Introduction
Chromobacterium violaceum (C.
violaceum) is a Gram-negative coccobacillus and an
environmental bacterium commonly found in soil and water,
particularly in tropical and subtropical regions. Infections with
C. violaceum are often linked to skin injuries, trauma, or
water exposure, which provide a route for the bacterium to enter
the body (1,2). A distinctive characteristic of C.
violaceum is its production of violacein, a violet pigment
regulated through a quorum sensing (QS) system (3,4).
C. violaceum, as with numerous other opportunistic
pathogens, forms biofilms with structured communities of bacterial
cells encased in a protective matrix (5). This biofilm formation enhances its
ability to produce various exotoxins, including hemolysins, which
can lyse red blood cells (RBCs) and other host cells (1,2).
Additionally, its outer membrane contains lipopolysaccharides
(LPS), potent endotoxins that are recognized by the host immune
system as danger signals, triggering an inflammatory response. In
systemic infections, the excessive release of LPS can lead to
septic shock a severe and life-threatening condition marked by
widespread inflammation and multi-organ failure (1,6).
C. violaceum possesses both type III and type
VI secretion systems (T3SS and T6SS), needle-like structures that
inject virulence proteins directly into host cells. These systems
are crucial for delivering effector proteins that manipulate host
cell functions, such as inhibiting immune responses or inducing
apoptosis, enhancing the ability of the bacterium to survive and
multiply within host tissues (7).
Additional virulence factors include motility via flagella,
siderophore production, antioxidant enzymes and proteases, all of
which contribute to rapid invasion, tissue destruction and
resistance to immune defenses, complicating treatment and
increasing the severity of infections (8).
One of the most alarming characteristics of C.
violaceum is its unexpected resistance to a number of commonly
used antibiotics, largely attributed to its QS mechanisms (9). The link between QS and pathogenesis
underscores the urgent need for the development of innovative
strategies to combat infections and mitigate their harmful effects
on human health (10). In light of
these challenges, early treatment of the pathogen, particularly
through the use of natural compounds, is vital for improving
patient outcomes (11). Given that
plant-based medicines are often reported to be reliable, effective
and relatively safe, they continue to be widely used in traditional
medicine worldwide (12).
Furthermore, as they are derived from natural sources, plant-based
treatments are considered to cause fewer side-effects than modern
synthetic drugs. A recent study demonstrated that natural
compounds, particularly plant-derived flavonoids, have greater
potential to combat dental bacterial biofilms; these compounds
exhibit promising antibiofilm properties, rendering them effective
alternatives for preventing and managing dental infections
(13).
Pithecellobium dulce (P. dulce), a
fruit of American origin from the Fabaceae family, is native
to tropical America and widely grown in India and the Andaman
Islands (14). Commonly referred
to as ‘Jungal Jalebi’ or ‘Black Bead Tree’ in English, ‘Vilayati
Babul’ in Hindi and ‘Kodukkapuli’ in Tamil, P. dulce is an
evergreen, medium-sized, spiny tree (15). Various parts of the plant have
notable medicinal uses, with the root extracts exhibiting
estrogenic activity (16).
Traditionally, different plant parts have been employed to treat
earaches, leprosy, peptic ulcers, toothaches and venereal diseases,
and serve as emollients, abortifacients, anodynes and larvicides
(17). The bark of P. dulce
is used as an astringent for dysentery and febrifuge, as well as to
treat dermatitis and eye inflammation. Polyphenols in the bark have
demonstrated anti-venomous properties (18). Additionally, ethanolic extracts
from the pod pulp of P. dulce have been shown to exhibit
antibacterial activity against both Gram-positive and Gram-negative
bacteria, including Bacillus subtilis and Klebsiella
pneumoniae, with secondary metabolites, such as flavonoids and
saponins contributing to this antibacterial effect (19).
To the best of our knowledge, the present study is
the first study to date aiming to investigate the effects of P.
dulce isolates on C. violaceum. The anti-QS functions of
P. dulce in relation to C. violaceum have not yet
been thoroughly investigated.
Materials and methods
Bacterial strains and growth
conditions
C. violaceum (CV12472) was generously
provided by Dr Busi Siddhartha from Pondicherry University,
Puducherry, Tamil Nadu, India.. The strain was cultured under
aerobic conditions at 30˚C in Luria-Bertani (LB) (HiMedia
Laboratories, LLC) broth to support optimal growth. For
experimental analysis, the bacterial culture was sub-cultured to
ensure optimal growth conditions. The identity of C.
violaceum was verified through the automated VITEK 2 system, as
previously detailed by David H. Pincus (BioMérieux, Inc.).,
delivering precise and reliable bacterial classification (20). Additionally, the initial
identification of the C. violaceum CV12472 was carried out
using standard microbiological methods, focusing on its
characteristic growth patterns on LB agar. These unique
characteristics facilitated its identification, aligning with
observations reported by August et al (21).
Collection of samples
Fruits and seeds from P. dulce were collected
for the present study from the Neelakudi Campus of the Central
University of Tamil Nadu, Thiruvarur, Tamil Nadu, India. The
collected plant parts were authenticated at the Indian Medical
Practitioner Co-operative Society (IMCOPS) herbarium in Chennai,
India.
Preliminary screening of herbal
derivatives
The P. dulce (fruits and seeds) were
collected, and following three rounds of washing with distilled
water, they were allowed to soak for 2 min in 70% ethanol (v/v).
The plant sections were then surface sterilized by immersing them
in 0.1% mercury chloride for 1 min and rinsing them three times
with sterile distilled water. The fruits were air-dried in the
shade after being sterilized. Subsequently, a mechanical grinder
was used to grind the dried fruits into a coarse powder.
A total of 10 g of coarsely ground fruit powder and
10 g of coarsely ground seed powder were immersed in 100 ml ethanol
and methanol (Rankem Laboratories, LLC), respectively, to carry out
the extraction process. These combinations were incubated in a
shaking incubator at 150 revolutions per minute (rpm) and 37˚C for
48-72 h. All extracts were filtered through Whatman (HiMedia
Laboratories, LLC) after 48 h.
Determination of minimum inhibitory
concentration (MIC)
The MIC values of P. dulce (fruits and seeds)
against C. violaceum CV12472 were determined using
previously established protocols (22). Briefly, 20 µl overnight cultures
were added to the LB broth with extracts at 20 to 0.039 mg/ml of
both seed and fruit extract of P. dulce (2-fold serial
dilution) and without extracts (control). The tubes containing
C. violaceum CV12472 culture were incubated at 30˚C and the
MIC values were observed and recorded.
Biofilm assay
A previously described protocol for the crystal
violet staining assay was followed, with slight modifications made
to suit the specific requirements of the experiment (9). In addition to a control without P.
dulce, C. violaceum CV12472 was cultured with P. dulce
fruit and seed extract at sub-MIC of 10 to 0.019 mg/ml. The
cultures were incubated in a microtiter plate at 30˚C for 48 h to
observe the effects. The planktonic cells were read at 600 nm using
optical density (OD) and were disposed of after 24 h without
causing any disturbance to the biofilm. Following the addition of
200 µl crystal violet (HiMedia Laboratories, LLC) to each well, the
plate was incubated for 15 min at room temperature to allow
staining. The unbound stain was eliminated from the wells
containing the crystal violet after 15 min of gentle washing with
sterile distilled water. At 520 nm, the absorbance was determined
using spectrophotometer (JASCO UV/Vis, India) after the adherent
biofilm-bound crystal violet was eluted in 70% ethanol.
Quantification of violacein production
in C. violaceum (CV12472)
The quantitative analysis of violacein production
was previously described by Venkatramanan et al (9), at a sub-lethal concentration of P.
dulce seed extract at 10 to 0.019 mg/ml, alongside a control
without P. dulce. Serial 2-fold dilutions of P. dulce
seed extract were loaded into test tubes containing LB broth,
facilitating a gradient of concentrations for further analysis.
Following the inoculation of 10 µl C. violaceum CV12472
overnight cultures into each test tube, the tubes were cultured for
18 h at 30˚C. The negative control, sterility control and positive
control (C. violaceum CV12472) were also maintained
throughout the assay. After incubation at 30˚C for 24 h, all tubes
were centrifuged at 5,724 x g for 10 min at 4˚C.. Once the culture
supernatant was disposed of, 200 µl DMSO (SRL Chemicals, Mumbai,
India) were added to the pellets and well mixed until the pigment
was extracted. The tubes were then centrifuged at 4,832 x g for 15
min at 4˚C. A 200-µl sample of the extracted violacein was added to
the microtiter plate and measured at 520 nm using spectrophotometer
(JASCO UV/Vis, India) By comparing the OD at 600 nm between the
treated strain and the untreated control, the percentage growth of
each was determined.
Bacterial growth curve
C. violaceum CV12472 growth curve was
examined both simultaneously with and without P. dulce seed
extract. Briefly, an overnight culture of C. violaceum
CV12472 was incubated into LB broth with seed extract of P.
dulce at 10 mg/ml and without seed extracts (control)
separately. The OD at 600 nm was measured every hour while the
culture setup was incubated at 37˚C for up to 24 h.
Statistical analysis
All experiments, including the biofilm assay,
violacein pigment assay, and growth curve analysis, were performed
in triplicate to ensure accuracy and reproducibility of the
results. Statistical significance was determined using one-way
ANOVA followed by Tukey's Honestly Significant Difference (HSD)
test, performed using GraphPad Prism 10.1.0 software (Dotmatics). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
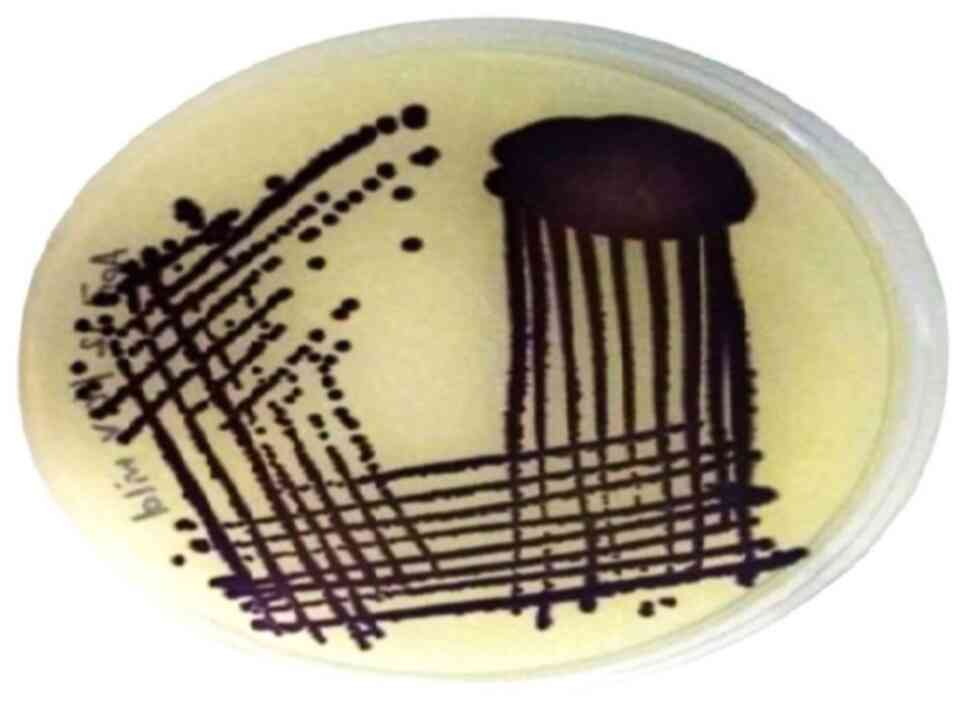
Identification of C. violaceum
The bacterial morphology was confirmed using the
VITEK 2 automated system. When cultured on LB agar, the isolate
formed colonies displaying a characteristic violet pigmentation, as
depicted in Fig. 1. This
distinctive chromogenic trait is a hallmark of C. violaceum,
aiding in its identification.
MIC evaluation
The P. dulce seed and fruit extract were
found to inhibit the growth of C. violaceum CV12472 at 20
mg/ml. The crude extracts anti-QS and antibiofilm activities were
then investigated at concentrations lower than their MIC values
(Table I).
 | Table IMinimum inhibitory concentration. |
Table I
Minimum inhibitory concentration.
| S. no | 2-fold dilution
concentration (mg/ml) | Growth
measureda; P.
dulce (seed and fruit) |
|---|
| 1 | 20 | - |
| 2 | 10 | + |
| 3 | 5 | + |
| 4 | 2.5 | + |
| 5 | 1.25 | + |
| 6 | 0.62 | + |
| 7 | 0.31 | + |
| 8 | 0.15 | + |
| 9 | 0.078 | + |
| 10 | 0.039 | + |
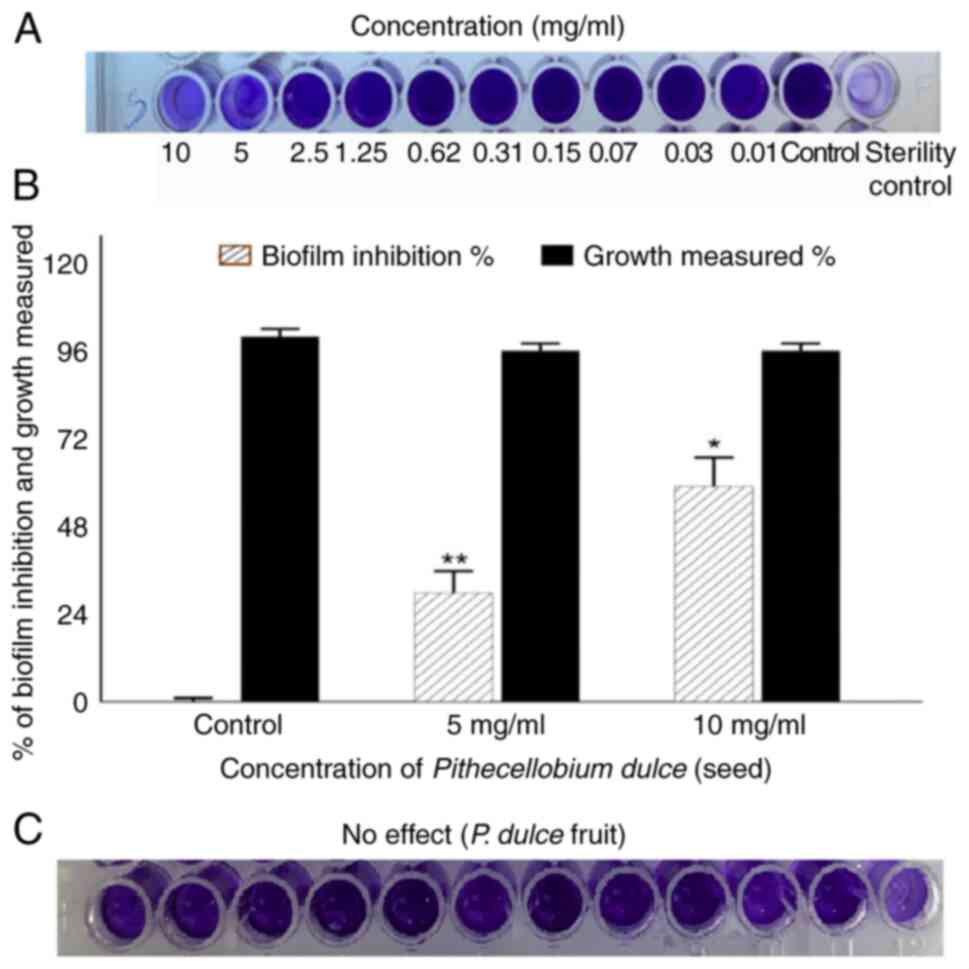
Effect of P. dulce extract on biofilm
inhibition in C. violaceum CV12472
The inhibition of biofilm formation in C.
violaceum (CV12472) was evaluated using the microtiter plate
method with 0.1% crystal violet staining. Compared with the
untreated controls, treatment with P. dulce seed extract
markedly reduced the biofilm-forming ability of C. violaceum
CV12472 (Fig. 2A).
Spectrophotometric analysis revealed maximum biofilm inhibition of
58.91 and 29.68% at concentrations of 10 and 5 mg/ml, respectively
(Fig. 2B). By contrast, the P.
dulce fruit extract had no notable effect on biofilm formation
(Fig. 2C). Notably, the seed
extract did not interfere with planktonic cell growth, indicating
biofilm inhibition was achieved at sub-MIC levels.
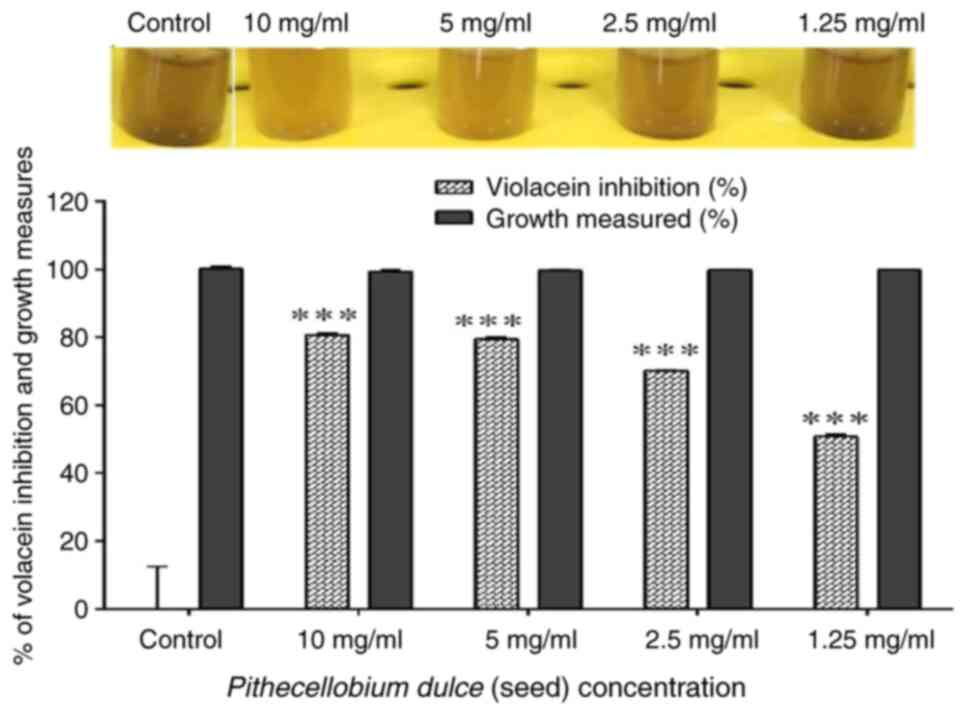
Quantification of violacein in C.
violaceum
C. violaceum CV12472 is commonly used for the
detection of QS signals. C6-HSL is a signaling molecule involved in
the production of violet color pigment of C. violaceum.
Thus, any disturbances occurring in C6-HSL molecule will affect the
ability of the organism to produce pigment (10). In the present study, C.
violaceum CV12472 was used as a control strain for the
qualitative and quantification of violacein pigment production.
Violacein pigment formation against C. violaceum CV12472 was
found to be inhibited by P. dulce (seed) extract in a
concentration-dependent manner and through qualitative analysis.
Only P. dulce (seed) exhibited a substantial reduction in
violacein production in C. violaceum CV12472 to the level of
80.66 and 79.5% when treated with P. dulce at 10 and 5
mg/ml, respectively (Fig. 3).
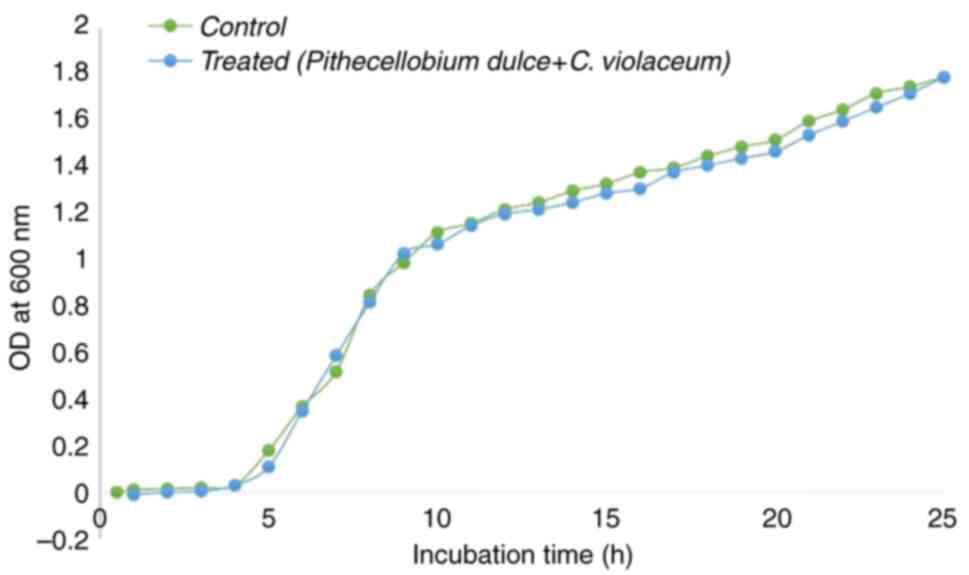
Bacterial growth curve analysis
In order to determine the growth inhibitory
activity, C. violaceum CV12472 was grown both with and
without P. dulce seed extract. As illustrated in Fig. 4, at a concentration of 10 mg/ml,
P. dulce seed extract did not inhibit planktonic growth.
This emphasizes that the extract specifically targets biofilm
formation rather than exhibiting general antibacterial
activity.
Discussion
C. violaceum is an opportunistic pathogen
that is usually linked to serious infections that occur after skin
injuries or water contamination exposure (23). Treatment is complex, and the risk
of systemic infections, such as septicemia and meningitis are
increased due to its capacity to produce virulence factors such as
violacein and different exotoxins, as well as its ability to form
biofilms. The growing resistance of bacteria to widely used
antibiotics has made treating infections increasingly challenging,
pushing the need for alternative solutions (12,24).
In this context, natural remedies are gaining recognition as a
promising approach for the future. As antibiotic resistance
escalates, natural compounds known for their diverse bioactive
properties offer the potential for safer and more effective
treatments. This marks a pivotal shift in the management of
infections, paving the way for innovative strategies in the coming
era.
The present study highlights the potent
antibacterial and antibiofilm properties of P. dulce,
demonstrating its effectiveness against C. violaceum
CV12472. The extracts from both the seeds and fruits exhibited
significant growth inhibition at concentrations as low as 20 mg/ml,
indicating that P. dulce may serve as a viable natural
alternative to conventional antibiotics. This is particularly
relevant given the increasing prevalence of antibiotic resistance
among pathogenic bacteria. Studies have demonstrated that P.
dulce exhibits significant antibacterial effects against
Streptococcus mutans at concentrations of 25, 50 and 100 µl
(25). These findings add to the
growing body of evidence supporting the potential of natural
substances as alternatives to conventional antibiotics.
Additionally, P. dulce has been shown to exhibit
bactericidal activity against Acinetobacter baumannii at a
concentration of 233 mg/ml, as well as against Staphylococcus
aureus and Escherichia coli at 300 mg/ml. These results
highlight the promising role of P. dulce as a natural
antimicrobial agent with broad-spectrum activity (26).
Additionally, the investigation into the anti-QS
properties of P. dulce is a novel aspect of the present
study, as QS plays a critical role in the virulence of many
bacteria, including C. violaceum CV12472. By inhibiting QS
mechanisms, P. dulce may disrupt biofilm formation and
reduce virulence factor production, thereby enhancing treatment
outcomes. Recent studies have highlighted a range of natural
compounds with notable anti-biofilm and QS inhibitory activities
(9,22,25).
One such compound is epigallocatechin gallate (EGCG), a polyphenol
derived from green tea. EGCG has demonstrated notable efficacy in
disrupting biofilms, achieving up to 95% inhibition in certain
bacterial strains, particularly when used in combination with
antibiotics. This underscores its potential as a valuable adjunct
in antimicrobial therapies aimed at overcoming biofilm-associated
infections (27). This synergistic
approach not only enhances the efficacy of existing antibiotics,
but also addresses the challenge posed by biofilm-associated
infections. The observed inhibition of biofilm formation in the
present study by P. dulce (seed) at sub-MIC level of 10
mg/ml, with a reduction of up to 58.91%, along with an impressive
80.66% decrease in violacein production in C. violaceum
CV12472, underscores its strong potential to disrupt key survival
mechanisms of the pathogen. By contrast, the P. dulce fruit
extract exhibited limited effects on biofilm formation. This
comparative insight suggests that the bioactive compounds in the
seed extract may target key pathways in biofilm-related infections
more effectively than those in the fruit extract. These results
position P. dulce (seed) as a promising candidate for
combating biofilm-related infections and QS-mediated virulence.
Similarly, research on QS inhibitors (QSIs) has shown significant
reductions in biofilm biomass when combined with antibiotics. For
instance, Brackman et al (28) reported that the co-administration
of QSIs alongside antibiotics resulted in a 68-90% reduction in
viable bacteria within biofilms. This demonstrates the
effectiveness of combination therapies in combating resistant
strains, such as P. aeruginosa and S. aureus,
offering a promising strategy to overcome biofilm-associated
infections (29). Additionally, a
previous study revealed that the synthesis and testing of
phytochemical tannic acid-mediated gold nanoparticles effectively
inhibited the biofilm of Streptococcus mutans at lowest
concentration range of 16 µg/ml (30). Furthermore, recent studies have
reported that the methanol extract of Actinidia deliciosa
(kiwi fruit) exhibits significant antibiofilm activity at a
concentration of 2.5 mg/ml (31).
These results are consistent with those obtained with P.
dulce, which likewise functions as an antibacterial agent and
an anti-QS compound. Furthermore, the present study (Fig. 4) demonstrated that P. dulce
seed extract did not inhibit planktonic growth at a concentration
of 10 mg/ml, underscoring its specific effect on biofilm formation
rather than broad antibacterial activity.
The findings of the present study indicated that
both seed and fruit extracts of Pithecellobium dulce
inhibited the growth of C. violaceum CV12472 at 20 mg/ml.
However, only the seed extract significantly reduced biofilm
formation and violacein production at a sub-MIC concentration of 10
mg/ml, suggesting the presence of bioactive compounds such as
flavonoids, anthocyanin, tannins, coumarin, triterpenoids,
saponins, alkaloids, sterols and fatty acids that likely target
bacterial adhesion and QS pathways essential for biofilm
development (32). By contrast,
the fruit extract demonstrated limited antibiofilm and anti-QS
effects, with no change in violacein production, potentially due to
the absence or lower concentrations of these specific
compounds.
Integrating P. dulce seed extract into
existing treatment regimens presents a promising strategy for
managing biofilm-associated infections, particularly as an adjunct
to conventional antibiotics. Its selective antibiofilm properties
highlight its potential as a natural agent in the fight against
biofilm formation and pathogen virulence, addressing the urgent
challenge of rising antibiotic resistance.
However, the present study focused solely on C.
violaceum CV12472; thus, while the results are promising, they
may not extend to other biofilm-forming bacteria. Furthermore,
these findings are based on in vitro assays, which may
produce different results in vivo, where complex host
factors can influence bioactivity. Variability in compound
concentrations across different P. dulce sources may also
affect the consistency of therapeutic effects.
In order to validate the efficacy and safety of
P. dulce seed compounds, in vivo studies are
essential. Animal models could provide insight into
pharmacokinetics, bioavailability and therapeutic potency in
physiological conditions, where host factors may modulate the
effects. Such studies would help determine optimal dosing
strategies and assess potential synergy when combined with
conventional antibiotics. Additionally, in vivo research
could reveal any anti-inflammatory or immunomodulatory effects of
P. dulce, further supporting its therapeutic potential for
biofilm-associated infections.
Future research is required to focus on elucidating
the precise mechanisms behind the antibiofilm and anti-QS
activities of key compounds in P. dulce seeds. Expanding
studies to include a broader range of pathogenic strains would also
help confirm the broader applicability of P. dulce seed
extract as a therapeutic agent in managing biofilm-associated
infections.
Taken together, the findings of the present study
indicate that integrating plant-based extracts into treatment
regimens could provide a dual advantage: Combating antibiotic
resistance, while simultaneously targeting bacterial virulence
mechanisms. This approach aligns with the increasing interest in
phytotherapy and the use of natural compounds as adjuncts or
alternatives to traditional antibiotics.
In conclusion, the present study made an attempt
towards screening edible fruits and seeds that inhibit the QS
regulated development of biofilms and virulence factors in
antibiotic resistant C. violaceum. Notably, to the best of
our knowledge, the present study is the first to demonstrate that
P. dulce seed extract effectively inhibits biofilm formation
of C. violaceum at a sub-MIC of 10 mg/ml, without affecting
planktonic cell growth. This highlights its targeted effect on
biofilm inhibition rather than exerting broad-spectrum
antibacterial activity. These findings suggest that P. dulce
seed extracts may serve as potent anti-QS agents, offering
potential for managing C. violaceum infections. Therefore,
the extracts, either alone or in combination with existing
antibiotics, could be effectively utilized as anti-infective agents
to help manage stubborn infections caused by C.
violaceum.
The exploration of the anti-QS properties of P.
dulce not only adds depth to its antimicrobial profile, but
also aligns with emerging strategies that leverage natural
compounds to combat antibiotic resistance and enhance treatment
outcomes against biofilm-associated infections. As research
continues to unveil the complexities of bacterial behavior and
resistance mechanisms, plant-derived compounds such as P.
dulce may play an integral role in future therapeutic
developments.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SFPM collected and managed the data and participated
in the writing of the manuscript. SFPM and NNP participated in
writing the proposal (objectives, methodology and scope of the
research project), performing data collection and in the writing of
the manuscript. GRV and PSG were involved in data curation, data
analysis and in revising the manuscript. GRV and PSG confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar MR: Chromobacterium violaceum: A
rare bacterium isolated from a wound over the scalp. Int J Appl
Basic Med Res. 2:70–72. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alim R, Safiullah SA, Munwar S, Mazhar I,
Zaman SU and Bari S: Wound Infection Caused by Chromobacterium
violaceum: A Case Report from a Tertiary Care Hospital in
Bangladesh. Adv Microbiol. 12:83–89. 2022.
|
|
3
|
Lee J, Kim JS, Nahm CH, Choi JW, Kim J,
Pai SH, Moon KH, Lee K and Chong Y: Two Cases of Chromobacterium
violaceum Infection after Injury in a Subtropical Region. J Clin
Microbiol. 37:2068–2070. 1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sharmin S, Jahan AA, Kamal SMM and Sarker
P: Fatal Infection Caused by Chromobacterium violaceum : A Case
Report from a Tertiary Care Hospital in Bangladesh. Case Rep Infect
Dis. 2019(6219295)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Siqueira IC, Dias J, Ruf H, Ramos EA,
Maciel EA, Rolim A, Labur L, Vasconcelos L and Silvany C:
Chromobacterium violaceum in Siblings, Brazil. Emerg Infect Dis.
11:1443–1445. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Park JW, Lee SJ, Kim JE, Kang MJ, Bae SJ,
Choi YJ, Gong JE, Kim KS, Jung YS, Cho JY, et al: Comparison of
response to LPS-induced sepsis in three DBA/2 stocks derived from
different sources. Lab Anim Res. 37(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Venkatramanan M and Nalini E: Regulation
of virulence in Chromobacterium violaceum and strategies to combat
it. Front Microbiol. 15(1303595)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Naga NG, El-Badan DE, Ghanem KM and
Shaaban MI: It is the time for quorum sensing inhibition as
alternative strategy of antimicrobial therapy. Cell Commun Signal.
21(133)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Venkatramanan M, Sankar Ganesh P, Senthil
R, Akshay J, Veera Ravi A, Langeswaran K, Vadivelu J, Nagarajan S,
Rajendran K and Shankar EM: Inhibition of Quorum Sensing and
Biofilm Formation in Chromobacterium violaceum by Fruit Extracts of
Passiflora edulis. ACS Omega. 5:25605–25616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mion S, Carriot N, Lopez J, Plener L,
Ortalo-Magné A, Chabrière E, Culioli G and Daudé D: Disrupting
quorum sensing alters social interactions in Chromobacterium
violaceum. NPJ Biofilms Microbiomes. 7(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sathishkumar K: Revitalising healthcare:
The role of natural products in modern medicine. Natural Product
Research. 1-3:2024.
|
|
12
|
Karunamoorthi K, Jegajeevanram K,
Vijayalakshmi J and Mengistie E: Traditional medicinal plants: A
source of phytotherapeutic modality in resource-constrained health
care settings. J Evid Based Complement Alternat Med. 18:67–74.
2013.
|
|
13
|
Venkatesan LS, Gunasekaran V and
Sathishkumar P: Combating dental biofilms using plant-derived
flavonoids: A simple and potential therapeutic approach. Nat Prod
Res: Oct 1, 2024 (Epub ahead of print).
|
|
14
|
Rao GN, Nagender A, Satyanarayana A and
Rao DG: Preparation, chemical composition and storage studies of
quamachil (Pithecellobium dulce L.) aril powder. J Food Sci
Technol. 48:90–95. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Orwa C, Mutua A, Kindt R, Jamnadass R and
Simons A: Agroforestree database: A tree species reference and
selection guide version 4.0. World Agroforestry Centre ICRAF,
Nairobi, KE, 2009.
|
|
16
|
Saxena VK and Singhal M: Novel prenylated
flavonoid from stem of Pithecellobium dulce. Fitoterapia.
70:98–100. 1999.
|
|
17
|
Govindarajan M, Sivakumar R, Rajeswari M
and Yogalakshmi K: Chemical composition and larvicidal activity of
essential oil from Mentha spicata (Linn.) against three mosquito
species. Parasitol Res. 110:2023–2032. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pithayanukul P, Ruenraroengsak P, Bavovada
R, Pakmanee N, Suttisri R and Saen-oon S: Inhibition of Naja
kaouthia venom activities by plant polyphenols. J Ethnopharmacol.
97:527–533. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pradeepa S, Subramanian S and Kaviyarasan
V: Evaluation of antimicrobial activity of Pithecellobium dulce pod
pulp extract. Asian J Pharm Clin Res. 7 (Suppl 1):S32–S37.
2014.
|
|
20
|
Pincus D.H: Microbial Identification Using
the Biomerieux VITEK 2 System. In: Encyclopedia of Rapid
Microbiological Methods. Miller MJ (Ed). pp1-32, 2013.
|
|
21
|
August PR, Grossman TH, Minor C, Draper
MP, MacNeil IA, Pemberton JM, Call KM, Holt D and Osburne MS:
Sequence analysis and functional characterization of the violacein
biosynthetic pathway from Chromobacterium violaceum. J Mol
Microbiol Biotechnol. 2:513–519. 2000.PubMed/NCBI
|
|
22
|
Soni M, Naseef Pathoor N, Viswanathan A,
Veeraragavan GR and Sankar Ganesh P: Exploring the antimicrobial
and antibiofilm activities of Artocarpus heterophyllus Lam. against
Pseudomonas aeruginosa PAO1. World Acad Sci J. 6(50)2024.
|
|
23
|
Barnes P, Gonzales J and Hammond D:
Chromobacterium violaceum : A rare opportunistic pathogen and clue
for pediatric chronic granulomatous disease. Pediatr Dermatol.
40:396–397. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Batista JH and Silva Neto JF:
Chromobacterium violaceum Pathogenicity: Updates and insights from
genome sequencing of novel chromobacterium species. Front
Microbiol. 8(2213)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sushma PG, Adimulapu Hima Sandeep, Saritha
Bhandari and Ashwini Anil Pokle: Evaluation of antibacterial
potential of Pithecellobium dulce against Streptococcus mutans. J
Pop Ther Clin Pharm. 30:58–64. 2023.
|
|
26
|
Aldarhami A, Bazaid AS, Alhamed AS,
Alghaith AF, Ahamad SR, Alassmrry YA, Alharazi T, Snoussi M, Qanash
H, Alamri A, et al: Antimicrobial potential of pithecellobium dulce
seed extract against pathogenic bacteria: In silico and in vitro
evaluation. Biomed Res Int. 2023(2848198)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shinde S, Lee LH and Chu T: Inhibition of
Biofilm Formation by the Synergistic Action of EGCG-S and
Antibiotics. Antibiotics (Basel). 10(102)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brackman G, Cos P, Maes L, Nelis HJ and
Coenye T: Quorum sensing inhibitors increase the susceptibility of
bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob
Agents Chemother. 55:2655–2661. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hawas S, Verderosa AD and Totsika M:
Combination therapies for biofilm inhibition and eradication: A
comparative review of laboratory and preclinical studies. Front
Cell Infect Microbiol. 12(850030)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Selvaraj K, Venkatesan LS, Ganapathy D and
Sathishkumar P: Treatment of dental biofilm-forming bacterium
Streptococcus mutans using tannic acid-mediated gold nanoparticles.
Microb Pathog. 189(106568)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mathew MZ, Arthanari A, Ganesh S, Naseef
Pathoor N, Ramalingam K and Ravindran V: Evaluating the efficacy of
actinidia deliciosa (Kiwi Fruit) extract in inhibiting pseudomonas
aeruginosa biofilm formation: An in vitro study with therapeutic
implications. Cureus. 16(e70082)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
El-hewehy A, Mohsen E, El-fishawy AM and
Fayed MAA: Traditional, Phytochemical, Nutritional and Biological
Importance of Pithecellobium dulce (Roxib.) Benth. Yüzüncü Yıl
Üniversitesi Tarım Bilimleri Dergisi. 34:354–380. 2024.
|