Introduction
Bone is the most common site for the settlement of
spreading of tumor cells in patients with breast cancer. The
treatment of bone metastasis is challenging, since several
complications can occur, thus affecting the lifestyle of patients
(1,2). The early detection of bone metastasis
could improve prevention, diagnosis and treatment. It is worth
noting that screening biomarkers in blood could be a useful
strategy for the early detection of bone metastasis, since this is
a low-cost and non-invasive method. Previous studies have
identified different biomarkers in diverse types of cancer
(3,4). However, the number of studies on the
identification of diagnostic and prediction biomarkers for cancer
metastasis is limited.
C-X-C motif chemokine ligand 12 (CXCL12), a
chemokine also known as stromal cell-derived factor-1 (SDF-1),
plays a crucial role in cancer metastasis (5). Previous studies have demonstrated
that CXCL12 is expressed in several types of cancer, such as
esophageal squamous cell carcinoma (6), epithelial ovarian cancer (7), renal cancer (8,9),
colorectal carcinoma (10) and
breast cancer (11). Furthermore,
another study revealed that CXCL12 was expressed in different sites
of metastasis, including the liver, bones, brain and lungs
(12). The content of CXCL12 has
also been determined in plasma samples from patients with different
types of cancer. The results demonstrated that the CXCL12 plasma
levels were significantly higher in patients with esophageal cancer
(13) and breast cancer (14). By contrast, it has also been
reported that patients with breast cancer exhibit lower plasma
CXCL12 and mRNA expression levels in the invasive tissues compared
with those in the control groups (14).
The carbohydrate antigen 15.3 (CA15.3) is a
glycoprotein with a molecular weight of 300-450 kDa. It is commonly
used as a common tumor marker for the spread of cancer cells to
lymph nodes in patients with metastatic breast cancer (15) and in monitoring adjuvant
chemotherapy following surgery in patients with human epidermal
growth factor receptor 2 (HER-2) negative breast cancer (16). A previous study demonstrated that
CA15.3 was associated with a poor prognosis in patients with breast
cancer (17). Furthermore, it was
previously found that CA15.3 levels were increased in the majority
of patients with metastatic breast cancer (18). However, the levels of CA15.3 have
also been found to be increased in other disorders, such as chronic
renal failure, colitis, and dermatological and liver diseases
(19). Therefore, it was
hypothesized that CA15.3 was not a specific biomarker for breast
cancer.
Emerging evidence has suggested that calcium
(Ca2+) and parathyroid hormone (PTH) play a critical
role in cancer progression by regulating cell proliferation,
migration and cell death-related pathways (20-23).
Bone is the most common site for Ca2+ storage.
Therefore, Ca2+ hemostasis may be affected when tumor
cells spread and settle into bones (24). A previous study demonstrated that
elevated Ca2+ levels were associated with a poor
prognosis of patients (25).
Furthermore, in another study, increased plasma Ca2+
levels in patients with bladder cancer and bone metastasis
displayed a high diagnostic accuracy and they were thus identified
as an independent risk factor for predicting bone metastasis in
these patients (26). PTH, a
critical hormone, regulates the absorption of Ca2+ from
the intestinal and its release from bones (27). In addition, PTH is involved in the
development of skeletal tumors. In previous study using a
preclinical model, the treatment of mice with PTH increased the
number of osteoblasts and tumor colonies in bones (28). There have been several attempts to
identify tumor markers for the diagnosis of different types of
cancer. However, only a limited number of studies have (29-31)
investigated the diagnostic accuracy of tumor markers and predicted
risk factors associated with bone metastasis in patients with
breast cancer.
Therefore, the present study aimed to investigate
the diagnostic accuracy of CXCL12 and CA15.3, and that of the
bone-related biomarkers, Ca2+ and PTH, and their
efficiency in predicting bone metastasis in patients with breast
cancer.
Patients and methods
Study design
In the present case control and cross-sectional
study, a total of 25 subjects were included in the control group,
25 patients were included in group newly diagnosed with breast
cancer, 25 patients were included in the treated group with primary
breast cancer, and 20 patients were included in the group with bone
metastasis. Bone metastasis occurred in patients with breast cancer
at an average of 4 months following diagnosis. The samples from
patients with primary breast cancer and those from patients with
bone metastasis were selected by a physician. Bone metastasis was
diagnosed by a computed tomography (CT) and positron emission
tomography-CT scan. The present study was conducted between
December, 2023 and May, 2024 at the Al-Amal National Hospital for
Cancer Management, Baghdad, Iraq. The breast cancer subtype was
identified from the pathological reports of patients. Additionally,
patients with osteoporosis, liver and kidney diseases, diabetes
mellitus, hypertension, and smoking and alcohol consumption history
were excluded from the study. The protocol of the present study
followed the Declaration of Helsinki and it was approved by College
of Science, Al-Nahrain University, Baghdad, Iraq (approval no.
4225/3/2, November 15, 2023). Informed consent was obtained from
all participants included in the present study.
Biomarker analysis
Peripheral blood samples were collected from
participants in lithium-heparin tubes. Plasma was separated
following blood centrifugation at 492 x g for 15 min at 4˚C and
stored at -20˚C. The CXCL12, CA15.3 and PTH levels were measured
using a sandwich ELISA assay, according to the manufacturer's
instructions (Sunlong Medical™ Human CXCL12/SDF-1 ELISA
kit; cat. no. EL0249Hu; Quick Step Human Carbohydrate Antigen 15-3
ELISA kit; cat. no. QS0383Hu; Quick Step Human Parathyroid Hormone
ELISA kit; cat. no. QS1342Hu; all from Sunlong Biotech Co., Ltd.).
The Ca2+ levels were determined using the
Elabscience® Calcium colorimetric assay kit (cat. no.
E-BC-K103M; Elabscience Biotechnology, Inc.).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8.4.3 software (Dotmatics). Data distributions were
analyzed using a Kolmogorov-Smirnov test. All continuous
parameters, which were not normally distributed, are expressed as
the median value with minimum and maximum values. Statistically
significant differences between continuous non-parametric variables
were assessed using the Kruskal-Wallis test, with Dunn's multiple
comparison post hoc tests among groups. Categorical variables are
presented as numbers and percentages. The significant differences
between categorical variables were determined using Fisher's exact
and Chi-squared tests. Receiver operating characteristic (ROC)
curves were used to evaluate the diagnostic accuracy of each
parameter via determining the area under the curve (AUC),
sensitivity and specificity values. The cut-off value of each
biomarker was calculated using Youden-J index. Multiple logistic
regression analyses were performed to identify the independent risk
factors associated with predicting bone metastasis in patients with
breast cancer. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological characteristics of
the patients
The clinicopathological characteristics of the
subjects in the control and breast cancer groups, including
patients with newly diagnosed with primary breast cancer, treated
patients with primary breast cancer and those with breast cancer
with bone metastasis, are presented in Table I. The subjects in each group were
age-matched (P=0.3046). In addition, no statistically significant
differences were obtained between the two groups in terms of breast
cancer types (P=0.2070). The majority of the patients with primary
breast cancer were of luminal A type (84%), while the remaining 12
and 4% were of HER-2 and the triple-negative breast cancer (TNBC)
subtype, respectively. None of the patients with primary breast
cancer were diagnosed with luminal B subtype. In addition, the
majority of the treated patients with primary breast cancer were of
luminal A subtype (68%), while 24, 4 and 4% of these patients had
the HER-2 type, TNBC and luminal B type breast cancer,
respectively. In the bone metastasis group, 90 and 10% of patients
were of luminal A and TNBC subtype, respectively. No patients with
HER-2 and luminal B breast cancer were enrolled. Furthermore, there
was a statistically significant difference among the different
groups in terms of the patients who underwent surgery and those who
did not (P<0.0001). All patients in the treated primary breast
cancer group underwent surgery, while none of the patients in the
primary breast cancer group underwent surgery. Additionally, the
majority of patients in the bone metastasis group (65%) also
underwent surgery. None of the participants in the control and
patient group had a history of smoking or alcohol abuse (Table I).
 | Table IClinical characteristic and
biochemical parameters of the patients with breast cancer. |
Table I
Clinical characteristic and
biochemical parameters of the patients with breast cancer.
| Variables | Control (n=25) | Patients with
primary breast cancer (n=25) | Treated patients
with primary breast cancer (n=25) | Patients with bone
metastasis (n=20) | P-value |
|---|
| Age (years), median
(min-max) | 48 (35-70) | 45 (24-70) | 50 (36-62) | 51 (43-78) | 0.3046 |
| Breast cancer
types, n (%) | | | | | 0.2070 |
|
Luminal
A | | 21(84) | 17(68) | 18(90) | |
|
Luminal
B | | 0 (0) | 1(4) | 0 (0) | |
|
HER-2 | | 3(12) | 6(24) | 0 (0) | |
|
TNBC | | 1(4) | 1(4) | 2(10) | |
| Surgery | | | | | <0.0001 |
|
Yes, n
(%) | | 0 (0) | 25(100) | 13(65) | |
|
No, n
(%) | | 25(100) | 0 (0) | 7(35) | |
| Smoking | | | | | >0.9999 |
|
Yes, n
(%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
|
No, n
(%) | 25(100) | 25(100) | 25(100) | 20(100) | |
| Alcohol
consumption | | | | | >0.9999 |
|
Yes, n
(%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
|
No, n
(%) | 25(100) | 25(100) | 25(100) | 20(100) | |
| CXCL12 (pg/ml),
median (min-max) | 1,296
(1137.5-637.5) | 1,371
(1179.2-1687.5) | 1,377
(1216.7-2470.8) | 1,485
(1220.8-2579.2) | >0.9999
a>0.9999b 0.0030c |
| CA15.3 (U/ml),
median (min-max) | 15.74
(11.6-27.04) | 14.54
(9.32-23.31) | 16.89
(10.23-57.65) | 21.78
(9.4-75.54) |
>0.9999a 0.0705b 0.0864c |
| Ca2+
(mmol/l), median (min-max) | 1.175
(0.831-1.247) | 1.199
(0.831-1.263) | 1.168
(0.851-1.297) | 1.239
(0.933-1.335) | >0.9999
a>0.9999b 0.0941c |
| PTH (pg/ml), median
(min-max) | 43.3
(30.39-79.52) | 44.27
(30.74-61.57) | 43.17
(27.9-78.01) | 35.09
(30.33-87.64) | >0.9999
0.6490b
0.0318c |
Comparison of biomarkers
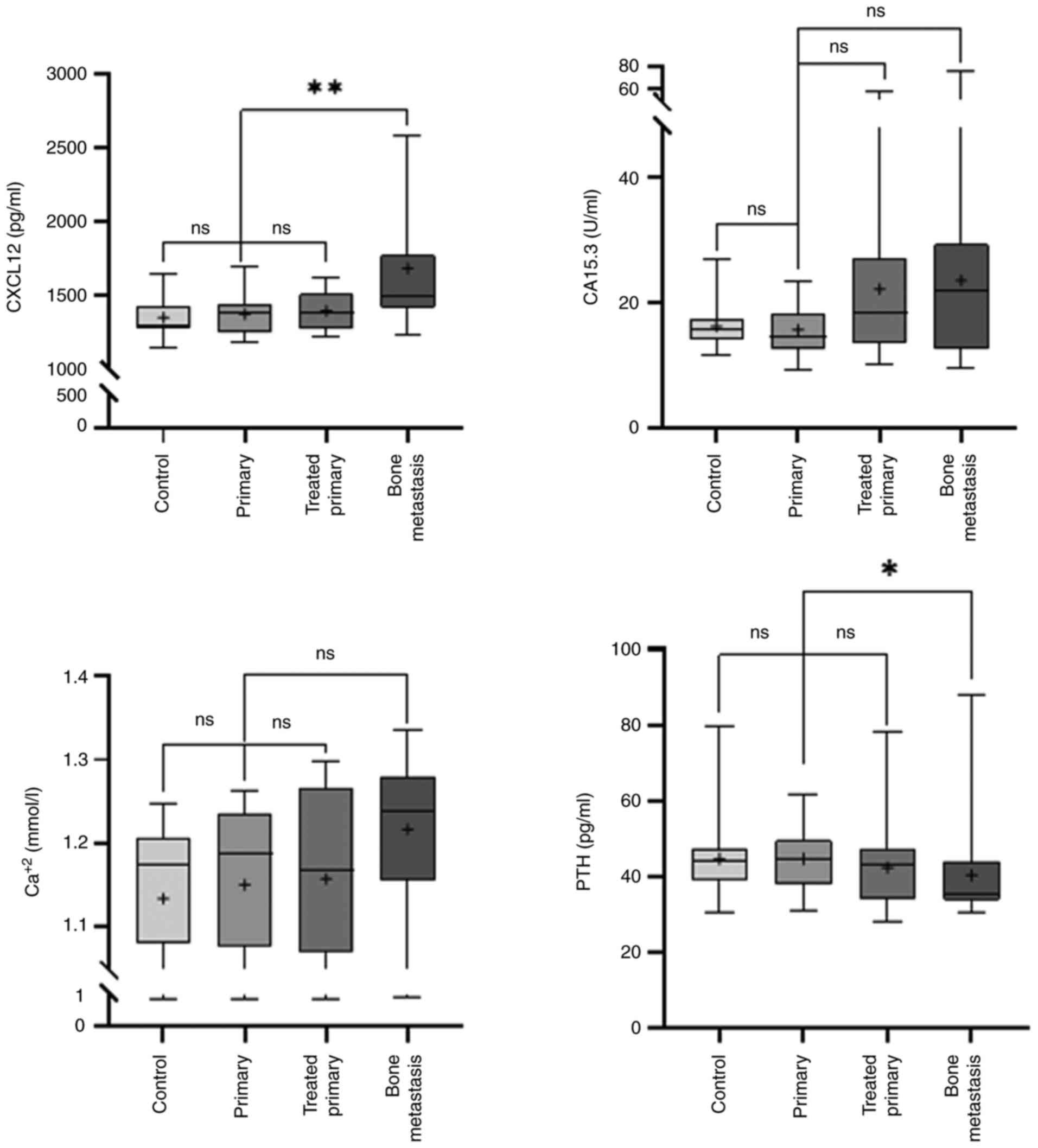
Subsequently, the secretion levels of CXCL12 and
CA15.3, as comparative biomarkers, and those of the bone-related
factors, Ca2+ and PTH, were compared (Table I). Of note, no significant
differences were observed in the CXCL12, CA 15.3, Ca2+
and PTH levels between the control and primary breast cancer groups
(P>0.9999), and primary breast cancer and treated primary breast
cancer groups (P>0.9999, P=0.0705, P>0.9999 and P=0.6490,
respectively). These findings indicated that treatment could not
affect the secretion levels of the candidate biomarkers. Notably,
the CXCL12 levels were significantly increased (P=0.0030) in the
primary breast cancer group compared with the bone metastasis
group. The Ca2+ levels were slightly increased
(P=0.0941), whereas those of PTH were notably decreased (P=0.0318)
in the primary breast cancer group compared with the bone
metastasis group. Finally, the CA15.3 levels were slightly
(P=0.0864) enhanced in the primary breast cancer group compared
with the bone metastasis group (Fig.
1).
Diagnostic accuracy and AUC values of
CXCL12, CA15.3, Ca2+ and PTH
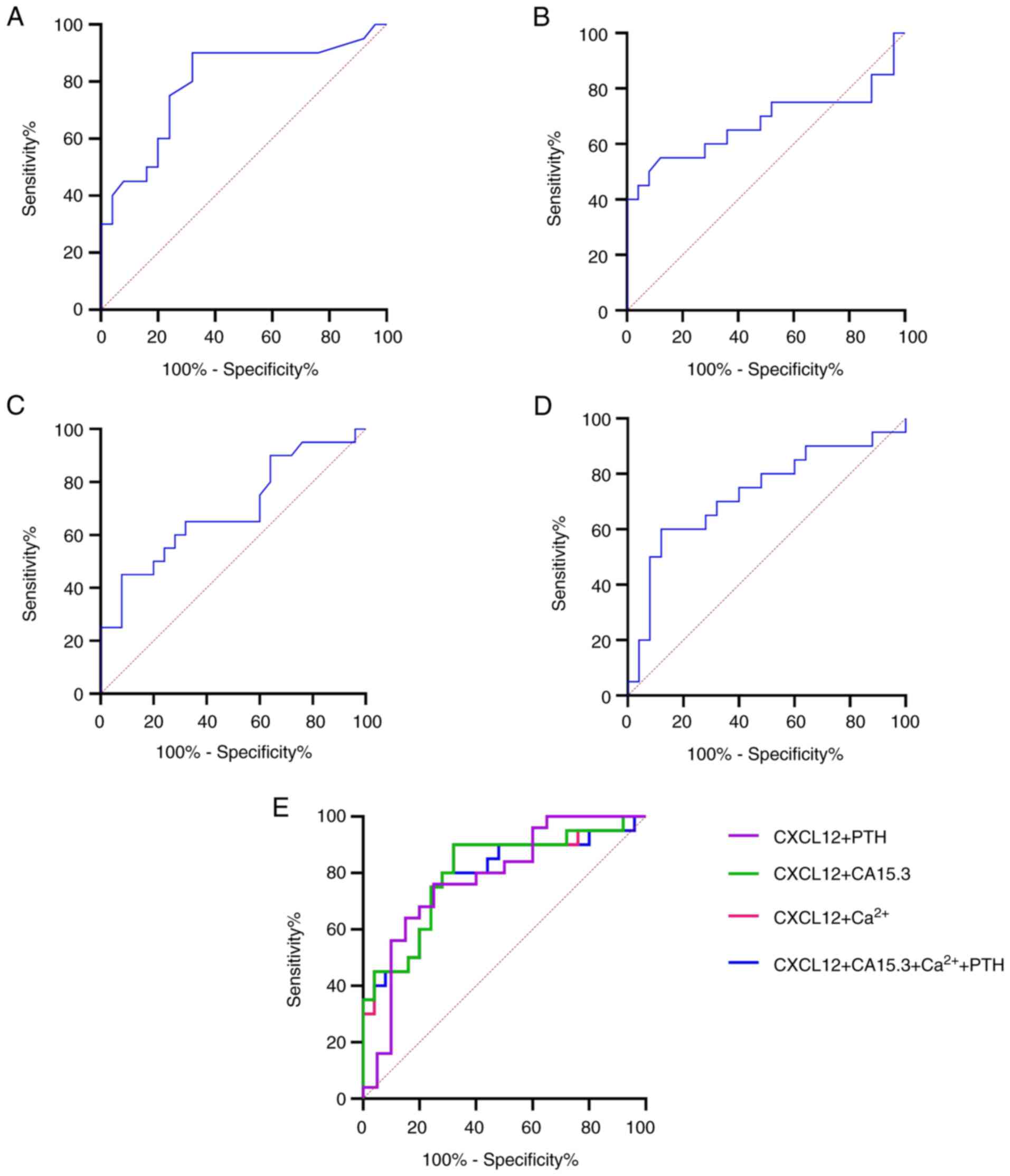
To evaluate the diagnostic reliability of CXCL12 and
CA15.3, as comparative biomarkers, and Ca2+ and PTH, as
bone-related biomarkers in bone metastasis in patients with breast
cancer compared with those with primary breast cancer, the
sensitivity, specificity and cut-off values were determined using
ROC analysis (Table II and
Fig. 2). The results revealed that
CXCL12 (AUC, 0.7940; P=0.0008; cut-off, >1,392 pg/ml) could be a
potential effective biomarker for predicting bone metastasis in
patients with breast cancer compared with CA15.3 (AUC, 0.6579;
P=0.0756; cut-off, >21.50 U/ml). The sensitivity and specificity
percentages for CXCL12 were 90 and 68%, respectively, while those
for CA13.5, 52 and 88%, respectively. In terms of bone-related
biomarkers, Ca2+ could discriminate patients with breast
cancer and bone metastasis from those with primary breast cancer
(AUC, 0.6792; P=0.0427; cut-off, >1.257 U/ml) with a sensitivity
of 45% and specificity of 91.6%. However, PTH (AUC, 0.7280;
P=0.0092; cut-off, <36.70 pg/ml) displayed a higher diagnostic
accuracy for predicting bone metastasis, with a sensitivity of 60%
and specificity of 88%, compared with Ca2+, but reduced
compared with CXCL12. These results indicated that CXCL12 could be
the optimal biomarker for distinguishing patients with breast
cancer and bone metastasis from those with primary breast cancer.
Notably, the diagnostic accuracy was increased after combining
CXCL12 and CA13.5 (Table II and
Fig. 2E; AUC=0.8040; P=0.0005),
with sensitivity and specificity rates of 90 and 68%, respectively,
which were higher compared with those of CXCL12 and CA13.5 alone.
Furthermore, the combination of CXCL12 + PTH and CXCL12 + CA15.3 +
Ca2+ + PTH notably increased the diagnostic accuracy
(AUC, 0.7800; P=0.0014 and AUC, 0.7820; P=0.0013), with a
specificity of 75 and 72%, respectively, for discriminating
patients with breast cancer and bone metastasis from those with
primary breast cancer.
 | Table IIEvaluation of CXCl12, CA15.3,
Ca2+ and PTH as diagnostic biomarkers. |
Table II
Evaluation of CXCl12, CA15.3,
Ca2+ and PTH as diagnostic biomarkers.
| Factors | AUC | Sensitivity
(%) | Specificity
(%) | 95% CI | Cut-off | P-value |
|---|
| CXCL12 (pg/ml) | 0.7940 | 90 | 68 | 0.6552-0.9328 | >1392 | 0.0008 |
| CA 15.3 (U/ml) | 0.6579 | 52 | 88 | 0.4750-0.8408 | >21.50 | 0.0756 |
|
Ca2+(mmol/l) | 0.6792 | 45 | 91.6 | 0.5154-0.8429 | >1.257 | 0.0427 |
| PTH (pg/ml) | 0.7280 | 60 | 88 | 0.5718-0.8842 | <36.70 | 0.0092 |
| CXCL12 +
CA15.3 | 0.8040 | 90 | 68 | 0.6709-.9371 | - | 0.0005 |
| CXCL12 +
Ca2+ | 0.8000 | 90 | 68 | 0.6650-0.9350 | - | 0.0006 |
| CXCL12 + PTH | 0.7800 | 76 | 75 | 0.6383-0.9217 | - | 0.0014 |
| CXCL12 +CA15.3
+Ca2+ + PTH | 0.7820 | 80 | 72 | 0.6415-0.9225 | - | 0.0013 |
CXCL12, CA15.3, Ca2+ and
PTH as independent risk factors of bone metastasis in patients with
breast cancer
To investigate whether CXCL12, CA15.3,
Ca2+ and PTH levels could serve as independent risk
factors for predicting bone metastasis in patients with breast
cancer, multiple logistic regression analysis was carried out
(Table III). The analysis
revealed that CXCL12 [β=0.01531; odds ratio (OR), 1.015; P=0.0261],
CA15.3 (β=0.3920; OR, 1.480; P=0.0226) and PTH (β=-0.4838; OR,
0.6164; P=0.0224), but not Ca2+ levels (β=7.515; OR,
1.835; P=0.1932) could be independent risk factors for predicting
bone metastasis.
 | Table IIIRisk factors for bone metastasis in
breast cancer. |
Table III
Risk factors for bone metastasis in
breast cancer.
| Factor | β | OR | OR (95% CI) | P-value |
|---|
| CXCL12 | 0.01531 | 1.015 | 1.005-1.033 | 0.0261 |
| CA15.3 | 0.3920 | 1.480 | 1.160-2.377 | 0.0226 |
|
Ca2+ | 7.515 | 1835 |
0.1275-3210486883 | 0.1932 |
| PTH | -0.4838 | 0.6164 | 0.3612-0.8378 | 0.0224 |
Discussion
Bone is considered as the most common site of
metastasis in patients with breast cancer. Emerging evidence has
suggested that ~65-70% of patients with breast cancer will
experience metastasis (32,33).
Nowadays, several attempts have been made to develop novel and
effective approaches for diagnosing and predicting the spread of
tumor cells in distant sites. Therefore, researches have focused on
the development of easy-to-apply, non-invasive and low-cost methods
for the early detection of bone metastasis, thus improving
treatment management. Therefore, the present study aimed to
identify tumor markers in the peripheral blood of patients with
breast cancer for the diagnosis and risk assessment of bone
metastasis to assist in its early detection and treatment
decision.
A previous study demonstrated that the mRNA
expression levels of CXCL12 in breast cancer tissues and plasma
content were decreased in patients with invasive breast carcinoma
compared with normal breast tissues and plasma from subjects in the
control group (34). On the other
hand, another study found that the plasma levels of CXCL12 were
significantly higher in diverse stages of breast cancer compared
with the healthy group (14).
However, herein, no significant changes in the CXCL12 levels were
observed between patients with primary breast cancer and control
subjects. This finding could be due to the inclusion of samples
from different types of breast cancer, since 84% of patients were
diagnosed with luminal A breast cancer, 12% with HER-2 breast
cancer and 4% with TNBC. By contrast, in the study by Motyka et
al (34), only patients with
luminal A and B breast cancer were included. Additionally, in the
study by Dabrowska et al (14), the types of breast cancer were not
defined.
Of note, in the present study, the plasma levels of
CXCL12 were significantly increased in patients with breast cancer
and bone metastasis compared with those with primary breast cancer.
In line with the present study, a previous study on patients with
non-small cell lung cancer, the plasma levels of CXCL12 were
notably increased in patients with bone metastasis, thus supporting
the vital role of this chemokine in metastasis (35). Other research has also highlighted
the key role of CXCL12 in intracellular pathways in cancer. A
previous study revealed that CXCL12 upregulation following its
binding to its receptor, CXCR4, stimulated different intracellular
pathways, thus ultimately promoting cell proliferation,
angiogenesis, survival and metastasis (5). Furthermore, in the present study, the
CXCL12 content displayed a high diagnostic accuracy (AUC, 0.7940)
and elevated plasma CXCL12 levels (>1,392 pg/ml) were considered
as a predictive biomarker for the development of bone metastasis in
patients with breast cancer. Additionally, CXCL12 was identified as
an independent risk factor (β=0.01531; P=0.0261) for predicting the
incidence of bone metastasis. To the best of our knowledge, the
present study is the first to investigate CXCL12 as a diagnostic
biomarker and prediction risk factor for patients with breast
cancer and bone metastasis.
In the present study, the CA15.3 levels did not
differ significantly compared with those in the control group. This
result was not in agreement with previous studies, indicating that
the levels of CA15.3 were higher in patients with breast cancer
compared with healthy subjects (14,34).
This finding may be due to the small sample size, which was one of
the limitations of the present study. Previously, it was suggested
that CA15.3 may be a good indicator for bone and liver metastasis
in patients with breast cancer (36,37).
In addition, the CA15.3 levels were enhanced in patients with
breast cancer and bone metastasis compared with those without bone
metastasis (38). The present
study demonstrated that the CA15.3 levels were slightly increased
in patients with bone metastasis compared with those with primary
breast cancer. Furthermore, the diagnostic accuracy of CA13.5 for
predicting bone metastasis was 0.6570, which was the lowest
compared with other candidate biomarkers in the present study.
However, the combination of CXCL12 and CA15.3 improved the
diagnostic accuracy to 0.8040, which was the highest among the
different combinations of biomarkers. This increase in predictive
accuracy following the combination of CXCL12 and CA15.3 was also
reported in previous studies investigating the accuracy of these
biomarkers in predicting breast cancer (14,34).
However, the combination of CXCL12 and CA15.3 in predicting bone
metastasis in patients with breast cancer has not been previously
investigated. Furthermore, increased CXCL12 and CA15.3 levels were
associated with cancer development and spread of tumor cells to
bones. In the present study, no significant differences in plasma
Ca2+ levels were obtained between patients with primary
breast cancer and the control group. However, plasma
Ca2+ level was slightly increased in patients with bone
metastasis compared with those with primary breast cancer. This
finding was consistent with that reported in a previous study,
demonstrating increased serum Ca2+ levels in patients
with bladder cancer and bone metastasis compared with those without
bone metastasis (26). The slight
elevation of Ca2+ levels in the bone metastasis group
could occur due to an imbalance between bone resorption and bone
formation, which could in turn lead to the release of
Ca2+ into the blood stream in bone metastasis (25). In addition, the results of the
present study illustrated that Ca2+ alone displayed a
moderate diagnostic accuracy (AUC, 0.6792; P=0.0427) with a
sensitivity and specificity of 45 of 91.6%, respectively. This
result was consistent with that reported in a previous study on
patients with bladder cancer and bone metastasis (26). Notably, the diagnostic accuracy was
increased (AUC, 0.8000, P=0.0006) after combining Ca2+
and CXCL12. In addition to the diagnostic accuracy, the combination
of Ca2+ and CXCL12 also improved the sensitivity to 90%,
but reduced the specificity to 68%.
A previous study found that elevated plasma PTH
levels were associated with the poor prognosis of patients with
advanced-stage prostate cancer experiencing bone metastasis
(23). Herein, the results were
inconsistent from those reported in the study by Schwartz (23), since plasma PTH levels were reduced
in patients with bone metastasis compared with those with primary
breast cancer. This contradiction in the results may be due to the
different cancer types included in these two studies. Therefore,
further studies are required in breast cancer to verify the results
of the present study. Enhanced Ca2+ levels could
mitigate the secretion levels of PTH in patients with bone
metastasis, since it has been reported that increased
Ca2+ levels can inhibit PTH secretion through a negative
feedback mechanism. Of note, PTH alone displayed a satisfactory
diagnostic accuracy (AUC, 0.7280; P=0.0092), which was higher
compared with that recorded for CA15.3 and Ca2+, but not
for CXCL12. The sensitivity was increased from 60% in PTH alone to
76% in the combination of PTH with CXCL12, while specificity
decreased from 88 to 75%. Nevertheless, the combination of CXCL12 +
CA15.3 + Ca2+ + PTH displayed an improved diagnostic
accuracy (AUC, 0.7820; P=0.0013), which was slightly lower than
that of CXCL12 alone, with a sensitivity and specificity of 80 and
72%, respectively. The combination of CXCL12 + CA15.3 exhibited an
enhanced diagnostic reliability for predicting bone metastasis in
patients with breast cancer compared with other candidate
biomarkers. More importantly, CXCL12, CA15.3 and PTH were
identified as possible independent risk factors for predicting of
bone metastasis. To the best of our knowledge, the present study
was the first to assess this finding.
Although several significant findings were reported
in the present study, there are some limitations that still need to
be addressed. Firstly, the sample size was small in each group.
Secondly, the majority of patients with breast cancer were
diagnosed with the luminal A type. Thirdly, the sensitivity and
specificity of the candidate biomarkers obtained were moderate.
Therefore, improvements are still warranted. Finally, different
tumor stages were not considered in this study. Overall, further
studies with a higher number of patients with different breast
cancer subtypes are required to provide more interesting results,
not only in patients with breast cancer with bone metastasis, but
also in those with liver metastasis. Additionally, different tumor
markers, such as calcitonin and calcitriol, could be assessed to
increase the sensitivity and specificity of the diagnostic
reliability.
Overall, the results of the present study suggested
that elevated plasma CXCL12 levels could be a novel potential
diagnostic biomarker and an independent risk factor for the
prediction of bone metastasis in patients with breast cancer.
CA15.3, Ca2+ and PTH levels also exhibited significant
diagnostic accuracy, but not as high as that obtained for the
combination of CXCL12 and CA15.3. CXCL12, CA15.3 and PTH levels
could be also considered as independent risk factors that could
promote the early detection of bone metastasis in patients with
breast cancer and improve the management of their treatment.
Acknowledgements
The authors would like to thank Dr Yaala Saady Raof,
a physician who works at Al-Amal National Hospital for Cancer
Patients (Baghdad, Iraq) and diagnosed the patients and provided
crucial advice throughout the study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BAA designed the concept of the study, carried out
the sample collection and performed the experiments. FAR supervised
the whole study, interpreted and statistically analyzed the data,
and wrote and edited the manuscript. BAA and FAR confirm the
authenticity of all the raw data. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocol of the present study followed the
Declaration of Helsinki and it was approved by the College of
Science, Al-Nahrain University, Baghdad, Iraq (approval no.
4225/3/2, November 15, 2023). Informed consent was obtained from
all participants included in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang H, Zuh W, Biskup E, Yang W, Yang Z,
Wang H, Qiu X, Zhang C and Hu G and Hu G: Incidence, risk factors
and prognostic characteristics of bone metastases and
skeletal-related events (SREs) in breast cancer patients: A
systematic review of the real world data. J Bone Oncol. 11:38–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243S–6249S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gambardella C, Mongardini FM, Paolicelli
M, Bentivoglio D, Cozzolino G, Ruggiero R, Pizza A, Tolone S, Del
Genio G, Parisi S, et al: Role of inflammatory biomarkers (NLR,
LMR, PLR) in the prognostication of malignancy in indeterminate
thyroid nodules. Int J Mol Sci. 24(6466)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tumer AA and Rashid FA: The relationship
between circulating irisin and oxidative stress in gastric and
colorectal cancer patients. Asian Pac J Cancer Prev. 23:2649–2654.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang J, Loberg R and Taichman RS: The
pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis.
Cancer Metastasis Rev. 25:573–587. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sasaki K, Natsugoe S, Ishigami S,
Matsumoto M, Okumura H, Setoyama T, Uchikado Y, Kita Y, Tamotsu K,
Hanazono K, et al: Expression of CXCL12 and its receptor CXCR4 in
esophageal squamous cell carcinoma. Oncol Rep. 21:65–71.
2009.PubMed/NCBI
|
|
7
|
Machelon V, Gaudin F, Camilleri-Broët S,
Nasreddine S, Bouchet-Delbos L, Pujade-Lauraine E, Alexandre J,
Gladieff L, Arenzana-Seisdedos F, Emilie D, et al: CXCL12
expression by healthy and malignant ovarian epithelial cells. BMC
Cancer. 11(97)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schrader AJ, Lechner O, Templin M, Dittmar
KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T,
Gatzlaff P, et al: CXCR4/CXCL12 expression and signalling in kidney
cancer. Br J Cancer. 86:1250–1256. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Potić Floranović M, Ristić Petrović A,
Veličković F and Janković Veličković L: Expression and prognostic
value of CXCL12/CXCR4/CXCR7 axis in clear cell renal cell
carcinoma. Clin Exp Nephrol. 25:1057–1069. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mousavi A, Hashemzadeh S, Bahrami T,
Estiar MA, Feizi MAH, Pouladi N, Rostamizadeh L and Sakhinia E:
Expression patterns of CXCL12 and its receptor in colorectal
carcinoma. Clin Lab. 64:871–876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S,
Jin Q, Li B, Yao F and Jin F: CXCL12-CXCR4 axis promotes the
natural selection of breast cancer cell metastasis. Tumour Biol.
35:7765–7773. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Łukaszewicz-Zając M, Mroczko B, Kozłowski
M and Szmitkowski M: The serum concentrations of chemokine CXCL12
and its specific receptor CXCR4 in patients with esophageal cancer.
Dis Markers. 2016(7963895)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dabrowska E, Przylipiak A, Zajkowska M,
Piskor BM, Sidorkiewicz I, Szmitkowski M and Lawicki S: Possible
diagnostic application of CXCL12 and CXCR4 as tumor markers in
breast cancer patients. Anticancer Res. 40:3221–3229.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fejzić H, Mujagić S, Azabagić S and Burina
M: Tumor marker CA 15-3 in breast cancer patients. Acta Med Acad.
44:39–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Zhao J, Wang Y, Cai W, Zhang X,
Li K, Liu W, Zhao Y and Kang H: Changes of tumor markers in
patients with breast cancer during postoperative adjuvant
chemotherapy. Dis Markers. 2022(7739777)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chu WG and Ryu DW: Clinical significance
of serum CA15-3 as a prognostic parameter during follow-up periods
in patients with breast cancer. Ann Surg Treat Res. 90:57–63.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yerushalmi R, Tyldesley S, Kennecke H,
Speers C, Woods R, Knight B and Gelmon KA: Tumor markers in
metastatic breast cancer subtypes: Frequency of elevation and
correlation with outcome. Ann Oncol. 23:338–345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sturgeon CM, Lai CL and Duffy MJ: Serum
tumour markers: How to order and interpret them. BMJ.
339(b3527)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prevarskaya N, Ouadid-Ahidouch H, Skryma R
and Shuba Y: Remodelling of Ca2+ transport in cancer: How it
contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol
Sci. 369(20130097)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Monteith GR, Davis FM and Roberts-Thomson
SJ: Calcium channels and pumps in cancer: Changes and consequences.
J Biol Chem. 287:31666–31673. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Monteith GR, McAndrew D, Faddy HM and
Roberts-Thomson SJ: Calcium and cancer: Targeting Ca2+ transport.
Nat Rev Cancer. 7:519–530. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Schwartz GG: Prostate cancer, serum
parathyroid hormone, and the progression of skeletal metastases.
Cancer Epidemiol Biomarkers Prev. 17:478–483. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Z, Yue Z, Ma X and Xu Z: Calcium
homeostasis: A potential vicious cycle of bone metastasis in breast
cancers. Front Oncol. 10(293)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Almuradova E and Cicin I: Cancer-related
hypercalcemia and potential treatments. Front Endocrinol.
14(1039490)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang P, Lan M, Peng AF, Yu QF, Chen WZ,
Liu ZL, Liu JM and Huang SH: Serum calcium, alkaline phosphotase
and hemoglobin as risk factors for bone metastases in bladder
cancer. PLoS One. 12(e0183835)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Potts JT and Gardella TJ: Chapter
6-Parathyroid Hormone and Calcium Homeostasis, in Pediatric Bone.
(Second Edition), Glorieux FH, Pettifor JM, and Jüppner H: Academic
Press, San Diego. pp109-140, 2012.
|
|
28
|
Brown HK, Allocca G, Ottewell PD, Wang N,
Brown NJ, Croucher PI, Eaton CL and Holen I: Parathyroid hormone
(PTH) increases skeletal tumour growth and alters tumour
distribution in an in vivo model of breast cancer. Int J Mol Sci.
19(2920)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zissimopoulos A, Stellos K, Matthaios D,
Petrakis G, Parmenopoulou V, Babatsikou F, Matthaiou E,
Theodosiadou E, Hountis P and Koutis C: Type I collagen biomarkers
in the diagnosis of bone metastases in breast cancer, lung cancer,
urinary bladder cancer and prostate cancer. Comparison to CEA, CA
15-3, PSA and bone scintigraphy. J BUON. 14:463–472.
2009.PubMed/NCBI
|
|
30
|
Tähtelä R and Thölix E: Serum
concentrations of type I collagen carboxyterminal telopeptide
(ICTP) and type I procollagen carboxy-and aminoterminal propeptides
(PICP, PINP) as biomarkers of metastatic bone disease in breast
cancer. Anticancer Res. 16:2289–2293. 1996.PubMed/NCBI
|
|
31
|
Wang W, Xu X, Tian B, Wang Y, Du L, Sun T,
Shi Y, Zhao X and Jing J: The diagnostic value of serum tumor
biomarkers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast
cancer. Clin Chim Acta. 470:51–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Manders K, van de Poll-Franse LV, Creemers
GJ, Vreugdenhil G, van der Sangen MJ, Nieuwenhuijzen GA, Roumen RM
and Voogd AC: Clinical management of women with metastatic breast
cancer: A descriptive study according to age group. BMC Cancer.
6(179)2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coleman RE, Smith P and Rubens RD:
Clinical course and prognostic factors following bone recurrence
from breast cancer. Br J Cancer. 77:336–340. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Motyka J, Gacuta E, Kicman A, Kulesza M,
Malinowski P and Ławicki S: CXCL12 and CXCR4 as potential early
biomarkers for luminal A and luminal B subtypes of breast cancer.
Cancer Manag Res. 15:573–589. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Neesanun S and Sriuranpong V: Association
of plasma CXCL12 level and bone metastasis in advance stage
non-small cell lung cancer patients in King Chulalongkorn Memorial
Hospital. J Clin Oncol. 33 (15_suppl)(e19099)2015.
|
|
36
|
Turanli S and Cetin A: Prognostic role of
serum cancer antigen 15-3 in breast cancer patients with isolated
bone metastases. Biomarkers. 15:418–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao R and Wang LP: Serological diagnosis
of liver metastasis in patients with breast cancer. Cancer Biol
Med. 9:57–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen WZ, Shen JF, Zhou Y, Chen XY, Liu JM
and Liu ZL: Clinical characteristics and risk factors for
developing bone metastases in patients with breast cancer. Sci Rep.
7(11325)2017.PubMed/NCBI View Article : Google Scholar
|