Introduction
Extra-articular organ involvement and symmetric
polyarticular invasive joint inflammation are hallmarks of
rheumatoid arthritis (RA), a chronic systemic inflammatory
disorder. RA is characterized by synovitis (1). At present, 0.5-1.0% of patients
present with RA worldwide, with greater prevalence rates observed
in females and the elderly (2). RA
involves immune cells and cytokines that trigger an inflammatory
response, leading to damage in the arthritic joint (3,4).
However, the pathophysiology of RA remains to be fully understood.
At present, research is focused on the involvement of the
inflammasome within rheumatic disorders. Pathogen
pattern-recognition receptors (PRRs) form multimeric complexes on
the inflammasome, and bind to damage-associated molecular patterns
and pathogen-associated molecular patterns to facilitate host
defense responses. In the majority of cases, pro-caspase (CASP)1,
PRR and a protein adapter form the inflammasome (5). Following activation, CASP1 inhibits
the binding between pro-IL-1, pro-IL-18 and gasdermin D (GSDMD),
resulting in pyroptosis and in an inflammatory response (6). Moreover, following the activation of
the inflammasome, GSDMD is cleaved into the GSDMD-N-terminal.
Cleavage is promoted canonically or non-canonically via external
stimuli or endogenous damage (7,8).
GSDMD-induced pyroptosis protects the host from bacterial invasion
(9). Numerous inflammatory
diseases exhibit persistent inflammation mediated by abnormal GSDMD
activation (10).
Temporomandibular joint disorders (11), and symptoms of dry mouth are oral
complications associated with RA (12,13).
In addition, patients with RA may experience extra-articular
involvement of the skin, eyes, heart, lungs, kidneys, neurological
system and gastrointestinal tract (14). Individuals with clinically
diagnosed RA may also experience changes in salivary function
(15). In the majority of the
affected organs, extra-articular symptoms of RA are caused by
vascular vasculitis, which may be followed by arterial occlusion
and vessel wall necrosis (14).
The present study aimed to evaluate the role of salivary CASP1 and
GSDMD in the pathophysiology of RA, and determine the potential
associations between RA, saliva pH and salivary flow rate.
Subjects and methods
Subject information
Patients with RA (aged 20-60 years) were divided
into two groups. The first group included 20 patients with a
clinical diagnosis of RA who were not receiving any type of
treatment from a rheumatologist (the newly diagnosed group). The
second group included 40 patients with RA who had previously been
treated with biological or non-biological Disease-modifying
antirheumatic drugs (DMARD) in the Rheumatology Unit of the Baghdad
Teaching Hospital (Baghdad, Iraq; treated group). Notably,
rheumatologists in this unit evaluated the disease activity of all
patients in each group using clinical disease activity score
(CDAI).
Patient data was obtained, including name, age, sex,
alcohol consumption, smoking history, family history, a history of
systemic disorders and previous medications. In total, the control
group included 16 male and 44 female participants. All patients in
the control group were in good overall health, with no systemic
disorders or immunological diseases and no previous medication. The
age and sex of the individuals in the control group were matched to
those of the RA groups. Patients were excluded from the present
study according to the following criteria: An age >60 years, a
history of smoking, alcoholism, or pregnancy at the time of the
study.
All groups were examined between December, 2022 and
mid-June, 2023. The present study was approved by the Institutional
Review Board Ethics Committee at the College of Medicine,
Al-Nahrain University, Baghdad, Iraq (ethics approval no.
20221029). The examination and collection of samples from patients
with RA was approved by The Ministry of Health, Iraq. Written
informed consent was obtained from all patients, and the
Declaration of Helsinki was followed.
Assessment of disease activity using
CDAI
In the Rheumatology Unit of The Baghdad Teaching
Hospital, rheumatologists used CDAI to evaluate the disease
activity of patients with RA (16).
Saliva collection
Unstimulated saliva collection was carried out
according to the guidelines described by Tenovuo and Lagerlof
(17). Samples were centrifuged
for 10 min at 804.96 x g at room temperature (~20˚C), and divided
into two groups for ELISA. Supernatants were stored at
-200˚C until use in further experiments.
Calculation of salivary flow rate
All unstimulated saliva was collected from patients
for 5 min. Volume was calculated and expressed as ml/min (18).
Determination of saliva PH
A digital pH meter with a single electrode was used
to measure saliva pH (Jenway pH meter 3320) (19).
Detection of salivary CASP1
Salivary CASP1 was evaluated using ELISA, following
the manufacturer's instructions (cat. no. ELK2076; ELK
Biotechnology). Briefly, a microtiter plate was pre-coated with
anti-CASP1 antibody (cat. no. ELK2076; ELK Biotechnology).
Following the addition of samples, a biotin-conjugated anti-CASP1
antibody was added to the microtiter plate. Following incubation
with primary antibodies for 50 min at 37˚C, the samples were
incubated with horseradish peroxidase (HRP)-conjugated avidin (part
number: ELK2076). TMB substrate solution (ELK Biotechnology, Co.,
Ltd.) was added to all wells, and color changes were observed in
wells containing CASP1, biotin-conjugated antibody and
enzyme-conjugated avidin. Changes in color were determined using a
spectrophotometer (Thermo Fisher Scientific, Inc.) at 450±10 nm,
and the enzyme-substrate reaction was terminated following the
addition of sulphuric acid solution (ELK Biotechnology, Co., Ltd.).
The optical density of the samples was read and compared with a
standard curve to determine the concentration of CASP1.
Detection of salivary GSDMD
Salivary GSDMD was evaluated using ELISA, following
the manufacturer's instructions (cat no. E6838Hu, Bioassay
Technology Laboratory). Briefly, a plate was pre-coated with the
anti-GSDMD antibody, and biotinylated human anti-GSDMD antibody was
subsequently added. Following incubation with primary antibodies
(provided with the kit) for 60 min at 37˚C, the samples were
incubated with HRP-conjugated streptavidin, washed and substrate
solution was added. Color development was indicative of human
GSDMD. An acidic stop solution was added to all samples, and the
absorbance was determined at 450 nm (using a spectrophotometer,
Thermo Fisher Scientific, Inc.).
Statistical analysis
SPSS (version, 26; IBM Corp.) was used for
statistical analysis. Differences between two groups were
determined using unpaired Student's t-tests, and differences
between multiple groups were determined using one-way ANOVA
followed by a post hoc test (Duncan's multiple range comparisons).
Percentage changes were determined using the Chi-squared test.
Pearson correlation coefficient (r) was used to calculate the
correlation between parameters. Receiving operating characteristics
curve (ROC) analysis was used to determine the diagnostic
capability of a binary discrimination system which plot the TPR
(true positive rate) sensitivity against the false positive rate
(1-specificity). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characteristics of the
study participants
The results of the present study revealed no
significant differences in the age and sex of the participants
between all groups. The mean disease duration in the treated RA
group was 8.95±5.123 years. In addition, there was no significant
difference in CDAI between the newly diagnosed and treated RA
groups. The results of the present study demonstrated significant
differences in the mean salivary flow rate and saliva pH between
all patients with RA and the control group (Table I).
 | Table IDemographic and clinical
characteristics of the patients (treated and newly diagnosed
patients) and the controls. |
Table I
Demographic and clinical
characteristics of the patients (treated and newly diagnosed
patients) and the controls.
| Variable | Treated group | Newly diagnosed
group | Control group | P-value | P-value (treated
group vs. newly diagnosed group) | P-value (treated
group vs. control group) | P-value (newly
diagnosed group vs. control group) |
|---|
| Age in years, median
(IQR) | 45.0000 (17.75) | 42.0000 (19.25) | 39.0000 (17.50) | 0.099 | | | |
| Sex, n (%) | | | | 0.200 | | | |
|
Male | 12 (30%) | 2 (10%) | 16 (26.7%) | | | | |
|
Female | 28 (70%) | 18 (90%) | 44 (73.3%) | | | | |
| Duration of disease
(years), mean ± SD | 8.95±5.123 | - | - | - | | | |
| CDAI, mean ± SD | 16.95±8.748 | 19.55±8.858 | | 0.200 | | | |
| Salivary flow rate
(ml/min), mean ± SD | 0.6518±0.42002 | 0.9105±0.70285 | 1.0402±0.48654 | 0.01 | 0.999 | 0.001a | 0.756 |
| pH of saliva, mean ±
SD | 7.5168±0.36498 | 7.3480±0.25345 | 7.1438±0.08501 | 0.01a | 0.999 | 0.001a |
0008a |
| Salivary CASP1
(ng/ml), mean ± SD | 2.0134±0.74364 | 1.8665±0.89211 | 1.5183±0.37402 | 0.01a | 0.457 | 0.002a | 0.999 |
| Salivary GSDMD
(ng/ml), mean ± SD | 3.1514±1.38895 | 3.4849±1.18221 | 3.2260±0.90858 | 0.50 | | | |
Detection of salivary CASP1and
GSDMD
The results of the present study revealed a
significant difference in the mean salivary CASP1 levels between
all three groups, with 2.0134, 1.8665 and 1.5183 ng/ml observed in
the treated RA, newly diagnosed and control groups, respectively.
In addition, the mean salivary CASP1 level was notably higher in
the newly diagnosed group compared with the control group. However,
there was no significant difference in the mean salivary CASP1
level between the treated RA and newly diagnosed groups (Table I).
In addition, the results of the present study
revealed that the mean salivary GSDMD levels were 3.4849, 3.1514
and 3.2260 ng/ml in the treated RA, newly diagnosed and control
groups, respectively; however, differences between groups were not
significant. In addition, there were no notable differences in the
means of GSDMD and CASP1 in the saliva of the two groups of RA when
these were divided according to disease activity score to low,
moderate and high activity (Table
II). Moreover, the results demonstrated no notable correlation
between CASP1, GSDMD, saliva pH or salivary flow rate (Table III).
 | Table IILevels of CASP1 and GSDMD, and the
disease activity score among treated and newly diagnosed
patients. |
Table II
Levels of CASP1 and GSDMD, and the
disease activity score among treated and newly diagnosed
patients.
| Groups | Disease activity | Salivary CASP1, mean
± SD | Salivary GSDMD, mean
± SD |
|---|
| Treated | Low | 2.0575±0.58939 | 2.9544±1.59425 |
| | Moderate | 2.1211±0.89705 | 3.1340±1.35189 |
| | High | 1.7050±0.53007 | 3.4509±1.27984 |
| | P-value | 0.4 | 0.7 |
| Newly diagnosed | Low | 1.6800±0.34496 | 3.4610±0.70885 |
| | Moderate | 1.7111±0.
50755 | 3.5117±1.40661 |
| | High | 2.1729±1.39582 | 3.4640±1.23875 |
| | P-value | 0.5 | 0.9 |
 | Table IIICorrelation between salivary
biomarker and oral manifestation. |
Table III
Correlation between salivary
biomarker and oral manifestation.
| Parameter | GSDMD | pH of saliva | Flow rate |
|---|
| CASP1 | | | |
|
Pearson's
correlation (r value) | 0.095 | 0.090 | .027 |
|
P-value
(two-tailed) | 0.380 | 0.404 | 0.803 |
| GSDMD | | | |
|
Pearson's
correlation (r value) | | 0.012 | -0.098 |
|
P-value
(two-tailed) | | 0.907 | .358 |
| pH of saliva | | | |
|
Pearson's
correlation (r value) | | | -0.167 |
|
P-value
(two-tailed) | | | 0.117 |
Diagnostic value of CASP1 and
GSDMD
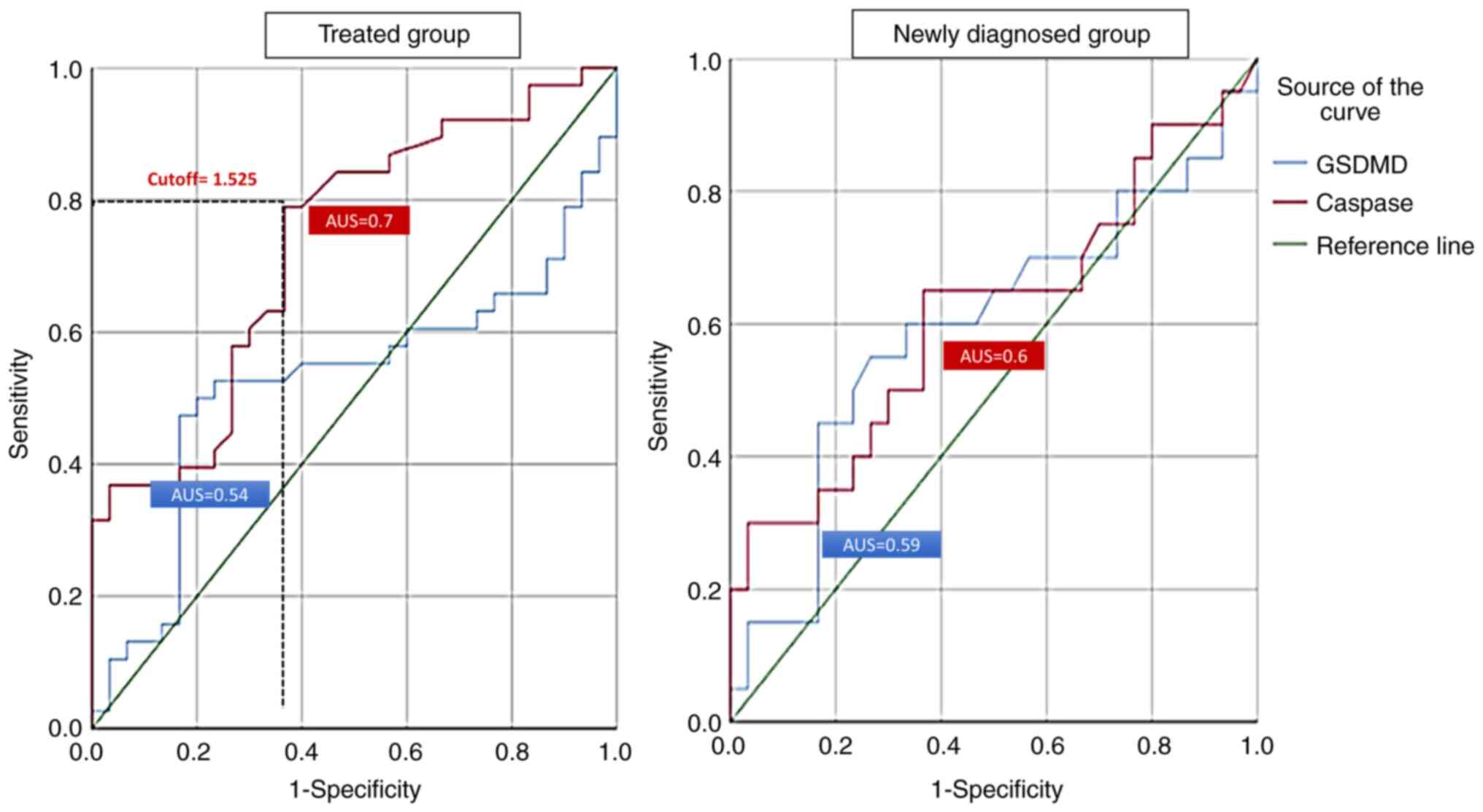
ROC analysis was used to determine the diagnostic
value of CASP1 and GSDMD in patients with RA. CASP1 in the treated
group revealed an area under the curve (AUC) of 0.7 (P=0.001),
while GSDMD was not found to be efficient. At a 1.525 cut-off,
CASP1 could predict the inflammation and disease activity in the
treated RA group at a sensitivity of 78% and specificity of 63%. By
contrast, the results of the ROC analysis demonstrated that CASP1
and GSDMD were not associated with disease activity in the newly
diagnosed group (Fig. 1).
Discussion
The results of the present study demonstrated that
RA often affects adults during their 40th decade of life, which is
consistent with the results of the study by Ranade and Doiphode
(20). The results of the present
study revealed no significant differences in age or sex between the
three study groups, which was comparable with the results of the
study by Al Ghuraibawi et al (21). In the present study, the majority
of patients with RA were female, which was also consistent with the
study by Alkazzaz (22). The
prevalence of RA may be higher among females due to gene silencing
on the X chromosome, which plays a key role in the development of
autoimmune disease. Notably, skewed inactivation may lead to
differences in gene silencing in the maternal and paternal X
chromosomes, resulting in distinct self-antigens that trigger a
greater immune response. The reactions between self-antigens
promote the development of auto-immune reactions, and the
development of disease, such as RA (23).
The present study revealed that the salivary flow
rate was significantly increased in patients with RA, compared with
the control group. This outcome is consistent with the study
conducted by Majid et al (24). This suggests that the salivary
glands are one of the main target organs of RA, since RA is
considered to be associated with the infiltration of lymphocytes of
affected glands, leading to decreased chemical and salivary changes
(25). In the present study, there
were no appreciable changes between the newly diagnosed and treated
patients with RA; however, the salivary pH increased significantly
in the two patient groups compared with the control group. The
study by Fadhil and Ahmed (26),
which discovered a substantial decrease in salivary pH levels
between the treated RA and control groups, is in conflict with this
conclusion. The findings of that study demonstrated that an
increase in the physiological salivary pH range corresponds to an
increase in saliva flow rate and vice versa (26). The results of the present study
revealed that patients with RA exhibited increased levels of
salivary CASP1 compared with the control group, and these results
were comparable with those obtained in the study by Kim et
al (27). However, Karabulut
et al (28) observed the
opposite result.
Kim et al (27) investigated the reason behind the
increase in CASP1 levels in the serum of patients receiving
treatment for RA. They found that CASP1 activation is a sign of
inflammasome activation, which is crucial for inducing an
inflammatory response in macrophages. suggests that the RA drug may
have an effect on CASP1 expression. The therapeutic strategy may
affect CASP1 expression and activity (29). In the present study, the median
value of salivary CASP1 in the newly diagnosed group was higher
than that in the control group, which suggests the involvement of
CASP1in the early stages of RA development. There were no
significant differences in the median value of salivary CASP1
between the treated and newly diagnosed groups. This result is in
accordance with the result presented in the study by Cascão et
al (30).
The results of the present study demonstrated that
there were no notable differences in the mean salivary GSDMD levels
between the three groups. To the best of our knowledge, the present
study is the first to investigate the levels of GSDMD in the saliva
of patients with RA. Notably, Zhang et al (31) investigated the synovial expression
of cleaved GSDMD using immunohistochemistry and multiplex
immunohistochemistry. The results of the study by Zhang et
al (31) demonstrated that
patients with RA exhibited increased inflammasomes and
GSDMD-N-terminal in the synovium, compared with patients with
osteoarthritis. This result is in disagreement with that of the
study conducted by Al Obaidi and Al Ghurabi (32), which discovered elevated levels of
NLRP3, which caused the release of GASDMD in the patient group
(periodontitis).
Of note, a limitation of the present study was that
the concentrations of CASP1 (inactive form) and GSDMD (inactive
form) were quantified in saliva. Nevertheless, the active forms
were not quantified. Thus, further studies are required to further
investigate these parameters.
In conclusion, the findings of the present study
provide valuable insight into the role of GSDMD and CASP1 in the
context of RA. The significance of these differences in the means
of CASP1 indicate that it could be a critical factor in the
severity and progression of RA. This aligns with existing
literature that highlights the importance of inflammatory mediators
in the pathogenesis of RA, suggesting that CASP1 may be a potential
target for therapeutic intervention.
Acknowledgements
The authors would like to thank Dr Ali Hussein and
Dr Adnan Sadkhan, Rheumatologists at the Rheumatology Unit of the
Baghdad Teaching Hospital for their great contribution in the
assessment of the severity of rheumatoid arthritis in patients
depending on the clinical disease activity score.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WLA was involved in the conception and design of the
study, in the literature search, in clinical analysis, data
analysis, statistical analysis, and in the preparation and
reviewing of the manuscript. AFAH was involved in the conception
and design of the study, in data analysis, and in the preparation
and reviewing of the manuscript. BHAG was involved in the
conception and design of the study and was also involved in the
preparation and reviewing of the manuscript. WLA and BHAG confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board Ethics Committee at the College of Medicine,
Al-Nahrain University, Baghdad, Iraq (ethics approval no.
20221029). The examination and collection of samples from patients
with RA was approved by The Ministry of Health, Iraq. Written
informed consent was obtained from all patients, and the
Declaration of Helsinki was followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scott DL, Wolfe F and Huizinga TWJ:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rudan I, Sidhu S, Papana A, Meng SJ,
Xin-Wei Y, Wang W, Campbell-Page RM, Demaio AR, Nair H, Sridhar D,
et al: Prevalence of rheumatoid arthritis in low- and middle-income
countries: A systematic review and analysis. J Glob Health.
5(010409)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alippe Y and Mbalaviele G: Omnipresence of
inflammasome activities in inflammatory bone diseases. Semin
Immunopathol. 41:607–618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aldhaher Z, Al-Ghurabi B and Alwan BH:
Serum levels of IL-22 and ACPA in patients with rheumatoid
arthritis. J Pure Appl Microbiol. 12:687–691. 2018.
|
|
5
|
Broz P and Dixit VM: Inflammasomes:
Mechanism of assembly, regulation and signalling. Nat Rev Immunol.
16:407–420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boucher D, Monteleone M, Coll RC, Chen KW,
Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, et
al: Caspase-1 self-cleavage is an intrinsic mechanism to terminate
inflammasome activity. J Exp Med. 215:827–840. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abdullah WL, Al-Hashimy AF, Al-Ghurabi BH
and Ramakrishnan M: Focus on the Function and mechanism of
pyroptosis in rheumatoid arthritis. Al-Salam J Med Sci. 3:28–36.
2024.
|
|
9
|
Wang J, Deobald K and Re F: Gasdermin D
protects from melioidosis through pyroptosis and direct killing of
bacteria. J Immunol. 202:3468–3473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang J, Yao J, Liu Y and Huang L:
Targeting the gasdermin D as a strategy for ischemic stroke
therapy. Biochem Pharmacol. 188(114585)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Garib BT and Qaradaxi SS:
Temporomandibular joint problems and periodontal condition in
rheumatoid arthritis patients in relation to their rheumatologic
status. J Oral Maxillofac Surg. 69:2971–2978. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guobis Z, Baseviciene N, Paipaliene P,
Niedzelskiene I and Januseviciūte G: Aspects of xerostomia
prevalence and treatment among rheumatic inpatients. Medicina
(Kaunas). 44:960–968. 2008.PubMed/NCBI
|
|
13
|
Abdulla WL, A-Ghurabi BH and Gathwan KH:
An Impairment of salivary gland function in rheumatoid arthritis:
Association with change in salivary biomarkers and disease
activity. J Bagh Coll Dent. 28:165–170. 2016.
|
|
14
|
Cojocaru M, Cojocaru IM, Silosi I, Vrabie
CD and Tanasescu R: Extra-articular manifestations in rheumatoid
arthritis. Maedica (Bucur). 5:286–291. 2010.PubMed/NCBI
|
|
15
|
Torres SR, Pedrazas CH, Correia MP, de
Azevedo MNL, Zamprogno T, Silva A Jr, Gonçalves LS and Papi JA:
Drugs or disease: Evaluating salivary function in RA patients. Bra
Oral Res. 30(e106)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Van der Heijde DM, Van't Hof M, Van Riel
PL and Van de Putte LB: Development of a disease activity score
based on judgment in clinical practice by rheumatologists. J
Rheumatol. 20:579–581. 1993.PubMed/NCBI
|
|
17
|
Tenovuo J and Lagerlof F: Saliva. In:
Thylstrup A, Fejerskov O (eds). Textbook of clinical cariology. 2nd
edition. Copenhagen: Munksgaard, pp17-43, 1994.
|
|
18
|
Navazesh M and Kumar SKS: University of
Southern California School of Dentistry. Measuring salivary flow:
Challenges and opportunities. J Am Dent Assoc. 139 (Suppl):35S–40S.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baliga S, Muglikar S and Kale R: Salivary
pH: A diagnostic biomarker. J Indian Soc Periodontol. 17:461–465.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ranade SB and Doiphode S: Is there a
relationship between periodontitis and rheumatoid arthritis? J
Indian Soc Periodontol. 16:22–27. 2012.
|
|
21
|
Al Ghuraibawi ZAG, Sharquie IK and Gorial
FI: A novel link of serum IL-39 levels in patients with rheumatoid
arthritis. Iraqi J Sci. 64:1651–1661. 2023.
|
|
22
|
Alkazzaz AMH: Incidence of rheumatoid
arthritis [2001 to 2011]. Iraqi Postgrad Med J. 12:568–572.
2013.
|
|
23
|
Valencia M: Sex and gender in rheumatoid
arthritis: considering a risk factor hierarchy. Cornell Undergrad
Res J. 1:23–29. 2022.
|
|
24
|
Majid AY, Talal S and Abdulla WL:
Estimation of some salivary elements in rheumatoid arthritis
patients. Int J Adv Res. 3:13–17. 2016.
|
|
25
|
Nagler RM, Salameh F, Reznick AZ, Livshits
V and Nahir AM: Salivary gland involvement in rheumatoid arthritis
and its relationship to induced oxidative stress. Rheumatology
(Oxford). 42:1234–1241. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fadhil HNM and Ahmed KM: Evaluation of
salivary anti-CCP in relation to some oral manifestations in
rheumatoid arthritis patients, Sulaimaniyah, Iraq. Res Sq: 1-18,
2023.
|
|
27
|
Kim SH, Lee JH, Jeong HJ, Kim JM, Baek WK,
Kim TH, Jun JB and Son CN: Clinical significance of elevated serum
caspase-1 levels in patients with ankylosing spondylitis. Ann Lab
Med. 42:293–295. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karabulut G, Kitapçıoğlu G, Özçaka Ö,
Alpöz E, Nalbantsoy A, Koçanaoğulları H, Gücenmez S, Keser G and
Kabasakal Y: Saliva levels of caspase-1, TNF-α, and IFN-γ in
primary Sjögren's syndrome: Oral mucosal abnormalities revisited.
Turk J Med Sci. 48:554–559. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
Resveratrol suppresses TNF-induced activation of nuclear
transcription factors NF-kappa B, activator protein-1, and
apoptosis: Potential role of reactive oxygen intermediates and
lipid peroxidation. J Immunol. 164:6509–6519. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cascão R, Polido-Pereira J, Canhão H,
Rodrigues AM, Navalho M, Raquel H, Neves-Costa A, Mourão AF,
Resende C, da Silva JA, et al: Caspase-1 is active since the early
phase of rheumatoid arthritis. Clin Exp Rheumatol.
30(144)2012.PubMed/NCBI
|
|
31
|
Zhang X, Wang Q, Cao G, Luo M, Hou H and
Yue C: Pyroptosis by NLRP3/caspase-1/gasdermin-D pathway in
synovial tissues of rheumatoid arthritis patients. J Cell Mol Med.
27:2448–2456. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Al Obaidi MJ and Al Ghurabi BH: Potential
role of NLRP3 inflammasome activation in the pathogenesis of
periodontitis patients with type 2 diabetes mellitus. J Med Chem
Sci. 6:522–531. 2023.
|