, with a significant difference (P<0.001).
Additionally, 165 patients did not have lymph node metastases,
including 19 with high PFKFB4 expression (19/165, 11.52%). Among
the 15 patients with lymph node metastases, 10 showed high PFKFB4
expression (10/15, 66.67%). The difference between the two groups
was statistically significant (P<0.001).
PFKFB4 expression increases in
endometrial cancer cells
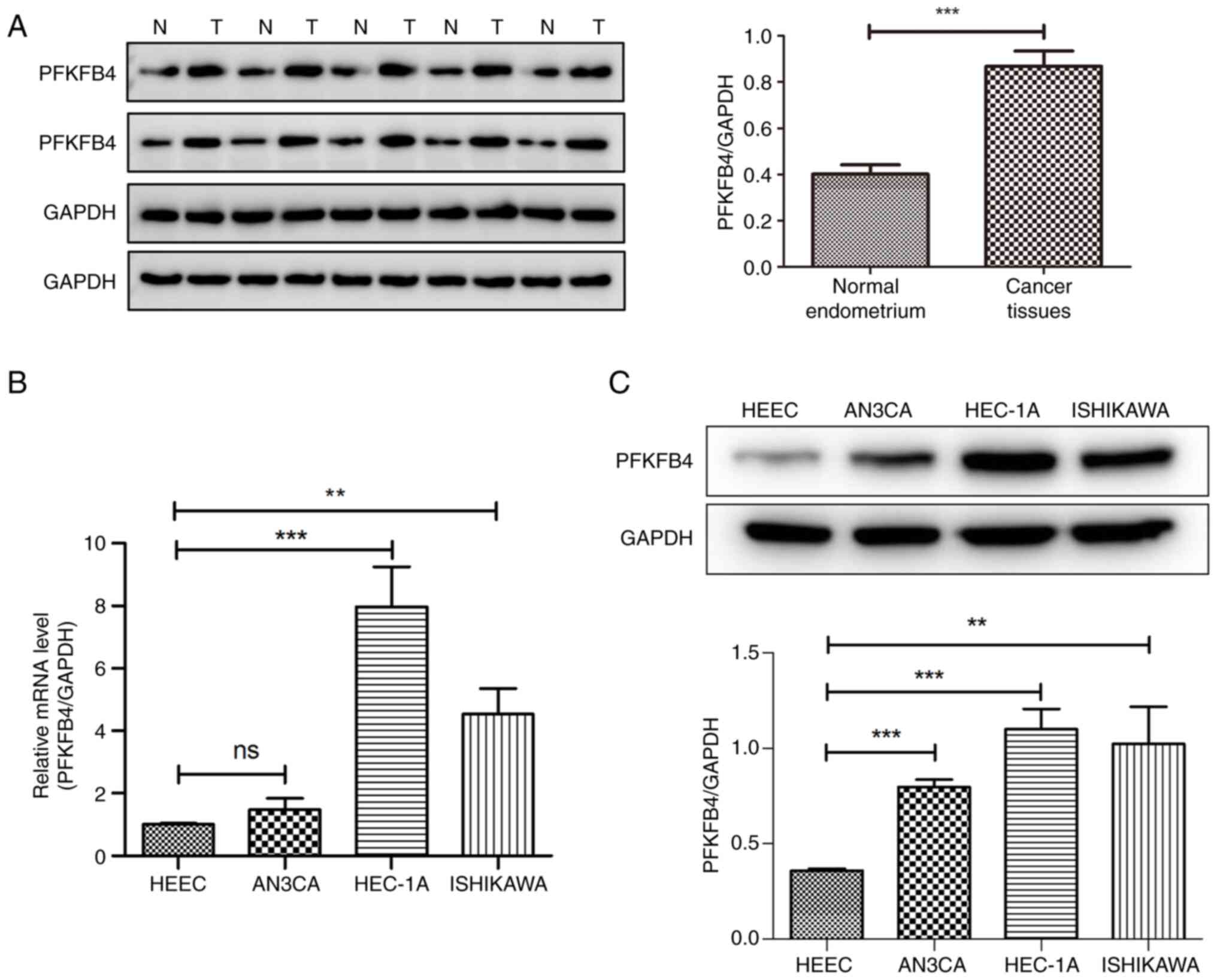
The protein expression of PFKFB4 in ten pairs of
endometrial cancer tissues and normal endometrium was measured by
western blotting. The protein expression of PFKFB4 was
significantly increased compared with that in the normal
endometrium (P<0.001; Fig. 2A).
The mRNA expression levels of PFKFB4 in the three
endometrial cancer cell lines and the human normal endometrial
epithelial cell line, HEEC, are shown in Fig. 2B. The mRNA levels of PFKFB4
in Ishikawa and HEC-1A cells were higher than those in HEECs
(P<0.01). The protein levels of PFKFB4 in the four cell lines
were measured. The protein levels of PFKFB4 in cancer cells were
significantly higher than in normal cells (P<0.01; Fig. 2C). Among the three cancer cell
lines, HEC-1A revealed the most significant upregulation;
therefore, HEC-1A cells were selected for subsequent
experiments.
PFKFB4 promotes proliferation and
migration and inhibits apoptosis of endometrial cancer cells in
vitro and in vivo
To further investigate the function of PFKFB4 in
endometrial cancer, a PFKFB4 knockdown plasmid was constructed and
the knockdown efficiency was determined by RT-qPCR. Compared with
the si-NC group, the mRNA levels of PFKFB4 in the
si-PFKFB4-1, si-PFKFB4-2, and si-PFKFB4-3 groups decreased
significantly (P<0.01), suggesting that the knockdown plasmids
were successfully constructed (Fig.
3A).
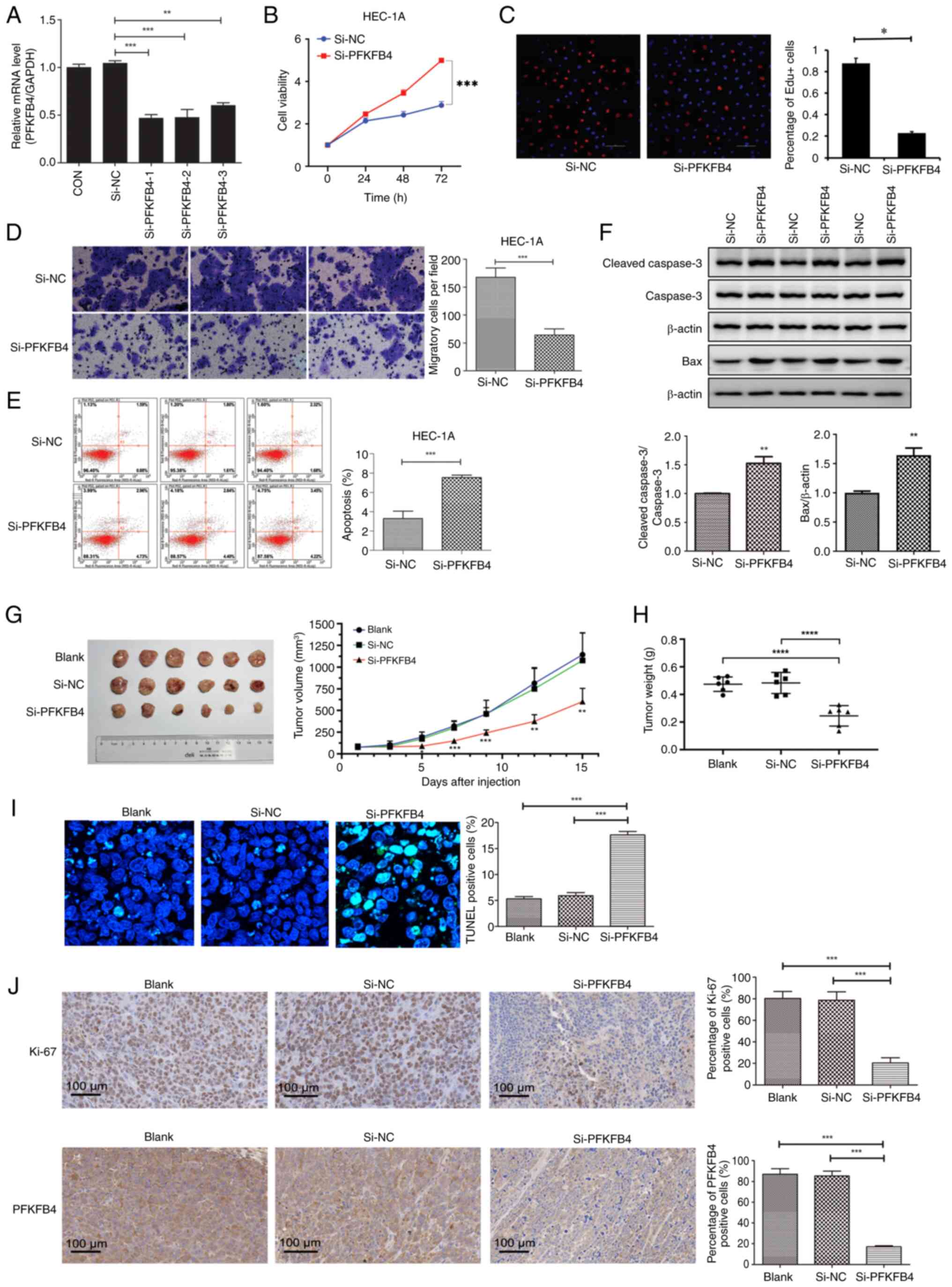 | Figure 3.PFKFB4 promotes proliferation and
migration and inhibits apoptosis of endometrial cancer cells in
vitro and in vivo. (A) PFKFB4 knockdown efficiency was
detected by reverse transcription-quantitative PCR. (B) The
viability of endometrial cancer cells after PFKFB4 knockdown was
detected by Cell Counting Kit-8. (C) The proliferation of
endometrial cancer cells was detected by EdU assay. (D) The
migration ability of endometrial cancer cells was tested in a
Transwell assay. (E) The apoptosis of endometrial cancer cells was
detected by flow cytometry. (F) The protein expression of apoptosis
indicators was analyzed by western blotting. (G) Tumor growth
curves of PFKFB4 knockdown cells or NC cells in the xenograft mouse
model. (H) Tumor weight. (I) TUNEL staining of tumor tissues
(magnification, ×400). (J) IHC staining of Ki-67, PFKFB4 and SRC-3
(magnification, ×400). *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 compared with control or si-NC. PFKFB4,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4; IHC,
immunohistochemistry; si-, small interfering; NC, negative
control. |
CCK-8 and EdU assays were performed to detect the
endometrial cancer cell proliferation following PFKFB4 knockdown.
PFKFB4 knockdown significantly decreased the proliferation of
endometrial cancer cells compared with that in the control
(P<0.001; Fig. 3B and C).
Transwell assays revealed that, compared with the control, PFKFB4
knockdown inhibited the migration of endometrial cancer cells
(P<0.001; Fig. 3D). Furthermore,
PFKFB4 knockdown significantly increased the apoptosis of cancer
cells (P<0.001; Fig. 3E) and
upregulated the protein expression of cleaved Caspase 3 and Bax
(Fig. 3F).
The effects of the PFKFB4 knockdown were further
investigated in vivo. The maximum tumor diameter measured
was 15.7 mm and maximum volume was 1478.9 mm3 in a mouse
in the blank group. The tumor volume and weight were significantly
lower in si-PFKFB4 group, compared with those in the blank and
si-NC groups (P<0.05; Fig. 3G and
H). TUNEL staining demonstrated that the percentage of TUNEL
positive cells was significantly higher in the si-PFKFB4 group,
compared with those in the blank and si-NC groups (P<0.05;
Fig. 3I). IHC staining demonstrated
that the protein expression of Ki-67 and PFKFB4 decreased in tumors
from the si-PFKFB4 group compared with those in the blank and si-NC
groups (Fig. 3J). Taken together,
these results suggest that PFKFB4 downregulation is beneficial for
the proliferation and tumorigenesis of endometrial cancer
cells.
PFKFB4 increases glycolysis in
endometrial cancer cells
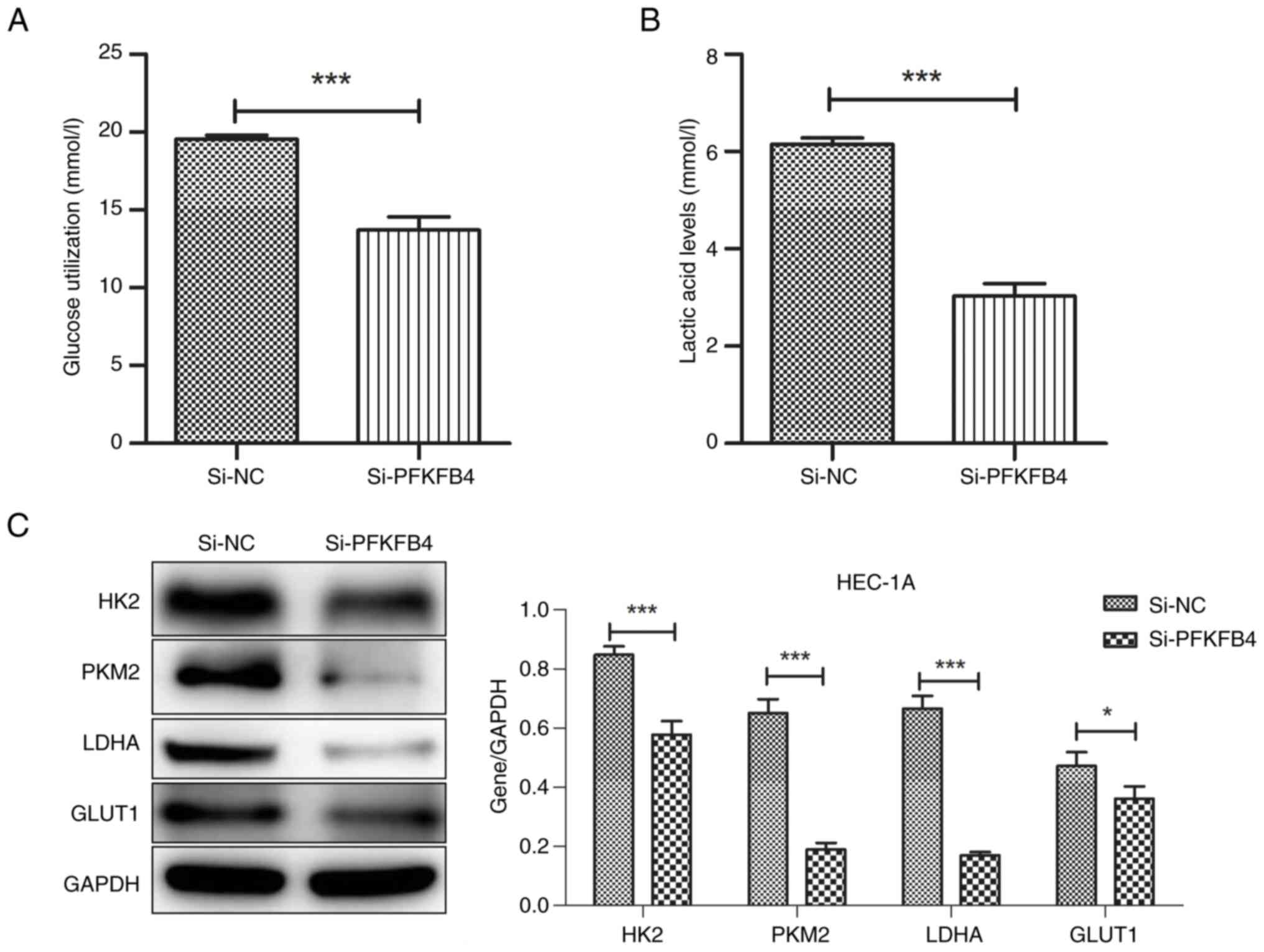
To analyze the effect of PFKFB4 on glycolysis in
endometrial cancer cells, an enzyme-linked immunosorbent assay was
used to measure glucose and lactic acid content after PFKFB4
knockdown. Compared with the control group, both glucose and lactic
acid levels decreased significantly in PFKFB4 knockdown group
(Fig. 4A and B). The levels of
glycolysis-related enzymes, including GLUT1, HK2, LDHA and PKM2,
were downregulated after PFKFB4 knockdown compared with those in
the control (Fig. 4C).
PFKFB4-phosphorylated SRC-3 regulates
glycolysis in endometrial cancer cells
SRC-3 may be a downstream target of PFKFB4 (15). Thus, the expression level of p-SRC-3
was measured after PFKFB4 knockdown using western blotting. The
results revealed that p-SRC-3 was significantly downregulated after
PFKFB4 knockdown compared with that in the control, indicating that
PFKFB4 could phosphorylate SRC-3 (Fig.
5A). SRC-3 overexpression induced changes in glycolysis in
endometrial cancer cells. The efficiency of SRC-3 overexpression is
shown in Fig. 5B. Compared with the
NC group, PFKFB4 expression in the NC + SRC-3-OE group revealed no
significant change, whereas SRC-3 expression was significantly
higher (P<0.001, Fig. 5C). In
the PFKFB4-KD + NC group, PFKFB4 and SRC-3 expression levels were
significantly lower than in those in the NC group (P<0.05,
Fig. 5C). Additionally, compared
with the PFKFB4-KD + NC group, PFKFB4 expression in the PFKFB4-KD +
SRC-3-OE group did not change significantly, whereas SRC-3
expression increased significantly (P<0.001, Fig. 5C). Subsequently, glucose and lactic
acid contents were detected after SRC-3 overexpression. Compared
with the NC group, glucose consumption and lactic acid production
after SRC-3 overexpression increased significantly (P<0.001),
whereas after PFKFB4 knockdown, they decreased significantly
(P<0.001). Compared with the PFKFB4-KD + NC group, glucose
consumption and lactic acid production in the PFKFB4-KD + SRC-3-OE
group increased (P<0.001), suggesting that SRC-3 overexpression
reversed the effect of PFKFB4 knockdown on glycolysis in cancer
cells (Fig. 5D and E).
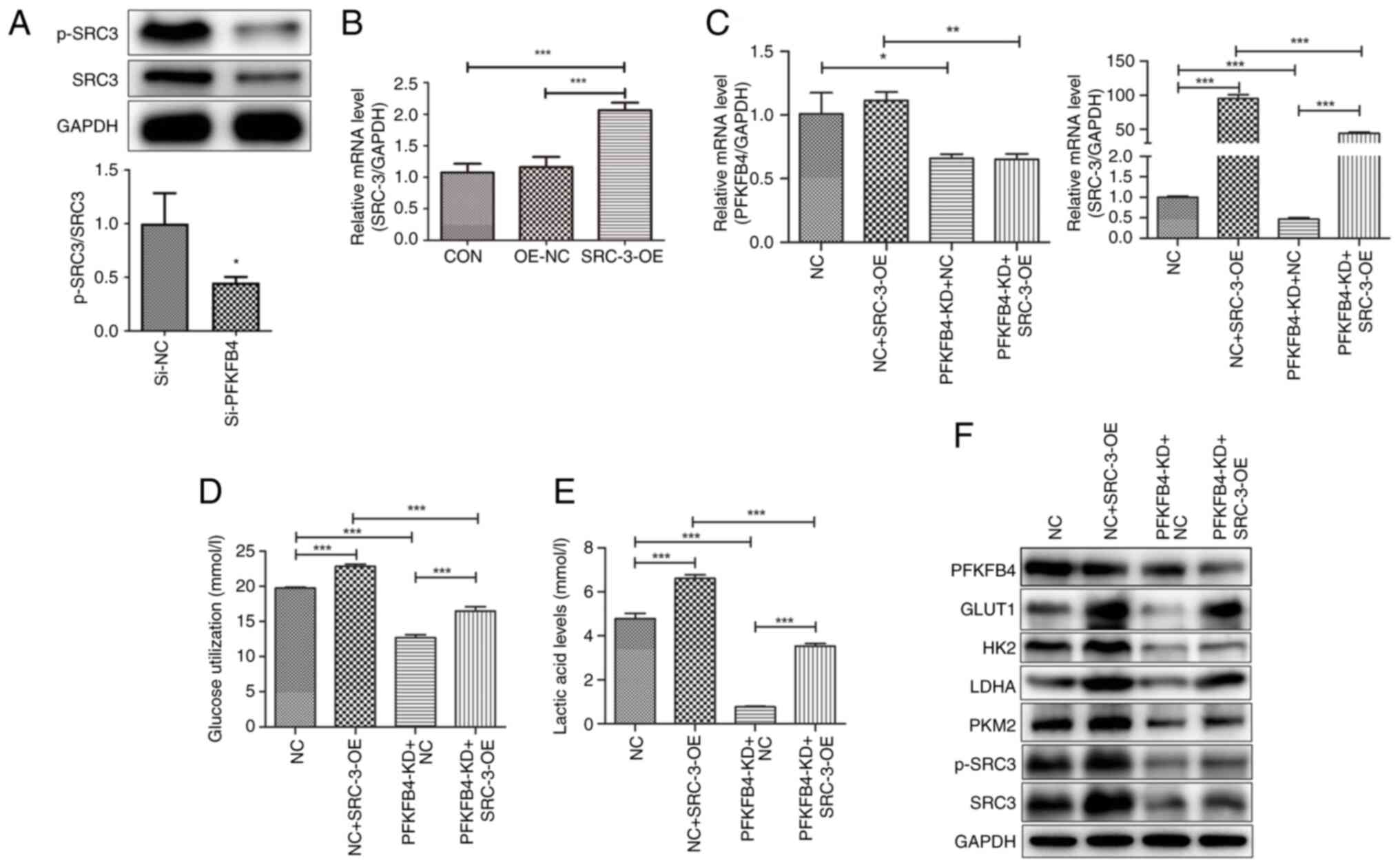 | Figure 5.PFKFB4-phosphorylated SRC-3 regulates
glycolysis in endometrial cancer cells. (A) The protein level of
p-SRC-3 was detected by western blotting. (B) The efficiency of
SRC-3 overexpression detected by RT-qPCR. (C) The effects of PFKFB4
KD and/or SRC-3 overexpression on the mRNA expression of PFKFB4 and
SRC-3 detected by RT-qPCR. (D and E) Glucose and lactic acid
production after PFKFB4 knockdown or SRC-3 overexpression measured
by ELISA. (F) The expression levels of glycolysis-related enzymes
detected by western blotting. *P<0.05, **P<0.01 and
***P<0.001. PFKFB4,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4; p-,
phosphorylated; SRC, steroid receptor coactivator-3; SRC-3-OE,
SRC-3 overexpression plasmid; RT-qPCR, reverse
transcription-quantitative PCR; KD, knockdown; p-, phosphorylated;
NC, negative control. |
In addition, the protein levels of
glycolysis-related enzymes (PKM2, GLUT1, HK2, LDHA and PFKFB4) and
p-SRC-3 were determined (Fig. 5F).
Compared with the NC group, PFKFB4 expression was downregulated
whereas PKM2, GLUT1, HK-II, LDHA and p-SRC-3 expression were
upregulated in the NC + SRC-3-OE group. The expression of PKM2,
GLUT1, HK2, LDHA, and p-SRC-3 was downregulated in the PFKFB4-KD +
NC group. Compared with the PFKFB4-KD+NC group, PFKFB4 expression
was downregulated, and PKM2, GLUT1, HK-II, LDHA and p-SRC-3
expression were upregulated in the PFKFB4-KD + SRC-3-OE group,
suggesting that SRC-3 overexpression reversed the effect of PFKFB4
knockdown on the expression of glycolysis-related enzymes.
Discussion
Metabolism is critical for the development of cancer
(31,32). The key enzymes involved in the core
metabolic processes of tumor cells play indispensable roles in
tumor cell survival and proliferation (33–35).
PFKFB4 is an important enzyme that regulates the energy pathways in
tumors and is involved in the movement of tumor and stem cells,
thereby acting as an energy regulator during tumor cell metastasis
(36). In the present study, the
protein level of PFKFB4 in endometrial cancer tissues and cells was
upregulated compared with that in normal endometrial tissues and
cells.
Dasgupta et al (15) demonstrated that PFKFB4 is a major
regulator of the SRC3-dependent cancer cell proliferation. PFKFB4
phosphorylates SRC-3, thereby promoting estrogen receptor
coactivation and cell proliferation. High expression of SRC-3 and
PFKFB4 indicates poor prognosis in patients with breast cancer. In
the present study, PFKFB4 was overexpressed in endometrial cancer
tissues compared with normal endometrial tissues, and its high
expression correlated with the depth of myometrial invasion, lymph
node metastasis, surgical pathological stage, vascular invasion,
and other adverse prognostic factors. Moreover, the present study
revealed that the higher the tumor malignancy, the higher the
PFKFB4 expression level, indicating that PFKFB4 may serve as a
potential target for treating patients with malignant endometrial
cancer. Yun et al (18)
reported that PFKFB4 is a prognostic biomarker of
non-muscle-invasive bladder cancer. Ros et al (37) also found that PFKFB4 upregulation
predicts shorter survival in patients with non-small cell lung or
breast cancers. Taken together, these results suggest that PFKFB4
is a potential therapeutic target for tumors.
PFKFB4 is also a key glycolytic enzyme that is
highly expressed in numerous tumors (38–41).
For instance, PFKFB4 is necessary in prostate cancer to maintain a
balance between energy generation and glycolytic activity (21). PFKFB4 is also a key metabolic enzyme
in pancreatic and breast cancers and acts as a regulator of the
glycolytic pathway in these tumors (15,42).
In the present study, the function of PFKFB4 was explored by
knocking down PFKFB4 in HEC-1A cells and found that PFKFB4
knockdown suppressed the proliferation and migration of endometrial
cancer cells and promoted their apoptosis, suggesting a
carcinogenic effect of PFKFB4 in endometrial cancer. The current
findings are consistent with those of PFKFB4 in other cancers
(35,43).
Even when oxygen is abundant in the
microenvironment, tumor cells metabolize glucose at a high rate, a
unique metabolic pattern known as aerobic glycolysis (Warburg
effect) (9). Metabolic
reprogramming induces changes in metabolic enzymes and patterns in
tumor cells. Glycolysis in tumor cells is regulated by different
enzymatic reactions that allow glucose-derived carbon units to
enter other anabolic pathways such as protein and nucleotide
biosynthesis. PFKFB4 balances anabolism and redox homeostasis
through allosteric regulation to control metabolic fluxes in the
glycolysis and pentose phosphate pathways. PFKFB4 plays a critical
role in regulating glucose metabolism and guiding metabolic
pathways required for macromolecular biosynthesis to maintain
cancer cell proliferation (44). In
addition, PFKFB4 has been reported to be necessary for
hypoxia-induced glycolysis (45).
The present study explored the effects of PFKFB4 knockdown on
glycolysis in endometrial cancer cells and revealed that PFKFB4
promotes glycolysis in cancer cells, which is consistent with
previous studies. Thus, PFKFB4 may increase the proliferation and
migration of endometrial cancer cells and inhibit apoptosis by
enhancing glycolysis.
Furthermore, PFKFB4 may be involved in cancer cell
metastasis via signal transduction. A previous study reported that
the interaction between PFKFB4 and SRC-3 is a key regulatory
mechanism in breast cancer (15).
The interaction between PFKFB4 and SRC-3 was further investigated
and it was found that PFKFB4 phosphorylated SRC-3, affected glucose
metabolism, and promoted glycolysis in endometrial cancer cells.
Improvement of glycolysis in cancer cells helps them cope with
hypoxia and nutritional deficiency, thus promoting their
proliferation and migration (46).
Overall, the current results indicated that PFKFB4 may serve as a
potential therapeutic target for cancer.
However, the present study had several limitations.
The role of PFKFB4 in regulating endometrial cancer glycolysis and
cell malignant behaviors was only investigated using the
loss-of-function method in one cell line owing to funding
limitations. Next, the effects of PFKFB4 on SRC-3 were
investigated; however, PFKFB4 can signal through additional the
downstream molecules of PFKFB4 besides SRC3. Thus, RNA sequencing
could be used in the future to more comprehensively identify
PFKFB4-regulated signaling pathways. Further in-depth experiments
in other endometrial cancer cells should be performed to
comprehensively confirm the function and molecular mechanism of
PFKFB4 in the regulation of glycolysis in endometrial
carcinoma.
In conclusion, the present study suggests that
PFKFB4 promotes proliferation and migration and inhibits apoptosis
in endometrial cancer cells. Furthermore, it was revealed that
PFKFB4 promotes glycolysis in endometrial cancer cells by
phosphorylating SRC-3, suggesting a pro-tumor role of the
PFKFB4-SRC-3-glycolysis axis in endometrial cancer. The present
study provides in vitro and in vivo evidence of the
potential application of PFKFB4 in endometrial cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Tianjin (grant no. 20JCZDJC00330), the Shanxi
Provincial Health Commission Scientific Research Project Plan (gran
no. 2020030), the Health Technology Project of Tianjin Municipal
Health Commission (grant no. TJWJ2022XK009) and the China
Anti-Cancer Association-Hengrui PARP Nicotinamide Cancer Research
Fund (grant no. CETSDHRCORP252007).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YW and YW were involved in the conception and
design, obtained the fundings. YW, JZ, SZ and JL performed the
experiments, analysis and interpretation of the data. JL performed
the statistical analysis. YW drafted the paper. YW and YW confirm
the authenticity of all the raw data. All authors revised the
manuscript critically for intellectual content, agree to be
accountable for all aspects of the work, and read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Human studies were approved by the Ethics Committee
of the People's Hospital of Shanxi (approval no. 13; Taiyuan,
China), and all participants provided written informed consent in
accordance with the Declaration of Helsinki. Animal experiments
were conducted according to the ARRIVE guidelines and approved by
the Ethics Committee of the People's Hospital of Shanxi (approval
no. 9; Taiyuan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EdU
|
-ethynyl-2′-deoxyuridine
|
|
GLUT1
|
glucose transporter 1
|
|
HK2
|
hexokinase 2
|
|
IHC
|
immunohistochemistry
|
|
LDHA
|
lactate dehydrogenase A
|
|
NC
|
negative control
|
|
PBS
|
phosphate-buffered saline
|
|
PFKFB4
|
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4
|
|
PKM2
|
pyruvate kinase M2
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
siRNA
|
small interfering RNA
|
|
SRC
|
steroid receptor coactivator-3
|
|
SRC-3-OE
|
SRC-3 overexpression plasmid
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick end labeling
|
References
|
1
|
Makker V, MacKay H, Ray-Coquard I, Levine
DA, Westin SN, Aoki D and Oaknin A: Endometrial cancer. Nat Rev Dis
Primers. 7:882021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vizza E, Bruno V, Cutillo G, Mancini E,
Sperduti I, Patrizi L, Certelli C, Zampa A, Giannini A and Corrado
G: Prognostic role of the removed vaginal cuff and its correlation
with L1CAM in low-risk endometrial adenocarcinoma. Cancers (Basel).
14:342021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henley SJ, Ward EM, Scott S, Ma J,
Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 126:2225–2249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arend RC, Jones BA, Martinez A and
Goodfellow P: Endometrial cancer: Molecular markers and management
of advanced stage disease. Gynecol Oncol. 150:569–580. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doll KM, Tseng J, Denslow SA, Fader AN and
Gehrig PA: High-grade endometrial cancer: Revisiting the impact of
tumor size and location on outcomes. Gynecol Oncol. 132:44–49.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeBerardinis RJ and Chandel NS: We need to
talk about the Warburg effect. Nat Metab. 2:127–129. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: The metabolism of carcinoma
cells. J Cancer Res. 9:148–163. 1925. View Article : Google Scholar
|
|
13
|
Warburg OH: Ueber den stoffwechsel der
tumoren. Springer; Berlin: 1926
|
|
14
|
Pilkis SJ, Claus TH, Kurland IJ and Lange
AJ: 6-Phosphofructo-2-kinase/fructose-2, 6-bisphosphatase: A
metabolic signaling enzyme. Annu Rev Biochem. 64:799–835. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dasgupta S, Rajapakshe K, Zhu B, Nikolai
BC, Yi P, Putluri N, Choi JM, Jung SY, Coarfa C, Westbrook TF, et
al: Metabolic enzyme PFKFB4 activates transcriptional coactivator
SRC-3 to drive breast cancer. Nature. 556:249–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Zeng F, Sun Y, Qiu Q, Zhang J,
Huang W, Huang J, Huang X and Guo L: Etk interaction with PFKFB4
modulates chemoresistance of small-cell lung cancer by regulating
AutophagyEtk controls PFKFB4 in promoting chemoresistance of SCLC.
Clin Cancer Res. 4:950–962. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Wu X, Li Y, Cao X, Zhang C and Gao
Y: PFKFB4 as a promising biomarker to predict a poor prognosis in
patients with gastric cancer. Oncol Lett. 21:2962021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yun SJ, Jo SW, Ha YS, Lee OJ, Kim WT, Kim
YJ, Lee SC and Kim WJ: PFKFB4 as a prognostic marker in
non-muscle-invasive bladder cancer. Urol Oncol. 30:893–899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi M, Ban Y, Tan Y, Xiong W, Li G and
Xiang B: 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and
4: A pair of valves for fine-tuning of glucose metabolism in human
cancer. Mol Metab. 20:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kotowski K, Rosik J, Machaj F, Supplitt S,
Wiczew D, Jabłońska K, Wiechec E, Ghavami S and Dzięgiel P: Role of
PFKFB3 and PFKFB4 in cancer: Genetic basis, impact on disease
development/progression, and potential as therapeutic targets.
Cancers (Basel). 13:9092021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Qian L, Lin J, Huang G, Hao N, Wei
X, Wang W and Liang J: CD44 regulates prostate cancer
proliferation, invasion and migration via PDK1 and PFKFB4.
Oncotarget. 8:65143–65151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu C, Qiao P, Fu R, Wang Y, Lu J, Ling X,
Liu L, Sun Y, Ren C and Yu Z: Phosphorylation of PFKFB4 by PIM2
promotes anaerobic glycolysis and cell proliferation in
endometriosis. Cell Death Dis. 13:7902022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Deng CX and Chen Q: SRC-3, a steroid
receptor coactivator: Implication in cancer. Int J Mol Sci.
22:47602021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han SJ, Jain P, Gilad Y, Xia Y, Sung N,
Park MJ, Dean AM, Lanz RB, Xu J, Dacso CC, et al: Steroid receptor
coactivator 3 is a key modulator of regulatory T cell-mediated
tumor evasion. Proc Natl Acad Sci USA. 120:e22217071202023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bijou I, Liu Y, Lu D, Chen J, Sloan S,
Alinari L, Lonard DM, O'Malley BW, Wang M and Wang J: Inhibition of
SRC-3 as a potential therapeutic strategy for aggressive mantle
cell lymphoma. PLoS One. 19:e02899022024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Varisli L, Dancik GM, Tolan V and
Vlahopoulos S: Critical roles of SRC-3 in the development and
progression of breast cancer, rendering it a prospective clinical
target. Cancers (Basel). 15:52422023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia Y, Liu J, Shi J, Zhang C, Wang X, Zhao
L, Lou Y, Guan X and Huangfu H: Epigenetic silencing of JAM3
promotes laryngeal squamous cell carcinoma development by
inhibiting the Hippo pathway. Oncol Rep. 53:282025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riener MO, Fritzsche FR, Clavien PA,
Pestalozzi BC, Probst-Hensch N, Jochum W and Kristiansen G: IMP3
expression in lesions of the biliary tract: A marker for high-grade
dysplasia and an independent prognostic factor in bile duct
carcinomas. Hum Pathol. 40:1377–1383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim J and DeBerardinis RJ: Mechanisms and
implications of metabolic heterogeneity in cancer. Cell Metab.
30:434–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wiel C, Le Gal K, Ibrahim MX, Jahangir CA,
Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, et al: BACH1
stabilization by antioxidants stimulates lung cancer metastasis.
Cell. 178:330–345. e222019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartrons R and Caro J: Hypoxia, glucose
metabolism and the Warburg's effect. J Bioenerg Biomembr.
39:223–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang JB, Erickson JW, Fuji R, Ramachandran
S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV and
Cerione RA: Targeting mitochondrial glutaminase activity inhibits
oncogenic transformation. Cancer Cell. 18:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goidts V, Bageritz J, Puccio L, Nakata S,
Zapatka M, Barbus S, Toedt G, Campos B, Korshunov A, Momma S, et
al: RNAi screening in glioma stem-like cells identifies PFKFB4 as a
key molecule important for cancer cell survival. Oncogene.
31:3235–3243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao R, Liu Y, Li D, Xun J, Zhou W, Wang P,
Liu C, Li X, Shen W, Su W, et al: PFKFB4 promotes breast cancer
metastasis via induction of hyaluronan production in a
p38-dependent manner. Cell Physiol Biochem. 50:2108–2123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ros S, Flöter J, Kaymak I, Da Costa C,
Houddane A, Dubuis S, Griffiths B, Mitter R, Walz S, Blake S, et
al: 6-Phosphofructo-2-kinase/fructose-2, 6-biphosphatase 4 is
essential for p53-null cancer cells. Oncogene. 36:3287–3299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chesney J, Clark J, Lanceta L, Trent JO
and Telang S: Targeting the sugar metabolism of tumors with a
first-in-class 6-phosphofructo-2-kinase (PFKFB4) inhibitor.
Oncotarget. 6:18001–18011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chesney J, Clark J, Klarer AC,
Imbert-Fernandez Y, Lane AN and Telang S: Fructose-2,
6-bisphosphate synthesis by 6-phosphofructo-2-kinase/fructose-2,
6-bisphosphatase 4 (PFKFB4) is required for the glycolytic response
to hypoxia and tumor growth. Oncotarget. 5:6670–6686. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun CM, Xiong DB, Yan Y, Geng J, Liu M and
Yao XD: Genetic alteration in phosphofructokinase family promotes
growth of muscle-invasive bladder cancer. Int J Biol Markers.
31:e286–e293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Lu C, Fang M, Yan W, Chen M, Ji
Y, He S, Liu T, Chen T and Xiao J: HIF-1α activates hypoxia-induced
PFKFB4 expression in human bladder cancer cells. Biochem Biophys
Res Commun. 476:146–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang P, Tao W, Lu C, Fan L, Jiang Q, Yang
C, Shang E, Cheng H, Che C, Duan J and Zhao M: Bruceine A induces
cell growth inhibition and apoptosis through PFKFB4/GSK3β signaling
in pancreatic cancer. Pharmacol Res. 169:1056582021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang G, Li S, Xue K and Dong S: PFKFB4 is
critical for the survival of acute monocytic leukemia cells.
Biochem Biophys Res Commun. 526:978–985. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dang CV: Cancer cell metabolism: There is
no ROS for the weary. Cancer Discov. 2:304–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Z and Hunter T: Metabolic kinases
moonlighting as protein kinases. Trends Biochem Sci. 43:301–310.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang J, Ren B, Yang G, Wang H, Chen G, You
L, Zhang T and Zhao Y: The enhancement of glycolysis regulates
pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. 2020.
View Article : Google Scholar : PubMed/NCBI
|