Introduction
Vitamin D is synthesized in the skin or acquired
through dietary intake. It is subsequently converted in the liver
to 25-hydroxyvitamin D [25(OH)D]. The conversion of 25(OH)D to its
active form, 1,25-dihydroxyvitamin D, occurs in the kidneys via the
enzyme, 1α-hydroxylase. The active form of vitamin D enhances
calcium and phosphorus absorption in the gastrointestinal tract,
promotes calcium reabsorption in the distal tubules and stimulates
osteoclastic activity (1,2).
In patients undergoing hemodialysis (HD), the
impaired ability of the kidneys to activate vitamin D leads to
significantly reduced circulating levels of 1,25-dihydroxyvitamin
D, and a deficiency in 25(OH)D is also prevalent (3,4). As
a result, in this population, active vitamin D receptor (VDR)
agonists are administered, instead of non-active forms of vitamin
D, primarily for the management of secondary hyperparathyroidism
(5). However, clinical data
indicate the beneficial effects of maintaining adequate serum
25(OH)D levels in patients undergoing HD. In these patients,
decreased serum levels of 25(OH)D are associated with increased
overall mortality rates (6), an
association on that has also been observed in the general
population (7). Based on such
observations, the Kidney Disease: Improving Global Outcomes (KDIGO)
clinical practice guidelines for the diagnosis, evaluation,
prevention and treatment of Chronic Kidney Disease-Mineral and Bone
Disorder (CKD-MBD) suggests that in patients with CKD stages 3-5D,
25(OH)D levels should be measured, with the frequency of repeated
testing determined by baseline values and therapeutic
interventions. The guidelines also suggest that vitamin D
deficiency and insufficiency should be addressed using treatment
strategies similar to those recommended for the general population.
However, this recommendation is classified as level 2, indicating
that different choices may be appropriate for different patients,
and is assigned a grade C, signifying that the quality of evidence
supporting it is low (8).
The beneficial effects of normal 25(OH)D levels may
be attributed to the expression of 1α-hydroxylase outside the
kidneys, which facilitates the local activation of 25(OH)D. This
local activation enables an autocrine or paracrine action at the
activation sites (9,10). In bone tissue, both osteoblasts and
osteoclasts express 1α-hydroxylase, allowing 25(OH)D to potentially
influence bone metabolism in an autocrine or paracrine manner,
independent of kidney-mediated activation (11,12).
Bone metabolism may also be indirectly influenced by 25(OH)D
through its effects on the parathyroid glands, which also express
1α-hydroxylase (13). As a result,
25(OH)D may suppress parathyroid hormone (PTH) production via
autocrine or paracrine mechanisms, a concept supported by some
clinical data (14,15). Additionally, dendritic cells,
macrophages, and activated T- and B-cells express both VDR and
1α-hydroxylase (16). During
inflammation, pro-inflammatory cytokines can upregulate
1α-hydroxylase in these cells, leading to the elevated local
conversion of 25(OH)D to 1,25-dihydroxyvitamin D. The
anti-inflammatory properties of vitamin D have been substantiated
(16,17), and in patients undergoing HD, an
inverse association between serum 25(OH)D levels and markers of
inflammation has been observed (18).
The present study evaluated the effects of 1-year
cholecalciferol supplementation in a cohort of patients with
vitamin D deficiency undergoing HD on bone mineral density (BMD),
serum intact PTH (iPTH) levels and markers of inflammation. The
KDIGO clinical practice guidelines for the diagnosis, evaluation,
prevention and treatment of CKD-MBD recommends that in patients
with CKD stages 3-5D who exhibit evidence of CKD-MBD and/or have
risk factors for osteoporosis, BMD testing should be considered to
assess the risk of fractures if the results are likely to influence
treatment decisions, particularly when the findings indicate
osteoporosis and the physician plans to initiate treatment with
anti-osteoporotic agents. This recommendation is classified as
level 2, and is assigned a grade B, signifying that the quality of
evidence supporting it is moderate (8). The exact KDIGO nomenclature and
description for rating guideline recommendations are provided in
the KDIGO guidelines (8). The
recommendation for BMD testing is based mainly on four prospective
cohort studies of dual-energy X-ray absorptiometry (DXA) measures
of BMD and incident fractures in adults with CKD G3a to G5D. One of
these studies measured BMD annually in 485 patients undergoing HD
and found that a lower baseline BMD at the femoral neck and total
hip was associated with an increased risk of fractures (19).
Patients and methods
Patients
Initially, 47 patients undergoing HD, who had
vitamin D deficiency, defined as serum 25(OH)D levels <20 ng/ml
(20), were identified. Patients
were recruited between October and November, 2017, and the study
was concluded between October and November, 2018. Following the
application of exclusion criteria, which disqualified smokers,
patients with active infections, malignancies, autoimmune diseases,
a history of parathyroidectomy, or those who had been using
corticosteroids, cytotoxic drugs, warfarin, anticonvulsants,
antidepressants, hormone replacement therapy, or bisphosphonates
within the 6 months preceding the study, 36 patients were
ultimately enrolled in the study. Over the course of the 1-year
study period, 8 patients were withdrawn from the study:
Specifically, 3 patients succumbed, 2 patients received kidney
transplants, and 3 patients were transferred to another renal unit.
Additionally, 8 patients were excluded due to non-compliance with
the study protocol, as indicated by the measurements of serum
25(OH)D levels. Consequently, of the initial 36 patients undergoing
HD enrolled in the study who were administered cholecalciferol,
only 20 patients completed the study. The analysis was conducted on
data from these 20 patients undergoing HD who successfully
completed the study. The mean age of these patients was 59.4±16.10
years, with 16 of the participants being male. The etiologies of
end-stage renal disease among the patients were as follows:
Diabetes mellitus in 7 patients, primary glomerulonephritis in 3
patients, hemolytic-uremic syndrome in 1 patient, interstitial
nephritis in 1 patient, hypertension in 1 patient, obstructive
nephropathy in 1 patient, autosomal dominant polycystic kidney
disease in 3 patients, nephrectomy in a patient with a dysplastic
contralateral kidney, and an unknown cause in 2 patients.
The patients underwent regular HD using polysulfone
dialyzers and a bicarbonate dialysate with a calcium concentration
of either 1.25 or 1.5 mmol/l. The HD sessions had a duration of 4
h, were performed three times per week, and had been ongoing for at
least 1 year prior to the study. The urea reduction rate
(66.7±7.2%), hemoglobin levels (11.62±0.83 g/dl) and albumin levels
(3.85±0.27) remained virtually unaltered at the end of the study
period compared to those measured at baseline (66.5±8.1%; 11.3±0.98
g/dl and 3.81±0.27, respectively). The laboratory values of
interest before and after cholecalciferol administration are
presented in Table I.
Nephrologists were allowed to independently decide on the use of
the phosphate binder sevelamer hydrochloride, the vitamin D analog
paricalcitol and the calcimimetic cinacalcet to meet the KDIGO
targets for serum PTH, calcium and phosphorus levels (8).
 | Table ILaboratory values before and after
the 1-year cholecalciferol administration. |
Table I
Laboratory values before and after
the 1-year cholecalciferol administration.
| Parameter | Before
cholecalciferol administration (n=20) | After
cholecalciferol administration (n=20) | P-value |
|---|
| URR (%) | 66.5±8.1 | 66.7±7.2 | 0.928 |
| Hemoglobin
(g/dl) | 11.3±0.98 | 11.62±0.83 | 0.273 |
| WBC (/µl) | 7,470±2,562 | 8,370±3,008 | 0.119 |
| Neutrophils
(/µl) | 5,118±1,969 | 5,835±2,218 | 0.159 |
| Lymphocytes
(/µl) | 1,393±640 | 1,550±645 | 0.272 |
| Albumin (g/dl) | 3.81±0.27 | 3.85±0.27 | 0.370 |
| Ca (mg/dl) | 8.99±0.10 | 8.99±0.11 | 1.000 |
| P (mg/dl) | 5.23±0.34 | 5.81±0.24 | 0.059 |
| iPTH (pg/ml) | 300.7±31.4 | 295.3±22.2 | 0.847 |
| 25(OH)D
(ng/ml) | 10.1±1.0 | 28.0±1.3 | <0.001 |
| CRP (mg/dl) | 0.69±0.77 | 0.48±0.39 | 0.418 |
Patients were enrolled in the study within a 2-week
period during the winter and completed the study in the same period
of the following winter. Each patient received one tablet of
cholecalciferol (1,200 IU; D3fix, Uni-Pharma) daily.
Cholecalciferol, also known as vitamin D3, is first converted in
the liver to 25(OH)D and then to its active form,
1,25-dihydroxyvitamin D in the kidneys (1,2).
Compliance was monitored by comparing the serum 25(OH)D levels at 6
months and at the end of the study period with the baseline levels.
In the case that the 25(OH)D levels increased by <50% following
the initiation of cholecalciferol, non-compliance was suspected,
and the patient was excluded from the study. BMD was assessed at
both the beginning and the end of the study.
The evaluation and administration of cholecalciferol
were carried out following the KDIGO guidelines, which advises that
for patients with CKD stages 3-5D, 25(OH)D levels should be
measured, and vitamin D deficiency or insufficiency should be
managed using treatment approaches similar to those recommended for
the general population (8).
Similarly, BMD assessment followed the KDIGO guidelines, which
recommend that in patients with CKD stages 3-5D who exhibit
evidence of CKD-MBD and/or possess risk factors for osteoporosis,
BMD testing should be considered to assess fracture risk if the
results are likely to influence treatment decisions (8). Informed consent was obtained from
each individual enrolled in the study, and the study protocol
received approval from the Ethics Committee of the Faculty of
Medicine at the University of Thessaly, Larissa, Greece (no. of
approval 558/10-2-2017).
Measurement of serum markers and
assessment of BMD and inflammation
Blood samples were drawn prior to the onset of the
second dialysis session of the week. Immunoassays for measuring
25(OH)D and intact PTH (iPTH) were performed in an ELECSYS 2010
automatic analyzer (Roche Diagnostics GmbH). C-reactive protein
(CRP) levels were measured using the Cobas Integra 400 automatic
analyzer (Roche Diagnostics GmbH). Serum phosphorus, calcium and
albumin levels, and complete blood count were determined and
documented as part of the routine laboratory assessments.
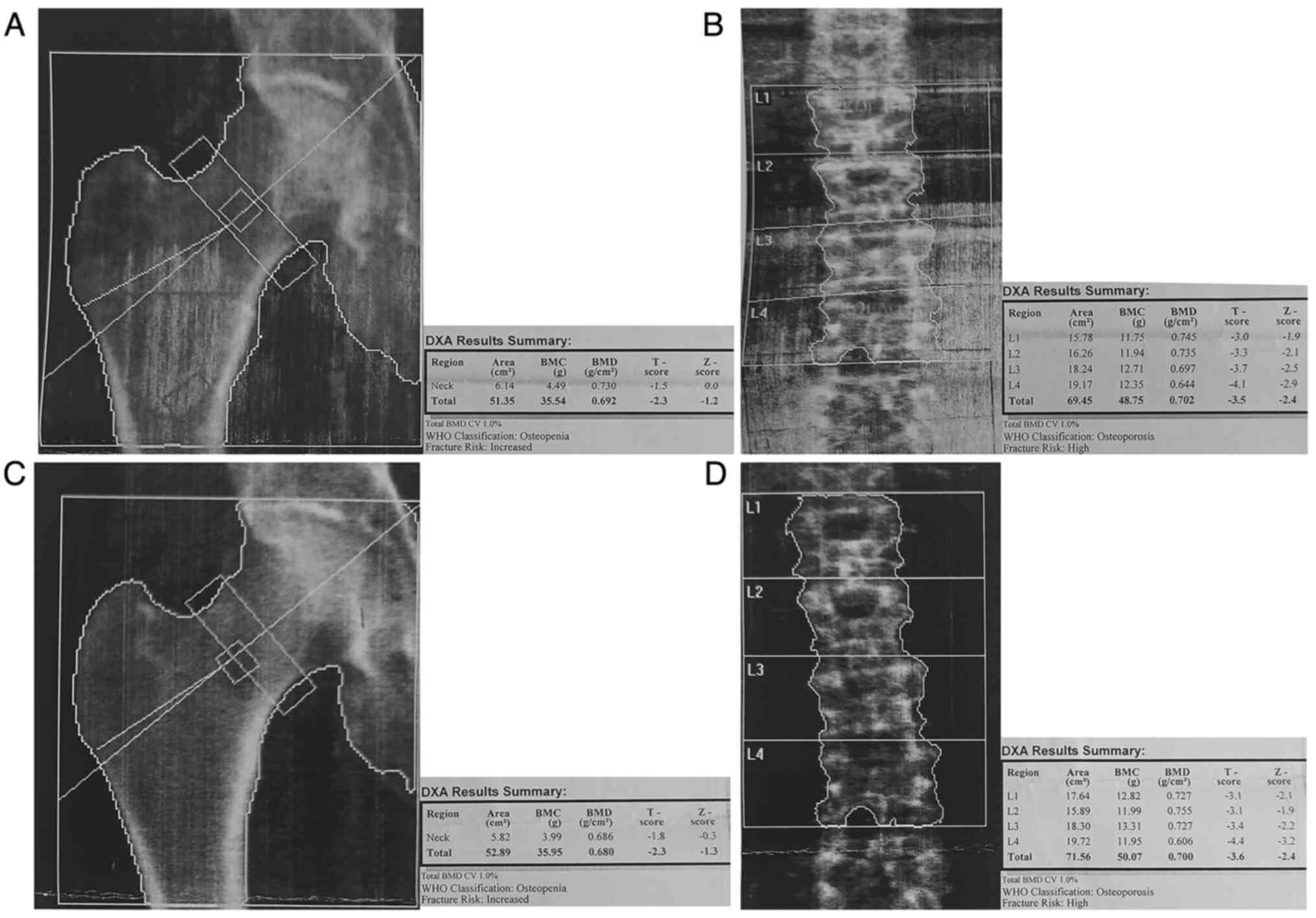
DXA BMD measurements were conducted using the
Hologic Discovery W Bone Densitometer (Hologic Inc.). The BMD,
T-score and Z-score of the lumbar spine and the right total hip
were documented. After obtaining the relevant consent for
publication, a representative measurement from a patient in the
study cohort is presented in Fig.
1.
Statistical analysis
To assess the normality of the variables, a
one-sample Kolmogorov-Smirnov test was employed. All variables,
apart from CRP, conformed to a normal distribution, and thus,
paired t-tests were utilized for mean comparisons. The results for
normally distributed variables are expressed as the mean ± standard
error of the mean. For CRP, which did not follow a normal
distribution, the Wilcoxon signed-rank test was applied, and the
results are presented as the median with interquartile range and
total range. A P-value <0.05 was considered to indicate a
statistically significant difference. Statistical analyses and
graphical representation were performed using IBM SPSS Statistical
software (version 29, IBM Corp.), while graphical representation
for one figure was generated using GraphPad Prism (Version 10.2.2,
Dotmatics).
Results
Effect of cholecalciferol on 25(OH)D
levels and BMD
In all patients who completed the study,
cholecalciferol supplementation led to a significant increase in
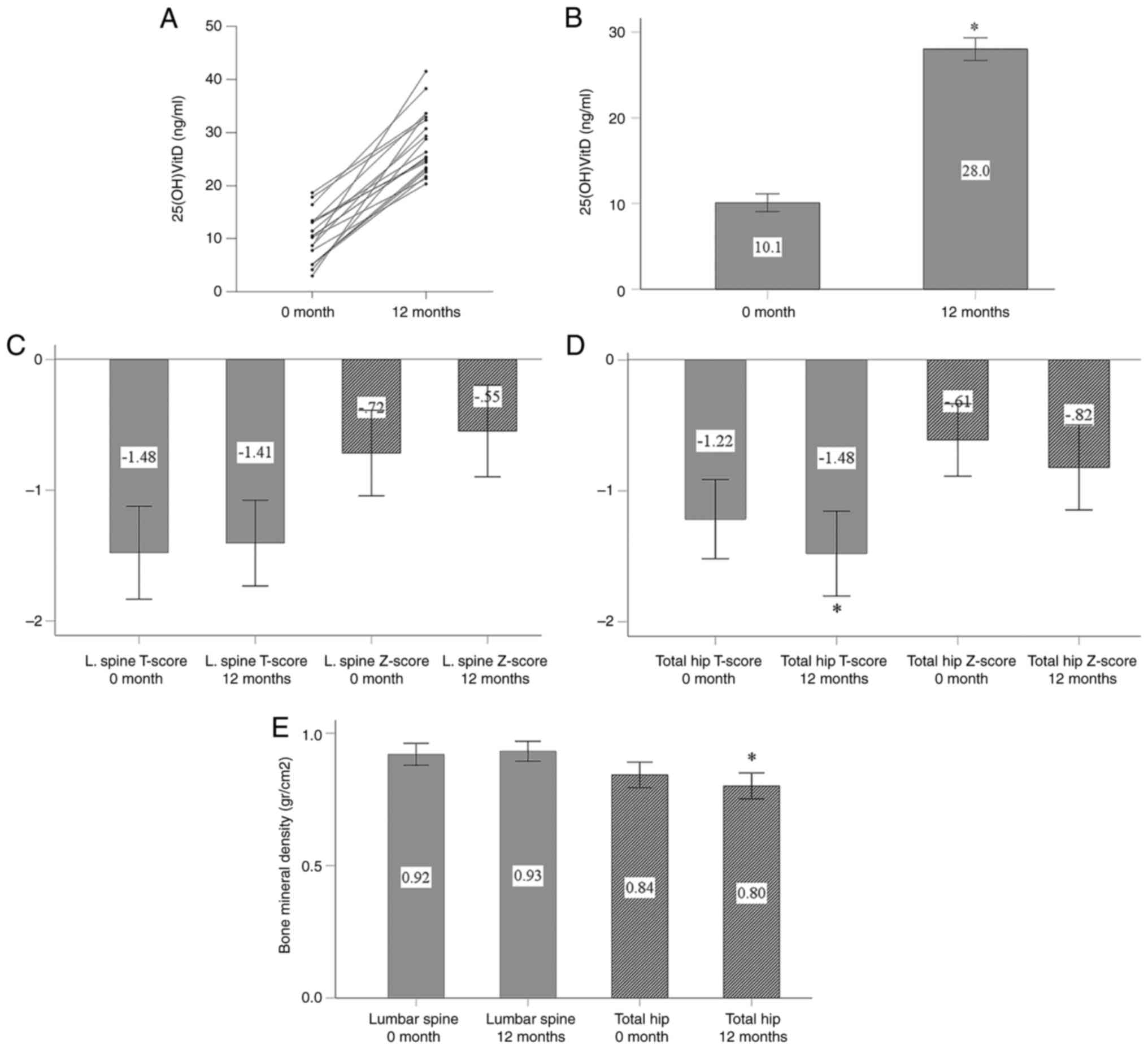
serum 25(OH)D levels (Fig. 2A).
The serum 25(OH)D concentration increased from 10.1±1.0 ng/ml
before cholecalciferol administration to 28.0±1.3 ng/ml at the end
of the study period (P<0.001; Fig.
2B).
Cholecalciferol supplementation did not
significantly affect the lumbar spine T-score, Z-score, or BMD. The
T-score was -1.48±0.36 before treatment and -1.41±0.33 after
treatment (P=0.471; Fig. 2C). The
Z-score also did not exhibit any significant change, from
-0.72±0.33 before treatment to -0.55±0.35 after treatment (P=0.174)
(Fig. 2C). The lumbar spine BMD
also remained stable, with measurements of 0.92±0.04 g/cm² before
treatment and 0.93±0.04 g/cm² after treatment (P=0.416; Fig. 2D).
In total hip measurements, both the T-score and BMD
exhibited a statistically significant decrease by the end of the
study period. The T-score decreased from -1.22±0.30 to -1.48±0.32
(P=0.007; Fig. 2D), and BMD
decreased from 0.84±0.05 g/cm² to 0.80±0.05 g/cm² (P<0.001;
Fig. 2E). The Z-score did not
exhibit a statistically significant decrease, changing
from-0.61±0.28 before treatment to -0.82±0.32 after treatment
(P=0.111; Fig. 2D).
Effects of cholecalciferol on serum
iPTH, calcium and phosphorus levels
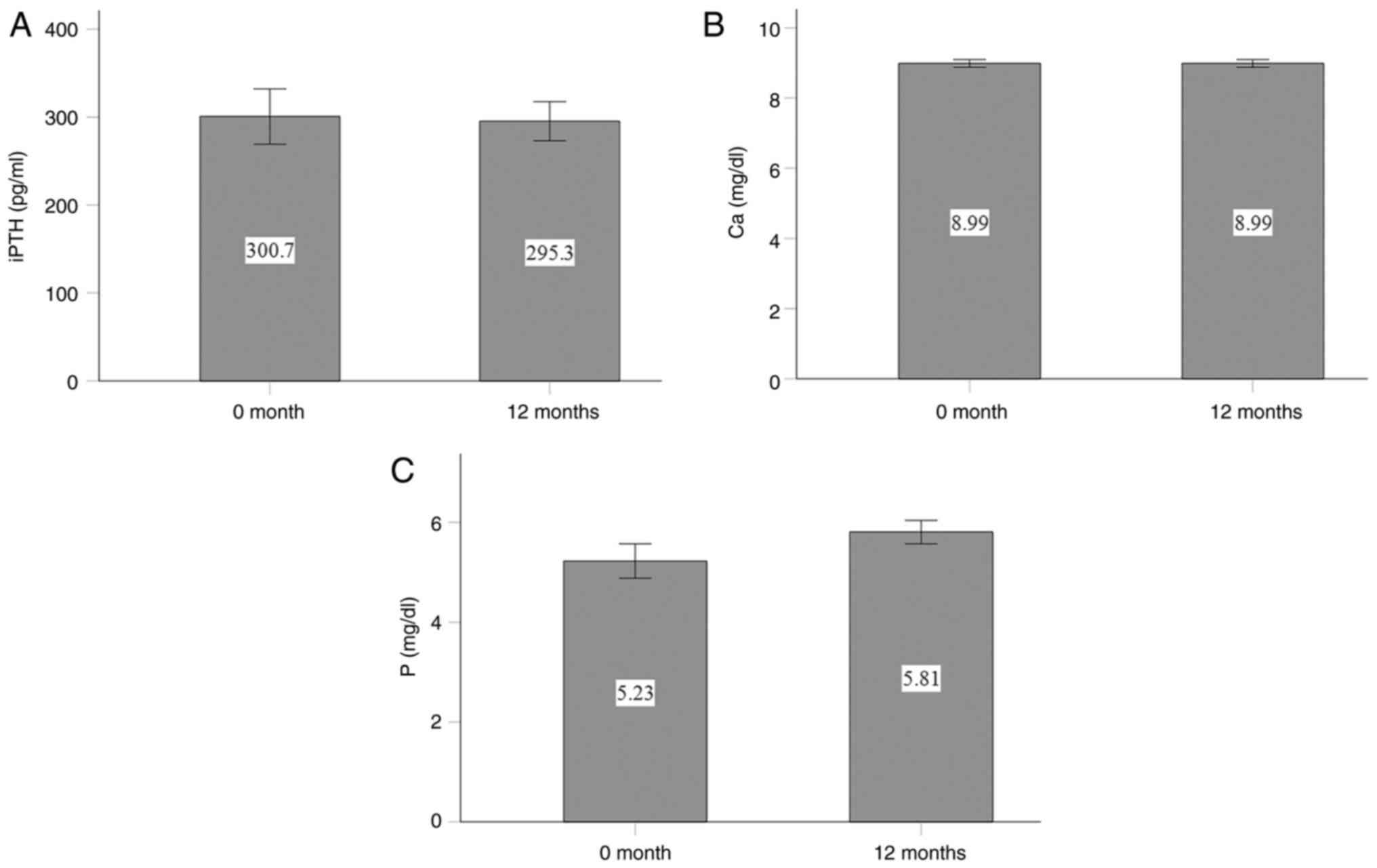
Cholecalciferol supplementation did not
significantly affect the serum iPTH, calcium, or phosphorus levels.
The serum iPTH levels were 300.7±31.4 pg/ml at the beginning of the
study and 295.3±22.2 pg/ml at the end of the study (P=0.847;
Fig. 3A). The serum calcium levels
remained unaltered, with values of 8.99±0.10 mg/dl at the start and
8.99±0.11 mg/dl at the end of the study (P=1.0; Fig. 3B). The serum phosphorus levels
exhibited a non-significant increase from 5.23±0.34 mg/dl before
treatment to 5.81±0.24 mg/dl after treatment (P=0.06; Fig. 3C).
Effects of cholecalciferol on CRP
levels and the neutrophil-to-lymphocyte ratio
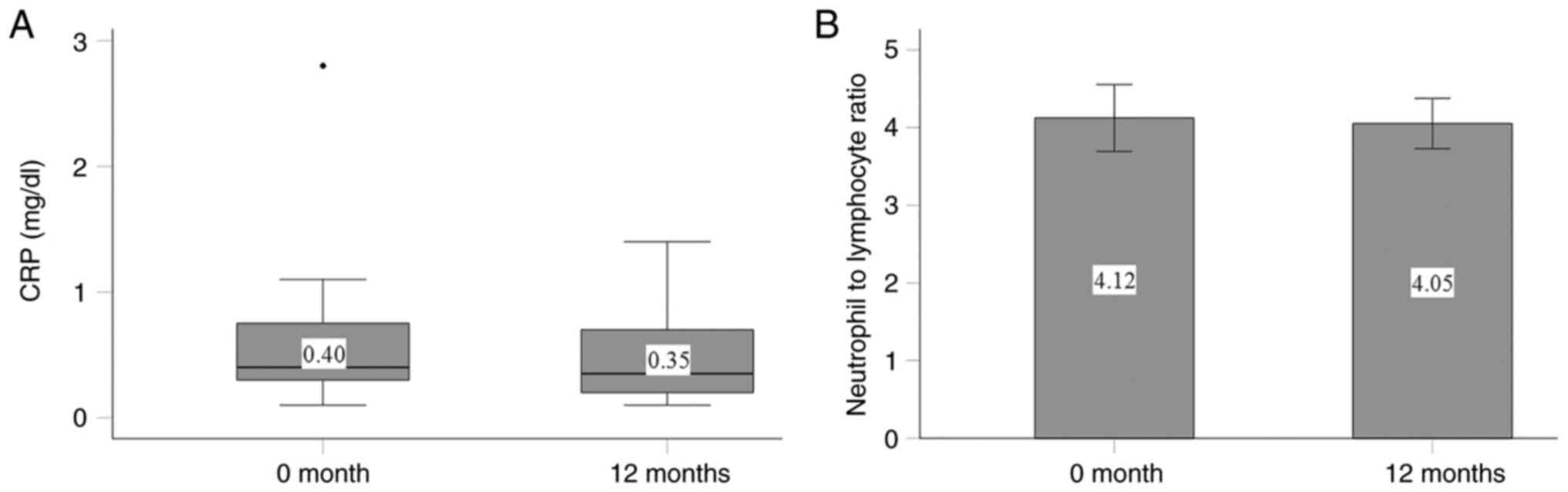
The administration of cholecalciferol did not
significantly affect the inflammatory markers, CRP and the
neutrophil-to-lymphocyte ratio. At the initiation of the study, the
median CRP value was 0.4 mg/dl (interquartile range, 0.30-0.78
mg/dl, with a minimum of 0.1 mg/dl and a maximum of 2.8 mg/dl), and
0.35 mg/dl (interquartile range, 0.20-0.75 mg/dl, with a minimum of
0.1 mg/dl and a maximum of 1.4 mg/dl) by the end of the study
(P=0.418; Fig. 4A). The
neutrophil-to-lymphocyte ratio was initially 4.12±1.92 and was
slightly altered to 4.05±1.45 by the end of the study (P=0.859;
Fig. 4B).
Discussion
Vitamin D deficiency, characterized by serum 25(OH)D
levels <20 ng/ml (20), is
prevalent among patients undergoing HD (3,4).
While the traditional model of vitamin D metabolism posits that
25(OH)D should be converted to its active form,
1,25-dihydroxyvitamin D, in the kidneys via the enzyme,
1α-hydroxylase (1,2), the KDIGO clinical practice guidelines
advise measuring serum 25(OH)D levels and administering 25(OH)D in
cases of vitamin D deficiency or insufficiency in patients with CKD
at stages 3-5D (8). This
recommendation is informed by observational studies, one of which
found that in patients with CKD, low serum 25(OH)D levels, but not
low 1,25-dihydroxyvitamin D levels, were associated with a higher
risk of developing progression to end-stage renal disease and
increased mortality (21). In
patients undergoing HD, low serum 25(OH)D levels have been linked
to an elevated mortality rate (6).
The beneficial effects of maintaining normal 25(OH)D levels may be
attributed to the expression of 1α-hydroxylase in extrarenal
tissues, which facilitates the localized activation of 25(OH)D.
This localized activation allows for autocrine or paracrine actions
at the sites where 25(OH)D is converted to its active form
(9,10).
At the bone level, the active form of vitamin D,
1,25-dihydroxyvitamin D, upregulates the expression of receptor
activator of nuclear factor-κB ligand (RANKL) in osteoblasts. RANKL
binds to its receptor RANK on preosteoclasts, promoting their
maturation into osteoclasts and subsequent bone resorption.
However, 1,25-dihydroxyvitamin D also stimulates osteoblasts to
produce osteoprotegerin, a decoy receptor that binds to and
neutralizes RANKL, thereby inhibiting osteoclastogenesis and
inducing osteoclast apoptosis. Both osteoblasts and osteoclasts
express 1α-hydroxylase, which allows for the local conversion of
25(OH)D to 1,25-dihydroxyvitamin D, independent of renal activation
(11,12). Additionally, PTH upregulates RANKL
and promotes osteoclastogenesis, and the role of
1,25-dihydroxyvitamin D in suppressing PTH has been
well-established (5). Notably, the
parathyroid glands express 1α-hydroxylase, enabling the local
conversion of 25(OH)D to 1,25-dihydroxyvitamin D, which can then
function in an autocrine or paracrine manner to suppress PTH
production independently of renal activation (13). Some clinical data support this
concept (14,15).
However, the data from the present study indicated
that although 1 year of cholecalciferol supplementation
significantly increased the serum 25(OH)D levels, it did not lead
to improvements in BMD in patients undergoing HD. In the general
population, the T-score measures the standard deviation of the bone
density of a patient relative to the mean of a young, healthy
reference population. A T-score <-2.5 indicates the highest risk
of fracture, is diagnostic for osteoporosis, and suggests the need
for pharmaceutical treatment. Scores between -2.5 and -1.0 indicate
an intermediate fracture risk, are diagnostic of osteopenia, and
may leave the best therapeutic approach uncertain. A T-score
>-1.0 is considered normal. T-scores are reliable for assessing
the risk of fractures in untreated post-menopausal females and
older males (22). The Z-score,
calculated as standard deviations from the mean of a reference
group matched by age, ethnicity and sex, should be used to assess
fracture risk in children, premenopausal females and in males
<50 of age. A Z-score <-2.0 indicates a lower-than-expected
bone mass, warranting further investigation when supported by the
clinical history of the patient (22). In the present study, DXA revealed
no significant changes in the lumbar spine T-score, Z-score, or BMD
before and after cholecalciferol administration. Notably, by the
end of the study period, there was a trend towards a decline in the
total hip Z-score that did not reach statistical significance,
while the decline in the T-score was statistically significant.
Additionally, total hip BMD exhibited a slight, yet statistically
significant decrease.
Of note, a previous study found that in patients
undergoing HD and peritoneal dialysis, BMD is not associated with
serum 25(OH)D levels (23). A post
hoc analysis of the IMPROVE-CKD study, which included patients with
stage 3b/4 CKD, also found no association between serum 25(OH)D
levels and lumbar spine BMD, as assessed by CT-derived Hounsfield
unit values (24). However, the
aforementioned findings are not universal. For instance, another
study detected a positive association between 25(OH)D levels and
BMD Z-scores in patients undergoing HD or peritoneal dialysis
(25). In addition to the
uncertainty regarding the association between serum 25(OH)D levels
and DXA-BMD, there are few studies that have evaluated the effects
of cholecalciferol administration, and their results are
controversial. In a post hoc analysis of the Vitamin D, Calcium,
Lyon Study II (DECALYOS II), which randomized 610 elderly females
to receive either cholecalciferol and calcium supplementation or a
placebo for 2 years, the participants overall exhibited a decrease
in BMD over time with active treatment. However, in a subgroup of
100 females on cholecalciferol and calcium with an estimated
glomerular filtration rate <45 ml/min, the rate of BMD loss
appeared slower compared to those on the placebo. It is important
to note, however, that women with a serum creatinine level >150
µmol/l were excluded from the trial (26). As regards patients undergoing HD, a
randomized trial involving 19 patients undergoing HD with serum
25(OH)D levels <20 ng/ml found no difference in BMD after 1 year
between those who received 2,000 IU of cholecalciferol three times
per week and those who received a placebo (27). Similar results were observed in
another small trial that included 12 patients undergoing HD
(28).
Likewise, in the present study, cholecalciferol
supplementation did not alter the serum iPTH, calcium or phosphorus
levels. A previous randomized trial reported comparable results,
demonstrating that oral ergocalciferol can elevate 25(OH)D levels
in patients undergoing HD without causing significant changes in
serum calcium, phosphorus, or iPTH levels over a 12-week period
(29). Collectively, the findings
of the present study indicate that cholecalciferol administration
may not significantly enhance bone health in patients undergoing
HD.
In addition to CKD-MBD, which is a known contributor
to increased mortality rates in patients undergoing HD (30), chronic inflammation is also
prevalent in this population and further exacerbates the risk of
mortality (31,32). Notably, dendritic cells,
macrophages, B-cells and T-cells express both the VDR and
1α-hydroxylase. This enables the conversion 25(OH)D to its active
form within these cells, allowing direct effects through the VDR
independent of renal activation (16). During inflammatory processes,
1α-hydroxylase expression is upregulated, and in extreme cases of
macrophage activation, such as in sarcoidosis, the produced
1,25-dihydroxyvitamin D can have systemic effects, including
hypercalcemia (33). VDR
activators have demonstrated anti-inflammatory properties (16), and in patients undergoing HD, serum
25(OH)D levels have been inversely associated with inflammation
markers (18). However, the data
of the present study indicated that 1 year of cholecalciferol
supplementation did not significantly affect the serum CRP levels.
Similarly, the neutrophil-to-lymphocyte ratio, another sensitive
marker of inflammation tested in patients undergoing HD (18,34),
was not affected by cholecalciferol. These findings suggest that
cholecalciferol supplementation may not effectively mitigate the
chronic inflammation characteristic of patients undergoing HD. The
data of the present study align with the findings of a large
randomized trial involving 746 patients undergoing HD with elevated
depressive scores, who were administered cholecalciferol at 50,000
IU/week vs. a placebo. The trial reported no difference in CRP
levels over the 12-month duration (35). A previous meta-analysis of 18
trials with 1834 patients also demonstrated that vitamin D
supplementation did not exert anti-inflammatory effects in patients
with CKD (36).
The present study critically evaluated the KDIGO
guidelines concerning the measurement and administration of 25(OH)D
in patients undergoing HD. It is important to highlight that this
recommendation is classified as level 2 and grade C. Notably, a
recent meta-analysis of 128 randomized trials, including 11,270
participants with stage 3-5 CKD or undergoing HD, found that
vitamin D supplements and activated vitamin D analogues had no
significant effect on the primary outcomes of all-cause mortality,
cardiovascular-related mortality, or fractures. The lack of
measurable clinical benefit was consistent across subgroup analyses
that distinguished between vitamin D supplementation and activated
vitamin D analogues (24).
Generally, there is a notable discrepancy between studies assessing
the impact of serum 25(OH)D levels on parameters, such as
inflammation, mortality, or fracture rates in patients undergoing
HD and those evaluating the effects of 25(OH)D supplementation. In
studies focusing on serum 25(OH)D levels, which often report
beneficial outcomes and suggest causality, numerous confounding
factors likely influence the results. For example, low serum
25(OH)D levels are associated with increased inflammatory markers
and mortality (6,18). However, this association does not
necessarily imply that low 25(OH)D levels directly cause
inflammation. Inflammation in patients undergoing HD can arise from
multiple factors, which in turn may lead to protein-energy wasting
(PEW) syndrome and increased mortality rates (37,38).
PEW is often characterized by a reduced food intake, resulting in
lower vitamin D consumption and subsequently, in reduced serum
25(OH)D levels. In this context, inflammation would be the cause of
low serum 25(OH)D levels rather than the consequence. Supporting
this finding, patients undergoing HD who have PEW syndrome
consistently exhibit low serum 25(OH)D levels (39).
The present study had certain limitations, which
should be mentioned. A limitation of the present study is the lack
of a placebo control group and the relatively small sample size of
patients undergoing HD. Nevertheless, these participants were
meticulously selected, and their adherence to the protocol was
confirmed through serum 25(OH)D level measurements. Another
limitation is that nephrologists had discretion in prescribing the
phosphate binder sevelamer hydrochloride, the vitamin D analog
paricalcitol, and the calcimimetic cinacalcet, all of which could
potentially influence the evaluated outcomes. Despite this, the use
of these medications aligns with KDIGO recommendations for managing
serum iPTH, calcium and phosphorus levels, thereby reflecting
actual clinical practice.
In conclusion, supplementation with cholecalciferol
over a 1-year period effectively increases serum 25(OH)D levels.
However, this intervention does not lead to significant
improvements in BMD or a reduction in inflammation among patients
undergoing HD who have vitamin D deficiency. Further research
involving larger patient cohorts is thus necessary to validate
these findings and, if warranted, to potentially revise the
relevant KDIGO guidelines.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TE and PM designed the study. MD, PM, GP, MT, CP,
MAPK, EL, IS and TE interpreted and analyzed the results, TE and MD
wrote the manuscript. TE and PM confirm the authenticity of all the
raw data. All authors drafted the manuscript, critically revised
the manuscript, agree to be fully accountable for ensuring the
integrity and accuracy of the work, and have read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol received approval from the Ethics
Committee of the Faculty of Medicine at the University of Thessaly,
Larissa, Greece (no. of approval 558/10-2-2017). Informed consent
was obtained from each individual enrolled in the study.
Patient consent for publication
Relevant consent for publication was obtained from
the patient whose representative measurements are presented in
Fig. 1.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gil Á, Plaza-Diaz J and Mesa MD: Vitamin
D: Classic and novel actions. Ann Nutr Metab. 72:87–95.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jean G, Souberbielle J and Chazot C:
Vitamin D in chronic kidney disease and dialysis patients.
Nutrients. 9(328)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sreevani M, Rao BS and Srivanvani S:
Vitamin D levels among chronic kidney disease patients at a
tertiary care hospital: A cross-sectional study. Natl J Lab Med.
13:BO01–BO04. 2024.
|
|
5
|
Franchi M, Gunnarsson J, Gonzales-Parra E,
Ferreira A, Ström O and Corrao G: Paricalcitol and extended-release
calcifediol for treatment of secondary hyperparathyroidism in
non-dialysis chronic kidney disease: Results from a network
meta-analysis. J Clin Endocrinol Metab. 108:e1424–e1432.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
da Silva Canhos MM, de Oliveira RC,
Modelli de Andrade LG, Caramori JCT, Barretti P and Martin LC:
Association between vitamin D levels and mortality in hemodialysis
patients: A cohort study. Ren Fail. 42:225–233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sutherland JP, Zhou A and Hyppönen E:
Vitamin D deficiency increases mortality risk in the UK biobank: A
nonlinear mendelian randomization study. Ann Intern Med.
175:1552–1559. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Kidney Disease, Improving Global Outcomes
(KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice
guideline update for the diagnosis, evaluation, prevention, and
treatment of chronic kidney disease-mineral and bone disorder
(CKD-MBD). Kidney Int Suppl (2011). 7:1–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bouillon R and Bikle D: Vitamin D
metabolism revised: Fall of dogmas. J Bone Miner Res. 34:1985–1992.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pike JW and Meyer MB: The unsettled
science of nonrenal calcitriol production and its clinical
relevance. J Clin Invest. 130:4519–4521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Driel M and van Leeuwen JPTM: Vitamin
D and bone: A story of endocrine and auto/paracrine action in
osteoblasts. Nutrients. 15(480)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Verlinden L and Carmeliet G: Integrated
view on the role of vitamin D actions on bone and growth plate
homeostasis. JBMR Plus. 5(e10577)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Segersten U, Correa P, Hewison M, Hellman
P, Dralle H, Carling T, Akerström G and Westin G: 25-Hydroxyvitamin
D(3)-1alpha-hydroxylase expression in normal and pathological
parathyroid glands. J Clin Endocrinol Metab. 87:2967–2972.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen X, Chu C, Doebis C, Xiong Y, Cao Y,
Krämer BK, von Baehr V and Hocher B: Vitamin D status and its
association with parathyroid hormone in 23,134 outpatients. J
Steroid Biochem Mol Biol. 220(106101)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eleftheriadis T, Antoniadi G, Liakopoulos
V, Stefanidis I and Galaktidou G: Inverse association of serum
25-hydroxyvitamin D with markers of inflammation and suppression of
osteoclastic activity in hemodialysis patients. Iran J Kidney Dis.
6:129–135. 2012.PubMed/NCBI
|
|
16
|
Ghaseminejad-Raeini A, Ghaderi A, Sharafi
A, Nematollahi-Sani B, Moossavi M, Derakhshani A and Sarab GA:
Immunomodulatory actions of vitamin D in various immune-related
disorders: A comprehensive review. Front Immunol.
14(950465)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eleftheriadis T, Antoniadi G, Liakopoulos
V, Kartsios C, Stefanidis I and Galaktidou G: Paricalcitol reduces
basal and lipopolysaccharide-induced (LPS) TNF-alpha and IL-8
production by human peripheral blood mononuclear cells. Int Urol
Nephrol. 42:181–185. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kara AV and Soylu YE: The relationship
between vitamin D and inflammatory markers in maintenance
hemodialysis patients. Int Urol Nephrol. 51:1659–1665.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iimori S, Mori Y, Akita W, Kuyama T,
Takada S, Asai T, Kuwahara M, Sasaki S and Tsukamoto Y: Diagnostic
usefulness of bone mineral density and biochemical markers of bone
turnover in predicting fracture in CKD stage 5D patients-a
single-center cohort study. Nephrol Dial Transplant. 27:345–351.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amrein K, Scherkl M, Hoffmann M,
Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, Martucci
G, Pilz S and Malle O: Vitamin D deficiency 2.0: An update on the
current status worldwide. Eur J Clin Nutr. 74:1498–1513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ravani P, Malberti F, Tripepi G, Pecchini
P, Cutrupi S, Pizzini P, Mallamaci F and Zoccali C: Vitamin D
levels and patient outcome in chronic kidney disease. Kidney Int.
75:88–95. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Williams S, Khan L and Licata AA: DXA and
clinical challenges of fracture risk assessment in primary care.
Cleve Clin J Med. 88:615–622. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yucel Kocak S and Ozdemir A: Comparison of
bone mineral density and biochemical factors in hemodialysis and
peritoneal dialysis patients. Clin Nephrol. 98:115–122.
2022.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Yeung WCG, Toussaint ND, Lioufas N, Hawley
CM, Pascoe EM, Elder GJ, Valks A and Badve SV: Vitamin D status and
intermediate vascular and bone outcomes in chronic kidney disease:
A secondary post hoc analysis of IMPROVE-CKD. Intern Med J.
54:1960–1969. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Elder GJ and Mackun K: 25-Hydroxyvitamin D
deficiency and diabetes predict reduced BMD in patients with
chronic kidney disease. J Bone Miner Res. 21:1778–1784.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bosworth C, de Boer IH, Targher G,
Kendrick J, Smits G and Chonchol M: The effect of combined calcium
and cholecalciferol supplementation on bone mineral density in
elderly women with moderate chronic kidney disease. Clin Nephrol.
77:358–365. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Mieczkowski M, Żebrowski P, Wojtaszek E,
Stompór T, Przedlacki J, Bartoszewicz Z, Sierdziński J, Wańkowicz
Z, Niemczyk S and Matuszkiewicz-Rowińska J: Long-term
cholecalciferol administration in hemodialysis patients: A
single-center randomized pilot study. Med Sci Monit. 20:2228–2234.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tsikliras N, Anagnostara A, Tsantekidou F
and Kyrgialanis A: #495 effect of Vit D supplementation on bone
mineral density in haemodialysis patients. A comparative study.
Nephrol Dial Transplant. 39 (Suppl 1)(gfae069-1604-495)2024.
|
|
29
|
Bhan I, Dobens D, Tamez H, Deferio JJ, Li
YC, Warren HS, Ankers E, Wenger J, Tucker JK, Trottier C, et al:
Nutritional vitamin D supplementation in dialysis: A randomized
trial. Clin J Am Soc Nephrol. 10:611–619. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fernández-Martín JL, Martínez-Camblor P,
Dionisi MP, Floege J, Ketteler M, London G, Locatelli F, Gorriz JL,
Rutkowski B, Ferreira A, et al: Improvement of mineral and bone
metabolism markers is associated with better survival in
haemodialysis patients: The COSMOS study. Nephrol Dial Transplant.
30:1542–1551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sasaki K, Shoji T, Kabata D, Shintani A,
Okute Y, Tsuchikura S, Shimomura N, Tsujimoto Y, Nakatani S, Mori
K, et al: Oxidative stress and inflammation as predictors of
mortality and cardiovascular events in hemodialysis patients: The
DREAM cohort. J Atheroscler Thromb. 28:249–260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Osawa H, Nakamura N, Tsutaya C, Saitoh H,
Shimada M, Murakami R, Fujita T, Narita-Kinjo I, Nagawa D, Nakata
M, et al: Role of high-sensitivity C-reactive protein in future
cardiovascular events in hemodialysis patients. In Vivo.
38:1351–1358. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mulkareddy V, Bhalla V, Upadhye S and
Siddam P: The diagnostic dilemma of sarcoidosis: A case of acute
hypercalcemia. Cureus. 12(e10399)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pineault J, Lamarche C, Bell R, Lafrance
JP, Ouellet G, Leblanc M, Pichette V, Bezzaoucha S and Vallée M:
Association of neutrophil-to-lymphocyte ratio with inflammation and
erythropoietin resistance in chronic dialysis patients. Can J
Kidney Health Dis. 4(2054358117735563)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Y, Liu Y, Lian Y, Li N, Liu H and Li
G: Efficacy of high-dose supplementation with oral vitamin d3 on
depressive symptoms in dialysis patients with vitamin D3
insufficiency: A prospective, randomized, double-blind study. J
Clin Psychopharmacol. 36:229–235. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao L, Zhu G, Wu L and Xie D: Effects of
vitamin D on inflammatory state in patients with chronic kidney
disease: A controversial issue. Ther Apher Dial. 27:383–393.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eleftheriadis T, Antoniadi G, Liakopoulos
V, Kartsios C and Stefanidis I: Basic science and dialysis:
Disturbances of acquired immunity in hemodialysis patients. Semin
Dial. 20:440–451. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hanna RM, Ghobry L, Wassef O, Rhee CM and
Kalantar-Zadeh K: A practical approach to nutrition, protein-energy
wasting, sarcopenia, and cachexia in patients with chronic kidney
disease. Blood Purif. 49:202–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Visiedo L, Pérez Abud R, Rivas-Ruiz F,
Payan JJ, Rey L, Tortajada B and Abilés J: Hypovitaminosis D and
its relationship with nutritional status and quality of life in
patients undergoing haemodialysis. Nutr Hosp. 40:144–150.
2023.PubMed/NCBI View Article : Google Scholar
|