1. Introduction
Ocular or eye cancer is defined as a malignant
development (uncontrolled cell proliferation) in any area of the
eye. Cancer can occur in various regions of the eye, including the
orbit, adnexal tissues and the eyeball. A previous study on 80
cases of ocular cancer discovered that eyelid tumors were the most
common (53.75%), followed by intraocular (21.25%),
conjunctival/corneal (20%) and orbital (5%) cancers (1). The three primary layers of the
eyeball are the sclera, uvea and retina. Cancer typically affects
the iris, ciliary body of the uvea and the choroid. The orbit is
the tissue that surrounds and protects the eyeball. Orbital
malignancies are tumors that reside in these tissues. Adnexal
structures cover the area containing tear glands and eyelids. These
are referred to as adnexal malignancies (2).
Types of eye cancer
Uveal melanoma. The uvea is the term for the
central layer of the eyeball. The ciliary body, choroid and iris
are its three main components. Uveal melanoma tumors originate from
melanocytes in the uveal or middle layer of the eye, where the
choroid is most frequently found, followed by the ciliary body and
iris (3). This is the most
prevalent and peculiar type of cancer, affecting primarily adults,
and up to half of its victims succumb due to liver metastases,
although it can also affect the skin, bones and lungs (4).
Retinoblastoma. Retinoblastoma affects the
retinal cells of the eye. It is an uncommon childhood eye cancer
that is usually hereditary and bilateral, resulting from a
mutation. Individuals who suffer from unilateral retinoblastoma do
not pass the illness on to their progeny, whereas people with
bilateral retinoblastoma have tumors that induce cancer in other
areas (5).
Conjunctival melanoma. Conjunctival melanoma
is derived from melanocytes and is an extremely uncommon type of
cancer, similar to uveal melanoma. Cancer spreads to other parts of
the body due to its rapid growth and propensity to migrate through
lymph and blood arteries (2,5).
Ocular surface squamous neoplasia (OSSN).
OSSN may culminate in basic dysplasia, invasive squamous cell
carcinoma and dysplastic and carcinomatous lesions in the cornea or
conjunctiva (6). It is most
typically detected in the limbal region, particularly in elderly
persons who live near the equator, where UV-B radiation is
excessive, making it a major etiological cause. The condition can
cause symptoms ranging from no pain to severe pain and vision loss,
emphasizing the significance of early diagnosis and treatment
(7).
Oncogenes and their types
A broad category of disorders known as cancer is
defined by genetic instability and altered DNA activities, which
can cause abnormalities in the cell cycle as well as unchecked
proliferation, dedifferentiation, and cellular immortalization. The
proto-oncogene group is a unique gene that controls the cell cycle.
They function as growth factors by acting as messengers, and they
are crucial for healthy cell division and growth (8,9).
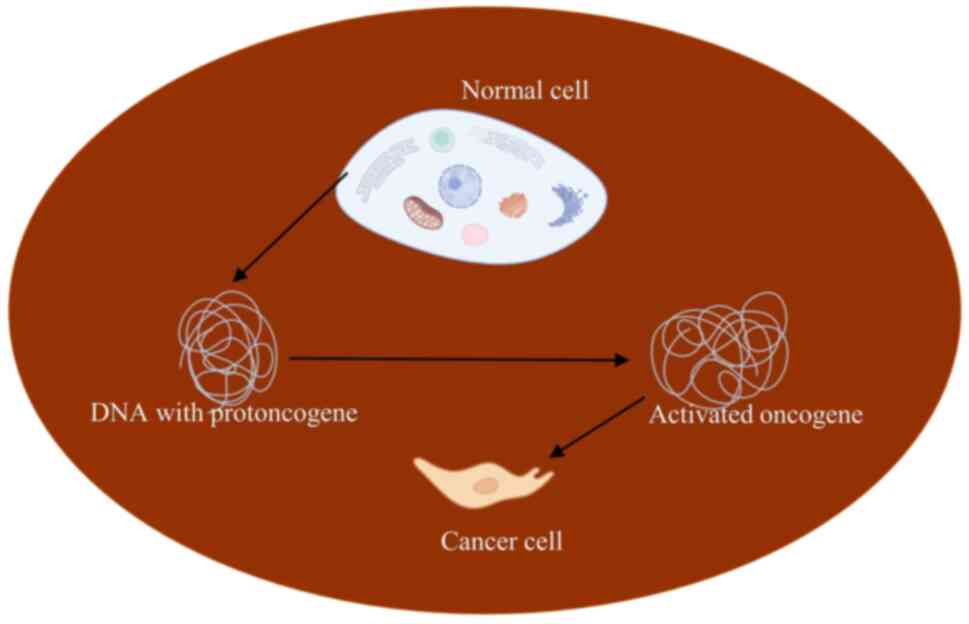
When proto-oncogenes interact with environmental carcinogens, they
undergo mutations and ramifications that activate or alter the new,
modified genes known as oncogenes (Fig. 1 and Table I).
 | Table ICertain oncogenes implicated in
cancer. |
Table I
Certain oncogenes implicated in
cancer.
| Target gene | Cancers caused |
|---|
| HER2 gene | Breast cancer |
| BCR/ABL1 gene | Chronic myeloid
leukemia |
| c-myc gene | Burkitt
lymphoma |
| N-myc gene | Small cell lung
cancer and neuroblastoma |
| EGFR and EML4AK
genes | Adenocarcinoma of
the lung |
| Kras gene | Pancreatic cancer
and lung cancer |
| GNA11 or GNAQ | Eye cancer (uveal
melanoma) |
| RB1 tumor
suppressor gene | Retinoblastoma |
2. Epidemiology
Ocular cancer is a relatively rare form of cancer
compared to other types of cancer. It involves tumors that form in
or around the eye. The epidemiology of ocular cancer varies
depending on the specific type of cancer and geographic region;
below are some general insights:
Age and sex
Some forms of ocular cancer, such as retinoblastoma
(a childhood cancer), primarily affect infants and young children.
Retinoblastoma often presents prior to the age of 5 years (10). However, other forms of ocular
cancer, such as uveal melanoma, typically occur in adults. There
may be variations in incidence based on gender and age groups for
different types of ocular cancer (11).
Risk factors
Several risk factors are associated with ocular
cancer, including exposure to ultraviolet (UV) radiation, certain
genetic factors (e.g., a family history of retinoblastoma), and
possibly, environmental factors, such as blue light exposure
(12) and human papilloma virus
(HPV) type 16 in synergy with UV exposure (13).
In individuals who have a fair skin and are
light-colored, the eyes may have a greater vulnerability to UV
light (14), which increases the
risk of developing ocular melanoma. Exposure to certain chemicals,
such as formaldehyde (15) and
pesticides such as acephate and bromacil (16) has been shown to be associated with
an increased risk of developing ocular cancer (17).
Geographic variation
The incidence of ocular cancer may vary according to
the geographic region. For instance, uveal melanoma is more common
in populations with lighter skin tones and may have higher rates in
regions with greater sun exposure (12,18-19).
Diagnosis and treatment
In cases of ocular cancer, prompt identification and
treatment are essential for optimal results. Depending on the type
and stage of the cancer, treatment options may include
chemotherapy, surgery, radiation therapy, targeted therapy and
immunotherapy (2). Due to the
relative rarity of ocular cancer and the diversity of its types,
ongoing research is essential to better understand its epidemiology
and risk factors, and develop optimal treatment strategies. Early
detection through regular eye examinations and the awareness of
risk factors can also contribute to improved outcomes for
individuals at risk of developing ocular cancer.
Epidemiological studies conducted by researchers and
institutions often include assessments of ocular cancer incidence,
risk factors and outcomes within specific populations. These
studies may use various methodologies, including surveys, medical
records reviews and population-based cancer registries. The fact
that uveal melanoma affects populations differently depending on
their place of residence is one of its main characteristics. Over
the past 30 years, the incidence of uveal melanoma has remained
constant, in contrast to that of cutaneous melanoma, which has
exhibited a notable increase in frequency (20). Examples of disparities in incidence
rates across Europe include Portugal, Spain, and Italy at two cases
per million, and Denmark, Sweden and Norway at nine cases per
million (21). While these sources
contribute to the understanding of ocular cancer epidemiology, the
study design, population characteristics and data quality all need
to be taken into consideration when interpreting the results.
Researchers continue to explore new methods and collaborate
internationally to improve cancer surveillance and advance
knowledge about ocular cancer prevention, diagnosis, and
treatment.
3. Oncogenes and their role in ocular
cancer
Oncogenes
An oncogene is a malfunctioning cellular gene
(proto-oncogene) caused by mutation, fusion with another gene, or
overexpression. Typically, oncogenes are considered to cause cancer
by inhibiting apoptosis or deregulating cell growth (22).
Oncogenes are genes that, when expressed abnormally,
can cause tumorigenic conversion. Under normal circumstances, their
proteins play essential roles in cellular signaling, growth, and
differentiation. Proto-oncogenes are the typical, appropriately
controlled forms of these genes. On the other hand, constitutive
activation of proto-oncogenes results in unchecked cell growth and
proliferation, which ultimately causes the development and spread
of cancer (23). Physical
mutations that cause changes in the structure of the encoded
protein and those that alter the appearance of proteins are two
well-known types of physical mutations that trigger proto-oncogenes
(24).
Function of oncogenes
Oncogenes are physically and functionally diverse.
They regulate the cell cycle, apoptosis and the growth,
differentiation, and proliferation of cells. Growth factors, growth
factor receptors, signal transducers, transcription factors,
apoptotic regulators and chromatin remodelers are among the
by-products of oncogenes (25).
Specific oncogenes implicated in
ocular cancer. Retinoblastoma (Rb) gene and retinoblastoma
There are 27 exons spread across roughly 200 kb of
DNA in the Rb gene. Mutations in the germline Rb gene typically
occur at 5'-C-phosphate-G-3' (CpG) dinucleotides and are
distributed throughout the whole gene (26). The Rb protein is functionally
inactive in nearly all human malignancies in which the Rb gene is
not altered, indicating that Rb is a tumor suppressor of
fundamental significance in cancer biology (27). Indeed, oncoproteins, which bind to
and inhibit Rb, are produced by tumor-causing viruses, such as
SV40, adenovirus and human papillomavirus. These proteins play a
crucial role in the tumorigenic potential of these viruses. The Rb
‘pocket’, which is produced by the interaction of the A and B boxes
along an extended interface, is essential to the ability of Rb to
suppress tumors, as shown by the fact that almost all mutations
that cause tumors disrupt it (5).
As the first cloned tumor suppressor gene, the Rb
gene is known for its ability to bind to the transcription factor
E2F and limit the transcription of genes required for the S phase,
thereby functioning as a negative regulator of the cell cycle. The
Rb gene is known to have biological impacts on tumor suppression,
cell cycle regulation, apoptosis and differentiation. These
functions are made possible by the interactions of the Rb gene with
a variety of cellular proteins. Of the >100 proteins that have
been demonstrated to interact with the Rb protein, most, if not
all, include the pocket domain. The transcription factors E2F are
the most studied Rb binding partners (28).
G protein subunit alpha Q (GNAQ)/guanine
nucleotide binding protein alpha 11 (GNA11) mutations in uveal
melanoma. The most frequent intraocular cancer in adults and
the most prevalent non-cutaneous melanoma is uveal melanoma
(29-31).
Uveal melanoma comprises 3-5% of all occurrences of melanoma; it
occurs in the uveal tract of the eye and mostly affects the choroid
(85-90%), ciliary body (5-8%) and iris (3-5%) (32).
Unlike other forms of melanoma, uveal melanomas do
not carry mutations in V-Raf murine sarcoma viral oncogene homolog
B1 (BRAF), neuroblastoma-RAS (NRAS), or neurofibromatosis type 1
(NF1) (33). Instead,
constitutively active mutations in GNAQ and GNA11, which encode the
closely related α subunits GQ and G11, account for >90% of uveal
melanomas. They belong to the Gαq family, which also includes G14
and G15/16. To generate heterotrimeric G proteins, individual α
subunits attach to β and γ subunits. The signals from Gαq-coupled
GPCRs are subsequently sent to effectors by these proteins. The
majority of uveal melanoma mutations affect codons Q209 and, less
frequently, codons R183 of GNA11 or GNAQ, which functionally
reduces their GTPase catalytic activity. In addition to GNAQQ209L
and GNAQQ209P having slightly different tertiary structures and
developing downstream signaling mutations, GNAQ and GNA11 have
somewhat different mutation spectra. Asp630 in phospholipase C β4
(PLCβ4) encoding, which is downstream of Gvq, or codon Leu129 in
CYSLTR2, a Gαq-coupled GPCR, were the sites of recurrent mutations
in 10% of uveal melanomas without GNAQ or GNA11 changes. Therefore,
the key activation of the Gαq pathway brought on by somatic
mutations can be considered the disease-defining feature of uveal
melanoma. Gαq pathway mutations are also linked to other neoplastic
conditions, such as mucosal melanoma, anastomosing and congenital
hemangiomas, capillary malformations, hepatic small artery
neoplasms, Sturge-Weber syndrome and port-wine stains (31). Additionally, mutations in the Gαq
pathway also produce phakomatosis pigmentovascularis and central
nervous system melanocytomas.
Alterations in ocular surface squamous
neoplasia. OSSN is a term used to describe a variety of tumors
that affect the ocular surface. These tumors can range in histology
from intraepithelial neoplasia to various stages of squamous cell
carcinoma. Early lesions that fluctuate in size typically begin at
the limbus, junction point of the cornea, and conjunctiva. Higher
or more advanced stages may infiltrate the orbit and affect the
eyelids. Oddly, OSSN usually only affects one eye (34).
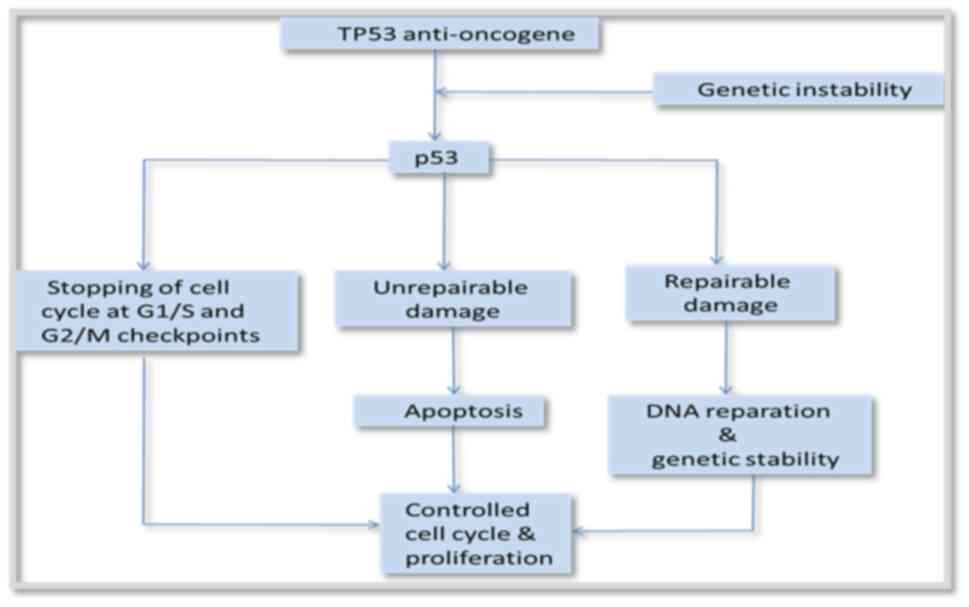
Various cellular functions are regulated by p53, a
crucial function that has been thoroughly investigated. p53 plays a
role in several biological processes, such as DNA repair,
apoptosis, senescence and homeostasis, in addition to cell cycle
arrest. As anticipated, p53 is expressed in every part of the eye
and plays a key role in the human body. Modified p53 signaling
pathways have been specifically linked to the development of
retinoblastoma, intraocular melanoma, uveal melanoma and
conjunctival malignancy. Since non-selective cancer chemotherapies
that may employ ionizing radiation may be linked to low efficacy or
dose-limiting toxicities in the eyes (35).
Other identified oncogenes. Conjunctival
melanoma accounts for ~5% of all ocular melanomas. Melanocytes in
the base layer of the conjunctival epithelium give rise to it. As
an early stage in the development of tumors, up to 50% of
conjunctival melanomas are characterized by the V600E mutation in
BRAF. Of note, ~30% of conjunctival melanomas have NF1 mutations
(36).
Molecular pathways and signaling
cascades
Oncogenic signaling in ocular malignancies
encompasses a wide spectrum of pathways and molecular changes.
Mutations in the GNAQ, GNA11, CYSLTR2, or PLCB4 genes increase G
protein-coupled receptor signaling in uveal melanoma, activating
the MAPK and protein kinase C pathways downstream (33). Other genetic alterations, such as
chromosome 3 monosomy and BRCA1 associated deubiquitinase 1 (BAP1)
mutations, also influence the risk of metastasis. MERTK, a receptor
tyrosine kinase overexpressed in a number of malignancies, promotes
cell migration and survival by activating the PI3K and MAPK
pathways (34). Chromosome changes
and oncogene mutations affect specific signaling pathways in ocular
cancers, such as retinoblastoma, uveal melanoma and squamous cell
carcinoma (39).
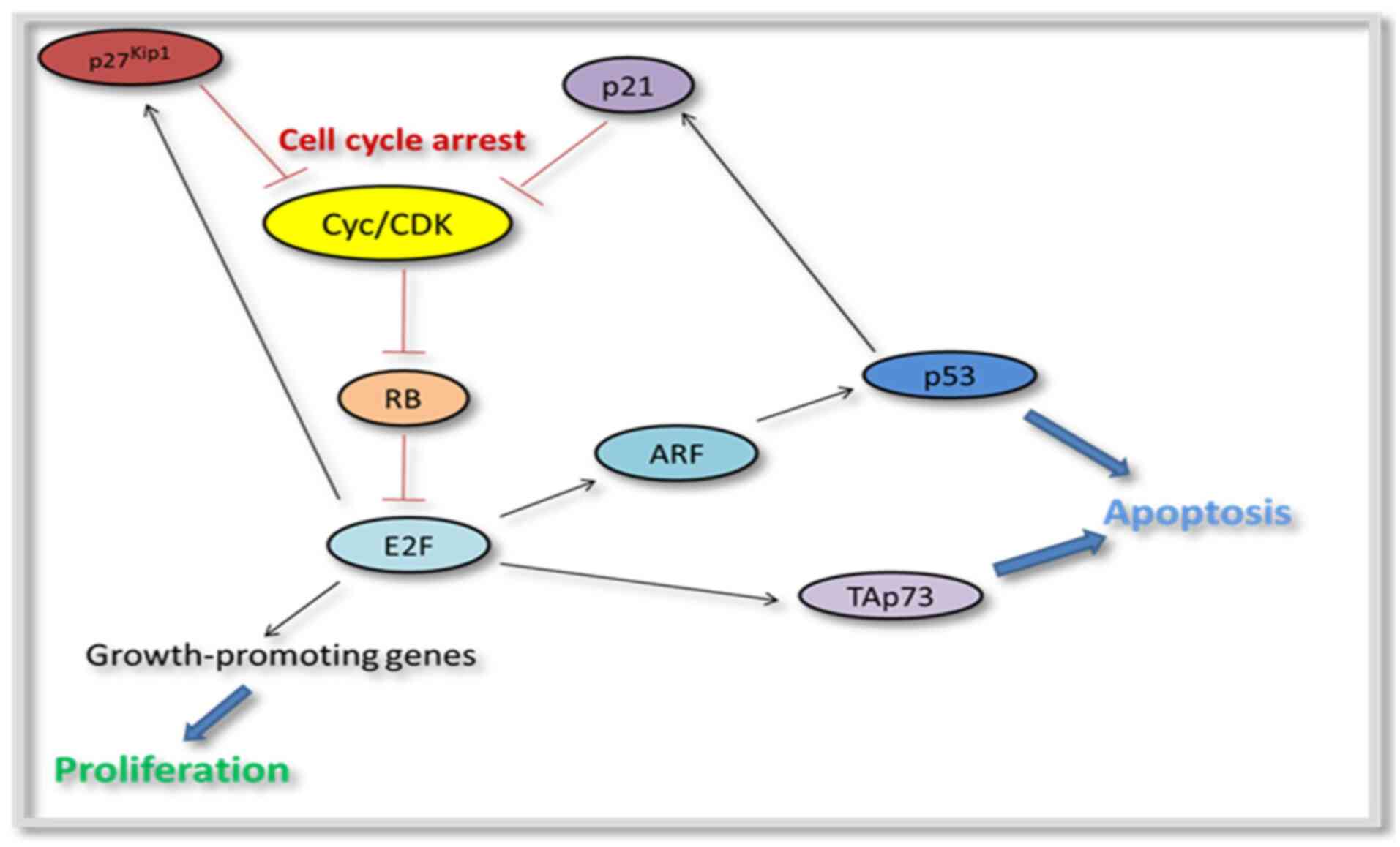
Cell cycle regulation. Cell cycle
dysregulation is crucial for ocular oncogenesis, particularly in
retinoblastoma and uveal melanoma. RNA sequencing in retinoblastoma
has revealed that cell cycle disruption plays a crucial role in
carcinogenesis (40), with six
genes (BUB1, RRM2, TPX2, UBE2C, NUSAP1 and DTL) identified as
possible targets for innovative therapeutics. Both retinoblastoma
and uveal melanoma rely on the retinoblastoma gene (RB1) and its
protein product (pRB) to suppress tumors and govern the cell cycle.
Cyclins D1 and E, as well as CDK inhibitors p16 and p27, are
overexpressed in primary uveal melanoma cell cultures, suggesting
aberrant cell cycle regulation (41) (Fig.
2).
Apoptosis. Ocular homeostasis and disease
processes depend heavily on apoptosis, a genetically programmed
type of cell death. Glaucoma, retinitis pigmentosa, cataract,
retinoblastoma and diabetic retinopathy are among the
ophthalmological disorders in which it is implicated (42). According to Tempestini et al
(43), there are two primary
apoptotic signaling pathways: The intrinsic (depending on
mitochondria) and extrinsic (dependent on death receptors)
pathways. Both pro- and anti-apoptotic factors are found in
mitochondria, and the main cause of cell death is the mitochondrial
permeability transition pore (43). Novel treatment approaches for
ophthalmology that prevent cellular death may result from an
understanding of the molecular pathways of apoptosis in ocular
illnesses (42) (Fig. 3).
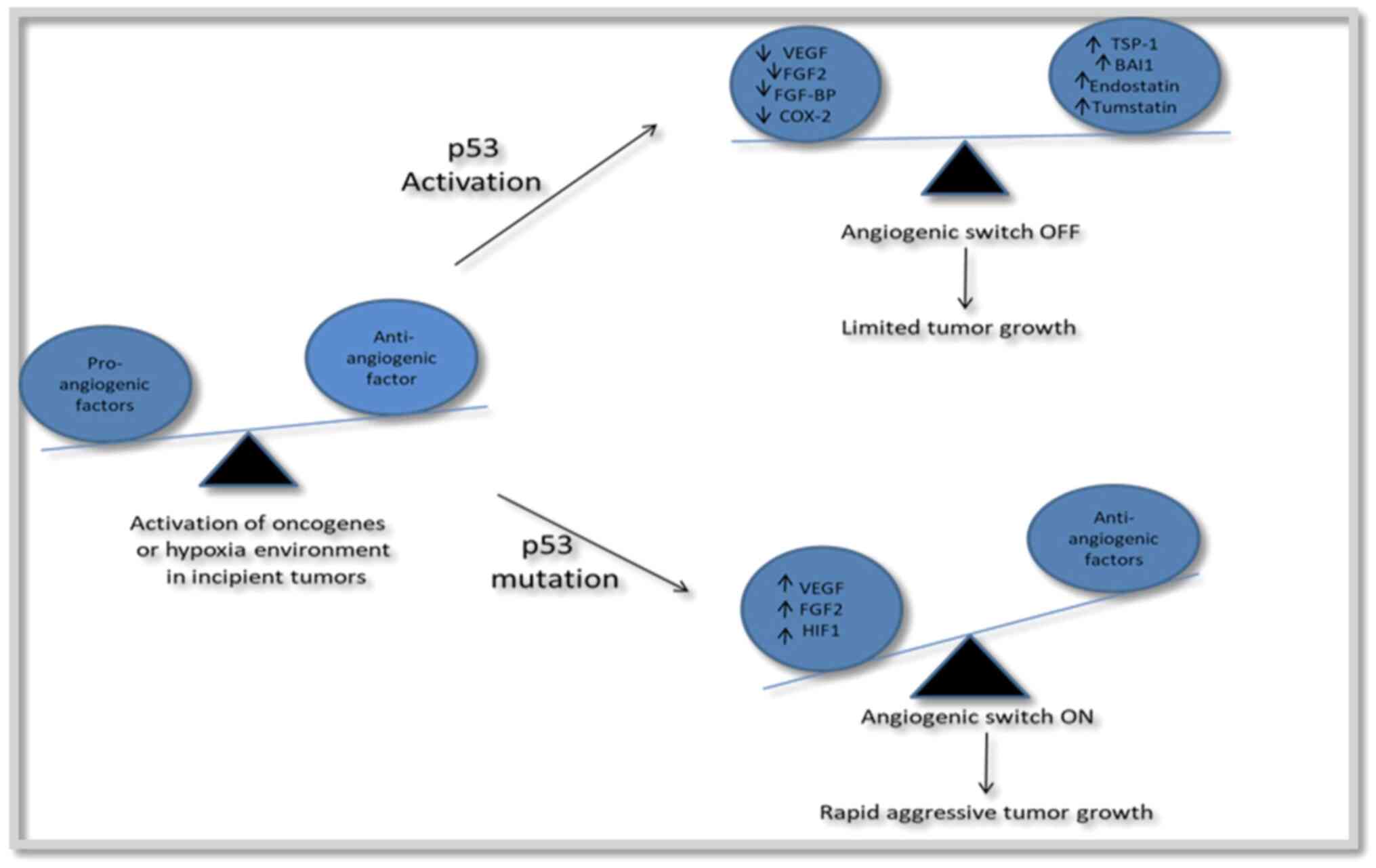
Angiogenesis. Angiogenesis, or the formation
of new blood vessels, is required for ocular tumor growth and
spread (44). The ‘angiogenic
switch’ enhances tumor vascularization when pro-angiogenic factors
exceed anti-angiogenic factors. Tumor blood vessels have a
different structure, permeability and maturation compared with
normal vessels (45). VEGF is a
key component of ocular angiogenesis (44). Anti-angiogenic medicines,
particularly anti-VEGF drugs, have shown promising results in the
treatment of ocular malignancies as they inhibit growth, reduce the
risk of metastasis and improve the effects of radiation therapy
(44,46). These therapies are being tested for
retinoblastoma, von Hippel Lindau disease and uveal melanoma, among
other ocular tumors (46)
(Fig. 4).
4. Mechanisms of oncogene activation
Epigenetic modifications
This type of mutation occurs in uveal melanoma. It
includes DNA methylation, histone modification and microRNAs
(miRNAs/miRs) (47).
DNA methylation. DNA methyltransferase
(DNMT)3A mediates the insertion or removal of a methyl group from a
DNA sequence. Transferases are responsible for writing DNA
methylation and are used as therapeutic targets to treat various
types of cancer (48,49).
i) The Ras association domain family 1 isoform A
(RASSF1A) gene, located on chromosome 3p21.3, significantly affects
the development and spread of uveal melanoma tumors when it is
absent or inactivated. It is essential for the control of the cell
cycle, apoptosis, and microtubule stability. RASSF1A methylation
may play a role in uveal melanoma carcinogenesis (3).
ii) Preferentially expressed antigen in melanoma
(PRAME) can occur regardless of the cancer progression stage. In
the instance of uveal melanoma, the hypo-methylation of locations
near the PRAME promoter has been demonstrated to promote PRAME
activation, with a concomitant increase in the risk of metastasis
(47-49).
c) DNMT1 and DNMT3B are key players in the
suppression of P161NK4A (also known as CDKN2A). It has been
demonstrated that P161NK4A and P14ARF gene epigenetic modifications
were frequently linked to uveal melanoma in addition to cutaneous
conditions (3).
d) The methylation of the telomerase reverse
transcriptase (TERT) promoter falls under uveal melanoma. The
oncogene human TERT has been shown to be overexpressed in uveal
melanoma (50).
Histone modification- Lysine residues of
histone tails are modified by enzymes including histone
acetyltransferase (HAT), histone deacetylase (HDAC) and histone
methyltransferase via acetylation, deacetylation, methylation,
ubiquitination and phosphorylation. Research indicates that the
loss of BAP1 has been linked to histone modification. Through the
hyperubiquitination of histone H2A, the depletion of the tumor
suppressor gene, BAP1, causes cancer cells to lose their ability to
differentiate. It is associated with other malignancies, as well as
an increased risk of developing uveal melanoma (51,52).
miRNAs. Non-coding RNAs known as miRNAs play
a regulatory role in the body. They regulate gene expression in
both physiological and pathological processes, including cell
proliferation, differentiation, death, organ development,
angiogenesis and extracellular matrix remodeling (53). Among the miRNAs involved in cell
motility and invasion that have been discovered abnormally
expressed in uveal melanoma, 65 were found to be downregulated, 28
were found to be upregulated, and three exhibited a distinct
expression pattern as demonstrated by Venza et al (54). Additionally, miRNAs control
immunological mediators, which can influence uveal melanoma
behavior (3,55) (Table
II).
 | Table IITypes of regulatory genes (52). |
Table II
Types of regulatory genes (52).
| Upregulated | Downregulated |
|---|
| miRNA-92a-3p | miR-137 |
| miRNA-181b | miR-144 |
| miR-20a | miR-145 |
| miR-155 | miR-296-3p |
| miR-454 | miR-23a |
| miR-367 | miR-23a |
| miR-21 | |
Genetic mutations
Mutations are defined as any alterations to the DNA
sequence of a gene. It is caused by changes in DNA replication that
occur during cell division and are exposed to environmental
triggers, such as viruses and mutagens. Eye cancer is caused by two
different types of mutations, namely point mutations and deletion
and insertion mutations (56).
Environmental factors
UV radiation. The mutation spectrum of TP53
has been used as a tool to predict the significance of UV
radiation. BRAF mutagenesis results from UV-related mechanisms,
including UVA photoreactions or error-prone translesion DNA
synthesis following non-specific mutations from UV-induced
oxidative stress (57). It has
been suggested that UV-induced DNA damage may play a role in the
formation of retinoblastoma (58).
Viral infections. One of the elements that
affects the eyes and can lead to cancer development is viral
infection. As an example, HIV specifically infects helper T-cells,
which results in apoptosis. Increased transmission to target cells
amplifies the carcinogenic effects of other viruses, including
Epstein-Barr virus, Kaposi sarcoma-associated herpes virus and
human papillomavirus (59,60).
5. Diagnostic approaches
Molecular profiling of ocular
tumors
Molecular profiling involves the use of various
technologies to understand the distinctive characteristics that are
found in cancer cells. It identifies the specific DNA, RNA, or
protein molecule that is associated with the disorder. There are
several examples of molecular profiling technologies are as
follows:
Next-generation sequencing (NGS). NGS is a
technology that enables the simultaneous sequencing of millions of
DNA or RNA sequences. It is sometimes referred to as massively
parallel sequencing or high-throughput sequencing. Second and
third-generation sequencing technologies have been developed as a
result of the rapid evolution of NGS technologies. Three steps are
involved in all sequencing technology types (52): i) Template preparation, which
involves extracting nucleic acids; ii) clonal amplification as part
of the library preparation process; and iii) short read alignment
and sequencing.
Additional research reveals that short sequence
reads that result in sequence gaps, PCR artifacts and pseudogenes
are only a few of the disadvantages associated with
second-generation sequencing technologies. Single-molecule
sequencing-based third-generation sequencing methods were
introduced to overcome over these restrictions (61).
This technique has been employed for germline RB1
detection in the majority of studies on NGS in retinoblastoma
(51). Furthermore, a pathogenic
mutation that is undetectable even by NSG may exist in the gene
promoter of RB1, non-coding region, or deep intronic region
(52). Although retinoblastomas
have not been found to exhibit p53 activity amplification, MDM4
encodes an E3 ubiquitin ligase that functions as a negative
regulator of p53 activity. The Rb gene has been extensively
studied, and the overexpression of this enzyme is expected to
activate the MAP kinase signaling pathway (51). Targeted therapy selection was aided
by the identification of several oncogenic driver gene changes by
the NSG-based integrative analysis system (49).
Expression profiling. Gene expression
profiling (GEP), which assesses the expression of several genes in
a tumor, has been proposed as an additional method to categorize
patients into prognostic risk groups in order to determine the risk
of an individual for developing metastasis (62). In addition to clinical and biopsy
data, GEP is a useful tool for classifying patients into low- and
high-risk groups. Gene expression analysis predicts the risk of
metastasis much more accurately than any other prognostic indicator
(48). A predictive strategy for
uveal melanoma has been created utilizing GEP. Tumors have been
categorized using these techniques into two classes as follows:
Class 1 (low risk) and class 2 (high risk) (63). The prognostic capacity of the
31-GEP test to distinguish between class 1 and class 2 CM (62). SF3B1, a gene whose mutation has
been found in late metastasizing tumors, is another gene implicated
in intermediate-risk uveal melanoma. The PRAME and EIF1AX genes
have also been linked to both a higher and lower incidence of
metastasis. Metastasizing uveal melanoma has also been linked to a
decrease in BAP1 expression. The PRAME gene can be used to
determine the percentage of class 1 tumors that result in
metastatic illness. Retinoic acid signaling is inhibited by the
protein PRAME, which inhibits cell division, proliferation and
apoptosis (64).
Biomarkers for oncogene
activation
A biomarker is a quantifiable trait or indicator
that can be utilized in tests or assessments of disease states,
biological processes, or treatment responses. Biomarkers help
forecast the prognosis and the likelihood of metastasis, which can
be employed for diagnostic purposes. Subgroups of biomarkers
include the following: i) Diagnostic; b) prognostic; c)
therapeutic; and d) preventative (65). Numerous biological samples,
including blood, urine, tissue and even genetic material such as
circulating tumor DNA (ctDNA), can contain biomarkers. These may
consist of substances, such as proteins, enzymes, hormones, genetic
material (DNA/RNA), or certain features of cells (66).
ctDNA. Tumor cells release highly fragmented
single or double-stranded DNA into the bloodstream, which is known
as ctDNA. A potent tool for studying the molecular heterogeneity
and clonal divergence of malignancy is the ctDNA assay. Tumor DNA
(ctDNA) in the circulation function as a substitute DNA source for
genomic research (67). It has
been suggested that ctDNA in retinoblastoma originates from the
aqueous and vitreous humor of the eye (68). When tumor cells undergo apoptosis,
or necrosis, or are actively secreted from their bodies, ctDNA is
formed. In metastatic uveal melanoma, ctDNA has been proven to be a
superior marker of survival prediction (69). Mutations that are mutually
exclusive and recurrent in GNA11, GNAQ, PLCβ, or CYSLTR2 are
observed in patients with uveal melanoma (70). Early-stage melanomas typically do
not exhibit any detectable ctDNA. The later stages of the disease
are linked to an immunotherapy response when BRAF and NRAS
mutations are present (69).
Within 8 weeks of commencing treatment, ctDNA in cutaneous melanoma
also exhibits a response to immunotherapy and combined BRAF and MEK
inhibition (69).
6. Therapeutic implications
Targeted therapies
Advanced-stage eye cancer may occasionally be
treated with targeted therapy. Drugs used in targeted therapy aim
to target molecules in cancer cells (71). Standard chemotherapy medications do
not function in the same way as targeted drugs. These drugs are
used in cases of chemoresistance and severe side-effects associated
with chemotherapy. Various types of therapies are described
below:
Surgery. Surgery for ocular melanoma may
involve removing only the tumors, removing parts of the eye, or
removing the entire eye in the event that it is severely damaged by
the tumors (72). Surgical options
include the following: i) Iridectomy (part of the iris is removed);
ii) iridocylectomy (removal of the iris and ciliary body); iii)
endoresection (removal of the choroidal tumor); iv) enucleation
(removal of the entire eye); v) orbital exenteration (eye and some
tissues are removed).
Radiation therapy. High-energy radiation is
used in radiation therapy to destroy cancer cells. It is currently
frequently selected over enucleation surgery for melanomas of the
right size and location for treating uveal melanoma (73). Proton beam radiation therapy,
stereotactic radiation therapy and plaque radiation therapy (also
known as plaque brachytherapy) are the three primary forms of
radiation therapy (74).
Laser treatment (transpupillary thermotherapy or
photodynamic therapy). The actions of photodynamic therapy are
is based on the selective elimination of cancer cells or diseased
arteries. The photodynamic therapy method employs light to activate
photosensitizers, which produce reactive oxygen species that
destroy cells (75). Photodynamic
therapy reduces harm to normal cells due to its unique methodology.
It is a widely utilized method for treating a variety of eye
diseases, including uveal melanoma (76).
Combination therapy
It has been demonstrated that epigenetic
medications, such as DNMT inhibitors, HDAC inhibitors, histone
methyltransferase inhibitors, such as enhancers of zeste homolog 2
inhibitors, and modifiers of miRNA expression, such as antagomirs,
can reduce the resistance of tumor cells to cytotoxic T-cells and
natural killer cells, while also improving the activity of
antigen-presenting cells (77). In
order to increase the effectiveness of cancer treatment, more focus
is currently being paid to recently developed combination
therapeutic techniques that use new molecular inhibitors or
epigenetic medicines in addition to other therapies (78).
Immunotherapy
A new, exciting area of cancer drug development is
immunotherapy. Immunotherapies aid in boosting the immune system to
invade cancer cells. Cytokines, monoclonal antibodies, cancer
vaccines and various other immunotherapies are considered highly
promising treatments for uveal melanoma (79). Among these, immune checkpoint
inhibitors and adoptive cell therapies stand out as notable
immunotherapy strategies.
Immune checkpoint inhibitors. Immune
checkpoint inhibitors are used to boost the immune response of the
body. It is helpful in the treatment of eye melanomas. It exhibits
great effect in advanced-stage cutaneous melanoma. However, it
exhibits minimal efficacy in metastatic uveal melanoma. Bispecific
T-cell engagers (BiTEs) are drugs that link T-cells to melanoma
cells, boosting the immunological response of the body. Tebentafusp
is one example of this type of medication (80).
Adoptive cell therapy (ACT). Treatment for
metastatic melanoma, particularly ocular forms, has shown promise
using ACT. Although tumor-infiltrating lymphocytes (TILs) have been
effectively employed for ACT, their restricted availability has
prompted the creation of other strategies (81). The technique that has demonstrated
efficacy in patients with cutaneous and ocular melanoma, is the
ex vivo growth of circulating antitumor T-cells from
peripheral blood mononuclear cells. A phase 2 trial has shown that
the adoptive transfer of autologous TILs can mediate remission of
metastatic uveal melanoma (82).
ACT is a highly tailored cancer treatment that targets somatic
mutations unique to each tumor, resulting in long-term, entire
regressions for patients with melanoma. The efficacy of ACT in the
treatment of cancer has been increased further by the ability to
genetically engineer lymphocytes (83).
7. Future directions
It appears that a wide range of medications and
treatments are helpful for ocular cancer. It is not required for
every treatment to be fully effective for each case of ocular
cancer. While some tumors may only operate locally, others may
specialize or become resistant to the treatments they receive.
Advances in diagnostic technique, such as NGS, expression
profiling, immunotherapy, biomarkers, targeted medicines, etc. have
come across relatively effectively in recent years. These
implications are applied not only to treatment, but also to the
identification of cancer linked to treatment resistance. Although
ocular cancer cannot be avoided, consuming a balanced diet can
reduce the risk of developing the disease.
Resistance is another issue that develops along with
the explosive expansion of therapies. The resistant gene renders
therapies or treatments ineffective. In such scenarios, preventive
medicine loses its significance. The challenges associated with
evaluating blood and tissues for biomarkers can be addressed with
concepts, such as liquid biopsy. Additionally, combination
medicines are employed when targeted therapy is ineffective. Upon
rational screening, certain drug combinations can provide
synergistic effects. In conclusion, knowledge of the role of
oncogenes in the genesis of ocular cancer and their applications in
the treatment of eye cancer is beneficial.
8. Conclusion
In conclusion, the present review aimed to provide a
detailed discussion of the role of oncogenes and summarizes genetic
predispositions, environmental factors and molecular pathways that
lead to the development of ocular cancer. The most prevalent eye
malignancies in children and adults, respectively, uveal melanoma
and retinoblastoma, have deepened the knowledge of researchers of
cell cycle mechanisms and cancer biology. Genetic similarities
between conjunctival and cutaneous melanoma, such as mutations in
the BRAF, NRAS and TERT genes, point to the possibility of
immunotherapy and targeted treatments. Early detection and
personalized treatments are the areas that hold vast potential in
terms of improved treatment strategies and efficacy from the
perspectives of patients and physicians. Collaboration among
researchers, physicians and patients may enhance the current
knowledge and management of ocular cancers, improving the prognosis
and standard of living for affected individuals. The combination of
customized medical procedures and cutting-edge genomic technologies
may also significantly improve future therapeutic
possibilities.
Acknowledgements
The authors would like to thank the University
Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur,
India for providing access to internet and e-library
facilities.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RP was involved in the conceptualization of the
study, in the writing of the original draft of the manuscript, and
in the writing, reviewing and editing of the manuscript. AD, KT, PT
and PS were involved in the writing, reviewing and editing of the
manuscript. PKS was involved in the conceptualization of the study,
in the writing of the original draft of the manuscript, in the
writing, reviewing and editing of the manuscript, and in study
supervision. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nandkar SJ and Sonwane BR: A study of
tumours and tumour like lesions in the eye and its Adnexa: Our
experience of 80 cases with review of literature. IP Int J Ocul
Oncol Oculoplasty. 6:1–9. 2020.
|
|
2
|
Wei L, Wang J and Yu W: The haematology
and eye disease. In: Integrative Ophthalmology. Springer,
Singapore, pp179-185, 2020.
|
|
3
|
Baradaran PC, Kozovska Z, Furdova A and
Smolkova B: Targeting epigenetic modifications in uveal melanoma.
Int J Mol Sci. 21(5314)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aughton K, Kalirai H and Coupland SE:
MicroRNAs and uveal melanoma: Understanding the diverse role of
these small molecular regulators. Int J Mol Sci.
21(5648)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Harbour JW: Eye cancer: Unique insights
into oncogenesis the Cogan lecture. Invest Ophthalmol Vis Sci.
47:1737–1745. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pe'er J: Ocular surface squamous
neoplasia: Evidence for topical chemotherapy. Int Ophthalmol Clin.
55:9–21. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee GA and Hirst LW: Ocular surface
squamous neoplasia. Surv Ophthalmol. 39:429–450. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–99. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Wang Z: Regulation of cell cycle
progression by growth factor-induced cell signaling. Cells.
10(3327)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kaliki S and Shields CL: Uveal melanoma:
Relatively rare but deadly cancer. Eye (Lond). 31:241–257.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brănișteanu DE, Porumb-Andrese E, Stărică
A, Munteanu AC, Toader MP, Zemba M, Porumb V, Cozmin M, Moraru AD,
Nicolescu AC and Brănișteanu DC: Differences and similarities in
epidemiology and risk factors for cutaneous and uveal melanoma.
Medicina (Kaunas). 59(943)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
O'shea JG: Environmental factors in the
epidemiology and aetiology of malignant tumours of the eye. Clin
Exp Optom. 79:177–185. 1996.
|
|
14
|
Houtzagers LE, Wierenga AP, Ruys AA,
Luyten GP and Jager MJ: Iris colour and the risk of developing
uveal melanoma. Int J Mol Sci. 21(7172)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Holly EA, Aston DA, Ahm DK and Smith AH:
Intraocular melanoma linked to occupations and chemical exposures.
Epidemiology. 7:55–61. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Thompson S, Ritz B, Cockburn M and Heck
JE: Prenatal ambient pesticide exposure and childhood
retinoblastoma. Int J Hyg Environ Health.
245(114025)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Behrens T, Lynge E, Cree I, Lutz JM,
Eriksson M, Guénel P, Merletti F, Morales-Suarez-Varela M, Afonso
N, Stengrevics A, et al: Occupational exposure to
endocrine-disrupting chemicals and the risk of uveal melanoma.
Scand J Work Environ Health. 38:476–483. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lopes FC, Sleiman MG, Sebastian K, Bogucka
R, Jacobs EA and Adamson AS: UV exposure and the risk of cutaneous
melanoma in skin of color: A systematic review. JAMA Dermatol.
157:213–219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chalada M, Ramlogan-Steel CA, Dhungel BP,
Layton CJ and Steel JC: The impact of ultraviolet radiation on the
aetiology and development of uveal melanoma. Cancers (Basel).
13(1700)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tarlan B and Kıratlı H: Uveal melanoma:
Current trends in diagnosis and management. Turk J Ophthalmol.
46:123–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu M, Yavuzyiğitoğlu S, Brosens E, Ramdas
WD and Kiliç E: Rotterdam Ocular Melanoma Study Group (ROMS).
Worldwide incidence of ocular melanoma and correlation with
pigmentation-related risk factors. Invest Ophthalmol Vis Sci.
64(45)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Botezatu A, Iancu IV, Popa O, Plesa A,
Manda D, Huica I, Vladoiu S, Anton G and Badiu C: Mechanisms of
oncogene activation. New Aspects in Molecular and Cellular
Mechanisms of Human Carcinogenesis. Bulgin D (ed). InTechOpen,
p192, 2016.
|
|
23
|
Kontomanolis EN, Koutras A, Syllaios A,
Schizas D, Kalagasidou S, Pagkalos A, Alatzidou D, Kantari P,
Ntounis T and Fasoulakis Z: Basic principles of molecular biology
of cancer cell-Molecular cancer indicators. J BUON. 26:1723–1734.
2021.PubMed/NCBI
|
|
24
|
Salem MS: Pathogenetics. An introductory
review. Egyptian Journal of Medical Human Genetics. 17:1–23.
2016.
|
|
25
|
Li Y, Giovannini S, Wang T, Fang J, Li P,
Shao C, Wang Y, TOR Centre, Shi Y, Candi E, et al: p63: A crucial
player in epithelial stemness regulation. Oncogene. 42:3371–3384.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hong D, Huang HJ, To H, Young LJ, Oro A,
Bookstein R, Lee YH and Lee WH: Structure of the human
retinoblastoma gene. Proc Natl Acad Sci USA. 86:5502–5506.
1989.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park JJ, Diefenbach RJ, Joshua AM, Kefford
RF, Carlino MS and Rizos H: Oncogenic signaling in uveal melanoma.
Pigment Cell Melanoma Res. 31:661–672. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Du W and Pogoriler J: Retinoblastoma
family genes. Oncogene. 25:5190–5200. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lapadula D and Benovic JL: Targeting
oncogenic gαq/11 in uveal melanoma. Cancers (Basel).
13(6195)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Silva-Rodríguez P, Fernández-Díaz D, Bande
M, Pardo M, Loidi L and Blanco-Teijeiro MJ: GNAQ and GNA11 genes: A
comprehensive review on oncogenesis, prognosis and therapeutic
opportunities in uveal melanoma. Cancers (Basel).
14(3066)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ma J, Weng L, Bastian BC and Chen X:
Functional characterization of uveal melanoma oncogenes. Oncogene.
40:806–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Barbagallo C, Stella M, Broggi G, Russo A,
Caltabiano R and Ragusa M: Genetics and RNA regulation of uveal
melanoma. Cancers (Basel). 15(775)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Van Raamsdonk CD, Bezrookove V, Green G,
Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS and Bastian
BC: Frequent somatic mutations of GNAQ in uveal melanoma and blue
naevi. Nature. 457:599–602. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gichuhi S, Ohnuma SI, Sagoo MS and Burton
MJ: Pathophysiology of ocular surface squamous neoplasia. Exp Eye
Res. 129:172–182. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Casciano F, Zauli E, Busin M, Caruso L,
AlMesfer S, Al-Swailem S, Zauli G and Yu AC: State of the art of
pharmacological activators of p53 in ocular malignancies. Cancers
(Basel). 15(3593)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rossi E, Schinzari G, Maiorano BA,
Pagliara MM, Di Stefani A, Bria E, Peris K, Blasi MA and Tortora G:
Conjunctival melanoma: Genetic and epigenetic insights of a
distinct type of melanoma. Int J Mol Sci. 20(5447)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chua V, Lapadula D, Randolph C, Benovic
JL, Wedegaertner PB and Aplin AE: Dysregulated GPCR signaling and
therapeutic options in uveal melanoma. Mol Cancer Res. 15:501–506.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Parinot C and Nandrot EF: A comprehensive
review of mutations in the MERTK proto-oncogene. Adv Exp Med Biol.
854:259–265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Croley CR, Pumarol J, Delgadillo BE, Cook
AC, Day F, Kaceli T, Ward CC, Husain I, Husain A, Banerjee S and
Bishayee A: Signaling pathways driving ocular malignancies and
their targeting by bioactive phytochemicals. Pharmacol Ther.
248(108479)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nie C, Ma H, Gao Y, Li J, Tang Z, Chen Y
and Lu R: RNA sequencing and bioinformatic analysis on
retinoblastoma revealing that cell cycle deregulation is a key
process in retinoblastoma tumorigenesis. Ophthalmologica.
244:51–59. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pardo M, Pineiro A, de la Fuente M, García
A, Prabhakar S, Zitzmann N, Dwek RA, Sánchez-Salorio M, Domínguez F
and Capeans C: Abnormal cell cycle regulation in primary human
uveal melanoma cultures. J Cell Biochem. 93:708–720.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Carella G: Introduction to apoptosis in
ophthalmology. Eur J Ophthalmol. 13 (Suppl 3):S5–S10.
2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tempestini A, Schiavone N, Papucci L,
Witort E, Lapucci A, Cutri M, Donnini M and Capaccioli S: The
mechanisms of apoptosis in biology and medicine: A new focus for
ophthalmology. Eur J Ophthalmol. 13 (Suppl 3):S11–S18.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Macovei L: Angiogenesis and
antiangiogenesis in intraocular tumors. Oftalmologia. 54:24–26.
2010.PubMed/NCBI(In Romanian).
|
|
45
|
Youssef PN, Sheibani N and Albert DM:
Retinal light toxicity. Eye (Lond). 25:1–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rosenblatt MI and Azar DT: Anti-angiogenic
therapy: Prospects for treatment of ocular tumors. Semin
Ophthalmol. 21:151–160. 2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gallenga CE, Franco E, Adamo GG, Violanti
SS, Tassinari P, Tognon M and Perri P: Genetic basis and molecular
mechanisms of uveal melanoma metastasis: A focus on prognosis.
Front Oncol. 12(828112)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sakai A, Tagami M, Kakehashi A,
Katsuyama-Yoshikawa A, Misawa N, Wanibuchi H, Azumi A and Honda S:
Expression, intracellular localization, and mutation of EGFR in
conjunctival squamous cell carcinoma and the association with
prognosis and treatment. PLoS One. 15(e0238120)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhong Y, Xu F, Wu J, Schubert J and Li MM:
Application of next generation sequencing in laboratory medicine.
Ann Lab Med. 41:25–43. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fallico M, Raciti G, Longo A, Reibaldi M,
Bonfiglio V, Russo A, Caltabiano R, Gattuso G, Falzone L and
Avitabile T: Current molecular and clinical insights into uveal
melanoma (Review). Int J Oncol. 58(10)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Francis JH, Richards AL, Mandelker DL,
Berger MF, Walsh MF, Dunkel IJ, Donoghue MT and Abramson DH:
Molecular changes in retinoblastoma beyond RB1: Findings from
next-generation sequencing. Cancers (Basel). 13(149)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Afshar AR, Pekmezci M, Bloomer MM, Cadenas
NJ, Stevers M, Banerjee A, Roy R, Olshen AB, Van Ziffle J, Onodera
C, et al: Next-generation sequencing of retinoblastoma identifies
pathogenic alterations beyond RB1 inactivation that correlate with
aggressive histopathologic features. Ophthalmology. 127:804–813.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li Z, Yu X, Shen J and Jiang Y: MicroRNA
dysregulation in uveal melanoma: A new player enters the game.
Oncotarget. 6:4562–4568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Venza M, Dell'Aversana C, Visalli M,
Altucci L, Teti D and Venza I: Identification of microRNA
expression patterns in cutaneous and uveal melanoma cell lines.
Tumori. 100:e4–e7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Achberger S, Aldrich W, Tubbs R, Crabb JW,
Singh AD and Triozzi PL: Circulating immune cell and microRNA in
patients with uveal melanoma developing metastatic disease. Mol
Immunol. 58:182–186. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Iengar P: An analysis of substitution,
deletion and insertion mutations in cancer genes. Nucleic Acids
Res. 40:6401–6413. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Goh AY, Ramlogan-Steel CA, Jenkins KS,
Steel JC and Layton CJ: Presence and prevalence of UV related
genetic mutations in uveal melanoma: Similarities with cutaneous
melanoma. Neoplasma. 67:958–971. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Soliman SE, Racher H, Zhang C, MacDonald H
and Gallie BL: Genetics and molecular diagnostics in
retinoblastoma-an update. Asia Pac J Ophthalmol (Phila). 6:197–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Di Girolamo N: Association of human
papilloma virus with pterygia and ocular-surface squamous
neoplasia. Eye (Lond). 26:202–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Alvarez OP, Monroy D, Thura S, Antonietti
M, Fasih-Ahmad S, Sepulveda-Beltran PA, Culbertson S, Dubovy SR,
Galor A and Karp CL: Infectious etiologies of conjunctival tumors.
Expert Rev Ophthalmol. 11:1–6. 2024.
|
|
61
|
Beasley AB, Chen FK, Isaacs TW and Gray
ES: Future perspectives of uveal melanoma blood based biomarkers.
Br J Cancer. 126:1511–1528. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Grossman D, Okwundu N, Bartlett EK,
Marchetti MA, Othus M, Coit DG, Hartman RI, Leachman SA, Berry EG,
Korde L, et al: Prognostic gene expression profiling in cutaneous
melanoma: Identifying the knowledge gaps and assessing the clinical
benefit. JAMA Dermatol. 156:1004–1011. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Aaberg TM, Covington KR, Tsai T, Shildkrot
Y, Plasseraud KM, Alsina KM, Oelschlager KM and Monzon FA: Gene
expression profiling in uveal melanoma: Five-year prospective
outcomes and meta-analysis. Ocul Oncol Pathol. 6:360–367.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Napolitano G, Tagliaferri D, Fusco S,
Cirillo C, De Martino I, Addeo M, Mazzone P, Russo NA, Natale F,
Cardoso MC, et al: A novel member of Prame family, Gm12794c,
counteracts retinoic acid differentiation through the
methyltransferase activity of PRC2. Cell Death Differ. 27:345–362.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Guan X, Pavani KC, Chunduru J, Broeckx BJ,
Van Soom A and Peelman L: Hsa-miR-665 is a promising biomarker in
cancer prognosis. Cancers (Basel). 15(4915)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rodríguez MF, Marta BF, Baameiro NL,
Santiago-Varela M, Silva-Rodríguez P, Blanco-Teijeiro MJ, Perez MP
and Ces AP: Blood biomarkers of uveal melanoma: Current
perspectives. Clin Ophthalmol. 20:157–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bustamante P, Tsering T, Coblentz J,
Mastromonaco C, Abdouh M, Fonseca C, Proença RP, Blanchard N, Dugé
CL, Andujar RA, et al: Circulating tumor DNA tracking through
driver mutations as a liquid biopsy-based biomarker for uveal
melanoma. J Exp Clin Cancer Res. 40(196)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Park JJ, Diefenbach RJ, Byrne N, Long GV,
Scolyer RA, Gray ES, Carlino MS and Rizos H: Circulating tumor DNA
reflects uveal melanoma responses to protein kinase C inhibition.
Cancers (Basel). 13(1740)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bidard FC, Madic J, Mariani P,
Piperno-Neumann S, Rampanou A, Servois V, Cassoux N, Desjardins L,
Milder M, Vaucher I, et al: Detection rate and prognostic value of
circulating tumor cells and circulating tumor DNA in metastatic
uveal melanoma. Int J Cancer. 134:1207–1213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Moore AR, Ceraudo E, Sher JJ, Guan Y,
Shoushtari AN, Chang MT, Zhang JQ, Walczak EG, Kazmi MA, Taylor BS,
et al: Recurrent activating mutations of G-protein-coupled receptor
CYSLTR2 in uveal melanoma. Nat Genet. 48:675–680. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Eagle RC Jr: Ocular tumors: Triumphs,
challenges and controversies. Saudi J Ophthalmol. 27:129–132.
2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shields CL, Kels JG and Shields JA:
Melanoma of the eye: Revealing hidden secrets, one at a time. Clin
Dermatol. 33:183–196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Tsotridou E, Loukovitis E, Tsiropoulos GN,
Zapsalis K, Pentara I, Tzima K, Eminidou V and Anogeianakis G:
Radiation treatment methods in uveal melanoma. Med Hypothesis
Discov Innov Ophthalmol. 10:32–42. 2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Finger PT, Rivard MJ, Chaugule SS,
Shanmugam PM, Saakyan S, Krema H, Sagoo MS and Sauerwein WA:
Ophthalmic radiotherapy: Plaques and implants. Surgical ophthalmic
oncology: A collaborative open access reference 2019:147-58.
|
|
75
|
Correia JH, Rodrigues JA, Pimenta S, Dong
T and Yang Z: Photodynamic therapy review: Principles,
photosensitizers, applications, and future directions.
Pharmaceutics. 13(1332)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bilmin K, Synoradzki KJ, Czarnecka AM,
Spałek MJ, Kujawska T, Solnik M, Merks P, Toro MD, Rejdak R and
Fiedorowicz M: New perspectives for eye-sparing treatment
strategies in primary uveal melanoma. Cancer (Basel).
14(134)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Xie Z, Zhou Z, Yang S, Zhang S and Shao B:
Epigenetic regulation and therapeutic targets in the tumor
microenvironment. Mol Biomed. 4(17)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Morel D, Jeffery D, Aspeslagh S, Almouzni
G and Postel-Vinay S: Combining epigenetic drugs with other
therapies for solid tumours-past lessons and future promise. Nat
Rev Clin Oncol. 17:91–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Basile MS, Mazzon E, Fagone P, Longo A,
Russo A, Fallico M, Bonfiglio V, Nicoletti F, Avitabile T and
Reibaldi M: Immunobiology of uveal melanoma: State of the art and
therapeutic targets. Front Oncol. 9(1145)2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hassel JC, Berking C, Forschner A,
Gebhardt C, Heinzerling L, Meier F, Ochsenreither S, Siveke J,
Hauschild A and Schadendorf D: Practical guidelines for the
management of adverse events of the T cell engager bispecific
tebentafusp. Eur J Cancer. 191(112986)2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mazzarella T, Cambiaghi V, Rizzo N, Pilla
L, Parolini D, Orsenigo E, Colucci A, Modorati G, Doglioni C,
Parmiani G and Maccalli C: Ex vivo enrichment of circulating
anti-tumor T cells from both cutaneous and ocular melanoma
patients: clinical implications for adoptive cell transfer therapy.
Cancer Immunol Immunother. 61:1169–1182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chandran SS, Somerville RP, Yang JC,
Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott
D, Paria BC, et al: Treatment of metastatic uveal melanoma with
adoptive transfer of tumour-infiltrating lymphocytes: A
single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol.
18:792–802. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015.PubMed/NCBI View Article : Google Scholar
|