1. Introduction
Prostate cancer (PCa) poses a serious global health
issue, particularly among older males (1-3)
and remains the second most common type of cancer diagnosed in
males following skin cancer. It is also the fifth leading cause of
cancer-related mortality worldwide, underscoring the critical need
for effective management and prevention strategies. Understanding
the complex interplay of risk factors, symptoms, treatment options
and patient outcomes is essential for improving the detection and
treatment of PCa. However, one of the key challenges lies in
addressing racial disparities in the development and prognosis of
PCa.
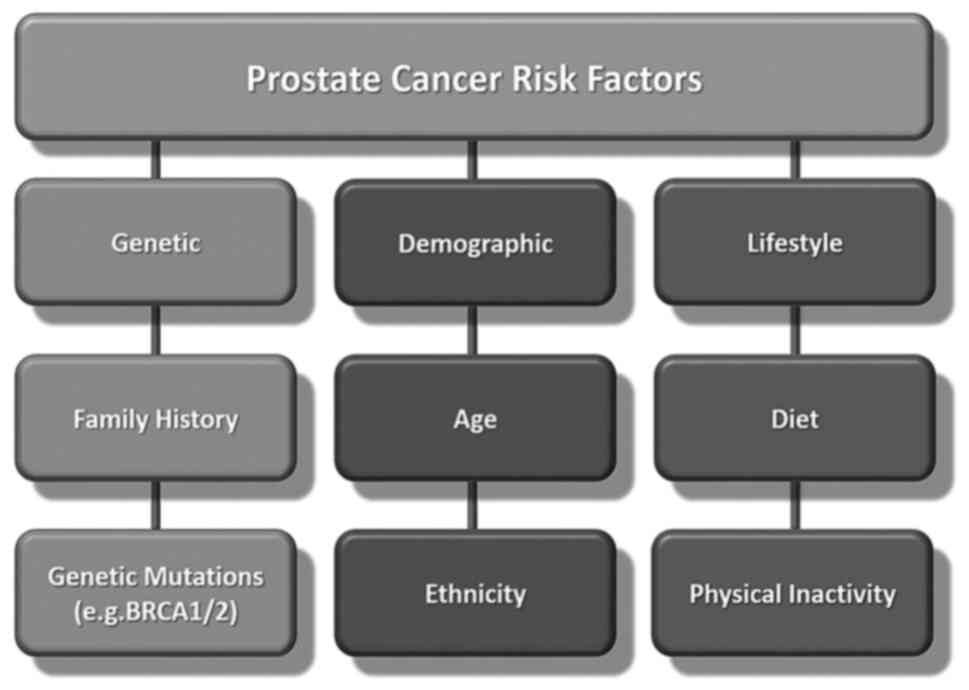
Several factors may increase the risk of developing
PCa, including age, race, family history and genetic
predispositions (4,5) (Fig.
1). Age is the most critical risk factor, with the likelihood
of developing PCa sharply increasing after the age of 50 years.
Genetic predisposition plays a critical role in the racial
disparities observed in the incidence of and mortality associated
with PCa (6). Another influential
factor is race (7). The impact of
race on PCa involves a multifaceted combination of genetic,
socioeconomic, environmental and healthcare access factors. These
variables contribute to observed racial disparities in both the
incidence of and mortality associated with PCa.
African American males, for instance, have a
markedly higher risk of developing PCa compared to other racial
groups (8,9). Studies have identified specific
genetic variants which are more prevalent among African American
males, such as those affecting the androgen receptor gene, which
may increase susceptibility to PCa (10,11).
A study published in Nature Genetics identified 187 new genetic
variants associated with an increased risk of developing PCa in
males (12). Notably, several of
these variants were exclusive to males of African ancestry,
shedding light on the disproportionately higher risk of developing
PCA in this population (12).
These findings indicate that African American males are more likely
to be diagnosed with aggressive forms of the disease and have
higher mortality rates compared with males of other racial and
ethnic groups (9,13). By contrast, Asian Americans and
Hispanics have lower incidence and mortality rates than
non-Hispanic Caucasian males (14,15).
Socioeconomic and healthcare access factors
significantly compound racial disparities in PCa outcomes,
particularly for African American males. These males are
disproportionately affected by structural barriers that limit their
access to timely, high-quality healthcare, which in turn
exacerbates the risk of poorer outcomes. Limited access to
healthcare services remains a major issue, with African American
males often facing challenges, such as fewer healthcare facilities
in their communities, longer waiting times for specialist
consultations, and fewer opportunities for preventive care and
early screening. Lower insurance coverage is another key factor.
African American males are more likely to be underinsured or
uninsured compared to their non-Hispanic Caucasian counterparts,
leading to delayed screenings and treatments. For a disease such as
PCa, where early detection is critical for an improved prognosis,
these delays often result in diagnosis at more advanced stages when
the cancer is more difficult to treat. Due to this lack of
insurance coverage, even in the case that PCa is detected at an
early stage, patients may not have access to optimal treatments,
including cutting-edge therapies or personalized treatment plans
based on genetic testing, which are typically costlier.
Delays in diagnosis and treatment are further
aggravated by socioeconomic factors, such as lower income levels,
which can make it challenging for patients to take time off work or
afford the high out-of-pocket costs associated with cancer care.
Additionally, the mistrust of the healthcare system, shaped by
historical injustices and experiences of bias in medical treatment,
can deter African American males from seeking timely care. Such a
combination of delayed diagnoses, inadequate treatment options and
a lack of trust in the healthcare system results in worse overall
prognoses for African American males with PCa.
Moreover, disparities in healthcare access are
compounded by differences in health literacy and awareness about
PCa. A number of males from racial minorities groups may not
receive adequate information about the importance of early
screening, family history, or available treatment options, which
limits their ability to make informed decisions about their
healthcare. Educational campaigns and culturally sensitive
healthcare initiatives are critical to improving awareness and
encouraging proactive health behaviours in at-risk populations. The
dual focus on genetic research and healthcare equity can help
reduce the disproportionately high mortality rates from PCa in this
population and may ultimately lead to more effective and equitable
cancer care for all racial groups.
Family history and genetic predisposition also play
a pivotal role, as males with a family history of PCa have a higher
risk of developing the disease (5,16).
In fact, understanding the role of family history and genetic
factors involved in the risk of developing PCa is critical for the
early detection of the disease, personalized screening and
prevention strategies (17). A
family history involves a history of PCa in close relatives, such
as fathers, brothers and sons. The risk increases further if
affected relatives were diagnosed at a younger age or if multiple
family members are affected. This suggests a hereditary component
to the risk of developing PCa, where genetic factors passed down
through generations contribute to susceptibility to the disease
(18,19).
Several genes have been identified that play a role
in susceptibility to PCa, including BRCA1, BRCA2, HOXB13 and others
(20,21). Mutations in these genes can
interfere with the normal process of cell growth and division,
leading to the uncontrolled growth of prostate cells and the
development of cancer. In addition, changes in other genes involved
in hormone regulation, DNA repair and inflammatory pathways may
also contribute to the risk of developing PCa.
Hereditary PCa accounts for 5-10% of all PCa cases
and is characterized by the strong familial clustering of the
disease. Inherited mutations in certain genes, such as BRCA1 and
BRCA2, are associated with an increased risk of developing PCa, as
well as other types of cancer, such as breast and ovarian cancer
(22,23). Males with hereditary PCa often
develop the disease at a younger age and may have more aggressive
forms of the disease compared to males without a family history of
the disease.
The present review aimed to address an critcal gap
in the literature by examining the complex role of racial and
genetic factors in PCa. While ample research has explored genetic
predisposition and family history, fewer studies have
comprehensively investigated the mechanisms through which race,
genetics and socioeconomic factors interact to influence PCa
outcomes. The present review hopes to add value by providing a
deeper understanding of the mechanisms through which these factors
contribute to disparities in PCa incidence, progression and
mortality. Moreover, the present review offers actionable
recommendations for integrating genetic screening, improving
healthcare access and tailoring treatment strategies to reduce
racial disparities in PCa outcomes.
By examining the intersections of race, genetics and
healthcare access, the present review seeks to inform future
research, policy initiatives and clinical practices aimed at
reducing disparities in the diagnosis and treatment of PCa.
2. Literature search strategies
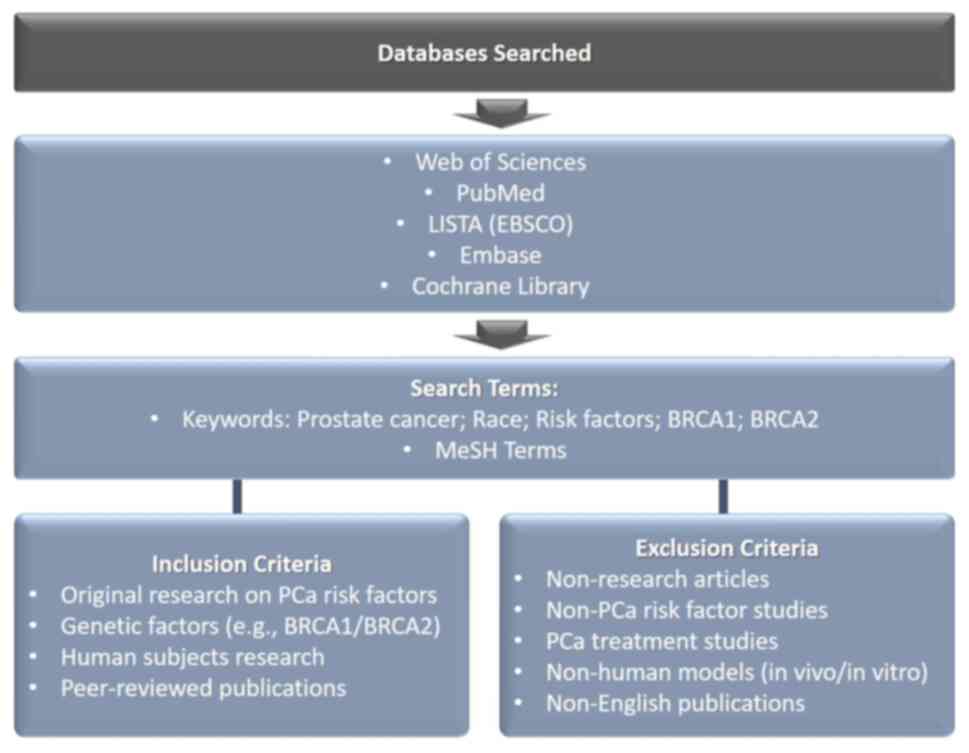
Open electronic databases, including Web of Science,
PubMed, LISTA (EBSCO), Embase and the Cochrane Library, were
searched using a combination of relevant keywords and Medical
Subject Headings (MeSH) terms. The literature search was limited to
articles published in the English language up until the date of
September, 2024. The scheme representing the method of the article
search is presented in Fig. 2.
The inclusion criteria for studies were as follows:
Publications were included if the analyses met the following
criteria: i) Original research studies that focused on the risk
factors of PCa development, particularly the impact of race; ii)
the studies included research on the genetic factors associated
with PCa, including mutations of the BRCA1 and BRCA2 genes; iii)
the studies involved human subjects; and iv) the studies were
published in peer-reviewed journals.
The following exclusion criteria were used: i)
Conference abstracts, case reports, editorials, letters or
comments; ii) the research was not specifically related to PCa risk
factors; iii) studies focused on PCa treatment; iv) PCa model
studies, including in vivo and in vitro studies; v)
Non-English language publications.
Quality assessment
The quality of each included study was evaluated
using the National Institutes of Health's Quality Assessment Tool
for Observational Cohort and Cross-Sectional Studies. This
assessment was performed by two independent reviewers, with any
disagreements resolved through discussion or, if necessary,
third-party adjudication.
3. Influence of race on the occurrence of
prostate cancer
PCa is one of the most common types of cancer among
males worldwide, and its incidence and outcomes can vary
significantly by race and ethnicity. Previous studies have strongly
suggested that race is a key factor in the development of PCa. For
example, African Americans have a higher risk of developing PCa
compared to males of other racial and ethnic groups (24). Other risk factors include obesity,
a diet high in red meat and processed foods and certain genetic
mutations.
In fact, the role of race in the development of PCa
is a complex and multifaceted issue that is still being carefully
investigated (25,26). Numerous studies have consistently
shown notable disparities in the incidence and mortality rates of
PCa among racial and ethnic groups (27). It has been shown that African
American males have the highest incidence of PCa worldwide,
followed by Caribbean males of African descent (28). Moreover, African American males are
more likely to be diagnosed with aggressive forms of the disease
and have higher mortality rates compared with males of other racial
and ethnic groups (9,13).
Ledet et al (25) found that, while genetic risk
factors for PCa were similar across African American and Caucasian
patients, African Americans had unique genetic variants and more
BRCA1 mutations. Zhang and Zhang (27) observed significant dietary
differences between African American and European American males,
with African Americans exhibiting a higher prevalence of PCa, but
limited direct dietary implications for cancer risk.
Epstein et al (29) found higher rates of biochemical
recurrence in African Americans post-prostatectomy, suggesting a
need for closer monitoring in this group. Owens et al
(13) reported similar emotional
and informational support needs across racial groups among PCa
survivors. In another study, Wu et al (14) identified factors, such as age,
tumor grade and marital status as significantly affecting mortality
in Asian-American patients, with surgery reducing the risk of
mortality. Deuker et al (30) reported that Native Hawaiians or
Pacific Islanders exhibited worse PCa characteristics at diagnosis
than Caucasians, although the mortality rates did not differ
significantly.
In a large cohort study on 4,282 patients,
Würnschimmel et al (15)
found that Asians with metastatic PCa (mPCa) had lower
cancer-specific mortality rates than other groups, while Hoeh et
al (31) reported no
significant difference in mortality rates between treatment types
in high-risk Hispanic patients.
Stern et al (32) found that South and East Asian males
had lower PCa mortality rates than Caucasians, while Whittemore
et al (33) linked family
history to an increased risk of developing PCa across ethnicities.
In a large study with 239,613 participants, El Khoury and Clouston
(34) observed that a lower
socioeconomic status increased PCa mortality rates across racial
groups, indicating the importance of tailored interventions
(34). Finally, Yamoah et
al (35) found that in African
American veterans, the incidence rate of PCa was almost 2-fold
higher compared with that of Caucasian veterans, pointing to
persistent disparities even with equal healthcare access.
It has been shown that African American males have a
higher prevalence of genetic variants associated with an increased
risk of developing aggressive forms of PCa, including mutations in
genes such as BRCA1, BRCA2 and HOXB13 (36,37).
These genetic differences may contribute to the higher morbidity
and mortality rates observed in this population (38,39).
In addition, differences in androgen receptor signaling pathways
and tumor micro-environmental factors may also contribute to racial
disparities in PCa.
A recent study by Gu et al (11) demonstrated that the West African
ancestry-specific single nucleotide polymorphism (SNP) rs7824364 on
8q24 independently predicted positive prostate biopsy in African
American males who were candidates for prostate biopsy following
PCa screening.
Thakker et al (40) used the data from the National
Cancer Database spanning 2004 to 2017, to analyze factors, such as
age, race, ethnicity, geographic location, education level, income
and insurance status. It was found that the percentage of patients
presenting with mPCa increased after the 2012 recommendation, with
Hispanics and non-Hispanic patients of African descent experiencing
a higher rate of increase compared to non-Hispanic Caucasians. The
insurance status significantly influenced the mPCa presentation
rates, with uninsured Hispanics and non-Hispanic patients of
African descent being more likely to be diagnosed with mPCa than
their insured counterparts. Additionally, a lower household income,
particularly among non-Hispanic subjects of African descent, was
linked to a higher likelihood of presenting with mPCa (40).
Furthermore, socioeconomic and environmental factors
also play a critical role in racial disparities in PCa. African
American males are more likely to experience social determinants of
health, such as poverty, limited access to healthcare, lower health
insurance rates, and barriers to screening and early detection.
These differences in access to and the utilization of health care
contribute to delays in diagnoses, resulting in more advanced
disease at diagnosis and poorer treatment outcomes.
Cultural and behavioral factors may also contribute
to racial disparities in PCa. African American males are less
likely to engage in preventive health measures, such as regular
screenings and healthy lifestyle choices, which can delay detection
of PCa and increase the risk of developing advanced disease at the
time of diagnosis. Moreover, cultural beliefs and attitudes toward
cancer and healthcare may influence treatment decisions and
adherence to medical recommendations.
Asian males exhibit distinct epidemiological
patterns of PCa compared with other racial and ethnic groups
(32,41). Historically, PCa is less common in
Asian countries, such as China, Japan and Korea compared to Western
countries. However, in recent years, there has been an increase in
the incidence of PCa among the Asian population, which is
associated with changes in lifestyle factors, dietary habits and
improvements in cancer detection methods (33,42).
Genetic factors play a crucial role in the development of PCa in
Asian males (14). Although
specific genetic mutations associated with the risk of developing
PCa have been extensively studied in other populations, such as
BRCA gene mutations in Ashkenazi Jews, the genetic landscape of PCa
in Asian males is less studied (43). However, recent studies have
identified several genetic variants unique to the Asian population
that may contribute to the susceptibility and aggressiveness of PCa
(44-46).
Environmental and lifestyle factors also influence
the development of PCa. In particular, African American males and
males of African descent, Asian males and Hispanic males exhibit
notable differences in PCa rates, suggesting that factors such as
diet, physical activity, socioeconomic conditions, and access to
healthcare may influence the development of the disease. However,
lifestyle factors, such as diet and access to healthcare
significantly affect the outcomes of African American males.
Studies have demonstrated that African American males are more
likely to have diets high in fat and processed foods, which have
been associated with an increased risk of developing PCa (47,48).
High-fat diets can lead to increased levels of testosterone, which
may promote the growth of PCa cells. Environmental stressors, such
as residing in communities with higher levels of pollution or lower
access to fresh, healthy foods, may also exacerbate the risk of
developing more aggressive forms of the disease (27). Physical inactivity and obesity,
both of which are more prevalent among African American males than
in other racial groups, are further environmental factors that have
been linked to an increased risk of developing PCa.
By contrast, Asian males, particularly those
residing in Asia, tend to exhibit lower incidence and mortality
rates of PCa (49). This
difference is often attributed to dietary factors and lifestyle
choices common in numerous Asian cultures. Diets rich in soy
products, green tea and fish, which are prevalent in countries,
such as Japan and China, have been associated with a lower risk of
developing PCa. Soy contains isoflavones, which are considered to
have a protective effect against PCa by inhibiting the growth of
cancer cells (50). However,
studies have shown that Asian males who migrate to Western
countries, where diets are often higher in fat and lower in
plant-based foods, exhibit a significant increase in the risk of
developing PCa (51,52). This suggests that lifestyle
factors, particularly diet, may have a greater influence on the
development of PCa than genetics alone.
Practices in screening and diagnosing PCa vary among
Asian countries and may influence disease detection and outcomes.
Although prostate-specific antigen (PSA) screening is widely used
in Western countries for the early detection of PCa, its utility
and effectiveness in Asian populations remains controversial
(53-55).
Cultural beliefs, healthcare infrastructure and resource
availability influence the implementation of PCa screening programs
in Asian countries, resulting in disparities in access to timely
diagnosis and treatment (10,56).
Cultural and social influences shape attitudes toward PCa
prevention, screening and treatment among Asian males.
Hispanic makes exhibit intermediate PCa incidence
rates compared to African American and Asian males; however, they
face unique environmental and lifestyle challenges that may
influence their risk. While genetic factors do play a role,
Hispanic males often face socioeconomic barriers similar to African
American males, such as lower access to healthcare, limited health
insurance coverage and delayed diagnosis due to fewer opportunities
for screening.
Dietary habits among Hispanic males may also
contribute to the risk of developing PCa. Traditional diets in
numerous Hispanic cultures can be high in red meat and
carbohydrates, which have been linked to an increased risk of
developing cancer. However, diets rich in fruits, vegetables and
fiber, also common in some Hispanic communities, may offer
protective effects. The variation in dietary habits within the
Hispanic population underscores the complexity of identifying
specific lifestyle factors that influence the risk of developing
PCa.
These differences highlight the importance of
considering environmental and lifestyle factors, alongside
genetics, when addressing PCa disparities among racial groups.
Public health initiatives should focus on improving access to
healthy foods, promoting regular physical activity and increasing
the awareness of PCa screening, particularly in underserved
populations. Tailoring prevention strategies to the specific needs
of each racial group can help reduce the burden of PCa and improve
outcomes for males globally.
4. Hereditary risk factors for the
development of prostate cancer
PCa is one of the most heritable cancers, and a
family history of PCa is a well-known risk factor (57-59).
Family history is a well-established risk factor for PCa. Studies
have demonstrated that males who have a first-degree relative
(father or brother) diagnosed with the disease have an ~2-fold
higher risk of developing PCa themselves compared with those
without a family history (60,61).
This familial aggregation of PCa suggests a hereditary component,
suggesting the involvement of genetic factors in the susceptibility
to the disease. Several genetic variants associated with the risk
of developing PCa have been identified through genome-wide
association studies, with common polymorphisms in genes such as
HOXB13, BRCA1 and BRCA2, and DNA repair genes contributing to the
familial clustering of the disease (62,63)). A summary of the roles of BRCA1 and
BRCA2 mutations in prostate cancer is presented in Table I.
 | Table IOverview of the roles of BRCA1 and
BRCA2 mutations in prostate cancer. |
Table I
Overview of the roles of BRCA1 and
BRCA2 mutations in prostate cancer.
| Gene | Role in prostate
cancer | Risk increase | Genetic
testing | Implications for
treatment | Screening
recommendations |
|---|
| BRCA1 | BRCA1 mutations are
less common in prostate cancer, yet still significant. They are
associated with a higher risk of developing aggressive forms of
prostate cancer. | Moderate increase
in risk. Individuals with BRCA1 mutations have a higher likelihood
of developing prostate cancer, especially aggressive types,
compared to the general population. | Recommended for
individuals with a family history of BRCA1-related cancers or
early-onset prostate cancer. Testing can help identify those at
risk and guide decisions on preventive measures. | May influence the
choice of treatment, including consideration of targeted therapies,
such as PARP inhibitors. BRCA1-related prostate cancers may respond
better to specific drugs. | Enhanced screening
protocols may be recommended for individuals with known BRCA1
mutations. This can include starting prostate cancer antigen
testing at an earlier age and conducting more frequent
screenings. |
| BRCA2 | BRCA2 mutations are
more commonly associated with prostate cancer and significantly
increase the risk. Males with BRCA2 mutations are more likely to
develop early-onset and aggressive prostate cancer. | Substantial
increase in risk. BRCA2 mutations are strongly linked to
early-onset prostate cancer and more aggressive forms, making it
critical for affected individuals to undergo regular and early
screenings. | Strongly
recommended for individuals with a family history of BRCA2-related
cancers, early-onset prostate cancer, or known BRCA2 mutations in
the family. Testing is crucial for early detection and prevention
strategies. | Significantly
influences treatment decisions, often leading to the use of PARP
inhibitors and other targeted therapies. BRCA2-related cancers may
also be more sensitive to certain chemotherapies. | Enhanced screening
protocols are strongly recommended for individuals with known BRCA2
mutations, including earlier and more frequent prostate cancer
antigen testing. MRI and other advanced imaging techniques may also
be used. |
PCa is known to have a strong familial component,
with studies suggesting that up to 5-10% of cases are due to
inherited genetic factors (62,64,65).
Mutations in specific genes, such as BRCA1, BRCA2 and HOXB13, and
DNA mismatch repair (MMR) genes (e.g., MutL homolog 1 (MLH1), MutS
homolog (MSH)2, MSH6 and PMS2) are associated with hereditary PCa
syndromes (17). These genetic
alterations can interfere with normal cell growth and
proliferation, increasing the risk of developing PCa.
In addition to BRCA1 and BRCA2 mutations, the HOXB13
gene has emerged as a crucial factor in hereditary PCa. The HOXB13
gene encodes a transcription factor that plays a pivotal role in
the regulation of genes involved in the development and maintenance
of prostate tissue (66,67). Mutations in this gene, particularly
the G84E variant, have been strongly linked to a significantly
increased risk of developing PCa, particularly in males with a
family history of the disease.
The G84E mutation in HOXB13 is relatively rare in
the general population; however, studies have found it to be more
prevalent among males of European ancestry (68). In particular, research has shown
that males carrying this mutation are at a 3-5-fold higher risk of
developing PCa compared to non-carriers. This elevated risk renders
HOXB13 G84E a key genetic marker for early detection and screening
in families with a history of PCa, allowing for more personalized
and proactive medical approaches (38).
The significance of the HOXB13 mutation extends
beyond familial cases. Although it is rare in the broader
population, its presence highlights the importance of genetic
screening for hereditary PCa (69). The identification of the HOXB13
G84E mutation can help clinicians and genetic counselors stratify
risk in patients with a family history of the disease, guiding
decisions regarding earlier and more frequent PCa screening.
Furthermore, recent studies suggest that males with
HOXB13 mutations may develop PCa at an earlier age and may be more
prone to aggressive forms of the disease (38,67,70).
This suggests that the gene may not only be used as a marker for an
increased risk of PCa, but also as a potential indicator for more
severe disease progression. Understanding the impact of HOXB13
mutations may improve both the prevention and management strategies
for those at a higher genetic risk.
Furthermore, mutations in DNA MMR genes, including
MLH1, MSH2, MSH6 and PMS2, are associated with Lynch syndrome, a
hereditary cancer syndrome that significantly increases the risk of
developing several types of cancer, including PCa. MMR genes are
responsible for correcting DNA replication errors that occur during
cell division. When these genes are mutated, replication errors are
uncontrolled, leading to genomic instability and an increased
likelihood of developing cancer. Males with Lynch syndrome not only
have an elevated risk of developing colorectal cancer, but also a
higher likelihood of developing PCa at an earlier age than those
without the syndrome.
The presence of these genetic mutations in the
BRCA1, BRCA2 and HOXB13, and MMR genes suggests that hereditary PCa
syndromes involve disruptions in crucial cellular pathways,
particularly those involved in DNA repair and genomic stability.
This increased understanding of the genetic basis of hereditary PCa
has critical implications for patient care. Males with a family
history of PCa, particularly those with known genetic mutations,
can benefit from genetic counselling and testing to assess their
risk. Early detection strategies, such as regular PSA testing and
digital rectal exams, can be tailored based on the genetic risk
profile of an individual, allowing for the more proactive and
personalized management of the risk of developing PCa.
Moreover, the identification of genetic mutations in
these high-risk families provides opportunities for targeted
therapies. For example, males with BRCA mutations may be candidates
for treatment with PARP inhibitors, a class of drugs that
specifically target cancer cells with defective DNA repair
mechanisms (71-73).
These therapies, which have shown promise in the treatment of
breast and ovarian cancers, are being explored for the treatment of
PCa, particularly for patients with advanced or metastatic disease
linked to genetic mutations.
Family history serves as a key component in PCa risk
assessment models, helping to identify individuals who are at an
increased risk and who may benefit from individualized screening
and preventative interventions. Males with a family history of PCa,
particularly those with multiple relatives with PCa or early-onset
disease, are considered to be at a high risk and may require
earlier and more frequent screening.
Current screening guidelines recommend that males
with a family history of PCa initiate a discussion with their
health care providers about the benefits and limitations of PSA
testing and digital rectal examinations commencing at the age of
40-45 years (or earlier if considered appropriate based on
individual risk factors). The recommendations can be tailored based
on individual risk factors, including family history, lifestyle and
genetic predisposition. Males who are at a higher risk may benefit
from earlier and more frequent screening with PSA testing and
digital rectal examinations to detect cancer at an earlier, more
treatable stage.
5. Mutations of the BRCA1 and BRCA2 genes as
a risk factor for the development of prostate cancer
According to previous studies and the Nordic Twin
study, up to 57% of the risk of developing PCa is due to hereditary
factors (74-76).
There are two types of hereditary factors: i) Common SNPs, which
themselves are associated with a slightly increased risk; and ii)
rare variants or mutations in genes, which significantly increase
the risk of developing PCa (for example, mutations in the BRCA1/2
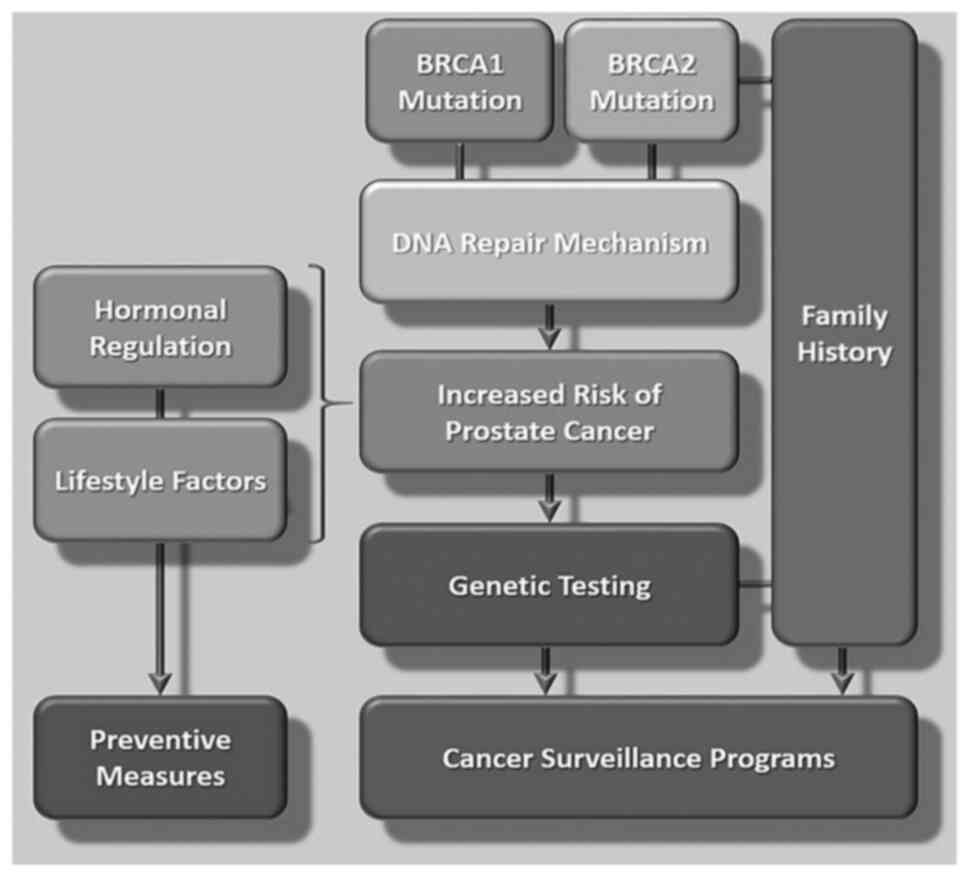
genes). Mutations in tumor suppressor genes disrupt their function
and lead to a significantly increased risk of developing cancer
(Fig. 1). For example, mutations
in the BRCA1 and BRCA2 genes increase susceptibility to the
autosomal dominant mode of inheritance of PCa and ovarian syndrome
(77) and manifest not only in the
form of these cancers, but also in the form of PCa and rectal
cancer.
BRCA1 and BRCA2 are two of the most well-known
tumour suppressor genes involved in the susceptibility to inherited
cancer (20,21). Mutations in these genes
significantly increase the risk of developing breast, prostate,
ovarian and other types of cancer (78-81).
BRCA1 and BRCA2 are large genes located on different
chromosomes (17q21 and 13q12-13, respectively) and encoding
proteins involved in maintaining genome stability through DNA
repair mechanisms. BRCA1 plays a critical role in DNA double-strand
break repair, transcriptional regulation and cell cycle control,
whereas BRCA2 is primarily involved in facilitating homologous
recombination, a process which is critical for DNA damage repair.
Mutations in BRCA1 and BRCA2 impair these DNA repair functions,
leading to genomic instability and an increased risk of developing
cancer (82,83). A schematic illustration of the
mutations of the BRCA1 and BRCA2 genes as a risk for developing
prostate cancer and patient surveillance is presented in Fig. 3.
Inherited mutations in BRCA1 and BRCA2 have been
shown to significantly increase the lifetime risk of developing
PCa, as well as breast and ovarian cancer, with carriers of
pathogenic variants having an up to 80% lifetime risk of developing
breast cancer and an up to 40% lifetime risk of developing ovarian
cancer (84,85). In addition, mutations in these
genes are associated with an increased risk of developing other
types of cancer, including PCa, as well as pancreatic and breast
cancer in males.
Genetic testing for BRCA1 and BRCA2 mutations plays
a critical role in identifying individuals who are at an increased
risk of hereditary cancer and in developing personalized risk
management strategies (21,86).
Genetic counselling is recommended for individuals with a personal
or family history suggestive of PCa (87,88).
Studies have shown that mutations in the BRCA1 and
BRCA2 genes not only increase the risk of developing PCa, but lead
to the development of more aggressive forms of the disease, which
are characterized by higher rates of disease relapse and increased
mortality rates (89,90). For example, studies have found that
the relative risk of PCa at an age ≤65 years is 1.8-4.5-fold higher
in BRCA1 carriers and 2.5-8.6-fold higher in BRCA2 carriers
(91-93).
Retrospective studies have found that younger carriers of BRCA2
mutations have an aggressive disease course, with high rates of
lymph node involvement and distant metastases, and higher mortality
rates compared to non-carriers (94-96).
The BRCA2 mutation status has now been validated as a prognostic
factor for worse outcomes. BRCA2 mutations have also been
associated with the early development of PCa (an age at diagnosis
of ≤55 years) (97,98).
Recent studies have highlighted the distinct impact
of BRCA1 and BRCA2 mutations on the risk of developing PCa, as well
as on progression and clinical outcomes, emphasizing the need for
personalized genetic screening and targeted treatment strategies.
Thorne et al (99) found
that BRCA2 mutation carriers had more locoregional and digestive
system involvement compared to non-carriers, suggesting distinct
disease characteristics in BRCA2-associated PCa. Similarly, Paulo
et al (100) analyzed
homologous recombination repair (HRR) gene mutations in early-onset
and familial PCa, identifying pathogenic variants in 3.9% of cases,
most frequently in the checkpoint kinase 2, ataxia telangiectasia
mutated and BRCA2 genes. This underlines the significance of HRR
genes in hereditary PCa predisposition.
Kwong et al (101) observed an increased risk of
developing breast cancer and PCa in high-risk male BRCA2 carriers,
supporting a potential role for male-specific screening. Nyberg
et al (96) further
demonstrated that BRCA1 mutations increase the incidence of PCa,
particularly in younger males, while BRCA2 mutations are associated
with aggressive disease and higher mortality rates, particularly in
those with a family history of PCa.
In the study by Fettke et al (102), BRCA2 alterations in DNA damage
repair genes were linked to poorer clinical outcomes in metastatic
castration-resistant PCa, emphasizing BRCA2 as a key prognostic
marker. Additionally, Barnes et al (103) reported that polygenic risk scores
could enhance personalized cancer risk estimation for BRCA1 and
BRCA2 male carriers, aiding clinical management.
Although there is debate about whether there is an
increased risk of developing PCa in BRCA1 carriers, there is
increasing evidence to indicate that PCa is aggressive in these
males (88,96). It has been shown that males who
carry a BRCA mutation may be at risk of developing other types of
cancer, such as breast cancer, pancreatic cancer and melanoma. In
addition, females who carry BRCA are at an increased risk of
developing breast and ovarian cancer (104,105).
The BRCA genes are known as tumor suppressor genes
(106-108).
Carcinogenesis occurs in carriers of germline BRCA mutations due to
loss of the wild-type allele (107). BRCA1 appears to play a major role
in DNA damage repair, protein transcription regulation and genomic
stability (109,110). The role of BRCA2 is most likely
limited to DNA damage repair (111,112). It is likely that mutations in
these genes, leading to dysfunction in the DNA repair pathway,
increase the risk of developing PCa; however, the mechanisms
through which they affect the course of the disease are not yet
fully understood. BRCA1 mutations have been identified in prostate
cell lines involving the androgen receptor and insulin-like growth
factor 1 receptor pathways (113,114). Additionally, the loss of the
suppressive function of BRCA2 has been shown to influence focal PCa
development, as well as disease progression through the regulation
of matrix metalloproteinase-9 by modulating the PI3-kinase/AKT and
MAP/ERK signaling pathways (115,116).
The association of BRCA mutations with aggressive
characteristics of PCa was identified in the aforementioned
retrospective studies (110,117). In their studies, Edwards et
al (118,119) found a 4-year difference in the
median overall survival between BRCA2 carriers and non-carriers in
patients diagnosed with PCa. Gallagher et al (120,121) compared clinical outcomes in 26
BRCA mutation carriers and 806 non-carriers and reported a 28%
increased risk of developing low-grade tumors (Gleason score ≥7)
compared with non-BRCA-associated PCa. Carriers of either mutation
were found to have a higher risk of relapse (hazard ratio (HR),
4.32; 95% confidence interval (CI), 1.31-13.62 for BRCA1; and
relative risk (RR), 2.41; 95% CI, 1.23-4.75 for BRCA2) and
mortality from PCa (RR, 5.16; 95% CI, 1.09-24.53 for BRCA1; and RR,
5.48; 95% CI, 2.03-14.79 for BRCA2) compared with non-carriers
(135).
Thorne et al (122) found that BRCA2 mutation carriers
had a worse PCa survival (HR, 4.97; 95% CI, 2.19-11.25), although
this may be due to a higher incidence of undifferentiated (Gleason
≥8) tumors (65.8 vs. 33.0%) and stage T3-T4 tumors (39.5 vs. 22.6%)
in carrier vs. non-carrier cohorts. In another study, Tryggvadottir
et al (123) identified
596 patients with PCa among 29,603 male relatives of unselected
probands with breast cancer. They staged and evaluated a subgroup
of 89 patients that included carriers of all mutations. Each
carrier was matched to two non-carriers according to year of
diagnosis and birth. Compared with non-carriers, BRCA2 999del5
mutation carriers had a younger mean age at diagnosis (69.0 vs.
74.0 years, P=0.002), a more advanced disease stage (79.3% with
stages 3 and 4 vs. 38.6%, P<0.001), a lower tumor grade (84.0%
grade 3-4 vs. 52.7%, P=0.007) and a shorter median survival time
(2.1 years; 95% CI, 1.4-3.6 years, vs. 12.4 years; 95%, CI 9.9-19.7
years). The BRCA2 999del5 mutation was also associated with an
increased risk of mortality from PCa (adjusted for year of
diagnosis and birth (HR, 3.42; 95% CI, 2.12-5.51); the association
remained following adjustment for stage and grade (RR, 2.35l 95%
CI, 1.08-5.11) (135). Narod
et al (124) noted that
the 5-year overall survival rate was shorter for BRCA2 than BRCA1
carriers (42 vs. 64%, respectively), with a median overall survival
of 15 years for BRCA1 mutation carriers and 5 years for BRCA2
mutation carriers.
Castro et al (125,126) examined the clinical
characteristics and outcomes of 2,181 patients with PCa, of whom 5
patients were BRCA1 carriers and 31 patients were BRCA2 mutation
carriers. They reported a spectrum of pathogenic mutations causing
a more aggressive PCa phenotype (Gleason score ≥8: 50% BRCA2
carriers, 20% BRCA1 carriers, 21% non-carriers; P=0.017), a higher
incidence of lymph node involvement (N1: 35% BRCA2 carriers, 50%
BRCA1 carriers, and 11% non-carriers, P<0.001) and a higher
likelihood of distant metastasis at diagnosis (M1: 21% BRCA2
carriers, 20% BRCA1 carriers, 9% non-carriers, P=0.034) (127). Additionally, compared with
non-carriers, patients with BRCA2 mutations had a significantly
shorter overall survival (10.8 vs. 13.3 years, respectively; HR,
2.5; P<0.001) and cancer-specific survival (8.6 vs. 16.3 years;
odds ratio, 2.8; P<0.001) (127).
According to the American National Comprehensive
Cancer Network (NCCN) guidelines, genetic counselling is
recommended for males (128-130).
In addition, genetic testing may be performed if the individual has
a Gleason score ≥7 and has one of the following criteria: i) One
close blood relative with ovarian cancer or breast cancer aged ≤50
years; or ii) two relatives with breast cancer, ovarian or prostate
(Gleason score ≥7 or higher) at any age.
Despite the studies and results obtained on the
association of BRCA 1/2 gene mutations and the risks of developing
certain types of cancer, the importance of genetic counselling and
testing in males remains underestimated (131-133).
Due to the autosomal dominant pattern of inheritance, transmission
of BRCA mutations through fathers or mothers occurs equally.
6. Synopsis
Genetic screening and testing are becoming
increasingly vital in oncology, providing insight into individuals
who are at risk of developing various types of cancers, including
PCa. Among the genetic factors associated with the risk of
developing PCa, mutations in the BRCA1 and BRCA2 genes have been
identified as key contributors. These mutations underscore the need
for integrating genetic testing into diagnostic processes, enabling
early detection and personalized care (20,21).
Mutations in the BRCA1 and BRCA2 genes, initially
identified as drivers of hereditary breast and ovarian cancer, are
increasingly implicated in PCa susceptibility (22,134). Males with inherited mutations in
these genes are known to have a significantly increased risk of
developing PCa compared to the general population. Research has
shown that males with BRCA2 mutations in particular have a 4-8-fold
higher risk of developing PCa, often at a younger age and with more
aggressive disease characteristics (61,86).
Given the clinical implications, genetic screening holds
significant promise for identifying at-risk individuals,
facilitating early detection and improving treatment outcomes.
The findings discussed in the present review suggest
clear public health implications. Integrating genetic testing for
BRCA mutations into routine PCa screening protocols, particularly
for high-risk groups, such as those with a family history of PCa or
belonging to certain racial groups, is a critical next step. The
early identification of mutation carriers could lead to the
adoption of tailored screening protocols that enable timely
intervention and more personalized treatment plans. For example,
evidence suggests that BRCA-mutated PCa may better respond to PARP
inhibitors, which opens the door to precision medicine approaches
that could markedly improve patient outcomes (135).
Ethnicity plays a critical role in the incidence and
mortality rates of PCa. African American males have the highest
incidence of PCa worldwide, followed by Caribbean males of African
descent (136,137). They also tend to be diagnosed
with more aggressive forms of the disease and have higher mortality
rates compared to men of other racial and ethnic groups (36,138,139). The disparities are partly due to
the higher prevalence of certain genetic variants, including BRCA1,
BRCA2 and HOXB13, which are more common in African American males.
The demonstrated racial disparities in PCa outcomes, particularly
the elevated risk faced by African American males, highlight the
need for targeted public health initiatives. Policymakers need to
prioritize expanding genetic testing and counseling services for
at-risk populations. Outreach programs should focus on increasing
awareness of genetic risk factors and providing accessible
screening options, particularly in underserved communities. For
example, expanding genetic screening programs in healthcare
settings that primarily serve African American populations could
help address the higher PCa mortality rates in this group.
By contrast, Asian Americans and Hispanics generally
exhibit lower incidence and mortality rates for PCa compared to
non-Hispanic Caucasian males (140-143).
These differences highlight the importance of considering ethnic
background in the risk assessment and management of prostate
cancer.
The findings underscore the necessity of integrating
genetic testing into routine PCa screening protocols, particularly
for high-risk groups identified through family history and ethnic
background. The early identification of BRCA mutation carriers can
facilitate more personalized and effective treatment plans,
potentially improving patient outcomes. Public health initiatives
should focus on raising awareness about the importance of genetic
testing for males, particularly those with a family history of
BRCA-related cancers. Efforts should also be made to expand access
to genetic testing and counselling services, ensuring that at-risk
populations receive appropriate screening and preventive care.
While the present review underscores the importance
of BRCA mutations in PCa, further research is required to explore
the mechanisms through which these mutations interact with other
genetic and environmental factors to influence PCa development. For
instance, studies are required to investigate how mutations in BRCA
genes may combine with other risk factors such as lifestyle, diet
and exposure to environmental carcinogens to impact disease
progression. Additionally, the role of epigenetic modifications in
PCa among different racial groups is an emerging area of research
that warrants further exploration. Understanding these complex
interactions could lead to more effective prevention strategies and
better-targeted therapies.
Additionally, policymakers should consider
revisiting current clinical guidelines for PCa screening. While
current guidelines often recommend PSA testing, incorporating
genetic screening into standard protocols, particularly for males
with a family history of BRCA-related cancers or those from
high-risk racial groups, could significantly improve early
detection efforts. Given the rapidly evolving landscape of genomic
medicine, existing guidelines may need to be updated to reflect the
growing role of genetic testing in PCa management.
Despite the clear benefits, there are challenges to
implementing genetic screening at a population level. Cost and
accessibility remain major barriers, particularly for individuals
residing in low-resource settings. Policymakers will need to
address how to fund and implement widespread genetic testing
without exacerbating existing healthcare disparities. Additionally,
ethical considerations surrounding genetic testing such as
patients' privacy and the potential for discrimination based on
genetic risk need to be carefully managed.
Expanding genetic testing would require a
coordinated effort between healthcare providers, insurance
companies and government bodies to ensure that the benefits of this
technology are equitably distributed. Ultimately, integrating
genetic screening into PCa prevention strategies could help shift
the focus from reactive to proactive healthcare, reducing the
overall burden of this disease on individuals, families and
healthcare systems. Continued efforts to promote genetic screening
and integration of genetic information into clinical practice are
essential to advancing prostate cancer treatment and ensuring
optimal outcomes for patients with cancer.
7. Conclusions and future perspectives
The present review underscores the significance of
ethnic and genetic factors in the development, progression and
clinical outcomes of PCa, particularly in the context of BRCA1 and
BRCA2 gene mutations. The impact of race on PCa risk and prognosis
is profound, with African American males displaying the highest
susceptibility and mortality rates among racial groups.
Contributing factors include genetic predispositions, socioeconomic
disparities and environmental influences that collectively worsen
the outcomes of patients with PCa. This is also evident in Asian
and Hispanic males, who experience intermediate risk levels, but
are affected by unique genetic profiles and lifestyle-related
factors, such as dietary patterns and access to healthcare.
Genetic predispositions, particularly BRCA1 and
BRCA2 mutations, significantly increase the riks of developing PCa
and are associated with aggressive forms of the disease. Given the
high mortality rates associated with BRCA-mutated PCa, the
integration of genetic screening into PCa prevention and management
is essential. The advent of genetic counselling and testing enables
the early identification of high-risk individuals, facilitating
personalized and proactive medical strategies. However, genetic
testing faces several obstacles, including costs, accessibility and
the need for supportive healthcare infrastructure. To address these
challenges, policy initiatives need to prioritize equitable access
to genetic counselling and testing, particularly for underserved
populations. Expanding public awareness campaigns, reducing
healthcare disparities, and advocating for culturally sensitive
healthcare practices will help bridge the gap in PCa diagnosis and
treatment. Additionally, the ethical considerations of genetic
testing such as patient privacy, potential discrimination and the
psychological impact of genetic risk knowledge must be carefully
managed to ensure that genetic testing is implemented responsibly
and equitably.
While the role of BRCA mutations in PCa is
increasingly recognized, further research is essential in order to
understand the interactions between genetic and environmental risk
factors. Studies focusing on how lifestyle, diet and environmental
carcinogens influence PCa progression in genetically predisposed
populations could provide insight into more effective prevention
strategies. Emerging research into the role of epigenetic
alterations in PCa among different racial groups also holds promise
for understanding the mechanisms driving these disparities.
To truly reduce the burden of PCa, healthcare
guidelines need to evolve. Current protocols predominantly
emphasize PSA testing, which, while beneficial, is less effective
in addressing the underlying genetic and ethnic complexities of PCa
risk. Integrating genetic testing and counselling into standard
screening practices, particularly for males with a family history
of BRCA-related cancers or those from high-risk racial groups would
enhance early detection and ultimately improve survival rates.
In conclusion, addressing the complex interplay of
ethnic, genetic and environmental factors in PCa requires a
multifaceted approach that combines scientific advancement with
social responsibility. Policymakers, healthcare providers and
researchers need to work collaboratively to promote personalized
medicine, expand genetic screening access and advocate for targeted
public health interventions. These efforts will help shift PCa
management from a reactive to a proactive model, improving outcomes
for all males, particularly those in high-risk groups. Continued
research, resource allocation and public health focus are essential
to transforming the landscape of PCa care and reducing the global
disparities that persist in this disease.
Acknowledgements
The authors are thankful to S.D. Asfendiyarov Kazakh
National Medical University, Almaty, Kazakhstan for the
administrative and technical support provided.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TS was involved in the writing, reviewing and
editing of the manuscript, as well as in study supervision, in the
writing of the original draft of the manuscript, and in project
administration. NA was involved in the data collection and analysis
of the literature. SA was involved in the in the writing of the
original draft of the manuscript, and preparing the figures. MN was
involved in in the collection and analysis of data from the
literature. AT was involved in the preparation of the figures and
in the analysis of the literature. ZD was involved in the writing
of the original draft of the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harborg S, Kjærgaard KA, Thomsen RW,
Borgquist S, Cronin-Fenton D and Hjorth CF: New horizons:
Epidemiology of obesity, diabetes mellitus, and cancer prognosis. J
Clin Endocrinol Metab. 109:924–935. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Varaprasad GL, Gupta VK, Prasad K, Kim E,
Tej MB, Mohanty P, Verma HK, Raju GSR, Bhaskar L and Huh Y: Recent
advances and future perspectives in the therapeutics of prostate
cancer. Exp Hematol Oncol. 12(80)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bergengren O, Pekala KR, Matsoukas K,
Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh
AN, Mucci L, et al: 2022 update on prostate cancer epidemiology and
risk factors-a systematic review. Eur Urol. 84:191–206.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mumuni S, O'Donnell C and Doody O: The
risk factors and screening uptake for prostate cancer: A scoping
review. Healthcare (Basel). 11(2780)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Berenguer CV, Pereira F, Câmara JS and
Pereira JAM: Underlying features of prostate cancer-statistics,
risk factors, and emerging methods for its diagnosis. Curr Oncol.
30:2300–2321. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Babatunde OA, Pearce JL, Jefferson MS,
Frey LJ, Angel PM, Drake RR, Allen CG, Lilly MB, Savage SJ and
Halbert CH: Racial distribution of neighborhood-level social
deprivation in a retrospective cohort of prostate cancer survivors.
Diseases. 10(75)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abashidze N, Stecher C, Rosenkrantz AB,
Duszak R Jr and Hughes D: Racial and ethnic disparities in the use
of prostate magnetic resonance imaging following an elevated
prostate-specific antigen test. JAMA Netw Open.
4(e2132388)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aristizabal C, Suther S, Yao Y,
Behar-Horenstein LS, Webb F, Stern MC and Baezconde-Garbanati L:
Training community African American and Hispanic/Latino/a advocates
on prostate cancer (PCa): A multicultural and bicoastal approach. J
Cancer Educ. 38:1719–1727. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang J, Xiong X, Zheng W, Liao X, Xu H,
Yang L and Wei Q: Evaluation of survival outcomes among black and
white patients with metastatic castration-resistant prostate
cancer: A systematic review and meta-analysis. Eur Urol Open Sci.
61:10–17. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee KY, Beatson EL, Steinberg SM, Chau CH,
Price DK and Figg WD: Bridging health disparities: A genomics and
transcriptomics analysis by race in prostate cancer. J Racial Ethn
Health Disparities. 11:492–504. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gu J, Chery L, González GMN, Huff C, Strom
S, Jones JA, Griffith DP, Canfield SE, Wang X, Huang X, et al: A
west African ancestry-associated SNP on 8q24 predicts a positive
biopsy in African American men with suspected prostate cancer
following PSA screening. Prostate. 84:694–705. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang A, Shen J, Rodriguez AA, Saunders EJ,
Chen F, Janivara R, Darst BF, Sheng X, Xu Y, Chou AJ, et al:
Characterizing prostate cancer risk through multi-ancestry
genome-wide discovery of 187 novel risk variants. Nat Genet.
55:2065–2074. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Owens OL, Dressler EV, Mayfield A,
Winkfield KM, Krane LS, Foust M and Sandberg JC: Considerations
from employed African-American and white prostate cancer survivors
on prostate cancer treatment and survivorship: A qualitative
analysis. Ethn Health. 29:309–327. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu D, Yang Y, Jiang M and Yao R: Competing
risk of the specific mortality among Asian-American patients with
prostate cancer: A surveillance, epidemiology, and end results
analysis. BMC Urol. 22(42)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Würnschimmel C, Wenzel M, Ruvolo CC,
Nocera L, Tian Z, Saad F, Briganti A, Shariat SF, Mirone V, Chun
FK, et al: Life expectancy in metastatic prostate cancer patients
according to racial/ethnic groups. Int J Urol. 28:862–869.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huynh-Le MP, Karunamuni R, Fan CC,
Thompson WK, Muir K, Lophatananon A, Tye K, Wolk A, Håkansson N,
Mills IG, et al: Common genetic and clinical risk factors:
Association with fatal prostate cancer in the Cohort of Swedish
Men. Prostate Cancer Prostatic Dis. 24:845–851. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abd Ellatif M, Gamal BE, Musaam AO, Malik
A and Tarique M: An update on genetic predisposition for prostate
cancer: Perspectives and prospects. Cell Mol Biol. 69:1–7.
2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Raghallaigh HN and Eeles R: Genetic
predisposition to prostate cancer: An update. Fam Cancer.
21:101–114. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Benafif S, Raghallaigh HN, McHugh J and
Eeles R: Genetics of prostate cancer and its utility in treatment
and screening. Adv Genet. 108:147–199. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Korneenko TV and Pestov NB: Oncogenic
BRCA1,2 mutations in the human lineage-A By-product of sexual
selection? Biomedicines. 12(22)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kalampokis N, Zabaftis C, Spinos T,
Karavitakis M, Leotsakos I, Katafigiotis I, van der Poel H, Grivas
N and Mitropoulos D: Review on the role of BRCA mutations in
genomic screening and risk stratification of prostate cancer. Curr
Oncol. 31:1162–1169. 2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Januskevicius T, Vaicekauskaite I,
Sabaliauskaite R, Matulevicius A, Vezelis A, Ulys A, Jarmalaite S
and Jankevicius F: Germline DNA damage response gene mutations in
localized prostate cancer. Medicina (Kaunas). 60(73)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Akhoundova D, Francica P, Rottenberg S and
Rubin MA: DNA damage response and mismatch repair gene defects in
advanced and metastatic prostate cancer. Adv Anat Pathol. 31:61–69.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Freedland SJ, Samjoo IA, Rosta E, Lansing
A, Worthington E, Niyazov A, Nazari J and Arondekar B: The impact
of race on survival in metastatic prostate cancer: A systematic
literature review. Prostate Cancer Prostatic Dis. 26:461–474.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ledet EM, Burgess EF, Sokolova AO, Jaeger
EB, Hatton W, Moses M, Miller P, Cotogno P, Layton J, Barata P, et
al: Comparison of germline mutations in African American and
Caucasian men with metastatic prostate cancer. Prostate.
81:433–439. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schiff J, Ledet EM, Ernst EM, Garvey CE,
Cotogno P and Sartor O: J Clin Oncol 35: 6 suppl, 2017. https://ascopubs.org/doi/10.1200/JCO.2017.35.6_suppl.225.
|
|
27
|
Zhang W and Zhang K: Quantifying the
contributions of environmental factors to prostate cancer and
detecting risk-related diet metrics and racial disparities. Cancer
Inform. 22(11769351231168006)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roach M III, Coleman PW and Kittles R:
Prostate cancer, race, and health disparity. Cancer J. 29:328–337.
2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Epstein M, Syed K, Danella J, Ginzburg S,
Belkoff L, Tomaszewski J, Trabulsi E, Singer EA, Jacobs BL, Raman
JD, et al: Model risk scores may underestimate rate of biochemical
recurrence in African American men with localized prostate cancer:
A cohort analysis of over 3,000 men. Prostate Cancer Prostatic Dis.
27:257–263. 2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deuker M, Stolzenbach LF, Pecoraro A,
Rosiello G, Luzzago S, Tian Z, Saad F, Chun FK and Karakiewicz PI:
PSA, stage, grade and prostate cancer specific mortality in Asian
American patients relative to Caucasians according to the United
States Census Bureau race definitions. World J Urol. 39:787–796.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hoeh B, Hohenhorst JL, Flammia R,
Horlemann B, Sorce G, Chierigo F, Tian Z, Saad F, Graefen M,
Gallucci M, et al: Cancer-specific mortality after radical
prostatectomy vs external beam radiotherapy in high-risk
Hispanic/Latino prostate cancer patients. Int Urol Nephrol.
54:81–87. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stern N, Ly TL, Welk B, Chin J, Ballucci
D, Haan M and Power N: Association of race and ethnicity with
prostate cancer-specific mortality in Canada. JAMA Netw Open.
4(e2136364)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Whittemore AS, Wu AH, Kolonel LN, John EM,
Gallagher RP, Howe GR, West DW, Teh CZ and Stamey T: Family history
and prostate-cancer risk in black, white, and Asian men in the
United-States and Canada. Am J Epidemiol. 141:732–740.
1995.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El Khoury CJ and Clouston SAP:
Racial/Ethnic disparities in prostate cancer 5-year survival: The
role of health-care access and disease severity. Cancers (Basel).
15(4284)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamoah K, Lee KM, Awasthi S, Alba PR,
Perez C, Anglin-Foote TR, Robison B, Gao A, DuVall SL, Katsoulakis
E, et al: Racial and ethnic disparities in prostate cancer outcomes
in the veterans affairs health care system. JAMA Netw Open.
5(e2144027)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stevens C, Hightower A, Buxbaum SG,
Falzarano SM and Rhie SK: Genomic, epigenomic, and transcriptomic
signatures of prostate cancer between African American and European
American patients. Front Oncol. 13(e2144027)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stangl A, Wilner C, Li P, Maahs L, Hwang C
and Pilling A: Molecular features and race-associated outcomes of
mutant metastatic castration-resistant prostate cancer. Prostate.
83:524–533. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kanayama M, Chen Y, Rabizadeh D, Vera L,
Lu C, Nielsen SM, Russell EM, Esplin ED, Wang H, Isaacs WB, et al:
Clinical and functional analyses of an African-ancestry
gain-of-function HOXB13 variant implicated in aggressive prostate
cancer. Eur Urol Oncol. 7:751–759. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wei J, Beebe-Dimmer J, Shi Z, Sample C,
Yan G, Rifkin AS, Sadeghpour A, Gielzak M, Choi S, Moon D, et al:
Association of rare, recurrent nonsynonymous variants in the
germline of prostate cancer patients of African ancestry. Prostate.
83:454–461. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Thakker PU, Perry AG, Hemal AK, Bercu CH,
Petrou SP, Pak RW, Broderick GA, Thiel DD, Dora CD, Lyon TD, et al:
Racial, ethnic, and socioeconomic disparities in rates of stage IV
prostate cancer after USPSTF category ‘D’ recommendation against
prostate-specific antigen screening: A retrospective cohort study.
Transl Androl Urol. 13:1093–1103. 2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ruvolo CC, Würnschimmel C, Nocera L,
Wenzel M, Tian Z, Shariat SF, Saad F, Verze P, Imbimbo C, Briganti
A, et al: Stage and cancer-specific mortality differ within
specific Asian ethnic groups for upper tract urothelial carcinoma:
North American population-based study. Int J Urol. 28:1247–1252.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lim J, Malek R, Jr S, Toh CC, Sundram M,
Woo SYY, Yusoff NAM, Teh GC, Chui BJT, Ng IS, et al: Prostate
cancer in multi-ethnic Asian men: Real-world experience in the
Malaysia prostate cancer (M-CaP) study. Cancer Med. 10:8020–8028.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stopsack KH, Nandakumar S, Arora K, Nguyen
B, Vasselman SE, Nweji B, McBride SM, Morris MJ, Rathkopf DE,
Slovin S, et al: Differences in prostate cancer genomes by
self-reported race: Contributions of genetic ancestry, modifiable
cancer risk factors, and clinical factors. Clin Cancer Res.
28:318–326. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sani MM, Kloping Y and Surahmad F: Benign
prostatic hyperplasia genetic variants in Asians. Clin Chim Acta.
565(119986)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Graham NJ, Souter LH and Salami SS: A
systematic review of family history, Race/Ethnicity, and genetic
risk on prostate cancer detection and outcomes: Considerations in
PSA-based screening. Urol Oncol. 15:S1078–S1143. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chiong E, Murphy DG, Buchan N, Chen K,
Chen SS, Chua MLK, Hamid AR, Kanesvaran R, Khochikar M, Letran J,
et al: Management of advanced prostate cancer in the Asia-Pacific
region: Summary of the Asia-Pacific advanced prostate cancer
consensus conference 2023. Asia Pac J Clin Oncol. 20:481–490.
2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu VN, Van Blarigan EL, Zhang L, Graff
RE, Loeb S, Langlais CS, Cowan JE, Carroll PR, Chan JM and Kenfield
SA: Plant-based diets and disease progression in men with prostate
cancer. JAMA Netw Open. 7(e249053)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mitchell E, Comerford K, Knight M,
McKinney K and Lawson Y: A review of dairy food intake for
improving health among black adults in the US. J Natl Med Assoc.
116:253–273. 2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kimura T and Egawa S: Epidemiology of
prostate cancer in Asian countries. Int J Urol. 25:524–531.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ge S, Zha L, Sobue T, Kitamura T, Ishihara
J, Iwasaki M, Inoue M, Yamaji T, Tsugane S and Sawada N: Dietary
consumption of antioxidant vitamins in relation to prostate cancer
risk in Japanese men: The Japan public health center-based
prospective study. J Epidemiol. 34:144–153. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Krishnan S, Kanthaje S, Punchappady DR,
Mujeeburahiman M and Ratnacaram CK: Circulating metabolite
biomarkers: A game changer in the human prostate cancer diagnosis.
J Cancer Res Clin Oncol. 149:951–967. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ko YH and Kim BH: Should contemporary
western guidelines based on studies conducted in the 2000s be
adopted for the prostate-specific antigen screening policy for
Asian men in the 2020s? World J Mens Health. 40:1–8.
2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang CM, Yuan L, Liu XH, Chen SQ, Wang HF,
Dong QF, Zhang B, Huang MS, Zhang ZY, Xiao J and Tao T: Developing
a diagnostic model for predicting prostate cancer: A retrospective
study based on Chinese multicenter clinical data. Asian J Androl.
26:34–40. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pandit AA, Halpern MT, Gressler LE, Kamel
M, Payakachat N and Li C: Association of race/ethnicity and patient
care experiences with receipt of definitive treatment among
prostate cancer survivors: A SEER-CAHPS study. Cancer Causes
Control. 35:647–659. 2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Alkhatib KY, Filipas DK, Briggs L, Frego
N, Koelker M, Lipsitz SR, Pierorazio PM, Rebbeck T, Kilbridge K,
Kibel AS, et al: Racial differences in knowledge, attitudes, and
sources of information about germline cancer genetic testing in the
USA: An analysis of the health information national trends survey
system. Prev Med. 178(107779)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sweis J, Ofori B and Murphy AB: Concerns
regarding prostate cancer screening guidelines in minority
populations. Prostate Cancer Prostatic Dis. 27:591–593.
2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sasaki T, Matsumoto R, Higashi S, Kato M,
Masui S, Yoshio Y, Nishikawa K and Inoue T: Impact of family
history on clinicopathological variables and disease progression in
Japanese prostate cancer patients undergoing robotic-assisted
radical prostatectomy. Int J Urol. 29:1339–1346. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xu J, Resurreccion WK, Shi Z, Wei J, Wang
CH, Zheng SL, Hulick PJ, Ross AE, Pavlovich CP, Helfand B, et al:
Inherited risk assessment and its clinical utility for predicting
prostate cancer from diagnostic prostate biopsies. Prostate Cancer
Prostatic Dis. 25:422–430. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Giri VN, Morgan TM, Morris DS, Berchuck
JE, Hyatt C and Taplin ME: Genetic testing in prostate cancer
management: Considerations informing primary care. CA Cancer J
Clin. 72:360–371. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cui H, Zhang W, Zhang L, Qu Y, Xu Z, Tan
Z, Yan P, Tang M, Yang C, Wang , et al: Risk factors for
prostate cancer: An umbrella review of prospective observational
studies and mendelian randomization analyses. PLoS Med.
21(e1004362)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Seibert TM, Garraway IP, Plym A, Mahal BA,
Giri V, Jacobs MF, Cheng HH, Loeb S, Helfand BT, Eeles RA and
Morgan TM: Genetic risk prediction for prostate cancer:
Implications for early detection and prevention. Eur Urol.
83:241–248. 2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Takeuchi T, Hattori-Kato M, Okuno Y,
Nomiya A, Fukuhara H, Zaitsu M and Azuma T: A genome-wide
association study of prostate cancer susceptibility using
occupational and environmental factors as confounding factors.
Cureus. 16(e52926)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Farashi S, Kryza T, Clements J and Batra
J: Post-GWAS in prostate cancer: From genetic association to
biological contribution. Nat Rev Cancer. 19:46–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Teerlink CC, Leongamornlert D, Dadaev T,
Thomas A, Farnham J, Stephenson RA, Riska S, McDonnell SK, Schaid
DJ, Catalona WJ, et al: Genome-wide association of familial
prostate cancer cases identifies evidence for a rare segregating
haplotype at 8q24.21. Hum Genet. 135:923–938. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Benafif S and Eeles R: Genetic
predisposition to prostate cancer. Br Med Bull. 120:75–89.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hall R, Bancroft E, Pashayan N, Kote-Jarai
Z and Eeles RA: Genetics of prostate cancer: A review of latest
evidence. J Med Genet. 61:915–926. 2024.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bıyıkoğlu M, Tanrıverdi R, Bozlu M, Şenel
S, Fidancı ŞB, Tamer L and Akbay E: Evaluation of homeobox protein
B13 (HOXB13) gene G84E mutation in patients with prostate cancer.
World J Urol. 42(476)2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Heise M, Jarzemski P, Nowak D, Bąk A,
Junkiert-Czarnecka A, Pilarska-Deltow M, Borysiak M, Pilarska B and
Haus O: Clinical significance of gene mutations and polymorphic
variants and their association with prostate cancer risk in Polish
men. Cancer Control. 29(10732748211062342)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nyberg T, Brook MN, Ficorella L, Lee A,
Dennis J, Yang X, Wilcox N, Dadaev T, Govindasami K, Lush M, et al:
CanRisk-Prostate: A comprehensive, externally validated risk model
for the prediction of future prostate cancer. J Clin Oncol.
41:1092–1104. 2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dairo O, DePaula Oliveira L, Schaffer E,
Vidotto T, Mendes AA, Lu J, Huynh SV, Hicks J, Sowalsky AG, De
Marzo AM, et al: Gene methylation is associated with fatty acid
synthase expression and clinical-genomic features of prostate
cancer. Cancer Res Commun. 4:152–163. 2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Panebianco M, Cereda V and D'Andrea MR:
Combination of the PARPi and ARSi in advanced castration resistant
prostate cancer: A review of the recent phase III trials. Explor
Target Antitumor Ther. 5:997–1010. 2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhao D, Wang A, Li Y, Cai X, Zhao J, Zhang
T, Zhao Y, Dong Y, Zhou F, Li Y and Wang J: Establishing the
homologous recombination score threshold in metastatic prostate
cancer patients to predict the efficacy of PARP inhibitors. J Natl
Cancer Cent. 4:280–287. 2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Castro E: PARP inhibitor addition to
androgen receptor pathway inhibitors in metastatic
castration-resistant prostate cancer should be limited to BRCA
mutation carriers. Eur Urol Focus. 10:504–505. 2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hassanin E, May P, Aldisi R, Spier I,
Forstner AJ, Nöthen MM, Aretz S, Krawitz P, Bobbili DR and Maj C:
Breast and prostate cancer risk: The interplay of polygenic risk,
rare pathogenic germline variants, and family history. Genet Med.
24:576–585. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Oliynyk RT: Evaluating the potential of
younger cases and older controls cohorts to improve discovery power
in genome-wide association studies of late-onset diseases. J Pers
Med. 9(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Graff RE, Möller S, Passarelli MN, Witte
JS, Skytthe A, Christensen K, Tan Q, Adami HO, Czene K, Harris JR,
et al: Familial risk and heritability of colorectal cancer in the
nordic twin study of cancer. Clin Gastroenterol Hepatol.
15:1256–1264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ponti G, De Angelis C, Ponti R, Pongetti
L, Losi L, Sticchi A, Tomasi A and Ozben T: Hereditary breast and
ovarian cancer: From genes to molecular targeted therapies. Crit
Rev Clin Lab Sci. 60:640–650. 2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Abdel-Razeq H, Sharaf B, Tamimi F, Hani
HB, Alsmadi O, Khalil H, Abunasser M, Edaily S and Mansour A:
Establishment of a clinical cancer genetics program for breast
cancer in a resource-limited country; challenges and opportunities.
Front Oncol. 14(1431985)2024.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Allen I, Hassan H, Walburga Y, Huntley C,
Loong L, Rahman T, Allen S, Garrett A, Torr B, Bacon A, et al:
Second primary cancer risks after breast cancer in BRCA1 and BRCA2
pathogenic variant carriers. J Clin Oncol.
29(JCO2401146)2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Fanale D, Corsini LR, Brando C, Randazzo
U, Bono M, Pedone E, Perez A, Sciacchitano R, Cancelliere D,
Piraino P, et al: BRCA-associated hereditary male cancers: Can
gender affect the prevalence and spectrum of germline pathogenic
variants? Front Oncol. 14(1414343)2024.PubMed/NCBI View Article : Google Scholar
|
|
81
|
John AO, Singh A, Yadav P, Joel A, Thumaty
DB, Ninan KF, Georgy JT, Cherian AJ, Thomas S, Thomas A, et al: The
BRCA mutation spectrum among breast and ovarian cancers in India:
Highlighting the need to screen BRCA1 185delAG among South Indians.
Eur J Hum Genet. 32:1319–1326. 2024.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Marino F, Totaro A, Gandi C, Bientinesi R,
Moretto S, Gavi F, Pierconti F, Iacovelli R, Bassi P and Sacco E:
Germline mutations in prostate cancer: A systematic review of the
evidence for personalized medicine. Prostate Cancer Prostatic Dis.
26:655–664. 2023.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Lukashchuk N, Barnicle A, Adelman CA,
Armenia J, Kang J, Barrett JC and Harrington EA: Impact of DNA
damage repair alterations on prostate cancer progression and
metastasis. Front Oncol. 13(1162644)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bugoye FC, Torrorey-Sawe R, Biegon R,
Dharsee N, Mafumiko FMS, Patel K and Mining SK: Mutational spectrum
of DNA damage and mismatch repair genes in prostate cancer. Front
Genet. 14(1231536)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zhang D, Xu X, Wei Y, Chen X, Li G, Lu Z,
Zhang X, Ren X, Wang S and Qin C: Prognostic role of DNA damage
response genes mutations and their association with the sensitivity
of olaparib in prostate cancer patients. Cancer Control.
29(10732748221129451)2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Reiter K and Hassler MR: Genetic testing
and management of prostate cancer patients with pathogenic germline
variants. Memo-Magazine of European Medical Oncology. 17:51–56.
2024.
|
|
87
|
Steinkellner L, Luger F and Loidl W:
Importance of genetic testing in prostate cancer. Urologie.
61:1392–1396. 2022.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
88
|
Rajwa P, Quhal F, Pradere B, Gandaglia G,
Ploussard G, Leapman MS, Gore JL, Paradysz A, Tilki D, Merseburger
AS, et al: Prostate cancer risk, screening and management in
patients with germline mutations. Nat Rev Urol. 20:205–216.
2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Inoue T, Sekito S, Kageyama T, Sugino Y
and Sasaki T: Roles of the PARP inhibitor in BRCA1 and BRCA2
pathogenic mutated metastatic prostate cancer: Direct functions and
modification of the tumor microenvironment. Cancers (Basel).
15(2662)2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Chen JY, Wang PY, Liu MZ, Lyu F, Ma MW,
Ren XY and Gao XS: Biomarkers for prostate cancer: From diagnosis
to treatment. Diagnostics (Basel). 13(3350)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Petrucelli N, Daly MB and Pal T: BRCA1-
and BRCA2-associated hereditary breast and ovarian cancer, In
GeneReviews((R)), M.P. Adam et al., Editors. 1993: Seattle
(WA).
|
|
92
|
Ragupathi A, Singh M, Perez AM and Zhang
D: Targeting the BRCA1/2 deficient cancer with PARP inhibitors:
Clinical outcomes and mechanistic insights. Front Cell Dev Biol.
11(1133472)2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Bychkovsky BL, Li T, Sotelo J, Tayob N,
Mercado J, Gomy I, Chittenden A, Kane S, Stokes S, Hughes ME, et
al: Identification and management of pathogenic variants in BRCA1,
BRCA2, and PALB2 in a tumor-only genomic testing program. Clin
Cancer Res. 28:2349–2360. 2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Nukaya T, Sumitomo M, Sugihara E, Takeda
M, Nohara S, Tanishima S, Takenaka M, Zennami K, Takahara K,
Shiroki R and Saya H: Estimating copy number to determine BRCA2
deletion status and to expect prognosis in localized prostate
cancer. Cancer Med. 12:8154–8165. 2023.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Foley GR, Marthick JR, Ostrander EA,
Stanford JL, Dickinson JL and FitzGerald LM: Association of a novel
BRCA2 mutation with prostate cancer risk further supports germline
genetic testing. Eur J Cancer. 180:155–157. 2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Nyberg T, Tischkowitz M and Antoniou AC:
BRCA1 and BRCA2 pathogenic variants and prostate cancer risk:
Systematic review and meta-analysis. Br J Cancer. 126:1067–1081.
2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Xie C, Luo J, He Y, Jiang L, Zhong L and
Shi Y: BRCA2 gene mutation in cancer. Medicine (Baltimore).
101(e31705)2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hongo H, Kosaka T, Hayashi H, Nakamura K,
Nishihara H, Mikami S, Beltran H and Oya M: Germline BRCA2 mutation
in a case of aggressive prostate cancer accompanied by spinal
bulbar muscular atrophy. Asian J Androl. 24:116–118.
2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Thorne H, Devereux L, Li J, Alsop K,
Christie L, van Geelen CT, Burdett N, Pishas KI, Woodford N,
Leditschke J, et al: BRCA1 and BRCA2 carriers with breast, ovarian
and prostate cancer demonstrate a different pattern of metastatic
disease compared with non-carriers: Results from a rapid autopsy
programme. Histopathology. 83:91–103. 2023.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Paulo P, Cardoso M, Brandão A, Pinto P,
Falconi A, Pinheiro M, Cerveira N, Silva R, Santos C, Pinto C, et
al: Genetic landscape of homologous recombination repair genes in
early-onset/familial prostate cancer patients. Genes Chromosomes
Cancer. 62:710–720. 2023.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kwong A, Ho CYS, Shin VY, Ng ATL, Chan TL
and Ma ESK: Molecular characteristics of Asian male-related
cancers. Breast Cancer Res Treat. 198:391–400. 2023.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Fettke H, Dai C, Kwan EM, Zheng T, Du P,
Ng N, Bukczynska P, Docanto M, Kostos L, Foroughi S, et al:
BRCA-deficient metastatic prostate cancer has an adverse prognosis
and distinct genomic phenotype. EbioMedicine.
95(104738)2023.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Barnes DR, Silvestri V, Leslie G, McGuffog
L, Dennis J, Yang X, Adlard J, Agnarsson BA, Ahmed M, Aittomäki K,
et al: Breast and prostate cancer risks for male BRCA1 and BRCA2
pathogenic variant carriers using polygenic risk scores. J Natl
Cancer Inst. 114:109–122. 2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Marchetti C, Ataseven B, Cassani C, Sassu
CM, Congedo L, D'Indinosante M, Cappuccio S, Rhiem K, Hahnen E,
Cordisco EL, et al: Ovarian cancer onset across different mutation
types: A view to a more tailored approach for BRCA mutated
patients. Int J Gynecol Cancer. 33:257–262. 2023.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Osman K, Ahmet K, Hilmi T, İlker NO, Ercan
Ö, Devrim Ç, Murat S, Emre Ç, İlhan H, Mustafa G, et al: BRCA
1/BRCA 2 pathogenic/likely pathogenic variant patients with breast,
ovarian, and other cancers. Balkan J Med Genet. 25:5–14.
2022.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Mitsueda R, Toda H, Shinden Y, Fukuda K,
Yasudome R, Kato M, Kikkawa N, Ohtsuka T, Nakajo A and Seki N:
Oncogenic targets regulated by tumor-suppressive miR-30c-1-3p and
miR-30c-2-3p: TRIP13 facilitates cancer cell aggressiveness in
breast cancer. Cancers (Basel). 15(4189)2023.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Loboda AP, Adonin LS, Zvereva SD, Guschin
DY, Korneenko TV, Telegina AV, Kondratieva OK, Frolova SE, Pestov
NB and Barlev NA: BRCA mutations-the achilles heel of breast,
ovarian and other epithelial cancers. Int J Mol Sci.
24(4982)2023.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lavoro A, Scalisi A, Candido S, Zanghì GN,
Rizzo R, Gattuso G, Caruso G, Libra M and Falzone L: Identification
of the most common BRCA alterations through analysis of germline
mutation databases: Is droplet digital PCR an additional strategy
for the assessment of such alterations in breast and ovarian cancer
families? Int J Oncol. 60(58)2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Werner H: BRCA1: An endocrine and
metabolic regulator. Front Endocrinol (Lausanne).
13(844575)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Russi M, Marson D, Fermeglia A, Aulic S,
Fermeglia M, Laurini E and Pricl S: The fellowship of the RING:
BRCA1, its partner BARD1 and their liaison in DNA repair and
cancer. Pharmacol Ther. 232(108009)2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Lee CL, Holter S, Borgida A, Dodd A,
Ramotar S, Grant R, Wasson K, Elimova E, Jang RW, Moore M, et al:
Germline BRCA2 variants in advanced pancreatic acinar cell
carcinoma: A case report and review of literature. World J
Gastroenterol. 28:6421–6432. 2022.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Boussios S, Rassy E, Moschetta M, Ghose A,
Adeleke S, Sanchez E, Sheriff M, Chargari C and Pavlidis N: BRCA
mutations in ovarian and prostate cancer: Bench to bedside. Cancers
(Basel). 14(3888)2022.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Werner H and Laron Z: Role of the GH-IGF1
system in progression of cancer. Mol Cell Endocrinol.
518(111003)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Sharon SM, Pozniak Y, Geiger T and Werner
H: TMPRSS2-ERG fusion protein regulates insulin-like growth
factor-1 receptor (IGF1R) gene expression in prostate cancer:
Involvement of transcription factor Sp1. Oncotarget. 7:51375–51392.
2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Alanee SR, Glogowski EA, Schrader KA,
Eastham JA and Offit K: Clinical features and management of BRCA1
and BRCA2-associated prostate cancer. Front Biosci (Elite Ed).
6:15–30. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
116
|
Moro L, Arbini AA, Yao JL, di Sant'Agnese
PA, Marra E and Greco M: Loss of BRCA2 promotes prostate cancer
cell invasion through up-regulation of matrix metalloproteinase-9.
Cancer Sci. 99:553–563. 2008.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Rein HL and Bernstein KA: Finding
significance: New perspectives in variant classification of the
RAD51 regulators, BRCA2 and beyond. DNA Repair (Amst).
130(103563)2023.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Edwards SM, Evans DG, Hope Q, Norman AR,
Barbachano Y, Bullock S, Kote-Jarai Z, Meitz J, Falconer A, Osin P,
et al: Prostate cancer in germline mutation carriers is associated
with poorer prognosis. Br J Cancer. 103:918–924. 2010.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi
R, Hope Q, Osin P, Jackson R, Southgate C, Singh R, Falconer A, et
al: Two percent of men with early-onset prostate cancer harbor
germline mutations in the gene. Am J Hum Genet. 72:1–12.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
120
|
Gallagher DJ, Cronin AM, Milowsky MI,
Morris MJ, Bhatia J, Scardino PT, Eastham JA, Offit K and Robson
ME: Germline mutation does not prevent response to taxane-based
therapy for the treatment of castration-resistant prostate cancer.
BJU Int. 109:713–719. 2012.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Gallagher DJ, Vijai J, Cronin AM, Bhatia
J, Vickers AJ, Gaudet MM, Fine S, Reuter V, Scher HI, Halldén C, et
al: Susceptibility loci associated with prostate cancer progression
and mortality. Clin Cancer Res. 16:2819–2832. 2010.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Thorne H, Willems AJ, Niedermayr E, Hoh
IM, Li J, Clouston D, Mitchell G, Fox S and Hopper JL: Kathleen
Cunningham Consortium for Research in Familial Breast Cancer
Consortium. Bolton D: Decreased prostate cancer-specific survival
of men with mutations from multiple breast cancer families. Cancer
Prev Res (Phila). 4:1002–1010. 2011.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Tryggvadóttir L, Vidarsdóttir L,
Thorgeirsson T, Jonasson JG, Olafsdóttir EJ, Olafsdóttir GH, Rafnar
T, Thorlacius S, Jonsson E, Eyfjord JE and Tulinius H: Prostate
cancer progression and survival in BRCA2 mutation carriers. J Natl
Cancer Inst. 99:929–935. 2007.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Narod SA, Neuhausen S, Vichodez G, Armel
S, Lynch HT, Ghadirian P, Cummings S, Olopade O, Stoppa-Lyonnet D,
Couch F, et al: Rapid progression of prostate cancer in men with a
mutation. Br J Cancer. 99:371–374. 2008.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Castro E, Jugurnauth-Little S, Karlsson Q,
Al-Shahrour F, Piñeiro-Yañez E, Van de Poll F, Leongamornlert D,
Dadaev T, Govindasami K, Guy M, et al: High burden of copy number
alterations and c-MYC amplification in prostate cancer from BRCA2
germline mutation carriers. Ann Oncol. 26:2293–2300.
2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Castro E, Salles DC, Lozano R, Thorne H,
Aragon I, Campos FL, Rubio J, Pecharroman AG, Prieto D, Lozano IG,
et al: Association between BRCA2 status and histologic variants
(intraductal (IDC) and cribriform (CRIB) histology) in prostate
cancer (PC). J Clin Oncol. 38:74–83. 2020.
|
|
127
|
Castro E, Goh C, Olmos D, Saunders E,
Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami
K, Guy M, et al: Germline BRCA mutations are associated with higher
risk of nodal involvement, distant metastasis, and poor survival
outcomes in prostate cancer. J Clin Oncol. 31:1748–1757.
2013.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Shee K, Cowan JE, Balakrishnan A, Escobar
D, Chang K, Washington SL III, Nguyen HG, Shinohara K, Cooperberg
MR and Carroll PR: Limited relevance of the very low risk prostate
cancer classification in the modern era: Results from a large
institutional active surveillance cohort. Eur Urol. 84:9–12.
2023.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Xie M, Gao XS, Ma MW, Gu XB, Li HZ, Lyu F,
Bai Y, Chen JY, Ren XY and Liu MZ: Population-based comparison of
different risk stratification systems among prostate cancer
patients. Front Oncol. 11(646073)2021.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Ellis SD, Blackard B, Carpenter WR, Mishel
M, Chen RC, Godley PA, Mohler JL and Bensen JT: Receipt of national
comprehensive cancer network guideline-concordant prostate cancer
care among African American and Caucasian American men in North
Carolina. Cancer. 119:2282–2290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Ueki A, Yoshida R, Kosaka T and
Matsubayashi H: Clinical risk management of breast, ovarian,
pancreatic, and prostatic cancers for variant carriers in Japan. J
Hum Genet. 68:517–526. 2023.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Militaru FC, Militaru V, Crisan N, Bocsan
IC, Udrea AA, Catana A, Kutasi E and Militaru MS: Molecular basis
and therapeutic targets in prostate cancer: A comprehensive review.
Biomol Biomed. 23:760–771. 2023.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Margel D, Benjaminov O, Ozalvo R, Grievink
LS, Kedar I, Yerushalmi R, Ben-Aharon I, Neiman V, Yossepowitch O,
Kedar D, et al: Personalized prostate cancer screening among men
with high risk genetic predisposition- study protocol for a
prospective cohort study. BMC Cancer. 14(528)2014.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Wan A, Zhang G, Ma D, Zhang Y and Qi X: An
overview of the research progress of BRCA gene mutations in breast
cancer. Biochim Biophys Acta Rev Cancer.
1878(188907)2023.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Slootbeek PHJ, Overbeek JK, Ligtenberg
MJL, van Erp NP and Mehra N: PARPing up the right tree; an overview
of PARP inhibitors for metastatic castration-resistant prostate
cancer. Cancer Lett. 577(216367)2023.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Xu J, Ma C, Hirschey R, Liu J, Neidre DB,
Nielsen ME, Keyserling TC, Tan X and Song L: Associations of role,
area deprivation index, and race with health behaviors and body
mass index among localized prostate cancer patients and their
partners. J Cancer Surviv.
18(10.1007/s11764-024-01625-z)2024.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Trendowski MR, Ruterbusch JJ, Baird TE,
Wenzlaff AS, Pandolfi SS, Hastert TA, Schwartz AG and Beebe-Dimmer
JL: Correlates of health-related quality of life in African
Americans diagnosed with cancer: A review of survivorship studies
and the Detroit research on cancer survivors cohort. Cancer
Metastasis Rev. 43:1373–1384. 2024.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Delgado D, Gillard M, Tong L, Demanelis K,
Oliva M, Gleason KJ, Chernoff M, Chen L, Paner GP, Griend DV and
Pierce BL: The impact of inherited genetic variation on DNA
methylation in prostate cancer and benign tissues of African
American and European American Men. Cancer Epidemiol Biomarkers
Prev. 33:557–566. 2024.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Tipre M, Hardy C, Bowman T, Glover M,
Gullet P, Baity D, Levy K and Baskin ML: Concept mapping with black
men: Barriers to prostate cancer screening and solutions. J Cancer
Educ. 38:1808–1815. 2023.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Wang EY, Borno HT, Washington Iii SL,
Friedlander T, Zhang S, Trejo E, Van Blarigan EL, Chan JM,
Shariff-Marco S, Beatty AL and Kenfield S: Engaging men of diverse
racial and ethnic groups with advanced prostate cancer in the
design of an mHealth diet and exercise intervention: Focus group
study. JMIR Cancer. 9(e45432)2023.PubMed/NCBI View
Article : Google Scholar
|
|
141
|
Lei F and Lee E: Cancer screening rates
among Asian Americans: A cross-sectional secondary data analysis
study. Cancer Control. 30(10732748231202462)2023.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Wadhwa A, Han K, Ramirez-Velandia F,
Alwakaa O, Riordan C, McNeil E, Granstein JH, Taussky P,
Enriquez-Marulanda A, Ogilvy CS, et al: Neighborhood deprivation,
race and ethnicity, and prostate cancer outcomes across California
health care systems. JAMA Netw Open. 7(e242852)2024.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Frego N, Beatrici E, Labban M, Stone BV,
Filipas DK, Koelker M, Lughezzani G, Buffi NM, Osman NY, Lipsitz
SR, et al: Racial disparities in prostate cancer screening: The
role of shared decision making. Am J Prev Med. 66:27–36.
2024.PubMed/NCBI View Article : Google Scholar
|