Introduction
Globally, breast cancer ranks highest among all
types of malignancies. It is the primary cause of
malignancy-related mortality among individuals between the ages of
50 and 55 years. Breast cancer worldwide accounts for ~22% of all
cancers affecting women (1). In
Iraq, breast cancer has exhibited a significant increase over the
past two decades, rendering it the most prevalent type of cancer
among women. It constitutes ~50% of all female cancers, followed by
thyroid, colon, central nervous system and lung cancers (2-4).
Triple-negative breast cancer (TNBC) constitutes 15 to 20% of all
cases of breast cancer. This subtype is characterized by the
absence of estrogen receptor (ER) and progesterone receptor (PR),
as well as human epidermal growth factor receptor-2 (HER-2)
(5). This subtype is distinguished
from others by a worse prognosis, a higher relapse probability and
an earlier age of onset (6,7).
Targeted therapies against HER-2 and endocrine medicines can be
beneficial for subtypes that test positive for HER-2 and hormone
receptors. However, adjuvant and neoadjuvant cytotoxic chemotherapy
is commonly employed for the treatment of patients with TNBC. Given
the low efficacy and high frequency of harmful side-effects of
chemotherapy, it is clear that more targeted therapeutic techniques
are required to treat this type of cancer (8). An increasingly common type of
immunotherapy, using immune checkpoint inhibitors (ICIs) is finding
an increasing number of applications in major therapeutic contexts.
Tumor cells can evade immune surveillance by employing
immunological checkpoints, the activation of coinhibitory signaling
pathways, and promoting immune tolerance. There is presently a
notable inclination towards investigating therapeutic alternatives
for TNBC through the utilization of ICIs, particularly those that
directly target the programmed cell death 1 (PD-1) and programmed
death ligand 1 (PD-L1) pathways. Depending on the expression of
PD-L1, the use of this type of therapy is commonly advised
(8-10).
Significant therapeutic advantages have been demonstrated in
bladder, lung, skin and kidney malignancies with PD-1/PD-L1
inhibitors (11). There is strong
evidence to indicate that immunotherapy has a higher response rate
in TNBC compared to other subtypes of breast cancer. This is due to
the fact that TNBC is usually characterized by an increased number
of mutations, a relatively high expression of PD-L1, and a larger
abundance of tumor-infiltrating lymphocytes (TILs) (12). In addition, improved results are
observed with higher TIL levels in TNBC (13). Using PD-1/PD-L1 inhibitors as a
tactic for combating TNBC is possible, as ICIs can accelerate the
elimination process of the immune system (14). Using atezolizumab combined with
nab-paclitaxel in treatment of the cases of metastatic TNBC (mTNBC)
cases that are positive for PD-L1 expression was approved in 2019
by the European Commission and the Food and Drug Administration
(FDA). Next to this license, the first immunotherapy protocol for
the treatment of breast cancer was authorized (15). After the promising outcomes in
treating mTNBC, researchers investigated the use of the PD-1/PD-L1
monoclonal antibodies for the treatment of early-stage TNBC. More
encouraging results have surfaced as of late (16-18).
A combination of basic research and clinical trials is required for
the effective use of PD-1/PD-L1 inhibitors in early-stage TNBC
(11).
Key tenets of PD-1/PD-L1
inhibition
PD-1 and PD-L1, which are transmembrane proteins,
have been classified as immunoglobulin (Ig) superfamily members.
The activated T-cell membrane surface exhibits the presence of
PD-1(13). The existence of PD-L1
in normal tissue has been well-reported, as it functions as the
ligand for PD-1. Interactions between PD-1 and PD-L1 decrease the
activity of T-cells, resulting in immunological tolerance. The
PD-1/PD-L1 pathway plays a central role in maintaining the
equilibrium in the body's immune system (19). The aberrant expression of PD-L1 has
been observed in several types of cancer, including lung,
colorectal and breast cancers, and melanoma. There is a potential
association between the cytokines present in the tumor
microenvironment (TME), specifically interferon (IFN) (20). IFN serves as the primary soluble
cytokine responsible for inducing the production of PD-L1 in tumor
cells as part of an immune response. The elevated production of
transcription factors in cancer cells leads to the upregulation of
PD-L1 transcription and translation, facilitated by the binding of
IFN to its receptor (20). The
activation of PD-1 and PD-L1 inhibits lymphocyte proliferation via
T-cell receptors. Immunosurveillance is initiated. As the
expression of PD-L1 increases in malignancies, the TME may become
more immunosuppressive (21).
Interfering with these co-inhibitory pathways has been shown to be
effective in the treatment of various types of cancer. For
instance, previous research has demonstrated that the inhibition of
the interaction between PD-1 and PD-L1 enhances the T-cell immune
response. This objective can be achieved through several
mechanisms: i) Stimulating and invigorating lymphocyte activity and
the secretion of cytotoxic cytokines; ii) proliferating and
activating CD8+ T-cells that exhibit specificity towards
tumor antigens; iii) inhibiting lymphocyte apoptosis induced by the
PD-1/PD-L1 interaction; and iv) augmenting the capacity of the
immune system to differentiate tumor cells (22).
Recent studies have provided evidence that the
inclusion of atezolizumab or pembrolizumab, which specifically
target PD-L1 or PD-1, in chemotherapeutic regimens leads to
enhanced outcomes in patients with both early-stage TNBC and mTNBC
(23,24). Among patients diagnosed with mTNBC,
the combined use of atezolizumab with nab-paclitaxel has
demonstrated a statistically significant benefit in
progression-free survival (PFS) and a clinically significant
benefit in overall survival. The observed advantage was found when
comparing the effects of nab-paclitaxel in patients who tested
positive for PD-L1(25), which
refers to tumor-infiltrating immune cells that express PD-L1 and
cover a minimum of 1% of the tumor area (19). The aforementioned discoveries have
resulted in a significant shift in the worldwide benchmarks of
healthcare. In patients with tumors positive for PD-L1, and who
exhibit a combined positive score (CPS) of at least 10, the
administration of pembrolizumab, either in combination with
gemcitabine or taxane plus carboplatin, has been demonstrated to
lead to a greater benefit in PFS when compared to a placebo
combination with chemotherapy. The calculation of the CPS score
involves dividing the aggregate count of tumor cells, lymphocytes
and macrophages expressing PD-L1 by the overall count of viable
tumor cells (23). By contrast, it
was observed that the occurrence of a pathological complete
response was not influenced by the presence of the PD-L1 status
when atezolizumab or pembrolizumab was administered alongside
neoadjuvant chemotherapy regimens in the early stage of TNBC
(24). This finding implies that
evaluating the PD-L1 status may have diminished importance in this
particular scenario (24).
Therefore, the assessment of PD-L1 expression in the
TNBC is increasingly being incorporated into normal pathological
procedures and has become a customary approach in the management of
mTNBC. Currently, there is a notable focus on the utilization of
PD-L1-targeted therapy in the clinical trials of the Iraqi nation
(not registered online yet), particularly in the context of ICIs.
The objective of the present study is to investigate the
immunohistological analysis of PD-L1 expression in TNBC and to
identify the clinicopathological characteristics that can be used
to predict positivity. This may enable the publication of an Iraqi
data-driven analysis on an intriguing and innovative subject in
clinical oncology.
Materials and methods
Study design
The present retrospective study included 40
histological materials provided by core needle biopsies under
ultrasound guidance from women with TNBC, together with their
relevant pathological reports. The samples were collected from the
Pathology Department of Al-Massa Private Center in Baghdad (Iraq)
during the period from January, 2021 to December, 2023. The
histological materials were formalin-fixed paraffin-embedded tissue
blocks of TNBC. The following data were extracted from the
pathological reports: The age of the patients, breast laterality,
histological grade, calcification, necrosis and cytological reports
provided by fine needle aspiration under an ultrasound guide for
any radiologically suspicious axillary lymph node status at
presentation. The present study was approved by the Al-Massa
Center's Ethical Committee (reference no. 141412-23), and it
followed its institutional policy. Consent to participate was
deemed not applicable as the present study was a retrospective
study using data with no violation of patient privacy.
Sample selection
The inclusion criterion was histological materials
of breast cancer that were triple-negative for immunohistochemical
tests (ER, PR and HER2\NEU) and had complete radiological,
histological and cytological reported data in the patient's
pathological report in a single center. The exclusion criteria
included the following: Histological materials of breast cancer
that were positive for one or more of the following
immunohistochemical tests: ER, PR and HER2\NEU; those with missing
or incomplete relevant data; invalid PDL1 tests; i.e., histological
samples that were not compatible with theappropriate PD-L1
test.
Immunohistochemistry
Formalin-fixed paraffin-embedded blocks were
submitted for immunohistochemistry. Fixation was performed with 10%
neutral-buffered formalin (Pandora Industries Pvt. Ltd.) was 48 h
at room temperature. A 4-µM-thick tissue section was created,
adhered to a charged slide, and then dried for 30 min at 62˚C. The
samples underwent standard heat epitope retrieval at pH 8.0 for 30
min in ethylene diamine tetraacetic acid (Unilong Industry Co.,
Ltd.). Subsequently, incubation with primary PD-L1 antibody using
anti-human PDL1 (PathnSitu Biotechnologies, PDL1-clone B7H1P;
isotype monoclonal rabbit IgG; cat. no. PR303) was performed,
followed by non-biotinylated anti-mouse immunoglobulin, and
peroxidase-labeled streptavidin (PathnSitu Biotechnologies; cat.
no. OSH001, 6 ml; ready-to-use). Harris hematoxylin (PathnSitu
Biotechnologies; cat. no. PS021 served as the counterstain for the
slides by covering tissue sections for 8-10 min at room
temperature. The optimal concentrations for the primary antibody
and the incubation time were applied according to the specific
instructions provided by PathnSitu Biotechnologies, the
manufacturer of the product. The recommended dilution was
1:50-1:100 and the incubation time was 30-60 min at room
temperature. Graded alcohols and xylenes (Greenwell Biotech/India)
were applied, followed by cover-slipping. Each run was conducted
with the inclusion of both positive and negative external controls;
the positive external control was splenic tissue, while the
negative external control was a known case of PD-L1-negative breast
cancer. The examination of the staining results was performed using
a light microscope (Leica Microsystem GmbH).
Data interpretation
The interpretation of the results was carried out by
two independent pathologists in a blinded manner. The assessment of
PD-L1 expression was achieved by using the CPS. It was obtained by
calculating the number of cells that stained positive (membranous
and/or cytoplasmic) with PD-L1, which included tumor cells,
lymphocytes and macrophages, divided by the total number of viable
tumor cells, then multiplied by 100. The specimen was regarded as
having PD-L1 expression if the CPS was ≥10. Accordingly, the total
sample was divided into two groups as follows: CPS <10 and CPS
≥10. Each histological sample should contain at least 100 viable
tumor cells; degenerated or necrotic cells were excluded (26). Photomicrographs were obtained using
a Leica ICC 50E camera (Leica Microsystem GmbH).
Statistical analysis
The analysis of the data in the present study was
performed using the Statistical Package for Social Sciences (SPSS)
version 25 (IBM Corp.). The range of the patient's age is presented
as the mean ± standard deviation (SD). Comparisons between PD-L1
expression and histological grade, calcification, necrosis and
axillary lymph node status at presentation were achieved using
Fisher's exact and Chi-squared tests. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
The present study used 40 histological samples of
TNBC; 20 were from the right breast and 20 were from the left one;
the age range of the patients was from 39 to 78 years. The mean age
was 62.825±3.12 years. Of the total number of samples, 16 (40%)
were grade I, 8 (20%) were grade II, and 16 (40%) were grade III.
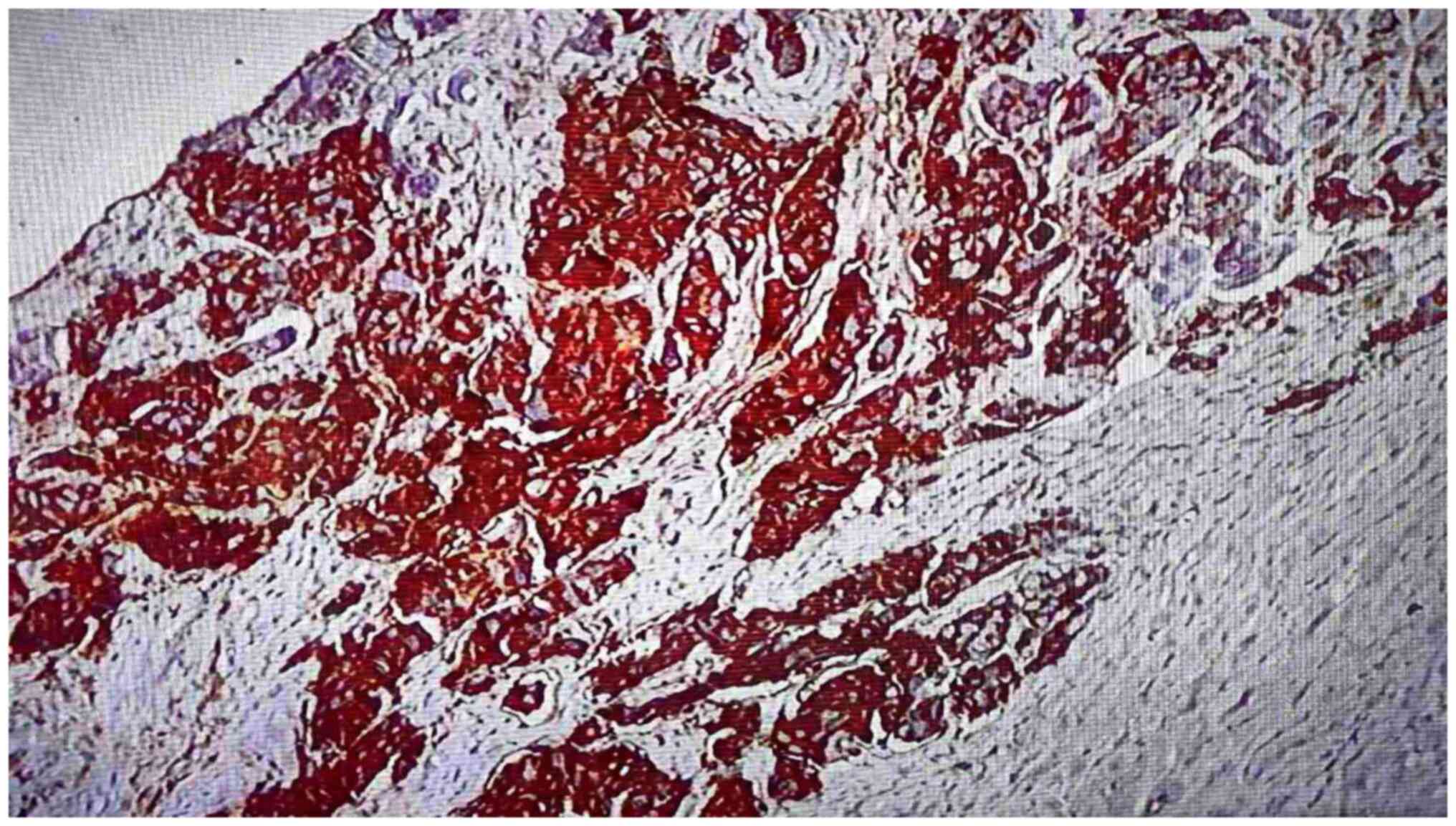
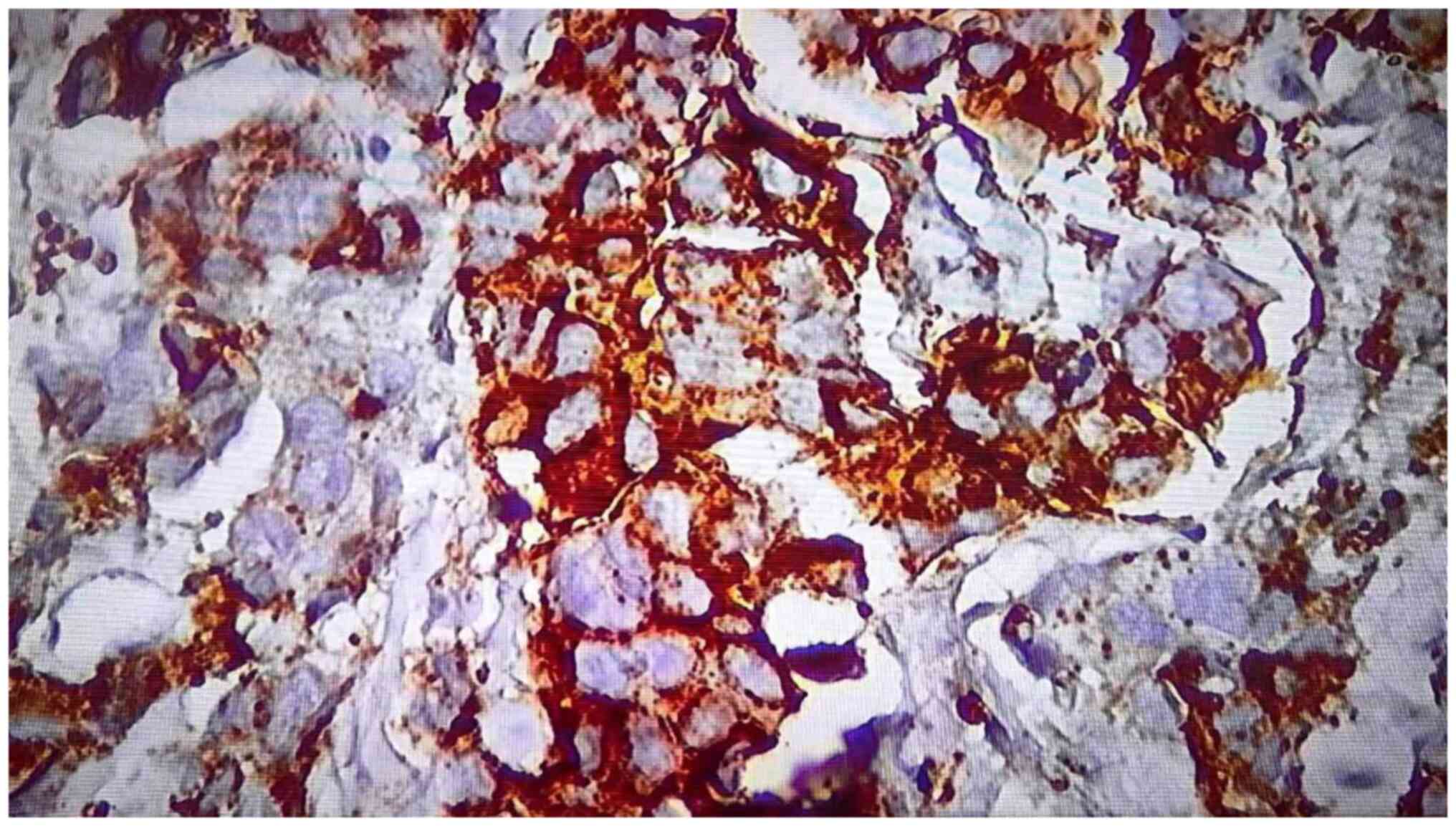
Positivity for PD-L1 was found in 24 (60%) of the total number of
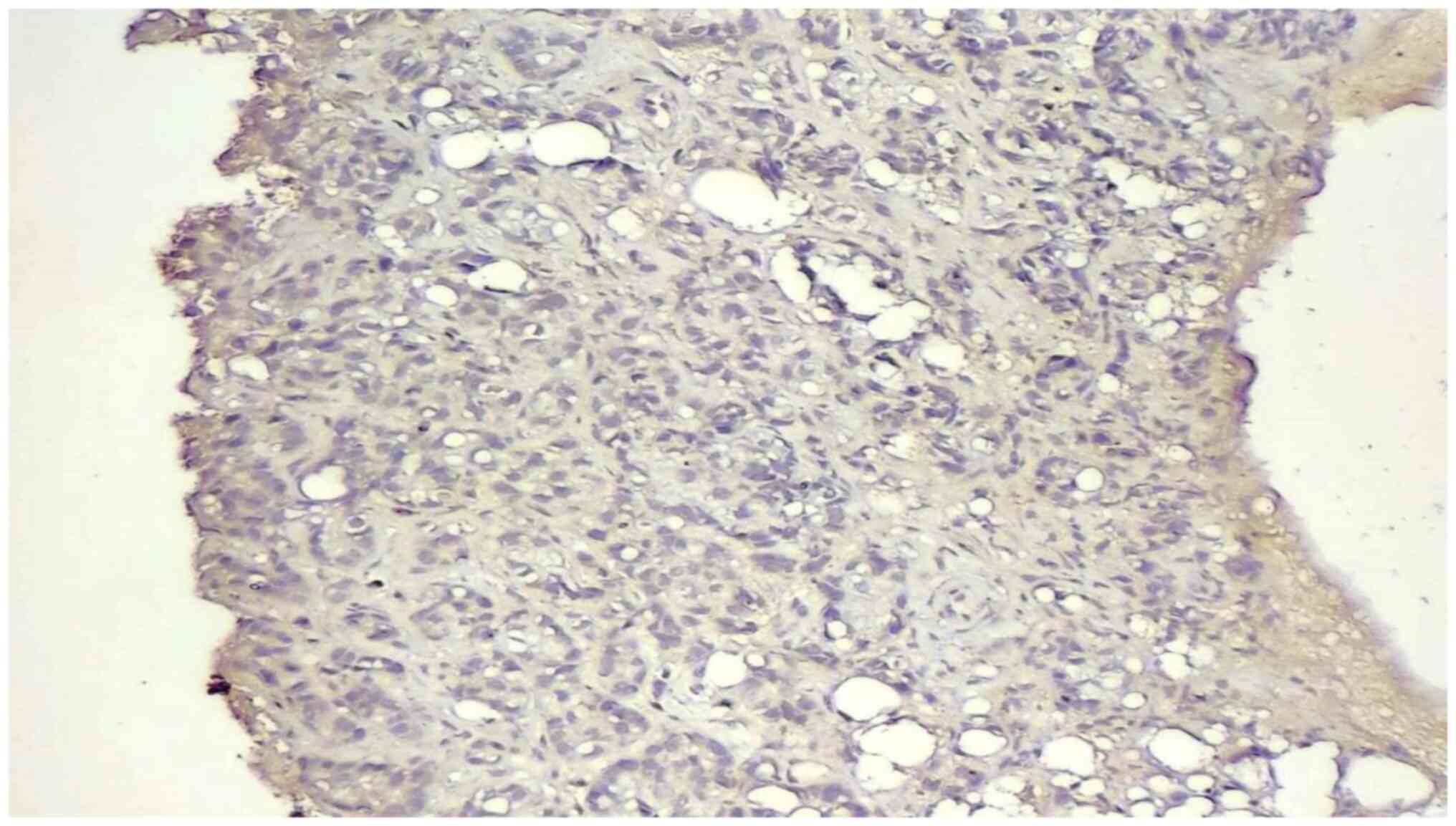
samples (Figs. 1 and 2). Negativity was observed in 16 (40%)
cases (Fig. 3). The mean number of
PD-L1-positive cases was 37.8333±21.85. There was a
non-statistically significant association between PD-L1 positivity,
histological grade and the presence of tissue necrosis. A
statistically significant association was found between PD-L1
positivity and the presence of calcification and positive axillary
lymph node status at presentation (Table I).
 | Table IAssociation between PD-L1 expression
and histological grade, necrosis, calcification and positive
axillary lymph node status at presentation in patients with
TNBC. |
Table I
Association between PD-L1 expression
and histological grade, necrosis, calcification and positive
axillary lymph node status at presentation in patients with
TNBC.
| | PD-L1 status | |
|---|
| Study variable | PD-L1 positive | PD-L1 negative | Total | P-value |
|---|
| Histological
grade | | | | |
|
Grade I | 8 (20%) | 8 (20%) | 16 (40%) | 0.286505a |
|
Grade
II | 4 (10%) | 4 (10%) | 8 (20%) | (Fisher's test) |
|
Grade
III | 12 (30%) | 4 (10%) | 16 (40%) | |
|
Total | 24 (60%) | 16 (40%) | 40 (100%) | |
| Necrosis | | | | |
|
Presence | 9 (22.5%) | 3 (7.5%) | 12 (30%) | 0.2969a |
|
Absence | 15 (37.5%) | 13 (32.5%) | 28 (70%) | (Chi-squared
test) |
|
Total | 24 (60%) | 16 (40%) | 40 (100%) | |
| Tissue
calcification | | | | |
|
Presence | 17 (42.5%) | 2 (5%) | 19 (47.5%) | 0. 0004b |
|
Absence | 7 (17.5%) | 14 (35%) | 21 (52.5%) | (Chi-squared
test) |
|
Total | 24 (60%) | 16 (40%) | 40 (100%) | |
| Axillary lymph node
status | | | | |
|
Positive for
metastasis | 16 (40%) | 2 (5%) | 18 (45%) | 0.001b |
|
Negative for
metastasis | 8 (20%) | 14 (35%) | 22 (55%) | (Chi-squared
test) |
|
Total | 24 (60%) | 16 (40%) | 40 (100%) | |
Discussion
Although PD-L1 expression is considered a biomarker
of response to anti-PD-L1 immunotherapy, the prognostic value of
PD-L1 expression in invasive mammary carcinoma remains an issue of
debate in clinical oncology, given the presence of a number of
different commercially available immunohistochemical clones,
variable cut-off points and scoring systems that have been used
among all the published data concerning PD-L1 expression in breast
cancer (27).
The present study demonstrated that PDL1 expression
was present in 60% (24 out of 40) of TNBC samples. There is
conflicting recorded data in the literature in this regard; thus,
it is irrational to compare the results across studies with a
similar aim (22,24,28-31).
Different results among these publications are due to variations in
methods of detection of PD-L1 expression, the use of microarray
techniques, and differences in the applied immunohistochemical
clones and scoring systems. In the literature, the recorded cut-off
was from 1 to 50%; 1% cut-off scores and (0-3 score) graded scoring
systems were frequently applied to test PD-L1 expression in human
cancers in the majority of published studies. This variation could
affect the prevalence of PD-L1 positivity among these studies
(32). Gonzalez-Ericsson et
al (33) and Vlajnic et
al (8) investigated the
differences in the results of PD-L1 expression among three
different PD-L1 immunohistochemical clones in TNBC, and confirmed
that the use of different PD-L1 immunohistochemical clones was
responsible for considerable discrepancies in the results of PD-L1
expression. The present study used a relatively low-cost,
frequently used commercially available clone in Iraq.
There is ample number of studies in the literature
focusing on the clinical characteristics of PD-L1 expression in
TNBC. The present study aimed to investigate the pathological
characterization of PD-L1 expression in this aggressive category of
breast cancer, which thus clarifies the novelty of the present
study. The present study demonstrated that there was a
non-statistically significant association between PD-L1 expression,
histological grade and the presence of tissue necrosis. However, a
statistically significant association was found between PD-L1
expression, tissue calcification and positive cytology for axillary
lymph node status at presentation. A similar Iraqi study performed
by Keorges (26), which used an
approximately similar sample size (n=44) and a similar score (CPS),
but with a different PD-L1 clone (Dako kits, PD-L1, clone 22C3),
demonstrated that there was a non-statistically significant
association between PD-L1 expression, histological grade and
positive axillary lymph node status. However, the criteria for the
inclusion of samples with associated positive axillary lymph node
status were not specified by the author in that study, whether by
radiology, fine needle aspiration cytology, or excisional biopsy.
This may contribute to the disagreement that was found with the
results of the present study. Furthermore, unlike the present
study, the study by Keorges (26)
found a low prevalence of PD-L1 positivity in TNBC at 25%, despite
using the same CPS cut-off value. There are two PD-L1 clones
commercially available and these are more frequently used in
routine testing (Dako, 22C3, and PathnSitu Biotechnologies, B7H1P).
Thus, based on the results, considerable disagreement was present
in the results of PD-L1 expression between these popular
clones.
The statistically significant association observed
in the present study between PD-L1 expression, the presence of
tissue calcification and positive axillary lymph node cytology
raises a concern about using pathological characteristics either to
select or to exclude patients from PD-L1 testing. However, to the
best of our knowledge, there are no published studies available to
date investigating the association of PD-L1 expression with tissue
necrosis and calcification for comparison and discussion.
Further and more extensive investigations are
warranted in order to determine the optimal cut-off value and
select the optimal clone to apply as a gold standard for PD-L1
testing in TNBC, following confirmation by future life expectancy
analyses.
The present study had certain limitations which
should be mentioned. The present study was a single-center study
with a small number of cases, and examined a cancer subtype with a
relatively low prevalence rate. In addition, an immunohistochemical
test was used that has a decreasing validity in a very old
specimen; very old blocks that were stored for a number of years
were avoided in such a retrospective analysis, which may have led
to a considerable effect on the sample size. Another limitation is
related to a lack of clinical information as with any retrospective
analysis.
In conclusion, the present study demonstrates that
PD-L1 expression is present at a relatively high prevalence rate in
TNBC; thus, it is rational to examine PDL1 expression in TNBC.
Pathological characteristics can be used for selecting and
excluding patients from PD-L1 testing.
Acknowledgements
The authors would like to thank the Pathology
Department of Al-Massa Private Center in Baghdad, Iraq for their
great support in data collection.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FFH, AFH and AQY were involved in the conception and
design of the study, and in the analysis and interpretation of the
results. MHF and FFH were involved in data collection. AQY, FFH and
AFH were involved in the preparation of the draft of the
manuscript. MHF and AFH confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
On December 19, 2023, the Al-Massa Center's Ethical
Committee approved the study (reference no. 141412-23), and it
followed its institutional policy. Consent to participate was
deemed not applicable as this was a retrospective study using data
with no violation of patient privacy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dawson AE and Mulford DK: Benign versus
malignant papillary neoplasms of the breast. Diagnostic clues in
fine-needle aspiration cytology. Acta Cytol. 80:23–28.
1994.PubMed/NCBI
|
|
2
|
Lafta RK: Health System in Iraq Post-2003
War. Al-Kindy Col Med J. 19:5–11. 2023.
|
|
3
|
Sahu N, Agrahari AK, Parida B and Das S:
Radiological Significance of Shear-Wave Elastography Technique for
Evaluation of Solid Breast Masses with Histopathological
Correlation. Al-Kindy Col Med J. 19:26–30. 2023.
|
|
4
|
Aswad N and Abedtwfeq RH:
Ultrasound-guided Core Needle Biopsy in the Diagnosis of Suspicious
Breast Lesions: Radiologist's Perspectives. Al-Kindy Col Med J.
19:22–29. 2023.
|
|
5
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borri F and Granaglia A: Pathology of
triple-negative breast cancer. Semin Cancer Biol. 72:136–145.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vlajnic T, Baur F, Soysal SD, Weber WP,
Piscuoglio S and Muenst S: PD-L1 expression in triple-negative
breast cancer: A comparative study of 3 different antibodies. Appl
Immunohistochem Mol Morphol. 30:726–730. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li CH, Karantza V, Aktan G and Lala M:
Current treatment landscape for patients with locally recurrent
inoperable or metastatic triple-negative breast cancer: A
systematic literature review. Breast Cancer Res.
21(143)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Galluzzi L, Humeau J, Buque A, Zitvogel L
and Kroemer G: Immunostimulation with chemotherapy in the era of
immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang T, Li W, Huang T and Zhou J:
Immunotherapy Targeting PD-1/PD-L1 in Early-stage triple-negative
breast cancer. J Pers Med. 13(526)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumor-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current research in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
14
|
Yarchoan M, Johnson BR III, Lutz ER,
Laheru DA and Jaffee EM: Targeting neoantigens to augment antitumor
immunity. Nat Rev Cancer. 17:209–222. 2017.
|
|
15
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced, or metastatic triple-negative
breast cancer (IMpassion130): Updated efficacy results from a
randomized, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 21:44–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Loibl S, Untch M, Burchardi N, Huober J,
Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C,
et al: A randomised phase II study investigating durvalumab in
addition to an anthracycline taxane-based neoadjuvant therapy in
early triple-negative breast cancer: Clinical results and biomarker
analysis of GeparNuevo study. Ann Oncol. 30:1279–1288.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Loibl S, Schneeweiss A, Huober J, Braun M,
Rey J, Blohmer JU, Furlanetto J, Zahm DM, Hanusch C, Thomalla J, et
al: Neoadjuvant durvalumab improves survival in early
triple-negative breast cancer independent of pathological complete
response. Ann. Oncol. 33:1149–1158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rizzo A, Cusmai A, Acquafredda S,
Giovannelli F, Rinaldi L, Misino A and Palmiotti G: KEYNOTE-522,
IMpassion031 and GeparNUEVO: Changing the paradigm of neoadjuvant
immune checkpoint inhibitors in early triple-negative breast
cancer. Future Oncol. 18:2301–2309. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ai L, Xu A and Xu J: Roles of the
PD-1/PD-L1 Pathway: Signaling, cancer, and beyond. Adv Exp Med
Biol. 1248:33–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Badve SS, Penault-Llorca F, Reis-Filho JS,
Deurloo R, Siziopikou KP, D'Arrigo C and Viale G: Determining PD-L1
Status in Patients With Triple-Negative Breast Cancer: Lessons
Learned From IMpassion 130. J Natl Cancer Inst. 114:664–675.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-Paclitaxel in Advanced Triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Keorges GJ: PD-L1 expression in Triple
Negative Breast Cancer: A study of an Iraqi population. J Med Sci.
92(e806)2023.
|
|
27
|
Erber R and Hartmann A: Understanding
PD-L1 testing in breast cancer: A practical approach. Breast Care
(Basel). 15:481–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marletta S, Fusco N, Munari E, Luchini C,
Cimadamore A, Brunelli M, Querzoli G, Martini M, Vigliar E,
Colombari R, et al: Atlas of PD-L1 for Pathologists: Indications,
scores, diagnostic platforms, and reporting systems. J Pers Med.
12(1073)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Beckers RK, Selinger CI, Vilain R, Madore
J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett
D, et al: Programmed death ligand 1 expression in triple-negative
breast cancer is associated with tumor-infiltrating lymphocytes and
improved outcome. Histopathology. 69:25–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oner G, Önder S, Karatay H, Ak N, Tükenmez
M, Müslümanoğlu M, İğci A, Dincçağ A, Özmen V, Aydiner A, et al:
Clinical impact of PD-L1 expression in triple-negative breast
cancer patients with residual tumor burden after neoadjuvant
chemotherapy. World J Surg Oncol. 19(264)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Doğukan R, Uçak R, Doğukan FM, Tanık C,
Çitgez B and Kabukcuoğlu F: Correlation between the expression of
PD-L1 and clinicopathological parameters in triple negative breast
cancer patients. Eur J Breast Health. 15:235–241. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gonzalez-Ericsson PI, Stovgaard ES, Sua
LF, Reisenbichler E, Kos Z, Carter JM, Michiels S, Le Quesne J,
Nielsen TO, Laenkholm AV, et al: The path to a better biomarker:
Application of a risk management framework for the implementation
of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer
clinical trials and daily practice. J Pathol. 250:667–684.
2020.PubMed/NCBI View Article : Google Scholar
|