1. Introduction
The study of nanotechnology is an intriguing field
of current research that is primarily concerned with the
production, alteration and application of minuscule particle
structures. These structures usually vary in size from ~1 to 100
nanometers. Nanotechnology presents a novel method of technological
progress that involves controlling materials on an extremely small
scale, which is equivalent to one billionth of a meter (1-3).
The field of nanotechnology is a captivating area of expertise that
encompasses physics, chemistry, engineering and biology. Over the
past few years, nanotechnologies have displayed encouraging
outcomes in the domain of human health, especially in the cure of
cancer (4).
Nanotechnology is dependent on the production and
modulation of nanoparticles (NPs); this process involves marked
changes in the properties of metals formed as by-products of
combustion reactions. The peculiar properties of NPs render them
ideally suited for the design of electrochemical sensors and
biosensors (5). At this size,
atoms and molecules function differently and have various
unexpected and fascinating applications. It provides resources for
the production of products, including medical applications
(6). NPs have been used in
numerous fields, including cosmetics, food medicine and genetics,
and have led to a number of discoveries, including fluorescent
biological markers, DNA structure testing, tissue engineering,
tumor destruction, separation and purification of biological
molecules, anticancer agents to tumor sites (7,8). NPs
also have antimicrobial activity against pathogenic bacteria, such
as multidrug-resistant pathogens (4,9,10).
NPs can be synthesized by using various methods,
including physical and chemical approaches. The key physical
methods employed to synthesize NPs are evaporation-condensation and
laser ablation. Physical synthesis methods provide advantages, such
as the absence of solvent contamination in the produced thin films
and the uniform distribution of NPs. However, physical methods can
be costly, and only a small quantity of powder is produced each
time. On the other hand, chemical reduction by organic and
inorganic reducing agents is the most common approach for
synthesizing NPs using chemical methods; while chemical methods can
be expensive, they have low yields and use toxic chemicals
(11). Due to issues associated
with physical and chemical methods, scientists have turned to
biological methods of synthase; biological methods (also known as
biosynthesis, green synthesis, biogenic synthesis and biofabricate)
provide an environmentally benign, low toxic, cost-effective and
efficient protocol to synthesize and fabricate NPs (9,10).
These methods employ microorganisms, such as bacteria, fungi,
viruses, yeast, actinomycetes, or their by-product and plant
extract (12,13).
Fungi are an extraordinary group of microorganisms
that are capable of producing a vast array of metabolites. This
renders them an ideal candidate for the biogenic synthesis of
nanoparticles. The reason behind this is that fungi secrete a
substantial amount of extracellular proteins that assist in
stabilizing the negative charge of NPs (14,15).
Fungal NPs can be effectively synthesized using Fusarium
species due to their filamentous nature and easy extraction from
plants and soil. This genus is well-studied and can grow on a
simple medium at moderate temperatures, rendering it a popular
choice for nanoparticle synthesis. The medical applications of NPs
synthesized through the Fusarium-mediated method are
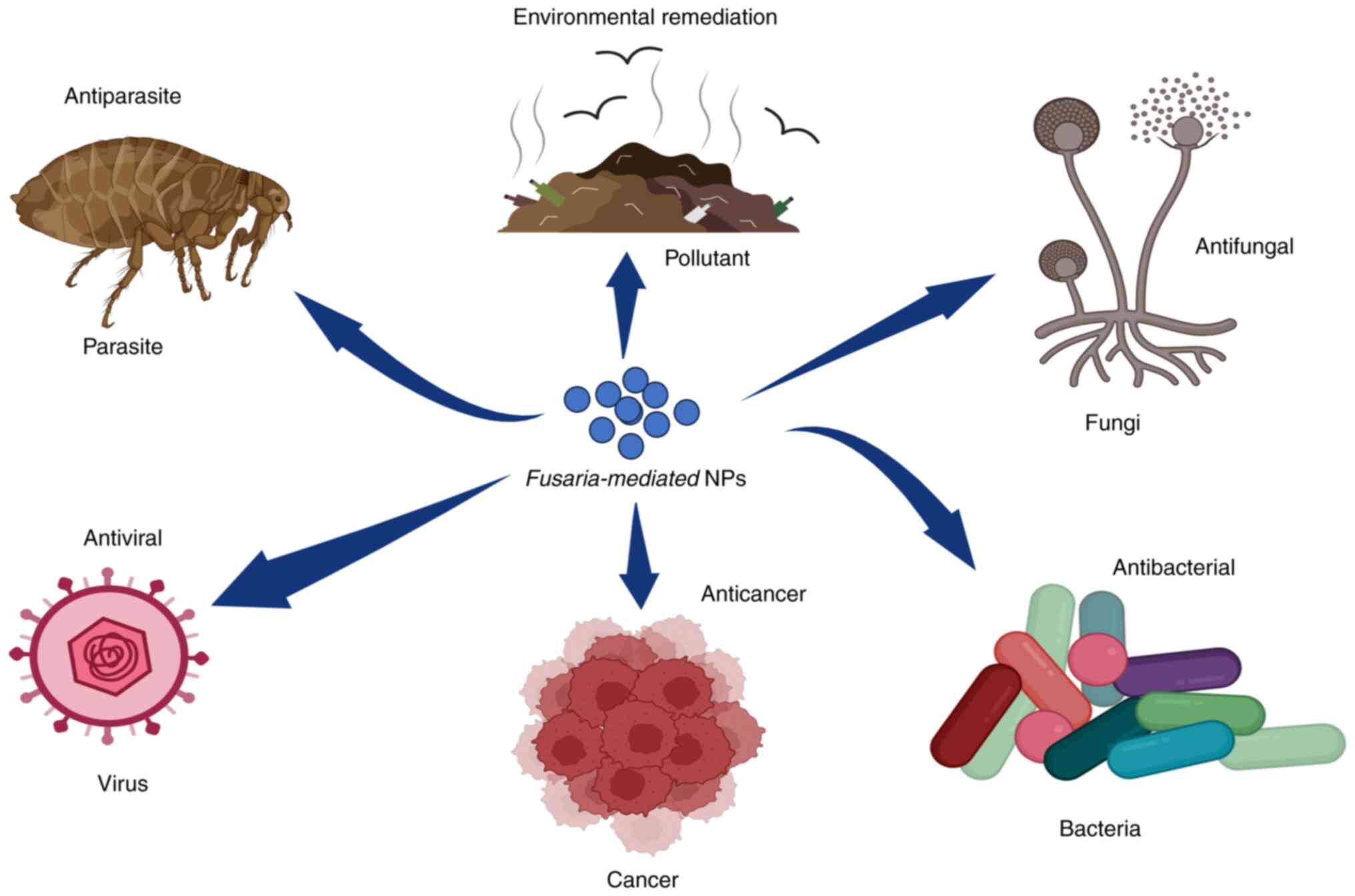
illustrated in (Fig. 1) (16,17).
The present review discusses the synthesis of NPs
using Fusarium species and their potential as antimicrobial
agents against multidrug-resistant Gram-negative bacteria. In
addition, the current methods for NP characterization are
summarized and the mechanisms through which these NPs exert their
antimicrobial activity are discussed.
2. Fusaria as a key element for the
construction of nanoparticles
NPs synthesized from fungal sources are used as
novel antibacterial and antifungal agents (18). Fusarium is a filamentous,
well-studied genus that is widely distributed on plants and in
soil. It is easy to isolate this bacterium from soil and it grows
on simple media at normal temperatures, as it is not a fastidious
microorganism (19,20). Of note, one method which can be
used to synthesize NPs from the mycelia of Fusarium spp.
fungus would involve growth in Erlenmeyer flasks filled with potato
dextrose broth. The flasks would be kept at an ideal temperature of
25±2˚C for 72 h. Once the growth is complete, the mycelia would be
collected by filtering them through Whatman filter paper to
separate them from the medium and other components. The collected
mycelia would then be purified and washed several times with
distilled water (21,22).
Subsequently, a suspension would be made by blending
the mycelia with distilled water and incubating it again at 25±2˚C
for 24 h. After the incubation period was complete, the cell
filtrate would be separated by filtration and then treated with
varying concentrations of metal salts. The mixture would be left to
incubate at room temperature until a noticeable color change was
observed (21,22). A list of the Fusarium
species that are used in the production of NPs are presented in
Table I.
 | Table ISynthesis of nanoparticles from
different Fusarium species. |
Table I
Synthesis of nanoparticles from
different Fusarium species.
| Species | Nanoparticles | Size | Shape | Application | (Refs.) |
|---|
| F.
acuminatum | Silver | 5-40 | Spherical | Antibacterial | (49) |
| F.
culmorum | Silver | 5-25 | Spherical | Antibacterial | (16) |
| F.
chlamydosporum | Silver | 6-26 | Spherical | Antifungal | (50) |
| F.
equiseti | Silver | 85.74 | Spherical | Antibacterial | (48) |
| F.
graminaerum | Silver | 40-50 | Spherical | Antibacterial | (51) |
| F.
keratoplasticum | Silver | 6-36 | Spherical | Antimicrobial
Protector for cotton fabric | (52) |
| F.
mangiferae | Silver | 25-52 | Spherical | Antigrowth Ant
biofilm Cytotoxicity | (26) |
| F.
oxysporum | Silver | 10-25 | Spherical | Antibacterial | (53) |
| | Silver | 20-50 | Spherical | - | (54) |
| | Silver | 5-13 | Spherical | Antibacterial
Cytotoxicity | (55) |
| | Silver | 21.3-37.3 | Spherical | Antimicrobial | (24) |
| | Silver | 8-25 | Spherical | Antibacterial
Antifungal | (21) |
| F.
oxysporum | Silver | 5-15 | - | - | (56) |
| | Silver | 1-50 | Spherical | Antibacterial | (57) |
| | Silver | 30-36.1 | Spherical | Antibacterial | (58) |
| | Platinum | 5-30 | Spherical | - | (59) |
| | Platinum
Platinum | 25 | - | Antimicrobial,
antioxidant photocatalytic | (60) |
| | Platinum | 10-100 | Hexagons pentagons
circle squares rectangles | - | (61) |
| | Zinc | 42 | Spherical | - | (62) |
| | Gold | - | Spherical
Hexagonal | Antibacterial | (63) |
| | Gold | 20-50 | Spherical,
hexagonal | Nano toxicity | (64) |
| | Gold | - | - | - | (23) |
| F.
pseudonygamai | Silver | 5-20 | Almost
spherical | Antibacterial
Anti-biofilm Antioxidant Cytotoxicity | (65) |
| | Gold | 8-60 | Spherical | Antibacterial
Anti-biofilm Antioxidant Cytotoxicity | |
| F.
scirpi | Silver | 2-20 | Quasispherical | Antibacterial | (66) |
| F.
solani | Gold | 40-45 | - | Anticancer
Biomedical applications | (67) |
| F.
solani | Gold | 20-50 | Spherical | - | (68) |
| | Silver | 5-35 | Spherical | - | (69) |
| | Silver | 130.6 | Spherical | - | (48) |
| | Silver | 8.27 | Spherical | Agriculture Seed
germination Seedling growth promoters | (70) |
| | Silver | 7.65-18.89 | Spherical | Antimicrobial | (71) |
| | Copper | 9.97-19.49 | Spherical | Antimicrobial | |
| | Zink | 8.55-21.76 | Spherical | Antimicrobial | |
| | Zink | 117.79-175.12 | Irregular | Agriculture Seed
germination Seedling growth promoters | (70) |
| F.
semitectum | Silver | 8-50 | Spherical
ellipsoidal | Antibacterial | (72) |
| | Silver | 10-60 | Spherical | - | (73) |
| | Silver | 5-30 | Spherical | Treatment of
grain-born fungi | (74) |
| | Silver | 130.6 | Spherical | Antibacterial | (48) |
| | Silver | 5-35 | Spherical | - | (69) |
| | Silver and
gold | 10-35 | Spherical | - | (75) |
| | Silver | 18-80 | Spherical | - | |
3. Characterization of nanoparticles
There are numerous techniques used to detect the
presence of NPs. The main techniques used for the characterization
of NPs are presented in Table II.
The primary technique for detecting the formation of silver NPs
(AgNPs) or gold NPs is visual observation. For instance, when the
fungal cell filtrate changes from yellowish to dark brown, it
indicates the formation of silver NPs. Similarly, the solution
color changes from yellow to dark red when gold NPs are formed
(18,23,24).
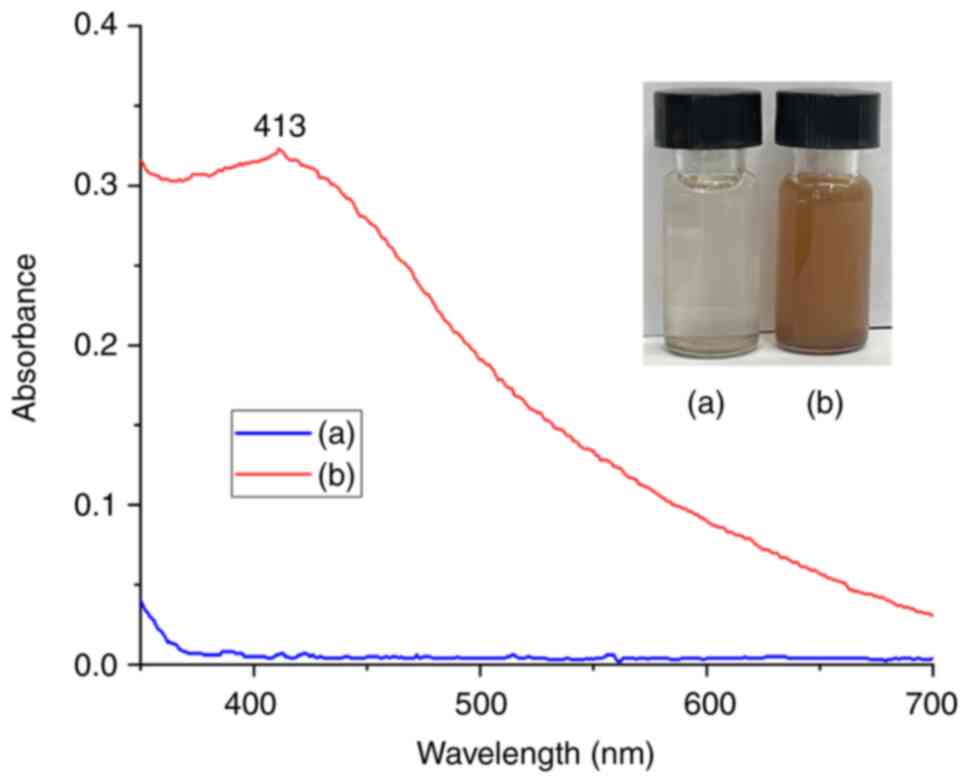
UV-visible absorption spectra of AgNPs are presented in Fig. 2 [only fungal extract and silver
nitrate (AgNO3) were used as controls in this case].
 | Table IIThe main techniques used for the
characterization of nanoparticles. |
Table II
The main techniques used for the
characterization of nanoparticles.
| Technique | Purpose | (Refs.) |
|---|
| TEM | It uses an electron
beam to image a nanoparticle sample | (76) |
| SEM | Scans a sample with
an electron beam to produce a magnified image for analysis | (25) |
| UV-Vis | Identifies the
absorption of ultraviolet light or visible light by chemical
compounds | (27) |
| XRD | Determines the
crystallographic structure of a material | (77) |
| FTIR | Identifies chemical
bonds in a molecule by producing an infrared absorption
spectrum | (78) |
| EDX | Identifies the
elemental composition of materials | (79) |
| Zeta potential | Determines the
surface charge of nanoparticles in a solution | (80) |
| AFM | Assists for the
visualization and measurement of nanostructures | (81) |
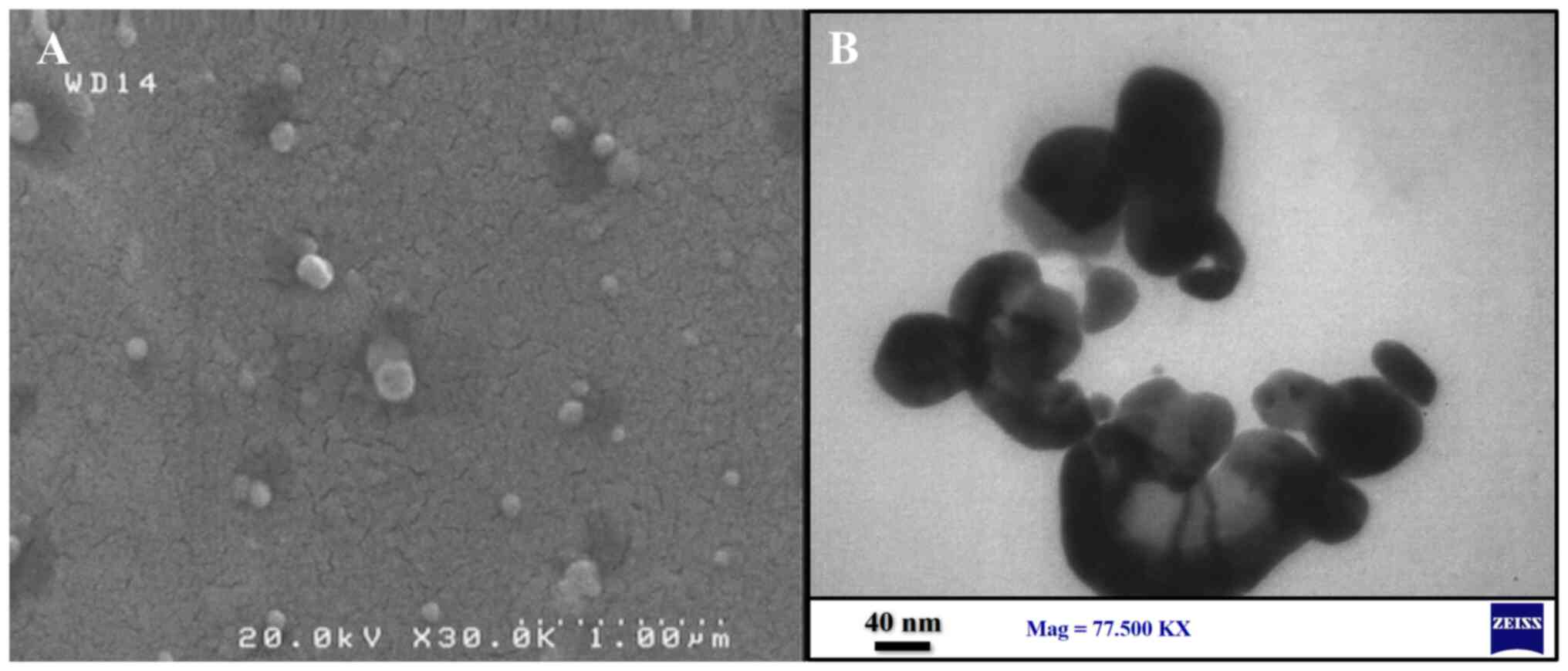
The scanning electron microscope (SEM) is a highly
versatile instrument that allows for the examination and analysis
of microstructure, morphology and chemical composition. The naked
eye can only distinguish objects subtending about 1/60˚ visual
angle, corresponds to a resolution of ~0.1 mm (when viewed from an
optimal distance of 25 cm). Optical microscopy can enlarge the
visual angle through its lens, although it has a resolution limit
of ~2,000 Å (25). The NPs are
observed by transmission electron microscopy (TEM) characterization
and are cleaned through plasma treatment using oxygen for <1
min. The sample is placed on the grid and allowed to dry at room
temperature. The samples are then inspected by operating at 120 KV
(16). An example of the
characterization of NPs using both SEM and TEM is illustrated in
Fig. 3.
The UV-visible spectrophotometer is a method used to
detect NPs shown in Fig. 2. To
analyze the NPs, their liquid samples were scanned in the range of
200-800 nm, with fungal filtrate and AgNO3 used as
controls. A scattering cell was used in this range, through which a
laser beam (~40 mW at k=635 nm) was passed. To observe the
nanoparticles via the path of the laser beam, a dedicated
no-microscope optical instrument (LM-20, NanoSight) was used, which
has a charge-coupled device camera. The motion of the particles in
the field of view (~100x100 µm) was recorded (at 30 fps), and the
subsequent video and images were analyzed to determine the size
distribution of the nanoparticles (26,27).
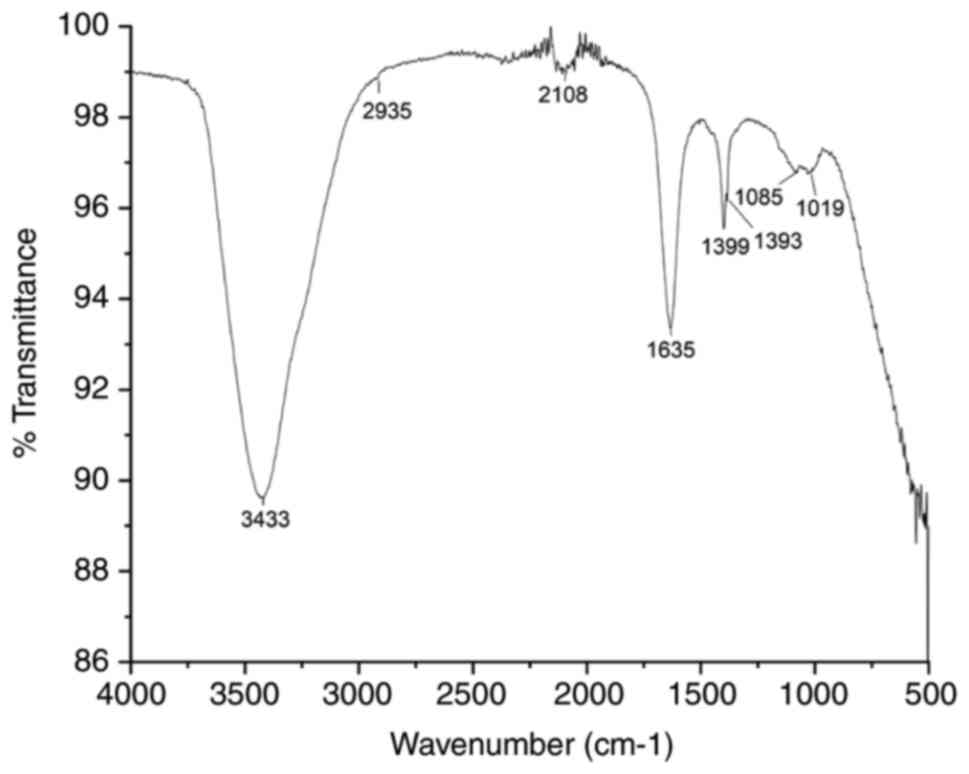
The characterization of NPs can also be carried out
using Fourier transform infrared spectroscopy (FTIR). FTIR aims to
analyze the biomolecules responsible for reducing silver ions and
stabilizing NPs in the solution, as illustrated in Fig. 4. For the sample preparation, a
colloidal NP solution would be mixed with potassium bromide (KBr)
in a clean crucible until a fine powder is produced. The dried
powder of NPs is then prepared and dried in an oven to remove any
traces of moisture and analyzed in the ranges of 1,000-2,000
cm-1 at a resolution of 4 cm-1 (16).
4. Fusaria nano-weapons against
Gram-negative pathogens
Due to the discovery that some NPs display
intriguing antibacterial properties, there has been an increased
interest in the manufacture and research of NPs in recent years
(8,28).
Antimicrobial resistance poses a major threat to
humanity and one of the most severe health crises of the current
era. Certain bacterial strains have become resistant to almost all
antibiotics, thus rendering it crucial to identify new
antibacterial drugs to fight these microorganisms (29,30).
In 2017, the World Health Organization (WHO) released a list of
priority antibiotic-resistant illnesses, divided into three
categories: Critical, high and medium. The majority of the bacteria
on the list are Gram-negative pathogens, which are more resistant
than Gram-positive bacteria due to their unique structure. This has
resulted in a significant global disease and mortality burden
(31-33).
Gram-negative bacteria have developed various
mechanisms to resist a wide range of antibiotics, such as
tetracycline, aminoglycosides and cotrimoxazole. The development of
nano-sized particles with antibacterial properties is highly
desirable for the creation of novel pharmaceuticals (28,34).
Consequently, scholars are actively exploring alternatives to
conventional antibiotics in response to rising antibiotic
resistance. Their investigations encompass a diverse range of
solutions, including plant extracts known for their antimicrobial
properties, the development of new antibiotic derivatives with
enhanced efficacy, and the use of NPs that can target bacteria more
precisely (35,36). However, there has been insufficient
research conducted on the toxicity of NPs, particularly regarding
their mechanisms of action. This is a matter of concern,
particularly as the field of nanomedicine continues to grow. In
recent decades, NPs have been widely used in various industries,
including as food additives and for drug delivery purposes
(37). With the persistent rise of
bacterial resistance, there is a growing need for the development
of new antibiotics. One of the most promising emerging antibiotic
drugs is metal NPs, which have demonstrated potent antibacterial
action in the majority of trials (7,38).
In general, smaller NPs tend to exhibit greater
antibacterial activity. However, there are conflicting findings
regarding the effectiveness of larger-sized NPs, and size alone is
not always the most critical factor in determining their toxicity.
Other factors that can affect the antibacterial properties of NPs
include the formulation process, the surrounding environment,
bacterial defense mechanisms and the physical properties of the NPs
themselves (39).
NPs that are smaller in size have a higher surface
area-to-volume ratio compared with larger NPs. This explains why
they are more toxic than larger ones. A larger surface area of
small NPs increases the proportion of contact with bacterial cells.
NPs <10 nm in size have a higher proportion of contact with
bacteria. The interaction between the NP and bacterial surface
causes an electrical impact that enhances the reactivity of NPs
(38,40,41).
Various-sized silver NPs have different antimicrobial activities
against different Gram-negative pathogenic bacteria, as illustrated
in Table III.
 | Table IIIDifferent Fusaria species
against Gram-negative pathogens. |
Table III
Different Fusaria species
against Gram-negative pathogens.
| Species | Nanoparticle | Gram-negative
pathogen | Size (nm) | Concentration
(µg/ml) | Inhibition zone
(mm) | (Refs.) |
|---|
| F.
acuminatum | Silver | Escherichia
coli | 5-40 | 20 | 10 mm | (49) |
| | | Salmonella
typhi | 5-40 | 20 | 17 mm | |
| F.
culmorum | Silver | Klebsiella
pneumoniae | 5-25 | 20 | 16 | (16) |
| | | Enterobacter
aerogenes | 5-25 | 20 | 17 | |
| F.
graminaerum | Silver | Pseudomonas
aeruginosa | 40-50 | 20-50 | 12-14.5 | (51) |
| | | Salmonella
sp. | 40-50 | 20-50 | 7.3-9.5 | |
| | | E. coli | 40-50 | 20-50 | 7.5-8 | |
| F.
oxysporum | Silver | E. coli | 10-25 | 100 | | (53) |
| F.
semitectum | Silver | K.
pneumonia | 8-50 | 50 | 16 | (24) |
| | | P.
aeruginosa | 8-50 | 50 | 15 | |
| | | K.
pneumonia | 8-50 | 50 | 16 | |
| | | P.
aeruginosa | 8-50 | 50 | 15 | (72) |
| F.
solani | Silver | E. coli | 130 | 50 | 7 | (48) |
| | | Pseudomonas
sp. | 130 | 50 | 11 | |
| | | Klebsiella
sp. | 130 | 50 | 15 | |
| F.
equiseti | Silver | E. coli | 85.74 | 50 | 7 | (48) |
| | | Pseudomonas
sp. | 85.74 | 50 | 11 | |
| | | Klebsiella
sp. | 85.74 | 50 | 7 | |
5. Effect of Fusaria
nanoparticles
There are various theories regarding the specific
mechanisms of the antibacterial action of AgNPs. However, the
precise mechanisms involved continue to be under investigation.
Some proposed possibilities include the generation of free metal
ion toxicity from the surface of synthesized nano-metals and
oxidative stress from the reactive oxygen species (ROS) on the
surface of NPs (42-44).
NPs can cause a deletion activity on the cell wall
of organisms, reduce oxidative stress, inactivate protein
synthesis, penetrate the cell membrane and modify essential
proteins, thereby increasing cell signal processes and hindering
the formation of biofilm (17,45).
The mode of action of NPs in damaging the bacterial membrane,
bacterial protein and bacterial DNA remains a topic of research.
The possible mechanism relies on the interaction of NPs with
bacteria, excessive ROS generation and the precipitation of NPs on
the bacterial exterior; which disrupts the cellular activities,
resulting in membrane disruption (44,46,47).
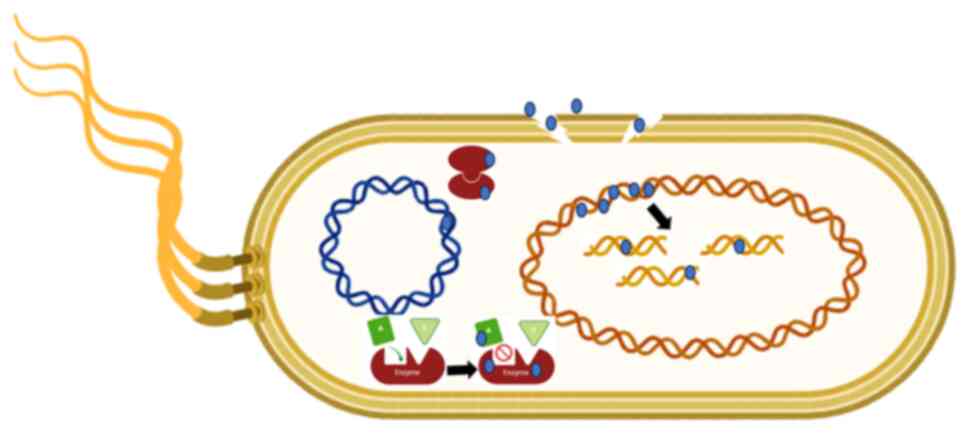
NPs can cause intracellular alterations by
inhibiting DNA replication ability. NPs which are <10 nm in size
can diffuse into the nucleus, causing DNA damage, chromosomal
abnormalities and cell cycle arrest, leading to genotoxicity in
human cell lines (17,48).
NPs have different mechanisms against Gram-negative
pathogens. Nano-silver can interact with the bacterial membrane,
which is considered the main mechanism for its antimicrobial
toxicity. NPs anchor themselves to the bacterial membrane,
penetrate it and trigger the destruction of the cell membrane. This
can lead to the release of ions into the intracellular medium,
which further increases the toxicity level. NPs located on an
Escherichia coli membrane can hold fast to the bacterial
cell wall, enter it, and cause structural changes in the cell film
such as the penetrability of the cell layer (17,48).
Copper NPs and zinc oxide NPs can also cause damage to the
bacterial membrane (17,43).
Gold NPs, for example, exploit their antibacterial
powers against multidrug-resistant (Gram-negative) bacteria via two
ways: Degrading membrane potential to lower ATP levels by reducing
ATPase activity and preventing ribosomal subunit interaction with
tRNA (43).
Metal reacts with sulfhydryl group proteins in
cells, inactivating the proteins. Silver ions and AgNPs can
interact with chemical groups like sulfide and chloride (43). NPs have different mechanisms of
action against Gram-negative pathogens, as illustrated in Fig. 5).
NPs derived from Fusarium species provide a
cost-effective and eco-friendly alternative to traditional physical
and chemical synthesis methods, demonstrating prominent
antimicrobial properties, particularly against Gram-negative
bacteria. They can be easily produced using simple media, rendering
them an appealing option for large-scale manufacturing. However,
challenges include variations in size and stability, the potential
for fungal contamination, and the necessity for comprehensive
toxicity assessments to guarantee safe clinical application.
6. Conclusion and future perspectives
The present mini-view demonstrates the immense
promise of biological synthesis for the production of NPs. In
particular, the production of NPs using fungi that are produced
from Fusarium species has a wide range of applications.
These NPs are noteworthy for their potent antibacterial action
against a range of pathogenic bacteria, including those that show
resistance to several drugs.
Further research on the mechanisms of action of NPs
produced from Fusarium against bacteria resistant to several
drugs is essential going forward. By conducting thorough research
in this field, it is possible to deepen the understanding of the
underlying mechanisms and develop more effective strategies to
address the increasing problem of antibiotic resistance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (RB, RT, HH and KQ) contributed equally
to the preparation and design of the manuscript. RB, RT and HH were
involved in the conception and design of the study. All authors
(RB, RT, HH and KQ) participated in articulating the content and in
drafting and editing the manuscript. Specifically, RB, HH, and KQ
focused on manuscript editing, while HH and KQ handled the
technical aspects. RB, RT and KQ organized the data included in the
review. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kader DA, Aziz DM, Mohammed SJ, Maarof
NNN, Karim WO, Mhamad SA, Rashid RM, Ayoob MM, Kayani KF and
Qurbani K: Green synthesis of ZnO/catechin nanocomposite:
Comprehensive characterization, optical study, computational
analysis, biological applications and molecular docking. Mater Chem
Phys. 319(129408)2024.
|
|
2
|
Qurbani KA, Amiri O, Othman GM, Fatah AA,
Yunis NJ, Joshaghani M, Ahmed S and Abdulrahman N: Enhanced
antibacterial efficacy through piezo memorial effect of CaTiO3/TiO2
nano-composite. Inorg Chem Commun. 165(112470)2024.
|
|
3
|
Ibrahim WM, Amiri O, Ahmed SS, Muhammed
HY, Mahmood PH, Qurbani KA, Abdulrahman NA, Younis KA and Omer PK:
Enhanced asphaltene degradation using piezocatalytic technology: A
novel approach for sustainable oilfield operations. Results Eng.
21(101938)2024.
|
|
4
|
Bayda S, Adeel M, Tuccinardi T, Cordani M
and Rizzolio F: The history of nanoscience and nanotechnology: From
chemical-physical applications to nanomedicine. Molecules.
25(112)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alagarasi A: Chapter-introduction to
nanomaterials. Indian Institute of Technology Madras, pp1-24,
2013.
|
|
6
|
Singh P, Kim YJ, Zhang D and Yang DC:
Biological synthesis of nanoparticles from plants and
microorganisms. Trends Biotechnol. 34:588–599. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Salata OV: Applications of nanoparticles
in biology and medicine. J Nanobiotechnology. 2(3)2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kyriacou SV, Brownlow WJ and Xu XHN: Using
nanoparticle optics assay for direct observation of the function of
antimicrobial agents in single live bacterial cells. Biochemistry.
43:140–147. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hussein S, Sulaiman S, Ali S, Pirot R,
Qurbani K, Hamzah H, Hassan O, Ismail T, Ahmed SK and Azizi Z:
Synthesis of silver nanoparticles from aeromonas caviae for
antibacterial activity and in vivo effects in rats. Biol Trace Elem
Res. 202:2764–2775. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qurbani K, Hussein S, Hamzah H, Sulaiman
S, Pirot R, Motevaseli E and Azizi Z: Synthesis of silver
nanoparticles by raoultella planticola and their potential
antibacterial activity against multidrug-resistant isolates. Iran J
Biotechnol. 20(e3121)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dhand C, Dwivedi N, Loh XJ, Ying AJ, Verma
NK, Beuerman RW, Lakshminarayanan R and Ramakrishna S: Methods and
strategies for the synthesis of diverse nanoparticles and their
applications: A comprehensive overview. RSC Adv. 5:105003–105037.
2015.
|
|
12
|
Hulkoti NI and Taranath TC: Biosynthesis
of nanoparticles using microbes-a review. Colloids Surf B
Biointerfaces. 121:474–483. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iravani S, Korbekandi H, Mirmohammadi SV
and Zolfaghari B: Synthesis of silver nanoparticles: Chemical,
physical and biological methods. Res Pharm Sci. 9:385–406.
2014.PubMed/NCBI
|
|
14
|
Molnár Z, Bódai V, Szakacs G, Erdélyi B,
Fogarassy Z, Sáfrán G, Varga T, Kónya Z, Tóth-Szeles E, Szűcs R and
Lagzi I: Green synthesis of gold nanoparticles by thermophilic
filamentous fungi. Sci Rep. 8(3943)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guilger-Casagrande M and de Lima R:
Synthesis of silver nanoparticles mediated by fungi: A review.
Front Bioeng Biotechnol. 7(287)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bawaskar M, Gaikwad S, Ingle A, Rathod D,
Gade A, Duran N, Marcato PD and Rai M: A new report on
mycosynthesis of silver nanoparticles by Fusarium culmorum.
Curr Nanosci. 6:376–380. 2010.
|
|
17
|
Rai M, Bonde S, Golinska P,
Trzcińska-Wencel J, Gade A, Abd-Elsalam KA, Shende S, Gaikwad S and
Ingle AP: Fusarium as a novel fungus for the synthesis of
nanoparticles: Mechanism and applications. J Fungi (Basel).
7(139)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Birla SS, Gaikwad SC, Gade AK and Rai MK:
Rapid synthesis of silver nanoparticles from Fusarium
oxysporum by optimizing physicocultural conditions.
ScientificWorldJournal. 2013(796018)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kalman B, Abraham D, Graph S, Perl-Treves
R, Meller Harel Y and Degani O: Isolation and identification of
Fusarium spp., the causal agents of onion (Allium cepa)
basal rot in Northeastern Israel. Biology (Basel).
9(69)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qurbani K and Hamzah H: Intimate
communication between Comamonas aquatica and Fusarium solani
in remediation of heavy metal-polluted environments. Arch
Microbiol. 202:1397–1406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Joshi P, Bonde S, Gaikwad S, Gade A,
Abd-Elsalam K and Rai M: Comparative studies on synthesis of silver
nanoparticles by Fusarium oxysporum and Macrophomina
phaseolina and it's efficacy against bacteria and Malassezia
furfur. J Bionanosci. 7:378–385. 2013.
|
|
22
|
Husseiny SM, Salah TA and Anter HA:
Biosynthesis of size controlled silver nanoparticles by
Fusarium oxysporum, their antibacterial and antitumor
activities. Beni Suef Univ J Basic Appl Sci. 4:225–231. 2015.
|
|
23
|
Mukherjee P, Senapati S, Mandal D, Ahmad
A, Khan MI, Kumar R and Sastry M: Extracellular synthesis of gold
nanoparticles by the fungus Fusarium oxysporum. Chembiochem.
3:461–463. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ahmed AA, Hamzah H and Maaroof M:
Analyzing formation of silver nanoparticles from the filamentous
fungus Fusarium oxysporum and their antimicrobial activity.
Turk J Biol. 42:54–62. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou W, Apkarian R, Wang ZL and Joy D:
Fundamentals of scanning electron microscopy (SEM). In: Scanning
Microscopy for Nanotechnology: Techniques and applications. Zhou W
and Wang ZL (eds). Springer, New York, NY, pp1-40, 2007.
|
|
26
|
Hamzah HM, Salah RF and Maroof MN:
Fusarium mangiferae as new cell factories for producing
silver nanoparticles. J Microbiol Biotechnol. 28:1654–1663.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Penner MH: Ultraviolet, visible, and
fluorescence spectroscopy. In: Nielsen SS (ed) Food Analysis. Food
Science Text Series. Springer, pp89-106, 2017.
|
|
28
|
Guzman M, Dille J and Godet S: Synthesis
and antibacterial activity of silver nanoparticles against
gram-positive and gram-negative bacteria. Nanomedicine. 8:37–45.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ahmed SK, Hussein S, Qurbani K, Ibrahim
RH, Fareeq A, Mahmood KA and Mohamed MG: Antimicrobial resistance:
Impacts, challenges, and future prospects. J Med Surg Public
Health. 2(100081)2024.
|
|
30
|
Qurbani K, Ali S, Hussein S and Hamzah H:
Antibiotic resistance in Kurdistan, Iraq: A growing concern. New
Microbes New Infect. 57(101221)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livermore DM: Current epidemiology and
growing resistance of gram-negative pathogens. Korean J Intern Med.
27:128–142. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
World Health Organization: Global
diffusion of eHealth: making universal health coverage achievable:
Report of the third global survey on eHealth. World Health
Organization, 2017.
|
|
33
|
Breijyeh Z, Jubeh B and Karaman R:
Resistance of gram-negative bacteria to current antibacterial
agents and approaches to resolve it. Molecules.
25(1340)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pfeifer Y, Cullik A and Witte W:
Resistance to cephalosporins and carbapenems in Gram-negative
bacterial pathogens. Int J Med Microbiol. 300:371–379.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Muhammed Aziz D, Hassan SA, Mamand DM and
Qurbani K: New azo-azomethine derivatives: Synthesis,
characterization, computational, solvatochromic UV-Vis absorption
and antibacterial studies. J Mol Struct. 1284(135451)2023.
|
|
36
|
Muhammed Aziz D, Hassan SA, Amin AAM,
Abdullah MN, Qurbani K and Aziz SB: A synergistic investigation of
azo-thiazole derivatives incorporating thiazole moieties: A
comprehensive exploration of their synthesis, characterization,
computational insights, solvatochromism, and multimodal biological
activity assessment. RSC Adv. 13:34534–34555. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Carvalho PM, Felício MR, Santos NC,
Gonçalves S and Domingues MM: Application of light scattering
techniques to nanoparticle characterization and development. Front
Chem. 6(237)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Slavin YN, Asnis J, Hńfeli UO and Bach H:
Metal nanoparticles: Understanding the mechanisms behind
antibacterial activity. J Nanobiotechnology. 15(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Osonga FJ, Akgul A, Yazgan I, Akgul A,
Eshun GB, Sakhaee L and Sadik OA: Size and shape-dependent
antimicrobial activities of silver and gold nanoparticles: A model
study as potential fungicides. Molecules. 25(2682)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rai MK, Deshmukh S, Ingle A and Gade A:
Silver nanoparticles: The powerful nanoweapon against
multidrug-resistant bacteria. J Appl Microbiol. 112:841–852.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Salomoni R, Léo P, Montemor A, Rinaldi B
and Rodrigues M: Antibacterial effect of silver nanoparticles in
Pseudomonas aeruginosa. Nanotechnol Sci Appl. 10:115–121.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gudikandula K and Charya Maringanti S:
Synthesis of silver nanoparticles by chemical and biological
methods and their antimicrobial properties. J Exp Nanosci.
11:714–721. 2016.
|
|
43
|
Nisar P, Ali N, Rahman L, Ali M and
Shinwari ZK: Antimicrobial activities of biologically synthesized
metal nanoparticles: An insight into the mechanism of action. J
Biol Inorg Chem. 24:929–941. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lamri M, Bhattacharya T, Boukid F, Chentir
I, Dib AL, Das D, Djenane D and Gagaoua M: Nanotechnology as a
processing and packaging tool to improve meat quality and safety.
Foods. 10(2633)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu L, Wang YY, Huang J, Chen CY, Wang ZX
and Xie H: Silver nanoparticles: Synthesis, medical applications
and biosafety. Theranostics. 10:8996–9031. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liao S, Zhang Y, Pan X, Zhu F, Jiang C,
Liu Q, Cheng Z, Dai G, Wu G, Wang L and Chen L: Antibacterial
activity and mechanism of silver nanoparticles against
multidrug-resistant Pseudomonas aeruginosa. Int J Nanomedicine.
14:1469–1487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shahzad K and Manzoor F: Nanoformulations
and their mode of action in insects: A review of biological
interactions. Drug Chem Toxicol. 44:1–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Saha P, Rajkumar K and Abraham J:
Comparative study on antimicrobial property of silver nanoparticles
synthesized by Fusarium equiseti and Fusarium solani.
Bionanoscience. 6:28–32. 2012.
|
|
49
|
Ingle A, Gade A, Pierrat S, Sonnichsen C
and Rai M: Mycosynthesis of silver nanoparticles using the fungus
Fusarium acuminatum and its activity against some human
pathogenic bacteria. Curr Nanosci. 4:141–144. 2008.
|
|
50
|
Khalil NM, Abd El-Ghany MN and
Rodríguez-Couto S: Antifungal and anti-mycotoxin efficacy of
biogenic silver nanoparticles produced by Fusarium
chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses.
Chemosphere. 218:477–486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shafiq SA, Al-Shammari RH and Majeed HZ:
Study of biosynthesis silver nanoparticles by Fusarium
graminaerum and test their antimicrobial activity. Int J Innov Appl
Stud. 15:43–50. 2016.
|
|
52
|
Mohmed AA, Fouda A, Elgamal MA, EL-Din
Hassan S, Shaheen TI and Salem SS: Enhancing of cotton fabric
antibacterial properties by silver nanoparticles synthesized by new
Egyptian strain Fusarium keratoplasticum A1-3. Egypt J Chem.
60:63–71. 2017.
|
|
53
|
El Domany E, Essam T, Ahmed A and Farghli
A: Biosynthesis, characterization, antibacterial and synergistic
effect of silver nanoparticles using Fusarium oxysporum. J
Pure Appl Microbiol. 11:1441–1446. 2017.
|
|
54
|
Marcato P, De Souza G, Alves O, Esposito E
and Durán N: Antibacterial activity of silver nanoparticles
synthesized by Fusarium oxysporum strain. In: Proceedings of
2nd Mercosur Congr on Chem. Eng, 4th Mercosur Congr on Process Sys
Eng, pp1-5, 2005.
|
|
55
|
Vijayan S, Divya K, George TK and Jisha
MS: Biogenic synthesis of silver nanoparticles using endophytic
fungi Fusarium oxysporum isolated from Withania somnifera
(L.), its antibacterial and cytotoxic activity. J Bionanosci.
10:369–376. 2016.
|
|
56
|
Ahmad A, Mukherjee P, Senapati S, Mandal
D, Khan MI, Kumar R and Sastry M: Extracellular biosynthesis of
silver nanoparticles using the fungus Fusarium oxysporum.
Colloids Surf B Biointerfaces. 28:313–318. 2003.
|
|
57
|
Srivastava S, Bhargava A, Pathak N and
Srivastava P: Production, characterization and antibacterial
activity of silver nanoparticles produced by Fusarium
oxysporum and monitoring of protein-ligand interaction through
in-silico approaches. Microb Pathog. 129:136–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kaabo HE, Saied E, Hassan SED, Mahdy HM
and Sultan MH: Penicillium oxalicum-mediated the green synthesis of
silica nanoparticles: characterization and environmental
applications. Biomass Conver Biorefin, pp1-18, 2024.
|
|
59
|
Syed A and Ahmad A: Extracellular
biosynthesis of platinum nanoparticles using the fungus
Fusarium oxysporum. Colloids Surf B Biointerfaces. 97:27–31.
2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gupta K and Chundawat TS: Bio-inspired
synthesis of platinum nanoparticles from fungus Fusarium
oxysporum: its characteristics, potential antimicrobial,
antioxidant and photocatalytic activities. Mater Res Express.
6(1050d6)2019.
|
|
61
|
Riddin TL, Gericke M and Whiteley CG:
Analysis of the inter- and extracellular formation of platinum
nanoparticles by Fusarium oxysporum f. sp. lycopersici using
response surface methodology. Nanotechnology. 17:3482–3489.
2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mirzadeh S, Darezereshki E, Bakhtiari F,
Fazaelipoor MH and Hosseini MR: Characterization of zinc sulfide
(ZnS) nanoparticles biosynthesized by Fusarium oxysporum.
Mater Sci Semicond Process. 16:374–378. 2013.
|
|
63
|
Naimi-Shamel N, Pourali P and Dolatabadi
S: Green synthesis of gold nanoparticles using Fusarium
oxysporum and antibac-terial activity of its tetracycline
conjugant. J Mycol Med. 29:7–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Pourali P, Badiee SH, Manafi S, Noorani T,
Rezaei A and Yahyaei B: Biosynthesis of gold nanoparticles by two
bacterial and fungal strains, Bacillus cereus and Fusarium
oxysporum, and assessment and comparison of their nanotoxicity in
vitro by direct and indirect assays. Electron J Biotechnol.
29:86–93. 2017.
|
|
65
|
Soliman MKY, Abu-Elghait M, Salem SS and
Azab MS: Multifunctional properties of silver and gold
nanoparticles synthesis by Fusarium pseudonygamai. Biomass
Convers Biorefin, pp1-18, 2022.
|
|
66
|
Rodríguez-Serrano C, Guzmán-Moreno J,
Ángeles-Chávez C, Rodríguez-González V, Ortega-Sigala JJ,
Ramírez-Santoyo RM and Vidales-Rodríguez LE: Biosynthesis of silver
nanoparticles by Fusarium scirpi and its potential as
antimicrobial agent against uropathogenic Escherichia coli
biofilms. PLoS One. 15(e0230275)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Clarance P, Luvankar B, Sales J, Khusro A,
Agastian P, Tack JC, Al Khulaifi MM, Al-Shwaiman HA, Elgorban AM,
Syed A and Kim HJ: Green synthesis and characterization of gold
nanoparticles using endophytic fungi Fusarium solani and its
in-vitro anticancer and biomedical applications. Saudi J Biol Sci.
27:706–712. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Gopinath K and Arumugam A: Extracellular
mycosynthesis of gold nanoparticles using Fusarium solani.
Appl Nanosci. 4:657–662. 2014.
|
|
69
|
Ingle A, Rai M, Gade A and Bawaskar M:
Fusarium solani: A novel biological agent for the
extracellular synthesis of silver nanoparticles. J Nanoparticle
Res. 11:2079–2085. 2009.
|
|
70
|
Trzcińska-Wencel J, Wypij M, Terzyk AP,
Rai M and Golińska P: Biofabrication of novel silver and zinc oxide
nanoparticles from Fusarium solani IOR 825 and their
potential application in agriculture as biocontrol agents of
phytopathogens, and seed germination and seedling growth promoters.
Front Chem. 11(1235437)2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
El Sayed MT and El-Sayed AS: Biocidal
activity of metal nanoparticles synthesized by Fusarium
solani against multidrug-resistant bacteria and mycotoxigenic
fungi. J Microbiol Biotechnol. 30:226–236. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shelar GB and Chavan AM: Fusarium
semitectum mediated extracellular synthesis of silver nanoparticles
and their antibacterial activity. Int J Biomed Adv Res. 5:20–24.
2014.
|
|
73
|
Basavaraja S, Balaji SD, Lagashetty A,
Rajasab AH and Venkataraman A: Extracellular biosynthesis of silver
nanoparticles using the fungus Fusarium semitectum. Mater
Res Bull. 43:1164–1170. 2008.
|
|
74
|
ABD El-Aziz ARM, Al-Othman MR, Mahmoud MA
and Metwaly HA: Biosynthesis of silver nanoparticles using
Fusarium solani and its impact on grain borne fungi. Dig J
Nanomater Biostruct. 10:655–662. 2015.
|
|
75
|
Dasaratrao Sawle B, Salimath B, Deshpande
R, Dhondojirao Bedre M, Krishnamurthy Prabhakar B and Venkataraman
A: Biosynthesis and stabilization of Au and Au-Ag alloy
nanoparticles by fungus, Fusarium semitectum. Sci Technol
Adv Mater. 9(035012)2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Evans JE, Jungjohann KL, Browning ND and
Arslan I: Controlled growth of nanoparticles from solution with in
situ liquid transmission electron microscopy. Nano Lett.
11:2809–2813. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Bunaciu AA, UdriŞTioiu EG and Aboul-Enein
HY: X-ray diffraction: instrumentation and applications. Crit Rev
Anal Chem. 45:289–299. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ismail AA, van de Voort FR and Sedman J:
Fourier transform infrared spectroscopy: Principles and
applications. Tech Instrum Anal Chem. 18:93–139. 1997.
|
|
79
|
Khan MSI, Oh SW and Kim YJ: Power of
scanning electron microscopy and energy dispersive X-ray analysis
in rapid microbial detection and identification at the single cell
level. Sci Rep. 10(2368)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang Y, Yang M, Portney NG, Cui D, Budak
G, Ozbay E, Ozkan M and Ozkan CS: Zeta potential: A surface
electrical characteristic to probe the interaction of nanoparticles
with normal and cancer human breast epithelial cells. Biomed
Microdevices. 10:321–328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Magonov SN and Reneker DH:
Characterization of polymer surfaces with atomic force microscopy.
Annu Rev Mater Res. 27:175–222. 1997.
|