Introduction
A progressive decline in muscle mass is associated
with ageing. This decrease in muscle mass begins at the age of ~40
years (1). The rate of muscle mass
loss accelerates with age, ranging from 4-5% per decade to as high
as 10% per decade in individuals >70 years of age (2,3).
Sarcopenia, which is defined as a syndrome characterized by a
decline of skeletal muscle plus low muscle strength and/or physical
performance, occurs at a higher prevalence with ageing, leading to
multiple adverse outcomes including falls, fractures, disability,
hospitalization or increased mortality rates (4-8).
Stroke significantly affects skeletal muscle.
Studies have demonstrated that muscle mass loss occurs early
following a stroke, and that the rate of decline is more rapid than
that observed with the normal ageing process (9,10).
In addition to muscle atrophy resulting from neuronal
deafferentation, other mechanisms accounting for the changes in
muscle mass, such as catabolic-anabolic imbalance, systemic
inflammatory activation and local muscle metabolic alterations,
have also been reported (11-13).
Furthermore, inactivity, fatigue, deconditioning and poor
nutrition, which are common in patients following a stroke, also
contribute to sarcopenia (11,13).
Since muscle is a critical organ for daily function, the loss of
muscle mass has a particularly detrimental impact on patients
following a stroke. For example, Jang et al (14) and Matsushita et al (15) found that sarcopenia occurring early
following stroke was associated with a poorer functional
outcome.
Patients following a stroke exhibit an increased
prevalence of sarcopenia when compared to age- and sex-matched
individuals who have not suffered a stroke (16,17).
According to a recent meta-analysis, the pooled prevalence of
sarcopenia in patients with chronic stroke, obtained from three
studies in the USA, Taiwan and Korea, was found to be 33.6 [95%
confidence interval (CI), 16.5-56.4] (18). The prevalence from each country was
16.8, 48.6 and 41.8%, respectively (14,18-20).
The authors of the meta-analysis also reported that there was a
limited number of studies focusing on sarcopenia in the stroke
population (18). Differences in
ethnicity, genetics, diet, lifestyle and living environment play a
role in the development of sarcopenia. Furthermore, since
sarcopenia and obesity can co-exist, the term sarcopenic obesity
(SO) has emerged. SO is characterized as a clinical condition of a
high body fat percentage and low lean mass; this combination is
considered to have a negative synergistic effect on patient
outcomes. SO and sarcopenia should be considered differently, since
individuals with obesity may have a comparable or even higher
absolute skeletal muscle mass due to a higher overall body mass.
The criteria for sarcopenia, which are based on appendicular
skeletal mass divided by height squared, were not considered
suitable for the diagnosis of SO. Instead, the relative reduction
in muscle mass per body mass, as demonstrated by appendicular
skeletal mass per body mass, was considered more appropriate
(21). SO has not yet been studied
in detail in patients with chronic stroke, and the consensus
criteria were only recently launched by the European Society for
Clinical Nutrition and Metabolism (ESPEN) and the European
Association for the Study of Obesity (EASO), in 2022(21). Thus, the aim of the present study
was to examine the prevalence of and predictive factors for
obesity, sarcopenia and SO in patients with chronic stroke in
Thailand.
Patients and methods
Patients and inclusion and exclusion
criteria
The present cross-sectional descriptive study was
conducted in patients with chronic stroke who attended the
Outpatient Rehabilitation Clinic at Srinagarind Hospital (the
university hospital of the Faculty of Medicine, Khon Kaen
University Khon Kaen, Thailand) between October, 2020 and January,
2022. Srinagarind Hospital is a super-tertiary care facility and
the largest university hospital in Northeastern Thailand. The
inclusion criteria were as follows: i) An age ≥18 years; ii)
ischaemic or haemorrhagic stroke with an onset >6 months; iii)
an ability to understand and follow commands; and iv) an ability to
provide consent to participate in the study. The exclusion criteria
were as follows: i) Unstable vital signs; ii) the use of medication
that may affect body mass or composition, e.g. steroids or
diuretics; and iii) having underlying diseases that may affect
walking performance, e.g., chronic joint pain conditions. The
present study was approved by the Khon Kaen University Ethics
Committee for Human Research (Ref. HE631455). The study was
conducted in accordance with local legislation and institutional
requirements. Each patient signed a written consent form prior to
participation.
Clinical assessment
Clinical assessment included body mass, height, body
mass index (BMI), handgrip strength, National Institutes of Health
Stroke Scale (NIHSS), upper and lower extremities Motricity Index
(MI) (22), Modified Rankin Scale
(MRS), Functional Ambulation Category (FAC), maximum calf
circumference, maximal grip strength (the maximum value achieved
from three attempts of forcefully grasping the device using the
Baseline Hydraulic Hand dynamometer), 10-metre walk test (a series
of 10-metre walks, with the average gait speed calculated from the
6 m covered between the 2-metre and 8-metre points divided by the
time to cover this distance), Oral Health Assessment Tool (OHAT),
and nutritional status (Mini Nutritional Assessment-Short Form:
MNA-SF). Cognitive impairment was defined as a score of ≤23 from
the Montreal Cognitive Assessment (23).
Muscle mass was measured using dual-energy X-ray
absorptiometry (DXA). Appendicular skeletal muscle mass measurement
was performed in a standard manner with a Lunar Prodigy DXA scanner
(GE Healthcare; Cytiva). The appendicular skeletal muscle index
(ASMI) was calculated as appendicular lean body mass (kg) divided
by the square of the height (m2).
Definition of obesity, sarcopenia and
SO. Obesity
Obesity was determined based on the BMI, which was
calculated as the body mass divided by the square of height
(kg/m2). Individuals classified as obese were defined
according to the Southeast Asia cut-off point of BMI ≥25
kg/m2 (24).
Sarcopenia. The diagnosis of sarcopenia was
based on the Asian Working Group for Sarcopenia (AWGS) 2019
consensus update. Sarcopenia was defined as a ‘low muscle mass’
plus ‘low hand grip strength’ and/or ‘low physical performance’.
The cut-off values of ASMI were as follows: Low muscle mass <7.0
kg/m2 in males and <5.4 kg/m2 in females
measured using DXA, low hand grip strength <28.0 kg for males
and <18.0 kg for females measured using the handgrip
dynamometer, reduced physical performance using gait speed <1.0
m/sec (25).
SO. SO was diagnosed according to the
European Society for Clinical Nutrition and Metabolism (ESPEN) and
the European Association for the Study of Obesity (EASO) consensus
(21). The diagnosis of SO was
determined if the participant fulfilled the following two criteria:
i) Altered skeletal muscle functional parameters assessed by hand
grip strength; and ii) altered body composition defined as an
increased fat mass (FM%) and reduced muscle mass assessed as
appendicular lean muscle divided by body mass (ALM/BM) (21). As regards FM%, the definition from
the study by Lee et al (26) we used, which identified high FM% in
the Asian population with an age ≥40 years as >25.8% for males
and >36.5% for females; these values represent the two highest
quintiles of the study population. The definition of a reduced
muscle mass based on ALM/BM was defined as <29.1% in males and
<23.0% for females, which are the values representing two
standard deviations (SDs) from the mean of the young reference
group aged 20-39 years (27).
Sample size calculation
The sample size was estimated using a single
population proportion formula, considering the proportion of
patients who had sarcopenia to be 35%. This proportion was obtained
from a previous study conducted in the able-bodied population in
Thailand, with a confidence level of 95% and a margin of error of
10% (28). The required sample
size was then 88 participants.
Statistical analysis
Outcomes were assessed for normality using the
Shapiro-Wilk test. Normally distributed, non-normally distributed,
and categorical data are presented as the mean ± SD, median (25 and
75th percentile; (p25, p75), and number (%), respectively.
Differences in the baseline characteristics were analysed using
one-way ANOVA for continuous variables and Fisher's exact test or a
Chi-squared test for categorical variables. When one-way ANOVA
indicated significant differences, Bonferroni post hoc tests were
performed to assess pairwise comparisons between groups. Univariate
logistic regression analysis was performed to evaluate individual
effect sizes and the statistical significance of each parameter,
reported in crude odds ratio and 95% confidence interval (CI).
Factors included in the model were established factors (age and
sex) (29,30) and factors that were found to have
P-values <0.25(31) (NIHSS,
OHAT, FAC, calf circumference and MNA-SF). BMI, maximal hand grip
and ASMI were not included as variables in the analysis as these
factors were used in the diagnosis of obesity, sarcopenia and SO.
Due to the exploratory nature of the study, a full-model approach
was used; thus, all factors were entered in a multivariable
logistic regression model (32).
The results are reported as adjusted odds ratios and 95% CI values.
A value of P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
Stata (Stata Statistical Software: Release 18. College Station, TX:
Stata Corp LLC).
Results
A total of 88 participants were initially included
in the study; however, following the exclusion of incomplete data,
the final sample consisted of 84 participants (58 men and 26
women). Their average age was 58.3 years. Almost two thirds (64.6%)
of the patients had ischaemic stroke, and more than half of the
patients had left hemiparesis (59.5%). The median duration
following the stroke was 2.2 years and ~14% had suffered from
recurrent strokes.
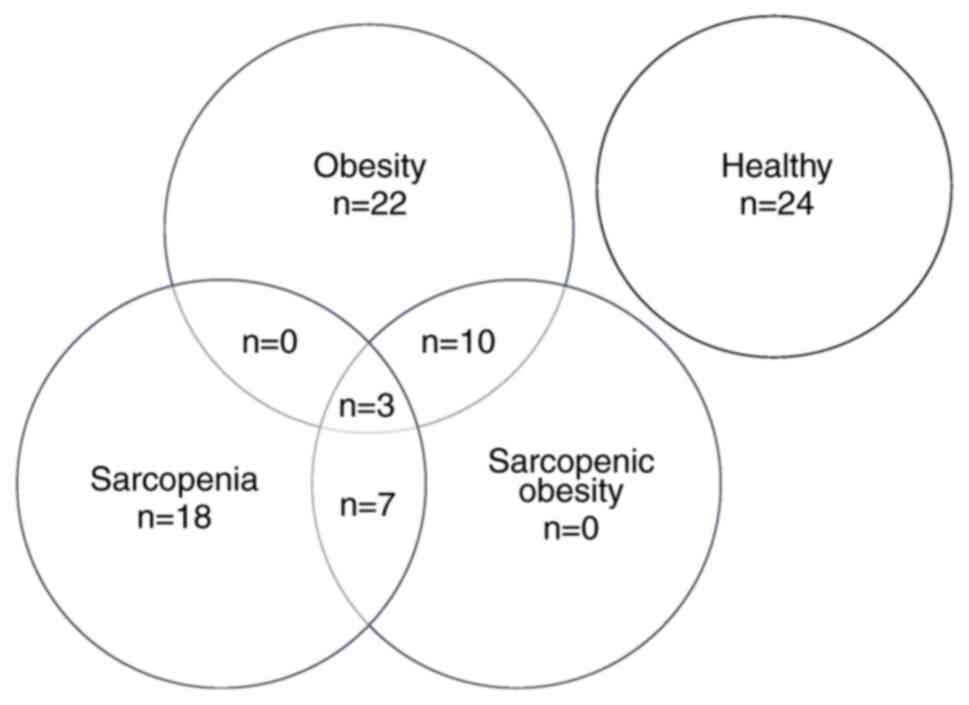
The prevalence of obesity, sarcopenia and SO for the
patients with chronic stroke was 26.2, 21.4 and 23.8%, respectively
(Fig. 1). A total of 7
participants met the criteria for both sarcopenia and SO, 10
participants met the criteria for both obesity and SO and 3
participants met the criteria for obesity, sarcopenia and SO. All
of them were included in the SO group, resulting in a final total
of 20 participants in the SO group (Fig. 1). Males were found to have
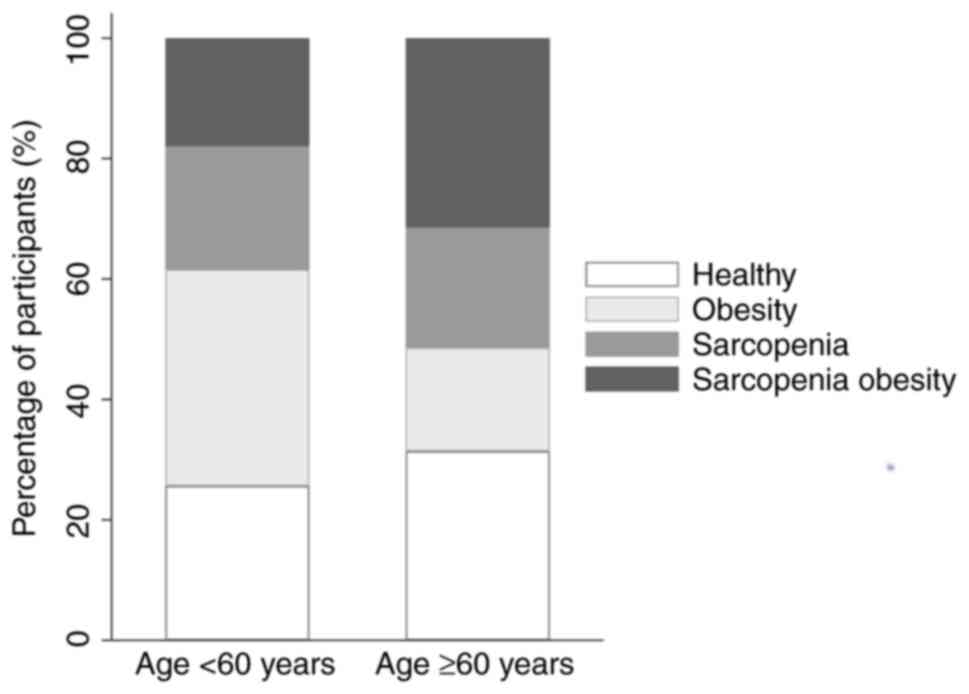
sarcopenia or SO (50%) more often than females (34.6%). The
prevalence of sarcopenia increased with age, with 39.5% of
individuals <60 years old affected, increasing to 51.2% in those
aged ≥60 years (Table I and
Fig. 2).
 | Table IBaseline demographic and clinical
characteristics of the patients with chronic stroke (n=84). |
Table I
Baseline demographic and clinical
characteristics of the patients with chronic stroke (n=84).
| Variables | Healthy (n=24) | Obesity (n=22) | Sarcopenia
(n=18) | Sarcopenic obesity
(n=20) | P-value |
|---|
| Age (years), mean
(SD) | 58.3 (9.3) | 55.0 (10.3) | 59.2 (7.5) | 61.0 (10.3) | 0.24 |
| Sex | | | | | |
|
Male | 13 (54.2) | 16 (72.7) | 14 (77.8) | 15 (75.0) | 0.34 |
|
Female | 11 (45.8) | 6 (27.3) | 4 (22.2) | 5 (25.0) | |
| BMI
(kg/m2), mean (SD) | 23.0 (1.9) | 27.1 (2.0) | 19.9 (2.5) | 26.3 (3.2) | 0.001a,b,c,d,f |
| Smoking | | | | | |
|
Yes | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (5.0) | 0.70 |
|
No | 24(100) | 21 (95.5) | 18(100) | 19 (95.0) | |
| Alcoholic
consumption | | | | | |
|
Yes | 2 (8.3) | 4 (18.2) | 1 (5.6) | 4 (20.0) | 0.48 |
|
No | 22 (91.7) | 18 (81.8) | 17 (94.4) | 16 (80.0) | |
| Educational
level/ | | | | | |
|
≤ Secondary
school | 11 (45.8) | 9 (40.9) | 4 (22.2) | 7 (35.0) | 0.54 |
|
>
Secondary school | 13 (44.2) | 13 (59.1) | 14 (77.8) | 13 (65.0) | |
| Comorbidities | | | | | |
|
Hypertension | | | | | |
|
Yes | 15 (62.5) | 15 (68.2) | 14 (77.8) | 12 (60.0) | 0.68 |
|
No | 9 (37.5) | 7 (31.8) | 4 (22.2) | 8 (40.0) | |
|
Diabetes
mellitus | | | | | |
|
Yes | 8 (33.3) | 8 (36.4) | 8 (44.4) | 7 (35.0) | 0.90 |
|
No | 16 (66.7) | 14 (63.6) | 10 (55.6) | 13 (65.0) | |
|
Dyslipidaemia | | | | | |
|
Yes | 6 (25.0) | 5 (22.7) | 5 (27.8) | 6 (30.0) | 0.95 |
|
No | 18 (75.0) | 17 (77.3) | 13 (72.2) | 14 (70.0) | |
| Charlson
Comorbidity Index (n=75), mean (SD) | 3.7 (1.9) | 3.0 (1.5) | 3.9 (1.6) | 4.1 (2.0) | 0.32 |
| Stroke type | | | | | |
|
Ischaemic | 16 (66.7) | 13 (59.1) | 9 (50.0) | 15 (75.0) | 0.48 |
|
Haemorrhagic | 8 (32.3) | 9 (40.9) | 9 (50.0) | 5 (25.0) | |
| Hemiparetic
side | | | | | |
|
Left | 12 (50.0) | 15 (68.2) | 10 (55.6) | 13 (65.0) | 0.60 |
|
Right | 12 (50.0) | 7 (31.8) | 8 (44.4) | 7 (35.0) | |
| Stroke
recurrence | | | | | |
|
First ever
stroke | 14 (70.8) | 18 (81.8) | 14 (77.8) | 14 (70.0) | 0.78 |
|
Recurrent
stroke | 7 (29.2) | 4 (18.2) | 4 (22.2) | 6 (30.0) | |
| NIHSS, mean
(SD) | 3.3 (2.6) | 4.3 (3.4) | 5.7 (4.4) | 6.7 (5.8) | 0.043 |
| OHAT (n=78), mean
(SD) | 1.2 (1.9) | 1.5 (1.3) | 1.1 (1.8) | 2.3 (1.8) | 0.12 |
| FAC (n=83), mean
(SD) | 3.6 (0.7) | 3.5 (0.8) | 3.1 (1.1) | 2.5 (1.2) | 0.001 |
| History of fall in
previous year | | | | | |
|
Yes | 9 (37.5) | 8 (36.4) | 6 (33.3) | 10 (50.0) | 0.80 |
|
No | 15 (62.5) | 14 (63.6) | 12 (66.7) | 10 (50.0) | |
| Calf circumference
(cm), mean (SD) | 33.8 (3.0) | 36.0 (3.7) | 30.5 (3.4) | 33.7 (3.1) |
<0.001b,d |
| MNA-SF, points,
mean (SD) | 11.2 (2.8) | 12.7 (1.7) | 8.4 (4.0) | 10.8 (2.7) |
<0.001b,d |
| Physical activity
level (SPAQ) (n=80) | | | | | |
| Moderate
(min/week), mean (SD) | 108.4 (195.9) | 84.8 (126.8) | 100.1 (160.1) | 145.3 (261.3) | 0.79 |
| Vigorous
(min/week), mean (SD) | 5.2 (17.5) | 22.6 (70.7) | 14.4 (40.3) | 20.8 (52.5) | 0.64 |
| MoCA (n=75), mean
(SD) | 21.3 (6.0) | 21.9 (4.5) | 19.9 (7.2) | 19.0 (6.4) | 0.46 |
| Maximal hand grip
(n=75) (kg), mean (SD) | 24.7 (9.4) | 30.6 (7.3) | 20.7 (8.3) | 23.5 (9.9) | 0.01d |
| Comfortable gait
speed (n=69) (m/sec), mean (SD) | 0.62 (0.38) | 0.59 (0.36) | 0.46 (0.33) | 0.51 (0.37) | 0.58 |
| ASMI
(kg/m2), mean (SD) | 7.0 (0.8) | 8.1 (0.9) | 5.8 (1.0) | 6.6 (0.9) |
<0.001a,b,d,e,f |
There were significant differences in BMI, NIHSS,
FAC, calf circumference, MNA-SF, maximal hand grip and ASMI among
the groups. Univariate logistic regression analysis revealed that
MNA-SF and calf circumference were associated with obesity.
However, only calf circumference [adjusted odds ratio (aOR), 1.38,
95% CI, 1.08-1.77] was a significant predictive factor for obesity
in multivariable logistic regression analysis.
As regards sarcopenia, two factors were significant
in the both univariate and multivariable logistic regression
analysis: The MNA-SF score (aOR, 0.70; 95% CI, 0.53-0.94), and calf
circumference (aOR, 0.66; 95% CI, 0.49-0.89).
OHAT, NIHSS and FAC were significant factors
associated with SO in the univariate logistic regression analysis.
However, the significant predictive factors for SO in the
multivariable logistic regression analysis were being male (aOR,
7.96; 95% CI, 1.05-60.49) and FAC (aOR, 0.15; 95% CI, 0.04-0.55)
(Table II).
 | Table IIFactors associated with obesity,
sarcopenia and sarcopenic obesity in patients with chronic
stroke. |
Table II
Factors associated with obesity,
sarcopenia and sarcopenic obesity in patients with chronic
stroke.
| Factors | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Obesity | | | | |
|
Age
(years) | 0.95
(0.90-1.00) | 0.069 | 0.99
(0.92-1.07) | 0.79 |
|
Male
sex | 1.27
(0.43-3.73) | 0.66 | 0.48
(0.09-2.44) | 0.38 |
|
MNA-SF
score | 1.57
(1.11-2.20) | 0.01 | 1.44
(0.95-2.18) | 0.086 |
|
OHAT
score | 0.97
(0.73-1.30) | 0.85 | 1.04
(0.67-1.63) | 0.86 |
|
NIHSS
score | 0.95
(0.84-1.09) | 0.47 | 1.09
(0.89-1.34) | 0.39 |
|
FAC
level | 1.41
(0.84-2.40) | 0.19 | 1.04
(0.41-2.62) | 0.93 |
|
Calf
circumference (cm) | 1.31
(1.08-1.59) | 0.006 | 1.38
(1.08-1.77) | 0.009 |
| Sarcopenia | | | | |
|
Age
(years) | 1.01
(0.96-1.07) | 0.65 | 1.06
(0.95-1.18) | 0.33 |
|
Male
sex | 1.75
(0.51-5.95) | 0.37 | 4.28
(0.48-38.20) | 0.19 |
|
MNA-SF
score | 0.75
(0.62-0.89) | 0.001 | 0.70
(0.53-0.94) | 0.017 |
|
OHAT
score | 0.80
(0.56-1.16) | 0.25 | 1.08
(0.64-182) | 0.77 |
|
NIHSS
score | 1.05
(0.94-1.17) | 0.38 | 1.02
(0.80-1.29) | 0.89 |
|
FAC
level | 0.91
(0.54-1.50) | 0.70 | 2.54
(0.72-8.91) | 0.15 |
|
Calf
circumference (cm) | 0.70
(0.56-0.88) | 0.002 | 0.66
(0.49-0.89) | 0.008 |
| Sarcopenic
obesity | | | | |
|
Age
(years) | 1.04
(0.98-1.10) | 0.15 | 1.05
(0.96-1.14) | 0.31 |
|
Male
sex | 1.47
(0.47-4.58) | 0.51 | 7.96
(1.05-60.49) | 0.045 |
|
MNA-SF
score | 0.98
(0.84-1.17) | 0.88 | 1.08
(0.82-1.44) | 0.57 |
|
OHAT
score | 1.40
(1.03-1.88) | 0.027 | 1.11
(0.73-1.68) | 0.63 |
|
NIHSS
score | 1.13
(1.00-1.28) | 0.047 | 0.84
(0.64-1.12) | 0.24 |
|
FAC
level | 0.43
(0.25-0.72) | 0.001 | 0.15
(0.04-0.55) | 0.004 |
|
Calf
circumference (cm) | 0.99
(0.85-1.16) | 0.95 | 0.93
(0.77-1.31) | 0.47 |
Discussion
The aim of the present study was to examine the
prevalence of and predictive factors for obesity, sarcopenia and SO
in patients with chronic stroke in Thailand. It was found that the
prevalence of obesity, sarcopenia and SO for patients with chronic
stroke was 26.2, 21.4 and 23.8%, respectively. Overall, the
prevalence of obesity, when including obesity cases that were
overlapping with SO and sarcopenia, was 41.6%, which was higher
than that in a previous study in Brazil (26.0%), but comparable to
that in a study in Korea (43.5%) (33,34).
It was found that the only predictive factor for obesity was calf
circumference.
Similar to multiple previous studies, males and
older-aged individuals exhibited a higher prevalence of sarcopenia
and SO (35,36). In able-bodied populations, the
prevalence of SO was reported to be less than half of the
prevalence of sarcopenia, although the age of inclusion into the
studies was higher than that in the present study (29,35,36).
Notably, patients with chronic stroke exhibited a similarly high
prevalence of sarcopenia and SO. This may suggest that following a
stroke, patients have a higher gain in body fat compared to the
able-bodied population. This notion is supported by a previous
systematic review demonstrated that fat mass significantly
increased between 6 and 12 months post-stroke with a pooled mean
increase of nearly 2 kg (37).
When focusing only on the diagnosis of sarcopenia,
it was noted that 28 patients fulfilled the criteria for sarcopenia
(10 patients were classified as SO in the present study), leading
to a prevalence of sarcopenia of 33.3%. The prevalence of
sarcopenia in patients following stroke in the present study was
comparable to a previous study on patients with acute stroke in
Japan (32.5%) (38), although the
age of the participants in the present study was lower (median age,
of 58.5 vs. 72 years). The prevalence of sarcopenia was lower in
the present study than that in previous studies in Korea (41.8%)
(14) and Taiwan (48.6%) (19), but higher than that in a study
conducted in the USA (16.8%) (20). The higher prevalence observed in
Korea and Taiwan may be due to the older age of the patients
recruited (age, 64.3±13.0 years) (14) and (64.6±15.1 years) (19), respectively (mean ± SD), compared
to the present study (age, 58.3±9.5 years). The lower prevalence in
the USA may be due to the inclusion criteria of that study, which
were limited to participants with stroke who had mild to moderate
hemiplegic gait deficits and had completed conventional
rehabilitation therapy. Different diagnostic criteria and
participant characteristics may also contribute to the differences
in prevalence. Previous studies in Japan and Korea used similar
criteria to define sarcopenia and found that the prevalence of
sarcopenia in patients with stroke was 39.7 and 53.5% (39,40).
The results of the present study are comparable to the pooled
prevalence estimate of sarcopenia in patients with chronic stroke
(≥6 months) in a recent systematic review and meta-analysis (34%)
(18). The lower prevalence of
sarcopenia in the present study may be attributed to the lower
average age of the participants.
It was found that calf circumference and nutritional
status determined using the MNA-SF score were predictive factors
for sarcopenia. Calf circumference was positively and moderately to
highly associated with appendicular skeletal muscle and skeletal
muscle index; thus, it was recommended as a surrogate marker of
muscle mass for the diagnosis of sarcopenia (41). This finding confirms the importance
of calf circumference and supports earlier studies that used calf
circumference as a screening test itself or as an additional part
of a screening test to increase the diagnostic accuracy for
sarcopenia (41,42). The importance of nutritional status
for sarcopenia, as highlighted in the present study, aligns with
findings from previous studies across various populations (43-47).
Several mechanisms may explain this. A reduced protein intake and
low vitamin D levels are associated with muscle mass loss (48,49).
Malnutrition leads to decreased levels of insulin-like growth
factor-1 and growth factors which are essential for muscle growth
(50). Malnutrition may increase
the production of reactive oxygen and nitrogen species, causing
oxidative stress, which reduces the regenerative potential of
skeletal muscle and leads to muscle dysfunction (51). Moreover, in patients with
disability, such as following a stroke, there may be a possible
inter-association between disability and malnutrition, which leads
to further detrimental effects (52). A recent longitudinal study
demonstrated that malnutrition preceded the onset of sarcopenia
(53). These findings, together
with those of multiple previous studies, highlight the importance
of screening the nutritional status in patients with stroke or
older adults who are at risk of developing sarcopenia, in order to
provide early intervention to prevent the progression of
malnutrition and sarcopenia (52-54).
It was found that MNA-SF was significantly associated with
confirmed and severe sarcopenia and it was noted that the
assessment of sarcopenia in individuals with a MNA-SF score <13
can be beneficial (46).
SO is an emerging clinical condition characterized
by excessive fat mass in the presence of reduced muscle mass
(55). SO produces a double
metabolic burden from low muscle mass and excess adiposity. This
negatively affects the muscle and gives rise to muscle anabolic
resistance, and to deteriorating cardio-metabolic and physical
function (55,56). Formerly, there was no universal
definition of SO. Researchers classify individuals as having SO if
they have appendicular mass fulfilling the criteria of sarcopenia
in the presence of a body mass index in the obese category
(55). SO has a negative effect on
patients following a stroke, as it has been shown to be a
predictive factor for physical limitations, falls and all-cause
mortality (57-59).
Additionally, it has been found to be associated with a lower level
of activities of daily living capability in patients with stroke
(60). Although the prevalence of
SO in participants with stroke was previously reported by
Matsushita et al (60),
that study was published before the standard criteria were
established and it was conducted in a cohort with sub-acute stroke.
Compared to the previous study by Matsushita et al (60), which demonstrated the prevalence of
simple obesity, sarcopenia and SO as 17, 32 and 28%, respectively
in 376 patients with sub-acute stroke, the present study revealed a
lower prevalence of sarcopenia and SO. However, the cut-off point
used herein for high body fat percentage (>25.8% for males and
>36.5% for females) (27) was
lower than the cut-off point used in the previous study (≥27% in
males and ≥38% in females) (60).
This may be due to the older age of their participants, with an
average age of 77.5 years. It should be noted that the criteria for
SO in the study by Matsushita et al (60) required the patient to fulfil the
criteria of sarcopenia and to have a higher cut-off body fat
percentage to define obesity. By contrast, SO, as defined in the
present study, was based on the ESPEN and the European EASO
consensus (21).
Being male and the ambulation status assessed by FAC
were found to be predictive factors for SO in the present study.
Being male increases the probability of developing SO, while a
higher FAC (indicating a better walking ability) decreases the
probability of having SO. The strong association with functional
ability was previously observed in the study by Matsushita et
al (60), which found that SO,
but not sarcopenia or obesity, was significantly associated with
FIM scores in participants with sub-acute stroke. Similarly,
Auyeung et al (57)
demonstrated that SO could predict the incidence of physical
limitations in older women. Furthermore, Broadwin et al
(61) demonstrated that an
increased percentage of fat mass and a decreased percentage of
fat-free mass were significantly associated with decreased
functional ability in both females and males.
A notable strength of the present study is that its
diagnosis of sarcopenia and SO followed the latest AWGS 2019
consensus, the ESPEN and the EASO consensus. However, the present
study had several limitations which should be mentioned. Although
DXA is considered highly accurate, fast and non-invasive, it cannot
delineate intramuscular fat and lean body mass. Thus, the lean body
mass measured from DXA may overestimate the real muscle mass in
patients with stroke (62,63). In addition, the present study was a
single-centre study; thus, the generalizability of the data may be
limited. The cross-sectional design of the study limits the ability
to claim causative associations between each factor and the
outcomes of obesity, sarcopenia and SO. The effect size of
association may have to be interpreted with caution. The sample
size calculation was based on a prevalence study design, which may
result in insufficient power for logistic regression analysis. It
is recommended that the sample size for logistic regression
analysis should be at least 500; small to moderate sample sizes may
overestimate the effect size (64,65).
Consequently, these findings should be considered to be
preliminary. In the future, incorporating additional predictors,
such as genetic markers, lifestyle factors, vitamin D levels,
micronutrient status and inflammatory biomarkers could lead to a
more comprehensive model (48,66-68).
A prospective study with a larger population could provide greater
statistical power to examine the associations between obesity,
sarcopenia and SO.
In conclusion, in the present study, sarcopenia and
SO were observed in almost half of the participants with chronic
stroke. Calf circumference was a predictor for both obesity and
sarcopenia. The nutritional status assessed using MNA-SF was a
predictor for sarcopenia. In addition, male sex and FAC were
predictive factors for SO.
Acknowledgements
Not applicable.
Funding
Funding: The present study received funding from the Faculty of
Medicine, Khon Kaen University, Khon Kaen, Thailand (grant no.
IN64215).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CN, PA, CS and JS were responsible for the research
conceptualization, study design and manuscript drafting. CN and JS
were involved in data collection, analysis and interpretation. CN
and JS confirm the authenticity of all the raw data All authors
(CN, PA, CS, and JS) contributed to the manuscript drafting and
read and approved the submitted version.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Khon Kaen University Ethics Committee for Human Research
(Ref. HE631455). The study was conducted in strict accordance with
the principles outlined in the Declaration of Helsinki and in
compliance with all relevant guidelines and regulations governing
human research ethics. Each patient provided written informed
consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
Guo SS, Zeller C, Chumlea WC and Siervogel
RM: Aging, body composition, and lifestyle: The fels longitudinal
study. Am J Clin Nutr. 70:405–411. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cameron J, McPhee JS, Jones DA and Degens
H: Five-year longitudinal changes in thigh muscle mass of
septuagenarian men and women assessed with DXA and MRI. Aging Clin
Exp Res. 32:617–624. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Auyeung TW, Lee SW, Leung J, Kwok T and
Woo J: Age-associated decline of muscle mass, grip strength and
gait speed: A 4-year longitudinal study of 3018 community-dwelling
older Chinese. Geriatr Gerontol Int. 14 (Suppl 1):S76–S84.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yeung SSY, Reijnierse EM, Pham VK,
Trappenburg MC, Lim WK, Meskers CGM and Maier AB: Sarcopenia and
its association with falls and fractures in older adults: A
systematic review and meta-analysis. J Cachexia Sarcopenia Muscle.
10:485–500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu J, Wan CS, Ktoris K, Reijnierse EM and
Maier AB: Sarcopenia is associated with mortality in adults: A
systematic review and meta-analysis. Gerontology. 68:361–376.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang X, Zhang W, Wang C, Tao W, Dou Q and
Yang Y: Sarcopenia as a predictor of hospitalization among older
people: A systematic review and meta-analysis. BMC Geriatr.
18(188)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang X, Wang C, Dou Q, Zhang W, Yang Y
and Xie X: Sarcopenia as a predictor of all-cause mortality among
older nursing home residents: A systematic review and
meta-analysis. BMJ Open. 8(e021252)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Janssen I: Influence of sarcopenia on the
development of physical disability: The cardiovascular health
study. J Am Geriatr Soc. 54:56–62. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jung HJ, Lee YM, Kim M, Uhm KE and Lee J:
Suggested assessments for sarcopenia in patients with stroke who
can walk independently. Ann Rehabil Med. 44:20–37. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jørgensen L and Jacobsen BK: Changes in
muscle mass, fat mass, and bone mineral content in the legs after
stroke: A 1 year prospective study. Bone. 28:655–659.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scherbakov N, von Haehling S, Anker SD,
Dirnagl U and Doehner W: Stroke induced Sarcopenia: Muscle wasting
and disability after stroke. Int J Cardiol. 170:89–94.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scherbakov N, Sandek A and Doehner W:
Stroke-related sarcopenia: Specific characteristics. J Am Med Dir
Assoc. 16:272–276. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Azzollini V, Dalise S and Chisari C: How
does stroke affect skeletal muscle? State of the art and
rehabilitation perspective. Front Neurol. 12(797559)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jang Y, Im S, Han Y, Koo H, Sohn D and
Park GY: Can initial sarcopenia affect poststroke rehabilitation
outcome? J Clin Neurosci. 71:113–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsushita T, Nishioka S, Taguchi S and
Yamanouchi A: Sarcopenia as a predictor of activities of daily
living capability in stroke patients undergoing rehabilitation.
Geriatr Gerontol Int. 19:1124–1128. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hunnicutt JL and Gregory CM: Skeletal
muscle changes following stroke: A systematic review and comparison
to healthy individuals. Top Stroke Rehabil. 24:463–471.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mas MF, González J and Frontera WR: Stroke
and sarcopenia. Curr Phys Med Rehabil Rep. 8:452–460.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Su Y, Yuki M and Otsuki M: Prevalence of
stroke-related sarcopenia: A systematic review and meta-analysis. J
Stroke Cerebrovasc Dis. 29(105092)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chang KV, Wu WT, Huang KC and Han DS:
Segmental body composition transitions in stroke patients: Trunks
are different from extremities and strokes are as important as
hemiparesis. Clin Nutr. 39:1968–1973. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ryan AS, Ivey FM, Serra MC, Hartstein J
and Hafer-Macko CE: Sarcopenia and physical function in middle-aged
and older stroke survivors. Arch Phys Med Rehabil. 98:495–499.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Donini LM, Busetto L, Bischoff SC,
Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y,
Cruz-Jentoft AJ, Dicker D, et al: Definition and diagnostic
criteria for sarcopenic obesity: ESPEN and EASO consensus
statement. Clin Nutr. 41:990–1000. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Collin C and Wade D: Assessing motor
impairment after stroke: A pilot reliability study. J Neurol
Neurosurg Psychiatry. 53:576–579. 1990.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thomann AE, Berres M, Goettel N, Steiner
LA and Monsch AU: Enhanced diagnostic accuracy for neurocognitive
disorders: A revised cut-off approach for the montreal cognitive
assessment. Alzheimers Res Ther. 12(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tham KW, Abdul Ghani R, Cua SC,
Deerochanawong C, Fojas M, Hocking S, Lee J, Nam TQ, Pathan F,
Saboo B, et al: Obesity in South and Southeast Asia-A new consensus
on care and management. Obes Rev. 24(e13520)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
working group for sarcopenia: 2019 Consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee J, Hong YP, Shin HJ and Lee W:
Associations of sarcopenia and sarcopenic obesity with metabolic
syndrome considering both muscle mass and muscle strength. J Prev
Med Public Health. 49:35–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS,
Hong D, Song G, Kim HJ, Choi YJ and Kim KM: Prevalence of
sarcopenia and sarcopenic obesity in the Korean population based on
the Fourth Korean national health and nutritional examination
surveys. J Gerontol A Biol Sci Med Sci. 67:1107–1113.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pongchaiyakul C, Limpawattana P, Kotruchin
P and Rajatanavin R: Prevalence of sarcopenia and associated
factors among Thai population. J Bone Miner Metab. 31:346–350.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gandham A, Scott D, Bonham MP, Kulkarni B,
Kinra S, Ebeling PR and Zengin A: Sex differences in bone health
among Indian older adults with obesity, sarcopenia, and sarcopenic
obesity. Calcif Tissue Int. 111:152–161. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F,
Zhao L, Shang Y, Ma Y and Ma B: Associated factors of sarcopenia in
community-dwelling older adults: A systematic review and
meta-analysis. Nutrients. 13(4291)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bursac Z, Gauss CH, Williams DK and Hosmer
DW: Purposeful selection of variables in logistic regression.
Source Code Biol Med. 3(17)2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Adulkasem N, Phinyo P, Khorana J,
Pruksakorn D and Apivatthakakul T: Prognostic factors of 1-year
postoperative functional outcomes of older patients with
intertrochanteric fractures in Thailand: A retrospective cohort
study. Int J Environ Res Public Health. 18(6896)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jang SY, Shin YI, Kim DY, Sohn MK, Lee J,
Lee SG, Oh GJ, Lee YS, Joo MC, Han EY, et al: Effect of obesity on
functional outcomes at 6 months post-stroke among elderly Koreans:
A prospective multicentre study. BMJ Open.
5(e008712)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vicente VS, Cabral NL, Nagel V, Guesser VV
and Safanelli J: Prevalence of obesity among stroke patients in
five Brazilian cities: A cross-sectional study. Arq Neuropsiquiatr.
76:367–372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Daskalopoulou C, Wu YT, Pan W, Giné
Vázquez I, Prince M, Prina M and Tyrovolas S: Factors related with
sarcopenia and sarcopenic obesity among low- and middle-income
settings: The 10/66 DRG study. Sci Rep. 10(20453)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu X, Hao Q, Yue J, Hou L, Xia X, Zhao W,
Zhang Y, Ge M, Ge N and Dong B: Sarcopenia, obesity and sarcopenia
obesity in comparison: prevalence, metabolic profile, and key
differences: Results from WCHAT study. J Nutr Health Aging.
24:429–437. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
English C, Thoirs K, Coates A, Ryan A and
Bernhardt J: Changes in fat mass in stroke survivors: A systematic
review. Int J Stroke. 7:491–498. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ikeji R, Nozoe M, Yamamoto M, Seike H,
Kubo H and Shimada S: Sarcopenia in patients following stroke:
Prevalence and associated factors. Clin Neurol Neurosurg.
233(107910)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yoshimura Y, Bise T, Nagano F, Shimazu S,
Shiraishi A, Yamaga M and Koga H: Systemic inflammation in the
recovery stage of stroke: Its association with sarcopenia and poor
functional rehabilitation outcomes. Prog Rehabil Med.
3(20180011)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shiraishi A, Yoshimura Y, Wakabayashi H
and Tsuji Y: Prevalence of stroke-related sarcopenia and its
association with poor oral status in post-acute stroke patients:
Implications for oral sarcopenia. Clin Nutr. 37:204–207.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kawakami R, Murakami H, Sanada K, Tanaka
N, Sawada SS, Tabata I, Higuchi M and Miyachi M: Calf circumference
as a surrogate marker of muscle mass for diagnosing sarcopenia in
Japanese men and women. Geriatr Gerontol Int. 15:969–976.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Barbosa-Silva TG, Menezes AM, Bielemann
RM, Malmstrom TK and Gonzalez MC: Grupo de Estudos em Composição
Corporal e Nutrição (COCONUT). Enhancing SARC-F: Improving
sarcopenia screening in the clinical practice. J Am Med Dir Assoc.
17:1136–1141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hai S, Cao L, Wang H, Zhou J, Liu P, Yang
Y, Hao Q and Dong B: Association between sarcopenia and nutritional
status and physical activity among community-dwelling Chinese
adults aged 60 years and older. Geriatr Gerontol Int. 17:1959–1966.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu J, Zhu Y, Tan JK, Ismail AH, Ibrahim R
and Hassan NH: Factors associated with sarcopenia among elderly
individuals residing in community and nursing home settings: A
systematic review with a meta-analysis. Nutrients.
15(4335)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ligthart-Melis GC, Luiking YC, Kakourou A,
Cederholm T, Maier AB and de van der Schueren MAE: Frailty,
sarcopenia, and malnutrition frequently (Co-)occur in hospitalized
older adults: A systematic review and meta-analysis. J Am Med Dir
Assoc. 21:1216–1228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shadmand Foumani Moghadam MR, Shahraki
Jazinaki M, Rashidipour M, Rezvani R, Pezeshki P, Ghayour Mobarhan
M and Hosseini Z: Mini nutrition assessment-short form score is
associated with sarcopenia even among nourished people-A result of
a feasibility study of a registry. Aging Med (Milton). 6:264–271.
2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Siotto M, Germanotta M, Guerrini A,
Pascali S, Cipollini V, Cortellini L, Ruco E, Khazrai YM, De Gara L
and Aprile I: Relationship between nutritional status, food
consumption and sarcopenia in post-stroke rehabilitation:
Preliminary data. Nutrients. 14(4825)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ganapathy A and Nieves JW: Nutrition and
sarcopenia-what do we know? Nutrients. 12(1755)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tieland M, Brouwer-Brolsma EM,
Nienaber-Rousseau C, van Loon LJ and De Groot LCPGM: Low vitamin D
status is associated with reduced muscle mass and impaired physical
performance in frail elderly people. Eur J Clin Nutr. 67:1050–1055.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bian A, Ma Y, Zhou X, Guo Y, Wang W, Zhang
Y and Wang X: Association between sarcopenia and levels of growth
hormone and insulin-like growth factor-1 in the elderly. BMC
Musculoskelet Disord. 21(214)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Damiano S, Muscariello E, La Rosa G, Di
Maro M, Mondola P and Santillo M: Dual role of reactive oxygen
species in muscle function: Can antioxidant dietary supplements
counteract age-related sarcopenia? Int J Mol Sci.
20(3815)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nishioka S: Current understanding of
sarcopenia and malnutrition in geriatric rehabilitation. Nutrients.
15(1426)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Vidaña-Espinoza HJ, López-Teros MT,
Esparza-Romero J, Rosas-Carrasco O, Luna-López A and Alemán Mateo
H: Association between the risk of malnutrition and sarcopenia at
4.2 years of follow-up in community-dwelling older adults. Front
Med (Lausanne). 11(1363977)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Darroch P, O'Brien WJ, Mazahery H and Wham
C: Sarcopenia prevalence and risk factors among residents in aged
care. Nutrients. 14(1837)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jean W: Sarcopenia. Clin Geriatr Med.
33:305–314. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Prado CMM, Wells JCK, Smith SR, Stephan
BCM and Siervo M: Sarcopenic obesity: A Critical appraisal of the
current evidence. Clin Nutr. 31:583–601. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Auyeung TW, Lee JSW, Leung J, Kwok T and
Woo J: Adiposity to muscle ratio predicts incident physical
limitation in a cohort of 3,153 older adults-an alternative
measurement of sarcopenia and sarcopenic obesity. Age (Dordr).
35:1377–1385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Atkins JL, Whincup PH, Morris RW, Lennon
LT, Papacosta O and Wannamethee SG: Sarcopenic obesity and risk of
cardiovascular disease and mortality: A population-based cohort
study of older men. J Am Geriatr Soc. 62:253–260. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Scott D, Seibel M, Cumming R, Naganathan
V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM and Hirani V:
Sarcopenic obesity and its temporal associations with changes in
bone mineral density, incident falls, and fractures in older men:
The concord health and ageing in men project. J Bone Miner Res.
32:575–583. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Matsushita T, Nishioka S, Taguchi S,
Yamanouchi A, Nakashima R and Wakabayashi H: Sarcopenic obesity and
activities of daily living in stroke rehabilitation patients: A
cross-sectional study. Healthcare (Basel). 8(255)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Broadwin J, Goodman-Gruen D and Slymen D:
Ability of fat and fat-free mass percentages to predict functional
disability in older men and women. J Am Geriatr Soc. 49:1641–1645.
2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Buckinx F, Landi F, Cesari M, Fielding RA,
Visser M, Engelke K, Maggi S, Dennison E, Al-Daghri NM, Allepaerts
S, et al: Pitfalls in the measurement of muscle mass: A need for a
reference standard. J Cachexia Sarcopenia Muscle. 9:269–278.
2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ryan AS, Dobrovolny CL, Smith GV, Silver
KH and Macko RF: Hemiparetic muscle atrophy and increased
intramuscular fat in stroke patients. Arch Phys Med Rehabil.
83:1703–1707. 2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Nemes S, Jonasson JM, Genell A and
Steineck G: Bias in odds ratios by logistic regression modelling
and sample size. BMC Med Res Methodol. 9(56)2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Bujang MA, Sa'at N, Sidik TMITAB and Joo
LC: Sample size guidelines for logistic regression from
observational studies with large population: emphasis on the
accuracy between statistics and parameters based on real life
clinical data. Malays J Med Sci. 25:122–130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
da Costa Teixeira LA, Avelar NCP, Peixoto
MFD, Parentoni AN, Santos JMD, Pereira FSM, Danielewicz AL,
Leopoldino AAO, Costa SP, Arrieiro AN, et al: Inflammatory
biomarkers at different stages of sarcopenia in older women. Sci
Rep. 13(10367)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Urzi F, Pokorny B and Buzan E: Pilot Study
on genetic associations with age-related sarcopenia. Front Genet.
11(615238)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jin H, Yoo HJ, Kim YA, Lee JH, Lee Y, Kwon
SH, Seo YJ, Lee SH, Koh JM, Ji Y, et al: Unveiling genetic variants
for age-related sarcopenia by conducting a genome-wide association
study on Korean cohorts. Sci Rep. 12(3501)2022.PubMed/NCBI View Article : Google Scholar
|