1. Introduction
Brain and other central nervous system tumors are
the leading causes of mortality due to cancer among females aged
<20 years and males aged <40 years (1). Glioblastoma multiforme (GBM) is the
most common malignant primary brain tumor affecting adults,
comprising 16% of all primary brain tumors and 54% of all gliomas
(2). GBM is the most aggressive
diffuse glioma of astrocytic origin and is classified as a grade IV
glioma based on the World Health Organization (WHO) classification
(3). GBM is extremely lethal,
being associated a 5-year survival rate of 5% and a median survival
rate of 16 months following diagnosis (4). GBM is classified into primary and
secondary subtypes, which develop through different genetic
pathways (5). Primary GBM
comprises ~90% of cases diagnosed, which arise de novo,
without evidence of a precursor tumor, and most commonly occurs
among older patients (6).
Secondary GBM arises from low-grade diffuse astrocytoma or
anaplastic astrocytoma and most commonly occurs in younger patients
(6). In 2021, the WHO reclassified
diffuse glioma based on the isocitrate dehydrogenase (IDH) mutation
status, categorizing it into IDH wild-type glioblastoma and
IDH-mutant glioblastoma (7). In
addition to the high mortality rate, patients with GBM are
characterized by a low health-related quality of life (QoL)
compared with patients with other types of (8).
Treatments for GBM are typically aggressive,
utilizing a combination of surgery resection, radiotherapy and
chemotherapy (9). Recent
advancements in the treatment of GBM, including targeted therapy
(e.g., monoclonal antibodies) and immunotherapy [programmed cell
death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1)
immune-checkpoint inhibitors] offer new hope for patients; however;
further research is required in order to fully understand and
optimize these approaches to improve the outcomes of patients
(10). Regrettably, standard GBM
treatment is associated with a wide range of side-effects and
secondary morbidity. Both tumor burden and treatment may result in
cognitive impairment, psychological distress, poor coordination and
motor control, and impairments in physical functioning, fatigue,
myopathy and neuropathy (11).
These side-effects impair the activities of daily living and
compromise the QoL of patients with GBM.
Due to toxicities from treatment and the high
mortality rate associated with GBM, there is a need for new
interventions to improve the QoL and reduce the mortality rates of
patients. Among the strategies being pursued, exercise has emerged
as an intervention that warrants further investigation. Exercise
has the potential for the direct and indirect prevention of both
cancer and comorbid diseases (12). Pre-clinical and clinical studies
have also shown that physical activity and structured exercise
improve the efficacy of cancer treatments as well as prognosis
(13,14). In addition, a previous
meta-analysis of randomized controlled trials revealed that
exercise during and after treatment in patients with cancer
improved physical and psychological parameters, and the QoL
(15).
The present review briefly discusses the genetic
pathogenesis of GBM. The findings of currently available literature
on physiological adaptation from exercise training in GBM are
summarized and the mechanisms through which exercise can improve
the QoL and reduce the mortality rates of patients with GBM are
also discussed. The present review also summarizes the underlying
mechanisms through which exercise may enhance the efficacy of
treatment, improve overall health and lower recurrence rates in
cancer populations. Finally, the updated exercise training
guidelines for patients with cancer are also discussed.
2. Literature search strategy
A literature search was carried out using the
MEDLINE/PubMed of the National Library of Medicine, Scopus and
Google Scholar databases to identify relevant articles. The search
methodology used a combination of the following key words:
‘cancer’, ‘brain tumor’, ‘glioma’, ‘glioblastoma’, ‘physical
capacity’, ‘exercise’, ‘training’ and ‘rehabilitation’. A search of
the reference lists of case studies and the clinical trials
included in the present review was also performed for the potential
to detect other relevant studies.
3. Genetic and molecular pathogenesis of
GBM
Over the past two decades, multiple molecular
variations have been identified in GBM, which have allowed for a
more comprehensive description of GBM, as well as an understanding
of the glioma landscape and pathways disrupted in this type of
cancer. The primary and secondary GBM subtypes appear to develop
through different genetic pathways and likely differ in prognosis
and response to therapy (5,6).
Primary GBM typically exhibits the overexpression of the epidermal
growth factor receptor (EGFR) gene, the loss of heterozygosity
(LOH) of chromosome 10q, which encodes phosphatase and tensin
homolog (PTEN), TERT promoter mutation, CDKN2A (p16) deletion, and
less frequently, mouse double minute 2 (MDM2) amplification
(5,6,16).
The mutations of secondary GBMs include the LOH of chromosome 19q,
the amplification of platelet-derived growth factor (PDGF)A and
PDGF receptor-α (PDGFR-α), retinoblastoma (RB), and mutations in
IDH1/2, p53 and alpha-thalassemia/mental retardation, X-linked
(16). These genetic mutations
result from the dysfunction of three main signaling pathways: The
receptor tyrosine kinase (RTK)/RAS/phosphatidylinositol 3-kinase
(PI3K) pathway, p53 pathway and RB signaling pathway (17). Other pathways implicated include
hypoxia-inducible factor (HIF), AMP-activated protein kinase (AMPK)
activity, anaerobic bioenergetics and inflammatory responses in the
brain (16-19).
Studies have also shown that epigenetic mechanisms, such as
promoter CpG island DNA hypermethylation, the aberrant expression
of microRNAs (miRNAs/miRs), and the post-translational modification
of histone protein also play a role in the development and
progression of GBM (20).
The RTK singling pathway is well-recognized as one
of the most common genetic alterations in malignant gliomas
(17). The EGFR and PDGFRA genes
are the most recurrently mutated and amplified (17). EGFR downstream signaling pathways
control a wide range of cellular activities, including growth,
migration and survival. This pathway further promotes cell
division, tumor invasiveness and resistance to chemotherapy in GBM
(21). EGFR activity is enhanced
by amplifying EGFR protein expression, deleting downstream pathway
inhibitors, and constitutively active EGFR (EGFRvIII), which is the
most common mutation among EGFRs amplified in GBM (17). This subsequently leads to the
activation of numerous downstream signaling pathways, such as the
PI3K/Akt/rapamycin-sensitive mTOR-complex (mTOR) pathway (22). Understanding the complex pathways
associated with the growth and progression of GBM will enhance
novel treatment options, such as targeted therapy via monoclonal
antibody administration.
HIFs and other metabolites play a critical
functional role in the tumor microenvironment (TME), which includes
cancer-associated fibroblasts, endothelial cells and immune cells,
and serves to foster a microenvironment structure for tumor growth
and proliferation (23). HIFs act
to promote angiogenesis, increasing the blood and nutrient supply
to cancer cells. The angiogenic switch found during cancer
progression is associated with exponential cancer progression
through HIF-1, activating an increase in vascular endothelial
growth factor (VEGF) expression (23). Previous research has demonstrated
an increase in VEGF expression in the TME, which supports the tumor
metabolically, further ingraining itself into the tissue and
eventually allowing intravasation into the systemic circulation and
metastasis (24). The previous
study by Cao et al (24)
demonstrated that the amplification of 14-3-3zeta (pro-apoptotic)
gene activated the PI3K/Akt pathway associated with the
overexpression of HIF-1a and VEGF in glioma. It is through this
mechanism that malignancy increases and prognosis decreases with
GBM diagnosis.
AMPK is an essential mediator in maintaining
cellular energy homeostasis and plays an essential role in
regulating growth, metabolism, autophagy and cell polarity in
healthy cells (25). For this
reason, the regulation of AMPK and its shift to promote tumor
development in cancer must be understood. A previous study
demonstrated that the high expression of AMPK plays a critical role
in bioenergetics pathways in GBM through interaction with the
HIF-VEGF pathway, aiding in tumor growth (26). AMPK also mediates glucose
transporter protein expression on the tumor cell, allowing for
increased glucose uptake for bioenergetic pathways and ATP
production at the tumor (26).
Cyclic AMP responsive element-binding protein 1 (CREB1) is
implicated as the primary factor that promotes this AMPK link to
HIF and glucose transporter protein activity (26). Previous research has explored the
role that these three factors play and the mechanisms through which
this pathway may be altered to combat this effect. It appears that
the inhibition of CREB1 and AMPK leads to a reduction in glucose
transporter protein and HIF expression, and a reduction in the
expression of GA-binding protein alpha chain, a transcription
factor responsible for the control of mitochondrial function
(26).
By-products of energy pathways are implicated in the
progression of GBM. The metabolic shift of cancer cells into a
glycolytic state leads to an increase in lactate production
(27). Lactate production and the
associated receptor hydroxycarboxylic acid receptor 1 (HCAR1) are
the primary factors that promote tumor growth and progression
(28). The TME cultivates
anaerobic pathways of energy production and therefore promotes
substantial lactate accumulation and upregulation of HCAR1
receptors (28). This upregulation
is associated with angiogenic signaling and VEGF overexpression,
specifically in the cerebral environment, which is highly unique to
GBM, as, to the best of our knowledge, there is no evidence in the
current literature that this receptor activity is upregulated in
the periphery in those diagnosed with GBM (28). Aldehyde dehydrogenase (ALDH) is
another glycolytic metabolite that is implicated in the metabolic
dysfunction of GBM. It has been shown that ALDH increases the
malignancy of GBM through the upregulation of its expression
(29). This association with an
increased malignancy is due to the promotion of glioma stem cells
(GSCs), which increases the aggressiveness of GBM cells (29). GSCs are resistant to temozolomide,
allowing them to survive therapy, leading to disease recurrence
(30). In addition, these cells
overexpress VEGF receptor 2 (VEGFR2) increasing endothelial cell
proliferation, migration, and blood vessel permeability leading to
increased edema in GBM (30).
Recent literature shows the selective deletion of stromal
cell-derived factor 1, which signals through CXCR4 expression on
GSCs, inhibits tumor growth and prolongs survival in GBM,
suggesting that targeting GSCs may inhibit tumor growth and limit
resistance to current anti-angiogenic therapies (31).
p53, as a tumor suppressor and transcription factor,
plays a crucial role in preventing tumor development by inducing
the apoptosis of damaged cells, maintaining genomic stability,
inhibiting angiogenesis and regulating cell metabolism and the TME
(32). p53 is also a key regulator
of cellular metabolism, stemness, autophagy, invasion, metastasis,
the TME and immunity (33). In
GBM, the p53 pathway is frequently deregulated, with research
demonstrating alterations in the ARF-MDM2-p53 pathway in 84% of GBM
cases according to The Cancer Genome Atlas (2013) and up to 94.1%
of GBM cell lines (34). In
secondary GBM, p53 mutations may occur alongside IDH1 mutations,
contributing to the complexity and controversy in p53 research in
GBM (35). PTEN a tumor suppressor
is a negative regulator of a major cell growth and survival
signaling pathway, namely the PI3K/Akt signaling pathway (36). The deregulation of PI3K signaling
pathways resulting from PTEN gene mutation on 10q23 at the level of
LOH in at least 60% of GBM (37).
PTEN genetic mutation is associated with the poor survival of
patients with GBM (38). The p53
and PTEN signaling pathways are critical targets for GBM therapy,
with advances in understanding their molecular mechanisms and
interactions with other signaling networks being essential for
improving treatment outcomes (39).
Inflammation associated with GBMs may induce
dysfunctional responses to the brain microenvironment (40). An increase in inflammation can
decrease the efficiency of cancer therapies and targeted therapies
such as anti-VEGF drugs. Chronic inflammation in the brain will
affect treatment delivery by altering the microenvironment and
stimulating inflammatory adaptations (40). Previous research has shown that the
Tie2-expressing monocyte population is pro-angiogenic, expressing
relevant gene transcripts such as VEGF (41). Myeloid-derived suppressor cells
(MDSCs) may also contribute to the integrity of the neo-endothelium
of tumor vessels due to their expression of endothelial markers,
such as CD31 and VEGF receptor, and their ability to
morphologically resemble endothelial cells (42). The mutation in p53 promotes chronic
inflammation in GBM, which is associated with a poor prognosis and
high mortality rates (43). The
primary target for upregulation by p53 gene mutation is the
upregulation of C-C motif chemokine ligand-2 and tumor necrosis
factor-α (TNF-α) expression. This upregulation is positively
related to increased microglia and monocyte-derived immune cell
infiltration, which may consequently promote inflammation in GBM,
but may also inhibit treatment delivery and action (43).
The role of inflammation in decreasing the efficacy
of targeted therapies may result from hypoxic conditions,
suppressing the expression of cylindromatosis (CYLD), a tumor
suppressor that regulates signaling pathways by acting as a
deubiquitinating enzyme (44). The
study conducted by Guo et al (44) found that suppression of CYLD was a
critical aspect of inflammatory responses in the GBM
microenvironment. Thus, CYLD may function as a tumor suppressor,
which unergoes a loss of function, followed by suppression, in the
presence of GBM. Furthermore, CYLD suppression may promote TNF-α
and NF-κB activity downstream and act in a paracrine fashion to
increase GBM tissue inflammation (44). This results in the promotion of
angiogenesis and a reduction in anti-angiogenic agents, leading to
the further supply of nutrients to the tumor and increasing its
malignancy.
4. Physiological and therapeutic mechanisms
of exercise in cancer
Physical activity and exercise decrease the risk of
developing several types of cancer, including breast, colon,
endometrial, kidney, bladder, esophageal and stomach cancers
(12,45). Therefore, increased attention has
been paid to exercise as a non-pharmacological intervention for
patients with cancer (46). While
the impact of exercise on the clinical outcomes of patients with
cancer is indispensable, understanding the cellular and molecular
mechanisms of exercise in cancer is warranted. Despite literature
advocating for the inclusion of exercise into treatment strategies
for patients with cancer across a variety of diagnoses, mechanistic
studies have not adequately assessed this response in the GBM
population, despite similarities in its pathology to other cancer
types.
Exercise and cancer prevention
Even though the mechanism of the reduced risk and
recurrence of cancer by exercise is not yet well known, it is
suggested that exercise has a positive impact on the TME through
the regulation of cellular processes and tumor growth (47). It is proposed that exercise exerts
several biological effects to moderate these processes, including
insulin/glucose metabolism, immune function, inflammation, sex
hormone concentration, oxidative stress, genomic instability and
myokine release (47,48). Regular exercise decreases plasma
insulin levels and insulin growth factor (IGF-1) which may reduce
cancer proliferation by decreasing the activation of receptor
tyrosine kinases (47). Obesity is
also associated with the development of several types of cancer by
the same biological mechanisms (48). Exercise plays a clear role in
weight management by reducing body fat and decreasing the incidence
of several types of cancer (48).
Research indicates that physical activity and regular exercise
decreases inflammation by reducing the levels of inflammatory
cytokines, circulating C-reactive protein and TNF-α, and increasing
the levels of anti-inflammatory myokines (49). Exercise also enhances immune system
function, which may play a role in cancer prevention (48). Further studies are required
however, to explore these mechanisms in the prevention of GBM.
Exercise and epigenetic
modification
A variety of physiological adaptations are induced
by exercise training, including the upregulation of signaling
mechanisms for DNA replication, transcription and protein synthesis
(50). Exercise may also play a
critical role in attenuating cancer progression through epigenetic
modification. A previous study demonstrated that anaerobic exercise
increased p53 and PTEN expression, and decreased MDM2 expression,
leading to the downregulation of the IGF-1 pathway in skin cancer
(51). A recent study demonstrated
that 4 weeks of high-intensity interval training (HIIT) reduced
tumor volume and upregulated the mRNA expression of p53 in mouse
models of breast cancer (52).
These studies suggest that exercise may play a therapeutic role by
enhancing the expression of the tumor-suppressor genes (TSGs), p53
and PTEN. The abnormal hypermethylation of TSGs is also a mediator
of cancer development and progression with carcinogenesis (53). A review article showed that
exercise adjusted the methylation status of TSGs and decreased
promoter hypermethylation in nonmalignant breast cancer (54). It was also demonstrated that 6
months of moderate exercise also decreased methylation of the TSG
L3MBTL1 in breast cancer, which is associated with a low risk of
recurrence and mortality (55). A
preclinical study revealed that regular exercise decreased
circulating miRNA levels, including those of miR-21 in mice
(56). An increase in in miR-21
expression is associated with the human estrogen receptor (ER)α in
breast cancer and induces HIF-1α and VEGF expression in prostate
cancer (57). Taken together,
these studies suggest that exercise may play a preventive role and
enhance targeted therapy in cancer by modifying epigenetic
mechanisms.
Several studies have reported that exercise
activates epigenetic mechanisms and regulates synaptic plasticity.
A previous study demonstrated that an acute single bout of exercise
has a positive impact on post-translational modifications of
histone by decreasing histone deacetylase enzyme and increasing
histone acetyltransferase enzyme in the hippocampus of rats
(58). Furthermore, several
studies have shown that exercise modulates epigenetic factors that
regulate brain-derived neurotrophic factor (BDNF) expression and
induces gene expression associated with synaptic plasticity in rats
(59). It was previously
demonstrated that 1-week wheel-running intervention increased the
global acetylation of histone 3 in the hippocampus of mice,
thereby, increasing the transcription of BDNF (60). Recent studies have demonstrated
that patients with GBM exhibited reduced levels of BDNF in both
their plasma and cerebrospinal fluid, potentially being associated
with cognitive decline in GBM (61,62).
Although several studies have shown that the adjuvant therapeutic
role of exercise functions through epigenetic modification in
patients with cancer (54), no
studies to date have examined the therapeutic effect of exercise on
epigenetic modification in patients with GBM, at least to the best
of our knowledge. Additional research to replicate animal model
findings in humans and explore the mechanistic effect of exercise
within the TME is critical for patients with GBM.
Exercise and the RTK singling
pathway
Previous studies have demonstrated that regular
exercise inhibits PI3K/Akt/mTOR signaling and attenauates tumor
growth in triple-negative breast cancer, which does not proliferate
in response to the estrogen receptor, progesterone receptor, or
EGFR/HER2/neu activation (63).
Exercise appears to modify several systemic signal inputs, which
leads to physiological adaptation of breast cancer TME and mTOR
inhibition (47). A preclinical
study revealed that exercise decreased tumor growth by inhibiting
of PI3K/Akt/mTOR signaling and enhancing apoptotic signaling via
caspase-3 and Bax in breast cancer models (64). Even though these data suggest that
exercise inhibits the PI3K/Akt/mTOR pathway and enhances the TME in
cancer, studies have not explored the effects of exercise on
PI3K/Akt/mTOR in GBM.
Exercise and angiogenesis
Anti-angiogenic therapy directed at VEGF or its
receptors has been approved for cancer treatment (65). However, despite their intended
function of impeding blood supply to tumors, these agents can lead
to hypoxia, potentially exacerbating tumor progression and
resistance to treatment. The most effective role of exercise on the
tumor is related to its impact on angiogenesis and vascular changes
in the TME. It has been shown that exercise enhances tumor VEGF
levels and angiogenesis (13).
Exercise training has been shown to increase VEGF expression and
tumor angiogenesis, and decrease tumor burden in a mammary cancer
mouse model (66). The increased
tumor vascularization and perfusion may decrease tumor hypoxia,
increase drug delivery to the tumor and increase tumor response to
radiation (13). A preclinical
study revealed that exercise with tamoxifen and letrozole treatment
reduced ERα, HIF-α, VEGF and miR-21 expression levels associated
with a decreased tumor growth and increased vascularization in
mouse breast cancer models (67).
Another preclinical study demonstrated that miR-21 induced tumor
vascularization by targeting PTEN, inducing the stimulation of AKT
and ERK1/2 signaling pathways and enhancing HIF-1 and VEGF
expression in prostate cancer cells (57). The anticipated outcome of
exercise-induced stabilization of HIF-1 was an increase in
metastatic spread. Contrary to expectations, voluntary wheel
running in mice with prostate or breast cancer has been found to
result in the opposite effect (68,69).
However, a recent meta-analysis demonstrated that regular exercise
did not significantly modify the number of metastatic foci or the
risk of developing metastasis in animal cancer models (70). Further research is warranted to
fully determine the impact of exercise on hypoxia, angiogenesis and
metastasis in cancer overall, with a specific focus on GBM.
Exercise and AMPK
It has been reported that regular exercise activates
the AMPK pathway, which plays a significant role in regulating
glucose uptake, glycogen synthesis and insulin sensitivity by
skeletal muscle (71). The
activation of AMPK may also suppress tumor growth by regulating
aerobic glycolysis, imposing metabolic checkpoints and inhibiting
cell growth (72). Further, it has
been shown that AMPK activation plays a significant role in
treating and preventing several types of cancer (73). For example, Lee et al
(19) demonstrated that wogonin
supplement, an AMPK activator, increased apoptosis and inhibited
cell proliferation by increasing p53 and p21 expression in GBM
cells. Another study demonstrated that regular exercise decreased
the number and volume of hepatocellular tumors by enhancing the
phosphorylation of AMPK and decreasing mTOR levels (74). However, several studies have found
that in the late stage of breast and colorectal cancer, AMPK
switches to a tumor promoter, enhancing cancer cell survival by
protecting against metabolic, oxidative and genotoxic stresses
(75-78).
Therefore, the AMPK pathway appears to play an essential role in
early-stage targeted therapy for patients with cancer. Further
research is warranted in order to examine the effects of exercise
on the AMPK pathway in cancer in general, and in GBM
specifically.
Exercise and lactate metabolism
Research has indicated that 7 weeks of aerobic
exercise reduces tumor growth, lactate concentration in the TME and
tumor monocarboxylate transporter expression by modifying ER
receptor α (79). Bacurau et
al (80) found that aerobic
exercise decreased carcinoma glucose consumption and lactate
concentration. Lactate accumulation in the TME leads to
angiogenesis and may inhibit cytotoxic immune T-cell activity
(48). Regular exercise also has a
positive effect on ALDH, which is implicated in the metabolic
dysfunction of GBM (81). The
effect of exercise on lactate and ALDH in cancer and GBM is not yet
fully understood. Future research aimed at determining this
relation is thus warranted.
Exercise and immune system
function
Recent literature highlights the role of exercise in
maintaining a healthy immune system in cancer (82). Regular exercise enhances immune
surveillance to detect and eliminate abnormal cells before they
develop into cancer (83,84). During acute exercise, tissue
macrophages exhibit an increased antipathogen activity and enhanced
recirculation of immunoglobulins, anti-inflammatory cytokines,
neutrophils, natural killer (NK) cells, cytotoxic T-cells and
immature B-cells, which are crucial for immune defense and
metabolic health (83-86).
Acute exercise facilitates the movement of innate immune cells and
components between lymphoid tissues and the blood compartment.
Although these changes are transient, their cumulative effect over
time improves immunosurveillance against pathogens and cancer
cells, while reducing systemic inflammation (83,84).
Regular exercise also enhances the immune system in cancer
indirectly by increasing vascularization, decreasing hypoxia,
decreasing glucose consumption and lowering lactate production
increasing infiltrating immune cells into the TME (82). Furthermore, infiltrating cytotoxic
immune cells into the TME is a positive prognostic marker for
cancer outcome and mortality (87). One potential mechanism through
which exercise improves immune system function in solid tumors is
by increasing NK cell recruitment and infiltration (88). A clinical study showed that acute
intermittent exercise mobilizes NK cells into circulation in breast
cancer patients to the same degree as age-matched healthy controls
(89). Another study demonstrated
that HIIT decreased tumor volume, enhanced metabolic health and
increased the NK cell number in breast cancer models (90). Pedersen et al (91) demonstrated that voluntary wheel
running in mice suppressed the tumor growth rate, associated with a
significant number of NK cells and the release of IL-6 from
exercising muscles and epinephrine from the adrenal glands. That
study suggested that IL-6 from exercising muscle may play a role in
recruiting NK cells to migrate into the TME. In addition, another
study demonstrated that a combination of exercise and PD-L1
inhibitor may delay tumor progression, decrease tumor burden,
decrease MDSCs, and increase NK cell activity in the preclinical
cancer model (92), where MDSCs
inhibit the infiltrating immune cells through PD-L1 that control
the activity of the cytotoxic immune cells (93). The impact of this dysfunction on
immune cell activity as a result of the tumor and TME requires
further research on order to fully understand the interplay and
regulation of cancer on immune health by exercise.
Exercise and cancer therapy
With an improvement in cancer management, the cancer
mortality rate has declined since 1990(1); however, this has led to an increase
in the number of patients treated with cancer therapy, resulting in
a greater number of reports of severe side-effects. As a result,
cancer survivors commonly report limitations from the adverse
side-effects of surgery, radiation and chemotherapy. Typical
side-effects include neutropenia, cardiac toxicity, skeletal muscle
dysfunction and fatigue (94),
which impair the activities of daily living and compromise the QoL
of patients with cancer. A previous study demonstrated that aerobic
exercise plays a potential role in preventing and treating the
cardiotoxic effects of doxorubicin through the improvement of
cardiorespiratory fitness (95).
Another study evaluating the effect of supervised exercise on
cancer survivors demonstrated improved cardiorespiratory fitness,
skeletal muscle strength and antioxidant capacity, and decreased
levels of oxidative stress in patients with cancer (96). Another study revealed that aerobic
exercise may reduce cognitive impairments by improving
neuroplasticity and mitochondrial function in the brains of rats
receiving doxorubicin treatment (97). Even though these data suggest that
the pleiotropic adjuvant therapeutic effect of exercise enhances
the pharmacodynamics of chemotherapy and alleviates the
side-effects of radiotherapy in GBM, to the best of our knowledge,
there are not studies available in the current literature which
have explored the effects of exercise on the side-effects of
temozolomide in GBM.
5. Role of exercise as an adjuvant therapy
in GBM
Literature reviews have indicated that
rehabilitation and physical activity have the potential to
ameliorate cognitive function, motor function and the QoL of
patients with brain tumors (98,99).
The European Association of Neuro-Oncology recommends that patients
with brain tumors exercise in a rehabilitative and supervised
setting (100); however, there
are few evidence-based guidelines for rehabilitative exercise to
reduce the loss of cognition, and improve the function and QoL of
patients with GBM (101).
Considering the abundance of evidence detailing the positive
benefit of physical activity and exercise in other forms of cancer,
the lack of evidence specific to GBM poses a significant challenge
within exercise oncology settings (102).
Feasibility of exercise in GBM
For those studies which have examined the role of
exercise as a non-pharmacological intervention in brain tumors, the
results are promising. For example, inpatient rehabilitation has
been shown to improve the functional performance, physical capacity
and daily activity of patients with brain tumors and GBM (103,104). The Exercise for Neuro and Head
and Neck Cancer Patients (ENHANCE) program revealed that 12 weeks
of aerobic and strength training was feasible and improved
psychological function, physical function and the QoL of patients
with brain tumors (105). A
controlled clinical trial demonstrated that 12 weeks of aerobic
exercise was an effective, safe, and low-cost therapy for enhancing
brain recovery by fostering white matter and hippocampal volume,
and improving the reaction time in children with brain tumors
(106). Similarly, a pilot
randomized controlled trial demonstrated that a home-based exercise
program was feasible and safe in patients with grade II and III
gliomas (107). A previous
clinical case report demonstrated that HIIT was feasible for a
patient with GBM undergoing multimodal therapy (108). Finally, a qualitative study also
demonstrated that combined exercise was a feasible and safe therapy
for patients with GBM undergoing chemoradiotherapy (109). Cumulatively, these data suggest
that exercise is safe, feasible and a potential adjuvant therapy
for patients with GBM.
Exercise improves performance and
quality of life of patients with GBM
A literature research revealed that rehabilitation
interventions can enhance the QoL and functional outcomes of
patients with glioma (98).
Recently, Gehring et al (107) demonstrated that 6 months of
home-based exercise training increased peak oxygen consumption by
7% in patients with brain tumors compared to a non-exercising
control group. Furthermore, another study demonstrated that 12
weeks of combined training reduced waist circumference and improved
grip strength and 30-sec sit-to-stand in brain tumor patients
(105). A previous case report
study revealed that a novel 60-week exercise training program
improved walking ability, exercise performance, muscle strength and
the QoL of patients with GBM actively undergoing radiotherapy
(110). A pilot study
demonstrated that intensive rehabilitation improved engagement in
activities of daily living and physical function scores in patients
who underwent the surgical resection of a brain tumor (111). Similarly, an observational
clinical trial revealed that 12 weeks of an inpatient or outpatient
rehabilitation program improved physical functioning scores in
daily activities, prevented functional disability, and reduced
symptoms in patients with GBM and brain tumors (112). These findings suggest that
improved functional performance and physical capacity may reduce
fatigue, promote psychological health and promote well-being,
subsequently improving the QoL of affected patients (113). While these data suggest that
exercise improves functional performance and the QoL of patients
with GBM, further studies are required to elucidate the exercise
effects on GBM (98). This
includes identifying optimal exercise models, assessing motor and
cognitive outcomes, evaluating the long-term training effects, and
measuring the influence of motor and cognitive rehabilitation on
the daily lives of patients.
Exercise improves the cognitive
function of patients with GBM
Regular exercise training improves cognitive
function and structure by enhancing neural plasticity, increasing
BDNF, decreasing endogenous corticosteroids and pro-inflammatory
cytokines, reducing oxidative stress, improving vascularization and
blood flow, and increasing the levels of hormones beneficial to
neural structure and function (114). However, the therapeutic role of
exercise in alleviating cognitive impairment is not yet well
understood in patients with GBM. A previous preclinical study
demonstrated that 4 weeks of exercise improved memory and cognitive
impairments in rats receiving oxaliplatin and 5-fluorouracil
chemotherapy (115). Another
preclinical study revealed that 5 weeks of aerobic exercise in
healthy rats treated with radiotherapy alleviated cognitive
impairment, relieved the impairment of hippocampal neurogenesis and
attenuated the downregulation of BDNF (114). These data suggest that exercise
aids in alleviating cognitive impairment in GBM and illuminates the
primary mechanisms of therapeutic efficacy of exercise on cognitive
function.
In humans, evidence exists to support the inclusion
of exercise in the GBM population. A previous study reported
significant improvements in various measures of cognitive function
following exercise training in patients with neurology disease
(116). Another study showcased
that 12 weeks of combined exercise improved mental health,
shortness of breath, psychological function, and symptom management
for depression and anxiety in patients with brain tumors (117). A randomized controlled trial
demonstrated that 6 months of home-based exercise training improved
cognitive test performance and the patient-reported outcomes of
patients with glioma (118).
Moreover, a recent randomized controlled trial demonstrated that
exercise coupled with monitor-augmented reality during radiotherapy
effectively mitigated the decline in muscle strength and cognitive
function in patients with high-grade gliomas (119). However, despite these positive
effects, that trial reported no significant impact of exercise on
BDNF levels (119). Given that
the majority of studies have been conducted using animal models,
further research is required to explore the effects of exercise on
cognitive function and BDNF in patients with GBM, and to further
elucidate the mechanisms contributing to the therapeutic effects of
exercise on cognitive impairments in humans with GBM.
Exercise and the prognosis of patients
with GBM
Initial research in preclinical animal models has
suggested that exercise improves survival in GBM. For example,
Lemke et al (120) found
that exercise with temozolomide therapy significantly prolonged the
survival of glioblastoma-bearing mice, reduced tumor volume and
invasiveness and prevented a significant loss of body mass. Another
recent preclinical study revealed that voluntary exercise decreased
the proliferation rate of tumor cells, delayed motor deterioration
and supported the ability for self-care in a mouse model of glioma
(121). It has been suggested
that exercise increases BDNF in circulation which may attenuate
tumor cell proliferation (60,114). The mechanisms of the protective
role of exercise in brain tumors and GBM, however, are not yet
fully understood, but may include physiological adaptations that
modulate tumor progression and cancer therapy (122). A preclinical study demonstrated
that aerobic exercise in mice infused with metastatic tumor cells
regulated tight junction proteins in brain microvessels during
metastasis and contributed to modulating BBB integrity, protecting
the brain during metastatic progression (123).
As these data have been translated to humans, a
previous observational study demonstrated that the performance
status has prognostic value, associated with the survival rate and
disease progression in patients with GBM (124). Moore et al (125) screened the health records of
>300,000 individuals and found that physically active
adolescents had a 35% lower risk of developing glioma than
physically inactive individuals. Another study reported that
patients with WHO grades III and IV malignant glioma who exercised
more than 9 metabolic-equivalent (MET) hours/week had a median
survival of 7.8 months longer than those who exercised <9 MET
hours/week, suggesting that exercise may improve survival duration
of patients with GBM (126).
Additionally, the National Walkers' and Runners' Health Studies
cohorts, which included >153,000 participants, demonstrated that
both general physical activity (i.e., walking 19-37 km/week) and
exercise (i.e., running 12-25 km/week) may reduce the risk of brain
tumor mortality by 43.2% (127).
These authors demonstrated that general exercise behavior is a
strong independent predictor of survival rate in recurrent glioma
(126). Preclinical studies have
laid the foundation of evidence for the utility of exercise
training to improve prognosis in animal models. The evidence
available in humans has also begun to shed light on the positive
effect of exercise training on prognosis in brain tumor patients.
However, the data are limited and gaps in scientific understanding
remain. Given what is known about other cancer types, additional
research focused on exploring the mechanisms and protective role of
exercise in GBM is required (45).
6. Exercise guidelines for cancer
survivors
The American College of Sports Medicine (ACSM) and
International Multidisciplinary Roundtable on Exercise and Cancer
have recommended that each cancer patient should be physically
active and avoid sedentary behavior (128). They concluded that physical
activity and exercise training play a role in preventing various
cancer types, improving longevity among cancer patients and
enhancing common cancer-related health outcomes including
cancer-related fatigue, physical functioning and health-related QoL
(12,128). Current programming for cancer
recommends moderate-intensity aerobic exercise for at least 30 min,
three times per week; and resistance exercise for two sets of 8-15
repetitions at 60% of one repetition maximum, twice per week.
However, these guidelines are created for cancer patients in
general, not specifically for patients with GBM. Patients with GBM
have a broad range of neurological and musculoskeletal impairments
that need to be considered before commencing an exercise training
program. Therefore, further studies are warranted to determine the
optimal mode, intensity and frequency of exercise in this
population, along with the appropriate time to initiate a program,
given the unique treatment considerations regarding surgery,
chemotherapy and radiation.
7. Conclusion and future perspectives
The pathogenesis of GBM has a variety of pathways
that can be targeted by traditional cancer treatment and may be
directly or indirectly influenced by exercise. The role of exercise
in the cancer population has already been established in various
other types of cancer, namely breast, prostate and colorectal
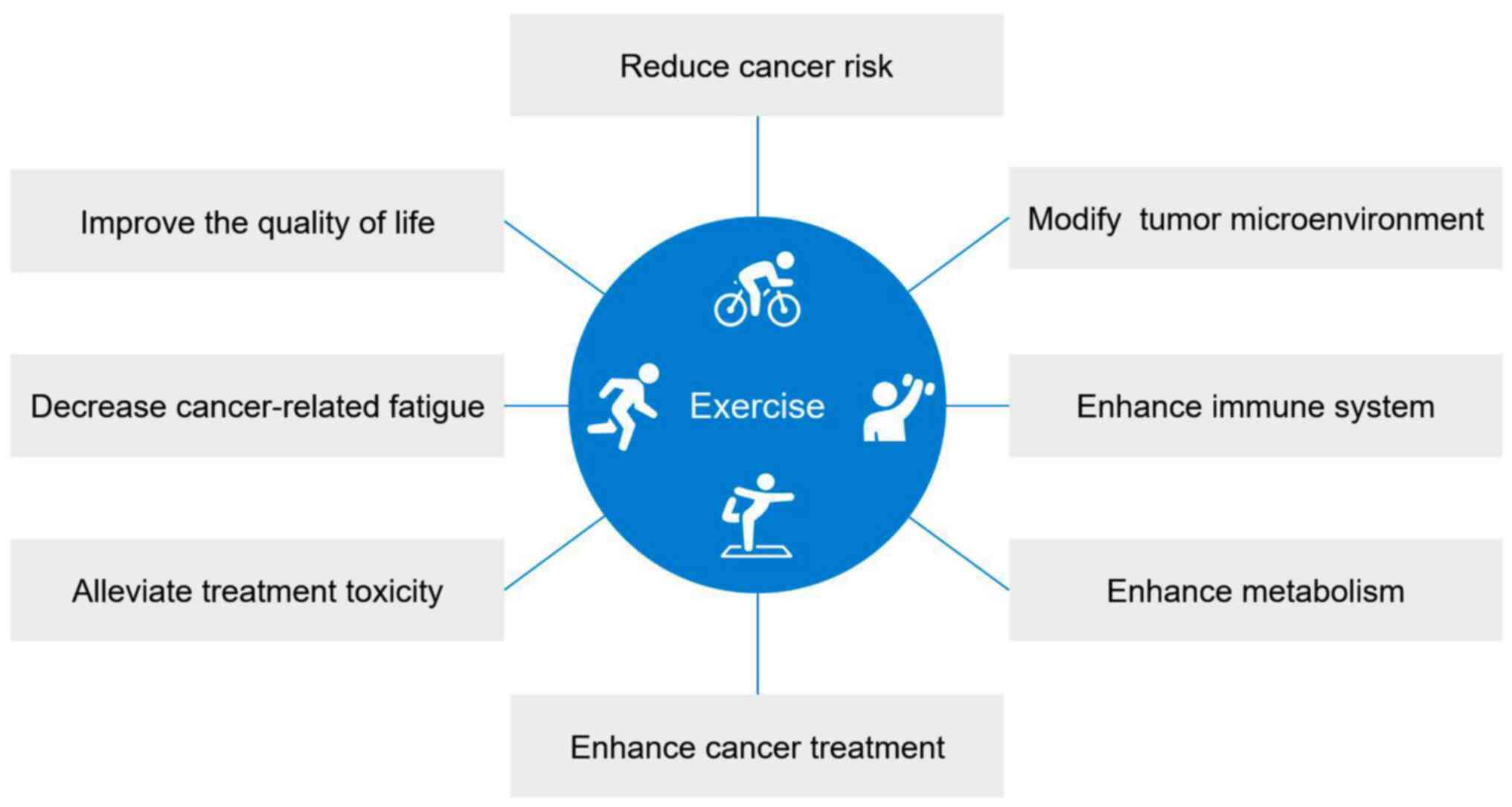
cancers (Fig. 1). Furthermore, the
complementary nature of exercise to traditional treatment renders
this a lucrative pursuit for this cancer population.
In summary, it is generally considered safe for
patients with GBM to participate in exercise and should be viewed
as a viable option by medical practitioners and patients. The
inclusion of this non-pharmacological form of therapy provides an
avenue for an improved prognosis and QoL, while instilling healthy
lifestyle behaviors in patients. Further research is warranted to
understand the mechanistic effect of exercise on cellular processes
impacted by cancer and its treatments, with a particular emphasis
on the GBM population. Concrete guidelines are also necessary to
guide clinicians and exercise physiologists on the most appropriate
exercise mode, intensity and frequency to implement for their
patients to maximize their rehabilitation. Finally, further
research is necessary in order to understand the impact of exercise
on novel forms of treatment in GBM, and cancer globally.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SS and NH conceptualized the study, engaged in
manuscript editing, reviewing and revision, and handled
communications with the journal. DH and TO assisted with manuscript
editing, reviewing and revision. All authors have reviewed and
endorsed the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peciu-Florianu I, Vannod-Michel Q, Vauleon
E, Bonneterre ME and Reyns N: Long term follow-up of patients with
newly diagnosed glioblastoma treated by intraoperative photodynamic
therapy: An update from the INDYGO trial (NCT03048240). J
Neurooncol: May 16, 2024 (Epub ahead of print) doi:
10.1007/s11060-024-04693-4, 2024.
|
|
5
|
Ohgaki H and Kleihues P: The definition of
primary and secondary glioblastoma. Clin Cancer Res. 19:764–772.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kleihues P and Ohgaki H: Primary and
secondary glioblastomas: From concept to clinical diagnosis. Neuro
Oncol. 1:44–51. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM and
Reifenberger G: , et al: The 2021 WHO classification of
tumors of the central nervous system: A summary. Neuro Oncol.
23:1231–1251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Taphoorn MJ and Bottomley A:
Health-related quality of life and symptom research in glioblastoma
multiforme patients. Expert Rev Pharmacoecon Outcomes Res.
5:763–774. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Caccese M, Indraccolo S, Zagonel V and
Lombardi G: PD-1/PD-L1 immune-checkpoint inhibitors in
glioblastoma: A concise review. Crit Rev Oncol Hematol.
135:128–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Golla H, Ahmad MA, Galushko M, Hampl J,
Maarouf M, Schroeter M, Herrlinger U, Hellmich M and Voltz R:
Glioblastoma multiforme from diagnosis to death: A prospective,
hospital-based, cohort, pilot feasibility study of patient reported
symptoms and needs. Support Care Cancer. 22:3341–3352.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Patel AV, Friedenreich CM, Moore SC, Hayes
SC, Silver JK, Campbell KL, Winters-Stone K, Gerber LH, George SM,
Fulton JE, et al: American college of sports medicine roundtable
report on physical activity, sedentary behavior, and cancer
prevention and control. Med Sci Sports Exerc. 51:2391–2402.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ashcraft KA, Warner AB, Jones LW and
Dewhirst MW: Exercise as adjunct therapy in cancer. Semin Radiat
Oncol. 29:16–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang L, Morielli AR, Heer E, Kirkham AA,
Cheung WY, Usmani N, Friedenreich CM and Courneya KS: Effects of
exercise on cancer treatment efficacy: A systematic review of
preclinical and clinical studies. Cancer Res. 81:4889–4895.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gerritsen JK and Vincent AJ: Exercise
improves quality of life in patients with cancer: A systematic
review and meta-analysis of randomised controlled trials. Br J
Sports Med. 50:796–803. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ohgaki H, Dessen P, Jourde B, Horstmann S,
Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM,
Maiorka PC, et al: Genetic pathways to glioblastoma: A
population-based study. Cancer Res. 64:6892–6899. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee DH, Lee TH, Jung CH and Kim YH:
Wogonin induces apoptosis by activating the AMPK and p53 signaling
pathways in human glioblastoma cells. Cell Signal. 24:2216–2225.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagarajan RP and Costello JF: Epigenetic
mechanisms in glioblastoma multiforme. Semin Cancer Biol.
19:188–197. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Wu C, Chen N, Gu H, Yen A, Cao L,
Wang E and Wang L: PI3K/Akt/mTOR signaling pathway and targeted
therapy for glioblastoma. Oncotarget. 7:33440–33450.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang Y, Lin D and Taniguchi CM: Hypoxia
inducible factor (HIF) in the tumor microenvironment: Friend or
foe? Sci China Life Sci. 60:1114–1124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cao WD, Kawai N, Miyake K, Zhang X, Fei Z
and Tamiya T: Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF
expression in human brain gliomas. Brain Tumor Pathol. 31:1–10.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chhipa RR, Fan Q, Anderson J,
Muraleedharan R, Huang Y, Ciraolo G, Chen X, Waclaw R, Chow LM,
Khuchua Z, et al: AMP kinase promotes glioblastoma bioenergetics
and tumour growth. Nat Cell Biol. 20:823–835. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alfarouk KO, Verduzco D, Rauch C,
Muddathir AK, Adil HH, Elhassan GO, Ibrahim ME, Orozco JD, Cardone
RA, Reshkin SJ and Harguindey S: Glycolysis, tumor metabolism,
cancer growth and dissemination. A new pH-based etiopathogenic
perspective and therapeutic approach to an old cancer question.
Oncoscience. 1:777–802. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Morland C, Andersson KA, Haugen ØP, Hadzic
A, Kleppa L, Gille A, Rinholm JE, Palibrk V, Diget EH, Kennedy LH,
et al: Exercise induces cerebral VEGF and angiogenesis via the
lactate receptor HCAR1. Nat Commun. 8(15557)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mao P, Joshi K, Li J, Kim SH, Li P,
Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, et al:
Mesenchymal glioma stem cells are maintained by activated
glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc
Natl Acad Sci USA. 110:8644–8649. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Codrici E, Enciu AM, Popescu ID, Mihai S
and Tanase C: Glioma stem cells and their microenvironments:
Providers of challenging therapeutic targets. Stem Cells Int.
2016(5728438)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guichet PO, Guelfi S, Teigell M, Hoppe L,
Bakalara N, Bauchet L, Duffau H, Lamszus K, Rothhut B and Hugnot
JP: Notch1 stimulation induces a vascularization switch with
pericyte-like cell differentiation of glioblastoma stem cells. Stem
Cells. 33:21–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng H, Ying H, Yan H, Kimmelman AC,
Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al: p53
and Pten control neural and glioma stem/progenitor cell renewal and
differentiation. Nature. 455:1129–1133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Dube C, Gibert M Jr, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 pathway in glioblastoma. Cancers (Basel).
10(297)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Brennan CW, Verhaak RGW, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wakimoto H, Tanaka S, Curry WT, Loebel F,
Zhao D, Tateishi K, Chen J, Klofas LK, Lelic N, Kim JC, et al:
Targetable signaling pathway mutations are associated with
malignant phenotype in IDH-mutant gliomas. Clin Cancer Res.
20:2898–2909. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9(338)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Koul D: PTEN signaling pathways in
glioblastoma. Cancer Biol Ther. 7:1321–1325. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Han F, Hu R, Yang H, Liu J, Sui J, Xiang
X, Wang F, Chu L and Song S: PTEN gene mutations correlate to poor
prognosis in glioma patients: A meta-analysis. Onco Targets Ther.
9:3485–3492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pearson JRD and Regad T: Targeting
cellular pathways in glioblastoma multiforme. Signal Transduct
Target Ther. 2(17040)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Galvão RP and Zong H: Inflammation and
gliomagenesis: Bi-directional communication at early and late
stages of tumor progression. Curr Pathobiol Rep. 1:19–28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Coffelt SB, Tal AO, Scholz A, De Palma M,
Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y and
Lewis CE: Angiopoietin-2 regulates gene expression in
TIE2-expressing monocytes and augments their inherent proangiogenic
functions. Cancer Res. 70:5270–5280. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gabrilovich D: Mechanisms and functional
significance of tumour-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK,
Shin YJ, Lee Y, Kim SH, Lee SY, Seo S, et al: TP53 gain-of-function
mutation promotes inflammation in glioblastoma. Cell Death Differ.
26:409–425. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo J, Shinriki S, Su Y, Nakamura T,
Hayashi M, Tsuda Y, Murakami Y, Tasaki M, Hide T, Takezaki T, et
al: Hypoxia suppresses cylindromatosis (CYLD) expression to promote
inflammation in glioblastoma: Possible link to acquired resistance
to anti-VEGF therapy. Oncotarget. 5:6353–6364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Moore SC, Lee IM, Weiderpass E, Campbell
PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, de Gonzalez AB,
Hartge P, et al: Association of leisure-time physical activity with
risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med.
176:816–825. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Galvão DA and Newton RU: Review of
exercise intervention studies in cancer patients. J Clin Oncol.
23:899–909. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Koelwyn GJ, Quail DF, Zhang X, White RM
and Jones LW: Exercise-dependent regulation of the tumour
microenvironment. Nat Rev Cancer. 17:620–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hojman P, Gehl J, Christensen JF and
Pedersen BK: Molecular mechanisms linking exercise to cancer
prevention and treatment. Cell Metab. 27:10–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Woods JA, Wilund KR, Martin SA and Kistler
BM: Exercise, inflammation and aging. Aging Dis. 3:130–140.
2012.PubMed/NCBI
|
|
50
|
Coffey VG and Hawley JA: The molecular
bases of training adaptation. Sports Med. 37:737–763.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yu M, King B, Ewert E, Su X, Mardiyati N,
Zhao Z and Wang W: Exercise ACTIVATES p53 and negatively regulates
IGF-1 pathway in epidermis within a skin cancer model. PLoS One.
11(e0160939)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nezamdoost Z, Saghebjoo M, Hoshyar R,
Hedayati M and Keska A: High-intensity training and saffron:
Effects on breast cancer-related gene expression. Med Sci Sports
Exerc. 52:1470–1476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zaidi SK, Van Wijnen AJ, Lian JB, Stein JL
and Stein GS: Targeting deregulated epigenetic control in cancer. J
Cell Physiol. 228:2103–2108. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ferioli M, Zauli G, Maiorano P, Milani D,
Mirandola P and Neri LM: Role of physical exercise in the
regulation of epigenetic mechanisms in inflammation, cancer,
neurodegenerative diseases, and aging process. J Cell Physiol.
234:14852–14864. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zeng H, Irwin ML, Lu L, Risch H, Mayne S,
Mu L, Deng Q, Scarampi L, Mitidieri M, Katsaros D and Yu H:
Physical activity and breast cancer survival: An epigenetic link
through reduced methylation of a tumor suppressor gene L3MBTL1.
Breast Cancer Res Treat. 133:127–135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Khori V, Shalamzari SA, Isanejad A,
Alizadeh AM, Alizadeh S, Khodayari S, Khodayari H, Shahbazi S,
Zahedi A, Sohanaki H, et al: Effects of exercise training together
with tamoxifen in reducing mammary tumor burden in mice: Possible
underlying pathway of miR-21. Eur J Pharmacol. 765:179–187.
2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: MiR-21 induced angiogenesis
through AKT and ERK activation and HIF-1α expression. PLoS One.
6(e19139)2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Elsner VR, Lovatel GA, Bertoldi K,
Vanzella C, Santos FM, Spindler C, de Almeida EF, Nardin P and
Siqueira IR: Effect of different exercise protocols on histone
acetyltransferases and histone deacetylases activities in rat
hippocampus. Neuroscience. 192:580–587. 2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z
and Fan G: Exercise impacts brain-derived neurotrophic factor
plasticity by engaging mechanisms of epigenetic regulation. Eur J
Neurosci. 33:383–390. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Abel JL and Rissman EF: Running-induced
epigenetic and gene expression changes in the adolescent brain. Int
J Dev Neurosci. 31:382–390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wójtowicz K, Czarzasta K, Przepiorka L,
Kujawski S, Cudnoch-Jedrzejewska A, Marchel A and Kunert P:
Brain-Derived neurotrophic factor (BDNF) concentration levels in
cerebrospinal fluid and plasma in patients with glioblastoma: A
prospective, observational, controlled study. Cureus.
15(e48237)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ng DQ, Cheng I, Wang C, Tan CJ, Toh YL,
Koh YQ, Ke Y, Foo KM, Chan RJ, Ho HK, et al: Brain-derived
neurotrophic factor as a biomarker in cancer-related cognitive
impairment among adolescent and young adult cancer patients. Sci
Rep. 13(16298)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Agostini D, Natalucci V, Baldelli G, De
Santi M, Zeppa SD, Vallorani L, Annibalini G, Lucertini F, Federici
A, Izzo R, et al: New insights into the role of exercise in
inhibiting mTOR signaling in triple-negative breast cancer. Oxid
Med Cell Longev. 2018(5896786)2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Alizadeh AM, Heydari Z, Rahimi M, Bazgir
B, Shirvani H, Alipour S, Heidarian Y, Khalighfard S and Isanejad
A: Oxytocin mediates the beneficial effects of the exercise
training on breast cancer. Exp Physiol. 103:222–235.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Faustino-Rocha AI, Silva A, Gabriel J, da
Costa RM, Moutinho M, Oliveira PA, Gama A, Ferreira R and Ginja M:
Long-term exercise training as a modulator of mammary cancer
vascularization. Biomed Pharmacother. 81:273–280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Isanejad A, Alizadeh AM, Shalamzari SA,
Khodayari H, Khodayari S, Khori V and Khojastehnjad N:
MicroRNA-206, let-7a and microRNA-21 pathways involved in the
anti-angiogenesis effects of the interval exercise training and
hormone therapy in breast cancer. Life Sci. 151:30–40.
2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jones LW, Antonelli J, Masko EM,
Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JR, Nagendran
J, Flores CT, et al: Exercise modulation of the host-tumor
interaction in an orthotopic model of murine prostate cancer. J
Appl Physiol (1985). 113:263–272. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Jones LW, Viglianti BL, Tashjian JA,
Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Moon EJ, Schroeder
T, Herndon JE II and Dewhirst MW: Effect of aerobic exercise on
tumor physiology in an animal model of human breast cancer. J Appl
Physiol. 108:343–348. 1985.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Rincón-Castanedo C, Morales JS,
Martín-Ruiz A, Valenzuela PL, Ramírez M, Santos-Lozano A, Lucia A
and Fiuza-Luces C: Physical exercise effects on metastasis: A
systematic review and meta-analysis in animal cancer models. Cancer
Metastasis Rev. 39:91–114. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
O'Neill HM: AMPK and exercise: Glucose
uptake and insulin sensitivity. Diabetes Metab J. 37:1–21.
2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li W, Saud SM, Young MR, Chen G and Hua B:
Targeting AMPK for cancer prevention and treatment. Oncotarget.
6:7365–7378. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kim I and He YY: Targeting the
AMP-activated protein kinase for cancer prevention and therapy.
Front Oncol. 3(175)2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Piguet AC, Saran U, Simillion C, Keller I,
Terracciano L, Reeves HL and Dufour JF: Regular exercise decreases
liver tumors development in hepatocyte-specific PTEN-deficient mice
independently of steatosis. J Hepatol. 62:1296–1303.
2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Vara-Ciruelos D, Russell FM and Hardie DG:
The strange case of AMPK and cancer: Dr Jekyll or Mr
Hyde?†. Open Biol. 9(190099)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hardie DG: AMPK: Positive and negative
regulation, and its role in whole-body energy homeostasis. Curr
Opin Cell Biol. 33:1–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Faubert B, Boily G, Izreig S, Griss T,
Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et
al: AMPK is a negative regulator of the Warburg effect and
suppresses tumor growth in vivo. Cell Metab. 17:113–124.
2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ponnusamy L, Natarajan SR, Thangaraj K and
Manoharan R: Therapeutic aspects of AMPK in breast cancer:
Progress, challenges, and future directions. Biochim Biophys Acta
Rev Cancer. 1874(188379)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Aveseh M, Nikooie R and Aminaie M:
Exercise-induced changes in tumour LDH-B and MCT1 expression are
modulated by oestrogen-related receptor alpha in breast
cancer-bearing BALB/c mice. J Physiol. 593:2635–2648.
2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Bacurau RF, Belmonte MA, Seelaender MC and
Costa Rosa LF: Effect of a moderate intensity exercise training
protocol on the metabolism of macrophages and lymphocytes of
tumour-bearing rats. Cell Biochem Funct. 18:249–258.
2000.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Campos JC, Fernandes T, Bechara LR, da
Paixão NA, Brum PC, de Oliveira EM and Ferreira JC: Increased
clearance of reactive aldehydes and damaged proteins in
hypertension-induced compensated cardiac hypertrophy: Impact of
exercise training. Oxid Med Cell Longev.
2015(464195)2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang X, Ashcraft KA, Warner AB, Nair SK
and Dewhirst MW: Can exercise-induced modulation of the tumor
physiologic microenvironment improve antitumor immunity? Cancer
Res. 79:2447–2456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Nieman DC and Wentz LM: The compelling
link between physical activity and the body's defense system. J
Sport Health Sci. 8:201–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Campbell JP and Turner JE: Debunking the
myth of exercise-induced immune suppression: Redefining the impact
of exercise on immunological health across the lifespan. Front
Immunol. 9(648)2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gupta P, Bigley AB, Markofski M, Laughlin
M and LaVoy EC: Autologous serum collected 1 h post-exercise
enhances natural killer cell cytotoxicity. Brain Behav Immun.
71:81–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Nieman DC, Henson DA, Austin MD and Brown
VA: Immune response to a 30-minute walk. Med Sci Sports Exerc.
37:57–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Idorn M and Hojman P: Exercise-dependent
regulation of NK cells in cancer protection. Trends Mol Med.
22:565–577. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Evans ES, Hackney AC, McMurray RG, Randell
SH, Muss HB, Deal AM and Battaglini CL: Impact of acute
intermittent exercise on natural killer cells in breast cancer
survivors. Integr Cancer Ther. 14:436–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Barra NG, Fan IY, Gillen JB, Chew M,
Marcinko K, Steinberg GR, Gibala MJ and Ashkar AA: High intensity
interval training increases natural killer cell number and function
in obese breast cancer-challenged mice and obese women. J Cancer
Prev. 22:260–266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Pedersen L, Idorn M, Olofsson GH,
Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC,
Pedersen KS, Dethlefsen C, et al: Voluntary running suppresses
tumor growth through epinephrine- and IL-6-dependent NK cell
mobilization and redistribution. Cell Metab. 23:554–562.
2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Wennerberg E, Lhuillier C, Rybstein MD,
Dannenberg K, Rudqvist NP, Koelwyn GJ, Jones LW and Demaria S:
Exercise reduces immune suppression and breast cancer progression
in a preclinical model. Oncotarget. 11:452–461. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Schirrmacher V: From chemotherapy to
biological therapy: A review of novel concepts to reduce the side
effects of systemic cancer treatment (Review). Int J Oncol.
54:407–419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Scott JM, Khakoo A, Mackey JR, Haykowsky
MJ, Douglas PS and Jones LW: Modulation of anthracycline-induced
cardiotoxicity by aerobic exercise in breast cancer: Current
evidence and underlying mechanisms. Circulation. 124:642–650.
2011.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Repka CP and Hayward R: Oxidative stress
and fitness changes in cancer patients after exercise training. Med
Sci Sports Exerc. 48:607–614. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Park HS, Kim CJ, Kwak HB, No MH, Heo JW
and Kim TW: Physical exercise prevents cognitive impairment by
enhancing hippocampal neuroplasticity and mitochondrial function in
doxorubicin-induced chemobrain. Neuropharmacology. 133:451–461.
2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Spina S, Facciorusso S, Cinone N,
Pellegrino R, Fiore P and Santamato A: Rehabilitation interventions
for glioma patients: A mini-review. Front Surg.
10(1137516)2023.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Sandler CX, Matsuyama M, Jones TL,
Bashford J, Langbecker D and Hayes SC: Physical activity and
exercise in adults diagnosed with primary brain cancer: A
systematic review. J Neurooncol. 153:1–14. 2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Pace A, Dirven L, Koekkoek JAF, Golla H,
Fleming J, Rudà R, Marosi C, Le Rhun E, Grant R, Oliver K, et al:
European association for neuro-oncology (EANO) guidelines for
palliative care in adults with glioma. Lancet Oncol. 18:e330–e340.
2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Piil K, Juhler M, Jakobsen J and Jarden M:
Controlled rehabilitative and supportive care intervention trials
in patients with high-grade gliomas and their caregivers: A
systematic review. BMJ Support Palliat Care. 6:27–34.
2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Vargo M: Brain tumor rehabilitation. Am J
Phys Med Rehabil. 90 (5 Suppl 1):S50–S62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Formica V, Del Monte G, Giacchetti I,
Grenga I, Giaquinto S, Fini M and Roselli M: Rehabilitation in
neuro-oncology: A meta-analysis of published data and a
mono-institutional experience. Integr Cancer Ther. 10:119–126.
2011.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Roberts PS, Nuño M, Sherman D, Asher A,
Wertheimer J, Riggs RV and Patil CG: The impact of inpatient
rehabilitation on function and survival of newly diagnosed patients
with glioblastoma. PM R. 6:514–521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Capozzi LC, Boldt KR, Easaw J, Bultz B and
Culos-Reed SN: Evaluating a 12-week exercise program for brain
cancer patients. Psychooncology. 25:354–358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Riggs L, Piscione J, Laughlin S,
Cunningham T, Timmons BW, Courneya KS, Bartels U, Skocic J, de
Medeiros C, Liu F, et al: Exercise training for neural recovery in
a restricted sample of pediatric brain tumor survivors: A
controlled clinical trial with crossover of training versus no
training. Neuro Oncol. 19:440–450. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Gehring K, Kloek CJ, Aaronson NK, Janssen
KW, Jones LW, Sitskoorn MM and Stuiver MM: Feasibility of a
home-based exercise intervention with remote guidance for patients
with stable grade II and III gliomas: A pilot randomized controlled
trial. Clin Rehabil. 32:352–366. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Troschel FM, Brandt R, Wiewrodt R, Stummer
W and Wiewrodt D: High-intensity physical exercise in a
glioblastoma patient under multimodal treatment. Med Sci Sports
Exerc. 51:2429–2433. 2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Halkett GKB, Cormie P, McGough S, Zopf EM,
Galvão DA, Newton RU and Nowak AK: Patients and carers'
perspectives of participating in a pilot tailored exercise program
during chemoradiotherapy for high grade glioma: A qualitative
study. Eur J Cancer Care (Engl). 30(e13453)2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Hansen A, Søgaard K and Minet LR:
Development of an exercise intervention as part of rehabilitation
in a glioblastoma multiforme survivor during irradiation treatment:
A case report. Disabil Rehabil. 41:1608–1614. 2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Yu J, Jung Y, Park J, Kim JM, Suh M, Cho
KG and Kim M: Intensive rehabilitation therapy following brain
tumor surgery: A pilot study of effectiveness and long-term
satisfaction. Ann Rehabil Med. 43:129–141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hojan K and Gerreth K: Can
multidisciplinary inpatient and outpatient rehabilitation provide
sufficient prevention of disability in patients with a brain
tumor?-A case-series report of two programs and a prospective,
observational clinical trial. Int J Environ Res Public Health.
17(6488)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Cormie P, Nowak AK, Chambers SK, Galvão DA
and Newton RU: The potential role of exercise in neuro-oncology.
Front Oncol. 5(85)2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ji JF, Ji SJ, Sun R, Li K, Zhang Y, Zhang
LY and Tian Y: Forced running exercise attenuates hippocampal
neurogenesis impairment and the neurocognitive deficits induced by
whole-brain irradiation via the BDNF-mediated pathway. Biochem
Biophys Res Commun. 443:646–651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Fardell JE, Vardy J, Shah JD and Johnston
IN: Cognitive impairments caused by oxaliplatin and 5-fluorouracil
chemotherapy are ameliorated by physical activity.
Psychopharmacology (Berl). 220:183–193. 2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Knöchel C, Oertel-Knöchel V, O'Dwyer L,
Prvulovic D, Alves G, Kollmann B and Hampel H: Cognitive and
behavioural effects of physical exercise in psychiatric patients.
Prog Neurobiol. 96:46–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Levin GT, Greenwood KM, Singh F, Tsoi D
and Newton RU: Exercise improves physical function and mental
health of brain cancer survivors: Two exploratory case studies.
Integr Cancer Ther. 15:190–196. 2016.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Gehring K, Stuiver MM, Visser E, Kloek C,
van den Bent M, Hanse M, Tijssen C, Rutten GJ, Taphoorn MJB,
Aaronson NK and Sitskoorn MM: A pilot randomized controlled trial
of exercise to improve cognitive performance in patients with
stable glioma: A proof of concept. Neuro Oncol. 22:103–115.
2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Pieczyńska A, Zasadzka E, Pilarska A,
Procyk D, Adamska K and Hojan K: Rehabilitation exercises supported
by monitor-augmented reality for patients with high-grade glioma
undergoing radiotherapy: Results of a randomized clinical trial. J
Clin Med. 12(6838)2023.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Lemke D, Pledl HW, Zorn M, Jugold M, Green
E, Blaes J, Löw S, Hertenstein A, Ott M, Sahm F, et al: Slowing
down glioblastoma progression in mice by running or the
anti-malarial drug dihydroartemisinin? Induction of oxidative
stress in murine glioblastoma therapy. Oncotarget. 7:56713–5625.
2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Tantillo E, Colistra A, Baroncelli L,
Costa M, Caleo M and Vannini E: Voluntary physical exercise reduces
motor dysfunction and hampers tumor cell proliferation in a mouse
model of glioma. Int J Environ Res Public Health.
17(5667)2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Betof AS, Dewhirst MW and Jones LW:
Effects and potential mechanisms of exercise training on cancer
progression: A translational perspective. Brain Behav Immun. 30
(Suppl):S75–S87. 2013.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Wolff G, Davidson SJ, Wrobel JK and
Toborek M: Exercise maintains blood-brain barrier integrity during
early stages of brain metastasis formation. Biochem Biophys Res
Commun. 463:811–817. 2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Michaelsen SR, Christensen IJ, Grunnet K,
Stockhausen MT, Broholm H, Kosteljanetz M and Poulsen HS: Clinical
variables serve as prognostic factors in a model for survival from
glioblastoma multiforme: An observational study of a cohort of
consecutive non-selected patients from a single institution. BMC
Cancer. 13(402)2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Moore SC, Rajaraman P, Dubrow R, Darefsky
AS, Koebnick C, Hollenbeck A, Schatzkin A and Leitzmann MF: Height,
body mass index, and physical activity in relation to glioma risk.
Cancer Res. 69:8349–8355. 2009.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Ruden E, Reardon DA, Coan AD, Herndon JE
II, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ,
Waner E, et al: Exercise behavior, functional capacity, and
survival in adults with malignant recurrent glioma. J Clin Oncol.
29:2918–2923. 2011.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Williams PT: Reduced risk of brain cancer
mortality from walking and running. Med Sci Sports Exerc.
46:927–932. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Campbell KL, Winters-Stone KM, Wiskemann
J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE,
Ligibel JA, Gerber LH, et al: Exercise guidelines for cancer
survivors: Consensus statement from international multidisciplinary
roundtable. Med Sci Sports Exerc. 51:2375–2390. 2019.PubMed/NCBI View Article : Google Scholar
|