Introduction
Quality antenatal monitoring of the newborn at birth
and timely medical intervention reduce perinatal mortality
(1). Perinatal mortality depends
on the condition of the newborn at birth, timely observation during
pregnancy and timely medical procedures (1). Perinatal mortality is common among
fetuses born with a low birth weight for their gestational age,
which can be caused by fetal growth retardation (FGR), even in
preterm newborns (2). Fetuses with
FGR do not reach to their intrauterine growth potential due to
multiple risk factors, and such infants have a high risk of
morbidity and mortality compared to healthy infants (3). Small for gestational age (SGA) is
most often defined as a birth weight <2,500 g (4). A newborn which is considered large
for gestational age (LGA) is most often defined to have a birth
weight >4,000 g (5,6). Often, a fetus with FGR is considered
to be one that weighs <10th percentile for gestational age;
however, this does not take into account the individual growth
potential of each fetus (7). FGR
can be present in a fetus that weights >10th percentile, it also
has not realized its growth potential. Conversely, constitutionally
small, yet healthy fetuses can have an overdiagnosis of FGR
(3). FGR is associated with
adverse perinatal outcomes (8).
Females with fetuses considered to be SGA and LGA require increased
attention in the management of pregnancy, as they may have various
pregnancy-related complications at birth. Timely and quality
obstetric care is the main and important condition in the detection
of pregnancy complications and reduces the risks of stillbirth and
perinatal mortality up to 28 days of life (9). Children who were considered to be SGA
and LGA at birth, have been observed as adults to have a higher
risk of developing diabetes, cardiovascular disease, certain types
of cancer, and a high risk of mortality from a variety of causes
(10).
Stillbirths are the result of FGR and reduced fetal
reserve from stress during labor and these fetuses often do not
reach their optimal birth weight (11). Therefore, it is crucial to perform
the timely early diagnosis of fetal FGR inn order to reduce the
number of stillbirths (12). Fetal
weight monitoring during pregnancy, taking into account the risks
that were present at the beginning of the pregnancy and that may
have occurred during the pregnancy, can reduce the incidence of
stillbirth and perinatal mortality (13,14).
Maternal age and parity should be considered when estimating fetal
weight, as well as other non-invasive methods of diagnosing the
gestational age of the fetus (14). The early diagnosis of FGR can
reduce the incidence of stillbirth and can allow for a reduction in
morbidity in full-term newborns (15). Other studies have noted that
newborns considered LGA were significantly less likely to be
referred for pre-testing and missed early detection of fetal
overgrowth (16,17). Maternal physiological factors, such
as weight at the beginning of pregnancy, age and parity can
influence the birth weight of the fetus and the risk of stillbirth
and perinatal mortality. In addition, the presence of maternal
pathological factors, such as hypertension, diabetes mellitus and
pre-eclampsia may increase the risk of stillbirth and perinatal
mortality. The present study investigated the effects of maternal
physiological and pathological characteristics on fetal birth
weight and on the risk of stillbirth and perinatal mortality.
Subjects and methods
Study population
The total number of charts analyzed was 9,580
individual charts and hospital records. Of these, 3,033 individual
charts and hospital records were not included in the study as they
had incomplete maternal and fetal data. The total of 6,457 the
individual cards and hospital records of pregnant females
hospitalized for delivery in women's consultations and maternity
hospitals in Semey city and nearby settlements in Zyryanovsk city,
Astana city, Aksu city, Almaty city, Atyrau city, in Kazakhstan
between January 1, 2016 and December 31, 2021. The populations
served in these counseling centers and maternity hospitals were of
similar ethnic and social backgrounds and had similar standards of
clinical management of pregnant women. The inclusion criteria were
the following: Females who gave consent for the study were between
the 22nd and 42nd weeks of gestation; i.e., they were term
singleton pregnant females with the presence of ultrasound
screening of the first trimester of pregnancy at 10-14 weeks. The
exclusion criteria included females with multiple pregnancies,
pregnancies complicated by neonatal chromosomal or structural
anomalies of the fetus. The gestation period was calculated from
the first day of the final menstrual period and corrected by the
index of the coccygeal-parietal size at the first screening
ultrasound according to the Clinical Protocol of the Ministry of
Health of the Republic of Kazakhstan ‘Management of physiological
pregnancy’ dated September 19, 2013. Individual charts and hospital
charts for pregnant women standardized at the national level across
the Kazakhstan. The Ethics Committee of Semey Medical University
approved the research protocol (#2 of from 10/25/2018), according
to the Declaration of Helsinki 1964. Informed consent was obtained
from each patient in writing.
Data collection
Maternal demographic and clinical characteristics
were recorded from individual pregnancy charts, including the
maternal age, height, weight, body mass index (BMI) at the
beginning of pregnancy, parity, presence of disease [a history of
arterial hypertension (AH), diabetes mellitus (DM), pre-eclampsia,
gestational hypertension (GH) and gestational DM (GDM) in the given
pregnancy] and the neonatal characteristics of the newborn. BMI was
defined as the weight (kg)/height in (m2). Pre-term
birth was defined as a birth between 22 and 37 full weeks of
gestation.
Concentration measurements
Newborns that were born weighing up to 2,500 g were
considered as newborns with a low birth weight. Newborns who were
born weighing >4,000 g were considered as overweight newborns.
Newborns born, but who succumbed before the period of 28 days were
recorded as perinatal mortality. Females with a maternal BMI at the
beginning of pregnancy up to and including 18.49 kg/m2
was considered as underweight. Those with a BMI between 18.5 and
24.9 kg/m2 was considered a normal weight. Those with a
BMI between 25 and 29.9 kg/m2 was considered as
overweight, and those with a BMI >30 kg/m2 was
considered obese.
Statistical analysis
All statistical analyses were performed using IBM
SPSS Statistics Version 26 (IBM Corp.) and the Stat Tech v. 3.0.9
program (developed by Stattech LLC). All variables were examined to
determine whether they were normally distributed. Descriptive
statistics included median (Q1-Q3) for non-normally distributed
continuous variables. The results were compared between fetal
weight groups and birth outcome groups. Non-parametric tests, such
as the Kruskal-Wallis test were used for non-normally distributed
for comparisons between three groups. The χ2 test or
Fisher's test were used for comparing differences in categorical
variables between groups. The diagnostic significance of maternal
physiological factors (age, parity and BMI) in predicting the birth
of a small or large fetus and birth outcomes was assessed using
receiver operating characteristic (ROC) analysis. All confidence
intervals (CIs) were 95%. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparisons of the subjects and
general information
The study population comprised 6,547 females aged
from 18 to 47 years. The total number of newborns born with a
normal weight was 5,253 newborns. A total of 566 newborns were born
weighing <2,500 g, and 728 newborns weighed >4,000 g. The
maternal demographic and neonatal characteristics of the study
population between the fetal weight group groups are presented in
Table I. Females with a newborn
weighing <2,500 g and those with a newborn weighing >4,000 g
were older in age than females who had newborns with a normal
weight (P=0.0001; Table I). As the
maternal age increased to >25 years, the risk of having a
newborn weighing <2,500 g and a newborn weighing >4,000 g
increased each year. Females aged 25-29 years gave birth to
newborns weighing <2,500 g 1.19-fold more often, those aged
30-34 years gave birth to newborns weighing <2,500 g 1.44-fold
more often, and females >35 years of age gave birth to newborns
weighing <2,500 g 1.82-fold more often than those aged 20-24
years (Table I). Females aged
25-29 years gave birth to newborns weighing >4,000 g 1.64-fold
more often, those aged 30-34 years gave birth to newborns weighing
>4,000 g 1.74-fold more often, and females >35 years of age
gave birth to newborns weighing >4,000 g 1.93-fold more often
than those aged 20-24 years (Table
I). Females <20 years of age gave birth to newborns weighing
<2,500 g less often than those aged 20-25 years.
 | Table IMaternal demographic and neonatal
characteristics by fetal weight group (n=6,547). |
Table I
Maternal demographic and neonatal
characteristics by fetal weight group (n=6,547).
| | Fetal weight
(g) | | P-values
(Kruskal-Wallis test) |
|---|
| | <2,500 | 2,500-4,000 | >4,000 | | |
|---|
| Parameters | n=566 | OR (95% CI) | n=5,253 | n=728 | OR (95% CI) | P-value
(χ2 test) | <2,500 vs.
2,500-4,000 g | <2,500 vs.
>4,000 g | 2,500-4,000 vs.
>4,000 g |
|---|
| Maternal age, years
Me (Q1-Q3) | 29 (25-33) | 27 (24-32) | 29 (25-33) | | 0.0001 | 0.9 | 0.0001 |
| Maternal age in
years, n (%) | | | | | | 0.0001 | | | |
|
<20 | 12 (0.18) | 0.8 (0.44-1.5) | 173 (2.64) | 9 (0.14) | 0.56
(0.28-1.12) | | | | |
|
20-24 | 122 (1.86) | 1.0 | 1,444 (22.05) | 134 (2.05) | 1.0 | | | | |
|
25-29 | 170 (2.6) | 1.19
(0.94-1.52) | 1,684 (25.72) | 257 (3.92) | 1.64
(1.32-2.52) | | | | |
|
30-34 | 146 (2.23) | 1.44
(1.12-1.86) | 1,197 (18.28) | 193 (2.95) | 1.74
(1.38-2.19) | | | | |
|
>35 | 116 (1.77) | 1.82
(1.39-2.38) | 755 (11.53) | 135 (2.06) | 1.93
(1.49-2.49) | | | | |
| BMI,
kg/м2) Me (Q1-Q3) | 23.44
(20.03-27.59) | 22.58
(20.28-25.71) | 24.98
(22.03-28.68) | | 0.004 | 0.0001 | 0.0001 |
| BMI, n (%) | | | | | | 0.0001 | | | |
|
WD | 65 (0.99) | 1.57
(1.18-2.1) | 467 (7.13) | 29 (0.44) | 0.59
(0.4-0.87) | | | | |
|
NW | 286 (4.37) | 1.0 | 3,236 (49.43) | 340 (5.19) | 1.0 | | | | |
|
OW | 125 (1.91) | 1.29
(1.04-1.61) | 1094 (16.71) | 220 (3.36) | 1.91
(1.59-2.3) | | | | |
|
Ob | 90 (1.37) | 2.23
(1.73-2.89) | 456 (6.96) | 139 (2.12) | 2.9
(2.33-3.62) | | | | |
| Parity, n (%) | | | | | | 0.0001 | | | |
|
0
births | 203 (3.1) | 1.0 | 1,774 (27.1) | 174 (2.66) | 1.0 | | | | |
|
1st
birth | 168 (2.57) | 0.81
(0.65-1.0) | 1,821 (27.81) | 230 (3.51) | 1.29
(1.05-1.58) | | | | |
|
2
births | 116 (1.77) | 0.9
(0.71-1.15) | 1,121 (17.12) | 194 (2.35) | 1.76
(1.42-2.19) | | | | |
|
≥3 | 79 (1.21) | 1.29
(0.97-1.7) | 537 (8.2) | 130 (1.98) | 4.47
(1.93-3.16) | | | | |
| Sex, n (%) | | | | | | 0.0001 | | | |
|
Boy | 293 (4.47) | 1,1
(0.93-1.31) | 2,591 (39.57) | 452 (6.9) | 1.68
(1.43-1.97) | | | | |
|
Girl | 273 (4.17) | 1.0 | 2,662 (40.66) | 276 (4.21) | 1.0 | | | | |
| Duration in days,
Me (Q1-Q3) | 235 (204-255) | 279 (273-285) | 285 (280-289) | | 0.0001 | 0.0001 | 0.0001 |
| Premature birth, n
(%) | | | | | | 0.0001 | | | |
|
No | 116 (1.77) | - | 5,100 (77.9) | 727 (11.1) | - | | | | |
|
Yes | 450 (6.87) | - | 153 (2.34) | 1 (0.01) | - | | | | |
Females with a newborn weighing <2,500 g
(P=0.004) and females with a newborn weighing >4,000 g had a
significantly higher BMI (P=0.0001). A total of 561 females were
underweight, 3,862 females had a healthy weight, 1,439 females were
overweight and 685 females were obese P=0.0001 (Table I). As demonstrated in Table I, maternal underweight, overweight
and obesity at the beginning of pregnancy increased the risk of
having a newborn weighing <2,500 g by 1.57-, 1.29- and
2.23-fold, respectively. Maternal underweight was associated with a
reduced risk of having a newborn weighing >4,000 g. In addition,
maternal overweight and obesity at the beginning of the pregnancy
increased the risk of having a newborn weighing >4,000 g by
almost 2-fold (Table I).
While examining the effect of parity on fetal
weight, it was demonstrated that newborns weighing <2,500 g were
more often born to mothers who had a history of three or more
births than mothers who had newborn weighing between 2,500-4,000 g
and mothers who had newborns weighing >4,000 g. The risk of
having a newborn weighing >4,000 g increased with each birth
from 1.29- to 4.47-fold (Table I).
In the present study sample, boys were born 1.1-fold more often
with a weight <2,500 g and 1.68-fold more often with a weight
>4,000 g compared to girls. Term fetuses were more likely to be
born at term, and newborns weighing <2,500 g were more likely to
be born prematurely (Table I).
The present study also examined the influence of
maternal pathological factors, such as a history of AH and DM,
preeclampsia, GH and GDM in a given pregnancy, on fetal weight at
birth. The maternal characteristics by extragenital diseases of the
study population between the fetal weight group groups are
presented in Table II. A maternal
history of AH increased the risk of having a newborn weighing
<2,500 g. The birth of a newborn weighing >4,000 g was less
common among females with AH and with preeclampsia (Table II). The risk of having a newborn
weighing <2,500 g increased in females with preeclampsia, and
this risk increased with the severity of preeclampsia (Table II). The presence of GH, on the
contrary, increased the risk of having a newborn weighing >4,000
g by 1.47-fold and reduced the risk of having a newborn weighing
<2,500 g (Table II). The
presence of GDM in a given pregnancy increased the risk of having a
newborn weighing >4,000 g by 2.26-fold. The presence of any type
of maternal DM in the present study sample did not affect fetal
weight at birth (Table II).
 | Table IIMaternal characteristics by
extragenital diseases in fetal weight groups (n=6,547). |
Table II
Maternal characteristics by
extragenital diseases in fetal weight groups (n=6,547).
| | Fetal weight
(g) | |
|---|
| | <2,500 | 2,500-4,000 | >4,000 | |
|---|
| Parameters | n=566 | OR (95% CI) | n=5,253 | n=728 | OR (95% CI) | P-value
(χ2 test or Fisher's testa) |
|---|
| Hypertension | | | | | | 0.0001 |
|
No | 521 (7.96) | 1.0 | 5,141 (78.52) | 717 (10.95) | 1.0 | |
|
1st
degree | 36 (0.55) | 4.61
(3.07-6.92) | 77 (1.18) | 9 (0.14) | 0.84
(0.42-1.68) | |
|
2
degrees | 9 (0.14) | 2.54
(1.21-5.31) | 35 (0.53) | 2 (0.03) | 0.41 (0.1-1.7) | |
| Pre-eclampsia | | | | | | 0.0001 |
|
No | 395 (6.03) | 1.0 | 5,022 (76.71) | 713 (10.89) | 1.0 | |
|
1st
degree | 19 (0.29) | 2.26
(1.37-3.72) | 107 (1.63) | 6 (0.09) | 0.39
(0.17-0.9) | |
|
2
degrees | 152 (2.32) | 15.58
(12.04-20.18) | 124 (1.89) | 9 (0.14) | 0.51
(0.26-1.01) | |
| Gestational
hypertension | | | | | | 0.029 |
|
No | 541 (8.26) | 1.0 | 4,986 (76.16) | 675 (10.31) | 1.0 | |
|
Yes | 25 (0.38) | 0.86
(0.57-1.31) | 267 (4.08) | 53 (0.81) | 1.47
(1.08-1.99) | |
| Diabetes
mellitus | | | | | | 0.12a |
|
No | 565 (8.63) | - | 5,242 (80.08) | 723 (11.04) | - | |
|
1 type | 1 (0.01) | - | 7 (0.11) | 4 (0.06) | - | |
|
2 type | 0 (0) | - | 4 (0.06) | 1 (0.01) | - | |
| Gestational
diabetes | | | | | | 0.0001 |
|
No | 561 (8.57) | 1.0 | 5,135 (78.43) | 692 (10.57) | 1.0 | |
|
Yes | 5 (0.08) | 0.39
(0.16-0.95) | 118 (1.8) | 36 (0.55) | 2.26
(1.55-3.32) | |
In the present study, birth outcomes were
categorized into three groups as follows: Live birth, stillbirth
and perinatal mortality up to 28 days of life. A total of 6,312
births were live births. Stillbirth accounted for 193 cases, and
the mortality of a newborn up to 28 days of life accounted for 42
cases. The results of analyzes by birth outcome group are
demonstrated in Tables III,
IV and V. The mean maternal age by birth outcome
differed significantly (Table
III). The risk of stillbirth and perinatal mortality increased
with age. The risk of stillbirth was almost 2-fold higher in
females <20 years of age compared to females aged 20-25 years.
This risk was highest in females >25 years of age. The risk of
stillbirth was 1.28-fold higher in females aged 25-29 years, it was
1.63-fold higher in females aged 30-35 years and was 1.92-fold
higher in females >35 years of age compared to females aged
20-25 years (Table III).
 | Table IIIMaternal demographic characteristics
in birth outcome groups (n=6,547). |
Table III
Maternal demographic characteristics
in birth outcome groups (n=6,547).
| | | | P-values
(Kruskal-Wallis test) |
|---|
| | Birth outcome | | |
|---|
| Parameters | Live birth n
(%) | Stillbirth n
(%) | OR (95% CI) | Perinatal mortality
n (%) | OR (95% CI) | P-value
(χ2 test) | Live birth vs.
stillbirth | Live birth vs.
perinatal mortality | Stillbirth vs.
perinatal mortality |
|---|
| Maternal age,
years; Me (Q1-Q3) | 28 (24-32) | 29 (25-33) | 30 (25-34.25) | | 0.004 | 0.02 | 0.4 |
| Maternal age in
years, n (%) | | | | | | 0.01 | | | |
|
<20 | 186 (2.84) | 8 (0.12) | 1.98
(0.91-4.32) | 0 | - | | | | |
|
20-24 | 1,655 (25.28) | 36 (0.55) | 1.0 | 9 (0.14) | 1.0 | | | | |
|
25-29 | 2,046 (31.25) | 57 (0.87) | 1.28
(0.84-1.95) | 8 (0.12) | 0.72
(0.28-1.87) | | | | |
|
30-34 | 1,469 (22.44) | 52 (0.79) | 1.63
(1.06-2.5) | 15 (0.23) | 1.88
(0.82-4.3) | | | | |
|
>35 | 956 (14.61) | 40 (0.61) | 1.92
(1.22-3.04) | 10 (0.15) | 1.92
(0.78-4.75) | | | | |
| BMI,
kg/м2) Me (Q1-Q3) | 22.85
(20.41-26.15) | 23.83
(20.31-27.59) | 25.08
(21.29-29.98) | | 0.1 | 0.05 | 0.25 |
| BMI, n (%) | | | | | | 0.01 | | | |
|
DW | 539 (8.24) | 17 (0.26) | 1.15
(0.68-1.93) | 5 (0.08) | 2.17
(0.79-5.95) | | | | |
|
NW | 3,743 (57.17) | 103 (1.57) | 1.0 | 16 (0.24) | 1.0 | | | | |
|
OW | 1,383 (21.12) | 45 (0.69) | 1.18
(0.83-1.69) | 11 (0.17) | 1.86
(0.86-4.02) | | | | |
|
Ob | 647 (9.88) | 28 (0.43) | 1.57
(1.03-2.41) | 10 (0.15) | 3.62
(1.63-8.0) | | | | |
| Parity, n (%) | | | | | | 0.13 | | | |
|
0
births | 2,071 (31.64) | 61 (0.93) | - | 19 (0.29) | - | | | | |
|
1st
birth | 2,149 (32.82) | 59 (0.9) | - | 11 (0.17) | - | | | | |
|
2
births | 1,382 (21.11) | 40 (0.61) | - | 9 (0.14) | - | | | | |
|
≥3 | 710 (10.85) | 33 (0.5) | - | 3 (0.04) | - | | | | |
 | Table IVMaternal characteristics by
extragenital diseases in birth outcome groups (n=6,547). |
Table IV
Maternal characteristics by
extragenital diseases in birth outcome groups (n=6,547).
| | Fetal weight
(g) | |
|---|
| Parameters | Live birth, n
(%) | Stillbirth, n
(%) | OR (95% CI) | Perinatal death n
(%) | OR (95% CI) | P-value
(χ2 test of Fisher's testa) |
|---|
| Hypertension | | | | | | 0.0001a |
|
No | 6,160 (94.09) | 184 (2.81) | 1.0 | 35 (0.53) | 1.0 | |
|
1st
degree | 106 (1.62) | 9 (0.14) | 2.84
(1.42-5.7) | 7 (0.11) | 11.62
(5.05-26.76) | |
|
2
degrees | 46 (0.7) | 0 | - | 0 | - | |
| Pre-eclampsia | | | | | | 0.0001a |
|
No | 5,951 (90.9) | 159 (2.43) | 1.0 | 20 (0.3) | 1.0 | |
|
1st
degree | 123 (1.88) | 6 (0.09) | 1.83
(0.79-4.21) | 3 (0.05) | 7.26
(2.13-24.75) | |
|
2
degrees | 238 (3.63) | 28 (0.43) | 4.4
(2.89-6.72) | 19 (0.29) | 23.75
(12.51-45.1) | |
| Gestational
hypertension | | | | | | 0.7 |
|
No | 5,982 (91.37) | 181 (2.76) | - | 39 (0.6) | - | |
|
Yes | 330 (5.04) | 12 (0.18) | - | 3 (0.05) | - | |
| Diabetes
mellitus | | | | | | 0.002a |
|
No | 6,299 (96.21) | 189 (2.89) | 1.0 | 42 (0.64) | 1.0 | |
|
1 type | 8 (0.12) | 4 (0.06) | 16.66
(4.97-55.82) | 0 | - | |
|
2 type | 5 (0.08) | 0 | - | 0 | - | |
| Gestational
diabetes | | | | | | 0.04a |
|
No | 6,154(94) | 193 (2.95) | - | 41 (0.63) | - | |
|
Yes | 158 (2.41) | 0 | - | 1 (0.01) | - | |
 | Table VNeonatal characteristics in birth
outcome groups (n=6,547). |
Table V
Neonatal characteristics in birth
outcome groups (n=6,547).
| | Birth outcome | | P-values
(Kruskal-Wallis test) |
|---|
| Parameters | Live birth. birth.
n (%) | Stillbirth. n
(%) | OR (95% CI) | Perinatal
mortality. n (%) | OR (95% CI) | P-value
(χ2 test or Fisher's testa) | Live birth vs.
stillbirth | Live birth vs.
perinatal mortality | Stillbirth vs.
perinatal mortality |
|---|
| Sex, n (%) | | | | | | 0.62 | | | |
|
Boy | 3,209 (49.01) | 104 (1.59) | - | 23 (0.35) | - | | | | |
|
Girl | 3,103 (47.4) | 89 (1.36) | - | 19 (0.29) | - | | | | |
| Fetal weight (g),
Me (Q1-Q3) | 3,420
(3,090-3,750) | 1,577
(758-2,750) | 800
(595-962.5) | | 0.0001 | 0.0001 | 0.0001 |
| Fetal weight
(g) | | | | | | 0.0001a | | | |
|
<2,500 | 389 (5.95) | 137 (2.09) | - | 40 (0.61) | - | | | | |
|
2,500-4,000 | 5201 (79.44) | 50 (0.76) | - | 2 (0.03) | - | | | | |
|
>4,000 | 722 (11.03) | 6 (0.09) | 0.86
(0.37-2.02) | 0 | - | | | | |
| Duration in days,
Me (Q1-Q3) | 279 (272-285) | 220 (188-265) | 194 (184-205) | | 0.0001 | 0.0001 | 0.0001 |
| Premature birth, n
(%) | | | | | | 0.0001 | | | |
|
No | 5,883(89.86) | 58 (0.89) | - | 2 (0.03) | - | | | | |
|
Yes | 429 (6.55) | 135 (2.06) | - | 40 (0.61) | - | | | | |
Higher mean BMI scores were observed in the
perinatal mortality groups compared with live births (Table III). The risk of stillbirth
increased in underweight, overweight and obese mothers by 1.15- to
1.57-fold. The risk of perinatal mortality was markedly higher in
underweight and obese mothers (Table
III). In the present study sample, parity did not affect fetal
mortality.
As demonstrated in Table IV, the presence of a maternal
history of AH and DM and the complication of this pregnancy with
preeclampsia increased the risk of stillbirth and perinatal
mortality in the present study sample. Perinatal mortality was
particularly high in mothers with AH and preeclampsia. However,
stillbirths were more common in mothers with type 1 DM. GH a given
pregnancy did not influence fetal mortality in the present study
sample (Table IV).
As demonstrated in Table V, stillbirth and perinatal
mortality were more likely to be observed when the fetal weight was
<2,500 g, which was expected. Premature birth often resulted in
stillbirth and perinatal fetal mortality, since a premature fetus
often cannot adapt to the external environment and may have various
congenital anomalies. However, stillbirth and perinatal mortality
also occur in full-term pregnancies, and the cause of mortality is
sometimes unclear. Fetal sex was not found to influence fetal
mortality in the present study.
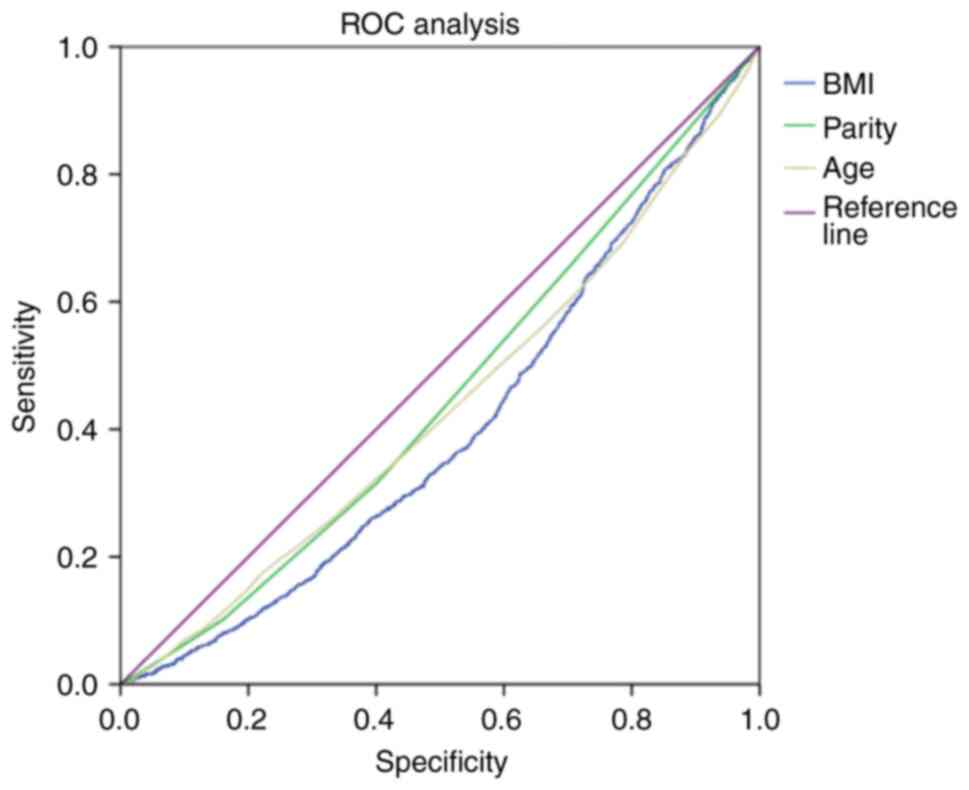
ROC analysis
The present study also assessed the diagnostic
significance of maternal physiological factors (age, parity and
BMI) in predicting the birth of a small or large fetus using ROC
curves (Fig. 1). The area of the
ROC curve corresponding to the association between the prediction
of small or large fetal weights and maternal age, maternal weight
and maternal BMI was 0.43±0.009 (95% CI, 0.42-0.45; P=0.0001);
0.45±0.009 (95% CI, 0.45-0.47; P=0.0001) and 0.4±0.009 (95% CI,
0.39-0.42; P=0 .0001), respectively (Fig. 1). The age threshold was 20.5 years
at the cut-off point. A higher risk of delivering a small or large
fetus was predicted for females ≥20.5 years of age. The sensitivity
and specificity of the method were 93.7 and 96.4%, respectively
(Table VI). The threshold value
of maternal parity at the cut-off point was 0.5. A high risk of
having a small or large fetus was predicted during the first
pregnancy, and this risk decreased with each birth. The sensitivity
and specificity of the method were 66.2 and 70.9%, respectively.
The threshold BMI value was 18.5 kg/m2 at the cut-off
point. A higher risk of having a small or large fetus was predicted
for females with a BMI ≥18.5 kg/m2. The sensitivity and
specificity of the method were 91.1 and 92.7%, respectively
(Fig. 1 and Table VI).
 | Table VISensitivity and specificity of
maternal demographic and neonatal characteristics in predicting the
birth of a small or large fetus. |
Table VI
Sensitivity and specificity of
maternal demographic and neonatal characteristics in predicting the
birth of a small or large fetus.
| | Crude | |
|---|
| Parameters | Cut-off point | Sensitivity
(%) | Specificity
(%) | P-value |
|---|
| Predicting the
birth of a small or large fetus | | | | |
|
Maternal
age, years | 20.5 | 93.7 | 96.4 | 0.0001 |
|
BMI
(kg/m2) | 18.5 | 91.1 | 92.7 | 0.0001 |
|
Parity, n
(%) | 0.5 | 66.2 | 70.9 | 0.0001 |
| Predicting
stillbirth or perinatal mortality | | | | |
|
Maternal
age, years | 21.5 | 92.3 | 90.2 | 0.0001 |
|
BMI
(kg/m2) | 18.5 | 90.6 | 91.4 | 0.0001 |
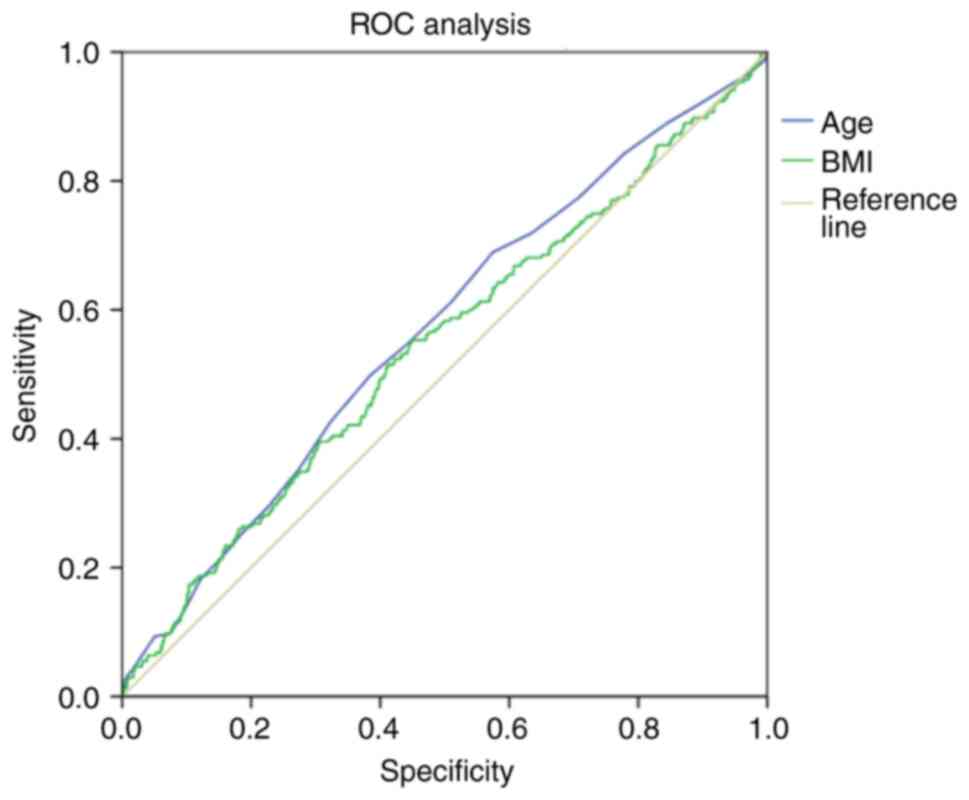
The present study further assessed the diagnostic
value of maternal physiological factors (age and BMI) in predicting
stillbirth or perinatal mortality using ROC curves (Fig. 2). The area of the ROC curve
corresponding to the association between the prognosis of fetal
mortality and maternal age and maternal BMI was 0.57±0.019 (95% CI,
0.53-0.61; P=0.0001) and 0.54±0 .02 (95% CI, 0.5-0.58; P=0.025),
respectively (Fig. 2). The age
threshold was 21.5 years at the cut-off point (Table VI). A higher risk of stillbirth or
perinatal mortality was predicted in females aged ≥21.5 years. The
sensitivity and specificity of the method were 92.3 and 90.2%,
respectively. The threshold BMI value is was 18.5 kg/m2
at the cut-off point. A higher risk of stillbirth and perinatal
mortality was predicted in females with a BMI ≥18.5
kg/m2. The sensitivity and specificity of the method
were 90.6 and 91.4%, respectively (Table VI).
Discussion
The present study aimed to investigate the
association between fetal weight and maternal physiological and
pathological characteristics. The results revealed that maternal
physiological and pathological characteristics were statistically
higher in the group of newborns considered SGA and LGA than the
group of newborns with normal weight. According to the results fo
the ROC analysis, maternal age, maternal weight and maternal BMI
were independently associated with risk of prediction of SGA or
LGA. In the present study, the maternal BMI at the beginning of
pregnancy was found to influence the birth weight of the newborns.
However, different studies have yielded varying results. In the
study by Cnattingius et al (18), the opposite result was found for
the association between maternal BMI and low fetal weight. The
authors of that study claimed that obesity protected from fetuses
with a low birth weight. However, they did not use individualized
centiles. Perhaps the individual physiological characteristics of
the mother were not taken into account (18). A newborn weighing 3,500 g would be
considered normal for the general population and for a pregnant
woman of normal weight. However, in a pregnant woman with a high
BMI, a fetus weighing 3,500 g may be below the 10th percentile
(19).
The maternal age and BMI were found to be
independently associated with risk of predicting of stillbirth or
perinatal mortality. In the present study, maternal weight at the
beginning of pregnancy was found to be associated with the risk of
perinatal mortality. Pregnant females who were underweight,
overweight and obese at the beginning of pregnancy were at a higher
risk of their newborns experiencing perinatal mortality. The study
by Gardosi et al (19)
analyzed the incidence of low birth weight fetuses in BMI groups
using an adapted individual standard and found a directly
proportional association between perinatal mortality and BMI.
Defined by individual percentiles, fetuses with a low birth weight
were more common in the group with a higher BMI, and also agreed
well with the trend in perinatal mortality rates (19). According to the result of the
unadapted standard, the incidence of fetuses with a low birth
weight was high in thin pregnant women, and on the contrary, it was
lower in women with a high BMI. This actually hides the fact that a
fetus can be relatively small compared to its optimal weight.
However, the adapted standard indicates that obese pregnant women
may be at an increased risk of having a fetus with a low birth
weight (19,20). Gardosi et al (19) argued that when maternal parity was
taken into account, the adapted low birth weight fetal standard
better reflected the risk of perinatal mortality, while the
unadapted standard overestimated the frequency of fetuses with a
low birth weight in females when parity was not taken into account,
which did not reflect the increased mortality.
In the present study, the risk of stillbirth and
perinatal mortality increased with age. Females with a newborn
weighing <2,500 g and those with newborns weighting >4,000 g
were older than females who had newborns with a normal weight. In
other studies, fetal weight at birth was influenced by maternal age
and parity (21). According to the
French College of Obstetricians and Gynecologists, the development
of FGR was influenced by a maternal age >35 years and parity
(22). In a previous study in
Slovenia, fetal weight at birth was found to be influenced by
maternal height, maternal weight at early gestation, maternal age
and parity, fetal sex and GDM (23).
In another study conducted in Iran, fetal birth
weight was shown to be influenced by maternal height, maternal
weight in early pregnancy, parity and the sex of the baby, as well
as paternal height and weight (24). In that study, fetal birth weight
was also shown to be influenced by rural residence, anemia,
pre-existing and GDM and pre-eclampsia (24). Another study reported an
association of fetal birth weight with maternal height, maternal
weight at the beginning of pregnancy, parity, ethnicity,
gestational age at delivery and fetal sex. Adverse pregnancy
outcomes were associated with FGR regardless of gestational age at
delivery (2). In a French study,
fetal height and birth weight were influenced by gestational age,
fetal sex, maternal height and weight at the beginning of
pregnancy, parity and ethnicity (25).
In the INTERGROWTH and NICHD studies, fetal weight
varied by fetal sex, maternal race and ethnicity (26,27).
Studies in recent years have shown that the fetus loses between 10
and 30% of its body weight between the time of intrauterine death
and the subsequent postnatal assessment. The majority of newborns
who are stillborn may have had a normal weight prior to mortality
and FGR may have appeared after birth. On the other hand, newborns
who are stillborn with FGR who were born with a normal birth weight
may be missed (27). In the
present study sample, boys were born with a weight <2,500 g and
with a weight >4,000 g compared to girls. Newborns weighing
<2,500 g were more likely to be born prematurely. This was
expected, since the pathological course of pregnancy could cause
various pregnancy complications, both for the fetus and for the
mother. The results obtained are similar to those of other studies.
In their study, Pritchard et al found that female infants
below the 10th percentile had no risk of stillbirth,
hospitalization to the intensive care unit, low Apgar scores, and
emergency caesarean section, but had increased risks associated
with medical interventions for the induction of labor. In addition,
newborn boys weighing below the 10th percentile had a high risk of
perinatal mortality, to the intensive care unit hospitalization,
Apgar scores <7 at 5 min and operative delivery (29). In another study, the authors
reported that male fetuses weighed more than female fetuses, and
that maternal height, early pregnancy weight, and maternal parity
influenced fetal birth weight (26). Another study found that male
infants considered to SGA were more likely to have a higher risk of
stillbirth, perinatal mortality, admission to the intensive care
unit, Apgar <7 at 5 min and emergency cesarean section compared
to female infants (29). Other
researchers suggest using fetal growth assessment standards
adjusted for the variable fetal sex to identify male infants at
increased risk of adverse outcomes, including stillbirth (25).
Maternal factors for the development of FGR include
GDM, renal failure, autoimmune diseases, erythematous heart
disease, cyanotic heart defects, hypertension, hyperglycemia or
preeclampsia, antiphospholipid syndrome, use and abuse of
psychoactive substances, multiple pregnancy, exposure to teratogens
and infectious diseases (3). The
present study confirmed the influence of maternal pathological
factors, such as a history of AH and DM, preeclampsia, GH and GDM
in a given pregnancy on fetal weight at birth. The maternal
characteristics by extragenital diseases of the study population
between the groups of fetal weight. A maternal history of AH
increased the risk of having a newborn weighing <2,500 g.
Females with AH and preeclampsia in were less likely to give birth
to a newborn weighing >4,000 g. The risk of having a newborn
weighing <2,500 g increased in females with preeclampsia during
this pregnancy, and this risk increased with the severity of
preeclampsia. During pre-eclampsia, there will be placental
ischemia, which leads to uteroplacental hypoperfusion and increases
the risk of stillbirth and perinatal death (30). In preeclampsia, placental lesions
are common as a consequence of maternal hypoperfusion (31). In the case that preeclampsia begins
early in pregnancy, it increases the likelihood of placental damage
associated with hypoperfusion. Other authors have stated that
pregnant women with pre-eclampsia in early pregnancy often had
vascular lesions and placental hypoplasia, and by contrast,
pregnant women with pre-eclampsia diagnosed at the end of the third
trimester had placental hyperplasia (32).
In the present study, the presence of GH in this
pregnancy increased the risk of having a newborn weighing >4,000
g and reduced the risk of having a newborn weighing <2,500 g. A
Chinese-American study reported that the early onset of GH and
preeclampsia was relatively more unfavorable for the fetus and
increased the risk of FGR (33).
Increased blood pressure during pregnancy leads to endothelial
dysfunction and reduced placental perfusion, this creates
conditions for reduced fetal growth and a low birth weight
(34).
In the present study, a significant association was
found between females with GDM in a given pregnancy and the risk of
having a newborn weighing >4,000 g. The presence of any type of
diabetes in the mother in the present study sample did not affect
fetal weight at birth. This result may be due to the fact that
diabetes has long been a known factor, which leads to the birth of
a newborn weighing >4,000 g; pregnancy management in such
females is always carried out in collaboration with
endocrinologists, and such females receive full observation,
treatment and prophylaxis to prevent the influence of DM on the
fetus. A previous study found that fetuses with FGR were more
likely to be born to women with coronary heart disease, AH and
insulin resistance (35).
Assessing the risk of adverse perinatal outcomes in GDM, the
authors of a previous cohort study reported that GDM was associated
with an increased risk of preterm labor, an increased risk of
stillborn fetuses and pre-eclampsia, fetal delivery with LGA
(36). Fetuses with LGA are common
in pregnant women with DM and GDM, as even moderate maternal
hyperglycemia increases the rate of accelerated fetal growth
(37).
The present study had certain limitations, which
should be mentioned. Firstly, the present study had a retrospective
design and the data collection was based on materials from the
medical history and individual cards of pregnant females. The
second limitation is that the diagnosis of FGR may have included
newborns that are physiologically small and may have missed large
fetuses that have not reached their physiological potential.
In conclusion, despite improvements in knowledge and
the understanding of pathological conditions during pregnancy,
there is still a high risk of stillbirth and perinatal mortality,
regardless of fetal weight. Therefore, it is necessary to prevent
the risk of stillbirth and perinatal mortality in healthy newborns,
as well as in small newborns who were born with a normal weight,
but have FGR. Thus, further studies which large sample sizes are
required in the future to investigate the issue of fetal FGR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS, KS, BI and GT were major contributors to the
study and made substantial contributions to the conception and
design of the study. MS and AS performed the data collection and
the statistical analyses of the data. MS and AS also participated
in the writing the manuscript. MS and AS confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Semey Medical University, which approved the
research protocol (Protocol # 2 of from 10/25/2018). Informed
consent was obtained from each patient in writing.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Millogo T, Ouédraogo GH, Baguiya A, Meda
IB, Kouanda S and Sondo B: Factors associated with fresh
stillbirths: A hospital-based, matched, case-control study in
Burkina Faso. Int J Gynecol Obstet. 135 (Suppl 1):S98–S102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bonellie S, Chalmers J, Gray R, Greer I,
Jarvis S and Williams C: Centile charts for birthweight for
gestational age for Scottish singleton births. BMC Pregnancy
Childbirth. 8(5)2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
ACOG: Fetal growth restriction. Pract
Bullettin. 204:97–109. 2019.
|
|
4
|
Ministry of Health of the Republic of
Kazakhstan. Care of low birth weight newborns. Clin Protocol
Diagnosis Treatment: 177, 2023.
|
|
5
|
Ministry of Health of the Republic of
Kazakhstan. Shoulder dystocia (difficulty in the shoulder girdle at
birth). Clin Protocol Diagnosis Treatment: 178, 2023.
|
|
6
|
Nguyen MT and Ouzounian JG: Evaluation and
Management of Fetal Macrosomia. Obstet Gynecol Clin North Am.
48:387–399. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beckmann CR, Ling FW, Herbert WN and Laube
DWSR: Obstetrics and gynecology. Lippincott Williams & Wilkins;
8th edition. Section II. 350–366. 2019.
|
|
8
|
Figueras F, Figueras J, Meler E, Eixarch
E, Coll O, Gratacos E, Gardosi J and Carbonell X: Customised
birthweight standards accurately predict perinatal morbidity. Arch
Dis Child Fetal Neonatal Ed. 92:F277–F280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barros FC, Bhutta ZA, Batra M, Hansen TN,
Victora CG and Rubens CE: GAPPS Review Group. Global report on
preterm birth and stillbirth (3 of 7): Evidence for effectiveness
of interventions. BMC Pregnancy Childbirth. 10 (Suppl
1)(S3)2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Risnes KR, Vatten LJ, Baker JL, Jameson K,
Sovio U, Kajantie E, Osler M, Morley R, Jokela M, Painter RC, et
al: Birthweight and mortality in adulthood: A systematic review and
meta-analysis. Int J Epidemiol. 40:647–661. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crotty M, Whitehead CH, Wundke R, Giles
LC, Ben-Tovim D and Phillips PA: Transitional care facility for
elderly people in hospital awaiting a long term care bed:
Randomised controlled trial. Br Med J. 331:1110–1113.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gardosi J, Madurasinghe V, Williams M,
Malik A and Francis A: Maternal and fetal risk factors for
stillbirth: Population based study. BMJ. 346(f108)2013.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
The Investigation and Management of the
Small-for-Gestational-Age Fetus. Green-top Guidel, 1-34, 2013.
|
|
14
|
Williams M, Turner S, Butler E and Gardosi
J: Fetal growth surveillance-Current guidelines, practices and
challenges. Ultrasound. 26:69–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ravula PC, Veluganti S, Gopireddy MMR and
Aziz N: Impact of introduction of the growth assessment protocol in
a South Indian tertiary hospital on SGA detection, stillbirth rate
and neonatal outcome. J Perinat Med. 50:729–736. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gelbaya TA and Nardo LG: Customised fetal
growth chart: A systematic review. J Obstet Gynaecol. 25:445–450.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gardosi J and Francis A: Controlled trial
of fundal height measurement plotted on customised antenatal growth
charts. Br J Obstet Gynaecol. 106:309–317. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cnattingius S, Bergström R, Lipworth L and
Kramer MS: Prepregnancy weight and the risk of adverse pregnancy
outcomes. N Engl J Med. 338:147–152. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gardosi J, Clausson B and Francis A: The
value of customised centiles in assessing perinatal mortality risk
associated with parity and maternal size. BJOG. 116:1356–1363.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gardosi J, Clausson B and Francis A: The
use of customised versus population-based birthweight standards in
predicting perinatal mortality. BJOG. 114:1301–1303.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kiserud T, Benachi A, Hecher K, Perez RG,
Carvalho J, Piaggio G and Platt LD: The World Health Organization
fetal growth charts: Concept, findings, interpretation, and
application. Am J Obstet Gynecol. 218 (Suppl 2):S619–S629.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vayssière C, Sentilhes L, Ego A, Bernard
C, Cambourieu D, Flamant C, Cambourieu D, Flamant C, Gascoin G,
Gaudineau A, et al: Fetal growth restriction and intra-uterine
growth restriction: Guidelines for clinical practice from the
French College of Gynaecologists and Obstetricians. Eur J Obstet
Gynecol Reprod Biol. 193:10–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Premru-Srsen T, Verdenik I, Mihevc
Ponikvar B, Hugh O, Francis A and Gardosi J: Customised birthweight
standard for a Slovenian population. J Perinat Med. 47:270–275.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nasri K, Hantoushzadeh S, Hugh O,
Heidarzadeh M, Habibelahi A, Shariat M, Tara F, Kashanian M,
Radmehr M, Yekaninejad MS, et al: Customized birthweight standard
for an Iranian population. J Matern Fetal Neonatal Med.
34:3651–3656. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Monier I, Blondel B, Ego A, Kaminski M,
Goffinet F and Zeitlin J: Does the presence of risk factors for
fetal growth restriction increase the probability of antenatal
detection? A French National Study. Paediatr Perinat Epidemiol.
30:46–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tarca AL, Romero R, Gudicha DW, Erez O,
Hernandez-Andrade E, Yeo L, Bhatti G, Pacora P, Maymon E and Hassan
SS: A new customized fetal growth standard for African American
women: The PRB/NICHD Detroit study. Am J Obstet Gynecol. 218 (Suppl
2):S679–S691.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Damhuis SE, Ganzevoort W and Gordijn SJ:
Abnormal Fetal Growth: Small for gestational age, fetal growth
restriction, large for gestational age: Definitions and
epidemiology. Obstet Gynecol Clin North Am. 48:267–79.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Poon LCY, Volpe N, Muto B, Syngelaki A and
Nicolaides KH: Birthweight with gestation and maternal
characteristics in live births and stillbirths. Fetal Diagn Ther.
32:156–165. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pritchard NL, Walker SP, Mitchell AR, Tong
S and Lindquist AC: Adjusting growth standards for fetal sex
improves correlation of small babies with stillbirth and adverse
perinatal outcomes: A state-wide population study. PLoS One.
17(e0274521)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mifsud W and Sebire NJ: Placental
pathology in early-onset and late-onset fetal growth restriction.
Fetal Diagn Ther. 36:117–128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ogge G, Chaiworapongsa T, Romero R,
Hussein Y, Kusanovic JP, Yeo L, Kim CJ and Hassan SS: Placental
lesions associated with maternal underperfusion are more frequent
in early-onset than in late-onset preeclampsia. J Perinat Med.
39:641–652. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nelson DB, Ziadie MS, McIntire DD, Rogers
BB and Leveno KJ: Placental pathology suggesting that preeclampsia
is more than one disease. Am J Obstet Gynecol. 210:66.e1–7.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Li N, An H, Li Z, Zhang L, Li H,
Zhang Y and Ye R: Impact of gestational hypertension and
preeclampsia on low birthweight and small-for-gestational-age
infants in China: A large prospective cohort study. J Clin
Hypertens (Greenwich). 23:835–842. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Everett TR and Lees CC: Beyond the
placental bed: Placental and systemic determinants of the uterine
artery Doppler waveform. Placenta. 33:893–901. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barker DJP, Bull AR, Osmond C and Simmonds
SJ: Fetal and placental size and risk of hypertension in adult
life. BMJ. 301:259–262. 1990.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Billionnet C, Mitanchez D, Weill A, Nizard
J, Alla F, Hartemann A and Jacqueminet S: Gestational diabetes and
adverse perinatal outcomes from 716,152 births in France in 2012.
Diabetologia. 60:636–644. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dittkrist L, Vetterlein J, Henrich W,
Ramsauer B, Schlembach D, Abou-Dakn M, Gembruch U, Schild RL,
Duewal A and Schaefer-Graf UM: Percent error of ultrasound
examination to estimate fetal weight at term in different
categories of birth weight with focus on maternal diabetes and
obesity. BMC Pregnancy Childbirth. 22(241)2022.PubMed/NCBI View Article : Google Scholar
|