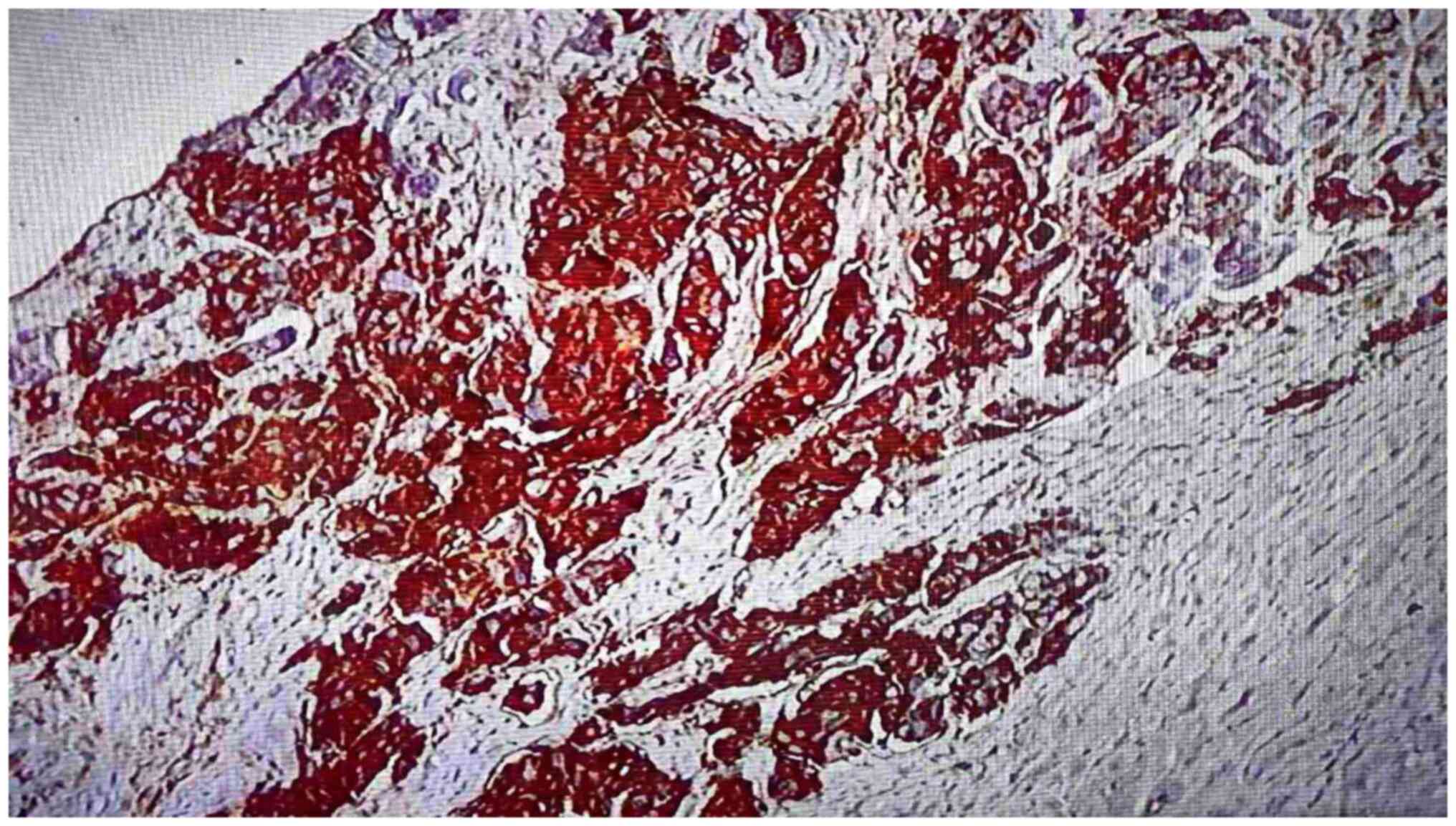
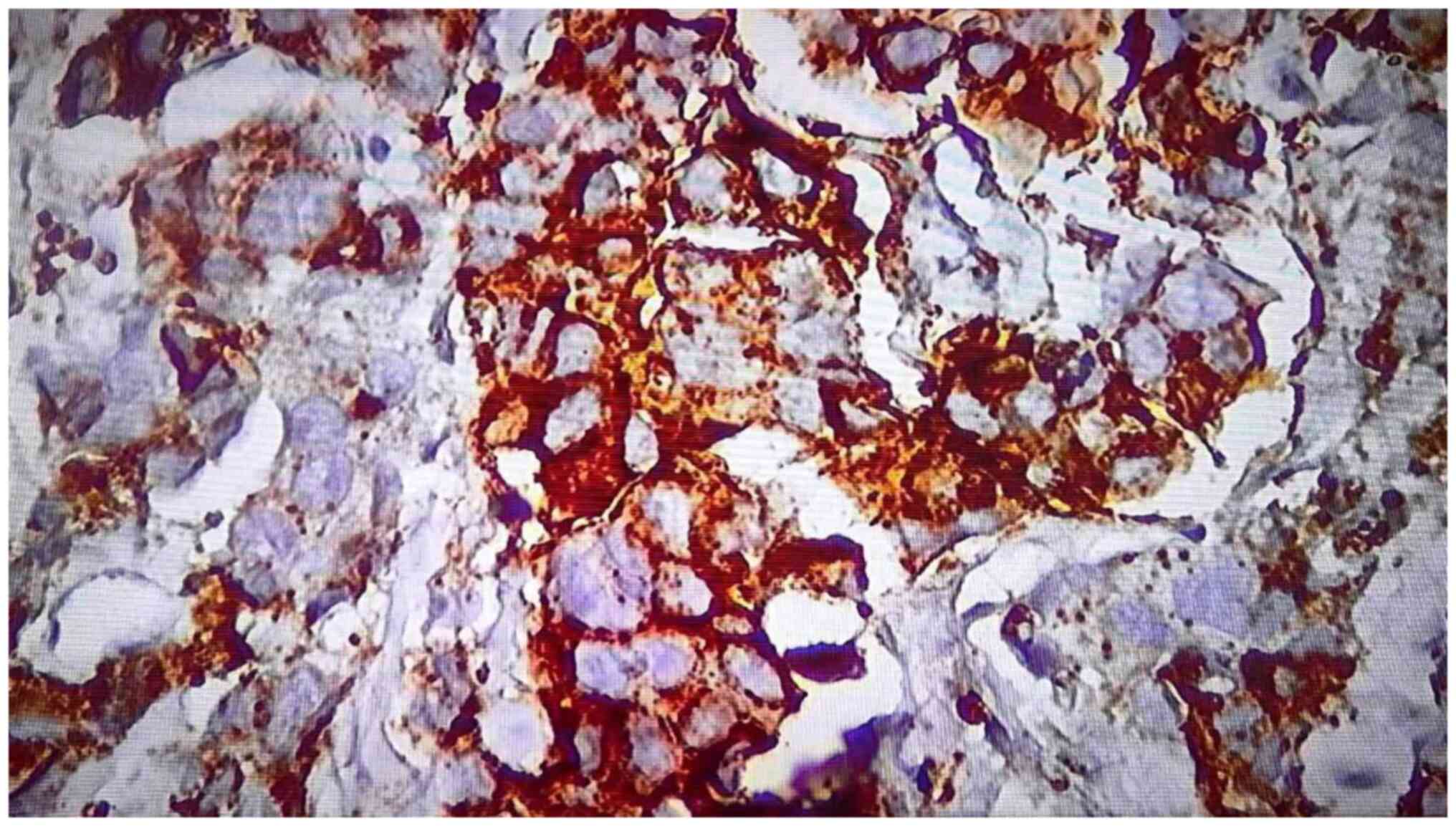
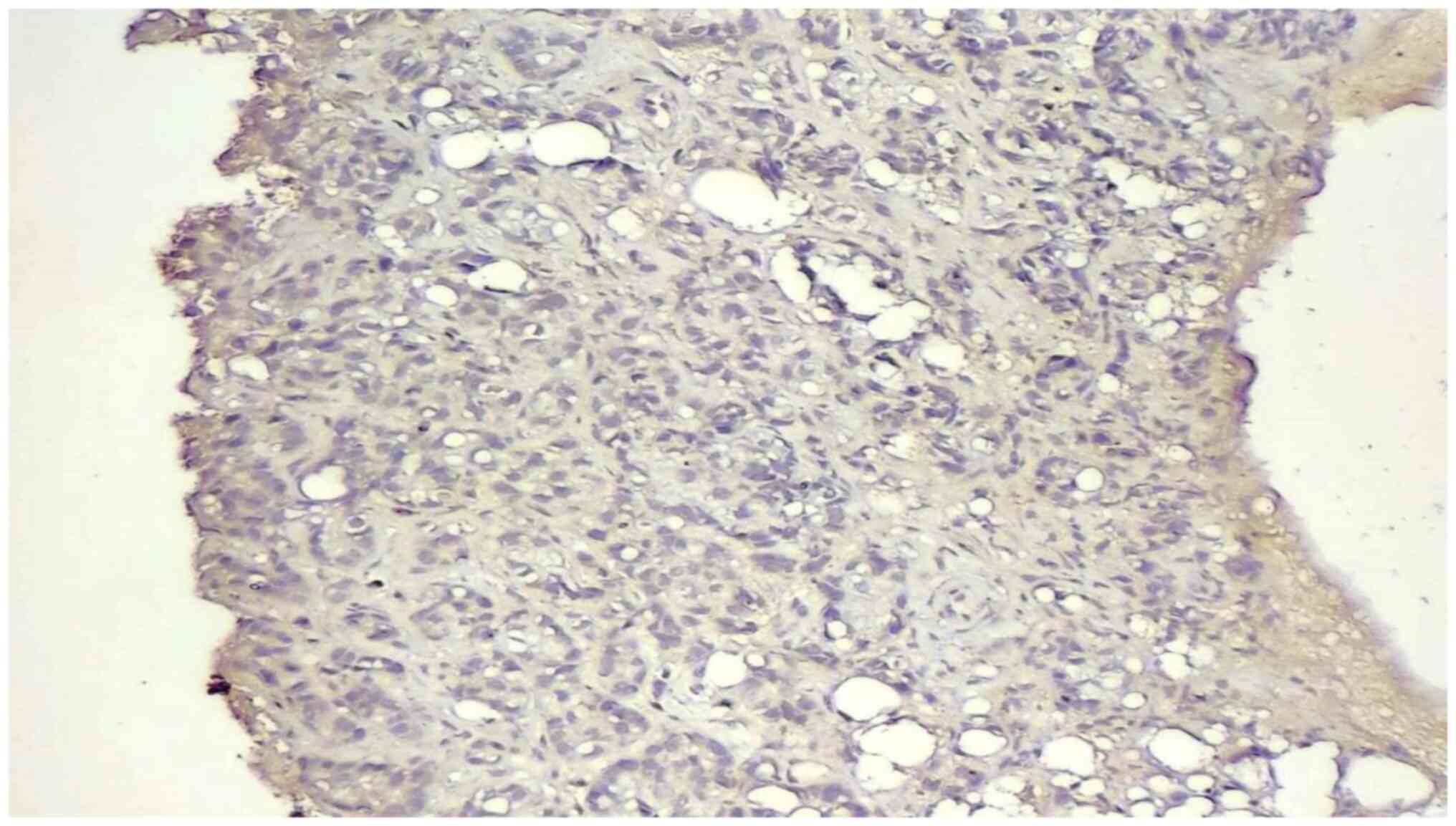
|
1
|
Dawson AE and Mulford DK: Benign versus
malignant papillary neoplasms of the breast. Diagnostic clues in
fine-needle aspiration cytology. Acta Cytol. 80:23–28.
1994.PubMed/NCBI
|
|
2
|
Lafta RK: Health System in Iraq Post-2003
War. Al-Kindy Col Med J. 19:5–11. 2023.
|
|
3
|
Sahu N, Agrahari AK, Parida B and Das S:
Radiological Significance of Shear-Wave Elastography Technique for
Evaluation of Solid Breast Masses with Histopathological
Correlation. Al-Kindy Col Med J. 19:26–30. 2023.
|
|
4
|
Aswad N and Abedtwfeq RH:
Ultrasound-guided Core Needle Biopsy in the Diagnosis of Suspicious
Breast Lesions: Radiologist's Perspectives. Al-Kindy Col Med J.
19:22–29. 2023.
|
|
5
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borri F and Granaglia A: Pathology of
triple-negative breast cancer. Semin Cancer Biol. 72:136–145.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vlajnic T, Baur F, Soysal SD, Weber WP,
Piscuoglio S and Muenst S: PD-L1 expression in triple-negative
breast cancer: A comparative study of 3 different antibodies. Appl
Immunohistochem Mol Morphol. 30:726–730. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li CH, Karantza V, Aktan G and Lala M:
Current treatment landscape for patients with locally recurrent
inoperable or metastatic triple-negative breast cancer: A
systematic literature review. Breast Cancer Res.
21(143)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Galluzzi L, Humeau J, Buque A, Zitvogel L
and Kroemer G: Immunostimulation with chemotherapy in the era of
immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang T, Li W, Huang T and Zhou J:
Immunotherapy Targeting PD-1/PD-L1 in Early-stage triple-negative
breast cancer. J Pers Med. 13(526)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumor-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current research in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
14
|
Yarchoan M, Johnson BR III, Lutz ER,
Laheru DA and Jaffee EM: Targeting neoantigens to augment antitumor
immunity. Nat Rev Cancer. 17:209–222. 2017.
|
|
15
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced, or metastatic triple-negative
breast cancer (IMpassion130): Updated efficacy results from a
randomized, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 21:44–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Loibl S, Untch M, Burchardi N, Huober J,
Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C,
et al: A randomised phase II study investigating durvalumab in
addition to an anthracycline taxane-based neoadjuvant therapy in
early triple-negative breast cancer: Clinical results and biomarker
analysis of GeparNuevo study. Ann Oncol. 30:1279–1288.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Loibl S, Schneeweiss A, Huober J, Braun M,
Rey J, Blohmer JU, Furlanetto J, Zahm DM, Hanusch C, Thomalla J, et
al: Neoadjuvant durvalumab improves survival in early
triple-negative breast cancer independent of pathological complete
response. Ann. Oncol. 33:1149–1158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rizzo A, Cusmai A, Acquafredda S,
Giovannelli F, Rinaldi L, Misino A and Palmiotti G: KEYNOTE-522,
IMpassion031 and GeparNUEVO: Changing the paradigm of neoadjuvant
immune checkpoint inhibitors in early triple-negative breast
cancer. Future Oncol. 18:2301–2309. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ai L, Xu A and Xu J: Roles of the
PD-1/PD-L1 Pathway: Signaling, cancer, and beyond. Adv Exp Med
Biol. 1248:33–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Badve SS, Penault-Llorca F, Reis-Filho JS,
Deurloo R, Siziopikou KP, D'Arrigo C and Viale G: Determining PD-L1
Status in Patients With Triple-Negative Breast Cancer: Lessons
Learned From IMpassion 130. J Natl Cancer Inst. 114:664–675.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-Paclitaxel in Advanced Triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Keorges GJ: PD-L1 expression in Triple
Negative Breast Cancer: A study of an Iraqi population. J Med Sci.
92(e806)2023.
|
|
27
|
Erber R and Hartmann A: Understanding
PD-L1 testing in breast cancer: A practical approach. Breast Care
(Basel). 15:481–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marletta S, Fusco N, Munari E, Luchini C,
Cimadamore A, Brunelli M, Querzoli G, Martini M, Vigliar E,
Colombari R, et al: Atlas of PD-L1 for Pathologists: Indications,
scores, diagnostic platforms, and reporting systems. J Pers Med.
12(1073)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Beckers RK, Selinger CI, Vilain R, Madore
J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett
D, et al: Programmed death ligand 1 expression in triple-negative
breast cancer is associated with tumor-infiltrating lymphocytes and
improved outcome. Histopathology. 69:25–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oner G, Önder S, Karatay H, Ak N, Tükenmez
M, Müslümanoğlu M, İğci A, Dincçağ A, Özmen V, Aydiner A, et al:
Clinical impact of PD-L1 expression in triple-negative breast
cancer patients with residual tumor burden after neoadjuvant
chemotherapy. World J Surg Oncol. 19(264)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Doğukan R, Uçak R, Doğukan FM, Tanık C,
Çitgez B and Kabukcuoğlu F: Correlation between the expression of
PD-L1 and clinicopathological parameters in triple negative breast
cancer patients. Eur J Breast Health. 15:235–241. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gonzalez-Ericsson PI, Stovgaard ES, Sua
LF, Reisenbichler E, Kos Z, Carter JM, Michiels S, Le Quesne J,
Nielsen TO, Laenkholm AV, et al: The path to a better biomarker:
Application of a risk management framework for the implementation
of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer
clinical trials and daily practice. J Pathol. 250:667–684.
2020.PubMed/NCBI View Article : Google Scholar
|