Introduction
The human body maintains homeostasis through the
filtration of blood via the kidneys. Any dysfunction in kidney
function poses a substantial threat to the life of an individual,
potentially leading to renal failure (1). Over the past two decades, there has
been a notable increase in patients with renal failure requiring
hemodialysis (2). These patients
are inherently immunocompromised due to dysfunctions in their
immune responses, rendering them more susceptible to opportunistic
infections, such as the Toxoplasma gondii (T. gondii)
protozoal parasite (3).
T. gondii, a protozoal parasite discovered in
1908, exhibits both sexual and asexual life cycles and has a broad
host range that includes humans, pets and wildlife. During the
emergence of AIDS in the 1980s, this parasite emerged as an
opportunistic pathogen, presenting a life-threatening risk to
immunocompromised individuals (4-6).
Infection with T. gondii activates the immune response of
the host, which often fails to control the infection. Host immune
cells recognize T. gondii-derived ligands, initiating
acquired immunity and ultimately curbing the proliferation of the
parasite in vivo. Innate immunity plays a pivotal role in
the immediate defense against pathogens, with the Toll-like
receptor (TLR) family serving as a vital component in detecting
pathogen-associated molecular patterns via its extracellular domain
and subsequently triggering inflammatory signaling pathways through
its intracellular domains (7).
Of particular interest, TLR-5 is considered to
mediate the recognition of T. gondii profilin. The
ectodomain of human TLR-5 harbors binding sites shared between
flagellin and profiling (8). The
TLR-5 gene is encoded by six exons and is located on the long arm
of human chromosome 1 (hCh1q). Notably, there are currently nine
reported polymorphisms in the promoter and coding regions of the
gene (9). Functional TLR gene
polymorphisms, such as rs2072493 and rs5744168, are found within
the exon regions (10) and have
significant immunological implications (11).
The present study aimed to explore the significance
of TLR-5 gene polymorphisms in individuals undergoing hemodialysis
and who are afflicted with toxoplasmosis. The present study aimed
to investigate the association between TLR-5 gene polymorphisms
that render individuals more susceptible to toxoplasmosis in a
sample of patients undergoing hemodialysis. Quantitative-polymerase
chain reaction (qPCR) techniques were employed to discern these
genetic associations.
Patients and methods
Recruitment of participants
All study participants were recruited from the
Department of Hemodialysis at Balad General Hospital, Salah Al-Din,
Iraq. Data collection was performed between January, 2022 and June,
2022. Ethics approval for the study was granted by the
Institutional Review Board of the College of Medicine, AL-Nahrain
University (Approval no. 20221152, Ref: IRB/221 on November 29,
2022). All patients provided written informed consent to
participate in the study.
Study design
The present case-control study included a total of
100 participants undergoing hemodialysis. Among the participants 50
patients were classified as seropositive for T. gondii.
[immunoglobulin (Ig)M- and IgG]-positive, group 1], while the
remaining 50 patients served as the control group and were
seronegative for T. gondii (IgM- and IgG-negative, group
2).
Blood sample collection
A total of 5 ml whole venous blood was collected
from each participant at the laboratory facility of the hospital.
Patient demographic information was recorded. Of the collected
blood, 2 ml were transferred to ethylenediaminetetraacetic acid
(EDTA) tubes and stored at -20˚C until further processing. The
remaining 3 ml were preserved in plain gel tubes for serum
preparation.
Serological testing
For the detection of human T. gondii, the
blood samples were tested for IgM and IgG antibodies using the
enzyme-linked immunosorbent assay (ELISA) method, following the
manufacturer's instructions (cat. no. ABIN367461, Biocompare).
Genetic analysis
The quick protocol SYNCTM DNA extraction kit
(Geneaid) was applied to obtain highly pure genomic DNA using
venous ethylene diamine tetra-acetic acid anticoagulated blood
samples. The absolute quantification real-time PCR system and the
commercially available TaqMan SNP Genotyping Assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) were used for the
genotype analysis. Real-time amplification was performed under the
following conditions: Denaturation at 95˚C for 10 min, followed by
40 cycles of denaturation at 95˚C for 15 sec and
annealing/extension at 60˚C for 60 sec. Allelic discrimination was
performed using SDS software (v.2.3).
The selected single nucleotide polymorphisms (SNPs)
in the TLR-5 gene (rs2072493 and rs5744168) were identified using
qPCR with specific primers. The primers used for TLR-5 SNPs
detection in present study are listed in Table I, that amplify the SNP region.
These primers are able to amplify wide spectrum of DNA of TLR-5
genotypes that extracted from blood. The pair of primers for SNP
(rs2072493) flanks have a sequence of approximately 140 bp, while
the pair of primers for SNP (rs5744168) flanks have a sequence of
approximately 187 bp. The primer sequences were designed based on
the SNP site of the TLR-5 gene, as sourced from the National Center
for Biotechnology Information (NCBI) and designed using the Primer3
program. The sequences of the primers used are listed in Tables I and II (12). Gene expression was analyzed using
the Livak method (13). The
comparative CT (∆∆Cq) approach was utilized to assess the relative
expression of two genes.
 | Table IPrimers for SNP2072493 (annealing
temperature, 60˚C). |
Table I
Primers for SNP2072493 (annealing
temperature, 60˚C).
| Primer name | Sequence |
|---|
| rs2072493-F |
5'-GAGGCCCAGCTATAGTGACATTG-3' |
| rs2072493-R |
5'-CCAGCTCCTAGCTCCTAATCCT-3' |
| rs2072493-P/C |
Hex-5'-TGTGATTAAGCCAACTGATA-3 |
| rs2072493-P/T |
Fam-5'-TGTGATTAAGCCAATTGATAA-3' |
 | Table IIPrimers for SNP5744168 (annealing
temperature, 60˚C). |
Table II
Primers for SNP5744168 (annealing
temperature, 60˚C).
| Primer name | Sequence |
|---|
| rs5744168-F |
5'-TTATTGCCACTCAAGAAGATATCGG-3 |
| rs5744168-R |
5'-ACAGTTCGAATTTCTATGGACTACC-3' |
| rs5744168-P/A |
Hex-5'-TAAGAGCATTGTCTCAGAGAT-3' |
| rs5744168-P/G |
Fam-5'-AGCATTGTCTCGGAGATC-3' |
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism version 7. To compare observed parameters and SNP numbers
between subgroups, one-way ANOVA analysis with Tukey's post hoc
test was employed. Descriptive statistics are presented as the mean
± standard error, and Chi-squared tests were used to analyze as
nominal variables. Frequencies and percentages (%) were recorded
and compared. Odds ratios (OR), 95% confidence intervals (CI) and
P-values were calculated using MedCalc to demonstrate the nominal
regression results. A value of P<0.05 was considered to indicate
a statistically significant difference. Pearson's correlation
analysis was used to determine the correlation of the polymorphisms
between the groups. Correlation coefficients were computed to
determine the correlations between markers using MegaStat (version
v 10.12) for Excel 2010.
Results
Patient demographics
The mean age of the patients in group 1 was 43.42
years, while that of the subjects in group 2 was 42.50 years, with
a standard deviation (SD) of 12.85. The ages of the participants
ranged from 19 to 68 years. The sex of the study groups was as
follows: Males, n=25 (50%); and females, n=25 (50%). The number of
patients with other diseases was 39 (78%) in group 1 and 31 (62%)
in group 2. Moreover, the number of patients with no other diseases
was 11 (22%) in group 1 and 19 (38%) in group 2. A large percentage
of patients did not have pets: 40 (80%) patients in group 1 and 42
(84%) patients in group 2. Consequently, the remaining patients had
pets: 10 (20%) in group 1 and 8 (16%) in group 2. The results also
revealed that the disease duration of the patients ranged from 1
month to 1 year: In group 1, there were 29 patients (42%), while
group 2 had 33 patients (66%). On the other hand, the duration from
1 to 3 years was observed in 21 patients (42%) in group 1 and 29
patients (38%) in group 2.
As regards the type of residence, 17 (34%) patients
in group 1 had an urban residence and 33 (66% had a rural
residence. In group 2, 31 (62%) of the participants had an urban
residence and 19 (38%) had a rural residence (Table III).
 | Table IIIDemographic data of the study
groups. |
Table III
Demographic data of the study
groups.
| Parameter | Group 1 | Group 2 |
|---|
| Age, years; median
(5-95 percentile) | 43.42 (19 to 68) | 42.50 (20-65) |
| Sex | | |
|
Female | 25 (50%) | 25 (50%) |
|
Male | 25 (50%) | 25 (50%) |
| Other diseases | | |
|
Yes | 39 (78%) | 31 (62%) |
|
No | 11 (22%) | 19 (38%) |
| Pet ownership | | |
|
Yes | 10 (20%) | 8 (12%) |
|
No | 40 (80%) | 42 (84%) |
| Residence | | |
|
Urban | 17 (34%) | 31 (62%) |
|
Rural | 33 (66%) | 19 (38%) |
| Hemolysis
duration | | |
|
From 1 month
to 1 year | 29 (48%) | 33 (66%) |
|
From 1 to 3
years | 21 (42%) | 29 (48%) |
| Total | 50 | 50 |
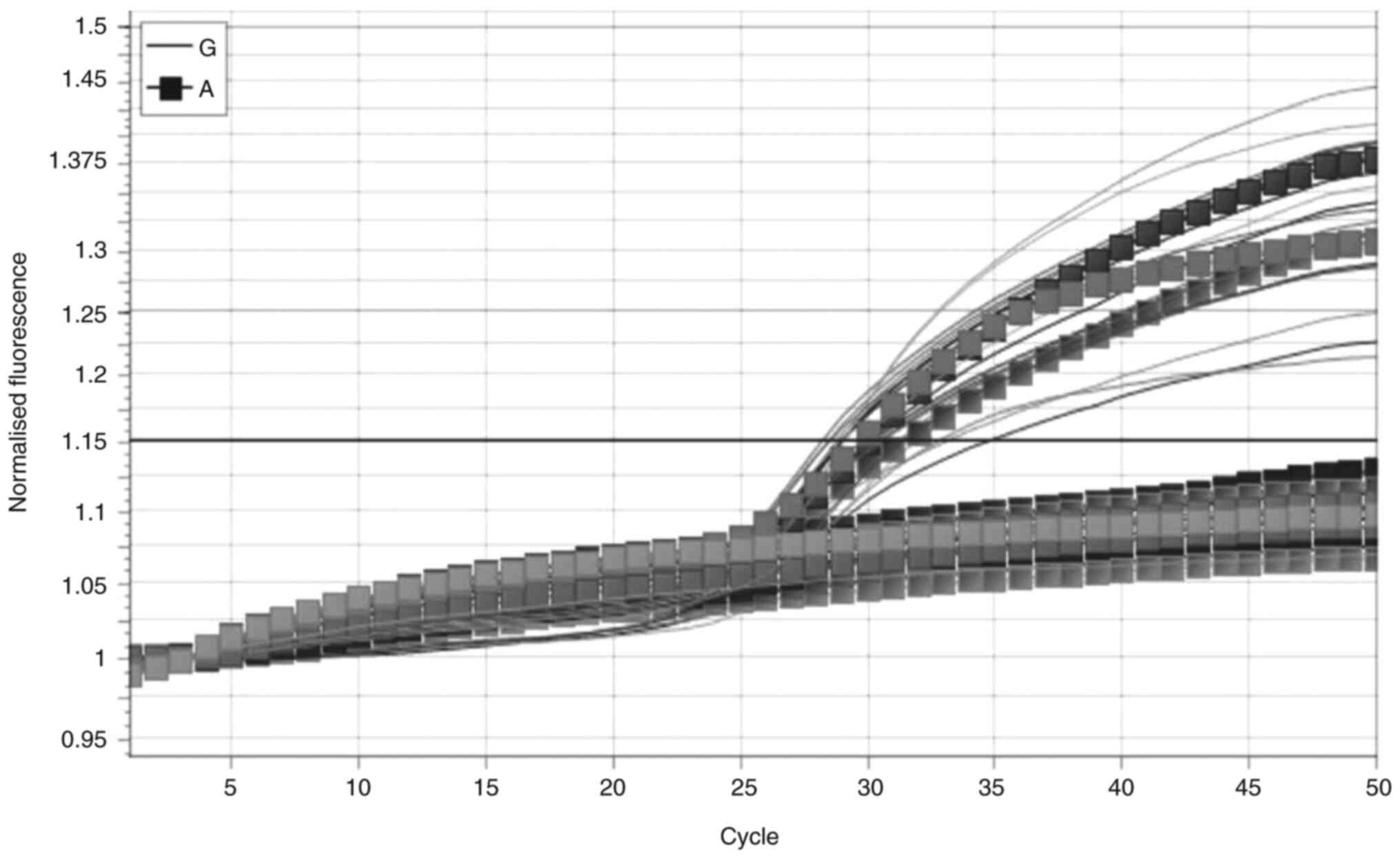
TLR-5 gene polymorphism rs5744168
In the case of the TLR-5 gene polymorphism
rs5744168, only two genotypes (GA and GG) were observed in the
study groups, as illustrated in Fig.
1. The homozygous genotype GG had a frequency of 41 (82%) in
group 1, and this frequency was not statistically significant
(P=0.7989) when compared to group 2, which had 40 (80%) patients
with this genotype. Conversely, the heterozygous genotype GA was
more prevalent in group 1, accounting for 18 (36%) individuals, in
contrast to group 2 with 10 (20%) individuals. This difference was
not statistically significant (P=0.0781). Further analysis revealed
an OR of 2.25 with a 95% CI ranging from 0.91 to 5.54 (Table IV). At the allelic level, the
frequency of the normal allele (allele G) in both groups was
comparable, with group 1 and group 2 displaying frequencies of 91
and 90%, respectively, with no significant difference (P=0.80).
Similarly, the frequency of the mutant allele (allele A) in groups
1 and 2 was 9 and 10%, respectively, with no significant
differences (P=0.80). The OR for this comparison was 0.89, with a
95% CI ranging from 0.34 to 2.29 (Table IV).
 | Table IVTLR-5 gene polymorphism rs5744168
genotypes and alleles in groups 1 and 2. |
Table IV
TLR-5 gene polymorphism rs5744168
genotypes and alleles in groups 1 and 2.
| rs5744168 | Group 1 (n=50) | Group 2 (n=50) | OR (95% CI) | P-value |
|---|
| Genotypes | | | | |
|
GG | 41 (82%) | 40 (80%) | 1.13 (0.41 to
3.09) | 0.7989 |
|
GA | 18 (36%) | 10 (20%) | 2.25 (0.91 to
5.54) | 0.0781 |
|
AA | 0 (0.0%) | 0 (0.0%) | | |
| Alleles | | | | |
|
G | 91 (91%) | 90 (90%) | 1.12 (0.43 to
2.89) | 0.80 |
|
A | 9 (9%) | 10 (20%) | 0.89 (0.34 to
2.29) | 0.80 |
TLR-5 gene polymorphism rs2072493
The PCR amplification of the TLR-5 gene polymorphism
rs2072493 is illustrated in Fig.
2. This polymorphism displayed three genotypes (TT, TC and CC).
In comparison to group 2, which had a homozygous genotype (TT)
frequency of 25 (50%) patients, group 1 had a homozygous genotype
(TT) with a frequency of 30 (60%) patients. This difference was not
statistically significant (P=0.3157), with an OR of 0.66 and a 95%
CI ranging from 0.30 to 1.47. The frequency of the heterozygous
genotype (TC) was 20 (40%) patients in group 1, the same as group
2, with no significant differences (P=1.000). Additionally, the
homozygous genotype CC had a frequency of 5 (10%) patients in group
1, whereas it was absent in group 2 (0%). This discrepancy was
significant (P=0.09), with an OR of 12.20 and a 95% CI ranging from
0.65 to 226.97 (Table V).
 | Table VTLR-5 gene polymorphism rs2072493
genotypes and alleles in groups 1 and 2. |
Table V
TLR-5 gene polymorphism rs2072493
genotypes and alleles in groups 1 and 2.
| rs2072493 | Group 1 (n=50) | Group 2 (n=50) | OR (95% CI) | P-value |
|---|
| Genotypes | | | | |
|
TT | 25 (50%) | 30 (60%) | 0.66 (0.30 to
1.47) | 0.3157 |
|
TC | 20 (40%) | 20 (40%) | 1.00 (0.44 to
2.22) | 1.000 |
|
CC | 5 (10%) | 0 (0.0%) | 12.2 (0.65 to
226.97) | 0.0934 |
| Alleles | | | | |
|
T | 70 (70%) | 80 (80%) | 0.58 (0.30 to
1.11) | 0.1043 |
|
C | 30 (30%) | 20 (20%) | 1.71 (0.89 to
3.28) | 0.1043 |
At the allelic level, the frequency of the wild-type
allele (allele T) reached 70% in group 1 and 80% in group 2, with
no significant difference (P=0.1043) and an OR of 0.58 and a 95% CI
ranging from 0.30 to 1.11. Conversely, the mutant allele (allele C)
had a frequency of 30% in group 1, as opposed to 20% in group 2,
indicating no significant difference (P=0.1043), with an OR of
1.7143 and a 95% CI ranging from 0.89 to 3.28 (Table V).
Correlation of rs5744168 between the
two groups
As shown in Table
VI, a positive correlation was found for rs5744168 between the
groups, with a significant difference (P<0.001) between the
groups.
 | Table VICorrelations of rs5744168 between
groups 1 and 2. |
Table VI
Correlations of rs5744168 between
groups 1 and 2.
| | Group 1 (n=50) | Group 2 (n=50) |
|---|
| rs5744168 | R value (Pearson's
correlation) | P-value | R value (Pearson's
correlation) | P-value |
|---|
| Group 1 | 1 | | 0.677a | 1b |
| Group 2 | 0.677a | 0.001 | 1 | |
Correlation of rs2072493 between the
two groups
As shown in Table
VII, a negative correlation was found for rs2072493 between the
groups, with no significant difference (P<0.660) between the
groups.
 | Table VIICorrelations of rs2072493 between
groups 1 and 2. |
Table VII
Correlations of rs2072493 between
groups 1 and 2.
| | Group 1 (n=50) | Group 2 (n=50) |
|---|
| rs207249 | R value (Pearson's
correlation) | P-value | R value (Pearson's
correlation) | P-value |
|---|
| Group 1 | 1 | | -0.064 | 0.66 |
| Group 2 | -0.064 | 0.66 | 1 | |
Discussion
Demographic data. The present case control
study revealed no differences in the mean age between the patients
and controls as the age of the control group was selected according
to patients group also the number of participants. In group 1 the
number of rural residents was higher than that of urban residents
as they are more likely to have a lower quality of life and a
substantial financial burden (14). The demographic data demonstrated
that the patients had other diseases and that many of them did not
have pets. However, the seropositivity in the patients may have
been due to the long duration of medication and other sources of
infection, rather than having pets, as the majority of patients did
not have pets.
TLR-5 gene polymorphisms. SNPs are prevalent
in the human genome, and they exert a significant influence on
innate immune responses by altering the amplitude and quality of
intracellular signaling cascades. These genetic variations can
affect susceptibility to infections and disease outcomes, and are
crucial in understanding the etiology of human diseases, clinical
characteristics, drug development and treatment strategies
(15).
In the present study, the TLR-5 gene polymorphism
rs5744168 was not significantly associated with susceptibility to
toxoplasmosis in group 1 (P>0.05). While there is a paucity of
studies directly comparable to the findings presented herein, SNP
rs5744168 has been linked to other infections, such as
Helicobacter pylori, as well as certain diseases such as
breast cancer, Crohn's disease and systemic lupus erythematosus
(16). The results of the present
study align with existing research, which generally suggests that
the gene polymorphism rs5744168, characterized by a stop codon and
low mutant allele frequencies, can reduce the interaction between
TLR-5 and pathogen-associated molecular patterns of T.
gondii, thereby limiting the immune response. This occurs due
to the truncation of TLR5 transmembrane signaling, inhibiting TLR5
homodimer assembly and localization, which, in turn, restricts the
immune response (17).
In the present study, as regards susceptibility to
toxoplasmosis, the TLR-5 gene polymorphism rs2072493 (P>0.05)
did not exhibit any significant associations. However, to the best
of our knowledge, there are a limited number of similar studies
available for a direct comparison. Globally, studies on TLR-5 gene
polymorphism and toxoplasmosis are relatively rare. The allele
frequency polymorphism for rs2072493 the present study (30% in
group 1) is in contrast to allele frequency percentages in various
populations, such as Caucasians (15%), Chinese (26%) and Northern
Indians (12%) (9,18,19).
These discrepancies may be attributed to differences in the study
population and genetic heterogeneity across diverse ethnicities.
Moreover, SNP rs2072493 has been linked to various diseases,
including colorectal cancer, Graves' disease and hepatitis B virus
infections (20-22).
Correlations of rs5744168 and rs2072493 between
groups 1 and 2. T. gondii has been implicated in
glomerular lesions and urinary abnormalities, often leading to
renal failure, as indicated by elevated creatinine levels in urine
(23). The present study confirmed
positive correlations of rs5744168 between groups 1 and 2. TLR-5
ligation triggers the production of pro-inflammatory cytokines,
such as TNF-α and IL-6, through the NF-κB pathway (24), which is consistent with elevated
pro-inflammatory responses observed in subjects undergoing
hemodialysis (25).
The transmission of the parasite relies on its
ability to establish long-lasting chronic infections. High levels
of T. gondii-specific IgG and cytokines in sera play a
pivotal role in limiting parasite growth, thereby perpetuating the
immune response to T. gondii through chronic infections
(26). The present study observed
negative correlations of rs2072493 between groups 1 and 2. The
ability of T. gondii to stimulate Th2-cytokines, including
IL-10, may explain the significant increase in IL-10 levels.
Additionally, IL-10 plays a critical role in suppressing the
release of various cytokines produced by T-cells, such as IL-12,
IL-6 and IFN-γ. It also inhibits natural killer cells from
producing cellular cytokines (27,28).
To the best of our knowledge, there are no prior studies
investigating the correlations of the TLR-5 gene polymorphisms,
rs5744168 and rs2072493, between patients infected with toxoplasma
undergoing hemodialysis.
In conclusion, the present study examined the TLR-5
gene polymorphisms (rs5744168 and rs2072493) and their association
with susceptibility to toxoplasmosis in patients undergoing
hemodialysis and yielded several key findings. Firstly, no
statistically significant differences were observed in the
susceptibility to toxoplasmosis between individuals with different
TLR-5 gene polymorphisms.
Furthermore, the present study revealed a notable
positive correlation between patients undergoing hemodialysis
carrying the rs5744168 polymorphism, both among seropositive and
seronegative individuals. By contrast, those with the rs2072493
polymorphism exhibited a negative correlation with susceptibility
to toxoplasmosis. These notable correlations point to the intricate
association between TLR-5 gene polymorphisms and the immune
response to toxoplasmosis in patients undergoing hemodialysis.
Further research in this area may shed light on the underlying
mechanisms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HDAM was involved in the conceptualization of the
study, as well as in the study methodology, formal analysis, in the
writing of the original draft of the manuscript, and in the
writing, reviewing and editing of the manuscript. KARM, ANAB and
NHM made substantial contributions to the conception and design of
the study, were responsible for the writing the of the manuscript,
and were involved in data curation, investigation and data
analysis. KARM, ANAB and NHM confirm the authenticity of all the
raw data All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was granted by the
Institutional Review Board of the College of Medicine, AL-Nahrain
University (Approval no. 20221152, Ref: IRB/221 on November 29,
2022). All patients provided written informed consent to
participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elmore SA, Jones JL, Conrad PA, Patton S,
Lindsay DS and Dubey JP: Toxoplasma gondii: Epidemiology, feline
clinical aspects, and prevention. Trends Parasitol. 26:190–196.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mcleod R, Van Tubbergen C, Montoya J and
Petersen E: Human toxoplasma infection. In: Toxoplasma Gondii: The
Model Apicomplexan-Perspectives and Methods. Elsevier, Amsterdam,
2013.
|
|
3
|
Sasai M and Yamamoto M: Innate, adaptive,
and cell-autonomous immunity against Toxoplasma gondii infection.
Exp Mol Med. 51:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kato S, Chmielewski M, Honda H,
Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P and
Lindholm B: Aspects of immune dysfunction in end-stage renal
disease. Clin J Am Soc Nephrol. 3:1526–1533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Omrani VF, Fallahi S, Rostami A,
Siyadatpanah A, Barzgarpour G, Mehravar S, Memari F, Hajialiani F
and Joneidi Z: Prevalence of intestinal parasite infections and
associated clinical symptoms among patients with end-stage renal
disease undergoing hemodialysis. Infection. 43:537–544.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tamura MK: Incidence, management, and
outcomes of end-stage renal disease in the elderly. Curr Opin
Nephrol Hypertens. 18:252–257. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
West AP, Koblansky AA and Ghosh S:
Recognition and signaling by toll-like receptors. Annu Rev Cell Dev
Biol. 22:409–437. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gonzalez RMS, Shehata H, O'connell MJ,
Yang Y, Moreno-Fernandez ME, Chougnet CA and Aliberti J: Toxoplasma
gondii-derived profilin triggers human toll-like receptor
5-dependent cytokine production. J Innate Immun. 6:685–694.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zeng HM, Pan KF, Zhang Y, Zhang L, Ma JL,
Zhou T, Su HJ, Li WQ, LI JY, Gerhard M, et al: Genetic variants of
toll-like receptor 2 and 5, helicobacter pylori infection, and risk
of gastric cancer and its precursors in a Chinese population.
Cancer Epidemiol Biomarkers Prev. 20:2594–2602. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Seok H, Lee BC, Kim YO and Chung JH:
Association between Exonic SNPs of TLR5 and Benign Prostate
Hyperplasia in Korean Population. Korean J Str Res. 21:331–337.
2013.
|
|
11
|
Rutkowski MR, Stephen TL, Svoronos N,
Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E,
Escovar-Fadul X, Nguyen JM, Cadungog MG, et al: Microbially driven
TLR5-dependent signaling governs distal malignant progression
through tumor-promoting inflammation. Cancer Cell. 27:27–40.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghasemali S, Nejati-Koshki K, Tafsiri E,
Rahmati-Yamchi M, Akbarzadeh A, Alizadeh E, Abbasi M, Barkhordari
A, Tozihi M, Kordi S and Zarghami N: Inhibitory effects of
beta-cyclodextrin-helenalin complexes on H-TERT gene expression in
the T47D breast cancer cell line-results of real time quantitative
PCR. Asian Pac J Cancer Prev. 14:6949–6953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jha V, Al-Ghamdi SMG, Li G, Wu MS,
Stafylas P, Retat L, Card-Gowers J, Barone S, Cabrera C and Sanchez
JJ: Global economic burden associated with chronic kidney disease:
A pragmatic review of medical costs for the Inside CKD research
programme. Adv Ther. 40:4405–4420. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Robert F and Pelletier J: Exploring the
impact of single-nucleotide polymorphisms on translation. Front
Genet. 9(507)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shuang C, Weiguang Y, Zhenkun F, Yike H,
Jiankun Y, Jing X, Xinghan L, Yue L and Dalin L: Toll-like receptor
5 gene polymorphism is associated with breast cancer
susceptibility. Oncotarget. 8:88622–88629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chaichana P, Chantratita N, Brod F,
Koosakulnirand S, Jenjaroen K, Chumseng S, Sumonwiriya M, Burtnick
MN, Brett PJ, Teparrukkul P, et al: A nonsense mutation in TLR5 is
associated with survival and reduced IL-10 and TNF-α levels in
human melioidosis. PLoS Negl Trop Dis. 11(e0005587)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hawn TR, Verbon A, Lettinga KD, Zhao LP,
LI SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, et
al: A common dominant TLR5 stop codon polymorphism abolishes
flagellin signaling and is associated with susceptibility to
legionnaires' disease. J Exp Med. 198:1563–1572. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meena NK, Ahuja V, Meena K and Paul J:
Association of TLR5 gene polymorphisms in ulcerative colitis
patients of North India and their role in cytokine homeostasis.
PLoS One. 10(e0120697)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klimosch SN, Foersti A, Eckert J, Knežević
J, Bevier M, von Schoenfels W, Heits N, Walter J, Hinz S, Lascorz
J, et al: Functional TLR5 genetic variants affect human colorectal
cancer SurvivalTLR variants affect colorectal cancer survival.
Cancer Res. 73:7232–7242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao W and Liu Z, Lin J, Xiong C, Li J, Wu
K, Ma Y, Gong Y and Liu Z: Association of TLR4 and TLR5 gene
polymorphisms with Graves' disease in Chinese Cantonese population.
Hum Immunol. 75:609–613. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cao L, Zhang T, Zhu J, Li A, Zheng K,
Zhang N, Su B, Xia W, Wu H, Li N and He Q: Polymorphism of TLR 5
rs5744174 is associated with disease progression in Chinese
patients with chronic HBV infection. APMIS. 125:708–716.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gharadaghi Y, Shojaee S, Khaki A,
Fathiazad F, Khaki AA, Ghdamkheir E and Rouhaninia M: Antiprotozoal
effect of Allium cepa on acute renal failure caused by Toxoplasma
gondii. Afr J Pharm Pharmacol. 6:771–777. 2012.
|
|
24
|
Dabagh-Gorjani F, Anvari F, Zolghadri J,
Kamali-Sarvestani E and Gharesi-Fard B: Differences in the
expression of TLRs and inflammatory cytokines in pre-eclamptic
compared with healthy pregnant women. Iran J Immunol. 11:233–245.
2014.PubMed/NCBI
|
|
25
|
Tbahriti HF, Meknassi D, Moussaoui R,
Messaoudi A, Zemour L, Kaddous A, Bouchenak M and Mekki K:
Inflammatory status in chronic renal failure: The role of
homocysteinemia and pro-inflammatory cytokines. World J Nephrol.
2:31–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao XY and Ewald SE: The molecular
biology and immune control of chronic Toxoplasma gondii infection.
J Clin Invest. 130:3370–3380. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meira CS, Pereira-Chioccola VL, Vidal JE,
de Mattos CCB, Motoie G, Costa-Silva TA, Gava R, Frederico FB, de
Mattos LC and Groups T: Cerebral and ocular toxoplasmosis related
with IFN-γ, TNF-α, and IL-10 levels. Front Microbiol.
5(492)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Salem MS, Shaker MJ and Mohammed NK:
Impact of toxoplasmosis in im-mune respons in hemodialysis
patients. Diyala J Medicine. 22:1–11. 2022.
|