1. Introduction
Gynecological cancers represent a significant health
burden for females worldwide. The three most common types of
gynecological cancer based on incidence include cervical,
endometrial and ovarian cancer. Data from the Global Cancer
Observatory indicate that cervical, endometrial and ovarian cancers
rank as the 2nd, 6th, and 7th most common types of gynecological
cancer among women, respectively (1); however, the prevalence varies
significantly by geographical region and they may be caused by
several factors, including obesity, availability of vaccines,
number of sexual partners, and hormonal factors, such as age of
menarche and oral contraceptive use (2-4).
The renin-angiotensin-aldosterone (RAA) system
regulates blood pressure and is involved in several diseases, such
as hypertension and heart failure. Studies have shown that this
system contributes to other functions besides perfusion and blood
pressure control (5). For example,
the main deleterious effects of the RAA system are mediated by the
action of angiotensin-2 on angiotensin-1 receptor (AT1R). The
activation of the angiotensin-2/AT1R arm, in turn, activates
pathways associated with cancer, such as the phosphoinositide
3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling
pathway, which results in cell proliferation and cancer cell
progression (5-7).
Several studies have found that the AT1R receptor pathway is
activated in several types of cancer, such as breast, pancreatic
and colorectal cancer (8-12).
However, these findings may not be surprising considering that the
AT1R receptor is involved in angiogenesis and fibrosis (13,14),
which are essential cancer processes (15). Epidemiological studies have
identified associations between angiotensin-converting enzyme (ACE)
inhibitors and angiotensin receptor blockers (ARBs) with a reduced
cancer incidence (16-20).
Other studies have found unclear or even inverse associations
between ARB use and cancer (21-25).
These findings may reflect the fact that hypertension usually
occurs concurrently with diabetes and obesity, with each disease
being a known risk factor for cancer development. Nevertheless,
early clinical studies evaluating ARBs as an adjunct treatment for
prostate cancer found beneficial effects on prostate and pancreatic
cancer (26,27). Notably, the activation of other
pathway arms through angiotensin derivatives, such as
angiotensin-(1-7) (28) and
angiotensin-(1-9)
(29) play a crucial role in
reducing cell proliferation and, possibly, cancer progression.
Therefore, it is important to determine whether this pathway plays
a role in gynecological cancers, particularly as the RAA system is
a shared pathway with several common risk factors for gynecological
cancers.
A common risk factor shared by gynecological cancers
and the RAA system is obesity (30), which is associated with
gynecological cancers due to a combination of hormonal factors,
such as increased estrogen levels, and an altered tumor and immune
microenvironment (30,31). Obesity may also influence cancer
development through the dysregulation of the RAA system (32,33).
The increased numbers of adipocytes in obese individuals cause an
increase in angiotensinogen production (34,35)
an essential pathology for developing obesity-induced hypertension
(36,37). This dysregulation of the RAA system
may lead to the development of cancer. Previous studies have found
that hypertension is associated with the increased development of
specific types of cancers, such as breast and endometrial cancer
(38-40).
These shared risk factors with gynecological cancers present an
interesting pathophysiological mechanism and potential treatment
strategy. A large epidemiological study of gynecological cancers
revealed a protective effect of ACE inhibitors and ARBs in ovarian
and cervical cancer (16);
however, the same study found that the protective effects of ACE-I
and ARB were associated with an increased risk of endometrial
cancer (16). These conflicting
findings necessitate the further exploration of the role of the RAA
system in the development of gynecological cancers, particularly to
determine whether RAA system blockade is effective for cancer
treatment. The present review discusses the connection between the
RAA system and gynecological cancers, specifically endometrial,
ovarian and cervical cancers.
2. Pathogenesis and burden of gynecological
cancers
Gynecological cancers constitute a significant
burden among women, with the incidence of cervical and endometrial
cancer reaching over one million cases worldwide and ranking as the
3rd and 4th most prevalent forms of cancer among women (1). Ovarian cancer, although much rarer,
has a higher risk of mortality, particularly compared with
endometrial cancer. The high prevalence of these gynecological
cancers is associated with several risk factors unique to specific
forms of cancers (41-43).
Despite their heterogeneity, these cancers contribute to
significant morbidity and a decreased quality of life; thus, they
deserve further scrutiny with respect to their molecular
characteristics (44).
Cervical cancers are unique among the gynecological
cancers due to their association with human papillomavirus (HPV)
infection (41). Perhaps the
strongest evidence of their association is the geographical
variations in cervical cancers that reflect a varying HPV
prevalence, and reduced access to vaccines, screening and
treatment. Of note, ~80% of all cervical cancers are squamous cell
carcinomas, whereas the remainder are adenocarcinomas (41). Other risk factors for cervical
cancer include smoking, oral contraceptive use, early sexual
activity, the number of sexual partners and other infections that
are transmitted sexually. HPV causes >90% of cervical cancers in
women and is a central mechanism in its pathophysiology (45,46)
HPV infection begins with a micro-abrasion leading to a
micro-wound. The virus then infects host epithelial cells, which
enables the virus to replicate. This establishes a suitable
environment for neoplastic progression (46). At this stage, the immune system may
eradicate the virus through the action of natural killer cells,
which constitute ~85% of cases, or the virus may evade the immune
system, which occurs in 15% of cases (47).
Cancer progression following HPV infection is
primarily caused by the viral oncoproteins. E5, E6 and E7 (45,46).
These proteins are initially responsible for suppressing the innate
immune response, thus enabling the infected cells to evade the
immune system. These proteins downregulate major histocompatibility
complex class 1 and inhibit the function of the Toll-like receptors
that produce interferon, a fundamental cytokine necessary for
effective immunity against viral infections (48,49).
The E6 and E7 oncoproteins further promote progression from
infection to neoplasia in situ. These proteins disrupt cell
cycle control typically regulated by cyclins and cyclin-dependent
kinases by degrading p53 and retinoblastoma protein (pRb) (46,50,51).
The disruption of p53 and pRb, two central tumor suppressor
proteins, promotes cell cycle progression and bypasses the normal
DNA damage checkpoints. This results in cell proliferation and the
accumulation of genomic alterations that promote cancer development
(46). To survive and invade
distant tissues, HPV-generated tumors must acquire nutrients
through the vascular network (52)
Gius et al (53) reported
that during the transition to a higher stage of cancer, cellular
stress resulting from cell overcrowding may trigger angiogenesis
and invasion through gene activation (53). Increased angiogenesis allows more
nutrients to be acquired by the tumor and is beneficial for further
invasion into tissues, as well as metastasis. Several genes play
crucial roles in this process, including
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (PIK3CA) (46,54). HPV infection alone cannot promote
cancer development. Some of these cases are HPV-negative, although
HPV-positive cases are more abundant (55). Nicolás et al (56) demonstrated that HPV-negative
cervical cancers have an aberrant p53 immunostaining pattern with
p16 overexpression. Previous research has revealed a strong link
between p53 mutation and a poor prognosis (57). Additional genetic mutations have
also been identified in HPV-negative cervical cancers, such as
those in the Kirsten rat sarcoma viral oncogene (KRAS), AT-rich
interaction domain-containing protein 1A (ARID1A) and phosphatase
and tensin homolog (PTEN) (55).
Similar to cervical cancer, endometrial cancer also
has a varying incidence based on geographical distribution;
however, it is not associated with a specific infection. Instead,
endometrial cancers are the most common gynecological cancers in
high-income countries, which have an increased prevalence of
obesity and metabolic syndromes (4,58,59).
Of the common types of cancer, no other type of cancer has the
highest association with obesity than endometrial cancer. In fact,
the association of obesity with endometrial cancer is equal to that
of smoking with lung cancer, indicating that it is the most
critical risk factor for endometrial cancer (60-62).
Endometrial cancers, such as the majority of cancers, have no clear
pathophysiology and are caused by multiple factors. Apart from
obesity, several risk factors associated with endometrial cancer
include an increased age, estrogen exposure, tamoxifen use, early
menarche, late menopause, low parity and genetic predisposition
(59). This type of cancer is
usually hormone-sensitive, reflecting the effects of excessive
estrogenic stimulation caused by either obesity, exogenous hormone
therapy, or prolonged estrogen stimulation caused by early
menarche, late menopause and low parity. Estrogen is highly
mitogenic and is involved in the physiological proliferation of the
endometrium (63). However,
unopposed estrogen therapy predisposes the endometrial tissue to
malignant transformation, which is known as atypical endometrial
hyperplasia (AEH). AEH is notorious for mutations in the PTEN gene,
a known tumor suppressor gene (59). Nonetheless, the loss of tumor
suppression alone is insufficient for the development of invasive
carcinoma. Another common mutation involved in endometrial cancer
is dysregulation of the PI3K/AKT pathway, which promotes cell
proliferation and possibly cancer growth (64).
Endometrial cancer pathogenesis may be divided into
two types. Clinicopathologically, type 1 is known as endometrioid
endometrial carcinoma, and type 2 is non-endometrial carcinoma.
Currently, >80% of the cases are diagnosed as type 1 endometrial
cancer, which develops from endometrial tissue secondary to
prolonged estrogen exposure. Of note, ~20% of cases are type 2
endometrial cancer developed from atrophic endometrial tissue that
does not involve estrogen exposure as the main risk factor
(65). Both types have different
prognoses, with type 1 carcinoma clinically proven to have a better
prognosis (66). Type 1
endometrial cancer accounts for 70-80% of cases with indolent
clinical behavior (67). Following
prolonged estrogen exposure, the endometrium may undergo
hyperplasia without atypia, leading to a more advanced stage with
cellular atypia, resulting in the development of endometrial
carcinoma (68). Genetically, type
1 endometrial carcinoma has several gene mutations, such as the
frequently encountered PTEN and KRAS mutations, as well as
microsatellite instability, to the less frequently encountered p16,
human epidermal growth factor receptor 2 (HER2) and E-cadherin
mutations (67). Type 2
endometrial cancer has a more aggressive clinical behavior
(69). It is not associated with
estrogen exposure (70). Frequent
genetic alterations in type 2 endometrial cancer include p53, HER2,
E-cadherin, p16, KRAS, β-catenin and PTEN, with 90% of cases
associated with p53 mutation (67).
Endometrial cancers may be further classified
according to their molecular profiles. Based on The Cancer Genome
Atlas (TCGA) data, there are four molecular subtypes of endometrial
cancers (71). The high copy
number group constitutes the most high-grade and aggressive type of
endometrial cancer. The previously mentioned PI3K/AKT mutations are
often mutated in this subgroup (59). The second tumor type is associated
with microsatellite instability caused by defects in the DNA
mismatch repair system (MMR). This defect results in a 10-fold
greater mutational burden compared with that of the general
mutational background. In addition, MMR defects are associated with
Lynch syndrome, and the National Institute for Health and Care
Excellence has recommended testing all endometrial cancers for this
syndrome (72,73). Lynch syndrome is found in up to 3%
of patients with endometrial cancers and is associated with an
increased risk of future cancers (58). The third subtype contains recurrent
mutations in the exonuclease domain of the polymerase-ε gene. This
mutation causes up to a 100-fold greater mutational burden, but is
associated with an improved prognosis. The fourth subtype is a
low-copy number alteration tumor, which consists of low-grade
endometrioid tumors. Although usually associated with an improved
prognosis, some tumors are caused by the activation of the
wingless-type MMTV integration site family (WNT)-β-catenin pathway
through the mutation of catenin beta 1 (CTNNB1) and have a worse
outcome (74,75). TCGA data reveal the highly
heterogeneous mutation pattern associated with endometrial cancers.
Further analyses of these mutations and potential treatments based
on these profiles are warranted.
Ovarian cancer is a heterogeneous group of cancers
with at least five known subtypes (76). This type of cancer, although rarer
compared with cervical and endometrial cancers, is the most common
cause of mortality from gynecological cancers (77). The primary subtype is high-grade
serous ovarian carcinoma (HGSOC), which represents 70% of the cases
(77). HGSOC usually presents at a
late stage, with 70% of cases diagnosed at stage III (43). The recurrence of this tumor
following surgery and chemotherapy is common, with a 5-year
survival of ~40%. These tumors present with a complex molecular
heterogeneity, copy number variations and multiple genomic variants
compared with the more stable mutation patterns observed in other
types of cancer (76,78). This heterogeneity is perhaps
reflected in the poor improvement in the survival rates of patients
with HGSOC over the past few decades (79). The whole genome sequencing of HGSOC
has revealed that the majority of mutations occur in tumor
suppressor genes, such as TP53, PTEN, retinoblastoma 1,
neurofibromin 1 and RAD51 paralog B (80). In addition, germline mutations
involving BRCA1 and BRCA2 and other homologous recombinant repair
(HRR) genes occur in approximately 50% of HGSOC cases (78). This heterogeneity makes therapy
targeting specific pathways challenging, which is why
platinum-based therapy continues to be the standard treatment
regimen (81). However, a defect
associated with the HRR genes has led to treatment with poly
ADP-ribose polymerase inhibitors with notable activity as a single
agent (43,77,81,82).
In the future, novel methods, such as machine learning may enable
the further characterization of these types of cancer (83,84).
Other ovarian cancer subtypes include endometrioid
ovarian cancer (EOC), clear-cell ovarian cancer (CCOC), low-grade
serous ovarian cancer (LGSOC), mucinous ovarian cancer (MOC) and
ovarian carcinosarcoma (OCS) (76,77).
Extensive literature covering their molecular heterogeneity can be
found in the review by Hollis (78); however, several notable
characteristics are discussed herein. The most common mutations for
the other ovarian cancer subtypes, apart from OCS, include a
typical mutation pattern also found in endometrial carcinoma.
Mutations in the WNT-β catenin pathway (CTNNB1), PI3K/AKT pathway
(PIK3CA), ARID1A and PTEN are often found in EOC and CCOC (78). Both subtypes are also associated
with MMR gene defects (Lynch syndrome). The most common mutations
involve genes involved in LGSOC and MOC, such as KRAS, BRAF and
NRAS, which are in the mitogen-activated protein kinase (MAPK)
pathway (78,85). Other genes also play a role in MOC,
such as ARID1A and PIK3CA. OCS is the least common, but the most
aggressive subtype of ovarian cancer. There has been minimal
molecular characterization, but TP53 mutations are present in ~90%
of the cases. Although there is significant heterogeneity of the
mutation patterns in ovarian cancers, two major pathways that may
interact with other pathways, such as the RAA system, include the
MAPK and the PI3K/AKT pathways.
3. The renin angiotensin aldosterone system
pathway
The RAA system affects multiple organs and systems
and is one of the most vital systems in the regulation of body
homeostasis (86). Its primary
function is to regulate blood pressure and consists of three main
components: Renin, angiotensin-2 and aldosterone, which are
interrelated (86,87).
The RAA system functions through a feedback
mechanism with several stimuli affecting its activity (88). First, a decrease in renal perfusion
can affect the juxtaglomerular apparatus, which secretes renin,
thus activating the RAA system. Another trigger of the RAA system
activity is mineral balance, such as sodium. Hyponatremia in the
blood is sensed by the macula densa followed by renin secretion.
The RAA system is activated in this scenario to retain sodium
through an increase in sodium absorption via the renal distal
convoluted tubule. The nervous system also plays a crucial role in
activating the RAA system through beta 1-adrenergic receptors,
which increase activity and blood pressure. Other substances also
contribute through feedback mechanisms, such as potassium levels,
the natriuretic peptide and angiotensinogen I, which decrease RAA
system activity (89). The main
RAA system pathway begins with renin. The juxtaglomerular apparatus
produces renin as its precursor, prorenin (90) which is then transformed into renin
by cathepsin-B and proconvertase-1. Renin is released into the
bloodstream and converts angiotensinogen, which is synthesized by
the liver, to angiotensin-1 (86,87,91).
However, circulating angiotensinogen-1 does not appear to have any
physiological activity. Pulmonary circulation in the lungs involves
an endothelial membrane containing ACE. This enzyme is primarily
located in the pulmonary circulation. ACE modifies the circulating
angiotensin-1, specifically at its C-terminus, into angiotensin-2,
the primary effector of the RAA system. Angiotensin-2 then
circulates and arrives at multiple sites throughout the body to
attenuate various physiological functions (87,89).
Angiotensin-2 exerts its effects by interacting with
its receptors, AT1R and angiotensin-2 type 2 receptor (AT2R). Each
receptor exerts a different range of effects. AT1R is a G-protein
coupled receptor distributed all over the body, but concentrated in
the heart, blood vessels, kidneys, adrenal glands and the central
nervous system (92). The main
functions of AT1R are inducing vasoconstriction resulting from
smooth muscle contraction, the release of vasopressin by the
hypothalamus, and increasing sodium and water reabsorption through
an increase of sodium absorption via the kidney proximal convoluted
tubule. The dysregulation of this receptor is associated with
several conditions, such as increased inflammation, fibrosis,
oxidative stress, tissue remodeling and chronic high blood pressure
that may eventually evolve into heart failure and chronic kidney
disease (93,94). Another angiotensin receptor, AT2R,
is also found throughout the human body. AT2R is also a G-protein
coupled receptor and is abundantly expressed in the uterus, fetal
tissue, heart, kidney, adrenal glands and brain. However, when
coupled with angiotensin-2, AT2R produces a different effect
compared with that of the AT1R receptor. AT2R induces vasodilation
and natriuresis, as well as the inhibition of inflammation,
fibrosis and sympathetic nerve activity (95,96).
A previous study on the RAA system revealed another
active receptor known as the MAS receptor (MASr) (97). This receptor is present in several
human organs, such as the brain, kidneys, adrenal glands, heart,
reproductive organs and intestines. However, instead of interacting
with angiotensin-2, MASr must be activated by its specific ligand,
angiotensin-(1-7). Angiotensin-(1-7) is a heptapeptide produced
from hydrolyzed angiotensin-1 and angiotensin-2. The production of
angiotensin-(1-7) from angiotensin-1 requires an intermediate
product, angiotensin-(1-9),
a nonapeptide that is subsequently converted to angiotensin-(1-7)
by ACE2. Angiotensin-2 can be transformed directly into
angiotensin-(1-7), which is a more favorable route. In the study by
Pawlik et al (98), it was
demonstrated that when bound to MASr, angiotensin-(1-7) induces the
production of prostaglandin E2. This generates nitric oxide and
inhibits cell growth which opposes the effect of vasoconstriction
and cell pro-proliferation from AT1R. Although it has its main
receptor, higher levels of angiotensin-(1-7) exhibit affinity for
AT1R and AT2R, whereas other researchers have shown that
angiotensin-(1-7) acts competitively to inhibit AT1R (99). In the study by Xu et al
(99) it was demonstrated that
MASr inhibits cancer cell growth through anti-proliferative
activity, the inhibition of cellular migration, invasion and
epithelial-to-mesenchymal transition, and anti-angiogenic activity.
Luo et al (100)
demonstrated that the decreased MASr activity in breast cancer
promoted cancer development. They concluded that MASr expression
functions as a tumor growth inhibitor. Alamandine, which is another
novel substance in the RAA system pathway, is a peptide formed
through the conversion of angiotensin-A by ACE2. It resembles
angiotensin-(1-7) action, but does not bind to MASr. Instead, it
acts on a different receptor, MAS-related G-protein coupled
receptor D (MrgD). Research on heart disease has indicated that
alamandine decreases blood pressure, cardiac remodeling,
reperfusion injury and ventricular hypertrophy (87). Qaradakhi et al (101) demonstrated that alamandine
exerted a similar effect as angiotensin-(1-7) by inhibiting cell
proliferation, exerting anti-fibrotic effects, and vasodilatation
resulting from the alteration of prostaglandin and endogenous
nitric oxide production. The study by da Silva et al
(102) suggested that alamandine
only affects tumor cells and reduces their mass and growth. Their
experiments indicated that alamandine exerts its effect through
activation of MrgD and MASr, which subsequently attenuates the
PI3K/AKT/mTOR pathway (102).
These novel pathways involving alamandine and
angiotensin-(1-7) represent promising targets to counteract the
effects of AT1R (103). The
angiotensin-2-angiotensin-(1-7)-MASr axis may be a promising
therapeutic target to prevent kidney disease progression because it
has a renoprotective effect on diabetic nephropathy (104). Adding the
angiotensin-A-alamandine-MrgD axis to the recognition of multiple
receptor-targeted therapies may lead to improvements in treatment
(105). Moreover, due to its
novel actions against processes, such as anti-proliferation,
anti-inflammation, and reduced angiogenesis, other prevention and
adjunctive strategies may be devised to overcome neoplastic
disease.
4. The renin angiotensin aldosterone system
pathway and cancers
The RAA system pathway exerts multiple effects
exerted through its receptors, AT1R and AT2R. Docked to its
different receptors, angiotensin-2 has a distinct effect on its
targets. The effects of activated AT1R are more detrimental at a
glance compared with AT2R. It similarly affects cancer-related
processes, such as inflammation, fibrosis, oxidative stress,
pro-proliferation and decreased apoptosis (93). It has been shown that AT1R is more
positively associated with neoplastic disease compared with AT2R,
which is frequently downregulated in specific cancers (12). Philippe (106) demonstrated that activated AT1R in
endothelial cells promotes angiogenesis. Other studies have also
demonstrated that AT1R activation induces the secretion of vascular
endothelial growth factor (VEGF), a potent inducer of endothelial
cell proliferation. Wagner et al (107) used MAP kinase kinase (MKK) and
VEGF inhibitors on angiosarcoma cells, which decreased tumor
activity and viability. Their findings indicate that the effect of
AT1R on angiogenesis is similar to that observed in cancer cells.
The review by Catarata et al (108) concluded that AT1R was
overexpressed in several neoplastic tissues, including endometrial
and breast cancer. Based on these findings, it can be concluded
that the activation of AT1R is associated with cancer, particularly
angiogenesis.
Cancer cells have the ability to metastasize. Tumor
cells can migrate from the primary tumor site through the
bloodstream or lymphatic vessels. The migration of cancer cells
followed by the development of new vascular growth of capillaries,
enables cancer cells to enter the circulation and subsequently
adapt to a new environment (109). Cancer tissue must have an
adequate blood supply and lymphatic tissue to survive and
metastasize. Therefore, molecules such as VEGF are essential
(110). Neoplastic tissue
secretes various VEGF forms, such as VEGF-A, which regulates
angiogenesis, tumor proliferation and metastasis, and VEGF-C and
VEGF-D, which promote lymphangiogenesis, cell migration and
invasion. These processes are activated through PI3K/AKT/mTOR, p38
MAPK and MKK, which are associated with AT1R activation (111). It has been shown that the
overexpression of AT1R in cancer cells may activate the
immunostimulatory pathway by secreting pro-inflammatory cytokines.
Therefore, RAA system activation promotes neoplastic-related
inflammation and the subsequent infiltration of inflammatory cells
into tumor tissue (112).
However, whether immune system activation during this condition is
beneficial remains unclear. Angiotensin-2 interaction with AT1R on
cancer cells produces pro-inflammatory cytokines, such as TGF-β,
interleukin (IL)-1α, IL-1β, IL-6, IL-8, cyclooxygenase (COX)-2 and
monocyte chemoattractant protein (MCP)-1(113). These pro-inflammatory cytokines
promote oxidative stress and impair myeloid and lymphoid immune
cell function. In addition, some of these cytokines are also
associated with increasing tumor aggression, such as high levels of
MCP-1(114). In addition, COX-2
suppresses antitumor immunity and contributes to resistance to
immunotherapy (115). Although at
a first glance, the activation of AT1R appears to convey
deleterious effects, this receptor is critical for immune cell
function, such as the differentiation and maturation of macrophages
(116,117). The overexpression of ACE in mice
has revealed that increasing angiotensin-2 increases the
pro-inflammatory and antitumor activity of macrophages compared
with wild-type mice (117). Taken
together, angiotensin-2 and AT1R interactions in cancer cells have
complex associations, with some studies (108,111-113)
supporting their role in cancer progression; however, they are also
an essential component of the antitumor activity of the immune
system.
The administration of ACE-I may interfere with the
interaction of RAA system with cancer cells (118). Introducing ACE-I to patients with
diabetic kidney disease may ameliorate the progression of
angiogenesis due to a reduction in circulating levels of
angiotensin-2(5). Wang et
al (119) found that the
administration of ACE-I reduced neovascularization in patients with
esophageal carcinoma, as evidenced by reduced CD31-positive vessel
density through immunochemical staining. The review by Bryniarski
et al (120) indicated
that administering ACE-I decreased pro-inflammatory cytokine
levels, improved immune cell function, modulated the immune
response and conferred an overall protective effect against cancer.
Therefore, it was hypothesized that tumor proliferation and
metastasis may be reduced by decreasing the interaction of
angiotensin-2 with AT1R. Therefore, lowering cytokine levels can
increase angiogenesis, survival and migration, functions that are
vital to cancer cells. ACE-I also shifts the conversion of
angiotensin-1 to form angiotensin-(1-7), which binds to MASr
(103). The increased quantity
and activity of angiotensin-(1-7) and MASr has an effect similar to
AT2R activity, such as decreasing angiogenesis, fibrosis,
inflammation and oxidative stress (121). Taken together, the use of ACE-I
may reduce cancer progression and represents a useful adjunct for
cancer treatment.
5. The renin angiotensin aldosterone system
pathway in gynecological cancers
ACE-I is widely used as an antihypertensive and
kidney protective agent that affects the RAA system pathway. The
RAA system pathway maintains homeostasis of the human body and may
specifically affect certain cellular functions, such as
proliferation, migration and angiogenesis (5). Thus, a strong association exists
between the RAA system pathway, ACE-I and malignancy. ACE-I
exhibits antiproliferative, antiangiogenetic, pro-apoptotic, and
anti-inflammatory capabilities associated with tumor cells that
overexpress AT1R (122).
In ovarian cancer, some genes, such as KRAS, PTEN,
PI3K and HER2, are usually mutated and promote cell overgrowth
rather than death (123). A loss
of PTEN activity resulting from mutated genes, and the activation
of KRAS and HER2 leads to the activation of the PI3K/AKT/mTOR and
ERK pathways (124-126).
These pathways increase cell proliferation, survival, protein
synthesis and transcription, and inhibit apoptosis via activated
B-cell lymphoma-extra-large (Bcl-xL)/B-cell lymphoma-2
(Bcl-2)-associated death promoters (125). Notably, angiotensin 2 plays a
crucial role in activating these pathways. Although ACE-I may not
directly inhibit these pathways, it may reduce angiotensin-2 to
limit its effects on cell proliferation. Nuclear factor (NF)-κB
plays a critical role in ovarian cancer metastasis. A previous
study found that the administration of ACE-I and AT1R blockers
inhibited NF-κB (127). NF-κB
activation promotes the epithelial-to-mesenchymal transition, an
essential process for the migration and metastasis of cancer cells.
Other mechanisms include an increase in angiogenesis and the
release of matrix-degrading enzymes that promote
epithelial-mesenchymal transition (128). Ovarian cancer cells express a
high number of angiotensin 1 receptors. Regulska et al
(129) found that at least 70% of
invasive ovarian carcinoma cases had confirmed AT1R expression by
immunohistochemistry. AT1R is beneficial for the growth of tumor
cells. Beyazit et al (130) found that ACE levels were
increased in patients with ovarian cancer. Increased serum levels
of ACE concomitantly increase the levels of angiotensin-2, which
exacerbates tumor growth, and suggests that ACE-I administration
may benefit patients. Harding et al (131) demonstrated that women who use
ACE-I have a lower mortality rate from ovarian cancer (14.8/100
person-years rate) compared with non-antihypertensive users
(17.7/100 person-years rate), with an adjusted hazard ratio of
0.76.
Cervical cancer is associated with infection by the
HPV. Several genes associated with the development of cervical
cancer include KRAS, ARID1A, PTEN, PIK3CA, fibronectin 1 (FN1) and
VEGF-A. The majority of these genes activate the PI3K pathway,
which increases cellular proliferation and growth. Cervical cancer
cells may proliferate abundantly in concomitant with VEGF-A
expression, which accommodates cell nourishment through
angiogenesis (46,54,132). The increased vascularization of
cervical cancer tissue facilitates cancer cell metastasis, further
promoting cancer-related mortality. Another gene usually affected
by cervical cancer is the FN1 gene, which plays a role in the
interaction of cells and the matrix, cellular migration, adhesion,
growth and differentiation. FN1 primarily affects FAK signaling, as
well as Bcl-2/Bax and N-cadherin (133). The focal adhesion kinase (FAK)
signaling pathway is a nonreceptor tyrosine kinase that affects
cellular adhesion, migration, proliferation, survival, and vascular
permeability, which are important for cancer cell growth (134). Bcl-2/Bax and N-cadherin promote
cell growth, metastasis, and anti-apoptotic activity (135). Therefore, inhibiting FN1 and the
FAK signaling pathway may be beneficial to cancer patients. The
mechanism associated with reduced FN1 activity remains unclear;
however, PI3K and integrins may promote FN1 expression (136). Garvin et al (137) demonstrated that the
administration of ACE-I reduces FN1 expression (137). FAK expression may also be
inhibited by ACE-I through ACE2 activity (138,139). A decrease in FAK expression may
be attributed to decreased interactions of FAK and its key
activating component, the integrins, which bind with ACE2(140). Brooks et al (141) found that lisinopril, which
belongs to ACE-I, can increase ACE2 expression in tissues (141). VEGF must also be inhibited to
prevent neoplastic tissue from overgrowth and metastasis. Radin
et al (142) found that
the inhibition of AT1R through ACE-I reduced VEGF expression and
angiogenesis. This suggests that introducing ACE-I in cervical
cancer may serve as an adjuvant for the primary treatment and
benefit of the patients. Nguyen et al (16) reported that RAA system inhibitors,
including ACE-I and ARB, are associated with a lower risk of
developing cervical cancer.
Molecular components play a key role in uterine
cancer in addition to estrogen exposure. Genetically, type 1
endometrial carcinoma is associated with several gene mutations,
from the more frequently encountered PTEN mutations, microsatellite
instability and KRAS to less frequently encountered p16, HER2 and
E-cadherin (67) PTEN acts as a
regulator for cell replication (143). It inhibits cell spreading and
migration, focal adhesion and MAPK activation, leading to tumor
cell overgrowth and angiogenesis. Therefore, the loss of PTEN is
advantageous for cancer cells to grow, spread, metastasize and
escape apoptosis (144). In
tumors, KRAS may function as a molecular recruiter to activate
proteins related to tumor growth and differentiation, and it is
associated with the Raf and PI3K signaling pathways (67,145). Type 2 endometrial cancer has a
more aggressive clinical behavior (69). This type of endometrial cancer is
not associated with prolonged exposure to estrogen (70). Genetic alterations in type 2
endometrial cancer include p53, HER2, E-cadherin, p16, KRAS,
β-catenin and PTEN. Οf note, ~90% of these tumors have p53
mutations (67). HER2 is
associated with the MAPK, PI3K, protein kinase C and signal
transducer and activator of transcription signaling pathways, which
promote cell growth and prevent apoptosis (146,147). E-cadherin is a transmembrane
protein involved in cell adhesion through calcium-dependent
interactions with cadherins. Mutations in E-cadherin result in
decreased cell cohesion and promote cell migration, invasion and
metastasis (148). The activities
of several proteins, such as KRAS, PTEN, HER2 and E-cadherin may be
blocked by ACE-I through inhibition of PI3K-MAPK signaling. Whether
other unknown pathways are not inhibited by ACE-I, the pathways
that are attenuated by ACE-I may be sufficient to prevent the
growth and spread of endometrial cancer. Delforce et al
(149) found that the
dysregulation of the RAA system may promote tumor progression in
endometrial cancer. This suggests that ACE-I may reduce the risk of
developing endometrial cancer. A summary of the interaction between
the RAA system and gynecological cancers that have been discussed
above is presented in Fig. 1.
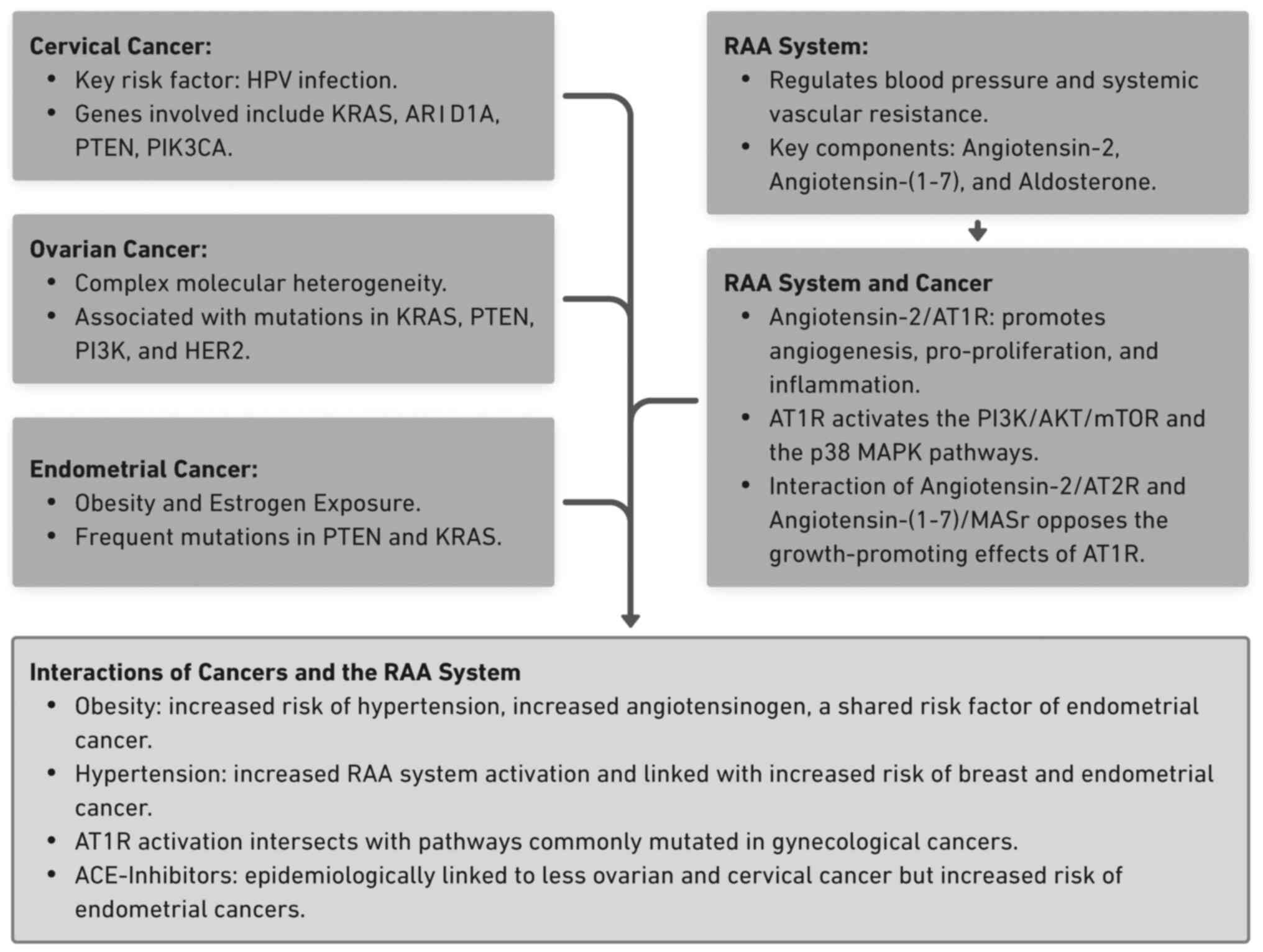 | Figure 1Summary of gynecological and RAA
system interactions. The key points highlighting the interaction
between cervical, ovarian and endometrial cancers and the RAA
system are shown. Several overlapping pathways, such as the
PI3K/AKT/mTOR, and shared risk factors, such as obesity and
hypertension, are described. RAA, renin-angiotensin-aldosterone;
HPV, human papillomavirus; KRAS, Kirsten rat sarcoma viral
oncogene; ARID1A, AT-rich interaction domain-containing protein 1A;
PTEN, phosphatase and tensin homolog; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha; PI3K, phosphoinositide 3-kinase; HER2, human epidermal
growth factor receptor 2; AT1R, angiotensin-1 receptor; mTOR,
mammalian target of rapamycin; MASr, MAS receptor; ACE,
angiotensin-converting enzyme; AT2R, angiotensin-2 receptor. |
6. Conclusion and future perspectives
The present review demonstrated that although
gynecological cancers present with significant molecular
heterogeneity, several genes are present, particularly growth
regulators, such as the PI3K and MAPK pathways. The majority of
these pathways interact with the RAA system through the well-known
angiotensin-2/AT1R pathway, to promote cancer growth. Notably, the
administration of ACE inhibitors may reduce their activation and,
therefore, decrease the effects of the RAA system on cancer, a
finding supported by several epidemiological studies. However, the
evidence remains inconclusive and although a number of in
vitro studies (7,9,10,14,100,102) have indicated that ACE
administration interferes with cancer cell growth through various
pathways, it is unclear which pathway dominates or influences
growth. Furthermore, to date, to the best of our knowledge, no
clinical trials have been performed to determine whether these
effects translate into practice. In addition, in individuals
without overactive RAA systems, such as those without hypertension,
it is unknown whether the concomitant administration of ACE
inhibitors with chemotherapy provides any benefit. Therefore,
further translational studies are required. Moreover, observational
studies examining whether the overactivation of the RAA system
influences cancer incidence are required to determine whether this
pathway significantly contributes to cancer development. Several
limitations are apparent in the present review article. The present
review is a narrative, and although the authors intended to be as
comprehensive as possible, some articles may have been missed. In
addition, the narrative may introduce bias in the interpretation of
the results. Nevertheless, the present review focused on studies
involving the interaction of the RAA system with cancers,
potentially improving the outcomes of cancer patients. Perhaps
future studies involving novel methods, such as machine learning,
will unravel the complex interactions between these molecular
pathways.
Acknowledgements
The authors would like to thank the Maranatha
Christian University for providing the facility and database access
during the preparation of the present review.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RFL and RSS conceived the idea for the present
review article and also wrote the initial draft of the manuscript.
RSS and AS collated the evidence to be included in the present
review, and AS revised and edited the manuscript. All authors
contributed to the final version, and all authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stewart SL, Lakhani N, Brown PM, Larkin
OA, Moore AR and Hayes NS: Gynecologic cancer prevention and
control in the national comprehensive cancer control program:
Progress, current activities, and future directions. J Womens
Health (Larchmt). 22:651–657. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
La Vecchia C: Ovarian cancer: Epidemiology
and risk factors. Eur J Cancer Prev. 26:55–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hassani B, Attar Z and Firouzabadi N: The
renin-angiotensin- aldosterone system (RAAS) signaling pathways and
cancer: Foes versus allies. Cancer Cell Int. 23(254)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
George AJ, Thomas WG and Hannan RD: The
renin-angiotensin system and cancer: Old dog, new tricks. Nat Rev
Cancer. 10:745–759. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao Y, Wang H, Li X, Cao M, Lu H, Meng Q,
Pang H, Li H, Nadolny C, Dong X and Cai L: Ang II-AT1R increases
cell migration through PI3K/AKT and NF-κB pathways in breast
cancer. J Cell Physiol. 229:1855–1862. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rasha F, Ramalingam L, Gollahon L, Rahman
RL, Rahman SM, Menikdiwela K and Moustaid-Moussa N: Mechanisms
linking the renin-angiotensin system, obesity, and breast cancer.
Endocr Relat Cancer. 26:R653–R672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coulson R, Liew SH, Connelly AA, Yee NS,
Deb S, Kumar B, Vargas AC, O'Toole SA, Parslow AC, Poh A, et al:
The angiotensin receptor blocker, Losartan, inhibits mammary tumor
development and progression to invasive carcinoma. Oncotarget.
8:18640–18656. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pei N, Mao Y, Wan P, Chen X, Li A, Chen H,
Li J, Wan R, Zhang Y, Du H, et al: Angiotensin II type 2 receptor
promotes apoptosis and inhibits angiogenesis in bladder cancer. J
Exp Clin Cancer Res. 36(77)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X, Meng Q, Zhao Y, Liu M, Li D, Yang
Y, Sun L, Sui G, Cai L and Dong X: Angiotensin II type 1 receptor
antagonists inhibit cell proliferation and angiogenesis in breast
cancer. Cancer Lett. 328:318–324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Almutlaq M, Alamro AA, Alamri HS, Alghamdi
AA and Barhoumi T: The effect of local renin angiotensin system in
the common types of cancer. Front Endocrinol (Lausanne).
12(736361)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okwan-Duodu D, Landry J, Shen XZ and Diaz
R: Angiotensin-converting enzyme and the tumor microenvironment:
Mechanisms beyond angiogenesis. Am J Physiol Regul Integr Comp
Physiol. 305:R205–R215. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fan F, Tian C, Tao L, Wu H, Liu Z, Shen C,
Jiang G and Lu Y: Candesartan attenuates angiogenesis in
hepatocellular carcinoma via downregulating AT1R/VEGF pathway.
Biomed Pharmacother. 83:704–711. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nguyen NTH, Nguyen PA, Huang CW, Wang CH,
Lin MC, Hsu MH, Bao HB, Chien SC and Yang HC:
Renin-angiotensin-aldosterone system inhibitors and development of
gynecologic cancers: A 23 million individual population-based
study. Int J Mol Sci. 24(3814)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee SH, Park J, Park RW, Shin SJ, Kim J,
Sung JD, Kim DJ and Yang K: Renin-angiotensin-aldosterone system
inhibitors and risk of cancer: A population-based cohort study
using a common data model. Diagnostics. 12(263)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Z, Wei L, Yin C, Li W and Wan B:
Angiotensin receptor blocker associated with a decreased risk of
lung cancer: An updated meta-analysis. J Pers Med.
13(243)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Christian JB, Lapane KL, Hume AL, Eaton CB
and Weinstock MA: VATTC Trial. Association of ACE inhibitors and
angiotensin receptor blockers with keratinocyte cancer prevention
in the randomized VATTC trial. J Natl Cancer Inst. 100:1223–1232.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chiang YY, Chen KB, Tsai TH and Tsai WC:
Lowered cancer risk with ACE inhibitors/ARBs: A population-based
cohort study. J Clin Hypertens (Greenwich). 16:27–33.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yarmolinsky J, Díez-Obrero V, Richardson
TG, Pigeyre M, Sjaarda J, Paré G, Walker VM, Vincent EE, Tan VY,
Obón-Santacana M, et al: Genetically proxied therapeutic inhibition
of antihypertensive drug targets and risk of common cancers: A
mendelian randomization analysis. PLoS Med.
19(e1003897)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Copland E, Canoy D, Nazarzadeh M, Bidel Z,
Ramakrishnan R, Woodward M, Chalmers J, Teo KK, Pepine CJ, Davis
BR, et al: Antihypertensive treatment and risk of cancer: An
individual participant data meta-analysis. Lancet Oncol.
22:558–570. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang S, Xie L, Zhuang J, Qian Y, Zhang G,
Quan X, Li L, Yu H, Zhang W, Zhao W and Qian B: Association between
use of antihypertensive drugs and the risk of cancer: A
population-based cohort study in Shanghai. BMC Cancer.
23(425)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Yi CH and Ya KG: Renin-angiotensin
system inhibitor use and colorectal cancer risk and mortality: A
dose-response meta analysis. J Renin Angiotensin Aldosterone Syst.
21(1470320319895646)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sipahi I: Risk of cancer with
angiotensin-receptor blockers increases with increasing cumulative
exposure: Meta-regression analysis of randomized trials. PLoS One.
17(e0263461)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang
W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, et al:
Total neoadjuvant therapy with FOLFIRINOX in combination with
losartan followed by chemoradiotherapy for locally advanced
pancreatic cancer: A phase 2 clinical trial. JAMA Oncol.
5:1020–1027. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Uemura H, Hasumi H, Kawahara T, Sugiura S,
Miyoshi Y, Nakaigawa N, Teranishi J, Noguchi K, Ishiguro H and
Kubota Y: Pilot study of angiotensin II receptor blocker in
advanced hormone-refractory prostate cancer. Int J Clin Oncol.
10:405–410. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Passos-Silva DG, Verano-Braga T and Santos
RAS: Angiotensin-(1-7): Beyond the cardio-renal actions. Clin Sci
(Lond). 124:443–456. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Flores-Munoz M, Work LM, Douglas K, Denby
L, Dominiczak AF, Graham D and Nicklin SA: Angiotensin-(1-9)
attenuates cardiac fibrosis in the stroke-prone spontaneously
hypertensive rat via the angiotensin type 2 receptor. Hypertension.
59:300–307. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wichmann IA and Cuello MA: Obesity and
gynecological cancers: A toxic relationship. Int J Gynaecol Obstet.
155 (Suppl 1):S123–S134. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Y, Yang J, Hilliard TS, Wang Z,
Johnson J, Wang W, Harper EI, Ott C, O'Brien C, Campbell L, et al:
Host obesity alters the ovarian tumor immune microenvironment and
impacts response to standard of care chemotherapy. J Exp Clin
Cancer Res. 42(165)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cabandugama PK, Gardner MJ and Sowers JR:
The renin angiotensin aldosterone system in obesity and
hypertension: Roles in the cardiorenal metabolic syndrome. Med Clin
North Am. 101:129–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Thethi T, Kamiyama M and Kobori H: The
link between the renin-angiotensin-aldosterone system and renal
injury in obesity and the metabolic syndrome. Curr Hypertens Rep.
14:160–169. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Boustany CM, Bharadwaj K, Daugherty A,
Brown DR, Randall DC and Cassis LA: Activation of the systemic and
adipose renin-angiotensin system in rats with diet-induced obesity
and hypertension. Am J Physiol Regul Integr Comp Physiol.
287:R943–R949. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schütten MTJ, Houben AJHM, De Leeuw PW and
Stehouwer CDA: The link between adipose tissue
renin-angiotensin-aldosterone system signaling and
obesity-associated hypertension. Physiology (Bethesda). 32:197–209.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yiannikouris F, Gupte M, Putnam K,
Thatcher S, Charnigo R, Rateri DL, Daugherty A and Cassis LA:
Adipocyte deficiency of angiotensinogen prevents obesity-induced
hypertension in male mice. Hypertension. 60:1524–1530.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yvan-Charvet L, Even P, Bloch-Faure M,
Guerre-Millo M, Moustaid-Moussa N, Ferre P and Quignard-Boulange A:
Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose
cell size and protects from diet-induced obesity and insulin
resistance. Diabetes. 54:991–999. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X
and He J: Hypertension and breast cancer risk: A systematic review
and meta-analysis. Sci Rep. 7(44877)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sun LM, Kuo HT, Jeng LB, Lin CL, Liang JA
and Kao CH: Hypertension and subsequent genitourinary and
gynecologic cancers risk: A population-based cohort study. Medicine
(Baltimore). 94(e753)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lindgren AM, Nissinen AM, Tuomilehto JO
and Pukkala E: Cancer pattern among hypertensive patients in North
Karelia, Finland. J Hum Hypertens. 19:373–379. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cibula D, Raspollini MR, Planchamp F,
Centeno C, Chargari C, Felix A, Fischerová D, Jahnn-Kuch D, Joly F,
Kohler C, et al: ESGO/ESTRO/ESP guidelines for the management of
patients with cervical cancer-update 2023. Int J Gynecol Cancer.
33:649–666. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Oaknin A, Bosse TJ, Creutzberg CL,
Giornelli G, Harter P, Joly F, Lorusso D, Marth C, Makker V, Mirza
MR, et al: Endometrial cancer: ESMO clinical practice guideline for
diagnosis, treatment and follow-up. Ann Oncol. 33:860–877.
2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Colombo N, Sessa C, Du Bois A, Ledermann
J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I,
Vergote I, et al: ESMO-ESGO consensus conference recommendations on
ovarian cancer: Pathology and molecular biology, early and advanced
stages, borderline tumours and recurrent disease. Ann Oncol.
30:672–705. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gil-Ibanez B, Davies-Oliveira J, Lopez G,
Díaz-Feijoo B, Tejerizo-Garcia A and Sehouli J: Impact of
gynecological cancers on health-related quality of life: Historical
context, measurement instruments, and current knowledge. Int J
Gynecol Cancer. 33:1800–1806. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Graham SV: The human papillomavirus
replication cycle, and its links to cancer progression: A
comprehensive review. Clin Sci (Lond). 131:2201–2221.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Balasubramaniam SD, Balakrishnan V, Oon CE
and Kaur G: Key molecular events in cervical cancer development.
Medicina (Kaunas). 55(384)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kurnia I, Rauf S, Hatta M, Arifuddin S,
Hidayat YM, Natzir R, Kaelan C, Bukhari A, Pelupessy NU and
Patelonggi IJ: Molecular Patho-mechanisms of cervical cancer
(MMP1). Ann Med Surg (Lond). 77(103415)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Park JS, Kim EJ, Kwon HJ, Hwang ES,
Namkoong SE and Um SJ: Inactivation of interferon regulatory
factor-1 tumor suppressor protein by HPV E7 oncoprotein.
Implication for the E7-mediated immune evasion mechanism in
cervical carcinogenesis. J Biol Chem. 275:6764–6769.
2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ashrafi GH, Haghshenas MR, Marchetti B,
O'Brien PM and Campo MS: E5 protein of human papillomavirus type 16
selectively downregulates surface HLA class I. Int J Cancer.
113:276–283. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bhattacharjee R, Das SS, Biswal SS, Nath
A, Das D, Basu A, Malik S, Kumar L, Kar S, Singh SK, et al:
Mechanistic role of HPV-associated early proteins in cervical
cancer: Molecular pathways and targeted therapeutic strategies.
Crit Rev Oncol Hematol. 174(103675)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pal A and Kundu R: Human papillomavirus E6
and E7: The cervical cancer hallmarks and targets for therapy.
Front Microbiol. 10(3116)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gius D, Funk MC, Chuang EY, Feng S,
Huettner PC, Nguyen L, Bradbury CM, Mishra M, Gao S, Buttin BM, et
al: Profiling microdissected epithelium and stroma to model genomic
signatures for cervical carcinogenesis accommodating for
covariates. Cancer Res. 67:7113–7123. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Vats A, Trejo-Cerro O, Thomas M and Banks
L: Human papillomavirus E6 and E7: What remains? Tumour Virus Res.
11(200213)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee JE, Chung Y, Rhee S and Kim TH: Untold
story of human cervical cancers: HPV-negative cervical cancer. BMB
Rep. 55:429–438. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nicolás I, Marimon L, Barnadas E, Saco A,
Rodríguez-Carunchio L, Fusté P, Martí C, Rodriguez-Trujillo A,
Torne A, Del Pino M and Ordi J: HPV-negative tumors of the uterine
cervix. Mod Pathol. 32:1189–1196. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zampronha Rde AC, Freitas-Junior R, Murta
EFC, Michelin MA, Barbaresco AA, Adad SJ, de Oliveira AM, Rassi AB
and Oton GJB: Human papillomavirus types 16 and 18 and the
prognosis of patients with stage I cervical cancer. Clinics (Sao
Paulo). 68:809–814. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lu KH and Broaddus RR: Endometrial cancer.
N Engl J Med. 383:2053–2064. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Makker V, MacKay H, Ray-Coquard I, Levine
DA, Westin SN, Aoki D and Oaknin A: Endometrial cancer. Nat Rev Dis
Primers. 7(88)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Crosbie EJ, Zwahlen M, Kitchener HC, Egger
M and Renehan AG: Body mass index, hormone replacement therapy, and
endometrial cancer risk: A meta-analysis. Cancer Epidemiol
Biomarkers Prev. 19:3119–3130. 2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Aune D, Navarro Rosenblatt DA, Chan DSM,
Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV and Norat
T: Anthropometric factors and endometrial cancer risk: A systematic
review and dose-response meta-analysis of prospective studies. Ann
Oncol. 26:1635–1648. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Renehan AG, Soerjomataram I, Tyson M,
Egger M, Zwahlen M, Coebergh JW and Buchan I: Incident cancer
burden attributable to excess body mass index in 30 European
countries. Int J Cancer. 126:692–702. 2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yu K, Huang ZY, Xu XL, Li J, Fu XW and
Deng SL: Estrogen receptor function: Impact on the human
endometrium. Front Endocrinol (Lausanne). 13(827724)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Joshi A and Ellenson LH: PI3K/PTEN/AKT
genetic mouse models of endometrial carcinoma. Adv Exp Med Biol.
943:261–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Terzic M, Aimagambetova G, Kunz J,
Bapayeva G, Aitbayeva B, Terzic S and Laganà AS: Molecular basis of
endometriosis and endometrial cancer: Current knowledge and future
perspectives. Int J Mol Sci. 22(9274)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ebring C, Marlin R, Macni J, Vallard A,
Bergerac S, Beaubrun-Renard M, Joachim C and Jean-Laurent M: Type
II endometrial cancer: Incidence, overall and disease-free survival
in Martinique. PLoS One. 18(e0278757)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ohgami T and Kato K: Pathogenesis of
endometrial cancer. In: Current Approaches to Endometrial Cancer.
Future Medicine Ltd, Unitec House, 2 Albert Place, London N3 1QB,
UK, pp18-32, 2014.
|
|
68
|
Miyamoto T and Shiozawa T: Two-sided role
of estrogen on endometrial carcinogenesis: stimulator or
suppressor? Gynecol Endocrinol. 35:370–375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lobo FD and Thomas E: Type II endometrial
cancers: A case series. J Midlife Health. 7:69–72. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Shen F, Gao Y, Ding J and Chen Q: Is the
positivity of estrogen receptor or progesterone receptor different
between type 1 and type 2 endometrial cancer? Oncotarget.
8:506–511. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Testing strategies for Lynch syndrome in
people with endometrial cancer. Diagnostics guidance, 2020.
|
|
73
|
Stinton C, Jordan M, Fraser H, Auguste P,
Court R, Al-Khudairy L, Madan J, Grammatopoulos D and
Taylor-Phillips S: Testing strategies for Lynch syndrome in people
with endometrial cancer: Systematic reviews and economic
evaluation. Health Technol Assess. 25:1–216. 2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu Y, Patel L, Mills GB, Lu KH, Sood AK,
Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, et al:
Clinical significance of CTNNB1 mutation and Wnt pathway activation
in endometrioid endometrial carcinoma. J Natl Cancer Inst.
106(dju245)2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kurnit KC, Kim GN, Fellman BM, Urbauer DL,
Mills GB, Zhang W and Broaddus RR: CTNNB1 (beta-catenin) mutation
identifies low grade, early stage endometrial cancer patients at
increased risk of recurrence. Mod Pathol. 30:1032–1041.
2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Prat J: Ovarian carcinomas: Five distinct
diseases with different origins, genetic alterations, and
clinicopathological features. Virchows Arch. 460:237–249.
2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253.
2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hollis RL: Molecular characteristics and
clinical behaviour of epithelial ovarian cancers. Cancer Lett.
555(216057)2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Irodi A, Rye T, Herbert K, Churchman M,
Bartos C, Mackean M, Nussey F, Herrington CS, Gourley C and Hollis
RL: Patterns of clinicopathological features and outcome in
epithelial ovarian cancer patients: 35 Years of prospectively
collected data. BJOG. 127:1409–1420. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Patch AM, Christie EL, Etemadmoghadam D,
Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey
PJ, et al: Whole-genome characterization of chemoresistant ovarian
cancer. Nature. 521:489–494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Oza AM, Tinker AV, Oaknin A,
Shapira-Frommer R, McNeish IA, Swisher EM, Ray-Coquard I,
Bell-McGuinn K, Coleman RL, O'Malley DM, et al: Antitumor activity
and safety of the PARP inhibitor rucaparib in patients with
high-grade ovarian carcinoma and a germline or somatic BRCA1 or
BRCA2 mutation: Integrated analysis of data from Study 10 and
ARIEL2. Gynecol Oncol. 147:267–275. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Boehm KM, Aherne EA, Ellenson L,
Nikolovski I, Alghamdi M, Vázquez-García I, Zamarin D, Long Roche
K, Liu Y, Patel D, et al: Multimodal data integration using machine
learning improves risk stratification of high-grade serous ovarian
cancer. Nat Cancer. 3:723–733. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Li W, Liu Y, Liu W, Tang ZR, Dong S, Li W,
Zhang K, Xu C, Hu Z, Wang H, et al: Machine learning-based
prediction of lymph node metastasis among osteosarcoma patients.
Front Oncol. 12(797103)2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bahar ME, Kim HJ and Kim DR: Targeting the
RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical
studies. Signal Transduct Target Ther. 8(455)2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Patel S, Rauf A, Khan H and Abu-Izneid T:
Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for
homeostasis and pathologies. Biomed Pharmacother. 94:317–325.
2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Martyniak A and Tomasik PJ: A new
perspective on the renin-angiotensin system. Diagnostics (Basel).
13(16)2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Mirabito Colafella KM, Bovée DM and Danser
AHJ: The renin-angiotensin-aldosterone system and its therapeutic
targets. Exp Eye Res. 186(107680)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Fountain JH, Kaur J and Lappin SL:
Physiology, renin angiotensin system. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing, 2024.
|
|
90
|
Sequeira-Lopez MLS and Gomez RA: Renin
cells, the kidney, and hypertension. Circ Res. 128:887–907.
2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lu H, Cassis LA, Kooi CWV and Daugherty A:
Structure and functions of angiotensinogen. Hypertens Res.
39:492–500. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Mascolo A, Scavone C, Rafaniello C, De
Angelis A, Urbanek K, di Mauro G, Cappetta D, Berrino L, Rossi F
and Capuano A: The role of renin-angiotensin-aldosterone system in
the heart and lung: Focus on COVID-19. Front Pharmacol.
12(667254)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kawai T, Forrester SJ, O'Brien S, Baggett
A, Rizzo V and Eguchi S: AT1 receptor signaling pathways in the
cardiovascular system. Pharmacol Res. 125:4–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Maranduca MA, Clim A, Pinzariu AC,
Statescu C, Sascau RA, Tanase DM, Serban DN, Branisteanu DC,
Branisteanu DE, Huzum B and Serban IL: Role of arterial
hypertension and angiotensin II in chronic kidney disease (review).
Exp Ther Med. 25(153)2023.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Sun Y, Li Y, Wang M, Yue M, Bai L, Bian J,
Hao W, Sun J, Zhang S and Liu H: Increased AT2R
expression is induced by AT1R autoantibody via two axes,
Klf-5/IRF-1 and circErbB4/miR-29a-5p, to promote VSMC migration.
Cell Death Dis. 11(432)2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Fatima N, Patel SN and Hussain T:
Angiotensin II type 2 receptor: A target for protection against
hypertension, metabolic dysfunction, and organ remodeling.
Hypertension. 77:1845–1856. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Alenina N and dos Santos RAS:
Angiotensin-(1-7) and Mas. In: The Protective Arm of the Renin
Angiotensin System (RAS). Elsevier, pp155-159, 2015.
|
|
98
|
Pawlik MW, Kwiecien S, Ptak-Belowska A,
Pajdo R, Olszanecki R, Suski M, Madej J, Targosz A, Konturek SJ,
Korbut R and Brzozowski T: The renin-angiotensin system and its
vasoactive metabolite angiotensin-(1-7) in the mechanism of the
healing of preexisting gastric ulcers. The involvement of Mas
receptors, nitric oxide, prostaglandins and proinflammatory
cytokines. J Physiol Pharmacol. 67:75–91. 2016.PubMed/NCBI
|
|
99
|
Xu J, Fan J, Wu F, Huang Q, Guo M, Lv Z,
Han J, Duan L, Hu G, Chen L, et al: The ACE2/angiotensin-(1-7)/Mas
receptor axis: Pleiotropic roles in cancer. Front Physiol.
8(276)2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Luo Y, Tanabe E, Kitayoshi M, Nishiguchi
Y, Fujiwara R, Matsushima S, Sasaki T, Sasahira T, Chihara Y, Nakae
D, et al: Expression of MAS1 in breast cancer. Cancer Sci.
106:1240–1248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Qaradakhi T, Apostolopoulos V and Zulli A:
Angiotensin (1-7) and alamandine: Similarities and differences.
Pharmacol Res. 111:820–826. 2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
da Silva FA, Rodrigues-Ribeiro L,
Melo-Braga MN, Passos-Silva DG, Sampaio WO, Gorshkov V, Kjeldsen F,
Verano-Braga T and Santos RAS: Phosphoproteomic studies of
alamandine signaling in CHO-MrgD and human pancreatic carcinoma
cells: An antiproliferative effect is unveiled. Proteomics.
22(e2100255)2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Patel VB, Zhong J-C, Grant MB and Oudit
GY: Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin
system in heart failure. Circ Res. 118:1313–1326. 2016.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Heyman SN, Walther T and Abassi Z:
Angiotensin-(1-7)-A potential remedy for AKI: Insights derived from
the COVID-19 pandemic. J Clin Med. 10(1200)2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Hrenak J, Paulis L and Simko F:
Angiotensin A/alamandine/MrgD axis: Another clue to understanding
cardiovascular pathophysiology. Int J Mol Sci.
17(1098)2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Philippe A: Unraveling the prevalence of
angiotensin II type 1 receptor antibodies in hypertension. Am J
Hypertens. 33:711–712. 2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Wagner MJ, Lyons YA, Siedel JH, Dood R,
Nagaraja AS, Haemmerle M, Mangala LS, Chanana P, Lazar AJ, Wang WL,
et al: Combined VEGFR and MAPK pathway inhibition in angiosarcoma.
Sci Rep. 11(9362)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Catarata MJ, Ribeiro R, Oliveira MJ,
Robalo Cordeiro C and Medeiros R: Renin-angiotensin system in lung
tumor and microenvironment interactions. Cancers (Basel).
12(1457)2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Perea Paizal J, Au SH and Bakal C:
Squeezing through the microcirculation: Survival adaptations of
circulating tumour cells to seed metastasis. Br J Cancer.
124:58–65. 2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Saman H, Raza SS, Uddin S and Rasul K:
Inducing angiogenesis, a key step in cancer vascularization, and
treatment approaches. Cancers (Basel). 12(1172)2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8(198)2023.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Pinter M and Jain RK: Targeting the
renin-angiotensin system to improve cancer treatment: Implications
for immunotherapy. Sci Transl Med. 9(eaan5616)2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Brasier AR, Recinos A III and Eledrisi MS:
Vascular inflammation and the renin-angiotensin system.
Arterioscler Thromb Vasc Biol. 22:1257–1266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Albulescu R, Codrici E, Popescu ID, Mihai
S, Necula LG, Petrescu D, Teodoru M and Tanase CP: Cytokine
patterns in brain tumour progression. Mediators Inflamm.
2013(979748)2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Liu B, Qu L and Yan S: Cyclooxygenase-2
promotes tumor growth and suppresses tumor immunity. Cancer Cell
Int. 15(106)2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Zhang RM, McNerney KP, Riek AE and
Bernal-Mizrachi C: Immunity and hypertension. Acta Physiol (Oxf).
231(e13487)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cao DY, Saito S, Veiras LC, Okwan-Duodu D,
Bernstein EA, Giani JF, Bernstein KE and Khan Z: Role of
angiotensin-converting enzyme in myeloid cell immune responses.
Cell Mol Biol Lett. 25(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Perini MV, Dmello RS, Nero TL and Chand
AL: Evaluating the benefits of renin-angiotensin system inhibitors
as cancer treatments. Pharmacol Ther. 211(107527)2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Wang Q, Lei X, Zhu S and Zhang S:
Angiotensin-I converting enzyme inhibitors suppress angiogenesis
and growth of esophageal carcinoma xenografts. Dis Esophagus.
25:757–763. 2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Bryniarski P, Nazimek K and Marcinkiewicz
J: Immunomodulatory activity of the most commonly used
antihypertensive drugs-angiotensin converting enzyme inhibitors and
angiotensin II receptor blockers. Int J Mol Sci.
23(1772)2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kunvariya AD, Dave SA, Modi ZJ, Patel PK
and Sagar SR: Exploration of multifaceted molecular mechanism of
angiotensin-converting enzyme 2 (ACE2) in pathogenesis of various
diseases. Heliyon. 9(e15644)2023.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Rosenthal T and Gavras I:
Renin-angiotensin inhibition in combating malignancy: A review.
Anticancer Res. 39:4597–4602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Budiana ING, Angelina M and Pemayun TGA:
Ovarian cancer: Pathogenesis and current recommendations for
prophylactic surgery. J Turk Ger Gynecol Assoc. 20:47–54.
2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Martins FC, Couturier DL, Paterson A,
Karnezis AN, Chow C, Nazeran TM, Odunsi A, Gentry-Maharaj A, Vrvilo
A, Hein A, et al: Clinical and pathological associations of PTEN
expression in ovarian cancer: A multicentre study from the ovarian
tumour tissue analysis consortium. Br J Cancer. 123:793–802.
2020.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Huang L, Guo Z, Wang F and Fu L: KRAS
mutation: From undruggable to druggable in cancer. Signal Transduct
Target Ther. 6(386)2021.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Fujimoto Y, Morita TY, Ohashi A, Haeno H,
Hakozaki Y, Fujii M, Kashima Y, Kobayashi SS and Mukohara T:
Combination treatment with a PI3K/Akt/mTOR pathway inhibitor
overcomes resistance to anti-HER2 therapy in PIK3CA-mutant
HER2-positive breast cancer cells. Sci Rep.
10(21762)2020.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Harrington BS and Annunziata CM: NF-κB
signaling in ovarian cancer. Cancers (Basel).
11(1182)2019.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Zhang T, Ma C, Zhang Z, Zhang H and Hu H:
NF-κB signaling in inflammation and cancer. MedComm (2020).
2:618–653. 2021.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Regulska K, Michalak M, Kolenda T,
Kozłowska-Masłoń J, Guglas K and Stanisz B: Angiotensin-converting
enzyme inhibitors for ovarian cancer? -A new adjuvant option or a
silent trap. Rep Pract Oncol Radiother. 28:551–564. 2023.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Beyazit F, Ayhan S, Celik HT and Gungor T:
Assessment of serum angiotensin-converting enzyme in patients with
epithelial ovarian cancer. Arch Gynecol Obstet. 292:415–420.
2015.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Harding BN, Delaney JA, Urban RR and Weiss
NS: Post-diagnosis use of antihypertensive medications and the risk
of death from ovarian cancer. Gynecol Oncol. 154:426–431.
2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Piškur I, Topolovec Z, Bakula M, Zagorac
I, Milić Vranješ I and Vidosavljević D: Expression of vascular
endothelial growth factor-A (VEGF-A) in adenocarcinoma and squamous
cell cervical cancer and its impact on disease progression: Single
institution experience. Medicina (Lithuania).
59(1189)2023.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Zhou Y, Shu C and Huang Y: Fibronectin
promotes cervical cancer tumorigenesis through activating FAK
signaling pathway. J Cell Biochem. 120:10988–10997. 2019.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Zhou J, Yi Q and Tang L: The roles of
nuclear focal adhesion kinase (FAK) on cancer: A focused review. J
Exp Clin Cancer Res. 38(250)2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Mrozik KM, Blaschuk OW, Cheong CM,
Zannettino ACW and Vandyke K: N-cadherin in cancer metastasis, its
emerging role in haematological malignancies and potential as a
therapeutic target in cancer. BMC Cancer. 18(939)2018.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Spada S, Tocci A, Di Modugno F and Nisticò
P: Fibronectin as a multiregulatory molecule crucial in tumor
matrisome: From structural and functional features to clinical
practice in oncology. J Exp Clin Cancer Res. 40(102)2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Garvin AM, De Both MD, Talboom JS, Lindsey
ML, Huentelman MJ and Hale TM: Transient ACE
(angiotensin-converting enzyme) inhibition suppresses future
fibrogenic capacity and heterogeneity of cardiac fibroblast
subpopulations. Hypertension. 77:904–918. 2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
McLachlan CS: The angiotensin-converting
enzyme 2 (ACE2) receptor in the prevention and treatment of
COVID-19 are distinctly different paradigms. Clin Hypertens.
26(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Wang R, Xu J, Wu J, Gao S and Wang Z:
Angiotensin-converting enzyme 2 alleviates pulmonary artery
hypertension through inhibition of focal adhesion kinase
expression. Exp Ther Med. 22(1165)2021.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Clarke NE, Fisher MJ, Porter KE, Lambert
DW and Turner AJ: Angiotensin converting enzyme (ACE) and ACE2 bind
integrins and ACE2 regulates integrin signalling. PLoS One.
7(e34747)2012.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Brooks SD, Smith RL, Moreira AS and
Ackerman HC: Oral lisinopril raises tissue levels of ACE2, the
SARS-CoV-2 receptor, in healthy male and female mice. Front
Pharmacol. 13(798349)2022.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Radin DP, Krebs A, Maqsudlu A and Patel P:
Our ACE in the HOLE: Justifying the use of angiotensin-converting
enzyme inhibitors as adjuvants to standard chemotherapy. Anticancer
Res. 38:45–49. 2018.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Stavropoulos A, Varras M, Vasilakaki T,
Varra VK, Tsavari A, Varra FN, Nonni A, Kavantzas N and Lazaris AC:
Expression of p53 and PTEN in human primary endometrial carcinomas:
Clinicopathological and immunohistochemical analysis and study of
their concomitant expression. Oncol Lett. 17:4575–4589.
2019.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Aslan O, Cremona M, Morgan C, Cheung LW,
Mills GB and Hennessy BT: Preclinical evaluation and reverse phase
protein Array-based profiling of PI3K and MEK inhibitors in
endometrial carcinoma in vitro. BMC Cancer. 18(168)2018.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Manning-Geist BL, Liu YL, Devereaux KA,
Paula ADC, Zhou QC, Ma W, Selenica P, Ceyhan-Birsoy O, Moukarzel
LA, Hoang T, et al: Microsatellite instability-high endometrial
cancers with MLH1 promoter hypermethylation have distinct molecular
and clinical profiles. Clin Cancer Res. 28:4302–4311.
2022.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Erickson BK, Najjar O, Damast S, Blakaj A,
Tymon-Rosario J, Shahi M, Santin A, Klein M, Dolan M,
Cimino-Mathews A, et al: Human epidermal growth factor 2 (HER2) in
early stage uterine serous carcinoma: A multi-institutional cohort
study. Gynecol Oncol. 159:17–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Elnashar AT, Hassab Elnaby NED, Abdellatef
AA and Salem MN: Her-2 neu expression in endometrial carcinoma. Med
J Cairo Univ. 86:69–76. 2018.
|
|
148
|
Florescu MM, Pirici D, Simionescu CE,
Stepan AE, Mărgăritescu C, Tudorache Ş and Ciurea RN: E-cadherin
and β-catenin immunoexpression in endometrioid endometrial
carcinoma. Rom J Morphol Embryol. 57:1235–1240. 2016.PubMed/NCBI
|
|
149
|
Delforce SJ, Lumbers ER, Corbisier de
Meaultsart C, Wang Y, Proietto A, Otton G, Scurry J, Verrills NM,
Scott RJ and Pringle KG: Expression of renin-angiotensin system
(RAS) components in endometrial cancer. Endocr Connect. 6:9–19.
2017.PubMed/NCBI View Article : Google Scholar
|















