1. Introduction
Cardiac arrhythmias pose a significant global
burden, resulting in cerebrovascular accidents, myocardial
infarction and sudden cardiac death (SCD) (1). The worldwide prevalence of atrial
fibrillation (AF), characterized by uncoordinated atrial activation
with the consequent deterioration of atrial mechanical function, is
37,574 million cases (0.51% of worldwide population) (2). The prevalence of AF is escalating in
middle socio-demographic index countries, and its absolute burden
is projected to increase by >60% in 2050, being one of the
largest epidemics (2). The
prevalence of AF is approximately 0.77% in China, with the mean age
of patients being 52.5±22.4 years (3), with a lifetime risk of 1/5 among
Chinese adults based on a medical insurance database involving 10
million in Southwest China (4).
Premature ventricular contractions (PVCs) manifest from being
asymptomatic to the development of cardiomyopathy, heart failure,
sustained ventricular tachycardia (VT) or even total mortality. In
Europe and North America, 50-100 unexpected SCDs occur per 100,000
population annually, half of these events owing to VT or
ventricular fibrillation (VF) (5).
Although classic Western anti-arrhythmics that tend
to interact with a specific or single target mostly via inhibition
possess potent pharmacologic activity, they portend risks of
breaking intracellular stability and have been reported to be
arrhythmogenic (6). Consequently,
the development of safe and effective regimens remains a major
focus of contemporary cardiovascular research. With globalization,
an integration of the traditional Chinese and Western medical
practices has begun in multiple medical centers (7). In recent years, network pharmacology
is an effective approach which can be used to address the
association between multiple TCM components and the synergistic
effect of drugs on a series of targets. Modern Chinese herbal
medicines manufactured according to traditional theory, play a
significant role in the treatment of cardiac arrhythmias (8).
Wenxin Keli (WXKL) is a first-state sanctioned
Traditional Chinese Medicine (TCM)-based anti-arrhythmic drug
(8). It was officially marketed in
1995 as it received new certification issued by the Ministry of
Health of the People's Republic of China (9). In China, it possesses a licensable
indication for PVCs, which was incorporated in the 2009 revision of
the National Reimbursement Drug List (10). As a sole anti-arrhythmic herbal
extract in the 2010 revision of Chinese Pharmacopoeia, almost 5
million Asians are currently being administered WXKL for the
treatment of ventricular arrhythmias (11). Wenxin Keli is composed of 5
constituents: Nardostachys jatamansi DC (Gansong), Radix
Notoginseng (Sanqi), Succinum (Hupo), Polygonatum
sibricum (Huangjing) and Codonopsis pilosula (Dangshen)
(4,6,12-14).
Dangshen, the ‘monarch’ in the regimen, characterized by a sweet
flavor, boosts ‘qi’ (nutrients), tranquilizes the mind and relieves
palpitations. Huangjing, the ‘minister’ herb, enhances the effects
of Dangshen, and also possesses a sweet flavor and fortifies the
spleen ‘qi’ to nourish the heart and lungs. The remaining 3
ingredients are assistant herbs. The bitter-flavored Sanqi removes
blood stasis, relieves pain and tonifies the system. Hupo, neutral
and sweet, pacifies the liver and tranquilizes the mind. Gansong is
warm and sweet, relieves depression and regulates the liver and
spleen ‘qi’. Its indications include palpitations, dyspnea,
fatigue, dizziness, chest tightness and pain, and insomnia to
improve the quality of life (9,15).
In a rat model of ischemia-reperfusion (I/R) injury,
pre-treatment with WXKL was shown to effectively improve cardiac
hemodynamics, accelerate coronary blood flow, enhance myocardial
contractility, and reduce the occurrence of arrhythmias (16,17).
WXKL reportedly increases coronary blood flow, reduces myocardial
oxygen consumption, enhances myocardial compliance, improves
myocardial hypoxia tolerance, relieves anterior and posterior
cardiac loading, and reduces arrhythmic occurrence (16). The aim of the present review
article was to review the effects of WXKL on cardiac
electrophysiology and how these can prevent cardiac
arrhythmias.
2. Roles of individual components of
WXKL
Nardostachys jatamansi DC (Gansong).
Nardostachys chinensis
batal extract (NcBe) at 10 g/l has been shown to
inhibit both peak INa and transient outward potassium current (Ito)
in rat ventricular myocytes. These actions were associated with the
suppression of acetylcholine-induced AF or atrial flutter, likely
by reducing both triggered activity and reentry (18,19).
Additionally, it has been shown to inhibit L-type calcium currents
(ICaL) and shift the current-voltage association upward without
changing its activation or reversal potential (9). Calaxin, one of its components, has
been predicted to be a CaV1.2 channel blocker (6). The dried roots of NcBe contain
sesquiterpenoids, which can lower the incidence of ventricular
arrhythmias and improve Cx43 redistribution in rats with
hyper-acute myocardial infarction (MI) (20). This is probably due to the lowering
of the heterogeneity of conduction (21). Moreover, NcBe contains valerian
ketones that can reduce myocardial cell automaticity, extend the
atrial action potential (AP) of the conduction system time and
interrupt reentry (15). The
volatile oil of Nardostachys can inhibit oxidative
stress-induced cell death via reactive oxygen species scavenging
and Akt activation in H9c2 cardiomyocytes (22). Since increased oxidative stress is
associated with arrhythmogenesis, this may act to suppress
arrhythmias (1). Nardosinone, one
of the major extracts from Nardostachys, has been shown to prevent
angiotensin II (AngII)-induced cardiac hypertrophy in embryonic rat
hearts, particularly at a concentration of ≥100 µmol/l by
inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt and MEK/ERK
pathways (23).
Radix Notoginseng (Sanqi).
Notoginseng
has been reported to antagonize
Na+/K+-ATPase activity (24) and the effect of acetylcholine on
various human organs (18). This
may reduce sinus node and ectopic pacemaker activity, and improve
microcirculation (15). It exerts
multiple cardio-protective effects, including pro-angiogenesis,
anti-apoptotic, inflammatory cascade repression and
endothelium-dependent vasodilation (22). It protects hypoxia-reoxygenation
induced H9c2 cardiomyocyte injury at the dose of 25 µg/ml
associated with apoptosis via the phosphatidylinositol 3-kinase
(PI3K)/AKT and mitogen-activated protein kinases (MAPKs) pathways
(25). Pre-treatment with
ginsenoside, an active ingredient of Notoginseng, has been
shown to protect rat cardiomyocytes from oxidative injury by the
expression of respiratory chain complexes via the RhoA/ROCK
signaling pathway (26) and
through an increase in the activity of endogenous antioxidants and
suppression of intracellular Ca2+ (27). According to a systematic review and
meta-analysis of 18 randomized controlled trials (RCTs),
Notoginseng was shown to reduce cardiovascular events, such
as acute myocardial infarction (AMI), severe arrhythmia, heart
failure and intractable angina pectoris (28). Through the integration of the gene
interaction network, the role of Notoginseng in the
treatment of coronary heart disease has been shown to involve the
cytokine-cytokine receptor interaction pathway (29). It has also been shown to mitigate
aging-induced cardiomyocyte apoptosis possibly by increasing
oxidative stress, and mitochondrial function-related signaling
pathways, such as the manganese superoxide dismutase (MnSOD)
signaling pathway, and mitochondrial-related signaling molecules,
such as peroxisome proliferator-activated receptor-gamma
coactivator (PGC)-1α, light chain (LC)3β and Beclin-1(30).
Succinum (Hupo)
No effect of succinum on cardiac physiology has been
found to date, at least to the best of our knowledge.
Polygonatum sibiricum (Huangjing).
Polygonatum sibiricum
exhibits lipid-lowering and anti-atherosclerotic
effects and tends to reduce blood pressure (15,20).
Apart from increasing Na+-K+-ATP and
Ca2+-ATP activity in mouse brain cells, Huanjing
enhances anti-aging effects by reducing lipid peroxide and B-type
monoamine oxidase levels. Its anti-atherosclerotic effect may be
associated with the reduction of foam cells and hypolipidemic
activity (31). In addition, its
protective effects on adriamycin-induced acute heart failure in
rats may be related to its anti-inflammatory effects and to the
inhibition of cardiomyocyte apoptosis (32).
Codonopsis pilosula (Dangshen)
The component codonopsine is predicted to interact
with KV7.1 that is significant in cardiac repolarization phases 2
and 3(6). It contains inulin and
amino acids, and exerts anti-platelet aggregation and enhances
immunity (15). It attenuates
calcium influx and apoptosis induced by angiotensin II in H9c2
cardiomyoblasts (22). It has been
reported to induce phosphodiesterase inhibition, leading to an
increase in cAMP levels in rat myocytes (24). Codonopsis has been shown to
reverse the AngII plus Leu27 insulin growth factor II
(IGFII)-induced caspase-3 activity and the apoptosis of H9c2
cardiomyoblasts (33) by reducing
Ca2+ influx and mitochondrial outer membrane
permeability (34) (Fig. 1).
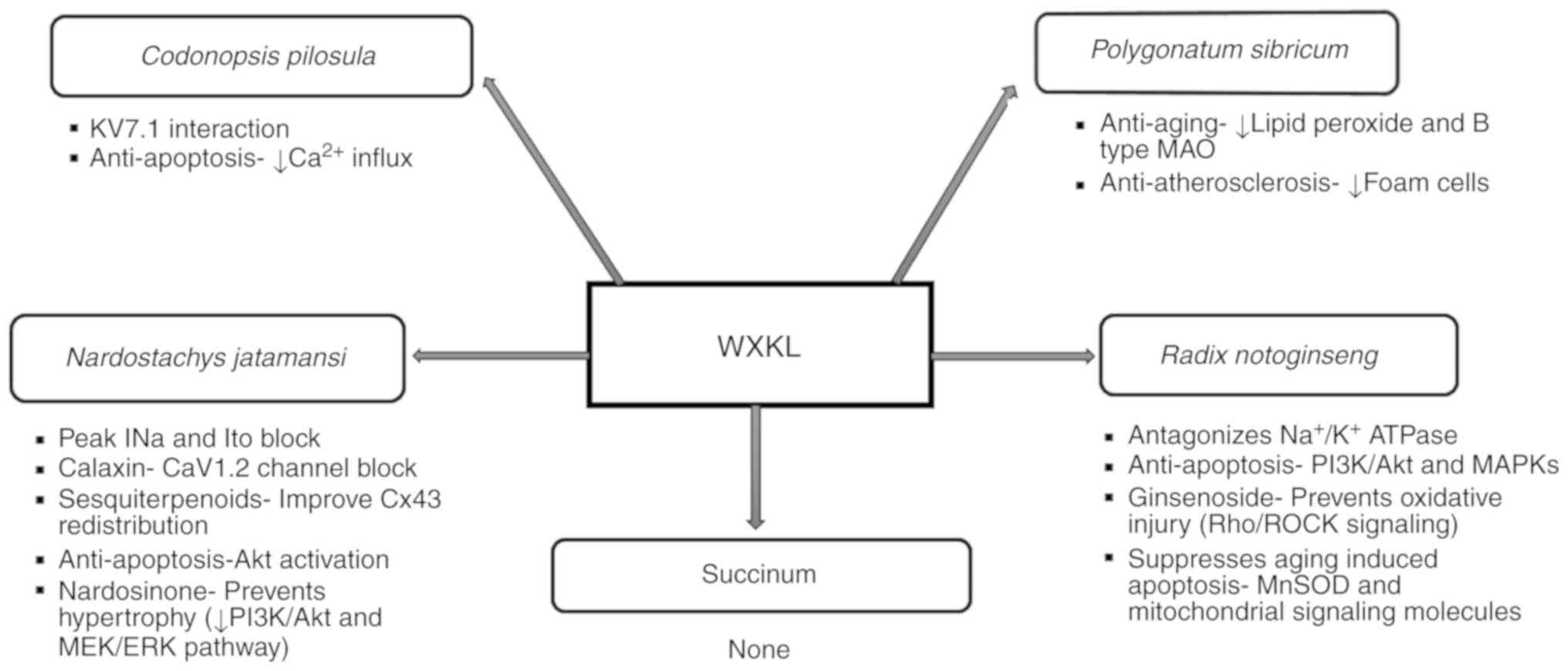 | Figure 1Diverse roles of individual
components of Wenxin Keli. AKT, protein kinase B; CaV1.2, L-type
voltage-dependent calcium channel 1.2; ERK, extracellular regulated
protein kinases; INa, fast sodium curent; Ito, transient outward
potassium current; KV7.1, voltage-activated K+ channel
7.1; MAO, monoamine oxidase; MAPKs, mitogen-activated protein
kinases; MEK, mitogen extracellular signal-regulated kinase;
Mn-SOD, manganese superoxide dismutase; PI3K, phosphatidylinositol
3-kinase; Rho/ROCK, ras homolog gene/a Rho-associated coiled
coil-forming proyein kinase; WXKL, Wenxin Keli. |
3. Effects of WXKL on atrial
electrophysiology
Sodium channel
The major atrial-ventricular Na channel disparities
are a more negative atrial INa steady-state inactivation and a
slower recovery from inactivation (35). Peak INa is responsible for phase 0
of the action potential. The density of peak INa is significantly
greater in atria than in ventricles, highest in Purkinje cells
(36).
The anti-AF effects of peak INa blockers are due
largely to the rate-dependent reduction of excitability, the
prolongation of the effective refractory period (ERP), and to the
conduction block in a critical part of reentrant circuit. The
reduction of peak INa can also significantly decrease intracellular
sodium (Nai) and, thus, intracellular calcium (Cai), which may
suppress Cai-mediated triggered activity (37). Severe structural remodeling,
resulting in a small number of connections between the
subendocardial and subepicardial layers of the atria, can prevent
termination of AF by Na-channel block (38).
While the atrial selectivity of other INa blockers
encompasses a preferential prolongation of action potential
duration (APD), WXKL at 5 g/l abbreviates APD90
predominantly in the atria (18).
It shifts the steady-state availability to more negative potentials
in atrial than in ventricular cells in a dose-dependent manner
(39). Such differing atrial and
ventricular effects may possibly be due to different ratios of
alpha and beta subunits. WXKL binds to the inactivated state of
peak INa and dissociates rapidly (39). Following ganglionated plexi (GP)
ablation, it has been shown to significantly attenuate the levels
of atrial natriuretic peptide (ANP), tumor necrosis factor (TNF)-α
and interleukin (IL)-6 within 10 weeks of oral administration at
0.25 g/kg/day, suggesting its anti-inflammatory effects (40).
The combination of 18 g 3 times daily of WXKL and
amiodarone has been shown to be safe and superior to amiodarone
monotherapy in the chemical conversion of recent-onset AF,
exhibiting a markedly shortened time in sinus rhythm restoration
(41). In another study, the same
dose of WXKL and 80 mg sotalol twice a day was shown to exhibit a
similar efficacy to assist sinus rhythm reversion from
hyperthyroidism-caused paroxysmal atrial fibrillation (PAF)
(42).
4. Effects of WXKL on ventricular
electrophysiology
Sodium channel
Late sodium channel (INaL) maintains phases 2 and 3
of cardiac AP. The rate-dependent reduction of INaL contributes to
rate-dependent APD abbreviation (43). The contribution of INaL is more
significant during prolonged APD and bradycardia. VT/VF most
commonly occur under the conditions of prolonged APD (44). The INaL inactivation occurs in
several hundreds of milliseconds and persists throughout the AP
plateau.
INaL suppresses VAs by reduction of repolarization
heterogeneity and intracellular Ca2+ secondary to the decrease of
late INa mediated Nai (37).
Increased intracellular Ca2+ alters repolarization such as T wave
alternans and causes DADs (45).
Its distribution is spatially heterogeneous in the ventricles
(46). INaL blockers such as
ranolazine effectively prevent or shorten rapid activation-induced
VT and VF in non-diseased ventricles (47).
In addition to its atrial selectivity, 5 g/l WXKL
has only a mild effect in reducing ventricular rapid INa. It binds
very rapidly to the inactivated sodium channel and dissociates
rapidly from the closed state (39). WXKL suppresses ventricular early
afterdepolarizations (EADs), delayed afterdepolarizations (DADs)
and triggered activities (TAs) by the selective inhibition of late
INa. The IC50 of the late INa by WXKL was approximately 3-fold
higher than that of the rapid INa in the rabbit left ventricle. In
addition, its weaker influence on rapid INa with little
use-dependence unlike classic INa blockers renders it less
proarrhythmic (48). WXKL at both
1 and 5 g/l exhibits anti-arrhythmic effects on Purkinje cells of
rabbit hearts via INaL inhibition by the shortening of APD and
attenuating ATX-II and ISO-induced EADs and DADs. The average
magnitude of INaL in these cells exceeded by 3-fold compared to
that in ventricular cells (12).
Potassium channel
Ito is the transient outward current resulting from
K+ efflux. It is a key regulator of phase one action
potential repolarization. Ito varies in early-onset lone AF,
Brugada syndrome and idiopathic VF (49). It is regulated by cold-inducible
RNA-binding protein (50).
Computational modeling predicts that in human ventricle, small
decreases in Ito slightly increase APD, while large increases can
shunt the AP and cause rapid repolarization (51).
The majority of SCD occurrences in patients with
heart failure (HF) is likely secondary to VAs. Heart failure with
preserved ejection fraction (HFpEF) increases the inducibility of
VA as a result of delayed repolarization and multiple reentry
circuits demonstrated by prolonged repolarization and Ito
downregulation, contributing to arrhythmogenesis (52).
WXKL at the dose of 8 g/kg daily has been shown to
prevent VAs in vivo following the long-term administration
in a rat model of MI associated with inhibition of ICaL and Ito. It
accelerates Ito inactivation without changing the activation
process or the recovery of its inactivation (8). The mutual mechanism in HF and
arrhythmia includes inflammation, oxidative stress and microRNA
regulation. Based on a systematic review and meta-analysis on 13
studies published in Chinese, WXKL plus amiodarone combination
group was shown to be superior in total effective rate, heart
function and reduction in PVCs compared to amiodarone alone. WXKL
downregulates genes associated with inflammation, neurohumoral
system and apoptosis (53,54).
When combined with a low concentration of 5 µM
quinidine, WXKL at 5 g/l inhibits Ito from epicardial and
endocardial sites of coronary-perfused canine right ventricular
wedge preparations in a dose-dependent manner (24). Consequently, it suppresses the
substrate and trigger for VT/VF in experimental models of Brugada
Syndrome (55,56).
5. Reactive oxygen species (ROS)
reduction
Oxidative stress leads to repolarization and
conduction abnormalities, eventually resulting in cardiac
arrhythmias (57). ROS abundance
increases cardiac fibrosis and hypertrophy and reduces inotropy.
Defective antioxidant mechanisms in the diabetic heart (58) are mitochondrial electron leakage,
NADPH oxidase and protein kinase C (PKC) activation, nitric oxide
synthase uncoupling (59) and a
common upstream pathway involving interaction of advanced glycation
end-products (AGEs) with their receptor (RAGE) to further promote
ROS synthesis (60). The
mitochondrial translocator protein (TSPO) links mitochondrial
instability to lethal arrhythmias through ROS-induced ROS release
(RIRR) (58).
Increased ROS production also activates the unfolded
protein response (UPR) that accordingly reduces ion channel
functionality and creates repolarization abnormalities (1). It decreases Cx43 protein levels in
the cell and perturbs gap junction conduction, resulting in
dysrhythmias and SCD. Additionally, intracellular Ca2+
homeostasis alters and causes fibrosis (61).
WXKL at at a dose of 3 g/kg has been shown to
improve intracellular mitochondrial membrane potential and oxygen
consumption in primary atrial fibroblasts by reducing ROS in
diabetic rats (62). WXKL has been
shown to lead to a recovery of the level of malondialdehyde (MDA),
the final product of lipid peroxidation, and superoxide dismutase
(SOD) in rats/rabbits subjected to I/R (63). WXKL also improves the secretions of
taurine and ketone bodies to overcome I/R-induced oxidative stress
(17).
6. Gap junction modulation
Gap junction proteins, connexins, mediate action
potential propagation between cells, coordinate mechanical and
electrical activity of heart muscle, and ensure simultaneous
cardiac electro-mechanical activity. The age-related
down-regulation of atrial Cx43 can facilitate the development of AF
in old guinea pig hearts (64) and
cause interstitial fibrosis and collagen deposition (65). In a canine model of sympathetic AF,
the low expression of Cx43 was shown to be involved in AF by
influencing inter-cellular channel conduction (66). Cx43 remodeling is associated with
atrial denervation following epicardial GP ablation and this is
ameliorated by WXKL (40)
(Table I).
 | Table IMechanisms of action of WXKL in
atrial arrhythmias. |
Table I
Mechanisms of action of WXKL in
atrial arrhythmias.
| Factors | Author/(Refs.) | Model | Study
characteristics | Mechanisms of
action of WXKL |
|---|
| Peak INa | Burashnikov et
al (18) | Canine right atrial
preparation | Ach-induced AF | Atrial selective
ERP prolongation Increase in DTE Ina block AF prevention |
| Mitochondrial
oxidative stress | Gong et al
(62) | Sprague-Dawley
rats |
H2O2 induced
oxidative stress and diabetic hearts | Regulation of
mitochondrial function Reduction of mitochondrial ROS |
| Gap junction | Xiao et al
(40) | Mongrel dogs | AF induced after
epicardial GP ablation | Inhibited Cx43
remodeling Attenuated ANP, IL-6 and TNF-α levels |
Cx43 is the primary gap junction protein that is
highly expressed within mammalian ventricular muscle at the
intercalated disk. A potential shift in its composition in the
ventricular conduction system may contribute to VAs in patients
with arrhythmogenic right ventricular cardiomyopathy (ARVC)
(67). Following MI, 2.7 g/kg WXKL
has been shown to protect the ultrastructure of gap junctions
within the intercalated disk, alleviate CX43 phosphorylation,
increase PKC and miRNA-1 levels, and increase the VF threshold
(22) (Table II).
 | Table IIMechanisms of action of WXKL in
ventricular arrhythmias. |
Table II
Mechanisms of action of WXKL in
ventricular arrhythmias.
| Factors | Author/(Refs.) | Model | Study
characteristics | Mechanisms of
action of WXKL |
|---|
| Ion channels
INaL | Xue et al
(48) | Rabbit LV
myocytes | Triggered VAs | Selective INaL
block Reduction of QT and Tp-e prolongation (1-3 mg/ml) Suppression
of EADs and DADs |
| | Hou et al
(12) | Rabbit hearts | ATX-II and ISO
induced arrhythmias in Purkinje cells | APD shortening
Suppression of EADs, DADs and TAs Selective INaL block |
| Ito | Wang et al
(8) | Rats in vivo | Ischemia induced
VAs | Reduced VT+VF
episodes ICaL and Ito block |
| | Minoura et
al (24) | Canine RV wedge
preparation | Experimental model
of Brugada syndrome | Suppression of
phase 2 reentry and polymorphic VT Ito and ICaL block |
| ICaL | Chen et al
(76) | Male rats | TAC-induced heart
failure | APD shortening ICaL
reduction by accelerating inactivation |
| | Minoura et
al (24) | Canine RV wedge
preparation | Experimental model
of Brugada syndrome | Suppression of
phase 2 reentry and polymorphic VT ICaL and Ito block |
| | Wang et al
(6) | Hartley guinea
pigs | Quinidine induced
arrhythmia | Alleviates
prolonged QT interval Cav1.2 channel block |
| | Xing et al
(69) | Sprague-Dawley
rats | MI induced
arrhythmia | Increased SR Ca2+
content and calcium transient amplitude |
| | Luo et al
(70) | Rabbit ventricular
myocytes |
Hypoxia/reoxygenation induced Ca2+
overload | Attenuation of Ca2+
overload APD shortening |
| | Hou et al
(12) | Rabbit hearts | ATX-II and ISO
induced arrhythmias in Purkinje cells | APD shortening Mild
reduction of ICaL |
| | Wang et al
(8) | Rats in vivo | Ischemia induced
VAs | Reduced VT + VF
episodes ICaL and Ito block |
| CaMKII | Xing et al
(69) | Sprague-Dawley
rats | MI-induced
arrhythmia | Reduced CaMKII and
Thr-286-phosphorylated CaMKII expression Increased RyR2
expression |
| | Yang et al
(14) | Sprague-Dawley
rats | TAC induced cardiac
hypertrophy | Reduced protein
level and phosphorylation of CaMKII Reduced Type III collagen
deposition |
| ANS | Minoura et
al (24) | Canine RV wedge
preparations | Experimental model
of Brugada syndrome | Increases ICaL via
indirect adrenergic stimulation Positive inotropic effect |
| | Zheng et al
(53) | Male adult
rabbits | MI | Downregulated genes
associated with neurohumoral system-endothelin 1 and ACE |
| Gap junctions | Wu et al
(22) | Sprague-Dawley
rats | MI | Alleviates Cx43
phosphorylation Increases miRNA-1 and PKC |
7. Calcium handling
Ca2+ plays a key role in the
excitation-contraction coupling and the activation of
Ca2+-dependent signaling pathways. APD prolongation as a
result of cardiac hypertrophy may increase the Ca2+
entry via ICaL during the long plateau phase resulting in
spontaneous sarcoplasmic reticulum (SR) Ca2+ release
(68).
WXKL has been shown to block ICaL reversibly in a
dose-dependent manner at 5 g/l in rabbit ventricular myocytes, in
rats with transverse aortic constriction (TAC)-induced heart
failure (19) and in an
experimental model of Brugada syndrome (24), by causing shift of ICaL activation
towards negative potentials (6).
In the TAC rat model of heart failure, WXKL was shown to reduce
ICaL by facilitating the steady-state inactivation and retarding
its recovery (19). In cardiac
myocytes from rats with MI, WXKL at 4 g/kg/day was shown to
significantly increase both the SR Ca2+ content and the
calcium transient amplitude (69).
WXKL inhibits hypoxia and the hypoxia/reoxygenation-induced
increase in diastolic [Ca2+]i and the
decrease in Δ[Ca2+]i. The effect of WXKL on
ICaL may be attributed to its suppressive effect on
[Ca2+]i transients (70). WXKL reduces the transmural
dispersion of repolarization and ameliorates calcium mishandling by
reducing [Ca2+]i overload among Purkinje
cells and in hypoxic/reoxygenated cardiomyocytes at 3 g/l in the
treatment of ventricular arrhythmias (12,70).
A decrease in both the expression of CaMKII and its phosphorylation
at Thr-286 may be the primary mechanism through which WXKL inhibits
heart failure and arrhythmia (69).
8. CaMKII signaling
CaMKII regulates SR Ca release and Ca reuptake
(71). It is an upstream regulator
of INaL in cardiac myocytes. The CaMKII-dependent phosphorylation
of ICaL attenuates its inactivation, increasing the ICaL window
current that plays a major role in the generation of EADs (72). Excess CaMKII activity due to HF
results in enhanced sympathetic excitation and hyperphosphorylation
of the ryanodine receptor (RyR), sarco/endoplasmic reticulum
Ca2+-ATPase (SERCA) and phospholamban (PLB) proteins,
which predisposes the cardiac myocytes to afterdepolarizations.
WXKL at 4 g/kg/day has been shown to significantly
reduce the expression of CaMKII, p-CaMKII (Thr-286) and PLB, and to
increase the expression of RyR2 and FKBP12.6 in rats with MI, to
inhibit arrhythmia (69). In rats
with TAC, the beneficial effects of WXKL and KN93, a pharmacologic
tool used to inhibit CaMKII, is attributed to the shortening of
APD90 and regulation of the CaMK II signal transduction pathway,
markedly decreasing the degree of collagen deposition and improving
fibrosis and hypertrophy (14).
9. Autonomic nervous system regulation
Shortened ventricular ER potential, APD,
fibrillation threshold, and triggered EAD and DAD by a high
sympathetic tone increase the risk of VAs (73). In canine models, the denervated
myocardium becomes hypersensitive to norepinephrine indicated by
the supersensitive shortening of ERP (74). In patients with long QT syndrome
(LQTS), VAs are triggered following a sympathetic burst from either
emotional stress or exercise. Experimentally, sympathetic
stimulation induces changes in ECG repolarization and the reduction
of the fibrillation threshold (75). The ischemic and infarcted
myocardium becomes a substrate sensitive to arrhythmia triggers due
to the heterogeneity of the sympathetic nervous system innervation
contributed by nerve sprouting (76).
Parasympathetic activation, on the other hand, is
considered to be anti-arrhythmogenic (77). In rodents, 100% VF episodes were
shown to be terminated with vagal nerve stimulation and the
efficacy was reduced in the presence of atropine (78,79).
The majority of episodes of VF in patients with Brugada syndrome
are observed during periods of high vagal tone, such as at rest,
during sleep, or from 12 to 6 a.m. (80,81).
The positive inotropic effect exerted by WXKL in
right ventricular wedge preparation is likely due principally to an
increase in ICaL secondary to the release of catecholamines. The
boost in ICaL also contributes to restoration of the action
potential dome and suppression of phase 2 reentry and pVT. WK
increases ICaL via indirect adrenergic stimulation (24). WXKL induces the release of
catecholamines from sympathetic nerve endings at the tissue level
exerting a tyramine-like effect (9). These ameliorative actions are
accompanied by normalization of the repolarization defects in the
epicardial AP and of the ECG by a diminution of the accentuated J
waves and ST segment elevation (24). The concentration of WXKL is
directly proportional to positive inotropy in wedge preparation,
contradicting to that in single myocytes (24). In rabbits with MI, treatment with
817 mg/kg/day WXKL downregulates genes associated with neurohumoral
system-endothelin 1 and angiotensin I converting enzyme (53).
10. Conclusion
Arrhythmia has multiple vulnerable facets that need
to be addressed individually for optimal patient management. To
date, WXKL has shown promising results in sinus rhythm reversion
and maintenance, the reduction of PVCs and the suppression of EADs
and DADs in a number of experimental and clinical models via
diverse effects on channels chiefly INa, ICaL and Ito, CaMKII and
its regulatory pathways, inflammatory mediators and P wave
dispersion. Its efficacy in suppressing arrhythmogenesis has been
compared to western drugs in several trials and the results have
been satisfactory. Its safety profile as compared to severe adverse
reactions and proarrhythmic tendencies of other western
antiarrhythmics has further validated its promising role. With
further identification of roles of gene and proteome-based
therapies, second messenger systems, intercellular conduction, NCX
inhibition, gating modifiers and ryanodine stabilizers, the scope
of managing arrhythmias has expanded as opposed to the sole
dependence of ion channel conductance-based therapies. Combining
these newer approaches with actions of WXKL under diverse
pathologies and circumstances could certainly fill the gaps in
treating inherited as well as acquired arrhythmic syndromes.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81970270 to
TL).
Availability of data and materials
Not applicable.
Authors' contributions
GT and TL put forward the conception and design of
this review. SD, MG and SG performed the review of the literature.
SD and MG contributed to the processing of the figure and tables.
SD, GT and TL were major contributors to the writing and revision
of the manuscript. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tse G, Yan BP, Chan YW, Tian XY and Huang
Y: Reactive oxygen species, endoplasmic reticulum stress and
mitochondrial dysfunction: The link with cardiac arrhythmogenesis.
Front Physiol. 7(313)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lippi G, Sanchis.Gomar F and Cervellin G:
Global epidemiology of atrial fibrillation: An increasing epidemic
and public health challenge. Int J Stroke: Jan 19, 2020 (Epub ahead
of print).
|
|
3
|
Murakoshi N and Aonuma K: Epidemiology of
arrhythmias and sudden cardiac death in Asia. Circ J. 77:2419–2431.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guo Y, Tian Y, Wang H, Si Q, Wang Y and
Lip GYH: Prevalence, incidence, and lifetime risk of atrial
fibrillation in China: New insights into the global burden of
atrial fibrillation. Chest. 147:109–119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
John RM, Tedrow UB, Koplan BA, Albert CM,
Epstein LM, Sweeney MO, Miller AL, Michaud GF and Stevenson WG:
Ventricular arrhythmias and sudden cardiac death. Lancet.
380:1520–1529. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang T, Lu M, Du Q, Yao X, Zhang P, Chen
X, Xie W, Li Z, Ma Y and Zhu Y: An integrated anti-arrhythmic
target network of compound Chinese medicine Wenxin Keli revealed by
combined machine learning and molecular pathway analysis
[corrected]. Mol Biosyst. 13:1018–1030. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kalifa J and Avula UM: The Chinese herb
extract Wenxin Keli: Atrial selectivity from the Far East. Hear
Rhythm. 9:132–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang X, Wang X, Gu Y, Wang T and Huang C:
Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in
rats: Involvement of L-type calcium and transient outward potassium
currents. Mol Med Rep. 7:519–524. 2012.
|
|
9
|
Tang Q: Effects of Nardostachys chinensis
Batal extract on sodium and calcium channels in rabbit ventricular
myocytes. Chin J Cardiol. 32:267–70. 2004.(In Chinese).
|
|
10
|
Brenyo A and Aktas MK: Review of
complementary and alternative medical treatment of arrhythmias. Am
J Cardiol. 113:897–903. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He M, Lv Z, Yang ZW, Huang JL and Liu F:
Efficacy and safety of Chinese herbal medicine Wenxin Keli for
ventricular premature be ats: A systematic review. Complement Ther
Med. 29:181–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hou J, Li W, Guo K, Chen XM, Chen YH, Li
CY, Zhao BC, Zhao J, Wang H, Wang YP and Li YG: Antiarrhythmic
effects and potential mechanism of WenXin KeLi in cardiac Purkinje
cells. Hear Rhythm. 13:973–982. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dong Y, Liao J, Yao K, Jiang W and Wang J:
Application of traditional Chinese medicine in treatment of atrial
fibrillation. Evid Based Complement Alternat Med.
2017(1381732)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang X, Chen Y, Li Y, Ren X, Xing Y and
Shang H: Effects of Wenxin Keli on Cardiac hypertrophy and
arrhythmia via regulation of the Calcium/Calmodulin dependent
Kinase II signaling pathway. Biomed Res Int.
2017(1569235)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang X, Wang Y, Feng X, Lu Y, Zhang Y,
Wang W and Zhu W: Systematic review and meta-analysis of randomized
controlled trials on Wenxin Keli. Drug Des Devel Ther.
10:3725–3736. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li J, Hu D, Song X, Han T, Gao Y and Xing
Y: The role of biologically active ingredients from natural drug
treatments for arrhythmias in different mechanisms. Biomed Res Int.
2017(4615727)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang M, Wang Q, Chen J, Wang Y, Fan G and
Zhu Y: Comparative metabonomics of Wenxin Keli and verapamil
reveals differential roles of gluconeogenesis and fatty acid
β-oxidation in myocardial injury protection. Sci Rep.
7(8739)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Burashnikov A, Petroski A, Hu D,
Barajas-Martinez H and Antzelevitch C: Atrial-selective inhibition
of sodium-channel current by Wenxin Keli is effective in
suppressing atrial fibrillation. Hear Rhythm. 9:125–131.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen Y, Li Y, Guo L, Chen W, Zhao M, Gao
Y, Wu A, Lou L, Wang J, Liu X and Xing Y: Effects of Wenxin Keli on
the action potential and L-type calcium current in rats with
transverse aortic constriction-induced heart failure. Evid Based
Complement Alternat Med. 2013(572078)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li M, Qiu R, Tian G, Zhang X, Li C, Chen
S, Zhang Q and Shang H: Wenxin Keli for Ventricular premature
complexes with Heart failure: A systematic review and meta-analysis
of randomized clinical trials. Complement Ther Med. 33:85–93.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tse G and Yeo JM: Conduction abnormalities
and ventricular arrhythmogenesis: The roles of sodium channels and
gap junctions. Int J Cardiol Heart Vasc. 9:75–82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu A, Zhao M, Lou L, Zhai J, Zhang D, Zhu
H, Gao Y, Shang H and Chai L: Effect of Wenxin Granules on Gap
Junction and miR-1 in rats with myocardial infarction. Biomed Res
Int. 2017(3495021)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Du M, Huang K, Gao L, Yang L, Wang WS,
Wang B, Huang K and Huang D: Nardosinone protects H9c2 cardiac
cells from angiotensin II-induced hypertrophy. J Huazhong Univ Sci
Technolog Med Sci. 33:822–826. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Minoura Y, Panama BK, Nesterenko VV,
Betzenhauser M, Barajas-Martínez H, Hu D, Di Diego JM and
Antzelevitch C: Effect of Wenxin Keli and quinidine to suppress
arrhythmogenesis in an experimental model of Brugada syndrome. Hear
Rhythm. 10:1054–1062. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun J, Sun G, Meng X, Wang H, Wang M, Qin
M, Ma B, Luo Y, Yu Y, Chen R, et al: Ginsenoside RK3 prevents
Hypoxia-Reoxygenation induced apoptosis in H9c2 Cardiomyocytes via
AKT and MAPK pathway. Evid Based Complement Alternat Med.
2013(690190)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li L, Pan CS, Yan L, Cui YC, Liu YY, Mu
HN, He K, Hu BH, Chang X, Sun K, et al: Ginsenoside Rg1 ameliorates
rat myocardial ischemia-reperfusion injury by modulating energy
metabolism pathways. Front Physiol. 9(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong
ZY, Zhao HB, Cui J, Xun SF, Huang XL, et al: Ginsenoside Rg1
protects rat cardiomyocyte from hypoxia/reoxygenation oxidative
injury via antioxidant and intracellular calcium homeostasis. J
Cell Biochem. 108:117–124. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song H, Wang P, Liu J and Wang C: Panax
notoginseng preparations for unstable angina pectoris: A systematic
review and meta-analysis. Phyther Res. 31:1162–1172.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu G and Wang J: Exploring mechanisms of
Panax notoginseng saponins in treating coronary heart disease by
integrating gene interaction network and functional enrichment
analysis. Chin J Integr Med. 22:589–596. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Z, Wang J, Song Y, He Y, Zhang C, Liu
C, Zhao H, Dun Y, Yuan D and Wang T: Panax notoginseng saponins
attenuate cardiomyocyte apoptosis through mitochondrial pathway in
natural aging rats. Phyther Res. 32:243–250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cui X, Wang S, Cao H, Guo H, Li Y, Xu F,
Zheng M, Xi X and Han C: A review: The bioactivities and
pharmacological applications of polygonatum sibiricum
polysaccharides. Molecules. 23(pii: E1170)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu X, Wu W, Chen X, Yang F, Zhang J and
Hou J: Protective effects of Polygonatum sibiricum polysaccharide
on acute heart failure in rats 1. Acta Cir Bras. 33:868–878.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chang KS, Lee NH, Kuo WW, Hu WS, Chang MH,
Tsai FJ, Tsai KH, Yang YS, Chen TS and Huang CY: Dung-Shen
downregulates the synergistic apoptotic effects of angiotensin II
plus Leu 27-IGF II on cardiomyoblasts. Acta Cardiol Sin. 30:56–66.
2014.PubMed/NCBI
|
|
34
|
Tsai KH, Lee NH, Chen GY, Hu WS, Tsai CY,
Chang MH, Jong GP, Kuo CH, Tzang BS, Tsai FJ, et al: Dung-Shen
(Codonopsis pilosula) attenuated the cardiac-impaired insulin-like
growth factor II receptor pathway on myocardial cells. Food Chem.
138:1856–1867. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Aguilar M and Nattel S: The past, present,
and potential future of sodium channel block as an atrial
fibrillation suppressing strategy. J Cardiovasc Pharmacol.
66:432–440. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Burashnikov A, Di Diego JM, Zygmunt AC,
Belardinelli L and Antzelevitch C: Atrium-selective sodium channel
block as a strategy for suppression of atrial fibrillation:
Differences in sodium channel inactivation between atria and
ventricles and the role of ranolazine. Circulation. 116:1449–1457.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Burashnikov A and Antzelevitch C: Role of
late sodium channel current block in the management of atrial
fibrillation. Cardiovasc Drugs Ther. 27:79–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gharaviri A, Verheule S, Eckstein J, Potse
M, Krause R, Auricchio A, Kuijpers NHL and Schotten U: Effect of
Na+-channel blockade on the three-dimensional substrate of atrial
fibrillation in a model of Endo-Epicardial dissociation and
transmural conduction. Europace. 20 (Suppl 3):iii69–iii76.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu D, Barajas-Martínez H, Burashnikov A,
Panama BK, Cordeiro JM and Antzelevitch C: Mechanisms underlying
atrial-selective block of sodium channels by Wenxin Keli:
Experimental and theoretical analysis. Int J Cardiol. 207:326–334.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xiao J, Zhao Q, Kebbati AH, Deng H, Wang
X, Dai Z, Yu S and Huang C: Wenxin Keli suppresses atrial substrate
remodeling after epicardial ganglionic Plexi ablation. Exp Clin
Cardiol. 18:153–157. 2013.PubMed/NCBI
|
|
41
|
Zhang N, Tse G, Dahal S, Yang Y, Gong M,
Chan CZY, Liu E, Xu G, Letsas KP, Korantzopoulos P, et al: Efficacy
of Wenxin Keli Plus Amiodarone versus Amiodarone Monotherapy in
treating recent-onset atrial fibrillation. Cardiol Res Pract.
2018(6047271)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Meng Z, Tan J, He Q, Zhu M, Li X, Zhang J,
Jia Q, Wang S, Zhang G and Zheng W: Wenxin Keli versus Sotalol for
paroxysmal atrial fibrillation caused by hyperthyroidism: A
prospective, open label, and randomized study. Evid Based
Complement Alternat Med. 2015(101904)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guo D, Lian J, Liu T, Cox R, Margulies KB,
Kowey PR and Yan GX: Contribution of late sodium current (INa-L) to
rate adaptation of ventricular repolarization and reverse
use-dependence of QT-prolonging agents. Hear Rhythm. 8:762–769.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Antzelevitch C: Electrical heterogeneity,
cardiac arrhythmias, and the sodium channel. Circ Res. 87:964–965.
2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sicouri S, Timothy KW, Zygmunt AC, Glass
A, Goodrow RJ, Belardinelli L and Antzelevitch C: Cellular basis
for the electrocardiographic and arrhythmic manifestations of
Timothy syndrome: Effects of ranolazine. Hear Rhythm. 4:638–647.
2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Qi D, Yang Z, Robinson VM, Li J, Gao C,
Guo D, Kowey PR and Yan GX: Heterogeneous distribution of INa-L
determines interregional differences in rate adaptation of
repolarization. Hear Rhythm. 12:1295–1303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Burashnikov A: Late INa Inhibition as an
Antiarrhythmic Strategy. J Cardiovasc Pharmacol. 70:159–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xue X, Guo D, Sun H, Wang D, Li J, Liu T,
Yang L, Shu J and Yan GX: Wenxin Keli suppresses ventricular
triggered arrhythmias via selective inhibition of late sodium
current. Pacing Clin Electrophysiol. 36:732–740. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xiao L, Koopmann TT, Ördög B, Postema PG,
Verkerk AO, Iyer V, Sampson KJ, Boink GJ, Mamarbachi MA, Varro A,
et al: Unique cardiac Purkinje fiber transient outward current
β-subunit composition: A potential molecular link to idiopathic
ventricular fibrillation. Circ Res. 112:1310–1322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li J, Xie D, Huang J, Lv F, Shi D, Liu Y,
Lin L, Geng L, Wu Y, Liang D and Chen YH: Cold-inducible
RNA-binding protein regulates cardiac repolarization by targeting
transient outward potassium channels. Circ Res. 116:1655–1659.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bohnen MS, Iyer V, Sampson KJ and Kass RS:
Novel mechanism of transient outward potassium channel current
regulation in the heart: Implications for cardiac electrophysiology
in health and disease. Circ Res. 116:1633–1635. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cho JH, Zhang R, Kilfoil PJ, Gallet R, de
Couto G, Bresee C, Goldhaber JI, Marbán E and Cingolani E: Delayed
repolarization underlies ventricular arrhythmias in rats with heart
failure and preserved ejection fraction. Circulation.
136:2037–2050. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zheng M, Liu Z, Liu N, Hou C, Pu J and
Zhang S: The effect of Wenxin Keli on the mRNA expression profile
of rabbits with myocardial infarction. Evid Based Complement
Alternat Med. 2016(2352614)2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zheng R, Tian G, Zhang Q, Wu L, Xing Y and
Shang H: Clinical safety and efficacy of Wenxin keli-amiodarone
combination on heart failure complicated by ventricular arrhythmia:
A systematic review and meta-analysis. Front Physiol.
9(487)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang G, Sau C, Lai W, Cichon J and Li W:
Sleep promotes branch-specific formation of dendritic spines after
learning. Science. 344:1173–1178. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Antzelevitch C and Patocskai B: Brugada
Syndrome: Clinical, Genetic, Molecular, Cellular, and Ionic
Aspects. Curr Probl Cardiol. 41:7–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
van Opbergen CJM, den Braven L, Delmar M
and van Veen TAB: Mitochondrial Dysfunction as Substrate for
Arrhythmogenic Cardiomyopathy: A search for new disease mechanisms.
Front Physiol. 10(1496)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ilkan Z and Akar FG: The mitochondrial
translocator protein and the emerging link between oxidative stress
and arrhythmias in the diabetic heart. Front Physiol.
9(1518)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ren X, Wang X, Yuan M, Tian C, Li H, Yang
X, Li X, Li Y, Yang Y, Liu N, et al: Mechanisms and treatments of
oxidative stress in atrial fibrillation. Curr Pharm Des.
24:3062–3071. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Faria A and Persaud SJ: Cardiac oxidative
stress in diabetes: Mechanisms and therapeutic potential. Pharmacol
Ther. 172:50–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Köhler AC, Sag CM and Maier LS: Reactive
oxygen species and excitation-contraction coupling in the context
of cardiac pathology. J Mol Cell Cardiol. 73:92–102.
2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gong M, Yuan M, Meng L, Zhang Z, Tse G,
Zhao Y, Zhang Y, Yuan M, Liang X, Fan G, et al: Wenxin Keli
regulates mitochondrial oxidative stress and homeostasis and
improves atrial remodeling in diabetic rats. Oxid Med Cell Longev.
2020(2468031)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Tian G, Sun Y, Liu S, Li C, Chen S, Qiu R,
Zhang X, Li Y, Li M and Shang H: Therapeutic effects of Wenxin Keli
in cardiovascular diseases: An experimental and mechanism overview.
Front Pharmacol. 9(1005)2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Nagibin V, Egan Benova T, Viczenczova C,
Szeiffova Bacova B, Dovinova I, Barancik M and Tribulova N: Ageing
related down-regulation of myocardial connexin-43 and up-regulation
of MMP-2 may predict propensity to atrial fibrillation in
experimental animals. Physiol Res. 65 (Suppl 1):S91–S100.
2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kato T, Iwasaki Y and Nattel S: Connexins
and atrial fibrillation. Circulation. 125:203–206. 2011.
|
|
66
|
Shu C, Huang W, Zeng Z, He Y, Luo B, Liu
H, Li J and Xu J: Connexin 43 is involved in the sympathetic atrial
fibrillation in canine and canine atrial myocytes. Anatol J
Cardiol. 18:3–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Paul M, Wichter T, Gerss J, Arps V,
Schulze-Bahr E, Robenek H, Breithardt G and Weissen-Plenz G:
Connexin expression patterns in arrhythmogenic right ventricular
cardiomyopathy. Am J Cardiol. 111:1488–1495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Milberg P, Fink M, Pott C, Frommeyer G,
Biertz J, Osada N, Stypmann J, Mönnig G, Koopmann M, Breithardt G
and Eckardt L: Blockade of I(Ca) suppresses early
afterdepolarizations and reduces transmural dispersion of
repolarization in a whole heart model of chronic heart failure. Br
J Pharmacol. 166:557–568. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Xing Y, Gao Y, Chen J, Zhu H, Wu A, Yang
Q, Teng F, Zhang DM, Xing Y, Gao K, et al: Wenxin-Keli regulates
the calcium/calmodulin-dependent protein kinase II signal
transduction pathway and inhibits cardiac arrhythmia in rats with
myocardial infarction. Evid Based Complement Alternat Med.
2013(464508)2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Luo A, Liu Z, Cao Z, Hao J, Wu L, Fu C,
Zeng M, Jiang W, Zhang P, Zhao B, et al: Wenxin Keli diminishes
Ca2+ overload induced by hypoxia/reoxygenation in
cardiomyocytes through inhibiting INaL and
ICaL. Pacing Clin Electrophysiol. 40:1412–1425.
2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Maier LS and Bers DM: Role of
Ca2+/calmodulin-dependent protein kinase (CaMK) in
excitation-contraction coupling in the heart. Cardiovasc Res.
73:631–640. 2007.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Heijman J, Voigt N, Wehrens XH and Dobrev
D: Calcium dysregulation in atrial fibrillation: The role of
CaMKII. Front Pharmacol. 5(30)2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lai Y, Yu L and Jiang H: Autonomic
neuromodulation for preventing and treating ventricular
arrhythmias. Front Physiol. 10(200)2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Inoue H and Zipes DP: Results of
sympathetic denervation in the canine heart: Supersensitivity that
may be arrhythmogenic. Circulation. 75:877–887. 1987.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yanowitz F, Preston JB and Abildskov JA:
Functional distribution of right and left stellate innervation to
the ventricles. Production of neurogenic electrocardiographic
changes by unilateral alteration of sympathetic tone. Circ Res.
18:416–428. 1966.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Chen PS, Chen LS, Cao JM, Sharifi B,
Karagueuzian HS and Fishbein MC: Sympathetic nerve sprouting,
electrical remodeling and the mechanisms of sudden cardiac death.
Cardiovasc Res. 50:409–416. 2001.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ng GA: Vagal modulation of cardiac
ventricular arrhythmia. Exp Physiol. 99:295–299. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Naggar I, Uchida S, Kamran H, Lazar J and
Stewart M: Autonomic boundary conditions for ventricular
fibrillation and their implications for a novel defibrillation
technique. J Physiol Sci. 62:479–492. 2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Meng L, Shivkumar K and Ajijola O:
Autonomic regulation and ventricular arrhythmias. Curr Treat
Options Cardiovasc Med. 20(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Takigawa M, Noda T, Shimizu W, Miyamoto K,
Okamura H, Satomi K, Suyama K, Aihara N, Kamakura S and Kurita T:
Seasonal and circadian distributions of ventricular fibrillation in
patients with Brugada syndrome. Hear Rhythm. 5:1523–1527.
2008.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Shen MJ and Zipes DP: Role of the
autonomic nervous system in modulating cardiac arrhythmias. Circ
Res. 114:1004–1021. 2014.PubMed/NCBI View Article : Google Scholar
|















