Introduction
Cardiovascular disease (CVD) is a major cause of
morbidity and mortality worldwide, remaining the leading cause of
mortality globally (1). The widely
accepted approach to disease prevention involves a strategy that
prioritizes prevention efforts towards individuals who are at a
higher risk, emphasizing a risk-based methodology (2,3). The
estimation of the probabilistic susceptibility of an individual to
disease, commonly referred to as risk prediction, holds paramount
importance in clinical decision-making, particularly as regards the
early detection and prevention of prevalent adult-onset conditions,
such as cardiovascular diseases (CVDs). Additionally, the effective
communication and comprehension of this information can render it a
potent asset in personal health management (4). Active management, which typically
includes lipid-lowering treatment, is recommended for individuals
whose 10-year risk is predicted to be above a certain threshold
based on current risk assessment tools (5).
The European Society of Cardiology (ESC) and the
American College of Cardiology/American Heart Association (ACC/AHA)
regularly issue guidelines for the prevention of atherosclerotic
cardiovascular disease (ASCVD) (6). These guidelines integrate standard
ASCVD risk factors, such as blood pressure, total cholesterol and
age to stratify individuals based on their 10-year risk of
developing ASCVD. However, almost 40% of ASCVD cases arise in
individuals classified as low or intermediate risk based on a
clinical assessment. Thus, these guidelines are not recommended for
preventive interventions, while they have reduced discriminative
power among younger adults and older adults (7).
Accurately assessing the risk of CVD manifestation
is thus vital in order to enable early detection and prevention
strategies, and guide clinical decision-making, to prevent both
future cardiovascular events and the associated deaths. Currently
in clinical practice, risk prediction primarily relies on
demographic characteristics, lifestyle factors, health parameters
and family history (8). However,
although there is a consensus among cardiologists and other health
professionals that genetics contribute significantly to common
adult-onset CVD appearance, routine genetic screening is currently
absent from clinical care, when genetics are the earliest
measurable contributor that can be assessed to estimate lifetime
predisposition to CVD (9).
In familial aggregation studies, it has been
observed that monogenic risk variants, typically rare, contribute
to a small proportion of heritable cardiovascular disease risk
(10,11). This finding provides evidence of
the polygenic nature of cardiometabolic disease development,
wherein common genetic variants (i.e., present in at least 1% of
the population) considerably contribute to the overall risk
(12).
Novel genetic profiling methods have been developed
to estimate the probabilistic susceptibility of an individual to
disease, based on their polygenic risk score (PRS) (13). That is a weighted sum of the number
of risk alleles carried by an individual, where the risk alleles
and their weights are defined by their measured effects as detected
by genome-wide association studies (GWAS) (14).
The authors aimed to develop a novel PRS for a
series of CVDs, and to further improve its risk estimation through
the incorporation of chronological age, behavioral parameters and
phenotypic characteristics, which then enable the calculation of a
composite marker, the adjusted PRS (Adj-PRS). Thus, a novel in
vitro diagnostic was developed, termed iDNA Cardio Health
(15,16). The objective was to provide a
genetic profiling tool for medical practice and facilitate the
non-invasive estimation of predisposition, thus further
personalizing cardiovascular disease prevention.
The present study aimed to address the limitations
of traditional clinical metrics known to underestimate the risk of
developing CVD for certain individuals with a higher genetic
susceptibility, by employing the Adj-PRS designed to incorporate
genetic predisposition along with the usual clinic cardiovascular
risk prediction parameters.
Materials and methods
Assessment tools
The present study developed a novel PRS to estimate
the comprehensive risk for six common cardiovascular conditions,
comprising coronary artery disease, dilated cardiomyopathy,
hypertrophic cardiomyopathy, atrial fibrillation, ischemic stroke
and heart failure (16).
Specifically, three unique algorithms were designed to: i) Search
for statistically significant single nucleotide polymorphisms
(SNPs) associated with disease predisposition in major databases
with published GWAS (PubMed and GWAS catalog); ii) detect the
appropriate SNPs by assessing P-value, beta coefficient, odds ratio
and linkage disequilibrium metrics; and iii) calculate PRS for each
cardiovascular condition under investigation.
An integrated risk assessment tool was created
(Adj-PRS), as the authors employed the American Heart Association's
Life's Simple 7 (LS7) lifestyle and phenotypic characteristics
scoring system (17), comprising
diet, physical activity, smoking, body mass index (BMI), total
cholesterol, blood glucose levels and blood pressure, to evaluate
the current cardiovascular health status of each individual and
dynamically calculate the combined risk of developing CVD.
Patient data
Buccal swab samples were collected from 287 healthy
individuals with the use of the iDNA Cardio Health kit and DNA was
isolated and genotyped. DNA isolation and genotyping was performed
by Eurofins employing BeadChips technology with Illumina PCR.
Subsequently, the calculation of PRS and Adj-PRS followed and
descriptive statistical measures were conducted. Further on, the
overall impact associated with gender, BMI and smoking was
investigated pre- and post-adjustment for coronary artery disease
(CAD) and ischemic stroke (IS) risk predisposition. Lastly, in a
separate sample of healthy individuals (n=291), the Adj PRS was
cross-compared among individuals categorized by blood pressure,
body mass index, salt consumption, exercise level, and smoking
status, following the described procedure.
Of note, the present study used pre-existing
anonymized data that does not allow for the identification of
individuals. The present study did not involve any invasive
procedures or direct interaction with patients. The present study
only utilized pre-existing anonymized genetic data. These factors
place the research outside the scope of requiring formal ethical
review, as it poses no foreseeable risks to individuals.
Nevertheless, it was ensured that the study adhered to the highest
ethical standards available and was carried out in accordance with
the Declaration of Helsinki. All participants were able to provided
informed consent, allowing for the use of their anonymized genetic
data for research and statistical purposes. This also applies to
the questionnaire survey for which all participants were capable of
and provided informed consent, allowing for the use of their
answers for research and statistical purposes. Moreover, all
participants had the right to withdraw their data at any time,
ensuring their autonomy and control over their information.
Statistical analysis
Data are presented as the mean ± SD and statistical
analysis was performed using GraphPad Prism 10 software
(Dotmatics). Statistical significance was calculated using
non-parametric one-way ANOVA of normally distributed data followed
by Tukey's post hoc test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
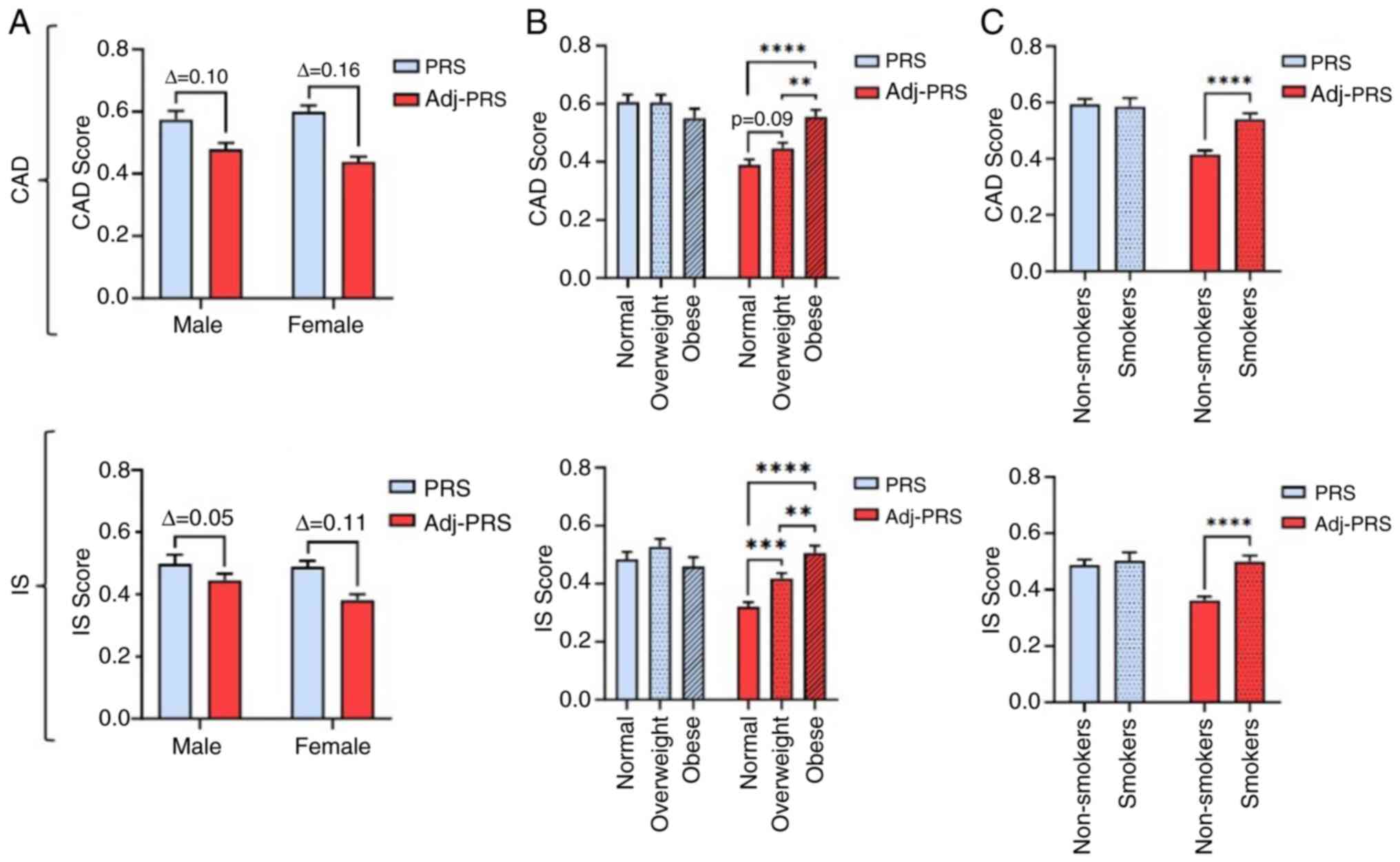
In the present study, CVD risk stratification was
examined in a randomly selected Greek population (n=287), employing
both PRS and Adj-PRS. In CAD, although no statistically significant
mean PRS differences were found between the sexes, a larger
reduction between PRS and Adj-PRS was observed in females,
putatively indicative of females adopting a healthier lifestyle and
thus an improved cardiovascular health status (Fig. 1A). As was expected for the mean
PRS, no marked differences were observed for the BMI and smoking
status categories, for both CAD and IS (Fig. 1B and C). However, for CAD, an increased BMI was
found to be significantly associated with a higher mean Adj-PRS in
overweight and obese individuals (Fig.
1B). Similarly, smokers were demonstrated to have a
significantly higher mean Adj- PRS comparing to non-smokers
(Fig. 1C). Similarly, for IS, a
larger reduction was observed in the mean Adj-PRS of females
compared to males (Fig. 1A), while
a significantly increased mean Adj-PRS was demonstrated both for
each increased BMI group (Fig. 1B)
and smokers (Fig. 1C).
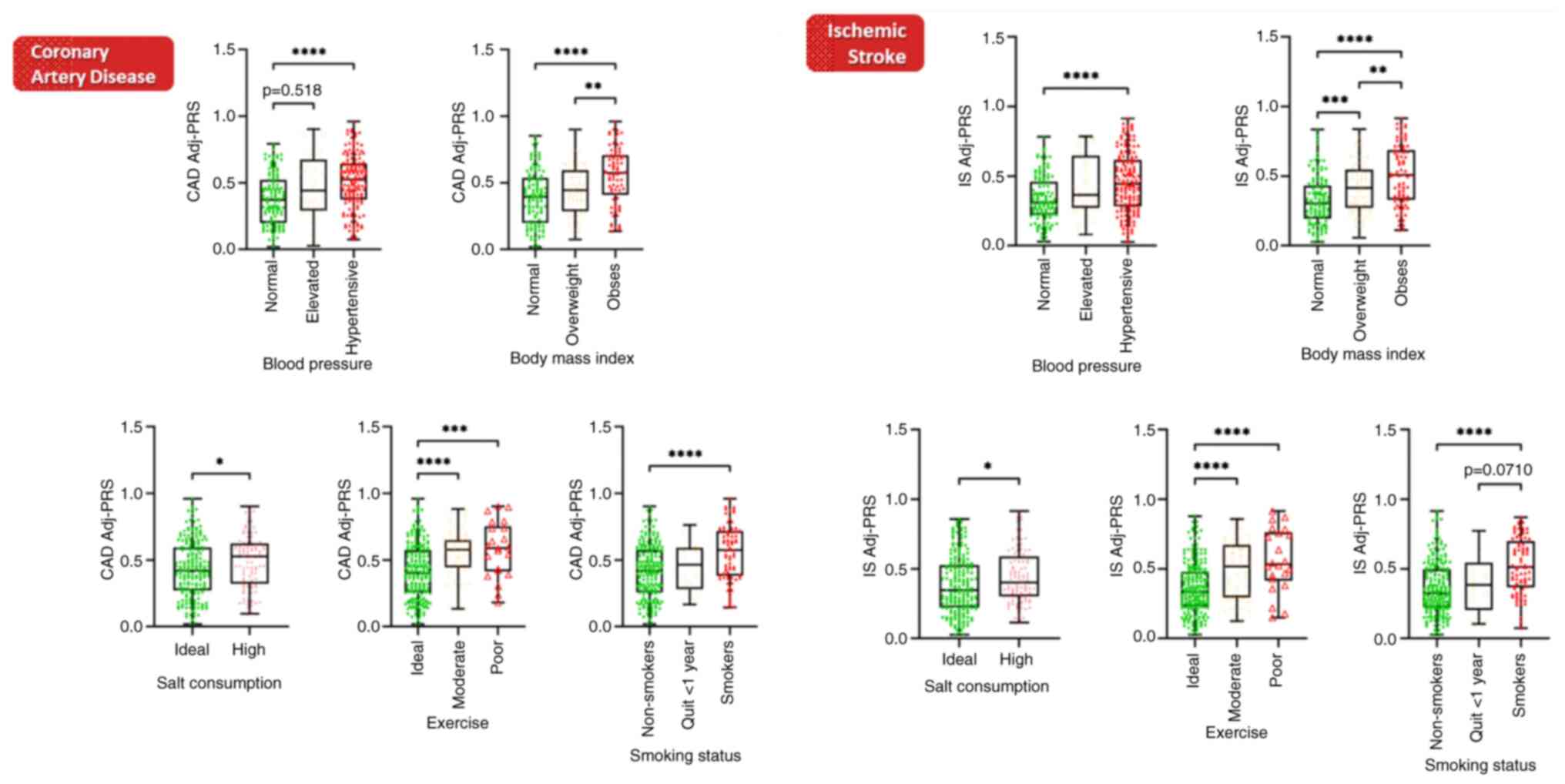
Further investigations, in a separate cohort of 291
healthy Greek individuals (Table
I), were carried out employing the Adj-PRS methodology to
dynamically fine tune risk prediction based on SNPs identified as
risk alleles, in combination with age and current cardiovascular
health status. Both for CAD and IS (Fig. 2), Adj-PRS was significantly
increased in hypertensive individuals, in overweight and obese
individuals, when the salt consumption was high (1,500 mg/day Na),
when the exercise level was recorded as moderate (150 min/week) or
poor (0 min/week), and in smokers. Notably, those who quit smoking
within the past year had improved their Adj-PRS, reaching levels of
significance in IS. Hence, Adj-PRS can reclassify underestimated
individuals from a marginal intermediate clinical risk to high
risk, when in the presence of underlying genetic predisposition
(i.e., high PRS).
 | Table IDemographic and clinical
characteristics of the 291 participants who completed the
questionnaire. |
Table I
Demographic and clinical
characteristics of the 291 participants who completed the
questionnaire.
| Characteristic | Total (n=291) |
|---|
| Age in years, mean
(SD) | 42.2 (10.8) |
| Sex, male, n (%) | 103 (35.3) |
| Overweight, BMI
>25 and <29.9, n (%) | 89 (30.5) |
| Obese, BMI >30, n
(%) | 82 (28.1) |
| Smoking, n (%) | 77 (26.4) |
| No exercise, n
(%) | 23 (7.9) |
| Cholesterol <200
mg/dl, n (%) | 210 (72.2) |
| Cholesterol ≥200 and
≤239 mg/dl, n (%) | 60 (20.6) |
| Cholesterol ≥240
mg/dl, n (%) | 21 (7.2) |
| Blood glucose ≤100
mm/dl, n (%) | 257 (88.3) |
| Blood glucose >100
and ≤125 mm/dl, n (%) | 29(10) |
| Blood glucose ≥126
mm/dl, n (%) | 5 (1.7) |
| Systolic blood
pressure <120 mm/Hg, n (%) | 149 (51.2) |
| Systolic blood
pressure ≥120 and <140 mm/Hg, n (%) | 131(45) |
| Systolic blood
pressure ≥140 mm/Hg, n (%) | 11 (3.8) |
Discussion
To the best of our knowledge, this is the first time
that a PRS has been employed to assess cardiovascular risk and its
interplay with environmental and lifestyle factors in a Greek
population. The findings of the present study suggest that while
PRS for CAD and IS is similarly distributed among females and
males, lifestyle choices which affect overall health, including
BMI, smoking, blood pressure, salt consumption and exercise
frequency, may stratify the risk, as assessed by the Adj-PRS. While
such evidence is currently lacking in the literature, similar
findings have been previously reported by Hasbani et al
(17), indicating that the adj-PRS
algorithm used herein may be able to refine risk as expected based
on environmental and lifestyle parameters. Nevertheless, future
research is essential to include performance metrics and provide
validation of our Adj PRS methodology in larger cohorts.
The evident underestimation of CVD risk (4,14)
through conventional clinical methodologies underscores the
necessity for more accurate assessment tools. The introduction of
the Adj-PRS presents a promising avenue for effectively
reclassifying individuals with marginal intermediate risk into a
high-risk category (18).
Identifying individuals with heightened genetic predisposition is
paramount, as noted in recent literature (5,8).
Individuals ranking in the top 5th percentile of a PRS exhibit a
3-fold elevated risk of developing CAD (12). Furthermore, disease onset tends to
occur 4.4 years earlier in individuals within the top 2.5% of their
PRS compared to those with an average PRS (7).
This innovative methodology harbors transformative
potential in the domain of CVD prevention, providing a
comprehensive framework encompassing screening, ongoing monitoring
and subsequent clinical interventions. These findings suggest that
PRS can be incorporated into current risk prediction frameworks to
calculate comprehensive risk scores for CVDs. This concept has key
implications for the wider use of genetic factors in the clinical
setting to refine risk stratification for a set of CVDs.
A growing body of evidence supports the integration
of PRS into existing risk assessment tools, as a means to enhance
predictive accuracy (19-21).
By incorporating genetic predisposition alongside traditional risk
factors, such as blood pressure, cholesterol levels and age, PRS
offers the potential to refine risk stratification and identify
individuals at heightened risk of cardiovascular events (19), thus improving the precision of
existing risk assessment models and better informing preventive
interventions.
Despite its potential, numerous challenges persist
in the broad adoption of this approach in the daily clinical
setting. These encompass concerns regarding the accessibility of
tests, clinician and patient education on results interpretation
and limitations as also reimbursement. Moreover, additional
research is required to clarify specific populations in whom
targeted genetic testing will impact management and future research
is required target the inclusion of PRSs within randomized
controlled trials (14). Ongoing
analyses have begun to address these issues, specifically noting
the lack of guidance in current guidelines for patients of high
polygenic risk (22,23).
The implementation of personalized medicine
strategies holds considerable promise in mitigating the burden of
CVDs and extending the human health span. By tailoring
interventions to individual genetic predispositions and lifestyle
factors, personalized medicine aims to optimize health outcomes and
enhance disease prevention efforts. The integration of both PRS and
adjusted PRS into clinical practice could proactively identify
at-risk individuals, thereby facilitating targeted interventions
and preventative measures.
Acknowledgements
Not applicable.
Funding
Funding: This study was privately funded by iDNA
Laboratories.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NP, EN and ES conceptualized the study. TF and AS
analyzed the data. ES, TF and NP wrote the manuscript. All authors
confirm the authenticity of all the raw data. All authors reviewed
and edited the manuscript, and all authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study used pre-existing anonymized data
that does not allow for the identification of individuals. The
present study did not involve any invasive procedures or direct
interaction with patients. The present study only utilized
pre-existing anonymized genetic data. These factors place the
research outside the scope of requiring formal ethical review, as
it poses no foreseeable risks to individuals. Nevertheless, it was
ensured that the study adhered to the highest ethical standards
available and was carried out in accordance with the Declaration of
Helsinki. All participants were able to provided informed consent,
allowing for the use of their anonymized genetic data for research
and statistical purposes. This also applies to the questionnaire
survey for which all participants were capable of and provided
informed consent, allowing for the use of their answers for
research and statistical purposes. Moreover, all participants had
the right to withdraw their data at any time, ensuring their
autonomy and control over their information.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Tsao CW, Aday AW, Almarzooq ZI, Alonso A,
Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP,
Commodore-Mensah Y, et al: Heart disease and stroke statistics-2022
update: A report from the American heart association. Circulation.
145:e153–e639. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: A report of the
American college of Cardiology/American Heart association task
force on clinical practice guidelines. Circulation. 140:e596–e646.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goff DC Jr, Lloyd-Jones DM, Bennett G,
Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy
D, O'Donnell CJ, et al: 2013 ACC/AHA guideline on the assessment of
cardiovascular risk: A report of the American College of
Cardiology/American Heart association task force on practice
guidelines. Circulation. 129:S49–S73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Torkamani A, Wineinger NE and Topol EJ:
The personal and clinical utility of polygenic risk scores. Nat Rev
Genet. 19:581–590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bebo A, Jarmul JA, Pletcher MJ, Hasbani
NR, Couper D, Nambi V, Ballantyne CM, Fornage M, Morrison AC, Avery
CL and de Vries PS: Coronary heart disease and ischemic stroke
polygenic risk scores and atherosclerotic cardiovascular disease in
a diverse, population-based cohort study. PLoS One.
18(e0285259)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Motamed N, Rabiee B, Perumal D, Poustchi
H, Miresmail SJ, Farahani B, Maadi M, Saeedian FS, Ajdarkosh H,
Khonsari MR, et al: Comparison of cardiovascular risk assessment
tools and their guidelines in evaluation of 10-year CVD risk and
preventive recommendations: A population based study. Int J
Cardiol. 228:52–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mars N, Koskela JT, Ripatti P, Kiiskinen
TTJ, Havulinna AS, Lindbohm JV, Ahola-Olli A, Kurki M, Karjalainen
J, Palta P, et al: Polygenic and clinical risk scores and their
impact on age at onset and prediction of cardiometabolic diseases
and common cancers. Nat Med. 26:549–557. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marston NA, Pirruccello JP, Melloni GEM,
Koyama S, Kamanu FK, Weng LC, Roselli C, Kamatani Y, Komuro I,
Aragam KG, et al: Predictive utility of a coronary artery disease
polygenic risk score in primary prevention. JAMA Cardiol.
8:130–137. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ashley EA, Hershberger RE, Caleshu C,
Ellinor PT, Garcia JG, Herrington DM, Ho CY, Johnson JA, Kittner
SJ, Macrae CA, et al: Genetics and cardiovascular disease: A policy
statement from the American Heart association. Circulation.
126:142–157. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aragam KG and Natarajan P: Polygenic
scores to assess atherosclerotic cardiovascular disease risk:
Clinical perspectives and basic implications. Circ Res.
126:1159–1177. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fahed AC, Wang M, Homburger JR, Patel AP,
Bick AG, Neben CL, Lai C, Brockman D, Philippakis A, Ellinor PT, et
al: Polygenic background modifies penetrance of monogenic variants
for tier 1 genomic conditions. Nat Commun. 11(3635)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khera AV, Chaffin M, Aragam KG, Haas ME,
Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT
and Kathiresan S: Genome-wide polygenic scores for common diseases
identify individuals with risk equivalent to monogenic mutations.
Nat Genet. 50:1219–1224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lambert SA, Abraham G and Inouye M:
Towards clinical utility of polygenic risk scores. Hum Mol Genet.
28:R133–R142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
O'Sullivan JW, Raghavan S, Marquez-Luna C,
Luzum JA, Damrauer SM, Ashley EA, O'Donnell CJ, Willer CJ and
Natarajan P: American Heart Association Council on Genomic and
Precision Medicine et al. Polygenic risk scores for
cardiovascular disease: A scientific statement from the American
heart association. Circulation. 146:e93–e118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Panagiotou N, Bersimis F, Fotis A, Sagonas
A, Ntoumou E, Salata E, Kallistratos M and Psychogios A: P273:
Adjusted polygenic score: Translation of a new concept for
cardiovascular disease prevention and management. Genetics in
Medicine Open. 1(100301)2023.
|
|
16
|
Panagiotou N and Chatziandreou E: MT5
adjusted polygenic risk score enables personalized cardiovascular
disease prevention and clinical management. Value in Health.
25:S378–S379. 2022.
|
|
17
|
Hasbani NR, Ligthart S, Brown MR, Heath
AS, Bebo A, Ashley KE, Boerwinkle E, Morrison AC, Folsom AR,
Aguilar D and de Vries PS: American heart association's life's
simple 7: Lifestyle recommendations, polygenic risk, and lifetime
risk of coronary heart disease. Circulation. 145:808–818.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mujwara D, Henno G, Vernon ST, Peng S,
Domenico PD, Schroeder B, Busby GB, Figtree GA and Bottà G:
Integrating a polygenic risk score for coronary artery disease as a
risk-enhancing factor in the pooled cohort equation: A
cost-effectiveness analysis study. J Am Heart Assoc.
11(e025236)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Riveros-Mckay F, Weale ME, Moore R, Selzam
S, Krapohl E, Sivley RM, Tarran WA, Sørensen P, Lachapelle AS,
Griffiths JA, et al: Integrated polygenic tool substantially
enhances coronary artery disease prediction. Circ Genom Precis Med.
14(e003304)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hindy G, Aragam KG, Ng K, Chaffin M, Lotta
LA and Baras A: Regeneron Genetics Center. Drake I, Orho-Melander
M, Melander O, et al: Genome-Wide polygenic score, clinical risk
factors, and long-term trajectories of coronary artery disease.
Arterioscler Thromb Vasc Biol. 40:2738–2746. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saadatagah S, Naderian M, Dikilitas O,
Hamed ME, Bangash H and Kullo IJ: Polygenic risk, rare variants,
and family history. JACC Adv. 2(100567)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Elliott J, Bodinier B, Bond TA,
Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC,
Elliott P and Tzoulaki I: predictive accuracy of a polygenic risk
score-enhanced prediction model vs a clinical risk score for
coronary artery disease. JAMA. 323:636–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Isgut M, Sun J, Quyyumi AA and Gibson G:
Highly elevated polygenic risk scores are better predictors of
myocardial infarction risk early in life than later. Genome Med.
13(13)2021.PubMed/NCBI View Article : Google Scholar
|