Introduction
Neonatal hyperglycaemia is a metabolic condition,
characterised by high blood glucose levels in newborn infants. It
can occur in neonates with a very low birth weight (VLBW) or an
extremely low birth weight (ELBW) and those who are critically ill
(1). There is no universally
accepted definition or consensus on blood glucose thresholds or
exposure time in the diagnosis of neonatal hyperglycaemia (2). However, it is commonly defined as
blood glucose levels exceeding 125 mg/dl (6.9 mmol/l) or plasma
glucose levels surpassing 150 mg/dl (8.3 mmol/l) (3).
The risk of developing hyperglycaemia increases with
a lower gestational age and birth weight. The cause of this
condition remains unknown; however, factors that may contribute
include a poorer insulin response to glucose in preterm infants,
delayed feeding and a decreased ability to suppress glucose
production. Pro-inflammatory cytokines and intensive care
interventions, such as the use of inotropes and corticosteroids,
may aggravate insulin resistance (2). The lack of a universally accepted
definition and blood glucose threshold makes it difficult to
determine the prevalence of neonatal hyperglycaemia. However,
recent studies conducted over the past 5 years have estimated the
incidence to range from 6 to 69% (4-8).
Neonatal hyperglycaemia is associated with negative consequences in
extremely preterm and VLBW/ELBW neonates, including sepsis,
intracranial haemorrhage, retinopathy of prematurity (ROP),
necrotising enterocolitis (NEC), the requirement for ventilator
support, growth retardation and increased mortality rates (9,10).
Currently, the long-term benefits of using insulin
therapy for managing neonatal hyperglycaemia are not well
supported, adding to the controversy surrounding its use (11). Insulin infusion may help with
maintaining nutritional intake and enhancing growth in neonates;
however, there is also evidence to suggest that it can further
complicate hypoglycaemia and hypokalaemia (12-14).
Further documentation is necessary to improve the effectiveness of
treatment approaches for neonatal hyperglycaemia. Given the
potential complications associated with this condition, it is
imperative to prioritise the timely assessment and implementation
of effective management strategies.
A number of previous studies on neonatal
hyperglycaemia have been published in Western countries (4-8,15-20).
The majority of the literature on this topic can be found in books
rather than clinical articles (21). Moreover, the majority of case
reports tend to focus only on neonatal hypoglycaemia (22-24).
Additionally, a critical gap emerges from the absence of
established guidelines for treating neonatal hyperglycaemia. The
current approach to defining and managing this condition heavily
relies on clinical judgment (13).
The absence of standardised protocols and treatment algorithms
emphasises the need for comprehensive research to develop
evidence-based guidelines, potentially starting with published case
reports. Moreover, neonatal hyperglycaemia is challenging to treat,
with numerous reports indicating poor outcomes associated with this
condition (4-8).
In an aim to bridge these critical gaps, the present
study describes a case of preterm ELBW neonate with hyperglycaemia.
To the best of our knowledge, this is the first case report of
neonatal hyperglycaemia in Indonesia. The objective is to emphasise
the importance of established guidelines for managing and
diagnosing hyperglycaemia, as well as to provide valuable insight
into comprehensive neonatal hyperglycaemia management. The present
study also discusses the effectiveness of insulin treatment in
managing hyperglycaemia and explores the associated morbidity
outcomes.
Case report
The patient in the present study was a female
neonate born extremely preterm with an ELBW. She was delivered at
25 weeks of gestation by a 31-year-old gravida 1, para 1 (G1P1)
woman via caesarean section at the Mother and Children Hospital in
Jakarta, Indonesia. The birth weight of the neonate was 550 g
(Fenton chart for preterm girls: between the 10 and 90th
percentile), with a length of 29 cm and a head circumference of 21
cm. During this pregnancy, the mother had experienced several
complications, including gestational hypertension, which was
managed with methyldopa and nifedipine and preeclampsia. Upon
admission, the blood pressure of the mother was 200/110 mmHg,
accompanied by proteinuria (3+) and signs of liver injury
(aspartate transaminase, 39 U/l; alanine transaminase, 25 U/l;
gamma-glutamyl transferase, 188 U/l). There was no reported history
of consanguinity. Prenatal tests for COVID-19, hepatitis B,
syphilis and human immunodeficiency virus all yielded negative
results. An analysis of the maternal family history revealed no
significant medical conditions, while that of the paternal family
revealed a history of hypertension and type 2 diabetes
mellitus.
Upon delivery, the patient displayed a low-pitched
cry, acrocyanosis, diminished muscle tone and a heart rate below
100 beats per minute (bpm). The appearance, pulse, grimace,
activity and respiration scores registered 6 and 7 at 1 and 5 min,
respectively (Fig. 1). Immediate
measures, including rapid assessment, warming, drying, stimulation,
airway positioning and suctioning, were undertaken. However, the
patient displayed no immediate response. Subsequent positive
pressure ventilation resulted in an increase in heart rate,
observable bilateral chest expansion and in an improved muscle
tone. Capillary refill time exceeded 3 sec, necessitating
intravenous fluid resuscitation prior to transfer to a level III
neonatal intensive care unit (NICU). Upon arrival at the NICU, the
condition of the patient remained stable, with minimal substernal
retraction noted during a physical examination. The transition from
positive pressure ventilation to non-invasive motion ventilation
was initiated, and the patient continued to maintain stability. The
placement of an umbilical venous catheter and blood sample
collection via the heel-prick method was then carried out.
A laboratory workup revealed a low erythrocyte
count, thrombocytopenia and leukopenia. At 1 h of life, her fasting
glucose levels were 33 mg/dl, which was defined as a hypoglycaemia
episode. To address this episode, the patient was administered a
10% bolus dextrose infusion (PT Otsuka Indonesia) with a dose of 2
ml/kg body weight (BW) or 1.1 ml at a rate of 1 ml/min, followed by
a continuous infusion of PG1 parenteral solution (PT Fresenius Kabi
Indonesia) at a dose of 60 ml/kg BW/day (volume, 30 ml). This was
administered along with lipids at 2 g/kg BW/day (volume, 5.5 ml)
and a maintenance dose of 10% dextrose (volume, 3 ml). The PG1
parenteral nutrition is the standard formula in the Indonesian
setting. It includes amino acids (17 ml), D40% (6.2 ml), D10% (5.3
ml), calcium (1.2 ml) and KCL (0.3 ml) (total volume, 30 ml). The
glucose infusion rate (GIR) was set at 4.2 mg/kg BW/min. By the 3rd
h of life, her blood glucose levels had risen to 71 mg/dl, leading
to an adjustment in the GIR to 6.9 mg/kg BW/min, following the
local guideline of 0-1 day of life (DOL) total parental nutrition,
which allows for an increase in GIR 4-6 mg/kg BW/min (25). Total parenteral nutrition is
adjusted daily based on glucose level and total solution target
determined in the local guideline.
Upon monitoring for 2 h, her blood pressure was
below the 3rd percentile and did not reach the target mean arterial
pressure (MAP) of >30 mmHg. Monitoring MAP is the main method
for assessing changes in circulatory function during the first DOL
in preterm infants (26). The most
commonly used criterion for hypotension is MAP #x003C;30 mmHg,
based on evidence that suggests the lower limit of the blood
pressure autoregulatory curve is ~28 to 30 mmHg in neonatal animal
models and preterm neonates (27).
It was assumed that there was a contractility disorder and it was
thus decided that dobutamine at 20 mcg/kg BW/min or 44 ml/kg BW/day
(rate of 1 ml/h) and dopamine 5 mcg/kg BW/min or 10.9 ml/kg BW/day
(rate of 0.25 ml/h) should be used in order to provide
cardiovascular support and improve blood pressure. During the
hourly cardiovascular monitoring, the condition of the patient
improved, prompting adjustments in medication doses according to
the hemodynamic status of the patient.
Following 24 h of monitoring, her blood glucose
surged to 451 mg/dl, indicating hyperglycaemia, requiring treatment
with an insulin bolus of 0.1 U and GIR adjustment to 10.9 mg/kg
BW/min to prevent sudden hypoglycaemia. At 1 h following the
administration of insulin, her blood glucose level remained high at
465 mg/dl; thus, parenteral nutrition adjustment was made by
lowering the GIR to 4.2 mg/kg BW/min, while closely monitoring the
glucose level. Following the direction of the neonatologists at the
hospital, if the blood glucose level of the patient were to exceed
250-350 mg/dl, bolus insulin would be administered at a dose of
0.05 U subcutaneously. If the blood glucose level was to exceed 350
mg/dl, 0.1 U insulin bolus would be administered. Blood glucose
monitoring should be carried out 4-6 times per day, 2 h following
insulin administration. Following insulin administration and GIR
adjustments, her blood glucose levels during the hospitalization
period remained #x003C;250 mg/dl; thus, no more insulin was
administered. The vital signs and glucose monitoring of the patient
during the hospitalization period in the NICU were recorded and are
presented in Fig. 2.
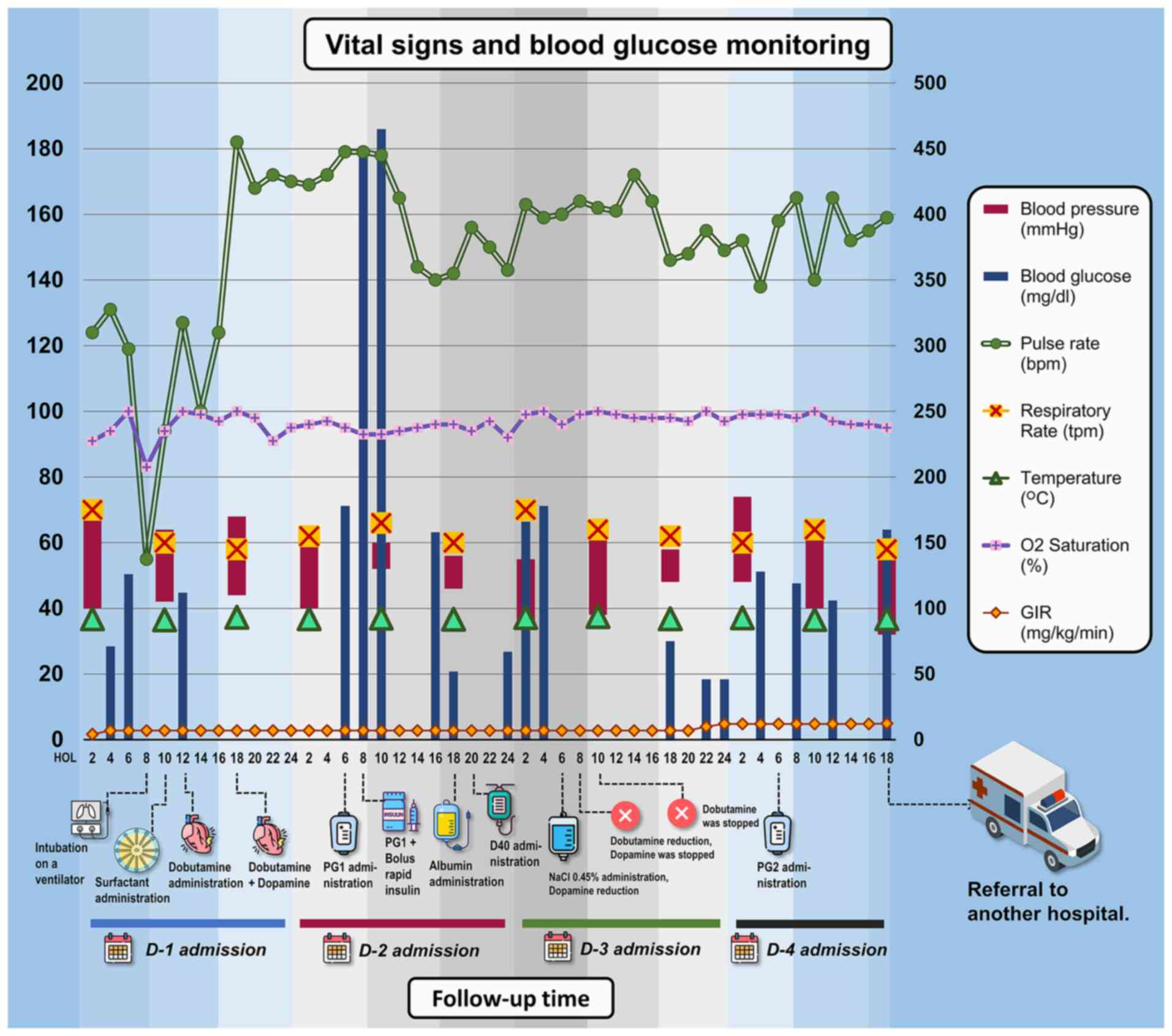 | Figure 2Vital sign monitoring of patients
during hospitalization in the NICU for 4-day monitoring. There was
a sudden surge in blood glucose levels during the first day of
admission to the NICU, worsening the condition of the patient. The
patient was admitted at 7 a.m. and received dobutamine at 10 h of
life and dopamine at 18 h of life on the first day to stabilise her
hemodynamic before being referred to another hospital on the 4th
day. An unstable condition occurred at the 8th h of life when the
patient was resuscitated and started using a ventilator. The
repeated episodes of hyperglycaemia (>125 mg/dl) mostly occurred
during the second day of admission, with a peak of 465 mg/dl. In
the image, the parameters of blood pressure, pulse rate,
respiratory rate, temperature, O2 saturation and GIR are plotted on
the left y-axis, while the blood glucose parameters are plotted on
the right y-axis. bpm, beats per minute; D, day; D40, dextrose 40%;
GIR, glucose infusion rate; HOL, hours of life; NaCl, sodium
chloride; NICU, neonatal intensive care unit; PG1/PG2, parental
nutrition; tpm, times per min. |
During her stay in the NICU, the patient experienced
a sudden apneic episode with marked substernal retraction,
requiring immediate intubation. Pulmonary surfactant was
administered, and a blood gas analysis revealed respiratory
alkalosis (Table I). While on a
ventilator, the patient remained stable with the following
settings: Assist control volume guaranteed mode, tidal volume of 6
ml/kg BW (~3.3 ml), respiratory rate of 60, positive end-expiratory
pressure of 5, a fraction of inspired oxygen of 21%, and an
inspiration:expiration (I:E) ratio of 1:2. Volume guaranteed is a
ventilation mode option that targets the tidal volume of the
patient and maintains it within a normal range to prevent volume
trauma. This mode of setting provides a consistent tidal volume
with every inflator cycle. The main advantage of this ventilation
mode is its ability to adapt to rapidly changing lung compliance
due to surfactant therapy. With this setting, the baby can maintain
good vital signs (oxygenation) and is well-adapted. The
neonatologist adjusted the ventilator settings based on the
condition of the patient, experience and clinical presentation
(28,29).
 | Table IBlood gas analysis results of the
patient (1 h after admission) in the NICU facility. |
Table I
Blood gas analysis results of the
patient (1 h after admission) in the NICU facility.
| Components | Results | Reference values of
premature babies |
|---|
|
PCO2 | 21.7 mmHg | 45-55 mmHg |
| PO2 | 82 mmHg | 40-60 mmHg |
| pH | 7.465 | 7.28-7.32 |
|
SaO2 | 97% | >80% |
|
HCO3- | 15.6 mmol/l | 22-24 mmol/l |
| BE | -8 mmol/l | ±2 mmol/l |
The patient also received intravenous meropenem (PT
Dexa Medica) at a dose of 40 mg/kg BW/dose (~22 mg twice a day) as
a prophylactic antibiotic. Additionally, micafungin supplied (PT
Combined Imperial Pharmaceuticals) was administered at a dose of
10-15 mg/kg BW/day (~4 mg/day) to prevent fungal infection and
early-onset neonatal sepsis. Neonatal infections can lead to
significant mortality and morbidity, particularly in ELBW infants,
and are challenging to treat. The high prevalence of enterobacteria
producing extended-range beta-lactamases in Indonesia makes
meropenem particularly advantageous due to its broader
antibacterial spectrum (30). This
enables the use of monotherapy instead of combination therapy.
Additionally, VLBW and ELBW infants have the highest incidence of
Candida spp. fungal infection. Micafungin is the only
medication approved for use in neonates by the European Medicine
Agency and is effective and safe (31-33).
The use of antibiotics was terminated once no infection was
detected based on a laboratory assessment. The choice of empirical
treatment depends on the clinical setting and local epidemiology,
making it difficult to standardise therapies for VLBW and ELBW
infants. The variability in clinical techniques used to treat
bacterial and fungal diseases may be influenced by the lack of
evidence-based guidelines.
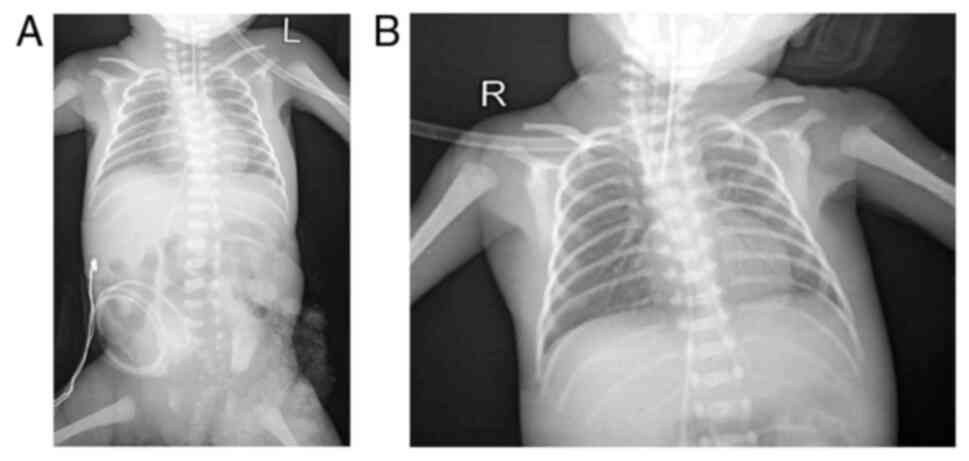
Thoraco-abdominal radiography was also performed in
the patient in the present study (Fig.
3), and this indicated grade IV hyaline membrane disease with
grade II NEC. An echocardiogram revealed a patent foramen ovale
with good heart contractility. Haematological analysis revealed
haemoglobin levels of 15.5 g/dl, a haematocrit of 44.1%, a
leukocyte count of 5.75x103/µl, and blood type O
positive.
On the third day in the NICU, the patient responded
well to the treatment provided. She received 80 ml/kg BW of PG2
solution, which is the same formula as PG1, but with additional
normal saline. This solution is only administered when the baby is
between 1 and 2 DOL. The blood glucose levels stabilised after
three consecutive monitoring sessions, with levels of 119, 106 and
160 mg/dl, respectively (with a GIR of 12.4 mg/kg BW/min). However,
due to socioeconomic and healthcare facility-related constraints,
the baby was referred to a public hospital while in stable
condition, with a blood glucose level of 78 mg/dl.
Discussion
Case reports on neonatal hyperglycaemia are limited,
particularly from Indonesia. The present study found six
international case reports that are summarised in Table II (15-20)
and Table III (4-8).
No definitive guidelines for neonatal hyperglycaemia have been
established to date, at least to the best of our knowledge
(10). Although the reports
presented in Tables II and
III did not consistently specify
the definitions used, all patients in those reports had a blood
glucose level >125 mg/dl (which aligns with the criteria for
hyperglycaemia that we referred to American Academy of Pediatrics
(AAP). The patient described herein has similarities with the cases
described in the studies by Hemachandra and Cowett (16), and Muzzy Williamson et al
(20), as all three cases involve
extremely preterm infants with VLBW. In the case in the present
study, the blood glucose levels were normal before escalating to
hyperglycaemic levels. However, the management approach used herein
aligns more closely with that used in the studies by Ferguson
(15), Yafi (17) and Fargas-Berríos et al
(19). In contrast to Hemachandra
and Cowett (16), Muzzy Williamson
et al (20) and Manzar
(18), who chose to reduce GIR to
initiate treatment, in the present study, the authors opted to
initiate insulin therapy from the beginning.
 | Table IISummary of findings of case reports
of hyperglycaemia among neonatal patients published worldwide. |
Table II
Summary of findings of case reports
of hyperglycaemia among neonatal patients published worldwide.
| Author(s), year of
publication (country) | Sex | Age | BW | Defining case | Treatment | Outcomes | (Refs.) |
|---|
| Ferguson, 1967
(UK) | M | Full term | 2,510 g | • Hyperglycaemia
onset: 5th DOL. • Post-FBG levels: 200-250 mg/dl, with concurrent
positive proteinuria. • FBG remained normal, leading to insulin
withholding. | On 10th DOL,
insulin initiation: 1 unit 3 times daily, escalating to 2 units 8
times daily after 1 week | Following the
initiation of insulin therapy, rapid weight gain occurred. After 11
days, a gradual reduction in insulin dosage was implemented. | (15) |
| Hemachandra and,
Cowett, 1999 (USA) | M | 26 weeks | 900 g | Serum glucose level
(unexplained: Fasting/random) on 3rd DOL exceeded 250 mg/dl, with
positive glycosuria. | Despite reducing
GIR by lowering dextrose concentration (from D10% to D5%) and
decreasing rate (from 16 mg/kg BW/min to 12 mg/kg BW/min), glucose
concentrations remained elevated at 320 and 410 mg/dl.
Consequently, insulin treatment initiated at 0.05 U/kg BW/h. | On 4th DOL, infant
received intravenous insulin, tolerating total parenteral nutrition
with 5% dextrose at 12 mg/kg BW/min and 2 g/kg BW/day amino acids
through a CVC. Glucose concentrations maintained within 100-159
mg/dl range. | (16) |
| Yafi, 2014
(USA) | M | 37 weeks | 1,565 g | • Initial blood
glucose levels (unexplained: Fasting/random): 85, 81 and 91 mg/dl.
• Initial central glucose level: 175 mg/dl. • By 24 HOL, central
glucose levels rose to 697 and 843 mg/dl. | Insulin
administration commenced at 25 HOL at a rate of 0.1 U/kg BW/hour.
On the 7th DOL, the patient initiated breastfeeding supplemented
with formula. The insulin drip was transitioned to a basal-bolus
subcutaneous insulin regimen normalized with feedings. | Blood glucose
levels were maintained within the range of 150-200 mg/dl. | (17) |
| Manzar, 2015
(USA) | M | 38 weeks | N/A | Initial blood
glucose (unexplained: fasting/random) exceeded 200 mg/dl. | The GIR was
subsequently reduced to 2.6 mg/kg BW/min. | The blood glucose
level normalized within 24 h. | (18) |
| Fargas-Berríos
et al, 2015 (Puerto Rico) | F | 39 weeks | 2,041 g | • Initial blood
glucose: 65 mg/dl (unexplained: fasting/random) • Following milk
formula feeding, blood glucose levels rose to 206-385 mg/dl | Continuous
intravenous regular insulin infusion commenced on the 13th HOL and
ceased upon achieving adequate glycemic control (104 mg/dl). | • In NICU, blood
glucose levels rose to 320-415 mg/dl, initiating continuous IV
regular insulin infusion (0.1 U/kg BW/h). • Due to hypoglycaemia,
insulin infusion discontinued, and carbohydrate infusion increased.
Subsequently, insulin lispro injection at 0.5 U given if
blood glucose exceeded 200 mg/dl. • Blood glucose ranged from 46 to
286 mg/dl over next 3 days, with adequate glycemia achieved at 5
WOL. | (19) |
| Muzzy Williamson
et al, 2020 (USA) | F | 23 weeks + 2
days | 520 g | • Blood glucose
(unexplained: fasting/random) on 3rd DOL: 180 mg/dl, with GIR of
4.2 mg/kg BW/min. • By 8th DOL, blood glucose levels persisted
above 190 mg/dl despite reduced GIR (3.6 mg/kg BW/min). | Insulin infusion
was initiated at a concentration of 0.25 U/ml in 0.9% NaCl,
administered at a rate of 0.02 U/kg BW/h. | On 9th DOL, blood
glucose reached 250 mg/dl despite continuous IV regular insulin at
0.12 U/kg BW/h and GIR of 4.1 mg/kg BW/min. | (20) |
 | Table IIISummary of findings of
epidemiological research on hyperglycaemia among neonatal patients
published worldwide in the last 5 years. |
Table III
Summary of findings of
epidemiological research on hyperglycaemia among neonatal patients
published worldwide in the last 5 years.
| Author(s), year of
publication (country) | Study design |
Population/sample | Hyperglycaemia
definition | Prevalence | Treatment | Outcomes | (Refs.) |
|---|
| Villamizar et
al, 2020 (USA) | Retrospective
cohort | 97 Infants
#x003C;32 weeks GA, #x003C;1,500 g BW, admitted to the NICU,
University of Minnesota Masonic Children's Hospital from February,
2012 to June, 2016. | • Blood glucose
levels >150 mg/dl (>8.3 mmol/l) • Patients grouped by
hyperglycaemia duration: 0 days, 1-4 days, ≥5 days. | 48.5% | Adjustment of GIR
without insulin mentioned. | ≥5 days of
hyperglycaemia negatively associated with fat mass and fat-free
mass z-scores at discharge, and fat-free mass z-scores at 4 months
corrected age. Persistent hyperglycaemia was also associated with
impaired neurodevelopment at 12 months corrected age. | (7) |
| Adeniji et
al, 2020 (Nigeria) |
Cross-sectional | 300 Neonates were
admitted to the special care baby unit at Wesley Guild
Hospital. | Blood glucose
levels ≥126 mg/dl (≥7.0 mmol/l). | 6.0% | N/A | Neonatal
hyperglycaemia was associated with parental low socioeconomic
class, maternal lack ofANC, vaginal delivery, grand multiparity,
outborn status, respiratory distress, probable sepsis, and neonatal
anaemia. Respiratory distress and probable sepsis independently
predicted hyperglycaemia. Hyper-glycaemia was significantly
associated with mortality. | (8) |
| Zamir et al,
2021 (Sweden) | Retrospective
cohort | 533 Preterm infants
#x003C;27 GA with complete glucose measurements and insulin therapy
data available during the first 28 DOL | Hyperglycaemia was
categorised as: • >8 mmol/l (>145 mg/dl), • >10 mmol/l
(>180 mg/dl), • >12 mmol/l (>216 mg/dl), or • >14
mmol/l (once or on 2 or 3 consecutive days during the first 28
DOL. | 69% | No standardised
insulin protocol; insulin treatment was given based on clinical
judgment. | Neonatal
hyperglycaemia (>8 mmol/l) was linked to lower intelligence
scores and worse motor outcomes at 6.5 years of age. Insulin
treatment was not associated with improved or worsened
neurodevelopmental outcomes. | (6) |
| Boscarino et
al, 2021 (Italy) | Prospective
cohort | 280 Preterm infants
#x003C;32 weeks GA or #x003C;1,500 g BW | Hyperglycaemia was
defined as 2 consecutive blood glucose levels >10 mmol/l
(>180 mg/dl) at least 3 h apart. | 29% | Moderate
hypergly-caemia was treated by reducing IV glucose concentration.
Severe hypergly-caemia was managed with insulin nfusion, titrated
every 4 h to maintain blood sugar #x003C;180 mg/dl. | Hyperglycaemia was
identified as a risk factor for motor delay. | (4) |
| El-Shimi et
al, 2022 (Egypt) | Prospective
cohort | 125 low birth
weight neonates (#x003C;2,500 g BW) | • Mild
hyperglycaemia 151-180 mg/dl • Moderate hyperglycaemia: 181-210
mg/dl • Severe hyperglycaemia: >210 mg/dl. | 24% | Moderate
hypergly-caemia was treated by decreasing GIR. Insulin therapy was
initiated if hypergly-caemia >200 mg/dl despite GIR
reductions. | Hyperglycaemia in
low-birth-weight infants was linked to morbidity and mortality.
Insulin treatment may be associated with hypoglycaemic episodes and
mortality. | (5) |
In the present study, it was not possible to
evaluate the long-term results of the patient as she needed to be
referred to another hospital. However, Gonzalez Villamizar et
al (7) discovered that infants
with a gestational age #x003C;32 weeks who have persistent
hyperglycaemia experience a decrease in lean mass at 4 months of
post-menstrual age and have unfavourable neurodevelopmental
outcomes at 12 months of post-menstrual age. Zamir et al
(6) also found that neonatal
hyperglycaemia was associated with lower intelligence scores and
poorer motor outcomes at 6.5 years of age. Boscarino et al
(4) concluded that hyperglycaemia
was a risk factor for motor delay.
Therapeutic approach for patients:
Highlighting the use of insulin
In clinical practice, managing neonatal
hyperglycaemia involves conservative and medical approaches.
Conservative therapy focuses on moderating glucose intake,
minimising predisposing factors, providing supportive care to
alleviate stress and ensuring adequate caloric intake (11-13).
On the other hand, medical therapy involves the administration of
insulin to achieve euglycaemia and improve nutrient absorption.
Medical intervention is necessary when blood glucose levels exceed
200 mg/dl, with a minimum 4-h interval between glucose measurements
and glucosuria exceeding +2 (11-13).
The strategy for treating neonatal hyperglycaemia used in the
patient described herein included adjusting the GIR to maintain
appropriate blood glucose levels, monitoring blood glucose and
administering insulin treatment. Following 2 h of insulin
treatment, the blood glucose level remained #x003C;250 mg/dl.
The initial step in addressing neonatal
hyperglycaemia is evaluating the GIR. The GIR can be lowered by
adjusting the infusion rate or the intravenous dextrose
concentration. With continuous glucose monitoring, reductions in
GIR typically occur gradually, ranging from 1-2 mg/kg BW/min every
2 h, until a GIR of 4 mg/kg/min is reached or until the dextrose
concentration is lowered to a minimum of 5% (11,12).
In the case that a low GIR (4 mg/kg BW/min) persists with
hyperglycaemia, this may indicate relative insulin shortage or
insulin resistance. The consideration for insulin administration
occurs when blood glucose levels exceed 250 mg/dl and urine glucose
has a reading of 2+ or higher in two samples collected at intervals
of at least 4 h (12). Insulin can
be administered by adding it to maintenance fluids or by
administering it independently. Bolus insulin administration is
rarely used due to the increased risk of hypoglycaemia (11). Initial insulin infusion dosages
typically range from 0.01 to 0.05 units/kg BW/h, with adjustments
made based on blood glucose levels in increments of 0.01 units/kg
BW/h. The maximum dosage allowed is 0.1 units/kg BW/h, with the
treatment goal being to maintain blood glucose levels between 100
and 150 mg/dl (11,34).
In neonates, the initial intravenous glucose
administration is maintained at a rate of 5-8 mg/kg BW/min to
maintain blood glucose levels within the normal range. Blood
glucose levels are typically checked every 4-6 h, although during
the initial insulin administration, they can be checked, every 2-3
h to prevent prolonged episodes of low blood sugar. In the case
that blood glucose levels exceed 145 mg/dl (8 mmol/l), a urine
glucose examination is necessary (12,35).
If glycosuria exceeds +1, the infant is at risk of increased
osmolality, which can lead to excessive urination and weight loss.
In such cases, adequate fluid therapy and frequent glucose
monitoring (every 1-2 h) are necessary (12). Recent studies have shown the
effectiveness of continuous glucose monitoring (CGM) in regulating
glucose levels and reducing the risk of both high and low blood
sugar in preterm infants (5,14).
In the case in the present study, CGM was commenced after a
hyperglycaemic episode that reached 451 mg/dl and peaked at 465
mg/dl. Following 3 h of CGM, the glucose levels stabilised. The
NICU team used CGM to prevent further hyperglycaemic events, in
line with the findings of the study by El-Shimi et al
(5) on the management of
hyperglycaemia.
Reflecting on the case described herein, caution
needs to be exercised due to the possibility of unexpected low
blood sugar levels (sudden episode of hypoglycaemia) when insulin
is administered continuously (3).
The risk of hypoglycaemia is a concern, particularly in ELBW
newborns, where insulin pharmacokinetics may be unpredictable
(35). This can be prevented by
regularly monitoring blood glucose levels. In the case that
hypoglycaemia occurs, insulin infusion must be discontinued and
treated with dextrose 10% boluses (2 ml/kg BW) (3). At this point, the strategy of
adjusting GIR to prevent hypoglycaemia during the initial
administration of insulin should also be considered. The rationale
for this lies in the ongoing debate surrounding the efficacy of
insulin therapy for neonatal hyperglycaemia. The justification for
administering insulin in the context of hyperglycaemia management
in newborns, while simultaneously adjusting GIR to prevent sudden
hypoglycaemic episodes, stems from the need to balance glycaemic
control without risking hypoglycaemia. Adjusting GIR allows for the
fine-tuning of glucose delivery to maintain blood glucose levels
within a safe range while insulin works to lower elevated levels.
This approach aims to prevent sudden drops in blood glucose
concentration, thereby minimising the risk of hypoglycaemia. By
closely monitoring blood glucose levels and adjusting GIR
accordingly, optimal glycaemic control can be achieved, while
mitigating the potential adverse effects of insulin therapy, such
as hypoglycaemia (35).
Hypokalaemia is also observed with insulin treatment and can be
prevented by periodic laboratory monitoring for electrolytes.
Hypokalaemia should be managed with adequate potassium
supplementation (3).
Risk factors of hyperglycaemia and its
impact on clinical outcomes
Previous studies have demonstrated that neonatal
hyperglycaemia is more common in newborns with a birth weight of
≤1,500 g (4,7,16,20),
with only two studies documenting this in a ELBW infant, similar to
the case in the present study (16,20).
The increased incidence of hyperglycaemia in relation to a lower
birth weight may be attributed to various factors, such as
increased glucose synthesis, low insulin levels, reduced insulin
receptor sensitivity or resistance, and the effects of
counter-regulatory hormones such as catecholamines (10). The study by Hays et al
(36) found that >50% of
neonates consistently had high blood glucose levels >150 mg/dl
during their first week of life (WOL). Additionally, the prevalence
of hyperglycaemia between 2 and 7 DOL was documented as 32% when
using a cut-off value of 250 mg/dl and increased to 57% when
considering a threshold of 150 mg/dl (5,36).
In the case described herein, the infant developed hyperglycaemia
on the second day after birth, which then decreased following
insulin administration. It is worth noting that the infant
continued to experience repeated episodes of hyperglycaemia until
the end of hospitalisation, although these were interspersed with
intermittent episodes of hypoglycaemia.
A study in Tokyo, Japan examined the factors
affecting hyperglycaemia in ELBW in the first 14 DOL (37). That study found significant
associations between hyperglycaemia and various parameters.
Specifically, factors such as gestational duration, birth weight,
chorioamnionitis and postnatal glucocorticoid use were identified
as key factors influencing the outcome (37). Additionally, maternal preeclampsia,
the premature rupture of membranes and antepartum haemorrhage have
also been found to be notable risk factors for hyperglycaemia
(5). The link between maternal
preeclampsia and elevated blood sugar levels in newborns may stem
from hypertensive mothers having a greater chance of delivering
infants with a lower birth weight and premature birth. These
conditions are known to be associated with hyperglycaemia, as also
observed in the case described herein (36). Both normoglycemic and
hyperglycaemic infants can experience respiratory distress and
sepsis. The latter often requires intensive medical intervention,
such as multiple antibiotics, inotropic support and reliance on
respiratory assistance, particularly mechanical ventilation. The
case described in the present study reinforces the understanding
that ELBW infants and those born extremely premature face increased
morbidity when hyperglycaemia is present.
It was hypothesised that refractory hyperglycaemia
in the patient in the present study was caused by the high-stress
metabolic and physical condition, which resulted in damage to the
β-pancreatic cells of the premature baby. Generating an adequate
reserve of β-pancreatic cells through neogenesis and proliferation
during the neonatal period is crucial for the long-term prevention
of the development of type 2 diabetes later in life (38). Preterm newborns have a higher
incidence of hyperglycaemia compared to full-term neonates. The
precise mechanisms involved are not yet fully understood, but they
may include issues such as insufficient insulin response to
glucose, failure to suppress gluconeogenesis, reduced glucose
transporter levels and insufficient protein intake leading to
decreased insulin-like growth factor-1 release. Insulin-like growth
factor-1 typically regulates blood glucose levels by enhancing
glucose utilisation in peripheral tissues, promoting glycogen
synthesis and inhibiting glucose production in the liver.
Inadequate amino acid intake hampers pancreas development and
insulin secretion. Ill neonates often exhibit reduced insulin
production and sensitivity due to immature or less responsive
peripheral receptors. Additionally, the increased production of
counter-regulatory hormones such as adrenaline and cortisol due to
heightened stress can contribute to hyperglycaemia in these
newborns (12).
Neonatal hyperglycaemia is a significant concern,
particularly for extremely preterm infants with a ELBW
(#x003C;1,000 g), as it has been linked to severe complications
that can markedly increase mortality rates. The study by Hays et
al (36) underscores the
substantial influence of blood glucose levels on both early
mortality and the occurrence of severe intraventricular haemorrhage
(IVH). A significant cerebral haemorrhage can disrupt normal brain
metabolism, leading to reduced glucose consumption and the
development of hyperglycaemia. Therefore it is clear that cerebral
bleeding may manifest both a cause and result of hyperglycaemia
(36). The impact of neonatal
hyperglycaemia extends beyond IVH and includes complications, such
as ROP, NEC, increased oxidative stress and susceptibility to
sepsis. These complications not only result in prolonged
hospitalisation but also have the potential to hinder physical
growth, which can be noticeable up to two years beyond the
corrected age (5). Hyperglycaemia
may also exacerbate the likelihood of developing sepsis and
dysfunction of multiple organs in infants with VLBW (5). For this reason, in the case described
herein, in addition to the management of hyperglycaemia, we
antibiotics and antifungals were also administered to prevent
neonatal sepsis, as well as to stabilise the haemodynamics of the
patient as the patient had a history of cardio-pulmonary disorder.
Moreover, in a 2021 Indonesian report, a significant prevalence of
early-onset neonatal sepsis was noted, with 52 out of 492 inborn
neonates (10.6%) diagnosed with culture-proven neonatal sepsis
(30). Additionally, a 2023
Indonesian study analysing 5,439 blood cultures found Gram-negative
bacteria, particularly Klebsiella spp. and
Acinetobacter spp., to be the predominant causative
pathogens for neonatal sepsis in Indonesia, with a prevalence of 35
and 19%, respectively (39).
Discussing the long-term impact of clinical
outcomes, ongoing debates exist regarding the associations between
hyperglycaemia in extremely preterm infants and their long-term
brain development. Subsequent studies conducted during their
school-age years indicate that children in the tightly regulated
blood glucose group have a similar survival rate and no
abnormalities in their brain development compared to those in the
standard blood glucose group (3,4,40).
However, noticeable differences in height and body composition are
evident, suggesting the multifaceted impact of hyperglycaemia on
various aspects of growth and development (3,4,40).
Clinical implications and prevention
strategies
Hyperglycaemia is a progressive disease and often
involves repeated episodes in extremely preterm newborns, with
negative clinical implications on their short and long-term health.
Preterm infants with a low birth weight are at higher risk of
developing hyperglycaemia, which is usually detected in the first
WOL. Although in the majority of cases, this is resolved within a
few days, some infants may experience hyperglycaemia for up to 10
days. While less common than hypoglycaemia, hyperglycaemia is
associated with increased morbidity and mortality rates (5,8).
Prolonged hyperglycaemia can worsen these outcomes; however, early
evaluation and treatment can help mitigate its effects. Neonatal
hyperglycaemia can lead to early mortality and is associated with a
higher risk of severe IVH, respiratory distress syndrome, sepsis,
NEC, bronchopulmonary dysplasia and periventricular leukomalacia
(41). In the present study, blood
glucose level of the newborn rose to 451 mg/dl on the first day of
life. Given these findings, it is critical for general
practitioners, paediatricians and neonatologists to be diligent in
identifying and managing hyperglycaemia in order to improve overall
prognosis.
To prevent the incidence and recurrence of
hyperglycaemia in neonates, several effective strategies have been
identified based on the literature. Increasing protein intake in
parenteral nutrition has been shown to be associated with a
decrease in the occurrence of hyperglycaemia in ELBW and VLBW
infants. Consuming amino acids at a daily rate of 4 g/kg BW/day can
reduce the likelihood of developing hyperglycaemia by 67% compared
to a daily intake of 2.5 g/kg BW (42). Amino acids, particularly arginine
and glutamine, have a positive impact on glucose control by
enhancing insulin secretion. Implementing strict glycaemic control,
increasing intravenous fat administration, initiating enteral
feeding early, and achieving full feeding faster all contribute to
reducing the likelihood of developing hyperglycaemia (43-47).
A 2011 Cochrane review advised against the use of
insulin as a preventive measure (48). In a multicentre study on 195 ELBW
infants (49), a reduction in the
risk of hyperglycaemia was observed when a continuous insulin
infusion at a rate of 0.05 units/kg BW/h was administered during
the initial week after birth. However, this approach also increased
the risk of hypoglycaemia and mortality within 28 days, when
compared to standard neonatal care (49). The criteria for initiating insulin
treatment vary significantly. The standard initial dosage ranges
from 0.05 to 0.1 units/kg BW of regular insulin. Neonatologists
typically begin with a concentrated dose (bolus) and then switch to
a continuous infusion of 0.01 to 0.05 units/kg BW per hour if high
blood glucose levels persist (3).
As part of clinical judgment, the metabolic clearance rate of
insulin is known to be higher in preterm newborns compared to
full-term infants or even adults, which may require higher rates of
insulin infusion. A previous study found that normal blood glucose
levels could be reached within an average of 31.4 h following the
initiation of insulin therapy (50).
The optimal target glucose range for extremely
preterm newborns is uncertain. A survey of clinical directors in
New Zealand and Australia revealed a wide range of desired blood
glucose levels, from 54 to 144 mg/dl (3 to 8 mmol/l) (50). In a randomised trial involving
premature infants born before at 30 weeks of gestation or with a
birth weight #x003C;1,500 g, maintaining blood glucose levels
between 72 and 108 mg/dl (4 and 6 mmol/l) led to improved weight
gain and head growth until 36 weeks of postmenstrual age (45). By contrast, maintaining blood
glucose levels between 144 and 180 mg/dl (8 and 10 mmol/l) has been
shown to not result in a similar improvement in linear growth
(50).
Limitations, challenges and future
directions
The present study was limited by the inability of
the clinical setting to fully identify the specific factors
contributing to neonatal hyperglycaemia. This limitation also
applies to diagnostic methods, such as insulin testing, pancreatic
radiological imaging, and, in some cases, even biopsy. As a result,
the patient was transferred to a public hospital offering better
care after achieving stabilisation at the authors' primary-level
healthcare institution. Another limitation was not having access to
the complete medical records of the referral hospital; only a brief
report was provided following the release of the patient. However,
it is noteworthy that this case report effectively depicts the
presentation of neonatal hyperglycaemia from the standpoint of
initial management and highlights the rare occurrence of such a
condition after birth. As a future direction, a prospective
follow-up study is warranted in order to determine the prognosis
for the development of very preterm infants with hyperglycaemia
(41).
In conclusion, the case described in the present
study highlights the importance of the early management of neonatal
hyperglycaemia. It is crucial to effectively treat hyperglycaemia
in neonates from the beginning, although it remains challenging to
manage. Treatment is primarily based on the clinical judgment of
the physician, and the most commonly used approach is to reduce the
GIR and/or administer insulin. It is hoped that this case serves as
a valuable lesson for general practitioners and paediatricians
treating ELBW hyperglycaemia cases, particularly in extremely
premature babies. Further extensive studies are required to develop
standardised protocols and treatment algorithms for neonatal
hyperglycaemia to enhance patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH served as the principal investigator for this
study, conceived the case report and made the decision to publish
it. SS, BA and MH took full responsibility for the work. SS, BA and
MH jointly designed the methodology and formal analysis. SS and BA
curated the data and conducted the investigation. SS, BA and MH had
complete access to the literature data, contributed to the
analysis, drafted the manuscript, and validated all evidence
analyses. MH utilised software to create visualizations of the
study findings, secured funding and managed the project.
Additionally, MH provided resources and supervised the study
process meticulously. All authors critically reviewed the
manuscript for significant intellectual content, and have read and
approved the final version for publication. SS, BA and MH confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Patient consent was obtained from the parents of the
infant described herein, and all images and data for publication
were consented according to the principles of the Declaration of
Helsinki.
Patient consent for publication
Patient consent was obtained from the parents of the
infant described herein, and consent was also obtained for the
publication of the present case report and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rath CP, Shivamallappa M, Muthusamy S, Rao
SC and Patole S: Outcomes of very preterm infants with neonatal
hyperglycaemia: A systematic review and meta-analysis. Arch Dis
Child Fetal Neonatal Ed. 107:269–280. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guiducci S, Meggiolaro L, Righetto A,
Piccoli M, Baraldi E and Galderisi A: Neonatal hyperglycemia and
neurodevelopmental outcomes in preterm infants: A review. Children
(Basel). 9(1541)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramel S and Rao R: Hyperglycaemia in
extremely preterm infants. Neoreviews. 21:e89–e97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boscarino G, Conti MG, Gasparini C, Onestà
E, Faccioli F, Dito L, Regoli D, Spalice A, Parisi P and Terrin G:
Neonatal hyperglycemia related to parenteral nutrition affects
long-term neurodevelopment in preterm newborn: A prospective cohort
study. Nutrients. 13(1930)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El-Shimi MS, El-Saoud PMA and Ismail RIH:
Risk factors and outcomes of hyperglycaemia in low birth weight
infants: A prospective observational study. Egypt J Hosp Med.
89:6473–6479. 2022.
|
|
6
|
Zamir I, Stoltz Sjöström E, Ahlsson F,
Hansen-Pupp I, Serenius F and Domellöf M: Neonatal hyperglycaemia
is associated with worse neurodevelopmental outcomes in extremely
preterm infants. Arch Dis Child Fetal Neonatal Ed. 106:460–466.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gonzalez Villamizar JD, Haapala JL,
Scheurer JM, Rao R and Ramel SE: Relationships between early
nutrition, illness, and later outcomes among infants born preterm
with hyperglycemia. J Pediatr. 223:29–33.e2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Adeniji EO, Kuti BP and E Elusiyan JB:
Prevalence, risk factors, and outcome of hospitalization of
neonatal hyperglycemia at a Nigerian health facility. Niger J Clin
Pract. 23:71–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Akmal DM, Razek ARAA, Musa N and El-Aziz
AGA: Incidence, risk factors and complications of hyperglycemia in
very low birth weight infants. Egypt Pediatr Assoc Gaz. 65:72–79.
2017.
|
|
10
|
Şimşek DG, Ecevit A, Hatipoğlu N, Çoban A,
Arısoy AE, Baş F, Mutlu GY, Bideci A and Özek E: Neonatal
hyperglycemia, which threshold value, diagnostic approach and
treatment?: Turkish neonatal and pediatric endocrinology and
diabetes societies consensus report. Turk Pediatri Ars. 53 (Suppl
1):S234–S238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Balasundaram P and Dumpa V: Neonatal
hyperglycaemia. In: Neonatal Hyperglycaemia or Name. Available
from: https://www.ncbi.nlm.nih.gov/books/NBK567769/.
Accessed September 1, 2023.
|
|
12
|
Parappil H, Gaffari M, Paramban R, Rijims
M, Skaria S and Ahmed SN: Management of hyperglycemia in the
neonatal unit A practical approach to diagnosis and management. J
Clin Neonatol. 11:38–44. 2022.
|
|
13
|
Kairamkonda VR and Khashu M: Controversies
in the management of hyperglycemia in the ELBW infant. Indian
Pediatr. 45:29–38. 2008.PubMed/NCBI
|
|
14
|
Beardsall K: Hyperglycaemia in the newborn
infant. Physiology verses pathology. Front Pediatr.
9(641306)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ferguson IC: Neonatal hyperglycaemia: Case
report with plasma insulin studies. Arch Dis Child. 42:509–513.
1967.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hemachandra AH and Cowett RM: Neonatal
Hyperglycemia. Pediatr Rev. 20:e16–e24. 1999.
|
|
17
|
Yafi M: A case of neonatal diabetes
presentation, diagnosis and management. Austin J Pediatr.
1(1004)2014.
|
|
18
|
Manzar S: Glucose 250, MD notified: A case
report. SunKrist J Neonat Pediatr. 2(1012)2020.
|
|
19
|
Fargas-Berríos N, García-Fragoso L,
García-García I and Valcárcel M: Neonatal hyperglycemia due to
transient neonatal diabetes mellitus in puerto rico. Case Rep
Pediatr. 2015(984214)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Muzzy Williamson JD, Thurlow B, Mohamed
MW, Yokom D and Casas L: Neonatal hyperglycemia in a preterm infant
managed with a subcutaneous insulin pump. Am J Health Syst Pharm.
77:739–744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gomella TL, Eyal FG and Bany-Mohammed F:
Gomella's Neonatology: Management, Procedures, On-Call Problems,
Diseases, and Drugs. 8th edition. McGraw Hill, New York, NY,
2020.
|
|
22
|
Consales A, Crippa BL, Colombo L, Villa R,
Menni F, Giavoli C, Mosca F and Bedeschi MF: CHARGE syndrome
presenting with persistent hypoglycemia: Case report and overview
of the main genetic syndromes associated with neonatal
hypoglycemia. Ital J Pediatr. 48(154)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cromer SJ, Sella AC, Rosenberg E, Scully
K, McDonnell M, Abreu AP, Weil M, Bernstein SN, Quinn M, Powe C, et
al: Report of prolonged neonatal hypoglycemia in three infants of
mothers with variants in HNF1A. AACE Clin Case Rep. 8:224–230.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Osada A, Arimitsu T, Kusakawa M, Kin T and
Hida M: A case of severe neonatal transient hyperinsulinemic
hypoglycaemia without identifiable risk factors: A case report. BMC
Pregnancy Childbirth. 22(423)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rundjan L, Tirtaningrum Y and Anindita C:
Penanganan paripurna bayi prematur di ruang perawatan dalam
(comprehensive management of premature infants in the nursing
room). In: Panduan Pelayanan Kesehatan Anak Terpadu (Integrated
Child Health Care Guidelines). Trihono PP, Windiastuti E, Pardede
SO, Endyarni B and Alatas FS (eds). Faculty of Medicine University
of Indonesia, Department of Pediatric, Jakarta, pp18-28, 2013.
|
|
26
|
da Costa CS, Czosnyka M, Smielewski P and
Austin T: Optimal mean arterial blood pressure in extremely preterm
infants within the first 24 h of life. J Pediatr. 203:242–248.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
St Peter D, Gandy C and Hoffman SB:
Hypotension and adverse outcomes in prematurity: Comparing
definitions. Neonatology. 111:228–233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mankoo PK, Shen R, Schultz N, Levine DA
and Sander C: Time to recurrence and survival in serous ovarian
tumors predicted from integrated genomic profiles. PLoS One.
6(e24709)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kamarudin AN, Cox T and Kolamunnage-Dona
R: Time-dependent ROC curve analysis in medical research: Current
methods and applications. BMC Med Res Methodol.
17(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Utomo MT, Sumitro KR, Etika R and Widodo
ADW: Current-proven neonatal sepsis in Indonesian tertiary neonatal
intensive care unit: A hematological and microbiological profile.
Iran J Microbiol. 13:266–273. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cuzzolin L: Antibiotics and antifungals in
VLBW infants. J Pediatr Neonat Individual Med. 4(e040214)2015.
|
|
32
|
Lutsar I, Chazallon C, Trafojer U, de
Cabre VM, Auriti C, Bertaina C, Calo Carducci FI, Canpolat FE,
Esposito S, Fournier I, et al: Meropenem vs standard of care for
treatment of neonatal late onset sepsis (NeoMero1): A randomised
controlled trial. PLoS One. 15(e0229380)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cao G, Zhou P, Zhang H, Sun B, Tong X and
Xing Y: Extended infusion of meropenem in neonatal sepsis: A
historical cohort study. Antibiotics (Basel).
11(341)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Binder ND, Raschko PK, Benda GI and
Reynolds JW: Insulin infusion with parenteral nutrition in
extremely low birth weight infants with hyperglycemia. J Pediatr.
114:273–280. 1989.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Decaro MH and Vain NE: Hyperglycaemia in
preterm neonates: What to know, what to do. Early Hum Dev. 87
(Suppl 1):S19–S22. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hays SP, Smith EO and Sunehag AL:
Hyperglycemia is a risk factor for early death and morbidity in
extremely low birth-weight infants. Pediatrics. 118:1811–1818.
2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Inage Y, Hirano D, Nakagawa A, Yamada S,
Kotake Y, Ikoma N, Kumazawa K, Hayashi S, Tanabe Y, Kobayashi M and
Shimizu M: Risk factors for hyperglycemia in extremely low birth
weight infants during the first 14 days. Pediatr Neonatol.
63:13–18. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang J and Wang H: Oxidative stress in
pancreatic beta cell regeneration. Oxid Med Cell Longev.
2017(1930261)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Putri ND, Dickson B, Baker J, Adrizain R,
Kartina L, Sukarja D, Cathleen F, Husada D, Utomo M, Yuniati T, et
al: Epidemiology of Neonatal Sepsis in Indonesia: High burden of
multidrug-resistant infections reveals poor coverage provided by
currently-recommended neonatal sepsis treatment regimens. SSRN:
Preprints with the Lancet. Available from: https://dx.doi.org/10.2139/ssrn.4519552. Accessed
April 15, 2024.
|
|
40
|
Gul R, Waheed KAI, Sheikh M, Javaid S,
Haroon F and Fatima ST: Is hyperglycemia a risk factor for neonatal
morbidity and mortality? Pak Armed Forces Med J. 67:621–626.
2017.
|
|
41
|
van der Lugt NM, Smits-Wintjens VE, van
Zwieten PH and Walther FJ: Short and long term outcome of neonatal
hyperglycemia in very preterm infants: A retrospective follow-up
study. BMC Pediatr. 10(52)2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Burattini I, Bellagamba MP, Spagnoli C,
D'Ascenzo R, Mazzoni N, Peretti A, Cogo PE and Carnielli VP: Marche
Neonatal Network. Targeting 2.5 versus 4 g/kg/day of amino acids
for extremely low birth weight infants: A randomized clinical
trial. J Pediatr. 163:1278–1282.e1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zamir I, Stoltz Sjöström E, Edstedt Bonamy
AK, Mohlkert LA, Norman M and Domellöf M: Postnatal nutritional
intakes and hyperglycemia as determinants of blood pressure at 6.5
years of age in children born extremely preterm. Pediatr Res.
86:115–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Stensvold HJ, Lang AM, Strommen K,
Abrahamsen TG, Ogland B, Pripp AH and Ronnestad AE: Strictly
controlled glucose infusion rates are associated with a reduced
risk of hyperglycaemia in extremely low birth weight preterm
infants. Acta Paediatr. 107:442–449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tottman AC, Bloomfield FH, Cormack BE,
Harding JE, Mohd Slim MA, Weston AF and Alsweiler JM: Relationships
between early nutrition and blood glucose concentrations in very
preterm infants. J Pediatr Gastroenterol Nutr. 66:960–966.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Burgess L, Morgan C, Mayes K and Tan M:
Plasma arginine levels and blood glucose control in very preterm
infants receiving 2 different parenteral nutrition regimens. JPEN J
Parenter Enteral Nutr. 38:243–253. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zamir I, Tornevi A, Abrahamsson T, Ahlsson
F, Engström E, Hallberg B, Hansen-Pupp I, Sjöström ES and Domellöf
M: Hyperglycemia in extremely preterm infants-insulin treatment,
mortality and nutrient intakes. J Pediatr. 200:104–110.e1.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sinclair JC, Bottino M and Cowett RM:
Interventions for prevention of neonatal hyperglycemia in very low
birth weight infants. Cochrane Database Syst Rev.
5(CD007615)2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Beardsall K, Vanhaesebrouck S,
Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, Midgley
P, Thompson M, Thio M, Cornette L, et al: Early insulin therapy in
very-low-birth-weight infants. N Engl J Med. 359:1873–1884.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Alsweiler JM, Kuschel CA and Bloomfield
FH: Survey of the management of neonatal hyperglycaemia in
Australasia. J Paediatr Child Health. 43:632–635. 2007.PubMed/NCBI View Article : Google Scholar
|