Introduction
Type 2 diabetes mellitus (Ty2-DM) is associated with
marked alterations in several parameters, including cellular,
metabolic, immunological and hematological abnormalities, which
eventually lead to vascular complications (1,2). The
most commonly encountered hematological abnormalities experienced
by patients with Ty2-DM are changes in the function, structure and
metabolism of red blood cells (RBCs), white blood cells (WBCs),
platelet counts and indices, and relevant hematological parameters
(3). Anemia is a widespread
blood-related condition which affects patients with diabetes
(4,5). It has been shown to be linked to
various diabetes complications, the main one being diabetic
nephropathy (6,7). The deterioration in kidney functions
leads to a decrease in erythropoietin (EPO) production, a hormone
responsible for the regulation of RBC production (8). Moreover, diabetic anemia can also
occur in the absence of nephropathy and may be related to other
factors, such as hypo-responsiveness to EPO, diabetic neuropathy,
uncontrol hyperglycemia, chronic inflammation, increased oxidative
stress, high levels of advanced glycation end products, deficiency
in vitamin B12, iron and folate, and antidiabetic agents (9). Some studies have provided evidence of
an association between antidiabetic agents and diabetic anemia in
certain cases; some antidiabetic agents can cause anemia as a
side-effect, some may increase the likelihood of developing anemia,
while others may not affect anemia (10,11).
Dipeptidyl peptidase 4 (DPP-4) inhibitors
(sitagliptin, linagliptin, saxagliptin, and alogliptin) are
examples of antidiabetic agents that are expected to produce a
lower incidence of anemia among patients with Ty2-DM (12). In general, DPP-4 decreases EPO
activity by breaking and negatively affecting colony-stimulating
factor potential and stress hematopoiesis. Based on these actions,
it is possible that DPP-4 inhibitors can increase EPO
hypo-responsiveness, which in turn controls hematopoietic stem
cells and progenitor cells, increasing EPO production and erythroid
colony formation (13). Despite
this promising effect of DPP-4 inhibitors on EPO and RBC
production, limited studies are available assessing these effects
on hematological parameters among patients with Ty2-DM. Therefore,
the principal aim of the present study was to investigate the
possible alterations in hematological parameters and EPO levels in
patients with Ty2-DM treated with sitagliptin and to estimate the
renal and liver functions in order to provide valuable insight into
its potential to protect patients with diabetes against
hematological, renal and liver abnormalities. The correlation
between the investigated parameters, if any, is also explored.
Materials and methods
Study design, study period and ethical
considerations
The present study is a retrospective cohort study
that was designed to investigate the hematological parameters, in
addition to renal and hepatic functions in patients with Ty2-DM
treated with sitagliptin. The patients were gathered from private
clinics, and the study was carried out during the period between
December, 2023 and April, 2024. The study was designed to adhere to
the World Medical Association's Declaration of Helsinki on the
moral conduct of trials on humans or animals. Ethical approval for
the conduction of the study was obtained from the Collegiate
Committee for Medical Research Ethics at the University of Mosul,
Mosul, Iraq (Code: CCMRE-phA-23-16). All participants had a clear
explanation about the purpose of the study and an informed consent
was requested to be signed by them. A study questionnaire was also
distributed to all participants, which included information about
age, weight, height, body mass index (BMI), the duration of
diabetes, the co-existence of diseases and co-existence
medications, and the duration of the sitagliptin and metformin
usage; this information facilitating the recognition of the
inclusion and exclusion criteria of the study before performing the
laboratory tests.
Study participants
The present study included 135 participants, who
were divided into three groups after being fully examined by the
specialists to detect their eligibility for inclusion in the study
and to exclude any abnormalities based on their medical, family and
bleeding disorders history. A control group of 22 males and 23
females with a mean age of 52.82±7.94 years, apparently healthy
individuals, who were not taking any medicine for any chronic
conditions was included as the first group (group 1). The
participants in the second group (group 2) had a mean age of
53.52±8.65 years, consisting of 23 male and 22 female patients with
Ty2-DM taking metformin at 1,000 mg daily. The third group (group
3) consisted of 22 male and 23 female patients with Ty2-DM who took
50 mg of sitagliptin and 1,000 mg of metformin daily; their mean
age was 54.64±8.66 years. Patients with type 1 diabetes mellitus,
those taking antidiabetic agents instead of sitagliptin and
metformin, those whose duration of medication use under
investigation was <3 months, pregnant females and lactating
mothers were not included in the patient groups. Individuals who
had chronic renal, liver diseases, or hematological diseases were
also excluded, as were those who took medications that affected
renal and liver functions, or hematological parameters. The healthy
control group establishes baselines for measured variables,
allowing researchers to assess how diabetic patients deviate from
normal physiological functions. This comparison helps to evaluate
the effectiveness of treatments, such as sitagliptin and metformin
and any adverse effects on hematological parameters, EPO levels and
renal and liver functions. Including a healthy group also controls
for confounding factors related to ethnicity, ensuring the groups
are comparable demographically. Moreover, having a healthy
reference helps to rule out influences from laboratory assessment
devices, enhancing the validity and reliability of the results.
Overall, the presence of a healthy control group is crucial to
attributing outcomes directly to the investigated drugs rather than
other confounding factors. The patients with Ty2-DM in group 2 who
were treated with metformin only served as the control group for
group 3, and the patients with Ty2-DM in group 3 who were treated
with sitagliptin plus metformin comprised the group which included
the drug under investigation.
Laboratory methods
A total of 7 ml venous blood were collected and 2 ml
were transferred into an anticoagulant EDTA tube to prevent
clotting before the blood tests were performed. Whole blood was
used to measure hemoglobin A1c (HbA1c) levels and complete blood
count (CBC). The turbidimetric inhibition immunoassay (TINIA),
which used a Cobas c 111 auto-analyzer and kit from Roche
Diagnostics was the basis for determining HbA1c levels (14). The CBC test was performed using the
Swelab Alpha Plus hematological analyzer; Sweden; Boule Medical AB
company; Swelab Alpha Plus is an automated hematological analyzer
whose measurement principles are based on the electric resistance
(impedance) of the cell counts and spectrophotometry of hemoglobin.
Hemoglobin, WBCs, RBCs, mean corpuscular volume (MCV), hematocrit
(Hct), mean corpuscular hemoglobin (MCH) and mean corpuscular
hemoglobin concentration (MCHC) were all estimated. Moreover, 5 ml
blood were transferred into a gel tube with a clotting activator to
obtain a serum after clotting and centrifugation at a speed of
3,000 rpm for 10 min at 20˚C. Serum ferritin levels were estimated
using a Cobas e 411 immunoassay analyzer (Roche Diagnostics); this
device depends on an electrochemiluminescence immunoassay (ECLIA)
method using kits supplied by Roche Diagnostics. Serum EPO was
measured following the manufacturer's instructions, using a
Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) with a ChroMate@
Microplate Reader manufactured by Awareness Technology, Inc. and a
kit supplied by ELK Biotechnology. Serum glucose, renal function
tests (including urea, creatinine and uric acid) and liver function
tests [including aspartate aminotransferase (AST), alanine
aminotransferase (ALT) and total bilirubin] were estimated
photometrically using a Cobas c 111 auto-analyzer and kits supplied
by Roche Diagnostics (15-17).
Statistical analysis
All data are presented as the mean values with
standard deviation (SD). Using one-way ANOVA followed by Tukey's
post hoc test, the statistically significant differences among the
studied groups were investigated. Pearson's correlation
coefficients, linear regression and 95% confidence intervals were
employed to examine the correlation between the variables being
studied. The studied groups were validated for the normal
distribution of data before any statistics, using normality tests
(Kolmogorov-Smirnov and Shapiro-Wilk tests). GraphPad Prism 8
software (Dotmatics) was used to perform the statistical analyses.
A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics of the study
participants
A significantly higher BMI value (P<0.001) was
observed in group 3 and group 2 compared with the control group,
however; however, there were no significant differences in BMI
values and in the duration of diabetes between groups 3 and 2. The
baseline characteristics of the study population are presented in
Table I.
 | Table IBaseline characteristics of the study
groups. |
Table I
Baseline characteristics of the study
groups.
| Variables | Group 1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| Age (years) | 52.82±7.9 | 53.52±8.65 | 54.64±8.66 |
| Number (M/F) | 45 (22/23) | 45 (23/22) | 45 (22/23) |
| BMI (kg
m-2) | 24.05±0.8 |
29.71±4.65a |
29.33±3.58a |
| Duration of diabetes
(years) | - | 4.22±3.10 | 5.54±2.63 |
Glycemic status
The levels of fasting serum glucose (FSG) exhibited
a significant difference (P<0.001) in group 3 group and group 2
compared with group 1. A significant decrease in FSG levels was
detected in group 2 compared with group 3 (P<0.005). Similarly,
the HbA1c levels in groups 3 and 2 exhibited a significant
difference (P<0.001) compared with those in group 1 (Table II).
 | Table IIGlycemic status of the study
groups. |
Table II
Glycemic status of the study
groups.
| Variables | Group 1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| FSG (mg/l) | 856.7±125 |
1,309±417.6a |
1,515±477.4a,b |
| HbA1c (%) | 5.44±0.35 |
6.71±1.41a |
7.10±1.16a |
Liver functions
The levels of liver function parameters, including
AST, ALT and total bilirubin, were within the normal range in all
of the studied groups. Liver function parameters in the patients
using sitagliptin were comparable to those of other groups, with no
significant differences observed (Table III). The assessment of liver
function parameters is crucial for excluding liver issues that may
affect EPO secretion and directly identify any blood abnormalities
linked to the examined drug.
 | Table IIILiver functions of the study
groups. |
Table III
Liver functions of the study
groups.
| Variables | Group1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| AST (U/l) | 18.37±4.69 | 20.59±5.01 | 19.57±6.97 |
| ALT (U/l) | 18.96±7.46 | 21.66±5.28 | 21.88±10.53 |
| Total bilirubin
(mg/l) | 4.5±2.0 | 4.9±2.3 | 4.8±3.2 |
Renal functions
The levels of renal function parameters exhibited
variable values among the study groups, as demonstrated in Table IV. The urea levels were
significantly increased in group 3 (P<0.001) and group 2
(P=0.014), when compared with those in group 1. The creatinine
levels were elevated in both group 3 (P<0.03) and group 2
(P<0.02) compared with group 1, although they remained within
the normal range. The uric acid levels were significantly higher
(P<0.003) in group 3 compared with both groups 1 and 2. However,
creatinine clearance (CrCl) showed no significant differences among
the study groups. This observation of renal parameters is critical
as it aids in ruling out potential renal abnormalities that could
affect EPO secretion, thereby establishing a clear connection
between any observed blood irregularities and the drug being
studied.
 | Table IVRenal functions of the study
groups. |
Table IV
Renal functions of the study
groups.
| Variables | Group 1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| Urea (mg/l) | 234.2±49 |
276.7±41.6a |
305.6±100c |
| Creatinine
(mg/l) | 7.1±1.2 |
8.4±2.1a |
8.5±2.2a |
| Uric acid
(mg/l) | 43.7±10.8 | 46.6±9.3 |
51.7±12.5b |
| CrCl (ml/min) | 103.60±23.18 | 112.70±29.57 | 110.30±29.08 |
Hematological parameters
Assays of hematological parameters in the present
study revealed notable changes and associations between the studied
groups, as summarized in Table V.
Hemoglobin levels were within the normal range for all groups;
however, they were significantly higher (P=0.006) in group 3
compared to group 2, and considerably lower (P=0.02) in group 2
compared to group 1. As regards serum ferritin levels, although all
participant groups had values within the normal range, a
significant increase (P=0.02) was found in patients on sitagliptin
plus metformin (group 3) compared to both the control and metformin
groups. Furthermore, the RBCs exhibited a significant increase
(P=0.004) in group 3 compared to group 2. Despite lower levels of
WBCs observed in group 2, a relatively significant increase in WBCs
(P=0.012) was observed in group 3 compared to group 2.
 | Table VHematological parameters of the study
groups. |
Table V
Hematological parameters of the study
groups.
| Variables | Group 1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| Hb (g/dl) | 138.9±13.3 |
128.7±12.9a |
139.9±16e |
| Ferritin
(ng/ml) | 100.50±49.79 | 99.45±55.62 |
150.90±99.98a,d |
| RBCs
(x106/µl) | 4.85±0.38 | 4.74±0.36 |
5.15±0.66e |
| WBCs
(x103/µl) | 7.50±1.66 | 6.80±1.40 |
8.06±2.11d |
| Hct (%) | 42.78±3.45 | 43.43±3.95 | 42.47±8.32 |
| MCV (fl) | 88.45±7.65 | 91.61±5.09 |
84.62±4.77a,f |
| MCH (pg) | 28.99±2.95 | 29.81±195 |
27.14±2.15b,f |
| MCHC (g/l) | 327.4±13.9 | 317.8±38.7 | 320.2±19.9 |
| EPO (pg/ml) | 17.28±3.12 | 18.37±1.37 | 17.90±1.98 |
There were no statistically significant differences
in Hct levels in groups 3 and 2. Moreover, the MCV of group 3 was
significantly lower (P=0.03) than that of group 1 and group 2
(P<0.001). Likewise, MCH was significantly lower (P=0.009) in
group 3 when compared with group 1 and a highly significant
reduction (P<0.001) was observed compared to group 2. When
comparing the two patient groups to the control group, there were
no statistically significant variations in MCHC or EPO.
Correlation between ferritin, and EPO,
Hb, RBCs, Hct and MCV
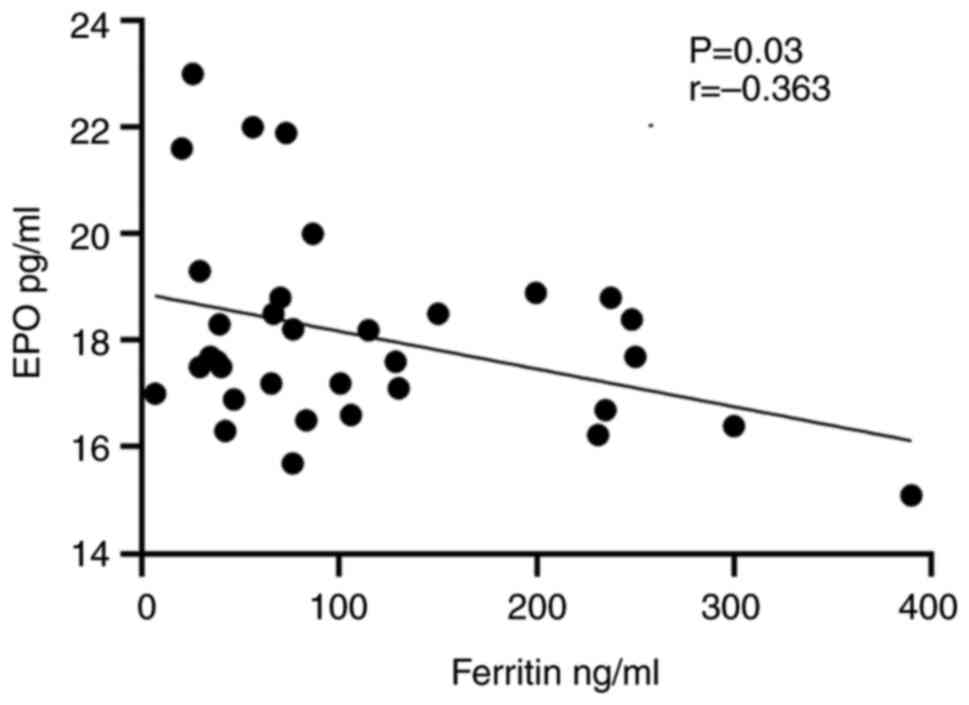
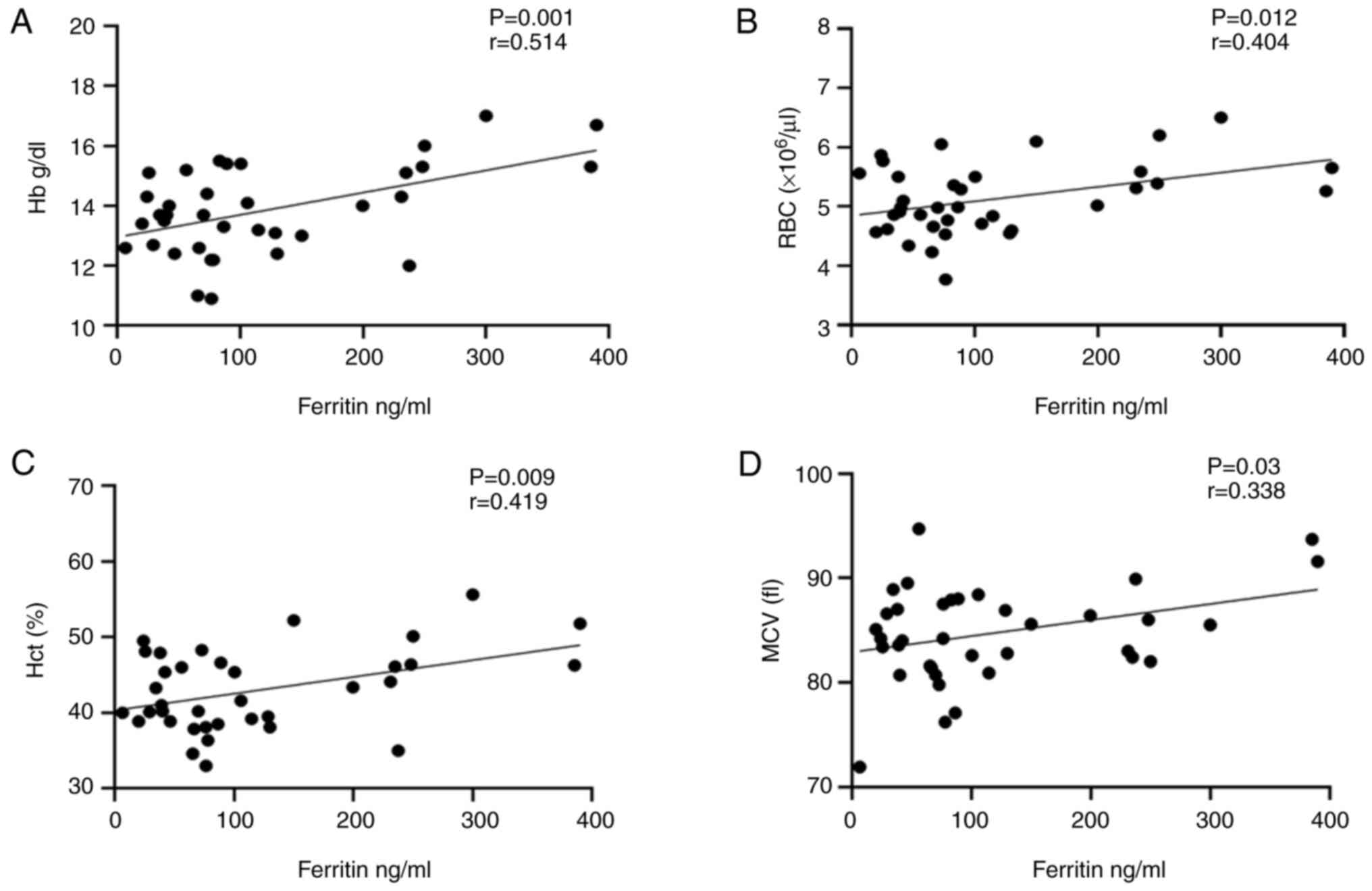
The results of the correlation analysis revealed a
strong inverse correlation between ferritin and EPO in group 3 who
took sitagliptin with metformin at P=0.03, as shown in Fig. 1. Conversely, in the same group, a
significant direct correlation was found between ferritin and Hb,
RBCs, Hct and MCV at (P=0.001, P=0.012, P=0.009 and P=0.03),
respectively as illustrated in Fig.
2.
Discussion
Since hematological, liver and kidney abnormalities
are increasingly common as a side-effect of antidiabetic agents, it
is critical to establish a strategy that treats diabetes, without
potentially aggravating any concomitant conditions. Despite the
abundance of existing studies about the action of DPP-4 inhibitors
on different body organs, there is limited information available of
how these inhibitors affect hematological parameters in patients
with diabetes. This gap inciates the importance of investigating
the potential of DPP-4 inhibitors in modulating hematological
parameters and EPO levels, as well as their effect on renal and
liver function in individuals with Ty2-DM. In the present study,
sitagliptin was used as a representative of DPP-4 inhibitors to
attain the desired aim.
The baseline characteristics of the study groups
were matched as regards age and sex. In the two patient groups, the
BMI values and the duration of diabetes were similar, eliminating
the impact of these factors on the assessed parameters. The
duration of the treatment in the two patient groups was over a
period of 6 months, and there was no significant difference in the
duration of the investigated drug among the patient groups. Thus,
any confounding factor regarding the duration that could affect the
examined parameters was ruled out.
The significant differences in FSG observed between
the metformin and sitagliptin plus metformin groups may be due to
the primary effects of sitagliptin on postprandial blood glucose
levels. This effect has been demonstrated by the addition of
sitagliptin to insulin plus metformin therapy, which provides
additional postprandial glycemic control (18). However, the non-significant
differences in HbA1c observed between the two patient groups may be
due to the metabolic memory phenomena, which refer to good or poor
blood glucose control that has a long-lasting effect on the
metabolic process of the body even after the blood glucose is
normalized (19).
Both metformin and sitagliptin clearly exhibited
their hepatoprotective effect, which is consistent with the
findings of previous studies (20,21),
thus, ruling out any liver issues that could lead to anemia and
directly ascertaining any blood abnormalities found with the
examined drug. Sitagliptin demonstrates its hepatic protection
through the suppression of NF-κB, a key mediator in the stimulation
of proinflammatory genes, thus suppressing inflammation (22). In addition, sitagliptin acts as a
scavenger for reactive oxygen species and has been shown to improve
diabetic liver inflammation in mice (23). Taken together, the abovementioned
results display the potential positive effects of sitagliptin when
combined with metformin on liver function (24).
Linked closely to the liver in maintaining
hematological balance, the kidney is another vital organ handling
blood abnormalities (25).
Notably, in the present study, renal function parameters in the
examined patients were notable for being higher than those in the
control group. These findings in renal parameters following
sitagliptin therapy are in accordance with those of previous
reports, where it was suggested that DDP-4 inhibitors, by
activating the glucagon-like peptide-1 (GLP-1) receptor, suppress
the type 3 transporter of the Na+/H+
exchanger, thereby promoting sodium excretion. Hence, the elevation
in creatinine levels, which is enhanced by sitagliptin, is linked
to the natriuretic effect of GLP-1. A slight increase in serum
creatinine levels was observed in a 2-year study of individuals
taking sitagliptin. A correlation was also observed between the
increase in creatinine and the decrease in HbA1c levels. These
findings suggest that the higher creatinine may be associated with
incretin activation, possibly due to the induction in GLP-1
diuretic activity, upregulation and subsequent dehydration
(26). Likewise, in a previous
study, the administration of GLP-1 agonist increased sodium
excretion, decreased urea excretion and increased urine pH levels,
which may be mediated by GLP-1 agonist-induced natriuresis in the
proximal tubule (27). Previously,
sitagliptin has been reported to increase serum uric acid levels
(28); however, the precise
mechanisms involved remain unknown. However, the imbalance between
uric acid production and excretion or indirect natriuresis activity
are suggested as possible mechanisms (28). Thus, such mechanisms are concisely
linked to the results in the present study regarding the elevated
serum urea, Cr and uric acid levels, compared to healthy
individuals. Notably, the non-significant results of creatinine
clearance were inexorably linked to normal kidney function and
health. However, the abnormalities of renal function can frequently
cause alterations in the hematological parameters. Notable findings
were obtained in the present study regarding hematological
parameters in the sitagliptin group compared to the control and
metformin groups. The significant reduction in the Hb level in the
metformin group was associated with the reduced levels of ferritin
due to reduced intestinal iron absorption or effects on iron
metabolism (29). In contrast to
the metformin group, sitagliptin users exhibited a significant
increase in Hb levels compared to the control group. Consequently,
the RBC counts were increased in this group compared to
metformin-only group. This is in line with the action of DPP-4
inhibitors in revealing the Hb decline in diabetic kidney disease
(12). Moreover, DPP-4 inhibitors
have not exhibited a direct linkage to hematological abnormalities
in Ty2-DM (30).
Although Hb and ferritin levels are associated with
iron status, the association between them can vary and depends on a
number of factors. Generally, elevated levels of serum ferritin are
typically caused by prolonged alcohol intake, cancer, metabolic
syndrome, renal or liver illness, and acute or chronic inflammation
rather than iron overload (31,32).
Herein, the strict inclusion criteria of the involved participants
excluded the possible pathological conditions that could elevate
serum ferritin levels. Moreover, a number of factors, including
iron, cytokines, oxidative stress and hormones, tightly control the
expression levels of ferritin. Surprisingly, the incretin hormone
can play a key role in the regulation of ferritin levels.
Furthermore, the nuclear factor-erythroid 2 related factor (Nrf2)
is regarded as a chief regulator of ferritin synthesis and
degradation to preserve iron homeostasis (33). Of note, the incretin hormone
upregulates the expression of Nrf2, which turns on the activation
of genes that code for antioxidant enzymes, including heme
oxygenase-1 (HO-1) which is a cytoprotective antioxidant protein
(34). Following the induction of
HO-1, this enzyme catalyzes heme oxygenation to produce a
physiologically active molecule, such as iron, resulting in the
high expression of ferritin for iron sequestration (35). Notably, sitagliptin has
hepatoprotective activity partly through the modulation of the
Nrf2/ HO-1 signaling pathway (36). Additionally, the result in the
study by Genc et al (37)
was consistent with the result of the present study; in their
study, the serum ferritin levels were higher before and after 1
year of treatment in both the metformin and gliptin plus metformin
groups. In the present study, serum ferritin levels were higher in
patients with Ty2-DM who were on sitagliptin plus metformin than
those on metformin alone. Thus, the aforementioned signaling
pathways were inevitably linked to the indirect effect of
sitagliptin on the elevation of serum ferritin levels through the
action of the incretin hormone, which is in line with the
significantly higher results of ferritin.
Additionally, due to the stored iron, higher
ferritin levels resulted in a greater number of RBCs with
sufficient Hb. Ferritin and EPO may be inversely associated due to
sufficient blood indices, balanced erythropoiesis and RBC
formation, by providing negative feedback to EPO induction. The
direct correlation between ferritin and blood indices in the
sitagliptin plus metformin group indicates that ferritin may be a
potential target to combat DM complications through the incretin
mimetic action of sitagliptin. Serum ferritin and WBCs are not
directly associated; however, inflammation may be the cause of an
indirect link. High ferritin levels during infection protect the
immune cells of the body, prevent bacteria from using iron, reduce
harmful free radicals, regulate the immune system and indicate
inflammation. Medical professionals use these values as a signpost
for therapeutic interventions (38). However, the statistically
significant results concerning WBCs in sitagliptin users elucidate
the capability of sitagliptin in directly affecting the
colony-stimulating factors (CSF) in the bone marrow, thus promoting
the production of blood cells regardless of its effect on
erythropoiesis. This is consistent with the captivating function of
DPP-4 in the regulation of proteins that govern different cell
types, such as more mature blood cells and hematopoietic stem
cells. It has been shown that a reduced DPP-4 activity is
associated with the increased ability of hematopoietic stem cells
to engraft (39). Furthermore,
DPP-4 truncates growth factors including granulocyte-CSF,
granulocyte macrophage-CSF and interleukin-3, which can drastically
lower the capacity of cells stimulated with them to form colonies
in comparison to the full-length proteins (40). Collectively, DPP-4 modifies
proteins and peptides that regulate hematopoietic cells; thus, by
targeting these proteins, a better understanding of blood cell
regulation and treatment of hematological-related diseases could be
attained (41). This precise
matching procedure enabled the immediate correlation of the
obtained results to the positive impact of sitagliptin on the
hematological parameters in patients with Ty2-DM.
Despite the significant findings on Hb, RBCs and MCV
counts, there were no significant changes in EPO levels in the
sitagliptin group. This was contradicted by the ability of another
member of DPP4 inhibitors, linagliptin, in reducing the dose of the
erythropoiesis-stimulating agent (ESA) darbepoetin alpha and
decreasing ESA resistance in patients undergoing hemodialysis
(42). Additionally, DPP-4
inhibitors have been reported to enhance the ESA-resistance index
in patients without iron deficiency undergoing hemodialysis
(43). Moreover, sitagliptin may
reduce oxidative stress in hematopoietic cells through its
therapeutic effect on hematopoietic injury (23). The appropriate explanation for the
unaltered EPO level in the present study was related to the
ferritin level in the sitagliptin plus metformin group compared to
metformin-only users. This speculation was based on the concept
that a high level of ferritin would activate the factor inhibiting
hypoxia-inducible factor 1 under normoxic and hypoxic conditions.
This factor is a co-regulator of the HIF gene, which is a master
manager of erythropoiesis. Thus, the high level of ferritin may
activate this factor and suppress the HIF gene, which will restrict
the production of EPO and erythropoiesis (44).
Even with the obtained result concerning EPO, both
serum ferritin and RBCs exhibited a statistically direct
correlation in sitagliptin users. Consequently, there was a direct
correlation between ferritin, Hb, Hct and MCV in this group of
patients. Alongside the inverse correlation between ferritin and
EPO, the results indicated the potential role of sitagliptin in
improving erythropoiesis through the direct inhibition of the DPP-4
enzyme, thereby reducing the degradation of EPO. Simultaneously,
sitagliptin may regulate the overproduction of EPO through indirect
incretin action on ferritin levels and may maintain EPO levels
within the normal range. Notably, correlation analyses were
performed among all the studied groups and on all the study
parameters in groups 2 and 3, and no significant associations were
found (data not shown). This indicates that the correlations
observed are related solely to groups 3 (patients on sitagliptin
only).
Nonetheless, it is essential to address the
limitations of the current study, such as the relatively small
sample size, which needs to be expanded in future studies.
Additionally, long-term prospective trials are recommended to
validate the results of this study. Even with these limitations,
the present study provided valuable insight into the potential of
sitagliptin on significant modulation of hematological indices,
while keeping normal renal and liver functions. This effect is
closely correlated with ferritin level rather than the impact of
the kidneys or the liver.
Acknowledgements
The authors appreciate the guidance and support from
the University of Mosul, College of Pharmacy and Nineveh Health
Directorate, Mosul, Iraq.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FAA and MNA conceived and designed the study. RIA
conducted the experiments. FAA and MNA analyzed data. RIA, FAA and
MNA drafted the manuscript. FAA and MNA confirm the authenticity of
all the raw data. All the authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was designed to adhere to the
World Medical Association's Declaration of Helsinki on the moral
conduct of trials on humans or animals. Ethical approval for the
conduction of the study was obtained from the Collegiate Committee
for Medical Research Ethics at the University of Mosul, Mosul, Iraq
(Code: CCMRE-phA-23-16). All participants had a clear explanation
about the purpose of the study and an informed written consent was
requested to be signed by them.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed GM, Abed MN and Alassaf FA: Impact
of calcium channel blockers and angiotensin receptor blockers on
hematological parameters in type 2 diabetic patients. Naunyn
Schmiedebergs Arch Pharmacol. 397:1817–1828. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alnaser RI, Alassaf FA and Abed MN:
Melatonin as a potential treatment option in diabetes
complications. Eur J Transl Clin Med: 7, 2024
doi:10.31373/ejtcm/192108.
|
|
3
|
Antwi-Baffour S, Kyeremeh R, Boateng SO,
Annison L and Seidu MA: Haematological parameters and lipid profile
abnormalities among patients with Type-2 diabetes mellitus in
Ghana. Lipids Health Dis. 17(283)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singh DK, Winocour P and Farrington K:
Erythropoietic stress and anemia in diabetes mellitus. Nat Rev
Endocrinol. 5:204–210. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ebrahim H, Fiseha T, Ebrahim Y and
Bisetegn H: Comparison of hematological parameters between type 2
diabetes mellitus patients and healthy controls at Dessie
comprehensive specialized hospital, Northeast Ethiopia: Comparative
cross-sectional study. PLoS One. 17(e0272145)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sahay M, Kalra S, Badani R, Bantwal G,
Bhoraskar A, Das AK, Dhorepatil B, Ghosh S, Jeloka T, Khandelwal D,
et al: Diabetes and anemia: International diabetes federation
(IDF)-Southeast asian region (SEAR) position statement. Diabetes
Metab Syndr. 11 (Suppl):S685–S695. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alnaser RI, Alassaf FA and Abed MN:
Adulteration of hypoglycemic products: The silent threat. Rom J Med
Pract. 18:202–205. 2023.
|
|
8
|
Bunn HF: Erythropoietin. Cold Spring Harb
Perspect Med. 3(a011619)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ahmed GM, Alassaf FA and Abed MN: The
interplay of the angiotensin receptor blockers and haematological
abnormalities: Insights and implications. J Ayub Med Coll
Abbottabad. 35 (Suppl 1)):S785–S792. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bell DSH: Metformin-induced vitamin B12
deficiency can cause or worsen distal symmetrical, autonomic and
cardiac neuropathy in the patient with diabetes. Diabetes Obes
Metab. 24:1423–1428. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ahmed G, Abed M and Alassaf F: An overview
of the effects of Sodium-glucose Cotransporter-2 inhibitors on
hematological parameters in diabetic patients. Iraqi J Pharm:
65-71, 2023 doi:10.33899/iphr.2023.137946.1041.
|
|
12
|
Zeng L, Chan GCK, Ng JKC, Fung WWS, Chow
KM and Szeto CC: The effect of Dipeptidyl peptidase 4 (DPP-4)
inhibitors on hemoglobin level in diabetic kidney disease: A
retrospective cohort study. Medicine (Baltimore).
102(e34538)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Broxmeyer HE, Hoggatt J, O'Leary HA,
Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag
S, Rohrabaugh SL, et al: Dipeptidylpeptidase 4 negatively regulates
colony-stimulating factor activity and stress hematopoiesis. Nat
Med. 18:1786–1796. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Alassaf FA, Jasim MHM, Alfahad M, Qazzaz
ME, Abed MN and Thanoon IAJ: Effects of bee propolis on FBG, HbA1c,
and insulin resistance in healthy volunteers. Turkish J Pharm Sci.
18:405–409. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Al-dabbagh BM, Abed MN, Mahmood NM,
Alassaf FA, Jasim MH, Alfahad MA and Thanoon IAJ:
Anti-inflammatory, antioxidant and hepatoprotective potential of
milk thistle in albino rats. Lat Am J Pharm. 41:1832–1841.
2022.
|
|
16
|
Abed MN, Qazzaz ME and Alassaf FA:
Investigating the nephrotoxic effects of medroxyprogesterone in
female albino rats. Ukr J Nephrol Dial. 2:25–33. 2024.
|
|
17
|
Imad AJT, Jasim MHM and Mohammed N:
Effects of omega-3 on renal function tests and uric acid level in
healthy volunteers. Lat Am J Pharm. 40:2319–2323. 2021.
|
|
18
|
Arnolds S, Dellweg S, Clair J, Dain MP,
Nauck MA, Rave K and Kapitza C: Further improvement in postprandial
glucose control with addition of exenatide or sitagliptin to
combination therapy with insulin glargine and metformin. Diabetes
Care. 33:1509–1515. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dong H, Sun Y, Nie L, Cui A, Zhao P, Leung
WK and Wang Q: Metabolic memory: Mechanisms and diseases. Signal
Transduct Target Ther. 9(38)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cozma GV, Apostu A, Macasoi I, Dehelean
CA, Cretu OM, Dinu S, Gaiță D and Manea A: In vitro and in ovo
evaluation of the potential hepatoprotective effect of metformin.
Medicina (Kaunas). 58(705)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ahmed A, Omar Z, El-Bakry MH and Ahmed MA:
Hepatoprotective effect of dipeptidyl peptidase-4 inhibitor
sitagliptin against carbon tetrachloride-induced liver fibrosis in
mice. Al-Azhar Assiut Med J. 19:459–468. 2021.
|
|
22
|
Jiang W, Wen D, Cheng Z, Yang Y, Zheng G
and Yin F: Effect of sitagliptin, a DPP-4 inhibitor, against
DENA-induced liver cancer in rats mediated via NF-κB activation and
inflammatory cytokines. J Biochem Mol Toxicol.
32(e22220)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang X, Ke J, Zhu YJ, Cao B, Yin RL, Wang
Y, Wei LL, Zhang LJ, Yang LY and Zhao D: Dipeptidyl peptidase-4
(DPP4) inhibitor sitagliptin alleviates liver inflammation of
diabetic mice by acting as a ROS scavenger and inhibiting the NFκB
pathway. Cell Death Discov. 7(236)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alnaser RI, Alassaf FA and Abed MN:
Incretin-based therapies: A promising approach for modulating
oxidative stress and insulin resistance in sarcopenia. J Bone
Metab. 31:251–263. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abed MN, Alassaf FA and Qazzaz ME:
Exploring the interplay between vitamin D, insulin resistance,
obesity and skeletal health. J Bone Metab. 31:75–89.
2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maeda H, Kubota A, Kanamori A, Tanaka Y,
Terauchi Y and Matsuba I: Study Group of Diabetes Committee,
Kanagawa Physicians Association. Effects of sitagliptin on the
serum creatinine in Japanese type 2 diabetes. Diabetes Res Clin
Pract. 108:e42–e45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tonneijck L, Smits MM, Muskiet MHA,
Hoekstra T, Kramer MHH, Danser AHJ, Diamant M, Joles JA and van
Raalte DH: Acute renal effects of the GLP-1 receptor agonist
exenatide in overweight type 2 diabetes patients: A randomised,
double-blind, placebo-controlled trial. Diabetologia. 59:1412–1421.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kutoh E, Wada A, Kuto AN, Hayashi J and
Kurihara R: Link between serum uric acid and pancreatic beta-cell
function in drug naïve subjects with type 2 diabetes treated with
sitagliptin. Hosp Pract (1995). 49:71–78. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Donnelly LA, Dennis JM, Coleman RL, Sattar
N, Hattersley AT, Holman RR and Pearson ER: Risk of anemia with
metformin use in Type 2 diabetes: A MASTERMIND study. Diabetes
Care. 43:2493–2499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Merdin FA and Merdin A: Do DPP-4 enzyme
inhibitors affect hemoglobin, leucocyte and thrombocyte levels in
patients with type 2 diabetes mellitus? Eur Rev Med Pharmacol Sci.
27:4614–4618. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cullis JO, Fitzsimons EJ, Griffiths WJ,
Tsochatzis E and Thomas DW: Investigation and management of a
raised serum ferritin. Br J Haematol. 181:331–340. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mahroum N, Alghory A, Kiyak Z, Alwani A,
Seida R, Alrais M and Shoenfeld Y: Ferritin-from iron, through
inflammation and autoimmunity, to COVID-19. J Autoimmun.
126(102778)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Anandhan A, Dodson M, Shakya A, Chen J,
Liu P, Wei Y, Tan H, Wang Q, Jiang Z, Yang K, et al: NRF2 controls
iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv.
9(ade9585)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oh Y and Jun HS: Effects of glucagon-like
peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci.
19(26)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Durante W: Targeting heme Oxygenase-1 in
the arterial response to injury and disease. Antioxidants (Basel).
9(829)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Abdel-Gaber SA, Geddawy A and Moussa RA:
The hepatoprotective effect of sitagliptin against hepatic ischemia
reperfusion-induced injury in rats involves Nrf-2/HO-1 pathway.
Pharmacol Rep. 71:1044–1049. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Genc FT, Nalbant A, Genc AC and Kaya T:
Effect of a combination of gliptin and metformin on serum vitamin
B12, folic acid, and ferritin levels. Rev Assoc Med Bras (1992).
69(e20230641)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Moreira AC, Mesquita G and Gomes MS:
Ferritin: An inflammatory player keeping iron at the core of
Pathogen-host interactions. Microorganisms. 8(589)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Farag SS, Nelson R, Cairo MS, O'Leary HA,
Zhang S, Huntley C, Delgado D, Schwartz J, Zaid MA, Abonour R, et
al: High-dose sitagliptin for systemic inhibition of
dipeptidylpeptidase-4 to enhance engraftment of single cord
umbilical cord blood transplantation. Oncotarget. 8:110350–110357.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
O'Leary HA, Capitano M, Cooper S, Mantel
C, Boswell HS, Kapur R, Ramdas B, Chan R, Deng L, Qu CK and
Broxmeyer HE: DPP4 truncated GM-CSF and IL-3 manifest distinct
receptor-binding and regulatory functions compared with their
full-length forms. Leukemia. 31:2468–2478. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ropa J and Broxmeyer HE: An expanded role
for dipeptidyl peptidase 4 in cell regulation. Curr Opin Hematol.
27:215–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aono M and Sato Y: Dipeptidyl peptidase 4
inhibitor linagliptin can decrease the dosage of
erythropoiesis-stimulating agents in patients on hemodialysis. Ren
Replace Ther. 2(44)2016.
|
|
43
|
Hasegawa T, Zhao J, Bieber B, Zee J,
Pisoni RL, Robinson BM, Hanafusa N and Nangaku M: Association
between dipeptidyl Peptidase-4 inhibitor prescription and
Erythropoiesis-stimulating agent hyporesponsiveness in hemodialysis
patients with diabetes mellitus. Kidney Blood Press Res.
46:352–361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jin P, Kang J, Lee MK and Park JW:
Ferritin heavy chain controls the HIF-driven hypoxic response by
activating the asparaginyl hydroxylase FIH. Biochem Biophys Res
Commun. 499:475–4781. 2018.PubMed/NCBI View Article : Google Scholar
|