Introduction
The potential long-term health effects of food-borne pathogens render them a serious public health concern. Pathogenic bacteria and their toxins are the primary factors responsible for food-borne diseases. These bacteria have the potential to pollute food at any stage of the food production and distribution process, ranging from the farm to the final consumer. Consuming raw or undercooked meat, poultry, fish, eggs, dairy products, and infected fruits and vegetables may lead to food-borne diseases. As a consequence of the growing prevalence of pathogens that are resistant to several drugs, it is crucial to actively seek for novel antimicrobial medicines. Several fruits have been examined for their ability to impede the proliferation of harmful bacteria. Fruits may potentially provide natural antibacterial agents which can be used to counteract the increase in drug-resistant bacterial strains (1,2).
Fruits and vegetables are widely consumed and play a vital role in diet; however, on the other hand, their waste has become a main concern worldwide. Horticulture waste refers to organic materials, such as leaves, stems, roots, flowers and fruit that are generated from the production and maintenance of horticultural crops (e.g., fruits, vegetables and ornamental plants) (3). These materials are considered as waste as they are removed from the crops as a part of pruning, thinning, harvesting, or other horticultural practices. Horticultural waste is a valuable resource for composting and soil improvement, and it can also be used for biogas production and as a feedstock for bioenergy production. Apart from this, horticultural waste can lead to issues for waste management and disposal, as it greatly contributes to the global output of organic waste. Furthermore, horticultural waste needs to be treated in a manner that is environmentally sound according to standards set forth by various nations. Pakistan solely generates ~49.6 million tons of solid waste a year, which has been increasing by >2.4% annually (4). The utilization of horticultural waste, such as lemon, banana and orange peels, for antimicrobial activity is a key issue due to the need for alternative and sustainable solutions against food-borne pathogens. The peel and pulp of these plants exhibit notable medicinal properties. Studies in the past have confirmed the presence of a substantial amount of bioactive compounds exhibiting potential benefits to human health as bactericidal, fungicidal and disease-controlling agents for food products (5,6).
Previously, the efficient antimicrobial potential of orange, yellow lemon and banana peel against various Gram-negative [Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae, Serratia marcescens, Escherichia coli (E. coli), Proteus vulgaris and Salmonella typhi (S. typhi)] and Gram-positive bacteria [Staphylococcus aureus (S. aureus), Enterococcus faecalis, Aeromonas hydrophila, Streptococcus pyogenes, Listeria monocytogenes and Lacticaseibacillus casei] was reported (7). Similarly, the antimicrobial and antioxidant potential of jackfruit waste parts (peel pomace, seed and fiber) was found to be effective against Xanthomonas axonopodis (8). The exploration of horticultural waste for its antibacterial properties has prospects for further investigations and advancement, since there are still numerous aspects to be comprehended. This may help enhance the understanding of harnessing the peel waste as a promising natural antimicrobial resource. Therefore, the present study aimed to assess the capacity of these organic chemicals to enhance the longevity of food items. The present study tested extracts and powders derived from several fruits to assess their efficacy as antioxidants and antibacterial agents against different food-related diseases. The tests conducted involved examining the impact of fruit extracts and powders on the growth of pathogenic microorganisms.
Materials and methods
Sample collection and processing
Fruit peel waste, including orange (Citrus sinensis), banana (Musa paradisiaca) and lemon (Citrus limonium) were obtained from local markets in Karachi, Pakistan. The obtained products were at the stage of becoming horticultural when they were secured from the market and were not appropriate for consumption in the fresh market. The fruit surfaces were thoroughly washed and rinsed with distilled water. The peels were separated and dried in a hot-air oven at 35˚C for 3 days until completely dry. After drying, the samples (orange and lemon) were crushed using a mortar and pestle and then grinded to a fine powder using an electric grinder (West point WF-9491). For the bananas, the peels were dried, chopped and weighed. A total of 30 g of banana peels were homogenized with 20 ml distilled water. The powder was stored in an airtight glass jar at 4˚C for use in subsequent analyses.
Preparation of extract
Methanol and ethanol (Sigma-Aldrich) were used as solvents for the extraction of bioactive compounds from the peel waste. The solvents are polar and effectively dissolve antimicrobial compounds, such as phenols and flavonoids commonly found in plant materials (9). The grounded material (5 g) was extracted with the solvents and placed in airtight glass jars. Extraction was carried out for 3 days at room temperature. The resulting extracts were filtered using Whattman filter paper and kept in a dark room for evaporation. The dried powder of each extract was stored in dark bottles and kept at 4˚C for further use. The stock solution was prepared by dissolving 0.1 g of each extract in 1 ml DMSO (Thermo Fisher Scientific, Inc.) to yield a concentration of 100 mg/ml.
Microorganism and cultural conditions
The Gram-negative bacteria used were P. aeruginosa, E. coli and S. typhi. The Gram-positive bacteria used during the study was S. aureus. The bacterial cultures were taken from the culture bank of Ziauddin University, Karachi, Pakistan. Bacterial cultures were sub-cultured for microbial cultivation in tryptic soy broth (Merck Millipore) at 37˚C for 24 h and used as an inoculum for antimicrobial analysis. The bacterial strains were maintained on tryptic soy agar (TSA) slants (Merck Millipore) at 4˚C during the study.
Determination of the antimicrobial activities of the extracts
The antimicrobial activity of the fruit peel extracts was estimated against food-borne pathogens with the agar well diffusion method. The log phase broth culture of the bacterial strain was swabbed evenly on the sterile surface of TSA plates. A total of 100 µl of each fruit peel solvent stock solution was loaded in the respective well. The plates were incubated at 37˚C for 24 h. Commercially available broad-spectrum antibiotics including 1.0% chloramphenicol and 0.3% gentamicin (Sigma-Aldrich) were used as a positive control, whereas a respective solvent without extract was used as a negative control. Antimicrobial activity was estimated by measuring the diameters of the inhibition zone with a millimeter ruler and tabulated.
Determination of the minimal inhibitory concentration
The minimum inhibitory concentration was calculated using the broth dilution method. Bacterial strains were sub-cultured into tryptic soy broth (TSB) (Merck Millipore) and incubated at 37˚C for 24 h. Dilutions were prepared and the concentration was adjusted to 106-107 microorganisms per ml to use as an inoculum. The microbial culture was added into 9 ml sterile broth containing various concentrations of extract ranging from 1 to 100 mg/ml. The cultures were incubated at 37˚C for 24 h and the growth of microorganisms was noted. The tube with no microorganism containing the lowest concentration of the test material was considered the minimal inhibitory concentration.
Determination of antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was performed to investigate the antioxidant activity of the fruit peels. The extract was mixed with DPPH solution (Merck Millipore); the sample extract included ~100 µl and 3.9 ml of ethanolic and methanolic DPPH solution. The samples were mixed by vortex and kept in a dark place at room temperature for 30 min. The absorbance was measured and the results were reported as the percentage inhibition of DPPH radicals (10).
Phytochemical analysis
Different biochemical tests including Dragendorff's, Wagner, Mayer, Hager test (alkaloid), ninhydrin test (glycoside), ferric chloride test (tannin), foam test (saponins) and alkaline reagent test (flavonoids) were performed for analyzing the extract chemical composition (11).
Test for alkaloids. Dragendorff's test was performed by the addition of 1 ml Dragendorff's reagent (Merck Millipore) to the 1 ml of each extract and the formation of an orange or reddish-brown precipitate indicates the presence of alkaloids. For Wagner's test, 1 ml of each extract was mixed with 2-3 drops of Wagner's reagent (Sigma-Aldrich) and a reddish-brown precipitate confirms the presence of alkaloids. 2 to 3 drops of Mayer's reagent (CDH Fine Chemicals) was added to the extract (1 ml) to examine the formation of a white or cream precipitate suggesting alkaloids are present. In the case of Hager's test, 1 ml of extract and Hager's reagent were mixed and a yellow precipitate indicates alkaloids in the sample.
Test for glycosides. The Ninhydrin test for glycosides involves adding a few drops of ninhydrin reagent (Thermo Fisher Scientific, Inc.) to the peel extract and heating the mixture for 2-3 min. The formation of a blue or violet color indicates the presence of glycosides.
Test for tannins. Ferric chloride test was performed by mixing the peel extract with 0.1% FeCl2 and the formation of brownish-green or bluish-black color indicates the presence of tannins.
Test for saponins. The foam test for saponins was performed by adding 10 ml sterile distilled water to 2.5 ml of each peel extract, followed by vigorous shaking for 30 sec. After shaking the sample was left for 30 min to settle. The formation of stable honeycomb-like froth indicated the presence of saponins.
Test for flavonoids. A total of 1 ml of extract was added to 2 ml of 2 % sodium hydroxide. The formation of a yellow color confirms the presence of flavonoids.
Antibiotic susceptibility test. Antibiotic tests can be conducted according to National Committee Laboratory Standards (12) for the following antibiotic discs: Chloramphenicol (20 µg), Amoxicillin (20 µg), Ciprofloxacin (5 µg), Gentamicin (10 µg), Tetracycline (30 µg) and Nalidixic acid (20 µg). Disc diffusion assay was performed. The bacterial culture was spread on the agar plates and antibiotic disc were placed and the plates were incubated at 37˚C for 24 h. The zone of inhibition was measured to assess the susceptibility of antibiotic against the bacterial strain.
FTIR analysis. The FTIR spectra was recorded to identify the functional groups using the FTS-65, Bio-Rad spectrophotometer (Bio-Rad Laboratories, Inc.). The FTIR spectrum was scanned from 4,000 cm-1 to 400 cm-1 with a resolution of 4cm-1.
Results
Antibiotic sensitivity of microbial strains
E. coli and P. aeruginosa exhibited resistance, and S. aureus and S. typhi exhibited sensitivity against chloramphenicol. P. aeruginosa, S. aureus and S. typhi exhibited sensitivity and E. coli exhibited resistance against amoxicillin. S. aureus, S. typhi and E. coli exhibited sensitivity and P. aeruginosa exhibited resistance against ciprofloxacin. E. coli, S. aureus and S. typhi exhibited sensitivity and P. aeruginosa exhibited resistance against gentamicin. E. coli and P. aeruginosa exhibited resistance, and S. aureus and S. typhi exhibited sensitivity against tetracycline. All three P. aeruginosa, E. coli and S. aureus exhibited resistance, and E. coli exhibited sensitivity against nalidixic acid (Table I). These data indicate that certain bacteria exhibit resistance to multiple antibiotics, which is a great concern in clinical settings as it limits treatment options. The presence of resistance in common pathogens highlights the need for the ongoing monitoring of antibiotic susceptibility patterns to guide effective treatment strategies and combat antibiotic resistance.
 |
Table I
Antibiotic susceptibility test.
|
Table I
Antibiotic susceptibility test.
| Antibiotic |
Escherichia coli |
Pseudomonas aeruginosa |
Staphylococcus aureus |
Salmonella typhi |
| Chloramphenicol |
R |
R |
S |
S |
| Amoxicillin |
R |
S |
S |
S |
| Ciprofloxacin |
S |
R |
S |
S |
| Gentamicin |
S |
R |
S |
S |
| Tetracycline |
R |
R |
S |
S |
| Nalidixic acid |
R |
S |
R |
R |
Antimicrobial activity of the extracts
The antimicrobial activity of orange peel along with two solvents was tested against four food-borne pathogens, namely E. coli, P. aeruginosa, S. typhi and S. aureus using the agar well diffusion method. Orange peel with ethanol exhibited an 18-mm inhibition zone for both S. typhi and P. aeruginosa. An inhibition zone of 12 mm was observed for E. coli and 8 mm for S. aureus. Orange peel with methanol exhibited a 17-mm inhibition zone for S. typhi, 10 mm for P. aeruginosa, and no inhibition zone was observed for E. coli and S. aureus. The antimicrobial activity of lemon peel along with two solvents was tested against four food-borne pathogens, namely E. coli, P. aeruginosa, S. typhi and S. aureus using the agar well diffusion method. Lemon peel with ethanol exhibited a 19-mm inhibition zone for S. typhi and 16 mm for P. aeruginosa. An inhibition zone of 13 mm was observed for E. coli and no inhibitory activity was noted against S. aureus. Lemon peel with methanol exhibited a 15-mm inhibition zone for S. typhi, 13 mm for P. aeruginosa, 12 mm for E. coli and 10 mm for S. aureus.
The antimicrobial activity of banana peel along with two solvents and the same concentration was tested against four food-borne pathogens, namely E. coli, P. aeruginosa, S. typhi and S. aureus using the agar well diffusion method. Banana peel with ethanol exhibited a 12-mm inhibition zone for S. typhi and an 8-mm inhibition zone was observed for E. coli. No notable activity was observed for P. aeruginosa and S. aureus. Banana peel with methanol exhibited a 15-mm inhibition zone for S. typhi, 9 mm was observed for E. coli and 11 mm for S. aureus (Table II).
 |
Table II
Antimicrobial potential of fruit peel waste against microbial strains.
|
Table II
Antimicrobial potential of fruit peel waste against microbial strains.
| |
Zone of inhibition (mm) |
| Peel extract |
Staphylococcus aureus |
Salmonella typhi |
Pseudomonas aeruginosa |
Escherichia coli |
| Orange (Et) |
8 |
18 |
18 |
12 |
| Orange (Mt) |
0 |
17 |
10 |
0 |
| Lemon (Et) |
0 |
19 |
16 |
13 |
| Lemon (Mt) |
10 |
15 |
13 |
12 |
| Banana (Et) |
0 |
12 |
0 |
8 |
| Banana (Mt) |
11 |
15 |
0 |
9 |
These results indicate that citrus peels, particularly lemon and orange, possess marked antimicrobial properties against food-borne pathogens, with lemon peel exhibiting the highest efficacy, particularly against S. typhi and P. aeruginosa, as evidenced by the largest zones of inhibition when using ethanol extracts. Orange peel also exhibited considerable activity, although this was slightly less effective than lemon peel. By contrast, banana peel demonstrated limited antimicrobial effects, particularly against E. coli and S. aureus, suggesting that while it has some potential, it is not as potent as the citrus alternatives. Overall, these findings highlight the promising role of natural extracts from citrus fruits as potential antimicrobial agents in food safety applications.
Estimated minimal inhibitory concentration of the extracts
The investigation of the minimal inhibitory concentration of various extracts revealed that orange peel extracts, both ethanol and methanol, demonstrated a notable ability to inhibit the growth of P. aeruginosa and S. typhi at concentrations of 50 mg/ml. Furthermore, both the orange ethanol and banana methanol extracts were effective against S. aureus, P. aeruginosa and E. coli at a lower concentration of 25 mg/ml. A notable finding was that several extracts, including orange and lemon in both the ethanol and methanol forms, exhibited minimal inhibitory concentration values as low as 12.5 mg/ml against E. coli, P. aeruginosa and S. aureus, indicating potent antimicrobial potential. The most potent activity was observed with the orange methanol, lemon methanol and banana ethanol extracts, which exhibited minimal inhibitory concentration values of 3.125 mg/ml against P. aeruginosa and E. coli. However, it is noteworthy that the lemon and banana methanol extracts did not demonstrate any inhibitory effect on S. aureus, S. typhi, P. aeruginosa, or E. coli, suggesting variability in the antimicrobial efficacy of these natural extracts depending on the pathogen and solvent used (Table III). Overall, these results underscore the potential of citrus and banana peels as sources of natural antimicrobial agents, with varying effectiveness based on the type of extract and target organism.
 |
Table III
Minimal inhibitory concentration of peel extract evaluated against microbial strains.
|
Table III
Minimal inhibitory concentration of peel extract evaluated against microbial strains.
| |
Minimal inhibitory concentration (mg/ml) |
| Peel extract |
Staphylococcus aureus |
Salmonella typhi |
Pseudomonas aeruginosa |
Escherichia coli |
| Orange (Et) |
25 |
6.25 |
50 |
12.5 |
| Orange (Mt) |
6.25 |
50 |
3.125 |
12.5 |
| Lemon (Et) |
6.25 |
3.125 |
12.5 |
6.25 |
| Lemon (Mt) |
- |
- |
3.125 |
- |
| Banana (Et) |
12.5 |
- |
- |
3.125 |
| Banana (Mt) |
12.5 |
- |
25 |
25 |
Antioxidant ability of the extracts
The antioxidant capability was tested on all samples of orange, lemon and banana peel with both ethanol and methanol solvents. Orange peel with the ethanol extract exhibited 59.8% antioxidant activity. Orange peel with the methanol extract exhibited 61.4% antioxidant activity. Lemon peel with ethanol exhibited 44.5% antioxidant activity. Lemon peel with the methanol extract exhibited 59.6% antioxidant activity. Banana peel along ethanol did not exhibit any antioxidant activity. Banana peel with methanol exhibited 13.9% antioxidant activity (Table IV). These findings suggest that citrus peels, particularly orange and lemon, are rich in antioxidants, which may be beneficial for health applications. Overall, these results highlight the varying degrees of antioxidant potential among different fruit peels and solvents, emphasizing the importance of selecting appropriate sources for antioxidant applications.
 |
Table IV
Antioxidant activity.
|
Table IV
Antioxidant activity.
| Peel extract |
Antioxidant activity (%) |
| Orange (Et) |
59.8 |
| Orange (Mt) |
61.4 |
| Lemon (Et) |
44.5 |
| Lemon (Mt) |
59.6 |
| Banana (Et) |
- |
| Banana (Mt) |
13.9 |
Phytochemical activity of the extracts
The phytochemical analysis of the ethanol and methanol extracts from orange, lemon and banana peels exhibited variations in the presence of bioactive compounds (Table V). Both the ethanol and methanol extracts exhibited a moderate presence of alkaloids across all three fruit peels. Notably, flavonoids were found in moderate amounts in the ethanol extracts of all three peels, with orange peel exhibiting a particularly high concentration, indicating its potential as a rich source of these beneficial compounds. Tannins also displayed a similar trend, with orange peel again demonstrating a high presence compared to the lemon and banana peels, which had moderate levels. Saponins were detected at moderate levels in both banana peel extracts, while the orange and lemon peels exhibited no saponin content in either solvent. Additionally, glycosides were present in moderate amounts in the ethanol extracts of the orange and banana peels, with the methanol extract of orange exhibiting a high concentration; however, both lemon peel extracts lacked glycosides. These findings suggest that orange peel is particularly rich in several phytochemicals, which may contribute to its antioxidant and antimicrobial properties, while lemon and banana peels exhibit a more limited profile, highlighting the potential for utilizing citrus peels in health-related applications.
 |
Table V
Phytochemical analysis of fruit peel.
|
Table V
Phytochemical analysis of fruit peel.
| Peel extract |
Alkaloids |
Flavonoids |
Tannins |
Saponins |
Glycosides |
| Orange (Et) |
+ |
+ |
+ |
- |
+ |
| Orange (Mt) |
+ |
++ |
++ |
- |
++ |
| Lemon (Et) |
+ |
+ |
+ |
- |
- |
| Lemon (Mt) |
+ |
+ |
+ |
- |
- |
| Banana (Et) |
+ |
+ |
+ |
+ |
+ |
| Banana (Mt) |
+ |
+ |
+ |
+ |
+ |
Results of FTIR analysis
The functional groups that are present on the surface of the peels were identified using FTIR spectroscopy. The results of the analysis of the banana peel methanol and ethanol extracts are illustrated in Figs. 1 and 2, which demonstrate the presence of numerous functional groups on the surface of the peel. From the spectra, band shifting and the possible involvement of O-H stretching were observed with the alcohol compound around the broad peak 3,700-3,584 cm-1. The absorption band at 3,350.4 cm-1 was attributable to the O-H stretching vibration of phenolic compounds, which specifically indicates the presence of hydroxyl groups in the extract. The peaks at 2,924.3 and 2,850.7 cm-1 were caused by the CH2 anti-symmetric stretch of methyl groups shown in Fig. 1; 2,400-2,000 cm-1 indicates potent O=C=O stretching with the carbon dioxide compound in both the extracts, respectively. Observations of the aromatic alcohol OH were made at 1,316 cm-1 and 1,231 cm-1. At 1,044 cm-1, a band corresponding to the C-O extension of an aromatic alcohol was observed. The bands caused by aromatic OH wags were observed between 900 and 750 cm-1, and their frequency depends on how the aromatic rings are substituted in both extracts (13).
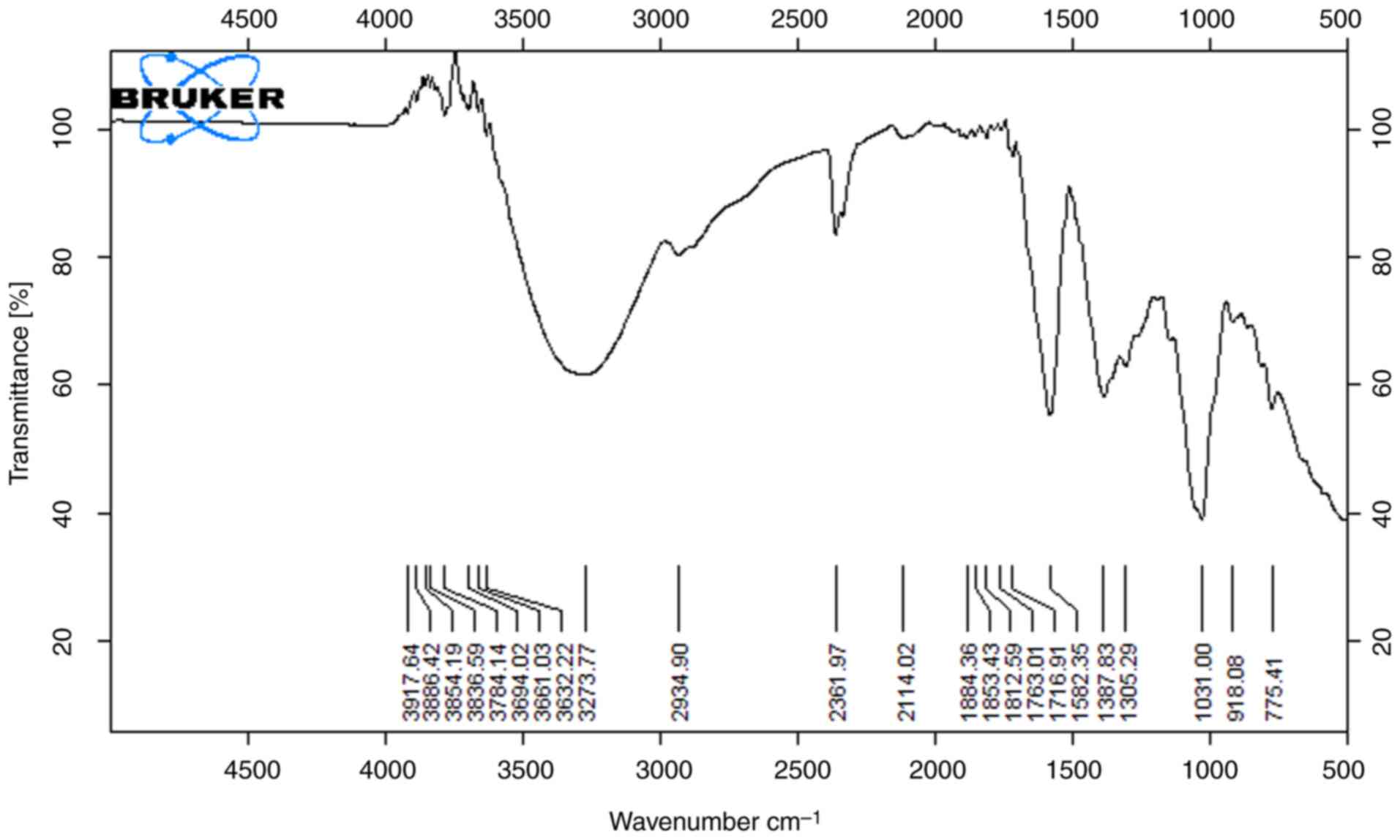 |
Figure 1
FTIR spectra of banana sample (methanol extract).
|
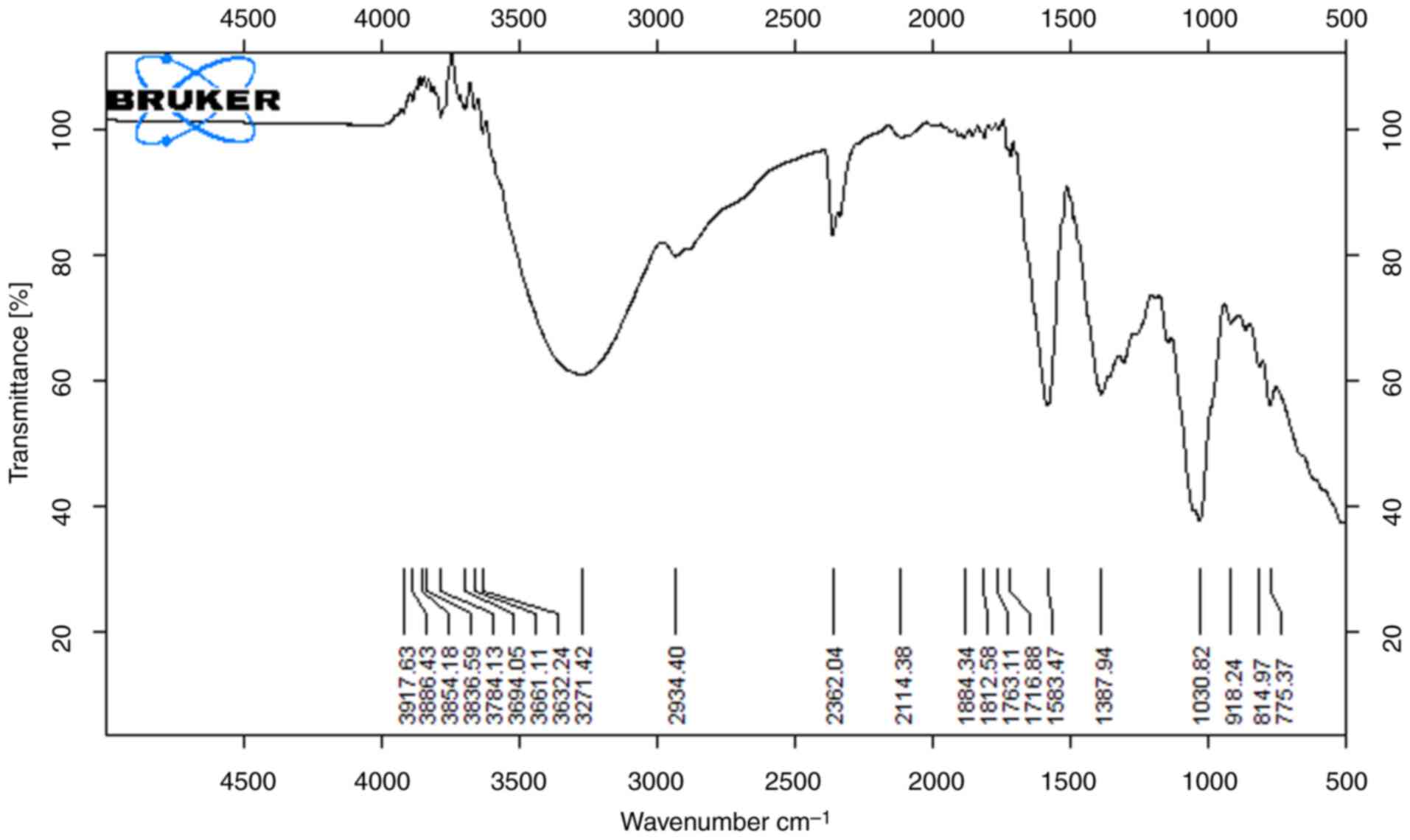 |
Figure 2
FTIR spectra of banana sample (ethanol extract).
|
The functional groups that are present on the surface of the peels were demonstrated using FTIR spectroscopy. The results of the analysis of the orange peel methanol and ethanol extracts are shown in Figs. 3 and 4, which demonstrate the presence of numerous functional groups on the surface of the peel. Both orange peel extracts, with peaks typical of lignocellulosic materials, demonstrated the primary functional groups contained in the material. The stretching of the O-H and N-H groups found in proteins, fatty acids and carbohydrates is related to the broadband with a center at 3,400 cm-1. The broad and strong absorption peaks (3,338 and 3,347 cm-1) are related to the O-H stretching vibrations brought on by the hydrogen bonding between intermolecular and intramolecular polymeric molecules. The carbonylic group of esters is associated with the band at 1.742 cm-1. The peaks at 1,098 cm-1 and 1023 cm-1, which are distinctive bands of cellulose and hemicellulose from lignocellulosic materials, are caused by the CO stretching (14). The most intense band in the high-energy region is caused by an abundance of OH groups from lignin and carbohydrates. The prominent band at 2,925 cm-1 is related to the presence of C-H stretching vibration along with bending vibrations around 1,428 cm-1 of aliphatic chains (-CH2- and -CH3-), forming the basic structure of this lignocellulosic material. The intense band at 1,045 cm-1 corresponds to the link C-O-H or C-O-R (alcohols or esters) (15).
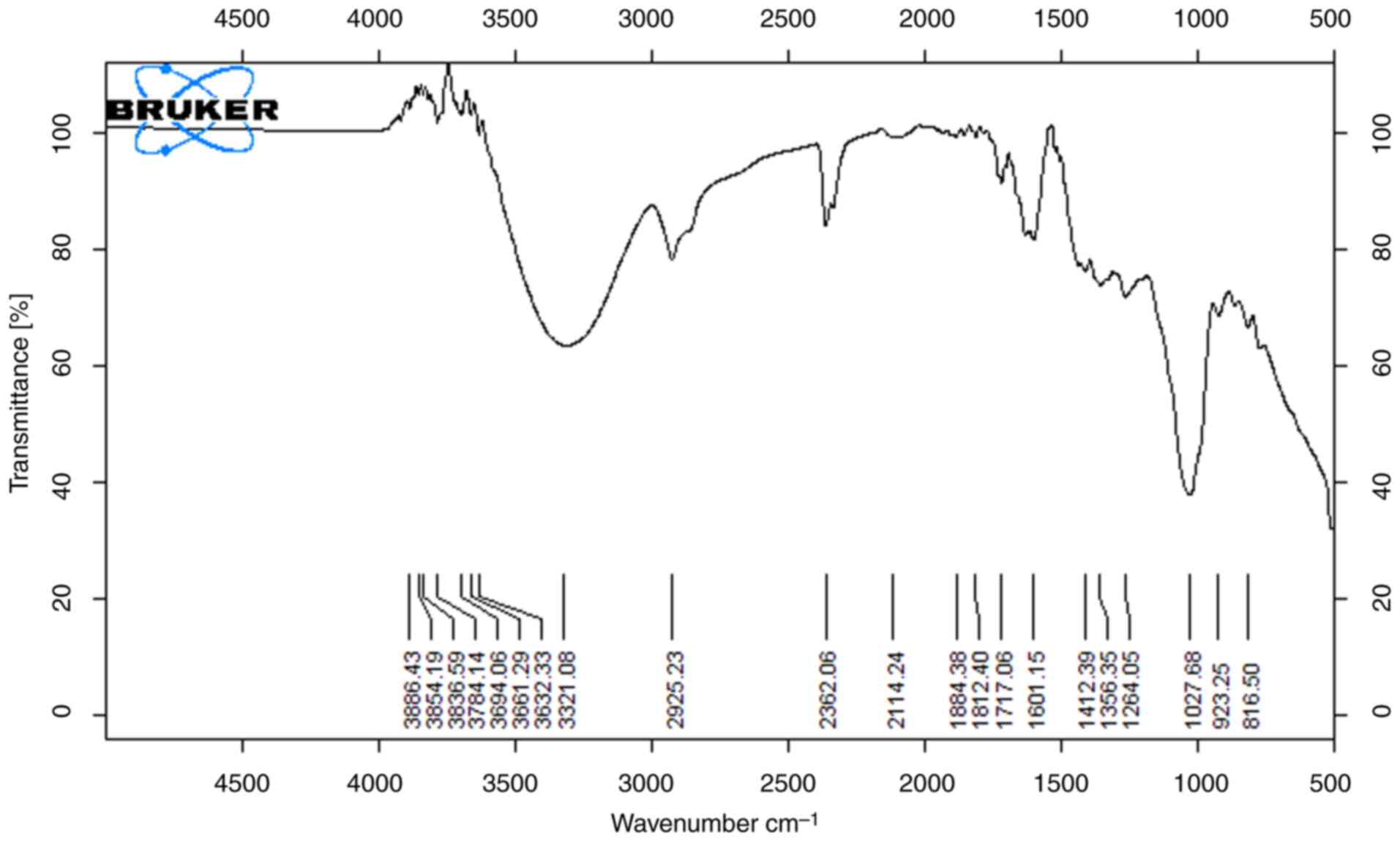 |
Figure 3
FTIR spectra of orange sample (methanol extract).
|
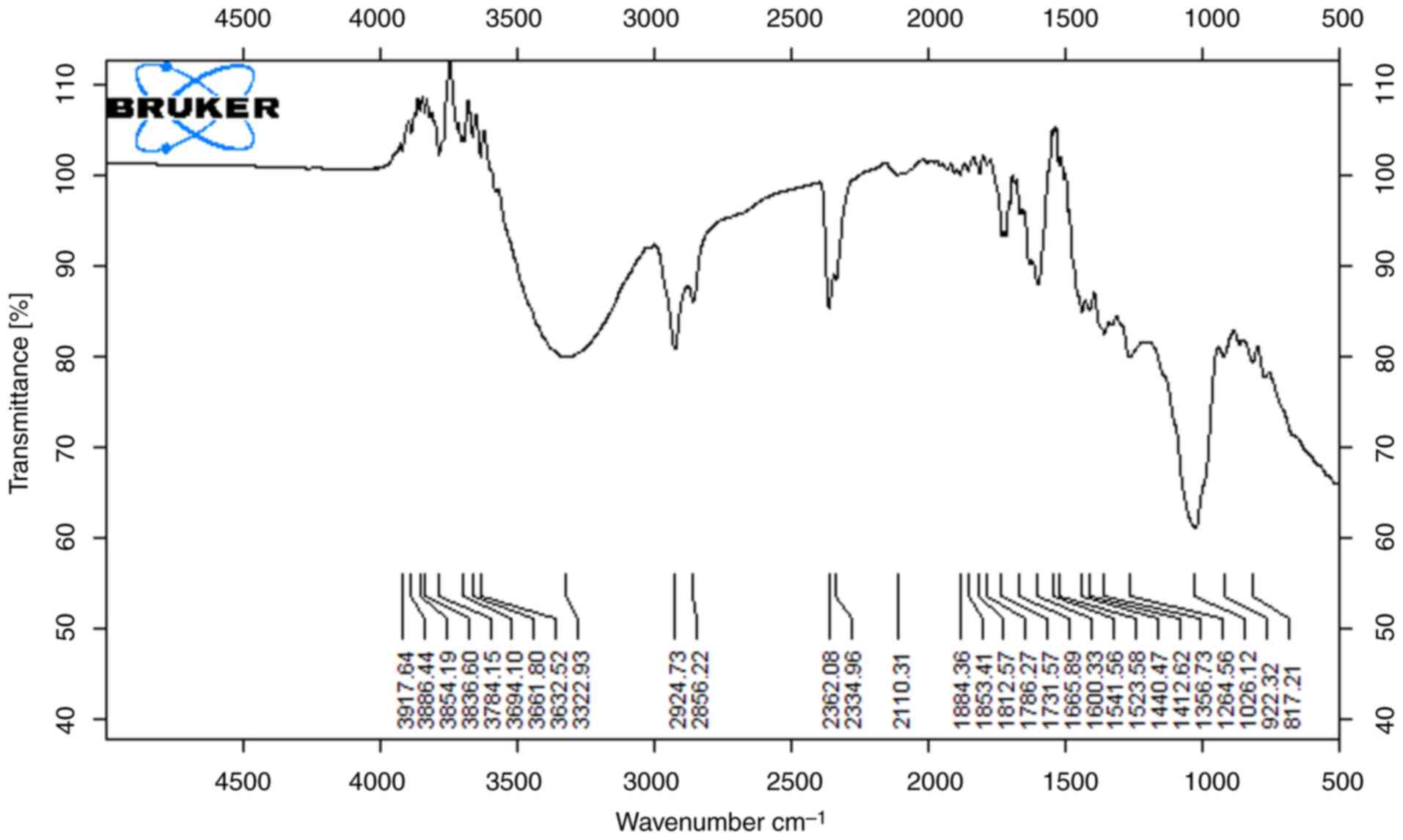 |
Figure 4
FTIR spectra of orange sample (ethanol extract).
|
The results of FTIR spectra analysis of lemon peel extracts are shown in Figs. 5 and 6. Indicative of the presence of hydroxyl groups of macromolecular association (cellulose, pectin, etc.) is the broad peak between 3,200 cm-1 and 3,600 cm-1 (14). In both extracts (Figs. 5 and 6), significant peaks are exhibited as a O-H stretch at 3,595.31 cm-1, C-H stretch at 2,830-2,695 cm-1, which is depicted as a carbohydrate ring, and a notable C=O stretch at 1,710-1665 cm-1. The strong peak in the range of 1,319.31 cm-1 implies that alcohols, carboxylic acids and esters are stretching vibrations (17). Bands around 1,650 and 1,750 cm-1 are indicative of free and esterified carboxyl groups shown in Fig. 5. Observed between spectra in Fig. 6 at 2,852 cm-1, 1,734.3 cm-1, 1,368.6 cm-1, 1,162 cm-1 and 572.72 m-1 are due to the slightly different solvating properties of ethanol as compared to Fig. 5. Lemon extract with ethanol as a solvent has the highest antimicrobial capability. The varying functional groups across these fruit peels suggest a rich chemical composition that may underlie their antioxidant and antimicrobial properties, particularly highlighting the lemon peel's superior antimicrobial capability when extracted with ethanol. Overall, these findings emphasize the potential of fruit peels as valuable sources of bioactive compounds for health-related applications.
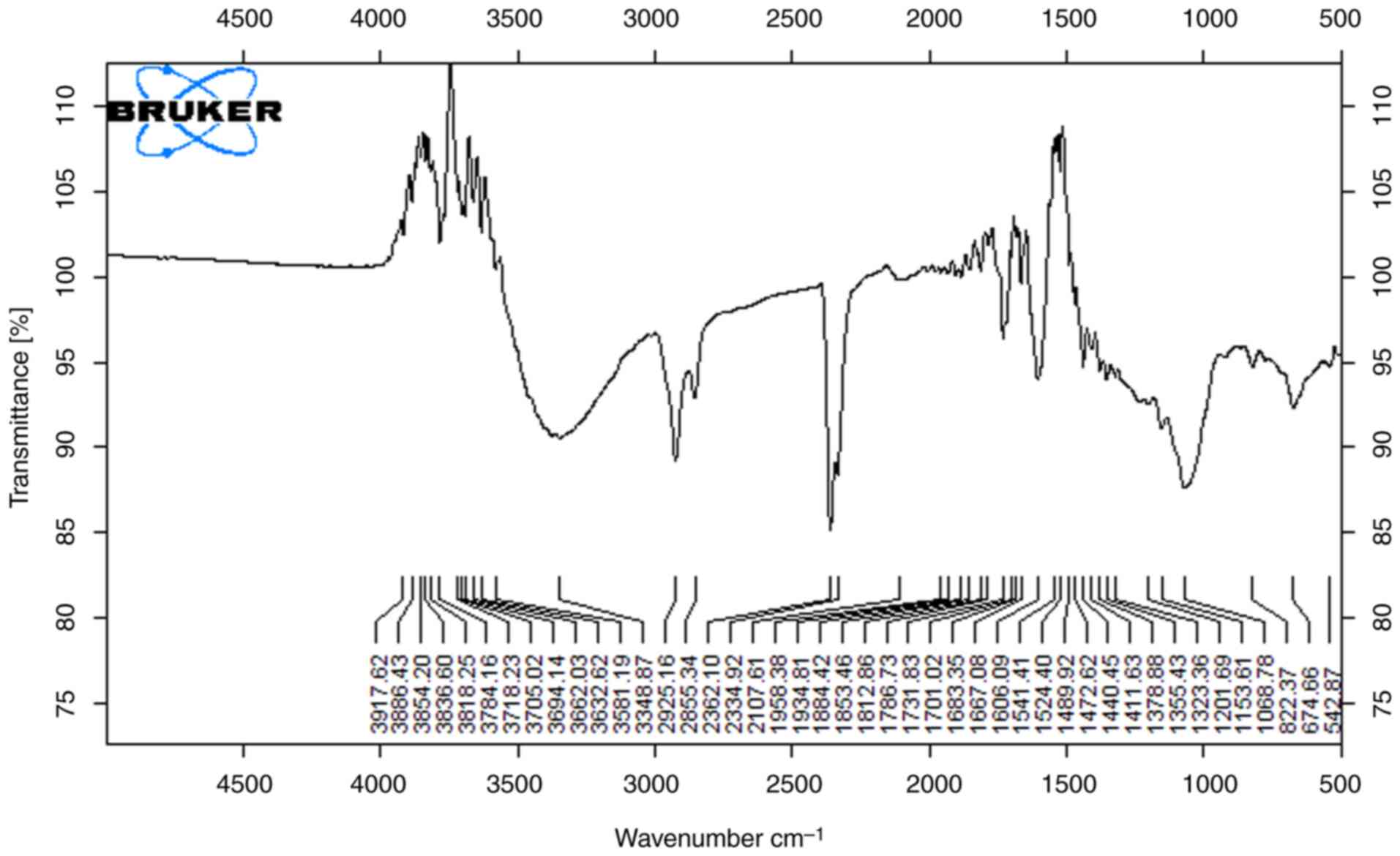 |
Figure 5
FTIR spectra of lemon sample (ethanol extract).
|
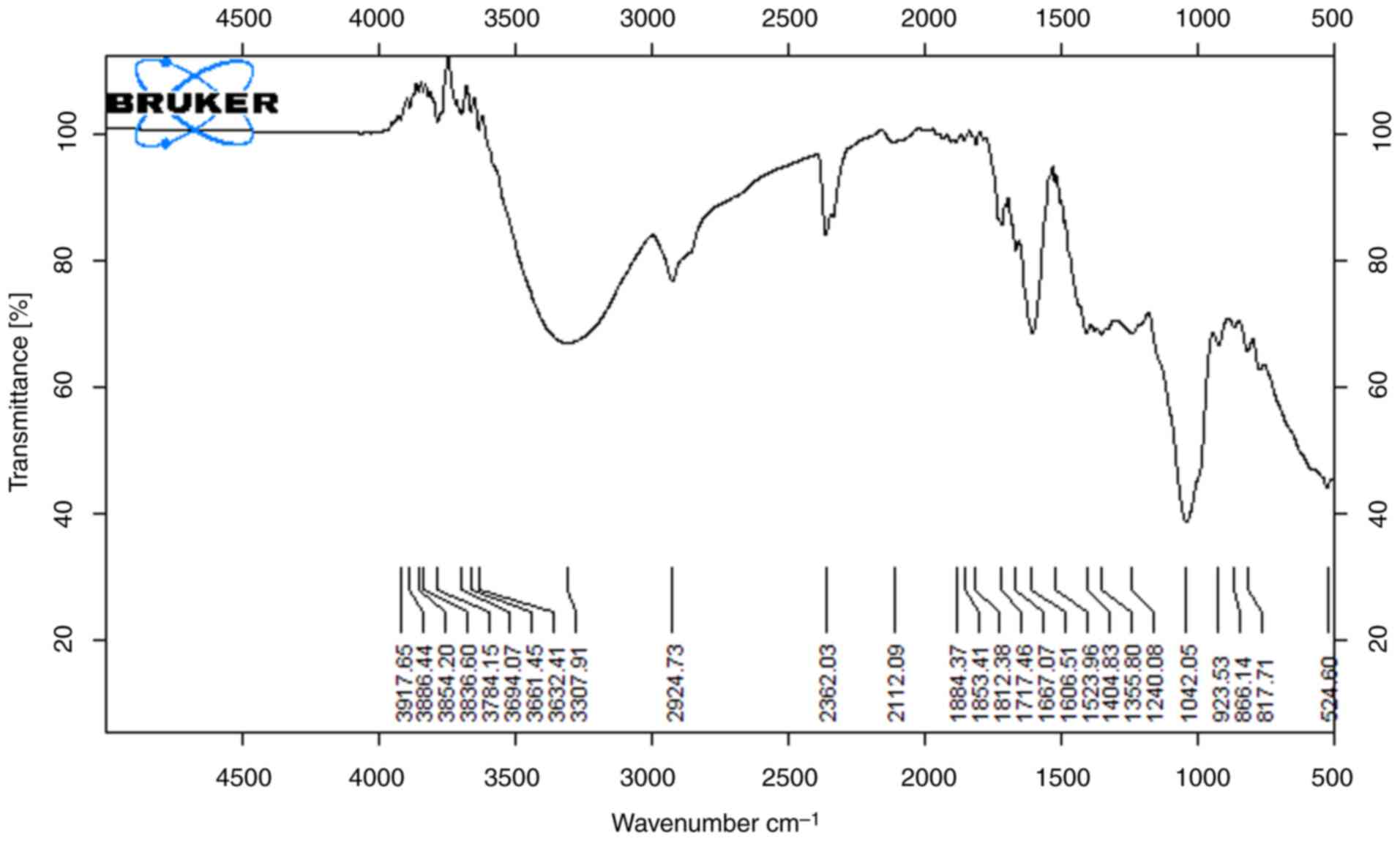 |
Figure 6
FTIR spectra of lemon sample (methanol extract).
|
Discussion
The pressing issue of global waste demands strategic management, particularly in the context of industrial and agricultural residues. The adherence to circular economy principles is pivotal for sustainable agriculture, minimizing, recycling and reusing agricultural waste to align with social, environmental and economic benefits (18,19). Addressing the substantial waste generated in fruit and vegetable processing poses a formidable challenge for the food industry, necessitating significant financial investment in proper disposal (8). However, these agricultural by-products harbor abundant phenolic compounds and bioactive chemicals that offer antioxidant, antimicrobial and health-enhancing properties (20). Leveraging these natural compounds as alternatives to synthetic additives not only yields beneficial compounds, but also mitigates environmental impacts associated with discarding substantial fruit residues (21).
In the present study, local varieties of fruit peels and their extracts were examined, which may exhibit different antimicrobial properties compared to those studied previously. Variations in geographical location, climate and cultivation practices can influence the phytochemical composition of these peels, potentially leading to different efficacy profiles against specific pathogens. The main aim of the present study was to assess the organic antimicrobial potential of orange, banana and lemon peels, emphasizing the repurposing of leftover fruit peels to manage solid waste, while aligning with environmental sustainability. The present study underscored the notable antibacterial efficacy of the tested fruit peel extracts against a spectrum of microorganisms. Utilizing the well diffusion method, the present study demonstrated substantial zones of growth inhibition, particularly noting the superior effectiveness of yellow lemon, followed by orange and then banana peel extracts, respectively, against Gram-negative and Gram-positive bacteria. The association between the yield of solvent extracts and antibacterial activity was evident, showcasing that yellow lemon and orange extracts paralleled the tested standard antibiotic, gentamicin, in their inhibitory potential against most bacteria. Previously, the antimicrobial activity of Citrus sinensis peel extracts against Streptococcus mutans and Lactobacillus acidophilus was found to be 8 to 13 mm with various concentrations of the extract (22). The antimicrobial activity of banana (Musa paradisiaca L.) peel against S. aureus and Candida krusei was previously found to be 30 and 10 mm, respectively (23). The antimicrobial activity of acetone, ethanol and ethyl acetate extract of Citrus limon against S. aureus, Enterococcus faecalis and S. typhi was previously estimated to be 9 to 16 mm in diameter (24). Another study revealed the potent antimicrobial activity in yellow banana fruit peels against Gram-positive and Gram-negative bacteria (25). Additionally, banana peels have been highlighted for their attributes in producing natural dyes and shielding cotton clothes from bacterial effects, owing to their rich reservoir of antioxidants and antibacterial elements (26). Lemon peel has been shown to exhibit efficacy against both Gram-positive and Gram-negative bacteria, with a heightened effect on Gram-positive strains, possibly due to the lipid content in the cell walls, inhibiting teichoic acid within the peptidoglycan layer (27). Similarly, banana peel extracts, owing to their phytochemical composition including tannins, glycosides, alkaloids and flavonoids, have been shown to display antibacterial properties against Gram-positive bacteria, suggesting potential in microbial infection treatment as a safer alternative to synthetic drugs. Combining multiple phytochemicals in banana peel extracts may produce synergistic effects, enhancing their antimicrobial potency compared to individual compounds. This multifaceted approach allows for a broader spectrum of activity against various pathogens, including both Gram-positive and Gram-negative bacteria (28,29). Notably, the ethanolic extract of banana peel has showcased superior antibacterial efficacy compared to the aqueous extract, attributed to the presence of specific compounds (8). Citrus peels, particularly from lemons and oranges, contain flavonoids, such as hesperidin and naringin, which have demonstrated antibacterial activity against foodborne pathogens, such as E. coli and Salmonella. Flavonoids can disrupt microbial cell membranes and interfere with their metabolic processes, leading to cell death (30,31).
These findings echo previous research, highlighting the substantial antimicrobial potential of citrus peel extracts attributed to their rich phenolic content, flavonoids and tannins. The observed inhibitory effects on bacterial growth suggest a synergistic action among various phytochemicals present in the extracts, possibly disrupting bacterial membranes and metabolic processes. The analysis of the minimum inhibitory concentration further supported these observations, affirming the ability of the extracts to inhibit microbial growth across various concentrations. Previous research has indicated that when natural preservatives derived from fruit peels are applied at appropriate concentrations, they can inhibit microbial growth without significantly altering the sensory attributes of the food (32). This shift towards natural preservatives aligns with the growing consumer demand for clean-label products, as it allows manufacturers to replace synthetic additives with safe, bio-based alternatives. Additionally, utilizing fruit peels contributes to sustainability by reducing food waste and enhancing the value of agricultural byproducts. The incorporation of natural preservatives may reduce reliance on synthetic chemicals, which can be costly and subject to regulatory scrutiny. By investing in the extraction and application of bioactive compounds from fruit peels, companies may lower production costs over time. This advancement could lead to improved food safety, greater consumer appeal and a more sustainable food supply chain. The present study highlights that methanolic and ethanolic extracts from orange, lemon and banana peels have potential as potent, cost-effective antimicrobial agents with broad applications. However, due to their natural abundance and lack of adverse reactions, their application is hindered by variability in composition, limited spectrum of activity, concentration-dependent efficacy, potential toxicity issues, stability concerns and regulatory challenges.
In conclusion, the findings of the present study signify the immense potential of fruit peels as organic antimicrobial agents, offering avenues for sustainable waste management and eco-friendly alternatives to synthetic additives in various industries. The present study underscores the relevance of harnessing natural compounds from agricultural waste for practical applications in the healthcare and food sectors, aligning with sustainable practices. Further research exploring formulation and application methods is warranted to optimize and commercialize these natural extracts effectively. In the future, these fruit peel extracts may be used as therapeutic agents. These fruit peel extracts may also be used to create antibiotic drugs.
Acknowledgements
The present study was conducted at the Pakistan Council of Scientific and Industrial Research (PCSIR), Karachi. Pakistan.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SBZ designed the study and finalized the manuscript. SI was involved in the conception of study and supervised the research work. SR performed the experiments and prepared the draft of the manuscript. IB performed the experiments and analyzed the results. BN performed the experiments. SK was involved in the interpretation of the results and revised the manuscript. All authors have read and approved the final manuscript. SBZ and SI confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Luo X, Song Y, Cao Z, Qin Z, Dessie W, He N and Tan Y: Evaluation of the antimicrobial activities and mechanisms of synthetic antimicrobial peptide against food-borne pathogens. Food Biosci. 49(101903): 2022. Brewer MS: Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf 10: 221-247, 2011.
|
|
2
|
Mane BG, Khurana SK, Choudhary S and Dhanze H: Effect of natural antimicrobials on foodborne pathogens and shelf life: A review. Biosci Bioeng Biotechnol. 1:22–31. 2014.
|
|
3
|
Zhu LD, Li ZH and Hiltunen E: Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed Res Int. 2016(8792548)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
González-Hernández AI, Gómez-Sánchez MÁ, Pérez-Sánchez R and Morales-Corts MR: Garden waste compost tea: A horticultural alternative to promote plant growth and root traits in tomato (Solanum lycopersicum L.) plants. Hortic. 9(1127)2023.
|
|
5
|
Tsegay ZT and Mulaw G: Antimicrobial activities and mode of action of bioactive substances from vegetable and fruit byproducts as a current option for valorization. Waste Biomass Valorization 1-28, 2024.
|
|
6
|
Chabi IB, Omiyalé OJ, Dèdéhou SECA, Ayégnon BP, Idrissou I, Boya B and Kayodé APP: Tomato seed (Solanum lycopersicum) meal derived from agrifood waste as functional ingredient: Nutritional value, antioxidant and antimicrobial activities, and functional properties. J Food Process Preserv. 2024(8824581)2024.
|
|
7
|
Saleem M and Saeed MT: Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J King Saud Univ Sci. 32:805–810. 2020.
|
|
8
|
Adan AA, Ojwang RA, Muge EK, Mwanza BK and Nyaboga EN: Phytochemical composition and essential mineral profile, antioxidant and antimicrobial potential of unutilized parts of jackfruit. Food Res. 4:1125–1134. 2020.
|
|
9
|
Mehmood A, Javid S, Khan MF, Ahmad KS and Mustafa A: In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 16(64)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mahloko LM, Silungwe H, Mashau ME and Kgatla TE: Bioactive compounds, antioxidant activity and physical characteristics of wheat-prickly pear and banana biscuits. Heliyon. 5:2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gillani SF, Ali S, Tahir HM, Shakir HA and Hassan A: Phytochemical analysis, antibacterial and antibiogram activities of fruits peels against human pathogenic bacteria. Int Food Res J. 27:963–970. 2020.
|
|
12
|
Kiehlbauch JA, Hannett GE, Salfinger M, Archinal W, Monserrat C and Carlyn C: Use of the national committee for clinical laboratory standards guidelines for disk diffusion susceptibility testing in New York state laboratories. J Clin Microbiol Rev. 38:3341–3348. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Niamah A: Determination, identification of bioactive compounds extracts from yellow banana peels and used in vitro as antimicrobial. Int J Phytomed. 6:625–632. 2014.
|
|
14
|
Cundari L, Fanneza Al and Arisma NC: Characterization of Biosorbent from Musa acuminata balbisian Peel using FTIR spectroscopy and its application to cadmium (Cd) removal: Effect of activator type, pH, and biosorbent ratio. CHEMICA: Jurnal Teknik Kimia. 9:142–152. 2022.
|
|
15
|
Zapata B, Balmaseda J, Fregoso-Israel E and Torres-García E: Thermo-kinetics study of orange peel in air. J Therm Anal Calorim. 98:309–315. 2009.
|
|
16
|
Šabanović E, Memić M, Sulejmanović J and Selović A: Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol J Chem Technol. 22:46–53. 2020.
|
|
17
|
Kumar H, Bhardwaj K, Sharma R, Nepovimova E, Kuča K, Dhanjal DS and Kumar D: Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Mo. 25(2812)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lobo MG and Dorta E: Utilization and management of horticultural waste. In postharvest technology of perishable horticultural commodities (pp. 639-666). Woodhead Publishing, 2019.
|
|
19
|
Adamu H, Bello U, Yuguda AU, Tafida UI, Jalam AM, Sabo A and Qamar M: Production processes, techno-economic and policy challenges of bioenergy production from fruit and vegetable wastes. Renew Sustain Energy Rev. 186(113686)2023.
|
|
20
|
Regolo L, Giampieri F, Battino M, Armas Diaz Y, Mezzetti B, Elexpuru-Zabaleta M and Mazzoni L: From by-products to new application opportunities: The enhancement of the leaves deriving from the fruit plants for new potential healthy products. Front Nutr. 11(1083759)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Martin JGP, Porto E, Corrêa CB, Alencar SM, Gloria EM, Cabral ISR and Aquino LM: Antimicrobial potential and chemical composition of agro-industrial wastes. J Nat Prod. 5:27–36. 2024.
|
|
22
|
Shetty SB, Mahin-Syed-Ismail P, Varghese S, Thomas-George B, Kandathil-Thajuraj P, Baby D and Devang-Divakar D: Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria: An in vitro study. J Clin Exp Dent. 8(e71)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sirajudin ZNM, Ahmed QU, Chowdhury AJK, Kamarudin EZ, Khan AV and Uddin ABMH: Antimicrobial activity of banana (Musa paradisiaca L.) peels against food borne pathogenic microbes. J Pure Appl Microbiol. 8:3627–3639. 2014.
|
|
24
|
Gurusiddappa LH, Varghese C, Gowda B and Kalikeri S: Antimicrobial activity and phytochemical analysis of solvent extraction of citrus limon peels. World J Environ Biosci. 12:1–6. 2023.
|
|
25
|
Helmy YA, Taha-Abdelaziz K, Hawwas HAEH, Ghosh S, AlKafaas SS, Moawad MM and Mawad AM: Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiot. 12(274)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hikal WM, Kačániová M and Said-Al Ahl HAH: Banana peels as possible antioxidant and antimicrobial agents. Asian J Res Rev Agriculture. 3:35–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lino PB, Corrêa CF, Archondo ME and Dellova DC: Evaluation of post- surgical healing in rats using a topical preparation based on extract of Musa sapientum L., Musaceae, epicarp. Revista Brasileira de Farmacognosia. 21:491–496. 2011.
|
|
28
|
Ehiowemwenguan G, Emoghene AO and Inetianbor JE: Antibacterial and phytochemical analysis of Banana fruit peel. IOSR J Pharm. 4:18–25. 2014.
|
|
29
|
Salah SM: Antibacterial activity and UV protection property of some Egyptian cotton fabrics treated with aqueous extract from banana peel. Int J Clothing Sci. 1:1–6. 2012.
|
|
30
|
Teshome E, Forsido SF, Rupasinghe HV and Olika Keyata E: Potentials of natural preservatives to enhance food safety and shelf life: A review. Sci World J. 2022(9901018)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Quinto EJ, Caro I, Villalobos-Delgado LH, Mateo J, De-Mateo-Silleras B and Redondo-Del-Río MP: Food safety through natural antimicrobials. Antibiot. 8(208)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Munekata PE, Pateiro M, Domínguez R, Nieto G, Kumar M, Dhama K and Lorenzo JM: Bioactive compounds from fruits as preservatives. Foods. 12(343)2023.PubMed/NCBI View Article : Google Scholar
|




















