Introduction
Uncontrolled diabetes is the primary cause for
glycation, which is capable of distorting the cellular skeleton and
architecture of vascular tissue, leading to microvascular and
macrovascular complications (1).
The persistent exposure of cells to the hyperglycemic milieu in
type 2 diabetes mellitus (T2DM) leads to the spontaneous
non-enzymatic glycation of major macromolecules, such as proteins
and lipids (2). The glycation of
proteins is termed as advanced glycation end products (AGEs);
similarly, poly unsaturated fatty acid (PUFA) glycation is termed
as advanced lipoxidation end products (ALEs) (1,3).
Therefore, circulating glucose during hyperglycemia affects
vascular integrity, causing damage to the end organ. One such end
organ examined in the present study is renal tissue, which is
affected by increased ALEs, such as carboxymethyl-lysine (CML). By
decreasing the action of PUFAs and increasing the levels of
pro-inflammatory factors, ALEs are thus formed. Microvascular
complications include diabetes with chronic kidney disease (CKD),
diabetic retinopathy (DR) and diabetic neuropathy (DN).
Macrovascular complications are usually observed in the majority of
cases of cardiovascular disease (CVD).
A well-established glycation marker is glycated
hemoglobin (HbA1c), which reflects the average glucose level for a
period of 120 days (life span of red blood cells) (4). Similarly, glycated albumin, known as
fructosamine, can reflect the average glucose level for a period of
21 days (5). Therefore, long- and
short-term glucose monitoring aids in the prognosis and management
of diabetes mellitus (DM). HbA1c and fructosamine are the
endogenously formed glycated products; nevertheless, CML is
produced in vivo due to the increased consumption of baked,
processed and fried fast food, and is hence known as exogenous CML
(6). Due to the rapid
transformation of lifestyle and food habits in this mechanical era,
the monitoring of AGE levels in blood is essential for preventing
the onset of aging-related disorders, not only diabetes but also
metabolic syndrome, obesity, cancer or thyroid issues.
The following routine parameters are considered for
the diagnosis of DM: Fasting blood glucose, post-prandial blood
glucose and HbA1c; these are considered as the diabetic profile. An
extended diabetic profile includes AGEs, fasting insulin, the
homeostasis model assessment for insulin resistance and the
quantitative insulin sensitivity check index (7). The recommended guidelines for the
diagnosis of diabetes with CKD include the measurement of blood
urea, serum creatinine, sodium and potassium levels, which are
included in renal function tests (8); cystatin C, urine albumin and the
estimated glomerular filtration rate are considered as extended
renal profiling to classify the CKD stage (7). The attrition of the vascular
structure increases following the increased exposure to glucose and
glucose-derived products (9). This
pathological condition causes aging, which is not physiological and
thereby, the reverting changes are not viable. To prevent
irreversible aging, it is recommended that the levels of AGEs are
estimated and markers of aging are determined.
DM is classified as one of the aging-related
disorders due to poor insulin production and reception (3). One of the markers related to aging,
the levels of which are upregulated during calorie restriction, is
Sirtuin1, which is a NAD+-dependent deacetylase enzyme
(10). In prolonged uncontrolled
hyperglycemia, NAD+ are depleted due to impaired insulin
action (11). Apart from glucose
and glucose-derived products, lipid parameters also need to be
managed and controlled to prevent arterial stenosis. Therefore,
lipid parameters and ratios derived from analyses, such as the
atherogenic index of plasma, atherogenic coefficient (AC), Castelli
Risk Index I and II and protein glycation index (PGI) serve as
surrogate markers for preventing either the onset or the
progression of diabetes to microvascular complications (12,13).
Dyslipidemia is either a cause or consequence of diabetic
complications; thus, the inclusion of lipid calculations may aid in
the selection of treatment modalities (13). Several physicians neglect routine
lipid profile analyses, such as HbA1c, which is one of the causes
for increased numbers of dyslipidemia and diabetic
complications.
As per the recent guidelines laid by the KDIGO for
the management of diabetes with CKD, dietary management serves as
the first line of treatment, as opposed to medication (8). The preferred medication is precision
medicine based on the response of the patient to the current
therapy prescribed by the treating clinician. Increased levels of
lipid components, such as triglycerides and low-density lipoprotein
affect the blood circulation to the renal tissue, causing renal
dysfunction (14). Apart from
lipid profiles, AGEs also play a crucial role in renal health.
Therefore, the aim of the present study was to derive a formula for
PGI and associate this with anti- and pro-aging molecules.
Materials and methods
Study design and focus
The present study was a comparative cross-sectional
study. Patients with T2DM attending the Outpatient Clinic, at the
Department of General Medicine and Diabetology at RL Jalappa
Hospital and Research Centre, Tamaka, India (constituent of Sri
Devaraj Urs Academy of Higher Education and Research) were
recruited for the study after fulfilling the inclusion and
exclusion criteria. Written informed consent was obtained from all
study subjects. The study groups were as follows: Group 1 (n=70),
included age- and sex-matched healthy controls; group 2 (n=70),
included patients with T2DM without CKD; and group 3 (n=70),
included patients with diabetes and CKD, based on the NKF EPI KDIGO
guidelines (8).
Inclusion and exclusion criteria
The following inclusion criteria were used: i)
Subjects clinically proven to suffer from T2DM, ii) subjects with
T2DM with CKD (diabetic kidney disease), and iii) subjects aged
between the ages of 35-70 years.
The exclusion criteria were as follows: Patients
with other types of DM; ii) patients taking drugs or other factors
known to cause diabetes and/or diabetes with CKD; iii) patients
undergoing renal dialysis; iv) patients with acute kidney injury
due to any cause and other renal pathologies.
Sample collection
After explaining the purpose of study in a language
that was understandable to the patient, a written informed consent
was obtained, designed according to the Declaration of Helsinki.
Under strict aseptic precautions, the study subjects were allowed
to be seated in a comfortable position. After the patients
underwent 8 h of fasting, blood samples were collected. The blood
samples obtained following fasting were divided into parts for
biochemical analyses. For the analysis of plasma glucose levels,
the samples were segregated into a NaF tube supplied by APR sales
(Ortho Clinical Diagnostics). For the analysis of HbA1c levels,
whole blood was collected into an EDTA tube supplied by APR sales
(Ortho Clinical Diagnostics). For the analysis of routine
biochemical parameters and research molecules, serum samples were
collected into plain tubes. Post-prandial blood (at 2 h) samples
were also collected. Corresponding urine samples were also
collected from the study subjects for urine fluoride analysis.
Study methodology
The present study was ethically approved by the
Central Ethics Committee of Sri Devaraj Urs Academy of Higher
Education and Research (Kolar, India) recognized by the Science and
Industrial Organization of the Ministry of Science and Technology
prior to the commencement of the study (ethics certificate no.
SDUAHER/KLR/CEC/35/2018-19). All the routine investigations were
carried out using a fully automated Vitro 5, 1 FS, a fusion
analyzer, maintained by Ortho Clinical Diagnostics. HbA1c levels
were estimated using a Bio-Rad D10 hemoglobin testing system
(Bio-Rad Laboratories, Inc.) based on the principle of HPLC at the
Biochemistry Section of Central Diagnostic Laboratory Services of
the study hospital. Manual parameters were analyzed at the Research
Laboratory of the Department of Biochemistry Sri Devaraj Urs
Medical College.
For the diagnosis of DM and one of its microvascular
complications, namely CKD, the following parameters were analyzed:
i) Diabetic profile: Fasting and post-prandial blood sugar levels
and glycated hemoglobin; ii) extended diabetic profile:
Carboxymethyl-lysine and fructosamine levels; iii) renal profile
parameters in serum: Creatinine and urea levels. These parameters
were analyzed using the following methods.
ELISA. Serum Sirtuin1 (ng/ml) levels were
measured using a kit procured from Sincere Biotech, Co., Ltd.
[E13651608 (Type II)]; serum CML levels (ng/ml) were measured using
a kit procured from Sincere Biotech, Co., Ltd. [E13651946 (Type
II)]; and serum fructosamine (ng/ml) levels were also measured
using a kit procured from Sincere Biotech (QY-E01291).
HPLC. HPLC was performed using a Bio-Rad D10
hemoglobin testing system (Bio-Rad Laboratories, Inc.) for
measuring the HbA1c levels (%).
Other parameters. An autoanalyzer (Vitros 5,1
Fs) (all reagents were procured from Ortho Clinical Diagnostics)
was used to measure the serum urea (mg/dl), serum creatinine
(mg/dl), serum triglycerides (TG; mg/dl), serum total cholesterol
(TC; mg/dl), serum high density lipoprotein (mg/dl) and serum
albumin (mg/dl) levels. The following parameters were also
calculated: i) Non-high-density lipoprotein (nHDL) (15)=total cholesterol-HDL; ii) AC
(16)=nHDL/HDL; and iii) PGI
(g/dl), which is defined as a marker for measuring aging and for
the prognosis of DM. The following equation was used to derive
value of the index, [(∑glycated proteins)-serum albumin]/anti-aging
molecule (Sirtuin1)].
Statistical analysis
SPSS version 20 software (IBM Corp.) was used to
perform the statistical analyses. The normality of distribution of
variables was assessed using the Kolmogorov-Smirnov test. All the
variables which are normally distributed (parametric) are presented
as the mean ± SD and those which are non-parametric are presented
as the median (25 to 75th percentile). Parametric tests were
carried out as follows: The data are presented as the mean ±
standard deviation (mean ± SD) for normally distributed data.
One-way analysis of variance (ANOVA) followed by the Tukey's and
post hoc test, was used to compared the three groups. In addition,
the following non-parametric tests were performed: Non-parametric
data, based on the frequency of data distribution, were divided
into quartiles with the 25 and 75th percentile and presented as the
median (25 to 75th percentile). The Bonferroni's correction was
applied after the Kruskal-Wallis test and Mann-Whitney U test to
determine any significant differences. Spearman's correlation
analysis [Rho (ρ)] was used for correlation analyses. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
The study comprised 115 males among 210 study
subjects, constituting ~55% of the total study population. Group 1
consisted of 31 (44.3%) males, group 2 included 47 (67%) males and
group 3 comprised of 37 (52.8%) male subjects.
The integration of mathematics with the markers of
diagnostic importance in DM may aid in improving the management of
the disease. The outcomes of treatment may help implement the same
prognostic tool in other aging-related disorders, such as cancer,
metabolic syndrome, CVD and Alzheimer's disease. Some researchers
have focused on atherogenic indices, since lipid deposition causes
various deleterious effects on organ systems (16,17).
Therefore, the consumption of foods rich in lipids causes arterial
changes and leads to diabetic microvascular complications.
The anthropometric measures and basic profiles for
diabetes and renal function of the subjects in the present study
are presented in Tables I and
II. Xie et al (17) demonstrated that blood pressure in
patients with diabetes and CKD was increased; this was also found
in the present study, with significant differences (P=0.08 for age
and P<0.001 for blood pressure) across groups 1, 2 and 3.
Similarly, the levels of other diabetic parameters and renal
profiles were significantly increased across the groups, indicating
the progression of T2DM. The levels of HbA1c were increased in
group 3 compared with the control.
 | Table IAnthropometric data of the study
subjects. |
Table I
Anthropometric data of the study
subjects.
| Parameter | Group 1 (n=70) | Group 2 (n=70) | Group 3 (n=70) | P-value |
|---|
| Age (years) | 50.71±9.2 | 53.1±8.2 | 56. 04±8.2 | 0.08 |
| SBP (mmHg) | 122.1±5.4 |
124.1±14.2a |
135.3±20.5a,b,c | <0.001 |
| DBP (mmHg) | 80.12±5.6 |
83.2±8.64a,c |
86.7±10.2a,b,c | <0.001 |
 | Table IIDiabetic and renal parameters of the
study subjects. |
Table II
Diabetic and renal parameters of the
study subjects.
| Parameter | Group 1 (n=70) | Group 2 (n=70) | Group 3 (n=70) | P-value |
|---|
| HbA1c (%) | 5.6±0.6 | 9.0±2.3a,c | 8.4±2.1a,b,c | <0.001 |
| Blood urea
(mg/dl) | 20.5±7.6 |
26.4±10.14a,c |
69.03±27.5a,b,c | <0.001 |
| Serum creatinine
(mg/dl) | 0.7±0.09 | 0.71±0.2 | 3.4±1.5a,b,c | <0.001 |
| Serum albumin
(g/dl) | 4.1±0.41 | 4.32±0.91 | 2.8±0.8a,b,c | <0.001 |
In order to evaluate the atherogenic status of the
study subjects, lipid profiles were analyzed (Table III). Statistically significant
differences were found (0.007, <0.001, 0.033 and <0.001)
across the groups for the levels of TC, HDL, TG and AC,
respectively, indicative of future diabetic complications due to
dyslipidemia, such as CVD.
 | Table IIILipid profiles of the study
subjects. |
Table III
Lipid profiles of the study
subjects.
| Parameter (reference
range) | Group 1 (n=70) | Group 2 (n=70) | Group 3 (n=70) | P-value |
|---|
| Total cholesterol,
mg/dl (120-200) | 172±39 | 161.6±60b,c | 184±59.3 | 0.007 |
| HDL, mg/dl
(40-60) | 40.01±09.1 | 28±10.5a,b,c | 41±14 | <0.001 |
| nHDL, mg/dl
(<130) | 132±40.01 | 126.1±59 | 145±56.9 | 0.077 |
| TG, mg/dl
(44-150)d | 141 (93-193.5) | 148 (114.5-211) | 184
(119.5-215.5)a,c | 0.033 |
| Atherogenic
coefficiente | 3.6±0.5 | 4±0.2 | 5.1±0.4a,b,c | <0.001 |
The data presented in Table IV depict the mean ± standard error
of the mean (SEM) for the entire population from which the study
subjects were recruited. It is the crucial part of the study which
portrays the importance of numerical values, which shall act a
surrogate marker during any type of paucity.
 | Table IVLipid indices of the study
subjects. |
Table IV
Lipid indices of the study
subjects.
| Parameter | Group 1 (n=70) | Group 2 (n=70) | Group 3 (n=70) | P-value |
|---|
| Atherogenic
index | 0.54±0.03 | 0.63±0.03 |
0.74±0.03a,c | <0.001 |
| Castelli risk index
1 (CRI 1) | 4.5±0.16 | 4.9±0.2 |
6.4±0.42a,b,c | 0.001 |
| Castelli risk index
2 (CRI 2) | 2.5±0.11 | 2.9±0.17 |
3.6±0.3a,b,c | 0.007 |
Currently, lipid ratios are gaining importance as
they can directly associate ‘bad’ with ‘good’ cholesterol. The
values of lipids and advanced biomarkers are presented in Tables IV and V, respectively. Mathematical indices
currently play a major role in planning strategies to manage
disorders and outbreaks. The reason behind this is that the number
implies a greater significance of the disease severity and
progression (18). There are
several mathematical designs, such as regression analysis, risk
ratios, etc., out of these the simplest one is the arithmetic
ratios of parameters whose values may serve a greater purpose when
correlated (18).
 | Table VSpecial parameters of the study
subjects. |
Table V
Special parameters of the study
subjects.
| Parameters | Group 1 (n=70) | Group 2 (n=70) | Group 3 (n=70) | P-value |
|---|
|
Sirtuin1(ng/ml) | 47.16 (12-97) | 33.5
(25.1-53)b,c | 50.1
(33.7-102.01) | 0.002 |
|
Carboxymethyl-lysine (ng/ml) | 900
(625.6-1,306) | 1,815
(1,100-2,592.03)a,c | 1,869
(1,155.1-2,272.5)a,c | <0.001 |
| Fructosamine
(ng/ml) (yet to be derived) | 100 (56-172) | 245.9
(98-341)a,c | 330
(131.2-418.2)a,c | <0.001 |
| PGI | 15.99
(8.1-71.4) | 45.01
(17.48-65.29)a,c | 58.2
(33.5-90.0)a,c | <0.001 |
In medicine, beginning from diagnosis to
therapeutics, mathematical interpretations play a vital role in
disease prognosis and management. Therefore, the present study
enumerates the ratios and calculated parameters, which shall be
considered in the prognosis of T2DM and DN. The values of
anti-aging molecules (e.g., Sirtuin1) and pro-aging molecules
(e.g., CML and fructosamine) are presented in Table V. These three are the major
determinants used to assess the magnitude of aging. The PGI, is the
novelty of the present study. PGI is defined as the index which
measures the amount of major protein glycated (pro-aging) alongside
the anti-aging protein. The median (25 to 75th percentile) of PGI
in the healthy controls was 15.99 (8.1-71.4); in the patients with
T2DM it was 45.01 (17.48-65.29); and in the patients with T2DM and
CKD it was 58.2 (33.5-90.0). When comparing the PGI between the
groups with the disease and controls, statistically significant
differences were found. Therefore, an increase in PGI increases
aging, which is well-defined and evident from the data presented in
Tables V and VI.
 | Table VICorrelation analysis of protein
glycation index with Sirtuin1 and CML. |
Table VI
Correlation analysis of protein
glycation index with Sirtuin1 and CML.
| Parameters | Group 1 (n=70) | P-value | Group 2 (n=70) | P-value | Group 3 (n=70) | P-value |
|---|
| Sirtuin1
(mg/ml) | -0.823a | <0.001 | -0.799a | <0.001 | -0.612a | <0.001 |
| CML (ng/ml) | 0.537a | <0.001 | 0.624a | <0.001 | 0.666a | <0.001 |
The results of Spearman's correlation analysis
revealed a significant an inverse correlation between PGI and the
anti-aging molecule, Sirtuin1 (-0.823, -0.799 and -0.612 in groups
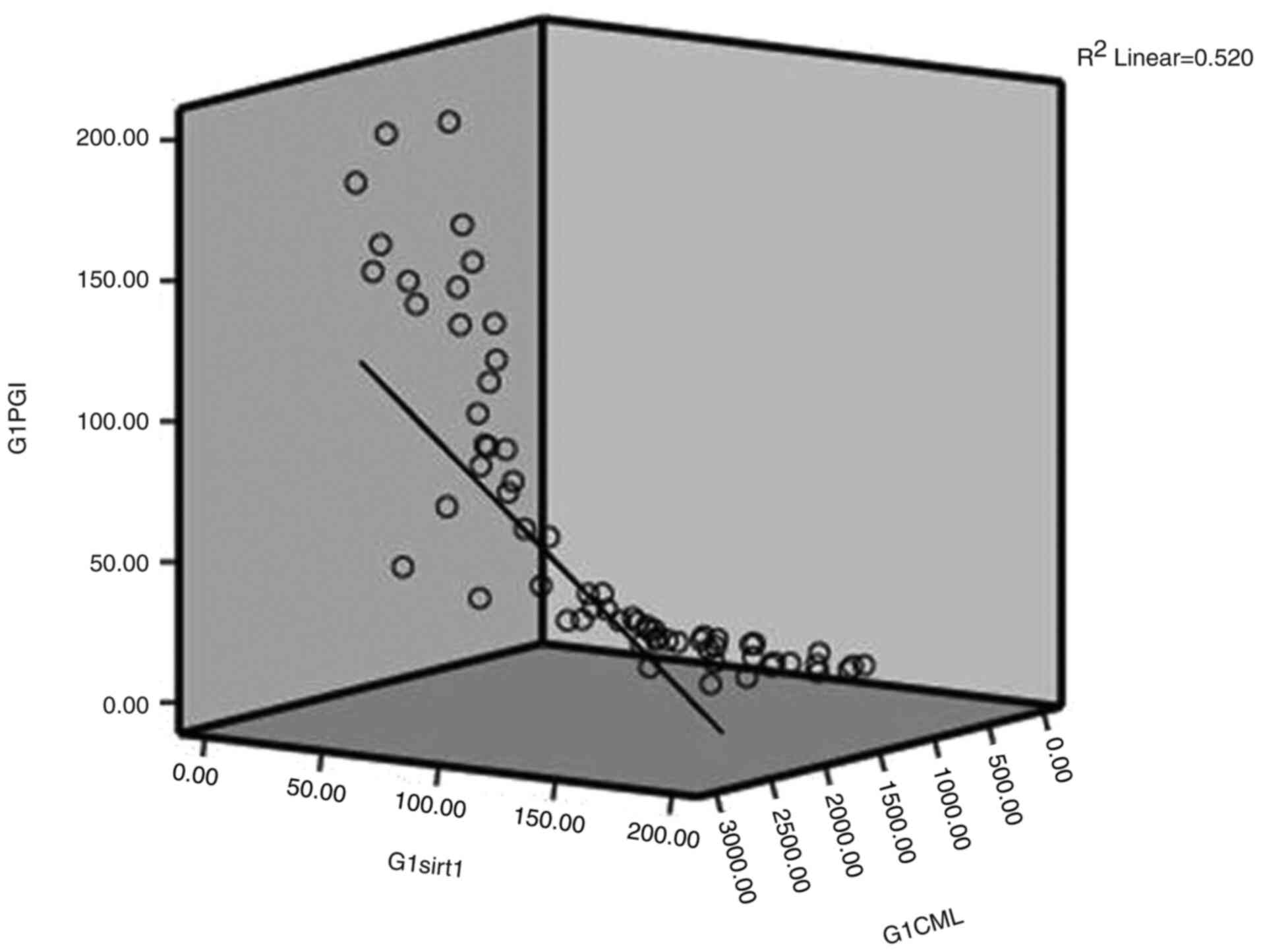
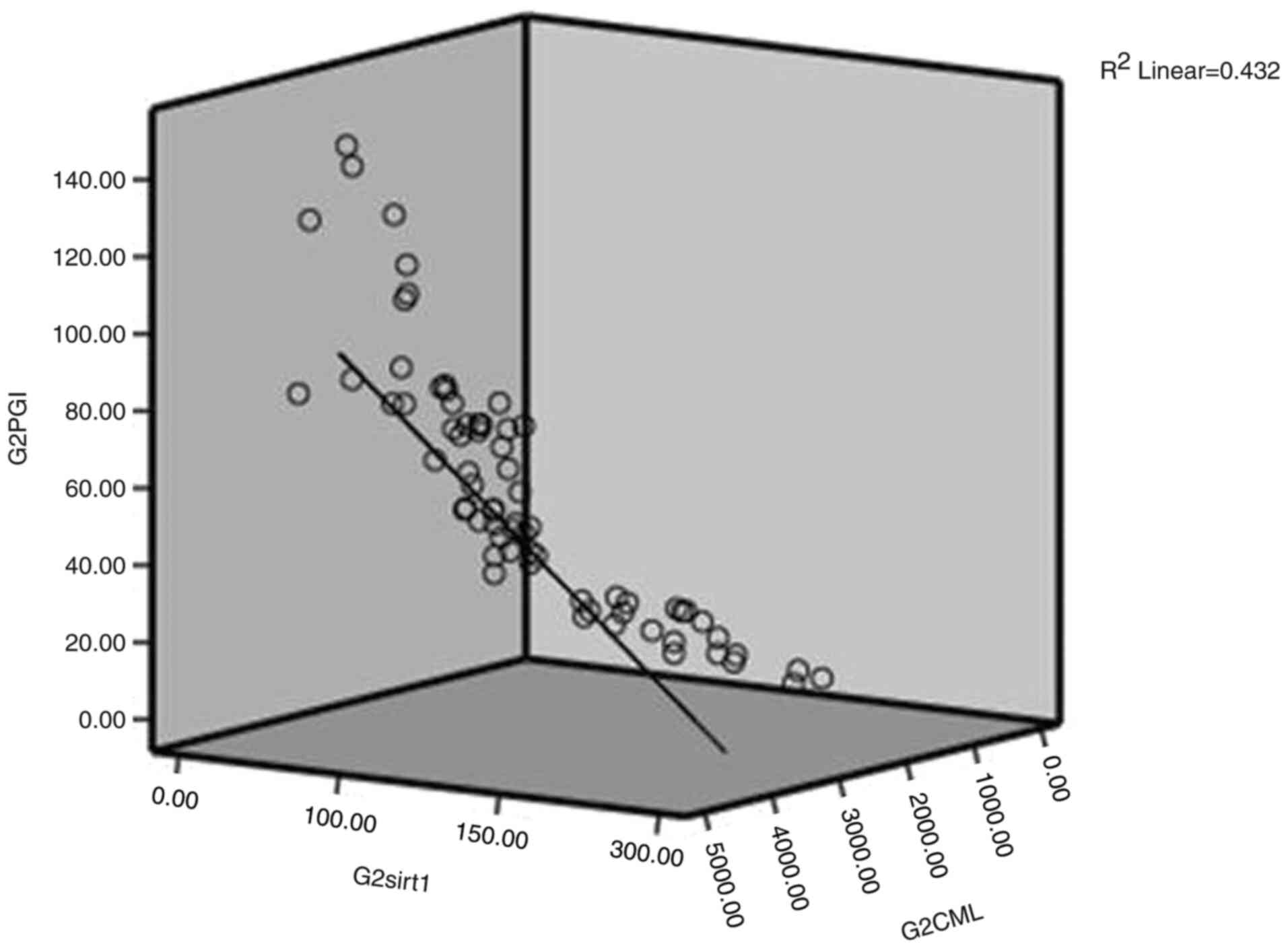
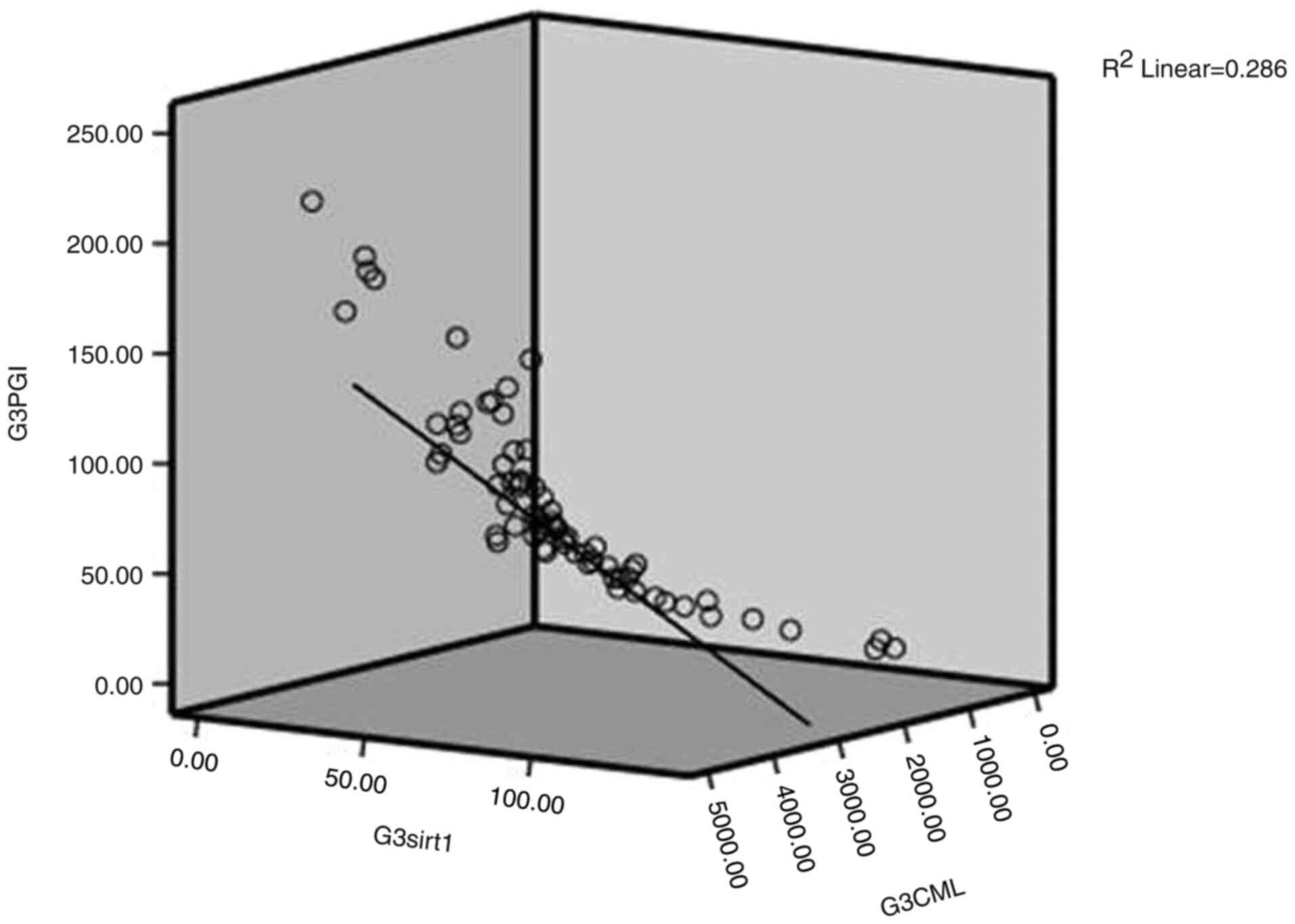
1, 2 and 3; shown in Fig. 1,
Fig. 2 and Fig. 3, respectively). Similarly, the
analysis of the correlation between PGI and CML revealed a
significant positive correlation (0.537, 0.624 and 0.666 in groups
1, 2 and 3; shown in Fig. 1,
Fig. 2 and Fig. 3, respectively). As a result, to
calculate PGI, the serum albumin concentration, and any one of the
glycated protein concentrations, an anti-aging molecule
concentration is required.
In conclusion, as per the American Diabetes
Association guidelines, fasting, post-prandial measurements and
HbA1c levels are of diagnostic significance as markers for DM.
Following several analyses and comparisons with other parameters,
any one of the AGE and calculated lipid indices (atherogenic
indices) may serve as prognostic tools in DM for the prevention of
DM-related complications, particularly diabetes with CKD and CVD.
PGI is a calculated parameter, which serves as an index not only
for DM, but also for other aging-related disorders, since it
includes pro- and anti-aging molecules. However, further studies
with larger cohorts examining different aging-related disorders in
the same population are required to confirm the findings of the
present study regarding PGI.
Acknowledgements
The authors would like to thank Sri Devaraj Urs
Academy of Higher Education and Research and Dr Bhuneshwar Yadav,
Mr. Manjunath, Mr. Srinivas and Mrs Kavitha who provided logistic
and technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SDRM was involved in sample collection, data
analysis, statistical analysis and interpretation of the data, and
in the writing of the manuscript. SKN was involved in subject
recruitment, sample collection and in the reviewing of the
manuscript. SDRM and SKN confirm the authenticity of all the raw
data which is preserved as an excel master chart. Both authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The Central Ethics Committee of Sri Devaraj urs
Academy of Higher Education and Research with it affiliated
institution Sri Devaraj Urs Medical College approved the study as
per the Declaration of Helsinki (approval no.
SDUAHER/KLR/CEC/35/2018-19). Informed consent was obtained from all
the study subjects after explaining them the study in
understandable language through patient information sheet.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garay-Sevilla ME, Rojas A, Portero-Otin M
and Uribarri J: Dietary AGEs as exogenous boosters of inflammation.
Nutrients. 13(2802)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cole JB and Florez JC: Genetics of
diabetes mellitus and diabetes complications. Nat Rev Nephrol.
16:377–390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asadipooya K and Uy EM: Advanced glycation
end products (AGEs), receptor for AGEs, diabetes, and bone: Review
of the literature. J Endocr Soc. 3:1799–1818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
ElSayed NA, Aleppo G, Aroda VR, Bannuru
RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D,
Johnson EL, et al: 2. Classification and diagnosis of diabetes:
Standards of care in diabetes-2023. Diabetes Care. 46 (Suppl
1):S19–S40. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
John J, Sakarde A, Chafle J, Amle D, Jose
J, Sakhare V and Rathod BD: An assessment of the utility of serum
fructosamine in the diagnosis and monitoring of diabetes mellitus.
Cureus. 15(e33549)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mengstie MA, Chekol Abebe E, Behaile
Teklemariam A, Tilahun Mulu A, Agidew MM, Teshome Azezew M, Zewde
EA and Agegnehu Teshome A: Endogenous advanced glycation end
products in the pathogenesis of chronic diabetic complications.
Front Mol Biosci. 9(1002710)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ram Mohan SD, Shashidhar KN, Anjanappa R
and Chandrappa M: Estimation of fluoride and sirtuin1 in patients
with diabetic nephropathy in Kolar District of Karnataka, India. J
Lab Physicians. 14:57–64. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Diabetes Work Group: KDIGO 2022 clinical practice guideline
for diabetes management in chronic kidney disease. Kidney Int. 102
(5S):S1–S127. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bertelli PM, Pedrini E, Hughes D,
McDonnell S, Pathak V, Peixoto E, Guduric-Fuchs J, Stitt AW and
Medina RJ: Long term high glucose exposure induces premature
senescence in retinal endothelial cells. Front Physiol.
13(929118)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ramsey KM, Mills KF, Satoh A and Imai S:
Age-associated loss of Sirt1-mediated enhancement of
glucose-stimulated insulin secretion in beta cell-specific
Sirt1-overexpressing (BESTO) mice. Aging Cell. 7:78–88.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chilelli NC, Burlina S and Lapolla A:
AGEs, rather than hyperglycemia, are responsible for microvascular
complications in diabetes: A ‘glycoxidation-centric’ point of view.
Nutr Metab Cardiovasc Dis. 23:913–919. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T,
Hu J, Xu W, Yi N and Lei S: Atherogenic index of plasma is a novel
and better biomarker associated with obesity: A population-based
cross-sectional study in China. Lipids Health Dis.
17(37)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Y, Feng Y, Li S, Ma Y, Lin J, Wan J and
Zhao M: The atherogenic index of plasma (AIP) is a predictor for
the severity of coronary artery disease. Front Cardiovasc Med.
10(1140215)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahmad S, Siddiqui Z, Rehman S, Khan MY,
Khan H, Khanum S, Alouffi S and Saeed M: A glycation angle to look
into the diabetic vasculopathy: Cause and cure. Curr Vasc
Pharmacol. 15:352–354. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu A and Reaven GM: Is measurement of
non-HDL cholesterol an effective way to identify the metabolic
syndrome? Nutr Metab Cardiovasc Dis. 23:1122–1127. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sujatha R and Kavitha S: Atherogenic
indices in stroke patients: A retrospective study. Iran J Neurol.
16:78–82. 2017.PubMed/NCBI
|
|
17
|
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y,
Li T, Maddukuri G, Tsai CY, Floyd T and Al-Aly Z: Analysis of the
global burden of disease study highlights the global, regional, and
national trends of chronic kidney disease epidemiology from 1990 to
2016. Kidney Int. 94:567–581. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martínez R and Sánchez-Soriano J:
Mathematical indices for the influence of risk factors on the
lethality of a disease. J Math Biol. 83(74)2021.PubMed/NCBI View Article : Google Scholar
|